Abstract
Background
Two antifibrotic medications, pirfenidone and nintedanib, are approved for the treatment of idiopathic pulmonary fibrosis (IPF). Little is known about their real-world adoption.
Research Question
What are the real-world antifibrotic utilization rates and factors associated with uptake among a national cohort of veterans with IPF?
Study Design and Methods
This study identified veterans with IPF who received care either provided by the Veterans Affairs (VA) Healthcare System or non-VA care paid for by the VA. Patients who had filled at least one antifibrotic prescription through the VA pharmacy or Medicare Part D between October 15, 2014, and December 31, 2019, were identified. Hierarchical logistic regression models were used to examine factors associated with antifibrotic uptake, accounting for comorbidities, facility clustering, and follow-up time. Fine-Gray models were used to evaluate antifibrotic use by demographic factors, accounting for the competing risk of death.
Results
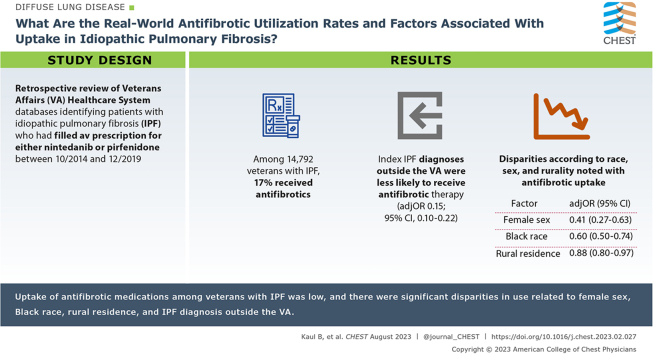
Among 14,792 veterans with IPF, 17% received antifibrotics. There were significant disparities in adoption, with lower uptake associated with female sex (adjusted OR, 0.41; 95% CI, 0.27-0.63; P < .001), Black race (adjusted OR, 0.60; 95% CI, 0.49-0.73; P < .001), and rural residence (adjusted OR, 0.88; 95% CI, 0.80-0.97; P = .012). Veterans who received their index diagnosis of IPF outside the VA were less likely to receive antifibrotic therapy (adjusted OR, 0.15; 95% CI, 0.10-0.22; P < .001).
Interpretation
This study is the first to evaluate the real-world adoption of antifibrotic medications among veterans with IPF. Overall uptake was low, and there were significant disparities in use. Interventions to address these issues deserve further investigation.
Key words: antifibrotics, disparities, idiopathic pulmonary fibrosis, interstitial lung disease, veterans
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 280
Take-home Points.
Study Question: What are the real-world antifibrotic utilizations rates and factors associated with uptake among a national cohort of veterans with IPF?
Results: Uptake of antifibrotic medications was low, with significant disparities according to race, sex, rurality, and VA vs non-VA care. Black, female, and rural veterans, as well as those who received their IPF care outside the VA, were less likely to receive antifibrotic therapy.
Interpretation: Overall uptake of antifibrotic medications was low, and there were significant disparities in use despite low medication co-pays among a national real-world cohort of veterans with IPF. We hypothesize that some of these disparities may be driven by differential access to medical specialists at tertiary care referral centers who are more likely to prescribe newer, evidence-based therapies. Future work that examines these access barriers and interventions to reduce disparities across the therapeutic spectrum of IPF care are needed.
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic interstitial lung disease associated with high morbidity and mortality.1, 2, 3 Historically, the median survival time has ranged from 2 to 5 years following diagnosis.4 In 2014, the US Food and Drug Administration (FDA) approved two novel antifibrotic medications, nintedanib and pirfenidone, for the treatment of IPF. Both medications have been shown to slow the rate of disease progression,5,6 reduce the frequency of exacerbations, with more recent meta-analysis data suggesting a mortality benefit,7 and are recommended as part of the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association treatment guidelines in a shared decision-making model that incorporates patient preference.2
Despite the demonstrated benefits in clinical outcomes, there is limited literature evaluating the real-world adoption of antifibrotic therapy. One study that examined antifibrotic utilization among Medicare beneficiaries found that since FDA approval, only 26% of patients with IPF had received antifibrotic medications, and there was heterogeneous uptake by age and sex, with older patients and female patients less likely to receive therapy.8 However, it is unclear whether low uptake was driven by high out-of-pocket medication costs or other factors such as lack of access to specialized care.
The Veterans Health Administration is the largest integrated health care system in the United States and consists of a network of 171 hospitals and more than 1,000 community-based outpatient clinics, which provide comprehensive care to approximately 9 million active or former-duty military personnel. It is unique in that medications are provided through a national drug formulary, and prescriptions for pirfenidone and nintedanib are covered benefits. Thus, out-of-pocket medication costs are low compared to most private insurance plans. The prevalence of IPF is also high among veterans relative to the general population, with recent data suggesting that military exposures may be associated with a greater risk of IPF.9, 10, 11
In this study, we used the strength of the Veterans Affairs (VA) electronic health record (EHR) system to examine the real-world adoption and disparities in uptake of antifibrotic medications among veterans with IPF.
Study Design and Methods
The University of California San Francisco Institutional Review Board (IRB 20-30063) and the San Francisco VA Research and Development Committee approved this study.
Data Source and Patient Identification
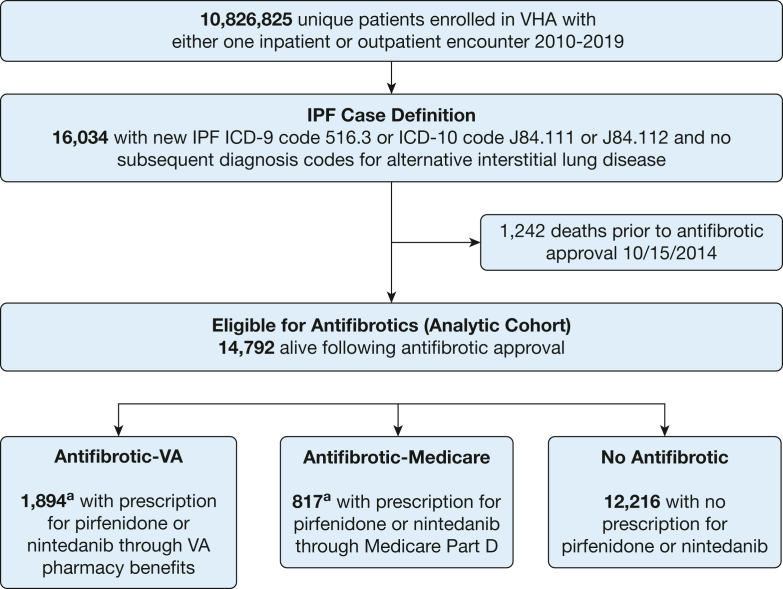
The VA EHR was used to identify patients enrolled in the VA Healthcare System who had at least one International Classification of Disease diagnosis code for IPF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 516.3 and 516.31 or International Classification of Diseases, Tenth Revision, Clinical Modification codes J84.111 and J84.112) recorded between January 1, 2010, and December 31, 2019. Prior IPF claims-based studies have discussed the utility of including the broader and more sensitive ICD-9-CM 515 code in addition to the narrower and more specific ICD-9-CM 516.3 code. The 515 codes are often used in the initial workup of IPF, and some patients with IPF may receive only these codes.12 In this medication uptake study, we chose to restrict our cohort to a more narrow case definition of disease as has been done with other medication uptake analyses in the literature8; this study thus included only patients with ICD codes 516.3 and 516.31, and their International Classification of Diseases, Tenth Revision, Clinical Modification equivalents J84.111, and J84.112, to optimize specificity. As in other large EHR-based studies, patients were considered to have IPF if they did not have any other diagnosis code for an alternative interstitial lung disease after the first code for IPF.9,12, 13, 14 We restricted the analytic cohort to veterans who were alive as of October 15, 2014, and would therefore have been eligible for antifibrotic therapy after FDA approval (Fig 1).
Figure 1.
Cohort identification.
VA = Veterans Affairs; VHA = Veterans Health Administration; ICD-9 = International Classification of Diseases, Ninth Revision; ICD-10 = International Classification of Diseases, Tenth Revision; IPF = idiopathic pulmonary fibrosis. aA total of 135 patients had received medications through both the VA and Medicare.
VA pharmacy data were used to identify patients who had filled a prescription for either nintedanib or pirfenidone between October 15, 2014, and December 31, 2019. We confirmed that all patients who had been prescribed an antifibrotic had an IPF diagnosis code. For each individual on antifibrotic therapy, the index initiation date was defined as the first fill date for either pirfenidone or nintedanib. Because veterans may also have co-insurance through Medicare, a universal federal public health insurance program that provides coverage for adults aged > 65 years, we cross-referenced Medicare’s prescription drug benefit plan (Medicare Part D) to identify veterans in our cohort who had received antifibrotic medications through Medicare.
Covariates including age, sex, race, ethnicity, rural vs urban residence at time of diagnosis, comorbidities, and VA medical facility where patients received care were extracted from VA EHR. Comorbid conditions were defined as one inpatient or two outpatient ICD codes. The VA defines rurality using the U.S. Department of Agriculture’s Rural-Urban Commuting Area system.15 The codes from this system are used to classify U.S. census tracts according to measures of population density, urbanization, and daily commuting. All veterans were categorized into rural vs urban residence based on home address at time of IPF diagnosis. Prior studies of pharmacoequity in the VA Healthcare System have noted that rural veterans have lower education and income than urban Veterans.16,17 Rurality is often also a marker of access to health care, with rural patients facing longer drive times to subspecialty care. We thus chose rurality as the primary marker of social disadvantage in our models.
Of note, veterans enrolled in the VA Healthcare System have several options for care, including receiving subspecialty care in the community (non-VA care) paid for by the VA if an appointment cannot be provided at a VA facility within a specified wait-time interval or drive-distance radius. Thus, we extracted information regarding whether the source of index IPF diagnosis was VA or non-VA care as a binary covariate in the model.
Statistical Analysis
The primary outcome of interest was antifibrotic uptake among veterans with IPF who were enrolled in the VA Healthcare System. To examine baseline characteristics of veterans with IPF who had received antifibrotic medications through either VA pharmacy benefits or Medicare Part D vs those who had not, we used t tests for continuous variables and χ2 tests for categorical variables. Hierarchical logistic regression models with cluster (VA facility) robust SEs were used to examine factors associated with antifibrotic uptake. The cluster robust SEs account for VA facility-level variability such as difference in local formulary administration or prescribing patterns. There were 817 VA facilities represented in the data set, and the number of patients with IPF per facility ranged from 1 to 214. We incorporated covariates, including patient characteristics (age, sex, race/ethnicity, rural residence, and comorbidities), year of index IPF diagnosis (to account for diffusion effect of treatment over time), and whether the source of index IPF diagnosis was VA or non-VA care paid for by VA, into regression models; this approach was based on previous literature that have investigated factors associated with novel therapeutic uptake of other medications.16,18 We identified a priori comorbidities commonly considered relative, although not absolute, contraindications to antifibrotic therapy and adjusted for them in our final model. To account for variable follow-up time, all regression models included a follow-up time variable defined as the latter of antifibrotic approval date (October 15, 2014) or index diagnosis date to end of study period (December 31, 2019) or death, whichever came first. In addition, we conducted a sensitivity analysis in which we restricted date of index IPF diagnosis to 2014 to 2019 to evaluate whether rate of antifibrotic uptake and factors associated with uptake changed when narrowing the analysis to a subgroup diagnosed after antifibrotic approval.
In addition to regression analysis, Fine-Gray models were constructed to account for the competing risk of death and to investigate differences in time trends of antifibrotic uptake by race, sex, and rurality, which are disparities of interest in the VA Healthcare System. Time to event was calculated from antifibrotic approval date or index diagnosis date (whichever came later) until date of antifibrotic prescription, death, or end of study period (December 31, 2019) by assuming independent left truncation. For all analyses, a two-tailed P value < .05 was used to define statistical significance. Analyses were conducted by using Stata version 16.1 (StataCorp).
Results
Among approximately 10.6 million veterans enrolled in the VA Healthcare System between 2010 and 2019, a total of 16,034 were newly diagnosed with IPF (Fig 1). A total of 1,242 patients died before FDA antifibrotic approval in 2014. Of the remaining 14,792 patients with IPF who would have been eligible for therapy, 2,576 (17%) received antifibrotics, 1,894 patients received medications through VA pharmacy benefits, and 817 patients received medications through Medicare. There were 135 patients who had received medications through VA and Medicare sequentially.
Veterans prescribed antifibrotic therapy were more likely to be male, White, and have been diagnosed with IPF within the VA as opposed to non-VA care paid for by the VA (Table 1). Patients receiving antifibrotic therapy had a lower prevalence of comorbidities, including ischemic heart disease, heart failure, and chronic liver or kidney disease. There were significant disparities in adoption of antifibrotic therapy (Table 2). In hierarchical logistic regression models, lower antifibrotic uptake was associated with female sex (adjusted OR, 0.41; 95% CI, 0.27-0.63; P < .001), Black race (adjusted OR, 0.60; 95% CI, 0.49-0.73; P < .001), and rural residence (adjusted OR, 0.88; 95% CI, 0.80-0.97; P = .012). Similar results were noted in a sensitivity analysis restricting the cohort to patients diagnosed with IPF between 2014 and 2019. Veterans who received their index diagnosis of IPF outside the VA Healthcare System (non-VA care paid for by the VA) were less likely to receive antifibrotic therapy (OR, 0.15, 95% CI, 0.10-0.22; P < .001). Similar point estimates were noted in Fine-Gray models that accounted for the competing risk of death.
Table 1.
Baseline Characteristics of Veterans With IPF by Use of Antifibrotic Medications
| Characteristic | No Antifibrotic (VA + Medicare) (n = 12,216) |
Any Antifibrotic (VA + Medicare) (n = 2,576) |
P Value |
|---|---|---|---|
| Age at diagnosis, mean ± SD, y | 73.1 ± 10.5 | 73.0 ± 7.3 | .59 |
| Sex | < .001 | ||
| Male | 11,665 (95.5%) | 2,549 (99.0%) | |
| Female | 551 (4.5%) | 27 (1.0%) | |
| Race | < .001 | ||
| White | 9,840 (80.6%) | 2,195 (85.2%) | |
| Black or African American | 966 (7.9%) | 119 (4.6%) | |
| Asian/Native Hawaiian or Pacific Islander/American Indian |
244 (2.0%) | 46 (1.8%) | |
| Unknown | 1,166 (9.5%) | 216 (8.4%) | |
| Ethnicity | .65 | ||
| Hispanic or Latino | 543 (4.4%) | 111 (4.3%) | |
| Not Hispanic or Latino | 10,629 (87.0%) | 2,282 (88.6%) | |
| Unknown | 1,044 (8.5%) | 183 (7.1%) | |
| Rural residence | 4,546 (37.2%) | 929 (36.1%) | .09 |
| Unknown | 910 (7.4%) | 154 (6.0%) | |
| Index year of IPF diagnosis | < .001 | ||
| 2010 | 268 (2.2%) | 17 (0.7%) | |
| 2011 | 346 (2.8%) | 30 (1.2%) | |
| 2012 | 600 (4.9%) | 51 (2.0%) | |
| 2013 | 903 (7.4%) | 103 (4.0%) | |
| 2014 | 1,504 (12.3%) | 262 (10.2%) | |
| 2015 | 1,840 (15.1%) | 381 (14.8%) | |
| 2016 | 1,791 (14.7%) | 458 (17.8%) | |
| 2017 | 1,719 (14.1%) | 486 (18.9%) | |
| 2018 | 1,648 (13.5%) | 466 (18.1%) | |
| 2019 | 1,597 (13.1%) | 322 (12.5%) | |
| Comorbidities | |||
| Ischemic heart disease | 3,787 (31.0%) | 672 (26.1%) | < .001 |
| Congestive heart failure | 1,711 (14.0%) | 151 (5.9%) | < .001 |
| Chronic liver disease | 464 (3.8%) | 36 (1.4%) | < .001 |
| Chronic kidney disease | 2,429 (19.9%) | 265 (10.3%) | < .001 |
| Atrial fibrillation | 1,649 (13.5%) | 191 (7.4%) | < .001 |
| DVT | 281 (2.3%) | 21 (0.8%) | < .001 |
| Pulmonary embolism | 224 (1.8%) | 20 (0.8%) | < .001 |
| IPF diagnosis within VA | 10,935 (89.5%) | 2,549 (99.0%) | < .001 |
IPF = idiopathic pulmonary fibrosis; VA = Veterans Affairs.
Table 2.
Odds of Antifibrotic Utilization Among Veterans With Idiopathic Pulmonary Fibrosis
| Variable | Primary Analysis |
Sensitivity Analysis |
||
|---|---|---|---|---|
| Multivariable ORa (95% CI) | P Value | Multivariable ORa (95% CI) | P Value | |
| Age, y | ||||
| ≤ 60 | Reference | Reference | ||
| > 60-70 | 1.97 (1.58-2.44) | < .001 | 2.28 (1.76-2.96) | < .001 |
| > 70–80 | 2.35 (1.90-2.90) | < .001 | 2.90 (2.25-3.75) | < .001 |
| > 80 | 1.18 (0.93-1.48) | .17 | 1.52 (1.15-1.99) | .003 |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.41 (0.27-0.63) | < .001 | 0.44 (0.27-0.70) | .001 |
| Race | ||||
| White | Reference | Reference | ||
| Black or African American | 0.60 (0.49-0.73) | < .001 | 0.57 (0.46-0.71) | < .001 |
| Asian/Pacific Islander/American Indian | 0.83 (0.56-1.22) | .34 | 0.83 (0.55-1.25) | .38 |
| Ethnicity | ||||
| Hispanic or Latino | Reference | Reference | ||
| Not Hispanic or Latino | 1.06 (0.84-1.35) | .62 | 1.08 (0.82-1.41) | .60 |
| Rurality | ||||
| Urban | Reference | Reference | ||
| Rural | 0.88 (0.80-0.97) | .01 | 0.90 (0.81-1.00) | .05 |
| Comorbidities | ||||
| Ischemic heart disease | 0.94 (0.85-1.05) | .281 | 0.95 (0.85-1.07) | .42 |
| Congestive heart failure | 0.64 (0.52-0.79) | < .001 | 0.60 (0.49-0.74) | < .001 |
| Chronic liver disease | 0.40 (0.29-0.56) | < .001 | 0.33 (0.22-0.49) | < .001 |
| Chronic kidney disease | 0.55 (0.48-0.64) | < .001 | 0.58 (0.50-0.67) | < .001 |
| DVT | 0.57 (0.36-0.89) | .014 | 0.44 (0.25-0.76) | .003 |
| Pulmonary embolism | 0.68 (0.46-1.01) | .057 | 0.75 (0.49-1.14) | .18 |
| Atrial fibrillation | 0.68 (0.57-0.80) | < .001 | 0.71 (0.59-0.86) | < .001 |
| VA vs non-VA care | ||||
| VA | Reference | Reference | ||
| Non-VA care paid for by VA | 0.15 (0.10-0.22) | < .001 | 0.17 (0.11-0.26) | < .001 |
Multivariable regression models were adjusted for patient demographic characteristics (age, sex, race/ethnicity), urban vs rural residence, Veterans Affairs (VA) or non-VA care, comorbidities, index year of diagnosis, follow-up time, and facility level clustering. The primary analysis included all patients diagnosed with idiopathic pulmonary fibrosis between 2010 and 2019 who were still alive at time of US Food and Drug Administration approval of antifibrotic medications on October 15, 2014. Sensitivity analysis restricted the cohort to patients with an index idiopathic pulmonary fibrosis diagnosis following the US Food and Drug Administration antifibrotic approval.
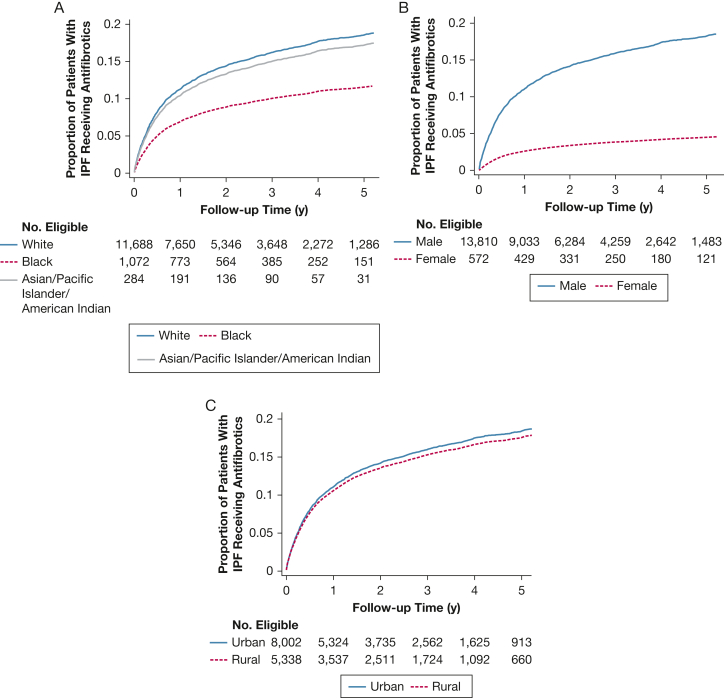
The proportion of patients with IPF receiving antifibrotic medications gradually increased over time for all sex, racial, and rural subgroups (Fig 2). However, there was uniformly lower uptake among female patients, non-White (Black, Asian, Pacific Islander, American Indian), and rural veterans; these disparities became more pronounced over time.
Figure 2.
Antifibrotic prescriptions by race (A), sex (B), and rural (C) subgroups.
Discussion
Clinical data sets from EHRs represent a robust means by which to evaluate real-world care patterns. Our study is the first to use clinical data sets to evaluate: (1) real-world adoption of antifibrotic medications among a national cohort of veterans with IPF and (2) to examine disparities in uptake. We found that among 14,792 veterans with a new diagnosis of IPF between 2010 and 2019, 17% received antifibrotic therapy. There were significant disparities in use, with female patients 59% less likely to receive antifibrotic therapy than male patients, Black patients 40% less likely to receive antifibrotic therapy than White patients, and rural patients 12% less likely to receive antifibrotic therapy than patients living in urban areas. Although uptake slowly increased across all subgroups, the disparities grew more pronounced over time. Patients who received their index IPF diagnosis through non-VA care paid for by the VA were less likely to receive antifibrotic medications. The results highlight the need for increased focus on the systemic uptake of novel therapies such as antifibrotic medications into routine clinical practice; in addition, as is the case in medicine more broadly, particular attention is needed to address inequities in IPF care.
Using data from the largest integrated health care system in the United States, our findings contrast with prior non-VA studies that have used clinical registries to evaluate antifibrotic uptake among patients with IPF. These registry-based studies have reported much higher antifibrotic utilization rates (approaching 70%).19, 20, 21, 22, 23, 24 Although registries play an important role in IPF research, they also have significant limitations when used to study health care utilization. IPF registry patients are commonly recruited from tertiary care referral centers, leading to selection bias of the underlying cohort, which is predominantly White, urban, of higher socioeconomic status, and highly motivated to pursue care for their disease. Tertiary care referral centers are also more likely to have the expertise and infrastructure to support rapid implementation of novel therapeutics into clinical practice for complex diseases such as IPF. Thus, the higher utilizations rates of antifibrotic medications in IPF registry studies likely represent the “upper limits” of uptake. We believe that our findings are more representative of the real-world population, and they are consistent with a real-world data study of Medicare Advantage beneficiaries.8
Racial and ethnic disparities in certain aspects of IPF care, such as lung transplantation, have been well described,25, 26, 27, 28 and higher mortality rates have recently been reported among patients with fibrotic lung disease living in disadvantaged neighborhoods, many of whom are people of color.28 However, there is very little literature examining pharmacoequity in IPF care. Pharmacoequity is defined as ensuring that all individuals, regardless of demographic characteristics, have access to high-quality medications required to manage their health needs.29 Inequities in access to prescription drugs for other chronic diseases have been well described, and the COVID-19 pandemic has further highlighted the substantial inequities by race in access to advanced therapies.30 Prior pharmacoequity literature has suggested that these disparities may be driven in part by insufficient insurance coverage leading to high co-pays among racial minorities. The fact that these disparities were still noted in the VA Healthcare System where there is a universal insurance plan, and where out-of-pocket costs range between $5 and $11 for a 30-day supply of medication (including antifibrotics), is therefore significant.31 We hypothesize that in the VA, these disparities are partly the result of by prescribing patterns at a clinician level driven by differential timely access to medical specialists at tertiary care referral centers who are more likely to prescribe newer, evidence-based therapies. Future work that examines these access barriers and how bridging them can reduce disparities across the therapeutic spectrum of IPF care is needed.
Veterans who received their IPF diagnosis outside the VA Healthcare System through non-VA care paid for by the VA were also significantly less likely to receive antifibrotic therapy. To meet the needs of veterans, the VA has a long-standing history of purchasing health-care services through community providers via a fee-for-service reimbursement model. Most recently, the VA Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSON) Act allows veterans who are unable to obtain a subspecialty appointment in < 28 days, or who have > 60 min drive time to the VA, to receive community care paid for by the VA.32 However, research has increasingly shown that outsourcing care may not be sufficient to improve access due to a combination of factors, including limited availability of specialists in the community and care fragmentation that occurs when patients and their health data leave the VA’s integrated system.33 Antifibrotic medications in particular require longitudinal laboratory monitoring of liver function; thus, care fragmentation, limited access to the VA EHR, or uncertainty about longitudinal follow-up with community providers may have contributed to the low uptake of antifibrotic medications among patients whose care was outsourced.
The 17% overall uptake of antifibrotic therapy is disappointing but perhaps not surprising. Prior implementation science studies have noted that, on average, it takes years for research evidence to reach clinical practice.34 There are several possible reasons for the low antifibrotic utilization rate, including patient and provider factors, medication costs, and lack of infrastructure needed to support uptake. Interviews from patient advocacy groups have reported that general practitioners and some pulmonologists have misconceptions about treatment guidelines, which can lead to delays in initiation of therapy. Other studies have noted that physicians are less likely to prescribe therapy to patients with IPF who they regard as having few symptoms, good quality of life, or “stable” disease, although this watch-and-wait approach has not been validated in the literature.22,23,35
Lastly, chronic disease management requires resources. The longitudinal and complex care needs of this patient population have led to the establishment of comprehensive interstitial lung disease clinics at large academic centers. Studies looking at the optimal components of these clinics have noted the importance of multidisciplinary teams, access to specialty services, efficient testing, support groups, and patient education programs.36 These centers are more likely to have the infrastructure to efficiently facilitate the uptake of antifibrotic medications. Helping patients connect with similar expert centers, many of which have a VA partner, in co-management models may better facilitate uptake and ultimately improve outcomes for veterans with IPF.
The current study has limitations. We used an ICD code-based algorithm to identify cases of IPF, which have not been individually case validated. Accurate identification of IPF cases using code-based algorithms depends on the characteristics of the underlying source population and the specificity of the algorithm used. We hypothesized that given the underlying demographic characteristics of the veteran population, the pretest probability of IPF was higher in our cohort than in cohorts with younger, more heterogeneous populations. In addition, to ensure a high level of specificity for this study, we used a fairly restrictive case definition of IPF. Second, we were not able to separate health care delivery variables from patient preferences in the determination of antifibrotic use. Recent IPF guidelines emphasize that individual patient preferences and values should be incorporated into a shared decision-making model regarding antifibrotic medication use.2 Although most patients who are offered antifibrotic therapy in tertiary care settings agree to take them, preference and values of the real-world patient population may be different. In the VA, the mean age at IPF diagnosis was 73 years. It is thus possible that competing priorities such as other chronic medical conditions that become more prevalent with age may have contributed to lower uptake. Lastly, we are unable to comment on the longitudinal use of antifibrotic medications; that is, whether veterans who started on these medications remained on them. Management of side effects and other treatment-related issues is critical to the successful longitudinal treatment of patients with IPF with antifibrotic medications. Otherwise, high rates of medication discontinuation may be observed. Future studies are needed to evaluate the persistence of antifibrotic use over time.
Interpretation
This study used data from the VA Healthcare System to evaluate uptake of antifibrotic medication in a real-world population and found that utilization was low despite minimal out-of-pocket medication costs. We further noted pronounced disparities by sex, race, rurality, and VA vs non-VA-based care. These findings have important implications for both the pulmonary fibrosis community at-large and the VA Healthcare System. Future work should focus on understanding why uptake is low, the barriers and facilitators to access, and designing interventions to improve their use as a critical component of comprehensive IPF care. Such intervention studies will benefit from the VA's Learning Healthcare System, which is implementing new care delivery models that seek to expand access to outpatient subspecialty services, integrate virtual care, and support ongoing process improvement studies to meet the demands of patients with complex care needs.
Funding/Support
The study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health [awards K12HL138046 and KL2TR001870], the VA Health Services Research and Development Quality Enhancement Research Initiative [I50-HX002756], and by grants from the Pulmonary Fibrosis Foundation and the Nina Ireland Foundation for Lung Health.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: B. K., J. S. L., L. A. P., C. M., I. O. R., V. D. B., A. M. D., H. R. C., and M. A. W. contributed to study conception, design, and interpretation. M. A. W. and N. Z. contributed to data acquisition. B. K., C. M., and M. A. W. contributed to analysis. B. K. drafted the report, and all authors revised it critically. All authors approved the final version.
Disclaimer: The views expressed in this article do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Roleofsponsors: The funders had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
References
- 1.Raghu G., Collard H.R., Egan J.J., et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G., Remy-Jardin M., Myers J.L., et al. An official American Thoracic Society/European Respiratory Society/Japenese Respiratory Society/Latin American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 3.Lederer D.J., Martinez F.J. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 4.Ley B., Collard H.R., King T.E., Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 5.King T.E., Jr., Bradford W.Z., Castro-Bernardini S., et al. the Group AS A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L., du Bois R.M., Raghu G., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 7.Petnak T., Lertjitbanjong P., Thongprayoon C., Moua T. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Chest. 2021;160:1751–1763. doi: 10.1016/j.chest.2021.06.049. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey T.M., Payne S., Sangaralingham L., Yao X., Shah N.D., Limper A.H. Adoption of the anti-fibrotic medications pirfenidone and nintedanib for patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2021;18(7):1121–1128. doi: 10.1513/AnnalsATS.202007-901OC. [DOI] [PubMed] [Google Scholar]
- 9.Kaul B., Lee J.S., Zhang N., et al. Epidemiology of idiopathic pulmonary fibrosis among US veterans, 2010-2019. Ann Am Thorac Soc. 2022;19:196–203. doi: 10.1513/AnnalsATS.202103-295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tighe R.M., Chaudhary S. Uncovering the epidemiology of idiopathic pulmonary fibrosis in the Veterans Affairs Health System. Ann Am Thorac Soc. 2022;19:161–162. doi: 10.1513/AnnalsATS.202108-972ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaul B., Lee J.S., Glidden D., et al. Agent Orange exposure and risk of idiopathic pulmonary fibrosis among US veterans. Am J Respir Crit Care Med. 2022;206(6):750–757. doi: 10.1164/rccm.202112-2724OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghu G., Chen S.Y., Yeh W.S., et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 13.Raghu G., Chen S.Y., Hou Q., Yeh W.S., Collard H.R. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179–186. doi: 10.1183/13993003.01653-2015. [DOI] [PubMed] [Google Scholar]
- 14.Ley B., Urbania T., Husson G., et al. Code-based diagnostic algorithms for idiopathic pulmonary fibrosis. Case validation and improvement. Ann Am Thorac Soc. 2017;14:880–887. doi: 10.1513/AnnalsATS.201610-764OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Department of Agriculture Economic Research Service Rural-Urban Commuting Codes. October 24, 2019. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
- 16.Essien U.R., Kim N., Hausmann L.R.M., et al. Disparities in anticoagulant therapy initiation for incident atrial fibrillation by race/ethnicity among patients in the Veterans Health Administration System. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Veterans Affairs Rural Veteran Health Care Challenges. July 20, 2022. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- 18.Essien U.R., Kim N., Magnani J.W., et al. Association of race and ethnicity and anticoagulation in patients with atrial fibrillation dually enrolled in Veterans Health Administration and Medicare: effects of Medicare Part D on prescribing disparities. Circ Cardiovasc Qual Outcomes. 2022;15(2) doi: 10.1161/CIRCOUTCOMES.121.008389. [DOI] [PubMed] [Google Scholar]
- 19.Wuyts W.A., Dahlqvist C., Slabbynck H., et al. Baseline clinical characteristics, comorbidities and prescribed medication in a real-world population of patients with idiopathic pulmonary fibrosis: the PROOF registry. BMJ Open Respir Res. 2018;5 doi: 10.1136/bmjresp-2018-000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salisbury M.L., Conoscenti C.S., Culver D.A., et al. the IPF-PRO Registry Principal Investigators Antifibrotic drug use in patients with idiopathic pulmonary fibrosis. Data from the IPF-PRO Registry. Ann Am Thorac Soc. 2020;17:1413–1423. doi: 10.1513/AnnalsATS.201912-880OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pesonen I., Carlson L., Murgia N., et al. Delay and inequalities in the treatment of idiopathic pulmonary fibrosis: the case of two Nordic countries. Multidiscip Respir Med. 2018;13:14. doi: 10.1186/s40248-018-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maher T.M., Swigris J.J., Kreuter M., et al. Identifying barriers to idiopathic pulmonary fibrosis treatment: a survey of patient and physician views. Respiration. 2018;96:514–524. doi: 10.1159/000490667. [DOI] [PubMed] [Google Scholar]
- 23.Maher T.M., Molina-Molina M., Russell A.M., et al. Unmet needs in the treatment of idiopathic pulmonary fibrosis—insights from patient chart review in five European countries. BMC Pulm Med. 2017;17:124. doi: 10.1186/s12890-017-0468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtze C.H., Freiheit E.A., Limb S.L., et al. Patient and site characteristics associated with pirfenidone and nintedanib use in the United States; an analysis of idiopathic pulmonary fibrosis patients enrolled in the Pulmonary Fibrosis Foundation Patient Registry. Respir Res. 2020;21:48. doi: 10.1186/s12931-020-1315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaffney A.W., Woolhander S., Himmelstein D., McCormick D. Disparities in pulmonary fibrosis care in the United States: an analysis from the Nationwide Inpatient Sample. BMC Health Serv Res. 2018;18:618. doi: 10.1186/s12913-018-3407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lederer D.J., Arcasoy S.M., Barr R.G., et al. Racial and ethnic disparities in idiopathic pulmonary fibrosis: a UNOS/OPTN database analysis. Am J Transplant. 2006;6:2436–2442. doi: 10.1111/j.1600-6143.2006.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lederer D.J., Caplan-Shaw C.E., O’Shea M.K., et al. Racial and ethnic disparities in survival in lung transplant candidates with idiopathic pulmonary fibrosis. Am J Transplant. 2006;6:398–403. doi: 10.1111/j.1600-6143.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 28.Goobie G.C., Ryerson C.J., Johannson K.A., et al. Neighborhood-level disadvantage impacts on patients with fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2022;205:459–467. doi: 10.1164/rccm.202109-2065OC. [DOI] [PubMed] [Google Scholar]
- 29.Essien U.R., Dusetzina S.B., Gellad W.F. A policy prescription for reducing health disparities—achieving pharmacoequity. JAMA. 2021;326:1793–1794. doi: 10.1001/jama.2021.17764. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez F., Solomon N., de Lemos J.A., et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143:2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Department of Veterans Affairs National Center for Veteran Analysis and Statistics. November 5, 2019. https://www.va.gov/vetdata/
- 32.S.2372 - 115th Congress (2017-2018): VA MISSION Act of 2018. Library of Congress, 6 June 2018. https://www.congress.gov/bill/115th-congress/senate-bill/2372/text
- 33.Kaul B., Hynes D.M., Hickok A., et al. Does community outsourcing improve timeliness of care for veterans with obstructive sleep apnea? Med. Care. 2021;59:111–117. doi: 10.1097/MLR.0000000000001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westfall J.M., Mold J., Fagnan L. Practice-based research—"Blue Highways" on the NIH roadmap. JAMA. 2007;297:403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 35.Bonella F., Wijsenbeek M., Molina-Molina M., et al. European IPF Patient Charter: unmet needs and a call to action for healthcare policymakers. Eur Respir J. 2016;47:597–606. doi: 10.1183/13993003.01204-2015. [DOI] [PubMed] [Google Scholar]
- 36.Graney B.A., He C., Marll, et al. and the Collaborators PCD Essential components of an interstitial lung disease clinic: results from a delphi survey and patient focus group analysis. Chest. 2021;159:1517–1530. doi: 10.1016/j.chest.2020.09.256. [DOI] [PMC free article] [PubMed] [Google Scholar]