Abstract
Objectives
Studies have suggested contradictory results on the relationship between chronic obstructive pulmonary disease (COPD) and periodontal disease (PD). The aim of this study was to determine whether PD increased the risk of COPD and COPD-related clinical events.
Design
A systematic review and meta-analysis.
Data sources
PubMed, Ovid EMBASE and Ovid CENTRAL were searched from inception to 22 February 2023.
Eligibility criteria for studies
We included trials and observational studies evaluating association of PD with the risk of COPD or COPD-related events (exacerbation and mortality), with statistical adjustment for smoking.
Data extraction and synthesis
Two investigators independently extracted data from selected studies using a standardised Excel file. Quality of studies was evaluated using the Newcastle-Ottawa Scale. OR with 95% CI was pooled in a random-effect model with inverse variance method.
Results
22 observational studies with 51 704 participants were included. Pooled analysis of 18 studies suggested that PD was weakly associated with the risk of COPD (OR: 1.20, 95% CI 1.09 to 1.32). However, in stratified and subgroup analyses, with strict adjustment for smoking, PD no longer related to the risk of COPD (adjusting for smoking intensity: OR: 1.14, 95% CI 0.86 to 1.51; smokers only: OR: 1.46, 95% CI 0.92 to 2.31; never smokers only: OR: 0.93, 95% CI 0.72 to 1.21). Moreover, PD did not increase the risk of COPD-related exacerbation or mortality (OR: 1.18, 95% CI 0.71 to 1.97) in the pooled result of four studies.
Conclusions
This study demonstrates PD confers no risk for COPD and COPD-related events when strictly adjusted by smoking. Large-scale prospective cohort studies with control of potential confounding factors are warranted to validate the present findings.
Keywords: chronic airways disease, respiratory medicine (see thoracic medicine), oral medicine, emphysema
Strengths and limitations of this study
This systematic review and meta-analysis only included studies with statistical adjustment for smoking, to adequately control the confounding by smoking.
We defined ‘periodontal disease’ as a wide variety of periodontal abnormalities according to clinical and radiographic assessments, which is not limited to periodontitis.
The language was restricted to English when conducting study searching, thus some literature might have been missed.
Clinical heterogeneity and publication bias compromised the evidence strength of this study, although subgroup and stratified analyses were performed.
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death, resulting in enormous economic burden.1 Commonly, COPD coexists with a variety of disorders, called comorbidities, which play significant roles in the progression and prognosis of COPD.2 3 Understanding the COPD–comorbidities relationship has been a momentous prerequisite for optimising disease prevention and management strategies.2 3
Given ageing and widespread use of inhaled corticosteroids in COPD, periodontal disease (PD) has been a common comorbidity of COPD.4 It is a chronic inflammatory condition of tissues surrounding and supporting the teeth, including gingiva, bone and ligament,5 with the prevalence estimates over 10% around the world and especially prevalent in elderly individuals.6 To date, diagnosis and assessment of PD are mostly based on periodontal measurements, including clinical attachment level (CAL), probing pocket depth (PPD) and alveolar bone loss (ABL).5 They are primary clinical manifestations of PD, reflecting the extent of periodontal tissue destruction.5
Based on the nature of inflammation,5 7 mounting evidence has shed light on the association between PD and development of COPD.8 9 Currently, three points are proposed. First, they share the same risk factors, such as age, gender, smoking and socioeconomic status.2 10 Second, they have similar pathogenetic mechanisms. Both diseases are characterised by host susceptibility to environmental factors, immune overreaction, oxidative stress and production of pro-inflammatory cytokines.7 8 Most importantly, neutrophilic inflammation plays a key role in both diseases.8 11 Third, oral bacteria released from the dental plaque in PD could trigger progression and acute exacerbation (AE) of COPD.12 13
Meanwhile, epidemiological evidence has indicated that PD increases risk of COPD11 14 15 and COPD-related events.13 16 Scannapieco et al revealed a 4.5-fold increased risk of COPD in patients with PD, compared with those without.14 A dose–response relationship was further implied between PD severity and lung function.15 Among patients with both diseases, COPD-related AE and mortality also significantly linked with periodontal status.13 16 Periodontal therapy, such as scaling and root planing treatment, may ameliorate lung function and decrease frequency of AE in COPD with chronic periodontitis.17 18 However, there were some other studies revealing opposite results, resulting in a long-standing controversy.19–21 It is worth noting that parameters used to determine PD apparently varied across studies, and these studies also failed to adequately control for confounders, especially smoking, the most important confounder for the COPD–PD relationship. Therefore, to provide the latest and most convincing evidence, we systematically reviewed current available literature to investigate whether PD increases the risk of COPD. The secondary objective was to evaluate the association between PD and the risk of COPD-related events. Subgroup and stratified analyses were also conducted to adjust for the confounding by smoking.
Methods
This systematic review and meta-analysis was conducted and reported in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline.22
Search strategy and selection criteria
We searched PubMed, Ovid EMBASE and Ovid Cochrane Central Register of Controlled Trials for records evaluating association between COPD and PD, from inception to 22 February 2023. The full search strategy was described in online supplemental table 1. The language was restricted to English, for the purpose of rapid review.23 Studies meeting the following criteria were included: (1) adult participants (≥18 years); (2) original studies with randomised controlled trial, cohort, case–control or cross-sectional study designs; (3) presenting clear diagnostic or assessment criteria for COPD and PD and (4) evaluating association between PD and the risk of COPD, or risk of COPD-related events (AE and mortality), with statistical adjustment for smoking, and providing the adjusted OR, relative risk (RR) or HR for the risk of COPD, AE and mortality in relation to PD. Given the inconsistent diagnostic criteria of PD across studies, we predefined PD as a wide variety of periodontal abnormalities according to clinical and radiographic assessments.24
bmjopen-2022-067432supp001.pdf (545.9KB, pdf)
According to the inclusion criteria, two independent investigators (MY and XL) performed systematical search, screened titles and abstracts of all retrieved studies to exclude duplicate or irrelevant records. For articles requiring further assessment, full-text reviews were carried out and references of retrieved articles and relevant reviews were also manually checked to identify additional eligible studies. Disagreements were resolved by discussion between the two reviewers or with the help of the third investigator (RP).
Data extraction and quality assessment
Two investigators (MY and RP) independently extracted data from selected studies using a standardised Excel (Microsoft Corporation) file. The following information was extracted: author, year of publication, country, study design, number of subjects (COPD and non-COPD), demographic characteristics of participants, diagnostic criteria for PD and COPD, definition of COPD-related AE and mortality, adjusted OR, RR or HR for the risk of COPD, AE and mortality in relation to PD, as well as adjustment for confounders. The primary outcome was the risk of COPD. Secondary outcome was the risk of COPD-related adverse events, including AE and mortality. Quality of studies was independently evaluated using the Newcastle-Ottawa Scale25 by two investigators (MY and XL). A score of ≥6 was considered a low risk, while <6 a high risk of bias. Both case–control and cohort studies had a maximum score of 9. Cross-sectional study was regarded as case–control study when performing quality assessment. Discrepancies regarding data extraction and quality assessment were resolved through discussion and consensus.
Data analysis
The final pooled estimate was expressed as OR with 95% CI. Considering CAL, ABL and PPD have been regarded as the primary parameters for PD,24 26 where more than one adjusted estimate was shown in the paper, we preferentially used the estimate regarding these parameters (CAL > ABL > PPD), or the estimate being better adjusted for tobacco smoking (never smokers > adjusting for smoking intensity (duration and dose) > adjusting for smoking status), or the estimate regarding more severe PD, where available. For case–control and cross-sectional studies, we estimated the OR, whereas for cohort studies we estimated the RR or HR. The random-effect model with inverse variance method were applied due to potential heterogeneity resulting from methodological differences. Heterogeneity across studies was identified with the I2 statistic. I2 statistic >50% indicated significant heterogeneity.
To explore heterogeneity, subgroup analyses were conducted based on study design (case–control, cross-sectional and cohort studies), geographical location (Asia, North America and Europe), assessment of PD (CAL, ABL and PPD), definition of COPD (Global Initiative for Chronic Obstructive Lung Diseases (GOLD) and non-GOLD criteria) and adjustment for smoking intensity (dose and duration of smoking). To better control the confounding effect of smoking, stratified analyses were performed in smokers and never smokers, respectively.
To test the robustness of study findings, we performed sensitivity analysis on studies with relatively large sample size (≥500 participants), which tended to be more representative of the general population and with smaller bias in the overall estimates in meta-analyses.27 Additionally, influence of a single study on the overall pooled estimate was tested by omitting one study in each turn. Publication bias was visually assessed using a funnel plot and quantitatively evaluated by the Egger’s tests. P <0.05 was considered statistically significant. All statistical analyses were performed using Stata V.16 (StataCorp) and Review manager V.5.4 (Cochrane Collaboration).
Patient and public involvement
No patients or other individuals are involved in the design, conduct, reporting or dissemination of this research.
Results
Study selection and characteristics
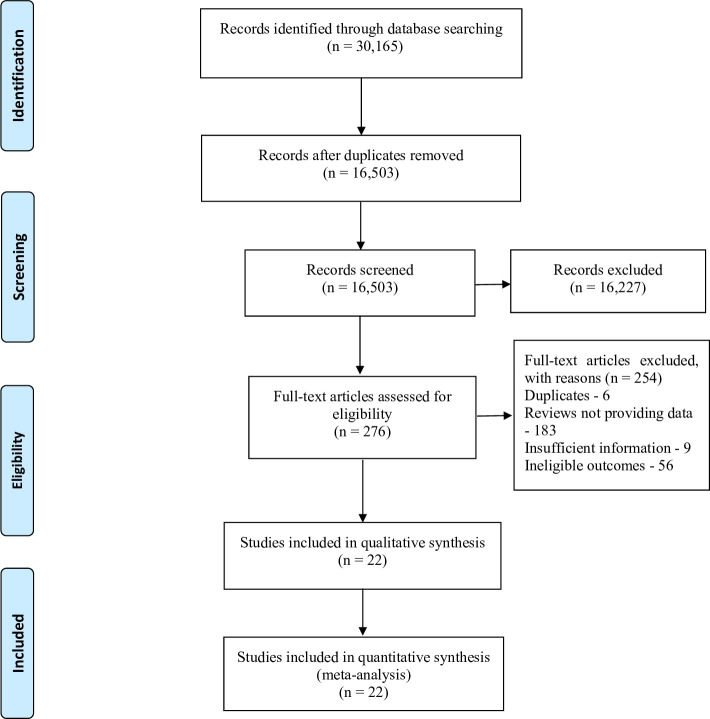
A total of 30 165 records were identified from the initial database search. 13662 records were removed for duplicates, and 16 227 records were excluded after titles and abstracts screening because of irrelevant content and animal studies. The remaining 276 full-text articles were identified for eligibility, of which 254 were excluded for reasons, including duplicates (6 studies), reviews (183 studies), insufficient information (9 studies) and ineligible designs and outcomes (56 studies). Finally, 22 studies14–16 19–21 28–43 were included in the review. The selection process is shown in figure 1.
Figure 1.
PRISMA flow diagram of study selection. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The characteristics of included 22 studies were given in table 1. The number of participants was 51 704 and there were 9973 (18.9%) patients with COPD. The mean age of patients with COPD was between 45.1 years and 83.1 years, while the mean age of the control subjects was between 42.2 years and 80.3 years. These studies were published between 1998 and 2021. The sample size ranged from 120 to 13 792. Nine studies were case–control studies15 19 28 29 32 33 36 40 42 and 10 studies were cross-sectional studies,14 20 30 31 34 35 38 39 41 43 only 3 studies with a cohort study design.16 21 37 Additionally, 11 studies were conducted in Asia,15 16 19 32 34 35 37 38 40–42 while 6 studies in the North America,14 20 21 28–30 4 studies in Europe31 33 36 39 and 1 study in Africa.43
Table 1.
Characteristics of included studies
| Year/study | Design | Location | No. COPD/control subjects | Age (COPD/ control subjects) |
Assessment of PD | Assessment of COPD |
| Hayes et al 28 1998 | Case–control | USA | 261/857 | 45.1±9.7/42.2±9.1 | ABL | FEV1 |
| Scannapieco et al 14 1998 | Cross-sectional | USA | 77/309 | NA | OHI | Self-reported |
| Garcia et al 29 2001 | Case–control | USA | 279/833 | NA | ABL, PPD | FEV1 |
| Scannapieco et al 30 2001 | Cross-sectional | USA | 810/12 982 | 51.2±17.9/43.9±17.7 | CAL, GB | Self-reported |
| Hyman et al 20 2004 | Cross-sectional | USA | 993/6 632 | 62.3±14.1/47.4±14.2 | CAL | GOLD |
| Leuckfeld et al 31 2008 | Cross-sectional | Norway | 130/50 | 54.9±4.9/47.0±9.8 | ABL | GOLD |
| Wang et al 19 2009 | Case–control | China | 306/328 | 63.9±9.8/63.3±9.0 | CAL, PLI | GOLD |
| Liu et al 42 2012 | Case–control | China | 183/209* | 64.3±10.1/63.6±9.7 | CAL, PPD, BI | GOLD |
| Si et al 15 2012 | Case–control | China | 581/438 | 63.9±9.4/62.8±9.5 | CAL, ABL, PPD, PLI, BI | GOLD |
| Zhou et al 32 2012 | Case–control | China | 193/181 | 63.6±10.3/62.1±9.1 | CAL, ABL, PPD, PLI, BI | GOLD |
| Barros et al 21 2013 | Cohort | USA | 399/1236† | 63.9±5.7/66.0±5.1 | CAL, PPD | GOLD |
| Ledić et al 33 2013 | Case–control | Croatia | 93/43 | 65.8±9.7/62.1±11.9 | CAL | GOLD |
| Chung et al 34 2016 | Cross-sectional | Korea | 697/5181 | 64.3±0.2/54.6±0.1 | PPD, GB | GOLD |
| AbdelHalim et al 43 2018 | Cross-sectional | Egypt | 134/116* | 56.8±10.4/55.3±9.1 | CAL, PPD, BI, PLI, OHI | GOLD |
| Harland et al 35 2018 | Cross-sectional | Japan | 149/1325 | 61.3±9.1/54.5±8.7 | PPD | GOLD |
| Lopez-de-Andrés et al 36 2018 | Case–control | Spain | 2699/2699 | 63±14/61±14 | Self-reported | Self-reported |
| Takeuchi et al 37 2019 | Cohort | Japan | 22/878 | NA | CAL, PPD | GOLD |
| Jung et al 38 2020 | Cross-sectional | Korea | 1134/6585 | 62.6±0.4/53.6±0.2 | PPD | FEV1/FVC |
| Qian et al 16 2020 | Cohort | China | 23‡/NA | 83.1±4.8/80.3±3.7 | ABL | NR |
| Winning et al 39 2020 | Cross-sectional | Sweden | 86/740 | NA | ABL | GOLD |
| Zhou et al 40 2020 | Case–control | China | 60/60 | 63.1±10.1/60.0±9.4 | CAL, PLI | GOLD |
| Kataoka et al 41 2021 | Cross-sectional | Japan | 464/249 | 54.1±9.4/NA | PPD | GOLD |
Continuous data are presented as mean±SD unless otherwise indicated.
*No. COPD subjects with frequent exacerbation (≥2 exacerbations in the last year)/infrequent exacerbation (<2 exacerbations in the last year).
†No. COPD subjects with events (hospitalisation for exacerbation or COPD-related death) in the 5-year follow-up visit/COPD subjects without events in the 5-year follow-up visit.
‡No. COPD-related mortality in a follow-up visit more than 5 years.
ABL, alveolar bone loss; BI, bleeding index; CAL, clinical attachment level; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GB, gingival bleeding; GOLD, Global Initiative for Chronic Obstructive Lung Disease; OHI, oral health index; PD, periodontal disease; PLI, plaque index; PPD, probing pocket depth.
All included articles performed multivariable analyses, in which the risk of COPD, or risk of COPD-related events (AE or mortality), was identified as the dependent variable and PD as the independent variable. Controlling for confounding by smoking included stratification (smokers and never smokers) or covariance adjustment in multivariable models (the degree of control: never smokers > adjusting for smoking intensity (duration and dose) > adjusting for smoking status).
The adjustment for confounders of included studies was detailedly presented in online supplemental table 2. Sixteen articles reported the adjusted ORs and four reported adjusted RRs, two studies reporting HRs. Definition of COPD comprised the GOLD criteria,2 FEV1 <65% of predicted volume, having a history of chronic bronchitis and/or emphysema, self-reported and others. Across almost all studies, periodontal examination was conducted by experienced or trained dentists. Periodontal parameters used for diagnosis of PD were CAL, ABL, PPD, gingival bleeding, bleeding index, plaque index (PLI) and oral health index (OHI). The detailed diagnostic criteria applied by included studies were presented in the online supplemental table 3.
Assessment of bias
Based on the Newcastle-Ottawa Scale, quality assessment for the 22 studies was shown in online supplemental table 4. Among them, 18 studies15 19–21 28–30 32–42 were rated as high quality with a total score of ≥6, whereas 4 studies14 16 31 43 with a score of <6, indicating a high risk of bias. The main reasons for lower scores were selection bias (representativeness of sample population), especially for control groups, and comparability of cases and control subjects.
Primary outcome
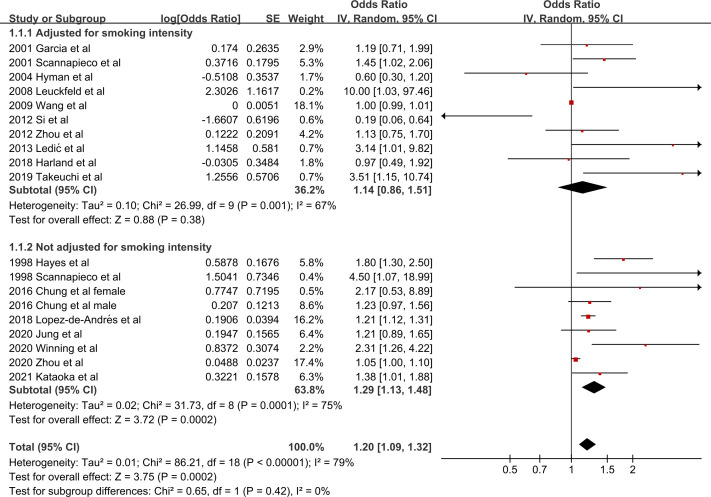
Eighteen studies14 15 19 20 28–41 provided data for the risk of COPD in relation to PD. Quantitative analysis demonstrated that after adjusting for smoking status, PD increased the risk of COPD, but only by a ratio of 1.20 (95% CI 1.09 to 1.32, p=0.0002, I2=79%) (figure 2). Further exclusion of any single study did not materially alter the overall pooled OR, with a range from 1.17 (95% CI 1.06 to 1.28) to 1.28 (95% CI 1.12 to 1.46). Sensitivity analysis limited to studies with larger sample size (≥500)15 19 20 28–30 34–39 41 revealed similar results (OR: 1.24, 95% CI 1.08 to 1.43, p=0.003, I2=82%) (online supplemental figure 1). However, significant publication bias was noted by visual inspections of the funnel plot (online supplemental figure 2) and the Egger’s test for small study effects (bias coefficient: 1.49, 95% CI 0.44 to 2.55, p=0.008).
Figure 2.
Forest plot of the risk of COPD by PD, subgroup analysis based on adjusted by smoking status and intensity versus by smoking status only. Values more than 1 indicate a higher risk in patients with PD. COPD, chronic obstructive pulmonary disease; PD, periodontal disease.
Subgroup analyses indicated that assessment parameters of PD (p=0.02), study design (p=0.05) and diagnosis of COPD (p=0.05) were the potential main causes of heterogeneity (table 2). Moreover, there were several findings in subgroup analyses. First, after further controlling for smoking intensity, PD did not increase the risk of COPD (OR: 1.14, 95% CI 0.86 to 1.51, p=0.38, 10 studies),15 19 20 29–33 35 37 similar to the subgroup applying a GOLD criterion (OR: 1.10, 95% CI 1.00 to 1.22, p=0.06, 12 studies).15 19 20 31–35 37 39–41 Second, among the parameters of CAL, ABL and PPD, only subgroup using the parameter of ABL showed a significant association between PD and the risk of COPD (OR: 1.98, 95% CI 1.32 to 2.97, p=0.001, 6 studies).15 28 29 31 32 39 Third, in the three geographical locations (Asia, North America and Europe), only the subgroup of Europe indicated that PD increased the risk of COPD (OR: 2.05, 95% CI 1.07 to 3.95, p=0.03, 4 studies).31 33 36 39
Table 2.
Subgroup analyses regarding the risk of COPD
| Subgroups | No. studies | No. participants/Cases | OR value (95% CI) | P value | I2, % |
| Adjusted for smoking intensity* | |||||
| Yes | 10 | 27 246/3556 | 1.14 (0.86 to 1.51) | 0.38 | 67 |
| No | 8 | 22 158/5478 | 1.29 (1.13 to 1.48) | 0.0002 | 75 |
| Assessment of PD | |||||
| CAL | 8 | 24 600/3058 | 1.04 (0.96 to 1.14) | 0.33 | 75 |
| ABL | 6 | 4629/1530 | 1.98 (1.32 to 2.97) | 0.001 | 56 |
| PPD | 8 | 19 189/3519 | 1.16 (0.89 to 1.51) | 0.27 | 63 |
| Geographical location | |||||
| Asia | 9 | 18 831/3606 | 1.07 (0.99 to 1.17) | 0.08 | 65 |
| North America | 5 | 24 033/2420 | 1.37 (0.93 to 2.01) | 0.11 | 63 |
| Europe | 4 | 6540/3008 | 2.05 (1.07 to 3.95) | 0.03 | 71 |
| Assessment of COPD | |||||
| GOLD | 12 | 19 879/3774 | 1.10 (1.00 to 1.22) | 0.06 | 71 |
| Non-GOLD | 6 | 29 525/5260 | 1.35 (1.14 to 1.61) | 0.0007 | 46 |
| Study design | |||||
| Case–control | 8 | 9 911/4472 | 1.12 (1.01 to 1.24) | 0.03 | 86 |
| Cross-sectional | 9 | 38 593/4540 | 1.34 (1.08 to 1.66) | 0.007 | 45 |
| Cohort | 1 | 878/22 | 3.51 (1.15 to 10.74) | 0.03 | – |
Bold: subgroups with positive results.
*Duration and dose of smoking.
ABL, alveolar bone loss; CAL, clinical attachment level; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; PD, periodontal disease; PPD, probing pocket depth.
Stratified analyses regarding smoking status revealed that PD did not increase the risk of COPD whether in smokers (OR: 1.46, 95% CI 0.92 to 2.31, p=0.11, 7 studies)15 19 20 29 31 32 35 or never smokers (OR: 0.93, 95% CI 0.72 to 1.21, p=0.58, 6 studies)15 19 20 29 32 35 (online supplemental figure 3).
Secondary outcome
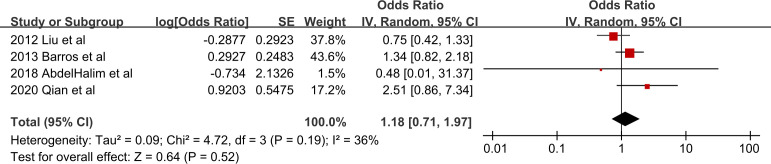
Only four studies evaluated the risk of COPD-related AE or mortality.16 21 42 43 Definition of AE was acute deterioration in clinical presentations according to the recommendation in GOLD guideline.21 42 43 Pooled analysis showed that after adjusting for smoking status, PD did not increase the risk of COPD-related AE or mortality (OR: 1.18, 95% CI 0.71 to 1.97, p=0.52, I2=36%) (figure 3).
Figure 3.
Forest plot of the risk of COPD-related events by PD. Values more than 1 indicate a higher risk in patients with PD. COPD, chronic obstructive pulmonary disease; PD, periodontal disease.
Discussion
This systematic review and meta-analysis identified 22 observational studies to investigate the association between COPD and PD. The results indicated that, after strictly adjusting for confounding by smoking, PD did not increase the risk of COPD, as well as the risk of COPD-related AE or mortality. Moreover, these findings were consistent across the subgroup and stratified analyses.
To the best of our knowledge, this is the first and largest meta-analysis investigating the association of PD with the risk of COPD and its clinical events, with adequately controlling the confounding effect of smoking. Besides, nearly all included articles were adjusted for age, except the study by Scannapieco et al.14 Prior publications have suggested that PD significantly increased the risk of COPD and COPD-related events. However, the majority of studies have non-negligible flaws, such as only performing univariate analyses, not controlling the confounding by smoking, and using parameters with relatively low specificity for determining PD.13 24 43 In the present study, to define PD as accurately as possible, we preferentially extracted data concerning the parameters of CAL, ABL and PPD rather than PLI, OHI or remaining teeth. CAL, ABL and PPD are clinical measurements reflecting the destruction of periodontal tissues and momentous parameters for diagnosis of PD.24 44 Meanwhile, compared with previous meta-analyses, we enrolled more studies, applied more rigorous screening criteria and most importantly, revealed opposite results. In the meta-analyses with incomplete adjustment for smoking, OR value for the risk of COPD ranged from 1.28 to 2.08.45–48 However, our findings were similar to studies conducted in never smokers,15 19 20 29 32 35 which showed that PD conferred no risk for COPD. Additionally, pooled analyses regarding parameters of CAL, ABL and PPD revealed that PD also did not increase the risk of COPD-related AE or mortality. These findings demonstrate that previously reported correlation between PD and COPD may be results of flawed study design, confounding by smoking and even other factors, such as age and living condition.
As a momentous inducer for inflammation-related pathological processes, tobacco is known to correlate with a variety of systemic disorders.49 It is also one of the foremost risk factors for both COPD and PD.5 10 From the epidemiological perspective, tobacco smoking is a confounder with spuriously inflated effect on the relationship between PD and systemic diseases.49 To investigate the true association between PD and COPD, it is of great importance to rigorously control the confounding effect of smoking, which means initiating research in never smokers. However, the majority of former studies failed to do that. After a wide search, only six studies focusing on never smokers were found, which unanimously indicated PD was not related with the risk of COPD. We also observed a decreased intensity of the association between both diseases with the increase of control for smoking. Therefore, it could be too early to make a certain conclusion on the COPD–PD relationship. Although interventional studies revealed that periodontal treatment reduced the risk of AE, a number of problems existed, including small sample size, limited study quality and unclear history of smoking or medication during the follow-up.17 18 For example, compared with control subjects, patients in treatment groups may reduce smoking intentionally, which could spuriously enhance the positive effect of periodontal treatment. Consequently, future researches need to take these problems into account.
It is worth noting that another possibility that smoking acts as an effect modifier in the COPD–PD relationship should not be ignored. Two observational studies performing stratified analyses concerning smoking status found that the strong correlation of PD with the risk of COPD was restricted to smokers.15 20 However, this was not revealed in the present study, thus more investigations in smokers and never smokers, respectively, are required.
Besides, current evidence has demonstrated several issues to be addressed in future study, comprising inconsistent diagnostic criteria of COPD and PD, the lack of prospective study design and differing adjustments for covariates. These contribute to substantial heterogeneity among studies.45 46 The present study indicated the heterogeneity was partly explained by study design, diagnostic criteria of COPD and PD. Significant association concerning PD and risk of COPD was only identified in subgroups lacking well designs, applying non-GOLD criteria or using ABL as the measure of PD. For one thing, this demonstrated that, as sources of bias, observational study design and non-standard diagnostic method for COPD could induce apparent deviations, confusing the true relationship between COPD and PD. For another, given undetermined diagnostic criteria for PD, discrepancies between ABL and other indexes cannot fully support the COPD–PD association. Notably, as a radiographic measure, although ABL has been widely considered to reflect cumulative effects of periodontal attachment loss over time by chronic inflammation,28 it does not only exist in PD. Non-PDs such as liver disorders, cancer and osteoporosis50 could also result in ABL. As mentioned previously,28 the observed correlation between ABL and risk of COPD may relate to those non-PDs.
Limitations
Several potential limitations should be taken into consideration when interpreting the present results. First, all included studies are observational, which are highly subject to selection bias and confounding by indication. Second, substantial heterogeneity was identified in the current study, though we conducted subgroup and stratified analyses to partly explain and reduce it. As stated above, several problems leading to heterogeneity need to be addressed in future researches. Third, the number of studies on risk of COPD-related events was limited, thus the result needs to be carefully understood. Limited number of studies in subgroup and stratified analyses suggested that more relevant studies with larger sample size are required. Fourth, although confounding effects of age and smoking were controlled by stratified analysis and statistical adjustment, other potential confounders such as gender, living condition and socioeconomic status10 could also reduce reliability of the results. Fifth, obvious publication bias was noted in relevant meta-analyses,45 46 including the present study. For the purpose of rapid review,23 we only included articles in English. There could exist non-English publications and unpublished evidence, although we searched English-language studies as much as possible. Finally, although smoking status and intensity were considered in subgroup analysis, information regarding tobacco content and chemical composition were not collected. This information is difficult to obtain, especially from self-reported smoking, leaving a residual smoking-related bias. Consequently, it is advisable to explore relationship between COPD and PD in never smokers.
Conclusion
In summary, this systematic review and meta-analysis suggests that PD is not associated with the risk of COPD and COPD-related events after strict adjustment for smoking, although the positive relationship between COPD and PD was previously reported. Large-scale prospective cohort studies with control of potential confounding factors are warranted to validate the present findings.
Supplementary Material
Footnotes
MY, RP and XL contributed equally.
Contributors: LC and LL designed the study, critically revised the paper and are the guarantors and responsible for the overall contents of this study. MY and XL screened and selected relevant studies. MY, RP and XL rated the study quality and extracted the data. MY, RP, XL and JP analysed the data and drafted the paper. All authors interpreted the data and acknowledged and agreed with the format and content of the paper before submission for publication.
Funding: This study was supported in part by grant 2016YFC0901100 from the National Key Research and Development Program of China.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. World Health Organization . The top 10 causes of death. 2020. Available: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death [Accessed 25 Feb 2023].
- 2. Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2023 report). 2023. Available: https://goldcopd.org/2023-gold-report-2/ [Accessed 25 Feb 2023].
- 3. Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: impact, measurement and mechanisms. Respirology 2015;20:1160–71. 10.1111/resp.12642 [DOI] [PubMed] [Google Scholar]
- 4. Tan L, Tang X, Pan C, et al. Relationship among clinical periodontal, microbiologic parameters and lung function in participants with chronic obstructive pulmonary disease. J Periodontol 2019;90:134–40. 10.1002/JPER.17-0705 [DOI] [PubMed] [Google Scholar]
- 5. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005;366:1809–20. 10.1016/S0140-6736(05)67728-8 [DOI] [PubMed] [Google Scholar]
- 6. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res 2014;93:1045–53. 10.1177/0022034514552491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sczepanik FSC, Grossi ML, Casati M, et al. Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontol 2000 2020;84:45–68. 10.1111/prd.12342 [DOI] [PubMed] [Google Scholar]
- 8. Usher AKH, Stockley RA. The link between chronic periodontitis and COPD: a common role for the neutrophil? BMC Med 2013;11:241. 10.1186/1741-7015-11-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong J, Li W, Wang Q, et al. Relationships between oral microecosystem and respiratory diseases. Front Mol Biosci 2021;8:718222. 10.3389/fmolb.2021.718222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000 2013;62:59–94. 10.1111/j.1600-0757.2012.00457.x [DOI] [PubMed] [Google Scholar]
- 11. Sapey E, Yonel Z, Edgar R, et al. The clinical and inflammatory relationships between periodontitis and chronic obstructive pulmonary disease. J Clin Periodontol 2020;47:1040–52. 10.1111/jcpe.13334 [DOI] [PubMed] [Google Scholar]
- 12. Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol 1999;70:793–802. 10.1902/jop.1999.70.7.793 [DOI] [PubMed] [Google Scholar]
- 13. Kelly N, Winning L, Irwin C, et al. Periodontal status and chronic obstructive pulmonary disease (COPD) exacerbations: a systematic review. BMC Oral Health 2021;21:425. 10.1186/s12903-021-01757-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scannapieco FA, Papandonatos GD, Dunford RG. Associations between oral conditions and respiratory disease in a national sample survey population. Ann Periodontol 1998;3:251–6. 10.1902/annals.1998.3.1.251 [DOI] [PubMed] [Google Scholar]
- 15. Si Y, Fan H, Song Y, et al. Association between periodontitis and chronic obstructive pulmonary disease in a Chinese population. J Periodontol 2012;83:1288–96. 10.1902/jop.2012.110472 [DOI] [PubMed] [Google Scholar]
- 16. Qian Y, Yuan W, Mei N, et al. Periodontitis increases the risk of respiratory disease mortality in older patients. Exp Gerontol 2020;133:110878. 10.1016/j.exger.2020.110878 [DOI] [PubMed] [Google Scholar]
- 17. Zhou X, Han J, Liu Z, et al. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol 2014;41:564–72. 10.1111/jcpe.12247 [DOI] [PubMed] [Google Scholar]
- 18. Kucukcoskun M, Baser U, Oztekin G, et al. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. J Periodontol 2013;84:863–70. 10.1902/jop.2012.120399 [DOI] [PubMed] [Google Scholar]
- 19. Wang Z, Zhou X, Zhang J, et al. Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. J Clin Periodontol 2009;36:750–5. 10.1111/j.1600-051X.2009.01448.x [DOI] [PubMed] [Google Scholar]
- 20. Hyman JJ, Reid BC. Cigarette smoking, periodontal disease: and chronic obstructive pulmonary disease. J Periodontol 2004;75:9–15. 10.1902/jop.2004.75.1.9 [DOI] [PubMed] [Google Scholar]
- 21. Barros SP, Suruki R, Loewy ZG, et al. A cohort study of the impact of tooth loss and periodontal disease on respiratory events among COPD subjects: modulatory role of systemic biomarkers of inflammation. PLoS One 2013;8:e68592. 10.1371/journal.pone.0068592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nussbaumer-Streit B, Klerings I, Dobrescu AI, et al. Excluding non-english publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol 2020;118:42–54. 10.1016/j.jclinepi.2019.10.011 [DOI] [PubMed] [Google Scholar]
- 24. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. 10.1038/nrdp.2017.38 [DOI] [PubMed] [Google Scholar]
- 25. Wells G, Shea B, O’Connell D, et al. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2021. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed 25 Feb 2023].
- 26. Farook FF, Alodwene H, Alharbi R, et al. Reliability assessment between clinical attachment loss and alveolar bone level in dental radiographs. Clin Exp Dent Res 2020;6:596–601. 10.1002/cre2.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin L. Bias caused by sampling error in meta-analysis with small sample sizes. PLoS One 2018;13:e0204056. 10.1371/journal.pone.0204056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hayes C, Sparrow D, Cohen M, et al. The association between alveolar bone loss and pulmonary function: the VA dental longitudinal study. Ann Periodontol 1998;3:257–61. 10.1902/annals.1998.3.1.257 [DOI] [PubMed] [Google Scholar]
- 29. Garcia RI, Nunn ME, Vokonas PS. Epidemiologic associations between periodontal disease and chronic obstructive pulmonary disease. Ann Periodontol 2001;6:71–7. 10.1902/annals.2001.6.1.71 [DOI] [PubMed] [Google Scholar]
- 30. Scannapieco FA, Ho AW. Potential associations between chronic respiratory disease and periodontal disease: analysis of national health and nutrition examination survey III. J Periodontol 2001;72:50–6. 10.1902/jop.2001.72.1.50 [DOI] [PubMed] [Google Scholar]
- 31. Leuckfeld I, Obregon-Whittle MV, Lund MB, et al. Severe chronic obstructive pulmonary disease: association with marginal bone loss in periodontitis. Respir Med 2008;102:488–94. 10.1016/j.rmed.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 32. Zhou X, Han J, Song Y, et al. Serum levels of 25-hydroxyvitamin D, oral health and chronic obstructive pulmonary disease. J Clin Periodontol 2012;39:350–6. 10.1111/j.1600-051X.2012.01852.x [DOI] [PubMed] [Google Scholar]
- 33. Ledić K, Marinković S, Puhar I, et al. Periodontal disease increases risk for chronic obstructive pulmonary disease. Coll Antropol 2013;37:937–42. [PubMed] [Google Scholar]
- 34. Chung JH, Hwang H-J, Kim S-H, et al. Associations between periodontitis and chronic obstructive pulmonary disease: the 2010 to 2012 Korean national health and nutrition examination survey. J Periodontol 2016;87:864–71. 10.1902/jop.2016.150682 [DOI] [PubMed] [Google Scholar]
- 35. Harland J, Furuta M, Takeuchi K, et al. Periodontitis modifies the association between smoking and chronic obstructive pulmonary disease in Japanese men. J Oral Sci 2018;60:226–31. 10.2334/josnusd.17-0225 [DOI] [PubMed] [Google Scholar]
- 36. Lopez-de-Andrés A, Vazquez-Vazquez L, Martinez-Huedo MA, et al. Is COPD associated with periodontal disease? A population-based study in Spain. Int J Chron Obstruct Pulmon Dis 2018;13:3435–45. 10.2147/COPD.S174898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takeuchi K, Matsumoto K, Furuta M, et al. Periodontitis is associated with chronic obstructive pulmonary disease. J Dent Res 2019;98:534–40. 10.1177/0022034519833630 [DOI] [PubMed] [Google Scholar]
- 38. Jung ES, Lee KH, Choi YY. Association between oral health status and chronic obstructive pulmonary disease in Korean adults. Int Dent J 2020;70:208–13. 10.1111/idj.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winning L, Polyzois I, Sanmartin Berglund J, et al. Periodontitis and airflow limitation in older Swedish individuals. J Clin Periodontol 2020;47:715–25. 10.1111/jcpe.13287 [DOI] [PubMed] [Google Scholar]
- 40. Zhou X, Wang J, Liu W, et al. Periodontal status and microbiologic pathogens in patients with chronic obstructive pulmonary disease and periodontitis: a case-control study. Int J Chron Obstruct Pulmon Dis 2020;15:2071–9. 10.2147/COPD.S266612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kataoka S, Kimura M, Yamaguchi T, et al. A cross-sectional study of relationships between periodontal disease and general health: the hitachi oral healthcare survey. BMC Oral Health 2021;21:644. 10.1186/s12903-021-01990-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Z, Zhang W, Zhang J, et al. Oral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbations. J Clin Periodontol 2012;39:45–52. 10.1111/j.1600-051X.2011.01808.x [DOI] [PubMed] [Google Scholar]
- 43. AbdelHalim HA, AboElNaga HH, Aggour RL. Chronic obstructive pulmonary disease exacerbations and periodontitis: a possible association. Egypt J Bronchol 2018;12:303–9. 10.4103/ejb.ejb_12_18 [DOI] [Google Scholar]
- 44. Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol 2018;45 Suppl 20:S162–70. 10.1111/jcpe.12946 [DOI] [PubMed] [Google Scholar]
- 45. Zeng X-T, Tu M-L, Liu D-Y, et al. Periodontal disease and risk of chronic obstructive pulmonary disease: a meta-analysis of observational studies. PLoS One 2012;7:e46508. 10.1371/journal.pone.0046508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gomes-Filho IS, Cruz SS da, Trindade SC, et al. Periodontitis and respiratory diseases: a systematic review with meta-analysis. Oral Dis 2020;26:439–46. 10.1111/odi.13228 [DOI] [PubMed] [Google Scholar]
- 47. Wu Z, Xiao C, Chen F, et al. Pulmonary disease and periodontal health: a meta-analysis. Sleep Breath 2022;26:1857–68. 10.1007/s11325-022-02577-3 [DOI] [PubMed] [Google Scholar]
- 48. Molina A, Huck O, Herrera D, et al. The association between respiratory diseases and periodontitis: a systematic review and meta-analysis. J Clin Periodontol 2023;50:842–87. 10.1111/jcpe.13767 [DOI] [PubMed] [Google Scholar]
- 49. Hujoel PP, Drangsholt M, Spiekerman C, et al. Periodontitis-systemic disease associations in the presence of smoking--causal or coincidental. Periodontol 2000 2002;30:51–60. 10.1034/j.1600-0757.2002.03005.x [DOI] [PubMed] [Google Scholar]
- 50. Intini G, Katsuragi Y, Kirkwood KL, et al. Alveolar bone loss: mechanisms, potential therapeutic targets, and interventions. Adv Dent Res 2014;26:38–46. 10.1177/0022034514529305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067432supp001.pdf (545.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.