Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) are associated with less favorable blood lipid profiles in epidemiological studies. However, little is known about the potential role of PFAS in longitudinal changes in lipids among midlife women even though women become more susceptible to metabolic alterations during the menopausal transition.
Objectives:
To examine associations of serum PFAS concentrations with longitudinal trajectories of blood total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides in midlife women undergoing menopausal transition.
Methods:
The sample included 1,130 women from the Study of Women’s Health Across the Nation 45–56 y of age at baseline (1999–2000). We measured serum PFAS concentrations including linear perfluorooctanoic acid (n-PFOA), perfluorononanoic acid (PFNA), linear and branched perfluorooctanesulfonic acid (n-PFOS and Sm-PFOS, respectively), and perfluorohexanesulfonic acid (PFHxS) at baseline. We used k-means clustering to identify subgroups with different patterns of PFAS mixture. Blood lipids were measured annually or biannually through 2016 with an average follow-up of 14.8 y. We identified longitudinal trajectories of each lipid using latent class growth models. We used multinomial log-linear models adjusted for covariates to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of lipid trajectory classes by PFAS and their mixtures.
Results:
Three distinct trajectories (low, middle, high) of total, LDL, and HDL cholesterol and two distinct trajectories (low and high) of triglycerides were identified. n-PFOS, Sm-PFOS, and PFHxS were positively associated with total and LDL cholesterol trajectories. n-PFOS was inversely associated with triglycerides trajectories. PFAS mixtures (high vs. low) showed positive associations with total and LDL cholesterol trajectories (high vs. low), showing ORs (95% CIs) of 1.69 (95% CI: 1.36, 2.12) and 1.79 (95% CI: 1.44, 2.22), respectively.
Discussion:
Concentrations of serum PFAS were positively associated with trajectories of total and LDL cholesterol, providing a line of evidence supporting adverse effects of PFAS on lipid homeostasis. https://doi.org/10.1289/EHP12351
Introduction
Per- and polyfluoroalkyl substances (PFAS) are manmade chemicals with a fully or partially fluorinated alkyl chain that is connected to different functional groups (e.g., carboxylate, sulfonate). PFAS have been used for various applications, including water-resistant fabrics, carpet, food packaging, and firefighting foams.1,2 Because of their industrial applications and chemically stable characteristics, PFAS have contaminated the global environment over decades.3,4 These environmentally persistent chemicals are detected in human populations5–8 and linked to adverse health effects such as liver injury,9 kidney and testicular cancer,2,10 and reproductive disorders.2,11
PFAS exposure has been associated with less-favorable blood lipids, including higher low-density lipoprotein (LDL) cholesterol and triglycerides concentrations in epidemiological studies including cross-sectional6,12–18 and longitudinal studies.6,17,19–22 Longitudinal studies of PFAS manufacturing workers19,20 and residents living near PFAS manufacturing plants21,22 reported positive associations between serum PFAS and cholesterol or triglycerides. Several recent studies have explored adults with prediabetes status17 and adults age 70 y6 and found associations of PFAS exposure with less-favorable lipids profiles (i.e., higher total cholesterol and triglycerides). The prospective epidemiologic studies on this topic to date have used a population-mean approach to analysis instead of a trajectory-based approach, which allows researchers to capture heterogeneity in lipid trajectory in the population.
Women in menopausal transition undergo important physiological changes (e.g., changes in hormones and lipids levels),23,24 and they have substantial heterogeneity in such changes.23,25 Because of the physiological changes across the menopausal transition, this life stage may be particularly vulnerable for exposure to factors that may lead to metabolic alterations. Moreover, serum PFAS concentrations increase in postmenopausal women because of cessation of menstruation.5,26,27 However, little is known about the potential role of PFAS in longitudinal changes in lipid profiles during this critical life stage except a study reporting a weak association of perfluorooctanoic acid (PFOA) exposure with hypercholesterolemia among women age 40–59 y.22 To address this data gap, we evaluated serum PFAS concentrations at baseline with trajectories of lipids measured for about 15 y of follow-up in a cohort of women 45–56 y of age, with a hypothesis that PFAS exposure has association with less-favorable lipid trajectories.
Methods
Study Population
The Study of Women’s Health Across the Nation (SWAN) is a multisite, multiethnic prospective cohort study designed to examine biological and psychosocial changes during menopausal transition and the association of this transition with age-related health in midlife women.28 In 1996–1997, SWAN recruited a total of 3,302 women from seven study sites in the United States, including Black women (from Boston, Massachusetts, Pittsburgh, Pennsylvania, Southeast Michigan, Michigan, and Chicago, Illinois), Hispanic women (from Newark, New Jersey), Chinese women (from Oakland, California), Japanese women (from Los Angeles, California), and White women (from all seven sites). Women were eligible to participate when they were 42–52 y of age, did not currently use exogenous hormone affecting ovarian function, had intact uterus and both ovaries, had at least one menstrual period in the previous 3 months, and self-identified as one of the above race or ethnicity categories at a given study site. During the follow-up in 2016–2017, participants nearly annually underwent questionnaire surveys, anthropometry, and collection of biological specimens (urine and serum) for the assessment of health status and related risk factors. The institutional review board at each study site approved the study protocol; all participants provided written informed consent at each study visit.
The SWAN-Multi-Pollutant Study (SWAN-MPS) was designed to evaluate the roles of various environmental pollutants, including PFAS, in metabolic and reproductive health outcomes during the menopausal transition.8 The SWAN-MPS measured environmental pollutants in archived urine or serum samples from the third SWAN follow-up (Visit 3, 1999–2000, ), which was set as SWAN-MPS baseline. Participants from the Chicago () and Newark () sites were not included in the SWAN-MPS because urine samples were not collected at these sites. Because chemicals of interest in the SWAN-MPS included urinary biomarkers, additional 648 women with insufficient serum or urine samples were excluded, yielding a base sample of 1,400 participants including 708 White women, 308 Black women, 177 Chinese women, and 207 Japanese women from five study sites (233 from Boston, 235 from Pittsburgh, 257 from Southeast Michigan, 309 from Oakland, 366 from Los Angeles). For the present study, we also excluded 270 participants having fewer than six repeated measurements of fasting blood lipids to better capture fluctuating lipid trajectories during the menopausal transition, yielding 1,130 SWAN-MPS women with at least six repeated fasting lipid measurements. These 1,130 participants had complete information on the covariates included in data analysis.
Measurement of Serum PFAS Concentrations
Quantification of PFAS in serum was conducted at the Division of Laboratory Sciences at the U.S. Centers for Disease Control and Prevention (U.S. CDC). The U.S. CDC laboratory’s involvement did not constitute engagement in human-subjects research. We used a modification of a previously described method.29 In brief, online solid-phase extraction coupled with high-performance liquid chromatography–isotope dilution tandem mass spectrometry was used to quantify serum concentrations of linear PFOA (n-PFOA), sum of branched PFOA isomers (Sb-PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoDA), perfluorohexane sulfonic acid (PFHxS), linear perfluorooctanesulfonic acid (n-PFOS), sum of perfluoromethylheptane sulfonic acid isomers (Sm-PFOS), 2-(N-methyl-perfluorooctane sulfonamido) acetic acid (MeFOSAA), and 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid (EtFOSAA). Internal standards, including , , , , , , , were used for quality assurance purposes. The coefficients of variation of quality control samples of low and high concentrations ranged from 5.9% to 12.1%, depending on the analyte. The limit of detection (LOD) was for all analytes. Concentrations below the LOD of PFAS with detection frequencies (n-PFOA, PFNA, PFHxS, n-PFOS, Sm-PFOS, MeFOSAA, EtFOSAA; Table S1) were imputed with LOD divided by square root of 2 for analysis.30 We did not include Sb-PFOA and PFDoDA in data analysis because of low detection frequencies (). PFDA and PFUnDA with detection frequencies of 40.4% and 31.2%, respectively, were included in data analysis as categorical variables (“detected” and “nondetected”). Previous studies have mainly focused on PFOA, PFNA, n-PFOS, Sm-PFOS, and PFHxS. Therefore, results of these PFAS were shown in the main text, and results for the other PFAS (i.e., PFDA, PFUnDA, MeFOSAA, EtFOSAA) were shown in the Supplemental Material.
Measurement of Blood Plasma Lipids
Methods for lipids measurements were previously described in detail.24,25 In brief, concentrations of lipids were determined in ethylenediamine tetraacetic acid–treated plasma. Total cholesterol and triglycerides were measured by enzymatic methods. Concentration of HDL cholesterol was determined by heparin-manganese precipitation procedure or the method of Izawa et al.31 LDL cholesterol was calculated by the Friedewald equation.32 Measurement of lipids were available only at visit 3 (SWAN-MPS baseline; 1999–2001), visit 4 (2000–2002), visit 5 (2001–2003), visit 6 (2002–2004), visit 7 (2003–2005), visit 9 (2005–2007), visit 12 (2009–2011), visit 13 (2011–2013), and visit 15 (2015–2016) because of fiscal limitations (Table S2).
Covariates
Sociodemographic factors including race or ethnicity, age (continuous, years), education [, some college (college attended but no degree obtained; associate degree), college graduate, postgraduate] were ascertained by self-administered questionnaire at SWAN baseline. Because race and ethnicity and study site may have interaction effects on PFAS concentrations or lipids, we combined race and ethnicity and study site into a 10-level variable (White participants from five sites; Black participants from three sites, Chinese and Japanese participants from one site each).8 Menopausal status (pre- or early perimenopause, late peri- or postmenopause, unknown due to hormone therapy, surgical menopause) was based on bleeding patterns, hormone use, and surgical history (bilateral salpingo-oophorectomy or hysterectomy). Pre- or early perimenopause was defined as having menses within the past 3 months. Late peri- or postmenopause was defined as 3 consecutive months of amenorrhea without other causes such as hormone therapy or surgical menopause. Smoking status (never, former, current smoker), alcohol consumption (0, drinks/wk), and lipid-lowering medication use (no use, statins use, nonstatin medication use, both statin and nonstatin medication use) were collected using standardized questionnaires at the visits when blood lipids were measured. Body mass index (BMI) was calculated as measured weight (kilograms per square meter) at visit 3. Physical activity score, which ranged from 3 to 15 (higher scores indicating higher activity), was assessed using a modified version of the Kaiser Physical Activity Survey.33,34 Physical activity included sports, exercise, household, childcare, and daily routine. Total energy intake was obtained from intake of each food item based on a modified Block food frequency questionnaire.35 For trajectory analyses, we included lipid-lowering medication use obtained at the visits when blood lipids were measured. For association analyses, we used covariates measured at visit 3 (SWAN-MPS baseline) but total energy intake measured at SWAN baseline (1996–1997), because dietary assessment was not conducted at visit 3 (Table S2). We did not consider time-varying BMI or menopausal status because serum PFAS concentrations were associated with longitudinal changes in body size/composition36 and age at menopause37 in the same cohort, suggesting that these factors could be mediators.
Statistical Analyses
All statistical analysis was performed using R (version 4.1.1; R Development Core Team). Multiple comparison was addressed by calculating adjusted -values using the Benjamini–Hochberg Method38 based on false discovery rate of 0.05. PFAS concentrations showed right-skewed distributions and were log-transformed with base 2 before analyses. Triglycerides were also log-transformed to normalize the distribution, but other lipids that were normally distributed were not log-transformed. PFAS concentrations were also categorized into tertiles to be associated with lipids trajectories.
Latent class growth model (LCGM) was used to identify trajectories of each lipid. LCGM is a group-based trajectory model, which allow us to cluster the individuals with similar trajectories of a given variable.39,40 Functions “stepFlexmix” and “FLXMRglmfix” in R package “flexmix” (version 2.3-17)39 was used for LCGM. We assumed a different number of latent classes, ranging from two to four. The data were randomly assigned to the classes, and a model was estimated for each class based on maximum likelihood and expectation-maximization algorithm with at most 100 iterations. To prevent local maxima, this process was repeated 100 times with different starting values.39 In the models, time since SWAN-MPS baseline was included as an independent variable and blood levels of each lipid as dependent variables. To account for the effect of lipid-lowering medications, we used two approaches. Wu et al. (2007)41 reviewed published clinical trials and proposed constant values to impute the original lipid levels without lipid-lowering medication. As a primary approach, we added the sensible constant values suggested by Wu et al. (2007) to the measured lipid levels of those with lipid-lowering medication (“Adding constant method”; Table S3). Then, the imputed lipid levels were included in the models as dependent variables. The secondary approach was to fit measured lipids levels as dependent variables and lipid-lowering medication use (no use, use of statins, use of nonstatin medication, use of both medications at each visit) as a covariate (“Covariate method”).42 To determine the best fitting LCGM models, we considered multiple models with different numbers of classes from two to four and different time terms (a linear and/or quadratic term or restricted cubic spline with 2–4 knots placed at quantile or equidistant points). Among the multiple models (total 27 models for each lipid; see Tables S4–S7), only models with posterior probability and a class size of the population remained.43 Among the remaining models, the best fitting model was selected based on Bayesian information criterion (BIC).43
Univariate statistics were calculated by lipid trajectory classification; participants having at least one of the less-favorable lipid trajectories (high total cholesterol trajectory, high LDL cholesterol trajectory, low HDL cholesterol trajectory, or high triglycerides trajectory) were classified as “less favorable lipid profile,” and those not having any of the less-favorable lipid trajectories were classified as “more favorable lipid profile.” For comparison, chi-square and Wilcoxon rank-sum tests were used for categorical and continuous variables, respectively. We calculated Spearman’s correlation coefficients between the serum concentrations of PFAS.
To examine association between PFAS serum concentrations and trajectories of each lipid, we estimated odds ratios (ORs) of latent class membership (the low class as the reference) using multinomial log-linear models with -transformed PFAS as exposure. To examine nonlinear association of PFAS, we categorized PFAS concentrations into tertiles. ORs and 95% confidence intervals (95% CIs) of latent class membership (medium and high) of lipids were calculated for a doubling of increase or tertiles of PFAS concentrations. The models were adjusted for a combination of race or ethnicity and study site (10 categories) to consider confounding by race or ethnicity and site effects as well as their interaction. Baseline characteristics including age, education, menopausal status, smoking status, alcohol consumption, BMI, physical activity, and total energy intake were also included as covariates in the models. These covariates were selected based on prior knowledge. Because BMI may mediate the associations between PFAS exposure and metabolic outcomes,44 we conducted a sensitivity analysis without BMI as a covariate to check the impact of BMI adjustment.
Given the high correlations between serum PFAS concentrations (Figure S1), we assessed effects of PFAS mixture in association with lipid trajectories using k-means clustering. As we examined multiple outcome variables (trajectories of four lipids), supervised methods such as weighted quantile sum regression or quantile g-computation are not appropriate in this study because the created chemical mixture would vary depending on the lipids. We chose k-means clustering, which is an unsupervised method and therefore creates a single mixture variable independent of outcome variables. K-means clustering is a nonparametric classification method that creates a categorical variable representing k clusters as minimizing within-cluster variances. K-means clustering was conducted for the PFAS with detection frequencies (n-PFOA, PFNA, n-PFOS, Sm-PFOS, PFHxS, MeFOSAA, EtFOSAA) after log-transformation and standardization of their concentrations. We used “kmeans” function in R package “stats.” The maximum number of iterations was set as 10, and Hartigan–Wong algorithm was used. The number of clusters (k) was chosen based on cubic clustering criterion, pseudo F statistics, elbow method, and interpretability. Associations of the clusters with lipid trajectories were examined by multinomial log-linear models adjusted for the same set of covariates.
We additionally assessed cross-sectional associations between serum PFAS concentrations with baseline blood lipid levels (Visit 3) or rate of change in lipid levels, and the results are provided as Supplemental Tables. Lipid levels imputed by “Adding constant method” were used for these analyses. Rate of change was calculated by the following equation:
| (1) |
where we considered the change in lipids from baseline (Visit 3) to Visit 6, Visit 9, and Visit 12, which, respectively, corresponded on average to 3.01, 5.98, and 10.69 y of follow-up. Linear regression was used to associate the blood lipid levels or rate of change in lipid levels with serum PFAS concentrations. The models were adjusted for the same sets of covariates included in the multinomial log-linear models. The threshold for statistical significance was set at 2-sided .
Results
Lipid Trajectories and Participants’ Characteristics
Three trajectories of total, LDL, and HDL cholesterol (denoted “Low,” “Middle.” and “High”), and two trajectories of triglycerides (denoted “Low” and “High”) were identified by latent class growth models with “adding constant method” (Figure 1). No trajectories overlapped during the follow-up. For total and LDL cholesterol, the high trajectory groups (19%) were characterized by an increasing trend at the beginning followed by decreasing and constant trajectories (Figure 1A,B). The middle (48%–49%) and low (32%–33%) trajectory groups started with an increasing trend and then remained constant. It is noteworthy that the high trajectory groups showed peak concentrations at the early period of the follow-up ( and y since baseline in total and LDL cholesterol trajectories, respectively), whereas there was no such peak in other groups. HDL cholesterol trajectories showed fluctuating trends over time (Figure 1C). The high trajectory group of triglycerides (37%) showed a decreasing trend, whereas the low trajectory group (63%) remained constant (Figure 1D). Similar trajectories were obtained using the “covariate method.” About 95% of the participants were classified into same group memberships when using these two different methods (Figure S2 and Table S8).
Figure 1.
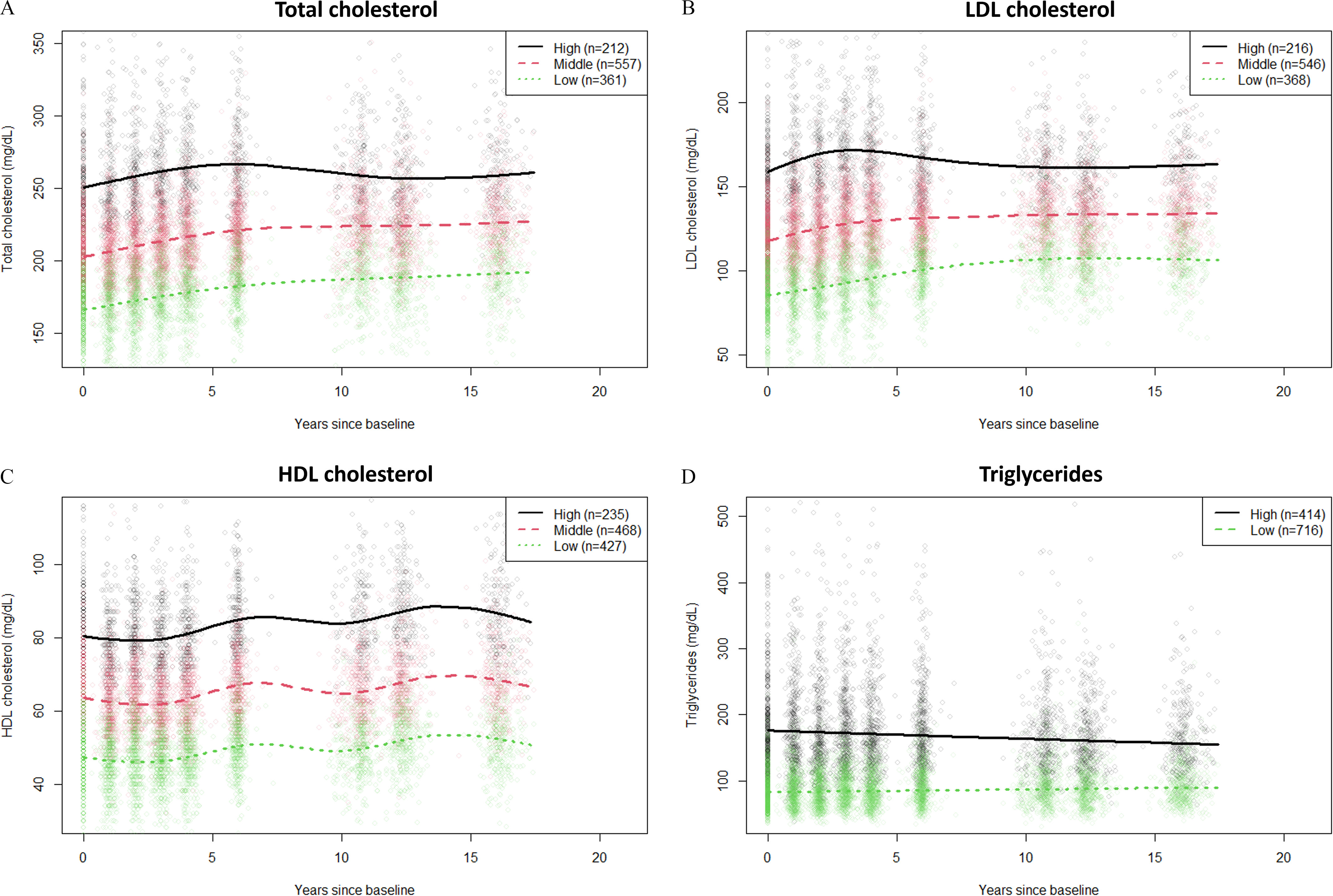
Trajectories of (A) total cholesterol, (B) LDL cholesterol, (C) HDL cholesterol, and (D) triglycerides identified by latent class growth models in the SWAN-MPS cohort (1999–2016, ). Lipid concentrations of participants taking lipid-lowering medication were imputed by the “adding constant method” described in the main text. Note: HDL, high-density lipoprotein; LDL, low-density lipoprotein; SWAN-MPS, SWAN-Multi-Pollutant Study.
Baseline characteristics of the study population are summarized in Table 1. Site and race/ethnicity were statistically significantly associated with less/more favorable lipid profile categorization. Women from the Oakland site and of Chinese race/ethnicity were more likely to have more favorable lipid profiles, whereas women from the Southeast Michigan site and of Black race/ethnicity were more likely to have less-favorable lipid profiles. Participants with more favorable lipid profiles tended to be of younger age, have higher education levels, have lower BMI, and reported no alcohol consumption in comparison with women with less-favorable lipid profiles.
Table 1.
Baseline characteristics of study participants in the SWAN-MPS cohort (1999–2016, ).
| Characteristics | (%) or median (interquartile range) | -Valuec | ||
|---|---|---|---|---|
| Total () | More-favorable lipid profile ()a |
Less-favorable lipid profile ()b | ||
| Site | 0.0008 | |||
| Los Angeles, CA | 320 (28) | 140 (29) | 180 (28) | |
| Oakland, CA | 249 (22) | 123 (26) | 126 (19) | |
| Southeast Michigan | 202 (18) | 64 (13) | 138 (21) | |
| Pittsburgh, PA | 188 (17) | 71 (15) | 117 (18) | |
| Boston, MA | 171 (15) | 83 (17) | 88 (14) | |
| Race/ethnicity | 0.007 | |||
| White | 579 (51) | 247 (51) | 332 (51) | |
| Black | 231 (20) | 81 (17) | 150 (23) | |
| Chinese | 138 (12) | 74 (15) | 64 (10) | |
| Japanese | 182 (16) | 79 (16) | 103 (16) | |
| Education | 0.01 | |||
| diploma | 186 (16) | 69 (14) | 117 (18) | |
| Some college | 355 (31) | 134 (28) | 221 (34) | |
| College | 287 (25) | 138 (29) | 149 (23) | |
| Postgraduate | 302 (27) | 140 (29) | 162 (25) | |
| Menopausal status | 0.65 | |||
| Pre-/early perimenopause | 717 (63) | 312 (65) | 405 (62) | |
| Late peri-/postmenopause | 214 (19) | 92 (19) | 122 (19) | |
| Unknown (hormone therapy) | 160 (14) | 63 (13) | 97 (15) | |
| Surgical menopause | 39 (3) | 14 (3) | 25 (4) | |
| Smoking | 0.08 | |||
| Never smoker | 721 (64) | 311 (65) | 410 (63) | |
| Former smoker | 307 (27) | 137 (28) | 170 (26) | |
| Current smoker | 102 (9) | 33 (7) | 69 (11) | |
| Alcohol consumption | ||||
| No (0 drinks/wk) | 600 (53) | 216 (45) | 384 (59) | |
| Yes ( drinks/wk) | 530 (47) | 265 (55) | 265 (41) | |
| Lipid-lowering medication | ||||
| No | 1,098 (97) | 479 (100) | 619 (95) | |
| Yes | 32 (3) | 2 (0) | 30 (5) | |
| Age (y) | 49.5 (47.4–51.5) | 49.2 (47.3–51.0) | 49.7 (47.4–51.8) | 0.009 |
| BMI () | 26.0 (22.4–31.5) | 23.6 (21.1–27.6) | 28.0 (24.0–33.2) | |
| Total energy intake (kcal) | 1,691 (1,341–2,170) | 1,666 (1,332–2,105) | 1,722 (1,358–2,228) | 0.07 |
| Total cholesterol () | 195 (174–219) | 184 (168–201) | 205 (184–233) | |
| LDL cholesterol () | 112 (93–131) | 100 (83–115) | 123 (104–144) | |
| HDL cholesterol () | 60 (50–71) | 68 (60–78) | 52 (46–61) | |
| Triglycerides () | 98 (73–142) | 76 (62–94) | 127 (94–180) | |
| n-PFOA () | 4.1 (2.8–5.8) | 4.0 (2.9–5.5) | 4.3 (2.8–6.2) | 0.02 |
| PFNA () | 0.6 (0.4–0.8) | 0.6 (0.4–0.8) | 0.6 (0.4–0.8) | 0.45 |
| n-PFOS () | 17.4 (12.5–24.4) | 16.8 (12.2–23.2) | 17.9 (12.7–25.4) | 0.07 |
| Sm-PFOS () | 7.4 (4.7–9.2) | 6.6 (4.5–9.7) | 7.7 (5.1–11.5) | 0.0008 |
| PFHxS () | 1.5 (1.0–2.4) | 1.5 (1.0–2.3) | 1.5 (1.0–2.5) | 0.38 |
Note: n (%) for categorical variables; median (interquartile range) for continuous variables. BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; n-PFOA, linear perfluorooctanoic acid; n-PFOS, linear perfluorooctanesulfonic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; Sm-PFOS, sum of perfluoromethylheptane sulfonic acid isomers; SWAN-MPS, SWAN-Multi-Pollutant Study.
Not having in any of the less-favorable trajectories (high total cholesterol, high LDL cholesterol, low HDL cholesterol, or high triglycerides).
Having in at least one of the less-favorable trajectories (high total cholesterol, high LDL cholesterol, low HDL cholesterol, or high triglycerides).
Chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables were used.
Associations of Lipid Trajectories with Serum Concentrations of Individual PFAS
When serum PFAS concentrations were considered continuously, ORs and their 95% CIs for high vs. low LDL cholesterol trajectories per doubling of PFAS serum concentrations were 1.28 (95% CI: 1.04, 1.57) for n-PFOS and 1.25 (95% CI: 1.05, 1.49) for Sm-PFOS (Table 2). Sm-PFOS and PFHxS concentrations were associated with total cholesterol trajectories [ORs and CIs for high vs. low triglycerides trajectories per doubling: 1.20 (95% CI: 1.00, 1.44) for Sm-PFOS; and 1.17 (95% CI: 1.00, 1.36) for PFHxS], but these associations became nonsignificant after controlling for multiple comparisons. On the contrary, a doubling of PFNA concentration was inversely associated, with (95% CI: 0.72, 0.98) for high vs. low triglycerides trajectories, though this association also became nonsignificant with multiple comparison corrections. Detection of PFDA and PFUnDA was positively associated with trajectories of total or LDL cholesterol, whereas MeFOSAA was positively associated with middle vs. low LDL cholesterol trajectories (Table S9). The detection of PFUnDA was also positively associated with middle vs. low HDL cholesterol trajectories. The detection of PFDA and doubling of EtFOSAA were positively associated with triglycerides trajectory.
Table 2.
Associations of serum PFAS concentrations with trajectories of total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides in the SWAN-MPS cohort (1999–2016, ).
| PFAS | Total cholesterol | LDL cholesterol | HDL cholesterol | Triglycerides | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trajectory | OR (95% CI)a | p-Valueb | Trajectory | OR (95% CI)a | p-Valueb | Trajectory | OR (95% CI)a | p-Valueb | Trajectory | OR (95% CI)a | p-Valueb | |
| n- PFOA | Low () | 1.00 | — | Low () | 1.00 | — | Low () | 1.00 | — | Low () | 1.00 | — |
| Middle () | 0.99 (0.84, 1.18) | 0.95 | Middle () | 1.03 (0.87, 1.22) | 0.74 | Middle () | 0.99 (0.84, 1.17) | 0.96 | High () | 0.86 (0.73, 1.02) | 0.16 | |
| High () | 0.96 (0.78, 1.18) | 0.70 | High () | 1.00 (0.82, 1.23) | 0.99 | High () | 1.02 (0.82, 1.26) | 0.95 | — | — | — | |
| PFNA | Low () | 1.00 | — | Low () | 1.00 | — | Low () | 1.00 | — | Low () | 1.00 | — |
| Middle () | 1.07 (0.91, 1.25) | 0.58 | Middle () | 1.10 (0.94, 1.29) | 0.27 | Middle () | 1.13 (0.96, 1.34) | 0.65 | High () | 0.84 (0.72, 0.98) | 0.12 | |
| High () | 1.21 (0.98, 1.49) | 0.11 | High () | 1.18 (0.96, 1.44) | 0.17 | High () | 1.13 (0.92, 1.39) | 0.62 | — | — | — | |
| n-PFOS | Low () | 1.00 | — | Low () | 1.00 | — | Low () | 1.00 | — | Low () | 1.00 | — |
| Middle () | 1.08 (0.92, 1.27) | 0.57 | Middle () | 1.14 (0.97, 1.34) | 0.17 | Middle () | 1.04 (0.88, 1.22) | 0.96 | High () | 0.86 (0.74, 1.01) | 0.16 | |
| High () | 1.22 (0.99, 1.49) | 0.11 | High () | 1.28 (1.04, 1.57) | 0.04 | High () | 0.99 (0.80, 1.23) | 0.95 | — | — | — | |
| Sm-PFOS | Low () | 1.00 | — | Low () | 1.00 | — | Low () | 1.00 | — | Low () | 1.00 | — |
| Middle () | 1.10 (0.96, 1.26) | 0.46 | Middle () | 1.19 (1.03, 1.36) | 0.15 | Middle () | 0.96 (0.83, 1.11) | 0.96 | High () | 0.97 (0.84, 1.10) | 0.72 | |
| High () | 1.20 (1.00, 1.44) | 0.11 | High () | 1.25 (1.05, 1.49) | 0.04 | High () | 0.95 (0.79, 1.13) | 0.91 | — | — | — | |
| PFHxS | Low () | 1.00 | — | Low () | 1.00 | — | Low () | 1.00 | — | Low () | 1.00 | — |
| Middle () | 1.16 (1.02, 1.31) | 0.12 | Middle () | 1.12 (0.99, 1.26) | 0.15 | Middle () | 1.07 (0.94, 1.21) | 0.85 | High () | 0.97 (0.86, 1.10) | 0.72 | |
| High () | 1.17 (1.00, 1.36) | 0.11 | High () | 1.11 (0.96, 1.30) | 0.20 | High () | 1.15 (0.98, 1.34) | 0.45 | — | — | — | |
Note: —, no data; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; n-PFOA, linear perfluorooctanoic acid; n-PFOS, linear perfluorooctanesulfonic acid; OR, odds ratio; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; Sm-PFOS, sum of perfluoromethylheptane sulfonic acid isomers; SWAN-MPS, SWAN-Multi-Pollutant Study: .
ORs and their 95% CIs per doubling of each PFAS concentration.
Adjusted -value based on false discovery rate. The models were adjusted for , age, education, menopausal status, smoking status, alcohol consumption, BMI, physical activity, and total energy intake.
When PFAS concentrations were categorized into tertiles, n-PFOS, Sm-PFOS, PFHxS, and MeFOSAA were positively associated with trajectories of total and LDL cholesterol, whereas EtFOSAA showed an inverse association with trajectories of total cholesterol (Figure 2A,B; Tables S10 and S11). The ORs (95% CIs) for high vs. low trajectories of total cholesterol were: 1.61 (1.27, 2.06) for n-PFOS, 1.65 (1.29, 2.11) for Sm-PFOS, 1.63 (1.29, 2.05) for PFHxS, 1.50 (1.18, 1.91) for MeFOSAA, and 0.76 (0.59, 0.97) for EtFOSAA. The OR (95% CI) for high vs. low trajectories of LDL cholesterol were: 1.89 (1.50, 2.38) for n-PFOS, 1.92 (1.52, 2.42) for Sm-PFOS, 1.41 (1.12, 1.78) for PFHxS, and 1.43 (1.13, 1.81) for MeFOSAA. PFNA showed nonmonotonous associations with total and LDL cholesterol trajectories (Figure 2A,B). Sm-PFOS was inversely associated with trajectories of HDL cholesterol with OR for high vs. low trajectories of 0.57 (95% CI: 0.44, 0.75) (Figure 2C; Table S12). n-PFOS was inversely associated with trajectories of triglycerides with OR for high vs. low trajectories of 0.71 (95% CI: 0.51, 0.99), but this association became nonsignificant after controlling for multiple comparisons (Figure 2D; Table S13). The sensitivity analysis without BMI as a covariate generally showed minuscule effects on the estimates, but the inverse associations of n-PFOS (tertile) and Sm-PFOS (continuous) with HDL cholesterol became significant after removing BMI from the models (Figure S3; Table S14).
Figure 2.
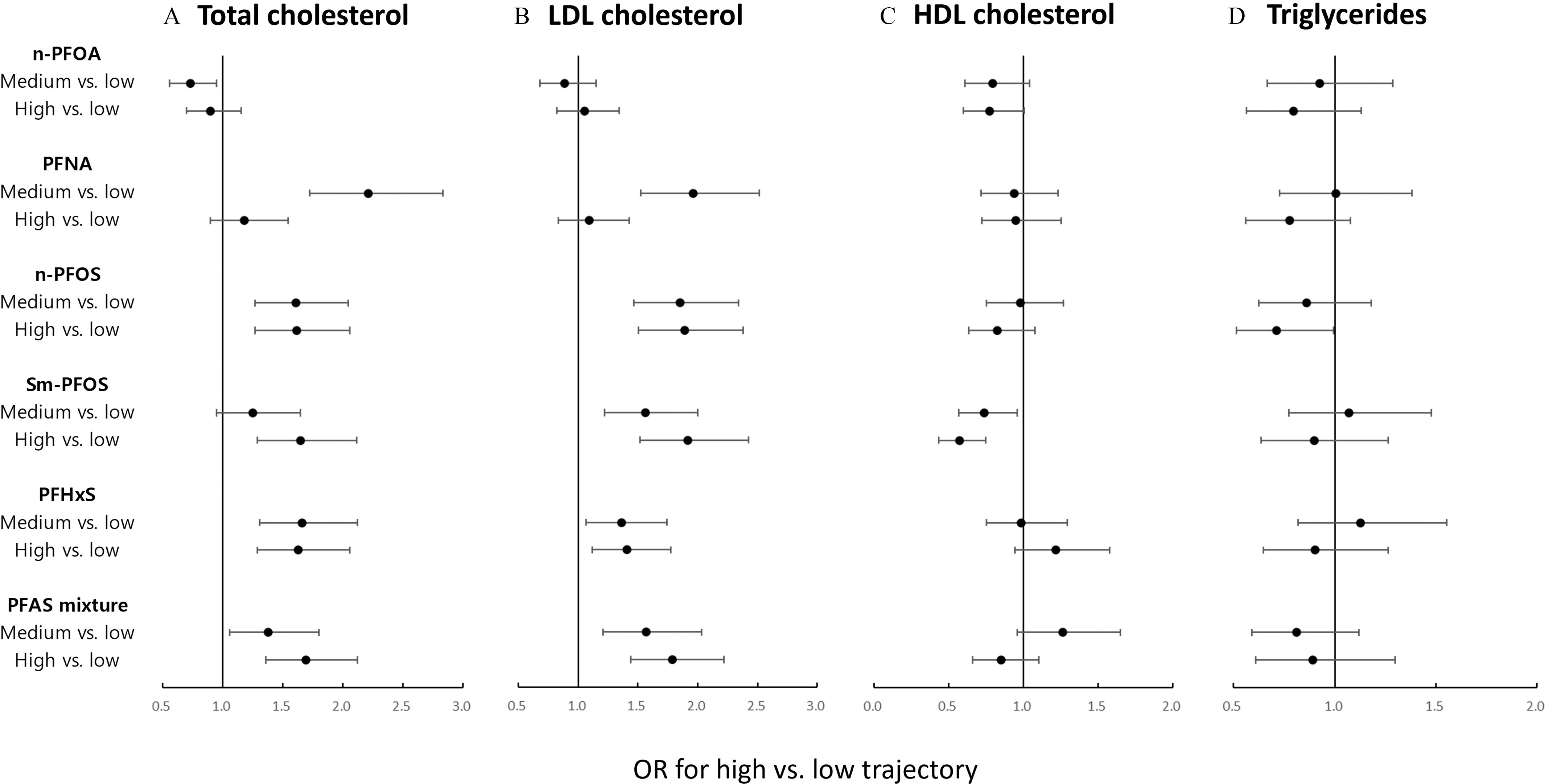
ORs (dot) and their 95% CIs (bar) for associations of tertiles of PFAS concentrations (vs. tertile 1) or PFAS mixture (vs. low concentration) with trajectories (high vs. low trajectories) of (A) total cholesterol, (B) LDL cholesterol, (C) HDL cholesterol, and (D) triglycerides in the SWAN-MPS cohort (1999–2016, ). The models were adjusted for , age, education, menopausal status, smoking status, alcohol consumption, BMI, physical activity, and total energy intake. Cutoff points for PFAS tertile groups: 3.4 and for n-PFOA, 0.6 and for PFNA, 14.2 and for n-PFOS, 5.7 and for Sm-PFOS, and 1.2 and for PFHxS. The ORs and their 95% CIs can also be found in Supplemental Tables S10, S11, S12, and S13. Note: CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; n-PFOA, linear perfluorooctanoic acid; n-PFOS, linear perfluorooctane sulfonic acid; OR, odds ratio; PFAS, per- and polyfluoroalkyl substances; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOS, perfluorooctanesulfonic acid; Sm-PFOS, sum of perfluoromethylheptane sulfonic acid isomers; SWAN-MPS, SWAN-Multi-Pollutant Study.
Mixture Effects of PFAS on Lipid Trajectories
K-means clustering identified three PFAS mixture clusters representing low (), medium (), and high concentrations () of individual PFAS, respectively (Figure S4). PFAS mixture was associated with higher trajectories of total and LDL cholesterol (Figure 2A,B). Comparing high PFAS concentrations with low PFAS concentrations, OR for high vs. low trajectories of total cholesterol was 1.69 (95% CI: 1.36, 2.12), and OR for high vs. low trajectories of LDL cholesterol was 1.79 (95% CI: 1.44, 2.22). The OR for high vs. low trajectories of HDL cholesterol was significant only when BMI was not included in the model [ (95% CI: 0.49, 0.81)] (Figure 2C; Figure S2C). Associations between PFAS mixture and triglycerides trajectories were not significant (Figure 2D).
Associations of Serum Concentrations of PFAS with Baseline Lipids and Rate of Change in Lipids
In the cross-sectional analysis at baseline (Visit 3), serum PFAS concentrations were not associated with total, LDL, or HDL cholesterol, whereas n-PFOA, PFNA, and n-PFOS were inversely associated with triglycerides (Table S15). Serum PFAS concentrations were not associated with rate of change in total or LDL cholesterol during follow-up, whereas n-PFOS was inversely associated with rate of change in HDL cholesterol from baseline to Visit 6 (Table S16). n-PFOS was also positively associated with rate of change in triglycerides from baseline to Visit 12.
Discussion
In this prospective study of a population of women age 45–56 y, we found serum concentrations of several PFAS (including PFAS mixture groups clustered by k-means algorithm) was predictive of higher total and LDL cholesterol trajectories, which were characterized by an early high peak in the trajectories. We also found that Sm-PFOS were inversely associated with HDL cholesterol trajectory.
The associations of several individual PFAS and PFAS mixture with total and LDL cholesterol trajectories observed in this study are generally in line with previous epidemiological findings (Table S17),2,10 providing a line of evidence that supports harmful effects of PFAS on blood cholesterol profile. Positive associations of blood concentrations of PFAS (e.g., PFOA, PFOS) with blood total or LDL cholesterol levels have been consistently reported in cross-sectional studies of general populations.12–14,16,45,46 Similar observations have been reported in longitudinal studies. Occupational exposure to PFOA was positively associated with higher level of total cholesterol.19,20 PFOA/PFOS exposure has been associated with higher levels of total and/or LDL cholesterol among residents living in a PFAS-contaminated area.21,22 Associations of PFAS exposure with higher cholesterol have been reported in recent longitudinal studies with general populations such as U.S. adults with prediabetes status () or Swedish older adults (),6,17 although insignificant associations have been also reported in Swedish adults ().47
Our findings on total and LDL cholesterol are supported by results from relevant experimental studies. Alterations in gene or protein expression regarding lipid metabolism was observed along with lipid accumulation in 3T3-L1 preadipocytes exposed to PFAS such as PFOA, PFOS, and PFHxS during their adipocyte differentiation.48,49 Although rodents studies generally showed lower serum cholesterol levels after PFAS exposure,50 results from mouse studies with human-relevant diet (i.e., high fat diet) were generally aligned with our observations, showing increased serum cholesterol levels after the exposure.51,52 Several mechanisms for PFAS-induced cholesterol dysregulation have been suggested. PFAS have been reported to be associated with liver injury, and PFAS-induced hepatic lipid accumulations have been shown in experiments with mice,53 including peroxisome proliferator-activated receptor or humanized mice,54,55 Because of the link between PFAS and liver injury,9 researchers have suggested that liver injury may be mediating the relationship between PFAS and increased cholesterol levels. Other possible mechanisms include epigenetic control of lipid metabolism56,57 and activation of nuclear receptors such as constitutive androstane receptor, pregnane X receptor, and liver X receptor.52,58
Sex hormone disrupting effects of PFAS may be another potential mechanism underlying their effects on lipid regulation. It is known that estrogen decreases circulating LDL cholesterol and increases HDL cholesterol.59 Ovariectomized rats showed increased cholesterol blood levels, which were decreased by estrogen treatment.60,61 Stimulation of hepatic LDL receptor62 and hepatic lipase63 and enhanced cholesterol efflux from peripheral tissues64 are proposed mechanisms for the effects of estrogen on cholesterol. Total and LDL cholesterol levels increase as estrogen decreases during the menopausal transition.23,24 Estrogen therapy in postmenopausal women increased their LDL cholesterol levels and decreased HDL cholesterol levels.65 On the other hand, PFOS has been inversely associated with estrogen levels in female populations.11 In a previous analysis of the SWAN-MPS PFAS data, PFOA and PFNA were inversely associated with estradiol, although the association between PFOS and estradiol was not significant.66 Given the association of PFAS exposure with estrogen and the effects of estrogen on cholesterol regulation, we hypothesize that PFAS exposure increases blood total and LDL cholesterol levels mediated by a PFAS-induced decrease in estrogen. However, exposure to PFOA or PFOS increased estrogen levels in rats67 and a human adrenal carcinoma cell line.68 Species difference in PFAS-induced estrogen change may be another reason for the discrepant associations of PFAS with cholesterol between humans and animals, but further investigation is needed.
The direction of the associations of PFAS with HDL cholesterol or triglycerides in previous epidemiological studies has been mixed (Table S17),2,10 but longitudinal studies mostly have shown primarily associations of serum PFAS with higher triglycerides levels. PFOA, PFNA, PFOS, and PFHxS were associated with hypertriglyceridemia among U.S. adults with prediabetes status ().17 Similar positive associations of serum PFAS and triglycerides were observed in longitudinal studies of Swedish older adults (),6 U.S. adults exposed to PFOA contaminated drinking water (),21 and occupationally exposed workers (),19 whereas a study of Swedish adults () reported inverse associations of PFNA and PFOS with triglycerides.47 It is unclear whether our observation of the inverse association of Sm-PFOS with HDL cholesterol trajectory was by chance or a result of a causal relationship. Further investigation may help to understand the reasons behind this observation.
It was an unexpected finding that the second tertile of n-PFOA (vs. first tertile) was inversely associated with the odds of high trajectory of total cholesterol. We do not have clear reasons for this observation. This inverse association with total cholesterol trajectory might be partly attributed to the inverse association of PFOA with HDL cholesterol, although it is known that the majority of total cholesterol consists of LDL cholesterol rather than HDL cholesterol.69 This might also be a result of chance, given that the association was nonlinear (only significant in the second tertile, not in the third tertile).
This study has numerous strengths. First, to the best of our knowledge, this is the first study investigating associations of PFAS exposure with lipids trajectories in midlife women. The trajectory analysis of this study allowed us to incorporate not only the levels of lipids but also the change patterns in lipids with PFAS exposure. For example, we identified that the high LDL cholesterol trajectory had an early rapid increase and a subsequent decrease, whereas the low trajectory had a slow increase. Because of such fluctuations in lipids in midlife women and individual differences in the patterns, a conventional population-mean approach with rate of changes in lipids levels during a certain period of time, as done in several previous studies, may not fully capture complex patterns in lipid change, which may vary between individuals. Our cross-sectional analyses using the population-mean approach did not show any significant associations of PFAS concentrations with baseline LDL cholesterol or the rate of change in LDL cholesterol during follow-up (Tables S15 and S16). These results emphasize the importance of using group-based trajectory analysis to secure a comprehensive understanding of the relationship between PFAS exposure and longitudinal changes in lipids.
Second, we accounted for lipid-lowering medication. Studies with lipid outcomes often exclude participants with lipid-lowering medication, which can, however, lead to selection bias.70 We accounted for the effects of lipid-lowering medication using two different methods, which yielded similar classifications of lipids trajectories. Third, we evaluated multiple PFAS compounds as a mixture. We used k-means clustering to identify three clusters of PFAS mixtures (low, medium, high). The observed monotonous positive associations between PFAS mixture clusters and total and LDL cholesterol trajectories further our understanding of the combined effects of PFAS on lipid disruption. In addition to previous studies using k-means clustering for PFAS mixture,37,71 our results suggest that k-means clustering is a useful method to cluster the participants with similar exposure status. Fourth, the present study was based on a multiethnic population with about half of the participants from minority race or ethnicity groups. There are limitations to this study. First, PFAS were measured only once at baseline. Although most PFAS considered in this study are biologically persistent with half-lives of the order of several years, our previous analysis of a subpopulation of SWAN-MPS () showed relatively low intraclass correlation coefficients among four repeated measurements of PFAS in serum collected 1999 through 2011, indicating relatively high within-subject variability over time.5 Therefore, a single measurement of PFAS at baseline might not be sufficient to represent overall PFAS exposure through follow-up. Second, serum PFAS concentrations can be affected by kidney function,27 which may lead to exposure assessment measurement error and associations toward null. However, even though we lacked information on kidney function, prevalence of kidney disease among women age 45–56 y is expected to be small. Third, although the present study was conducted in a racially/ethnically diverse female population, the results of this study may not be generalizable to males and other racial/ethnic groups, in particular Hispanic populations. Finally, we cannot rule out the possibility of unmeasured residual confounding.
In this first study examining longitudinal associations of PFAS with lipids trajectories among midlife women, we showed that PFAS serum concentrations were associated with less-favorable lipids trajectories, particularly total and LDL cholesterol trajectories. These findings suggest that PFAS exposure is a potential modifiable risk factor for lipid metabolic disorders, even though the underlying mechanisms of action are still poorly understood. Further studies investigating potential mechanisms of PFAS-induced lipid alteration (e.g., estrogen-mediated pathway) would expand the scientific knowledge in this area.
Supplementary Material
Acknowledgments
The authors thank the study staff at each site and all the women who participated in the SWAN.
The authors also thank the late X. Ye at the U.S. CDC for her support in PFAS assessment.
The SWAN has grant support from the National Institutes of Health (NIH), the Department of Health and Human Services (DHHS), through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The study was also supported by the SWAN Repository (U01AG017719). This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through UCSF-CTSI Grant Number UL1 RR024131.
This study was also supported by grants from the National Institute of Environmental Health Sciences R01-ES026578, R01-ES026964, R01-ES035087, and P30-ES017885, and by the U.S. CDC/National Institute for Occupational Safety and Health grant T42-OH008455. H.K. was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1A6A3A03037876).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Clinical Centers: University of Michigan, Ann Arbor—C. Karvonen-Gutierrez, PI 2021–present, S. Harlow, PI 2011–2021, and M. Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, Massachusetts—J. Finkelstein, PI 1999–present, and R. Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, Illinois—H. Kravitz, PI 2009–present, L. Powell, PI 1994–2009; University of California, Davis/Kaiser—E. Gold, PI; University of California, Los Angeles, Los Angeles, California—G. Greendale, PI; Albert Einstein College of Medicine, Bronx, New York—C. Derby, PI 2011–present, R. Wildman, PI 2010–2011, and N. Santoro, PI 2004–2010; University of Medicine and Dentistry–New Jersey Medical School, Newark—G. Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, Pennsylvania—K. Matthews, PI.
NIH Program Office: NIA, Bethesda, Maryland—R. Correa-de-Araujo 2020–present, C. Dutta 2016–2020, W. Rossi 2012–2016, S. Sherman 1994–2012, and M. Ory 1994–2001; NINR, Bethesda, Maryland–program officers.
Central Laboratory: University of Michigan, Ann Arbor–D. McConnell (Central Ligand Assay Satellite Services).
U.S. CDC Laboratory: Division of Laboratory Sciences, National Center for Environmental Health, U.S. CDC, Atlanta, Georgia.
NIA Biorepository: R. Correa-de-Araujo 2019–present; SWAN Repository: University of Michigan, Ann Arbor—S. Harlow 2013–2018, D. McConnell 2011–2013, and M. Sowers 2000–2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, Pennsylvania—M. Mori Brooks, PI 2012–present, K. Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, Massachusetts—S. McKinlay, PI 1995–2001.
Steering Committee: S. Johnson, current chair; C. Gallagher, former chair.
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the U.S. CDC. Use of trade names is for identification only and does not imply endorsement by the U.S. CDC, the Public Health Service, or the DHHS.
References
- 1.KEMI (Swedish Chemicals Agency). 2015. Report 7/15: Occurrence and Use of Highly Fluorinated Substances and Alternatives. https://www.kemi.se/en/publications/reports/2015/report-7-15-occurrence-and-use-of-highly-fluorinated-substances-and-alternatives [accessed 1 July 2023].
- 2.Agency for Toxic Substances and Disease Registry (ATSDR). 2021. Toxicological Profile for Perfluoroalkyls. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. 10.15620/cdc:59198. [DOI] [PubMed] [Google Scholar]
- 3.Ahrens L, Bundschuh M. 2014. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: a review. Environ Toxicol Chem 33(9):1921–1929, PMID: , 10.1002/etc.2663. [DOI] [PubMed] [Google Scholar]
- 4.Garg S, Kumar P, Mishra V, Guijt R, Singh P, Dumée LF, et al. 2020. A review on the sources, occurrence and health risks of per-/poly-fluoroalkyl substances (PFAS) arising from the manufacture and disposal of electric and electronic products. J Water Process Eng 38:101683, 10.1016/j.jwpe.2020.101683. [DOI] [Google Scholar]
- 5.Ding N, Harlow SD, Batterman S, Mukherjee B, Park SK. 2020. Longitudinal trends in perfluoroalkyl and polyfluoroalkyl substances among multiethnic midlife women from 1999 to 2011: The Study of Women′s Health Across the Nation. Environ Int 135:105381, PMID: , 10.1016/j.envint.2019.105381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunder L, Lind PM, Salihovic S, Stubleski J, Kärrman A, Lind L. 2022. Changes in plasma levels of per- and polyfluoroalkyl substances (PFAS) are associated with changes in plasma lipids – a longitudinal study over 10 years. Environ Res 211:112903, PMID: , 10.1016/j.envres.2022.112903. [DOI] [PubMed] [Google Scholar]
- 7.Tsai MS, Miyashita C, Araki A, Itoh S, Bamai Y, Goudarzi H, et al. 2018. Determinants and temporal trends of perfluoroalkyl substances in pregnant women: the Hokkaido Study on Environment and Children’s Health. Int J Environ Res Public Health 15(5):989, PMID: , 10.3390/ijerph15050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SK, Peng Q, Ding N, Mukherjee B, Harlow SD. 2019. Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: evidence of racial/ethnic and geographic differences in PFAS exposure. Environ Res 175:186–199, PMID: , 10.1016/j.envres.2019.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello E, Rock S, Stratakis N, Eckel SP, Walker DI, Valvi D, et al. 2022. Exposure to per- and polyfluoroalkyl substances and markers of liver injury: a systematic review and meta-analysis. Environ Health Perspect 130(4):46001, PMID: , 10.1289/EHP10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steenland K, Fletcher T, Stein CR, Bartell SM, Darrow L, Lopez-Espinosa MJ, et al. 2020. Review: evolution of evidence on PFOA and health following the assessments of the C8 Science Panel. Environ Int 145:106125, PMID: , 10.1016/j.envint.2020.106125. [DOI] [PubMed] [Google Scholar]
- 11.Ding N, Harlow SD, Randolph JF Jr, Loch-Caruso R, Park SK. 2020. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum Reprod Update 26(5):724–752, PMID: , 10.1093/humupd/dmaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, et al. 2013. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS One 8(2):e56969, PMID: , 10.1371/journal.pone.0056969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher M, Arbuckle TE, Wade M, Haines DA. 2013. Do perfluoroalkyl substances affect metabolic function and plasma lipids?—analysis of the 2007–2009, Canadian Health Measures Survey (CHMS) Cycle 1. Environ Res 121:95–103, PMID: , 10.1016/j.envres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Skuladottir M, Ramel A, Rytter D, Haug LS, Sabaredzovic A, Bech BH, et al. 2015. Examining confounding by diet in the association between perfluoroalkyl acids and serum cholesterol in pregnancy. Environ Res 143(pt A):33–38, PMID: , 10.1016/j.envres.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. 2009. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol 170(10):1268–1278, PMID: , 10.1093/aje/kwp279. [DOI] [PubMed] [Google Scholar]
- 16.Cong J, Chu C, Li QQ, Zhou Y, (Min) Qian Z, Dee Geiger S, et al. 2021. Associations of perfluorooctane sulfonate alternatives and serum lipids in Chinese adults. Environ Int 155:106596, PMID: , 10.1016/j.envint.2021.106596. [DOI] [PubMed] [Google Scholar]
- 17.Lin PID, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert M-F, et al. 2019. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults—longitudinal analysis of the diabetes prevention program outcomes study. Environ Int 129:343–353, PMID: , 10.1016/j.envint.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canova C, Barbieri G, Zare Jeddi M, Gion M, Fabricio A, Daprà F, et al. 2020. Associations between perfluoroalkyl substances and lipid profile in a highly exposed young adult population in the Veneto Region. Environ Int 145:106117, PMID: , 10.1016/j.envint.2020.106117. [DOI] [PubMed] [Google Scholar]
- 19.Olsen GW, Burris JM, Burlew MM, Mandel JH. 2003. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J Occup Environ Med 45(3):260–270, PMID: , 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- 20.Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR. 2007. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med 49(8):872–879, PMID: , 10.1097/JOM.0b013e318124a93f. [DOI] [PubMed] [Google Scholar]
- 21.Fitz-Simon N, Fletcher T, Luster MI, Steenland K, Calafat AM, Kato K, et al. 2013. Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology 24(4):569–576, PMID: , 10.1097/EDE.0b013e31829443ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winquist A, Steenland K. 2014. Modeled PFOA exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts. Environ Health Perspect 122(12):1299–1305, PMID: , 10.1289/ehp.1307943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tepper PG, Randolph JF, McConnell DS, Crawford SL, El Khoudary SR, Joffe H, et al. 2012. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health Across the Nation (SWAN). J Clin Endocrinol Metab 97(8):2872–2880, PMID: , 10.1210/jc.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. 2009. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 54(25):2366–2373, PMID: , 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badon SE, Gabriel KP, Karvonen-Gutierrez C, Sternfeld B, Gold EB, Waetjen LE, et al. 2021. Dual trajectories of physical activity and blood lipids in midlife women: The Study of Women’s Health Across the Nation. Maturitas 146:49–56, PMID: , 10.1016/j.maturitas.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong F, MacLeod M, Mueller JF, Cousins IT. 2014. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: evidence from population-based pharmacokinetic modeling. Environ Sci Technol 48(15):8807–8814, PMID: , 10.1021/es500796y. [DOI] [PubMed] [Google Scholar]
- 27.Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K. 2017. A study of reverse causation: examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect 125(3):416–421, PMID: , 10.1289/EHP273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sowers M, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, et al. 2000. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. In: Menopause. Lobo RA, Kelsey J, Marcus R, eds. Elsevier, 175–188. [Google Scholar]
- 29.Kato K, Basden BJ, Needham LL, Calafat AM. 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218(15):2133–2137, PMID: , 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 30.Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- 31.Izawa S, Okada M, Matsui H, Horita Y. 1997. A new direct method for measuring HDL-cholesterol which does not produce any biased values. J Med Pharm Sci 37:1385–1388. [Google Scholar]
- 32.Friedewald WT, Levy RI, Fredrickson DS. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502, PMID: , 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 33.Sternfeld B, Ainsworth BE, Quesenberry CP. 1999. Physical activity patterns in a diverse population of women. Prev Med 28(3):313–323, PMID: , 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 34.Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. 2000. Evaluation of the Kaiser Physical Activity Survey in women. Med Sci Sports Exerc 32(7):1327–1338, PMID: , 10.1097/00005768-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. 1986. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 124(3):453–469, PMID: , 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 36.Ding N, Karvonen-Gutierrez CA, Herman WH, Calafat AM, Mukherjee B, Park SK. 2021. Perfluoroalkyl and polyfluoroalkyl substances and body size and composition trajectories in midlife women: the Study of Women’s Health Across the Nation 1999–2018. Int J Obes (Lond) 45(9):1937–1948, PMID: , 10.1038/s41366-021-00848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding N, Harlow SD, Randolph JF, Calafat AM, Mukherjee B, Batterman S, et al. 2020. Associations of perfluoroalkyl substances with incident natural menopause: the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab 105(9):e3169–e3182, PMID: , 10.1210/clinem/dgaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57(1):289–300, 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 39.Leisch F. 2004. FlexMix: a general framework for finite mixture models and latent glass regression in R. J Stat Softw 11(8):1–18, 10.18637/jss.v011.i08. [DOI] [Google Scholar]
- 40.Jung T, Wickrama KAS. 2008. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass 2(1):302–317, 10.1111/j.1751-9004.2007.00054.x. [DOI] [Google Scholar]
- 41.Wu J, Province MA, Coon H, Hunt SC, Eckfeldt JH, Arnett DK, et al. 2007. An investigation of the effects of lipid-lowering medications: genome-wide linkage analysis of lipids in the HyperGEN study. BMC Genet 8(1):60, PMID: , 10.1186/1471-2156-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhana K, van Rosmalen J, Vistisen D, Ikram MA, Hofman A, Franco OH, et al. 2016. Trajectories of body mass index before the diagnosis of cardiovascular disease: a latent class trajectory analysis. Eur J Epidemiol 31(6):583–592, PMID: , 10.1007/s10654-016-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kupsco A, Wu H, Calafat AM, Kioumourtzoglou MA, Cantoral A, Tamayo-Ortiz M, et al. 2022. Prenatal maternal phthalate exposures and trajectories of childhood adiposity from four to twelve years. Environ Res 204(pt B):112111, PMID: , 10.1016/j.envres.2021.112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue K, Goto A, Sugiyama T, Ramlau-Hansen CH, Liew Z. 2020. The confounder-mediator dilemma: should we control for obesity to estimate the effect of perfluoroalkyl substances on health outcomes? Toxics 8(4):125, PMID: , 10.3390/toxics8040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Château-Degat ML, Pereg D, Dallaire R, Ayotte P, Dery S, Dewailly É. 2010. Effects of perfluorooctanesulfonate exposure on plasma lipid levels in the Inuit population of Nunavik (Northern Quebec). Environ Res 110(7):710–717, PMID: , 10.1016/j.envres.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Liu HS, Wen LL, Chu PL, Lin CY. 2018. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013–2014. Environ Pollut 232:73–79, PMID: , 10.1016/j.envpol.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Donat-Vargas C, Bergdahl IA, Tornevi A, Wennberg M, Sommar J, Koponen J, et al. 2019. Associations between repeated measure of plasma perfluoroalkyl substances and cardiometabolic risk factors. Environ Int 124:58–65, PMID: , 10.1016/j.envint.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Modaresi SMS, Wei W, Emily M, DaSilva NA, Slitt AL. 2022. Per- and polyfluoroalkyl substances (PFAS) augment adipogenesis and shift the proteome in murine 3T3-L1 adipocytes. Toxicology 465:153044, PMID: , 10.1016/j.tox.2021.153044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watkins AM, Wood CR, Lin MT, Abbott BD. 2015. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol Cell Endocrinol 400:90–101, PMID: , 10.1016/j.mce.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 50.Andersen ME, Hagenbuch B, Apte U, Corton JC, Fletcher T, Lau C, et al. 2021. Why is elevation of serum cholesterol associated with exposure to perfluoroalkyl substances (PFAS) in humans? A workshop report on potential mechanisms. Toxicology 459:152845, PMID: , 10.1016/j.tox.2021.152845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rebholz SL, Jones T, Herrick RL, Xie C, Calafat AM, Pinney SM, et al. 2016. Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicol Rep 3:46–54, PMID: , 10.1016/j.toxrep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlezinger JJ, Puckett H, Oliver J, Nielsen G, Heiger-Bernays W, Webster TF. 2020. Perfluorooctanoic acid activates multiple nuclear receptor pathways and skews expression of genes regulating cholesterol homeostasis in liver of humanized PPARα mice fed an American diet. Toxicol Appl Pharmacol 405:115204, PMID: , 10.1016/j.taap.2020.115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan X, Xie G, Sun X, Li Q, Zhong W, Qiao P, et al. 2013. High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One 8(4):e61409, PMID: , 10.1371/journal.pone.0061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakagawa T, Ramdhan DH, Tanaka N, Naito H, Tamada H, Ito Y, et al. 2012. Modulation of ammonium perfluorooctanoate-induced hepatic damage by genetically different PPARα in mice. Arch Toxicol 86(1):63–74, PMID: , 10.1007/s00204-011-0704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Das KP, Wood CR, Lin MJ, Starkov AA, Lau C, Wallace KB, et al. 2017. Perfluoroalkyl acids-induced liver steatosis: effects on genes controlling lipid homeostasis. Toxicology 378:37–52, PMID: , 10.1016/j.tox.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Zhang Y, Zhang W, Jin Y, Dai J. 2012. Association of perfluorooctanoic acid with HDL cholesterol and circulating miR-26b and miR-199–3p in workers of a fluorochemical plant and nearby residents. Environ Sci Technol 46(17):9274–9281, PMID: , 10.1021/es300906q. [DOI] [PubMed] [Google Scholar]
- 57.Kim S, Thapar I, Brooks BW. 2021. Epigenetic changes by per- and polyfluoroalkyl substances (PFAS). Environ Pollut 279:116929, PMID: , 10.1016/j.envpol.2021.116929. [DOI] [PubMed] [Google Scholar]
- 58.Bjork JA, Butenhoff JL, Wallace KB. 2011. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology 288(1–3):8–17, PMID: , 10.1016/j.tox.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 59.Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JÅ. 2012. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol 212(1):3–12, PMID: , 10.1530/JOE-11-0044. [DOI] [PubMed] [Google Scholar]
- 60.Liu D, Bachmann KA. 1998. An investigation of the relationship between estrogen, estrogen metabolites and blood cholesterol levels in ovariectomized rats. J Pharmacol Exp Ther 286(1):561–568, PMID: . [PubMed] [Google Scholar]
- 61.Sock ETN, Mayer G, Lavoie JM. 2016. Combined effects of rosuvastatin and exercise on gene expression of key molecules involved in cholesterol metabolism in ovariectomized rats. PLoS One 11(7):e0159550, PMID: , 10.1371/journal.pone.0159550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parini P, Angelin B, Rudling M. 1997. Importance of estrogen receptors in hepatic LDL receptor regulation. Arterioscler Thromb Vasc Biol 17(9):1800–1805, PMID: , 10.1161/01.atv.17.9.1800. [DOI] [PubMed] [Google Scholar]
- 63.Jones DR, Schmidt RJ, Pickard RT, Foxworthy PS, Eacho PI. 2002. Estrogen receptor-mediated repression of human hepatic lipase gene transcription. J Lipid Res 43(3):383–391, PMID: , 10.1016/S0022-2275(20)30144-9. [DOI] [PubMed] [Google Scholar]
- 64.Wang H, Liu Y, Zhu L, Wang W, Wan Z, Chen F, et al. 2014. 17β-estradiol promotes cholesterol efflux from vascular smooth muscle cells through a liver X receptor α-dependent pathway. Int J Mol Med 33(3):550–558, PMID: , 10.3892/ijmm.2014.1619. [DOI] [PubMed] [Google Scholar]
- 65.Darabi M, Rabbani M, Ani M, Zarean E, Panjehpour M, Movahedian A. 2011. Increased leukocyte ABCA1 gene expression in post-menopausal women on hormone replacement therapy. Gynecol Endocrinol 27(9):701–705, PMID: , 10.3109/09513590.2010.507826. [DOI] [PubMed] [Google Scholar]
- 66.Harlow SD, Hood MM, Ding N, Mukherjee B, Calafat AM, Randolph JF, et al. 2021. Per- and polyfluoroalkyl substances and hormone levels during the menopausal transition. J Clin Endocrinol Metab 106(11):e4427–e4437, PMID: , 10.1210/clinem/dgab476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han X, Alam MN, Cao M, Wang X, Cen M, Tian M, et al. 2022. Low levels of perfluorooctanoic acid exposure activates steroid hormone biosynthesis through repressing histone methylation in rats. Environ Sci Technol 56(9):5664–5672, PMID: , 10.1021/acs.est.1c08885. [DOI] [PubMed] [Google Scholar]
- 68.Kang JS, Choi JS, Park JW. 2016. Transcriptional changes in steroidogenesis by perfluoroalkyl acids (PFOA and PFOS) regulate the synthesis of sex hormones in H295R cells. Chemosphere 155:436–443, PMID: , 10.1016/j.chemosphere.2016.04.070. [DOI] [PubMed] [Google Scholar]
- 69.Fragki S, Dirven H, Fletcher T, Grasl-Kraupp B, Bjerve Gützkow K, Hoogenboom R, et al. 2021. Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: what do we know and what not? Crit Rev Toxicol 51(2):141–164, PMID: , 10.1080/10408444.2021.1888073. [DOI] [PubMed] [Google Scholar]
- 70.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. 2005. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 24(19):2911–2935, PMID: , 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 71.Wen X, Wang M, Xu X, Li T. 2022. Exposure to per- and polyfluoroalkyl substances and mortality in U.S. adults: a population-based cohort study. Environ Health Perspect 130(6):67007, PMID: , 10.1289/EHP10393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


