Abstract
Background
At present, the only definitive treatment for adult phimosis is circumcision, which is a surgical removal of the prepuce. Novoglan is a novel device that could offer patients with phimosis an alternative to surgery. It is based on application of custom-moulded balloons for gradual skin remodelling and prepuce dilatation. This open-label clinical trial aimed to investigate the safety, efficacy and tolerability of the Novoglan treatment.
Methods
A prospective trial was conducted on 20 patients with adult phimosis recruited at Macquarie University Hospital and Princess Alexandra Hospital. After eligibility screening and enrolment, patients were provided with the Novoglan product and training. The treatment involved twice daily 10-minute applications for a duration of 4–8 weeks with patient’s degree of phimosis assessed before and at 6–8 weeks after the initiation of the treatment. Participants were also asked to complete questionnaires aimed to assess the safety and tolerability of the Novoglan treatment.
Results
The treatment was successful with improved foreskin retraction in 90% of patients and all patients achieving full foreskin retraction after the treatment. Ninety-five percent of patients reported reduced level of anxiety, and over 60% of patients reported reduced pain/discomfort during sexual activity or in general. Similarly, 95% of patients were moderately-to-very satisfied with the treatment and would recommend Novoglan to others. No adverse events were observed and only 15% of participants reported minor side effects.
Conclusions
The Novoglan-01 trial demonstrated high safety, efficacy and tolerability of the Novoglan treatment for adult phimosis and its high potential as a conservative alternative to circumcision or steroid cream treatment.
Trial Registration
The Novoglan-01 study has been registered with the Australia and New Zealand Clinical Trial Registry under the reference ACTRN 1262 10009 24853, dated 15 July 2021.
Keywords: Phimosis, adult male phimosis, circumcision, conservative treatment of phimosis
Highlight box.
Key findings
• The Novoglan treatment is safe, effective and highly tolerable alternative to circumcision in adults with phimosis with all patients achieving foreskin retraction after the treatment.
What is known and what is new?
• Circumcision remains the standard of care for treatment of adult phimosis, however many patients would prefer non-surgical approach.
• Novoglan-01 clinical trial has shown that non-surgical Novoglan treatment could improve foreskin retraction in adults with phimosis without significant side effects or discomfort.
What is the implication, and what should change now?
• The findings of the Novoglan-01 clinical trial demonstrate high potential of Novoglan as a conservative first line treatment in adults with phimosis.
Introduction
Male phimosis is characterised by stenosis of the foreskin resulting in the inability of the prepuce to retract over the glans penis or tightness of the foreskin when retracted. Physiological phimosis is a condition in infants and boys which usually disappears by the age of 10 (1). Pathological phimosis can occur at any age in uncircumcised adult men and is frequently associated with lichen sclerosus (also known as Balanitis Xerotica Obliterans) or balanitis/balanoposthitis. Phimosis can be graded according to a scale originally developed by Kikiros, Beasley and Woodward (2). While the data on the prevalence of adult phimosis is limited, a recent systematic review by Morris et al. (1) estimated the risk of phimosis in males over 18 years of age to be 3.4% [95% confidence interval (CI): 1.8–6.6%] and its prevalence is expected to rise in the near future given decline in the rate of newborn circumcision (3). Adult phimosis often requires treatment as it can cause significant and often painful symptoms such as recurrent infections and ballooning of the foreskin during urination or pain during sexual intercourse (4).
Phimosis has been traditionally managed by circumcision. While this is effective and provides a permanent solution, it is not without complications. These can include pain, bleeding, swelling, inadequate skin removal, wound infection, oedema, urinary retention, meatal stenosis, foreskin adhesion and fistulas and have been reported to reach 2.4% to 17.7% (5-8). Patients with phimosis, therefore, often wish to trial other methods of treatment before proceeding with circumcision. These methods include preputioplasty, or surgical treatment of phimosis with preservation of the foreskin (9) or conservative treatments, such as use of corticosteroid creams (2,10) with progressive stretching of the prepuce and application of stretching devices (11,12).
Recently, a new conservative treatment option, the Novoglan product, has shown promising results, with a high success rate and satisfaction reported by users. Novoglan is a novel medical device for conservative treatment for adult phimosis that involves the use of a balloon that can be inserted underneath the opening of the foreskin (between the glans and the preputial mucosa), progressively inflated by a user and kept in place. The inflation is controlled by the user to ensure safe and painless application. The patient is advised to use the device twice daily for a period of 4 to 8 weeks until full normal prepuce retraction is achieved. The Novoglan-01 clinical trial aimed to confirm the safety, efficacy, and tolerability of the Novoglan product as a conservative treatment for adult phimosis. We present this article in accordance with the TREND reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-91/rc).
Methods
Novoglan device
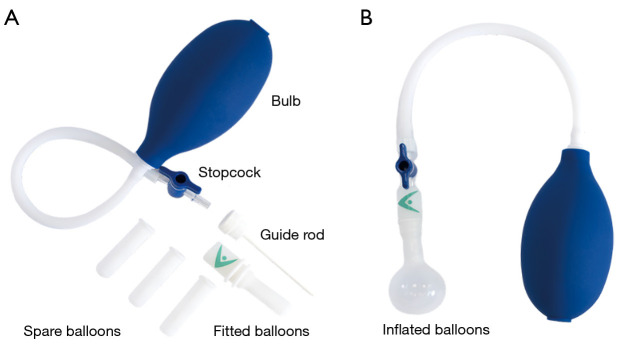
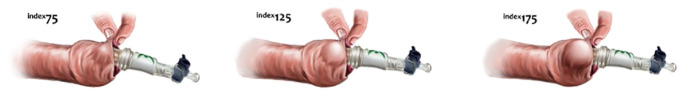
The Novoglan product is a medical device commercialised in Australia since 2006 and listed with the Australian Therapeutics Goods Administration (ARTG 168962) as a conservative treatment option designed for adult patients with phimosis. As shown at Figure 1A, its main component is a small balloon that can be inserted underneath the opening of the foreskin and progressively inflated causing skin stretching by providing gentle and even pressure. The Novoglan balloon is guided under the foreskin between the glans and the prepuce using a purpose moulded rod (Figure 1A) and placed in the same position every time. Once the balloon is in place the rod is removed, a stopcock is attached, and balloon is inflated by the patient using an inflation bulb. The assembled Novoglan device with inflated balloon can be seen at Figure 1B. The pressure is under control of the user to ensure foreskin stretching with no discomfort (Figure 2). The Novoglan device is applied for 10 minutes twice daily for a total duration of 4–8 weeks. The Novoglan kits were provided for the study free of charge by Platigo Solutions Pty Ltd.
Figure 1.
The components of the Novoglan device (A) and assembled device with balloon inflated (B).
Figure 2.
Application of the Novoglan device with patient-controlled foreskin stretching.
Ethics
The Novoglan-01 clinical trial was approved by the Human Research Ethics Committees at Macquarie University (Macquarie University HREC, Reference Nos. 520193 3799 8816 and 52020 3799 21701) and Royal Alexandra Hospital (Queensland Health Metro South HREC, Reference No. HREC/2020/QMS/66682). Both Macquarie University HREC and Queensland Health Metro South HREC follow the Australian Code for the Responsible Conduct of Research and the National Statement on Ethical Conduct in Human Research. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants gave informed consent before taking part in the study.
Participants
The Novoglan-01 clinical trial has been conducted at the Urology Department at Macquarie University Hospital, Sydney, NSW and at Princess Alexandra Hospital, Brisbane, QLD, Australia, where a total of 20 consecutive phimosis patients have been screened and enrolled. To take part in the study, these patients had to be male 18 or older, referred to the urology clinic, have symptoms of adult phimosis, report pain or discomfort, and be willing to provide informed consent as well as participate and comply with the study requirements. The exclusion criteria were: bleeding, ulcer or active infection of the penis, prior prepuce surgery, except for frenuloplasty, hypospadias, severe scaring of the glans or foreskin, history of penile cancer, and history of a psychological illness or other conditions which may interfere with their ability to understand the study requirements. Based on a power calculation, we required at least 18 participants to complete the treatment. Following an interim analysis after recruitment of the first 20 participants, a decision was made to analyse and publish the results given compliance and the size of the effect achieved.
Objectives and trial design
The hypothesis for the Novoglan-01 study is that the Novoglan product is an alternative conservative treatment for adult phimosis that is safe, effective and well tolerated by participants. The primary study objective was to determine the efficacy of the Novoglan treatment by measuring the degree of phimosis before and after the treatment. The first of secondary objectives was to assess the safety of the Novoglan treatment by observing and reporting any treatment discomfort or adverse event experienced by the participants. The adverse events were defined as per Therapeutic Goods Administration Medical device incident reporting & investigation scheme and included serious conditions that require hospitalisation, have risk of permanent damage, life-threatening or leading to death. (please see https://www.tga.gov.au/resources/resource/guidance/medical-device-incident-reporting-investigation-scheme-iris). The other secondary objective was to confirm the participant’s satisfaction with the Novoglan treatment by assessing the quality-of-life improvement and treatment tolerability as reported by the participant at the end of the trial. The Novoglan-01 clinical trial is an investigator-initiated, multicentre, single arm, non-randomised, observational, prospective study.
Intervention
The interventions of the Novoglan-01 study included 5 contacts (Table 1), where every step concluded with confirmation of date and time for the next contact.
Table 1. The Novoglan-01 trial timelines.
| Study visit | Timeline | Interventions in summary |
|---|---|---|
| Enrolment Visit | As scheduled with Site Investigator | Eligibility screening and enrolment |
| Study Visit 1 | Minimum 1 week following Enrolment Visit | Phimosis measurement, quality of life questionnaire, Novoglan product supply and training |
| Study Visit 2 (phone) | 1 week following Study Visit 1 | Treatment progress and adverse events |
| Study Visit 3 (phone) | 4 weeks following Study Visit 1 | Treatment progress and adverse events |
| Final Visit | 6–8 weeks following Study Visit 1 | Phimosis measurement, quality of life questionnaire, tolerability questionnaire, trial completion |
The first contact was the Enrolment Visit, where the Site Investigator collected patient’s demographics and medical history with a particular focus on phimosis and conducted a general medical examination and penile examination to confirm phimosis. The Site Investigator then confirmed patient’s eligibility for the study and invited them to participate. If the patient agreed to participate in the study, they were then asked to sign the Novoglan-01 Participant Informed Consent Form and assigned a Novoglan-01 Study unique participant identifier.
The second contact was the Study Visit 1, scheduled at a minimum of 1 week following the Enrolment Visit. During the Study Visit 1, the degree of patient’s phimosis was determined using a revised measurement scale based on Kikiros et al. (2), an evaluation of the participant quality of life was carried out using the Novoglan-01 Quality-of-Life Questionnaire, and the Novoglan investigative product as well as printed how-to-use guide were supplied to the participant. The participants were then explained the various components of the Novoglan product and trained in its appropriate and safe use. The Novoglan-01 Quality-of-Life Questionnaire has been specifically designed for this study.
The third and fourth contacts were the Study Visits 2 and 3 conducted by telephone and scheduled at 1 and 4 weeks, respectively, following the Study Visit 1. During these contacts, the participants were offered the option to schedule an additional face-to-face training and asked about the overall progress with the treatment, problems in using the Novoglan product, adverse events, and changes to the concomitant medications.
The fifth and final contact was the Final Visit scheduled at 6–8 weeks following Study Visit 1. During that visit, the assessment of the participant’s phimosis was conducted, the participant’s quality of life was assessed using the Novoglan-01 Quality-of-Life Questionnaire, and participant’s perceived tolerability of the Novoglan product was assessed using the Novoglan-01 Treatment Tolerability Questionnaire, both of which were specifically designed for the Novoglan-01 clinical study. The final outcomes of the treatment were then reviewed and discussed with participant by a Site Investigator.
The provisions for modifying allocated interventions included the option of organising additional training session of the use of Novoglan device either by phone or in person at Study Visits 2 or 3. Participants could terminate their participation in the trial at any time. The strategies to ensure adherence to the intervention protocols included offering additional training sessions on the Novoglan device to participants identified as in need of it during Study Visits 2 or 3. There were no other specific concomitant care or interventions permitted or prohibited during the trial other than those specified in the eligibility criteria.
While at the Princess Alexandra Hospital participants were assessed by the site investigator at every step, at Macquarie University Hospital step 1 was conducted by the Site Investigator, steps 2–4 were conducted by a Clinical Trial Nurse and step 5 was conducted by both site investigator and the clinical trial nurse.
Outcome measures
The expected outcomes from the Novoglan-01 study were as follows:
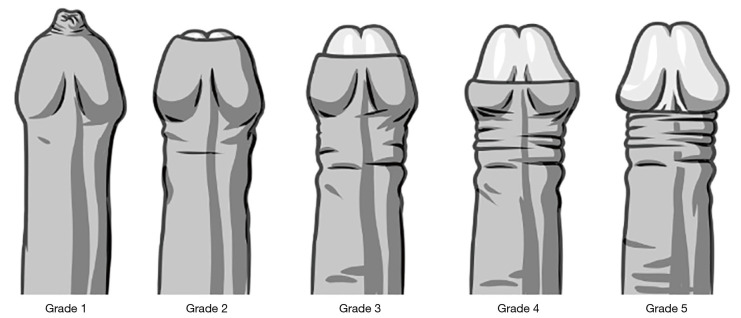
❖ Confirmation of the ability of the Novoglan product to effectively relieve phimosis as measured by foreskin retraction before and after treatment against the Phimosis Measurement Scale (Table 2, Figure 3) at Study Visit 1 and Final Study Visit.
❖ Confirmation of the safety of the Novoglan treatment as evidenced by the lack of any adverse events or significant side effects during treatment.
❖ Confirmation of the satisfaction of participants with the Novoglan treatment as measured by the Quality-of-Life Questionnaire completed before and after treatment.
❖ Confirmation of the tolerability of the Novoglan treatment as measured by the treatment tolerability questionnaire filled in by the participants during the Final Visit.
❖ Confirmation of the Novoglan treatment as a valid conservative first line treatment alternative to steroid creams in adult males presenting with phimosis.
Table 2. The phimosis measurement scale used to assess the efficacy of the treatment.
| Phimosis grade | Description |
|---|---|
| 1 | Absolutely no retraction |
| 2 | Slight retraction leaving a gap between the tip of the prepuce and the glans, i.e., neither meatus nor glans are exposed |
| 3 | Partial retraction, just sufficient to see the glandular meatus |
| 4 | Partial retraction, exposing part of the glans |
| 5 | Full retraction of the foreskin, tight behind the glans |
| 6 | Full and free retraction of the foreskin, no tightness behind the glans |
Figure 3.
The phimosis measurement scale used to assess foreskin retraction and the efficacy of the treatment.
Statistical analysis
The Novoglan-01 data involved Phimosis assessment and the Quality-of-Life Questionnaire (both ordinal, scalar) completed pre- and post-treatment and treatment tolerability questionnaire completed by the participants after the treatment. The statistical analysis of the Novoglan-01 trial has also involved descriptive variables collected for each patient such as physical parameters (e.g., age, weight, height—numerical variables), medical history (categorical, binary) and record of adverse events (categorical, binary). Univariate analysis was used for these data.
The sample size was determined using a power analysis to achieve a power of 0.8 in a paired-samples t-test with an alpha level of 0.05. Based on an expected standard deviation of 1.5 for the paired differences, the computed Cohen’s d for the size effect of the paired-samples t-test was 0.67. Using all the above inputs, the minimum required sample size for the paired-samples t-test was calculated to be 18.
Results
Patient recruitment and clinical characteristics
From September 2019 to May 2022, a total of 20 consecutive patients were screened as potential participants for this study. All 20 study participants met the inclusion criteria and decided to proceed with the study. As shown in Tables 3,4, the participant population was a mix of 65% Caucasians and 35% Asians of a mean age of 32.10 years and a mean body mass index (BMI) of 25.16 kg/m2 which is considered as overweight by the Heart Foundation (see: https://www.heartfoundation.org.au). Eighty percent of participants had no relevant medical history, but 4 participants had some other condition or illness. There were 7 participants with evidence of lichen sclerosus and no evidence of prior frenuloplasty in any participant. It is important to note that the diagnosis of lichen sclerosus was based only on clinical examination, which has been shown to be inaccurate in some instances (13). In terms of concomitant drug use, one participant reported use of statin and one reported use of statin and a blood pressure medication.
Table 3. Descriptive characteristics of the participants recruited in the Novoglan-01 clinical trial.
| Characteristic | N | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Age (years) | 20 | 20.00 | 63.00 | 32.10 | 12.20 |
| Weight (kg) | 20 | 62.80 | 126.00 | 79.40 | 15.90 |
| Height (cm) | 19 | 164.00 | 189.00 | 178.70 | 6.60 |
| BMI (kg/m2) | 19 | 20.30 | 40.70 | 25.16 | 5.30 |
SD, standard deviation; BMI, body mass index.
Table 4. Characteristics of participants recruited in the Novoglan-01 clinical trial.
| Characteristic | Frequency | Percent |
|---|---|---|
| Ethnicity | ||
| Caucasian | 13 | 65 |
| Asian | 7 | 35 |
| Relevant medical history | 4 | 20 |
| Evidence of lichen sclerosus | 7 | 35 |
| Prior frenuloplasty | 0 | 0 |
Treatment efficacy
The degree of phimosis was assessed before the treatment and at the final visits at 6 to 8 weeks after the initiation of the treatment as a measurement of its efficacy. The findings can be seen in Table 5. Of note, while at the first visit 17 patients were classified as grades 1–4, three patients had grade 5 and no patients had their phimosis assessed as grade 6, all 20 patients have been assessed as either grade 5 or 6 at the final visit with 10 patients (50%) in each group.
Table 5. The number of participants with phimosis measurement rank 1–6 at first visit and their respective distribution at final visit (count and percent).
| Score at Visit 1 | Number of patients, Visit 1 | Score at Final Visit | ||
|---|---|---|---|---|
| 1–4 | 5 | 6 | ||
| 1 | 1 (5%) | – | 1 | – |
| 2 | 7 (35%) | – | 2 | 5 |
| 3 | 6 (30%) | – | 3 | 3 |
| 4 | 3 (15%) | – | 2 | 1 |
| 5 | 3 (15%) | – | 2 | 1 |
| 6 | – | – | – | – |
| Total | – | – | 10 (50%) | 10 (50%) |
As shown at Table 5 and Figure 4, 6 participants (30%) had a four-degree improvement with improvement from phimosis measurement 1 to 5 (1 participant) and from 2 to 6 (5 participants). Five participants (25%) experienced a three-degree improvement with their phimosis score changing from 2 to 5 (2 participants) and from 3 to 6 (3 participants). Four participants (20%) had a two-degree improvement from phimosis measurement changing from 3 to 5 (3 participants) and from 4 to 6 (1 participant). Three participants (15%) had a one-degree improvement with phimosis score changing from 4 to 5 and from 5 to 6 in two and one participants, respectively. Only 2 participants (10%) had no improvement of the phimosis measurement, which remained at 5.
Figure 4.
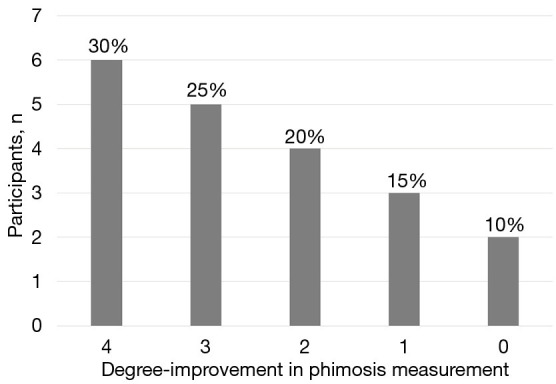
Bar-chart representing the number of participants with 4-, 3-, 2- and 1-degree improvement or no change of their phimosis measurement.
Quality of life
The effect of phimosis on patient’s quality of life was assessed before and after Novoglan treatment in terms of general pain/discomfort, pain/discomfort during sexual activity, impact on usual activities, and anxiety by using specifically designed questionnaires (Table 6). The level of pain or discomfort of participants was none to moderate at Visit 1 and only none or slight at the Final Visit. Out of 8 participants reporting slight or moderate general pain/discomfort at the First Visit, 6 participants reported reduction in general pain/discomfort, while 2 participants reported their level of pain/discomfort remained unchanged at the Final Visit. Two participants (10%) reported new slight general pain or discomfort at the Final Visit. Nineteen participants (95%) reported pain (slight-to-severe) during sexual activity at Visit 1. Following Novoglan treatment 13 participants (68%) observed reduction to slight or no pain at all with no severe pain reported at the final visit. Phimosis did not appear to significantly affect usual activities with only one patient reporting slight problem at Visit 1. This patient reported no impact on usual activities after the treatment, while one other patient developed slight problems doing usual activities. In terms of anxiety or depression, all patients reported it at slight (16 participants, 80%) or moderate (4 participants, 20%) level at Visit 1 with 19 participants (95%) reporting reduced level of anxiety/depression following the treatment.
Table 6. The quality-of-life assessment before and after the Novoglan treatment.
| Ranking [scale 1 to 5] | Visit 1 | Final Visit | |||
|---|---|---|---|---|---|
| Count | Percent | Count | Percent | ||
| General pain/discomfort | |||||
| None [1] | 12 | 60 | 15 | 75 | |
| Slight [2] | 5 | 25 | 5 | 25 | |
| Moderate [3] | 3 | 15 | 0 | 0 | |
| Pain/discomfort during sexual activity | |||||
| No pain [1] | 1 | 5 | 13 | 65 | |
| Slight [2] | 8 | 40 | 5 | 25 | |
| Moderate [3] | 10 | 50 | 2 | 10 | |
| Severe [4] | 1 | 5 | 0 | 0 | |
| Impact of usual activities | |||||
| No problem doing usual activities [1] | 19 | 95 | 19 | 95 | |
| Slight problems doing usual activities [2] | 1 | 5 | 1 | 5 | |
| Anxiety/depression | |||||
| Not anxious/depressed [1] | 0 | 0 | 17 | 85 | |
| Slightly anxious/depressed [2] | 16 | 80 | 3 | 15 | |
| Moderately anxious/depressed [3] | 4 | 20 | 0 | 0 | |
Overall, Novoglan treatment was found to reduce the level of anxiety in 95% of participants, level of general pain/discomfort during sexual activity in 68% of participants and in general pain/discomfort in 63% of participants, with no worsening anxiety or pain during sexual activity reported. Only 2 participants (10%) reported new slight general pain/discomfort after the treatment, however, no significant impact on usual activities was observed. Such slight pain/discomfort could be attributed to a period of desensitization needed for a newly exposed glans or to a short-term discomfort observed in a small minority of patients after skin stretching.
Treatment tolerability and satisfaction
As part of the Novoglan treatment assessment, at the final visit patients were asked to provide feedback on usefulness of the Novoglan demonstration and user guide, ease of understanding how Novoglan works, ease and confidence in Novoglan application, pain associated with Novoglan use, the overall satisfaction with the treatment and whether they would recommend it to others.
All participants found the demonstration and the user guide useful or very useful (Table 7). Seventeen participants (85%) found understanding how to use Novoglan easy or very easy and 18 participants (90%) felt confident they had used the product correctly. Similarly, 18 participants (90%) had only slight or no pain in using Novoglan and a total of 16 participants (80%) were satisfied or very satisfied with the treatment. While 3 participants (15%) were moderately satisfied and 1 participant (5%) reported dissatisfaction, a total of 19 participants (95%) would recommend the Novoglan treatment to others. The Novoglan treatment was reported as progressing well by 95% of participants with no participant requiring additional training.
Table 7. Novoglan tolerability and satisfaction.
| Ranking [scale 1 to 4] | Count | Percent |
|---|---|---|
| Usefulness of demonstration | ||
| Very useful [1] | 16 | 80 |
| Useful [2] | 4 | 20 |
| Usefulness of User Guide | ||
| Very useful [1] | 16 | 80 |
| Useful [2] | 4 | 20 |
| How easy to understand the use of Novoglan? | ||
| Very easy [1] | 15 | 75 |
| Easy [2] | 2 | 10 |
| Moderately easy [3] | 3 | 15 |
| How easy to use did you find Novoglan? | ||
| Very easy [1] | 13 | 65 |
| Easy [2] | 4 | 20 |
| Moderately easy [3] | 3 | 15 |
| How confident do you feel that you used Novoglan correctly? | ||
| Very confident [1] | 13 | 65 |
| Confident [2] | 5 | 25 |
| Somewhat confident [3] | 2 | 10 |
| How painful was it to use Novoglan? | ||
| Not painful at all [1] | 12 | 60 |
| Slightly [2] | 6 | 30 |
| Moderately [3] | 1 | 5 |
| Moderately-to-quite painful [3 to 4] | 1 | 5 |
| How overall satisfied are you with your Novoglan treatment? | ||
| Very satisfied [1] | 12 | 60 |
| Satisfied [2] | 4 | 20 |
| Moderately satisfied [3] | 3 | 15 |
| Quite dissatisfied [4] | 1 | 5 |
| Novoglan treatment recommendation to others | ||
| Maybe | 1 | 5 |
| Yes | 19 | 95 |
Within the Quality-of-Life Questionnaire, participants were also asked about their perception of circumcision as a treatment option before and after the Novoglan treatment. The number of patients considering circumcision as a near future treatment option dropped by 69.2% following the treatment from 13 participants at Visit 1 to 4 participants at the Final Visit.
Side effects and adverse events
There was no adverse event reported during the trial period by any participant. The side effects assessed at Visits 2, 3 and Final Visit were pain in using Novoglan, foreskin itching or discoloration or other side effects (Table 8). Pain was reported by 2 patients at each visit. Similarly, 2 participants (10%) reported itching of the foreskin at Visit 2, however, no participants reported it at Visit 3 and only one at the Final Visit. Foreskin discoloration was experienced by one participant at Visit 2 and has not been reported by anyone at following visits. Foreskin discomfort has been reported by only three participants at Visit 2 and by one and two participants at third and final visits, respectively. Only one participant reported other side effect (discharge under foreskin) at the Final Visit with none reported at Visits 2 and 3.
Table 8. Side effects reported by the participants.
| Side effect | Visit 2 | Visit 3 | Final Visit | |||||
|---|---|---|---|---|---|---|---|---|
| Count | Percent | Count | Percent | Count | Percent | |||
| Any pain in using Novoglan | ||||||||
| No | 18 | 90 | 18 | 90 | 18 | 90 | ||
| Yes | 2 | 10 | 2 | 10 | 2 | 10 | ||
| Any itching of the foreskin in using Novoglan | ||||||||
| Missing | 1 | 5 | 0 | 0 | 0 | 0 | ||
| No | 17 | 85 | 20 | 100 | 19 | 95 | ||
| Yes | 2 | 10 | 0 | 0 | 1 | 5 | ||
| Any foreskin discoloration in using Novoglan | ||||||||
| Missing | 1 | 5 | 0 | 0 | 0 | 0 | ||
| No | 18 | 90 | 20 | 100 | 20 | 100 | ||
| Yes | 1 | 5 | 0 | 0 | 0 | 0 | ||
| Any foreskin discomfort in using Novoglan | ||||||||
| No | 17 | 85 | 19 | 95 | 18 | 90 | ||
| Yes | 3 | 15 | 1 | 5 | 2 | 10 | ||
| Any other side effect in using Novoglan | ||||||||
| No | 20 | 100 | 20 | 100 | 19 | 95 | ||
| Yes | 0 | 0 | 0 | 0 | 1 | 5 | ||
Only one participant reported other side effect—discharge under foreskin.
Overall, the vast majority of participants did not report any side effect in using Novoglan, with only 1 or 2 participants reporting side effect. Added to the lack of any adverse event, this confirms the strong safety profile of Novoglan.
Discussion
Circumcision offers a definitive treatment for adult phimosis. While it has a low rate of complications and they tend to be moderate and transient, patients may experience pain, bleeding, swelling, inadequate skin removal, wound infection, oedema, urinary retention, meatal stenosis, foreskin adhesion and fistulas (5-7). Of note, in a recent literature review, Bossio and colleagues noted that complications following circumcision are more frequent in adult than in children or infants and have been reported to reach 2.4% to 17.7% (8). A study by El Bcheraoui et al. later showed a similar trend with a very low rate of complications of 0.40% (95% CI: 0.39–0.41%) in infants, going up to 9.06% for circumcision performed between the ages of one and nine and 5.31% for males over 10 years of age (6). Additionally, several methods of preputioplasty, or surgical treatment of phimosis with preservation of the foreskin have been described (9).
Corticosteroid therapy has been introduced by Kikiros and colleagues (2) in the early 1990s as a conservative alternative treatment for phimosis. Such treatment is performed by the patient over a period of 4 to 8 weeks with application of the cream on the tip of the foreskin opening with gentle and progressive stretching of the prepuce over the glans, with an aim to stimulate the growth of the foreskin and its expansion over time (14,15). This form of phimosis treatment has been extensively studied and applied over the past three decades and has been effective in 71.1–91.1% of cases with a recurrence rate of up to 29.5% (10,14-17). Other methods of conservative treatment include recently developed devices for phimosis treatment, however, data on their application is very limited with some devices being suitable only for patients with low-grade phimosis, who can at least partially retract the foreskin and expose the glans (11,12). Due to higher prevalence of phimosis in children, some methods of phimosis treatment were validated in paediatric populations only and require careful interpretation and further assessment in adults.
The Novoglan treatment appears to be a potential alternative to existing methods of phimosis treatment with high levels of safety and efficacy and very low rate of side effects. It demonstrated outstanding efficacy in terms of the improvement of foreskin retraction with all patients achieving full foreskin retraction after the treatment. Unlike other methods, Novoglan has been most effective in patients with more advanced phimosis who had either no foreskin retraction or could only partially expose the meatus (grades 1–3) before the treatment and were all able to achieve full retraction (grades 5–6) after the Novoglan treatment. Grade 6 foreskin retraction has been achieved by 57% of patients with grades 1–3 disease and by only 33.3% of patients with grades 4 or 5 phimosis. The Novoglan treatment demonstrated significant improvement in quality of life of the participants reducing anxiety in 95% of participants, pain/discomfort in 63% of participants who initially reported general pain/discomfort and 68% of patients who reported pain/discomfort during sexual activity before the treatment. Only 2 participants (10%) reported new slight general pain/discomfort after the treatment. Similarly, Novoglan demonstrated high levels of tolerability and satisfaction with only 2 participants (10%) reporting more than slight pain during its use, only 1 participant (5%) reporting overall dissatisfaction and no participants requiring additional training on the use of Novoglan. Importantly, the Novoglan treatment demonstrated excellent safety with no adverse events reported and side effects reported by only 10–15% of participants throughout the visits.
This study successfully demonstrated the efficacy and safety of Novoglan treatment, its tolerability and patient’s satisfaction. The study population was well-representative of phimosis patients as it included patients with all degrees of phimosis from absolutely no retraction (grade 1) to males whose foreskin was fully retractable, but tight behind the glans (grade 5). The main limitations of this study were relatively low number of participants, and lack of long-term follow up data available at this stage as other conservative methods of phimosis treatment have been reported to suffer from phimosis recurrence. Another potential limitation is that all participants of this study were recruited in Australia and could not be representative of populations in other parts of the world, although participants were recruited across 2 states with multiethnic demographics. To further address these limitations, the recruitment for a study with a larger number of participants and longer follow up is planned to commence with the aim to collect data needed for consideration of the Novoglan product as part of the conservative treatment guidelines for adult phimosis.
Conclusions
The Novoglan-01 study has demonstrated Novoglan device to be a safe and effective treatment for adult phimosis with all patients being able to retract foreskin after the treatment. While larger studies and head-to-head comparison to current surgical and conservative methods of phimosis treatments are needed, results from the Novoglan-01 study clearly demonstrate the high potential of Novoglan as first-line phimosis treatment.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by Platigo Solutions Pty Ltd.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Novoglan-01 clinical trial was approved by the Human Research Ethics Committees at Macquarie University (Macquarie University HREC, Reference Nos. 520193 3799 8816 and 52020 3799 21701) and Royal Alexandra Hospital (Queensland Health Metro South HREC, Reference No. HREC/2020/QMS/66682). Both Macquarie University HREC and Queensland Health Metro South HREC follow the Australian Code for the Responsible Conduct of Research and the National Statement on Ethical Conduct in Human Research. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants gave informed consent before taking part in the study.
Footnotes
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-91/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-91/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-91/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-91/coif). EC serves as an unpaid editorial board member of Translational Andrology and Urology from August 2018 to July 2024. EC reports that Platigo Solutions Pty Ltd. provided investigative product for the study. HM is a consultant of Platigo Solutions Pty Ltd. and received assistance from Platigo Solutions for the manuscript medical writing. AJ is an employee of Platigo Solutions Pty Ltd. and reports that Platigo Solutions Pty Ltd. provided investigative product for the study. DC is an employee of Minomic International Ltd. and received support from Platigo Solutions Pty Ltd. to attend the USANZ 2022 Conference. DG reports that Platigo Solutions Pty Ltd. provided investigative product for the study. The other authors have no conflicts of interest to declare.
References
- 1.Morris BJ, Matthews JG, Krieger JN. Prevalence of Phimosis in Males of All Ages: Systematic Review. Urology 2020;135:124-32. 10.1016/j.urology.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Kikiros CS, Beasley SW, Woodward AA. The response of phimosis to local steroid application. Pediatr Surg Int 1993;8:329-32. 10.1007/BF00173357 [DOI] [Google Scholar]
- 3.Owings M, Uddin S, Williams S. Trends in Circumcision for Male Newborns in U.S. Hospitals: 1979-2010. 2013. Available online: http://www.cdc.gov/nchs/nhds.htm [Google Scholar]
- 4.Czajkowski M, Czajkowska K, Zarańska K, et al. Male Circumcision Due to Phimosis as the Procedure That Is Not Only Relieving Clinical Symptoms of Phimosis But Also Improves the Quality of Sexual Life. Sex Med 2021;9:100315. 10.1016/j.esxm.2020.100315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman B, Khoury J, Petersiel N, et al. Pros and cons of circumcision: an evidence-based overview. Clin Microbiol Infect 2016;22:768-74. 10.1016/j.cmi.2016.07.030 [DOI] [PubMed] [Google Scholar]
- 6.El Bcheraoui C, Zhang X, Cooper CS, et al. Rates of adverse events associated with male circumcision in U.S. medical settings, 2001 to 2010. JAMA Pediatr 2014;168:625-34. 10.1001/jamapediatrics.2013.5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung YC, Chang DC, Westfal ML, et al. A Longitudinal Population Analysis of Cumulative Risks of Circumcision. J Surg Res 2019;233:111-7. 10.1016/j.jss.2018.07.069 [DOI] [PubMed] [Google Scholar]
- 8.Bossio JA, Pukall CF, Steele S. A review of the current state of the male circumcision literature. J Sex Med 2014;11:2847-64. 10.1111/jsm.12703 [DOI] [PubMed] [Google Scholar]
- 9.Osmonov D, Hamann C, Eraky A, et al. Preputioplasty as a surgical alternative in treatment of phimosis. Int J Impot Res 2022;34:353-8. 10.1038/s41443-021-00505-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia de Freitas R, Nobre YD, Demarchi GT, et al. Topical treatment for phimosis: time span and other factors behind treatment effectiveness. J Pediatr Urol 2006;2:380-5. 10.1016/j.jpurol.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 11.Yue YW, Chen YW, Deng LP, et al. Design and development of a new type of phimosis dilatation retractor for children. World J Clin Cases 2021;9:4159-65. 10.12998/wjcc.v9.i17.4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carilli M, Asimakopoulos AD, Pastore S, et al. Can circumcision be avoided in adult male with phimosis? Results of the PhimoStop™ prospective trial. Transl Androl Urol 2021;10:4152-60. 10.21037/tau-21-673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czajkowski M, Żawrocki A, Czajkowska K, et al. Lichen Sclerosus and Phimosis – Discrepancies Between Clinical and Pathological Diagnosis and Its Consequences. Urology 2021;148:274-9. 10.1016/j.urology.2020.11.027 [DOI] [PubMed] [Google Scholar]
- 14.Reddy S, Jain V, Dubey M, et al. Local steroid therapy as the first-line treatment for boys with symptomatic phimosis – a long-term prospective study. Acta Paediatr 2012;101:e130-3. 10.1111/j.1651-2227.2011.02534.x [DOI] [PubMed] [Google Scholar]
- 15.Zhou G, Jiang M, Yang Z, et al. Efficacy of topical steroid treatment in children with severe phimosis in China: A long-term single centre prospective study. J Paediatr Child Health 2021;57:1960-5. 10.1111/jpc.15628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zavras N, Christianakis E, Mpourikas D, et al. Conservative treatment of phimosis with fluticasone proprionate 0.05%: a clinical study in 1185 boys. J Pediatr Urol 2009;5:181-5. 10.1016/j.jpurol.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 17.ter Meulen PH, Delaere KP. A conservative treatment of phimosis in boys. Eur Urol 2001;40:196-9; discussion 200. 10.1159/000049772 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as