Highlights
-
•
In 2015–2016 flu season, A/H1N1/pdm09 was predominant all around the world and in our region.
-
•
Software including MEGA-X, MODELLER, UCSF ChimeraX, auto-dock 4.2, and other online tools were used to analyze the phylogenetic relationship, antiviral resistance and vaccine efficiency of selected NA proteins.
-
•
Iranian isolates were located in clade 6B similar to A/Michigan/45/2015 reference sequence.
-
•
The phylogenetic study showed that most Iranian NA sequences (between 2015 and 2016) were located in a single clade and following years were located in its subclade by 3 major mutations (G77R/K, V81A, and J188T).
-
•
Resistant mutations in drug targets of NA including I117M, D151E, I223V, and S247N were ascertained in 10 isolates during the 2015–2016 flu seasons.
Keywords: Neuraminidase, Phylogenetic analysis, Antiviral resistance, Bioinformatics, Vaccine efficiency
Abstract
Influenza A viruses (H1N1) have been consistently one of the most evolving viruses that escape from vaccine-induced immunity. Although there has been a rapid rise in human influenza virus knowledge since the 2009 pandemic, the molecular information about Iranian strains is still inadequate. The aim of this study was to analyze the neuraminidase (NA) segment of the Iranian isolates in terms of phylogenetic, antiviral resistance, and vaccine efficiency. Ninety-three NA sequences collected among 1758 nasopharyngeal swab samples during the 2015–2016 influenza season were sequenced and submitted to NCBI. Moreover, all the submitted Iranian influenza H1N1 NA sequences since 2010 till 2019 were included in the study.
Software including MEGA-X, MODELLER, UCSF ChimeraX, Auto-Dock 4.2, and other online tools were used to analyze the phylogenetic relationship, vaccine efficiency, and binding affinity to sialic acid of the selected NA proteins. Moreover, the information about antiviral drug resistance mutations of NA were gathered and compared to the Iranian NA segments to check the presence of antiviral drug-resistant strains.
The phylogenetic study showed that most Iranian NA sequences (between 2015 and 2016) were located in a single clade and following years were located in its subclade by 3 major mutations (G77R/K, V81A, and J188T). Resistant mutations in drug targets of NA including I117M, D151E, I223V, and S247N were ascertained in 10 isolates during the 2015–2016 flu seasons.
Investigation of vaccination effect revealed that Iranian isolates in 2017 and 2018 were best matched to A/Brisbane/02/2018 (H1N1), and in 2019 to A/Guangdong-Maonan/SWL1536/2019 (H1N1).
Furthermore, we performed an in-silico analysis of NA enzymatic activity of all Iranian sequences by assessment of enzyme stability, ligand affinity, and active site availability. Overall, the enzyme activity of four Iranian strains (AUG84119, AUG84157, AUG84095, and AUG84100) was assumed as the maximum enzyme activity. This study highlighted the evolutionary trend of influenza A virus/H1N1 circulating in Iran, which provides a preliminary viewpoint for a better comprehension of new emerging strains’ virulence and thus, more appropriate monitoring of influenza virus A/H1N1 during each outbreak season.
1. Introduction
According to WHO reports the influenza A virus is responsible for the severe illness of 3 to 5 million cases, and an average of about 500,000 deaths annually. Due to antigenic changes on the influenza virus, periodic update of the strains involved in influenza vaccines is of importance.
One of the major antigens on the surface of influenza A viruses is neuraminidase (NA) with a homotetramer structure. Each monomeric subunit of NA type I is formed of approximately 470 amino acids, which consist of a short cytoplasmic tail (6 amino acids), followed by a transmembrane domain containing 29 amino acids (Blok and Air, 1982), a stalk domain with 46 amino acids, and a globular head domain containing the enzyme active site (Blok and Air, 1982; McAuley et al., 2019). The NA active site consists of conserved residues that either interact directly with sialic acid or has a structural role that holds the catalytic residues (McAuley et al., 2019).
The NA protein as a receptor-destroying enzyme cleaves the sialic acid attached to the hemagglutinin (HA) from the host cell membrane, thereby facilitating the release of progeny viruses and spreading to new cells in the final stage of infection (Wagner et al., 2002). Besides, it was hypothesized that NA may facilitate infection with penetration of the virus into the mucus layer in the host respiratory epithelia by cleaving sialic acids on mucins (Zanin et al., 2016; Clark et al., 2017). Therefore, inhibiting NA enzymatic activity through antiviral drugs such as oseltamivir, zanamivir and peramivir has attracted attention as an effective method to cope with influenza infection progression (Sautto et al., 2018).
Accumulation of point mutations and re-assortment lead to the evolution of influenza virus strain, which can result in either reduced sensitivity to antivirals or enzyme activity changes in balance with HA changes (Wagner et al., 2002; McKimm‐Breschkin, 2013). Therefore, NA sequence and structure analysis is of importance in terms of influenza virus susceptibility to antiviral drugs and also its activity level. On the other hand, it is proved that influenza virulence, host range and infectivity are determined by the HA and NA coordination. In fact, there should be a balance between binding the virus to and releasing it from the cell receptor to have an efficient viral life cycle. One of the most important mediating factors in the HA binding to the specific cell receptors is the number of glycosylated sites. The lesser HA glycosylation, leads to stronger binding to the cell receptor. Accordingly, increasing the action of NA is necessary to release the progeny virus from the host cell. In other words, if HA is highly glycosylated, the binding to the cell receptor is weaker and less NA activity is required (Sun et al., 2012).
Immunity against NA plays a crucial role in influenza vaccination and confers comprehensive protection, particularly in case of antigenic shift or drift in HA (Eichelberger and Monto, 2019). Therefore, activation of the immune system against the influenza virus through NA antigen (in addition to HA antigens) would be a critical parameter for vaccine efficacy. The more extensive correspondence between recommended vaccine strains and circulating influenza isolates provides more protection against the influenza virus (Sautto et al., 2018).
The National Collaborating Laboratory of Influenza, Pasteur Institute of Iran, investigates the frequency of influenza subtypes of circulating viruses, annually. In the 2015–2016 flu season, 1758 respiratory samples were received and confirmed as A/H1N1 viruses. Phylogenetic analysis of the HA genes of 100 positive specimens revealed the circulation of clade 6B1, characterized by amino acid substitutions S84N, S162N and I216T. Position 162 was predicted to become glycosylated which affected the evolution of influenza viruses (Mohebbi et al., 2019).
In this sturdy, full NA sequences of 97 samples isolated in the 2015–2016 flu season in Iran were amplified. The amino acid substitutions, their putative effects on 3D structure, and antiviral drug susceptibility were assessed. Moreover, the sequences of Iranian influenza NA genes, which were submitted on the NCBI between 2010 and 2019 were included for further study and analysis.
2. Materials and methods
2.1. Study population
The National Collaborating Laboratory of Influenza, Pasteur Institute of Iran, receives nasopharyngeal swab samples from the inpatients with severe acute respiratory illness and investigates the frequency of influenza type/subtype of circulating viruses, annually. During the 2015–2016 influenza season (from October 2015 till March 2016), we received 1758 nasopharyngeal swab samples in virus transport medium (VTM) from 3 provinces of Iran (Markazi, Semnan and Zanjan). The samples were transported at 4 °C and kept aliquoted at −70 °C. The sample collection methodology was approved by the Research Ethics Committee of the Pasteur Institute of Iran (IR.PII.REC.1395.94).
2.2. Molecular diagnosis, gene amplification and sequencing
The real-time RT-PCR assay was performed using SuperScript III Platinum ® One-Step qRT-PCR kit (Invitrogen, USA) to detect the positive samples for influenza virus typing/subtyping according to the world health organization (WHO) guide line (WHO. 2009). The samples confirmed positive for A/H1N1 were propagated in Madin-Darby Canine Kidney (MDCK) cell cultures as described previously (Mohebbi et al., 2019).
The NA genes of influenza A/H1N1 viruses were amplified by RT-PCR and sequenced. Briefly, the extracted viral RNA of the viruses propagated in MDCK cell was transcribed into cDNA by ThermoScript RT-PCR System for First-Strand cDNA Synthesis, using a universal primer (U12: AGCGAAAGCAGG) (Hoffmann et al., 2001).
Full NA sequence was amplified by specific primers designed using Primer 3 software and synthesized by TIB MOLBIOL, Germany (Table 1). To ensure that the PCR amplifies the sequences successfully, two pairs of primers were designed to make two PCR products of 763 bp and 1042 bp with overlapping of 372 bp.
Table 1.
Sequences and positions of the NA specific primers used to amplify two overlapping fragments of circulating influenza A/H1N1 viruses in Iran, 2015–2016.
| Influenza subtype | Primer sequence (5′−3′) | Target | Position (Nt) | PCR Product (bp) |
|---|---|---|---|---|
| H1N1 | F: CCAAACCAAAAGATAATAACC | NA1 | 1- 21 | 1042 |
| R: CTTTTACTCCATTTGCTCC | 1024–1043 | |||
| H1N1 | F: AGAACACAAGAGTCTGAATG | NA2 | 671–691 |
763 |
| R: AGTAGAAACAAGGAGTTTTTT | 1413–1434 |
PCR technique with specific primers was performed using Platinum® Taq DNA Polymerase High Fidelity kit (Invitrogen, UK). The designed primers were used at a final concentration of 0.5 μM. Finally, the thermal cycler was programmed for amplification as follow: incubation at 94 °C, 2 min, and then 35 cycles of denaturation at 95 °C, 15 s, annealing at 43 °C, 30 s, and extension at 68 °C, 2 min. This was followed by a final elongation step at 68 °C for 15 min. PCR products were confirmed using gel electrophoresis and purified using GenElute™ PCR Clean-up Kit. Amplified samples subjected to sequencing using an ABI Sequence Genetic Analyzer (Applied Biosystems, Foster City, CA) at Sequence Laboratories of First Base Company, Malaysia.
2.3. Phylogenetic analyses
All the isolated sequences in the present study were submitted in GenBank. Iranian isolates available in data bank (during 2010 to 2019) including this study isolates (2015–2016) were compared with the most related sequences available in NCBI Influenza Virus Database (http://www.ncbi.nlm.nih.gov/genomes/FLU). The amino acid sequences corresponding to complete NA of the recommended vaccine strains by WHO and Centers for Disease Control and Prevention (CDC) during 2009 – 2020 seasonal flu were also included in the phylogenetic tree. The sequences were downloaded from the NCBI Influenza Resource Database and the Global Initiative on Sharing All Influenza Data (GISAID; http://platform.gisaid.org/) databases (Liechti et al., 2010; Clark et al., 2017). Data are shown in supplementary file 1.
The total NA protein sequences of the isolated influenza A/H1N1 viruses were aligned and edited using BioEdit software version 7.0 (https://www.bioedit.com/) and Mega-X (MEGA, version X; http://www.megasoftware.net/) by ClustalW multiple alignment system (Hall et al., 2011; Kumar et al., 2018).
A phylogenetic tree for the NA amino acid was constructed using the maximum likelihood method inferred based on the best-fit amino acid substitution model (Neighbor-joining) for the NA within MEGA software. The reliability measure of tree topology was assessed by bootstrap analysis with 1000 replications. Clades were analyzed based on the clustering patterns in the NA phylogeny (Jiménez-Alberto et al., 2013; Clark et al., 2017; Kumar et al., 2018).
2.4. Prediction of potential N-glycosylation sites
The amino acid sequences of NA (full length) from patient and refseq from NCBI were used to predict N-glycosylation sites by the NetNGlyc 1.0. (http://www.cbs.dtu.dk/services/ NetNGlyc/). The NetNGlyc 1.0 analyzes the sequence context of Asn-Xaa-Ser/Thr-sequons (Gupta and Brunak, 2002). The number of glycosylation sites was obtained for a single monomer of NA and locations of the glycosylation sites were numbered according to the full- length NA sequence of A/California/04/2009. The complete NA amino acid of A/H1N1/pdm09 isolate from the present study, was used for three-dimensional (3D) structure analysis. The crystal structures of A/California/07/ 2009 NA were downloaded from PDB database (http://www.rcsb.org). Then glycan was added onto the potential N-glycosites of NA using with the Glyprot Server (http://www.glycosciences.de/modeling/glyprot/).
2.5. Comparative protein modeling and molecular docking analyses
2.5.1. Homology protein modeling and model validation
To assess the vaccine efficiency against circulating influenza A H1N1 isolates in Iran, predominant NA sequences were selected (KY328789 Khorasan-Shomali 2014, AUG84119 Markazi 2015, AUG84157 Zanjan 2015, AUG84132 Markazi 2015, AUG84095 Markazi 2016, AUG84100 Markazi 2016, 291,678 Iran 2017, 338,058 Iran 2018, 406,135 Iran 2019) and protein homology modeling was done to analyze their antigenic similarity with NA antigen of recommended strains by WHO.
Firstly, a sequence similarity search based on BlastP algorithm in non-redundant protein sequences database was carried out in Protein Data Bank (PDB) to find the best-related protein templates for building models of the selected sequences. Five structures were identified as homologous from Protein Data Bank (PDB) (PDB ID: 6LXK, 4B7Q, 4B7J, 4B7M, 4B7N). According to the protein sequence identity equal to 98%, E-Value: 0, and the full length covering, the three-dimensional structure of the protein (PDB ID: 6LXK) was chosen as the template for subsequent homology modeling simulations.
Consequently, MODELLER 9.21 (Webb and Sali, 2016) was used to predict protein models. To select satisfactory models, the validation process was carried out through the SAVES server (https://saves.mbi.ucla.edu/) (Colovos and Yeates, 1993; Eisenberg et al., 1997). The UCSF chimaera program was used to display and visualize the structures (Pettersen et al., 2004).
2.5.2. In silico docking predictions
Molecular docking studies were performed to investigate the binding mode of NA protein and ligands (either antiviral drugs or sialic acid) (Du et al., 2016). The eight different molecules docking affinity to the NA models, selected randomly from different branches of phylogenetic tree, were investigated using the Graphical User Interface program “Auto-Dock 4.2″ (Morris et al., 2009). The cocrystal ligand of the ligand of the neuraminidase (6LXK.pdb) was removed and docked again with the neuraminidase. Then, by comparing the location and conformation of docked cocrystal ligand to the neuraminidase and the complex form, docking validation was examined. Docking operations have also been defined to compare the affinity of ligands and antiviral drugs, including Oseltamivir, Zanamivir and Peramivir.
3. Results and discussion
In 2015–2016 Influenza season, the 1758 samples were referred to the National Collaborating Laboratory of Influenza, Pasteur Institute of Iran from three provinces. Seasonal influenza peaks occurred from October through December. This seasonal outbreak was the same as what happened to the other regions of the world in autumn and winter. During this period, from 566 (30.8%) detected positive samples (Influenza A and B viruses), 504 cases (89%) were A/H1N1/pdm09. Few circulations of A/H3N2 virus (3 cases) and influenza B virus (59 cases) were also found. We observed a notable increase in the number of A/H1N1 circulating viruses in our region. According to the routine yearly detection of the WHO and CDC, predominant A/H1N1 subtypes in the European and Asian regions besides the United States region were reported in the 2015–2016 season (WHO and CDC, 2016).
As shown in Fig. 1, among five consecutive flu seasons from 2014 till 2019, the distribution and frequency of influenza virus A/H1N1/pdm09 in 2015–2016 showed significant increment based on the data from the National Collaborating Laboratory of Influenza, Pasteur Institute of Iran.
Fig. 1.
Influenza virus subtypes frequency in Iran during the 2014–2019 influenza seasons.
3.1. Analysis of the genetic characteristics of NA
The H1N1 positive samples (with CT values less than 25) were propagated in MDCK cell culture and used for the NA gene amplification. To comprehensively analyze the NA genetic variation of A/H1N1 viruses, the amplified NA genes (93 cases) were sequenced and submitted to GenBank (MG572244.1 to MG572338.1). Compared to the vaccine strain A/California/07/2009(H1N1) (ACQ63272.1), isolated Iranian influenza A H1N1 strains showed more than 97% homology. Iranian NA sequences (2015–2016) contained 127 mutations at the nucleotide level resulted in 49 amino acid substitutions (Supplementary file 2). However, 74 nucleic acid mutations were synonymous.
3.2. Phylogenetic analysis
Phylogenetic analysis of Iranian NA sequences (Fig. 2) revealed that all A/H1N1 strains were descended from A/California/2009‐like viruses. Circulating A/H1N1 strains during 2015 and 2016 were mostly similar to A/Michigan/45/2015 reference sequence located in clade 6B In this clade, all sequences carried N248D, N369K, V241I, N44S, N200S, I321V, I34V, K432E, N386K, and L40I substitutions compared to the influenza A/California/07/2009(H1N1). A greater number of sequences also contained L264I, N270K/E/R, I314M and V13I amino acid substitutions, which isolated them from previous generations in clade 6B1 and transmitted them to the next generations. However, other mutations led to amino acid changes such as I117M, N341S, V67I and S35N were detected in clade 6B which sound reduced virus fitness, due to the lack of transmission. Furthermore, one of the 2015–2016 samples, was clustered in genetic clade 5, carrying V106I substitution. Position 248, which is correlated with the conformation of the active site and position 106, which is an inner residue, were located near both the primary calcium ion-binding site that enhanced virus replication with low-pH stability of NA (Takahashi et al., 2013; Tolentino‐Lopez et al., 2013). This finding suggests that the acquisition of both the V106I and N248D replacements allows for the efficient adaptation of the pandemic (H1N1) 2009 virus to humans. However, V106I returned to I106V after a while, implying that V106I reduced virus fitness when other beneficial amino acid substitutions were added to A/H1N1 strains NA gene.
Fig. 2.
Phylogenetic tree based on the maximum likelihood. Iranian's NA sequences and reference sequences are coded by color and symbol. Each clade is remarked by mutations that led to genetic distance. Similar sequences frequencies are collapsed and indicated.
Regarding the clade 6B.1A1, G77R/K, V81A, and I188T substitutions were added to the previous mutations and created a subclade including 8 Iranian strains isolated in 2018–2019 along with Influenza A/Brisbane/02/2018 reference virus, suggesting a close relatedness with Iranian influenza virus isolates during 2017 and 2018. In the next year (2019), at least 5 amino acid substitutions (D416N, Q51K, F74S, T452I, and I388K) were found in Iranian influenza virus isolates. The reference influenza virus A/Guangdong-Maonan/SWL1536/2019 was also grouped in this subclade, but this sequence contained one more mutation (S52N).
3.3. Drug resistance analysis
Regarding antiviral drug-resistant mutations reported by WHO, we found 10 amino acid substitutions in the critical positions causing susceptibility alteration in 2014–2016 isolates (Table 2). These substitutions included I117M (6), D151E (1) I223V (2), and S247N (1). D151E substitution was previously reported as an inhibitory mutation in H3N2 and considered as reduced inhibitory effect on zanamivir and peramivir (Okomo-Adhiambo et al., 2010; Orozovic et al., 2011), while other substitutions, which occurred in the hotspots, were reported as safe with no effect in the favor of drug resistance (Choi et al., 2013; Prachanronarong et al., 2016). However, some of these mutations were reported to have reduced and highly reduced inhibitory effects on oseltamivir, zanamivir and peramivir when they appeared in the conjunction with other amino acid replacements such as E119V, H275Y, N295S, and Q136K ((GISRS), 2018) (Paradis et al., 2015). Moreover, most of the Iranian isolates contained substitutions such as V241I, N369K, N386K and K432E, which were reported as the fitness improvement mutations in the conjunction with H275Y or changed the binding pattern and affinity of oseltamivir for just NA, resulting in the reduced inhibitory effect of oseltamivir (Butler et al., 2014; Liu et al., 2017). These findings suggest that these strains are prone to acquire mutations such as H275Y and become resistant to the oseltamivir and Peramivir. Noticeably, inferring susceptibility to NA inhibitors based on the sequence information alone should be precautioned and experimentally determined (Tewawong et al., 2018).
Table 2.
Amino acid substitutions related to the antiviral drug resistance and their location on NA protein of circulating influenza A H1N1 viruses in Iran, 2010–2019.
 |
Green substitutions are reported as safe, but they can lead to a highly reduced inhibitory effect of some antiviral drugs. Yellow substitution is reported as a reduced inhibitory effect on zanamivir and peramivir.
Green substitutions are reported as safe, but they can lead to a highly reduced inhibitory effect of some antiviral drugs. Yellow substitution is reported as a reduced inhibitory effect on zanamivir and peramivir.
3.4. Vaccine efficiency
Neuraminidase as a surface antigen plays a critical role in immunogenicity and therefore, it would be of importance in vaccination strategies. The efficiency of vaccines was investigated against Iranian isolates (Fig. 3).
Fig. 3.
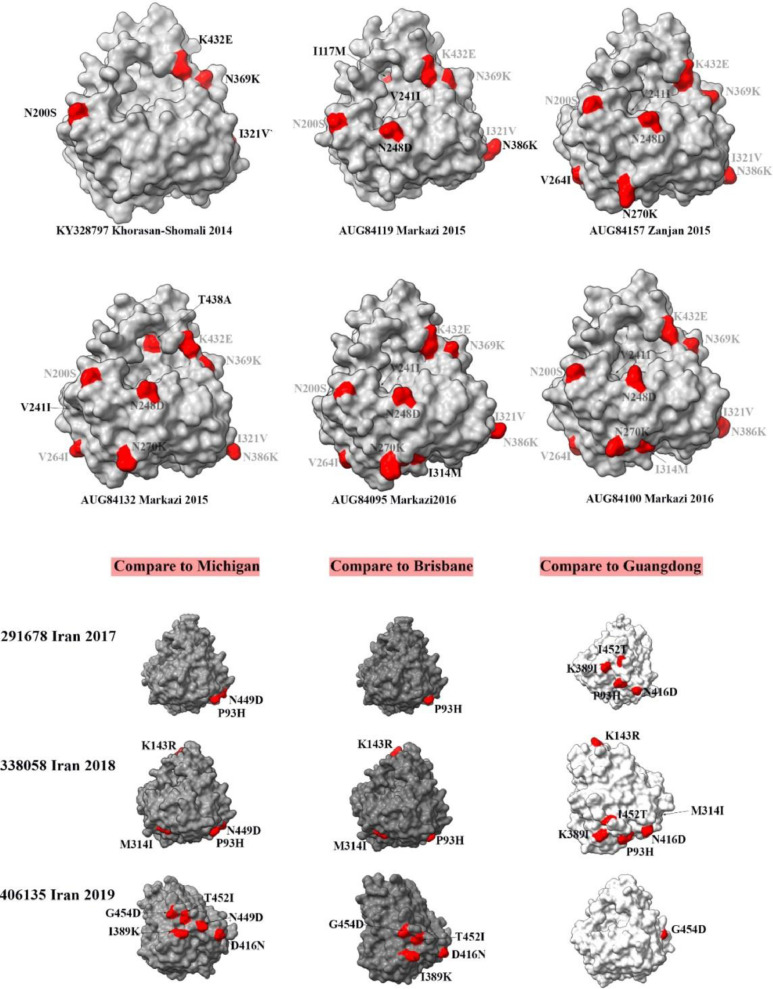
Schematic picture of NA antigens of circulating influenza viruses in Iran during different years and their antigenic sites mutations in comparison with vaccine strains. Mutations compared with vaccine strains are highlighted by red color. The first six models (2014–2016) are compared with California vaccine strains. Iranian isolates from 2017 to 2019 are compared with Michigan, Brisbane, and Guangdong vaccine strains at the bottom of the figure.
Firstly, we demonstrated amino acid substitutions (compared to the California 2009) in the surface level of 2014 to 2016 isolates to investigate the NA antigenic diversity during these years. It resulted in a progressive increase in the distance between Iranian isolates NA and California 2009 NA. These mutations included K432E, N369K, I321V, N200S, I117M, V241I, N248D, N386K, V264I, N270K, T438A, V241I, and I314M. A/California/7/2009 (H1N1) pdm09-like virus was recommended as a vaccine for use in the 2016–2017 season. It can be inferred that it was an improper recommendation for the immunization against NA of circulating strains between 2014 and 2016 in Iran. However, comparing 2014 to 2016 Iranian isolates with Michigan vaccine strain revealed an increasing correlation in surface level. 2014 candidate strain carried 6 amino acid replacement, 2015 strains showed between 1 and 3 amino acid substitutions and two frequent 2016 strains (AUG84095 and AUG84100) showed identical surface antigens compare to Michigan vaccine strain (Fig. 3)
Secondly, the efficacy of three vaccine strains including Michigan, Brisbane and Guangdong was assessed against Iranian isolates during 2017–2019. The vaccine strain of Michigan was recommended by WHO in the 2017–2018 and 2018–2019 seasons. During these seasons, Iranian isolates showed two point mutations of P93H and N449D for the 2017 isolate and four point mutations caused P93H, N449D, K143R, and M314I compared to the Michigan vaccine strain. While there was a difference between Iranian isolates and Michigan strain during the two seasons, it was clear that vaccination against NA was improved compared to the previous years. However, when it comes to comparing with Brisbane, it can be seen that NA similarity between Iranian isolates and Brisbane strain was more and this strain would be a better choice for vaccination against NA during 2017 and 2018. Brisbane vaccine strain was recommended by WHO in the next season (2019–2020). Our investigation suggests that the Brisbane vaccine strain was closer to the Iranian isolates during 2017 and 2018 compared to the Michigan strain in terms of NA antigen.
Finally, A/Guangdong-Maonan/SWL1536/2019 strain was recommended by WHO for the 2020–2021 season, which its NA structure was very close to the Iranian isolate of 2019. Comparison of this vaccine strain with the Iranian isolate of 2019 showed only one amino acid substitution, suggesting that this vaccine strain was a powerful recommendation to vaccinate Iranians against circulating H1N1 isolates.
3.5. Enzyme stability assessment
To assess the enzyme stability, the glycosylation profile is one of the important parameters. Glycosylation occurs on HA and NA antigen protein structures which are subjected to N-linked glycosylation. The addition or deletion of a functional N-glycosylation site can lead to the evolutionary changes in the host specificity, protein stability, virus growth rate, and vaccine yield. These mutations, can either alter the biological properties of HA and NA directly, or reduce receptor binding activity indirectly by masking antigenic regions of the HA protein, or regulating catalytic activity, or preventing proteolytic cleavage of the stalk of NA (Zanin et al., 2016; She et al., 2017).
Eight glycosylation sites have been found on NA of A/California/07/2009(H1N1) at positions 50, 58, 63, 68, 88, 146, 235 and 386 (Sun et al., 2011). While several glycosylation sites of Iranian strains are affected by mutations such as S35N, N44S and N386K, there were only 3 strains (AUG84132, AUG84151, AUG84184) out of 95 isolates during 2015–2016, which were affected by the change of S35N mutation. The substitution at position N44S, which is transmitted in all Iranian samples since 2013 is a removed glycosylation site in the stalk region by inserting the new amino acid. Moreover, the substitution of D416N was observed in 2019 isolates and made a potential to introduce a new glycosylation site.
Based on our results, since 2014, N386K substitution occurred in all samples except for AUG84188 and KY328797. It is proved that the presence of N386K can restore NA functionality when there is another mutation such as P431S, but the presence of N386K can finally result in decreased susceptibility to oseltamivir and generate resistant viruses to this NA inhibitory drug (Romero-Beltran et al., 2016). It means that N386K viruses are prone to P431S mutation under the pressure of oseltamivir treatment. Moreover, the co-presence of N386K and H275Y does not modify or affect the affinity of the virus neither to the SA-like substrates nor to the oseltamivir (Romero-Beltran et al., 2016).
In addition to four glycosylations at positions 50, 58, 63, 68 in the stalk region of NA monomer that was seen in 1918 and 2009 pandemic flu viruses, two glycosylation sites including 42 and 44 occurred in this region on the seasonal influenza viruses. The presence of this cluster of glycosylation sites may protect the stalk region of NA from the host protease attack (Sun et al., 2012). The presence of at least one cysteine residue and a glycosylated site in the NA stalk is of necessity. On the one hand, there is evidence that shows an enhanced tetramerization in the stalk region by enabling disulfide bonds (induced by cysteine residues) to form between each NA chain. Moreover, the more stable stalk contributes further to the greater NA activity. Therefore, firstly, we assessed the existence or non-existence of the cysteine residues in two positions of 14 and 49 in which the California 2009 strain contains cysteine. Secondly, we checked for the presence of cysteine residues in other positions of the stalk region. The assessments showed these two regions are highly conserved in all sequences since California 2009 pandemic, and there has not been any further cysteine in the stalk region.
From this evidence, we can infer that the numbers and the positions of cysteine residues were sufficient for the NA evolution since 2009. On the other hand, there is a reverse correlation between glycosylated sites in the stalk region and virulence, meaning the fewer glycosylated regions in the stalk, the more lethality and virulence of the influenza virus (Park et al., 2017).
Accordingly, we analyzed the existence of potent regions in which glycosylation may occur using NetNGlyc 1.0 Server. This analysis was done in comparison with California 2009 strain which its stalk likely includes four glycosylation sites in the positions of 50, 58, 63, and 68. A potent glycosylated region appeared in AUG84132 Markazi 2015 at the position of 35, which shows reduced fitness of virus because it disappeared in the next generations.
Glycosylation in the position of 42 appeared since 2010 and has remained steady so far. While this glycosylated position reduced the stalk stability, it could increase virus fitness. The loss of glycosylation at the position of 63 in the AUG84177 Markazi 2015 strain may increase the stalk stability and therefore the virulence. However, the mutation that caused glycosylation loss was not stable and disappeared in the next generations.
Double mutations of V241I and N369K enhance the stability and compatibility of the virus and increase the surface expression of the enzymatic function of NA (Butler et al., 2014). They were found in all Iranian sequences (2010–2019) except for two cases (AUG84188 Tehran 2015 and KC842137 Ardebil 2011).
Another amino acid substitution, Q51P, which occurred in the NA protein in the strains AUG84182 (Markazi 2015) and KY634459 (Tehran 2015) was reported as a cause of a reduction in potential glycosylation sites. This mutation can be important if the virus is established in new hosts (Zhao et al., 2012).
The active site of the NA enzyme is located on the head domain; therefore, analyzing the head domain would be an excellent parameter to get a better understanding of NA functionality. To fulfill this aim, we selected some sequences in each clade (including the most repetitive ones or a random sequence) and analyzed the sialic acid-binding affinity to the enzyme, distances between reactive amino acids, the position in the sialic acid, and also homo-tetramer stability.
It was proved that there was a coevolved relationship between the transmembrane region and the head domain of neuraminidase in a way that the transmembrane region tends to increase polarity (da Silva et al., 2015). In the case of candidate sequences, we analyzed the transmembrane region to assess their amino acid mutations towards more polarity. Most of the mutations in the transmembrane region including M151I, M19I, S31L, I34V, V13I, I32M, and M19L were the transition of hydrophobic/hydrophilic to hydrophobic amino acids led to ineffective or reduced polarity. However, some hydrophobic/hydrophilic to polar mutations (N21K, S35N, V13T, M19T) may be compatible with the evolving enzymatic head domain.
The frequency of three out of them were remarkably low and disappeared in the next generations, but the fourth mutation, M19T, appeared in four sequences in the last season, which suggests that it could increase the virus fitness. To assess the tetramer structure stability of NA proteins, which is a critical parameter of the enzyme activity, we calculated the Gibbs free energy of their structure (Supplementary file 3). The lowest Gibbs free energy reflects the highest structure stability and therefore stronger function (Jacobs and Dallakyan, 2005; Du et al., 2016). According to the results, approximately all strains showed a free Gibbs energy of below −50,000 except one strain in 2015 (AUG84114), which was about −48,700. This strain NA protein likely has the most unstable neuraminidase structure resulting in less functionality. The lowest Gibbs free energy amounts, which belonged to the isolated strains in 2014 (KY328797) and 2015 (AUG84127), were −54,989 KJ/mol and −55,377 KJ/mol, respectively.
Affinity analysis showed a range of different ΔG from 1.1 to 3.9 kcal/mol (this value does not include the effect of satisfying hydrogen bonds and salt bridges across the interface) and the numbers of potential hydrogen bonds across the interface (NHB) were between 5 and 12 in each docking model (Each hydrogen bond contributes about 0.5 kcal/mol into the free energy of protein binding). The P-values for ΔG of all docking models were higher than 0.5, which means that the interface was less hydrophobic than it could be. The P>0.5 was the sign of the fact that the interface was likely to be an artefact of the crystal packing. Moreover, we added a column of totally free energy of the protein binding which was calculated by the sum of the ΔG value and the number of potential hydrogen bonds at the interface area (Supplementary file 4).
The strains AUG84119, AUG84157, AUG84095, AUG84100, AUG84114, and 291678 are probably the strongest NA enzymes according to their tetramer stability and affinity to sialic acid. The number of hydrogen bonds and their lengths and hydrophobic contacts of NA amino acids with sialic acid confirm this supposition. For instance, strain AUG84119, the strongest strain, contains 4 hydrophobic contacts and 10 hydrogen bonds, while other docking models showed fewer contacts and bonds. Comparing the tetramer structure Gibbs free energy and free energy of protein binding of each docking model refers to the fact that there is often a negative correlation between these two values. More total affinity is necessary to compensate for poor enzyme function under the influence of low structure stability. According to Supplementary file 4, for instance, strain AUG84127 has the least value of tetramer structure Gibbs free energy and the greatest affinity, while AUG84114, conversely, has the weakest tetramer structure with a strong affinity to sialic acid. On the other hand, the trends of affinity and tetramer stability reflect the fact that the NA protein of Iranian H1N1 influenza viruses is increasing in the terms of tetramer stability and decreasing the sialic acid affinity. According to the bioinformatic studies on stability, it is necessary to focus on the experimental studies of the neuraminidase molecule stability, which are under consideration.
4. Conclusion
This study was performed to analyze the neuraminidase segment of Iranian isolates from 2010 in the terms of phylogenetic, antiviral resistance, and vaccine efficacy. To sum up, the results of our study showed the evolutionary trend of influenza A/H1N1 virus circulating in Iran, which provide a preliminary analysis for a better comprehension of new emerging Iran strain's virulence and thus, more appropriate monitoring of influenza virus A/H1N1 during each outbreak season.
Funding
This work was funded by grant No.942 from the Pasteur Institute of IRAN.
Author statement
Conceived and designed the experiments: FF; performed the experiments: SM, AM; Analyzed the data: SM, BF; Contributed reagents/materials: FF; Wrote the paper: SM, FF, PM; Comprehensive reading the revised manuscript: all authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199182.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- (GISRS) Summary of Neuraminidase Amino Acid Substitutions Associated With Reduced Inhibition by Neuraminidase Inhibitors. 2018. EWGoASftWHOGISaRS. Accessed 25 July 2020. [Google Scholar]
- Blok J., Air G.M. Variation in the membrane-insertion and" stalk" sequences in eight subtypes of influenza type A virus neuraminidase. Biochemistry. 1982;21:4001–4007. doi: 10.1021/bi00260a015. [DOI] [PubMed] [Google Scholar]
- Butler J., Hooper K.A., Petrie S., Lee R., Maurer-Stroh S., Reh L., Guarnaccia T., Baas C., Xue L., Vitesnik S. Estimating the fitness advantage conferred by permissive neuraminidase mutations in recent oseltamivir-resistant A (H1N1) pdm09 influenza viruses. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W., Shin J.Y., Jeong H.E., Jeong M.J., Kim S.J., Lee J.Y., Kang C. Generation and characterization of recombinant influenza A(H1N1) viruses resistant to neuraminidase inhibitors. Osong Public Health Res. Perspect. 2013;4:323–328. doi: 10.1016/j.phrp.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.M., DeDiego M.L., Anderson C.S., Wang J., Yang H., Nogales A., Martinez-Sobrido L., Zand M.S., Sangster M.Y., Topham D.J. Antigenicity of the 2015–2016 seasonal H1N1 human influenza virus HA and NA proteins. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colovos C., Yeates T.O. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci.e. 1993;2:1511–1519. doi: 10.1002/pro.5560020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva D.V., Nordholm J., Dou D., Wang H., Rossman J.S., Daniels R. The influenza virus neuraminidase protein transmembrane and head domains have coevolved. J. Virol. 2015;89:1094–1104. doi: 10.1128/JVI.02005-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Li Y., Xia Y.L., Ai S.M., Liang J., Sang P., Ji X.L., Liu S.Q. Insights into protein-ligand interactions: mechanisms, models, and methods. Int. J. Mol. Sci. 2016;17:1–34. doi: 10.3390/ijms17020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelberger M.C., Monto A.S. Neuraminidase, the forgotten surface antigen, emerges as an influenza vaccine target for broadened protection. J. Infect. Dis. 2019;219:S75–S80. doi: 10.1093/infdis/jiz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Lüthy R., Bowie J.U. [20]VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol. 1997;277:396–404. doi: 10.1016/s0076-6879(97)77022-8. [DOI] [PubMed] [Google Scholar]
- Hall T., Biosciences I., Carlsbad C. BioEdit: an important software for molecular biology. GERF Bull. Biosci. 2011;2:60–61. [Google Scholar]
- Hoffmann E., Stech J., Guan Y., Webster R., Perez D. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- Jacobs D.J., Dallakyan S. Elucidating protein thermodynamics from the three-dimensional structure of the native state using Network Rigidity. Biophys. J. 2005;88:903–915. doi: 10.1529/biophysj.104.048496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Alberto A., Alvarado-Facundo E., Ribas-Aparicio R.M., Castelán-Vega J.A. Analysis of adaptation mutants in the hemagglutinin of the influenza A (H1N1) pdm09 virus. PLoS One. 2013;8:e70005. doi: 10.1371/journal.pone.0070005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti R., Gleizes A., Kuznetsov D., Bougueleret L., Le Mercier P., Bairoch A., Xenarios I. OpenFluDB, a database for human and animal influenza virus. Database. 2010 doi: 10.1093/database/baq004. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-s, Jiao X-y, Wang S., Su W-z, Jiang L-z, Zhang X., Ke C-w, Xiong P. Susceptibility of influenza A (H1N1)/pdm2009, seasonal A (H3N2) and B viruses to Oseltamivir in Guangdong, China between 2009 and 2014. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-08282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley J.L., Gilbertson B.P., Trifkovic S., Brown L.E., McKimm-Breschkin J.L. Influenza virus neuraminidase structure and functions. Front. Microbiol. 2019;10:39. doi: 10.3389/fmicb.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm-Breschkin J.L. Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza Other Respir. Viruses. 2013;7:25–36. doi: 10.1111/irv.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebbi A., Fotouhi F., Jamali A., Yaghobi R., Farahmand B., Mohebbi R. Molecular epidemiology of the hemagglutinin gene of prevalent influenza virus A/H1N1/pdm09 among patient in Iran. Virus Res. 2019;259:38–45. doi: 10.1016/j.virusres.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okomo-Adhiambo M., Nguyen H.T., Sleeman K., Sheu T.G., Deyde V.M., Garten R.J., Xu X., Shaw M.W., Klimov A.I., Gubareva L.V. Host cell selection of influenza neuraminidase variants: implications for drug resistance monitoring in A(H1N1) viruses. Antiviral Res. 2010;85:381–388. doi: 10.1016/j.antiviral.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Orozovic G., Orozovic K., Lennerstrand J., Olsen B. Detection of resistance mutations to antivirals oseltamivir and zanamivir in avian influenza A viruses isolated from wild birds. PLoS One. 2011;6:e16028. doi: 10.1371/journal.pone.0016028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E.G., Pinilla L.T., Holder B.P., Abed Y., Boivin G., Beauchemin C.A. Impact of the H275Y and I223V mutations in the neuraminidase of the 2009 pandemic influenza virus in Vitro and evaluating experimental reproducibility. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Kim J.I., Lee I., Bae J.-Y., Yoo K., Nam M., Kim J., Park M.S., Song K.-J., Song J.-W. Adaptive mutations of neuraminidase stalk truncation and deglycosylation confer enhanced pathogenicity of influenza A viruses. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-11348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Prachanronarong K.L., Özen A., Thayer K.M., Yilmaz L.S., Zeldovich K.B., Bolon D.N., Kowalik T.F., Jensen J.D., Finberg R.W., Wang J.P., Kurt-Yilmaz N., Schiffer C.A. Molecular basis for differential patterns of drug resistance in influenza N1 and N2 neuraminidase. J. Chem. Theory Comput. 2016;12:6098–6108. doi: 10.1021/acs.jctc.6b00703. [DOI] [PubMed] [Google Scholar]
- Romero-Beltran L., Baker S.F., Puerto-Solís M., González-Losa R., Conde-Ferraez L., Alvarez-Sánchez L.C., Martínez-Sobrido L., Ayora-Talavera G. Mutations at highly conserved residues in influenza A (H1N1) pdm09 virus affect neuraminidase activity. Virus Res. 2016;225:1–9. doi: 10.1016/j.virusres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Sautto G.A., Kirchenbaum G.A., Ross T.M. Towards a universal influenza vaccine: different approaches for one goal. Virol. J. 2018;15:1–12. doi: 10.1186/s12985-017-0918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She Y.-M., Farnsworth A., Li X., Cyr T.D. Topological N-glycosylation and site-specific N-glycan sulfation of influenza proteins in the highly expressed H1N1 candidate vaccines. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-10714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Wang Q., Zhao F., Chen W., Li Z. Glycosylation site alteration in the evolution of influenza A (H1N1) viruses. PLoS One. 2011;6:e22844. doi: 10.1371/journal.pone.0022844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Wang Q., Zhao F., Chen W., Li Z. Prediction of biological functions on glycosylation site migrations in human influenza H1N1 viruses. PLoS One. 2012;7:e32119. doi: 10.1371/journal.pone.0032119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Song J., Suzuki T., Kawaoka Y. Mutations in NA that induced low pH-stability and enhanced the replication of pandemic (H1N1) 2009 influenza A virus at an early stage of the pandemic. PLoS One. 2013;8:e64439. doi: 10.1371/journal.pone.0064439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewawong N., Marathe B.M., Poovorawan Y., Vongpunsawad S., Webby R.J., Govorkova E.A. Neuraminidase inhibitor susceptibility and neuraminidase enzyme kinetics of human influenza A and B viruses circulating in Thailand in 2010–2015. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolentino-Lopez L., Segura-Cabrera A., Reyes-Loyola P., Zimic M., Quiliano M., Briz V., Muñoz-Fernández A., Rodríguez-Pérez M., Ilizaliturri-Flores I., Correa-Basurto J. Outside-binding site mutations modify the active site's shapes in neuraminidase from influenza A H1N1. Biopolymers. 2013;99:10–21. doi: 10.1002/bip.22130. [DOI] [PubMed] [Google Scholar]
- Wagner R., Matrosovich M., Klenk H.D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 2002;12:159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- Webb B., Sali A. Comparative protein structure modeling using MODELLER. Curr. Protocols Bioinform. 2016;54 doi: 10.1002/cpbi.3. 5.6. 1-5.6. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin M., Baviskar P., Webster R., Webby R. The interaction between respiratory pathogens and mucus. Cell Host Microbe. 2016;19:159–168. doi: 10.1016/j.chom.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Fan Q., Zhong L., Li Y., Liu W., Liu X., Gao S., Peng D., Liu X. Isolation and phylogenetic analysis of pandemic H1N1/09 influenza virus from swine in Jiangsu province of China. Res. Vet. Sci. 2012;93:125–132. doi: 10.1016/j.rvsc.2011.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.