We would like to issue a correction for a mistaken phrase ‘time from symptom onset to treatment exposure’ in our published study and replace it with ‘time from symptom onset to admission’ as we stated in Data Source and Supplementary Fig. 1. We provide the detailed instructions for the modifications and the revised version of Fig. 3.
-
(1)
Six modifications (replacing “time from symptom onset to treatment exposure” with “time from symptom onset to admission”) are needed which appeared in Summary/Methods, Baseline covariates, Statistical analysis, Results, Table 1 and Supplementary Table 1.
-
(2)
Similarly, “About 87.3% patients were treated with Azvudine above 5 days of the symptom's onset” in Results should be changed to “About 87.3% patients were admitted above 5 days of the symptom's onset”.
-
(3)
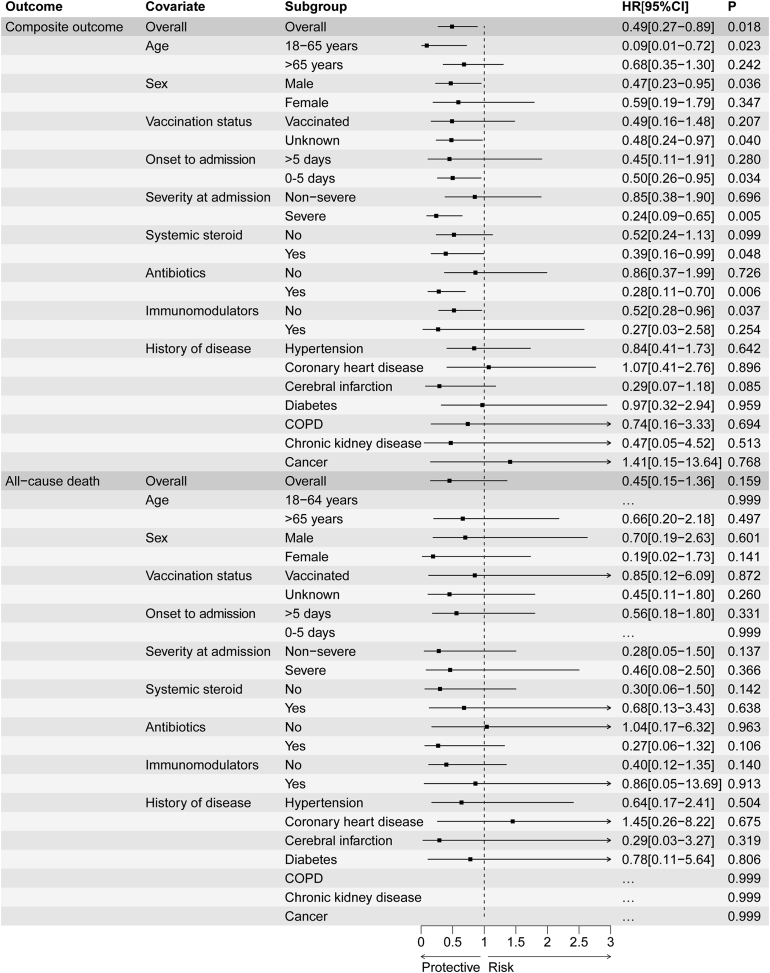
“Onset to exposure” in Fig. 3 should be changed to “Onset to admission”
Fig. 3.
The effectiveness of Azvudine in reducing the risk of composite disease progression outcome and risk of all-cause death by subgroups of selected baseline characteristics. Abbreviation, COPD, chronic obstructive pulmonary disease. Ellipsis (…) means that the model does not converge due to few outcomes; Horizontal lines indicate the ranges of the 95% CIs and the vertical dash lines indicate the hazard ratio of 1.
Table 1.
Baseline characteristics of the participants.
| Baseline characteristics | Before matching |
After 1:1 propensity-score matching |
||||
|---|---|---|---|---|---|---|
| Azvudine (n = 245) | Controls (n = 722) | SMD | Azvudine (n = 245) | Matched controls (n = 245) | SMD | |
| Age (years), mean (SD) | 69.13 (13.4) | 66.89 (14.8) | 0.159 | 69.13 (13.4) | 69.25 (14.0) | 0.022 |
| Sex, n (%) | 0.079 | 0.025 | ||||
| Men | 154 (62.9) | 426 (59.0) | 154 (62.9) | 157 (64.1) | ||
| Women | 91 (37.1) | 296 (41.0) | 91 (37.1) | 88 (35.9) | ||
| COVID-19 vaccination status | 0.010 | 0.016 | ||||
| Vaccinated | 118 (48.2) | 344 (47.6) | 118 (48.2) | 120 (49.0) | ||
| Unknown | 127 (51.8) | 378 (52.4) | 127 (51.8) | 125 (51.0) | ||
| Time from symptom onset to admission, n (%) | 0.334 | 0.012 | ||||
| 0–5 days | 214 (87.3) | 537 (74.4) | 214 (87.3) | 215 (87.8) | ||
| >5 days | 31 (12.7) | 185 (25.6) | 31 (12.7) | 30 (12.2) | ||
| Severity at admission, n (%) | 0.189 | 0.060 | ||||
| Non-severe | 88 (35.9) | 196 (27.1) | 88 (35.9) | 81 (33.1) | ||
| Severe | 157 (64.1) | 526 (72.9) | 157 (64.1) | 164 (66.9) | ||
| Concomitant treatments initiated at admission, n (%) | ||||||
| Systemic steroid | 64 (26.1) | 152 (21.1) | 0.119 | 64 (26.1) | 56 (22.9) | 0.076 |
| Antibiotics | 130 (53.1) | 265 (36.7) | 0.333 | 130 (53.1) | 138 (56.3) | 0.065 |
| Immunomodulators | 25 (10.2) | 57 (7.9) | 0.080 | 25 (10.2) | 22 (9.0) | 0.042 |
SMD, Standard mean difference.
Supplementary Table 1.
Propensity-score model conditional on baseline covariates.
| Covariates | Coefficients | Standard Errors | P values |
|---|---|---|---|
| Intercept | −2.185619 | 0.522340 | 2.86e-05 |
| Age (years) | 0.004412 | 0.005592 | 0.430118 |
| Gender (female vs. male) | −0.077771 | 0.157773 | 0.622062 |
| Severity at admission (severe vs. non-severe) | −0.146920 | 0.166242 | 0.376818 |
| COVID-19 vaccination status (vaccinated vs. unknown) | 0.149249 | 0.154748 | 0.334811 |
| Time from symptom onset to admission (0–5 vs. >5 days) | 0.725780 | 0.218937 | 0.000916 |
| Systemic steroid (yes vs. no) | 0.035365 | 0.181723 | 0.845697 |
| Antibiotics (yes vs. no) | 0.544309 | 0.155723 | 0.000473 |
| Immunomodulators (yes vs. no) | 0.550108 | 0.194569 | 0.004694 |
The phrase “time from symptom onset to treatment exposure” initially used in the paper refers to the duration from the onset of COVID-19 symptoms to the period of treatment exposure, while the patients in the control group did not take any antiviral agents during the observation period. In fact, the variable we used for propensity-score matching in the study is the time from symptom onset to admission, but we mistakenly used the expression ‘time from symptom set to treatment exposure’. We want to correct this error in the article and it did not change the results, direction, or conclusions of our study.
Contributor Information
Minxue Shen, Email: shenmx1988@csu.edu.cn.
Furong Zeng, Email: zengflorachn@hotmail.com.
Xiang Chen, Email: chenxiangck@126.com.
Guangtong Deng, Email: dengguangtong@outlook.com.