Abstract
Background
One-half of all people who undergo lung cancer screening (LCS) currently use tobacco. However, few published studies have explored how to implement effective tobacco use treatment optimally during the LCS encounter.
Research Question
Was the Optimizing Lung Screening intervention (OaSiS) effective at reducing tobacco use among patients undergoing LCS in community-based radiology facilities?
Study Design and Methods
The OaSiS study (National Cancer Institute [NCI] Protocol No.: WF-20817CD) is an effectiveness-implementation hybrid type II cluster randomized trial of radiology facilities conducted in partnership with the Wake Forest National Cancer Institute Community Oncology Research Program research base. We randomly assigned 26 radiology facilities in 20 states to the intervention or usual care group. Staff at intervention facilities implemented a variety of strategies targeting the clinic and care team. Eligible patient participants were aged 55 to 77 years undergoing LCS and currently using tobacco. Of 1,094 who completed a baseline survey (523 intervention group, 471 control group) immediately before the LCS appointment, 956 completed the 6-month follow-up (86% retention rate). Fifty-four percent of those who reported not using tobacco at 6 months completed biochemical verification via mailed cotinine assay. Generalized estimating equation marginal models were used in an intention-to-treat analysis to predict 7-day tobacco use abstinence.
Results
The average self-reported abstinence among participants varied considerably across facilities (0%-27%). Despite a significant increase in average cessation rate over time (0% at baseline to approximately 13% at 6 months; P < .0001), tobacco use did not differ by trial group at 14 days (OR, 0.96; 95% CI, 0.46-1.99; P = .90), 3 months (OR, 1.17; 95% CI, 0.69-1.99; P = .56), or 6 months (OR, 0.97; 95% CI, 0.65-1.43; P = .87).
Interpretation
The OaSiS trial participants showed a significant reduction in tobacco use over time, but no difference by trial arm was found.
Trial Registry
ClinicalTrials.gov; No.: NCT03291587; URL: www.clinicaltrials.gov
Key Words: cessation, implementation, lung cancer screening, NCORP, smoking
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 292
Take-home Points.
Study Question: Are radiology facilities able to promote tobacco use cessation effectively among individuals who undergo lung cancer screening using provider- and systems-level implementation of tobacco use treatment?
Results: Thirteen percent of participants who smoke in the Optimizing Lung Screening Trial quit using tobacco at 6 months after implementation (P < .0001), but tobacco use did not differ by trial group at 14 days, 3 months, or 6 months.
Interpretation: Radiology facilities varied widely in their adoption and sustainability of evidence-based tobacco use treatment, and this heterogeneity may have influenced site-level variation in quit rates among patients undergoing lung cancer screening.
Lung cancer accounts for almost 25% of cancer deaths in the United States.1 In 2011, the US National Lung Screening Trial (NLST) demonstrated that annual low-dose CT imaging reduced lung cancer mortality by 20% compared with chest radiography.2 Lung cancer screening (LCS) has the greatest public health benefit when coupled with tobacco use cessation.3,4 Offering moderately effective tobacco use treatments (TUTs) at the time patients who use tobacco undergo LCS could lead to 13% of individuals quitting,5 with an additional 1.2% reduction in lung cancer incidence and more life-years saved vs screening alone.6 Meza et al4 further demonstrated that TUT with low-dose CT screening substantially reduces lung cancer deaths and increases life-years. For example, adding a cessation intervention of modest effectiveness (15%) to low-dose CT screening results in life-year gains that are comparable with increasing screening uptake from 30% to 100%.
In 2015, the Centers for Medicare and Medicaid Services (CMS) required that all patients who use tobacco and undergo LCS receive information on the importance of tobacco use cessation and TUT.7 CMS also advised that TUT be offered at a shared decision-making visit to discuss screening and at the time of screening. In 2022, CMS removed the requirement that radiology facilities make tobacco use cessation interventions available to those who use tobacco. Nonetheless, LCS serves as an excellent opportunity to expand reach of TUT, given that one-half of all those who undergo LCS currently use tobacco. Few published studies have explored how to optimize the implementation and effectiveness of cessation support during the LCS encounter.
The Optimizing Lung Screening Intervention (OaSiS) Trial is a cluster randomized trial to reduce tobacco use among participants undergoing LCS at community-based radiology facilities affiliated with the National Cancer Institute Community Oncology Research Program (NCORP).8 This trial is part of the Smoking Cessation at Lung Examination Collaboration.9,10 The Smoking Cessation at Lung Examination Collaboration is an effort combining eight federally funded research studies targeting how best to support tobacco use treatment for patients undergoing LCS.
Study Design and Methods
Study Design
The OaSiS study (Identifier: WF-20817CD) is an effectiveness-implementation hybrid (type II) cluster randomized trial of radiology facilities conducted in partnership with the Wake Forest NCORP Research Base.8 A hybrid type II design was chosen because it places equal value on examining effectiveness and implementation outcomes in a single trial. Given that TUT is an evidence-based strategy that has not yet been tested rigorously and repeatedly in the LCS environment, this design gave us the opportunity to asses whether it works in a new setting and under what conditions.11
We sent all NCORP community site principal investigators and cancer care delivery research leaders an e-mail outlining eligibility for the trial and soliciting their potential interest. Twenty-eight NCORP community sites with radiology facilities completed a brief survey that assessed LCS volume, the racial and ethnic composition of LCS participants, and availability of tobacco cessation support services within the radiology facility. Cancer care delivery research leaders obtained information from the director of the radiology facility and from health system leaders and data from the electronic health record to complete the survey. To be included in the trial, radiology facilities: (1) had to report an LCS volume of at least 50 screenings within the prior 6 months, (2) had to be willing to be randomized, and (3) had to be able to identify a champion(s) who would serve as the study liaison and organize each facility’s efforts to promote tobacco use cessation. Radiology facilities were not excluded from eligibility based on existing cessation support services. A health system with more than one radiology facility chose one location to participate in this trial. This study was approved by the National Cancer Institute Cancer Prevention and Control CIRB on December 10, 2019.
Within facilities, we included patients who were referred for LCS and: (1) were aged 55 to 77 years per Medicare reimbursement guidelines for LCS at the time the trial was initiated, (2) self-reported as using tobacco every day or some days at baseline, and (3) had not received tobacco dependence treatment within the last 30 days unless using bupropion for depression. We excluded individuals with any of the following criteria: prior 30-day use of a tobacco dependence treatment, (2) use of e-cigarettes only (dual e-cigarette and cigarette users were eligible), (3) presence of a cognitive or physical impairment that would prevent the person from completing surveys, and (4) did not speak English.
Sample
Of the 28 NCORP community sites that initially expressed interest and were eligible for the OaSiS trial, 26 radiology facilities per protocol sample size requirements were selected randomly from 20 states. They predominantly were privately owned (88.5%), were urban (73.1%), and had been in operation for an average of 4 years, and one-half of the sites had a lung screening coordinator or navigator who performed shared decision-making for LCS.12 No differences were found across intervention and control sites regarding these characteristics (Table 1). Radiology facilities were matched in pairs based on lung screening volume and racial and ethnic diversity, and then were assigned randomly to the intervention or usual care group. Facilities were expected to recruit up to 50 eligible participants for the trial. Table 1 provides site-level data on facility ownership (private, public, or university), payor mix of patients seeking LCS (eg, Medicare, Medicaid), duration of the LCS program, number of new LCS screenings in the prior 6 months, and American College of Radiology designation for LCS. Table 2 provides self-reported cessation services offered by the radiology facility (eg, referral to quit line, pharmacotherapy, counseling) at baseline. Champions served as a study liaison and organizer of efforts to promote tobacco use cessation services at the radiology facility. Sites could choose more than one representative to fill these roles. They included LCS program coordinators, lung nodule nurse practitioners, CT scan imaging leaders and technicians, physicians (radiologic medical director, pulmonary), tobacco use cessation counselors, research nurses, and NCORP personnel.
Table 1.
Baseline Characteristics of Radiology Facilities Participating in the OaSiS Trial
| Variable | Overall (N = 26) | Control (n = 13) | Intervention (n = 13) | P Value |
|---|---|---|---|---|
| Health system ownership | .99 | |||
| Private | 23 (88.5) | 11 (84.6) | 12 (92.3) | |
| Public | 2 (7.7) | 1 (7.7) | 1 (7.7) | |
| University | 1 (3.9) | 1 (7.7) | 0 (0.0) | |
| LCS program duration, y | 4.0 (3.0–5.0) | 3.8 (3.0–4.5) | 4.0 (3.0–5.5) | .55 |
| No. of LCSs in the prior 6 mo | 230 (144–442) | 296 (147–485) | 230 (139–429) | .78 |
| Type of navigator for SDM | .15 | |||
| Registered nurse | 8 (30.8) | 3 (23.1) | 5 (38.5) | |
| Nurse practitioner | 3 (11.5) | 3 (23.1) | 0 (0.0) | |
| Medical doctor | 1 (3.9) | 1 (7.7) | 1 (7.7) | |
| Research technician | 1 (3.9) | 0 (0.0) | 0 (0.0) | |
| Payor mix of patient undergoing LCS | ||||
| Medicare | 54.0 (36.0–65.0) | 52.5 (35.0–64.0) | 56.0 (40.0–65.0) | .82 |
| Medicaid | 7.5 (4.5–14.5) | 10.0 (7.0–20.0) | 5.0 (0.9–8.0) | .06 |
| Private | 30.0 (20.0–39.0) | 26.0 (20.0–40.0) | 30.0 (20.0–39.0) | .82 |
| No insurance | 0.0 (0.0–2.0) | 0.0 (0.0–18.0) | 0.0 (0.0–0.0) | .20 |
| Other insurance | 0.0 (0.0–2.5) | 0.0 (0.0–1.0) | 0.0 (0.0–3.0) | .47 |
| American College of Radiology designation for LCS | 11 (42.3) | 7 (53.8) | 4 (30.8) | .43 |
| Current tobacco use, % | 59.5 (50.0–70.0) | 63.0 (50.0–72.0) | 54.0 (50.0–60.0) | .40 |
Data are presented as No. (%) or median (interquartile range), unless otherwise indicated. LCS = lung cancer screening; OaSiS = Optimizing Lung Screening Trial; SDM = shared decision-making.
Table 2.
Self-reported Tobacco Use Treatment Services Offered by Radiology Facilities in the OaSiS Trial at Baseline
| Variable | Overall (N = 26) | Control (n = 13) | Intervention (n = 13) | P Value |
|---|---|---|---|---|
| Individual provider discussion about tobacco use cessation tailored to the LCS context | 16 (57.7) | 9 (69.2) | 7 (53.8) | .34 |
| Individual provider discussion about tobacco use cessation not tailored to the LCS context | 15 (55.6) | 7 (53.8) | 8 (61.5) | .50 |
| Group tobacco use cessation classes or support at the clinic | 7 (26.9) | 3 (23.1) | 4 (30.8) | .50 |
| Group tobacco use cessation classes or support outside of the clinic | 10 (38.5) | 6 (46.2) | 4 (30.8) | .34 |
| Individual tobacco use cessation classes or support at the clinic | 14 (53.8) | 5 (38.5) | 9 (69.2) | .12 |
| Individual tobacco use cessation classes or support outside of the clinic | 11 (42.3) | 6 (46.2) | 5 (38.5) | .50 |
| Fax referral to state quit line | 9 (34.6) | 4 (30.8) | 5 (38.5) | .50 |
| E-referral to state quit line | 11 (42.3) | 5 (38.5) | 6 (46.2) | .50 |
| Pamphlet or brochure given on quit line | 20 (76.9) | 12 (92.3) | 8 (61.5) | .08 |
| Online tobacco use cessation classes or support | 5 (19.2) | 2 (15.4) | 3 (23.1) | .50 |
| Referral to text-to-quit or other text messaging services to help patients quit using tobacco | 6 (23.1) | 2 (15.4) | 4 (30.8) | .32 |
| Enrollment in text-to-quit or other text messaging services to help patients quit using tobacco | 3 (11.5) | 1 (7.7) | 2 (15.4) | .50 |
| Referral to online, web-based apps to help patients quit using tobacco | 5 (19.2) | 1 (7.7) | 4 (30.8) | .16 |
| Enrollment in online, web-based apps to help patients quit using tobacco | 3 (11.5) | 0 (0.0) | 3 (23.1) | .11 |
| Other | 4 (16.0) | 2 (15.4) | 2 (15.4) | .67 |
| Sum of services by site | 5.0 (2.8–7.3) | 5.0 (2.5–6.5) | 6.0 (3.0–8.0) | .56 |
Data are presented as No. of sites (%) or median (interquartile range), unless otherwise indicated. LCS = lung cancer screening; OaSiS = Optimizing Lung Screening Trial.
Procedures
Participants were screened for eligibility before the LCS appointment via telephone or in person on the same day but before the LCS examination. Eligible patients were invited to participate, administered a written informed consent form, and completed an in-person baseline survey immediately before the LCS scan. Participants were contacted by telephone within 14 days and approximately 3 and 6 months after the LCS examination for follow-up surveys.
OaSiS Implementation Strategies
We previously published a description of the intervention strategies.8 In brief, the strategies included: (1) virtual training for LCS staff and leadership on the 5As of tobacco cessation counseling (Ask, Advise, Assess, Assist, and Arrange), and pharmacotherapy,13 shared decision-making and lung screening, motivational interviewing, and the role of CT scan imaging technicians in promoting tobacco use cessation; (2) an in-person strategic planning session on the opportunities and barriers to implementing cessation support; (3) codevelopment of an action plan to implement cessation support into the LCS workflow; (4) performance coaching and audit and feedback to support implementation of cessation support during the LCS visit; and (5) provision of health promotion materials for patients and the clinic. Team members from the radiology facilities in the intervention arm participated in all of the intervention activities. We required that a tobacco cessation champion and members of the imaging team participate in strategies 1, 2, 3, and 4. However, other individuals who participated in these strategies varied by site based on personnel and resources at the radiology facility and health system. Other participants included, for example, lung navigators and centralized tobacco cessation, pharmacy, and marketing personnel. Radiology facilities in the usual care arm were offered all implementation activities after data collection was completed. Receipt of the strategies was voluntary for usual care clinics and not an expectation of trial participation.
Measurement
The primary outcome was self-reported past-7-day tobacco use at the 6-month follow-up survey. Participants who indicated not using tobacco for the past 7 days at the 6-month telephone follow-up were mailed a saliva collection kit (SalivaBio Oral Swab; Salimetrics, Inc.) to validate self-report biochemically. Cotinine levels were determined using the high-sensitivity Salivary Cotinine Quantitative Enzyme Immunoassay kit (item number 1-2002; Salimetrics, Inc.), a competitive immune assay kit with a determination range from 0.8 to 200 ng/mL. Saliva samples were analyzed in duplicate without dilution. Salivary cotinine levels of < 15 ng/mL were deemed consistent with no tobacco use for the prior 7 days. Secondary outcomes included self-reported tobacco use at 14 days and 3 months, quit attempts at 3 and 6 months, and the self-reported number of cessation services received at baseline and 14 days. Participants received $10 gift cards for completion of each survey and a $20 gift card for returning the saliva kit.
Sociodemographic characteristics included sex (male, female, or nonbinary), age (55-64 years, 65-74 years, or ≥ 75 years), race or ethnicity (White/non-Hispanic, Black/non-Hispanic, American Indian/non-Hispanic, or Hispanic/all races), residence (metropolitan or nonmetropolitan according to the Rural-Urban Commuting Area coding system), household income (< $15,000, $15,000-$34,999, $35,000-$64,999, or ≥ $65,000), household income or poverty status (≤ 200% vs > 200% of the 2019 US Department of Health and Human Services poverty guidelines), marital status (married or not married), employment status (working, retired, disabled, or not employed for pay), and health insurance coverage (private, Medicaid, Medicare, dual-enrollment in Medicaid and Medicare, military, or not available).14
Participants self-rated their health as poor or fair, good, or very good or excellent. They also indicated whether they had a family history (yes or no) or personal history (yes or no) of cancer. Participants rated their worry about lung cancer developing (not at all, a little, somewhat, or extremely) and their perceived impact of tobacco use treatment on lung cancer risk (none, a little, somewhat, or very much).15 Other tobacco-related measures included a single, validated item for nicotine dependence from the Fagerstrom test for nicotine dependence (smokes one cigarette within the first 30 min of waking: yes or no); cigarettes per day; pack-years smoked; another tobacco user in the household (yes or no); use of other tobacco products in the past 30 days; readiness to quit tobacco use (scale of 1-10, with 10 being the highest readiness); and self-efficacy to quit tobacco use (scale of 1-10, with 10 being the highest).16, 17, 18, 19
Statistical Considerations
A sample size of 836 participants (26 sites with 32 participants each, assumed intraclass correlation of 0.03) yielded 80% power to detect a 10% difference in self-reported 7-day tobacco use abstinence at 6 months between arms, assuming a 10% abstinence rate in the control arm. To allow for 25% loss to follow-up at 6 months, we planned to enroll 1,114 participants.
Sociodemographic, health- and tobacco-related characteristics, and receipt of cessation services were summarized using mean ± SD and No. (%) for continuous and categorical variables, respectively. To account for the nested cluster structure within participants and within facilities, we used generalized estimating equation marginal models in an intention-to-treat analysis as well as sensitivity analyses to predict 7-day tobacco use abstinence. A binomial distribution with logit link was specified with group, time, and the interaction of group by time included as factors while allowing intercept and time to vary by participants within facility with an exchangeable working correlation matrix designation.
Sensitivity analysis assessed robustness to missing responses. Restricted maximum likelihood estimation was used to impute missing responses under a missing-at-random assumption via a data augmentation algorithm combined with Markov chain Monte Carlo method with 500 imputations. The variables group, age, sex, marital status, employment, and time were included. After imputation, generalized estimating equation models were performed as described above in each imputed dataset. Results were pooled to obtain summary estimates.
Cotinine-verified tobacco use abstinence sensitivity analyses included three models that build on prior generalized estimating equation model assumptions: (1) 6-month self-reported tobacco use abstinence with intervention group as a single factor; (2) with facility clustering, changing participants with cotinine values of > 15 ng/mL without disclosed nicotine replacement therapy (NRT) to still using tobacco at 6 months with all other responses unchanged; and (3) reclassifying participants who self-reported tobacco use abstinence who did not return a sample (or one that could not be processed) as still using tobacco at 6 months.
Results
Twenty-six radiology facilities were randomized either to the intervention or to the usual care arm. One control radiology facility withdrew from the study after randomization, but before participant recruitment; the facility was not replaced. One intervention radiology facility withdrew after accruing one participant to the trial and did not obtain any follow-up data after baseline assessments. Therefore, 25 radiology facilities were included in the analysis with 24 having follow-up data (Table 3). Of 1,550 participants assessed for eligibility, 450 were excluded (Fig 1, Consolidated Standards of Reporting Trials diagram). Of the 1,100 participants enrolled, 1,094 eligible participants completed the baseline survey (523 in the intervention arm, 471 in the control arm); 956 completed 6-month surveys for an 86% retention rate.
Table 3.
Demographic Characteristics of Participants in the OaSiS Trial Undergoing LCS
| Variable | Overall (N = 1,094) | Control (n = 571) | Intervention (n = 523) | Adjusted P Valuea |
|---|---|---|---|---|
| Sex | .69 | |||
| Male | 530 (48.8) | 281 (49.6) | 249 (47.9) | |
| Female | 552 (50.8) | 283 (49.9) | 269 (51.7) | |
| Nonbinary | 5 (0.5) | 3 (0.5) | 2 (0.4) | |
| Age, y | .10 | |||
| 55-64 | 619 (56.6) | 333 58.3) | 286 (54.7) | |
| 65-74 | 433 (39.6) | 220 (38.5) | 213 (40.7) | |
| 75+ | 42 (3.8) | 18 (3.2) | 24 (4.6) | |
| Race or ethnicity | .66 | |||
| White, non-Hispanic | 875 (81.9) | 452 (80.4) | 423 (83.4) | |
| Black, non-Hispanic | 142 (13.3) | 74 (13.2) | 68 (13.4) | |
| Hispanic, all races | 28 (2.6) | 23 (4.1) | 5 (1.0) | |
| American Indian, non-Hispanic | 24 (2.3) | 13 (2.3) | 11 (2.2) | |
| Residence | .06 | |||
| Nonmetropolitan | 220 (20.2) | 165 (29.1) | 55 (10.5) | |
| Metropolitan | 870 (79.8) | 403 (71.0) | 467 (89.5) | |
| Education | .17 | |||
| < HS, HS, or HS Equivalency | 555 (51.1) | 307 (54.1) | 248 (47.7) | |
| Some after HS or college graduate | 532 (48.9) | 260 (45.9) | 272 (52.3) | |
| Household income/y | .18 | |||
| < $15,000 | 232 (21.2) | 137 (24.2) | 94 (18.1) | |
| $15,000-34,999 | 246 (22.5) | 133 (23.5) | 113 (21.7) | |
| $35,000-64,999 | 234 (21.3) | 116 (20.5) | 116 (22.3) | |
| $65,000+ | 227 (20.7) | 109 (19.2) | 117 (22.5) | |
| Unknown | 155 (14.2) | 72 (12.7) | 80 (15.4) | |
| Poverty level | .46 | |||
| Yes | 248 (22.7) | 145 (25.4) | 103 (19.7) | |
| No | 686 (62.6) | 349 (61.1) | 337 (64.4) | |
| Unknown | 160 (14.6) | 77 (13.5) | 83 (15.9) | |
| Marital status | .89 | |||
| Married | 549 (50.6) | 284 (50.1) | 265 (51.1) | |
| Not currently married | 537 (49.5) | 283 (49.9) | 254 (48.9) | |
| Employment status | .69 | |||
| Working full or part time | 360 (33.1) | 187 (32.9) | 173 (33.2) | |
| Retired | 452 (41.5) | 226 (39.8) | 226 (43.4) | |
| Disabled | 222 (20.4) | 124 (21.8) | 98 (18.8) | |
| Homemaker, caregiver, student, or unemployed | 55 (5.1) | 31 (5.5) | 24 (4.6) | |
| Health insurance coverage | .44 | |||
| At least private insurance | 513 (47.2) | 254 (45.0) | 259 (49.6) | |
| At least Medicaid | 216 (19.9) | 121 (21.5) | 95 (18.2) | |
| At least Medicare | 282 (26.0) | 151 (26.8) | 131 (25.1) | |
| At least military | 30 (2.8) | 11 (2.0) | 19 (3.6) | |
| None | 45 (4.1) | 27 (4.8) | 18 (3.5) |
Data are presented as No. (%), unless otherwise indicated. HS = high school; LCS = lung cancer screening; OaSiS = Optimizing Lung Screening Trial.
Adjusted for correlation within site.
Figure 1.
Optimizing Lung Screening Trial Consolidated Standards of Reporting Trials diagram.
Study participants were 51% female, aged 43% 65 years or older, and 82% White. Approximately 20% lived in a nonmetropolitan area, and 44% had a household income of < $35,000. More than one-third of participants described their health as fair or poor and 7.5% and 28% had a personal or family history of cancer, respectively (Table 3). Most participants (72%) reported smoking their first cigarette of the day within 30 min of waking and smoked an average of 17 ± 9.6 cigarettes/d. Most participants had tried to quit previously (84%), and the readiness to quit score was an average of 5.9 ± 2.1. Respondents on average were moderately confident they could quit (mean ± SD, 5.7 ± 2.6); 59% reported being somewhat or extremely worried about lung cancer developing, yet 21% said quitting tobacco use would have no or little impact on lung cancer risk (Table 4). After adjusting for correlation within the facility, no differences were found in respondent characteristics between the intervention and control groups (Tables 3, 4).
Table 4.
Health- and Tobacco-Related Characteristics of Participants in the OaSiS Trial Undergoing LCS
| Variable | Overall (N = 1,094) | Control (n = 571) | Intervention (n = 523) | Adjusted P Valuea |
|---|---|---|---|---|
| Kessler score | 4.4 ± 4.7 | 4.6 ± 4.6 | 4.2 ± 4.7 | 0.28 |
| Perceived health | 0.97 | |||
| Poor or fair | 391 (36) | 199 (35.2) | 192 (36.8) | |
| Good | 485 (44.6) | 265 (46.8) | 220 (42.2) | |
| Very good or excellent | 212 (19.5) | 102 (18.0) | 110 (21.1) | |
| Self-reported cancer history | 81 (7.5) | 40 (7.1) | 41 (7.9) | 0.68 |
| Self-reported family cancer history | 301 (28.0) | 165 (29.2) | 136 (26.6) | 0.40 |
| Lung cancer worry | 0.93 | |||
| Not at all or a little | 438 (40.7) | 225 (40.1) | 213 (41.4) | |
| Somewhat or extremely | 637 (59.3) | 336 (59.9) | 301 (58.6) | |
| Perceived impact of cessation on lung cancer risk | 0.48 | |||
| Not at all or a little | 227 (20.8) | 120 (21.2) | 106 (20.4) | |
| Somewhat or very much | 777 (71.0) | 409 (72.1) | 364 (70.0) | |
| Unknown | 90 (8.2) | 38 (6.7) | 50 (9.6) | |
| Smoke cigarette within 30 min of waking | 775 (71.8) | 403 (71.3) | 372 (72.4) | 0.88 |
| No. of cigarettes/d | 17.2 ± 9.6 | 17.1 ± 8.9 | 17.3 ± 10.4 | 0.89 |
| Pack-y of smoking | 46.1 ± 25.0 | 46.5 ± 24.3 | 45.7 ± 25.7 | 0.63 |
| Another using tobacco in the household | 324 (29.8) | 158 (27.9) | 166 (31.9) | 0.29 |
| Use of other tobacco products in last 30 d | 145 (13.3) | 78 (13.8) | 67 (12.8) | 0.77 |
| Last quit attempt | 0.47 | |||
| Never | 171 (15.6) | 92 16.2) | 78 (15.0) | |
| Within the past year | 274 (25.1) | 164 (28.9) | 109 (21.0) | |
| > 1 y | 579 (52.9) | 280 (49.4) | 296 (56.9) | |
| Unknown | 70 (6.4) | 31 (5.5) | 37 (7.1) | |
| Cessation readiness | 5.9 ± 2.1 | 6 ± 2.0 | 5.8 ± 2.2 | 0.53 |
| Quitting self-efficacy | 5.7 ± 2.6 | 5.8 ± 2.7 | 5.5 ± 2.6 | 0.40 |
Data are presented as No. (%) or mean ± SD, unless otherwise indicated. LCS = lung cancer screening; OaSiS = Optimizing Lung Screening Trial.
Adjusted for correlation within site.
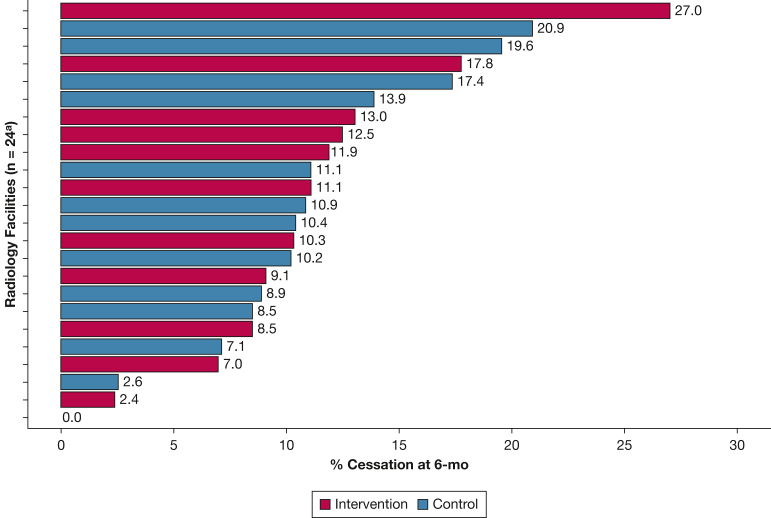
Self-reported 7-day quit rates at 6 months varied considerably across facilities, ranging from 0% to 27% (Fig 2). In adjusted models, an average of 13% of participants reporting no tobacco use at 6 months. Overall, tobacco use cessation rates increased over time (0% at baseline to 13% at 6 months; P < .0001), but tobacco use did not differ by trial group at 14 days (OR, 0.96; 95% CI, 0.46-1.99; P = .90), 3 months (OR, 1.17; 95% CI, 0.69-1.99; P = .56), or 6 months (OR, 0.97; 95% CI, 0.65-1.43; P = .87) (Table 5). Results from the sensitivity analysis showed that intention-to-treat model estimates were robust to missing data (e-Table 1).
Figure 2.
Bar graph showing Optimizing Lung Screening Trial cessation rate. aOne facility withdrew at baseline and another facility withdrew immediately after baseline with no follow-up data obtained.
Table 5.
Modeled Probability Estimates of 7-D Point Prevalence of Tobacco Use Abstinence After Date of the LCS
| Time | % Cessation (95% CI) |
Difference, OR (95% CI) | P Value | |
|---|---|---|---|---|
| Intervention | Control | |||
| 14 d | 2.8 (1.6-0.4.8) | 3.0 (2.0-4.9) | 0.95 (0.45-1.99) | .90 |
| 3 mo | 6.5 (4.4-9.5) | 5.5 (3.8-8.0) | 1.17 (0.69-1.99) | .56 |
| 6 mo | 13.0 (9.7-17.4) | 13.5 (10.3-17.6) | 0.97 (0.65-1.43) | .87 |
All participants currently used tobacco at time of study entry; as such, the preintervention starting rate of cessation is 0% and was not included in the model. LCS = lung cancer screening.
Of the participants who reported not using tobacco at 6 months, 60 of 112 participants (54%) returned a sample, 27 participants (53%) from the intervention group and 33 participants (54%) from the control group (P = .99). Two samples (3%) were misplaced and could not be processed, both from the intervention group. Of the 58 analyzable samples, 36 participants (62%) who self-reported not using tobacco at 6 months showed cotinine values of < 15 ng/mL in the intervention group and 23 participants (70%) who did so in control group showed such values (P = .19). Results from the sensitivity analysis showed that the self-report model estimates are robust to cotinine-verified abstinence (e-Table 2).
The number of cessation services received by participants at 14 days was similar between the intervention and control groups (mean, 7.68 vs 7.51; P = .85) (Table 6). In both groups, participants were more likely to report being asked and advised to quit and receive self-help materials than to receive the most effective TUT (pharmacotherapy or counseling). Overall, 36% of participants reported that they were prescribed or recommended an NRT such as nicotine gum, patch, or spray and 27% said they were prescribed or recommended bupropion or varenicline to help them quit. Additionally, 32% said they were referred to group or individual counseling to support cessation.
Table 6.
Participant Self-reported Receipt of Tobacco Use Treatment Services at the Time of LCS Measured Within 14 Days After the Scan
| Variable | Overall (N = 1,093) | Control (n = 571) | Intervention (n = 522) | P Value | Adjusted P Valuea |
|---|---|---|---|---|---|
| Total no. of services received per participant | 7.69 ± 6.19 | 7.86 ± 6.07 | 7.51 ± 6.33 | .35 | .85 |
| Asked if willing to try to quit | 661 (60.5) | 361 (63.2) | 300 (57.5) | .10 | .65 |
| Advised to quit | 614 (56.2) | 344 (60.2) | 270 (51.7) | .01 | .47 |
| Asked if ready to reduce or stop | 601 (55.0) | 313 (54.8) | 288 (55.2) | .74 | .92 |
| Talked to about benefits of quitting | 582 (53.2) | 332 (58.1) | 250 (47.9) | .01 | .47 |
| Asked if confident ready to reduce or stop | 552 (50.5) | 302 (52.9) | 250 (47.9) | .07 | .63 |
| Asked how important for you to change | 541 (49.5) | 301 (52.7) | 240 (46.0) | .09 | .67 |
| Talked to about dangers | 537 (49.1) | 306 (53.6) | 231 (44.3) | .01 | .47 |
| Provided booklets, brochures | 523 (47.8) | 249 (43.6) | 274 (52.5) | < .001 | .30 |
| Saw signs or brochures | 522 (47.8) | 266 (46.6) | 256 (49.0) | .02 | .30 |
| Arranged follow-up | 435 (39.8) | 231 (40.5) | 204 (39.1) | .86 | .96 |
| Offered to assist with quit plan | 404 (37.0) | 217 (38.0) | 187 (35.8) | .64 | .90 |
| Prescribed or recommend nicotine replacement therapyb | 392 (35.9) | 231 (40.5) | 161 (30.8) | .00 | .44 |
| Asked to describe positive and negative aspects of tobacco use | 331 (30.3) | 173 (30.3) | 158 (30.3) | .89 | .96 |
| Suggested quit line | 370 (33.9) | 164 (28.7) | 206 (39.5) | < .001 | .27 |
| Suggested class or counseling | 352 (32.2) | 187 (32.7) | 165 (31.6) | .84 | .96 |
| Asked to describe positive and negative aspects of reducing or stopping | 380 (34.8) | 211 (37.0) | 169 (32.4) | .11 | .59 |
| Prescribed or recommended medication to help you quitc | 296 (27.1) | 157 (27.5) | 139 (26.6) | .98 | 1.00 |
| Suggested enroll in text-to-quit | 170 (15.6) | 69 (12.1) | 101 (19.3) | < .001 | .26 |
| Worked with to set date to stop | 145 (13.3) | 75 (13.1) | 70 (13.4) | .76 | .87 |
Data are presented as No. (%) or mean ± SD, unless otherwise indicated. LCS = lung cancer screening.
Adjusted for correlation within site.
Prescribed or recommended any kind of nicotine replacement therapy to help you quit, such as nicotine gum, patch, or spray?
Prescribed or recommended any kind of medicine to help you quit (such as bupropion and varenicline)?
In univariate analyses, participants in the intervention group were more likely to report observing signs and receiving brochures at the 14-day follow-up survey. They also were more likely to have quit line or text-to-quit recommended. Participants in the control group were more likely to be advised to quit, talked to about the benefits of quitting, and offered NRT. No statistically significant differences were found in site-reported cessation offerings at baseline (Table 2).
Discussion
In the OaSiS trial, no significant differences were found in 6-month self-reported 7-day abstinence rates between participants in the intervention radiology facilities and those in the control facilities, with an overall quit rate of 13%. Nonetheless, considerable heterogeneity in cessation rates was observed across facilities, suggesting that high levels of tobacco use cessation can be achieved under optimal implementation conditions (eg, staffing, prioritization, and resources). In a systematic review of randomized controlled trials evaluating tobacco use treatment during LCS, four studies found no significant difference in quit rates among participants in treatment vs usual care arms, whereas one study found a difference among participants who received telephone cessation counseling in the intervention arm.20 Three of these trials had fewer than 100 participants and none were cluster randomized trials. The Smoking Cessation at Lung Examination Collaboration trials, with their dual emphasis on implementation and effectiveness, are intended to close the gap in knowledge about how best to reach patients undergoing LCS with TUT.
Several explanations may apply for the overall significant reduction in tobacco use among study participants, but the lack of differential effect by trial arm. First, intervention radiology facilities were encouraged to adopt strategies that were feasible and sustainable as part of their LCS workflow using existing facility and health system resources. Services were expected to be applied to all patients who use tobacco and undergo LCS, not just those being evaluated as part of the OaSiS trial. No financial or personnel support was provided by the research team. The research team guided the intervention radiology facilities in a strategic planning process to implement cessation support services into the workflow. The research team also provided remote implementation support via performance coaching and audit and feedback to intervention facilities (providing facility staff patient-level data on TUT services that patients reported receiving during the LCS visit). However, it ultimately was the responsibility of the radiology facility staff to choose and implement cessation strategies that were most feasible. Facilities often adopted cessation support services that were minimally disruptive to the LCS workflow and cost neutral, many of which are known to be minimally efficacious (eg, brochures and passive referrals to external cessation support services). Moreover, the most efficacious treatments, including TUT medication and counseling, would not be feasible in imaging facilities without additional or different personnel. Patients undergoing LCS will encounter a CT scan imaging technician or manager who guides them through the LCS process, and that is routinely the only clinical person with whom they interact. Some may have contact with a patient navigator, who can provide brief motivational counseling to support cessation, but rarely has prescribing privileges.
Second, facilities were not excluded from the OaSiS trial if they offered cessation services at baseline. Imaging personnel reported offering some form of cessation support to meet the CMS guidelines for reimbursement at the time the trial was launched. Excluding facilities based on existing offerings would be inconsistent with a trial to implement evidence-based cessation support in real-world settings. Overall, participants in the intervention arm reported receiving a higher number of cessation support services relative to control facilities 14 days after the LCS visit, but the difference was not significant.
A third explanation for the nondifferential treatment effects may be differences in being offered NRT as a part of routine LCS care, which was not measured at baseline. Key informant interviews after the trial was completed elucidated important information about cessation offerings. Three facilities in the control arm showed tobacco use cessation rates ranging from 17% to 21% at 6 months. In two of the three radiology facilities, NRT was offered routinely to patients undergoing LCS as part of routine LCS care. One of the facilities received tobacco Master Settlement Agreement funds and used these funds to purchase NRT, whereas another facility supported all patients undergoing any type of cancer screening with NRT and counseling.21 Although these control facilities did not cross over to the intervention arm of the trial in a traditional sense, they nonetheless were engaged in robust cessation support independent of the OaSiS trial. We did not systematically evaluate cessation support offerings in the radiology facilities of the control arm during the course of the trial, a study limitation. Nor did we differentiate between offering and providing NRT in intervention sites. Importantly, we did not exclude sites based on baseline cessation support offerings. Another important aspect of the OaSiS trial is that NCORP community sites and Cancer Care Delivery Research leaders within these sites had access to the full protocol regardless of trial arm. Implementation strategies and primary and secondary outcomes were known to all sites before study initiation. Participants were not randomized; radiology facilities were. Therefore, facilities were not blind to the study or their trial arm. Although multiple efforts were made to match and randomize clinics appropriately based on LCS volume and patient demographics, pre-existing tobacco cessation practices between the intervention and control clinics may have favored control clinics. We compared NCORP population-level data from the Landscape 2017 survey to determine if any differences in practice characteristics occurred between the sites that participated in the OaSiS trial and nonparticipating sites. The 2017 (NCORP) Landscape Assessment (funding support from National Cancer Institute Grant 2UG1CA189824) provides important information about available services and capacity to engage in cancer care delivery research among the seven research bases and 46 parent NCORPs (accounting for approximately 1,000 discrete oncology practice sites). No significant differences were found in terms of practice characteristics between the different cohorts (e-Table 3), suggesting that the sites were similar at baseline regarding organizational characteristics such as health system ownership and payor mix. However, the Landscape survey measured only three TUT services (eg, group tobacco use cessation and referral to the quit line) (e-Table 4). On these three items, the NCORP sites were no different than the NCORP sites suggesting that practice-level randomization worked.
The absence of masking, a common element of cluster randomized trials, increases the likelihood that control clinics mimic intervention clinics. Known participation in a trial might have created an incentive to engage in cessation support behaviors that otherwise may not have happened. In the future, it would be advantageous to consider matching on baseline cessation offerings, to engage in a high degree of preimplementation planning that allows for tailored and optimized implementation strategies to the radiology facilities context, or both.
Relative to the general population and participants in the NLST, OaSiS trial participants used tobacco more cigarettes per day and were nicotine dependent.22 As observed by Rojewski et al,23 NLST participants who used tobacco within the first 30 min of waking were significantly less likely to quit compared with those who used tobacco after 60 min (OR, 0.66; 95% CI, 0.56-0.78). Seventy-two percent of OaSiS participants fell within the category of people who use tobacco within 30 min of waking up. In the general population, 7.5% of adults who use tobacco successfully quit doing so over the course of 1 year.24 Among participants in the NLST, abstinence from tobacco use cessation at 6 months was 5.6% among Black participants and 7.2% among White participants undergoing LCS. Thus, a self-reported 13% overall quit rate among OaSiS participants is promising. We performed cotinine validation on one-half of the patient sample and confirmed the overall findings, but we were unable to control for NRT use for most of the respondents and had to rely on mailed saliva kits, which is an additional limitation of the study. Moreover, one-half of study participants did not return saliva kits; however, sensitivity analyses showed that results are robust to missing data. Helping people heavily dependent on using tobacco to quit is critical given that they have higher risk of lung cancer developing with increased mortality rates compared with those who do not use tobacco and lower success in quitting. The LCS setting seems to be a good venue for maximizing reach into this population.
Interpretation
Important lessons can be learned from the OaSiS trial about the trade-offs of engaging in a cluster randomized trial for tobacco use treatment and support during LCS. Encouraging radiology facilities to adopt locally feasible cessation services for patients undergoing LCS and using existing (rather than extramural, research-funded) resources may lead to the adoption of low-cost, low-yield cessation services for many clinics, as well as a high degree of heterogeneity of cessation support services across radiology facilities. The lack of prescribed, highly efficacious interventions often used in randomized control trials (eg, pharmacotherapy or counseling) decreases the potential impact in the short run, but may have maximized reach and sustainability in the long run. An important next step that optimizes the LCS encounter to promote tobacco use treatment should consider pragmatic trials that allow site-level adaptation of evidence-based cessation support that is tailored to the facility and health system resources. Moreover, a more explicit and deliberative effort is needed to remind clinical partners about trade-offs and health system leaders about the trade-offs in adopting easy-to-implement services (eg, health promotion brochures) vs robust referral processes to high-yield services (eg, pharmacologic support and counseling). Radiology facility staff can be coached on the most efficacious TUT and supported during their implementation.
Funding/Support
This study was supported by the National Cancer Institute [Grants R01CA207158 and 2UG1CA189824].
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: K. L. F. and C. C. take responsibility for (are the guarantors of) the content of the manuscript, including the data and analysis. K. L. F., C. C., K. E. W., E. L. S., and D. P. M. contributed to conception and design of the study. E. V. D., C. K., K. L. F., and C. C. contributed to acquisition, analysis, or interpretation of data. All authors drafted the work or revised it critically for important intellectual content. All authors gave final approval of the version to be published.
Role of sponsors: The National Cancer Institute reviewed this manuscript before submission for adherence to NCORP guidelines.
Other contributions: The authors thank the following NCORP sites and primary investigators for their participation in the OaSiS trial: Beaumont NCORP, Shaker Dakhil, MND; Columbus NCORP, Timothy D. Moore, MD; Gulf South Minority Underserved NCORP, Augusto C. Ochoa, MD; Hawaii Minority Underserved NCORP, Jeffrey L. Berenberg, MD; Nevada Cancer Research Foundation NCORP, John A. Ellerton, MD; Sanford NCORP of the North Central Plains, Preston D. Steen, MD; Southeast Clinical Oncology Research Consortium NCORP, Judith O. Hopkins, MD; VCU Massey Cancer Center Minority Underserved NCORP, Khalid Matin, MD; and Wisconsin NCORP, Adedayo A. Onitilo, MD, PhD.
∗ OaSiS Study Team Collaborators: Wake Forest NCORP Research Base: Glenn J. Lesser, MD (principal investigator), Kathryn E. Weaver, PhD, MPH (principal investigator), Karen Craver (administrator), Renee Glenn, Eden Gurganus, Bill Stanfield; OaSiS Study Team: Kristie Foley, PhD (principle investigator), Caroline Chiles, MD (principal investigator), Christina Bellinger, MD, Emily Dressler, PhD, William Jeffrey Petty, MD, David Miller, MD, John Spangler, MD, Erin Sutfin, PhD, Kathryn E. Weaver, PhD, MPH, Carol Kittel (staff), Rebecca Stone (staff). Aurora NCORP: Saint Luke’s Medical Center, Thomas J. Saphner, MD. Baptist Memorial Health Care/Mid South Minority Underserved NCORP: Baptist Memorial Hospital-Memphis and Baptist Cancer Center Tipton, Raymond U. Osarogiagbon, MD, Robert Optican, MD, MSHA. Cancer Research Consortium of West Michigan NCORP: Spectrum Health at Butterworth Campus, Kathleen J. Yost, MD. Cancer Research for the Ozarks NCORP: CoxHealth South Hospital, Judy S. Hancock, MHA, CCRP. Carle Cancer Center NCORP: Carle Foundation Hospital, Kendrith M. Rowland, MD, Karina Parke, FNP-BC. Catholic Health Initiatives NCORP: Mercy Medical Center, Richard L. Deming, MD, Jessica Ellensohn, DNP, ARNP, NP-C, Kacey Strovers, BS, RT(R)(CT)(M). Delaware/Christiana Care NCORP: Helen F Graham Cancer Center, Gregory A. Masters, MD, Nora C. Katurakes, MSN, RN, OCN. Geisinger Cancer Institute NCORP: Geisinger Health System, Rajiv Panikkar, MD. Georgia CaRes Minority Underserved NCORP: Augusta University; Georgia Cancer Center, Martha S. Tingen, PhD, RN, FAAN. Georgia NCORP: Lewis Cancer and Research Pavilion at Saint Joseph’s/Candler, Howard Zaren, MD, FACS, Stephanie Smith, RN, MSN, OCN. Heartland Cancer Research NCORP: Missouri Baptist Medical Center, Bryan A. Faller, MD. Metro Minnesota Community Oncology Research Consortium: Ridgeview Medical Center, Daniel M. Anderson, MD, Heather Kehn, RN, MPH, Cindy Steenstra. Michigan Cancer Research Consortium NCORP: Saint Joseph Mercy Hospital, Melanie Edwards, MD, Joey Parins, MA, CTTS, NCTTS, Anthony J. Galati, MS. Montefiore Minority Underserved NCORP: Montefiore Medical Center-Moses Campus, H. Dean Hosgood, PhD. NCORP of the Carolinas (Greenville Health System NCORP): Greenville Health System Cancer Institute, Ki Y. Chung, MD.
Additional information: The e-Tables are available online under “Supplemental Data.”
Footnotes
DISCLAIMER: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Data
References
- 1.American Cancer Society Key statistics for lung cancer: how common is lung cancer? American Cancer Society website. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
- 2.Aberle D.R., Adams A.M., Berg C.D., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanner N.T., Kanodra N.M., Gebregziabher M., et al. The association between smoking abstinence and mortality in the National Lung Screening Trial. Am J Respir Crit Care Med. 2016;193(5):534–541. doi: 10.1164/rccm.201507-1420OC. [DOI] [PubMed] [Google Scholar]
- 4.Meza R., Cao P., Jeon J., et al. Impact of joint lung cancer screening and cessation interventions under the new recommendations of the US Preventive Services Task Force. J Thorac Oncol. 2022;17(1):160–166. doi: 10.1016/j.jtho.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans W.K., Gauvreau C.L., Flanagan W.M., et al. Clinical impact and cost-effectiveness of integrating smoking cessation into lung cancer screening: a microsimulation model. CMAJ Open. 2020;8(3):e585–e592. doi: 10.9778/cmajo.20190134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadham C.J., Cao P., Jayasekera J., et al. Cost-effectiveness of smoking cessation interventions in the lung cancer screening setting: a simulation study. J Natl Cancer Inst. 2021;113(8):1065–1073. doi: 10.1093/jnci/djab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tammemägi M.C., Berg C.D., Riley T.L., Cunningham C.R., Taylor K.L. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106(6):dju084. doi: 10.1093/jnci/dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley K.L., Miller D.P., Jr., Weaver K., et al. The OaSiS trial: a hybrid type II, national cluster randomized trial to implement smoking cessation during CT screening for lung cancer. Contemp Clin Trials. 2020;91 doi: 10.1016/j.cct.2020.105963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph A.M., Rothman A.J., Almirall D., et al. Lung cancer screening and smoking cessation clinical trials. SCALE (Smoking Cessation within the Context of Lung Cancer Screening) Collaboration. Am J Respir Crit Care Med. 2018;197(2):172–182. doi: 10.1164/rccm.201705-0909CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cancer Institute Smoking cessation at lung examination: the SCALE Collaboration. National Cancer Institute website. https://cancercontrol.cancer.gov/brp/tcrb/scale-collaboration
- 11.Curran G.M., Bauer M., Mittman B., Pyne J.M., Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. Mar. 2012;50(3):217–226. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellinger C., Foley K.L., Dressler E.V., et al. Organizational characteristics and smoking cessation support in community-based lung cancer screening programs. J Am Coll Radiol. 2022;19(4):529–533. doi: 10.1016/j.jacr.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore M.C., Jaen C.R., Baker T.B., et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update—a US public health service report. Am J Prev Med. 2008;35(2):158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Human Resources and Services Administration Defining rural population. Human Resources and Services Administration website. https://www.hrsa.gov/rural-health/about-us/what-is-rural
- 15.Park E.R., Ostroff J.S., Rakowski W., et al. Risk perceptions among participants undergoing lung cancer screening: baseline results from the National Lung Screening Trial. Ann Behav Med. 2009;37(3):268–279. doi: 10.1007/s12160-009-9112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerstrom K.O. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 17.Apodaca T.R., Abrantes A.M., Strong D.R., Ramsey S.E., Brown R.A. Readiness to change smoking behavior in adolescents with psychiatric disorders. Addict Behav. 2007;32(6):1119–1130. doi: 10.1016/j.addbeh.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latimer-Cheung A.E., Fucito L.M., Carlin-Menter S., et al. How do perceptions about cessation outcomes moderate the effectiveness of a gain-framed smoking cessation telephone counseling intervention? J Health Commun. 2012;17(9):1081–1098. doi: 10.1080/10810730.2012.665420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biener L., Abrams D.B. The contemplation ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 20.Iaccarino J.M., Duran C., Slatore C.G., Wiener R.S., Kathuria H. Combining smoking cessation interventions with LDCT lung cancer screening: a systematic review. Prev Med. 2019;121:24–32. doi: 10.1016/j.ypmed.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Public Health Law Center at Mitchell Hamline School of Law . 2022. Commercial tobacco control: countering the tobacco epidemic.https://www.publichealthlawcenter.org/topics/commercial-tobacco-control/master-settlement-agreement Public Health Law Center at Mitchell Hamline School of Law website. [Google Scholar]
- 22.Centers for Disease Control and Prevention National Center for Health Statistics: data, questionnaires, and related documentation. Centers for Disease Control and Prevention website. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- 23.Rojewski A.M., Tanner N.T., Dai L., et al. Tobacco dependence predicts higher lung cancer and mortality rates and lower rates of smoking cessation in the National Lung Screening Trial. Chest. 2018;154(1):110–118. doi: 10.1016/j.chest.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creamer M.R., Wang T.W., Babb S., et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(45):1013. doi: 10.15585/mmwr.mm6845a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.