Abstract
Background
Declines in the prevalence of cigarette smoking, advances in targeted therapies, and implementation of lung cancer screening have changed the clinical landscape for lung cancer. The proportion of lung cancer deaths is increasing in those who have never smoked cigarettes. To better understand contemporary patterns in survival among patients with lung cancer, a comprehensive evaluation of factors associated with survival, including differential associations by smoking status, is needed.
Methods
Patients diagnosed with lung cancer between January 1, 2010, and September 30, 2019, were identified. We estimated all-cause and lung cancer-specific median, 5-year, and multivariable restricted mean survival time (RMST) to identify demographic, socioeconomic, and clinical factors associated with survival, overall and stratified by smoking status (never, former, and current).
Results
Analyses included 6813 patients with lung cancer: 13.9% never smoked, 54.2% formerly smoked, and 31.9% currently smoked. All-cause RMST through 5 years for those who never, formerly, and currently smoked was 32.1, 25.9, and 23.3 months, respectively. Lung cancer–specific RMST was 36.3 months, 30.3 months, and 26.0 months, respectively. Across most models, female sex, younger age, higher socioeconomic measures, first-course surgery, histology, and body mass index were positively associated, and higher stage was inversely associated with survival. Relative to White patients, Black patients had increased survival among those who formerly smoked.
Conclusions
We identify actionable factors associated with survival between those who never, formerly, and currently smoked cigarettes. These findings illuminate opportunities to address underlying mechanisms driving lung cancer progression, including use of first-course treatment, and enhanced implementation of tailored smoking cessation interventions for individuals diagnosed with cancer.
Lung cancer is the leading cause of cancer-related deaths in the United States, with an estimated 131 811 deaths expected in 2021 (1). Cigarette smoking is the primary cause of lung cancer in the United States (1-3). As cigarette smoking prevalence declines in the United States, the proportion of lung cancer patients who have never smoked cigarettes has been increasing (1,4-8). Approximately 15% of all lung cancer deaths occur in people who have never smoked. Thus, the burden of nonsmoking-related lung cancer is an important health issue (1).
Several studies describe clinical and demographic characteristics associated with lung cancers diagnosed in patients who never smoked (4-24). Compared with adults with a history of smoking, patients who never smoked and develop lung cancer are more likely to be female, identify as Asian, have a lower comorbidity burden, and have lung cancers with adenocarcinoma histology (5-7,9,11,13,15,24-26). Most of these studies did not describe differences in socioeconomic measures (SES), such as rurality, poverty levels, or education, between lung cancer patients who never smoked and those who ever smoked (4-6,9,11-13,16-22,24,25). Some studies that did report SES did not report survival (19), and other studies did not include tumor specific factors (5,11,16,21). All studies that did report SES and survival are from data collection ending prior to 2017 (9,10,12,16-18,20-22).
Given the high burden of lung cancer on the United States and that the lung cancer landscape is rapidly changing (27-30), it is important to describe contemporary, comprehensive characteristics and patterns among lung cancer patients by smoking status who receive health-care services in diverse community settings. Our objective was to fill this gap by describing and quantifying survival differences between lung cancer patients by smoking status in a contemporary sample.
Methods
Study setting and data sources
This retrospective analysis was completed within the Population-based Research to Optimize the Screening Process (PROSPR) Lung consortium (31). As described in detail elsewhere, the PROSPR-Lung Common Data Model is a standardized resource containing data on individuals from 5 diverse health-care systems: Henry Ford Health, Kaiser Permanente Colorado, Kaiser Permanente Hawaii, Marshfield Clinic Health System, and the University of Pennsylvania Health System (31,32). The PROSPR-Lung Common Data Model includes data on patient demographics, census-based measures of SES, procedures, diagnoses, vital status, and tumor data (collected from each site’s tumor registry).
Study population
The study population included patients aged 35-89 years diagnosed with lung cancer between January 1, 2010, and September 30, 2019. We calculated the Yost Index, an area-level composite measure of SES, and rural-urban commuting area (RUCA) codes, a census-based characterization of rural and urban and journey-to-work commuting status, for the census tract of residence for each individual (33-35).The Yost Index was chosen to characterize overall SES because it is a robustly validated metric to measure SES that is comprised of household income, poverty, rent, home value, employment, education, and working class, and RUCA was added to control for variation in rurality (36,37). We collected information on sex, self-reported race and ethnicity, tumor characteristics, and first-course treatment. Those identifying in a racial or ethnic group that comprised less than 1% of the study population and those with unknown race were categorized as “Unknown/another race.” A modified Charlson Comorbidity Index was calculated for each person in the year prior to lung cancer diagnosis (38,39). We identified the closest recorded smoking status and body mass index (BMI) (kg/m2) before lung cancer diagnosis. Smoking status was categorized as never, current, and former.
Statistical analysis
Differences in patient characteristics stratified by smoking status were evaluated using χ2 tests. Overall survival was assessed by restricted mean survival time (RMST) through 5 years (40-44). Overall survival was defined as the time from lung cancer diagnosis to death from any cause. Patients who were alive on the date of their last documented encounter within the health system were censored on that date (45). A multivariable adjusted model was estimated to quantify the effect of the following covariates on survival: Yost Index, RUCA, smoking status, sex, race and ethnicity, age, American Joint Committee on Cancer (AJCC) stage, histology, previously diagnosed nonlung cancer, Charlson Comorbidity Index, BMI, first-course radiation, surgery, and systemic therapy indicators from each site’s tumor registry, year of diagnosis, and health system. All variables were selected a priori based on clinician confirmation of variables that have shown an association in the prior literature (4,7,9,10,12,23,25) and were included regardless of statistical significance. We also estimated models stratified by smoking status and calculated interaction P values to determine if the effect of each of these covariates varied by smoking status. Median survival and 5-year survival probabilities were estimated to facilitate comparability with other published literature. Kaplan-Meier curves were created to graphically depict unadjusted survival and compared with a log-rank test. Additional multivariable RMST models were estimated: 1) a model excluding stage to address the prognostic value of histology alone (46); and 2) a model based on the subset of patients with adenocarcinoma histology to address variation by smoking status.
Lung cancer–specific RMST was estimated on patients from 4 of the 5 health systems where the ascertainment of cause of death was available through linkages to state death registries. Lung cancer–specific survival was defined as the time from lung cancer diagnosis to death from lung cancer (International Classification of Diseases, Tenth Revision [ICD-10] code: C34*). Patients who were alive on the date of their last documented encounter within the health system were censored on that date. The competing event of death from nonlung cancer causes was treated as a censored observation at the date of death (47). All covariates used in the all-cause multivariable–adjusted model were also used in this model.
Analyses were performed using SAS Software version 9.4M6 (SAS Institute Inc, Cary, NC, USA). Strengthening the Reporting of Observational Studies in Epidemilogy (STROBE) criteria were followed (48). P values were 2-sided, and P less than .05 was considered statistically significant. Study protocols and human participant protection considerations were reviewed and approved by the institutional review board at Kaiser Permanente Colorado (KPCO), which is the institutional review board of record for PROSPR-Lung.
Results
Patient characteristics
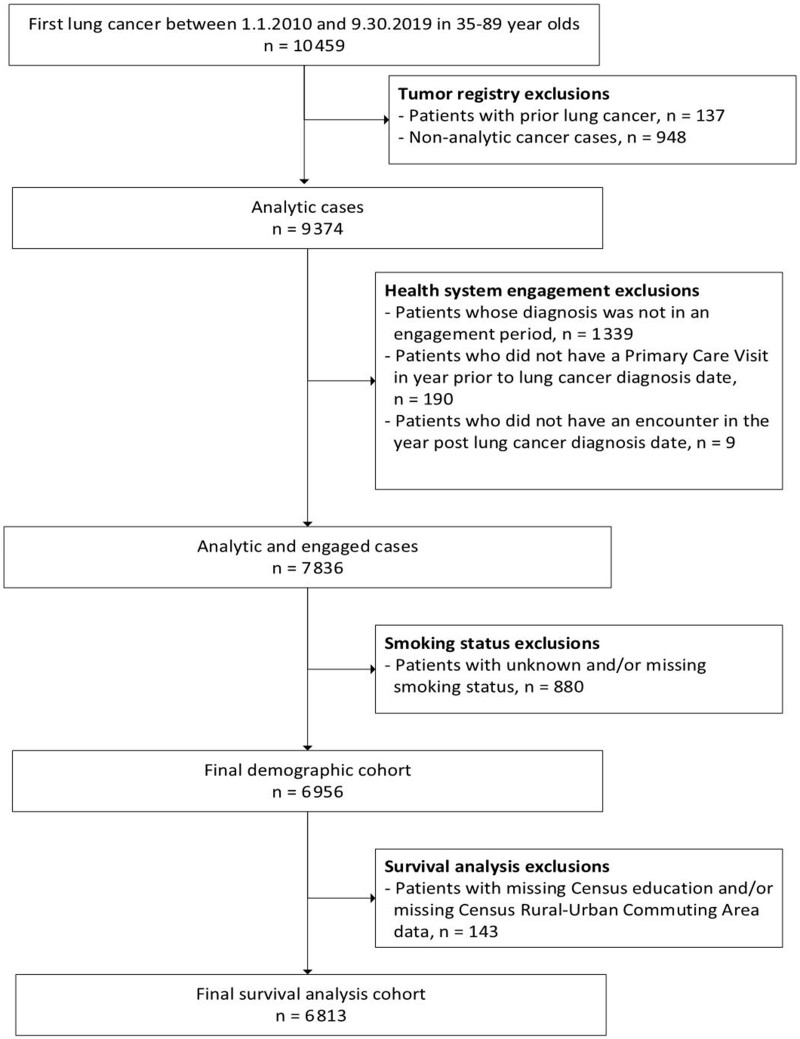
A total of 6813 patients with lung cancer were identified: 946 (13.9%) patients who never smoked, 3695 formerly smoked (54.2%), and 2172 (31.9%) currently smoked (Table 1; Figure 1). Compared with patients who formerly or currently smoked, patients who never smoked were more likely to be female than male (68.1% vs 51.0% vs 52.9%) and more likely to identify as Asian than White (14.9% vs 6.3% vs 3.5%), have adenocarcinomas than other histologies (64.0% vs 47.5% vs 40.3%), and have the highest Yost index (ie, most affluent; 27.1% vs 20.9% vs 14.1%). The largest proportion of those who never and formerly smoked was 75-89 years (39.0% and 43.4%, respectively), whereas the largest proportion of those who currently smoked was 65-74 years (37.2%). Compared with patients who never smoked, those who formerly or currently smoked were more likely to have 2 or more comorbid conditions (63.7% vs 76.6% vs 69.8%). Patients who currently smoked were more likely to have a BMI less than 25 kg/m2 compared with patients who never and formerly smoked (51.7% vs 37.9% vs 36.8%, respectively).
Table 1.
Demographic and clinical characteristics of patients diagnosed with lung cancer
| Characteristic | Never | Former | Current | Total | P a |
|---|---|---|---|---|---|
| Total, no. (%) | 946 (13.9) | 3695 (54.2) | 2172 (31.9) | 6813 | |
| Sex, no. (%) | <.001 | ||||
| Female | 644 (68.1) | 1883 (51.0) | 1148 (52.9) | 3675 (53.9) | |
| Male | 302 (31.9) | 1812 (49.0) | 1024 (47.1) | 3138 (46.1) | |
| Race and ethnicity, no. (%) | <.001 | ||||
| Asian | 141 (14.9) | 231 (6.3) | 75 (3.5) | 447 (6.6) | |
| Black | 90 (9.5) | 485 (13.1) | 386 (17.8) | 961 (14.1) | |
| Hispanic | 40 (4.2) | 122 (3.3) | 89 (4.1) | 251 (3.7) | |
| Native Hawaiian/Pacific Islander | 31 (3.3) | 129 (3.5) | 85 (3.9) | 245 (3.6) | |
| White | 604 (63.8) | 2578 (69.8) | 1443 (66.4) | 4625 (67.9) | |
| Another race/unknown race | 40 (4.2) | 150 (4.1) | 94 (4.3) | 284 (4.2) | |
| Age at diagnosis, no. (%), y | <.001 | ||||
| 35-54 | 107 (11.3) | 124 (3.4) | 214 (9.9) | 445 (6.5) | |
| 55-64 | 186 (19.7) | 611 (16.5) | 695 (32.0) | 1492 (21.9) | |
| 65-74 | 284 (30.0) | 1358 (36.8) | 807 (37.2) | 2449 (35.9) | |
| 75-89 | 369 (39.0) | 1602 (43.4) | 456 (21.0) | 2427 (35.6) | |
| Yost State quintile (census based), no. (%) | <.001 | ||||
| Quintile 1 (lowest) | 166 (17.5) | 808 (21.9) | 640 (29.5) | 1614 (23.7) | |
| Quintile 2 | 177 (18.7) | 737 (19.9) | 445 (20.5) | 1359 (19.9) | |
| Quintile 3 | 164 (17.3) | 733 (19.8) | 454 (20.9) | 1351 (19.8) | |
| Quintile 4 | 183 (19.3) | 643 (17.4) | 326 (15.0) | 1152 (16.9) | |
| Quintile 5 (highest) | 256 (27.1) | 774 (20.9) | 307 (14.1) | 1337 (19.6) | |
| RUCA (census based), no. (%) | <.001 | ||||
| Urban focused | 798 (84.4) | 3244 (87.8) | 1836 (84.5) | 5878 (86.3) | |
| Large rural city/town | 77 (8.1) | 226 (6.1) | 167 (7.7) | 470 (6.9) | |
| Small rural town/isolated small rural town | 71 (7.5) | 225 (6.1) | 169 (7.8) | 465 (6.8) | |
| AJCC stage, no. (%) | <.001 | ||||
| I | 294 (31.1) | 1121 (30.3) | 442 (20.3) | 1857 (27.3) | |
| II | 72 (7.6) | 290 (7.8) | 179 (8.2) | 541 (7.9) | |
| III | 107 (11.3) | 633 (17.1) | 433 (19.9) | 1173 (17.2) | |
| IV | 426 (45.0) | 150 (4.1) | 999 (46.0) | 1575 (23.1) | |
| Other/unknown | 47 (5.0) | 151 (4.1) | 119 (5.5) | 317 (4.7) | |
| Histology, no. (%) | <.001 | ||||
| Adenocarcinoma | 605 (64.0) | 1756 (47.5) | 875 (40.3) | 3236 (47.5) | |
| Large cell | 11 (1.2) | 27 (0.7) | 28 (1.3) | 66 (1.0) | |
| Non-small cell/other | 130 (13.7) | 640 (17.3) | 337 (15.5) | 1107 (16.2) | |
| Squamous | 74 (7.8) | 775 (21.0) | 487 (22.4) | 1336 (19.6) | |
| Small cell | 35 (3.7) | 412 (11.2) | 419 (19.3) | 866 (12.7) | |
| Carcinoids | 91 (9.6) | 85 (2.3) | 26 (1.2) | 202 (3.0) | |
| Year of diagnosis, no. (%) | .10 | ||||
| 2010-2012 | 169 (17.9) | 662 (17.9) | 440 (20.3) | 1271 (18.7) | |
| 2013-2015 | 329 (34.8) | 1256 (34.0) | 758 (34.9) | 2343 (34.4) | |
| 2016-2019 | 448 (47.4) | 1777 (48.1) | 974 (44.8) | 3199 (47.0) | |
| Previously diagnosed non-lung cancer, no. (%) | 118 (12.5) | 510 (13.8) | 238 (11.0) | 866 (12.7) | .007 |
| Modified Charlson Co-morbidity Indexb, no. (%) | <.0001 | ||||
| 0-1 condition | 343 (36.3) | 866 (23.4) | 657 (30.2) | 1866 (27.4) | |
| 2 or more conditions | 603 (63.7) | 2829 (76.6) | 1515 (69.8) | 4947 (72.6) | |
| First course therapy (tumor registry based), no. (%) | |||||
| Radiation | 255 (27.0) | 1356 (36.7) | 893 (41.1) | 2504 (36.8) | <.001 |
| Surgery | 331 (35.0) | 928 (25.1) | 446 (20.5) | 1705 (25.0) | <.001 |
| Systemic therapy | 413 (43.7) | 1638 (44.3) | 1148 (52.9) | 3199 (47.0) | <.001 |
| BMI status at time of diagnosis, no. (%) | <.001 | ||||
| <25 (underweight/normal) | 359 (37.9) | 1359 (36.8) | 1122 (51.7) | 2840 (41.7) | |
| 25-29 (overweight) | 289 (30.5) | 1274 (34.5) | 612 (28.2) | 2175 (31.9) | |
| 30+ (obese) | 290 (30.7) | 1045 (28.3) | 411 (18.9) | 1746 (25.6) | |
| Unknown | 8 (0.8) | 17 (0.5) | 27 (1.2) | 52 (0.8) |
Differences in patient characteristics between smoking status categories were evaluated using χ2 tests. AJCC = American Joint Committee on Cancer; BMI = body mass index; NSCLC = non–small-cell lung cancer; RUCA = rural-urban commuting area.
Modified Charlson Comorbidity Index excludes HIV/AIDS diagnoses.
Figure 1.
Flow diagram of lung cancer patients included in these analyses.
All cause survival
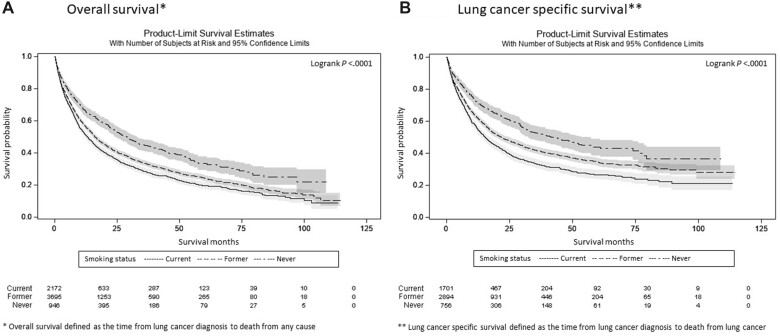
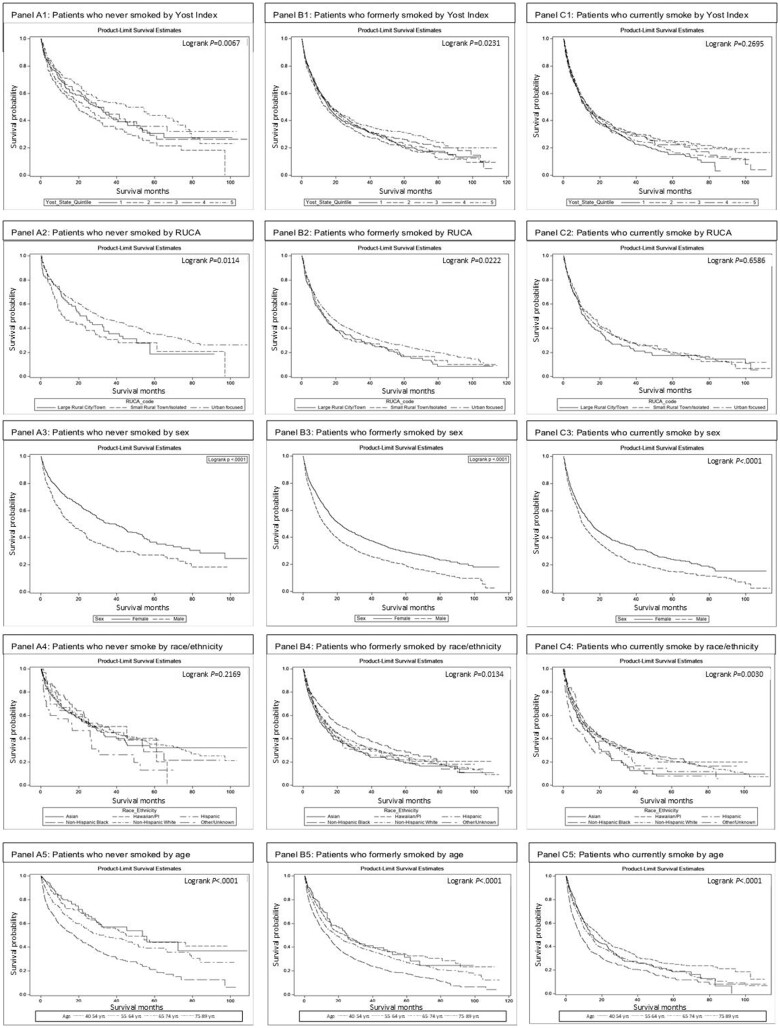
Median follow-up time was 36.2 months (95% confidence interval [CI] = 34.2 to 40.0 months), 40.2 months (95% CI = 38.4 to 42.3 months), and 39.7 months (95% CI = 36.6 to 43.6 months) for patients who never, formerly, and currently smoked, respectively. Patients who never smoked had the longest median time from diagnosis to death (29.0 months, 95% CI = 24.2 to 33.2 months), followed by those who formerly smoked (16.1 months, 95% CI = 15.3 to 17.4 months) and those who currently smoked (13.0 months, 95% CI = 11.9 to 14.6 months) (Figure 2, A; Supplementary Table 1, available online). Unadjusted RMST was 32.1, 25.9, and 23.3 months for patients who never, formerly, and currently smoked, respectively. Kaplan-Meier plots depict unadjusted survival for each smoking status stratified by Yost Index, RUCA, sex, race and ethnicity, and age (Figure 3).
Figures 2.
A) and B) Kaplan-Meier plots and 95% confidence intervals of survival following lung cancer diagnosis through 5 years of follow-up stratified by smoking status.
Figure 3.
Kaplan-Meier plots of overall survival from diagnosis through 5 years of follow-up for patients diagnosed with lung cancer stratified by patients who never smoked (A) to patients who formerly smoked (B) to patients who currently smoked (C).
After adjusting for all factors, patients who formerly and currently smoked had decreased survival compared with patients who never smoked (−3.3 and −2.6 months, respectively; both P < .001). Multivariable analysis identified 10 factors associated with RMST (Table 2): higher stage, male sex, small cell histology, and a higher comorbid burden were statistically significantly associated with decreased survival, whereas highest quintile Yost Index, identifying as Black, younger age, adenocarcinoma, carcinoid histologies, receipt of first-course systemic therapy, radiation and/or surgery, and higher BMI were associated with increased survival.
Table 2.
Multivariable model of restricted mean survival time (RMST) through 5 years from time of diagnosis for all patients with lung cancera
| Characteristic | Coefficient (95% CI)b | P |
|---|---|---|
| Intercept | 35.7 (32.4 to 38.9) | <.001 |
| Smoking status | ||
| Patients who never smoked | Ref | |
| Patients who currently smoked | −3.3 (−4.9 to −1.7) | <.001 |
| Patients who formerly smoked | −2.6 (−4.1 to −1.2) | <.001 |
| Sex | ||
| Female | Ref | |
| Male | −3.4 (−4.4 to −2.4) | <.001 |
| Race and ethnicity | ||
| Non-Hispanic White | Ref | |
| Asian | −1.3 (−3.7 to 1.1) | .28 |
| Black | 1.7 (0.01 to 3.3) | .048 |
| Hispanic | −0.1 (−2.7 to 2.4) | .91 |
| Native Hawaiian/Pacific Islander | 1.3 (−3.7 to 1.1) | .28 |
| Another race/unknown race | −2.1 (−4.2 to 0.1) | .06 |
| Age at lung cancer diagnosis (years) | ||
| 75-89 | Ref | |
| 40-54 | 5.1 (2.9 to 7.2) | <.001 |
| 55-64 | 4.6 (3.2 to 5.9) | <.001 |
| 65-74 | 2.1 (1.0 to 3.2) | <.001 |
| Yost State Quintile (census based) | ||
| Yost 1 (lowest) | Ref | |
| Yost 2 | 0.3 (−1.2 to 1.7) | .72 |
| Yost 3 | 0.4 (−1.1 to 1.8) | .61 |
| Yost 4 | 1.4 (−0.1 to 2.9) | .08 |
| Yost 5 (highest) | 2.3 (0.7 to 3.8) | .004 |
| RUCA (census based) | ||
| Urban focused | Ref | |
| Large rural city/town | −0.6 (−3.1 to 1.9) | .63 |
| Small rural town focused/isolated small rural | 1.2 (−1.7 to 4.0) | .42 |
| AJCC stage at diagnosis | ||
| I | Ref | |
| II | −11.4 (−13.6 to −9.2) | <.0001 |
| III | −18.4 (−20.3 to −16.5) | <.0001 |
| IV | −28.9 (−30.5 to −27.3) | <.0001 |
| Other/unknown | −15.7 (−18.4 to −13.0) | <.0001 |
| Histology | ||
| NSCLC/other | Ref | |
| Adenocarcinoma | 3.2 (1.8 to 4.5) | <.001 |
| Carcinoids | 4.2 (1.4 to 7.1) | .004 |
| Large cell | −3.0 (−7.3 to 1.3) | .17 |
| Small cell | −2.7 (−4.4 to −1.1) | .001 |
| Squamous | −0.7 (−2.3 to 0.8) | .36 |
| Clinical conditions | ||
| Previous non-lung cancer | −1.4 (−2.8 to 0.05) | .06 |
| Modified Charlson Comorbidity Indexc | ||
| 0-1 condition | Ref | |
| 2 or more conditions | −2.0 (−3.1 to −0.8) | <.001 |
| Receipt of other first-course therapyd | ||
| No radiation | Ref | |
| Radiation | 2.1 (0.9 to 3.2) | <.001 |
| No surgery | Ref | |
| Surgery | 15.1 (13.5 to 16.8) | <.001 |
| No systemic therapy | Ref | |
| Systemic therapy | 8.5 (7.4 to 9.6) | <.001 |
| BMI status at diagnosis | ||
| <25 (underweight/normal) | Ref | |
| 25-29 (overweight) | 1.3 (0.2 to 2.4) | .03 |
| 30 + (obese) | 2.1 (0.9 to 3.4) | <.001 |
| Unknown | −5.2 (−9.4 to −0.9) | .02 |
RUCA = Rural-Urban Commuting Area; AJCC = American Joint Committee on Cancer; BMI = body mass index; CI = confidence interval; NSCLC = non–small-cell lung cancer; Ref = reference group. Results mutually adjusted for year of diagnosis and health-care system in addition to all variables listed in table.
The coefficient value represents the number of additional months of survival gained or lost if the corresponding characteristics were present relative to the reference group for that covariate.
Modified Charlson Comorbidity Index excludes HIV/AIDS diagnoses.
First-course therapy as documented in tumor registry data.
Interaction analyses revealed the effect of histology and surgery as part of first-course therapy on survival varied by smoking status (Table 3). Among patients who never smoked, those with squamous cell histology had −7.2 (95% CI = −12.9 to −1.5) months decreased survival relative to those with non–small-cell lung cancer or other, whereas this estimate was −0.9 (95% CI = −1.2 to 3.0) and −2.8 (95% CI = −5.5 to −0.2) months among those who formerly and currently smoked, respectively (Pinteraction = .007). Among patients who formerly and currently smoked, those who received surgery had 16.8 (95% CI = 14.6 to 19.0) and 15.0 (95% CI = 12.0 to 18.0) months increased survival, whereas those who never smoked had 8.1 (95% CI = 3.3 to 12.8) months (Pinteraction = .01). Although not statistically significant, we observed relatively large differences in the effect of age by smoking status. Relative to the oldest age, patients who never and formerly smoked had similar survival that was significant (8.7 [95% CI = 3.7 to 13.7] and 7.0 [95% CI = 3.2 to 10.8] months, respectively), whereas survival among those who currently smoked was null (2.3 [95% CI = −0.9 to 5.5] months).
Table 3.
Multivariable models of restricted mean all-cause survival time through 5 years from time of diagnosis stratified by smoking statusa
| Characteristic | Patients who never smoked | Patients who formerly smoked | Patients who currently smoked | Smoking status comparisons |
|---|---|---|---|---|
| Coefficient (95% CI)b | Coefficient (95% CI)b | Coefficient (95% CI)b | P interaction | |
| Intercept | 37.0 (28.0 to 45.9) | 33.8 (29.5 to 38.0) | 32.0 (27.3 to 36.7) | |
| Sex | .24 | |||
| Female | Ref | Ref | Ref | |
| Male | −4.6 (−7.4 to −1.7) | −2.9 (−4.2 to −1.6) | −3.8 (−5.4 to −2.2) | |
| Race and ethnicity | .79 | |||
| White | Ref | Ref | Ref | |
| Asian | −6.0 (−11.0 to −1.1) | −0.9 (−4.3 to 2.4) | −1.1 (−5.9 to 3.7) | |
| Black | 2.3 (−2.5 to 7.0) | 2.5 (−2.2 to 6.5) | −0.1 (−2.7 to 2.4) | |
| Hispanic | −3.3 (−10.7 to 4.0) | 0.7 (−2.7 to 4.2) | −0.6 (−4.4 to 3.2) | |
| Native Hawaiian/Pacific Islander | −6.2 (−14.6 to 2.3) | 2.2 (−2.2 to 6.5) | 3.5 (−1.5 to 8.5) | |
| Another race/Unknown race | −0.5 (−6.4 to 5.5) | −0.7 (−3.8 to 2.4) | −4.6 (−7.9 to −1.3) | |
| Age at lung cancer diagnosis (years) | .05 | |||
| 75-89 | Ref | Ref | Ref | |
| 40-54 | 8.7 (3.7 to 13.7) | 7.0 (3.2 to 10.8) | 2.3 (−0.9 to 5.5) | |
| 55-64 | 7.7 (4.0 to 11.4) | 3.1 (1.1 to 5.0) | 4.3 (2.0 to 6.7) | |
| 65-74 | 2.0 (−1.2 to 5.2) | 2.4 (1.0 to 3.9) | 0.8 (−1.3 to 3.0) | |
| Yost State Quintile (census based) | .10 | |||
| Yost 1 (lowest) | Ref | Ref | Ref | |
| Yost 2 | −1.6 (−5.6 to 2.5) | −0.9 (−2.8 to 1.0) | 2.7 (0.2 to 5.1) | |
| Yost 3 | 1.0 (−3.4 to 5.5) | 0.1 (−1.9 to 2.0) | 0.7 (−1.7 to 3.0) | |
| Yost 4 | 1.0 (−3.1 to 5.0) | 1.1 (−0.9 to 3.2) | 2.1 (−0.5 to 4.7) | |
| Yost 5 (highest) | 3.7 (−0.4 to 7.7) | 2.5 (0.4 to 4.7) | 0.3 (−2.4 to 3.0) | |
| RUCA (census based) | ||||
| Urban focused | Ref | Ref | Ref | .89 |
| Large rural city/town | −3.4 (−9.8 to 3.0) | −1.0 (−4.5 to 2.6) | 1.4 (−2.8 to 5.7) | |
| Small rural town focused/isolated small rural | −0.9 (−7.9 to 6.0) | 0.6 (−3.4 to 4.6) | 3.2 (−1.6 to 8.1) | |
| AJCC stage at diagnosis | .72 | |||
| I | Ref | Ref | Ref | |
| II | −8.0 (−13.2 to −2.8) | −12.1 (−15.0 to −9.2) | −11.5 (−15.6 to −7.3) | |
| III | −19.6 (−25.1 to −14.1) | −17.2 (−19.7 to −14.7) | −19.8 (−23.1 to −16.5) | |
| IV | −32.6 (−37.7 to −27.5) | −27.1 (−29.2 to −25.0) | −31.0 (−33.9 to −28.1) | |
| Other/unknown | −13.7 (−21.1 to −6.3) | −14.8 (−18.4 to −11.1) | −18.7 (−23.4 to −14.0) | |
| Histology | .008 | |||
| NSCLC/other | Ref | Ref | Ref | |
| Adenocarcinoma | 3.6 (−0.1 to 7.3) | 3.3 (1.4 to 5.1) | 2.5 (0.1 to 4.8) | |
| Large cell | −7.3 (−17.9 to 3.4) | −5.1 (−11.8 to 1.6) | 0.8 (−5.6 to 7.3) | |
| Squamous | −7.2 (−12.9 to −1.5) | 0.9 (−1.2 to 3.0) | −2.8 (−5.5 to −0.2) | |
| Small cell | −6.7 (−12.7 to −0.7) | −3.6 (−5.8 to −1.4) | −1.8 (−4.5 to 0.9) | |
| Carcinoids | 6.1 (1.02 to 11.2) | 3.5 (−0.6 to 7.7) | 3.9 (−3.9 to 11.6) | |
| Clinical conditions | .34 | |||
| No previous non-lung cancer | Ref | Ref | Ref | |
| Previous non-lung cancer | −3.4 (−7.1 to 0.4) | −0.3 (−2.2 to 1.6) | −2.6 (−5.1 to −0.1) | |
| Modified Charlson Comorbidity Indexc | .15 | |||
| 0-1 condition | Ref | Ref | Ref | |
| 2 or more conditions | −1.3 (−4.4 to 1.7) | −3.2 (−4.8 to −1.6) | −0.3 (−2.1 to 1.5) | |
| Receipt of other first course therapyd | ||||
| No radiation | Ref | Ref | Ref | |
| Radiation | −0.8 (−4.2 to 2.7) | 2.8 (1.3 to 4.3) | 2.5 (0.6 to 4.3) | .64 |
| No surgery | Ref | Ref | Ref | |
| Surgery | 8.1 (3.3 to 12.8) | 16.8 (14.6 to 19.0) | 15.0 (12.0 to 18.0) | .045 |
| No systemic therapy | Ref | Ref | Ref | |
| Systemic therapy | 7.8 (4.6 to 11.0) | 7.9 (6.3 to 9.4) | 9.3 (7.4 to 11.1) | .79 |
| BMI status at diagnosis | .60 | |||
| <25 (underweight/normal) | Ref | Ref | Ref | |
| 25-29 (overweight) | 0.2 (−3.0 to 3.3) | 2.2 (0.7 to 3.7) | −0.3 (−2.2 to 1.6) | |
| 30+ (obese) | 2.2 (−1.1 to 5.4) | 2.9 (1.2 to 4.6) | 0.8 (−1.5 to 3.1) | |
| Unknown | 3.2 (−11.3 to 17.7) | −6.9 (−13.9 to 0.1) | −7.0 (−12.7 to −1.2) |
RUCA = Rural-Urban Commuting Area; AJCC = American Joint Committee on Cancer; Ref = Reference group in multivariable model. Results mutually adjusted for year of diagnosis and health-care system in addition to all variables listed in the table. BMI = body mass index; CI = confidence interval; NSCLC = non–small-cell lung cancer.
The coefficient value represents the number of additional months of survival gained or lost if the corresponding characteristics were present relative to the reference group for that covariate.
Modified Charlson Comorbidity Index excludes HIV/AIDS diagnoses.
First-course therapy as documented in tumor registry data.
Multivariable models show the factor with the largest association with increased survival for both patients who formerly and currently smoked was surgery as part of first course therapy (16.8 and 15.0 months, respectively). For patients who never smoked, the factor with the largest association with increased survival was younger age (8.7 months for those aged 40-54 years). Across all patients, a diagnosis of stage IV disease had the largest association with decreased survival: −32.6, −27.1, and −31.0 months for patients who never, formerly, and currently smoked, respectively.
Lung cancer–specific survival
Similar survival trends were observed in those for whom we had cause of death data (N = 5351). Consistent with all-cause death results noted above, patients who never smoked had the longest median time from diagnosis to death from lung cancer compared with patients who formerly and currently smoked (43.3, 21.7, and 15.3 months, respectively) (Figure 2, A). In the multivariate-adjusted models (Supplementary Table 3, available online), relative to White patients, a statistically significant protective survival effect was found for Black patients who never smoked (RMST = 7.4 months, P = .01). The effect of sex varied by smoking status (Pinteraction = .047). Among those who currently smoked, males had decreased survival relative to females (−4.6 [95% CI = −6.7 to −2.5]) months, whereas the effect of sex was not statistically significant among those that never and formerly smoked: −1.3 (95% CI = −4.9 to 2.2) and −1.6 (95% CI = −3.3 to 0.1) months, respectively. The effect of histology did not vary across smoking status (Pinteraction = .15).
Additional models
The multivariable RMST model excluding stage showed an anticipated increase in the role of histology (data not shown). Specifically, survival for those with small cell histology worsened. Adjusted months lost were 10.6, 4.5, and 5.9 for patients who never, formerly, and currently smoked, respectively.
Among patients with adenocarcinoma histology, the difference in RMST persisted and increased between smoking statuses (Supplementary Table 2, available online). The effect of age on survival varied by smoking status (Pinteraction = .003). Among patients who never smoked and formerly smoked, those aged 40-54 years had 7.7 (95% CI = 1.5 to 14.0) and 8.0 (95% CI = 2.6 to 13.3) months of increased survival relative to patients 75-89 years whereas those who currently smoked had 1.6 (95% CI = −3.4 to 6.6) months of increased survival. The statistically significant interacting effect of surgery as part of first-course treatment with all histologies was abated.
Discussion
In the changing landscape of lung cancer, our study uniquely provides comprehensive data documenting the association and variation of key socioeconomic, demographic, clinical, and tumor-specific factors for patients diagnosed with lung cancer between 2010 and 2019 in 5 heterogeneous community health-care settings, overall and by smoking status. This is the first study, to our knowledge, to provide comprehensively adjusted RMST estimated differentials for patients with lung cancer who currently smoked (−3.3, P = .001) or formerly smoked (−2.6, P = .001) relative to patients who never smoked. We found that even in an insured group with relatively homogenous health-care access, smoking status still predicts survival after diagnosis. After controlling for a comprehensive set of patient-level demographic and clinical characteristics, higher SES, Black race, older age at diagnosis, adenocarcinoma and carcinoid histologies, receipt of first-course therapy, and higher BMI were associated with better survival, whereas male sex, higher stage, small cell histology, and higher comorbid burden were associated with worse survival.
In contrast to literature that has examined disparities in cancer survival by race and ethnicity (49,50), in our diverse, insured cohort of patients with lung cancer, our findings were robust to model specifications and found that relative to White patients, survival was not less favorable among Asian, Native Hawaiian or Pacific Islander, Black, or Hispanic patients. Census tract measures of SES were consistently positively associated with survival and were correlated with smoking status. We found patients who never smoked were more likely to have larger proportions of patients with higher SES. Consistent with the findings of Lofling and Clement-Duchene who found patients who never smoked more likely to live in neighborhoods with higher education levels and less poverty, we found a similar result using a composite index that encompasses 7 different census variables (9,10).
Consistent with results from previous studies, we found patients who never smoked were more likely to be female (4-6,9,10,13,24), have a higher proportion of Asian patients (5,9), and have a higher proportion of patients younger than 55 years or older than 74 years at lung cancer diagnosis (10). Most patients who never smoked in our cohort had adenocarcinoma histology (64.0%), which is consistent with what is well established in the literature, with proportions at 54%-93% (4,6,9-11,13,24). In contrast to previous studies, we found patients who never smoked had a higher proportion of early-staged lung cancers (9,10,13,24,25). This may be explained by our insured cohort that may be clinically and demographically different from those who were diagnosed in other countries (9,10,13,24,25) and/or were diagnosed in years prior to our study (9,25).
Supporting the large and robust literature on smoking as a primary mortality risk factor, we found overall survival was higher in patients who never smoked (9,10,13,14,25). However, our survival estimates were longer than those previously reported in some studies (9,10,25), with a median survival for patients who never, formerly, and currently smoked of 29.0, 16.1, and 13.0 months, respectively. Although we adjusted for receipt of systemic therapy, longer survival may be partly explained by the shift since 2015 in systemic therapy from platinum-based chemotherapy to new immunotherapy and targeted therapies that have shown improved survival in clinical trials (51). Targeted therapies have shown prolonged survival, particularly among patients who never smoked, females, and/or Asian patients with EGFR driver mutations. New immunotherapy treatments have shown improved survival among patients with smoking-related cancers (28,52).
In multivariable analyses, we found 6 factors associated with longer survival across all smoking statuses: female sex, decreased age, earlier AJCC stage, adenocarcinoma or carcinoid histology, first-course systemic therapy, and first-course surgery. This is consistent with some studies but is in contrast with others. Clement-Duchene did not observe a significant longer survival with respect to sex, age, and adenocarcinoma histology (9). We also found that BMI categories of overweight (BMI = 25-30) and obese (BMI > 30) provided a protective survival effect for patients who formerly smoked.
Our findings show that although never smoking is best, quitting smoking as early as possible is still beneficial relative to current smoking, as suggested by the reduced survival times (53). This reinforces the call for enhanced smoking cessation efforts as a key strategy for increasing survival among lung cancer patients. Our cohort consisted of insured patients with access to high-quality health-care services; therefore, our findings suggest that much of the survival deficit observed among non-White persons as shown in other studies can potentially be alleviated with access to insurance and high-quality health care. We observed a slight survival advantage among Black patients who formerly smoked relative to White patients. This finding aligns with the previous literature that found often Black patients who do smoke tend to smoke fewer cigarettes per day and start later in life (54).
A key strength of this study is the large, population-based cohort from which we obtained our data. The PROSPR-Lung cohort is racially, ethnically, and geographically diverse and speaks to the generalizability of our results. The calculation of survival in terms of a more clinically relevant measure split out by specific demographic, and clinical factors associated with survival is rarely reported.
Although we report novel survival estimates across multiple factors, it is not without limitations. We did not have access to individual-level SES data but reported Census tract–level data (55,56). However, area-level variables themselves are informative because they capture structural effects of SES inequities and are a widely used and accepted proxy (57). We did not have access to molecular marker mutation data, nor did we stratify by specific type of first-course systemic therapy (eg, cytotoxic, targeted, or immunotherapy), which could have affected our survival measures; however, we did adjust our model for stage, histology, and receipt of first-course therapy. Receipt of first-course therapy had large impacts on increased survival; however, this finding is difficult to interpret because there is little surgery among patients with stage IIIB/IV and systemic therapy is not standard treatment for stage I. We did not model small cell and non–small cell cancers separately. However, our subanalysis analyzing adenocarcinoma cancers covered 54.4% of all non–small cell cancers (58). Changes in smoking status after cancer diagnosis were not ascertained, which could have affected survival. Lastly, smoking status was collected as documented by providers in the electronic health record. Smoker misclassification is generally thought to be small (59-62), and our estimate of the proportion of those who never smoke is consistent with other reported proportions (1,4-6,10,14,24). However, the true smoking prevalence may be underestimated in our cohort.
This study supported prior findings showing statistically significantly longer unadjusted and adjusted survival among patients who never smoke, while finding potentially actionable and observed non–tobacco-related risk factors. Illuminating targets for interventions and advancing evidence and awareness on rates and predictors of survival within different patient groups may be key to optimizing survival as evidence emerges that lung cancer increasingly becomes a heterogeneous disease with increased availability of novel therapeutics.
Supplementary Material
Acknowledgements
The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Contributor Information
Nikki M Carroll, Institute for Health Research, Kaiser Permanente Colorado, Denver, CO, USA.
Andrea N Burnett-Hartman, Institute for Health Research, Kaiser Permanente Colorado, Denver, CO, USA; Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA, USA.
Katharine A Rendle, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Christine M Neslund-Dudas, Henry Ford Health and Henry Ford Cancer Institute, Detroit, MI, USA.
Robert T Greenlee, Marshfield Clinic Research Institute, Marshfield, WI, USA.
Stacey A Honda, Hawaii Permanente Medical Group, Center for Integrated Healthcare Research, Kaiser Permanente Hawaii, Honolulu, HI, USA.
Anil Vachani, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Debra P Ritzwoller, Institute for Health Research, Kaiser Permanente Colorado, Denver, CO, USA.
Data availability
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Author contributions
Nikki M Carroll, MS (Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Software; Validation; Visualization; Writing—original draft; Writing—review & editing), Andrea N. Burnett-Hartman, PhD (Conceptualization; Methodology; Writing—review & editing), Katharine A. Rendle, PhD, MSW, MPH (Methodology; Writing—review & editing), Christine M. Neslund-Dudas, PhD (Methodology; Writing—review & editing), Robert T. Greenlee, PhD, MPH (Methodology; Writing—review & editing), Stacey A. Honda, MD, PhD (Methodology; Writing—review & editing), Anil Vachani, MD, MS (Methodology; Writing—review & editing), Debra P. Ritzwoller, PhD (Conceptualization; Methodology; Writing—original draft; Writing—review & editing).
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (R50CA251966 to [NMC] and UM1CA221939 [KAR, CND, ABH, RTG, SAH, AV, DPR]).
Conflicts of interest
NMC and DPR report research support from Pfizer paid to their institution outside of the submitted work. KAR reports research support from Pfizer and AstraZeneca paid to her institution and personal fees as a scientific advisor to Merck, all outside of the submitted work. ABH reports research support from Biodesix paid to her institution outside of the submitted work. AV reports personal fees as a scientific advisor to the Lung Cancer Initiative at Johnson & Johnson and grants to his institution from MagArray, Inc, Broncus Medical, Inc, and Precyte, Inc outside of the submitted work. AV is an advisory board member of the Lungevity Foundation (unpaid). CND reports research support from Genentech paid to her institution. All other authors reported no conflicts of interest.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2. Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31-54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 3. Secretan B, Straif K, Baan R, et al. ; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033-1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 4. Siegel DA, Fedewa SA, Henley SJ, Pollack LA, Jemal A. Proportion of never smokers among men and women with lung cancer in 7 US States. JAMA Oncol. 2021;7(2):302-304. doi: 10.1001/jamaoncol.2020.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pelosof L, Ahn C, Gao A, et al. Proportion of never-smoker non-small cell lung cancer patients at three diverse institutions. JNCI J Natl Cancer Inst. 2017;109(7):djw295. doi: 10.1093/jnci/djw295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cufari ME, Proli C, De Sousa P, et al. Increasing frequency of non-smoking lung cancer: presentation of patients with early disease to a tertiary institution in the UK. Eur J Cancer (Oxford, England: 1990). 2017;84:55-59. doi: 10.1016/j.ejca.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 7. Stiles BM, Rahouma M, Hussein MK, et al. Never smokers with resected lung cancer: different demographics, similar survival. Eur J Cardio-Thorac Surg. 2018;53(4):842-848. doi: 10.1093/ejcts/ezx390. [DOI] [PubMed] [Google Scholar]
- 8. Bryant A, Cerfolio RJ. Differences in epidemiology, histology, and survival between cigarette smokers and never-smokers who develop non-small cell lung cancer. Chest. 2007;132(1):185-192. doi: 10.1378/chest.07-0442. [DOI] [PubMed] [Google Scholar]
- 9. Clément-Duchêne C, Stock S, Xu X, et al. Survival among never-smokers with lung cancer in the cancer care outcomes research and surveillance study. Ann Am Thorac Soc. 2016;13(1):58-66. doi: 10.1513/AnnalsATS.201504-241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Löfling L, Karimi A, Sandin F, et al. Clinical characteristics and survival in non-small cell lung cancer patients by smoking history: a population-based cohort study. Acta Oncol (Stockholm, Sweden). 2019;58(11):1618-1627. doi: 10.1080/0284186x.2019.1638521. [DOI] [PubMed] [Google Scholar]
- 11. Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25(5):472-478. doi: 10.1200/jco.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Booth CM, Li G, Zhang-Salomons J, Mackillop WJ. The impact of socioeconomic status on stage of cancer at diagnosis and survival: a population-based study in Ontario, Canada. Cancer. 2010;116(17):4160-4167. doi: 10.1002/cncr.25427. [DOI] [PubMed] [Google Scholar]
- 13. Korpanty GJ, Kamel-Reid S, Pintilie M, et al. Lung cancer in never smokers from the Princess Margaret Cancer Centre. Oncotarget. 2018;9(32):22559-22570. doi: 10.18632/oncotarget.25176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerrigan K, Wang X, Haaland B, et al. Real world characterization of advanced non-small cell lung cancer in never smokers by actionable mutation status. Clin Lung Cancer. 2021;22(4):260-267.e2. doi: 10.1016/j.cllc.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 15. Nemesure B, Albano D, Nemesure A. Short- and long-term survival outcomes among never smokers who developed lung cancer. Cancer Epidemiol. 2021;75:102042. doi: 10.1016/j.canep.2021.102042. [DOI] [PubMed] [Google Scholar]
- 16. Lee H, Singh GK. Disparities in all-cancer and lung cancer survival by social, behavioral, and health status characteristics in the United States: a longitudinal follow-up of the 1997-2015 National Health Interview Survey-National Death Index Record Linkage Study. J Cancer Prev. 2022;27(2):89-100. doi: 10.15430/jcp.2022.27.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tannenbaum SL, Koru-Sengul T, Zhao W, Miao F, Byrne MM. Survival disparities in non-small cell lung cancer by race, ethnicity, and socioeconomic status. Cancer J. 2014;20(4):237-245. doi: 10.1097/ppo.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 18. Shah M, Parmar A, Chan KKW. Socioeconomic disparity trends in diagnostic imaging, treatments, and survival for non-small cell lung cancer 2007-2016. Cancer Med. 2020;9(10):3407-3416. doi: 10.1002/cam4.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hovanec J, Siemiatycki J, Conway DI, et al. Lung cancer and socioeconomic status in a pooled analysis of case-control studies. PLoS One. 2018;13(2):e0192999. doi: 10.1371/journal.pone.0192999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hastert TA, Beresford SA, Sheppard L, White E. Disparities in cancer incidence and mortality by area-level socioeconomic status: a multilevel analysis. J Epidemiol Community Health. 2015;69(2):168-176. doi: 10.1136/jech-2014-204417. [DOI] [PubMed] [Google Scholar]
- 21. Denton EJ, Hart D, Russell PA, Wright G, Conron M. Lung cancer and socio-economic status: Inextricably linked to place of residence. Intern Med J. 2017;47(5):563-569. doi: 10.1111/imj.13376. [DOI] [PubMed] [Google Scholar]
- 22. Yang X, Deng L, Li M, Zhou Y, Wang G. Impact of socioeconomic status on cancer staging, survival in non-small cell lung cancer. Front Public Health. 2022;10:992944. doi: 10.3389/fpubh.2022.992944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Japuntich SJ, Kumar P, Pendergast JF, et al. Smoking status and survival among a National cohort of lung and colorectal cancer patients. Nicotine Tob Res. 2019;21(4):497-504. doi: 10.1093/ntr/nty012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dias M, Linhas R, Campainha S, Conde S, Barroso A. Lung cancer in never-smokers - what are the differences? Acta Oncologica (Stockholm, Sweden). 2017;56(7):931-935. doi: 10.1080/0284186x.2017.1287944. [DOI] [PubMed] [Google Scholar]
- 25. Toh CK, Ong WS, Lim WT, et al. A decade of never-smokers among lung cancer patients-increasing trend and improved survival. Clin Lung Cancer. 2018;19(5):e539-e550. doi: 10.1016/j.cllc.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 26. Couraud S, Zalcman G, Milleron B, Morin F, Souquet PJ. Lung cancer in never smokers--a review. Eur J Cancer (Oxford, England: 1990). 2012;48(9):1299-1311. doi: 10.1016/j.ejca.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 27. Vachani A, Sequist LV, Spira A. AJRCCM: 100-year anniversary. The shifting landscape for lung cancer: past, present, and future. Am J Respir Crit Care Med. 2017;195(9):1150-1160. doi: 10.1164/rccm.201702-0433CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet (London, England). 2017;389(10066):299-311. doi: 10.1016/s0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 29. Tsao AS, Scagliotti GV, Bunn PA Jr, et al. Scientific advances in lung cancer 2015. J Thorac Oncol. 2016;11(5):613-638. doi: 10.1016/j.jtho.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 30. Balata H, Fong KM, Hendriks LE, et al. Prevention and early detection for NSCLC: advances in thoracic oncology 2018. J Thorac Oncol. 2019;14(9):1513-1527. doi: 10.1016/j.jtho.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 31. Rendle KA, Burnett-Hartman AN, Neslund-Dudas C, et al. Evaluating lung cancer screening across diverse healthcare systems: a process model from the lung PROSPR consortium. Cancer Prev Res (Philadelphia, PA). 2020;13(2):129-136. doi: 10.1158/1940-6207.capr-19-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burnett-Hartman AN, Carroll NM, Honda SA, et al. Community-based lung cancer screening results in relation to patient and radiologist characteristics: the PROSPR consortium. Ann Am Thorac Soc. 2022;19(3):433-441. doi: 10.1513/AnnalsATS.202011-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81-92. doi: 10.1007/s10552-013-0310-1. [DOI] [PubMed] [Google Scholar]
- 34. Morrill R, Cromartie J, Hart G. Metropolitan, urban, and rural commuting areas: toward a better depiction of the United States settlement system. Urban Geogr. 1999;20(8):727-748. doi: 10.2747/0272-3638.20.8.727. [DOI] [Google Scholar]
- 35. Rural Health Research Center Rural-Urban Commuting Areas (RUCAS). http://depts.washington.edu/uwruca/ruca-maps.php. Accessed September 22, 2021.
- 36. Oppong BA, Rolle AA, Ndumele A, et al. Are there differences in outcomes by race among women with metastatic triple-negative breast cancer? Breast Cancer Res Treat. 2022;196(2):399-408. doi: 10.1007/s10549-022-06736-8. [DOI] [PubMed] [Google Scholar]
- 37. Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMS (Washington, DC). 2016;4(3):1238. doi: 10.13063/2327-9214.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. [DOI] [PubMed] [Google Scholar]
- 39. Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584-590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 40. Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol . 2014;32(22):2380-2385. doi: 10.1200/jco.2014.55.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim DH, Uno H, Wei LJ. Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol. 2017;2(11):1179-1180. doi: 10.1001/jamacardio.2017.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conner SC, Sullivan LM, Benjamin EJ, LaValley MP, Galea S, Trinquart L. Adjusted restricted mean survival times in observational studies. Stat Med. 2019;38(20):3832-3860. doi: 10.1002/sim.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Trinquart L, Jacot J, Conner SC, Porcher R. Comparison of treatment effects measured by the hazard ratio and by the ratio of restricted mean survival times in oncology randomized controlled trials. J Clin Oncol. 2016;34(15):1813-1819. doi: 10.1200/jco.2015.64.2488. [DOI] [PubMed] [Google Scholar]
- 44. Hassett MJ, Uno H, Cronin AM, Carroll NM, Hornbrook MC, Ritzwoller DP. Comparing survival after recurrent vs De Novo Stage IV advanced breast, lung, and colorectal cancer. JNCI Cancer Spectr. 2018;2(2):pky024. doi: 10.1093/jncics/pky024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Masoudi FA, Go AS, Magid DJ, et al. Age and sex differences in long-term outcomes following implantable cardioverter-defibrillator placement in contemporary clinical practice: findings from the Cardiovascular Research Network. J Am Heart Assoc. 2015;4(6):e002005. doi: 10.1161/jaha.115.002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun Z, Aubry MC, Deschamps C, et al. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: an analysis of 5018 hospital- and 712 population-based cases. J Thorac Cardiovasc Surg. 2006;131(5):1014-1020. doi: 10.1016/j.jtcvs.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 47. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/circulationaha.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England). 2007;370(9596):1453-1457. doi: 10.1016/s0140-6736(07)61602-x. [DOI] [PubMed] [Google Scholar]
- 49. Islami F, Guerra CE, Minihan A, et al. American Cancer Society's report on the status of cancer disparities in the United States, 2021. CA Cancer J Clin. 2022;72(2):112-143. doi: 10.3322/caac.21703 [DOI] [PubMed] [Google Scholar]
- 50. Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi: 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640-649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gandara DR, Riess JW, Kelly K, Li T, Mack PC, Lara PN Jr. Evolution and increasing complexity of the therapeutic landscape in advanced non-small-cell lung cancer. Clin Lung Cancer. 2017;18(1):1-4. doi: 10.1016/j.cllc.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 53. Wang X, Romero-Gutierrez CW, Kothari J, Shafer A, Li Y, Christiani DC. Prediagnosis smoking cessation and overall survival among patients with non-small cell lung cancer. JAMA Netw Open. 2023;6(5):e2311966. doi: 10.1001/jamanetworkopen.2023.11966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Royce JM, Hymowitz N, Corbett K, Hartwell TD, Orlandi MA. Smoking cessation factors among African Americans and whites. COMMIT Research Group. Am J Public Health. 1993;83(2):220-226. doi: 10.2105/ajph.83.2.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kostelanetz S, Di Gravio C, Schildcrout JS, Roumie CL, Conway D, Kripalani S. Should we implement geographic or patient-reported social determinants of health measures in cardiovascular patients. Ethn Dis. 2021;31(1):9-22. doi: 10.18865/ed.31.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chetty R, Stepner M, Abraham S, et al. The association between income and life expectancy in the United States, 2001-2014. JAMA. 2016;315(16):1750-1766. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703-710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12-24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 60. Wells AJ, English PB, Posner SF, Wagenknecht LE, Perez-Stable EJ. Misclassification rates for current smokers misclassified as nonsmokers. Am J Public Health. 1998;88(10):1503-1509. doi: 10.2105/ajph.88.10.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Groenhof TKJ, Koers LR, Blasse E, et al. ; UCC-CVRM Study Groups. Data mining information from electronic health records produced high yield and accuracy for current smoking status. J Clin Epidemiol. 2020;118:100-106. doi: 10.1016/j.jclinepi.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 62. Caraballo RS, Giovino GA, Pechacek TF, Mowery PD. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2001;153(8):807-814. doi: 10.1093/aje/153.8.807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.