Abstract
Background and Aims
Coronavirus disease (COVID‐19) is a major danger to world health and has been linked to periodontitis in a number of epidemiological observational studies. However, it is unclear whether COVID‐19 causes periodontitis. COVID‐19's causal influence on periodontitis was determined using bidirectional Mendelian randomization (MR).
Methods
Large‐scale COVID‐19 and periodontitis genome wide association study data were analyzed. Inverse variance weighting, MR‐Egger, weighted median, and MR‐PRESSO were used to estimate causal effects. Sensitivity studies were conducted using the Cochran's Q test, the MR‐Egger intercept test, the MR‐PRESSO, and the leave‐one‐out (LOO) analysis. Further investigation of potential mediating factors was performed using risk factor analysis.
Results
The MR presented no causal relationship between periodontitis and hospitalization for COVID‐19 (odds ratio [OR] = 0.97, 95% confidence interval [CI] 0.78–1.20; p = 0.76), vulnerability to COVID‐19 (OR = 1.04, 95% CI 0.88–1.21; p = 0.65), COVID‐19 disease severity (OR = 1.01, 95% CI 0.92–1.11; p = 0.81). Meanwhile, a noncausal effect of genetic hospitalization for COVID‐19, illness severity, and vulnerability to periodontitis was detected. Other MR methods yielded identical results to inverse variance weighting. According to sensitivity analysis, horizontal pleiotropy is unlikely to affect causal estimation.
Conclusion
Periodontitis had no link to the risk of COVID‐19 hospitalization, susceptibility, or severity. However, the substance in COVID‐19 that is responsible for this effect must be studied further.
Keywords: COVID‐19, Mendelian randomization, periodontitis
1. INTRODUCTION
During Coronavirus Disease 2019 (COVID‐19), individuals all over the world were infected with the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 , 2 WHO declared COVID‐19 a pandemic due to its rapid spread worldwide in March 2020. 3 , 4 In 2019, COVID‐19, initially discovered in Wuhan, China, quickly spread over the globe, causing over 160 million cases and nearly 2,000 deaths by May 25, 2021. 1 A public health emergency burdens healthcare systems worldwide, threatening individuals' well‐being and disrupting economies. 5 , 6 The key to treating and managing COVID‐19 is to understand how serious COVID‐19 is linked to other health issues. Several studies have found that serious COVID‐19 is linked to being older, being male, and having a number of other health problems, such as obesity, type 2 diabetes, heart disease, and high blood pressure. 7 , 8 , 9 , 10 , 11 , 12
Periodontitis is a bacterial infection of the periodontal tissues that causes bone resorption and tooth loss in the oral cavity. 13 This chronic inflammatory noncommunicable disease, the sixth most prevalent globally, affects approximately 50% of adults. Periodontitis affects approximately 11% of the population. 14 , 15 Public health and socioeconomic problems remain a major concern due to periodontitis' high prevalence and consequences. 16 Several consequences can arise from periodontitis, including tooth loss and disability, affecting the patient's quality of life and overll‐being. 17 , 18 In addition to diabetes, cardiovascular disease, and increased mortality, severe periodontitis is associated with other systemic diseases. 19 , 20 Periodontitis was considered aggressive periodontitis (AgP) in 1999, whereas chronic periodontitis (CP) is a slower‐progressing form of periodontitis. 21
A link between periodontitis and COVID‐19 has long been recognized. 22 , 23 , 24 Innate immune system activation and the creation of inflammatory cytokines such interleukin‐6 and tumour necrosis factor play an important role in both disorders. 25 Several observational studies have also demonstrated a link between COVID‐19 and periodontitis. 25 , 26 , 27 A causal link between periodontitis and COVID‐19 must be established to understand periodontitis and COVID‐19 pathogenesis better. Thus, we hypothesize that COVID‐19 could be caused by periodontitis progression. With the emergence of Mendelian randomization (MR) and large‐scale genome wide association study (GWAS), it is now feasible to establish a causal relationship between complex characteristics and illnesses. MR, a novel epidemiological approach, may be considered conceptually as natural RCTs because allocating genotypes from parent to offspring is random. In contrast to observational studies, the use of genetic variations as instrumental variables (IVs) in MR is an efficient way to prevent the effects of confounding factors such measurement error, reverse causality, and underlying bias. Therefore, we investigate the causal link between COVID‐19 and periodontitis using MR analysis with genetic variation as an IVs. 28 In addition, we employ reverse MR to examine the bidirectional causal effect of COVID‐19 on periodontitis.
2. METHODS
2.1. Data sources
Data from the GLIDE consortium's most recent meta‐analysis of GWAS of gene‐lifestyle interactions were used to summarize the findings for periodontitis (https://data.bris.ac.uk/data/dataset/2j2rqgzedxlq02oqbb4vmycnc2). The data set included 12,289 clinically identified cases and 22,326 controls with European ancestry. 29 Seventeen IV SNPs were identified as having a p value > 5 × 10−6, as used in other literature. 30
A GWAS meta‐analysis conducted previously for COVID‐19, Round 5, was used to obtain genetic statistics for the COVID‐19 phenotype (https://www.covid19hg.org/results/). 31 A total of 2,586,691 European‐ancestry participants participated in the study. Three phenotypes, including susceptibility, hospitalization, and severe clinical consequences, were linked to COVID‐19. The diagnosis of SARS‐CoV‐2 infection was made through a laboratory confirmation, electronic health records, patient‐reported COVID‐19 infection, or a physician diagnosis of COVID‐19. COVID‐19 patients (N = 112,612) and disease‐free population controls were compared to determine susceptibility. According to WHO guidelines, patients hospitalized for COVID‐19‐related symptoms or a confirmed SARS‐CoV‐2 infection were considered hospitalized COVID‐19 patients. The study compared COVID‐19 hospitalization outcomes between two groups: those with COVID‐19 (N = 24,274) and those without. Severe COVID‐19 patients were characterized as those who needed respiratory support while in the hospital, including interventions such as Bilevel positive airway pressure, continuous external negative pressure, intubation, continuous positive airway pressure, or high‐flow nasal cannulation. The number of severe cases was 8779 for COVID‐19 severity. 32 , 33 IV SNPs were identified with the p > 5 × 10−8.
2.2. MR analyses
To ensure that the genetic IVs met the three key MR assumptions, we applied the following criteria: (i) a GWAS‐related p value less than 5 × 10−6, ii) a linkage disequilibrium [LD] r2 < 0.001, and iii) a proximity of <1 MB from the index variant. The potential effects of variance heterogeneity and pleiotropy were assessed using three different MR methods: inverse variance weighting (IVW), MR Egger, and weighted median. 34 , 35 The main analysis involved applying standard IVW estimates, which integrated the Wald ratios of each SNP of the outcome and calculated summary causal estimates. If only one SNP was available, the causality of the outcome exposure was estimated using the Wald ratio. Additional MR analyses were conducted using several methods, such as MR‐Egger regression, weighted median, and MR‐PRESSO (MR‐PESO) to strengthen the MR results. An empirical distribution was considered in the weighted median method, with each variant receiving a weight corresponding to the inverse of the variance of the ratio estimate. 36 MR‐Egger regression offers the advantage of being able to examine for unbalanced multi‐effects and significant heterogeneity, though it needs a larger sample size to detect similar under‐exposure variation. 37 The weighted median technique revealed that horizontal pleiotropy contributed at least 50% of the weighted variance. 36
2.3. Sensitivity analysis
Horizontal pleiotropy is characterized by genetic variations that are linked to the exposure of interest and impact the outcome (i.e., severe COVID‐19) via other pathways beyond the hypothesized exposure. To evaluate the robustness of the results and detect potential pleiotropy, several tests were conducted, including Cochran's Q statistic, funnel plots, leave‐one‐out (LOO) tests, and MR‐Egger intercept tests. In the Cochran's Q test, a p value less than 0.05 suggest the presence of heterogeneity. Additionally, horizontal pleiotropy in MR‐Egger regression was calculated using the intercept term. LOO analysis was performed, in which each exposure‐associated SNP was removed separately to determine whether any single SNP was responsible for the causal estimate.
2.4. Risk factors
Several potential co‐variables that could affect the genetic relationship between periodontitis and COVID‐19 were explored further. Additionally, the PhenoScanner platform, which provides comprehensive information on genotype‐phenotype associations, was utilized to check whether any of the SNPs used in the analysis were linked to potential risk factors such as body mass index, obesity, smoking, drinking, and hypertension. 38 Any SNPs found to be associated were removed from the analysis to prevent confounding and ensure the validity of the results.
2.5. Statistical analysis
The MR analysis findings were presented as odds ratios (OR) with accompanying 95% confidence intervals (CI). Each standard deviation (SD) increment in COVID‐19 risk caused an estimate of the relative periodontitis risk. This study was conducted using TwoSampleMR (version 4.25) and MR‐PRESSO (version 1.0) in R (version 3.6.1). 39
2.6. Ethical approval
This study's data were publicly available, and the studies from which they were obtained had previously obtained ethical approval and participant consent. 29 , 31 , 40
3. RESULTS
3.1. The causal effect of periodontitis on COVID‐19
This study used 17 SNPs to predict periodontitis genetically, with no genetic variant being palindromic or having intermediate allele frequencies. The genetic instrument used in the MR analysis had all F statistics greater than the typically recommended value of 10, indicating that it was a robust and strong instrument. 41
Figure 1 and Table 1 present the MR estimates for the different methods. The genetically predicted periodontitis risk and the COVID‐19 risk appeared unrelated. The increased risk of periodontitis due to MR was not statistically significant with increased COVID‐19 risk (vulnerability to COVID‐19: SNP: 3, odds ratio [OR] = 1.03, 95% CI: 0.79–1.34, p = 0.82; hospitalization for COVID‐19: SNP: 4, OR = 1.34, 95% CI: 0.98–1.85, p = 0.07; the severity of COVID‐19 illness: SNP: 2, OR = 1.01, 95% CI: 0.61–1.66, p = 0.97). However, MR‐Egger, the Weighted Median, and the Weighted Mode demonstrated similar findings. Figure 2 illustrates a scatter plot of SNP effect sizes for periodontitis and COVID‐19. The heterogeneity test revealed no heterogeneity among individual SNPs. An MR‐Egger regression and MR‐PRESSO test indicate that horizontal pleiotropy has not affected these findings (Table 2). LOO analysis revealed that no single SNP contributed significantly to periodontitis causal estimates. Figures 3 and 4 depict a funnel plot and a LOO analysis plot, respectively.
Figure 1.
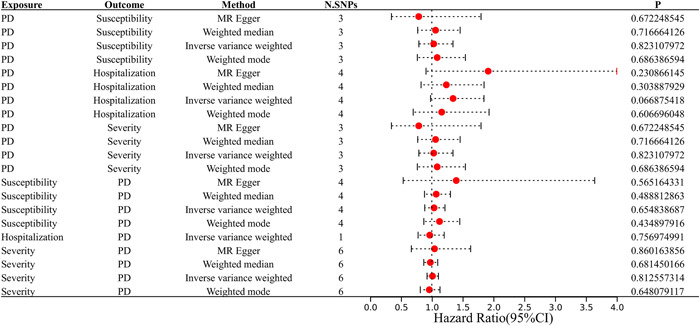
Estimated causal effects between periodontitis and COVID using different MR methods. MR, Mendelian randomization.
Table 1.
Mendelian randomization estimates of the causal association between periodontitis and COVID‐19.
| Exposure | Outcome | OR (95% CI) | p Value |
|---|---|---|---|
| Periodontitis | Susceptibility | 1.03 (0.79−1.34) | 0.82 |
| Hospitalization | 1.34 (0.98−1.85) | 0.07 | |
| Severity | 1.01 (0.61−1.66) | 0.97 | |
| Susceptibility | periodontitis | 1.04 (0.88−1.21) | 0.65 |
| Hospitalization | 0.97 (0.78−1.20) | 0.76 | |
| Severity | 1.01 (0.92−1.11) | 0.81 |
Figure 2.
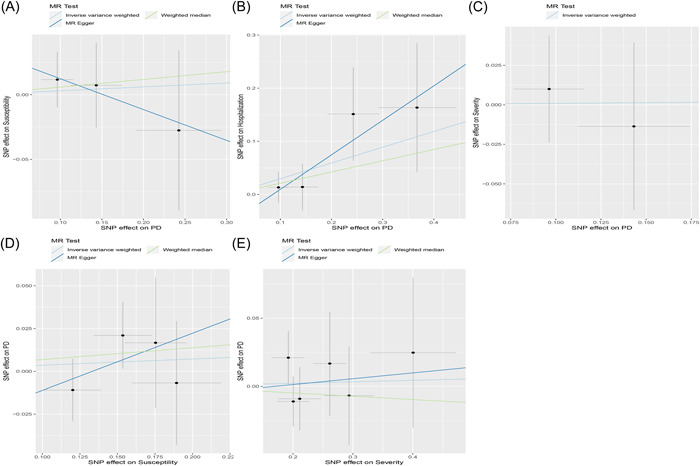
Scatter plot of the causal relationships between periodontitis and COVID using different MR methods. (A) Causal estimates for periodontitis on COVID susceptibility. (B) Causal estimates for periodontitis on COVID hospitalization. (C) Causal estimates for periodontitis on COVID severity. (D) Causal estimates for COVID susceptibility on periodontitis. (E) Causal estimates for COVID severity on periodontitis. MR, Mendelian randomization.
Table 2.
Sensitivity analysis of the causal association between periodontitis and the risk of COVID‐19.
| Exposure | Outcome | Cochrane Q test | MR‐Egger | MR‐PRESSO p value | ||
|---|---|---|---|---|---|---|
| Q value | p | Intercept | p | |||
| Periodontitis | Susceptibility | 0.49 | 0.78 | 0.036 | 0.62 | 0.52 |
| Hospitalization | 1.71 | 0.63 | −0.055 | 0.41 | 0.71 | |
| Severity | 0.15 | 0.69 | NA | NA | NA | |
| Susceptibility | Periodontitis | 1.54 | 0.67 | −0.044 | 0.59 | 0.55 |
| Hospitalization | NA | NA | NA | NA | NA | |
| Severity | 2.05 | 0.84 | −0.007 | 0.89 | 0.53 | |
Figure 3.
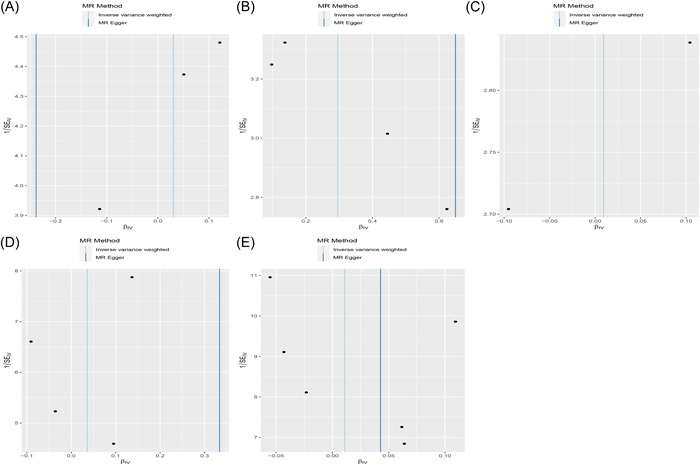
The funnel plots from genetically periodontitis on COVID‐19 hospitalization, susceptibility and severity. (A) Causal estimates for periodontitis on COVID susceptibility. (B) Causal estimates for periodontitis on COVID hospitalization. (C) Causal estimates for periodontitis on COVID severity. (D) Causal estimates for COVID susceptibility on periodontitis. (E) Causal estimates for COVID severity on periodontitis.
Figure 4.
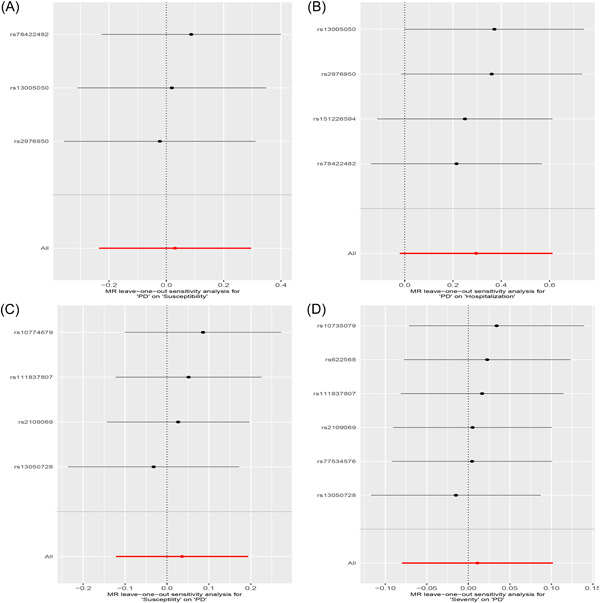
The leave‐one‐out analysis plots between periodontitis and COVID. (A) Causal estimates for periodontitis on COVID severity. (B) Causal estimates for periodontitis on COVID susceptibility. (C) Causal estimates for COVID susceptibility on periodontitis. (D) Causal estimates for COVID severity on periodontitis.
3.2. Causal effects of COVID‐19 on periodontitis
The 167, 637, and 550 SNPs with p < 5 × 10−8 were considered IV SNPs for COVID‐19 hospitalization, susceptibility, and disease severity, respectively. Identifying any palindromes with moderate allele frequencies was impossible, and the average F values for COVID‐19 hospitalization, susceptibility, and illness severity were 22.22, 21.32, and 23.15, respectively, with all genetic variations having F values higher than 10.
Figure 1 compares the MR estimates generated by the different methods. Genetically predicted periodontitis and COVID‐19 risk were not causally associated. The IVW demonstrated no statistically significant link between COVID‐19 and periodontitis (COVID‐19 hospitalization: SNP: 1, OR = 0.97, 95% CI: 0.78–1.20, p = 0.76; vulnerability to COVID‐19: SNP: 4, OR = 1.04, 95% CI: 0.88–1.21, p = 0.65), COVID‐19 seriousness (SNP: 6, OR = 1.01, 95% CI: 0.92–1.11, p = 0.81). Meanwhile, the weighted median, the MR‐Egger, and the weighted mode methods were similar. Figure 2 presents the scatter plots for periodontitis and COVID‐19. The heterogeneity test revealed that the SNPs were not heterogeneous. Based on the results of the MR‐Egger regression and MR‐PRESSO tests, there is strong evidence to suggest that horizontal pleiotropy did not affect the causal relationship between periodontitis and COVID‐19. (Table 2). LOO analysis indicated that no single SNP contributed significantly to causal estimates of periodontitis. Figures 3 and 4 illustrate the funnel plots and the LOO.
4. DISCUSSION
Oral health is impacted by broader health issues. Numerous studies have shown a connection between issues with dental health and systemic illnesses such diabetes, obesity, atherosclerotic heart disease, Alzheimer's disease, and the aftereffects of those illnesses. 42 Periodontitis is an inflammatory condition caused by physiological disruptions that elevate the host's inflammatory response 43 and lead to systemic disease. 44 Periodontitis has been associated with various diseases, including stroke, 45 cardiovascular disease, 46 malignant tumor, 47 and pneumonia. 48 “Cytokine storm,” caused by cytokine release from host cells, has been linked to SARS‐CoV‐2 infection. 49 This association between periodontitis and systemic diseases is partially due to cytokines that stimulate bacteria and destroy tissues. 50 Consequently, purely observational studies cannot establish causality between periodontitis and COVID‐19. Therefore, we investigated the causal link between periodontitis and COVID‐19 using MR. We discovered no evidence that genetically predicted periodontitis in individuals of European descent is associated with COVID‐19 in our two‐sample MR analysis.
Periodontitis and COVID‐19 have previously been linked in epidemiological studies. Evidence suggests that COVID patients have a higher risk of developing periodontitis. COVID‐19 has been linked to severe periodontitis 25 and a higher risk of serious COVID‐19 complications. 51 , 52 , 53 A cross‐sectional clinical study by Gupta et al. indicated that COVID‐19 pneumonia and hospitalization increased with periodontitis realities. 26 A recent meta‐analysis found that while COVID‐19 was not significantly linked to periodontal symptoms (OR = 1.1; 95% CI, 0.73–1.65) or mortality among patients with periodontitis (OR = 2.71; 95% CI, 0.64–11.51), there was an observed link between COVID‐19 severity and the presence of periodontal disease (OR = 3.18; 95% CI, 1.81–5.58). 54 In Qatar, a case‐control study involving 568 patients examined COVID‐19 complications and the presence of periodontitis. 25 Another study of 137 COVID‐19 patients (aged 20 to 65) revealed that patients with signs of oral disease were more likely to be infected by COVID‐2019 than those without oral disease. 55
Aging, obesity, diabetes, hypertension, and cardiovascular disease are associated with COVID‐19 severity 56 , 57 and are also significantly associated with periodontitis. 16 , 46 Due to this uncertainty, it is unknown if periodontitis increases the severity of Coronavirus through comorbidities or if particular mechanisms or pathways are involved. 58 , 59 The included research suggested a link between COVID‐19 infection and periods of poor oral health. However, there are several causes of poor dental health, including COVID‐19‐related stress and/or emotional responses, intubation, medications, and immunological problems. 60 , 61 Therefore, whether the oral health problem is produced directly by SARS‐CoV‐2, is connected to antibiotic therapy, or if an immunity to COVID‐19 has been badly impaired is unknown. 62 It has been hypothesized that symptoms of oral lesions may suggest that immune storm‐related damage has persisted after the eradication of SARS‐CoV‐2, and SARS‐CoV‐2‐related inflammatory markers may have been upregulated during this period. 60 COVID‐19 patients treated with antibiotics are often prophylactic and therapeutic measures. Several infectious agents, including SARS‐CoV‐2, were detected in the oral cavity, suggesting a potential co‐infection. As a result, antibiotic therapy may raise the risk of oral health issues by altering the oral microbiota because it is difficult to confirm a causal relationship. 63 The SARS‐COV‐2 pathogenesis and its direct role in oral homeostasis, the effects of antibiotics or other treatments on these processes, and the impact of the inflammatory response must be considered. More research is required to determine whether COVID‐19 infection causes oral health problems. Therefore, we performed a reverse MR to determine whether COVID‐19 also causally affects periodontitis in the opposite direction.
Several strengths can be identified in the current bidirectional MR. First, Using the MR design, our investigation may mimic a randomized controlled trial in an observational situation. Randomized control features are widely accepted in the study of causality but are costly, expensive, and frequently impractical. However, MR studies can effectively avoid confounding bias in assigning SNPs randomly at conception. In contrast to other observational studies, MR also avoids reverse causality effects. Second, we analyzed the biggest GWAS data of periodontitis to determine the causal association between COVID‐19 and periodontitis. Our study supports the causal link between periodontitis and COVID‐19.
This MR analysis has some flaws that need to be considered when interpreting the results. Firstly, the study population was of European ancestry, so the findings may not be generalizable to other populations from different regions. Secondly, the IVs used in the analysis were selected based on SNPs with p values less than 5 × 10−6 in GWAS data of periodontitis. This threshold is lower than the commonly accepted threshold of 5 × 10−8 and may have affected the statistical power of the causal estimates. Therefore, caution is required when interpreting the results, and further research is needed to validate the findings and confirm their generalizability to different populations.
In summary, our study demonstrated that the risk of COVID‐19 is not causally related to genetic periodontitis and did not genetically predict COVID‐19 susceptibility and hospitalization on periodontitis, except for COVID‐19 severity.
AUTHOR CONTRIBUTIONS
Jukun Song: Conceptualization; data curation; software; writing—original draft. Yadong Wu: Formal analysis; investigation; methodology. Xinhai Yin: Methodology; project administration; validation. Junmei Zhang: Software; visualization; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The present study was a secondary analysis based on publicly available data. Informed consent was obtained for all the participants according to the original GWAS protocol, and ethical approval for GWAS was obtained from the original GWAS authors.
TRANSPARENCY STATEMENT
The lead author Junmei Zhang affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
We wish to acknowledge the participants and investigators of the COVID19 and the GLIDE consortium. The works was supported by Guizhou Medical University Affiliated Stomatological Hospital Doctoral Fund (Hospital‐Doctor Joint J‐2023‐4).
Song J, Wu Y, Yin X, Zhang J. Relationship between periodontitis and COVID‐19: a bidirectional two‐sample Mendelian randomization study. Health Sci Rep. 2023;6:e1413. 10.1002/hsr2.1413
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Data can be downloaded from the ‘GLIDE' database (https://data.bris.ac.uk/data/dataset/2j2rqgzedxlq02oqbb4vmycnc2) and COVID19‐hg GWAS meta‐analyses round 5 (https://www.covid19hg.org/results/).
REFERENCES
- 1. Iacobucci G. Covid‐19: new UK variant May be linked to increased death rate, early data indicate. BMJ. 2021;372:n230. [DOI] [PubMed] [Google Scholar]
- 2. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jin YH, Zhan QY, Peng ZY, et al. Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID‐19: an evidence‐based clinical practice guideline (updated version). Mil Med Res. 2020;7(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang D, Hu M, Ji Q. Financial markets under the global pandemic of COVID‐19. Finance Res Lett. 2020;36:101528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenberg N, Docherty M, Gnanapragasam S, Wessely S. Managing mental health challenges faced by healthcare workers during covid‐19 pandemic. BMJ. 2020;368:m1211. [DOI] [PubMed] [Google Scholar]
- 7. Rapp JL, Lieberman‐Cribbin W, Tuminello S, Taioli E. Male sex, severe obesity, older age, and chronic kidney disease are associated with COVID‐19 severity and mortality in New York city. Chest. 2021;159(1):112‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ebinger JE, Achamallah N, Ji H, et al. Pre‐existing traits associated with Covid‐19 illness severity. PLoS One. 2020;15(7):e0236240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao F, Zheng KI, Wang XB, et al. Obesity is a risk factor for greater COVID‐19 severity. Diabetes Care. 2020;43(7):e72‐e74. [DOI] [PubMed] [Google Scholar]
- 10. Clift AK, Coupland CAC, Keogh RH, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in wuhan. J Allergy Clin Immunol. 2020;146(1):110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. 2019;394(10194):249‐260. [DOI] [PubMed] [Google Scholar]
- 14. Ramseier CA, Anerud A, Dulac M, et al. Natural history of periodontitis: disease progression and tooth loss over 40 years. J Clin Periodontol. 2017;44(12):1182‐1191. [DOI] [PubMed] [Google Scholar]
- 15. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- 16. Billings M, Holtfreter B, Papapanou PN, Mitnik GL, Kocher T, Dye BA. Age‐dependent distribution of periodontitis in two countries: findings from NHANES 2009 to 2014 and SHIP‐TREND 2008 to 2012. J Periodontol. 2018;89:S140‐S158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tonetti MS, Jepsen S, Jin L, Otomo‐Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. 2017;44(5):456‐462. [DOI] [PubMed] [Google Scholar]
- 18. Tonetti MS, Sanz M. Implementation of the new classification of periodontal diseases: decision‐making algorithms for clinical practice and education. J Clin Periodontol. 2019;46(4):398‐405. [DOI] [PubMed] [Google Scholar]
- 19. Balan H, Popescu E, Angelescu G. Pathologic crossroads: cardio‐vascular diseases, periodontal diseases and calcium antagonists. J Med Life. 2011;4(1):2‐10. [PMC free article] [PubMed] [Google Scholar]
- 20. Romandini M, Baima G, Antonoglou G, Bueno J, Figuero E, Sanz M. Periodontitis, edentulism, and risk of mortality: a systematic review with meta‐analyses. J Dent Res. 2021;100(1):37‐49. [DOI] [PubMed] [Google Scholar]
- 21. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45(suppl 20):S149‐S161. [DOI] [PubMed] [Google Scholar]
- 22. Campisi G, Bizzoca ME, Lo Muzio L. COVID‐19 and periodontitis: reflecting on a possible association. Head Face Med. 2021;17(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aquino‐Martinez R, Hernández‐Vigueras S. Severe COVID‐19 lung infection in older people and periodontitis. J Clin Med. 2021;10(2):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jafer MA, Hazazi MA, Mashi MH, et al. COVID‐19 and periodontitis: a reality to live with. J Contemp Dent Pract. 2020;21(12):1398‐1403. [PubMed] [Google Scholar]
- 25. Marouf N, Cai W, Said KN, et al. Association between periodontitis and severity of COVID‐19 infection: a case‐control study. J Clin Periodontol. 2021;48(4):483‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta S, Mohindra R, Singla M, et al. The clinical association between periodontitis and COVID‐19. Clin Oral Investig. 2022;26(2):1361‐1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang C, Sun Y, Xu M, et al. Potential links between COVID‐19 and periodontitis: a bioinformatic analysis based on GEO datasets. BMC Oral Health. 2022;22(1):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richmond RC, Davey Smith G. Mendelian randomization: concepts and scope. Cold Spring Harbor Perspect Med. 2022;12(1):a040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shungin D, Haworth S, Divaris K, et al. Genome‐wide analysis of dental caries and periodontitis combining clinical and self‐reported data. Nat Commun. 2019;10(1):2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nolde M, Holtfreter B, Kocher T, et al. No bidirectional relationship between depression and periodontitis: a genetic correlation and Mendelian randomization study. Front Immunol. 2022;13:918404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Initiative C‐HG. The COVID‐19 host genetics initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS‐CoV‐2 virus pandemic. Eur J Human Genet. 2020;28(6):715‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Au Yeung SL, Li AM, He B, Kwok KO, Schooling CM. Association of smoking, lung function and COPD in COVID‐19 risk: a two‐step Mendelian randomization study. Addiction. 2022;117(7):2027‐2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen X, Hong X, Gao W, et al. Causal relationship between physical activity, leisure sedentary behaviors and COVID‐19 risk: a Mendelian randomization study. J Transl Med. 2022;20(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slob EAW, Burgess S. A comparison of robust Mendelian randomization methods using summary data. Genet Epidemiol. 2020;44(4):313‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Staley JR, Blackshaw J, Kamat MA, et al. PhenoScanner: a database of human genotype‐phenotype associations. Bioinformatics. 2016;32(20):3207‐3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Team RJC. R: A Language and Environment for Statistical Computing. 14. R Foundation for Statistical Computing; 2009:12‐21. [Google Scholar]
- 40. Jiang L, Zheng Z, Fang H, Yang J. A generalized linear mixed model association tool for biobank‐scale data. Nature Genet. 2021;53(11):1616‐1621. [DOI] [PubMed] [Google Scholar]
- 41. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tavares M, Lindefjeld Calabi KA, San Martin L. Systemic diseases and oral health. Dent Clin North Am. 2014;58(4):797‐814. [DOI] [PubMed] [Google Scholar]
- 43. Winning L, Linden GJ. Periodontitis and systemic disease: association or causality? Current Oral Health Reports. 2017;4(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21(7):426‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baniulyte G, Piela K, Culshaw S. How strong is the link between periodontitis and stroke? Evid Based Dent. 2021;22(1):10‐11. [DOI] [PubMed] [Google Scholar]
- 46. Sanz M, Marco Del Castillo A, Jepsen S, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. 2020;47(3):268‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu‐chao W, Yafei W, Lei Z. [Research progress on the relationship between Porphyromonas gingivalis and oral squamous cell carcinoma]. West China J Stomatol. 2015;33(6):651‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mammen MJ, Scannapieco FA, Sethi S. Oral‐lung microbiome interactions in lung diseases. Periodontol 2000. 2020;83(1):234‐241. [DOI] [PubMed] [Google Scholar]
- 49. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respirat Med. 2020;8(6):e46‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patel J, Woolley J. Necrotizing periodontal disease: oral manifestation of COVID‐19. Oral Dis. 2021;27(suppl 3):768‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vieira AR. Oral manifestations in coronavirus disease 2019 (COVID‐19). Oral Dis. 2021;27(suppl 3):770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Botros N, Iyer P, Ojcius DM. Is there an association between oral health and severity of COVID‐19 complications? Biomed J. 2020;43(4):325‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qi X, Northridge ME, Hu M, Wu B. Oral health conditions and COVID‐19: a systematic review and meta‐analysis of the current evidence. Agi Health Res. 2022;2(1):100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sirin DA, Ozcelik F. The relationship between COVID‐19 and the dental damage stage determined by radiological examination. Oral Radiol. 2021;37(4):600‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu H, Chen S, Liu M, Nie H, Lu H. Comorbid chronic diseases are strongly correlated with disease severity among COVID‐19 patients: a systematic review and Meta‐Analysis. Aging Dis. 2020;11(3):668‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 2000. 2020;83(1):90‐106. [DOI] [PubMed] [Google Scholar]
- 59. Kara C, Çelen K, Dede FÖ, Gökmenoğlu C, Kara NB. Is periodontal disease a risk factor for developing severe Covid‐19 infection? The potential role of Galectin‐3. Exp Biol Med. 2020;245(16):1425‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sampson V, Kamona N, Sampson A. Could there be a link between oral hygiene and the severity of SARS‐CoV‐2 infections? Br Dent J. 2020;228(12):971‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral mucosal lesions in a COVID‐19 patient: new signs or secondary manifestations? Int J Infect Dis. 2020;97:326‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2021;73(11):e4208‐e4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gherlone EF, Polizzi E, Tetè G, et al. Frequent and persistent salivary gland ectasia and oral disease after COVID‐19. J Dent Res. 2021;100(5):464‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Data can be downloaded from the ‘GLIDE' database (https://data.bris.ac.uk/data/dataset/2j2rqgzedxlq02oqbb4vmycnc2) and COVID19‐hg GWAS meta‐analyses round 5 (https://www.covid19hg.org/results/).


