Abstract
The immune system plays a key role in the suppression and progression of basal cell carcinoma (BCC). The primary aetiological factor for BCC development is exposure to ultraviolet radiation (UVR) which, particularly in lighter Fitzpatrick skin types, leads to the accumulation of DNA damage. UVR has roles in the generation of an immunosuppressive environment, facilitating cancer progression. Rates of BCC are elevated in immunosuppressed patients, and BCC may undergo spontaneous immune-mediated regression. Histologic and immunohistochemical profiling of BCCs consistently demonstrates the presence of an immune infiltrate and associated immune proteins. Early studies of immune checkpoint inhibitors reveal promising results in BCC. Therefore, the host immune system and tumor responses to it are important in BCC pathogenesis. Understanding these interactions will be beneficial for disease prognostication and therapeutic decisions.
Keywords: Basal cell, Carcinoma, Neoplasms, Skin neoplasms, Tumor-infiltrating lymphocytes
EPIDEMIOLOGY AND CLINICAL FEATURES
Basal cell carcinoma (BCC) is the most common malignancy, and its overall incidence continues to rise1,2. Several studies suggest incidence is increasing in Asian populations with lighter Fitzpatrick skin types3,4. Overall, the disease is most prevalent in older age groups and men5. Skin, hair, and eye phenotype are independent risk factors for the development of BCC, with the disease occurring more frequently in people with lighter skin (i.e. Fitzpatrick skin type I-III), red or blonde hair color, and light eye colour6,7,8,9. The most common site for BCC, in all ethnicities, is the head and neck10,11,12,13.
Patients typically present with slow-growing lesions (frequently on sun-damaged skin), that may be ulcerated, bleeding, pruritic, or entirely asymptomatic6. Pigmented BCC represents 50% to 75% of tumors in Asians; more than 10 times the number observed in Caucasians10,11,14. Pigmented BCC is less aggressive. These lesions require fewer Mohs stages for excision, have reduced subclinical infiltration and are associated with less aggressive histologic subtypes15,16. Nodular BCC is the most common histological subtype across all ethnicities—representing up to 80% of cases, followed by superficial BCC10,12,17. Less frequent histologic subtypes are associated with clinically aggressive behavior and recurrent disease18. These include—morpheaform, sclerosing, infiltrative, micronodular, and basosquamous18. The presence of perineural invasion is also a high-risk finding18. High-risk clinical factors include—location on the head and neck, size ≥2 cm, poorly defined borders, recurrent tumors, and lesions at sites of previous radiation therapy18.
LYMPHOMA TO LYMPHOPROLIFERATIVE DISORDERS
BCC appears to be chiefly caused by ultraviolet radiation (UVR)19. BCC occurs most frequently on sun-exposed sites (i.e. head and neck) and rates are higher in individuals with greater susceptibility to ultraviolet (UV)-induced DNA damage (i.e. lighter Fitzpatrick skin types)6,7. BCC is characterized by a typical UV mutation signature, namely C>T transitions occurring at dipyrimidine sites, and sporadic BCC has the highest mutation burden of any malignancy20,21.
Iatrogenic radiation and arsenic exposure have also been established as causes of BCC22,23,24. Iatrogenic radiation to the head and neck leads to a relative risk for BCC development of 3.623. Arsenic-induced BCC often develops after a long latency period and frequently occurs on non-sun exposed sites24.
Commonly mutated genes in BCC have been described. Genetic profiling of patients with Basal Cell Naevus Syndrome, who develop multiple BCCs from a young age, identified mutations in the PTCH1 gene25,26 which encodes a transmembrane receptor involved in the Hedgehog signaling pathway27. Studies of sporadic BCC have mapped driver mutations to PTCH1 and other components of the Hedgehog pathway including SMO and GLI 28,29,30. Other recurrently mutated driver genes include TP53 and members of the RAS protooncogene family21.
UVR suppresses cutaneous immunity. Early studies by Kripke31 demonstrated that transplantation of UV-induced tumors into immunocompetent mice resulted in the immune rejection of tumors. Similar transplantation experiments with immunosuppressed or UVB-irradiated mice maintained tumor survival31. The induction and elicitation of contact hypersensitivity are suppressed when hapten is applied to a site irradiated by either UVB or UVA radiation32,33.
There are many mechanisms of UV-mediated immunosuppression. Locally, UVR affects chromophores in the skin, changing the molecular configuration and altering function. Urocanic acid is found predominantly in the stratum corneum34,35. It is a chromophore that undergoes UV-mediated conversion from trans-urocanic acid to cis-urocanic acid36. The roles of these isomers differ. While trans-urocanic acid appears to have a photoprotective role—there is an accumulation of UVB-mediated DNA damage in mice deficient in the enzyme necessary for its production37—cis-UCA has deleterious effects through its promotion of cutaneous immunosuppression. Cis-UCA increases TNFα levels38. TNFα traps Langerhans cells within the epidermis, impairing migration to draining lymph nodes and subsequent generation of specific T cells38. Cis-UCA has roles in promoting mast cell degranulation39.
UVR promotes the formation of reactive oxygen species, which oxidize esterified fatty acyl residues and create platelet-activating factor-like (PAF-like) ligands40. These stimulate the PAF pathway, producing cyclooxygenase (COX) 2 and mast cell activation41.
Experimental studies have implicated COX2 overexpression in BCC cell lines with increased angiogenesis and resistance to regulated cell death following UVR42. Higher expression of COX2 in BCC has been associated with increased invasion and angiogenesis43.
Nucleotide lesions, specifically UVR-induced cyclobutane pyrimidine dimers (CPDs), may have immunosuppressive properties44,45. In mouse models and human skin, repair of CPD lesions by endonucleases facilitated systemic and local hypersensitivity reactions46,47, prevented erythema and sunburn, and increased production of interferon (IFN)-γ-mediated cell adhesion molecule ICAM-146.
Vitamin D (activated by UVR) has roles in immunosuppression48,49. When applied topically in high doses, calcitriol, the active form of vitamin D, suppresses delayed-type hypersensitivity reactions48. Calcitriol binds dendritic cells and suppresses their maturation50. The outcome is a reduced antigen-presenting capability and an increased differentiation of T-regulatory cells51. Vitamin D intake has been reported as modestly associated with BCC but not cutaneous squamous cell carcinoma (cSCC) risk52,53.
IMMUNOSUPPRESSION AND BCC
Immunosuppression is associated with an elevated risk of keratinocyte cancer (KC) development. Organ-transplant recipients (OTRs) are a high-risk group for the development of KC54. cSCC incidence in OTRs is up to 250 times that of the general population55. BCC incidence is around 10 times that of the general population55. This may be explained by the differences in immunogenicity of each tumor. The cumulative dose of immunosuppressive medication in OTRs has a greater effect on cSCC risk than BCC risk56,57. Heart/lung and renal transplant recipients have higher KC rates compared to liver transplant recipients58,59,60 and the BCC:cSCC in liver transplant patients appears to be closer to that of the general population60,61. This may be due to reduced cumulative immunosuppressive doses in liver transplant recipients60.
Unlike cSCC, there is no strong evidence supporting that BCCs in OTRs display aggressive disease. A study examining 176 cases of BCC from OTRs identified certain features unique to OTR BCCs, including the development of lesions at a significantly younger age, presence of significantly more lesions on extra-cephalic locations, and identification of lesions at unusual sites including genitalia and axillae62. A retrospective study of 69 renal transplant recipients did not identify differences in localization or clinicopathologic presentation of BCCs63.
LYMPHOMA
Non-Hodgkin lymphoma (NHL) encompasses a group of lymphoproliferative disorders which induce immunosuppression. The neoplastic immune cells alter the expression of cell surface markers, leading to reduced recognition and inhibition of effector cells64,65. Locally and systemically B-cell NHL patients have an expansion of CD14+ HLA-DRlow monocytes with immunosuppressive properties and elevated CD4+ CD25+ T-regulatory cells66,67. Chronic lymphocytic leukemia (CLL) patients are at an increased risk of developing many cancers68. Compared to the general population, the standardized cancer incidence ratio (all cancers) is 2.0 for men and 1.2 for women68. Rates of KC (both cSCC and BCC) are elevated in patients with NHL68,69,70,71. In a recent study, BCC incidence was calculated for CLL and non-CLL-NHL72. Both groups demonstrated an elevated incidence of BCC compared to the general population72, with a greater difference observed in the CLL group72.
The clinical behavior of KC in NHL patients is more aggressive, and KC is associated with a poorer NHL prognosis73. Non-melanoma skin cancer may be a marker of poor prognosis in patients with non-Hodgkin’s lymphoma74. This relates to both BCC and cSCC. In patients with CLL and BCC managed with Mohs micrographic surgery (MMS), 5-year local recurrence was 22%; 14 times higher than in patients without CLL75. Higher post-treatment recurrence rates were identified in another study assessing this population72.
HUMAN IMMUNODEFICIENCY VIRUS
HIV status has been associated with elevated rates of BCC and cSCC development76,77,78. In one large study HIV, positive individuals had a 2.1-fold increase in BCC76. Similar results were identified by Burgi et al.77. Crum-Cianflone et al.79 evaluated incidence rates and risk factors for the development of cutaneous malignancies in 4,490 HIV-infected patients. In HIV-positive individuals, risk factors for the development of BCC, including skin, hair, and eye phenotype, are unchanged from those seen in the general population79. In their study, participants developed BCC at a significantly younger age than that observed in the general population79. They also noted a raised BCC:cSCC ratio, unlike the converse observed in OTRs where cSCC is the predominant KC. The most common location for BCCs was the head and neck79.
Studies have not identified an association between lower CD4+ T cell counts and higher viral load with increased risk of BCC76,77,79,80.
IMMUNE INFILTRATE
T lymphocytes
Early studies consistently demonstrated a predominantly T cell infiltrate in BCC81,82,83,84,85,86 (Fig. 1)85. These tumor infiltrating lymphocytes (TILS) have various functions and may promote or inhibit tumor survival. Normal skin appears to have a paucity of infiltrating T cells—this includes a lack of CD8 and CD3 expression87.
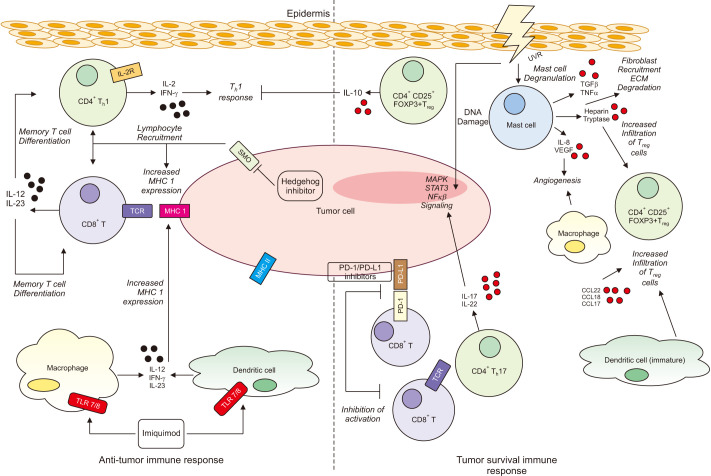
Fig. 1. The immune response (tumor promoting and tumor inhibiting) to basal cell carcinoma (BCC). Tumor promoting: Ultraviolet radiation (UVR) damages DNA leading to mutagenesis. UVR suppresses cutaneous immunity through many mechanisms including promotion of mast cell degradation. Mast cells have roles in angiogenesis through expression of interleukin (IL)-8 and VEGF. Other mast cell mediators include heparin, tryptase, TGFβ and TNFα. These promote a Th2 environment, induce T-regulatory cells and recruit fibroblasts. CD4+ FOXP3+ T-regulatory cells are found in the immune infiltrate. These cells release suppressive cytokines and inhibit the Th1 response. IL-17 and IL-22 cytokines promote MAPK, NFκβ and STAT3 signaling. Chemokines CCL17, CCL18, CCL20 and immature dendritic cells have roles in attracting T-regulatory cells. The PD-1/PD-L1 pathway is an immune resistance mechanism promoting apoptosis of effector cells. High levels of PD-1 and PD-L1 are found on tumor cells and immune cells. This interaction is inhibited by immune checkpoint inhibitors. Tumor inhibiting: CD4+ and CD8+ helper T cells are present in the BCC immune infiltrate. These effector cells secrete Th1 cytokines including IL-2 and interferon (IFN)-γ. IL-12 and IL-23, cytokines involved in the regulation of the Th1 response, promote memory T cell differentiation and the anti-tumor response. MHC expression is low in BCC. Expression is increased after Imiquimod treatment and Hedgehog Inhibitor treatment. Imiquimod increases levels of the anti-tumor cytokines IL-12 and IFN-γ. Mature dendritic cells have tumor inhibiting roles. Macrophages appear to have conflicting roles in BCC pathogenesis. ECM: extracellular matrix.
A small proportion of BCCs undergo either partial or complete spontaneous regression. Several immunologic differences have been described in this population. CD3+ and CD4+ cells are present in significantly higher numbers in the overlying epidermis of regressing BCC88. A case report of a regressing BCC described elevated levels of CD3+, CD4+, CD8+, and TIA-1 (a marker of NK cells) in the immune infiltrate89. A case series of periorbital regressing BCC also demonstrated increased infiltration of CD4+ T cells around and into the tumor nests90. In addition, the IL-2 receptor, a marker of activated T cells, was elevated in regressing tumors88,91. These findings suggest that particular phenotypes of CD4+ expressing T cells may mediate tumor regression88. These may likely be Th1 CD4+ T cells—reflected by the cytokine profile of regressing BCCs91.
CD4+ CD25+ FOXP3+ T-regulatory cells have been detected around BCC tumor aggregates87.
In facial BCC, nearly half of CD4+ cells were identified as being CD4+ FOXP3+ regulatory T cells86. This is in comparison to UV-protected skin which has a paucity of T-regulatory cells86. T-regulatory cells have immunosuppressive functions87. Elevated expression of CCL22, CCL18, and CCL17, chemokines that attract T-regulatory cells, have also been described in BCC87.
Kaporis et al.87 have described elevated levels of CD8+ T cells in BCC compared to normal skin, with high levels of IFN-associated gene interleukin (IL)-12 and IL-23 expression which together promote memory T cells and have anti-tumor properties. Other studies have reported the presence of these cytokines in BCC92,93. CD8 expression levels were reduced in primary BCC tumors of patients who then went on to have recurrent disease94. In summary, there is relatively sparse knowledge of T cell infiltration in BCC lesions and how they affect clinicopathologic presentation and prognosis.
Mast cells
There is a significant mast cell infiltrate in BCC, especially at the periphery of tumors85,95,96,97. The number of mast cells is inversely proportional to TILs85, more aggressive tumors have greater mast cell numbers85,96.
While smoking does not appear to be a risk factor for the development of BCC98, it is associated with greater peritumoral mast cell numbers, which may explain the higher prevalence of aggressive morpheaform BCC variants in this population99.
Mast cells express VEGF and IL-8, suggesting that mast cells may have roles in the regulation of the immune cell infiltrate and angiogenesis95. Similarly, BCCs with greater microvessel density are more aggressive100. Mast cell-deficient mice inoculated with an aggressive melanoma variant have slower angiogenic responses and reduced rates of metastasis101.
Mast cell granules contain various substances including histamine, heparin, leukotrienes, prostaglandins, tryptase, TNFα, TGFβ, IL-3, and IL-4102. Histamine and TNFα have roles in local and systemic immunosuppression96,102,103,104. Heparin and tryptase are mitogens for fibroblasts and endothelial cells and increase regulatory T cell function105. IL-4 levels have been demonstrated to be upregulated in BCC and facilitate a Th2, immunosuppressive environment87.
Dermal mast cell prevalence is elevated in sun-protected skin of patients with a history of BCC, suggesting increasing mast cell density may be a risk factor for BCC development106. The authors proposed that given mast cells are facilitators of cutaneous immunosuppression, higher baseline dermal mast cell density may increase susceptibility for BCC development106. Previously, the authors reported that UVB-induced systemic suppression of contact hypersensitivity is determined by dermal mast cell prevalence107.
Thus, mast cells may promote UVB-mediated immunosuppression, angiogenesis, and extracellular matrix degradation102. UVR itself also affects mast cells. UVR-induced mediators including cis-urocanic acid and endothelin-1 promote mast cell degranulation104,108.
Macrophages and dendritic cells
Macrophages and dendritic cells are antigen-presenting cells. They are consistently reported to be present in the BCC immune infiltrate, but to a lesser extent than T cells81,109.
Macrophages can broadly be classified into two phenotypic subsets, M1 and M2. M1 macrophages have traditionally been viewed to have anti-tumor properties—i.e. phagocytosis of tumor cells, secretion of cytokines promoting cytotoxic lymphocytes within the tumor microenvironment (TME)110,111. However, secreted reactive oxidative species also cause tissue damage and promote malignancy112. M2 macrophages may release tumor-promoting growth factors and have roles in angiogenesis and cell proliferation113,114,115.
Beksaç et al.94 identified a predominance of M2 macrophages in BCC. They did not identify an association of M2 macrophage level with recurrence94. No association was identified between macrophage subtype or amount in recurrent vs non-recurrent BCC in another study116. However, contrasting findings have been reported by Tjiu et al.43, who identified the presence of M2 macrophages in BCC. Higher numbers of these TAMs were significantly associated with more aggressive disease—i.e. greater depth of invasion and higher microvessel density43. Exposure of BCC cell lines to M2 macrophages enhances invasion and angiogenesis in vitro43. Depletion of dermal dendritic cells, Langerhans cells and M1 macrophages in a PTCH-deficient mouse model resulted in enlargement of BCC lesions117.
In contrast, nicotinamide (NAM), a KC chemo-preventive agent, results in a significant reduction of CD68+ macrophages (a marker of M1 and M2 cells), but not of the M2-specific marker CD163+, in the tumor infiltrate118. Therefore, NAM may selectively deplete M1 macrophages118. The authors postulated that this may be one mechanism of its anti-tumor effect118.
CD1a is a marker of dendritic cells including Langerhans cells94. CD1a expression is relatively low in BCC94. Lower levels have been identified in primary tumors of patients who developed recurrent disease94. In one study, there were fewer CD1a expressing cells in the epidermis adjacent to the tumor versus normal epidermis87. Higher levels of Langerhans cells, identified by the S-100 marker, have been associated with less aggressive BCC119. Therefore, the presence of mature dendritic cells in the BCC TME may have a protective role.
Dendritic cells lacking markers of maturation (i.e. CD40, CD83, and LAMP) have been described in BCC87. These immature dendritic cells may have pro-tumorigenic functions by induction of T cell tolerance and production of suppressive cytokines87,120,121.
MHC molecules
The significantly elevated rates of cSCC in OTRs compared to BCC may be in part explained by reduced MHC1 levels in BCC, implying that the CD8+ cytotoxic immune response has a lesser role in BCC than in cSCC122.
If cancer is to survive it must evolve mechanisms to evade the immune response. MHC class 1 molecules are expressed on antigen-presenting cells123. They present abnormal peptides synthesized by the cell itself and are presented to CD8+ T cells123. BCC has a relative lack of MHC expression122,124. Normal human skin constitutively expresses MHC 1124. Expression of β2-microglobulin, a component of the MHC 1 molecule, is low in BCC125. Lower class 1 antigen expression in BCC is correlated with more aggressive tumors and lack of differentiation status124. Treatment with imiquimod increases MHC 1 expression in BCC126. Treatment with hedgehog pathway inhibitors also increases MHC 1 levels and infiltration of CD8+ T cells in BCC127. MHC class 2 expression is variable in BCC85,89 and is more commonly present on infiltrating T cells compared to tumor cells128,129.
HLA-G is a type of MHC class 1b antigen. Under physiological conditions, it occurs in immune-privileged sites. It has been characterized in some tumors and has immunosuppressive functions130,131. In BCC it is expressed on both neoplastic cells and inflammatory cells132.
Cytokine profile
The dominant cytokine expression profile in most untreated BCCs facilitates local immunosuppression and tumor survival133. This suppressive cytokine profile is altered during treatment and in tumors which display spontaneous regression91,93.
Most studies report that BCC is characterized by the expression of Th2 cytokines87,133,134, including IL-4, IL-5, IL-13, and IL-10132,133. Compared to cSCC, BCC has significantly higher levels of Th2 cytokines: IL-4, IL-5, IL-6, and IL-1β133. ELISA assay of a BCC cell line demonstrated high levels of IL-10 and IL-4 production134. Tumor cell production of suppressive cytokines is a mechanism of tumor survival in BCC; however, anti-tumor cytokines are also present in lesions87,91. Head and neck BCC exhibits a more aggressive and treatment-resistive clinical course133. Higher numbers of suppressive cytokines are found in head and neck BCC133.
IL-17, IL-22, and IL-23 are present in higher levels in BCC compared to normal skin93. Exposure of BCC cell lines to IL-17 and IL-22 cytokines results in cellular proliferation in vitro92. These results were replicated in xenograft tumor mouse models92. Prior studies have demonstrated slower tumorigenesis in IL-17 deficient mice135. Exposure of BCC cell lines to IL-22 results in increased amount and duration of phosphorylated products within the STAT3 and MAPK pathways92. Constitutive p65 phosphorylation, a proxy of NFκβ signaling , was identified following IL-17 exposure92. These findings imply that IL-17 and IL-22 play a role in BCC pathogenesis and likely promote tumor survival92.
IFN-γ is a Th1 cytokine with roles in promoting Th1 differentiation136. Most studies report low IFN-γ levels in BCC, implying a reduced role for Th1 mediated immunity92,134. Flow cytometry analysis of isolated BCC immune infiltrates demonstrated reduced IFN-γ-positive and CD8+ T cell levels compared to peripheral blood mononuclear cells92.
Elevated IFN-γ in the tumor infiltrate is associated with regressing tumors91. A recent study has described presence of Th1 cytokines in BCC and peritumoral skin, however non-irradiated (by UVR) skin lacked expression of these cytokines86.
Imiquimod is a standard treatment for superficial BCC. It has both direct and indirect effects on the skin immune system which leads to immune-mediated destruction of neoplastic cells. It binds to toll-like receptor 7/8 on inflammatory cells leading to the release of pro-inflammatory mediators, including IL-12 and IFN-γ, and activation of the cell-mediated immune response pathway126,137. It also results in elevation of CD68+ macrophages and plasmacytoid predendritic cell levels in the intra and peritumoral infiltrate138, and an increase in CD4+ T cells levels and MHC 1 expression93,126,139. These findings suggest the immune mechanisms by which imiquimod induces an anti-tumor response.
IMMUNE CHECKPOINT INHIBITORS
The programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) signaling pathway is an adaptive immune resistance mechanism that promotes apoptosis of effector immune cells140. In a series of 40 BCC’s, 22% of tumors demonstrated PD-L1 expression on tumor cells and 82% demonstrated PD-L1 expression on TILS or macrophages141. PD-1 expression was demonstrated on TILS in 100% of cases141. 82% of cases with PD-L1 expression on infiltrating immune cells were near a PD-1 expressing cell141. Case reports describe mixed, but predominantly favorable, responses to immunotherapy142,143,144.
A patient with metastatic BCC demonstrated a favorable response after 14 months of pembrolizumab143. Ikeda et al.142 present a Hedgehog-inhibitor-resistant metastatic BCC treated with nivolumab. A near-complete remission at 4 months was achieved142. Amplification of a chromosomal region containing PDL1/PDL2/JAK2 genes and a high mutation burden was detected142. A vismodegib-resistant metastatic BCC attained partial response to a trial PD-1 inhibitor REGN2810145. Goodman et al.146 reported on four patients with locally advanced/metastatic BCC, with three of four demonstrating complete or partial responses to anti-PD-1 therapies. A recent phase 2 trial of the PD-1 antibody cemiplimab demonstrated clinically significant anti-tumor activity in locally advanced and metastatic BCC, with 21% of patients demonstrating a partial response and 6% of patients demonstrating complete response147. In late 2021, the FDA approved cemiplimab for use in locally advanced and metastatic BCC148.
The role of CTLA4-inhibitors in BCC is poorly characterized. A case report of a locally advanced BCC of the head and neck in a patient with BRAF-negative metastatic melanoma was commenced on ipilimumab with subsequent shrinkage of the BCC149.
Inoperable or metastatic BCC may be a good candidate for immune checkpoint inhibitors; however, understanding the prognostic role of the immune infiltrate is imperative in the selection of these agents.
CONCLUSION
There are multiple mechanisms by which BCC evades the anti-tumor immune response. UVR facilitates the creation of an immunosuppressive environment. BCC tumors express suppressive cytokines and may downregulate MHC expression on tumor cells. BCC may undergo spontaneous regression and the immune profile of regressing tumors differs from that of progressing tumors. Treatments alter the immune infiltrate and cytokine profile of BCC, promoting an anti-TME.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: None.
References
- 1.Asgari MM, Moffet HH, Ray GT, Quesenberry CP. Trends in basal cell carcinoma incidence and identification of high-risk subgroups, 1998-2012. JAMA Dermatol. 2015;151:976–981. doi: 10.1001/jamadermatol.2015.1188. [DOI] [PubMed] [Google Scholar]
- 2.Verkouteren JAC, Ramdas KHR, Wakkee M, Nijsten T. Epidemiology of basal cell carcinoma: scholarly review. Br J Dermatol. 2017;177:359–372. doi: 10.1111/bjd.15321. [DOI] [PubMed] [Google Scholar]
- 3.Kantor J. The epidemiology of skin cancer in Asia: September 2021. J Am Acad Dermatol. 2021;85:569. doi: 10.1016/j.jaad.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Ichihashi M, Naruse K, Harada S, Nagano T, Nakamura T, Suzuki T, et al. Trends in nonmelanoma skin cancer in Japan. Recent Results Cancer Res. 1995;139:263–273. doi: 10.1007/978-3-642-78771-3_20. [DOI] [PubMed] [Google Scholar]
- 5.Wu S, Han J, Li WQ, Li T, Qureshi AA. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am J Epidemiol. 2013;178:890–897. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron MC, Lee E, Hibler BP, Barker CA, Mori S, Cordova M, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303–317. doi: 10.1016/j.jaad.2018.03.060. Erratum in: J Am Acad Dermatol 2021;85:535. [DOI] [PubMed] [Google Scholar]
- 7.Khalesi M, Whiteman DC, Tran B, Kimlin MG, Olsen CM, Neale RE. A meta-analysis of pigmentary characteristics, sun sensitivity, freckling and melanocytic nevi and risk of basal cell carcinoma of the skin. Cancer Epidemiol. 2013;37:534–543. doi: 10.1016/j.canep.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Chinem VP, Miot HA. Prevalence of actinic skin lesions in patients with basal cell carcinoma of the head: a case-control study. Rev Assoc Med Bras (1992) 2012;58:188–196. [PubMed] [Google Scholar]
- 9.Oh CC, Jin A, Koh WP. Trends of cutaneous basal cell carcinoma, squamous cell carcinoma, and melanoma among the Chinese, Malays, and Indians in Singapore from 1968-2016. JAAD Int. 2021;4:39–45. doi: 10.1016/j.jdin.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi A, Shimizu H, Nishikawa T. Clinical histopathological characteristics of basal cell carcinoma in Japanese patients. Arch Dermatol. 1996;132:320–324. [PubMed] [Google Scholar]
- 11.Moore MG, Bennett RG. Basal cell carcinoma in asians: a retrospective analysis of ten patients. J Skin Cancer. 2012;2012:741397. doi: 10.1155/2012/741397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41–47. doi: 10.1046/j.1365-2133.2002.04804.x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta R, Gordon SL, Council ML, Hurst EA. Clinical characteristics of basal cell carcinoma in African Americans: a 10-year retrospective review at a single academic institution. Dermatol Surg. 2019;45:660–665. doi: 10.1097/DSS.0000000000001744. [DOI] [PubMed] [Google Scholar]
- 14.Cho S, Kim MH, Whang KK, Hahm JH. Clinical and histopathological characteristics of basal cell carcinoma in Korean patients. J Dermatol. 1999;26:494–501. doi: 10.1111/j.1346-8138.1999.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 15.Moon HR, Park TJ, Ro KW, Ryu HJ, Seo SH, Son SW, et al. Pigmentation of basal cell carcinoma is inversely associated with tumor aggressiveness in Asian patients. J Am Acad Dermatol. 2019;80:1755–1757. doi: 10.1016/j.jaad.2018.06.059. [DOI] [PubMed] [Google Scholar]
- 16.Ro KW, Seo SH, Son SW, Kim IH. Subclinical infiltration of Basal cell carcinoma in asian patients: assessment after mohs micrographic surgery. Ann Dermatol. 2011;23:276–281. doi: 10.5021/ad.2011.23.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raasch BA, Buettner PG, Garbe C. Basal cell carcinoma: histological classification and body-site distribution. Br J Dermatol. 2006;155:401–407. doi: 10.1111/j.1365-2133.2006.07234.x. [DOI] [PubMed] [Google Scholar]
- 18.Bichakjian CK, Olencki T, Aasi SZ, Alam M, Andersen JS, Berg D, et al. Basal cell skin cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:574–597. doi: 10.6004/jnccn.2016.0065. [DOI] [PubMed] [Google Scholar]
- 19.Kricker A, Armstrong BK, English DR. Sun exposure and non-melanocytic skin cancer. Cancer Causes Control. 1994;5:367–392. doi: 10.1007/BF01804988. [DOI] [PubMed] [Google Scholar]
- 20.Bonilla X, Parmentier L, King B, Bezrukov F, Kaya G, Zoete V, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48:398–406. doi: 10.1038/ng.3525. [DOI] [PubMed] [Google Scholar]
- 21.Jayaraman SS, Rayhan DJ, Hazany S, Kolodney MS. Mutational landscape of basal cell carcinomas by whole-exome sequencing. J Invest Dermatol. 2014;134:213–220. doi: 10.1038/jid.2013.276. [DOI] [PubMed] [Google Scholar]
- 22.Tran H, Chen K, Shumack S. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2003;149 Suppl 66:50–52. doi: 10.1046/j.0366-077x.2003.05622.x. [DOI] [PubMed] [Google Scholar]
- 23.Shore RE, Moseson M, Xue X, Tse Y, Harley N, Pasternack BS. Skin cancer after X-ray treatment for scalp ringworm. Radiat Res. 2002;157:410–418. doi: 10.1667/0033-7587(2002)157[0410:scaxrt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Yu HS, Liao WT, Chai CY. Arsenic carcinogenesis in the skin. J Biomed Sci. 2006;13:657–666. doi: 10.1007/s11373-006-9092-8. [DOI] [PubMed] [Google Scholar]
- 25.Chenevix-Trench G, Wicking C, Berkman J, Sharpe H, Hockey A, Haan E, et al. Further localization of the gene for nevoid basal cell carcinoma syndrome (NBCCS) in 15 Australasian families: linkage and loss of heterozygosity. Am J Hum Genet. 1993;53:760–767. [PMC free article] [PubMed] [Google Scholar]
- 26.Bialer MG, Gailani MR, McLaughlin JA, Petrikovsky B, Bale AE. Prenatal diagnosis of Gorlin syndrome. Lancet. 1994;344:477. doi: 10.1016/s0140-6736(94)91810-4. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrini C, Maturo MG, Di Nardo L, Ciciarelli V, Gutiérrez García-Rodrigo C, Fargnoli MC. Understanding the molecular genetics of basal cell carcinoma. Int J Mol Sci. 2017;18:2485. doi: 10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 29.Dahmane N, Lee J, Robins P, Heller P, Ruizi Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. Erratum in: Nature 1997;390:536. [DOI] [PubMed] [Google Scholar]
- 30.Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, Glynn M, Zaphiropoulos PG, Pressman C, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 31.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 32.Damian DL, Matthews YJ, Halliday GM. Topical riboflavin attenuates ultraviolet B- and ultraviolet A-induced immunosuppression in humans. Photodermatol Photoimmunol Photomed. 2010;26:66–69. doi: 10.1111/j.1600-0781.2010.00486.x. [DOI] [PubMed] [Google Scholar]
- 33.Matthews YJ, Halliday GM, Phan TA, Damian DL. A UVB wavelength dependency for local suppression of recall immunity in humans demonstrates a peak at 300 nm. J Invest Dermatol. 2010;130:1680–1684. doi: 10.1038/jid.2010.27. [DOI] [PubMed] [Google Scholar]
- 34.Gibbs NK, Norval M. Urocanic acid in the skin: a mixed blessing? J Invest Dermatol. 2011;131:14–17. doi: 10.1038/jid.2010.276. [DOI] [PubMed] [Google Scholar]
- 35.Tabachnick J. Urocanic acid, the major acid-soluble, ultraviolet-absorbing compound in guinea pig epidermis. Arch Biochem Biophys. 1957;70:295–298. doi: 10.1016/0003-9861(57)90107-8. [DOI] [PubMed] [Google Scholar]
- 36.Kurogochi Y, Fukui Y, Nakagawa T, Yamamoto I. A note on cisurocanid acid. Jpn J Pharmacol. 1957;6:147–152. doi: 10.1254/jjp.6.147. [DOI] [PubMed] [Google Scholar]
- 37.Barresi C, Stremnitzer C, Mlitz V, Kezic S, Kammeyer A, Ghannadan M, et al. Increased sensitivity of histidinemic mice to UVB radiation suggests a crucial role of endogenous urocanic acid in photoprotection. J Invest Dermatol. 2011;131:188–194. doi: 10.1038/jid.2010.231. [DOI] [PubMed] [Google Scholar]
- 38.Kurimoto I, Streilein JW. cis-urocanic acid suppression of contact hypersensitivity induction is mediated via tumor necrosis factor-alpha. J Immunol. 1992;148:3072–3078. [PubMed] [Google Scholar]
- 39.Pham DL, Lim KM, Joo KM, Park HS, Leung DYM, Ye YM. Increased cis-to-trans urocanic acid ratio in the skin of chronic spontaneous urticaria patients. Sci Rep. 2017;7:1318. doi: 10.1038/s41598-017-01487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marathe GK, Johnson C, Billings SD, Southall MD, Pei Y, Spandau D, et al. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J Biol Chem. 2005;280:35448–35457. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- 41.Pei Y, Barber LA, Murphy RC, Johnson CA, Kelley SW, Dy LC, et al. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J Immunol. 1998;161:1954–1961. [PubMed] [Google Scholar]
- 42.Tjiu JW, Liao YH, Lin SJ, Huang YL, Tsai WL, Chu CY, et al. Cyclooxygenase-2 overexpression in human basal cell carcinoma cell line increases antiapoptosis, angiogenesis, and tumorigenesis. J Invest Dermatol. 2006;126:1143–1151. doi: 10.1038/sj.jid.5700191. [DOI] [PubMed] [Google Scholar]
- 43.Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129:1016–1025. doi: 10.1038/jid.2008.310. Erratum in: J Invest Dermatol 2018;138:471. [DOI] [PubMed] [Google Scholar]
- 44.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci U S A. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yarosh DB, Canning MT, Teicher D, Brown DA. After sun reversal of DNA damage: enhancing skin repair. Mutat Res. 2005;571:57–64. doi: 10.1016/j.mrfmmm.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 46.Stege H, Roza L, Vink AA, Grewe M, Ruzicka T, Grether-Beck S, et al. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc Natl Acad Sci U S A. 2000;97:1790–1795. doi: 10.1073/pnas.030528897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yarosh D, Bucana C, Cox P, Alas L, Kibitel J, Kripke M. Localization of liposomes containing a DNA repair enzyme in murine skin. J Invest Dermatol. 1994;103:461–468. doi: 10.1111/1523-1747.ep12395551. [DOI] [PubMed] [Google Scholar]
- 48.Damian DL, Kim YJ, Dixon KM, Halliday GM, Javeri A, Mason RS. Topical calcitriol protects from UV-induced genetic damage but suppresses cutaneous immunity in humans. Exp Dermatol. 2010;19:e23–e30. doi: 10.1111/j.1600-0625.2009.00955.x. [DOI] [PubMed] [Google Scholar]
- 49.Hanneman KK, Scull HM, Cooper KD, Baron ED. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332–1334. doi: 10.1001/archderm.142.10.1332. [DOI] [PubMed] [Google Scholar]
- 50.Ferreira GB, Overbergh L, Verstuyf A, Mathieu C. 1α,25-Dihydroxyvitamin D3 and its analogs as modulators of human dendritic cells: a comparison dose-titration study. J Steroid Biochem Mol Biol. 2013;136:160–165. doi: 10.1016/j.jsbmb.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 51.van der Aar AM, Sibiryak DS, Bakdash G, van Capel TM, van der Kleij HP, Opstelten DJ, et al. Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J Allergy Clin Immunol. 2011;127:1532–1540.e7. doi: 10.1016/j.jaci.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 52.Park SM, Li T, Wu S, Li WQ, Qureshi AA, Cho E. Vitamin D intake and risk of skin cancer in US women and men. PLoS One. 2016;11:e0160308. doi: 10.1371/journal.pone.0160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahamat-Saleh Y, Aune D, Schlesinger S. 25-Hydroxyvitamin D status, vitamin D intake, and skin cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Sci Rep. 2020;10:13151. doi: 10.1038/s41598-020-70078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mittal A, Colegio OR. Skin cancers in organ transplant recipients. Am J Transplant. 2017;17:2509–2530. doi: 10.1111/ajt.14382. [DOI] [PubMed] [Google Scholar]
- 55.Hartevelt MM, Bavinck JN, Kootte AM, Vermeer BJ, Vandenbroucke JP. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation. 1990;49:506–509. doi: 10.1097/00007890-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Fortina AB, Piaserico S, Caforio AL, Abeni D, Alaibac M, Angelini A, et al. Immunosuppressive level and other risk factors for basal cell carcinoma and squamous cell carcinoma in heart transplant recipients. Arch Dermatol. 2004;140:1079–1085. doi: 10.1001/archderm.140.9.1079. [DOI] [PubMed] [Google Scholar]
- 57.Glover MT, Deeks JJ, Raftery MJ, Cunningham J, Leigh IM. Immunosuppression and risk of non-melanoma skin cancer in renal transplant recipients. Lancet. 1997;349:398. doi: 10.1016/S0140-6736(97)80015-3. [DOI] [PubMed] [Google Scholar]
- 58.Krynitz B, Olsson H, Lundh Rozell B, Lindelöf B, Edgren G, Smedby KE. Risk of basal cell carcinoma in Swedish organ transplant recipients: a population-based study. Br J Dermatol. 2016;174:95–103. doi: 10.1111/bjd.14153. [DOI] [PubMed] [Google Scholar]
- 59.Frezza EE, Fung JJ, van Thiel DH. Non-lymphoid cancer after liver transplantation. Hepatogastroenterology. 1997;44:1172–1181. [PubMed] [Google Scholar]
- 60.Perera GK, Child FJ, Heaton N, O'Grady J, Higgins EM. Skin lesions in adult liver transplant recipients: a study of 100 consecutive patients. Br J Dermatol. 2006;154:868–872. doi: 10.1111/j.1365-2133.2006.07154.x. [DOI] [PubMed] [Google Scholar]
- 61.Funk-Debleds P, Ducroux E, Guillaud O, Ursic-Bedoya J, Decullier E, Vallin M, et al. Subsequent nonmelanoma skin cancers and impact of immunosuppression in liver transplant recipients. J Am Acad Dermatol. 2018;79:84–91. doi: 10.1016/j.jaad.2017.12.063. [DOI] [PubMed] [Google Scholar]
- 62.Kanitakis J, Alhaj-Ibrahim L, Euvrard S, Claudy A. Basal cell carcinomas developing in solid organ transplant recipients: clinicopathologic study of 176 cases. Arch Dermatol. 2003;139:1133–1137. doi: 10.1001/archderm.139.9.1133. [DOI] [PubMed] [Google Scholar]
- 63.Mertz KD, Proske D, Kettelhack N, Kegel C, Keusch G, Schwarz A, et al. Basal cell carcinoma in a series of renal transplant recipients: epidemiology and clinicopathologic features. Int J Dermatol. 2010;49:385–389. doi: 10.1111/j.1365-4632.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 64.Laurent C, Charmpi K, Gravelle P, Tosolini M, Franchet C, Ysebaert L, et al. Several immune escape patterns in non-Hodgkin's lymphomas. Oncoimmunology. 2015;4:e1026530. doi: 10.1080/2162402X.2015.1026530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nijland M, Veenstra RN, Visser L, Xu C, Kushekhar K, van Imhoff GW, et al. HLA dependent immune escape mechanisms in B-cell lymphomas: implications for immune checkpoint inhibitor therapy? Oncoimmunology. 2017;6:e1295202. doi: 10.1080/2162402X.2017.1295202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111:5359–5370. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 67.Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/-monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117:872–881. doi: 10.1182/blood-2010-05-283820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mellemgaard A, Geisler CH, Storm HH. Risk of kidney cancer and other second solid malignancies in patients with chronic lymphocytic leukemia. Eur J Haematol. 1994;53:218–222. doi: 10.1111/j.1600-0609.1994.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 69.Brewer JD, Habermann TM, Shanafelt TD. Lymphoma-associated skin cancer: incidence, natural history, and clinical management. Int J Dermatol. 2014;53:267–274. doi: 10.1111/ijd.12208. [DOI] [PubMed] [Google Scholar]
- 70.Levi F, Randimbison L, Te VC, La Vecchia C. Non-Hodgkin's lymphomas, chronic lymphocytic leukaemias and skin cancers. Br J Cancer. 1996;74:1847–1850. doi: 10.1038/bjc.1996.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232:267–269. [PubMed] [Google Scholar]
- 72.Brewer JD, Shanafelt TD, Khezri F, Sosa Seda IM, Zubair AS, Baum CL, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester Epidemiology Project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302–309. doi: 10.1016/j.jaad.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agnew KL, Ruchlemer R, Catovsky D, Matutes E, Bunker CB. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129–1135. doi: 10.1111/j.1365-2133.2004.05982.x. [DOI] [PubMed] [Google Scholar]
- 74.Hjalgrim H, Frisch M, Storm HH, Glimelius B, Pedersen JB, Melbye M. Non-melanoma skin cancer may be a marker of poor prognosis in patients with non-Hodgkin's lymphoma. Int J Cancer. 2000;85:639–642. doi: 10.1002/(sici)1097-0215(20000301)85:5<639::aid-ijc7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 75.Mehrany K, Weenig RH, Pittelkow MR, Roenigk RK, Otley CC. High recurrence rates of Basal cell carcinoma after mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985–988. doi: 10.1001/archderm.140.8.985. [DOI] [PubMed] [Google Scholar]
- 76.Silverberg MJ, Leyden W, Warton EM, Quesenberry CP, Jr, Engels EA, Asgari MM. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350–360. doi: 10.1093/jnci/djs529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burgi A, Brodine S, Wegner S, Milazzo M, Wallace MR, Spooner K, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005;104:1505–1511. doi: 10.1002/cncr.21334. [DOI] [PubMed] [Google Scholar]
- 78.Zhao H, Shu G, Wang S. The risk of non-melanoma skin cancer in HIV-infected patients: new data and meta-analysis. Int J STD AIDS. 2016;27:568–575. doi: 10.1177/0956462415586316. [DOI] [PubMed] [Google Scholar]
- 79.Crum-Cianflone N, Hullsiek KH, Satter E, Marconi V, Weintrob A, Ganesan A, et al. Cutaneous malignancies among HIV-infected persons. Arch Intern Med. 2009;169:1130–1138. doi: 10.1001/archinternmed.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Asgari MM, Ray GT, Quesenberry CP, Jr, Katz KA, Silverberg MJ. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892–896. doi: 10.1001/jamadermatol.2017.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Habets JM, Tank B, Vuzevski VD, van Reede EC, Stolz E, van Joost T. Characterization of the mononuclear infiltrate in basal cell carcinoma: a predominantly T cell-mediated immune response with minor participation of Leu-7+ (natural killer) cells and Leu-14+ (B) cells. J Invest Dermatol. 1988;90:289–292. doi: 10.1111/1523-1747.ep12456065. [DOI] [PubMed] [Google Scholar]
- 82.Dellon AL, Potvin C, Chretien PB, Rogentine CN. The immunobiology of skin cancer. Plast Reconstr Surg. 1975;55:341–354. [PubMed] [Google Scholar]
- 83.Viac J, Bustamante R, Thivolet J. Characterization of mononuclear cells in the inflammatory infiltrates of cutaneous tumours. Br J Dermatol. 1977;97:1–10. doi: 10.1111/j.1365-2133.1977.tb15421.x. [DOI] [PubMed] [Google Scholar]
- 84.De Panfilis G, Colli V, Manfredi G, Misk I, Rima S, Zampetti M, et al. In situ identification of mononuclear cells infiltrating cutaneous carcinoma: an immuno-histochemical study. Acta Derm Venereol. 1979;59:219–222. [PubMed] [Google Scholar]
- 85.Deng JS, Brod BA, Saito R, Tharp MD. Immune-associated cells in basal cell carcinomas of skin. J Cutan Pathol. 1996;23:140–146. doi: 10.1111/j.1600-0560.1996.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 86.Omland SH, Nielsen PS, Gjerdrum LM, Gniadecki R. Immunosuppressive environment in basal cell carcinoma: the role of regulatory T cells. Acta Derm Venereol. 2016;96:917–921. doi: 10.2340/00015555-2440. [DOI] [PubMed] [Google Scholar]
- 87.Kaporis HG, Guttman-Yassky E, Lowes MA, Haider AS, Fuentes-Duculan J, Darabi K, et al. Human basal cell carcinoma is associated with FOXP3+ T cells in a Th2 dominant microenvironment. J Invest Dermatol. 2007;127:2391–2398. doi: 10.1038/sj.jid.5700884. [DOI] [PubMed] [Google Scholar]
- 88.Hunt MJ, Halliday GM, Weedon D, Cooke BE, Barnetson RS. Regression in basal cell carcinoma: an immunohistochemical analysis. Br J Dermatol. 1994;130:1–8. doi: 10.1111/j.1365-2133.1994.tb06873.x. [DOI] [PubMed] [Google Scholar]
- 89.Fujimura T, Kakizaki A, Kambayashi Y, Aiba S. Basal cell carcinoma with spontaneous regression: a case report and immunohistochemical study. Case Rep Dermatol. 2012;4:125–132. doi: 10.1159/000339621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herwig-Carl MC, Loeffler KU. Regression of periocular basal cell carcinoma: a report of four cases with clinicopathologic correlation. Ocul Oncol Pathol. 2020;6:107–114. doi: 10.1159/000501370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong DA, Bishop GA, Lowes MA, Cooke B, Barnetson RS, Halliday GM. Cytokine profiles in spontaneously regressing basal cell carcinomas. Br J Dermatol. 2000;143:91–98. doi: 10.1046/j.1365-2133.2000.03596.x. [DOI] [PubMed] [Google Scholar]
- 92.Nardinocchi L, Sonego G, Passarelli F, Avitabile S, Scarponi C, Failla CM, et al. Interleukin-17 and interleukin-22 promote tumor progression in human nonmelanoma skin cancer. Eur J Immunol. 2015;45:922–931. doi: 10.1002/eji.201445052. [DOI] [PubMed] [Google Scholar]
- 93.Pellegrini C, Orlandi A, Costanza G, Di Stefani A, Piccioni A, Di Cesare A, et al. Expression of IL-23/Th17-related cytokines in basal cell carcinoma and in the response to medical treatments. PLoS One. 2017;12:e0183415. doi: 10.1371/journal.pone.0183415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beksaç B, İlter N, Erdem Ö, Çakmak P, Çenetoğlu S, Yapar D. Sparsity of dendritic cells and cytotoxic T cells in tumor microenvironment may lead to recurrence in basal cell carcinoma. Int J Dermatol. 2020;59:1258–1263. doi: 10.1111/ijd.15065. [DOI] [PubMed] [Google Scholar]
- 95.Aoki M, Pawankar R, Niimi Y, Kawana S. Mast cells in basal cell carcinoma express VEGF, IL-8 and RANTES. Int Arch Allergy Immunol. 2003;130:216–223. doi: 10.1159/000069515. [DOI] [PubMed] [Google Scholar]
- 96.Erkiliç S, Erbağci Z. The significance of mast cells associated with basal cell carcinoma. J Dermatol. 2001;28:312–315. doi: 10.1111/j.1346-8138.2001.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 97.Diaconu NC, Kaminska R, Naukkarinen A, Harvima RJ, Nilsson G, Harvima IT. Increase in CD30 ligand/CD153 and TNF-alpha expressing mast cells in basal cell carcinoma. Cancer Immunol Immunother. 2007;56:1407–1415. doi: 10.1007/s00262-007-0290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dusingize JC, Olsen CM, Pandeya NP, Subramaniam P, Thompson BS, Neale RE, et al. Cigarette smoking and the risks of basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2017;137:1700–1708. doi: 10.1016/j.jid.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 99.Erbagci Z, Erkiliç S. Can smoking and/or occupational UV exposure have any role in the development of the morpheaform basal cell carcinoma? A critical role for peritumoral mast cells. Int J Dermatol. 2002;41:275–278. doi: 10.1046/j.1365-4362.2002.01487.x. [DOI] [PubMed] [Google Scholar]
- 100.Staibano S, Boscaino A, Salvatore G, Orabona P, Palombini L, De Rosa G. The prognostic significance of tumor angiogenesis in nonaggressive and aggressive basal cell carcinoma of the human skin. Hum Pathol. 1996;27:695–700. doi: 10.1016/s0046-8177(96)90400-1. [DOI] [PubMed] [Google Scholar]
- 101.Starkey JR, Crowle PK, Taubenberger S. Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer. 1988;42:48–52. doi: 10.1002/ijc.2910420110. [DOI] [PubMed] [Google Scholar]
- 102.Ch'ng S, Wallis RA, Yuan L, Davis PF, Tan ST. Mast cells and cutaneous malignancies. Mod Pathol. 2006;19:149–159. doi: 10.1038/modpathol.3800474. [DOI] [PubMed] [Google Scholar]
- 103.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 104.Wille JJ, Kydonieus AF, Murphy GF. cis-urocanic acid induces mast cell degranulation and release of preformed TNF-alpha: a possible mechanism linking UVB and cis-urocanic acid to immunosuppression of contact hypersensitivity. Skin Pharmacol Appl Skin Physiol. 1999;12:18–27. doi: 10.1159/000029842. [DOI] [PubMed] [Google Scholar]
- 105.Yamamoto T, Katayama I, Nishioka K. Expression of stem cell factor in basal cell carcinoma. Br J Dermatol. 1997;137:709–713. [PubMed] [Google Scholar]
- 106.Grimbaldeston MA, Skov L, Finlay-Jones JJ, Hart PH. Increased dermal mast cell prevalence and susceptibility to development of basal cell carcinoma in humans. Methods. 2002;28:90–96. doi: 10.1016/s1046-2023(02)00213-x. [DOI] [PubMed] [Google Scholar]
- 107.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Metz M, Lammel V, Gibbs BF, Maurer M. Inflammatory murine skin responses to UV-B light are partially dependent on endothelin-1 and mast cells. Am J Pathol. 2006;169:815–822. doi: 10.2353/ajpath.2006.060037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smolle J, Soyer HP, Ehall R, Bartenstein S, Kerl H. Langerhans cell density in epithelial skin tumors correlates with epithelial differentiation but not with the peritumoral infiltrate. J Invest Dermatol. 1986;87:477–479. doi: 10.1111/1523-1747.ep12455529. [DOI] [PubMed] [Google Scholar]
- 110.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 111.Ma PF, Gao CC, Yi J, Zhao JL, Liang SQ, Zhao Y, et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J Hepatol. 2017;67:770–779. doi: 10.1016/j.jhep.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 112.Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–120. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 113.Hwang I, Kim JW, Ylaya K, Chung EJ, Kitano H, Perry C, et al. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med. 2020;18:443. doi: 10.1186/s12967-020-02618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 115.Tu D, Dou J, Wang M, Zhuang H, Zhang X. M2 macrophages contribute to cell proliferation and migration of breast cancer. Cell Biol Int. 2021;45:831–838. doi: 10.1002/cbin.11528. [DOI] [PubMed] [Google Scholar]
- 116.Padoveze EH, Chiacchio ND, Ocampo-Garza J, Cernea SS, Belda W, Sotto MN. Macrophage subtypes in recurrent nodular basal cell carcinoma after Mohs micrographic surgery. Int J Dermatol. 2017;56:1366–1372. doi: 10.1111/ijd.13790. [DOI] [PubMed] [Google Scholar]
- 117.König S, Nitzki F, Uhmann A, Dittmann K, Theiss-Suennemann J, Herrmann M, et al. Depletion of cutaneous macrophages and dendritic cells promotes growth of basal cell carcinoma in mice. PLoS One. 2014;9:e93555. doi: 10.1371/journal.pone.0093555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Minocha R, Martin AJ, Chen AC, Scolyer RA, Lyons JG, McKenzie CA, et al. A reduction in inflammatory macrophages may contribute to skin cancer chemoprevention by nicotinamide. J Invest Dermatol. 2019;139:467–469. doi: 10.1016/j.jid.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 119.Santos I, Mello RJ, Santos IB, Santos RA. Quantitative study of Langerhans cells in basal cell carcinoma with higher or lower potential of local aggressiveness. An Bras Dermatol. 2010;85:165–171. doi: 10.1590/s0365-05962010000200006. [DOI] [PubMed] [Google Scholar]
- 120.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang H, Guo Z, Zhang M, Wang J, Chen G, Cao X. Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood. 2006;108:1189–1197. doi: 10.1182/blood-2006-01-007187. [DOI] [PubMed] [Google Scholar]
- 122.Walter A, Barysch MJ, Behnke S, Dziunycz P, Schmid B, Ritter E, et al. Cancer-testis antigens and immunosurveillance in human cutaneous squamous cell and basal cell carcinomas. Clin Cancer Res. 2010;16:3562–3570. doi: 10.1158/1078-0432.CCR-09-3136. [DOI] [PubMed] [Google Scholar]
- 123.Cornel AM, Mimpen IL, Nierkens S. MHC class I downregulation in cancer: underlying mechanisms and potential targets for cancer Immunotherapy. Cancers (Basel) 2020;12:1760. doi: 10.3390/cancers12071760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cabrera T, Garrido V, Concha A, Martín J, Esquivias J, Oliva MR, et al. HLA molecules in basal cell carcinoma of the skin. Immunobiology. 1992;185:440–452. doi: 10.1016/s0171-2985(11)80086-0. [DOI] [PubMed] [Google Scholar]
- 125.Hua LA, Kagen CN, Carpenter RJ, Goltz RW. HLA and beta 2-microglobulin expression in basal and squamous cell carcinomas of the skin. Int J Dermatol. 1985;24:660–663. doi: 10.1111/j.1365-4362.1985.tb05719.x. [DOI] [PubMed] [Google Scholar]
- 126.Walter A, Schäfer M, Cecconi V, Matter C, Urosevic-Maiwald M, Belloni B, et al. Aldara activates TLR7-independent immune defence. Nat Commun. 2013;4:1560. doi: 10.1038/ncomms2566. [DOI] [PubMed] [Google Scholar]
- 127.Otsuka A, Levesque MP, Dummer R, Kabashima K. Hedgehog signaling in basal cell carcinoma. J Dermatol Sci. 2015;78:95–100. doi: 10.1016/j.jdermsci.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 128.García-Plata D, Mozos E, Carrasco L, Solana R. HLA molecule expression in cutaneous squamous cell carcinomas: an immunopathological study and clinical-immunohistopathological correlations. Histol Histopathol. 1993;8:219–226. [PubMed] [Google Scholar]
- 129.Kohchiyama A, Oka D, Ueki H. Expression of human lymphocyte antigen (HLA)-DR on tumor cells in basal cell carcinoma. J Am Acad Dermatol. 1987;16:833–838. doi: 10.1016/s0190-9622(87)70109-1. [DOI] [PubMed] [Google Scholar]
- 130.Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N. HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol. 2003;81:199–252. doi: 10.1016/s0065-2776(03)81006-4. [DOI] [PubMed] [Google Scholar]
- 131.Urosevic M, Dummer R. Human leukocyte antigen-G and cancer immunoediting. Cancer Res. 2008;68:627–630. doi: 10.1158/0008-5472.CAN-07-2704. [DOI] [PubMed] [Google Scholar]
- 132.Westin AT, Gardinassi LG, Soares EG, Da Silva JS, Donadi EA, Da Silva Souza C. HLA-G, cytokines, and cytokine receptors in the non-aggressive basal cell carcinoma microenvironment. Arch Dermatol Res. 2022;314:247–256. doi: 10.1007/s00403-021-02218-x. [DOI] [PubMed] [Google Scholar]
- 133.Elamin I, Zecević RD, Vojvodić D, Medenica L, Pavlović MD. Cytokine concentrations in basal cell carcinomas of different histological types and localization. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17:55–59. [PubMed] [Google Scholar]
- 134.Kim J, Modlin RL, Moy RL, Dubinett SM, McHugh T, Nickoloff BJ, et al. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 135.Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smeltz RB, Chen J, Ehrhardt R, Shevach EM. Role of IFN-gamma in Th1 differentiation: IFN-gamma regulates IL-18R alpha expression by preventing the negative effects of IL-4 and by inducing/maintaining IL-12 receptor beta 2 expression. J Immunol. 2002;168:6165–6172. doi: 10.4049/jimmunol.168.12.6165. [DOI] [PubMed] [Google Scholar]
- 137.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002;27:571–577. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 138.Urosevic M, Maier T, Benninghoff B, Slade H, Burg G, Dummer R. Mechanisms underlying imiquimod-induced regression of basal cell carcinoma in vivo. Arch Dermatol. 2003;139:1325–1332. doi: 10.1001/archderm.139.10.1325. [DOI] [PubMed] [Google Scholar]
- 139.De Giorgi V, Salvini C, Chiarugi A, Paglierani M, Maio V, Nicoletti P, et al. In vivo characterization of the inflammatory infiltrate and apoptotic status in imiquimod-treated basal cell carcinoma. Int J Dermatol. 2009;48:312–321. doi: 10.1111/j.1365-4632.2009.03916.x. [DOI] [PubMed] [Google Scholar]
- 140.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727–742. [PMC free article] [PubMed] [Google Scholar]
- 141.Lipson EJ, Lilo MT, Ogurtsova A, Esandrio J, Xu H, Brothers P, et al. Basal cell carcinoma: PD-L1/PD-1 checkpoint expression and tumor regression after PD-1 blockade. J Immunother Cancer. 2017;5:23. doi: 10.1186/s40425-017-0228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ikeda S, Goodman AM, Cohen PR, Jensen TJ, Ellison CK, Frampton G, et al. Metastatic basal cell carcinoma with amplification of PD-L1: exceptional response to anti-PD1 therapy. NPJ Genom Med. 2016;1:16037. doi: 10.1038/npjgenmed.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fischer S, Hasan Ali O, Jochum W, Kluckert T, Flatz L, Siano M. Anti-PD-1 therapy leads to near-complete remission in a patient with metastatic basal cell carcinoma. Oncol Res Treat. 2018;41:391–394. doi: 10.1159/000487084. [DOI] [PubMed] [Google Scholar]
- 144.Sabbatino F, Marra A, Liguori L, Scognamiglio G, Fusciello C, Botti G, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126. doi: 10.1186/s40425-018-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Falchook GS, Leidner R, Stankevich E, Piening B, Bifulco C, Lowy I, et al. Responses of metastatic basal cell and cutaneous squamous cell carcinomas to anti-PD1 monoclonal antibody REGN2810. J Immunother Cancer. 2016;4:70. doi: 10.1186/s40425-016-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goodman AM, Kato S, Cohen PR, Boichard A, Frampton G, Miller V, et al. Genomic landscape of advanced basal cell carcinoma: implications for precision treatment with targeted and immune therapies. Oncoimmunology. 2017;7:e1404217. doi: 10.1080/2162402X.2017.1404217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stratigos AJ, Sekulic A, Peris K, Bechter O, Prey S, Kaatz M, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:848–857. doi: 10.1016/S1470-2045(21)00126-1. [DOI] [PubMed] [Google Scholar]
- 148.United States Food and Drug Administration. FDA approves cemiplimab-rwlc for locally advanced and metastatic basal cell carcinoma. United States Food and Drug Administration; 2021. [Google Scholar]
- 149.Mohan SV, Kuo KY, Chang AL. Incidental regression of an advanced basal cell carcinoma after ipilimumab exposure for metastatic melanoma. JAAD Case Rep. 2016;2:13–15. doi: 10.1016/j.jdcr.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]