Abstract
Epidemiology studies demonstrate that women are at a significantly lower risk of developing type 2 diabetes (T2D) compared to men. However, the molecular basis of this risk difference is not well understood. In this study, we examined the sex differences in the genetic programs of pancreatic endocrine cells. We combined pancreas perifusion data and single-cell genomic data from our laboratory and from publicly available data sets to investigate multiple axes of the sex differences in the human pancreas at the single-cell type and single-cell level. We systematically compared female and male islet secretion function, gene expression program, and regulatory principles of pancreatic endocrine cells. The perifusion data indicate that female endocrine cells have a higher secretion capacity than male endocrine cells. Single-cell RNA-sequencing analysis suggests that endocrine cells in male controls have molecular signatures that resemble T2D. In addition, we identified genomic elements associated with genome-wide association study T2D loci to have differential accessibility between female and male delta cells. These genomic elements may play a sex-specific causal role in the pathogenesis of T2D. We provide molecular mechanisms that explain the differential risk of T2D between women and men. Knowledge gained from our study will accelerate the development of diagnostics and therapeutics in sex-aware precision medicine for diabetes.
Keywords: type 2 diabetes, sex differences, human pancreatic islets, perifusion, single-cell RNA-seq, single-cell ATACseq, precision medicine
Sex is increasingly being recognized as one of the major factors affecting health outcomes. Sex differences are particularly prominent in metabolic disorders including type 2 diabetes (T2D). Epidemiology studies indicate that men are at higher risk of developing diabetes than women (1). Besides sexual dimorphic traits for how the body stores and uses energy, recent studies indicate that women and men have intrinsic differences in their pancreas biology (2). Specifically, women appear to have higher capacities for insulin secretion than men. This phenomenon has been reported both in vivo (3, 4) and in perfused islets in vitro (5). Sex-specific endocrine properties may partially explain the lower prevalence of diabetes observed in women, despite their higher frequency of obesity, compared with men (6).
The functional unit of the endocrine pancreas is the islet of Langerhans. Within the islets, there are at least 5 endocrine cell types (alpha, beta, delta, epsilon, and pancreatic polypeptide [PP] cells) and multiple stromal cell types (endothelial, fibroblast, neuronal, and immune cells). In addition, ductal and acinar cells in the exocrine pancreas have been shown to physically contact islets and affect islet secretion. Sex differences in the endocrine pancreas have been investigated at the physiology (1, 7), histology (8), genetics (9), genomics (10, 11), and epigenomic (5) levels. However, to our knowledge, no study to date has examined the sex differences in the molecular landscapes of the human pancreas at the single-cell level. Given the complexity of the cellular composition and molecular programs of the endocrine pancreas, the single-cell approach is critical to revealing the most granular details of the differences in the genetic underpinnings between women and men.
In this study, we investigated multiple axes of the sex differences in the pancreas at the single-cell type and single-cell level. Our data confirm the higher secretion capacities of female endocrine cells compared with male endocrine cells and suggest that endocrine cells in male controls (defined as donors without diabetes, age ≥ 17 years) have molecular signatures resembling T2D. Our data provide the molecular framework that helps to decipher the sex-specific principles in glucose sensing and hormone secretion. These results will pave the way for precision medicine to further optimize the prevention and treatment of T2D.
Material and Methods
Data Sets
Perifusion data sets
Perifusion data and corresponding donor metadata were downloaded from PANC-DB (https://hpap.pmacs.upenn.edu/), the open-source data repository for cellular and molecular data sets generated by the Human Islet Research Network's Human Pancreas Analysis Program (HPAP) (data accessed April 2022) (12). Donor selection criteria included (1) adults (age ≥ 17 years), (2) no clinical type 1 diabetes or T2D diagnosis, (3) glycated hemoglobin A1c (HbA1c) less than 6.5%, and (4) no autoantibody positivity. Following this selection strategy, perifusion data associated with 18 women and 12 men were downloaded and processed. The combined perifusion data and donor metadata are reported in Supplementary Table S1 (13).
Single-cell RNA-sequencing data sets
Raw FASTQ files and metadata of 3 data sets from our laboratory and 7 from other laboratories were combined. The 10 single-cell RNA-sequencing (RNA-seq) studies were downloaded from the following depositories: Baron (GEO: GSE84133) (14), Enge (GEO: GSE81547) (15), Lawlor (GEO: GSE86473) (16), Segerstolpe (EMBL-EBI: E-MTAB-5061) (17), Muraro (GEO: GSE85241) (18), Wang_C1 (GEO: GSE83139 and GSE154126) (19, 20), Wang_C1HT (https://hpap.pmacs.upenn.edu/) (12), Wang_10x (https://hpap.pmacs.upenn.edu/) (12), Xin (GEO: GSE81608) (21), and Xin_10x (GEO: GSE114297) (22). Raw reads were aligned to the GRCh38 genome assembly with STAR (version 020201) with default parameters. Gene expression matrices associated with individual single-cell RNA-seq data sets were further quality-controlled and processed using Seurat pipeline (V4) (23). The combined reprocessed single-cell RNA-seq data were deposited as a Seurat object (Supplementary Data S1) (13).
Single-cell ATAC-sequencing data sets
Raw FASTQ files of the single-cell ATAC-sequencing (ATAC-seq) data were downloaded from PANC-DB (https://hpap.pmacs.upenn.edu/; data accessed April 2021). Only those data associated with adult control donors without autoantibody positivity were included for downstream processing. Reads were aligned to the GRCh37/hg19 genome using the CellRanger ATAC pipeline (version 2.1.0) with the cellranger-atac count command with default parameters. The resulting fragment files were used as input in ArchR (version 1.0.1) for further quality control and processing (24).
Bulk RNA-sequencing data sets
Data from bulk RNA-seq from pancreatic islets were used to visualize the transcriptions from the rs12789028 locus. These data were downloaded from GSE76268 (25). Raw reads were aligned to GRCh37/hg19 assembly with STAR with default parameters.
Perifusion Data Analysis
The 160 minutes of perifusion experiment were divided into 7 conditions based on the stimuli applied: (1) Baseline1, 20 to 29 minutes, no stimuli were introduced; (2) 4 mM amino acid mixture (AAM), 30 to 60 minutes, 4 mM AAM were introduced; (3) 3 mM G, 61 to 80 minutes, 4 mM AAM + 3 mM glucose (G) were introduced; (4) 16.7 mM G, 81 to 100 minutes, 4 mM AAM + 16.7 mM G were introduced; (5) 0.1 mM isobutylmethylxanthine (IBMX), 101 to 120 minutes, 4 mM AAM + 16.7 mM G + 0.1 mM IBMX were introduced; (6) Baseline2, 121 to 140 minutes, no stimuli were introduced; (7) 30 mM potassium chloride (KCl), 141 to 160 minutes, 30 mM KCl were introduced. The detailed perifusion design is included in Supplementary Table S1 (13). Areas under the curve (AUCs) within each condition were computed separately between women and men. The differences between the 2 sexes in AUCs in each condition were evaluated using a t test. The resulting raw P values were corrected by false discovery rate (FDR) to control for false positives with multiple testing. An FDR threshold of 10% was used to determine the statistical significance.
Insulin secretions under 16.7 mM G were further divided into first phase (81-88 minutes) and second phase (89-100 minutes). The stimulation indices (SIs) for both phases were calculated by dividing the peak insulin secretion in the first phase or average insulin secretion in the second phase against the average insulin secretion under 3 mM G condition. The differences between the 2 sexes in their SIs were evaluated using a t test.
Glucagon secretions under 4 mM AAM were further divided into first phase (31-38 minutes) and second phase (39-60 minutes). The SI was computed as the peak (phase 1) or average (phase 2) secretion fold changes (FCs) over baseline 1. The differences between the 2 sexes in their SIs were evaluated using a t test.
To identify perifusion changes with age, the female and male donors were divided into quartiles. Data associated with the youngest quartile and the oldest quartile within each sex were then compared using the same procedure as described earlier.
Single-Cell RNA-Sequencing Data Analysis
Data integration and cell type identification
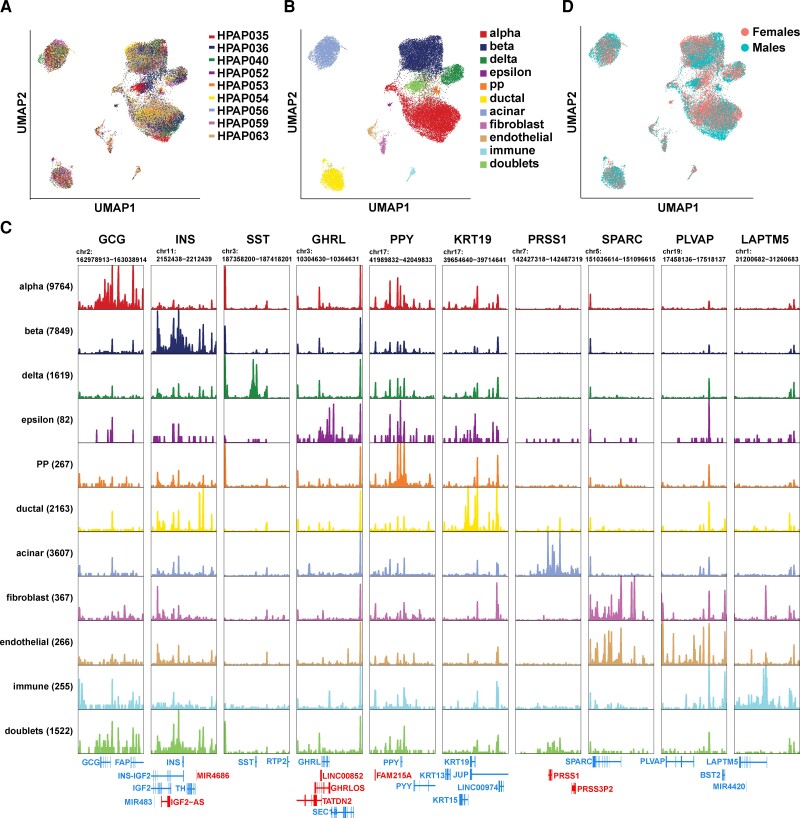
Single cells were filtered with minimal reads of 200. Single-cell data sets were integrated similarly to previously described (26). Briefly, different single-cell data were integrated with the SCTransform integration pipeline with Seurat V4.0.6 (27). A resolution of 0.4 was used for cell clustering based on the stability of the resulting clusters visualized with clustree (28). Principal component analysis (PCA) and Uniform Manifold Approximation and Projection (UMAP) were used to reduce the data dimensions and embed the single cells (29). Cell type classification was based on the expression of canonical pancreatic markers in each cluster: GCG for alpha cells, INS for beta cells, SST for delta cells, GHRL for epsilon cells, PPY for PP cells, KRT18 for ductal cells, PRSS1 for acinar cells, SPARC for fibroblasts, VWF for endothelial cells, and LAPTM5 for immune cells. One population exhibited coexpression of multiple cell type markers and was thus categorized as doublets.
Differential expression analysis and pathway annotation
Limma trend (30) was used for differential expression analysis to (1) compare female and male gene expression values in each pancreatic cell type in control donors; (2) compare gene expression differences between T2D and control in each sex in each pancreatic cell type; and (3) compare gene expression differences between T2D and control in each cell type independent of donor sexes. To control for batch effects, the source of single-cell data (1 of the 10 data sets) was included as a covariate in the design matrix in all 3 comparison groups. In addition, in comparison group 3, the design matrix was set as condition × sex + source to include the interactions between conditions (T2D vs control) and sex (female or male) to derive sex-independent molecular changes associated with T2D pathology.
For gene set enrichment analysis (GSEA) comparing the gene expression differences in beta cells of control women and men with the signature genes related to beta-cell exocytosis, we created custom gene sets using the top correlated and anticorrelated genes to total exocytosis in beta cells from control donors described in the Patch-Seq study (31). FDR less than 10% was used as the threshold to select statistically significant enrichment.
For gene ontology (GO) pathway overrepresentation analysis, FDR less than 1% was used as a threshold to select differentially expressed genes. These genes were further stratified based on the direction of FCs and then served as input in the clusterProfiler enrichGO function for pathway enrichment computation with the GO aspect restricted to biological process (32). FDR less than 10% was used as the threshold to select statistically significant enrichment. For those analyses with more than 10 enriched GO terms, we further performed the following 2 procedures: (1) To identify the relationship between the top 50 most significantly enriched GO terms (ordered by FDR), we applied the emapplot function from clusterProfiler; and (2) to summarize the GO terms, we used REVIGO TreeMap view to visualize superclusters and selected representative terms (33).
For GSEA comparing gene expression differences between women and men with T2D, differentially expressed genes between T2D and control in females within each pancreatic cell type were filtered with FDR less than 1% and absolute FC greater than 1.5 to create custom gene sets. The gene lists derived from differential expression analysis between men with T2D and male controls in the corresponding cell types were then ranked by FC and used as input for GSEA. FDR less than 10% was used as the threshold to select significant enrichment.
Rank-rank hypergeometric overlap
Rank–rank hypergeometric overlap (RRHO) aims to compare gene expression profiles across 2 gene lists that were ranked by the degree of differential expression from 2 independent differential expression analyses without a rigid threshold cutoff (34). The RRHO method applied in our study is an updated version of RRHO (implemented by the RRHO2 R package) (35) that improves the interpretability of RRHO when the differential expression patterns are discordant in the 2 gene lists. The input score was calculated as −log10(adjusted P value) * (log2FC). Genes within each of the 2 gene lists were ranked by the input score and served as input for RRHO2.
Single-Cell ATACA-Sequencing Data Analysis
Cell type calling
All aligned single-cell ATAC-seq data were input as fragment files in ArchR (version 1.0.1) to create an ArchR project (24). Cells were filtered with a minimal transcription start site enrichment score of 4 and minimal unique nuclear fragments of 1000. Gene scores were calculated with a tile size of 500 bp, a gene window of 100 kb on either side of the gene, and a default exponential weighting function of e(−abs(distance)/5000) + e−1). Putative doublets were identified and removed with the addDoubletScores and filterDoublets functions in ArchR with default parameters. Dimension reduction was performed by ArchR's implementation of latent semantic indexing (LSI) using the addIterativeLSI function with iterations = 4, varFeatures = 15 000, resolution = 0.2, and maxClusters = NULL. Other parameters were default. Harmony was then used to correct batch effects using the ArchR wrapper function addHarmony. Clustering was performed with addClusters with method = Seurat and resolution = 0.8. Single-cell embeddings were visualized with addUMAP with reducedDims = Harmony, nNeighbors = 30, minDist = 0.5. Clusters were annotated based on the gene scores of marker genes: GCG for alpha cells, INS for beta cells, SST for delta cells, GHRL for epsilon cells, PPY for PP cells, KRT19 for ductal cells, PRSS1 for acinar cells, SPARC for fibroblasts, PLVAP for endothelial cells, and LAPTM5 for immune cells. Local chromatin accessibility at marker genes was plotted using the plotBrowserTrack function.
Pseudo-bulk replicates and peak calling
To increase the power to detect sex-specific peaks, we split each cell-type cluster into female or male specific. For each of the resulting female or male cell types, we generated pseudo-bulk replicates with addGroupCoverages with default parameters. Peak calling was performed with Macs2 with an iterative overlap peak merging procedure by first calling peaks for each pseudo-bulk replicate. The resulting peaks were then sequentially merged across pseudo-bulks within each sex-specific cell type, and then between all cell types across both sexes. This process was implemented by invoking the function addReproduciblePeakSet with default parameters. The peak set was then added to the ArchR project with addPeakMatrix.
ChromVAR motif deviation analysis
Motif annotations were added to the ArchR project with the addMotifAnnotations function with motifSet = cisbp. ChromVAR was used to identify motif deviation between female and male endocrine cells of the same cell type. Per-cell deviation scores were computed with the addDeviationsMatrix function with default parameters. Differential deviation analysis was performed with the getMarkerFeatures function with useMatrix = MotifMatrix, useSeqnames = z, testMethod = wilcoxon, and default values for the other parameters. Female cells were chosen as the use group and male cells were used as the background group.
Integration with single-cell RNA-sequencing data
To take advantage of the single-cell RNA-seq data in order to examine the correlations between peak accessibility and gene expression (peak to gene linkage), we projected the single-cell RNA-seq data from the 10 data sets described earlier to the single-cell ATAC-seq data with unconstrained integration by addGeneIntegrationMatrix with reducedDims = Harmony and default values for the other parameters. The peak-to-gene linkage was computed with the addPeak2GeneLinks function with reducedDims = Harmony.
Identification of positive transcription factor regulators
Positive transcription factor (TF) regulators were defined as those TFs with gene expression positively correlated to the changes in the accessibility of their corresponding motifs. Deviant TF motifs were identified with the getGroupSE function with useMatrix = MotifMatrix. The correlation of motif deviation scores and their expression values were then computed with the correlateMatrices function and filtered based on a threshold of correlation greater than 0.5 and adjusted P value less than .01.
Identification of sex-biased differential accessible peaks and type 2 diabetes genome-wide association study enrichment analysis
To identify open chromatin peaks that were differentially accessible between different sexes, the getMarkerFeatures function was invoked with useMatrix = PeakMatrix, testMethod = wilcoxon, and default values for the other parameters. Female cells were chosen as the use group and male cells were used as the background group. FDR less than 20% was used to select statistically significant differential peaks. To investigate whether T2D risk loci overlap with sex-biased differential accessible sites, we used 338 independent genome-wide association study (GWAS) enrichment signals from DIAMANTE that were fine-mapped to 99% credible sets with multiancestry meta-regression (36). Each differential accessible peak in each cell type was then intersected with the T2D GWAS intervals.
Results
Female Islets Have Higher Insulin Secretion Capacity Than Male Islets
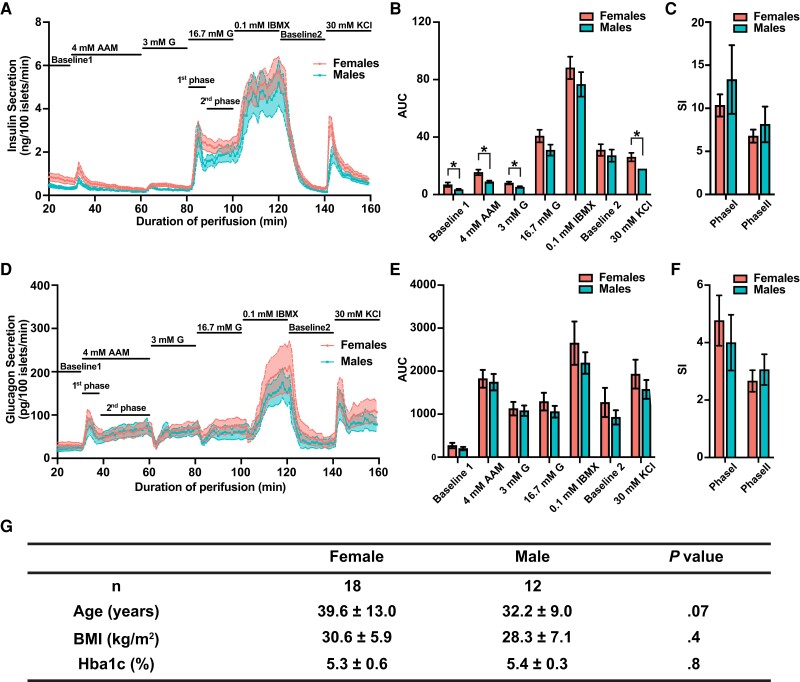
To assess whether control, nondiabetic donors display sexual differences in islet secretion, we turned to islet perifusion data deposited by the HPAP (12). Islet perifusion system provides a well-controlled experimental setting where the secretory responses of isolated islets to various external stimuli can be evaluated in a time-resolved manner and cross-compared. We collected data from 18 adult control women and 12 adult control men (Supplementary Table S1) (13). We partitioned the perifusion data into 7 different experimental conditions corresponding to different stimuli that included Baseline1, 4 mM AAM, 3 mM G, 16.7 mM G, 0.1 mM IBMX, Baseline2, and 30 mM KCl. We then compared the AUC between females and males for each condition (Fig. 1). For insulin secretion, under the Baseline1, 4 mM AAM, 3 mM G, and 30 mM KCl conditions, female islets demonstrated significantly increased insulin secretion compared with males (FDR < 10%) (Fig. 1A and 1B). To further derive mechanistic insight on insulin secretion, we separated the insulin secretion under 16.7 mM G into 2 phases corresponding to the releasing of the docking granules (first phase) and progressive recruiting and releasing of newcomer granules (second phase) (37). We calculated the SIs of both phases. We found no statistical differences between women and men in their SIs (Fig. 1C). This result argues against a difference in G-stimulated insulin secretion between women and men. Rather, female islets appear to function at a higher baseline, are more responsive to amino acids, and contain more membrane-docked granules (released by KCl-induced depolarization) (37). Large variations were observed for glucagon secretion and no statistically significant differences were detected in AUCs of all conditions between the 2 sexes (Fig. 1D and 1E). However, in all conditions, female islets had trends of increased glucagon secretion (see Fig. 1E). Furthermore, we observed biphasic dynamics of glucagon secretion under AAM stimulus (Fig. 1D). Such dynamics have been reported before but were less characterized, although presumably following a similar mechanistic process compared to insulin secretion (38–40). We again quantified the SIs of the 2 phases and observed no differences (Fig. 1F).
Figure 1.
Sex differences in human islet functional responses in adult control donors. A, Insulin perifusion data from control women and control men. Solid lines indicate data average, with female data shown in pink and male data shown in blue. Shaded areas represent SEs of the distribution. Conditions include Baseline1 (no stimuli), 4 mM AAM (4 mM amino acid mixture [AAM]), 3 mM G (4 mM AAM + 3 mM glucose [G]), 16.7 mM G (4 mM AAM + 16.7 mM glucose), 0.1 mM IBMX (4 mM AAM + 16.7 mM Glucose + 0.1 mM isobutylmethylxanthine [IBMX], Baseline2 [no stimuli], and 30 mM KCl (30 mM potassium chloride [KCl]). B, Quantification of area under the curve (AUC) in insulin secretion in each of the stimulus conditions. Error bar indicates ± SE. Differences between women and men in AUC were tested by unpaired t test. *False discovery rate less than 0.1. C, Quantification of stimulation indices (SIs) for the first and second phase of insulin secretion. Differences between women and men were tested by unpaired t test. No statistically significant difference is observed. D, Glucagon perifusion data from the same donors as shown in A. E, Quantification of AUC in glucagon secretion in each of the stimulus conditions. F, SIs for the first and second phase of glucagon secretion. Differences between women and men were tested by unpaired t test. No statistically significant difference is observed. G, Donor characteristics. Data are shown as mean ± SD. P values are based on Mann-Whitney U test.
No differences in body mass index and HbA1c were detected between the 2 sexes (Fig. 1G). However, in our data set, female donors were slightly older than men although this difference was not statistically significant (see Fig. 1G). Given the abundant evidence of age-related islet functional alteration in the literature (41–43), we regrouped the perifusion data and compared the insulin and glucagon secretion capacities between the youngest and oldest quartiles of women or men. We observed statistically significant decreases in insulin and glucagon secretion in female islet cells with aging under various conditions (Supplementary Fig. S1) (13). Conversely, in men, there is a trend for increased insulin secretion with aging (significant at 4 mM AAM and 3 mM G conditions) (see Supplementary Fig. S1) (13). This result implies a potential sex-specific aging effect in the islets. It is important to note that the oldest quartile of the female donors had an average age of 56.3 years, overlapping with the average age span for menopausal transition. Hence, when comparing this group with the youngest quartile, we cannot exclude the compound effects both of age and hormonal changes on islet function. However, given the apparent reduction in insulin secretion with age in women, our result strongly suggests a higher insulin secretion capacity in female islets compared with male islets even though female donors were slightly but not significantly older.
Single-Cell RNA-Sequencing Data Integration
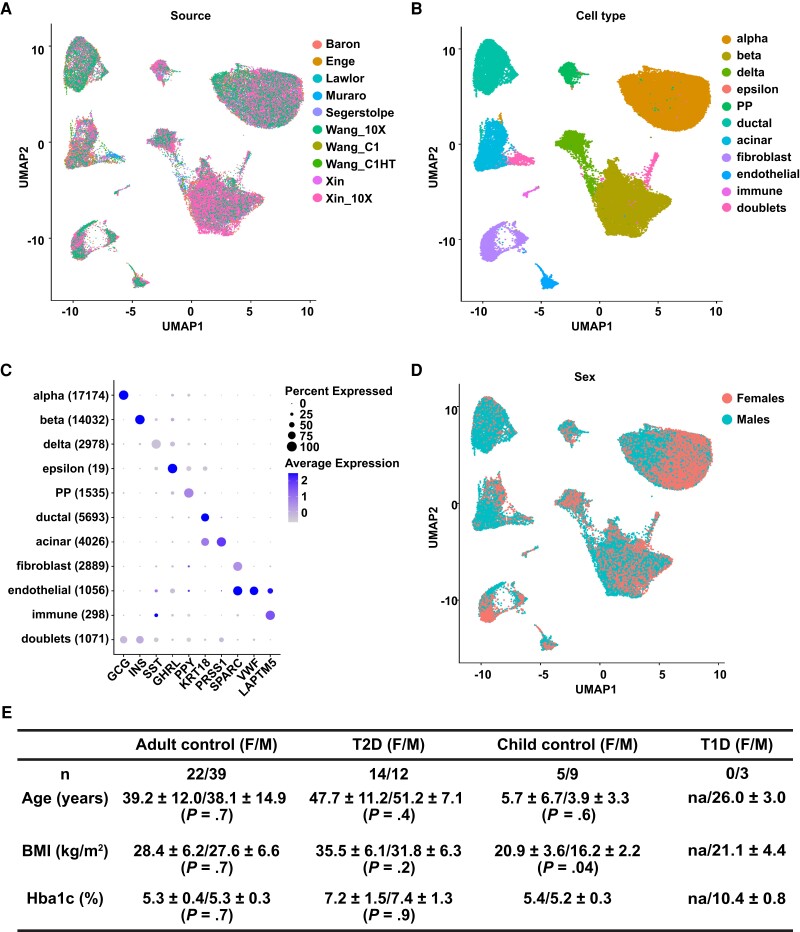
To systematically investigate the molecular mechanism underlying the differences in islet secretion in different sexes, we turned to single-cell RNA-seq data to characterize the transcriptional landscape of pancreatic cells in a cell type–specific and sex-specific manner. We retrieved 10 published single-cell RNA-seq data sets including 3 of our own on human islets cells (14–22). We performed quality control on each data set separately and then integrated these data sets using the Seurat integration pipeline (44). This integration method successfully corrected for batch effect. Post integration, cells from different sources superimposed each other and clustered by cell type (Fig. 2A and 2B and Supplementary Fig. S2) (13). We identified 10 major clusters corresponding to 10 pancreatic cell types (Fig. 2B and 2C). One additional cluster (3% of cells) that showed expression of multiple cell type markers was likely to contain doublets and was thus removed from further analysis. In total, we annotated 48 931 single cells from 104 donors (Supplementary Table S2) (13). Among them, 6743 cells carried cell-type labels from the original publications, and 97% of those cells were assigned the same cell-type label in the integrated data set, validating our integration and cell-type calling pipeline. The cell-type clusters did not separate based on sex (Fig. 2D). The summary demographic information of all donors in the integrated single-cell RNA-seq data set is shown in Fig. 2E. No differences in age, body mass index, and HbA1c were detected between the 2 sexes among adult control and T2D donors—the 2 conditions we focused on in this research.
Figure 2.
Integration of single-cell RNA sequencing (RNA-seq) data from 10 studies. A, Uniform Manifold Approximation and Projection (UMAP) embedding with cells colored according to study source. B, UMAP embedding with cells colored according to cell type. C, Dot plot illustrating how the expression of marker genes changes across different cell types. The size of the dot represents the percentage of cells that express the marker genes, while the color represents the average expression level of the marker genes across all cells within the same cell type. The number of cells in each cell type is shown inside parentheses. D, UMAP embedding with cells colored according to sex. E, Summary demographic information of donors in each category in the integrated single-cell RNA-seq data. P values are based on Mann-Whitney U test. na, not applicable.
Cell Type-Specific Expression of Sex-Biased Genes in Control Donors Without Diabetes
Pancreatic cells physically occupy the same environments and functionally cooperate to regulate glucose homeostasis. In fact, it has been recognized that almost all major pancreatic cell types contribute to the pathogenesis of diabetes (45–48). Single-cell RNA-seq technology enables us to compare the genetic mechanisms regulating pancreas biology in women and men in a cell type–specific manner both in beta and non–beta islet cells. To explore the differences in the metabolic regulation of women and men, we performed differential expression analysis between these 2 sexes in the 3 major pancreatic endocrine cell types in adult control donors (Supplementary Table S3) (13). Genes expressed at higher levels in women compared to men are henceforth referred to as female-biased genes; genes expressed at higher levels in men compared to females are henceforth referred to as male-biased genes.
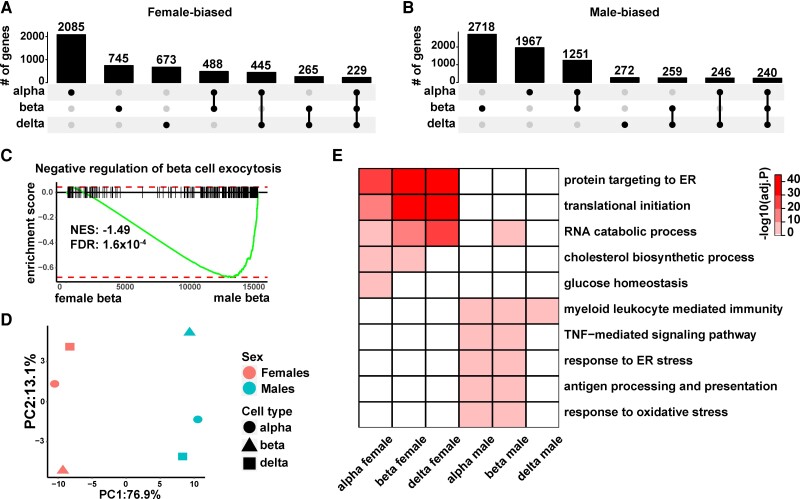
Within the endocrine cell types, alpha cells contained the highest number of female biased-genes, followed by beta cells (an FDR cutoff of 1% was used for all comparisons) (Fig. 3A). In contrast, beta cells had the highest number of male-biased genes, followed by alpha cells (Fig. 3B). There were a total of 4930 female-biased genes and 6953 male-biased genes. The majority of the sex-biased genes were cell type specific, consistent with previous reports (11, 49). However, 229 (4.7% of the total) female-biased genes and 240 (3.5% of the total) male-biased genes were shared among all three endocrine cell types (see Fig. 3A and 3B). Sixteen of the shared female-biased genes are located on the X chromosome and among them, 10 are known constitutive X-chromosome inactivation (XCI) escapees (50). These include 2 master regulators of XCI: XIST and JPX (51, 52). Among the shared male-biased genes, 12 are located on the Y chromosome, including SRY, the master regulator of sex determination (53). The expression of SRY in pancreatic cells is intriguing because its expression has been considered to be restricted to cells within the genital ridge (54). Nonetheless, the existence of master regulators controlling sex-specific biological processes in the upregulated or downregulated genes shared among all 3 endocrine cell types confirms the validity of our differential expression analysis pipeline in uncovering sex differences in gene expressions. The other prominent feature of the 229 shared female-biased genes was that 41 of them (18%) encode ribosomal proteins. Of noteworthy relevance, the female-dominant ribosomal protein signature was recently reported in mouse islet cells (55). The higher expression of ribosomal proteins indicates that female endocrine cells may have higher activities in protein synthesis.
Figure 3.
Sex-biased gene expression in human alpha, beta, and delta cells. A, Upset plot summarizing the number of female-biased genes unique to and shared between different endocrine cell types. The dot plot at the bottom indicates the intersection groups and the bar plot at the top corresponds to the number of unique genes (labeled on top of each bar) in each intersection. B, Upset plot summarizing the number of male-biased genes unique to and shared between different endocrine cell types. C, Gene set enrichment analysis (GSEA) shows that male-biased genes in beta cells are significantly associated with negative regulation of beta-cell exocytosis. D, Principal component analysis (PCA) projection of female and male endocrine cells. Log2 fold change in average gene expression between female and male cells of the same cell type was used as input for the PCA. The color of the symbols indicates sex (pink, female; blue, male), while the shape of the symbols indicates cell type (circle, alpha cells; triangle, beta cells; square, delta cells). E, Enrichment of GO terms among genes with differential expression between females and males. GO terms displayed are parent terms selected based on REVIGO TreeMap.
In light of our finding that female beta cells have significantly higher insulin secretion compared with men (see Fig. 1), we asked whether we could find molecular features reflecting this sex difference in beta-cell physiology. We derived signature genes positively or negatively associated with beta-cell exocytosis from the recent Patch-Seq study, in which beta-cell vesicle exocytosis and gene expression were jointly measured at the single-cell level (31). We used these signature genes as the reference gene sets for GSEA. We found that male-biased genes in beta cells were significantly associated with negative regulation of beta-cell exocytosis (Fig. 3C). This result is in line with our perifusion data. Moreover, our result suggests that the differential genetic underwirings between female and male endocrine cells drive their secretion phenotypes.
We next performed PCA aiming to systematically explore the structure of the sex-biased genes in the 3 endocrine cell types. For each gene, log2 FC in average gene expression between female and male cells of the same type was used as input for the PCA. This approach effectively “normalized” the cell type–dependent gene expression values. Through this analysis, we found that principal component 1, which explained 76.9% of the variance, separated female and male cells regardless of their cell types (Fig. 3D). This result suggests that there are common molecular changes between the 2 sexes in all 3 endocrine cell types. To further investigate the biological pathways implicated in the sex-dependent gene expressions, we performed GO enrichment analysis using sex-biased genes from each cell type. We found significant overlap in the GO terms among different cell types (Fig. 3E). The enriched GO terms in female-biased genes were related to transcription, translation, and other metabolism pathways, indicating increased cellular activities in female endocrine cells; while male-biased genes were related to immune processes, endoplasmic reticulum (ER) stress, and oxidative stress, reflecting a deviation from homeostasis and increased cellular stress in male endocrine cells (Fig. 3E and Supplementary Table S3) (13). The sexually divergent molecular signatures of pancreatic endocrine cells are consistent with our other findings and supports the central concept of this work—differences in the molecular programs of female and male pancreatic endocrine cells determine their different secretory capacities, which in turn lead to the differential risks of diabetes in different sexes.
Molecular Changes in Females and Males With Type 2 Diabetes
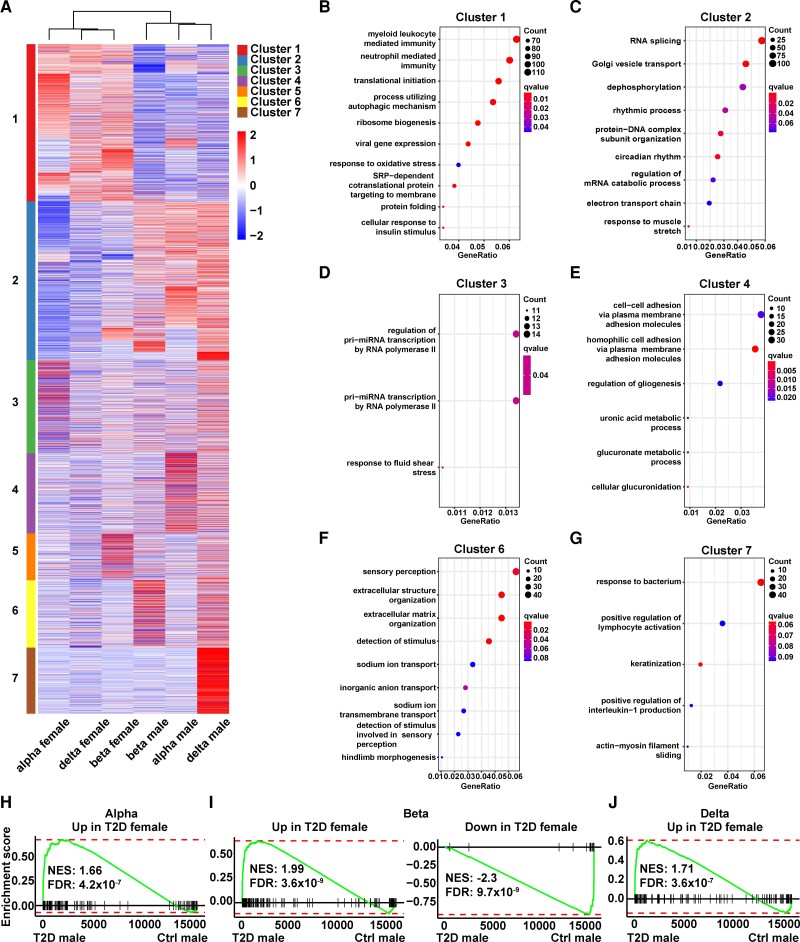
We next investigated whether there were sex differences in diabetes pathogenesis. Toward this goal, we performed differential expression analysis comparing gene expression values of female and male donors with T2D to their corresponding sex-matched controls in each of the 3 major pancreatic endocrine cell types (Supplementary Table S4) (13). We then compiled all the differential expressed genes associated with T2D and visualized them in a heat map (Fig. 4A). For each gene, the input value for the heat map was the log2 FC in the average gene expressions between T2D and control of the same sample type. Hierarchical clustering of all the samples shows that the 2 top-level clusters corresponded to female or male partitions (see Fig. 4A). This result indicates there are extensive sex-dependent T2D gene expression changes. Furthermore, this finding also points to the shared molecular processes of T2D pathogenesis among all major endocrine cell types. K means clustering on the genes revealed 7 major clusters (see Fig. 4A). Cluster 1 contained genes that were commonly upregulated in women with T2D across all 3 endocrine cell types. GO analysis showed that cluster 1 genes were enriched for cellular immunity, autophagic mechanism, translation, secretion, and protein folding (Fig. 4B). Cluster 2 contained genes that were commonly upregulated in males with T2D across the 3 endocrine cell types. These genes were enriched for RNA splicing, circadian rhythm, and other cellular processes (Fig. 4C). Cluster 3 consisted of genes upregulated in alpha cells of females with T2D. Pri-miRNA transcription was one of the top GO terms associated with cluster 3 genes (Fig. 4D). Cluster 4 had genes that were upregulated in alpha cells of men with T2D. The glucuronic acid pathway, a relatively minor route of glucose metabolism (56), was enriched in cluster 4 genes (Fig. 4E). Cluster 5 genes were upregulated in beta cells of women with T2D. No GO terms were significantly enriched in cluster 5 genes, although a large number of kinases were present in this list. Cluster 6 included genes that were upregulated in beta cells of men with T2D. Processes associated with extracellular matrix organization were enriched in cluster 6 genes (Fig. 4F). Cluster 7 genes were upregulated in delta cells of men with T2D. A number of immune-related processes including response to bacteria, lymphocyte activation, as well as interleukin-1 production were enriched in cluster 7 genes (Fig. 4G). No unique gene cluster was associated with delta cells of females with T2D. All of the GO terms in different gene clusters did not overlap. Overall, this analysis highlights the existence of diverse cellular and sex-specific trajectory of T2D development.
Figure 4.
Molecular changes in alpha, beta, and delta cells of women and men with type 2 diabetes (T2D). A, Heat map summarizes the expression pattern of T2D-related genes in different cell types both in women and men. Heat map input values are log2 fold change of T2D vs controls within the same sample type (same cell type and sex as indicated in the column names). Columns of the heat map are clustered based on hierarchical clustering with Ward's method and Pearson correlation as the distance measurement. Rows of the heat map are clustered with K-means clustering with the resulting clusters color-coded. B, Dot plot displaying the gene ontology (GO) terms related to cluster 1 genes that are up in all the 3 endocrine cell types of women with T2D. C, GO terms related to cluster 2 genes that are up in all the 3 endocrine cell types of men with T2D. D, GO terms related to cluster 3 genes that are up in alpha cells of women with T2D. E, GO terms related to genes that are up in alpha cells of T2D males. F, GO terms related to genes that are up in beta cells of T2D males. G, GO terms related to genes that are up in delta cells of T2D males. H, GSEA indicates that in alpha cells, the gene set “Up in T2D women” is significantly enriched in T2D men. I, In beta cells, the gene sets “Up in T2D women” and “down in T2D women” are significantly enriched in T2D men and control men, respectively. J, In delta cells, the gene set “up in T2D women” is significantly enriched in T2D men.
The analysis shown here aims to identify the differences in T2D-related molecular processes across samples; next we sought to investigate whether there were similarities in these processes. Toward this, we created female T2D signature gene sets by thresholding and filtering the female-specific differential expression gene lists (see “Methods”). We then performed GSEA, mapping T2D expression changes in men to the female T2D signature gene sets. We observed that, in a cell-specific manner, most signature genes upregulated in women with T2D were also upregulated in men with T2D (Fig. 4H-4J). In beta cells, genes downregulated in women with T2D were also enriched in genes downregulated in men with T2D (Fig. 4I). Together, results from GSEA and hierarchical clustering indicate that in all major pancreatic endocrine cell types, women and men share similar changes in the molecular landscape of T2D pathogenesis, albeit cell type, as well as sex-specific differences, exist.
Taking advantage of the increased statistical power gained from a large number of cells in the integrated single-cell RNA-seq data sets, we next derived molecular signatures related to T2D that are shared between the 2 sexes in each of 3 endocrine cell types (Supplementary Table S4) (13). GO enrichment analysis shows that genes involved in the autophagic mechanism, cell growth, and protein folding were upregulated in all 3 endocrine cell types in T2D, whereas adenosine triphosphate synthesis and other cellular metabolic processes were downregulated in T2D compared with controls (Supplementary Fig. S4) (13). These biological processes altered in T2D are largely in line with what has been reported in previous studies (17, 57).
Endocrine Cells From Male Controls Harbor Gene Signatures Resembling Type 2 Diabetes
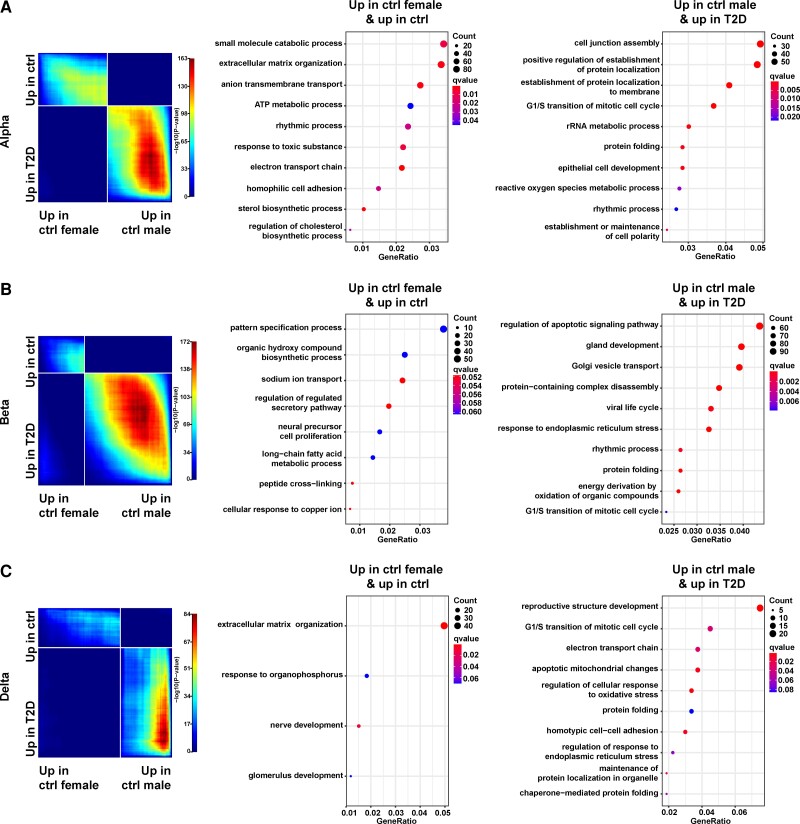
We hypothesized that differential gene expression between women and men during homeostasis led to their differential risks in the development of T2D. To test this hypothesis, we used a stratified RRHO method to compare the gene expression profiles across 2 gene lists that were ranked by (1) the degree of differential expression of female controls vs male controls, and (2) the degree of differential expression of T2D vs controls (contained differentially expressed genes shared between the 2 sexes), respectively (34, 35). This approach in effect projects the differential gene expression between sexes during homeostasis into the context of pathogenesis of diabetes. This analysis revealed a significant reverse correlation pattern between the 2 gene lists in all the 3 endocrine cell types: female controls bore more resemblance to controls, while male controls were more similar in gene expression to T2D (Fig. 5A-5C, left panels). Looking closer at the overlapping genes contributing to the significant reverse correlation pattern, we found that in all 3 endocrine cell types, genes up in female controls (vs male controls) and up in controls (vs T2D) (these genes are located in the upper left quadrants of the RRHO plots) were highly enriched in GO terms related to cellular metabolic pathways. For example, in alpha cells, the significant GO terms associated with these genes included adenosine triphosphate metabolic process, sterol biosynthetic process, and regulation of cholesterol metabolic process (Fig. 5A, middle panel). In contrast, genes up in male controls (vs female controls) and up in T2D (vs controls) (these genes are located in the lower right quadrants of the RRHO plots) were enriched in GO terms related to cellular stress (see Fig. 5A-5C, middle and right panels). For example, in alpha cells, GO terms associated with these genes included reactive oxygen species metabolic process (see Fig. 5A, right panel). Our findings support the recurring notion in this study that endocrine cells of male controls present with “impaired” cellular/molecular profiles compared with female controls.
Figure 5.
Male controls have gene expression patterns similar to type 2 diabetes (T2D). A, Results are shown for alpha cells. Left, rank-rank hypergeometric overlap (RRHO) analysis comparing 2 gene lists containing the differentially expressed genes from female controls vs male controls (x axis) and the differentially expressed genes from T2D vs controls (y axis). White strips represent the transition points in the directions of differential expression in the corresponding gene list. Middle, dot plot displaying the gene ontology (GO) terms related to genes located in the upper left quadrant of RRHO plot. These genes are up in female controls (compared with male controls) and up in controls (compared with T2D). Representative GO terms were selected based on superclusters from REVIGO (32). Right, dot plot displaying the GO terms related to genes located in the lower right quadrant of RRHO plot. These genes are up in male controls (compared with female controls) and up in T2D (compared with controls). B, Same layout as A except that the results are shown for beta cells. C, Same layout as A except that the results are shown for delta cells.
Genomic Regulatory Process of Sex-Biased Gene Expression
To uncover the regulatory principles underlying the sex-specific gene expression in pancreatic endocrine cells, we obtained single-cell ATAC-seq data from 9 adult control donors (4 women and 5 men) without diabetes from HPAP (12). Using the ArchR algorithm (24), we performed quality control to filter out low-quality nuclei and putative doublets. Post filtering, we obtained a total of 27 761 cells, with median unique fragments per cell of 11 123, and an average transcription start site enrichment score of 14.1 (±3.4) (Supplementary Fig. S5A) (13). All samples showed characteristic fragment size distribution corresponding to nucleosomal periodicity (Supplementary Fig. S5B) (13).
We next employed term frequency-inverse document frequency normalization (58) followed by graph-based clustering and UMAP nonlinear dimension reduction to visualize the cellular groups driven by differential open/close chromatin structures. We observed 11 distinct clusters that were shared between different donors (Fig. 6A and 6B). Each cluster was annotated based on the local chromatin accessibility of the marker genes. In this way, we could assign 10 of the clusters to unique pancreatic cell types (Fig. 6B and 6C, and Supplementary Fig. S5C) (13). One cluster (5.5% of total cells) contained cells that displayed increased accessibility to multiple pancreatic cell type markers. Hence, we categorized this cluster as potential doublets (see Fig. 6B and 6C). Female and male pancreatic cells largely overlapped in the UMAP space (Fig. 6D).
Figure 6.
Integration of single-cell ATAC-seq data from 9 control donors. A, Uniform Manifold Approximation and Projection (UMAP) embedding with cells colored according to the donor. B, UMAP embedding with cells colored according to cell type. C, Genome browser tracks displaying local chromatin accessibility at marker genes in each cell type. In the line diagrams of genes at the bottom, blue-colored genes are on the minus strand and red-colored genes are on the plus strand. The marker genes are denoted on top of each column. The number of cells in each cell type are shown inside parentheses. D, UMAP embedding with cells colored according to donor sex.
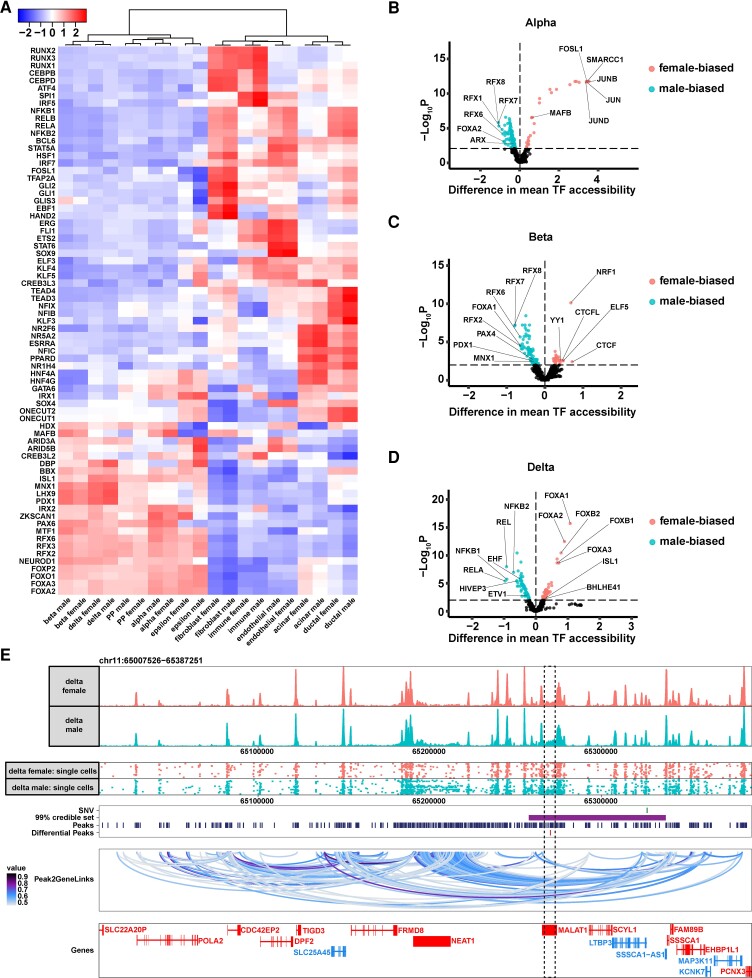
TFs are the main regulators that govern cell-specific gene activities. To parse the regulatory roles of the accessible chromatin revealed by single-cell ATAC-seq in a sex-specific manner, we next carried out TF motif analysis in each cell type stratified by female or male. To ensure that the motifs and corresponding TFs were relevant to our biological system, we filtered the TFs and retained only those that displayed concordant behavior between motif accessibility and gene activity (defined as positive transcription factor regulators; see “Methods”). We observed that, overall, female and male cells shared global patterns in transcriptional regulation in a cell type–specific manner (Fig. 7A). Hierarchical clustering revealed 2 top-level clusters, corresponding to endocrine and exocrine/mesenchymal partitions (see Fig. 7A). Furthermore, beta/delta cells, alpha/PP cells, and ductal/acinar cells had many similar active TFs and clustered together into subclusters, indicating the close ontogeny and gene expression programs of the 2 cell types within each group. The pattern of motif enrichments in the different pancreatic cell types resembled what was reported recently (59). This motif analysis also unveiled known and novel TFs that likely play important regulatory roles in each pancreatic cell type. For example, beta cells were highly enriched in the binding motifs of MNX1, LHX9, and PDX1 (see Fig. 7A). MNX1 and PDX1 are well characterized and are considered upstream regulators of beta-cell fate (60–62). The role of LHX9 in beta-cell transcriptional regulation has not been reported, although its binding partner LDB1 has been shown to be critical in maintaining the differentiated state of beta cells (63, 64). In alpha cells, the most accessible binding motifs were associated with IRX2, ZKSCAN1, and PAX6. IRX2 and PAX6 but not ZKSCAN1 have been reported to be key regulators of alpha-cell identity (65–68). In delta cells, the most enriched binding motif was from DBP. DBP (D-box binding PAR BZIP transcription factor) is involved in circadian clock regulation. DBP was shown to be expressed at higher levels in pancreatic islets compared with other tissues, although its cell-type specificity has not been reported (69).
Figure 7.
Differential accessible sites in female and male endocrine cells. A, Row-normalized chromVAR motif enrichment z scores for positive transcription factor regulator motifs with variable enrichment across different cell types. Hierarchical clustering was performed with Ward's algorithm with Pearson correlation as the distance measurement. B, ChromVar differential deviation analysis comparing female and male motifs in open chromatin regions in alpha cells. The top 5 female- or male-biased motifs are labeled, together with known transcription factors (TFs) that are important for alpha-cell fate determination. C, ChromVar differential deviation analysis in beta cells. D, ChromVar differential deviation analysis in delta cells. E, A Type 2 diabetes (T2D) genome-wide association study (GWAS) signal resides in a differential accessible peak between female and male delta cells. Top panel, aggregated peaks of delta cells from females and males. Second from the top panel, single-cell peaks from female and male delta cells. 100 cells from each population were randomly selected for display. Third from top panel, line indicators of GWAS index single-nucleotide variation (SNV) rs12789028 (row 1), 99% credible set associated with the GWAS signal (row 2), single-cell ATAC-seq peaks (row 3), and differential peaks between female and male delta cells (row 4). Second from bottom panel, peak to gene links. Bottom panel, line diagrams of genes: blue-colored genes are on the minus strand and red-colored genes are on the plus strand. The boxed region contains the differential peak.
Next, we performed a pairwise differential motif analysis comparing the differences in motif accessibilities in women and men of the same cell type (Supplementary Table S5) (13). Motifs with increased accessibility in women compared to men are henceforth referred to as female-biased motifs; motifs with increased accessibility in men compared to women are henceforth referred to as male-biased motifs. Within the endocrine cell types, alpha cells contained the most differential accessible motifs: With an FDR less than 0.01, we identified 31 female-biased and 147 male-biased motifs in alpha cells (Fig. 7B). The top 5 female-biased motifs are associated with JUNB, SMARCC1, FOSL1, JUN, and JUND (see Fig. 7B). JUNB, FOSL1, JUN, and JUND are part of the AP-1 transcription factor complex that plays important roles in cell proliferation, cell death, and inflammatory response (70, 71). The top 5 male-biased motifs are associated with RFX7, RFX8, RFX1, RFX6, and FOXA2 (see Fig. 7B). Among them, RFX6 and FOXA2 are key in endocrine-cell fate including alpha cell-fate specification (72–74). Motifs associated with canonical alpha-cell TFs were variably enriched in the open chromatin regions in females (MAFB) or males (ARX) (see Fig. 7B). In beta cells, there were 33 female-biased and 120 male-biased motifs (Fig. 7C). The top 5 female-biased motifs are related to CTCF, NRF1, ELF5, CTCFL, and YY1 (see Fig. 7C). Among them, CTCF is an important chromatin architecture protein that regulates the 3-dimensional structure of chromatin (75); and both NRF1 and YY1 have been reported to play a role in chromatin remodeling (76–79). The male-biased motifs in beta cells are similar to male-biased motifs in alpha cells. The top 5 male-biased motifs are bound by RFX7, RFX8, RFX6, FOXA1, and RFX2 (see Fig. 7C). In addition, motifs associated with PAX4, PDX1, and MNX1, TFs that play important roles in beta-cell specification and function (60–62, 80), were found to be more male biased (see Fig. 7C). In delta cells, 83 female-biased and 69 male-biased motifs were found (Fig. 7D). The top 5 female-biased motifs are associated with FOXB1, HNF4G, FOXC2, TCF4, and CLOCK (see Fig. 7D). The top 5 male-biased motifs are associated with ATOH1, HNF4A, ZNF683, ETV6, and IRF3 (see Fig. 7D). Motifs of some of the well-recognized pan-endocrine TFs including the FOXAs and ISL1 were female-biased (see Fig. 7D). Other novel delta cell–specific TFs, as reported in a recent single-cell meta-analysis (81), were variably enriched in the open chromatin fragments in women (BHLHE41) or men (ETV1, EHF) (see Fig. 7D). Most of the female-biased differential motifs within each endocrine cell type were not shared among the 3 endocrine cells; however, alpha and beta cells shared a large proportion of male-biased motifs (Supplementary Fig. S6A and S6B) (13). The majority of TFs associated with female- and male-biased motifs were not hormone related, in contrast to reported sex-biased TFs shared across multiple tissues (11). Together, these results suggest an endocrine cell type–specific mechanism in controlling sex-specific genetic programs and indicate that hormones do not play a key role in the process.
We next examined the expressions of the TFs related to the sex-biased motifs in our single-cell RNA-seq data. We found that the majority of these TFs did not show sex-biased expressions (Supplementary Fig. S6C-S6 E) (13). This result concurs with previous reports that indicate sex-biased TF targeting is not dependent on sex-biased gene expression (11, 49).
The majority of T2D-associated variants identified through GWAS fall into noncoding but functional genomic elements, which present as accessible chromatin sites (82–84). We reasoned that our cell type- and sex-specific chromatin accessibility information could help to associate the GWAS signals with their potential genomic targets. Toward this goal, we intersected the differential accessible peak sets between women and men in alpha, beta, and delta cells with the latest T2D GWAS meta-analysis generated by the DIAMANTE consortium (13, 36). Intriguingly, we observed multiple instances where T2D GWAS signals localized in the differential accessible peaks between women and men in delta cells (Supplementary Fig. S7 and Supplementary Table S6) (13). For example, one 99% GWAS-credible set associated with the index single-nucleotide variation rs12789028 overlapped with one differential peak that was more accessible in delta cells of men compared with women (Fig. 7E). By integrating the single-cell ATAC-seq and the single-cell RNA-seq data, we created peak-to-gene lineage by computing the correlation between peak accessibility and gene expression. This approach associated the ATAC-seq peaks near the GWAS signal, including the peaks associated with the index single-nucleotide variation rs12789028, to the promoters of NEAT1. Additional evidence supports the likely functional role of NEAT1 in pancreatic endocrine cells: (1) NEAT1 is 1 of the 2 genes (the other is MALAT1) within this genomic region that have detectable expression from bulk RNA-seq of human islets (Supplementary Fig. S8) (13); and (2) NEAT1 is located within an islet super enhancer that is bound by multiple endocrine-specific TFs including NKX2.2, FOXA2, and MAFB (82, 85) (see Supplementary Fig. S8) (13). Thus, contrary to the previous thought that this GWAS signal was associated with LTBP3 (86), our study nominated NEAT1 as the GWAS target. To determine whether the sex-biased chromatin accessibility translates to differential gene expression, we examined the NEAT1 levels in our integrated single-cell RNA-seq data set. We found that NEAT1 trended toward higher expression in delta cells of male controls compared with female controls, consistent with the direction in their corresponding chromatin accessibility changes, although the sex differences in expression did not reach statistical significance (Supplementary Table S3) (13). Further establishing the potential causal role of NEAT1 in the pathogenesis of T2D, NEAT1 was significantly upregulated in delta cells of donors with T2D compared with controls (FDR < 10%) (Supplementary Table S4) (13). This result is additionally supported by recent literature indicating a higher NEAT1 levels in serums in individuals with T2D compared with control individuals without T2D, although the tissue and cell-type specificity in NEAT1 expression was not examined in these studies (87–89). Together, our study points to the possibility that women and men exhibit differential diabetes risk conferred by differential chromatin accessibilities in this region through a NEAT1-delta cell-pancreatic islet axis.
Discussion
T2D is one of the most prevalent chronic disorders, and its frequency is on the rise. Sex affects the presentation, diagnosis, progression, and drug response of T2D. However, our understanding of how sex contributes to these processes remains limited. Closing this knowledge gap will lead to a leap forward in the precision medicine of diabetes. Our work aims to address the sex specificity in the molecular programs of pancreatic cells. Recognizing and understanding the sex-specific genetic underwirings can help us understand the differential trajectory of diabetes in women and men, which can then lead to sex-specific strategies in precision therapeutics.
Our study confirmed the higher insulin secretion of islets from women compared with men in response to various stimuli (see Fig. 1). The difference in pancreatic endocrine secretion capacity may partially explain the distinctive risk of T2D between the 2 sexes.
To further explore the molecular determinants of the sex differences in endocrine function, we employed high-cellular resolution single-cell-omic technologies. Through single-cell transcriptomic analysis, we identified hundreds to thousands of genes displaying sex-biased expression patterns in each endocrine cell type of control donors (see Fig. 3A and 3B). The majority of the sex-biased genes are cell type specific. This result concurs with a recent large-scale cataloging study that identified most sex-biased genes as tissue specific (11). Of particular note, compared with bulk analysis that have been applied in previous studies (11, 49), single-cell RNA-seq analysis provides a major advantage in overcoming confounding effects such as the effects of sex differences in tissue cellular composition on gene expression. It is foreseeable that a further increase in the power to identify disease stage-specific and sex-biased gene expressions in subgroups of cells can be achieved by improvement in single-cell RNA-seq cell numbers, sequencing depth, RNA library complexity, and sophistication in computational tools, all areas that are in active development (90).
Despite the cell type-specificity in the expression of sex-biased genes, pathway analysis revealed that these genes share common molecular pathways in all 3 endocrine cell types analyzed (see Fig. 3D and 3E). All 3 endocrine cells also shared a large number of common gene expression changes with T2D pathogenesis (see Fig. 4A). In fact, hierarchical clustering revealed that the predominant separation in gene expression was between women and men during T2D transition (see Fig. 4A). Nevertheless, in all 3 endocrine cell types, GSEA analysis indicates that diabetes pathogenesis on the whole follows a common molecular pathway both in women and men (see Fig. 4H-4J). We would like to emphasize that the GSEA results do not contradict the results from hierarchical clustering because while the clustering method aimed to identify the differences between samples, the GSEA analysis underlies the similarities of different expression profiles.
Direct comparison of differential expression gene lists revealed that male control individuals have a gene expression signature resembling T2D (Fig. 5). This finding suggests that men are predisposed to diabetes in their genetic programs. Indeed, male-biased genes are frequently associated with immune response as well as cellular stress-related pathways including ER stress and oxidative stress (see Fig. 3E). All of these processes have been extensively studied and found to be involved in the development of T2D (91–93). Collectively, these data suggest a model in which female endocrine cells are “healthier” and male endocrine cells present with “impaired” cellular/molecular profiles even in control donors. Intriguingly, in animal models of diabetes in which elevated ER stress is genetically, pharmacologically or dietarily induced, there are consistently higher instances of hyperglycemia in males compared with females (9, 94–101). This result suggests that sex differences in pancreatic endocrine tissues share conserved cellular/molecular processes in diverse species.
Analyzing single-cell ATAC-seq data from control donors, we identified hundreds of TF motifs that were differentially accessible between women and men (Fig. 7). Notably, the majority of the sex-biased TFs were not hormone related, arguing that other factors, possibly epigenetic mechanisms such as DNA methylation, may in turn play dominant roles in the sex-specific genetic program of pancreatic endocrine cells (5). It is worth noting that all the islet data (including perifusion, single-cell RNA-seq, and single-cell ATAC-seq) were collected on islets that had been isolated and cultured in vitro for at least 3 days. Thus, it is possible that all the transient and directly hormone-related genetic/genomic changes were erased during this culture and what we detected were more stable molecular differences between female and male endocrine cells.
Integrating single-cell ATAC-seq data with single-cell RNA-seq data, we found that most sex-biased motifs were not differentially expressed between female and male endocrine cells (see Supplementary Fig. S6) (13). This finding is consistent with recent reports that sex-biased regulatory network structures originate from sex-biased differential targeting rather than sex-biased expression of TFs themselves (11, 49). Alternatively, it may also be that the expression bias of TFs exists during early development and this bias leads to differential binding in women and men. The differential binding sites can then be propagated by epigenetic mechanisms such as histone modifications and DNA methylations during later cellular stages (102).
To put the sexual divergent activities in the genomic regulatory regions in the context of T2D causality, we found multiple instances in which T2D GWAS loci overlapped with the differential accessible peaks in delta cells. One such example is located near NEAT1 (see Fig. 7E). NEAT1 was discovered in 2007 as one of the most abundant long noncoding RNAs in the nucleus of mammalian cells (103). It is transcribed from multiple endocrine neoplasia type 1 locus in the human chromosome 11q13 (104). Traditionally, multiple endocrine neoplasia type 1, a mendelian disorder characterized by varying combinations of tumors of multiple endocrine cell types including pancreatic islets, has been attributed to the mutations on the MEN1 gene (Online Mendelian Inheritance in Man, OMIM. Johns Hopkins University, Baltimore, MD. MIM No. 131100, last edited: December 23, 2021, https://omim.org/). However, because NEAT1 was not identified until much later, mutations on NEAT1 have not been examined in the families with multiple endocrine neoplasia type 1. Our work put NEAT1 as one of the promising candidates in regulating sex-specific endocrine disfunction. We reason that NEAT1 perhaps provides the missing heritability of patients with multiple endocrine neoplasia type 1 but bear no coding mutations on the MEN1 gene. Further functional validation is needed to confirm the sex-specific roles of NEAT1 in pancreatic islets and in the pathogenesis of endocrine diseases including T2D.
In summary, our results support the notion that intrinsic genetic wiring of female and male endocrine cells contributes to the differential risk of T2D between the 2 groups. Moreover, our analysis redefined the T2D-associated genetic variants in the context of cell type as well as sex-specific genomic changes. Our work thus provides novel insights into T2D etiology and indicates new treatment opportunities. Importantly, we demonstrated the utility of single-cell-omics to discover disease mechanisms. We believe our work will serve as a solid foundation for ultimately progressing toward sex-aware precision medicine in the prevention and treatment of diabetes and other diseases.
Acknowledgments
This manuscript used data acquired from the Human Pancreas Analysis Program (HPAP-RRID:SCR_016202) Database (https://hpap.pmacs.upenn.edu), a Human Islet Research Network (RRID:SCR_014393) consortium (UC4-DK-112217, U01-DK-123594, UC4-DK-112232, and U01-DK-123716). The authors thank Dr Terra Bradley for the careful editing of the manuscript.
Abbreviations
- AAM
amino acid mixture
- AUC
area under the curve
- DBP
D-box binding PAR BZIP transcription factor
- ER
endoplasmic reticulum
- FC
fold change
- FDR
false discovery rate
- G
glucose
- GO
gene ontology
- GSEA
gene set enrichment analysis
- GWAS
genome-wide association study
- HbA1c
glycated hemoglobin A1c
- HPAP
Human Pancreas Analysis Program
- IBMX
isobutylmethylxanthine
- KCl
potassium chloride
- PC
principal component
- PCA
principal component analysis
- PP
pancreatic polypeptide
- RNA-seq
RNA-sequencing
- RRHO
rank-rank hypergeometric overlap
- SI
stimulation index
- T2D
type 2 diabetes
- TF
transcription factor
- UMAP
Uniform Manifold Approximation and Projection
Contributor Information
Hyo Jeong Yong, Department of Biomedical Sciences, College of Medicine, Florida State University, Tallahassee, Florida 32306, USA.
Maria Pilar Toledo, Department of Biomedical Sciences, College of Medicine, Florida State University, Tallahassee, Florida 32306, USA.
Richard S Nowakowski, Department of Biomedical Sciences, College of Medicine, Florida State University, Tallahassee, Florida 32306, USA.
Yue J Wang, Department of Biomedical Sciences, College of Medicine, Florida State University, Tallahassee, Florida 32306, USA.
Financial Support
Y.J.W. was supported by grants from the JDRF (1-INO-2022-1129-A-N) and the Helmsley Charitable Trust George Eisenbarth nPOD Award for Team Science (2018PG-T1D060).
Disclosures
The authors declare no conflict of interest.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Tramunt B, Smati S, Grandgeorge N, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63(3):453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gannon M, Kulkarni RN, Tse HM, Mauvais-Jarvis F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol Metab. 2018;15:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basu R, Dalla Man C, Campioni M, et al. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006;55(7):2001–2014. [DOI] [PubMed] [Google Scholar]
- 4. Horie I, Abiru N, Eto M, et al. Sex differences in insulin and glucagon responses for glucose homeostasis in young healthy Japanese adults. J Diabetes Investig. 2018;9(6):1283–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall E, Volkov P, Dayeh T, et al. Sex differences in the genome-wide DNA methylation pattern and impact on gene expression, microRNA levels and insulin secretion in human pancreatic islets. Genome Biol. 2014;15(12):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varlamov O, Bethea CL, Roberts CT Jr. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne). 2014;5:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marchese E, Rodeghier C, Monson RS, et al. Enumerating β-cells in whole human islets: sex differences and associations with clinical outcomes after islet transplantation. Diabetes Care. 2015;38(11):e176–e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walker EM, Cha J, Tong X, et al. Sex-biased islet β cell dysfunction is caused by the MODY MAFA S64F variant by inducing premature aging and senescence in males. Cell Rep. 2021;37(2):109813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayne BT, Bianco-Miotto T, Buckberry S, et al. Large scale gene expression meta-analysis reveals tissue-specific, sex-biased gene expression in humans. Front Genet. 2016;7:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oliva M, Muñoz-Aguirre M, Kim-Hellmuth S, et al. The impact of sex on gene expression across human tissues. Science. 2020;369(6509):eaba3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaestner KH, Powers AC, Naji A; HPAP Consortium; Atkinson MA. NIH Initiative to improve understanding of the pancreas, islet, and autoimmunity in type 1 diabetes: the Human Pancreas Analysis Program (HPAP). Diabetes. 2019;68(7):1394–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yong HJ, Toledo MP, Nowakowski RS, Wang YJ. Supplementary data for “Sex differences in the molecular programs of pancreatic cells contribute to the differential risks of type 2 diabetes.” Mendeley Data. Deposited July 8, 2020. https://data.mendeley.com/datasets/s8bsv4cp23/1. [DOI] [PMC free article] [PubMed]
- 14. Baron M, Veres A, Wolock SL, et al. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst. 2016;3(4):346–360.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Enge M, Efsun Arda H, Mignardi M, et al. Single-cell analysis of human pancreas reveals transcriptional signatures of aging and somatic mutation patterns. Cell. 2017;171(2):321–330.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lawlor N, George J, Bolisetty M, Kursawe R. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type–specific expression changes in type 2 diabetes. Genome Res. 2017;27(2):208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Segerstolpe Å, Palasantza A, Eliasson P, et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 2016;24(4):593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muraro MJ, Dharmadhikari G, Grün D, et al. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 2016;3(4):385–394.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang YJ, Schug J, Won KJ, et al. Single-cell transcriptomics of the human endocrine pancreas. Diabetes. 2016;65(10):3028–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Avrahami D, Wang YJ, Schug J, et al. Single-cell transcriptomics of human islet ontogeny defines the molecular basis of β-cell dedifferentiation in T2D. Mol Metab. 2020;42:101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xin Y, Kim J, Okamoto H, et al. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab. 2016;24(4):608–615. [DOI] [PubMed] [Google Scholar]
- 22. Xin Y, Dominguez Gutierrez G, Okamoto H, et al. Pseudotime ordering of single human β-cells reveals states of insulin production and unfolded protein response. Diabetes. 2018;67(9):1783–1794. [DOI] [PubMed] [Google Scholar]
- 23. Hao Y, Hao S, Andersen-Nissen E, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573–3587.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Granja JM, Corces MR, Pierce SE, et al. ArchR is a scalable software package for integrative single-cell chromatin accessibility analysis. Nat Genet. 2021;53(3):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ackermann AM, Wang Z, Schug J, Naji A, Kaestner KH. Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes. Mol Metab. 2016;5:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yong HJ, Xie G, Liu C, et al. Gene signatures of NEUROGENIN3+endocrine progenitor cells in the human pancreas. Front Endocrinol (Lausanne). 2021;12:736286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zappia L, Oshlack A. Clustering trees: a visualization for evaluating clusterings at multiple resolutions. Gigascience. 2018;7(7):giy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McInnes L, Healy J, Melville J. UMAP: uniform manifold approximation and projection for dimension reduction. arXiv [stat.ML] 2018. Accessed May 6, 2022. http://arxiv.org/abs/1802.03426
- 30. Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Camunas-Soler J, Dai X-Q, Hang Y, et al. Patch-Seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab. 2020;31(5):1017–1031.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu T, Hu E, Xu S, et al. Clusterprofiler 4.0: a universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2(3):100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6(7):e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plaisier SB, Taschereau R, Wong JA, Graeber TG. Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 2010;38(17):e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cahill KM, Huo Z, Tseng GC, Logan RW, Seney ML. Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Sci Rep. 2018;8(1):9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahajan A, Spracklen CN, Zhang W, et al. Multi-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translation. Nat Genet. 2022;54(5):560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohara-Imaizumi M, Nakamichi Y, Tanaka T, Ishida H, Nagamatsu S. Imaging exocytosis of single insulin secretory granules with evanescent wave microscopy: distinct behavior of granule motion in biphasic insulin release. J Biol Chem. 2002;277(6):3805–3808. [DOI] [PubMed] [Google Scholar]
- 38. Shackman JG, Reid KR, Dugan CE, Kennedy RT. Dynamic monitoring of glucagon secretion from living cells on a microfluidic chip. Anal Bioanal Chem. 2012;402(9):2797–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wewer Albrechtsen W, Kuhre RE, Windeløv JA, et al. Dynamics of glucagon secretion in mice and rats revealed using a validated sandwich ELISA for small sample volumes. Am J Physiol Endocrinol Metab. 2016;311(2):E302–E309. [DOI] [PubMed] [Google Scholar]
- 40. Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The new biology and pharmacology of glucagon. Physiol Rev. 2017;97(2):721–766. [DOI] [PubMed] [Google Scholar]
- 41. Muller DC, Elahi D, Tobin JD, Andres R. The effect of age on insulin resistance and secretion: a review. Semin Nephrol. 1996;16(4):289–298. [PubMed] [Google Scholar]
- 42. Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52(7):1738–1748. [DOI] [PubMed] [Google Scholar]
- 43. Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284(1):E7–E12. [DOI] [PubMed] [Google Scholar]
- 44. Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–1902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gromada J, Chabosseau P, Rutter GA. The α-cell in diabetes mellitus. Nat Rev Endocrinol. 2018;14(12):694–704. [DOI] [PubMed] [Google Scholar]
- 46. Huising MO, van der Meulen T, Huang JL, Pourhosseinzadeh MS, Noguchi GM. The difference δ-cells make in glucose control. Physiology. 2018;33(6):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pierzynowski SG, Gregory PC, Filip R, Woliński J, Pierzynowska KG. Glucose homeostasis dependency on acini-islet-acinar (AIA) axis communication: a new possible pathophysiological hypothesis regarding diabetes mellitus. Nutr Diabetes. 2018;8(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dirice E, De Jesus DF, Kahraman S, et al. Human duct cells contribute to β cell compensation in insulin resistance. JCI Insight. 2019;4(8):e99576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lopes-Ramos CM, Chen CY, Kuijjer ML, et al. Sex differences in gene expression and regulatory networks across 29 human tissues. Cell Rep. 2020;31(12):107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tukiainen T, Villani AC, Yen A, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550(7675):244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown CJ, Hendrich BD, Rupert JL, et al. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71(3):527–542. [DOI] [PubMed] [Google Scholar]
- 52. Tian D, Sun S, Lee JT. The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell. 2010;143(3):390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kashimada K, Koopman P. Sry: the master switch in mammalian sex determination. Development. 2010;137(23):3921–3930. [DOI] [PubMed] [Google Scholar]
- 54. Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121(6):1603–1614. [DOI] [PubMed] [Google Scholar]
- 55. Liu G, Li Y, Zhang T, et al. Single-cell RNA sequencing reveals sexually dimorphic transcriptome and type 2 diabetes genes in mouse islet β cells. Genomics Proteomics Bioinformatics. 2021;19(3):408–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eisenberg F Jr, Dayton PG, Burns JJ. Studies on the glucuronic acid pathway of glucose metabolism. J Biol Chem. 1959;234(2):250–253. [PubMed] [Google Scholar]
- 57. Ngara M, Wierup N. Lessons from single-cell RNA sequencing of human islets. Diabetologia. 2022;65(8):1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cusanovich DA, Daza R, Adey A, et al. Multiplex single cell profiling of chromatin accessibility by combinatorial cellular indexing. Science. 2015;348(6237):910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chiou J, Zeng C, Cheng Z, et al. Single-cell chromatin accessibility identifies pancreatic islet cell type- and state-specific regulatory programs of diabetes risk. Nat Genet. 2021;53(4):455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. β-Cell-specific inactivation of the mouseIpf1/Pdx1 gene results in loss of the β-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12(12):1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Harrison KA, Thaler J, Pfaff SL, Gu H, Kehrl JH. Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9-deficient mice. Nat Genet. 1999;23(1):71–75. [DOI] [PubMed] [Google Scholar]
- 62. Li H, Arber S, Jessell TM, Edlund H. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat Genet. 1999;23(1):67–70. [DOI] [PubMed] [Google Scholar]
- 63. Hunter CS, Dixit S, Cohen T, et al. Islet α-, β-, and δ-cell development is controlled by the Ldb1 coregulator, acting primarily with the islet-1 transcription factor. Diabetes. 2013;62(3):875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ediger BN, Lim HW, Juliana C, et al. LIM domain-binding 1 maintains the terminally differentiated state of pancreatic β cells. J Clin Invest. 2017;127(1):215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387(6631):406–409. [DOI] [PubMed] [Google Scholar]
- 66. Petri A, Ahnfelt-Rønne J, Frederiksen KS, et al. The effect of neurogenin3 deficiency on pancreatic gene expression in embryonic mice. J Mol Endocrinol. 2006;37(2):301–316. [DOI] [PubMed] [Google Scholar]
- 67. Soyer J, Flasse L, Raffelsberger W, et al. Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development. Development. 2010;137(2):203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dorrell C, Schug J, Lin CF, et al. Transcriptomes of the major human pancreatic cell types. Diabetologia. 2011;54(11):2832–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Allaman-Pillet N, Roduit R, Oberson A, et al. Circadian regulation of islet genes involved in insulin production and secretion. Mol Cell Endocrinol. 2004;226(1-2):59–66. [DOI] [PubMed] [Google Scholar]
- 70. Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4(5):E131–E136. [DOI] [PubMed] [Google Scholar]
- 71. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee CS, Sund NJ, Behr R, Herrera PL, Kaestner KH. Foxa2 is required for the differentiation of pancreatic α-cells. Dev Biol. 2005;278(2):484–495. [DOI] [PubMed] [Google Scholar]
- 73. Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22(24):3435–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Smith SB, Qu HQ, Taleb N, et al. Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463(7282):775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137(7):1194–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cai Y, Jin J, Yao T, et al. YY1 Functions with INO80 to activate transcription. Nat Struct Mol Biol. 2007;14(9):872–874. [DOI] [PubMed] [Google Scholar]
- 77. Wu S, Shi Y, Mulligan P, et al. A YY1–INO80 complex regulates genomic stability through homologous recombination-based repair. Nat Struct Mol Biol. 2007;14(12):1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sherwood RI, Hashimoto T, O’Donnell CW, et al. Discovery of directional and nondirectional pioneer transcription factors by modeling DNase profile magnitude and shape. Nat Biotechnol. 2014;32(2):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Domcke S, Bardet AF, Adrian Ginno P, Hartl D, Burger L, Schübeler D. Competition between DNA methylation and transcription factors determines binding of NRF1. Nature. 2015;528(7583):575–579. [DOI] [PubMed] [Google Scholar]
- 80. Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386(6623):399–402. [DOI] [PubMed] [Google Scholar]
- 81. Van Gurp L, Fodoulian L, Oropeza D, et al. Generation of human islet cell type-specific identity genesets. Nature. 2022;13(1):2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pasquali L, Gaulton KJ, Rodríguez-Seguí SA, et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet. 2014;46(2):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Varshney A, Scott LJ, Welch RP, et al. Genetic regulatory signatures underlying islet gene expression and type 2 diabetes. Proc Natl Acad Sci USA. 2017;114(9):2301–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thurner M, van de Bunt M, Torres JM, et al. Integration of human pancreatic islet genomic data refines regulatory mechanisms at type 2 diabetes susceptibility loci. Elife. 2018;7:e31977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mularoni L, Ramos-Rodríguez M, Pasquali L. The pancreatic islet regulome browser. Front Genet. 2017;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vujkovic M, Keaton JM, Lynch JA, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. 2020;52(7):680–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shao K, Xi L, Cang Z, Chen C, Huang S. Knockdown of NEAT1 exerts suppressive effects on diabetic retinopathy progression via inactivating TGF-β1 and VEGF signaling pathways. J Cell Physiol. 2020;235(12):9361–9369. [DOI] [PubMed] [Google Scholar]
- 88. Alfaifi M, Ali Beg MM, Alshahrani MY, et al. Circulating long non-coding RNAs NKILA, NEAT1, MALAT1, and MIAT expression and their association in type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2021;9(1):e001821. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89. Jia D, He Y, Wang Y, et al. NEAT1: a novel long non-coding RNA involved in mediating type 2 diabetes and its various complications. Curr Pharm Des. 2022;28(16):1342–1350. [DOI] [PubMed] [Google Scholar]
- 90. Wang YJ, Kaestner KH. Single-Cell RNA-seq of the pancreatic islets—a promise not yet fulfilled? Cell Metab. 2019;29(3):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia. 2005;48(6):1038–1050. [DOI] [PubMed] [Google Scholar]
- 92. Scheuner D, Vander Mierde D, Song B, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11(7):757–764. [DOI] [PubMed] [Google Scholar]
- 93. Eguchi N, Vaziri ND, Dafoe DC, Ichii H. The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int J Mol Sci. 2021;22(4):1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Leiter EH. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Natl Acad Sci U S A. 1982;79(2):630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kava RA, West DB, Lukasik VA, Greenwood MR. Sexual dimorphism of hyperglycemia and glucose tolerance in Wistar fatty rats. Diabetes. 1989;38(2):159–163. [DOI] [PubMed] [Google Scholar]
- 96. Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46(5):887–894. [DOI] [PubMed] [Google Scholar]
- 97. Herbach N, Rathkolb B, Kemter E, et al. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes. 2007;56(5):1268–1276. [DOI] [PubMed] [Google Scholar]
- 98. Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P. Endoplasmic reticulum stress and eIF2α phosphorylation: the Achilles heel of pancreatic β cells. Mol Metab. 2017;6(9):1024–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ingvorsen C, Karp NA, Lelliott CJ. The role of sex and body weight on the metabolic effects of high-fat diet in C57BL/6N mice. Nutr Diabetes. 2017;7(4):e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Deeds MC, Anderson JM, Armstrong AS, et al. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45(3):131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Austin ALF, Daniels Gatward LF, Cnop M, et al. The KINGS Ins2+/G32S mouse: a novel model of β-cell endoplasmic reticulum stress and human diabetes. Diabetes. 2020;69(12):2667–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Spitz F, Furlong EEM. Transcription factors: from enhancer binding to developmental control. Nat Rev Genet. 2012;13(9):613–626. [DOI] [PubMed] [Google Scholar]
- 103. Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB, Chess A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics. 2007;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Guru SC, Agarwal SK, Manickam P, et al. A transcript map for the 2.8-Mb region containing the multiple endocrine neoplasia type 1 locus. Genome Res. 1997;7(7):725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in “References.”