Abstract
Objectives
Chronic pain results in significant impairment in older adults, yet some individuals maintain adaptive functioning. Limited research has considered the role of positive resources in promoting resilience among older adults. Likewise, these factors have largely been examined independently. We aimed to identify resilience domains based on biopsychosocial factors and explore whether resilience phenotypes vary across sleep disturbance, fatigue, and cognitive function.
Methods
Sixty adults (ages ≥60 years) with chronic low back pain completed measures of psychological, health, and social functioning. On the basis of previously published analyses, principal-components analysis was conducted to create composite domains for these measures, followed by cluster analysis to identify phenotypes.
Results
Four profiles emerged: Cluster 1, with high levels of psychosocial and health-related functioning; Cluster 2, with high health-related functioning and low psychosocial functioning; Cluster 3, with high psychosocial functioning and poorer health; and Cluster 4, with low levels of functioning across all domains. Significant differences across cluster membership emerged for sleep disturbance (ηp2 = 0.29), fatigue (ηp2 = 0.29), and cognitive abilities (ηp2 = 0.47). Individuals with the highest levels of resilience demonstrated more optimal outcomes in sleep and fatigue (P values ≤0.001) than did individuals with a less resilient phenotype. Furthermore, the High-Resilience group (Cluster 1) and the High Psychosocial / Low Health group (Cluster 3) had lower cognitive impairment than did the High Health / Low Psychosocial group (Cluster 2) and the Low-Resilience group (Cluster 4) (P values ≤0.009).
Conclusions
A higher array of protective resources could buffer against the negative sequelae associated with chronic low back pain. These exploratory findings support the multidimensional nature of resilience and suggest that targeting resilience from a multisystem perspective might help to optimize interventions for older adults with chronic pain.
Keywords: Resilience, Back Pain, Fatigue, Sleep, Cognitive Function, Chronic Low Back Pain, Low Back Pain, Older Adults, Multisystem
Introduction
Chronic low back pain (cLBP) is one of the most common, poorly understood, and disabling pain conditions, affecting 36.1% of individuals 60 years of age or older [1]. cLBP rarely occurs alone and is often accompanied by other interrelated clinical symptoms that have an impact on general health and psychological well-being [2]. For instance, fatigue is a common concern among older adults with pain, and more than 50% of patients with cLBP report concomitant sleep disturbances, with pain and fatigue associated with decrements in quality of life [3]. Likewise, cognitive deficits, including impairments in attentional capacity and memory, have been observed [4], and pain predicts greater cognitive decline over time [5].
Although research has focused predominantly on vulnerability factors (e.g., pain catastrophizing, fear-avoidance) related to the maintenance and exacerbation of pain [6, 7], a growing body of literature supports the protective effects of positive, resilience factors in the pain experience. Resilience is broadly conceptualized as adaptive functioning in the face of adversity or systemic challenges [8], with several factors contributing to resilience. Several studies have found that psychosocial factors, such as positive affect [9], hope [10], and perceived social support [11–13], are associated with better pain-associated outcomes, including lower pain severity, depression, and pain-related disability. In addition, numerous lifestyle and health-related factors (e.g., physical activity) have emerged as important contributors to health and improved pain outcomes [14, 15].
Although existing studies have supported the adaptive benefits of psychological, social, and lifestyle factors in the experience of pain, historically these factors have been examined independently, with a predominant focus on psychological facets. However, such approaches might overlook potential additive contributions among protective factors, thereby failing to account for the multidimensional nature of resilience. Few studies have attempted to examine resilience from a broader framework in the context of aging and chronic pain and to take into consideration the multiple sources that promote adaptive function. Among older adults with knee pain, Johnson et al. [16] found that a resilience index comprised of psychological and biobehavioral resilience factors was more predictive of longer telomere length than were psychological factors alone. Furthermore, our previous work identified phenotypic patterns of resilience based on psychological, social, and health-related functioning in older adults with cLBP, with greater psychological and physical functioning observed among those with a more resilient phenotype [17]. Thus, combining multiple protective factors to conceptualize resilience could improve our understanding of adaptive function, and subsequently examining relationships among these multidimensional phenotypic profiles and modifiable pain-related outcomes might have greater clinical relevance than examining resilience factors in isolation.
Given these considerations, the aim of the present study was to examine whether phenotypic profiles of resilience (as defined from our previous work) among older adults with cLBP [17] differ across measures of sleep disturbance, fatigue, and cognitive abilities. These outcomes were selected because they are modifiable (and thus can be improved through therapeutic interventions) [18–20] and are often comorbid with chronic pain. We hypothesized that individuals with a more resilient phenotype (i.e., higher psychological, social, and health-related function) would demonstrate lower levels of sleep disturbance and fatigue and higher cognitive function relative to individuals lower in resilience.
Methods
Participants
Sixty-nine older adults (60 years of age or more) with cLBP were recruited from the community via posted fliers, radio and print media announcements, and word-of-mouth referral. All participants provided verbal and written informed consent. Participants were included if they reported at minimum mild low back pain (≥2/10 on the Numerical Pain Rating Scale) occurring on at least half of the days during the preceding 3 months. Given the prevalence of pain comorbidities among older adults and to generalize results more broadly, the presence of other musculoskeletal pain conditions did not preclude enrollment in the study, as long as low back pain was reported as an individual’s primary pain condition. Participants were excluded for recent vertebral fracture, back surgery within the preceding 6 months, diagnosis of cauda equina syndrome, uncontrolled hypertension (≥150/90 mm Hg), current cardiovascular disease, neurological disease associated with somatosensory abnormalities (e.g., neuropathy, seizures, Parkinson’s disease), current major medical illness (e.g., metastatic or visceral disease), chronic opioid use, or systemic inflammatory disease (e.g., spondyloarthropathies including ankylosing spondylitis, systemic lupus erythematosus, etc.). Participants were provided with an honorarium (up to $100) upon completion of the study.
Procedure
The University of Florida Institutional Review Board approved all study procedures. An initial phone screen was conducted to determine study inclusion. Eligible participants attended two 2- to 3.5-hour laboratory sessions scheduled approximately 1 week apart. During the first visit, a thorough demographic and medical history was obtained via self-report, anthropometric tests were conducted (i.e., calculation of body mass index [BMI]), and psychosocial questionnaires and functional performance measures were completed. Participants also completed questionnaires from home during the time between visits 1 and 2. Additional psychosocial questionnaires were completed at visit 2.
Measures
Predictors of Multisystem Resilience
Positive and Negative Affect Schedule (PANAS)
Positive affect and negative affect were assessed with the Positive and Negative Affect Schedule (PANAS) [21]. Respondents were presented with a 20-item scale consisting of 10 positively valenced and 10 negatively valenced terms. Each term is rated on a five-point scale ranging from 1 (very slightly or not at all) to 5 (extremely), resulting in scale scores for positive affect and negative affect. Higher scale scores correspond to increased positive and negative affect (only positive affect scores were included in the present analysis). Reliability tests indicated high internal consistency of items on the positive affect scale (α = 0.90).
Adult Dispositional Hope Scale (ADHS)
The 12-item Adult Dispositional Hope Scale (ADHS) includes eight statements measuring two aspects of hope: pathways (e.g., “There are lots of ways around a problem”) and agency (e.g., “I energetically pursue my goals”), as well as four “filler” items that are not included in scoring [22]. Participants indicated the degree to which each statement best described them by rating each item on a scale from 1 (definitely false) to 8 (definitely true). Higher scores indicate greater trait levels of hope. Reliability analyses from the present investigation revealed a Cronbach’s α of 0.92 for the ADHS, indicating high internal consistency.
PROMIS Positive Affect and Well-Being Scale
The Patient-Reported Outcomes Measurement Information System (PROMIS) Positive Affect and Well-Being Scale is a 23-item measure that assesses positive affect and overall sense of satisfaction with life [23]. Each item is rated on a scale ranging from 1 (never) to 5 (always) to indicate how often respondents experienced positive emotion and/or purpose and meaning in life (e.g., “[Lately], I had a sense of balance in my life”). Higher scores indicate greater positive affect and well-being (Cronbach’s α = 0.97).
Life-Orientation Test–Revised (LOT-R)
Dispositional optimism was evaluated with the Life-Orientation Test–Revised (LOT-R), which consists of 10 items (including four unscored items and three reverse-scored items). Participants were asked to use a five-point scale ranging from 0 (strongly disagree) to 4 (strongly agree) and rate the degree to which they agreed with the presented statements (e.g., “In uncertain times, I usually expect the best”) [24]. Higher LOT-R scores reflect greater optimism. This measure demonstrated adequate reliability in the sample (α = 0.73).
PROMIS Support (Emotional, Instrumental, Informational)
The PROMIS Support scales were used to measure social functioning. Specifically, the short forms of the emotional scale (eight items; e.g., “I have someone who makes me feel appreciated”), instrumental scale (four items; e.g., “Do you have someone to take you to the doctor if you need it?”), and informational scale (four items; e.g., “I have someone to turn to for suggestions about how to deal with a problem”) were administered [25]. Items are rated on a scale ranging from 1 (never) to 5 (always) for all three domains, with higher scores indicating greater social support. High internal consistency was found for all three scales, and they also demonstrated high reliability with each other: emotional, α = 0.97; instrumental, α = 0.96; informational, α = 0.96; and all support measures combined, α = 0.97.
Anthropometric Tests: Body Composition
During visit 1, each participant’s waist circumference (5 cm above the navel) and hip circumference (widest part of the hips) were calculated (in centimeters) with a measuring tape, with waist-to-hip ratio determined by dividing the waist circumference by the hip circumference. Body weight was measured to the nearest 0.1 kg on a digital scale (Healthometer), and height was assessed to the nearest centimeter on a wall stadiometer. Calculation of BMI was determined by weight in kilograms divided by height in meters squared.
Health Comorbidities
A health status questionnaire was used to determine the presence of physical health comorbidities. Participants reported current medical conditions by placing an “X” next to applicable items (e.g., high blood pressure, heart disease, diabetes, asthma or breathing problems, kidney/renal disease, thyroid problem, neurological disorder, or other self-reported health conditions). Medical diagnoses were placed into International Classification of Diseases, 10th Edition (ICD-10) diagnostic categories for reporting purposes.
Smoking Status
Current cigarette smoking status was assessed with the following question: “How would you describe your cigarette smoking?” Possible responses included: “never smoked,” “used to smoke but have now quit,” and “current smoker,” and individuals were categorized as either current smokers (yes) or nonsmokers (no).
Outcome Questionnaires
PROMIS Sleep Disturbance
The short form of the PROMIS Sleep Disturbance measure includes eight questions (e.g., “I had a problem with my sleep”) examining insomnia-like items and assesses a person’s perceived sleep quality and restoration associated with sleep, perceived sleep difficulties and concerns with falling and staying asleep, and perceptions of adequate and satisfactory sleep over the previous 7 days [26]. Higher scores indicate greater sleep disturbance. The Sleep Disturbance scale had excellent internal consistency in the present sample (α = 0.93).
PROMIS Fatigue (8a)
The eight-item PROMIS Fatigue–Short Form (8a) was used to evaluate fatigue (e.g., “How run down did you feel on average?”) and fatigue-related interference in functioning (e.g., “How often did you have trouble finishing things because of your fatigue?”) over the previous 7 days [27]. Ratings are made on a five-point scale ranging from 1 (not at all / never) to 5 (very much / always). Higher scores suggest greater fatigue. This measure demonstrated high reliability in the sample (α = 0.96).
PROMIS Applied Cognitive Abilities (4a)
Participants completed the four-item PROMIS Applied Cognitive Abilities questionnaire as a measure of self-reported cognitive functioning (e.g., “My mind has been as sharp as usual”) [28]. Items are rated from 1 (not at all) to 5 (very much), with higher scores reflecting better subjective experience of cognitive abilities. This psychometrically sound measure had an internal consistency reliability of 0.93.
All PROMIS measures were transformed into T-scores, with norms based on a U.S. general population mean of 50 (standard deviation of 10). Although the minimal clinically important difference has not been established for all PROMIS measures, a range between 2 and 6 T-score points has been recommended [29]. Thus, we used a conservative T-score change of ≥6 points to designate clinically meaningful differences across cluster groups.
Statistical Analysis
All analyses were conducted in SPSS (IBM SPSS Statistics for Windows, Version 27.0, Armonk, NY), and the significance level was set at P < 0.05 (two tailed). Means, standard deviations, and frequencies for demographic characteristics were calculated with descriptive statistics. Zero-order correlations were conducted between sociodemographic characteristics and outcome variables (i.e., sleep disturbance, fatigue, cognitive abilities). A principal-components analysis with oblique rotation was conducted with the following variables to characterize the dimensionality of each resilience measure: positive affect, dispositional hope, positive well-being, optimism, waist-to-hip ratio, BMI, number of physical health comorbidities, smoking status, emotional support, instrumental support, and informational support. Per guidelines, three items were required to load on a factor, with a difference of ≥0.20 observed between cross-loadings [30]. Components with eigenvalues greater than 1 were selected for further analysis, and the scree plot was inspected to confirm the number of factors to be retained. To identify subgroups of individuals that differed across resilience domains, hierarchical cluster analysis using Ward’s clustering method with squared Euclidean distances as the similarity measures was conducted. Agglomeration coefficients were examined to identify the cluster solution that best represented the data, with the optimal number being chosen on the basis of the point at which the percentage change was the largest between the clusters [31]. As a measurement of internal validity, a cross-validation was conducted by creating a subsample of the data through a random splitting method (removing 30% of the cases) and then comparing this cluster solution with the original sample. No differences were observed in the cluster solution across both samples. Multivariate analysis of variance was then conducted to examine cluster group differences in sleep disturbance, fatigue, and cognitive abilities. To obtain effect size estimates associated with F tests, partial eta-squared (ηp2) was calculated (small = 0.01, medium = 0.06, large = 0.14). Cohen’s d was reported as the effect size for mean comparisons (small = 0.20, medium = 0.50, large = 0.80).
Results
Participant Characteristics
Table 1 presents sociodemographic characteristics (means and standard deviations) of the sample. Participants predominantly were female (57%), were White/Caucasian (70%), had a college degree (50%), were married or partnered (52%), and were not employed (85%). Their average age was 68 years (range: 60–93 years), the duration of their back pain was 16.4 years (range: 1–56 years), and their mean BMI was 29.3 kg/m2. Back pain was rated as being of moderate intensity (mean [M] = 5.5, range = 2–10). Two of the 69 participants discontinued after the first session because of time constraints, and seven participants who were initially eligible were excluded during their first appointment (n = 1 for the use of exclusion medications, n = 3 for an exclusionary medical condition, n = 3 for not meeting pain duration criteria), thereby leaving 60 participants.
Table 1.
Demographic and clinical characteristics
| Characteristic | Value |
|---|---|
| Age, years, mean±SD | 68.1±7.0 |
| Sex, n (%) | |
| Male | 26 (43.3) |
| Female | 34 (56.7) |
| Race, n (%) | |
| White/Caucasian | 42 (70.0) |
| Black / African American | 12 (20.0) |
| Other | 6 (10.0) |
| Education, n (%) | |
| ≤HS diploma | 13 (21.7) |
| Some college / technical degree | 17 (28.3) |
| Associate’s/Bachelor’s degree | 18 (30.0) |
| Graduate/professional degree | 12 (20.0) |
| Marital status, n (%) | |
| Married/partnered | 31 (51.7) |
| Not married/partnered | 29 (48.3) |
| Employment, n (%) | |
| Employed | 9 (15.0) |
| Not employed | 51 (85.0) |
| Income, n (%)* | |
| <$20,000 | 21 (35.0) |
| $20,000–$39,999 | 10 (16.7) |
| $40,000–$59,999 | 11 (18.3) |
| $60,000–$99,999 | 8 (13.3) |
| >$99,999 | 7 (11.7) |
| Back pain duration, years, mean±SD | 16.4±14.2 |
| BMI, kg/m2, mean±SD | 29.3±5.8 |
Note: Some data not reported. HS= high school; SD= standard deviation.
Zero-Order Correlations
Zero-order correlations were analyzed across sociodemographic variables and study outcomes (Table 2). Greater levels of sleep disturbance, fatigue, and cognitive dysfunction were reported among individuals who were unmarried/unpartnered (P values ≤0.004). A higher level of fatigue was associated with younger age, lower income, and greater BMI (P values ≤0.04). Furthermore, greater cognitive function was reported among those who were White/Caucasian and individuals with higher income (P values ≤0.04). Sleep disturbance, fatigue, and cognitive abilities were all significantly correlated (P values ≤0.001).
Table 2.
Zero-order correlations across demographic characteristics and study outcomes
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | — | |||||||||||
| 2. Sex | 0.18 | — | ||||||||||
| 3. Race | –0.29* | 0.16 | — | |||||||||
| 4. Education | 0.04 | –0.19 | –0.45** | — | ||||||||
| 5. Marital status | –0.29* | –0.11 | 0.31* | –0.14 | — | |||||||
| 6. Employment | 0.26* | 0.18 | 0.17 | –0.11 | –0.06 | — | ||||||
| 7. Income | 0.36** | –0.09 | –0.34** | 0.28* | –0.41** | –0.23 | — | |||||
| 8. Pain duration | 0.06 | 0.01 | –0.16 | 0.06 | –0.14 | –0.02 | 0.09 | — | ||||
| 9. BMI | –0.12 | 0.02 | 0.29* | –0.21 | 0.13 | –0.02 | –0.26 | –0.07 | — | |||
| 10. PROMIS Sleep | –0.25 | 0.06 | 0.18 | –0.19 | 0.36** | 0.09 | –0.23 | –0.06 | 0.24 | — | ||
| 11. PROMIS Fatigue | –0.27* | –0.13 | 0.03 | –0.04 | 0.36** | 0.04 | –0.28* | –0.21 | 0.26* | 0.59** | — | |
| 12. PROMIS Cognitive | 0.18 | –0.15 | –0.26* | 0.11 | –0.37** | –0.23 | 0.35** | 0.11 | –0.14 | –0.72** | –0.57** | — |
Note: *P < 0.05, **P ≤ 0.01.
Sex coded: 0 = male, 1 = female; race coded: 0 = White, 1 = Black/Other; education coded: 0 = ≤high school degree, 1 = >high school degree; marital status coded: 0 = married/partnered, 1 = not married/partnered; employment coded: 0 = employed, 1 = not employed; income coded: 0 = <$20,000, 1 = ≥$20,000; PROMIS = Patient-Reported Outcomes Measurement Information System.
Principal-Components Analysis and Cluster Analysis
Results for the principal-components analysis and cluster analysis are reported in a previous article; however, we briefly detail them here. We refer readers to Bartley et al. (2019) [17] for a full description of the results. First, a principal-components analysis was conducted with 11 items (i.e., positive affect, dispositional hope, positive well-being, optimism, waist-to-hip ratio, BMI, number of physical health comorbidities, smoking status, emotional support, instrumental support, informational support) through the use of oblique rotation (direct oblimin). A three-factor solution with eigenvalues greater than Kaiser’s criterion of 1 was derived, accounting for 72.4% of the variance in scores (Component 1: positive, psychological factors; Component 2: health-related functioning; Component 3: social support). These three domains were then used in cluster analysis to identify empirically derived classifications based on profiles of psychological, health, and social support. Four clusters emerged from the analysis: 1) Cluster 1 (n = 25, 41.7%), with high levels of psychological, health, and social support functioning (High-Resilience group); 2) Cluster 2 (n = 13, 21.7%), with high health-related functioning and low levels of psychosocial function (High Health / Low Psychosocial group); 3) Cluster 3 (n = 15, 25.0%), with poor health-related functioning, high psychological functioning, and moderate-to-high social support (High Psychosocial / Low Health group); and 4) Cluster 4 (n = 7, 11.7%), with low levels of functioning across psychological, social, and health-related factors (Low-Resilience group).
Physical and Cognitive Function Profiles Across Cluster Groups
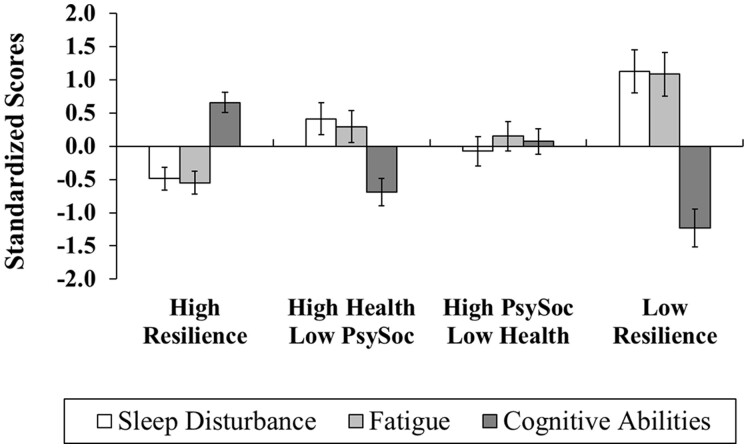
Significant differences across cluster membership emerged for sleep disturbance [F(3,56) = 7.67, P ≤ 0.001, ηp2 = 0.29], fatigue [F(3,56) = 7.70, P ≤ 0.001, ηp2 = 0.29], and cognitive abilities [F(3,56) = 16.49, P ≤ 0.001, ηp2 = 0.47] (Figure 1, Table 3). Specifically, sleep disturbance was lowest for the High-Resilience (Cluster 1) group when compared with the High Health / Low Psychosocial (Cluster 2) and Low-Resilience (Cluster 4) groups (P = 0.003, d = 1.01 and P ≤ 0.001, d = 1.79, respectively). Furthermore, individuals in the Low-Resilience (Cluster 4) group reported a significantly higher degree of sleep disturbance than did those in the High Psychosocial / Low Health (Cluster 3) group (P = 0.004, d = 1.24), as well as the highest level of fatigue compared with Cluster 1 (P ≤ 0.001, d = 1.67) and Cluster 3 (P = 0.02, d = 0.87). When compared with individuals with higher health-related function (Cluster 2), the effect for higher fatigue in the Low-Resilience group (Cluster 4) approached significance (P = 0.05, d = 0.73). Higher levels of fatigue were also observed among the High Health / Low Psychosocial (Cluster 2) and High Psychosocial / Low Health (Cluster 3) groups (P = 0.006, d = 1.04 and P = 0.016, d = 0.87, respectively) relative to individuals in the High-Resilience (Cluster 1) group. With regard to cognitive abilities, participants in the High-Resilience (Cluster 1) group had higher cognitive function relative to all groups (all P values <0.01, all d values= 0.84 to 3.09). Cognitive function was also higher among participants with greater psychosocial functioning (Cluster 3) than among those in the High Health / Low Psychosocial (Cluster 2) and Low-Resilience (Cluster 4) groups (P = 0.009, d = 0.88 and P ≤ 0.001, d = 1.98, respectively).
Figure 1.
Physical and cognitive functioning outcomes across multisystem resilience profiles. Relative to the Low-Resilience (Cluster 4) group, individuals with the highest levels of resilience demonstrated more optimal outcomes in sleep and fatigue. Furthermore, the High-Resilience (Cluster 1) and High Psychosocial / Low Health (Cluster 3) groups had lower cognitive impairment than did the High Health / Low Psychosocial (Cluster 2) and Low-Resilience (Cluster 4) groups. *Error bars represent standard error of the mean.
Table 3.
Descriptive statistics for physical and cognitive measures across cluster groups
| Cluster 1 |
Cluster 2 |
Cluster 3 |
Cluster 4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | High Health | High Psychosocial | Low | |||||||||
| Resilience | Low Psychosocial | Low Health | Resilience | |||||||||
| (n = 25) |
(n = 13) |
(n = 15) |
(n = 7) |
|||||||||
| M | SD | 95% CI | M | SD | 95% CI | M | SD | 95% CI | M | SD | 95% CI | |
| Sleep disturbance | 45.1 | 6.9 | 41.7–48.6 | 54.1 | 10.6 | 49.3–58.9 | 49.3 | 8.5 | 44.8–53.7 | 61.3 | 10.7 | 54.7–67.8 |
| Fatigue | 44.5 | 6.7 | 41.0–47.9 | 52.9 | 9.3 | 48.2–57.8 | 51.5 | 9.2 | 47.1–55.9 | 60.8 | 12.1 | 54.3–67.4 |
| Cognitive abilities | 56.6 | 6.6 | 53.6–59.6 | 43.1 | 9.7 | 38.9–47.3 | 50.7 | 7.4 | 46.8–54.6 | 37.7 | 5.6 | 32.1–43.4 |
Note: Means are presented as T-scores (mean of 50, SD of 10). M= mean; SD = standard deviation; CI= confidence interval.
Discussion
Resilience is increasingly recognized as a significant predictor of pain-related outcomes, but there remains a poor understanding of the heterogeneity of resilience. To address this, our study sought to analyze subgroups of older adults characterized by distinct profiles of psychosocial, sociological, and health-related resilience factors and to identify whether these phenotypic profiles differed across symptoms of sleep disturbance, fatigue, and cognitive abilities.
On the basis of previous findings [17], we observed four different profiles of resilience factors, with approximately 42% of the sample characterized as highly resilient (High-Resilience cluster group). As hypothesized, individuals with a higher phenotypic profile of resilience (i.e., greater psychological, social, and health-related function) exhibited lower self-reported sleep disturbance, fatigue, and cognitive dysfunction, thereby supporting previous research suggesting that a unique combination of resources might better characterize resilience and account for adaptive outcomes, relative to examining factors in isolation [16, 17]. Notably, we found that participants with lower levels of psychological and social functioning (Clusters 2 and 4) reported greater impairments in sleep, energy, and cognition than did participants with a higher degree of psychosocial functioning (Clusters 1 and 3). Although speculative, these findings suggest that a greater degree of supportive resources and positive psychological attributes (e.g., hope, optimism) might facilitate greater self-efficacy in managing back pain, including increased agency to engage in health-promoting strategies (e.g., physical activity, dietary modification, stress management, sleep hygiene) [9, 13]. Positive psychosocial resources could also exert their effects on health via biological pathways. Indeed, there is growing evidence that positive emotions could have distinct physiological correlates that mediate protective effects on health [32]. For instance, Costello et al. (2002) found that less optimistic individuals experienced greater pain sensitivity and had higher levels of norepinephrine and interleukin-6 than did those with higher optimism [33]. Future research examining the behavioral, psychosocial, and biological pathways that underpin resilient functioning is an important directive and could be a step toward advancing the development of individually tailored mechanism-based therapies for chronic pain.
Together, our data are in line with prior research supporting the heterogeneity of chronic pain; however, previous attempts to identify clinical phenotypes in cLBP have targeted primarily somatosensory function and prognostic risk factors [34–39]. Of the few studies that integrated resilience-based mechanisms in their phenotyping, these focused on a narrow range of psychological factors [34, 38, 39]. Similarly, much of the broader literature has accounted for only a small number of factors to characterize resilience, many of which have been examined in isolation from a single aspect of functioning (e.g., psychological function). Though still informative, individual approaches overlook potential synergistic contributions among protective factors, which might not fully represent resilient capacity. Given the growing burden of cLBP among older adults, a more comprehensive, systemic understanding of the factors that promote resilience could be key to buffering the impact of pain among this cohort.
Clinical Implications and Future Directions
Results from the present study have potential clinical implications for the assessment and management of chronic pain. In particular, our findings support the consideration of resilience from a multidimensional perspective—a deeper understanding of which could allow for directed therapeutic interventions to improve patients’ ability to cope with and more effectively manage cLBP. For instance, methods that promote optimal social engagement and positive psychological health, such as cognitive-behavior therapy, couples-focused interventions, and social intelligence training [40], might be advantageous for mitigating physical and cognitive impairments for individuals in Cluster Group 2. Given the negative impact of stigma in populations with chronic pain and in aging populations, acceptance-based approaches and structural interventions that target negative stereotypes and discrimination at the individual (self-stigma) and socio-political (societal) levels might be particularly warranted [41]. For individuals with a Lower-Resilience profile (Cluster 4), broadening the therapeutic regimen to incorporate both psychotherapy (e.g., cognitive-behavior therapy) and lifestyle modification strategies for weight and disease management could be justified. Likewise, positive psychological interventions that focus on bolstering positive emotions, cognitions, and behaviors (e.g., gratitude expression, pleasant activity engagement, using personal strengths) might be an avenue toward enhancing treatment outcomes, with preliminary findings supporting their efficacy in chronic pain [42–47], including a recent meta-analysis demonstrating the beneficial effects of positive psychological interventions on pain intensity and emotional functioning [48]. Thus, capitalizing on positive psychological resources through positive psychological interventions could be a promising target for pain management among aging adults.
Strengths and Limitations
The present study has some strengths that merit acknowledgment. First, we present one of the first studies to examine resilience from a multidimensional perspective in older adults with chronic pain [16, 17], with large effect sizes and clinically meaningful group differences observed across study outcomes. Second, we used a robust statistical approach to empirically derive resilience indices and cluster subgroups. Third, several validated and reliable measures were incorporated to examine psychosocial and health-related functioning. Fourth, we extend our previous findings [17] by highlighting the role that resilience has on behavioral and cognitive processes.
Despite these strengths, some limitations should be noted. Although our effect sizes were large and there were clinically meaningful differences in outcomes across groups (based on a T-score difference of 6 points), our sample size was small, with low participant numbers comprising clusters. This could have influenced the classification of study participants, thereby impacting external validity and the generalizability of findings. Given the nature of cluster analysis and our modest sample size, replication in a larger sample is needed before definitive conclusions about clinical and statistical significance can be drawn. Thus, these findings should be considered exploratory and interpreted cautiously. In addition, our study was cross-sectional in nature. Given the recognition of resilience as a dynamic and fluid process [49], the allocation of participants into subgroups might limit the ability to address the dynamic nature of resilience. Studies with multiple time points would support a richer understanding of resilience, and in particular, phenotypic variations that might be temporally related to changes in physical and cognitive functioning. In addition, our study population consisted of older adults who were predominantly White/Caucasian. Given the limited diversity in the sample, future studies with more adequate representation of other demographic groups, including persons of color, are recommended. Finally, although several psychosocial and health constructs were used to form participant subgroups, we acknowledge that this was not an exhaustive list of resources that characterize resilience. Although there are varying conceptual and methodological approaches in the operationalization of resilience, recent theoretical conceptualizations highlight the consideration and inclusion of multilevel and integrated processes that cut across biological, psychological, and environmental systems [49, 50]. Supporting this, dietary intake (i.e., increased consumption of plant-based foods) and higher levels of physical activity are associated with diminished risk of pain [51, 52], whereas environmental and sociological factors (e.g., socioeconomic status) are known contributors to pain and interference [53], with a higher degree of deprivation associated with poorer physical function [54]. Furthermore, the effect of positive psychological resources on pain is differentially expressed across race [55, 56], with recent evidence supporting associations between ethnic identity and pain-specific resilience [57]. This underscores the importance of contextual and cultural factors of resilience, including social determinants of adaptive function. Thus, investigating a broad range of promotive biopsychosocial factors might provide important predictive insights into the unique combination of resources that account for resilient responses.
Conclusions
Together, the results of the present study highlight the multicomponent nature of resilience and suggest that individuals with a greater degree of protective psychological, social, and health-related resources could be at lower risk of impairments in sleep, fatigue, and cognition. Findings support the consideration of resilience from a multisystem perspective, including the additive benefit of modifiable protective factors across multiple domains of functioning. In line with the tenants of precision medicine [58], the assessment and inclusion of resilience factors in phenotyping might facilitate a greater understanding of adaptive functioning among aging adults and enhance future tailoring of therapies that improve pain management. Given the exploratory nature of these findings, future studies with a larger sample size are warranted.
Acknowledgments
The authors thank Ralisa Pop, Stephanie Hersman, Morgan Ingram, Jordan McGee, Kylie Broskus, Paige McKenzie, and Michelle Jacomino for their assistance with data collection.
Contributor Information
Emily J Bartley, Department of Community Dentistry and Behavioral Science, University of Florida, Gainesville, Florida, USA.
Melissa Makhoul, Hariri School of Nursing, American University of Beirut, Beirut, Lebanon.
Shreela Palit, Nemours Children’s Health, Center for Healthcare Delivery Science, Jacksonville, Florida, USA.
Michael E Robinson, Department of Clinical and Health Psychology, University of Florida, Gainesville, Florida, USA.
Roger B Fillingim, Department of Community Dentistry and Behavioral Science, University of Florida, Gainesville, Florida, USA.
Funding sources: Research reported in this publication was supported by the National Institutes of Health/National Institute on Aging grant (K99AG052642) awarded to EJB. Dr. Bartley is currently funded by the National Institutes of Health/National Institute on Aging (R00AG052642, R21AG070642).
Conflicts of interest: There are no conflicts of interest to report.
References
- 1. Wong CKW, Mak RYW, Kwok TSY, et al. Prevalence, incidence, and factors associated with non-specific chronic low back pain in community-dwelling older adults aged 60 years and older: A systematic review and meta-analysis. J Pain 2022;23(4):509–34. [DOI] [PubMed] [Google Scholar]
- 2. Miaskowski C, Blyth F, Nicosia F, et al. A biopsychosocial model of chronic pain for older adults. Pain Med 2020;21(9):1793–805. [DOI] [PubMed] [Google Scholar]
- 3. Jakobsson U. A literature review on fatigue among older people in pain: Prevalence and predictors. Int J Older People Nurs 2006;1(1):11–6. [DOI] [PubMed] [Google Scholar]
- 4. Roth RS, Geisser ME, Theisen-Goodvich M, Dixon PJ. Cognitive complaints are associated with depression, fatigue, female sex, and pain catastrophizing in patients with chronic pain. Arch Phys Med Rehabil 2005;86(6):1147–54. [DOI] [PubMed] [Google Scholar]
- 5. Rouch I, Edjolo A, Laurent B, et al. Association between chronic pain and long-term cognitive decline in a population-based cohort of elderly participants. Pain 2021;162(2):552–60. [DOI] [PubMed] [Google Scholar]
- 6. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 2001;17(1):52–64. [DOI] [PubMed] [Google Scholar]
- 7. Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000;85(3):317–32. [DOI] [PubMed] [Google Scholar]
- 8. Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, Yehuda R. Resilience definitions, theory, and challenges: Interdisciplinary perspectives. Eur J Psychotraumatol 2014;5(1):25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finan PH, Garland EL. The role of positive affect in pain and its treatment. Clin J Pain 2015;31(2):177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Long KNG, Kim ES, Chen Y, et al. The role of hope in subsequent health and well-being for older adults: An outcome-wide longitudinal approach. Global Epidemiol 2020;2:100018. [Google Scholar]
- 11. Lee JE, Kahana B, Kahana E. Social support and cognitive functioning as resources for elderly persons with chronic arthritis pain. Aging Ment Health 2016;20(4):370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oraison HM, Kennedy GA. The effect of social support in chronic back pain: Number of treatment sessions and reported level of disability. Disabil Rehabil 2021;43(11):1526–31. [DOI] [PubMed] [Google Scholar]
- 13. Che X, Cash R, Ng SK, Fitzgerald P, Fitzgibbon BM. A systematic review of the processes underlying the main and the buffering effect of social support on the experience of pain. Clin J Pain 2018;34(11):1061–76. [DOI] [PubMed] [Google Scholar]
- 14. Geneen LJ, Moore RA, Clarke C, et al. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst Rev 2017;4(1):Cd011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tse MM, Wan VT, Ho SS. Physical exercise: Does it help in relieving pain and increasing mobility among older adults with chronic pain? J Clin Nurs 2011;20(5-6):635–44. [DOI] [PubMed] [Google Scholar]
- 16. Johnson AJ, Terry E, Bartley EJ, et al. Resilience factors may buffer cellular aging in individuals with and without chronic knee pain. Mol Pain 2019;15:1744806919842962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartley EJ, Palit S, Fillingim RB, Robinson ME. Multisystem resiliency as a predictor of physical and psychological functioning in older adults with chronic low back pain. Front Psychol 2019;10:1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emerson C, Barhoun P, Olive L, et al. A systematic review of psychological treatments to manage fatigue in patients with inflammatory bowel disease. J Psychosom Res 2021;147:110524. [DOI] [PubMed] [Google Scholar]
- 19. Pressler SJ, Titler M, Koelling TM, et al. Nurse-enhanced computerized cognitive training increases serum brain-derived neurotropic factor levels and improves working memory in heart failure. J Card Fail 2015;21(8):630–41. [DOI] [PubMed] [Google Scholar]
- 20. McCurry SM, Zhu W, Von Korff M, et al. Effect of telephone cognitive behavioral therapy for insomnia in older adults with osteoarthritis pain: A randomized clinical trial. JAMA Intern Med 2021;181(4):530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 1988;54(6):1063–70. [DOI] [PubMed] [Google Scholar]
- 22. Snyder CR, Harris C, Anderson JR, et al. The will and the ways: Development and validation of an individual-differences measure of hope. J Pers Soc Psychol 1991;60(4):570–85. [DOI] [PubMed] [Google Scholar]
- 23. Salsman JM, Victorson D, Choi SW, et al. Development and validation of the positive affect and well-being scale for the Neurology Quality of Life (Neuro-QOL) measurement system. Qual Life Res 2013;22(9):2569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herzberg PY, Glaesmer H, Hoyer J. Separating optimism and pessimism: A robust psychometric analysis of the revised Life Orientation Test (LOT-R). Psychol Assess 2006;18(4):433–8. [DOI] [PubMed] [Google Scholar]
- 25. Hahn EA, DeWalt DA, Bode RK, et al. ; PROMIS Cooperative Group. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol 2014;33(5):490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ Sleep Disturbance and Sleep-Related Impairment item banks. Behav Sleep Med 2011;10(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ameringer S, Elswick RK Jr, Menzies V, et al. Psychometric evaluation of the Patient-Reported Outcomes Measurement Information System Fatigue–Short Form across diverse populations. Nurs Res 2016;65(4):279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saffer BY, Lanting SC, Koehle MS, Klonsky ED, Iverson GL. Assessing cognitive impairment using PROMIS® applied cognition-abilities scales in a medical outpatient sample. Psychiatry Res 2015;226(1):169–72. [DOI] [PubMed] [Google Scholar]
- 29. Terwee CB, Peipert JD, Chapman R, et al. Minimal important change (MIC): A conceptual clarification and systematic review of MIC estimates of PROMIS measures. Qual Life Res 2021;30(10):2729–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Howard MC. A review of exploratory factor analysis decisions and overview of current practices: What we are doing and how can we improve? Int J Hum Comput Interact 2016;32(1):51–62. [Google Scholar]
- 31. Milligan GW, Cooper MC. An examination of procedures for determining the number of clusters in a data set. Psychometrika 1985;50(2):159–79. [Google Scholar]
- 32. Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull 2005;131(6):925–71. [DOI] [PubMed] [Google Scholar]
- 33. Costello NL, Bragdon EE, Light KC, et al. Temporomandibular disorder and optimism: Relationships to ischemic pain sensitivity and interleukin-6. Pain 2002;100(1-2):99–110. [DOI] [PubMed] [Google Scholar]
- 34. Carlesso LC, Raja Rampersaud Y, Davis AM. Clinical classes of injured workers with chronic low back pain: A latent class analysis with relationship to working status. Eur Spine J 2018;27(1):117–24. [DOI] [PubMed] [Google Scholar]
- 35. Carlesso LC, Tousignant-Laflamme Y, Shaw W, Larivière C, Choinière M. Exploring pain phenotypes in workers with chronic low back pain: Application of IMMPACT recommendations. Can J Pain 2021;5(1):43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coronado RA, Bialosky JE, Robinson ME, George SZ. Pain sensitivity subgroups in individuals with spine pain: Potential relevance to short-term clinical outcome. Phys Ther 2014;94(8):1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rabey M, Slater H, O’Sullivan P, Beales D, Smith A. Somatosensory nociceptive characteristics differentiate subgroups in people with chronic low back pain: A cluster analysis. Pain 2015;156(10):1874–84. [DOI] [PubMed] [Google Scholar]
- 38. Rabey M, Smith A, Beales D, Slater H, O’Sullivan P. Differing psychologically derived clusters in people with chronic low back pain are associated with different multidimensional profiles. Clin J Pain 2016;32(12):1015–27. [DOI] [PubMed] [Google Scholar]
- 39. Viniol A, Jegan N, Hirsch O, et al. Chronic low back pain patient groups in primary care—A cross sectional cluster analysis. BMC Musculoskelet Disord 2013;14(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sturgeon JA, Zautra AJ. Social pain and physical pain: Shared paths to resilience. Pain Manag 2016;6(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bean DJ, Dryland A, Rashid U, Tuck NL. The determinants and effects of chronic pain stigma: A mixed methods study and the development of a model. J Pain 2022;23(10):1749–64. [DOI] [PubMed] [Google Scholar]
- 42. Hausmann LR, Parks A, Youk AO, Kwoh CK. Reduction of bodily pain in response to an online positive activities intervention. J Pain 2014;15(5):560–7. [DOI] [PubMed] [Google Scholar]
- 43. Hausmann LRM, Youk A, Kwoh CK, et al. Testing a positive psychological intervention for osteoarthritis. Pain Medicine 2017;18(10):1908–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muller R, Gertz KJ, Molton IR, et al. Effects of a tailored positive psychology intervention on well-being and pain in individuals with chronic pain and a physical disability: A feasibility trial. Clin J Pain 2016;32(1):32–44. [DOI] [PubMed] [Google Scholar]
- 45. Müller R, Segerer W, Ronca E, et al. Inducing positive emotions to reduce chronic pain: A randomized controlled trial of positive psychology exercises. Disabil Rehabil 2022;44(12):2691–704. [DOI] [PubMed] [Google Scholar]
- 46. Peters ML, Smeets E, Feijge M, et al. Happy despite pain: A randomized controlled trial of an 8-week internet-delivered positive psychology intervention for enhancing well-being in patients with chronic pain. Clin J Pain 2017;33(11):962–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Janevic M, Robinson-Lane SG, Courser R, Brines E, Hassett AL. A community health worker–led positive psychology intervention for African American older adults with chronic pain. Gerontologist 2022;62(9):1369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Braunwalder C, Müller R, Glisic M, Fekete C. Are positive psychology interventions efficacious in chronic pain treatment? A systematic review and meta-analysis of randomized controlled trials. Pain Medicine 2022;23(1):122–36. [DOI] [PubMed] [Google Scholar]
- 49. Liu JJW, Reed M, Girard TA. Advancing resilience: An integrative, multi-system model of resilience. Pers Individ Diff 2017;111:111–8. [Google Scholar]
- 50. Ungar M, Theron L, Murphy K, Jefferies P. Researching multisystemic resilience: A sample methodology. Front Psychol 2020;11:607994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Strath LJ, Brooks MS, Sorge RE, Judd SE. Relationship between diet and relative risk of pain in a cross-sectional analysis of the REGARDS longitudinal study. Pain Manage 2022;12(2):168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pinto RZ, Ferreira PH, Kongsted A, et al. Self-reported moderate-to-vigorous leisure time physical activity predicts less pain and disability over 12 months in chronic and persistent low back pain. Eur J Pain 2014;18(8):1190–8. [DOI] [PubMed] [Google Scholar]
- 53. Jackson P, Goodin BR, Long DL, et al. The area deprivation index corresponds effectively with other measures of objective socioeconomic status in adults with chronic low back pain. J Nurs Meas 2022;30(3):433–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jenkins PJ, Perry PR, Yew Ng C, Ballantyne JA. Deprivation influences the functional outcome from total hip arthroplasty. Surgeon 2009;7(6):351–6. [DOI] [PubMed] [Google Scholar]
- 55. Bartley EJ, Hossain NI, Gravlee CC, et al. Race/ethnicity moderates the association between psychosocial resilience and movement-evoked pain in knee osteoarthritis. ACR Open Rheumatol 2019;1(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morais CA, Fullwood D, Palit S, et al. Race differences in resilience among older adults with chronic low back pain. J Pain Res 2021;14:653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Halfon M, Guanhong M, Bartley EJ, Fillingim RB, Morais CA. Exploring the association of ethnic identity and pain resilience: A pilot study in the Latinx/Hispanic community. J Pain 2022;23(5):36. [Google Scholar]
- 58. Edwards RR, Dworkin RH, Turk DC, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain Rep 2021;6(1):e896. [DOI] [PMC free article] [PubMed] [Google Scholar]