Abstract
CSF1R expression modulates tumor-associated macrophages, making CSF1R blockade an appealing immune-modulating therapeutic target. We evaluated the correlation between CSF1R tumor RNA expression and outcome (pan-cancer setting). RNA expression was ranked as a percentile (0-100) using a standardized internal reference population (735 tumors; 35 histologies). Among 514 patients, there was no difference in survival from biopsy between high and low CSF1R expressors (< 50 percentile versus ≥ 50 percentile rank). There was also no significant difference in median progression-free or overall survival (from treatment) based on CSF1R expression in 21 patients who received CSF1R inhibitors (all p values ≥ 0.08). Concurrent upregulation of ≥ 2 additional immune checkpoint markers (e.g. PD-L1, BTLA, CTLA4, LAG3, TIM3) was observed in all tumor samples with CSF1R expression ≥ 50th percentile. Pending further large prospective studies, patients with high tumor CSF1R expression may need treatment that co-targets the specific immune checkpoint pathways activated in order to impact outcome.
Keywords: CSF1R, targeted therapy, cancer, biomarker, checkpoints
Introduction
Colony stimulating factor-1 receptor (CSF1R) acts as the sole receptor for the ligand colony stimulating factor-1 (CSF1), which facilitates macrophage differentiation, growth, survival, and migration [1]. It is also known as macrophage colony-stimulating factor receptor (M-CSFR), CD115, and c-FMS. Signal transduction of CSF1 through its transmembrane receptor CSF1R occurs through several key intracellular signaling pathways, including FAK, RAS, STAT3, and PI3K [2]. CSF1 mediates signaling pathways that activate macrophage differentiation [3]. M1 macrophages have a pro-inflammatory, immunostimulatory anti-tumor effect via cytokine production. Conversely, M2 macrophages display an anti-inflammatory phenotype, promoting tissue remodeling and tumor progression [3]. Programming of the M2 phenotype macrophages is induced by the CSF/CSF1R axis [4]. CSF1 also facilitates macrophage motility as a chemokine.
In addition to macrophage activity, CSF1R also regulates myeloid cell lineage, promoting the differentiation of myeloid progenitors [2]. Proliferation and differentiation of bone-resorbing osteoclasts is facilitated by CSF1R [5]. CSF1R is also detected on the surface of other myeloid cells, as well as dendritic cells. CSF1R is expressed among neural progenitor cells [6]. Microglia, the primary immune cells of the central nervous system, depend on CSF1R-mediated signaling pathways for survival. CSF1R knockout mice were noted to have skeletal malformations, decreased lifespan, and impairment of neurologic development [7].
In malignancy, tumor-associated macrophages (TAMs) have been induced to display M2 characteristics, thereby suppressing the immune response. Within tumor tissues, elevated numbers of macrophages have been associated with increased spread of cancer and disease progression. Activation of the CSF/CSF1R axis leads to recruitment of these CSF1R-expressing macrophages. Elevated CSF1R correlates with poor prognosis in multiple tumor types, including breast, ovarian, uterine, colorectal, and prostate cancer [8]. This has been attributed to the recruitment of TAMs via the CSF1/CSF1R signaling pathway, promoting progression of disease and metastasis [9]. CSF1R overexpression is associated with high-grade tumors, and high concentrations of CSF-1 and TAMs have been identified at the invasive sites of breast tumors [8]. This suggests that CSF1R plays an important role in both the migration as well as the activation of TAMs. In mouse models of pancreatic ductal adenocarcinoma, inhibiting signaling by the myeloid growth factor receptor CSF1R can functionally reprogram macrophage responses that enhance antigen presentation and productive antitumor T-cell responses [10].
Tenosynovial giant cell tumors (TGCTs) are of special interest in regard to the CSF1/CSF1R pathway. These tumors can be associated with translocations that fuse CSF1 to COL6A and result in overexpression of the CSF1/CSF1R axis [11,12]. Pexidartinib, a potent CSF1R inhibitor, demonstrated clinical benefit in the treatment of tenosynovial giant cell tumors, with a 39% overall response rate in Phase III trial data, leading to FDA approval [13].
Despite promising preclinical data, the performance of CSF1R inhibitors in other tumor types has been otherwise limited thus far. One potential reason for the negative clinical studies to date could be attributed to the heterogeneity of cancer, as targeting one marker could have limitations. In small studies, patients have been treated with CSF1R inhibitors in combination with cytotoxic therapy or immunotherapy. Among patients with high grade gliomas treated with pexidartinib in combination with temozolomide and radiation, the addition of CSF1R inhibition added toxicity without improvement in clinical outcomes [14]. Pexidartinib was studied in combination with eribulin in triple negative breast cancer patients (n = 31) with only a 16% response rate (NCT01596751). In a small phase 1 study of pancreatic and colorectal cancer patients (n = 19), pexidartinib and anti-PDL1 antibody therapy demonstrated a 21% response rate [15]. Early phase clinical trials investigating combination therapy with CSF1R inhibitors in combination with PD1 and PD-L1 inhibitors are ongoing in intrahepatic cholangiocarcinoma, peripheral T cell lymphoma, and soft tissue sarcomas (ClinicalTrials.gov; NCT04301778, NCT03927105, NCT02584647).
To date, there are no clinical trials in which CSF1R inhibitor therapies were selected based on CSF1R expression. To further investigate the role of CSF1R inhibition and clinical outcomes depending on CSF1R expression level, we sought to determine if patients with high CSF1R RNA expression had improved clinical outcomes when treated with a CSF1R inhibitor.
Materials and methods
Subjects
All patients were evaluated and treated at the University of California San Diego Moores Cancer Center. Using the CLIA-certified and CAP-accredited laboratory Omniseq, comprehensive immune profiling was obtained for patients with solid tumor malignancies. We examined the electronic medical record to determine which patients received the drugs of interest as well as the clinical characteristics.
Ethics
The patients were analyzed according to the guidelines of the PREDICT (Profile Related Evidence Determining Individualized Cancer Therapy) protocol (NCT02478931) and any investigational interventions/therapies for which the patients gave written informed consent; all ethical approvals from the University of California San Diego Internal Review Board.
RNA sequencing
All biopsies were obtained prior to initiation of CSF1R inhibitor therapy. Tumor genomic profiling was performed for all patients. Pathology specimens were provided to Omniseq laboratory as formalin-fixed, paraffin-embedded (FFPE) blocks for comprehensive genomic profiling via multiplex PCR-based RNA sequencing. Using truXTRAC FFPE extraction kit (Covaris, Inc., Woburn, MA), RNA was extracted from FFPE. RNA was purified and diluted in 50 uL water. Quant-iT RNA HS assay (Thermo Fisher Scientific, Waltham, MA) was utilized to quantify yield. Designated RNA titer cutoff for use was 10 ng. RNA sequence was read using Torrent Suite’s plugin immuneResponseRNA (v5.2.0.0) 34 and then transcription was normalized against internal housekeeping gene profiles. Using a standardized internal reference population of 735 tumors from 35 different histologies, RNA expression was ranked as “Low” (0-24), “Intermediate” (25-74), or “High” (75-100). Expression of CSF1R as well as nine other checkpoint markers (PD-1, PD-L1, PD-L2, BTLA, CTLA4, LAG3, TIM3, TNFRSF14, VISTA) were compared.
Statistical analysis
All statistical analyses were conducted using SPSS Version 27.0. Progression-free survival (PFS) was defined as the time from initiation of CSF1R inhibitor therapy to documented disease progression or death. Overall survival (OS) was defined as the time from initiation of CSF1R inhibitor therapy until death or last date of contact. Patients still progression-free at time of last contact (for PFS) and those still alive (for OS), respectively, were censored at that date.
Data availability
Available upon reasonable request.
Results
Five hundred fourteen patients underwent comprehensive transcriptomic tumor immune profiling, of which 41 patients received a tyrosine kinase inhibitor with CSF1R inhibitory properties. Patients were excluded if tumor biopsy was performed over 24 months prior to initiation of CSF1R inhibitor (Figure S1). Twenty-one patients received a tyrosine kinase inhibitor with CSF1R inhibition within 24 months of a tissue biopsy on which immune profiling was performed.
Baseline patient demographics and characteristics are summarized in Table 1. Fifteen patients (71.4%) were men. The median age at the time of biopsy sent for immune profiling was 61 years (range, 29-76 years). Gastrointestinal malignancies were the most common histologic subtype. Overall, 95.3% of patients had an ECOG of 0 or 1 at the time of initiation of CSF1R inhibition. Patients were treated with six different tyrosine kinase inhibitors with CSF1R inhibition (Tables 1 and S1). Among 21 patients, 11 (52.4%) received pazopanib (IC50 for CSF1R = 146 nM). The median number of prior systemic lines of therapy was four. Seven patients (33.3%) were treated with a tyrosine kinase inhibitor as monotherapy. Comparing clinical characteristics between patients with CSF1R RNA rank < 50 percentile versus ≥ 50 percentile, there was no statistically significant difference in age, sex, prior systemic lines of therapy, or median number of drugs administered.
Table 1.
Demographics and baseline clinical characteristics of patients who received tyrosine kinase inhibitors with CSF1R inhibitory action (N = 21)
| Demographics | N (%) | ||
|
| |||
| Age1 | < 61 years old | 10 (47.6) | |
| ≥ 61 years old | 11 (52.4) | ||
| Gender | Male | 15 (71.4) | |
| Female | 6 (28.6) | ||
| Diagnosis | Gastrointestinal cancer | 7 (33.3) | |
| Sarcoma | 4 (19.1) | ||
| Gynecologic cancer | 3 (14.3) | ||
| Lung cancer | 2 (9.5) | ||
| Thyroid cancer | 2 (9.5) | ||
| Other malignancy2 | 3 (14.3) | ||
| ECOG3 | 0 | 6 (28.6) | |
| 1 | 14 (66.7) | ||
| 2 | 1 (4.7) | ||
| CSF1R RNA expression rank | 0-49 percentile | 7 (33.3) | |
| 50-74 percentile | 8 (38.1) | ||
| ≥ 75 percentile | 6 (28.6) | ||
| CSF1R inhibitor4 | Pazopanib | 11 (52.4) | |
| Sorafenib | 4 (19.1) | ||
| Regorafenib | 3 (14.3) | ||
| Sunitinib | 1 (4.7) | ||
| Crizotinib | 1 (4.7) | ||
| Entrectinib | 1 (4.7) | ||
|
| |||
| Comparison of patients with CSF1R RNA rank < 50 percentile (N = 7) versus ≥ 50 percentile (N = 14) | |||
|
| |||
| CSF1R < 50 (n = 7) | CSF1R ≥ 50 (n = 14) | P-value | |
|
| |||
| Median age-year (range) | 61 (41-67) | 59 (29-76) | 0.915 |
| Number men (%) | 5 (71.4) | 10 (71.4) | 16 |
| Number ECOG 0-1 (%) | 7 (100) | 13 (92.9) | 0.476 |
| Number with gastrointestinal cancer (%) | 3 (42.9) | 4 (28.6) | 0.516 |
| Median number of lines of therapy prior to CSF1R inhibitor (range) | 3 (1-4) | 4 (1-5) | 0.095 |
| Median number of drugs administered along with CSF1R inhibitor (range) | 1 (0-2) | 2 (0-2) | 0.165 |
There was no statistically significant difference in age, sex, primary tumor histology, prior systemic lines of therapy, or the median number of drugs administered between patient tumors with low CSF1R RNA expression < 50 percentile (N = 7) and intermediate to high CSF1R expression ≥ 50 percentile.
Median age 61 years of was used as a cutoff. Age range 29-76 years.
Other malignancies include adrenocortical carcinoma (N = 1), renal cell carcinoma (N = 1), and neuroendocrine (N = 1).
ECOG denotes Eastern Cooperative Oncology Group.
See Table S1 for anti-CSF1R 50% inhibitory concentration (IC50) activities among different tyrosine kinase inhibitors.
Student’s T-test performed.
Chi-squared test performed.
When comparing all 514 patients, the median overall survival (OS) from time of biopsy in patients with CSF1R RNA expression rank < 50 percentile was 28.07 months, which was not statistically different from patients with CSF1R ≥ 50 percentile whose median overall survival was 38.04 months (P = 0.23) (Figure 1).
Figure 1.
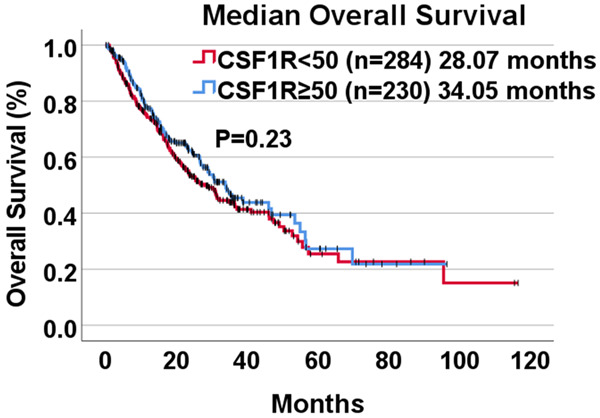
Kaplan-Meier analysis of overall based on RNA expression rank of CSF1R (n = 514). There is no significant difference between mOS based on CSF1R expression rank ≥ 50 percentile (34.05 months) compared to CSF1R < 50 percentile (28.07 months) (P = 0.23) among all patients whose tumor tissue was sent for genomic profiling. Survival is calculated from date of biopsy to date of death or last contact (if patient still alive at last contact, survival was censored on that date).
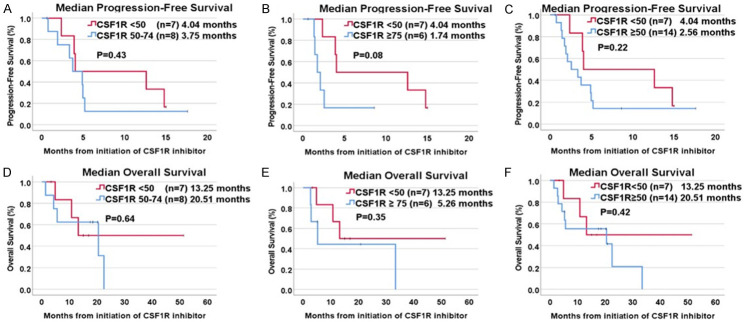
Among twenty-one patients treated with a CSF1R inhibitor, seven (33.3%) had tumors with CSF1R RNA expression rank < 50 percentile, eight (38.1%) had tumor tissue with CSF1R RNA expression rank 50-74 percentile, and six patient samples (28.6%) had CSF1R expression ≥ 75 percentile. The median progression-free survival (PFS) from time of CSF1R inhibitor initiation was 1.74 months (95% CI, 0.95-2.53 months) for CSF1R expression ≥ 75, and 4.04 months (95% CI, 0-14.50 months) for CSF1R expression < 50 percentile (P = 0.08) (Figure 2). Median PFS between CSF1R 50-74 percentile (3.75 months) and CSF1R < 50 percentile (4.04 months) showed no difference (P = 0.43) (Table 2). There was no significant difference in PFS between the groups.
Figure 2.
Kaplan-Meier analysis of progression-free survival and overall survival from initiation of tyrosine kinase inhibitor with CSF1R inhibition based on RNA expression rank of CSF1R. A. Comparison of median PFS between CSF1R 50-74 percentile (3.75 months) and CSF1R < 50 percentile (4.04 months) showed no difference (P = 0.43). B. Median PFS showed a trend towards worse PFS in patients with CSF1R expression ≥ 75 percentile at 1.74 months compared to 4.04 months for CSF1R < 50 percentile (P = 0.08). C. Median PFS was not statistically different between CSF1R < 50 percentile (4.04 months) and CSF1R ≥ 50 (2.56 months) (P = 0.22). D. OS was compared between CSF1R 50-74 percentile (20.51 months) and CSF1R < 50 percentile (13.25 months) with no statistically significant difference (P = 0.64). E. No statistically significant difference in OS was observed based on CSF1R ≥ 75 percentile compared to CSF1R < 50 percentile (5.26 months vs 13.25 months) (P = 0.35). F. Median OS was not significantly prolonged among patients with CSF1R ≥ 50 percentile treated with a CSF1R inhibitor (20.51 months) compared to CSF1R < 50 percentile (13.25 months), although there was a trend toward a longer OS (P = 0.42). 1(+) indicates censored event.
Table 2.
Clinical outcomes among patients who received tyrosine kinase inhibitors with CSF1R inhibition (N = 21)
| Characteristics | N | PFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Median (months) (95% CI) | Univariate | Median (months) (95% CI) | Univariate | |||||
|
|
|
|||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |||||
| CSF1R (RNA expression rank) | < 50 | 7 | 4.04 (0-14.50) | Reference | - | 13.25 (CI not calculable) | Reference | - |
| ≥ 50-74 | 8 | 3.75 (1.61-5.89) | 1.6 (0.50-5.15) | 0.43 | 20.51 (0-42.69) | 0.71 (0.17-2.99) | 0.64 | |
| CSF1R (RNA expression rank) | < 50 | 7 | 4.04 (0-14.50) | Reference | - | 13.25 (CI not calculable) | Reference | - |
| ≥ 75 | 6 | 1.74 (0.95-2.53) | 3.79 (0.86-16.67) | 0.08 | 5.26 (0.69-9.83) | 2.06 (0.45-9.35) | 0.35 | |
| CSF1R (RNA expression rank) | < 50 | 7 | 4.04 (0-14.50) | Reference | - | 13.25 (CI not calculable) | Reference | - |
| ≥ 50 | 14 | 2.56 (0.27-4.85) | 1.93 (0.66-5.65) | 0.22 | 20.51 (0-53.77) | 0.58 (0.16-2.18) | 0.42 | |
| Gastrointestinal cancer | Yes | 7 | 3.91 (3.49-4.33) | Reference | - | 13.24 (4.14-22.35) | Reference | - |
| No | 14 | 3.35 (0-6.71) | 1.47 (0.53-4.04) | 0.45 | 20.51 (0-47.40) | 0.61 (0.19-1.94) | 0.40 | |
| Number of prior lines of therapy | ≥ 4 | 9 | 2.56 (1.22-3.91) | Reference | - | 22.52 (4.00-41.04) | Reference | - |
| < 4 | 12 | 4.04 (2.31-5.78) | 0.60 (0.22-1.62) | 0.31 | 20.51 (6.11-34.92) | 1.20 (0.38-3.79) | 0.75 | |
| Single agent therapy | Yes | 7 | 3.35 (0.79-5.92) | Reference | - | 22.52 (5.40-39.64) | Reference | - |
| No | 14 | 3.75 (1.28-6.22) | 0.71 (0.26-1.92) | 0.49 | 13.25 (0.15-26.34) | 2.51 (0.67-9.43) | 0.17 | |
Median progression-free survival (PFS) and overall survival (OS) were assessed. Univariate Cox regression analysis was performed for each variable to calculate hazard ratios (HR) with 95% confidence intervals (CI).
Median OS from time of CSF1R inhibitor initiation among patients with CSF1R expression ≥ 75 percentile was 5.26 months (95% CI, 0.69-9.83 months) and 13.25 months (95% CI not calculable) for CSF1R expression < 50 percentile (P = 0.35). Overall survival was numerically better in patients with CSF1R expression 50-74 percentile at 20.51 months (95% CI, 0-42.69 months) compared to CSF1R expression < 50 percentile, but the difference in median OS was not statistically significant (P = 0.64) (Table 2). There was no statistically significant difference in clinical outcomes (PFS and OS) based on type of cancer, number of prior lines of therapy or single or combination therapy approach (Table 2). Moreover, among 14 patients with CSF1R expression ≥ 50 percentile, only 1 patient (7.1%) (clear cell renal cell carcinoma) had a partial response to pazopanib on radiographic imaging (duration of response = 18 months).
Since treatment with CSF1R inhibition in patients with increased CSF1R expression was not correlated with a significant benefit on clinical outcomes (PFS and OS), we constructed a heat map of other immune markers (PD-1, PD-L1, PD-L2, BTLA, CTLA4, LAG3, TIM3, TNFRSF14, VISTA) evaluated on immune profiling (Figure 3). Of the 14 patients with CSF1R ≥ 50 percentile, all had intermediate or high expression of two or more additional immune checkpoints that were upregulated.
Figure 3.
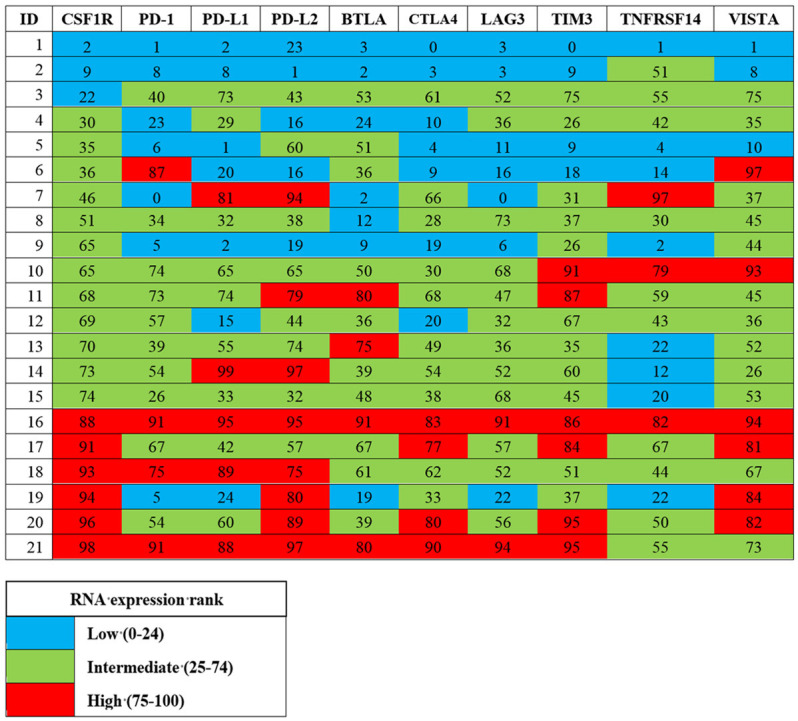
Expression pattern of multiple checkpoint markers among patients who received tyrosine kinase inhibitor with CSF1R inhibition (N = 21). Numbers indicate RNA expression rank, color coding was based on RNA expression rank of low, intermediate, or high, corresponding to values of 0-24, 25-74, and 75-100 percentile, respectively. Patients with intermediate or high CSF1R expression all demonstrated simultaneous intermediate or high expression of two or more other immune checkpoint proteins.
Discussion
CSF1R plays a pivotal role in the M2 polarization of macrophages as well as the recruitment and survival of TAMs [1]. This axis impedes the immune surveillance mechanism. Inhibition of the CSF1R pathway depletes TAMs from the tumor and surrounding microenvironment, offering a potential therapeutic target to activate anti-tumor immunity.
We hypothesized that malignancies with high CSF1R RNA expression may demonstrate better clinical outcome with CSF1R inhibition when compared to CSF1R low tumors. To our surprise, in the current study, we did not observe a statistically significant PFS or OS difference between patients with low or high CSF1R expression who were treated with CSF1R inhibitors. Previous clinical studies have demonstrated a correlation between high CSF1R expression and inferior outcomes. In an evaluation of breast cancer patients, CSF1R positive tumors were more frequently identified among node positive patients, and there was an inferior OS in breast cancer patients with high CSF1R expression [16,17]. High CSF1R expression via IHC in gastric cancer patients was also associated with lymph node and peritoneal metastases, where patients with CSF1R overexpression were also found to have inferior disease-free survival and overall survival [18]. Depending on tumor histology type, CSF1R has been of mixed prognostic significance, but in our analysis, CSF1R expression in 514 patients had no impact on survival. This might explain why there was no survival advantage to CSF1R inhibition.
It may also be that targeting CSF1R alone is insufficient. Previous in vitro studies have shown that single-agent CSF1R inhibition had a modest response to tumor growth. CSF1R inhibition depleted TAMs, but monotherapy did not improve survival in mesothelioma mouse models [19]. However, data showed a synergistic effect between anti-PD-1 therapy and CSF1R blockade in lung squamous cell carcinoma mouse models with improved response rates with the addition of pexidartinib compared to single agent PD-1 blockade [20]. All patients in our study appeared to have moderate or high expression of other immune markers in addition to CSF1R. Given the co-expression of other checkpoint markers, it is likely that use of CSF1R inhibitors in combination with other checkpoint inhibitors that are simultaneously upregulated are needed to derive a clinical benefit [21]. Early phase clinical trial data previously examined combination therapy with CSF1R inhibitor carbiralizumab and nivolumab in melanoma, lung cancer, and renal cell carcinoma with favorable safety data and pharmacodynamic activity, suggesting feasibility of combination therapy [22].
Therefore, we believe a rationally selected combination, based on genomic profiling results, is crucial for optimizing therapeutic outcomes. An individualized approach to therapy based on genomic sequencing previously demonstrated improved clinical outcomes when selected by a molecular tumor board [23,24]. High matching of therapies to genomic alterations correlated with better survival outcomes [25]. Targeting multiple immune markers based on RNA expression markers may be important to identifying the optimal population to derive clinical benefit from a combination approach with CSF1R inhibitors.
There were several limitations to our study. The sample size was small with multiple primary tumor histologies. Therefore, our sample population was heterogenous. In addition, multiple CSF1R inhibitors were used with varying inhibitory concentrations of CSF1R (Table S1). Drugs such as pexidartinib, a highly potent CSF1R inhibitor, were not available at the time of study. More potent inhibitors for CSF1R may be needed to see clinical benefit. In addition, data analysis was performed retrospectively, although immune profiling, used in selecting patients for treatment was performed prospectively. Other limitations include the fact that RNA expression does not necessarily correlate with the protein/receptor level and our study was unable to evaluate CSF1R protein expression. Additional studies correlating RNA sequencing and immunohistochemistry data are needed.
In conclusion, although our study did not see a clinical benefit from CSF1R inhibition among patients with high CSF1R RNA expression, the co-expression of other checkpoint markers is interesting and would benefit from further exploration to confirm if optimized combination immune therapy based on RNA expression levels may elicit improved clinical outcomes.
Disclosure of conflict of interest
Ann Moeller, Suzanna Lee, and Hitendra Patel declare that they have no competing interests. Sarabjot Pabla, Mary K. Nesline, Jeffrey Conroy are employees of Omniseq, Inc. Jason K. Sicklick receives research funding from Amgen, Foundation Medicine is a consultant for Deciphera, Ethicon and speaker for Bayer, Deciphera, Foundation Medicine, La Hoffman-Roche, Merck, QED. Gregory P. Botta serves on the Advisory Board of Natera and as a consultant to TumorGen Inc. and CEND Therapeutics.Shumei Kato serves as a consultant for Foundation Medicine, NeoGenomics and CureMatch. He receives speaker’s fee from Roche and advisory board for Pfizer. He has research funding from ACT Genomics, Sysmex, Konica Minolta and OmniSeq.Jacob J. Adashek serves on the advisory board of CureMatch, Inc. Razelle Kurzrock has received research funding from Biological Dynamics, Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, and TopAlliance; as well as consultant and/or speaker fees and/or advisory board for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Biological Dynamics, Daiichi Sankyo, Inc., EISAI, EOM Pharmaceuticals, Iylon, Merck, NeoGenomics, Neomed, Pfizer, Prosperdtx, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch Inc., CureMetrix, and IDbyDNA; serves on the Board of CureMatch and CureMetrix, and is a co-founder of CureMatch.
Supporting Information
References
- 1.Tamimi RM, Brugge JS, Freedman ML, Miron A, Iglehart JD, Colditz GA, Hankinson SE. Circulating colony stimulating factor-1 and breast cancer risk. Cancer Res. 2008;68:18–21. doi: 10.1158/0008-5472.CAN-07-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumari A, Silakari O, Singh RK. Recent advances in colony stimulating factor-1 receptor/c-FMS as an emerging target for various therapeutic implications. Biomed Pharmacother. 2018;103:662–679. doi: 10.1016/j.biopha.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 3.Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5:53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai H, Zhang Y, Wang J, Gu J. Defects in macrophage reprogramming in cancer therapy: the negative impact of PD-L1/PD-1. Front Immunol. 2021;12:690869. doi: 10.3389/fimmu.2021.690869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gyori DS, Mocsai A. Osteoclast signal transduction during bone metastasis formation. Front Cell Dev Biol. 2020;8:507. doi: 10.3389/fcell.2020.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chitu V, Gokhan S, Stanley ER. Modeling CSF-1 receptor deficiency diseases - how close are we? FEBS J. 2022;289:5049–5073. doi: 10.1111/febs.16085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green KN, Crapser JD, Hohsfield LA. To kill a microglia: a case for CSF1R inhibitors. Trends Immunol. 2020;41:771–784. doi: 10.1016/j.it.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dwyer AR, Greenland EL, Pixley FJ. Promotion of tumor invasion by tumor-associated macrophages: the role of CSF-1-activated phosphatidylinositol 3 kinase and src family kinase motility signaling. Cancers (Basel) 2017;9:68. doi: 10.3390/cancers9060068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan AR, Pixley FJ. CSF-1R signaling in health and disease: a focus on the mammary gland. J Mammary Gland Biol Neoplasia. 2014;19:149–159. doi: 10.1007/s10911-014-9320-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West RB, Rubin BP, Miller MA, Subramanian S, Kaygusuz G, Montgomery K, Zhu S, Marinelli RJ, De Luca A, Downs-Kelly E, Goldblum JR, Corless CL, Brown PO, Gilks CB, Nielsen TO, Huntsman D, van de Rijn M. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc Natl Acad Sci U S A. 2006;103:690–695. doi: 10.1073/pnas.0507321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, Ries C, Brillouet A, Muller C, Jegg AM, Broske AM, Dembowski M, Bray-French K, Freilinger C, Meneses-Lorente G, Baehner M, Harding R, Ratnayake J, Abiraj K, Gass N, Noh K, Christen RD, Ukarma L, Bompas E, Delord JP, Blay JY, Ruttinger D. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 13.Tap WD, Gelderblom H, Palmerini E, Desai J, Bauer S, Blay JY, Alcindor T, Ganjoo K, Martin-Broto J, Ryan CW, Thomas DM, Peterfy C, Healey JH, van de Sande M, Gelhorn HL, Shuster DE, Wang Q, Yver A, Hsu HH, Lin PS, Tong-Starksen S, Stacchiotti S, Wagner AJ ENLIVEN investigators. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet. 2019;394:478–487. doi: 10.1016/S0140-6736(19)30764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colman H, Raizer JJ, Walbert T, Plotkin SR, Chamberlain MC, Wong ET, Puduvalli VK, Reardon DA, Iwamoto FM, Mrugala MM, Johnson B, Sonty K, Karlin DA, Pelayo M, Hutchinson M, Hsu H. Phase 1b/2 study of pexidartinib (PEX) in combination with radiation therapy (XRT) and temozolomide (TMZ) in newly diagnosed glioblastoma. J. Clin. Oncol. 2018;36:2015–2015. [Google Scholar]
- 15.Cassier PA, Garin G, Eberst L, Delord JP, Chabaud S, Terret C, Montane L, Bidaux AS, Laurent S, Jaubert L, Ferlay C, Bernardin M, Tabone-Eglinger S, Gilles-Afchain L, Menetrier-Caux C, Caux C, Treilleux I, Pérol D, Gomez-Roca CA. MEDIPLEX: a phase 1 study of durvalumab (D) combined with pexidartinib (P) in patients (pts) with advanced pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer (CRC) J. Clin. Oncol. 2019;37:2579–2579. [Google Scholar]
- 16.Riaz N, Burugu S, Cheng AS, Leung SCY, Gao D, Nielsen TO. Prognostic significance of CSF-1R expression in early invasive breast cancer. Cancers (Basel) 2021;13:5769. doi: 10.3390/cancers13225769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kluger HM, Dolled-Filhart M, Rodov S, Kacinski BM, Camp RL, Rimm DL. Macrophage colony-stimulating factor-1 receptor expression is associated with poor outcome in breast cancer by large cohort tissue microarray analysis. Clin Cancer Res. 2004;10:173–177. doi: 10.1158/1078-0432.ccr-0699-3. [DOI] [PubMed] [Google Scholar]
- 18.Okugawa Y, Toiyama Y, Ichikawa T, Kawamura M, Yasuda H, Fujikawa H, Saigusa S, Ohi M, Araki T, Tanaka K, Inoue Y, Tanaka M, Miki C, Kusunoki M. Colony-stimulating factor-1 and colony-stimulating factor-1 receptor co-expression is associated with disease progression in gastric cancer. Int J Oncol. 2018;53:737–749. doi: 10.3892/ijo.2018.4406. [DOI] [PubMed] [Google Scholar]
- 19.Dammeijer F, Lievense LA, Kaijen-Lambers ME, van Nimwegen M, Bezemer K, Hegmans JP, van Hall T, Hendriks RW, Aerts JG. Depletion of tumor-associated macrophages with a CSF-1R kinase inhibitor enhances antitumor immunity and survival induced by DC immunotherapy. Cancer Immunol Res. 2017;5:535–546. doi: 10.1158/2326-6066.CIR-16-0309. [DOI] [PubMed] [Google Scholar]
- 20.Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guerin M, Biton J, Ouakrim H, Regnier F, Lupo A, Alifano M, Damotte D, Donnadieu E. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci U S A. 2018;115:E4041–E4050. doi: 10.1073/pnas.1720948115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adashek JJ, Goloubev A, Kato S, Kurzrock R. Missing the target in cancer therapy. Nat Cancer. 2021;2:369–371. doi: 10.1038/s43018-021-00204-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss SA, Djureinovic D, Jessel S, Krykbaeva I, Zhang L, Jilaveanu L, Ralabate A, Johnson B, Levit NS, Anderson G, Zelterman D, Wei W, Mahajan A, Trifan O, Bosenberg M, Kaech SM, Perry CJ, Damsky W, Gettinger S, Sznol M, Hurwitz M, Kluger HM. A phase I study of APX005M and cabiralizumab with or without nivolumab in patients with melanoma, kidney cancer, or non-small cell lung cancer resistant to anti-PD-1/PD-L1. Clin Cancer Res. 2021;27:4757–4767. doi: 10.1158/1078-0432.CCR-21-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, De P, Krie A, Piccioni DE, Miller VA, Ross JS, Benson A, Webster J, Stephens PJ, Lee JJ, Fanta PT, Lippman SM, Leyland-Jones B, Kurzrock R. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med. 2019;25:744–750. doi: 10.1038/s41591-019-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato S, Kim KH, Lim HJ, Boichard A, Nikanjam M, Weihe E, Kuo DJ, Eskander RN, Goodman A, Galanina N, Fanta PT, Schwab RB, Shatsky R, Plaxe SC, Sharabi A, Stites E, Adashek JJ, Okamura R, Lee S, Lippman SM, Sicklick JK, Kurzrock R. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat Commun. 2020;11:4965. doi: 10.1038/s41467-020-18613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razak AR, Cleary JM, Moreno V, Boyer M, Calvo Aller E, Edenfield W, Tie J, Harvey RD, Rutten A, Shah MA, Olszanski AJ, Jager D, Lakhani N, Ryan DP, Rasmussen E, Juan G, Wong H, Soman N, Smit MD, Nagorsen D, Papadopoulos KP. Safety and efficacy of AMG 820, an anti-colony-stimulating factor 1 receptor antibody, in combination with pembrolizumab in adults with advanced solid tumors. J Immunother Cancer. 2020;8:e001006. doi: 10.1136/jitc-2020-001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available upon reasonable request.