Abstract
The increasing evaporative demand due to climate change will significantly affect the balance of carbon assimilation and water losses of plants worldwide. The development of crop varieties with improved water-use efficiency (WUE) will be critical for adapting agricultural strategies under predicted future climates. This review aims to summarize the most important leaf morpho-physiological constraints of WUE in C3 plants and identify gaps in knowledge. From the carbon gain side of the WUE, the discussed parameters are mesophyll conductance, carboxylation efficiency and respiratory losses. The traits and parameters affecting the waterside of WUE balance discussed in this review are stomatal size and density, stomatal control and residual water losses (cuticular and bark conductance), nocturnal conductance and leaf hydraulic conductance. In addition, we discussed the impact of leaf anatomy and crown architecture on both the carbon gain and water loss components of WUE. There are multiple possible targets for future development in understanding sources of WUE variability in plants. We identified residual water losses and respiratory carbon losses as the greatest knowledge gaps of whole-plant WUE assessments. Moreover, the impact of trichomes, leaf hydraulic conductance and canopy structure on plants’ WUE is still not well understood. The development of a multi-trait approach is urgently needed for a better understanding of WUE dynamics and optimization.
Keywords: Crown architecture, leaf anatomy, mesophyll conductance, minimal conductance, respiration, rubisco, stomata, WUE
Climate-change-induced evaporative demand will impact carbon assimilation and water losses in plants. Developing water-efficient plant varieties is crucial. This review summarizes leaf morpho-physiological constraints on water-use efficiency (WUE) in C3 plants, highlighting knowledge gaps. The understanding of WUE dynamics needs further exploration of residual water losses, respiratory carbon losses, trichomes, leaf hydraulic conductance, and canopy structure. A multi-trait approach is needed for understanding WUE optimizing in plants.
Introduction
Water-use efficiency (WUE) reflects a balance between carbon gain and water loss in plants, introduced more than 100 years ago by Briggs and Shantz (1913). Since then, multiple ways and methods to assess WUE at a different level of organization and temporal resolution were developed and conceptualized (Vadez et al. 2014, 2023; Hatfield and Dold 2019; Brendel 2021). Two WUE parameters reflect a momentary state of leaf carbon and water fluxes: intrinsic water-use efficiency (WUEi) as a ratio of CO2 assimilation rate (An) to water vapour stomatal conductance (gs), obtained during gas-exchange measurements at leaf level (Petrik et al. 2022a). Another closely related variant, instantaneous WUEi, is calculated as a ratio of An and leaf transpiration (Bacon et al. 2004). Other WUE parameters capture the long-term balance between carbon fixation and transpiratory water losses. Biomass-based indices include whole-plant WUEbio as the ratio of biomass accumulation to cumulative transpiration of the plants (Condon et al. 2004; Brendel 2021). Furthermore, yield WUE is usually calculated as crop yield per hectare divided by total transpiration or evapotranspiration (Hatfield and Dold 2019; Zahoor et al. 2019). The use of growth-based WUE calculated as the ratio of annual basal area increment and cumulative annual transpiration is used in dendrobiology (Szatniewska et al. 2022). Moreover, the carbon isotope ratio (δ 13C) has been extensively used as a proxy of long-term WUE13C, because of the preference for the lighter isotope during physical and chemical processes involved in CO2 uptake and assimilation (Farquhar et al. 1989; Frank et al. 2015; Ma et al. 2023). Ecosystem-wide WUE derived from eddy-covariance measurements (WUEGPP) is a ratio between gross primary production (GPP) of the ecosystem and total cumulative transpiration or evapotranspiration (Yi et al. 2019). WUEGPP can be also derived from remote sensing data as the GPP to evapotranspiration ratio (Ahmadi et al. 2019). Overall, the individual-level, long-term (vegetation season) based WUEbio is the most precise assessment of real resource utilization of plants as they capture both assimilatory and respiratory balance with productive and unproductive water losses (Brendel and Epron 2022). WUEbio should thus be more commonly used as the standard WUE estimates in agricultural and plant sciences, instead of the WUEi, which is much easier to measure but represents only one point in time.
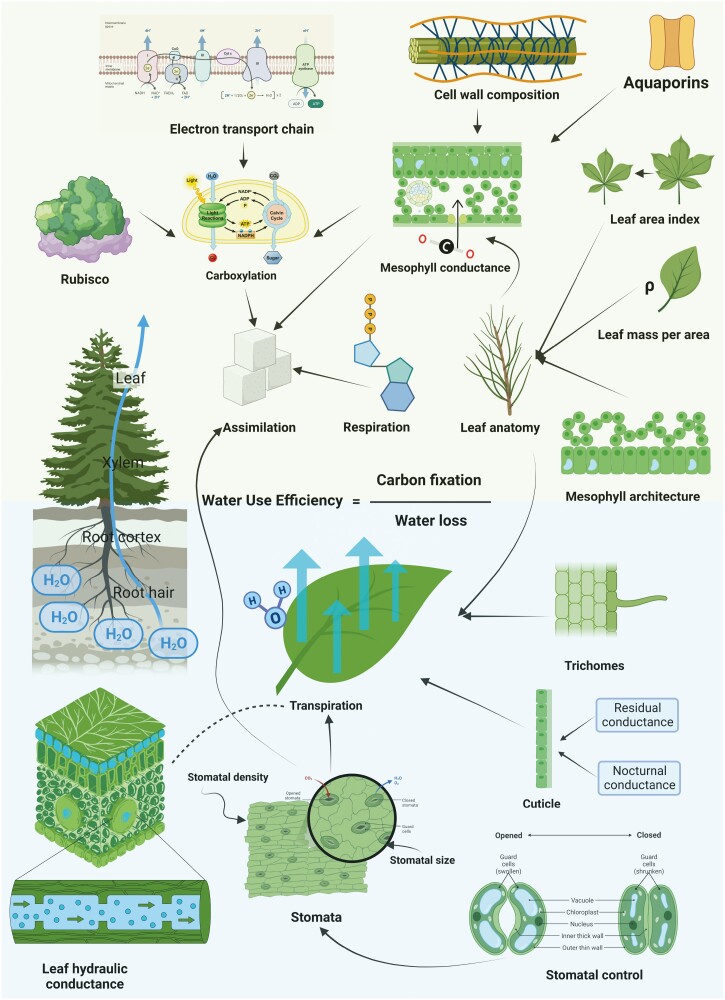
The importance of WUE acclimation in plants is due to raising evaporation demands caused by climate change and possible frequent water-deficit stress during seasonal droughts (Ponce-Campos et al. 2013; Schuldt et al. 2020). Plants with higher WUE will have a competitive advantage in natural ecosystems and economic significance for agricultural production. The momentary WUEi of plants can be improved either by lower transpiration losses or higher efficiency of carbon assimilation (Flexas et al. 2016; Hatfield et al. 2019). Understanding of constraining factors of WUE is crucial for crop optimization efforts and the correct assessment of adaptive responses of plant communities (Quan et al. 2020; Kang et al. 2021). WUE variability is affected by multiple morphological and physiological traits (Figure 1). The size and density of stomata affect the maximal stomatal conductance and stomatal responsiveness to environmental changes (Nunes et al. 2022; Pitaloka et al. 2022). As stomatal morphology and anatomy can be altered with biotechnological methods for improved WUE, it is a great target for future research (Caine et al. 2019; Li et al. 2020). The responsiveness of stomata to fluctuating light and drought can also improve long-term WUEbio (Xylogiannis et al. 2020; Zhao et al. 2021a). Several studies have found a negative correlation between WUE estimates and leaf hydraulic conductance (Wedegaertner et al. 2022; Barrera-Ayala et al. 2023; Liu et al. 2023), but these findings are still inconclusive (Corcuera et al. 2012; Sellin et al. 2014; Jin et al. 2016) and we need a better causal explanation of the relationship. Another important constraint of WUE is the mesophyll conductance (gm) of CO2 towards Rubisco (Flexas et al. 2016; Zhu et al. 2021). Maximization of the gm/gs ratio was suggested as a possible goal for improving WUE of crops (Flexas et al. 2013a; Fullana-Pericàs et al. 2017). The next step of WUE improvement is an optimization of Rubisco carboxylation efficiency (Flexas et al. 2016). The long-term WUEbio enhancement could be further achieved by the reduction of respiratory losses and residual water losses during night or drought (Escalona et al. 2012; Coupel-Ledru et al. 2016). Finally, leaf anatomy, which influences both mesophyll conductance CO2 and transpiratory losses, can also alter plant WUE (Bramley et al. 2013; Trueba et al. 2022).
Figure 1.
Overview of mechanisms and traits which affect the carbon fixation (upper half) and water loss (lower half) components of water-use efficiency in C3 plants. Created with BioRender.com and adapted with Canva.com.
The objective of this review paper was to summarize various morphological and physiological factors, which influence WUE in plants, as a stepping stone for a more holistic approach to the multi-factor assessment of WUE constraints (Figure 1). We also focused on identifying under-represented physiological and morphological traits in current research, which are needed for understanding WUE optimization in plants. Moreover, this review focuses specifically on WUEi, WUE13C and WUEbio to provide the most possibly concise overview of this complex topic at a similar spatial scale. It is worth pointing out that environmental factors such as water availability (Amitrano et al. 2019; Zhao et al. 2021b), soil structure (Hatfield et al. 2001; Rabarijaona et al. 2022), air pollution (Hatfield et al. 2001; Rabarijaona et al. 2022) and nutrients (Dijkstra et al. 2016; Gharun et al. 2021; Song et al. 2022) can also have a significant impact on WUE. However, this falls beyond the scope of the study and is therefore not further discussed.
Water Side of WUE
Stomatal density and trichomes
Plants can influence their transpiratory losses and therefore potentially their WUE via stomatal regulation (Hetherington and Woodward 2003; Bertolino et al. 2019). The stomatal adjustment could include changes in stomatal density (SD), stomatal anatomy (size, shape) and stomatal control mechanisms (Sack and Buckley 2016; Petrik et al. 2022b). Multiple recent studies, which used genetic manipulation methods to alter SD, have reported improved WUEi connected to the reduction of SD. A genetic manipulation (EPF2OE) approach in a study by Franks et al. (2015) led to Arabidopsis mutants with lower SD that showed higher WUEi and long-term WUE13C due to lower stomatal conductance of water vapour (gs) but unchanged photosynthetic capacity. Similarly, a combination of high-yield rice cultivars with overexpressed OsEPF1 epidermal patterning factor (EPF) led to a reduction of SD, lower gs, improved WUEi and overall drought tolerance (Caine et al. 2019). The EPF overexpression in bread wheat has led to similar results of reduced SD and improved WUEi, without yield losses (Dunn et al. 2019). Guo et al. (2019a) have reported the genetic pathway of EDT1/HDG11, ERECTA, and E2Fa loci, which regulates WUEi of Arabidopsis via modulation of SD. Overexpression of SlTLFP8 (Tubby-like F-box protein 8) reduced SD by 10–20 % in tomatoes and was connected to enhanced WUEi (Li et al. 2020). Similarly, repression of PuGTL1 via Pu-miR172d overexpression led to a reduction of SD and higher WUEi in Populus ussuriensis (Liu et al. 2021). On the other hand, overexpression of STOMAGEN led to higher SD, greater photosynthetic activity (+30 %), but also greater transpiration (+100 %), which resulted in reduced WUEi (Tanaka et al. 2013). Contrary, the study by Bhaskara et al. (2022) also reported a positive relationship between SD and WUEbio derived from natural variation in Arabidopsis accessions. Moreover, other leaf structures such as trichomes (trichomes/SD ratio) can play a significant role in WUEi and WUEbio enhancement via lower transpiratory losses due to leaf–air boundary layer resistance (Mo et al. 2016; Galdon-Armero et al. 2018). For example, Chen et al. (2022) observed a doubling in trichome density and a decline in gs by 85 % between droughted and well-watered Shepherdia × utahensis plants. Single gene manipulation efforts, such as EPF2OE, could have negative pleiotropic effects on other metabolic processes and should be further explored to avoid these negative side effects (Flexas et al. 2016; Husaini et al. 2022). It seems that the reduction of SD for improving WUEi and WUE13C/bio could be a viable option for plant breeding initiatives. Additionally, the incorporation of further leaf structures, such as trichomes, in combination with SD can improve our understanding of WUE constraints.
Stomatal size and responsiveness
Stomatal control mechanisms include reaction to atmospheric vapour pressure deficit (Grossiord et al. 2020), plant water potential (Buckley 2005, 2019; Dayer et al. 2020), light conditions (Lawson et al. 2010; McAusland et al. 2013) and CO2 concentration (Franks and Beerling 2009). Photosynthetic activity of C3 plants can adjust in seconds to changes in irradiance, but the lag in stomatal responses limits the CO2 uptake and therefore constrains photosynthesis and limits WUE (Lawson et al. 2012). Several studies have reported that smaller stomata respond faster than larger stomata to changes in environmental conditions (Drake et al. 2013; Lawson et al. 2014; Kardiman and Raebild 2018; Durand et al. 2019). Faster stomatal response in the study by Lawson et al. (2014) has been linked to higher WUEi values under naturally changing irradiance levels. Theoretical maximal stomatal conductance (gmax) showed a negative correlation with stomatal size, but smaller stomata showed faster response time to variable irradiance in five Banksia species (Drake et al. 2013). A study by Lei et al. (2023) also found that larger stomata of domesticated rice showed slower response time to fluctuating light and overall lower WUE13C. The genetic manipulation study in rice has found that mutants with small stomatal size showed higher WUEi, in comparison to mutants with greater stomatal size (Pitaloka et al. 2022). Des Marais et al. (2014) found that Arabidopsis genotypes with larger stomata due to AtMPK12 substitution showed lower WUEi compared with the common allele. The improved WUEi of wheat cultivars under water-deficit stress was linked to smaller stomatal size, lower SD and reduced transpiration rates (Li et al. 2017). A study by Amitrano et al. (2021) showed that a 49 % increase in WUEi and WUEbio of lettuce has been associated with a reduction of stomatal size under different vapour pressure deficit (VPD) treatments. Moreover, drought stress exposure inhibited stomatal development (smaller stomata) and increased the WUEi in cotton (Dubey et al. 2023). On the other hand, a study by Xiong and Flexas (2020) on ferns, gymnosperms and angiosperms found a negative correlation between stomatal size and gm, therefore possibly limiting WUE. A comparison of Quercus robur genotypes has found a positive correlation between guard cell length and WUE13C, contradicting the majority of results suggesting that smaller stomata promote higher WUE13C (Roussel et al. 2009). Liu et al. (2018a) have found a quadratic relationship between stomatal size and WUEi at the community level, across forest ecosystems along the latitudinal transect, with an optimal stomatal size of approximately 400 μm2. Smaller stomatal size could be connected to higher WUE in plants, presumably due to faster response to environmental conditions. Nevertheless, there is probably an optimal stomatal size and further reduction can be detrimental due to CO2 limitations of photosynthesis.
Stomatal control and light sensitivity
Excessive water loss under an impaired state of photosynthetic apparatus (drought, salinity stress) can negatively affect the WUE of plants. Timely stomatal closure is then another major component of WUE optimization of plants under water-deficit stress (Yang et al. 2016; Hartmann et al. 2021). A study by Yi et al. (2019) showed that WUE13C of isohydric species was generally more sensitive to environmental change due to their conservative water potential regulation strategy than WUE13C of the anisohydric species and increased significantly with rising VPD during periods of water stress. The accumulation of abscisic acid (ABA), which drives the stomatal closure of plants under water deficit, can be considered a key factor for both WUEi and WUE13C/bio improvement in plants (Negin and Moshelion 2016; Guo et al. 2019b; Mukarram et al. 2021). Plants capable of fine-tuning their stomatal control with ABA can possess an enhanced WUEi with sustained biomass or yield gains (Yoo et al. 2009; Yao et al. 2021). Improved WUEi in the presence of elevated ABA levels has been demonstrated in transgenic Arabidopsis (Zhang et al. 2008) and tomato (Thompson et al. 2007; Lamarque et al. 2020). Exogenous application of ABA showed enhanced WUEi and WUE13C in Populus davidiana (Li et al. 2004) and Marsilea crenata fern (Tai-Chung et al. 2020). French bean and sugar beet plants pretreated with ABA also showed improved WUEi under water-deficit stress (Pospíšilová and Baťková 2004). Enhanced stimulation of ABA signalling of Arabidopsis via distinct ABA receptors can result in constitutively high WUEi (Yang et al. 2016). WUE13C of Arabidopsis and wheat was also enhanced by modulating ABA responses either by using overexpression of specific ABA receptors or deficiency of ABA coreceptors (Yang et al. 2019). ABA receptors from Populus canescens were stably introduced into Arabidopsis in a study by Papacek et al. (2019), which led to enhanced WUEi. Moreover, overexpression of PeJAZ2 increased WUEi of poplar under drought stress by regulating ABA signalling rather than ABA synthesis (Rao et al. 2023). Partial root-zone drying can generate a root-to-shoot pressure signal from the dry part of the root zone that also promotes stomatal closure via a drop in cell turgor and enhances WUEi via ABA utilization (Davies et al. 2002; Pérez-Pérez et al. 2012; McAdam and Brodribb 2016; Zhang et al. 2018; Xylogiannis et al. 2020). These results, therefore, suggest great opportunities for WUE optimization in crops with the use of transgenic methods, breeding efforts and biotechnological tools for ABA utilization.
Stomatal sensitivity to light could be another important determinant of plant WUEi by adjusting the magnitudes of change in gs as a function of the environment (Vialet-Chabrand et al. 2016). Part of the stomatal response involves the balance between photosynthetic electron transport and carbon reduction either in guard cells, chloroplasts, or in the mesophyll (Messinger et al. 2006). Overexpression of Photosystem II Subunit S in tobacco led to lower stomatal opening in response to light, which resulted in a 25 % reduction of water loss and improved WUEi (Glowacka et al. 2018). The desynchronization of An and gs can lead to a surplus in transpiration when An is low but gs is high (e.g. transition from high to low light), hence reducing WUEi (McAusland et al. 2016; Coupel-Ledru 2021). The introduction of a blue light-activated K+ ion channel, named BLINK1, to Arabidopsis, led to a faster reaction of stomatal aperture under both increasing and decreasing irradiance, which ultimately enhanced the plants’ biomass accumulation and WUEbio (Papanatsiou et al. 2019). Dynamic plant response to VPD and light fluctuations under natural conditions were suggested to increase plants WUEbio (Gosa et al. 2019). Lower stomatal openness and lower gs under short-term light transitions led to higher WUEi in chilli pepper treated with “smart glass” compared to the control group (Zhao et al. 2021a). A study by Li et al. (2023) found that overexpression of OE-PtrVCS2 in Populus trichocarpa led to smaller stomatal aperture under drought stress and overall higher WUEi than in the wild type. Greater WUEi of isohydric Pine species has been also linked to lower stomatal openness under increasing light, while anisohydric Oak species behaved more opportunistically with lower WUEi (Renninger et al. 2015). Reduction of stomatal openness as a reaction to light changes can probably improve the WUE of plants but can lead to a reduction of the total growth and yield of crops. Nevertheless, improving stomatal response time to changing irradiance levels can improve the plants’ WUE without a negative impact on assimilation and growth.
Residual and nocturnal conductance
When the stomata are closed (night, drought), plants are still losing water via their cuticle, bark or incompletely closed stomata (Duursma et al. 2019; Lintunen et al. 2021). Cuticular transpiration has been recognized as a significant factor affecting drought survival rates (Duursma et al. 2019) and might affect WUE13C/bio due to residual transpiration (Ni et al. 2012; Ávila-Lovera et al. 2019). Minimum leaf conductance (gmin) incorporates water loss across the leaf cuticle, bark and through the incompletely closed stomata (Schuster et al. 2017; Blackman et al. 2019; Duursma et al. 2019; Lintunen et al. 2021). Minimization of these residual losses during periods of reduced assimilation rate due to stomatal limitations can therefore lead to improved long-term WUE13C/bio (Sevanto 2020). The water loss from leaves of plants under drought is dominated by gmin after stomatal closure. This has been related to the thickness of the cuticular wax layer, which increases in response to water deficit (Jeffree 2006; Shepherd and Wynne Griffiths 2006; Bueno et al. 2020). However, a relationship between the thickness of the cuticular wax layer and gmin can be insignificant, both within (Anfodillo et al. 2002; Bueno et al. 2020) and across species (Riederer and Schreiber 2001). The variability of gmin can be also driven by stomatal morphology (leaky stomata) or chemical composition of cuticle (Duursma et al. 2019; Machado et al. 2021). In a recent study across 23 genotypes of wheat, cuticular transpiration showed a strong positive correlation with water loss per dry mass unit, which the authors considered as a proxy for WUEbio (Gašparovič et al. 2021). A modelling simulation approach by Duursma et al. (2019) revealed a theoretical reduction of WUEi under increasing gmin of plants using the general Ball-Berry model of stomatal conductance. Moreover, hydroponically grown Festuca arundinacea exposed to salinity treatment showed enhanced WUEi and lower gmin compared to the control group (Vandegeer et al. 2021). On the other hand, eucalyptus clones under water-deficit treatment showed significant intra-specific differences in cuticular conductance but not in WUEi (Carignato et al. 2019). A study by Clarke et al. (1991) also found no significant correlation between minimal conductance and long-term WUEbio in wheat under drought stress. The impact of cuticular conductance or gmin on WUE has not been yet properly quantified and is therefore a great target for future research.
The analogical parameter, nocturnal conductance, is also critical for optimization of long-term WUE13C/bio (Coupel-Ledru et al. 2016; Even et al. 2019). Excessive water losses during the night (Dawson et al. 2007; Forster 2014) decrease long-term WUE as there is no photosynthetic gain during the night. It has been suggested that the low nocturnal conductance of shade-tolerant plant species is consistent with their conservative water-use strategy (Resco de Dios et al. 2019). Nocturnal conductance is usually dominated by cuticular transpiration, but incomplete stomatal closure during the night has been observed in C3 plants (Caird et al. 2007; Escalona et al. 2012). Reduction of night transpiration can theoretically improve the WUEbio of crops without growth penalties (Tardieu et al. 2022). A study by Dayer et al. (2021) has shown that night transpiration was linked more to the specific circadian rhythm of the wine cultivars rather than environmental conditions, suggesting strong genetic control. Night transpiration also had a significant impact on total transpiration and WUEbio in a study by Medrano et al. (2017) and was recognized as one of the under-explored factors affecting whole-plant WUE. Nocturnal conductance also showed a significant negative correlation with WUEbio among black poplar genotypes exposed to drought stress (Bogeat-Triboulot et al. 2019). Differences in the night transpiration between Pinus contorta thinning treatments corresponded to differences in WUE under water-deficit stress (Wang et al. 2020). Further quantification of the night transpiration effect on the long-term WUE of plants is needed for a proper understanding of the phenomenon. Selection for plants with low cuticular conductance and conservative stomatal control (avoiding leaky stomata) can greatly improve their WUE and drought resistance.
Leaf hydraulic conductance
Leaf hydraulic conductance (Kleaf) can be coordinated with higher WUEi, as observed in several studies (Fichot et al. 2009; Andrade et al. 2022; Wedegaertner et al. 2022). Nevertheless, it is still unknown if the plants with higher WUE develop smaller xylem vessels causing lower Kleaf (but greater xylem embolism resistance, cf. Isasa et al. 2023) as they have lower hydraulic requirements to maintain leaf gas exchange, or the lower Kleaf leads to greater WUE by constraining water supply in leaves. Kleaf is tied to leaf assimilation and stomatal conductance rate in a positive linear fashion (Santiago et al. 2004; Sellin et al. 2014). Reduction of leaf hydraulic conductance via gene manipulation can lead to lower water losses but is also tied with a proportional reduction of assimilation rates and therefore non-significant changes in WUEbio (Zsögön et al. 2015). The environmental response of Kleaf and its impact on WUE has received more attention in recent studies and has been identified as a major trait that could constrain WUE under changing VPD (Flexas et al. 2013a; Xiong et al. 2018). However, no consensus has been reached to date regarding the direction of the relationship between Kleaf and WUE. On one hand, Yao et al. (2021) reported that raising WUEi of Caragana sp. with decreasing water potential was coordinated with decreasing Kleaf but also rapid biosynthesis of ABA. The Solanum species with significantly lower Kleaf showed also significantly higher WUE13C under well-watered conditions (Barrera-Ayala et al. 2023), while WUEi of Ginkgo biloba was also negatively correlated with Kleaf (Liu et al. 2023). Warming treatment in four subtropical tree species led to higher Kleaf but lower WUE13C (Wu et al. 2020). On the other hand, Jin et al. (2016) reported a positive relationship between Kleaf and WUEi among 10 temperate tree species. Similarly, a positive correlation between WUE13C and Kleaf was reported for Pinus pinaster populations exposed to drought stress (Corcuera et al. 2012). Moreover, Sellin et al. (2013, 2014) found no significant correlation between WUEi and Kleaf in birch and aspen trees. In conclusion, the direction of the Kleaf-WUE relationship is unclear, and further work must be conducted to assess whether breeding for lower Kleaf to reduce water losses possibly leads to improved WUE without a significant reduction of growth. Future experiments with gene manipulation techniques that will not affect other physio-morphological traits are needed to understand the causal link of these correlations.
Carbon Side of WUE
Mesophyll conductance
Improving CO2 diffusion to the sites of carboxylation without increasing stomatal conductance can enhance WUEi. This requires improving mesophyll conductance to CO2 (gm) and it has been proposed that the ratio gm/gs is a relevant breeding trait for improving WUE (Galmés et al. 2011; Flexas et al. 2013b; Tomás et al. 2014a; Flexas 2016). The gm has been recognized as one of the main limiting factors of WUE in both crops (Leakey et al. 2019) and tree species (Zhu et al. 2021), potentially due to the close coupling of gm and Kleaf as both share the same pathways of water movement in leaves (Flexas et al. 2013b; Xiong et al. 2017). A close positive relationship has also been observed between gm and gs although the reason for this remains speculative (Guiliani et al. 2013; Barbour and Kaiser 2016). However, a study by Fullana-Pericas et al. (2017) showed a strong positive correlation between gm/gs and WUEi in Mediterranean tomato landraces. Similarly, WUEi showed a strong positive correlation with gm/gs in tobacco under chloride nutrient treatments (Franco‐Navarro et al. 2019). The variability of gm has been linked to leaf anatomy, where cell wall thickness, membrane permeabilities, cytosol and stromal conductance were constraining factors of gm (Terashima et al. 2011; Tomás et al. 2013; Ouyang et al. 2017). The cell wall conductance to CO2 can be influenced by cell wall thickness, porosity and tortuosity (Evans et al. 2009; Ellsworth et al. 2018). A study by Roig-Oliver et al. (2020) found a strong negative correlation between cellulose and gm in grapevine. The hemicellulose to pectin ratio of the cell wall correlated positively with the gm of tobacco exposed to drought and salinity stress (Clemente-Moreno 2019). Tholen et al. (2008) manipulated the chloroplast arrangement in Arabidopsis and thus modified gm through changes in the surface of chloroplasts exposed to the intercellular air spaces (Sc/S). The positive impact of Sc/S on gm and An has been observed also for Mediterranean oak species (Peguero-Pina et al. 2017), rice (Xiong et al. 2017) and tobacco (Clarke et al. 2021). A recent study by Baillie and Fleming (2020) has found that coordination of stomatal and mesophyll development is crucial for the optimization of gm and therefore WUE. Findings to date suggest that certain stomatal development signalling components, such as TMM, ER and STOMAGEN, may be required for interlayer coordination, and that gas exchange may also regulate mesophyll structure (Dow et al. 2017). Acclimation of gm to changing environmental conditions has been linked to aquaporins and carbonic anhydrase (Flexas et al. 2006; Warren 2007). The gm can be affected by specific genes (e.g. aquaporin NtAQP1, HvPIP2, AtBBX21) and thus targeted by genetic manipulation of crops (Evans et al. 2009). Overexpression of aquaporin genes led to increased gm (Hanba et al. 2004) and inhibition of lower gm in various crops (Flexas et al. 2006). Tobacco aquaporin NtAQP1 aids the trans-membrane transport of CO2 in plants and thus contributes to the CO2 permeability of the plasma membrane of the mesophyll cells (Uehlein et al. 2003). Carbonic anhydrase activity has been positively correlated to gm (Price et al. 1994; Momayyezi and Guy 2017) and chloroplast fraction of gm (Gillon et al. 2000). Carbon anhydrase accelerates the interconversion of the dissolved inorganic carbon species, CO2 and HCO3-, which helps optimize the initial stages of photosynthesis. A recent study by Gómez-Ocampo et al. (2021) found that overexpression of AtBBX21 led to enhanced gm and Jmax, coupled with higher WUE in potato plants under drought. Moreover, manipulation of heterotrimeric G protein signalling can improve plants’ WUEi and productivity due to higher gm rates under drought conditions (Zait et al. 2021). More specifically, the canonical Gα (RGA1) subunit gene of G protein regulated gm in rice, which was reflected in improved photosynthetic capacity and overall WUE (Wang and Botella 2022). The optimization of gm and therefore WUE is multifaceted and incorporates multiple organizational levels from cell biochemistry to whole leaf anatomy. There is also great intra-specific variability of gm across crops (Tomás et al. 2014a; Chen et al. 2021) and trees (Momayyezi and Guy 2017; Peguero-Pina et al. 2017) and therefore, it is a reasonable target for breeding efforts which aim at maximizing WUE. Nevertheless, the practical performance of the population/individual’s selection could be hindered by the low reliability of current gm measurements (Pons et al. 2009; Lundgren and Fleming 2020). The development of more precise gm measurement techniques (Márquez et al. 2023) could greatly improve the understanding of WUE constraint by gm. Furthermore, the strong coupling of gm with Kleaf (Flexas et al. 2013; Xiong et al. 2017) and gs (Guiliani et al. 2013; Barbour and Kaiser 2016) might impede efforts to improve WUEi through modification of gm. As shown by Pathare et al. (2023) using rice cell wall mutants, modifying gm indeed increases photosynthetic capacity but at the cost of simultaneously increasing gs, resulting in no overall change in WUEi.
Carboxylation rate
Another target to achieve improved photosynthesis is to improve the biochemical capacity for CO2 assimilation, that is, improving the carboxylation efficiency of Rubisco for C3 species (Gago et al. 2014; Flexas et al. 2016). Optimizing the efficiency of RuBP carboxylation by Rubisco has the potential of improving WUE by decreasing the concentration of CO2 required to achieve high photosynthetic rates (Carmo-Silva et al. 2015). The maximum carboxylase activity of Rubisco (Vcmax) and the capacity for photosynthetic electron transport (Jmax) can constrain the WUE from the carbon assimilation side. Maintenance of functional electron transport under drought stress led to higher WUEi in Magnolia grandiflora (Vastag et al. 2020). Reduction of Vcmax under ozone treatment caused decoupling of photosynthesis and stomatal conductance, which led to lowered WUEi in rice (Masutomi et al. 2019) and poplar clones (Xu et al. 2022). Vcmax and therefore photosynthetic capacity increases with leaf maturation, thus young spring foliage can experience reduced WUE13C, which can be critical, especially during spring droughts (Cernusak 2020). Enhanced WUEi of common bean genotypes under heat stress was linked to higher Vcmax (Suárez et al. 2021). Additionally, Vcmax/gs ratio has been suggested as a useful trait to characterize WUEi variability (positive correlation) across multiple plant species (Flexas et al. 2014). Acclimation of WUEi and WUE13C was coupled to Vcmax and Jmax across Arabidopsis genotypes in a study by Easlon et al. (2014). Moreover, the improvement of WUEi in Brassica juncea was linked to higher carboxylation efficiency (A/Ci) under biochar treatment (Silva Gonzaga et al. 2019). Photosynthesis and therefore WUEi can be limited by Rubisco and RuBP regeneration, especially under high irradiance conditions (Galmés et al. 2014). Plants with simultaneous stimulation of RuBP regeneration and electron transport can improve their WUEi due to better photosynthetic capacity (López-Calcagno et al. 2020). Other alternatives to improve the WUE13C/bio would be decreasing photorespiration by means of higher Rubisco efficiency for CO2 (Whitney et al. 2011; Parry et al. 2013) or altering the photorespiratory CO2 release by adjusting metabolic pathways in leaves (Peterhansel and Maurino 2011). Total leaf N content shows a significant positive impact on the carboxylation capacity of plants (Wright et al. 2003; Paillassa et al. 2020). The identification of specific amino acids affecting Rubisco kinetics (Orr et al. 2016) may provide suitable targets for improving CO2 assimilation and consequently WUEi (Nadal and Flexas 2019). Further exploration of optimization of Rubisco activity can positively influence the WUE of plants without any direct trade-off with growth capacity and yield of crops.
Respiration
Carbon loss through respiration is another process that decreases WUEbio (Seibt et al. 2008; Gago et al. 2014; Tortosa et al. 2016). Plants with lower maintenance respiration rates can maintain higher WUEbio. Moreover, respiration could be considered the main factor behind the gap between WUEi and whole-plant WUEbio (Medrano et al. 2017). High respiratory losses were linked to lower WUEbio of C4Miscanthus x gigantus located in USA drylands (Maleski et al. 2019). Greater night-time respiration (i.e. high nocturnal transpiration) has been also recognized as one of the major factors behind the reduction of WUEbio under magnesium deficiency of barley (Tränkner et al. 2016). High VPD fluxes led to larger reductions in photosynthesis in comparison to respiration, which decreased the overall productivity and WUEbio of plants from a semi-arid ecosystem (Roby et al. 2020). The higher stability of mitochondria and susceptibility of chloroplasts, especially PSII, to abiotic stress can negatively influence the balance between carbon assimilation and respiration towards lower WUEi (Dahal and Vanlerberghe 2017). Root respiration explained around 40 % of WUEbio reduction in both well-irrigated and non-irrigated treatments of grapevine (Tomás et al. 2014b). Root respiration might be a major component of total plant respiration and thus an important target for further exploration for WUEbio optimization (Escalona et al. 2012). Leaf development (maturation) connected with greater respiratory losses could be seen as an additional constraint to long-term WUE13C (Zufferey 2016; Hernández-Montes et al. 2019). There is a still lack of precise quantification of day respiration or night-time respiration effect on whole-plant WUEbio and further research is needed. Nevertheless, respiration is connected with plant growth and fruit ripening. Therefore, plant breeding or genetic manipulation efforts that would aim at reducing respiration rates would probably lead to a significant reduction of growth and/or yield. Higher respiratory losses could be also linked to the upregulation of antioxidant systems and artificial reduction of respiration could be therefore defective. The inclusion of respiration for WUE calculation creates a more robust estimate, which improves the correlation with whole-plant WUEbio (Cernusak et al. 2007; Zhang et al. 2019). For example, Senbayram et al. (2015) have shown that the 9.8–48.6 % beneficiary effect of nitrogen fertilization on daytime WUEi was lost when nocturnal stomatal conductance and night-time respiration were taken into consideration. Therefore, the respiratory aspect of carbon balance should not be neglected for correct total plant WUEbio estimates.
Leaf Anatomy and Plant Crown Architecture
Leaf anatomy can affect the mesophyll diffusion conductance to CO2, carboxylation capacity and ultimately WUE in plants (Tomás et al. 2013; Carriquí et al. 2015). Increasing internal air volume might have positive effects on WUEi (Mediavilla et al. 2001), probably due to enhanced internal CO2 conductance to the site of carboxylation. Similarly, Guerfel et al. (2009) reported more efficient water use associated with thicker palisade parenchyma in olive trees. The leaves’ architecture can influence the WUEi due to variable mesophyll porosity and SD to intercellular airspace volume ratio in coniferous tree species (Trueba et al. 2022), and cell wall properties such as cell wall thickness (Tcw) might influence gm and thus WUEi (Flexas et al. 2021; Pathare et al. 2023). Mutant rice populations with higher leaf mass per area (LMA) showed improved whole-plant WUEbio under both control and water-limited conditions (Reddy et al. 2020a). In the study by Horike et al. (2021), WUEi of five shrub species covaried with LMA under drought stress. LMA differences explained WUE13C variance across rice mutants through its influence on carbon gain (Reddy et al. 2020b). A study by Medrano et al. (2009) also reported a positive correlation between WUEi and LMA in Mediterranean herbs and shrubs. Similarly, LMA was positively correlated with WUE13C among trees (Betula, Larix, Pinus) in the boreal forest (Ge et al. 2022). A thicker leaf can be associated with a thicker boundary layer, which lowers transpiratory losses and ultimately improves WUEbio (Bramley et al. 2013). The manipulation of leaf anatomy has been proposed as a potential theoretical target for improving photosynthetic capacity and WUE in plants (Tholen et al. 2012). The development of plants with thicker leaves and high internal air volume can theoretically improve their WUE.
Further macro-morphological constraint, which affects the whole-plant WUEbio, is plant crown architecture (Christina et al. 2016; Medrano et al. 2017; McNeil et al. 2023). A more complex crown architecture creates shade for inner leaves, which can reduce evaporative demand and therefore improve WUE balance. A positive effect of shading treatment on leaf-level WUEi has been observed for Actinidia chinensis (Chartzoulakis et al. 1993), A. deliciosa (Montanaro et al. 2009), Citrus aurantium (García-Sánchez et al. 2015), C. sinensis (Jifon et al. 2003; Syvertsen et al. 2003), Coffea arabica (Liu et al. 2018b) and Fragaria ×ananassa (Cordoba-Novoa et al. 2022). It is notable to say that shade leaves are optimized for low irradiance and if exposed to direct sunlight (crown damage) they can show decreased WUEi (Dai et al. 2009). Moreover, the leaves of Pinus taeda in the lower parts of the crown showed significantly higher WUEi in comparison to the upper part during the peak of the vegetation season (Blazier et al. 2004). The WUE13C derived from wood in Fagus crenata and Quercus crispula showed a positive correlation with tree height, crown depth and crown width (Osada et al. 2004). Furthermore, Glenn et al. (2015) showed that the less complex pillar form of Prunus persica had lower WUEi due to higher canopy transpiration in comparison to the common crown form. Leaf area index (LAI) as an indicator of crown density also shows a positive impact on WUEbio across various terrestrial ecosystem types (Li et al. 2018; Luo et al. 2022). The raising WUEbio of Alpine grasslands has been also linked to increasing LAI (Ma and Zhang 2022). Nevertheless, higher LAI and therefore greater total transpiration can be detrimental for arid regions where it can have a negative impact on WUEbio (Malone et al. 2016). More complex crown architecture and higher LAI can enable plants to optimize and improve their whole-plant WUE due to the shading effect and probably also due to better microclimatic conditions within the crown.
Conclusion and Future Prospects
The WUE balance of plants is multifaceted and affected at multiple levels of organization from molecular to whole-plant level. The main constraining factors identified in this review were stomatal morphology and control, minimal and nocturnal conductance, mesophyll conductance, carboxylation efficiency, respiration rates, leaf anatomy and crown architecture. The traits are usually analysed in research papers separately or in specific combinations (e.g. stomatal morphology and gas exchange). We suggest that future research should include multi-trait analyses with the aim of WUE optimization, thereby deepening our understanding of the coupling and decoupling of carbon uptake and water-use traits. The technological progress of phenotyping platforms can lead to more robust experimental designs that could handle multi-trait analysis. The night-time transpiration and respiration seem to be under-developed major aspects of long-term WUE optimization, which could be further investigated. The effect of leaf hydraulic conductance and canopy structure on WUE is also not very well understood and can be improved. A better understanding of morpho-physiological constraints of WUE can help us to effectively develop more drought-resilient crop and tree species.
Sources of Funding
PP was supported by the Federal Ministry of Education and Research, BMBF project BioWaWi grant number 16LW0093.
Contributions by the Authors
PP conceived the paper idea. PP, APP and MM wrote the first draft. BS and LJL supervised the process and helped with the editing of the manuscript.
Conflict of Interest Statement
None declared.
Supporting Information
The following additional information is available in the online version of this article –
Plants, Ecosystems & Climate. Chief Editor: Mary Heskel
Contributor Information
Peter Petrík, Karlsruhe Institute of Technology (KIT), Institute of Meteorology and Climate Research-Atmospheric Environmental Research (IMK-IFU), Kreuzeckbahnstraße 19, 82467 Garmisch-Partenkirchen, Germany.
Anja Petek-Petrik, Institute of Botany, Czech Academy of Sciences, Lidická 971, 602 00 Brno, Czech Republic.
Mohammad Mukarram, Department of Phytology, Faculty of Forestry, Technical University in Zvolen, T.G. Masaryka 24, 960 01 Zvolen, Slovakia.
Bernhard Schuldt, Chair of Forest Botany, Institute of Forest Botany and Forest Zoology, Technical University of Dresden (TUD), Pienner Str. 7, 01737 Tharandt, Germany.
Laurent J Lamarque, Département des Sciences de l’environnement, Université du Québec à Trois-Rivières, Trois-Rivières, QC G8Z 4M3, Canada.
Data Availability
No original data was used in this commentary. The discussion and synthesis are based on already published studies.
References
- Ahmadi B, Ahmadalipour A, Tootle G, Moradkhani H.. 2019. Remote sensing of water use efficiency and terrestrial drought recovery across the contiguous United States. Remote Sensing 11:731. [Google Scholar]
- Amitrano C, Arena C, Rouphael Y, De Pascale S, De Micco V.. 2019. Vapour pressure deficit: the hidden driver behind plant morphofunctional traits in controlled environments. Annals of Applied Biology 175:313–325. [Google Scholar]
- Amitrano C, Rouphael Y, Pannico A, De Pascale S, De Micco V.. 2021. Reducing the evaporative demand improves photosynthesis and water use efficiency of indoor cultivated lettuce. Agronomy 11:1396. [Google Scholar]
- Andrade MT, Oliveira LA, Pereira TS, Cardoso AA, Batista-Silva W, DaMatta FM, Zsögön A, Martins SCV.. 2022. Impaired auxin signaling increases vein and stomatal density but reduces hydraulic efficiency and ultimately net photosynthesis. Journal of Experimental Botany 73:4147–4156. [DOI] [PubMed] [Google Scholar]
- Anfodillo T, Di Bisceglie DP, Urso T.. 2002. Minimum cuticular conductance and cuticle features of Picea abies and Pinus cembra needles along an altitudinal gradient in the Dolomites (NE Italian Alps). Tree Physiology 22:479–487. [DOI] [PubMed] [Google Scholar]
- Ávila-Lovera E, Haro R, Ezcurra E, Santiago LS.. 2019. Costs and benefits of photosynthetic stems in desert species from southern California. Functional Plant Biology 46:175–186. [DOI] [PubMed] [Google Scholar]
- Bacon MA. 2004. Water use efficiency in plant biology. Boca Raton, FL: Blackwell CRC Press. [Google Scholar]
- Baillie AL, Fleming AJ.. 2020. The developmental relationship between stomata and mesophyll airspace. New Phytologist 225:1120–1126. [DOI] [PubMed] [Google Scholar]
- Barbour MM, Kaiser BN.. 2016. The response of mesophyll conductance to nitrogen and water availability differs between wheat genotypes. Plant Science 251:119–127. [DOI] [PubMed] [Google Scholar]
- Barrera-Ayala D, Tapia G, Ferrio JP.. 2023. Leaf carbon and water isotopes correlate with leaf hydraulic traits in three Solanum species (S. peruvianum, S. lycopersicum and S. chilense). Agriculture 13:525. [Google Scholar]
- Bertolino LT, Caine RS, Gray JE.. 2019. Impact of stomatal density and morphology on water-use efficiency in a changing world. Frontiers in Plant Science 10:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara GB, Lasky JR, Razzaque S, Zhang L, Haque T, Bonnette JE, Civelek GZ, Verslues PE, Juenger TE.. 2022. Natural variation identifies new effectors of water-use efficiency in Arabidopsis. Proceedings of the National Academy of Sciences 119:e2205305119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman CJ, Li X, Choat B, Rymer PD, De Kauwe MG, Duursma RA, Tissue DT, Medlyn BE.. 2019. Desiccation time during drought is highly predictable across species of Eucalyptus from contrasting climates. New Phytologist 224:632–643. [DOI] [PubMed] [Google Scholar]
- Blazier MA, Hennessey TC, Lynch TB, Wittwer RF, Payton ME.. 2004. Productivity, crown architecture, and gas exchange of North Carolina and Oklahoma/Arkansas loblolly pine families growing on a droughty site in southeastern Oklahoma. Forest Ecology and Management 194:83–94. [Google Scholar]
- Bogeat-Triboulot MB, Buré C, Gerardin T, Chuste PA, Le Thiec D, Hummel I, Durand M, Wildhagen H, Douthe C, Molins A, et al. 2019. Additive effects of high growth rate and low transpiration rate drive differences in whole plant transpiration efficiency among black poplar genotypes. Environmental and Experimental Botany 166:103784. [Google Scholar]
- Bramley H, Turner NC, Siddique KHM.. 2013. Water use efficiency. In: Kole C, ed. Genomics and breeding for climate-resilient crops. Berlin, Heidelberg: Springer Berlin Heidelberg, 225–268. [Google Scholar]
- Brendel O. 2021. The relationship between plant growth and water consumption: a history from the classical four elements to modern stable isotopes. Annals of Forest Science 78:47. [Google Scholar]
- Brendel O, Epron D.. 2022. Are differences among forest tree populations in carbon isotope composition an indication of adaptation to drought? Tree Physiology 42:26–31. [DOI] [PubMed] [Google Scholar]
- Briggs LJ, Shantz HL. 1913. The water requirement of plants. In: Bureau of plant industry bulletin. Washington, DC: US Department of Agriculture; 282–285. [Google Scholar]
- Buckley TN. 2005. The control of stomata by water balance. New Phytologist 168:275–292. [DOI] [PubMed] [Google Scholar]
- Buckley TN. 2019. How do stomata respond to water status? New Phytologist 224:21–36. [DOI] [PubMed] [Google Scholar]
- Bueno A, Sancho-Knapik D, Gil-Pelegrín E, Leide J, Peguero-Pina JJ, Burghardt M, Riederer M.. 2020. Cuticular wax coverage and its transpiration barrier properties in Quercus coccifera L. leaves: does the environment matter? Tree Physiology 40:827–840. [DOI] [PubMed] [Google Scholar]
- Caine RS, Yin X, Sloan J, Harrison EL, Mohammed U, Fulton T, Biswal AK, Dionora J, Chater CC, Coe RA, et al. 2019. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytologist 221:371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caird MA, Richards JH, Donovan LA.. 2007. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiology 143:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignato A, Vázquez-Piqué J, Tapias R, Ruiz F, Fernández M.. 2019. Variability and plasticity in cuticular transpiration and leaf permeability allow differentiation of eucalyptus clones at an early age. Forests 11:9. [Google Scholar]
- Carmo-Silva E, Scales JC, Madgwick PJ, Parry MAJ.. 2015. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant, Cell & Environment 38:1817–1832. [DOI] [PubMed] [Google Scholar]
- Carriquí M, Cabrera HM, Conesa MA, Coopman RE, Douthe C, Gago J, Gallé A, Galmés J, Ribas-Carbo M, Tomás M, et al. 2015. Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study: photosynthetic comparison in ferns and angiosperms. Plant, Cell & Environment 38:448–460. [DOI] [PubMed] [Google Scholar]
- Cernusak LA. 2020. Gas exchange and water‐use efficiency in plant canopies. Plant Biology 22:52–67. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Aranda J, Marshall JD, Winter K.. 2007. Large variation in whole-plant water-use efficiency among tropical tree species. New Phytologist 173:294–305. [DOI] [PubMed] [Google Scholar]
- Chartzoulakis K, Therios I, Noitsakis B.. 1993. Effects of shading on gas exchange, specific leaf weight and chlorophyll content in four kiwifruit cultivars under field conditions. Journal of Horticultural Science 68:605–611. [Google Scholar]
- Chen L, Luo W, Huang J, Peng S, Xiong D.. 2021. Leaf photosynthetic plasticity does not predict biomass responses to growth irradiance in rice. Physiologia Plantarum 173:2155–2165. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Sun Y, Kopp K, Oki L, Jones SB, Hipps L.. 2022. Effects of water availability on leaf trichome density and plant growth and development of Shepherdia × utahensis. Frontiers in Plant Science 13:855858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christina M, Nouvellon Y, Laclau JP, Stape JL, Campoe OC, le Maire G.. 2016. Sensitivity and uncertainty analysis of the carbon and water fluxes at the tree scale in Eucalyptus plantations using a metamodeling approach. Canadian Journal of Forest Research 46:297–309. [Google Scholar]
- Clarke JM, Richards RA, Condon AG.. 1991. Effect of drought stress on residual transpiration and its relationship with water use of wheat. Canadian Journal of Plant Science 71:695–702. [Google Scholar]
- Clarke VC, Danila FR, von Caemmerer S.. 2021. CO2 diffusion in tobacco: a link between mesophyll conductance and leaf anatomy. Interface Focus 11:20200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente‐Moreno MJ, Gago J, Díaz‐Vivancos P, Bernal A, Miedes E, Bresta P, Liakopoulos G, Fernie AR, Hernández JA, Flexas J.. 2019. The apoplastic antioxidant system and altered cell wall dynamics influence mesophyll conductance and the rate of photosynthesis. The Plant Journal 99:1031–1046. [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD.. 2004. Breeding for high water-use efficiency. Journal of Experimental Botany 55:2447–2460. [DOI] [PubMed] [Google Scholar]
- Corcuera L, Gil-Pelegrin E, Notivol E.. 2012. Differences in hydraulic architecture between mesic and xeric Pinus pinaster populations at the seedling stage. Tree Physiology 32:1442–1457. [DOI] [PubMed] [Google Scholar]
- Cordoba-Novoa HA, Pérez-Trujillo MM, Cruz Rincón BE, Flórez-Velasco N, Magnitskiy S, Moreno Fonseca LP.. 2022. Shading reduces water deficits in strawberry (Fragaria X Ananassa) plants during vegetative growth. International Journal of Fruit Science 22:725–740. [Google Scholar]
- Coupel-Ledru A, Lebon E, Christophe A, Gallo A, Gago P, Pantin F, Doligez A, Simonneau T.. 2016. Reduced nighttime transpiration is a relevant breeding target for high water-use efficiency in grapevine. Proceedings of the National Academy of Sciences 113:8963–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupel-Ledru A. 2021. Plant water-use efficiency. In: John Wiley & Sons, Ltd., ed. eLS. Wiley, 1–8. [Google Scholar]
- Dahal K, Vanlerberghe GC.. 2017. Alternative oxidase respiration maintains both mitochondrial and chloroplast function during drought. New Phytologist 213:560–571. [DOI] [PubMed] [Google Scholar]
- Dai Y, Shen Z, Liu Y, Wang L, Hannaway D, Lu H.. 2009. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environmental and Experimental Botany 65:177–182. [Google Scholar]
- Davies WJ, Wilkinson S, Loveys B.. 2002. Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytologist 153:449–460. [DOI] [PubMed] [Google Scholar]
- Dawson TE, Burgess SSO, Tu KP, Oliveira RS, Santiago LS, Fisher JB, Simonin KA, Ambrose AR.. 2007. Nighttime transpiration in woody plants from contrasting ecosystems. Tree Physiology 27:561–575. [DOI] [PubMed] [Google Scholar]
- Dayer S, Herrera JC, Dai Z, Burlett R, Lamarque LJ, Delzon S, Bortolami G, Cochard H, Gambetta GA.. 2020. The sequence and thresholds of leaf hydraulic traits underlying grapevine varietal differences in drought tolerance. Journal of Experimental Botany 71:4333–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer S, Herrera JC, Dai Z, Burlett R, Lamarque LJ, Delzon S, Bortolami G, Cochard H, Gambetta GA.. 2021. Nighttime transpiration represents a negligible part of water loss and does not increase the risk of water stress in grapevine. Plant, Cell & Environment 44:387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais DL, Auchincloss LC, Sukamtoh E, McKay JK, Logan T, Richards JH, Juenger TE.. 2014. Variation in MPK12 affects water use efficiency in Arabidopsis and reveals a pleiotropic link between guard cell size and ABA response. Proceedings of the National Academy of Sciences 111:2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra FA, Carrillo Y, Aspinwall MJ, Maier C, Canarini A, Tahaei H, Choat B, Tissue DT.. 2016. Water, nitrogen and phosphorus use efficiencies of four tree species in response to variable water and nutrient supply. Plant and Soil 406:187–199. [Google Scholar]
- Dow GJ, Berry JA, Bergmann DC.. 2017. Disruption of stomatal lineage signaling or transcriptional regulators has differential effects on mesophyll development, but maintains coordination of gas exchange. New Phytologist 216:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake PL, Froend RH, Franks PJ.. 2013. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. Journal of Experimental Botany 64:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey R, Pandey BK, Sawant SV, Shirke PA.. 2023. Drought stress inhibits stomatal development to improve water use efficiency in cotton. Acta Physiologiae Plantarum 45:30. [Google Scholar]
- Dunn J, Hunt L, Afsharinafar M, Meselmani MA, Mitchell A, Howells R, Wallington E, Fleming AJ, Gray JE.. 2019. Reduced stomatal density in bread wheat leads to increased water-use efficiency. Journal of Experimental Botany 70:4737–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand M, Brendel O, Buré C, Le Thiec D.. 2019. Altered stomatal dynamics induced by changes in irradiance and vapour‐pressure deficit under drought: impacts on the whole‐plant transpiration efficiency of poplar genotypes. New Phytologist 222:1789–1802. [DOI] [PubMed] [Google Scholar]
- Duursma RA, Blackman CJ, Lopéz R, Martin‐StPaul NK, Cochard H, Medlyn BE.. 2019. On the minimum leaf conductance: its role in models of plant water use, and ecological and environmental controls. New Phytologist 221:693–705. [DOI] [PubMed] [Google Scholar]
- Easlon HM, Nemali KS, Richards JH, Hanson DT, Juenger TE, McKay JK.. 2014. The physiological basis for genetic variation in water use efficiency and carbon isotope composition in Arabidopsis thaliana. Photosynthesis Research 119:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth PV, Ellsworth PZ, Koteyeva NK, Cousins AB.. 2018. Cell wall properties in Oryza sativa influence mesophyll CO2 conductance. New Phytologist 219:66–76. [DOI] [PubMed] [Google Scholar]
- Escalona JM, Tomàs M, Martorell S, Medrano H, Ribas-Carbo M, Flexas J.. 2012. Carbon balance in grapevines under different soil water supply: importance of whole plant respiration. Australian Journal of Grape and Wine Research 18:308–318. [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I.. 2009. Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany 60:2235–2248. [DOI] [PubMed] [Google Scholar]
- Even M, Sabo M, Meng D, Kreszies T, Schreiber L, Fricke W.. 2019. Night-time transpiration in barley (Hordeum vulgare) facilitates respiratory carbon dioxide release and is regulated during salt stress. Annals of Botany 123:223–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Hubick KT, Condon AG, Richards RA.. 1989. Carbon isotope fractionation and plant water-use efficiency. In: Rundel PW, Ehleringer JR, Nagy KA, eds. Ecological studies. Stable isotopes in ecological research. New York, NY: Springer New York, 21–40. [Google Scholar]
- Fichot R, Laurans F, Monclus R, Moreau A, Pilate G, Brignolas F.. 2009. Xylem anatomy correlates with gas exchange, water-use efficiency and growth performance under contrasting water regimes: evidence from Populus deltoides x Populus nigra hybrids. Tree Physiology 29:1537–1549. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R.. 2006. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2in vivo. The Plant Journal 48:427–439. [DOI] [PubMed] [Google Scholar]
- Flexas J, Niinemets U, Gallé A, Barbour MM, Centritto M, Diaz-Espejo A, Douthe C, Galmés J, Ribas-Carbo M, Rodriguez PL, et al. 2013a. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynthesis Research 117:45–59. [DOI] [PubMed] [Google Scholar]
- Flexas J, Scoffoni C, Gago J, Sack L.. 2013b. Leaf mesophyll conductance and leaf hydraulic conductance: an introduction to their measurement and coordination. Journal of Experimental Botany 64:3965–3981. [DOI] [PubMed] [Google Scholar]
- Flexas J, Carriquí M, Coopman RE, Gago J, Galmés J, Martorell S, Morales F, Diaz-Espejo A.. 2014. Stomatal and mesophyll conductances to CO2 in different plant groups: underrated factors for predicting leaf photosynthesis responses to climate change? Plant Science 226:41–48. [DOI] [PubMed] [Google Scholar]
- Flexas J, Díaz-Espejo A, Conesa MA, Coopman RE, Douthe C, Gago J, Gallé A, Galmés J, Medrano H, Ribas-Carbo M, et al. 2016. Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant, Cell & Environment 39:965–982. [DOI] [PubMed] [Google Scholar]
- Flexas J, Clemente-Moreno MJ, Bota J, Brodribb TJ, Gago J, Mizokami Y, Nadal M, Perera-Castro AV, Roig-Oliver M, Sugiura D, et al. 2021. Cell wall thickness and composition are involved in photosynthetic limitation. Journal of Experimental Botany 72:3971–3986. [DOI] [PubMed] [Google Scholar]
- Forster MA. 2014. How significant is nocturnal sap flow? Tree Physiology 34:757–765. [DOI] [PubMed] [Google Scholar]
- Franco‐Navarro JD, Rosales MA, Cubero‐Font P, Calvo P, Álvarez R, Diaz‐Espejo A, Colmenero‐Flores JM.. 2019. Chloride as macronutrient increases water use efficiency by anatomically‐driven reduced stomatal conductance and increased mesophyll diffusion to CO2. The Plant Journal 99:tpj.14423. [DOI] [PubMed] [Google Scholar]
- Frank DC, Poulter B, Saurer M, Esper J, Huntingford C, Helle G, Treydte K, Zimmermann NE, Schleser GH, Ahlström A, et al. 2015. Water-use efficiency and transpiration across European forests during the Anthropocene. Nature Climate Change 5:579–583. [Google Scholar]
- Franks PJ, Beerling DJ.. 2009. CO2 -forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology 7:227–236. [DOI] [PubMed] [Google Scholar]
- Franks PJ, W. Doheny‐Adams T, Britton‐Harper ZJ, Gray JE.. 2015. Increasing water‐use efficiency directly through genetic manipulation of stomatal density. New Phytologist 207:188–195. [DOI] [PubMed] [Google Scholar]
- Fullana-Pericàs M, Conesa MA, Soler S, Ribas-Carbó M, Granell A, Galmés J.. 2017. Variations of leaf morphology, photosynthetic traits and water-use efficiency in Western-Mediterranean tomato landraces. Photosynthetica 55:121–133. [Google Scholar]
- Gago J, Douthe C, Florez-Sarasa I, Escalona JM, Galmes J, Fernie AR, Flexas J, Medrano H.. 2014. Opportunities for improving leaf water use efficiency under climate change conditions. Plant Science 226:108–119. [DOI] [PubMed] [Google Scholar]
- Galdon-Armero J, Fullana-Pericas M, Mulet PA, Conesa MA, Martin C, Galmes J.. 2018. The ratio of trichomes to stomata is associated with water use efficiency in Solanum lycopersicum (tomato). The Plant Journal 96:607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J, Conesa MA, Ochogavía JM, Perdomo JA, Francis DM, Ribas-Carbó M, Savé R, Flexas J, Medrano H, Cifre J.. 2011. Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum. Plant, Cell & Environment 34:245–260. [DOI] [PubMed] [Google Scholar]
- Galmés J, Conesa MA, Díaz-Espejo A, Mir A, Perdomo JA, Niinemets U, Flexas J.. 2014. Rubisco catalytic properties optimized for present and future climatic conditions. Plant Science 226:61–70. [DOI] [PubMed] [Google Scholar]
- García-Sánchez F, Simón I, Lidón V, Manera FJ, Simón-Grao S, Pérez-Pérez JG, Gimeno V.. 2015. Shade screen increases the vegetative growth but not the production in ‘Fino 49’ lemon trees grafted on Citrus macrophylla and Citrus aurantium L. Scientia Horticulturae 194:175–180. [Google Scholar]
- Gašparovič K, Živčák M, Brestič M, Hauptvogel P.. 2021. Diversity of leaf cuticular transpiration and growth traits in field-grown wheat and aegilops genetic resources. Agronomy 11:522. [Google Scholar]
- Ge Z, Man X, Cai T, Duan B, Xiao R, Xu Z.. 2022. Environmental factors at different canopy heights had significant effects on leaf water-use efficiency in cold-temperate larch forest. Sustainability 14:5126. [Google Scholar]
- Gharun M, Klesse S, Tomlinson G, Waldner P, Stocker B, Rihm B, Siegwolf R, Buchmann N.. 2021. Effect of nitrogen deposition on centennial forest water-use efficiency. Environmental Research Letters 16:114036. [Google Scholar]
- Gillon JS, Yakir D.. 2000. Internal conductance to CO2 Diffusion and C18OO discrimination in C3 leaves. Plant Physiology 123:201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani R, Koteyeva N, Voznesenskaya E, Evans MA, Cousins AB, Edwards GE.. 2013. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (Genus Oryza). Plant Physiology 162:1632–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn DM, Bassett CB, Tworkoski T, Scorza R, Miller SS.. 2015. Tree architecture of pillar and standard peach affect canopy transpiration and water use efficiency. Scientia Horticulturae 187:30–34. [Google Scholar]
- Glowacka K, Kromdijk J, Kucera K, Xie J, Cavanagh AP, Leonelli L, Leakey ADB, Ort DR, Niyogi KK, Long SP.. 2018. Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop. Nature Communications 9:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Ocampo G, Ploschuk EL, Mantese A, Crocco CD, Botto JF.. 2021. BBX21 reduces abscisic acid sensitivity, mesophyll conductance and chloroplast electron transport capacity to increase photosynthesis and water use efficiency in potato plants cultivated under moderated drought. The Plant Journal 108:1131–1144. [DOI] [PubMed] [Google Scholar]
- Gosa SC, Lupo Y, Moshelion M.. 2019. Quantitative and comparative analysis of whole-plant performance for functional physiological traits phenotyping: new tools to support pre-breeding and plant stress physiology studies. Plant Science 282:49–59. [DOI] [PubMed] [Google Scholar]
- Grossiord C, Buckley TN, Cernusak LA, Novick KA, Poulter B, Siegwolf RTW, Sperry JS, McDowell NG.. 2020. Plant responses to rising vapor pressure deficit. New Phytologist 226:1550–1566. [DOI] [PubMed] [Google Scholar]
- Guerfel M, Baccouri O, Boujnah D, Chaïbi W, Zarrouk M.. 2009. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Scientia Horticulturae 119:257–263. [Google Scholar]
- Guo X, Wang Y, Zhao P, Xu P, Yu G-H, Zhang L-Y, Xiong Y, Xiang C-B.. 2019a. AtEDT1/HDG11 regulates stomatal density and water-use efficiency via ERECTA and E2Fa. New Phytologist 223:1478–1488. [DOI] [PubMed] [Google Scholar]
- Guo T, Wang N, Xue Y, Guan Q, van Nocker S, Liu C, Ma F.. 2019b. Overexpression of the RNA binding protein MhYTP1 in transgenic apple enhances drought tolerance and WUE by improving ABA level under drought condition. Plant Science 280:397–407. [DOI] [PubMed] [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M.. 2004. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant and Cell Physiology 45:521–529. [DOI] [PubMed] [Google Scholar]
- Hartmann H, Link RM, Schuldt B.. 2021. A whole-plant perspective of isohydry: stem-level support for leaf-level plant water regulation. Tree Physiology 41:901–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield JL, Dold C.. 2019. Water-use efficiency: advances and challenges in a changing climate. Frontiers in Plant Science 10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield JL, Sauer TJ, Prueger JH.. 2001. Managing soils to achieve greater water use efficiency: a review. Agronomy Journal 93:271–280. [Google Scholar]
- Hernández-Montes E, Tomás M, Escalona JM, Bota J, Medrano H.. 2019. Leaf growth rate and nitrogen content determine respiratory costs during leaf expansion in grapevines. Physiologia Plantarum 165:746–754. [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI.. 2003. The role of stomata in sensing and driving environmental change. Nature 424:901–908. [DOI] [PubMed] [Google Scholar]
- Horike H, Kinoshita T, Kume A, Hanba YT.. 2021. Responses of leaf photosynthetic traits, water use efficiency, and water relations in five urban shrub tree species under drought stress and recovery. Trees 37:53–67. [Google Scholar]
- Husaini AM. 2022. High-value pleiotropic genes for developing multiple stress-tolerant biofortified crops for 21st-century challenges. Heredity 128:460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isasa E, Link RM, Jansen S, Tezeh FR, Kaack L, Sarmento Cabral J, Schuldt B.. 2023. Addressing controversies in the xylem embolism resistance–vessel diameter relationship. New Phytologist 238:283–296. [DOI] [PubMed] [Google Scholar]
- Jeffree CE. 2006. The fine structure of the plant cuticle. In: Riederer M, Mller C, eds. Biology of the plant cuticle. Oxford, UK: Blackwell Publishing Ltd.; 11–125. [Google Scholar]
- Jifon JL, Syvertsen JP.. 2003. Moderate shade can increase net gas exchange and reduce photoinhibition in citrus leaves. Tree Physiology 23:119–127. [DOI] [PubMed] [Google Scholar]
- Jin Y, Wang C, Zhou Z, Li Z.. 2016. Co-ordinated performance of leaf hydraulics and economics in 10 Chinese temperate tree species. Functional Plant Biology 43:1082–1090. [DOI] [PubMed] [Google Scholar]
- Kang J, Hao X, Zhou H, Ding R.. 2021. An integrated strategy for improving water use efficiency by understanding physiological mechanisms of crops responding to water deficit: present and prospect. Agricultural Water Management 255:107008. [Google Scholar]
- Kardiman R, Ræbild A.. 2018. Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiology 38:696–705. [DOI] [PubMed] [Google Scholar]
- Lamarque LJ, Delzon S, Toups H, Gravel A-I, Corso D, Badel E, Burlett R, Charrier G, Cochard H, Jansen S, et al. 2020. Over-accumulation of abscisic acid in transgenic tomato plants increases the risk of hydraulic failure. Plant, Cell & Environment 43:548–562. [DOI] [PubMed] [Google Scholar]
- Lawson T, Blatt MR.. 2014. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiology 164:1556–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, von Caemmerer S, Baroli I.. 2010. Photosynthesis and stomatal behaviour. Progress in Botany 72. Berlin, Heidelberg: Springer Berlin Heidelberg, 265–304. [Google Scholar]
- Lawson T, Kramer DM, Raines CA.. 2012. Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Current Opinion in Biotechnology 23:215–220. [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Ferguson JN, Pignon CP, Wu A, Jin Z, Hammer GL, Lobell DB.. 2019. Water use efficiency as a constraint and target for improving the resilience and productivity of C3 and C4 crops. Annual Review of Plant Biology 70:781–808. [DOI] [PubMed] [Google Scholar]
- Lei Z, He Y, Li X, He Z, Zhang Y, Zhang W, Liu F, Zhang Y.. 2023. Domestication has reduced leaf water use efficiency associated with the anatomy of abaxial stomata in cotton. Journal of Experimental Botany 74:878–888. [DOI] [PubMed] [Google Scholar]
- Li C, Yin C, Liu S.. 2004. Different responses of two contrasting Populus davidiana populations to exogenous abscisic acid application. Environmental and Experimental Botany 51:237–246. [Google Scholar]
- Li Y, Li H, Li Y, Zhang S. 2017. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. The Crop Journal 5:231–239. [Google Scholar]
- Li Y, Shi H, Zhou L, Eamus D, Huete A, Li L, Cleverly J, Hu Z, Harahap M, Yu Q, et al. 2018. Disentangling climate and Lai effects on seasonal variability in water use efficiency across terrestrial ecosystems in China. Journal of Geophysical Research, Biogeosciences 123:2429–2443. [Google Scholar]
- Li S, Zhang J, Liu L, Wang Z, Li Y, Guo L, Li Y, Zhang X, Ren S, Zhao B, et al. 2020. SlTLFP8 reduces water loss to improve water-use efficiency by modulating cell size and stomatal density via endoreduplication. Plant, Cell & Environment 43:2666–2679. [DOI] [PubMed] [Google Scholar]
- Li M, Dong H, Li J, Dai X, Lin J, Li S, Zhou C, Chiang VL, Li W.. 2023. PtrVCS2 regulates drought resistance by changing vessel morphology and stomatal closure in Populus trichocarpa. International Journal of Molecular Sciences 24:4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintunen A, Preisler Y, Oz I, Yakir D, Vesala T, Hölttä T.. 2021. Bark transpiration rates can reach needle transpiration rates under dry conditions in a semi-arid forest. Frontiers in Plant Science 12:790684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, He N, Zhang J, Li Y, Wang Q, Sack L, Yu G.. 2018a. Variation of stomatal traits from cold temperate to tropical forests and association with water use efficiency. Functional Ecology 32:20–28. [Google Scholar]
- Liu X, Qi Y, Li F, Yang Q, Yu L.. 2018b. Impacts of regulated deficit irrigation on yield, quality and water use efficiency of Arabica coffee under different shading levels in dry and hot regions of southwest China. Agricultural Water Management 204:292–300. [Google Scholar]
- Liu Q, Wang Z, Yu S, Li W, Zhang M, Yang J, Li D, Yang J, Li C.. 2021. Pu-miR172d regulates stomatal density and water-use efficiency via targeting PuGTL1 in poplar. Journal of Experimental Botany 72:1370–1383. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang C, Meng Y, Zhang F, Huang N, Wang J, Li Y.. 2023. Hydraulic and economical traits in short- and long-shoot leaves of Ginkgo biloba males and females. Forests 14:535. [Google Scholar]
- López-Calcagno PE, Brown KL, Simkin AJ, Fisk SJ, Vialet-Chabrand S, Lawson T, Raines CA.. 2020. Stimulating photosynthetic processes increases productivity and water-use efficiency in the field. Nature Plants 6:1054–1063. [DOI] [PubMed] [Google Scholar]
- Lundgren MR, Fleming AJ.. 2020. Cellular perspectives for improving mesophyll conductance. The Plant Journal 101:845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Bie X, Yi G, Zhou X, Zhang T, Li J, Lai P.. 2022. dominant impacting factors on water-use efficiency variation in inner Mongolia from 2001 to 2018: vegetation or climate? Remote Sensing 14:4541. [Google Scholar]
- Ma N, Zhang Y.. 2022. Contrasting trends in water use efficiency of the alpine grassland in Tibetan plateau. Journal of Geophysical Research: Atmospheres 127:1–19. [Google Scholar]
- Ma WT, Yu YZ, Wang X, Gong XY.. 2023. Estimation of intrinsic water-use efficiency from δ13C signature of C3 leaves: assumptions and uncertainty. Frontiers in Plant Science 13:1037972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado R, Loram‐Lourenço L, Farnese FS, Alves RDFB, de Sousa LF, Silva FG, Filho SCV, Torres-Ruiz JM, Cochard H, Menezes-Silva PE.. 2021. Where do leaf water leaks come from? Trade-offs underlying the variability in minimum conductance across tropical savanna species with contrasting growth strategies. New Phytologist 229:1415–1430. [DOI] [PubMed] [Google Scholar]
- Maleski JJ, Bosch DD, Anderson RG, Coffin AW, Anderson WF, Strickland TC.. 2019. Evaluation of miscanthus productivity and water use efficiency in southeastern United States. Science of the Total Environment 692:1125–1134. [DOI] [PubMed] [Google Scholar]
- Malone SL, Tulbure MG, Pérez‐Luque AJ, Assal TJ, Bremer LL, Drucker DP, Hillis V, Varela S, Goulden ML.. 2016. Drought resistance across California ecosystems: evaluating changes in carbon dynamics using satellite imagery. Ecosphere 7:1–19. [Google Scholar]
- Márquez DA, Stuart‐Williams H, Cernusak LA, Farquhar GD.. 2023. Assessing the CO2 concentration at the surface of photosynthetic mesophyll cells. New Phytologist 238:1446–1460. [DOI] [PubMed] [Google Scholar]
- Masutomi Y, Kinose Y, Takimoto T, Yonekura T, Oue H, Kobayashi K.. 2019. Ozone changes the linear relationship between photosynthesis and stomatal conductance and decreases water use efficiency in rice. Science of the Total Environment 655:1009–1016. [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ.. 2016. Linking turgor with ABA biosynthesis: implications for stomatal responses to vapor pressure deficit across land plants. Plant Physiology 171:2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Davey PA, Kanwal N, Baker NR, Lawson T.. 2013. A novel system for spatial and temporal imaging of intrinsic plant water use efficiency. Journal of Experimental Botany 64:4993–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Vialet‐Chabrand S, Davey P, Baker NR, Brendel O, Lawson T.. 2016. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytologist 211:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil BE, Fahey RT, King CJ, Erazo DA, Heimerl TZ, Elmore AJ.. 2023. Tree crown economics. Frontiers in Ecology and the Environment 21:40–48. [Google Scholar]
- Mediavilla S, Escudero A, Heilmeier H.. 2001. Internal leaf anatomy and photosynthetic resource-use efficiency: interspecific and intraspecific comparisons. Tree Physiology 21:251–259. [DOI] [PubMed] [Google Scholar]
- Medrano H, Flexas J, Galmés J.. 2009. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant and Soil 317:17–29. [Google Scholar]
- Medrano H, Escalona JM, Flexas J, Martorell S, Tomás M.. 2017. From leaf to plant water use efficiency: solving the gaps for a whole plant evaluation. Acta Horticulturae 1157:167–176. [Google Scholar]
- Messinger SM, Buckley TN, Mott KA.. 2006. Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiology 140:771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y, Yang R, Liu L, Gu X, Yang X, Wang Y, Zhang X, Li H.. 2016. Growth, photosynthesis and adaptive responses of wild and domesticated watermelon genotypes to drought stress and subsequent re-watering. Plant Growth Regulation 79:229–241. [Google Scholar]
- Momayyezi M, Guy RD.. 2017. Substantial role for carbonic anhydrase in latitudinal variation in mesophyll conductance of Populus trichocarpa Torr. & Gray: mesophyll conductance of cottonwood. Plant, Cell & Environment 40:138–149. [DOI] [PubMed] [Google Scholar]
- Montanaro G, Dichio B, Xiloyannis C.. 2009. Shade mitigates photoinhibition and enhances water use efficiency in kiwifruit under drought. Photosynthetica 47:363–371. [Google Scholar]
- Mukarram M, Choudhary S, Kurjak D, Petek A, Khan MMA.. 2021. Drought: sensing, signalling, effects and tolerance in higher plants. Physiologia Plantarum 172:1291–1300. [DOI] [PubMed] [Google Scholar]
- Nadal M, Flexas J.. 2019. Variation in photosynthetic characteristics with growth form in a water-limited scenario: implications for assimilation rates and water use efficiency in crops. Agricultural Water Management 216:457–472. [Google Scholar]
- Negin B, Moshelion M.. 2016. The evolution of the role of ABA in the regulation of water-use efficiency: from biochemical mechanisms to stomatal conductance. Plant Science 251:82–89. [DOI] [PubMed] [Google Scholar]
- Ni Y, Guo YJ, Guo YJ, Han L, Tang H, Conyers M.. 2012. Leaf cuticular waxes and physiological parameters in alfalfa leaves as influenced by drought. Photosynthetica 50:458–466. [Google Scholar]
- Nunes TDG, Slawinska MW, Lindner H, Raissig MT.. 2022. Quantitative effects of environmental variation on stomatal anatomy and gas exchange in a grass model. Quantitative Plant Biology 3:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr D, Alcântara A, Kapralov MV, Andralojc J, Carmo-Silva E, Parry MAJ.. 2016. Surveying Rubisco diversity and temperature response to improve crop photosynthetic efficiency. Plant Physiology 172:pp.00750.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada N, Tateno R, Hyodo F, Takeda H.. 2004. Changes in crown architecture with tree height in two deciduous tree species: developmental constraints or plastic response to the competition for light? Forest Ecology and Management 188:337–347. [Google Scholar]
- Ouyang W, Struik PC, Yin X, Yang J.. 2017. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. Journal of Experimental Botany 68:5191–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillassa J, Wright IJ, Prentice IC, Pepin S, Smith NG, Ethier G, Westerband AC, Lamarque LJ, Wang H, Cornwell WK, et al. 2020. When and where soil is important to modify the carbon and water economy of leaves. New Phytologist 228:121–135. [DOI] [PubMed] [Google Scholar]
- Papacek M, Christmann A, Grill E.. 2019. Increased water use efficiency and water productivity of arabidopsis by abscisic acid receptors from Populus canescens. Annals of Botany 124:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanatsiou M, Petersen J, Henderson L, Wang Y, Christie JM, Blatt MR.. 2019. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 363:1456–1459. [DOI] [PubMed] [Google Scholar]
- Parry MAJ, Andralojc PJ, Scales JC, Salvucci ME, Carmo-Silva AE, Alonso H, Whitney SM.. 2013. Rubisco activity and regulation as targets for crop improvement. Journal of Experimental Botany 64:717–730. [DOI] [PubMed] [Google Scholar]
- Pathare VS, Panahabadi R, Sonawane BV, Apalla AJ, Koteyeva N, Bartley LE, Cousins AB.. 2023. Altered cell wall hydroxycinnamate composition impacts leaf and canopy-level CO2-uptake and water use in rice. Plant Physiology: kiad428. doi: 10.1093/plphys/kiad428. [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sisó S, Flexas J, Galmés J, García-Nogales A, Niinemets U, Sancho-Knapik D, Saz MA, Gil-Pelegrín E.. 2017. Cell-level anatomical characteristics explain high mesophyll conductance and photosynthetic capacity in sclerophyllous Mediterranean oaks. New Phytologist 214:585–596. [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez JG, Dodd IC, Botía P.. 2012. Partial rootzone drying increases water-use efficiency of lemon Fino 49 trees independently of root-to-shoot ABA signalling. Functional Plant Biology 39:366. [DOI] [PubMed] [Google Scholar]
- Peterhansel C, Maurino VG.. 2011. Photorespiration redesigned. Plant Physiology 155:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik P, Petek-Petrik A, Konôpková A, Fleischer P, Stojnic S, Zavadilova I, Kurjak D.. 2022a. Seasonality of PSII thermostability and water use efficiency of in situ mountainous Norway spruce (Picea abies). Journal of Forestry Research 34:197–208. [Google Scholar]
- Petrik P, Petek‐Petrik A, Kurjak D, Mukarram M, Klein T, Gömöry D, Střelcová K, Frýdl J, Konôpková A.. 2022b. Interannual adjustments in stomatal and leaf morphological traits of European beech (Fagus sylvatica L.) demonstrate its climate change acclimation potential. Plant Biology 24:1287–1296. [DOI] [PubMed] [Google Scholar]
- Pitaloka MK, Caine RS, Hepworth C, Harrison EL, Sloan J, Chutteang C, Phunthong C, Nongngok R, Toojinda T, Ruengphayak S, et al. 2022. Induced genetic variations in stomatal density and size of rice strongly affects water use efficiency and responses to drought stresses. Frontiers in Plant Science 13:801706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-Campos GE, Moran MS, Huete A, Zhang Y, Bresloff C, Huxman TE, Eamus D, Bosch DD, Buda AR, Gunter SA, et al. 2013. Ecosystem resilience despite large-scale altered hydroclimatic conditions. Nature 494:349–352. [DOI] [PubMed] [Google Scholar]
- Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E.. 2009. Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. Journal of Experimental Botany 60:2217–2234. [DOI] [PubMed] [Google Scholar]
- Pospíšilová J, Baťková P.. 2004. Effects of pre-treatments with abscisic acid and/or benzyladenine on gas exchange of French bean, sugar beet, and maize leaves during water stress and after rehydration. Biologia plantarum 48:395–399. [Google Scholar]
- Price GD, von Caemmerer S, Evans JR, Yu J-W, Lloyd J, Oja V, Kell P, Harrison K, Gallagher A, Badger MR.. 1994. Specific reduction of chloroplast carbonic anhydrase activity by antisense RNA in transgenic tobacco plants has a minor effect on photosynthetic CO2 assimilation. Planta 193:331–340. [Google Scholar]
- Quan Q, Zhang F, Meng C, Ma F, Zhou Q, Sun F, Niu S.. 2020. Shifting biomass allocation determines community water use efficiency under climate warming. Environmental Research Letters 15:094041. [Google Scholar]
- Rabarijaona A, Ponton S, Bert D, Ducousso A, Richard B, Levillain J, Brendel O.. 2022. Provenance differences in water-use efficiency among sessile oak populations grown in a mesic common garden. Frontiers in Forests and Global Change 5:914199. [Google Scholar]
- Rao S, Tian Y, Zhang C, Qin Y, Liu M, Niu S, Li Y, Chen J.. 2023. The JASMONATE ZIM-domain–OPEN STOMATA1 cascade integrates jasmonic acid and abscisic acid signaling to regulate drought tolerance by mediating stomatal closure in poplar. Journal of Experimental Botany 74:443–457. [DOI] [PubMed] [Google Scholar]
- Reddy SH, Da Costa MVJ, Kambalimath SK, Rajanna Mavinahalli P, Muthurajan R, Chinnusamy V, Sevanthi AM, Neelamraju S, Gopala Krishnan S, Singh AK, et al. 2020a. Relative contribution of stomatal parameters in influencing WUE among rice mutants differing in leaf mass area. Plant Physiology Reports 25:483–495. [Google Scholar]
- Reddy SH, Singhal RK, DaCosta MVJ, Kambalimath SK, Rajanna MP, Muthurajan R, Sevanthi AM, Mohapatra T, Sarla N, Chinnusamy V, et al. 2020b. Leaf mass area determines water use efficiency through its influence on carbon gain in rice mutants. Physiologia Plantarum 169:194–213. [DOI] [PubMed] [Google Scholar]
- Renninger HJ, Carlo NJ, Clark KL, Schäfer KVR.. 2015. Resource use and efficiency, and stomatal responses to environmental drivers of oak and pine species in an Atlantic Coastal Plain forest. Frontiers in Plant Science 6:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resco de Dios V, Chowdhury FI, Granda E, Yao Y, Tissue DT.. 2019. Assessing the potential functions of nocturnal stomatal conductance in C3 and C4 plants. New Phytologist 223:1696–1706. [DOI] [PubMed] [Google Scholar]
- Riederer M, Schreiber L.. 2001. Protecting against water loss: analysis of the barrier properties of plant cuticles. Journal of Experimental Botany 52:2023–2032. [DOI] [PubMed] [Google Scholar]
- Roby MC, Scott RL, Moore DJP.. 2020. High vapor pressure deficit decreases the productivity and water use efficiency of rain-induced pulses in semiarid ecosystems. Journal of Geophysical Research, Biogeosciences 125:1–14.36158138 [Google Scholar]
- Roig-Oliver M, Nadal M, Clemente-Moreno MJ, Bota J, Flexas J.. 2020. Cell wall components regulate photosynthesis and leaf water relations of Vitis vinifera cv. Grenache acclimated to contrasting environmental conditions. Journal of Plant Physiology 244:153084. [DOI] [PubMed] [Google Scholar]
- Roussel M, Le Thiec D, Montpied P, Ningre N, Guehl J-M, Brendel O.. 2009. Diversity of water use efficiency among Quercus robur genotypes: contribution of related leaf traits. Annals of Forest Science 66:408–408. [Google Scholar]
- Sack L, Buckley TN.. 2016. The developmental basis of stomatal density and flux. Plant Physiology 171:2358–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago LS, Goldstein G, Meinzer FC, Fisher JB, Machado K, Woodruff D, Jones T.. 2004. Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 140:543–550. [DOI] [PubMed] [Google Scholar]
- Schuldt B, Buras A, Arend M, Vitasse Y, Beierkuhnlein C, Damm A, Gharun M, Grams TEE, Hauck M, Hajek P, et al. 2020. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic and Applied Ecology 45:86–103. [Google Scholar]
- Schuster AC, Burghardt M, Riederer M.. 2017. The ecophysiology of leaf cuticular transpiration: are cuticular water permeabilities adapted to ecological conditions? Journal of Experimental Botany 68:5271–5279. [DOI] [PubMed] [Google Scholar]
- Seibt U, Rajabi A, Griffiths H, Berry JA.. 2008. Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia 155:441–454. [DOI] [PubMed] [Google Scholar]
- Sellin A, Niglas A, Õunapuu E, Karusion A.. 2013. Impact of phloem girdling on leaf gas exchange and hydraulic conductance in hybrid aspen. Biologia plantarum 57:531–539. [Google Scholar]
- Sellin A, Niglas A, Õunapuu-Pikas E, Kupper P.. 2014. Rapid and long-term effects of water deficit on gas exchange and hydraulic conductance of silver birch trees grown under varying atmospheric humidity. BMC Plant Biology 14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senbayram M, Tränkner M, Dittert K, Brück H.. 2015. Daytime leaf water use efficiency does not explain the relationship between plant N status and biomass water‐use efficiency of tobacco under non‐limiting water supply. Journal of Plant Nutrition and Soil Science 178:682–692. [Google Scholar]
- Sevanto S. 2020. Why do plants have waxy leaves? Do we know after all? Tree Physiology 40:823–826. [DOI] [PubMed] [Google Scholar]
- Shepherd T, Wynne Griffiths D.. 2006. The effects of stress on plant cuticular waxes. New Phytologist 171:469–499. [DOI] [PubMed] [Google Scholar]
- Silva Gonzaga MI, Oliveira da Silva PS, Carlos de Jesus Santos J, Ganassali de Oliveira Junior LF.. 2019. Biochar increases plant water use efficiency and biomass production while reducing Cu concentration in Brassica juncea L. in a Cu-contaminated soil. Ecotoxicology and Environmental Safety 183:109557. [DOI] [PubMed] [Google Scholar]
- Song W, Loik ME, Cui H, Fan M, Sun W.. 2022. Effect of nitrogen addition on leaf photosynthesis and water use efficiency of the dominant species Leymus chinensis (Trin.) Tzvelev in a semi-arid meadow steppe. Plant Growth Regulation 98:91–102. [Google Scholar]
- Suárez JC, Urban MO, Contreras AT, Noriega JE, Deva C, Beebe SE, Polanía JA, Casanoves F, Rao IM.. 2021. Water use, leaf cooling and carbon assimilation efficiency of heat resistant common beans evaluated in Western Amazonia. Frontiers in Plant Science 12:644010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvertsen JP, Goni C, Otero A.. 2003. Fruit load and canopy shading affect leaf characteristics and net gas exchange of ‘Spring’ navel orange trees. Tree Physiology 23:899–906. [DOI] [PubMed] [Google Scholar]
- Szatniewska J, Zavadilova I, Nezval O, Krejza J, Petrik P, Čater M, Stojanović M.. 2022. Species-specific growth and transpiration response to changing environmental conditions in floodplain forest. Forest Ecology and Management 516:120248. [Google Scholar]
- Tai-Chung WU, Bai-Ling LIN, Wen-Yuan KAO.. 2020. Active stomatal control of Marsilea crenata, an amphibious fern, in response to CO2 and exogenous application of ABA. TAIWANIA 65:431–437. [Google Scholar]
- Tanaka Y, Sugano SS, Shimada T, Hara-Nishimura I.. 2013. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytologist 198:757–764. [DOI] [PubMed] [Google Scholar]
- Tardieu F. 2022. Different avenues for progress apply to drought tolerance, water use efficiency and yield in dry areas. Current Opinion in Biotechnology 73:128–134. [DOI] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tholen D, Niinemets U.. 2011. Leaf functional anatomy in relation to photosynthesis. Plant Physiology 155:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen D, Boom C, Noguchi K, Ueda S, Katase T, Terashima I.. 2008. The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant, Cell & Environment 31:1688–1700. [DOI] [PubMed] [Google Scholar]
- Tholen D, Boom C, Zhu X-G.. 2012. Opinion: prospects for improving photosynthesis by altering leaf anatomy. Plant Science 197:92–101. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Andrews J, Mulholland BJ, McKee JMT, Hilton HW, Horridge JS, Farquhar GD, Smeeton RC, Smillie IRA, Black CR, et al. 2007. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiology 143:1905–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Ribas-Carbó M, Tosens T, Vislap V, Niinemets U.. 2013. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. Journal of Experimental Botany 64:2269–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás M, Medrano H, Brugnoli E, Escalona JM, Martorell S, Pou A, Ribas-Carbó M, Flexas J.. 2014a. Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency: genotypic variability of mesophyll conductance. Australian Journal of Grape and Wine Research 20:272–280. [Google Scholar]
- Tomás M, Medrano H, Escalona JM, Martorell S, Pou A, Ribas-Carbó M, Flexas J.. 2014b. Variability of water use efficiency in grapevines. Environmental and Experimental Botany 103:148–157. [Google Scholar]
- Tortosa I, Escalona JM, Bota J, Tomás M, Hernández E, Escudero EG, Medrano H.. 2016. Exploring the genetic variability in water use efficiency: evaluation of inter and intra cultivar genetic diversity in grapevines. Plant Science 251:35–43. [DOI] [PubMed] [Google Scholar]
- Tränkner M, Jákli B, Tavakol E, Geilfus C-M, Cakmak I, Dittert K, Senbayram M.. 2016. Magnesium deficiency decreases biomass water-use efficiency and increases leaf water-use efficiency and oxidative stress in barley plants. Plant and Soil 406:409–423. [Google Scholar]
- Trueba S, Théroux‐Rancourt G, Earles JM, Buckley TN, Love D, Johnson DM, Brodersen C.. 2022. The three‐dimensional construction of leaves is coordinated with water use efficiency in conifers. New Phytologist 233:851–861. [DOI] [PubMed] [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R.. 2003. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425:734–737. [DOI] [PubMed] [Google Scholar]
- Vadez V, Kholova J, Medina S, Kakkera A, Anderberg H.. 2014. Transpiration efficiency: new insights into an old story. Journal of Experimental Botany 65:6141–6153. [DOI] [PubMed] [Google Scholar]
- Vadez V, Pilloni R, Grondin A, Hajjarpoor A, Belhouchette H, Brouziyne Y, Chehbouni G, Kharrou MH, Zitouna-Chebbi R, Mekki I, et al. 2023. Water use efficiency (WUE) across scales: from genes to landscape. Journal of Experimental Botany: erad052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegeer RK, Zhao C, Cibils‐Stewart X, et al. 2021. Silicon deposition on guard cells increases stomatal sensitivity as mediated by K+ efflux and consequently reduces stomatal conductance. Physiologia Plantarum 171:358–370. [DOI] [PubMed] [Google Scholar]
- Vastag E, Orlović S, Konôpková A, Kurjak D, Cocozza C, Pšidová E, Lapin K, Kesić L, Stojnić S.. 2020. Magnolia grandiflora L. shows better responses to drought than Magnolia × soulangeana in urban environment. iForest - Biogeosciences and Forestry 13:575–583. [Google Scholar]
- Vialet-Chabrand S, Matthews JSA, Brendel O, Blatt MR, Wang Y, Hills A, Griffiths H, Rogers S, Lawson T.. 2016. Modelling water use efficiency in a dynamic environment: an example using Arabidopsis thaliana. Plant Science 251:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Botella JR.. 2022. Heterotrimeric G protein signaling in abiotic stress. Plants 11:876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, del Campo AD, Wei X, Winkler R, Liu W, Li Q.. 2020. Responses of forest carbon and water coupling to thinning treatments from leaf to stand scales in a young montane pine forest. Carbon Balance and Management 15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CR. 2007. Stand aside stomata, another actor deserves centre stage: the forgotten role of the internal conductance to CO2 transfer. Journal of Experimental Botany 59:1475–1487. [DOI] [PubMed] [Google Scholar]
- Wedegaertner K, Shekoofa A, Purdom S, Walters K, Duncan L, Raper TB.. 2022. Cotton stomatal closure under varying temperature and vapor pressure deficit, correlation with the hydraulic conductance trait. Journal of Cotton Research 5:20–30. [Google Scholar]
- Whitney SM, Houtz RL, Alonso H.. 2011. Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiology 155:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M.. 2003. Least-cost input mixtures of water and nitrogen for photosynthesis. The American Naturalist 161:98–111. [DOI] [PubMed] [Google Scholar]
- Wu T, Tissue DT, Li X, Liu S, Chu G, Zhou G, Li Y, Zheng M, Meng Z, Liu J.. 2020. Long-term effects of 7-year warming experiment in the field on leaf hydraulic and economic traits of subtropical tree species. Global Change Biology 26:7144–7157. [DOI] [PubMed] [Google Scholar]
- Xiong D, Flexas J.. 2020. From one side to two sides: the effects of stomatal distribution on photosynthesis. New Phytologist 228:1754–1766. [DOI] [PubMed] [Google Scholar]
- Xiong D, Flexas J, Yu T, Peng S, Huang J.. 2017. Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 in Oryza. New Phytologist 213:572–583. [DOI] [PubMed] [Google Scholar]
- Xiong D, Douthe C, Flexas J.. 2018. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species: coordination of CO2 diffusion and H2O transport inside leaves. Plant, Cell & Environment 41:436–450. [DOI] [PubMed] [Google Scholar]
- Xu Y, Feng Z, Peng J, Tarvainen L.. 2022. Elevated ozone decreases the activity of Rubisco in poplar but not its activation under fluctuating light. Tree Physiology 42:1762–1775. [DOI] [PubMed] [Google Scholar]
- Xylogiannis E, Sofo A, Dichio B, Montanaro G, Mininni AN.. 2020. Root-to-shoot signaling and leaf water-use efficiency in peach trees under localized irrigation. Agronomy 10:437. [Google Scholar]
- Yang Z, Liu J, Tischer SV, Christmann A, Windisch W, Schnyder H, Grill E.. 2016. Leveraging abscisic acid receptors for efficient water use in Arabidopsis. Proceedings of the National Academy of Sciences 113:6791–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Liu J, Poree F, Schaeufele R, Helmke H, Frackenpohl J, Lehr S, von Koskull-Döring P, Christmann A, Schnyder H, et al. 2019. Abscisic acid receptors and coreceptors modulate plant water use efficiency and water productivity. Plant Physiology 180:1066–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao G, Nie Z, Turner NC, Li F-M, Gao T-P, Fang X-W, Scoffoni C.. 2021. Combined high leaf hydraulic safety and efficiency provides drought tolerance in Caragana species adapted to low mean annual precipitation. New Phytologist 229:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Maxwell JT, Wenzel MK, Roman DT, Sauer PE, Phillips RP, Novick KA.. 2019. Linking variation in intrinsic water-use efficiency to isohydricity: a comparison at multiple spatiotemporal scales. New Phytologist 221:195–208. [DOI] [PubMed] [Google Scholar]
- Yoo CY, Pence HE, Hasegawa PM, Mickelbart MV.. 2009. Regulation of transpiration to improve crop water use. Critical Reviews in Plant Sciences 28:410–431. [Google Scholar]
- Zahoor SA, Ahmad S, Ahmad A, et al. 2019. Improving water use efficiency in agronomic crop production. In: Hasanuzzaman M, ed. Agronomic crops. Singapore: Springer Singapore, 13–29. [Google Scholar]
- Zait Y, Ferrero‐Serrano A, Assmann SM.. 2021. The α subunit of the heterotrimeric G protein regulates mesophyll CO2 conductance and drought tolerance in rice. New Phytologist 232:2324–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wollenweber B, Jiang D, Liu F, Zhao J.. 2008. Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis thaliana constitutively expressing ABP9, a bZIP transcription factor. Journal of Experimental Botany 59:839–848. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Sussmilch F, Nichols DS, Cardoso AA, Brodribb TJ, McAdam SAM.. 2018. Leaves, not roots or floral tissue, are the main site of rapid, external pressure-induced ABA biosynthesis in angiosperms. Journal of Experimental Botany 69:1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu X, Chen L, Jia G.. 2019. Whole-plant instantaneous and short-term water-use efficiency in response to soil water content and CO2 concentration. Plant and Soil 444:281–298. [Google Scholar]
- Zhao C, Chavan S, He X, Zhou M, Cazzonelli CI, Chen Z-H, Tissue DT, Ghannoum O.. 2021a. Smart glass impacts stomatal sensitivity of greenhouse Capsicum through altered light. Journal of Experimental Botany 72:3235–3248. [DOI] [PubMed] [Google Scholar]
- Zhao J, Feng H, Xu T, Xiao J, Guerrieri R, Liu S, Wu X, He X, He X.. 2021b. Physiological and environmental control on ecosystem water use efficiency in response to drought across the northern hemisphere. Science of the Total Environment 758:143599. [DOI] [PubMed] [Google Scholar]
- Zhu L, Li H, Thorpe MR, Hocart CH, Song X.. 2021. Stomatal and mesophyll conductance are dominant limitations to photosynthesis in response to heat stress during severe drought in a temperate and a tropical tree species. Trees 35:1613–1626. [Google Scholar]
- Zsögön A, Alves Negrini AC, Peres LEP, Nguyen HT, Ball MC.. 2015. A mutation that eliminates bundle sheath extensions reduces leaf hydraulic conductance, stomatal conductance and assimilation rates in tomato (Solanum lycopersicum). New Phytologist 205:618–626. [DOI] [PubMed] [Google Scholar]
- Zufferey V. 2016. Leaf respiration in grapevine (Vitis vinifera ’Chasselas’) in relation to environmental and plant factors. VITIS - Journal of Grapevine Research 55:65–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No original data was used in this commentary. The discussion and synthesis are based on already published studies.