Introduction:
The early randomized controlled trials revealed no differences in survival between breast-conserving surgery (BCS) and mastectomy. However, breast cancer treatment has undergone changes, and the results of recent population-based registry studies suggest superior long-term survival after BCS. To explore the current evidence, a systematic review and meta-ana lysis of population-based observational studies from 2010 and onward was conducted.
Methods:
A literature search was conducted in the PubMed, Embase, and Cochrane databases to identify relevant literature. Keywords included “mastectomy,” “breast conserving surgery,” and “survival.” The identified studies were narratively reviewed and effect sizes (hazard ratios [HRs]) for overall (OS) and breast cancer-specific survival (BCSS) were combined with random-effects models.
Results:
A total of 30 reports were included in the review, and results from 25 studies were included in the meta-analyses. Compared with mastectomy, BCS was associated with better OS (HR = 1.34 [1.20–1.51]; N = 1,311,600) and BCSS (HR = 1.38 [1.29–1.47]; N = 494,267). Selected subgroups of patients, based on lymph node status, age (<50 years/≥50 years), and radiation therapy after mastectomy (±), all showed better overall survival after BCS. The number (range 4–12) and type of prognostic variables adjusted for in the survival analyses of the studies did not statistically significantly moderate the differences in survival between BCS and mastectomy.
Conclusions:
The combined findings from large population-based studies indicate that BCS is associated with survival benefit compared with mastectomy, suggesting that BCS be the recommended treatment of early breast cancer (T1-2N0-1M0) if a radical lumpectomy can be performed.
Keywords: breast conserving surgery, mastectomy, survival, breast cancer specific survival, breast conserving surgery vs. mastectomy, BCS
Mini-Abstract: A systematic review and meta-analysis of population-based observational studies comparing the survival after breast-conserving surgery and mastectomy in patients with breast cancer. The review comprises 30 papers from 2010 to 2021. More than 1.3 million patients are included in the meta-analyses.
Breast-conserving surgery (BCS) was introduced in the 1980s after randomized controlled trials (RCTs) had documented adequate local control and equivalent survival.1 Long-term follow-up studies confirmed the initial results.2–5 Although the long-term follow-up studies were published relatively recently in 2002,2,3 2008,4 and 2016,5 the comparable survival of BCS and mastectomy has generally been observed for patients treated several decades ago. Breast cancer treatments have since improved, and in the recent decades, BCS combined with radiation therapy (RT) to the residual breast has become the gold standard in the treatment of early breast cancer, used in approximately 7 of 10 patients.6
Although there are no new RCTs comparing BCS with mastectomy, several single, multicenter, and population-based registry studies have evaluated the outcome of the type of surgery in recent years.7–10 Even though the level of evidence from such studies is lower than from RCTs, the results provide important information about the treatment of unselected patients. Some population-based observational studies confirm that the outcome after BCS is at least as favorable as after mastectomy,11,12 but most studies suggest that long-term survival after BCS may even be superior to survival after mastectomy.7,13,14 Furthermore, it is unclear whether treatment-associated differences in survival may be moderated by differences between patients in demographic and disease characteristics, for example, age, lymph node involvement, and whether mastectomy is combined with RT or not. It could also be of interest to compare possible differences in survival across regions, as treatment standards may vary between, for example, North America and Europe. Increased knowledge about possible differences between BCS and mastectomy in both overall and breast cancer-specific survival and possible moderators of such differences is of urgent interest to clinicians.
To the best of our knowledge, no systematic review and meta-analysis has included the recently published, population-based studies. Our aim was, therefore, to fill this gap in our knowledge by conducting a systematic review and meta-analysis of the available population-based observational studies published from 2010 and onward.
METHODS
This systematic review and meta-analysis was preregistered with PROSPERO (reg. no. CRD42021272711) and is reported in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.15
Search Strategy and Selection Criteria
A systematic keyword-based search was conducted by the investigators in the Embase, PubMed, and Cochrane databases for the period from January 1, 2010, to June 30, 2021 (final search July 3, 2021) using a combination of MESH-terms and keywords referring to “mastectomy,” “breast conserving surgery,” and “survival.” No further restrictions were applied. The search was supplemented by hand-searching for potentially relevant articles in the reference lists of retrieved articles. Based on the population, intervention, comparator, outcome (PICO) approach,16 the studies were eligible for inclusion if (1) the population studied included patients with breast cancer, (2) a proportion of the sample had been treated with BCS, (3) were compared with patients treated with mastectomy, and (4) provided data on overall survival (OS) and breast cancer-specific survival (BCSS). Studies had to be population-based observational studies, and we excluded RCTs and hospital-level single- or multicenter studies. Only English-language reports in peer-reviewed journals were considered, and we excluded the “grey literature,” for example, conference abstracts and dissertations. Study selection was performed independently by 2 authors (PC, AB). Data extraction was performed independently by pairs of authors from a group of 3 (PC, AB, MM). Disagreements were resolved through negotiation. When studies presented results for the same or overlapping samples, the study with the largest number of patients was included in the meta-analyses.
Data Extraction
The data extracted were hazard ratios (HR) and their 95% confidence intervals for (a) OS and (b) BCSS of BCS versus mastectomy. If the original publications reported the results as mastectomy versus BCT, the reciprocal values were calculated (1/original value) and used in the meta-analyses. Additional data extracted were (c) the number of patients in each analysis, (d) the prognostic covariates adjusted for in the survival analysis, for example, tumor characteristics (T-stage, N-stage, localization, type, grade, hormone- and HER2-receptor status, lymphovascular invasion), patient characteristics (age, comorbidity), and treatments (RT, chemotherapy [CT], endocrine therapy [ET], anti-HER2 treatment), (e) whether patients treated with mastectomy had received RT or not, (f) any special restrictions of the sample (e.g., nodal status [N0, N+], age group, triple negative breast cancer [TNBC]), (g) follow-up time (months), and (h) region (North America, Europe, Asia, Oceania).
We tried to obtain additional information from a single study about the number of patients who had received radiation treatment.17 Unfortunately, the researchers were not able to supply the requested information.
Study Quality and Certainty of Evidence
The Newcastle-Ottawa Quality Assessment Scale (NOQAS) for cohort studies18 was used to evaluate the risk of bias in the included studies. The studies were rated independently by 2 authors (NR, MM), and disagreements resolved through negotiation. The robvis tool19 was used to provide a visual summary of the risk of bias. The certainty of available evidence was assessed with the GRADE method.20
Meta-analytical Strategy
Observational cohort studies were subjected to random effects meta-analysis to ascertain the pooled overall effect estimate and its precision. To aid the interpretation of the results, we conducted, as a supplement to the conventional frequentist meta-analysis, a Bayesian Model-Averaged meta-analysis.21
Pooling Effect Sizes
An inverse variance-weighted random-effects model considering the precision of each study was used in all analyses, with hazard ratios larger than 1.0 taken to indicate an effect in the direction of BCS associated with increased OS and BCSS. For studies reporting relevant data, results of comparisons in separate subgroups, for example, between BCS and mastectomy plus RT or BCS and mastectomy minus RT (Mx+RT and/or Mx-RT), in lymph node negative (N0) and lymph node positive (N+) patients, in younger (age <50 years) and older patients (age ≥50 years), and in North American and European studies, were analyzed separately. The individual and pooled hazard ratios are presented in forest plots. Sensitivity analyses were planned for the evaluation of the influence of possible outliers (defined as ± 2 standard deviations from the pooled estimate).
Heterogeneity
Heterogeneity was investigated using Q and I2 statistics.22 Heterogeneity tests aim at determining to which degree the variation in effect sizes reflects true differences (heterogeneity) or sampling error. The I2 value is an estimate of the between-study variance in a pooled effect estimate that is accounted for by heterogeneity of the effect sizes in the included studies and is assumed to be relatively unaffected by the number of studies.23 If the results indicated heterogeneity (I2 > 0.0), we calculated the 95% prediction interval, which estimates the expected range of true effects in 95% of future studies.24
Publication Bias
The possibility of publication bias was assessed using funnel plots and Egger’s test for pooled results of 10 or more effect sizes.25 If results were suggestive of possible publication bias, we planned to conduct sensitivity analyses by imputing the “missing studies” and calculating adjusted effect estimates using the Duval and Tweedie trim-and-fill method.26
Moderator Analyses
To explore possible sources of heterogeneity (I2 > 0.0), we used meta-regression (random-effects, maximum likelihood method) to examine the associations between the effect size and a number of possible categorical and continuous moderators, including (a) lymph node positive status (referent: lymph node negative), (b) older age (≥ 50) (referent: age < 50), (c) number of demographic, tumor-, and treatment-related factors adjusted for in the survival analysis, (d) studies conducted in North America (referent: Europe), (e) median follow-up time in months, and (f) high risk of bias (referent: low risk of bias). The frequentist analyses were performed using Comprehensive Meta-Analysis, version 3.27
Supplementary Bayesian Analysis
A supplementary Bayesian Model-Averaged meta-analysis21 of the associations between surgery type and survival examined the results of 4 models: (a) fixed-effect null hypothesis (fH0), (b) fixed-effect alternative hypothesis (fH1), (c) random-effects null hypothesis (rH0), and (d) random-effects alternative hypothesis (rH1). Bayesian Model-Averaged analysis thus avoids selecting either a fixed- or random-effects model and addresses 2 questions in light of the observed data: What is the plausibility that the overall effect is nonzero and is there between-study variability in the effect size? We chose an uninformed prior probability, that is, 25%, of each of the 4 models and 2,000 iterations. Concerning parameter distributions, we chose previously recommended defaults.21 We thus used a zero-centered Cauchy prior with a scale of 0.707 for the effect size. To have zero indicating the null effect, the hazard ratios and the upper and lower limits were log-transformed. For the between-study variation, we used an empirically informed prior distribution of nonzero between-study deviation estimates based on effect sizes from 705 meta-analyses published in Psychological Bulletin between 1990 and 2013.28 This distribution has been approximated by an Inverse-Gamma (1, 0.15) prior on the standard deviation (Tau).21 The supplementary Bayesian analyses were conducted with JASP, version 0.14.1.29
RESULTS
Study Characteristics
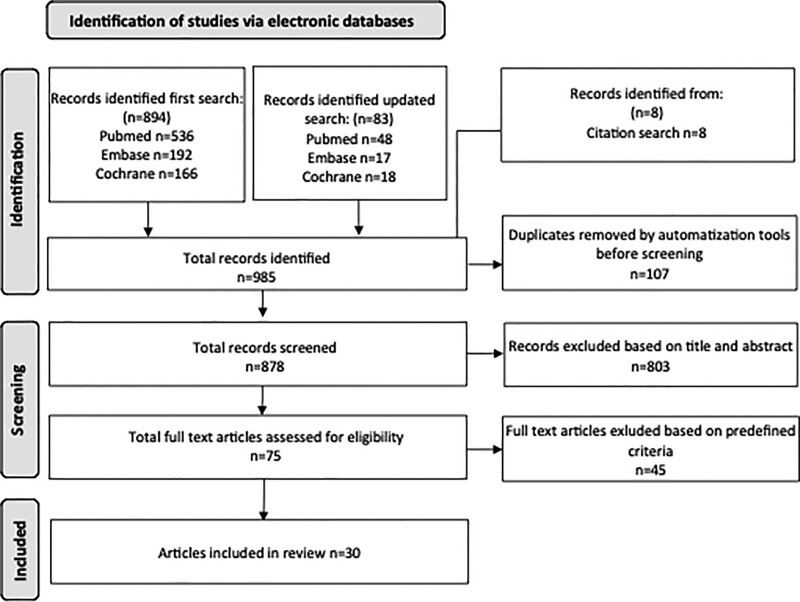
A total of 878 studies were found after removal of duplicates and screened for eligibility by title and abstract leaving 75 for full-text assessment. A total of 30 study reports were included in the review (Fig. 1), with 20 from North America, 7 from Europe, 2 from Asia, and 1 from Oceania. The studies reported survival data for patients treated between 1990 and 2014 (Table 1). One study9 reported on 2 separate populations. The population sizes ranged from 1,784 to 845,136 patients with a total of 2,343,878 (BCS, mastectomy) patients in the included studies. There is considerable overlap with several studies based on cohorts from the same registries. Thus, there were several publications from the US based on the database from the Surveillance, Epidemiology, and End Results (SEER) Program30–38 and the National Cancer Database (NCDB),6–21 and there were also several studies based on the national registries in Norway8,39,40 and the Netherlands.9,13,41
FIGURE 1.
Flowchart describing the literature search through the databases of PubMed, Embase, and Cochrane.
TABLE 1.
Description of the 30 Population-based Studies Included in the Review
| Author, year | Population, Country | Number of Patients | Included Patients | Tumor Characteristics | Treatment Data | Comorbidity | Follow-up Months | Survival Data | Stratified Analysis |
|---|---|---|---|---|---|---|---|---|---|
| Mahmood et al 201230 |
SEER, USA 1990–2009 |
14,764: B 6640, M 8124 |
T1-2N0-1M0 Age 20–39 |
TS (10 mm groups), LN, type, grade, ER, PgR |
RT | No | 68 mo | 5-yr OS: B 92.5% M 83.5% |
Age |
| Hwang et al 20137 |
California, USA 1990–2004 |
112,514: B 61,771, M 50,383 |
T1-2N0-1M0 | TS, LN, grade | RT | No | 110 mo | 10-yr OS: age <50/HR– B 81%, M 75% Age ≥50/HR+ B 92%, M 87% |
<50HR–, <50HR+, ≥50HR–, ≤50HR+ |
| Jeon et al 201342 | South Korea 1988–2006 |
3512 B 1951 M 1561 |
T1N0-1M0 Age ≤40 |
T-stage (T1a, T1b, T1c), N-stage (N0, N1) | CT ET |
No | 111 | 10-yr BCSS B 96.9%, M 94.9% |
N-stage (N0, N1) |
| Agarwal et al 201431 |
SEER, USA 1998–2009 |
132,149: B 92,671, M 34,999 (4479) |
T≤40 mm, N0-1M0 |
TS (0-2, 2-4), LN ER, PgR, grade |
No | No | ? | 5-yr BCSS: B 97%, M 94%, M+RT 90% 10-yr BCSS: B 95%, M 90%, M+RT 83% |
Mx-RT, Mx+RT |
| Hartmann-Johnsen et al 20158 |
Norway 2002–2010 |
13,015: B 8065, M 4950 |
T1-2N0-1M0 | TNM-stage, type, grade | No | No | 87–104 mo | 5-yr OS: B 95% M 80% |
Age < 50 |
| Fisher et al 201517 |
Alberta, Canada 2005–2011 |
14,939: B alone 805, B 5722, M 8412 (?) |
T1-4N0-3M0 | T-stage, N-stage, ER, PR | CT (adjuvant, neoadjuvant), ET; (RT) |
No | 50 mo | 5-yr OS: B alone 74% B 94% M 83% |
Stage (I, II, III), Mx-RT, Mx+RT |
| Hofvind et al 201539 |
Norway 2005–2011 |
9547: B 5906, M 3641 |
T1-4N0-3M0 | T-stage, N-stage, molecular subtypes, grade | CT, ET, RT | No | ? | 6-yr OS: B 97.1% M 89.3% |
No |
| Chen, Liu et al 201543 |
NCDB, USA 2004–2011 |
160,880: B 126,569, M 34,311 (8181) |
T1-2N0-3M0 | T-stage, N-stage, ER, PR, grade, localization, LVI | CT | Yes | 43.4 mo | 5-yr OS: B 93.2% M 83.5% |
N-stage (N0, N1, N2), Age (<50; ≥) and N-stage, CCI and N-stage, Mx-RT, Mx+RT |
| Ye et al 201532 |
SEER, USA 1998–2003 |
6671: B 3249, M 3422 (795) |
T1-3N0-1M0 Age < 40 |
Stage, T-stage (T1-T3), LN (N0, N1), ER | CT, ET | No | 111 MO | 10-yr OS: B 82% M 65% |
Age, stage, Mx-RT, Mx+RT |
| van Maaren et al 201613 |
Netherlands 2000–2004 |
37,207: B 21,734, M 15,473 |
T1-2N0-1M0 | Size, LN, ER, PR, grade, localization | CT, ET | No | 10-yr OS: B 82% M 65% |
T1N0, T1N1, T2N0, T2, N1 | |
| van Maaren et al 201641 |
Netherlands 2000–2004 |
3071: B 1055, M 2016 (2016) |
T1-2N2M0 | Size, LN, type, grade, ER | CT, ET | No | 126 MO | 10-yr OS: B 63% M 52% |
T1N2, T2N2 |
| Bleicher et al 201634 |
SEER, USA 1992–2009 |
5685: B 887, M 4798 |
T3N0-3M0 Age 65 |
Stage II-III, LN, type, grade, size, ER | CT, RT; NACT | Yes | 5-yr OS: B 51.8 M 49.6 |
||
| Chen, Wang et al 201733 |
SEER, USA 2010–2013 |
11,514: B 5469, M 6045 |
T1-4N0-3M0 TNBC |
Grade, stage, TNM | RT | No | 22 mo | 4-yr OS: B 91% M 82% |
Grade, stage, T-stage, N-stage, age |
| Hartmann-Johnsen et al 201740 |
Norway 1998–2009 |
6387: B 4449, M 1938 |
T1-2N0-1M0 | Size, LN, type, grade, ER-stratification in TNM | No | No | 72 mo | 10-yr OS: B 96% M 92% |
T1N0, T2N0, T1N1, T2N1 |
| Lagendijk et al* 2017(1)9 |
Netherlands 1998–2005 |
60,381: B 31,413, M 4950 |
T1-2N0-2M0 | Tumor localization, T-stage, N-stage, type, grade | CT, ET, RT | Yes | 144 mo | T1-2N0-1 10-yr OS: B 89% M 78% |
T1-2N0-1, T1-2N2, T1-2N0-1; age; ER/PgR; adjuvant therapy; comorbidity |
| Lagendijk et al* 2017(2)9 |
Netherlands 2006–2012 |
69,311: B 41,580, M 27,731 |
T1, 2N0-2M0 | Tumor localization, T-stage, N-stage, type, grade. HR, HER2, focality | CT, ET, RT | Yes | 84 mo | T1-2N0-1 10-yr OS: B 94% M 87% |
T1-2N0-1, T1-2N2, T1-2N0-1; age; ER/PgR; adjuvant therapy; comorbidity |
| Mogal et al 201736 |
SEER, USA 1998–2009 |
1784: B alone 270, B 918, M 596 |
T1-2N01M0 Age 70+ |
Size, LN | RT | Yes | BCS+RT 10-yr OS: B alone 63% B 73% M 63% |
||
| Grover et al 201737 |
SEER, USA 1995–2009 |
150,171: B 94,477 M 51,219 |
T1-2 (≤30 mm), N0M0 Age ≥50 |
Size (5 mm groups), ER, PgR | RT | No | 61/63 | 10-yr OS B 79.5 B (APBI) 78.8 M 67.4 |
|
| Christiansen et al 201810 |
DBCG, Denmark 1995–2012 |
58,331: B 26,958, M 27,143 (6556) |
T1-3N1-3M0 | Size, LN, type, grade, ER, HER2, LVI, focality | CT, ET, RT | Yes | 138 mo | 10-yr OS: B 82% M 57% |
N-stage (N0, N1, N2, N2; age, year of incl. CCl, adjuvant treatment, Mx-RT, Mx+RT |
| Lazow et al 201944 |
NCDB, USA 2004–2014 |
11,859: B 5074, M 6785 |
T1N0M0 Age <40 |
Grade, “subtypes” based on HR and HER2 | CT, ET, anti-HER2, RT, bilat mastectomy, reconstruction | Yes | 62 mo | ||
| Mazor et al 201911 |
NCDB, USA 2004–2011 |
30,324: B 3296, M 27,028 |
T3N0-3M0 | Size, LN, grade, type | CT, RT | Yes | ? | 5-yr OS: B 68% M 69% |
|
| Landercasper et al 201912 |
NCDB, USA 2004–2013 |
845,136: B 464,053, M 381,084 |
T0-4N0-3M0 | T-stage, N-stage, grade, type, ER | CT, RT | Yes | ? | 5-yr OS: B 88.6% M 83% |
Stage (I, II, III); HR (positive, negative) |
| Li et al 201935 |
SEER, USA 2010–2014 |
14,910: B 7381, M 7529 (562) |
T1-2N0M0 TNBC |
Size, grade, stage, type (HR, HER2) | CT, (RT) | No | ? | 5-yr OS: B 88.6% M 83% |
Age; size; grade; stage, Mx-RT, Mx+RT |
| Almahariq et al 202045 |
NCDB, USA 2006–2014 |
231,642: B 144,263, M 87,379 |
T1-2N0M0 | T-stage, ER, PgR, HER2, grade, LVl, no LN evaluated, localization, RS | CT, ET | Yes | B 49 M 47 |
5-yr OS: B 94.4% M 91.8% |
Age, RS |
| Wrubel et al 202046 |
NCDB, USA 2004-2014 |
202,376: B 101,188, M 101,188 |
T1-2N0-1M0 | Size, No pos. nodes, type, grade, ER, PgR, HER2, LVl | CT, ET | No | B 43 M 41 |
5-yr OS: B 92.9% M 89.7% |
|
| de Boniface et al 202114 |
NKBD, Sweden 2008–2017 |
48,986: B 29,367, M 19,616 (7206) |
T1-2N0-2M0 | T-stage (T1mi, T1a etc.), N-stage, ER, PR, HER2, type, grade, | RT, CT, ET, HER2, (NACT) | Yes | 75 | 5-yr OS B 95.1% M 84.5% M+R 86.0 |
T1N0, T1N1, T1N2, T2N0, T2N1, T2N2, Mx-RT, Mx+RT |
| Guo et al 202138 |
SEER, USA 2010–2015 |
13,986: B 6116, M 7146 (2663) |
T1-4N0-3M0 TNBC |
T-stage, N-stage, grade, laterality, tumor site (HR, HER2) | RT | No | ? | 5-yr OS B 87.9% M 79.6% M+R 65.5% |
Age, T-stage, N-stage, Mx-RT, Mx+RT |
| Zhang et al 202147 |
SEER, USA 2001–2016 |
2412: B 881 M 1531 |
T1-3N0-3M0 Metaplastic BC |
T-stage, N-stage, HR, grade | RT, CT | No | 73 | 5-yr OS 84.3 vs 62.5 | |
| Chu et al 202148 |
Lousianna, USA 2004–2016 |
18,260: B 9968, M 8292 |
T1-2N0-1M0 T3N0M0 | T-stage, N-stage, grade, type, ER, focality | (RT), CT, ET | Yes | 81 | 5-yr OS B 92.0% M 84.8% |
|
| Abrahimi et al 202149 |
New Zealand 2000–2015 |
6384: B 4608, M 1776 (269) |
T1-2 (size < 30 mm) N1-2M0 | T-stage (T1. T2), N-stage, ER, PgR, type, grade, LVI | RT, CT, ET | No | 106 | Mx-RT, Mx+RT | |
| Kim et al 202150 |
South Korea 1998–2012 |
45,770: B 28,623, M 17,147 |
T1-2N0-1M0 | T-stage, N-stage, grade, LVI, subtype (ER HER2) | No | No | 68 | 10-yr OS B 93.2% 87.9% |
*Lagendijk et al includes 2 distinct periods and is therefore included as 2 separate studies. Numbers in parentheses represent subgroups of patients having mastectomy plus radiation therapy included in separate comparison with BCS plus radiation therapy.
B, breast-conserving surgery + RT; BCSS, breast cancer-specific survival; CT, chemotherapy; DBCG, Database of Danish Breast Cancer Group; ER, estrogen receptor; ET, endocrine therapy; HR, hormone receptor status; LN, lymph node status; LVI, lymphovascular invasion; M, Mastectomy; M+R, mastectomy + RT; MO, months; NCDB, National Center Data Base; OS, overall survival; PgR, progesteron receptor; RS, recurrence score; RT, radiation therapy; SEER, Database of Surveillance, Epidemiology, and End Results Program; TNBC, triple negative breast cancer; Type, histological type.
The tumor characteristics varied considerable between studies. All included information on tumor size and nodal status, but information on hormone receptor status was lacking in 7 reports7–9,11,33,36,42 and HER2-status was only available for 10 studies.9,10,14,35,38,39,44–46,50 Data on systemic treatment were sparse and completely lacking in 10 studies,7,8,30,31,35–38,40,50 and HER2-directed treatment was only reported in a single study.14
Follow-up times ranged from 22 to 144 months and was not reported in 9 studies (Table 1). Five-year OS was reported in 15 studies. Most studies reported a better 5-year OS after BCS compared with mastectomy (range 2–22%). One study11 reported on a cohort restricted to tumors larger than 5 cm, where the OS was similar in the two groups (1% improved survival after mastectomy). Likewise, the 10-year OS was better after BCS (range 4–25%) in the 10 studies reporting on this outcome.
Three SEER-studies focused on triple negative breast cancer (TNBC) alone. Chen, Wang et al33 looked at a population treated 2010–13, and Li et al35 reported on a material from 2010 to 2014. These 2 materials have some overlap, but Li et al restricted the material to node negative patients (N = 14,910), whereas Chen, Wang et al also included N1–4 patients (N = 11,514). Very recently, Guo et al published on a SEER population from 2010 to 2015 (N = 13,262) overlapping both the previous studies. All 3 studies showed a better outcome after BCS with reported differences in OS: 9% (4 years), 5% (5 years), and 8% (5 years), respectively (Table 1). One further study reported on metaplastic breast cancer (N = 2,412) and described a remarkable 22% better 5-year OS after BCS.47
When assessing the risk of bias using NOQAS (Figure S1, see http://links.lww.com/AOSO/A171), no studies were considered of high risk of bias. In 9 studies, the risk was found unclear, mainly because the studies lacked treatment data. In 22 studies, the risk of bias was considered low. The full assessment is described in the supplementary materials (Table S1, see http://links.lww.com/AOSO/A171).
The number of prognostic demographic, disease, and treatment-related variables adjusted for in the survival analyses ranged from 436 to 12.9,10 Of the studies included in the overall analyses, all 16 had adjusted for tumor stage, lymph node status, and age, and 14 studies had adjusted for tumor grade. Fewer studies had adjusted for factors such as hormone receptors (K = 11), HER2 status (K = 6), comorbidity (K = 8), CT (K = 10), and ET (K = 7). Older studies had generally adjusted for fewer variables than more recent studies (r = 0.59; P = 0.016). An overview of the covariates adjusted for in the analyses is provided in the supplementary materials (Table S2, see http://links.lww.com/AOSO/A171).
Associations between surgery type and overall survival
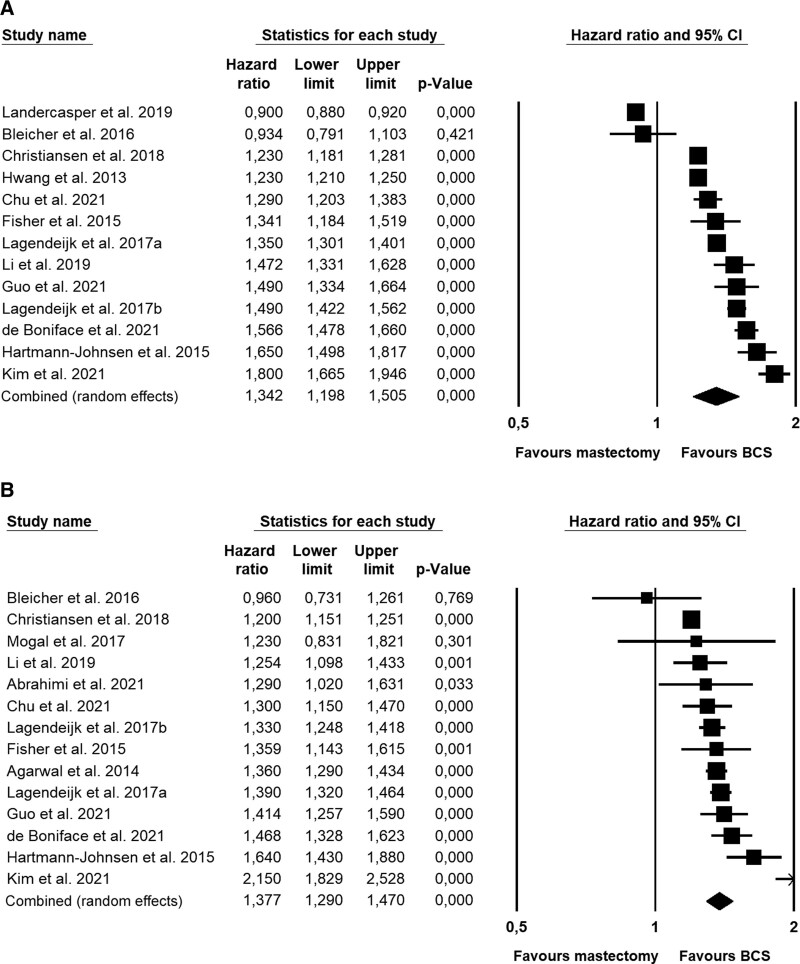
Thirteen independent studies with a total of 1,311,600 breast cancer patients provided data on OS with a median follow-up of 75 months. The patients who had received BCS had better overall survival than patients who had been treated with mastectomy (Fig. 2A, Table 2), with the difference corresponding to a HR of 1.34. The results of the individual studies varied considerably and were highly heterogeneous, that is, with almost all the variation (98.9%) estimated to be due to systematic differences in effect sizes rather than random error. The considerable variation in effect sizes explains the broad 95% prediction interval, signaling that in 95% of future similar studies, the hazard ratios are expected to fall between 0.84 and 2.15. There was no evidence of publication bias (Egger’s test, P = 0.120).
FIGURE 2.
Forest plots showing meta-analysis of survival data in population-based independent cohorts of breast cancer patients. (A) Overall survival. The 13 studies included 1,311,600 patients. (B) Breast cancer-specific survival. Fourteen studies with 494,267 patients.
TABLE 2.
OS and BCSS of BCS Compared With Mx—Meta-analyses of Results of Studies Using Data From Population-based Samples
| BCS vs Mx | K* | Heterogeneity | Pooled effect size | 95% PI # | |||||
|---|---|---|---|---|---|---|---|---|---|
| Q† | P | I 2 ‡ | Tau2 § | HR∥ | 95% CI | P ¶ | |||
| OS** | 13 | 1045.7 | <0.001 | 98.9 | 0.042 | 1.34 | 1.20–1.51 | <0.001 | 0.84–2.15 |
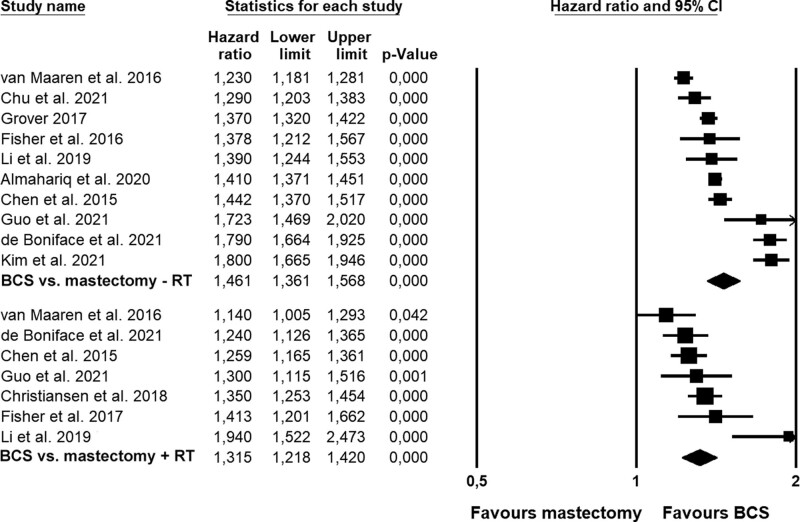
| OS, Mx– RT ** †† ‡‡ | 10 | 137.4 | <0.001 | 93.4 | 0.011 | 1.46 | 1.36–1.57 | <0.001 | 1.13–1.89 |
| OS, Mx+ RT ** †† ‡‡ | 7 | 18.2 | 0.006 | 67.0 | 0.007 | 1.32 | 1.22–1.42 | <0.001 | 1.04–1.67 |
| OS, N0§§ | 10 | 45.6 | <0.001 | 80.3 | 0.004 | 1.39 | 1.33–1.46 | <0.001 | 1.19–1.63 |
| OS, N+∥∥ | 7 | 39.5 | <0.001 | 84.8 | 0.011 | 1.38 | 1.26–1.50 | <0.001 | 1.02–1.85 |
| OS, age <50 | 6 | 42.1 | <0.001 | 88.1 | 0.048 | 1.27 | 1.05–1.54 | 0.015 | 0.65–2.47 |
| OS, age ≥50 | 5 | 68.1 | <0.001 | 94.1 | 0.009 | 1.40 | 1.28–1.54 | <0.001 | 1.00–1.97 |
| OS, North America | 7 | 581.6 | <0.001 | 99.0 | 0.045 | 1.22 | 1.04–1.43 | 0.017 | 0.68–2.19 |
| OS, Europe | 5 | 73.6 | <0.001 | 94.6 | 0.011 | 1.44 | 1.31–1.59 | <0.001 | 0.99–2.09 |
| OS, TNBC | 2 | 0.0 | 0.873 | 00 | 0.0 | 1.48 | 1.37–1.60 | <0.001 | NA |
| OS, Low study quality (score 0–6) | 3 | 219.4 | <0.001 | 96.4 | 0.049 | 1.04 | 0.80–1.35 | 0.773 | 0.04–27.7 |
| OS, High study quality (score 7–10) | 10 | 229.5 | <0.001 | 96.1 | 0.014 | 1.44 | 1.33–1.55 | <0.001 | 1.08–1.92 |
| Breast cancer–specific survival (BCSS) ** | 14 | 81.1 | <0.001 | 84.0 | 0.011 | 1.38 | 1.29–1.47 | <0.001 | 1.09–1.75 |
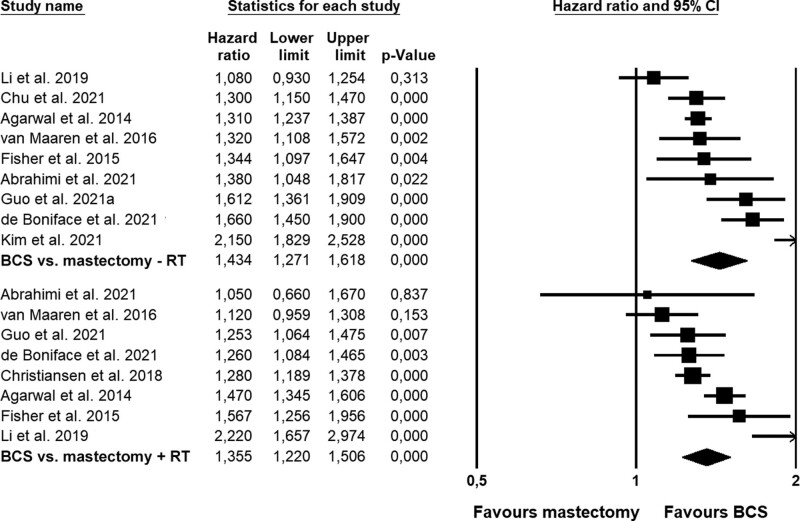
| BCSS, Mx–RT †† ‡‡ | 9 | 54.0 | <0.001 | 85.2 | 0.027 | 1.43 | 1.27–1.62 | <0.001 | 0.94–2.16 |
| BCSS, Mx + RT†† ‡‡ | 8 | 26.5 | <0.001 | 73.6 | 0.014 | 1.36 | 1.22–1.51 | <0.001 | 0.99–1.87 |
| BCSS, N0§§ | 8 | 15.1 | 0.034 | 53.8 | 0.004 | 1.30 | 1.21–1.39 | <0.001 | 1.09–1.55 |
| BCSS, N+∥∥ | 7 | 10.1 | 0.120 | 40.6 | 0.002 | 1.31 | 1.23–1.39 | <0.001 | 1.13–1.52 |
| BCSS, age <50 | 5 | 9.8 | 0.044 | 59.1 | 0.006 | 1.16 | 1.05–1.28 | 0.002 | 0.75–1.79 |
| BCSS, age ≥50 | 5 | 21.1 | <0.001 | 81.0 | 0.009 | 1.24 | 1.11–1.38 | <0.001 | 0.88–1.75 |
| BCSS, North America | 7 | 8.2 | 0.222 | 27.1 | 0.002 | 1.32 | 1.24–1.40 | <0.001 | 1.15–1.52 |
| BCSS, Europe | 5 | 37.6 | <0.001 | 89.4 | 0.009 | 1.38 | 1.26–1.51 | <0.001 | 0.99–1.93 |
| OS, Low study quality (score 0–6) | 3 | 4.4 | 0.018 | 55.0 | 0.023 | 1.19 | 0.94–1.49 | 0.145 | 0.10–13.33 |
| OS, High study quality (score 7–10) | 11 | 75.3 | <0.001 | 86.7 | 0.011 | 1.40 | 1.31–1.50 | <0.001 | 1.09–1.80 |
*K = number of studies/independent samples in the analysis.
†Q statistic: P values <0.1 taken to suggest heterogeneity.
‡I2 statistic: the proportion of the variance explained by differences in effect sizes beyond random error (heterogeneity).
§Tau2: the between-study variance in effect sizes.
∥Pooled effect size (random-effects model): HR.
¶P values (2-tailed): Statistically significant (P < 0.05) in bold. HR > 1 indicates an association in the hypothesized direction, that is, BCS is associated with improved survival compared with mastectomy.
#95% PI, that is, the interval in which 95% of future observations from the same family of studies will fall, given the observed data, calculated for heterogeneous ESs (I2 > 0).
**For pooled estimates from K ≥ 10, the possibility of publication bias was explored with funnel plots and Egger’s test. No indications of publication bias were found (Egger’s test > 0.05).
††Mx ± RT = Mastectomy with and without radiotherapy.
‡‡Number of studies/independent samples for Mx ± RT do not add up with overall OS and BCSS analyses due to omission of overlapping samples to ensure independence.
§§N0 = lymph node-negative breast cancer.
∥∥N+ = lymph node-positive breast cancer.
95% PI, 95% prediction interval; BCS, breast-conserving surgery; HR, hazard ratio; Mx, mastectomy; OS, overall survival.
The overall findings were supported by the supplementary Bayesian meta-analysis, which provided very strong evidence for a nonzero difference in overall survival between BCS and mastectomy in favor of BCS corresponding to a Bayes Factor (BF)51 of 179, that is, indicating that the alternative hypothesis is 179 times more likely than the null hypothesis. Likewise, the Bayesian analysis provided extremely strong evidence concerning heterogeneity of the effect sizes with a BF for heterogeneity of 5.72e+210. The combined HR found in the Bayesian meta-analysis was 1.34, which is identical to the effect found with the frequentist approach (1.34). The credible interval, that is, the interval that the true effect sizes are assumed to lie within with 95% probability, was 1.17 to 1.51 and similar to the confidence interval (1.20–1.51).
In subgroup analyses (Table 2), the difference in OS in favor of BCS was larger when compared to mastectomy without RT (HR = 1.46; Fig. 3) than when compared with mastectomy with RT (HR = 1.32). Larger differences were also found for older patient samples (≥50 year; Figure S2, see http://links.lww.com/AOSO/A171), European samples, and samples in high-quality studies. The magnitude of the difference did not appear to be associated with lymph node status (Figure S3, see http://links.lww.com/AOSO/A171). When using meta-regression (Table 3) to explore the moderating influence of lymph node status, age group, region, number of relevant prognostic factors adjusted for in the analysis, time-to follow-up, and study quality, only time-to-follow-up (P = 0.027) and study quality (P < 0.001) emerged as a statistically significant moderators, with shorter time-to follow-up and high study quality being associated with greater differences in OS in the favor of BCS, and the models explaining 40% and 53% of the variation in hazard ratios, respectively. When examining the role of adjusting for individual prognostic variables, which exhibited sufficient variation (i.e., tumor type, hormone receptor status, HER2 status, ET, and comorbidity), no results reached statistical significance (data not shown). As the samples overlap with the same patients receiving BCS being compared to the 2 groups receiving mastectomy, we were unable to statistically compare BCS versus mastectomy with and without RT.
FIGURE 3.
Forest plots showing comparisons in overall survival between BCS and mastectomy without (–RT) or with radiation therapy (+RT). BCS indicates breast-conserving surgery; RT, radiation therapy.
TABLE 3.
Results of Meta-regression-based Analyses of Possible Categorical and Continuous Moderators of the Difference in Overall and Breast Cancer-specific Survival Between BCS and Mx
| Moderator | Outcome | K | Slope | 95% CI | P | R 2 |
|---|---|---|---|---|---|---|
| N+ (referent: N0)* | OS | 17 | –0.01 | –0.109 to 0.089 | 0.840 | 0.01 |
| BCSS | 15 | 0.013 | –0.039 to 0.065 | 0.623 | 0.00 | |
| Age ≥ 50 (referent: age < 50) | OS | 11 | 0.082 | –0.192 to 0.357 | 0.556 | 0.07 |
| BCSS | 10 | 0.056 | –0.098 to 0.210 | 0.478 | 0.19 | |
| Number of covariates adjusted for (range: 5–12) | OS | 13 | –0.011 | –0.054 to 0.032 | 0.616 | 0.02 |
| BCSS | 14 | –0.023 | –0.049 to 0.003 | 0.080 | 0.32 | |
| North America (referent: Europe) | OS | 12 | –0.173 | –0.351 to 0.005 | 0.057 | 0.25 |
| BCSS | 12 | –0.046 | –0.149 to 0.057 | 0.379 | 0.00 | |
| Time-to-FU (months) (range: 43–138 months) | OS | 9 | –0.002 | –0.005 to –0.000 | 0.027 | 0.40 |
| BCSS | 10 | –0.002 | –0.005 to 0.001 | 0.186 | 0.26 | |
| High study quality (referent: low) | OS | 13 | 0.332 | 0.152 to 0.512 | <0.001 | 0.53 |
| BCSS | 14 | 0.166 | –0.056 to 0.388 | 0.142 | 0.10 |
* N0/N+ = lymph node-negative and lymph node-positive breast cancer.
Moderators with significant influence on survival are marked with bold P values.
BCS indicates breast-conserving surgery; BCSS, breast cancer-specific survival; Mx, mastectomy; Mx ± RT, mastectomy with or without radiotherapy; OS, overall survival.
Associations Between Surgery Type and Breast Cancer-specific Survival
Fourteen independent studies with a total of 494,267 breast cancer patients provided data on BCSS across a median follow-up of 78 months. The patients who had received BCS had better BCSS compared with patients who were treated with mastectomy, with the difference corresponding to a HR of 1.38 (Fig. 2B, Table 2). The results of the individual studies were highly heterogeneous, with 84.0% of the variation estimated to be due to systematic between-study differences. The 95% prediction interval for BCSS was 1.09 to 1.75, and thus narrower than for OS. The results were not suggestive of publication bias (Egger’s test: P = 0.29).
Bayesian meta-analysis provided extremely strong evidence for a nonzero difference in BCSS between BCS and mastectomy in favor of BCS corresponding to a BF of 4010, that is, indicating the alternative hypothesis to be 4010 times more likely than the null hypothesis. The Bayesian analysis also provided extremely strong evidence concerning heterogeneity of the effect sizes with a BF for heterogeneity of 2.43e+09. The combined HR found in the Bayesian meta-analysis was 1.38, similar to the effect found with the frequentist approach (1.38). The credible interval was 1.25 to 1.51 and only slightly broader than the confidence interval (1.29–1.48).
As seen for OS, the differences in BCSS in favor of BCS were somewhat larger when compared to mastectomy without RT (HR = 1.43) than when compared with mastectomy and RT (HR = 1.36; Fig. 4). Likewise, larger differences were found for older patient samples (≥50 year; Figure S4, see http://links.lww.com/AOSO/A171), European samples, and samples in high-quality studies, whereas the difference did not appear to be associated with lymph node status (Table 2; Figure S5, see http://links.lww.com/AOSO/A171). When exploring the effects of moderators with meta-regression (Table 3), no associations reached statistical significance. This was also the case, when examining the role of adjusting for the individual prognostic covariates, which exhibited sufficient variation (i.e., tumor type, hormone receptor status, HER2 status, ET, and comorbidity—data not shown). Due to partly overlapping samples, we were unable to statistically compare BCS versus mastectomy with and without radiotherapy.
FIGURE 4.
Forest plots showing comparisons in breast cancer-specific survival between BCS and mastectomy without (–RT) or with radiation therapy (+RT). BCS indicates breast-conserving surgery; RT, radiation therapy.
Propensity score matching (PSM) or adjustment
Five studies used propensity score matching. 12,31,34,45,46 Wrubel et al performed PSM in a 1:1 fashion leading to 2 groups of 101.118 subjects each. Five-year OS was significantly better after BCS than after mastectomy (92.9% vs 89.7%, P < 0.001). Hazard ratios were not calculated. Landercasper et al12 included a sub-analysis based on PSM 1:1 with 124.139 patients in each group and reported an overall HR = 0.98 (0.96–0.99) in favor of mastectomy. Further stratification by stage gave the following results: stage I HR = 0.78 (0.76–0.81); stage II HR = 1.02 (0.99–1.05), and stage III HR = 1.20 (1.16–1.25), with mastectomy leading to a more favorable outcome in early-stage breast cancer in contrast to in more advanced disease stages. Agarwal et al31 presented a Cox multivariate analysis on 2 groups of patients with a similar likelihood for a given treatment based on propensity scores. The resulting hazard ratios were in agreement with those from the general multivariate model depicted in Figure 2 (BCS vs mastectomy alone: HR = 1.23 [1.25–1.39]; BCS vs mastectomy + RT: HR = 1.90 [1.73–2.08]). Almahariq et al45 and Bleicher et al34 also used propensity score adjustment in their multivariate model, which are included in the present meta-analyses.
DISCUSSION
The results of the present systematic review and meta-analysis of recently published populations-based observational cohort studies of more than 1.3 million breast cancer patients provide compelling documentation that early breast cancer patients treated with BCS and RT, have, on average, 34% better OS and 38% better BCSS than patients treated with mastectomy. The results of the conventional frequentist meta-analyses were supported by the Bayesian analyses, which indicated strong support for nonzero differences in favor of BCS. The results in favor of BCS hold true regardless of whether mastectomy was combined with RT or not, and for all analyzed subgroups: lymph node negative, lymph node positive, those younger than 50 years, those 50 years or older, TNBC patients, and patients treated in Europe as well as in North America.
There are currently no agreed upon explanations for the observed differences in survival between BCS and mastectomy in breast cancer and for why the recent observational studies report a more favorable outcome after BCS in contrast to the earlier randomized trials.5 First, it is important to note that in the studies included in the present review, it cannot be ruled out that patients who receive BCS differ from those treated with mastectomy. Due to the nature of the observational data, we do not know the many different reasons for the actual choice made in each of the individual clinical situations. When interpreting the results, it is therefore important to note that all the included studies had adjusted for a number of prognostic and treatment variables. However, the number of covariates varied considerably between studies, ranging from 5 to 12. Almost all studies had adjusted for age, tumor stage, tumor grade, and nodal stage, and several had adjusted for hormone receptor status, comorbidity, RT, and CT. Still, relatively few had adjusted for HER2 status and ET, almost none for lymphovascular invasion and focality, and no studies had adjusted for anti-HER2 treatment. When we used meta-regression to explore whether the difference found between BCS and mastectomy was influenced by the number of prognostic covariates adjusted for in the analyses, this did not appear to be the case for neither OS nor BCSS. When examining the possible role of adjustment for a number of individual prognostic factors, including comorbidity, tumor type, hormone receptor status, HER2-status, ET, none of the associations reached statistical significance. Taken together, the data suggest that the differences observed in survival are not sufficiently explained by differences in prognostic characteristics.
A possible explanation for the difference in outcome between the RCTs and the newer observational studies could be changes in treatment over time which have led to better loco-regional control after BCS and RT. In the early study by Veronesi et al,2 local recurrence was observed in 8.5% after BCS in contrast to 2.3% after mastectomy, and such results were typical for that period. Since then, the occurrence of local recurrence has been halved,52 and today, the incidences of local recurrence after BCS is around 2% over 5 years.53 According to the 2011 EBCTCG meta-analysis,54 the lower local recurrence rates after BCS should translate into a better survival. Although the mastectomy group probably also will benefit from fever local recurrences, the absolute numbers are lower, and the impact on survival will be less pronounced. Thus, the development has favored the outcome after BCS in comparison with mastectomy. Second, there have also been speculations that RT makes the difference,9,55 but this was not supported in a stratified analysis included in the Danish study.10 In a subgroup analysis of patients with macrometastases, who all had loco-regional RT irrespective of type of surgery, better relative survival (28%) was still found after BCS compared with mastectomy. A third explanation could be that the surgical trauma is more marked after mastectomy resulting in more pronounced immuno-suppression, which, in turn, may promote growth of residual local tumor cells, circulating tumor cells, and micrometastases.56 Finally, we would also like to draw attention to the so-called abscopal effect,57 which has been extensively discussed in relation to breast cancer.58 This proposed mechanism involves RT and the immune system. RT to the residual breast may destroy small foci of cancer cells left behind after the breast-conserving procedure. RT will not only induce immunosuppressive effects but also, during the process of destroying these cancer cells, mobilize host immune effector mechanisms involving pro-immunogenic effects leading to the inactivation and destruction of remaining tumor cells and micrometastases in the body. Such a mechanism could perhaps explain a proportion of the difference in outcome between mastectomy and lumpectomy, even when mastectomy is combined with RT, as remaining tumor foci, apart from within lymph nodes, would be very rare after mastectomy.
The results for TNBC should be interpreted with caution. First, the prevailing data do not include information on BRCA-mutations. Second, in the study which only included patients under 40,32 where the proportion of BRCA1-positive is expected to be significant, the benefit seems smaller. Third, the finding of a less favorable outcome after mastectomy and RT in one study38 indicates that the groups in the comparison are not congruent, even in the adjusted comparison. On the other hand, a previous meta-analysis from 2015 by Vila et al,59 restricted to patients younger than 40 years, came to the conclusion that BCS was at least as safe as mastectomy (HR = 0.90 [0.81–1.00]). Very recently, a meta-analysis confined to BRCA1 and BRCA2,60 concluded that survival outcomes following BCS is comparable to mastectomy in BRCA carriers. However, only few studies with a small number of patients were included in the meta-analysis, and as the study also reports a more than 400% increase in local recurrence after BCS, the results are difficult to interpret. Taken together, more studies including information on BRCA-status are needed before conclusions can be drawn regarding the safety of BCS in TNBC and in the very young patients.
Breast-conserving therapy is not always applicable,61 and it is argued that breast cancer in the very young women (<35 years) may have a survival benefit after mastectomy,61 and that could be related to a higher risk of loco-regional recurrence after BCS in this age-group.53 The recommendations to these patients are therefore more complicated. Breast cancer in combination with extensive ductal carcinoma in situ (DCIS), particularly in women under 40 yrs., may also require mastectomy.61 BCS is also not an option if RT cannot be offered. The present meta-analysis and the currently available literature do not indicate that BCS should be omitted because of nodal status N0-1, but when it comes to more advanced nodal stages, it is more difficult to come to a uniform conclusion.10,43 For T3 tumors, the sparse data indicate that there could be a small benefit in survival of mastectomy compared to BCS.11,34 On the other hand, if clear margins and an acceptable cosmetic result can be achieved, multifocal and multicentric breast cancer as well as central location of the cancer in the breast should not be considered as an indication for mastectomy instead of BCS.61
All but one of the reviewed population-based cohort studies point in the same direction. In contrast, Landercasper et al12 found results that stand out in several ways. First, in contrast to all remaining studies, this study showed a more favorable outcome after mastectomy. Second, it was found in the propensity matched cohorts that the benefit of mastectomy was most pronounced among patients with an early tumor stage. Third, a considerable difference was observed between the unadjusted and the adjusted hazard ratios which changed from 0.6 to 1.1. Fourth, there is a pronounced difference in the size of the population between this study and other comparable studies from NCDB. Chen et al43 report on a cohort from 2004 to 2011 that included only T1-2N0-1 patients, which must be the bulk of patients with breast cancer from that period. Mazor et al11 report on the population of patients with T3N0-3 from the same period (2004–2011), and together these 2 studies thus report on a total population of 180,309 T1-3N0-3M0 patients. Although they included a two year longer observational period, it is unclear how Landercasper et al could include a population from NCDB that is more than four times larger (N = 845,136). Furthermore, the proportion of patients treated with BCS differs considerably between these studies. Landercasper et al report 54.9% receiving BCS, whereas Chen et al and Mazor et al together report 71.8% patients treated with BCS. There is also considerable overlap between the studies of Landercasper et al12 and the studies by Almahariq et al,45 and Wrubel et al,46 who report on NCDB populations treated between 2006 and 2014 and between 2006 and 2015, respectively. Both studies were restricted to T1-2M0. One was further restricted to N0,45 and the other46 included N0-1. The populations contained 231,642 and 431,899 patients, respectively, and among those 62.3% and 70.0% were treated by BCS. Compared with these studies, the Landercasper study includes a population almost twice the size the number reported by Wrubel et al. Neither Landercasper et al12 nor the two most recently published papers45,46 provide any information on the considerable differences in population sizes and proportions of BCS between the NCDB studies.11,43 Although the results reported by Landercasper et al are based on by far the largest cohort, the discrepancies in study population sizes need to be clarified, and the results confirmed.
Strengths and limitations
Our systematic review and meta-analysis has several strengths. First, the numbers of patients in the population-based independent cohorts included in the meta-analyses are very large, and all studies, but one, show the same tendency in favor of BCS for both overall and breast cancer-specific survival. Second, the meta-analyses are based on studies which all have adjusted for a number of relevant prognostic factors. Third, no study was considered being at high risk of bias and we found no clear indication of publication bias. Fourth, we were able to perform a number of stratified analyses showing comparable results across comparisons between BCS and mastectomy with and without post-mastectomy radiation therapy, as well as across different subgroups of patients, including node negative and node positive, young and older age groups. Finally, the results were supported by results of supplementary Bayesian meta-analyses indicating very strong support for nonzero differences in survival.
Although the differences are thus robust, a number of possible limitations should also be noted. First, the included studies are not randomized controlled trials. The validity of the comparisons is therefore dependent on proper adjustment for patient, tumor, and treatment variables, and these variables are limited or missing in several of the studies. Second, the included studies could also suffer from confounding by indication. Comorbidity is strongly associated with poorer survival,62 and it has previously been shown that patients with more comorbidity were more likely to be treated with mastectomy.10 The risk of selection bias was demonstrated in the Danish study,10 where patients who were initially assigned to BCS, but ended up being treated with mastectomy, had significantly better survival than patients for whom mastectomy was decided up front. This could artificially reduce survival after mastectomy in studies which have not adjusted for comorbidity. Even with these limitations, the results are robust, when it comes to patients with the most frequent stages at presentation (T1-2N0-1M0). The only clear outlier is the study by Landercasper et al,12 where discrepancies in study population sizes and proportions of patients receiving BCS are found, when compared to other studies from NCDB, and this raise some concerns. Even so, it does not alter the overall result of the meta-analysis.
Conclusions
The combined findings from large population-based studies indicate that BCS is associated with survival benefit compared with mastectomy, suggesting that BCS be the recommended treatment of early breast cancer (T1-2N0-1M0) if a radical lumpectomy can be performed.
ACKNOWLEDGMENTS
This study was performed by researchers at Aarhus University Hospital, Copenhagen University Hospital and University Hospital Federico II, Naples without further external funding.
Supplementary Material
Funding Statement
Disclosure: The authors declare that they have nothing to disclose.
Footnotes
Published online 5 October 2022
Disclosure: The authors declare that they have nothing to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and surgery in early breast cancer—an overview of the randomized trials. N Engl J Med. 1995;333:1444–1456. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. [DOI] [PubMed] [Google Scholar]
- 4.Blichert-Toft M, Nielsen M, Düring M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008;47:672–681. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Jiang L, Gao B, et al. Survival and disease-free benefits with mastectomy versus breast conservation therapy for early breast cancer: a meta-analysis. Breast Cancer Res Treat. 2016;157:517–525. [DOI] [PubMed] [Google Scholar]
- 6.Jensen M, Laenkholm A, Offersen BV, et al. The clinical database and implementation of treatment guidelines by the Danish Breast Cancer Cooperative Group in 2007 – 2016. Acta Oncol (Madr). 2017;0:1–6. [DOI] [PubMed] [Google Scholar]
- 7.Hwang ES, Lichtensztajn DY, Gomez SL, et al. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer. 2013;119:1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann-Johnsen OJ, Kåresen R, Schlichting E, et al. Survival is better after breast conserving therapy than mastectomy for early stage breast cancer: a registry-based follow-up study of Norwegian Women primary operated between 1998 and 2008. Ann Surg Oncol. 2015;22:3836–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagendijk M, van Maaren MC, Saadatmand S, et al. Breast conserving therapy and mastectomy revisited: breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int J Cancer. 2018;142:165–175. [DOI] [PubMed] [Google Scholar]
- 10.Christiansen P, Carstensen SL, Ejlertsen B, et al. Breast conserving surgery versus mastectomy: overall and relative survival-a population based study by the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol. 2018;57:19–25. [DOI] [PubMed] [Google Scholar]
- 11.Mazor AM, Mateo AM, Demora L, et al. Breast conservation versus mastectomy in patients with T3 breast cancers (> 5 cm): an analysis of 37,268 patients from the National Cancer Database. Breast Cancer Res Treat. 2019;173:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landercasper J, Ramirez LD, Borgert AJ, et al. A reappraisal of the comparative effectiveness of lumpectomy versus mastectomy on breast cancer survival: a propensity Score-Matched Update From the National Cancer Data Base (NCDB). Clin Breast Cancer. 2019;19:e481–e493. [DOI] [PubMed] [Google Scholar]
- 13.van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17:1158–1170. [DOI] [PubMed] [Google Scholar]
- 14.de Boniface J, Szulkin R, Johansson ALV. Survival after breast conservation vs mastectomy adjusted for comorbidity and socioeconomic status. JAMA Surg. 2021;56:S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 16.Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher S, Gao H, Yasui Y, et al. Survival in stage I-III breast cancer patients by surgical treatment in a publicly funded health care system. Ann Oncol. 2015;26:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 8, 2022.
- 19.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gronau QF, Van Erp S, Heck DW, et al. A Bayesian model-averaged meta-analysis of the power pose effect with informed and default priors: the case of felt power. Compr Results Soc Psychol. 2017;2:123–138. [Google Scholar]
- 22.JPT H, J T, J C, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, Ltd; 2019. [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.IntHout J, Ioannidis JP, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 27.Borenstein M, Hedges L, Higgins J, et al. Comprehensive Meta-Analysis Version 3. Biostat, Englewood, NJ; 2013. [Google Scholar]
- 28.Van Erp S, Verhagen J, Grasman RPPP, et al. Estimates of between-study heterogeneity for 705 meta-analyses reported in psychological bulletin from 1990–2013. J Open Psychol Data. 2017;5:4. [Google Scholar]
- 29.van Doorn J, van den Bergh D, Dablander F, et al. The JASP guidelines for conducting and reporting a Bayesian analysis. Psychon Bull Rev. 2021;28:813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmood U, Morris C, Neuner G, et al. Similar survival with breast conservation therapy or mastectomy in the management of young women with early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;83:1387–1393. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149:267–274. [DOI] [PubMed] [Google Scholar]
- 32.Ye JC, Yan W, Christos PJ, et al. Equivalent survival with mastectomy or breast-conserving surgery plus radiation in young women aged < 40 years with early-stage breast cancer: a national registry-based stage-by-stage comparison. Clin Breast Cancer. 2015;15:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen QX, Wang XX, Lin PY, et al. The different outcomes between breast-conserving surgery and mastectomy in triple-negative breast cancer: a population-based study from the SEER 18 database. Oncotarget. 2017;8:4773–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bleicher RJ, Ruth K, Sigurdson ER, et al. Breast conservation versus mastectomy for patients with T3 primary tumors (>5 cm): a review of 5685 medicare patients. Cancer. 2016;122:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Chen Y, Wang X, et al. T1-2N0M0 triple-negative breast cancer treated with breast-conserving therapy has better survival compared to mastectomy: a SEER population-based retrospective analysis. Clin Breast Cancer. 2019;19:e669–e682. [DOI] [PubMed] [Google Scholar]
- 36.Mogal HD, Clark C, Dodson R, et al. Outcomes after mastectomy and lumpectomy in elderly patients with early-stage breast cancer. Ann Surg Oncol. 2017;24:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grover S, Nurkic S, Diener-West M, et al. Survival after breast-conserving surgery with whole breast or partial breast irradiation in women with early stage breast cancer: a SEER data-base analysis. Breast J. 2017;23:292–298. [DOI] [PubMed] [Google Scholar]
- 38.Guo L, Xie G, Wang R, et al. Local treatment for triple-negative breast cancer patients undergoing chemotherapy: breast-conserving surgery or total mastectomy? BMC Cancer. 2021;21:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofvind S, Holen Å, Aas T, et al. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur J Surg Oncol. 2015;41:1417–1422. [DOI] [PubMed] [Google Scholar]
- 40.Hartmann-Johnsen OJ, Kåresen R, Schlichting E, et al. Better survival after breast-conserving therapy compared to mastectomy when axillary node status is positive in early-stage breast cancer: a registry-based follow-up study of 6387 Norwegian women participating in screening, primarily operated between 1998. World J Surg Oncol. 2017;15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Maaren MC, de Munck L, Jobsen JJ, et al. Breast-conserving therapy versus mastectomy in T1-2N2 stage breast cancer: a population-based study on 10-year overall, relative, and distant metastasis-free survival in 3071 patients. Breast Cancer Res Treat. 2016;160:511–521. [DOI] [PubMed] [Google Scholar]
- 42.Jeon YW, Choi JE, Park HK, et al. Impact of local surgical treatment on survival in young women with T1 breast cancer: long-term results of a population-based cohort. Breast Cancer Res Treat. 2013;138:475–484. [DOI] [PubMed] [Google Scholar]
- 43.Chen K, Liu J, Zhu L, et al. Comparative effectiveness study of breast-conserving surgery and mastectomy in the general population: a NCDB analysis. Oncotarget. 2015;6:40127–40140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazow SP, Riba L, Alapati A, et al. Comparison of breast-conserving therapy vs mastectomy in women under age 40: national trends and potential survival implications. Breast J. 2019;25:578–584. [DOI] [PubMed] [Google Scholar]
- 45.Almahariq MF, Quinn TJ, Siddiqui Z, et al. Breast conserving therapy is associated with improved overall survival compared to mastectomy in early-stage, lymph node-negative breast cancer. Radiother Oncol. 2020;142:186–194. [DOI] [PubMed] [Google Scholar]
- 46.Wrubel E, Natwick R, Wright GP. Breast-Conserving therapy is associated with improved survival compared with mastectomy for early-stage breast cancer: a propensity score matched comparison using the national cancer database. Ann Surg Oncol. 2021;28:914–919. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Yang C, Lei C, et al. Survival outcomes after breast-conserving therapy compared with mastectomy for patients with early-stage metaplastic breast cancer: a population-based study of 2412 patients. Breast. 2021;58:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu QD, Hsieh MC, Lyons JM, et al. 10-Year survival after breast-conserving surgery compared with mastectomy in Louisiana women with early-stage breast cancer: a population-based study. J Am Coll Surg. 2021;232:607–621. [DOI] [PubMed] [Google Scholar]
- 49.Abrahimi MS, Elwood M, Lawrenson R, et al. Associated factors and survival outcomes for breast conserving surgery versus mastectomy among New Zealand women with early-stage breast cancer. Int J Environ Res Public Health. 2021;18:2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H, Lee SB, Nam SJ, et al. Survival of breast-conserving surgery plus radiotherapy versus total mastectomy in early breast cancer. Ann Surg Oncol. 2021;28:5039–5047. [DOI] [PubMed] [Google Scholar]
- 51.Goodman SN. Toward evidence-based medical statistics. 2: the Bayes factor. Ann Intern Med. 1999;130:1005–1013. [DOI] [PubMed] [Google Scholar]
- 52.Bouganim N, Tsvetkova E, Clemons M, et al. Evolution of sites of recurrence after early breast cancer over the last 20 years: implications for patient care and future research. Breast Cancer Res Treat. 2013;139:603–606. [DOI] [PubMed] [Google Scholar]
- 53.Bodilsen A, Bjerre K, Offersen BV, et al. Importance of margin width in breast-conserving treatment of early breast cancer. J Surg Oncol. 2016;113:609–615. [DOI] [PubMed] [Google Scholar]
- 54.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onitilo AA, Engel JM, Stankowski RV, et al. Survival comparisons for breast conserving surgery and mastectomy revisited: community experience and the role of radiation therapy. Clin Med Res. 2015;13:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Retsky M, Demicheli R, Hrushesky WJ, et al. Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Curr Med Chem. 2013;20:4163–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–241. [DOI] [PubMed] [Google Scholar]
- 58.Jatoi I, Benson JR, Kunkler I. Hypothesis: can the abscopal effect explain the impact of adjuvant radiotherapy on breast cancer mortality? NPJ Breast Cancer. 2018;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vila J, Gandini S, Gentilini O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: a systematic meta-analysis comparing breast-conserving surgery versus mastectomy. Breast. 2015;24:175–181. [DOI] [PubMed] [Google Scholar]
- 60.Davey MG, Davey CM, Ryan ÉJ, et al. Combined breast conservation therapy versus mastectomy for BRCA mutation carriers - a systematic review and meta-analysis. Breast. 2021;56:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nijenhuis MV, Rutgers EJ. Who should not undergo breast conservation? Breast. 2013;22 Suppl 2:S110–S114. [DOI] [PubMed] [Google Scholar]
- 62.Land LH, Dalton SO, Jørgensen TL, et al. Comorbidity and survival after early breast cancer. A review. Crit Rev Oncol Hematol. 2012;81:196–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.