Abstract
Objectives
To scope published reviews addressing fatigue in rheumatoid arthritis (RA), spondyloarthritis, osteoarthritis and fibromyalgia in areas relevant for clinical practice: (1) definition, (2) measurement instruments and diagnosis, (3) determinants, (4) consequences and (5) effectiveness of interventions.
Methods
A systematic literature search of reviews was performed in five bibliographical databases. A hierarchical data extraction was applied based on review type (Cochrane reviews (CRs), followed by non-Cochrane systematic reviews (SRs) and narrative reviews (NRs)) and year of publication. Extracted data were summarised in elaborated narrative syntheses. Results were discussed with a patient panel.
Results
One hundred and thirty-four reviews were included (19 CRs, 44 SRs, 71 NRs). No agreed on definition was reported for general fatigue, nor for types of fatigue. Twenty-five measurement instruments were found, all self-reported. Five instruments proposed a threshold for excessive fatigue. Pain, physical function and depressive symptoms were the most frequently studied disease-related determinants of fatigue; female sex and stress the most frequent contextual determinants. Work performance, followed by impact on pain, physical activity and social roles were the most frequently studied consequences. Whenever quantified, associations between fatigue with determinants and consequences were on average small. For non-pharmacological interventions, if effect sizes were reported, these were negligible to small and for pharmacological interventions negligible to moderate. Patients recommended actions for research and practice.
Conclusion
Syntheses of reviews point to the complexity of fatigue. The extensive amount of evidence could be used to offer tailored management plans to patients in clinical practice and inform future research agendas.
Keywords: Arthritis, Rheumatoid; Arthritis, Psoriatic; Arthritis; Osteoarthritis; Spondylitis, Ankylosing
WHAT IS ALREADY KNOWN ON THIS TOPIC
Fatigue is a prominent symptom in rheumatic and musculoskeletal diseases (RMDs) but is insufficiently addressed in clinical practice.
WHAT THIS STUDY ADDS
This scoping review shows that patients with RMDs experience different types of fatigue and that a large amount of disease-related but also contextual factors are associated with fatigue.
A broad range of non-pharmacological interventions for fatigue have been evaluated, but effect sizes, whenever quantified, were generally negligible to small across RMDs.
Effect sizes of pharmacological interventions on fatigue, if reported, were small to moderate for rheumatoid arthritis, negligible to small for fibromyalgia, but not synthesised in systematic reviews for spondyloarthritis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The extensive amount of evidence summarised in this scoping review can inform future research agendas to ultimately improve management of this complex symptom.
Introduction
Over two-thirds of patients with RMDs experience severe or very severe fatigue and patients with RMDs are more affected by fatigue compared with the general population.1–4 Many patients feel that fatigue surpasses pain as a source of disability and that this symptom is insufficiently addressed by healthcare providers.2
In continuous efforts to improve quality of care for patients with rheumatic and musculoskeletal diseases (RMDs), the Dutch Arthritis Society organised panel discussions among patients with RMDs to gain insight into the knowledge gaps that should be addressed to improve daily care. Patients ranked ‘fatigue and its treatment’ as the area with the highest priority.5
To further specify the knowledge gap related to managing fatigue in clinical practice, the patient panel formulated 15 research questions that were subsequently summarised in 5 research areas including: (1) the definitions of fatigue; (2) measurement instruments to quantify and diagnose fatigue; (3) determinants of fatigue; (4) consequences of fatigue and (5) the effect of interventions on fatigue (online supplemental file S1).
rmdopen-2023-003056supp001.pdf (996.3KB, pdf)
The number of peer-reviewed clinical studies addressing fatigue in RMDs is substantial and many studies have already been summarised in literature reviews. Notwithstanding, knowledge across various research areas remains fragmented, as studies/reviews frequently focus on one rheumatic condition or address a specific topic in a larger research area. As a result, the available knowledge from various areas is insufficiently integrated and fails to recognise differences and similarities related to fatigue across RMDs. This fragmentation also hampers translation of knowledge into the management of fatigue and hinders identification of potentially unaddressed research questions. It was, therefore, decided to perform a scoping review of all available reviews that addresses the five agreed on research areas.
A scoping review is a relatively new approach for mapping the existing literature in a given field.6 Scoping reviews can be performed to summarise and disseminate research findings, to identify research gaps, and to make recommendations for future research. Quality assessments of underlying studies are no part of scoping reviews, as they aim to map the availability of these studies but not their robustness or generalisability.6
The objective of this study was to perform a scoping review of published literature reviews addressing the five preidentified research areas on fatigue in patients with rheumatoid arthritis (RA), spondyloarthritis (SpA, including psoriatic arthritis (PsA)), osteoarthritis (OA) and fibromyalgia (FM).
Methods
This scoping review was performed according to the methodological framework for scoping reviews by Arksey and O’Malley.6 The research protocol was registered in the Registry for Scoping Reviews (OSF, https://osf.io/3dr7b/). This paper was written in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews Checklist.7
Literature search
A systematic literature search was performed in December 2020 and updated in December 2021 in the following five electronic bibliographical databases: MEDLINE (PubMed), EMBASE, the Cochrane Library for Reviews, CINAHL and PsycINFO. The search string contained the following search terms: (1) ‘review’, (2) ‘fatigue’, (3) ‘rheumatoid arthritis’ or ‘spondyloarthritis’ or ‘psoriatic arthritis’ or ‘osteoarthritis’ or ‘fibromyalgia’. These search terms were specified by including synonyms and by transforming all relevant search terms to be compatible with each database (online supplemental file S2). The search was restricted to English and Dutch language. Reference lists of included reviews were screened for additional eligible reviews.
Eligibility criteria
Reviews were eligible if they considered adult patients with RA, SpA, OA or FM (by clinical diagnosis or by fulfilling classification criteria), and reported a quantitative or narrative synthesis of studies addressing one of the 5 research areas (15 research questions, online supplemental file S1). No restrictions were applied for the year of publication or type of review, and thus included Cochrane reviews (CRs), as well as non-Cochrane systematic reviews (SRs) and narrative reviews (NRs). Also, for underlying studies within the reviews, no restrictions were formulated concerning their setting (eg, population surveys, rheumatology clinic), study design (eg, quantitative or qualitative; prospective or retrospective; observational or experimental study design) or fatigue being a primary or concomitant objective of the reviews.
Review selection
Records were imported into Rayyan software and duplicates were removed.8 Two reviewers (EB and KH) independently screened all selected records based on titles and abstracts for eligibility (online supplemental file S3). Next, one reviewer (EB) screened the full text articles and decided whether the eligibility criteria were met. Arguments for exclusion were checked by the second reviewer (KH). Disagreement was resolved by consensus in the presence of a third reviewer (AVT).
Data extraction
Standardised data extraction forms were in line with the Cochrane Collaboration’s recommendations for SRs for each of the five research areas.9 Extraction forms were piloted and adapted for the purpose of evaluating reviews (eg, the number of underlying studies, availability and results of pooled estimates for associations or effect sizes). Data extraction was performed by one reviewer (EB) and was checked by the second reviewer (KH) for 50% of the reviews. The data extraction was performed in a hierarchical approach based on review type (CRs followed by SRs, followed by NRs) and year of publication (from most recent to least recent). For example, SRs and NRs were not considered if a CR on a similar research question was more recently published. In addition, reviews were excluded when there was (partial) overlap in underlying studies with other reviews, in that case, the most complete review was included.
Data synthesis and reporting
Extracted data of each review were reported in an elaborated narrative synthesis stratified per research area and for each RMD separately (online supplemental file S4–S18).
To facilitate synthesis for the research areas ‘determinants’ and ‘consequences’, individual ‘determinants’ or ‘consequences’ were categorised using the International Classification of Functioning, Disability and Health (ICF) as guidance.10 The formal ICF linking rules could not be strictly applied, because determinants that actually belonged to separate ICF categories were often grouped for the purpose of the included reviews. Therefore, determinants and consequences were classified into the main ICF components (Body functions combined with Body Structures, Activities, Participation, Contextual personal factors and Contextual environmental factors) while further keeping the terminology (of grouped determinants/consequences) as in the reviews. For some studies within the reviews, it was unclear whether the factor studied was a ‘determinant’ or ‘consequence’, especially when underlying studies had a cross-sectional design. Whenever insufficiently reported in the review, factors were classified as determinants.
For each determinant of fatigue, bubble plots were computed per RMD of interest to summarise the number of unique underlying studies across reviews (bubble size) together with the overall direction of the association (positive, negative, absent or inconsistent association with fatigue).
For interventions, findings were reported separately for non-pharmacological and pharmacological interventions. Non-pharmacological interventions were reported per intervention type and pharmacological interventions were reported per drug class.
Whenever available, quantitative findings were reported as formulated in each review (eg, characteristics of measurement instruments, strength of associations (weak, moderate or strong) or effect sizes (small, moderate or large)).
Patient and public involvement
Two meetings were organised to discuss the results of this study with the patient discussion panel on fatigue from the Dutch Arthritis Society. In preparation, all participants received summaries of (preliminary) findings. At the first meeting, the types of fatigue most frequently encountered within reviews were preliminarily classified and subsequently discussed with the patient panel (as that part of the data extraction was finished). In the second meeting, the final results were presented, and the patient panel helped interpreting our findings on the research questions and identifying new knowledge gaps.
Results
Overall, 134 reviews were included (19 CRs, 44 SRs and 71 NRs (online supplemental file S4)). Of these, 54/134 (40%) reviews addressed fatigue as the primary objective, and 45/134 (34%) reviews considered fatigue in RA. Table 1 shows the total number of included reviews per different review type for each research area and RMD of interest. CRs only reported on non-pharmacological and pharmacological interventions, whereas SRs and NRs also addressed other research areas.
Table 1.
Included reviews covering one or more research areas and/or RMDs
| Research areas | Cochrane reviews n=19 | Systematic reviews n=44 | Narrative reviews n=71 |
| Definition of fatigue n=16* | |||
| RA | – | – | 4 (4) |
| SpA | – | – | 2 (2) |
| OA | – | – | 2 (2) |
| FM | – | – | 4 (3) |
| Mixed RMDs | – | – | 5 (5) |
| Measurement instruments for fatigue n=26* | |||
| RA | – | 2 (1) | 7 (5) |
| SpA | – | 2 (0) | 7 (3) |
| OA | – | 1 (0) | 1 (1) |
| FM | – | 1 (0) | 5 (2) |
| Mixed RMDs | – | – | 1 (1) |
| Determinants of fatigue n=28* | |||
| RA | – | 4 (4) | 9 (7) |
| SpA | – | 1 (0) | 6 (3) |
| OA | – | – | 3 (2) |
| FM | – | – | 6 (3) |
| Mixed RMDs | – | – | – |
| Consequences of fatigue n=21* | |||
| RA | – | 4 (3) | 11 (7) |
| SpA | – | 1 (0) | 3 (1) |
| OA | – | – | 3 (2) |
| FM | – | – | – |
| Mixed RMDs | – | – | – |
| Non-pharmacological interventions n=39 | |||
| RA | 1 (1) | 1 (0) | 2 (2) |
| SpA | 1 (1) | 1 (0) | 2 (1) |
| OA | – | 2 (0) | 1 (1) |
| FM | 10 (5) | 14 (5) | 4 (2) |
| Mixed RMDs | – | – | – |
| Pharmacological interventions n=39 | |||
| RA | 1 (1) | 3 (1) | 9 (3) |
| SpA | – | 2 (0) | 5 (1) |
| OA | – | – | – |
| FM | 6 (1) | 8 (1) | 5 (0) |
| Mixed RMDs | – | – | – |
Number of included reviews (number of reviews including fatigue in their primary objective).
*Reported sum of reviews is not equal to the individual number of reviews per research area and review type, because some reviews cover one or more research areas and/or RMDs. References of all included reviews can be found in online supplemental file S4.
FM, fibromyalgia; OA, osteoarthritis; RA, rheumatoid arthritis; RMDs, rheumatic and musculoskeletal diseases; SpA, spondyloartritis.
Definition of fatigue
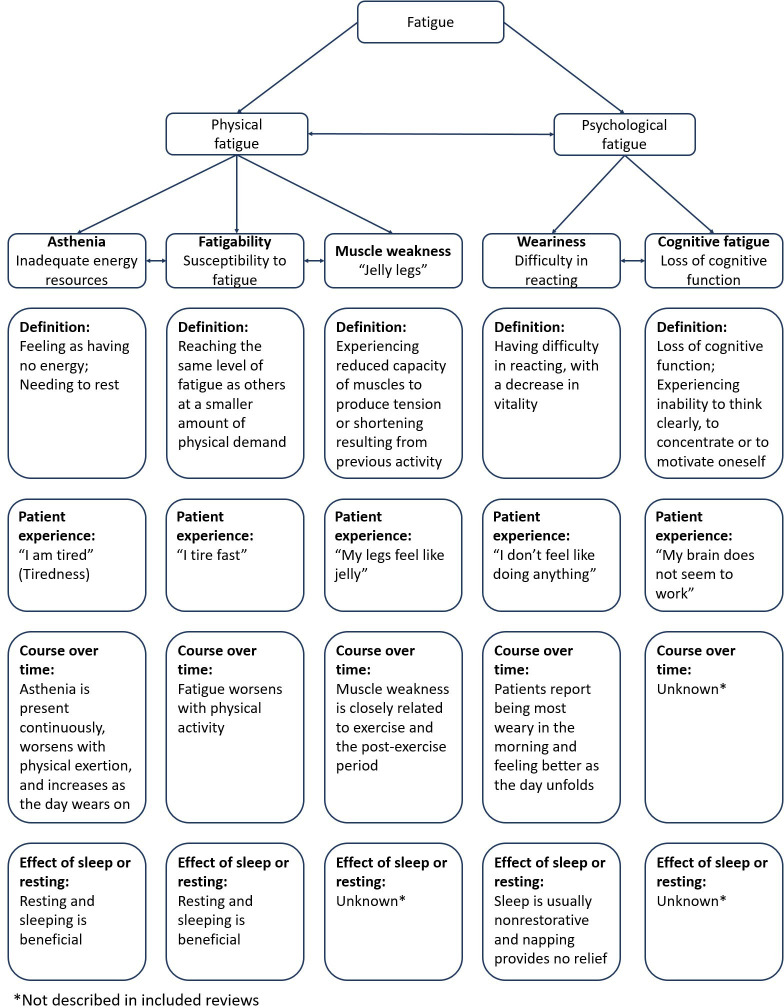
Fatigue in RMDs was defined in 16 NRs. Across reviews, there was agreement that fatigue is a complex, highly subjective symptom, including various types with specific characteristics that can occur simultaneously or alternatingly in daily life.2 11–14 Fatigue can, therefore, be defined and expressed differently over time within one person, among persons with the same RMD or different RMDs. The reviews differentiate fatigue in several ways, including acute versus chronic fatigue, central versus peripheral and spinal fatigue, normal versus pathological fatigue and various definitions have been provided for fatigue in general (table 2) and different types of fatigue. However, no agreed on definition for fatigue or (any of the) different types of fatigue were found for any RMD (online supplemental file S5). Figure 1 attempts to synthesise the types of fatigue identified in studies in RMDs. Many papers distinguish between physical and mental fatigue. Described subtypes for physical fatigue include asthenia, fatigability and muscle weakness, and for mental fatigue this includes weariness and cognitive fatigue (figure 1).
Table 2.
Definitions of general fatigue reported in the included reviews
| Included reviews* | Year of publication | Review type | Population | Reported definitions of fatigue in the included reviews† |
| Seifert et al | 2019 | NR | RMDs |
|
| Dupond et al2 | 2011 | NR | RMDs |
|
| Stebbings et al11 | 2010 | NR | RA and OA |
|
| Marrelli et al12 | 2018 | NR | RA |
|
| Balsamo et al | 2014 | NR | RA |
|
| Rosen et al | 2016 | NR | SpA (PsA) |
|
| Hackney et al | 2019 | NR | OA |
|
| Casale et al | 2011 | NR | FM |
|
*Complete references are provided in online supplemental file S5, as well as definitions of different types of fatigue.
†Minor textual adaptations were made for consistency reasons.
FM, fibromyalgia; OA, osteoarthritis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; RMDs, rheumatic and musculoskeletal diseases; SpA, spondyloarthritis.
Figure 1.
Schematic representation of the construct fatigue in RMDs based on included reviews.
Measurement instruments for fatigue
Measurement instruments for fatigue and their characteristics were addressed in 26/134 (19%) of the included reviews (6 SRs and 20 NRs). The majority of the information was retrieved from one NR by Elera-Fitzcarrald et al that describes instruments used to assess fatigue in patients with RMDs.15 References of all included reviews for this research area are available in online supplemental file S4.
Across reviews, 3 disease-specific (2 for RA and 1 for RA, SpA, OA and FM) and 22 generic self-reported measurement instruments were described (online supplemental file S6). Of these, 10/25 (40%) instruments aimed to be used in research settings, 7 (28%) were validated for use in both clinical and research settings and for the remaining 8 (32%) instruments this was not reported in the reviews. More than half of the available instruments (13/25; 52%) were single questions assessing overall fatigue, while the other instruments were multidimensional, that is, assessing one or more types of fatigue. Fatigue as a single item was sometimes part of patient-reported outcomes assessing other health domains, for example, a question on fatigue is part of the Rheumatoid Arthritis Impact of Disease, the Bath Ankylosing Spondylitis Disease Activity Index, the Psoriatic Arthritis Impact of Disease and the Fibromyalgia Impact Questionnaire.16–19 One NR reported that the most frequently used measurement instruments for assessing fatigue in RMDs were the Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F), Fatigue Severity Scale (FSS), Multidimensional assessment of fatigue and fatigue on a Visual Analogue Scale.15
For 5 instruments (5/25; 25%), validated cut-off values to diagnose or classify ‘excessive fatigue’ were available. Of note, this was the case for only one instrument (single-item 0–10 rating scale) that was proposed for use in clinical practice. Both reliability (internal consistency and/or test–retest) and validity (content, construct and/or criterion validity) were reported for 17/25 (68%) instruments, and were mostly rated as moderate to strong. Overall, all disease-specific instruments, several generic multidimensional questionnaires (ie, Short Form 36 (SF36) vitality subscale, Multidimensional Fatigue Inventory, Multidimensional Fatigue Symptom Inventory Short Form, FACIT-F, Checklist Individual Strength fatigue, Profile of Fatigue, Fatigue Severity Inventory (FSI), FSS and the single item questions (Fatigue Numeric Rating Scales (Fatigue NRS)) to assess severity or impact of fatigue had sufficient construct validity and reliability. Comparative validity was not reported in reviews.
Determinants of fatigue
Determinants of fatigue in RMDs of interest were addressed in 28/134 reviews (21%; 5 SRs and 23 NRs, table 1). Of these, 18/28 reviews (64%) addressed fatigue as their primary objective and 13/28 reviews (46%) concerned determinants of fatigue specifically in RA.
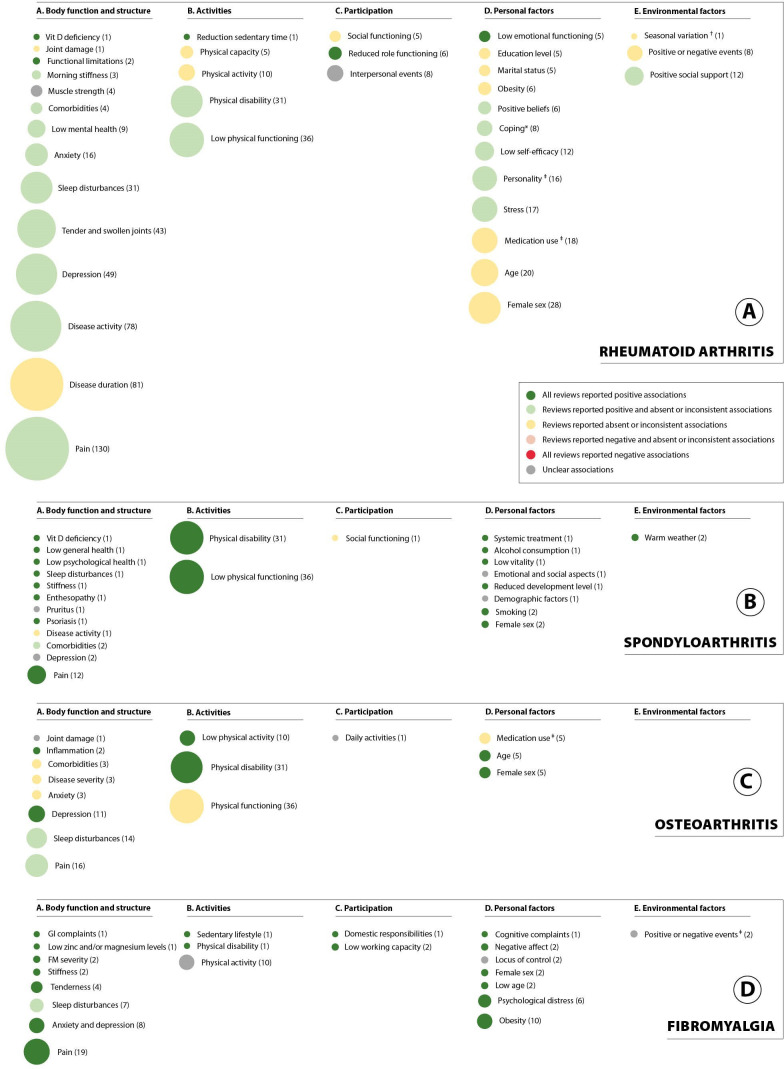
An overview of types of determinants per RMD of interest is available in online supplemental file S7–S10. There was a broad range in the number of underlying studies across reviews for each determinant (range 1–130, median 3 and IQR 3–8, see figure 2). Reviews sparsely reported relevant methodological aspects of underlying studies (eg, design and setting; whether or not adjusted for confounders) and relevant aspects related to synthesis or findings (eg, direction and strength of association; pooled effect) were often absent.
Figure 2.
Identified determinants of fatigue categorised within the ICF model. The overall direction of associations between determinants and fatigue across reviews were summarised and colour-coded for (A) rheumatoid arthritis, (B) spondyloarthritis, (C) osteoarthritis and (D) fibromyalgia. A positive association indicates that an increase in the factors contributes to more severe experiences of fatigue. These summaries are reported independent of strength (weak, moderate or strong) and statistical significance of the associations. The bubble size represents the number of underlying studies according to the reviews that studied these associations. *In multivariable analyses, only worrying coping retained its significant association with fatigue. †Higher fatigue during winter was suggested, but multivariable analyses were inconsistent. ‡Variable summarising a concept that includes ≥2 dimensions: for details, see online supplemental file S7–S10. FM, fibromyalgia; ICF, international classification of functioning; GI, gastrointestinal.
Clearly, determinants belonging to the ICF components ‘disability and health’ were more frequently studied than determinants belonging to the components ‘contextual factors’. Across reviews, pain, sleep disturbances, physical function/disability and depressive symptoms/anxiety were the most frequently studied health-related determinants of fatigue. Of note, pain was generally positively associated with fatigue in most reviews although some reviews in RA and OA reported inconsistent results. For disease activity, reviews in RA repeated generally positive findings while in SpA associations were inconsistent in all reviews. Whenever provided, strength of associations were generally small. A positive association between sleep disturbances and fatigue was reported in SpA, while both positive and inconsistent associations were reported for RA, OA and FM.
Female sex was consistently positively associated with (higher) fatigue in SpA, OA and FM, but inconsistent associations were found for RA. Inconsistent associations were reported for medication use in RA and OA.
Consequences of fatigue
The consequences of fatigue on health outcomes were addressed in 21/134 reviews (16%, 5 SRs and 16 NRs) for RA, SpA and OA, but not for FM (table 1). Of these, 12/21 reviews (57%) addressed fatigue as a primary objective. Of note, 15/21 reviews (71%) concerned consequences of fatigue specifically in RA.
Twenty-one types of consequences had been reported, among which 8 were studied in at least 1 SR and 15 types of consequences were exclusively addressed in NRs in 1 or more of the RMDs (table 3). Overall, 14 types of consequences were also reported as determinants. Again, methodological aspects of underlying studies and numeric findings of statistical analyses were sparsely reported.
Table 3.
Consequences of fatigue reported by included reviews
| ICF-model component | RA | SpA | OA | FM | ||
| Functional perspective | Body function and structure | Pain | X* | X* | ||
| Disease activity/severity | X* | X* | ||||
| Fatigue | X† | |||||
| Overall health/health-related quality of life | X | X | X | |||
| Depression | X* | X* | X* | |||
| Sleep (disturbances) | X* | |||||
| Activities | Physical functioning‡ | X*† | ||||
| Physical activity‡ | X*† | X† | X* | |||
| Physical impairment/disability‡ | X* | |||||
| Sexual activities | X | |||||
| Participation | Work performance | X† | X | X | ||
| Social activities and household chores | X* | X | ||||
| Role limitations (general) | X* | |||||
| Daily self-care and socially relevant tasks | X | |||||
| Contextual perspective | Personal factors | Stress | X*† | |||
| Parenting and family size | X† | |||||
| Physical and mental or emotional well-being | X* | |||||
| Coping | X* | |||||
| Environmental factors | Social support | X*† | ||||
| Partner relationships | X | |||||
| Relational and socioeconomic variables | X*† | |||||
*Also reported as determinants for fatigue by included reviews.
†Reported in at least one systematic review, excluding those that were unclear as to whether variable was considered a determinant or consequence.
‡Conceptual difference between these consequences was not clear based on the reviews.
FM, fibromyalgia; ICF, International Classification of Functioning; OA, osteoarthritis; RA, rheumatoid arthritis; SpA, spondyloarthritis.
Across reviews, consistent associations were found between more fatigue and impairments of body functions (eg, pain, disease activity and depression), limitations in the performance of activities and restrictions in the level of participation (eg, social activities) (online supplemental file S11–S13). In RA, work performance was the most frequently reported consequence of fatigue, including presenteeism, absenteeism and work productivity loss (two SRs and three NRs).
Consequences of fatigue on aspects belonging to the ICF components ‘contextual factors’ were only reported for RA (eg, family size, social support and socioeconomic variables). Findings on the influence of fatigue on contextual factors in RA revealed that fatigue negatively influences experiences of stress, coping strategies and feelings of having adequate social support.
Effect of non-pharmacological interventions on fatigue
The effect of non-pharmacological interventions on fatigue in RMDs was addressed in 39/134 reviews (29%) (12 CRs, 18 SRs and 9 NRs, table 1). Of these, 18 reviews (46%) addressed fatigue in their primary objective.
The 39 reviews summarised 75 interventions comprising exercise (n=28); psychotherapy and education (n=16); lifestyle behaviour (n=5); electrical nerve stimulation (n=10); complementary and alternative medicine (n=7); or other interventions (n=9) (eg, nurse-led care or massages) (online supplemental file S14–S17). Of these, 14/75 interventions were exclusively discussed in NRs. An overview of interventions for which the effects were reported in CRs is provided in table 4.
Table 4.
Effect of non-pharmacological interventions on fatigue in patients with RMDs, as reported in Cochrane reviews
| Cochrane review* | Year of publication | RMD | Type of intervention | Reported effect of intervention on fatigue† (reported quality of evidence) |
| Physical exercise interventions | ||||
| Cramp et al | 2013 | RA |
|
Small effect (M) |
| Regnaux et al20 | 2019 | SpA |
|
No effect (VL) Reduction in fatigue (one study) (VL) |
| Resistance exercise therapy | ||||
| Busch et al | 2013 | FM |
|
Large effects (NR) |
| FM |
|
No effect (NR) | ||
| Whole body vibration (WBV) therapy | ||||
| Bidonde et al | 2017 | FM |
|
Reduction that met the threshold for clinical relevance (VL) |
| Meditative movement therapies therapy (MMT) (eg, Ai Chi, Tai Chi, Yoga awareness, Bat, Qi-Gong, Water yoga) | ||||
| Theadom et al | 2015 | FM |
|
Advantageous (VL) |
| Mixed exercise training (two or more components of physical exercise) | ||||
| Bidonde et al | 2019 | FM |
|
More improvement postintervention, but at long-term follow-up only one-third studies showed an effect (M) |
| FM |
|
No effect (VL) | ||
| FM |
|
No effect (VL) | ||
| FM |
|
No effect (VL) | ||
| FM |
|
No effect (VL) | ||
| Aerobics exercise (eg, cycling, walking, regardless of frequency, duration or intensity) | ||||
| Bidonde et al | 2017 | FM |
|
No effect (one study) (VL) Significant effect (two studies) at long-term follow-up (VL) |
| FM |
|
No effect (L) | ||
| FM |
|
No effect (L) | ||
| Busch et al | 2007 | FM |
|
No effect (VL) |
| Aquatic exercise therapy | ||||
| Bidonde et al | 2014 | FM |
|
No effect (NR) |
| FM |
|
No effect (NR) | ||
| FM |
|
No effect (NR) | ||
| FM |
|
No effect (NR) | ||
| Flexibility exercise therapy | ||||
| Kim et al | 2019 | FM |
|
No effect (VL) |
| Psychosocial interventions | ||||
| Cramp et al | 2013 | RA |
|
Small effect (L) |
| Mind and body therapy | ||||
| Theadom et al | 2015 | FM |
|
No effect (VL) |
| FM |
|
No effect (VL) | ||
| Complementary interventions and complementary medicine | ||||
| Cramp et al | 2013 | RA |
|
No effect (L) |
| RA |
|
Greater mean reduction (L) | ||
| Acupuncture | ||||
| Deare et al | 2013 | FM |
|
Significant difference (L) |
| FM |
|
No effect (M) | ||
| FM |
|
No effect (NR) | ||
| Transcutaneous electrical nerve stimulation (TENS) | ||||
| Johnson et al | 2017 | FM |
|
Reduced fatigue with movement, but not at rest (VL) |
| FM |
|
Clinically important improvements (VL) | ||
| FM |
|
Clinically important improvements (VL) | ||
| Lifestyle interventions | ||||
| Cramp et al | 2013 | RA |
|
Improvement in intervention group‡ (L) |
| RA |
|
Improvements between baseline and follow-up (L) | ||
| RA |
|
Small improvements between baseline and follow-up (L) | ||
*Complete references are provided in online supplemental file S14–S17, as well as results of non-Cochrane systematic reviews and narrative reviews.
†Effect always refers to a reduction of fatigue compared to controls, unless otherwise indicated.
‡Between-arm comparisons were not reported.
FM, fibromyalgia; L, low; M, moderate; NR, not reported; RA, rheumatoid arthritis; RMDs, rheumatic and musculoskeletal diseases; SpA, spondyloarthritis; VL, very low.
Across RMDs, non-pharmacological interventions had generally no or a small positive effect on fatigue compared with usual care (online supplemental file S18–S20). The effectiveness of interventions on fatigue was inconsistent across RMDs, for example, two CRs summarised that aerobic exercise compared with usual care has a small effect on fatigue in RA, but no effect in SpA.20 21
Effect of pharmacological interventions on fatigue
The effect of pharmacological interventions on fatigue in patients with RMDs was addressed in 39/134 reviews (29%, 7 CRs, 13 SRs and 19 NRs). Of these, 8 reviews (21%) included fatigue as the primary objective. No review on pharmacological interventions in OA reported effects on fatigue (table 1). An overview of pharmacological interventions on fatigue in RA and FM for which the effects were reported in CRs is provided in tables 5 and 6. No CRs addressed the effects of pharmacological interventions on fatigue in SpA or OA.
Table 5.
Effect of pharmacological interventions on fatigue in patients with rheumatoid arthritis, as reported in Cochrane reviews
| Cochrane review* | Year of publication | Pharmacological interventions | Reported effect of intervention on fatigue† (reported quality of evidence) |
| Biological DMARDs (bDMARDs) | |||
| Almeida et al22 | 2016 |
|
Small to moderate improvement in patients with active RA and moderate to high levels of fatigue (M) |
|
Moderate effect (M) | ||
|
Moderate effect (M) | ||
*Complete references are provided in online supplemental file S18, as well as results of non-Cochrane systematic reviews and narrative reviews.
†Effect always refers to a reduction of fatigue compared with controls.
‡These drugs are not prescribed in patients with RA.
DMARD, disease-modifying anti-rheumatic drugs; L, Low; M, moderate; NR, not reported; TNF, tumour necrosis factor; VL, very low.
Table 6.
Effect of pharmacological interventions on fatigue in patients with fibromyalgia, as reported in Cochrane reviews
| Cochrane review* | Year of publication | Pharmacological interventions | Reported effect of intervention on fatigue† (reported quality of evidence) |
| Anti-depressant class serotonin and norepinephrine reuptake inhibitors (SNRIs) | |||
| Welsch et al34 | 2018 |
|
Overall effect not substantial (L) |
| Anti-depressant class selective serotonin reuptake inhibitors (SSRIs) | |||
| Walitt et al35 | 2015 |
|
Not statistically significantly superior (VL) |
|
Not statistically significantly superior (VL) | ||
| Anti-depressant class tricyclic antidepressants (TCAs) | |||
| Tofferi et al49 | 2004 |
|
No improvement (NR) |
| Welsch et al33 | 2018 |
|
No statistically significant benefit (L) |
| Antipsychotics | |||
| Walitt et al38 | 2016 |
|
Significant improvement (VL) |
|
No statistically significant difference (L) | ||
| Cannabinoids | |||
| Walitt et al38 | 2016 |
|
Did not convincingly relieve fatigue (VL) |
| Combinations of pharmacological interventions for fatigue | |||
| Thorpe et al37 | 2018 |
|
No statistically significant effect (VL) |
|
Amitriptyline alone or in combination with naproxen: significantly larger improvements in VAS scores of sleep difficulty, fatigue and morning tiredness (VL) Naproxen: no statistically significant effect (VL) |
||
|
No statistically significant change (VL) | ||
|
Melatonin (low/high dose) with fluoxetine: significant improvement (VL) Melatonin (high dose) monotherapy: no improvement (VL) |
||
| Comparative efficacy of pharmacological interventions for fatigue | |||
| Welsch et al 34 | 2018 |
|
No significant differences (NR for subgroup analyses) |
*Complete references are provided in online supplemental file S17, as well as results of non-Cochrane systematic reviews and narrative reviews.
†Effect always refers to a reduction of fatigue compared with controls.
‡Cyclobenzaprine is a muscle relaxant, structurally related to TCAs.
FM, fibromyalgia; L, low; M, moderate; NR, not reported; VAS, Visual Analogue Scale; VL, very low.
In RA, the effect of 12 biological disease-modifying anti-rheumatic drugs (bDMARDs), 2 targeted synthetic DMARDs (tsDMARDs) and a cannabinoid on fatigue were summarised in 1 CR, 3 SRs and 9 NRs (online supplemental file S18). In patients with active RA and moderate to high levels of fatigue, 1 CR and 1 SR reported that bDMARDs as a group have a small to moderate positive effect on fatigue compared with placebo or usual care.22 23 Additionally, for tocilizumab, another SR reported clinically important improvements in fatigue compared with placebo.24 Two bDMARDs (sarilumab and anakinra) and both tsDMARDs (baricitinib and tofacitinib) were exclusively discussed in NRs.25–28 In several intervention studies reported in CR or SR, methotrexate was an active comparator, but effects in this treatment arm or compared with placebo were not synthesised. One SR reported that the cannabinoid nabilone has no superiority in reducing fatigue compared with placebo.29
In SpA, the effect of NSAIDs, one conventional synthetic DMARD (csDMARD), four bDMARDs and two tsDMARDs on fatigue were reported in two SRs and five NRs (online supplemental file S19). Overall, ‘improvements’ (without effect size) of fatigue were reported in SRs and NRs for NSAIDs and bDMARDs in axial SpA (axSpA) and one csDMARD (methotrexate) in PsA. For tofacitinib in PsA, no improvement in fatigue was found according to one NR.30 Effects on fatigue were quantified in two NRs only. One NR discussed a pooled analysis of three randomised controlled trials in which apremilast resulted in clinically important reductions of fatigue in 51% of patients with PsA.31 One other NR reported that infliximab and etanercept reduced fatigue levels by more than 50% in studies among patients with axSpA.32
In FM, the effect of 12 anti-depressants, 1 anticonvulsant, 1 antipsychotic, 2 dietary supplements and 10 ‘other’ pharmacological interventions, such as a dopaminergic agonist (pramipexole), a central stimulant (modafinil) or hypnotics (zopiclone and zolpidem), on fatigue were reported in 6 CRs, 8 SRs and 5 NRs (online supplemental file S20). Five CRs reported that almost all antidepressants have no or a small positive effect on fatigue compared with control interventions.33–37 One CR cautioned about the very low quality of evidence for effect of antipsychotics on fatigue in FM.38 One SR reported a significant reduction of fatigue for the dietary supplement Coenzyme Q10 compared with control.39 A second dietary supplement (s-adenosylmethionine) and nine ‘other’ pharmacological interventions were exclusively discussed in NRs (online supplemental file S20).40–43
Patient panel discussion feedback
At the first meeting with the patient discussion panel, participants discussed the proposed schematic classification for types of fatigue and their descriptions (ie, figure 1), and consented to the final version. When discussing the full results in the second meeting, participants felt that the findings overall confirmed their experience in daily life. They were impressed by the large amount of available knowledge on fatigue, which contrasted with the limited attention paid to fatigue in daily clinical practice. In addition, participants pointed to factors related to fatigue that were not discussed in the included reviews, such as the effect of specific lifestyle interventions on fatigue (eg, two patients participated in the lifestyle intervention ‘plants for joints’, of which findings were not yet available at time of our literature searches and therefore not included).44 Some participants felt that it was stigmatising that the majority of reviews of non-pharmacological interventions were performed in FM. Overall, the patient panel advised to translate the findings into points to be considered for clinical practice and to define a research agenda with specific attention for diagnosing and treating excessive fatigue in RMDs.
Discussion
A panel of patients with RMDs prioritised fatigue as the most important topic that should be addressed to improve daily clinical care. As a first step, this scoping review summarised systematic and non-systematic reviews on aspects of fatigue that are relevant for clinical practice, addressing five predefined research areas in four RMDs.
Although no consensus definition exists for fatigue in RMDs, the reviews were in agreement that patients with RMDs can experience several types of fatigue that can occur simultanously or alternatingly in patients’ lives. Notwithstanding, no agreement exists on which types should be distinguished. It is therefore not suprising that measurement instruments summarised in reviews, even if multidimensional, differed largely on the number and type of dimensions adressed. Importantly, all instruments were patient reported and only a small proportion of these instruments were specifically developed and/or validated for use in clinical care or included cut-off values to identify persons with excessive fatigue.
Numerous reviews showed that a large number of health-related and contextual factors were associated with fatigue as either a determinant or a consequence, but overall the strength of assocations was small. Whenever quantified, pharmacological interventions had a small to moderate effect on fatigue in RA, but no to a small positive effect in FM. No SRs reported effect sizes of pharmacological interventions on fatigue in SpA but narrative summaries frequently reported improvements on fatigue following drug treatment. A large variety of non-pharmacological interventions (including cognitive behavioural therapy and dietary changes) had generally no to a small positive effect on fatigue across RMDs, with most reviews focusing specifically on FM.
Whenever reported, strength of associations and effects of interventions were overall weak or small, with the exception of some pharmacological interventions in RA that showed a moderate effect size. Partly, this could be explained by methodological issues. First, fatigue was not always the primary objective of the review, and therefore, effects were not always quantified, even not in SRs. Also, in the underlying studies, fatigue was rarely the primary endpoint. Consequently, effects from intervention and association studies might be underestimated as study populations were often not selected on the presence of (a specific type or specified level of) fatigue, reducing potential for improvement and lacking power to adequately determine strength of associations. Finally, the synthesis and interpretation of aggregated data in reviews is likely complicated by the heterogeneity of study designs (eg, head-to-head comparisons or placebo-controlled interventions) and measurement of fatigue.
Multiple variables were reported both as a potential determinant (ie, predicting fatigue) ánd a potential consequence (ie, predicted by fatigue). This notably includes variables such as pain, disease activity/severity, physical functioning and depression, as well as factors related to social functioning. Unfortunately, findings from studies reporting on associations with or consequences of fatigue often relied on bivariate correlations and the majority of included reviews did not explicitly report whether underlying studies involved cross-sectional and/or longitudinal analyses, nor whether they were adjusted for confounders, which precludes firm conclusions on the direction of causal relationships. Most likely, however, fatigue in RMDs is determined by numerous multidirectional and/or circular pathways. As an example, while pain was positively correlated with fatigue in RA, SpA, OA and FM, there were reviews describing an indirect effect of sleep disturbances on fatigue by lowering pain thresholds in these RMDs, with some studies indicating that the effect of sleep disturbance on fatigue might even be fully mediated by pain. Similarly, it seems plausible that the effects of interventions on fatigue might—at least in part—be indirect and/or mediated by effects of these interventions on, for example, pain and physical or emotional functioning.
The patient panel questioned whether findings could be translated to clinical practice. Currently, patients may struggle to communicate their fatigue with their care provider and as a result may feel misunderstood or isolated.2 13 Our scoping review indicates fatigue is a complex symptom, and patients clearly recognise different types of fatigue. Using clinical reasoning, the information retrieved about type(s) of fatigue experienced, determinants and consequences can subsequently be used to compose a personalised treatment plan together with the patient. Such proposal might vary from spreading activities throughout the day to save energy, to increasing physical fitness, practicing mindfulness or focusing rather on patients’ acceptance of fatigue. The European League Against Rheumatism (EULAR) recommendations for core competences of health professionals in rheumatology advise to stimulate patients’ self-management for fatigue, and our review can also help to identify factors and treatment options to consider when discussing self-management.45
An important aim of scoping reviews is to identify potential knowledge gaps and highlight areas that are in need of further inquiry. Our results underline the importance of establishing consensus on an overarching definition of fatigue and different types of fatigue in RMDs. An operable construct that comprehensively captures the various experiences of fatigue among patients with RMDs could not only serve as a framework to identify or develop/adapt measurement instruments in alignment with types of fatigue, but could also support communication between patients and care providers in clinical practice. Ideally, this should be developed in cooperation with patients, based on the available evidence. The schematic synthesis of fatigue proposed in this scoping review (figure 1), verified and supported by a patient panel, illustrates a possible approach and potential starting point for such an endeavour. As for clinical trials, the EULAR/American College of Rheumatology collaborative recommendations for reporting disease activity in clinical trials in RA, already advised to include fatigue when evaluating effectiveness of interventions.46 Our findings suggest that a comprehensive understanding of fatigue would benefit from high quality studies which include fatigue as a specific research objective. Given the complex multidimensional nature of fatigue, the development of a conceptual framework for fatigue in RMDs would be beneficial. Conceptual models have previously been proposed for fatigue in RA and other inflammatory rheumatic diseases, but were primarily focused on pathogenesis.47 48 Similar conceptual models to understand the experience of health could be proposed. Overall, as an essential next step to unravel and ultimately improve fatigue in RMDs, the development of an agreed research agenda on fatigue is warranted.
Our review has several limitations. First, in line with the methodology of scoping reviews, we did not perform quality assessments of the reviews. Clearly, NRs have higher risk of bias in the conclusions. Second, relevant determinants, consequences and interventions might have been missed when they have not (yet) been the objective of a published review. Third, reviews sparsely reported whether fatigue was assessed as one general construct or as one or more types of fatigue, which hampers the translation of these research results into clinical practice. Fourth, pathophysiological pathways of fatigue were no research area of this scoping review as we focused on relevant areas for clinical practice. The clinical value of potential (laboratory or imaging) biomarkers for various types of fatigue could be added to the research agenda.
Strengths of this scoping review are that it addresses areas that are typically relevant for clinical care. Furthermore, this project was initiated by patients and all results were discussed with a patient panel to include patients’ interpretations, verifying that results are relatable from the patient perspective.
In conclusion, many reviews have been published on fatigue in RMDs, but fatigue was often addressed as a secondary objective in these studies. The extensive amount of evidence synthesised in this scoping review can be translated to clinical care in order to support clinical reasoning and to compose a tailored treatment plan for fatigue in an individual patient. More important, the findings should stimulate the development of a research agenda as a logical next step. That process should emphasise collaboration between research areas to efficiently develop more insights into and solutions for this complex symptom.
Acknowledgments
The authors gratefully thank the patient discussion panel of the Dutch Arthritis Society for their valuable contribution to this study.
Footnotes
Contributors: AB designed the study protocol. EB and KH performed the data selection, extraction and reporting. All authors contributed to interpretation of the data. All authors were involved in drafting and critically revising the article and gave final approval of the submitted manuscript. EB and KH contributed equally to this work. Guarantor: AB.
Funding: This study was funded by the Dutch Arthritis Society (20-001).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Data are available on reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Katz P. Causes and consequences of fatigue in rheumatoid arthritis. Curr Opin Rheumatol 2017;29:269–76. 10.1097/BOR.0000000000000376 [DOI] [PubMed] [Google Scholar]
- 2.Dupond JL. Fatigue in patients with rheumatic diseases. Joint Bone Spine 2011;78:156–60. 10.1016/j.jbspin.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 3.Dagfinrud H, Vollestad NK, Loge JH, et al. Fatigue in patients with Ankylosing Spondylitis: A comparison with the general population and associations with clinical and self-reported measures. Arthritis Rheum 2005;53:5–11. 10.1002/art.20910 [DOI] [PubMed] [Google Scholar]
- 4.Pilgaard T, Hagelund L, Stallknecht SE, et al. Severity of fatigue in people with rheumatoid arthritis, Psoriatic arthritis and Spondyloarthritis - results of a cross-sectional study. PLoS One 2019;14. 10.1371/journal.pone.0218831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ReumaNederland . Route23. 2022. Available: https://reumanederland.nl/route23/ [Accessed 5 Feb 2023].
- 6.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 7.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for Scoping reviews (PRISMA-SCR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 8.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile App for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for systematic reviews of interventions. In:. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019. 10.1002/9781119536604 [DOI] [Google Scholar]
- 10.World Health Organization . International classification of functioning, disability, and health (ICF). 2017. Available: https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health [Accessed 1 Nov 2021].
- 11.Stebbings S, Treharne GJ. Fatigue in rheumatic disease: an overview. International Journal of Clinical Rheumatology 2010;5:487–502. 10.2217/ijr.10.30 [DOI] [Google Scholar]
- 12.Marrelli K, Cheng AJ, Brophy JD, et al. Perceived versus performance fatigability in patients with rheumatoid arthritis. Front Physiol 2018;9:1395. 10.3389/fphys.2018.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaime-Lara RB, Koons BC, Matura LA, et al. A qualitative Metasynthesis of the experience of fatigue across five chronic conditions. J Pain Symptom Manage 2020;59:1320–43. 10.1016/j.jpainsymman.2019.12.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent A, Benzo RP, Whipple MO, et al. Beyond pain in Fibromyalgia: insights into the symptom of fatigue. Arthritis Res Ther 2013;15:221. 10.1186/ar4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elera-Fitzcarrald C, Rocha J, Burgos PI, et al. Measures of fatigue in patients with rheumatic diseases: A critical review. Arthritis Care Res (Hoboken) 2020;72 Suppl 10(Suppl 10):369–409. 10.1002/acr.24246 [DOI] [PubMed] [Google Scholar]
- 16.Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in Ankylosing Spondylitis: the development of the bath Ankylosing Spondylitis functional index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- 17.Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing Psoriatic arthritis: elaboration and preliminary validation of the Psoriatic arthritis impact of disease (Psaid) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 2014;73:1012–9. 10.1136/annrheumdis-2014-205207 [DOI] [PubMed] [Google Scholar]
- 18.Bennett RM, Friend R, Jones KD, et al. The revised Fibromyalgia impact questionnaire (FIQR): validation and Psychometric properties. Arthritis Res Ther 2009;11:R120. 10.1186/ar2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gossec L, Paternotte S, Aanerud GJ, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis 2011;70:935–42. 10.1136/ard.2010.142901 [DOI] [PubMed] [Google Scholar]
- 20.Regnaux J-P, Davergne T, Palazzo C, et al. Exercise programmes for Ankylosing Spondylitis. Cochrane Database Syst Rev 2019;10. 10.1002/14651858.CD011321.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almeida C, Choy EHS, Hewlett S, et al. Non-pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev 2013;8. 10.1002/14651858.CD008322.pub2 [DOI] [PubMed] [Google Scholar]
- 22.Almeida C, Choy EHS, Hewlett S, et al. Biologic interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev 2016. 10.1002/14651858.CD008334.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauffier K, Salliot C, Berenbaum F, et al. Effect of Biotherapies on fatigue in rheumatoid arthritis: a systematic review of the literature and meta-analysis. Rheumatology (Oxford) 2012;51:60–8. 10.1093/rheumatology/ker162 [DOI] [PubMed] [Google Scholar]
- 24.Townes SV, Furst DE, Thenkondar A. The impact of Tocilizumab on physical function and quality of life in patients with rheumatoid arthritis: a systematic literature review and interpretation. Open Access Rheumatol 2012;4:87–92. 10.2147/OARRR.S14563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atzeni F, Nucera V, Masala IF, et al. Il-6 involvement in pain, fatigue and mood disorders in rheumatoid arthritis and the effects of Il-6 inhibitor Sarilumab. Pharmacol Res 2019;149. 10.1016/j.phrs.2019.104402 [DOI] [PubMed] [Google Scholar]
- 26.Choy EH. Effect of Biologics and targeted synthetic disease-modifying anti-rheumatic drugs on fatigue in rheumatoid arthritis. Rheumatology (Oxford) 2019;58(Suppl 5):v51–5. 10.1093/rheumatology/kez389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleischmann R, Stern R, Iqbal I. Anakinra: an inhibitor of IL-1 for the treatment of rheumatoid arthritis. Expert Opin Biol Ther 2004;4:1333–44. 10.1517/14712598.4.8.1333 [DOI] [PubMed] [Google Scholar]
- 28.Pope JE. Management of fatigue in rheumatoid arthritis. RMD Open 2020;6. 10.1136/rmdopen-2019-001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzcharles M-A, Baerwald C, Ablin J, et al. Efficacy, tolerability and safety of Cannabinoids in chronic pain associated with rheumatic diseases (Fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): A systematic review of randomized controlled trials. Schmerz 2016;30:47–61. 10.1007/s00482-015-0084-3 [DOI] [PubMed] [Google Scholar]
- 30.Paik J, Deeks ED. Tofacitinib: A review in Psoriatic arthritis. Drugs 2019;79:655–63. 10.1007/s40265-019-01091-3 [DOI] [PubMed] [Google Scholar]
- 31.Keating GM. Apremilast: a review in psoriasis and Psoriatic arthritis. Drugs 2017;77:459–72. 10.1007/s40265-017-0709-1 [DOI] [PubMed] [Google Scholar]
- 32.Missaoui B, Revel M. Fatigue in Ankylosing Spondylitis. Ann Readapt Med Phys 2006;49:305–8. 10.1016/j.annrmp.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 33.Welsch P, Bernardy K, Derry S, et al. Mirtazapine for Fibromyalgia in adults. Cochrane Database Syst Rev 2018;8. 10.1002/14651858.CD012708.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welsch P, Üçeyler N, Klose P, et al. Serotonin and noradrenaline reuptake inhibitors (Snris) for Fibromyalgia. Cochrane Database Syst Rev 2018;2. 10.1002/14651858.CD010292.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walitt B, Urrútia G, Nishishinya MB, et al. Selective serotonin reuptake inhibitors for Fibromyalgia syndrome. Cochrane Database Syst Rev 2015. 10.1002/14651858.CD011735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Häuser W, Bernardy K, Uçeyler N, et al. Treatment of Fibromyalgia syndrome with antidepressants: a meta-analysis. JAMA 2009;301:198–209. 10.1001/jama.2008.944 [DOI] [PubMed] [Google Scholar]
- 37.Thorpe J, Shum B, Moore RA, et al. Combination Pharmacotherapy for the treatment of Fibromyalgia in adults. Cochrane Database Syst Rev 2018;2018. 10.1002/14651858.CD010585.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walitt B, Klose P, Üçeyler N, et al. Antipsychotics for Fibromyalgia in adults. Cochrane Database Syst Rev 2016;2016. 10.1002/14651858.CD011804.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehrabani S, Askari G, Miraghajani M, et al. Effect of coenzyme Q10 supplementation on fatigue: a systematic review of Interventional studies. Complement Ther Med 2019;43:181–7. 10.1016/j.ctim.2019.01.022 [DOI] [PubMed] [Google Scholar]
- 40.Gerardi MC, Batticciotto A, Talotta R, et al. Novel pharmaceutical options for treating Fibromyalgia. Expert Rev Clin Pharmacol 2016;9:559–65. 10.1586/17512433.2016.1145052 [DOI] [PubMed] [Google Scholar]
- 41.Guymer EK, Clauw DJ. Treatment of fatigue in Fibromyalgia. Rheum Dis Clin North Am 2002;28:367–78. 10.1016/s0889-857x(01)00007-2 [DOI] [PubMed] [Google Scholar]
- 42.Staud R. Pharmacological treatment of Fibromyalgia syndrome: new developments. Drugs 2010;70:1–14. 10.2165/11530950-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 43.Staud R. Sodium Oxybate for the treatment of Fibromyalgia. Expert Opin Pharmacother 2011;12:1789–98. 10.1517/14656566.2011.589836 [DOI] [PubMed] [Google Scholar]
- 44.Walrabenstein W, Wagenaar CA, van der Leeden M, et al. A Multidisciplinary lifestyle program for rheumatoid arthritis: the "plants for joints" Rheumatology (Oxford) 2023. 10.1093/rheumatology/keac693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edelaar L, Nikiphorou E, Fragoulis GE, et al. EULAR recommendations for the generic core Competences of health professionals in rheumatology. Ann Rheum Dis 2020;79:53–60. 10.1136/annrheumdis-2019-215803 [DOI] [PubMed] [Google Scholar]
- 46.Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis 2008;67:1360–4. 10.1136/ard.2008.091454 [DOI] [PubMed] [Google Scholar]
- 47.Davies K, Dures E, Ng W-F. Fatigue in inflammatory rheumatic diseases: Current knowledge and areas for future research. Nat Rev Rheumatol 2021;17:651–64. 10.1038/s41584-021-00692-1 [DOI] [PubMed] [Google Scholar]
- 48.Hewlett S, Chalder T, Choy E, et al. Fatigue in rheumatoid arthritis: time for a conceptual model. Rheumatology (Oxford) 2011;50:1004–6. 10.1093/rheumatology/keq282 [DOI] [PubMed] [Google Scholar]
- 49.Tofferi JK, Jackson JL, O’Malley PG. Treatment of Fibromyalgia with Cyclobenzaprine: A meta-analysis. Arthritis Rheum 2004;51:9–13. 10.1002/art.20076 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003056supp001.pdf (996.3KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Data are available on reasonable request to the corresponding author.