Abstract
Background:
In an earlier study, a scaffold-free tissue-engineered construct (TEC) derived from autologous synovial membrane mesenchymal stromal cells (MSCs) was developed and demonstrated to be safe and effective for cartilage repair at 2 years postoperatively.
Purpose:
To investigate clinical outcomes and magnetic resonance imaging (MRI) findings at 5 years after implantation.
Study Design:
Case series; Level of evidence, 4.
Methods:
This was an observational first-in-human study limited to 5 patients (age, 28-46 years) with symptomatic knee chondral lesions (size, 1.5-3.0 cm2) on the medial femoral condyle, lateral femoral condyle, or femoral groove. Synovial MSCs were isolated from arthroscopic biopsy specimens and cultured to develop a TEC that matched the lesion size. The TECs were then implanted into chondral defects without fixation and assessed at up to 5 years postoperatively. The patients were clinically evaluated using the visual analog scale for pain, Lysholm score, Tegner score, and Knee injury and Osteoarthritis Outcome Score. An MRI scan evaluation was also performed for morphologic and compositional quality of the repair tissue at both 2 and 5 years of follow-up.
Results:
All clinical scores were significantly improved from the preoperative evaluation to the 2- and 5-year follow-ups and the results were stable over time. The MRI scan evaluation showed cartilage defects filled with newly generated tissues with good tissue integration to adjacent host cartilage over time. The cartilage thickness and surface smoothness of the repair cartilage were maintained up to 5 years postoperatively. The MOCART (magnetic resonance observation of cartilage repair tissue) 2.0 Knee Scores remained high at 5 years, although the total points decreased slightly.
Conclusion:
The results highlight the efficacy and feasibility of autologous scaffold-free TEC derived from synovial MSCs for regenerative cartilage repair via a sutureless and simple implantation procedure, showing good clinical outcomes and MRI findings with stable results at midterm follow-up. Further follow-up will be needed to assess the long-term quality of the repair tissue.
Keywords: cartilage repair, clinical study, mesenchymal stromal cell, scaffold free, synovium
Injured articular cartilage does not usually heal spontaneously, due to its avascular and aneural surroundings. 7 Over time, such injuries can progress to osteoarthritis due to the inability of chondral lesions to heal effectively. This progression can lead to significantly reduced physical activity and substantial lifestyle modifications, often at a young age. Therefore, a variety of approaches have been assessed to improve cartilage healing. Since the first results with autologous chondrocyte implantation, 5 cell-based approaches have been studied extensively with a variety of cell sources, including chondrocytes and mesenchymal stromal cells (MSCs). 24
MSCs have the potential to differentiate into a variety of cells, including bone, cartilage, tendon, muscle, and adipose tissue. 3,4 Specifically, MSCs isolated from synovium are well suited for cartilage repair because of their ease of harvest and excellent capacity for chondrogenic differentiation. 8,21 Using such cells, a 3-dimensional (3-D) tissue-engineered construct (TEC) has been developed through simple-cell culture methods. 3,4 The TEC contains undifferentiated synovium-derived MSCs surrounded by the extracellular matrices synthesized only by the cells. 3,24 With an abundance of fibrillar collagen and adhesion molecules, these TECs are pliable and highly adherent to normal cartilage; therefore, sutureless implantation to damaged chondral surfaces is readily achieved. 3,25
In our earlier study, the safety and efficacy of TECs for cartilage repair at 2 years postimplantation was documented. 27 In the present study, we aimed to further evaluate the clinical outcomes and magnetic resonance imaging (MRI) findings at 5 years postimplantation in the same cohort to assess the continued efficacy of the TEC treatment.
Methods
An observational first-in-human study limited to 5 patients was performed at Osaka University Hospital. The Ministry of Health, Labour and Welfare of Japan and our institutional review board restricted approval to just 5 patients as an “early proof of concept” trial; thus, 5 patients (4 men and 1 woman; age range, 28-46 years) were enrolled between February 1, 2013, and April 30, 2014. All patients had isolated full-thickness cartilage defects of the knee (<5 cm2, International Cartilage Regeneration and Joint Preservation Society grade 3 or 4). Patients with joint instability and/or abnormal alignment were excluded from this study. The present study was designed in accordance with the Declaration of Helsinki, and informed consent was obtained from all included patients.
Procedure
As addressed in our earlier study, 27 a 2-step procedure was performed: the first for arthroscopic evaluation and a synovial tissue biopsy and the second for the implantation surgery. For isolation of autologous synovial MSCs, synovium (>1 g) was aseptically taken from an anterior part of the knee joint arthroscopically and under general anesthesia. Care was taken to remove only synovium and exclude fat from the samples. All procedures for cell culture were performed at the cell-processing center in the Medical Center for Translational Research of Osaka University Hospital under International Organization for Standardization (ISO9001) certification.
The cell isolation protocol was essentially the same as that used in our previous studies. 3,4 Briefly, the synovium was minced meticulously and digested enzymatically with animal-origin-free collagenase (Worthington), and the isolated cells were cultured until passage 1 or 2 with growth medium containing high-glucose Dulbecco’s modified Eagle’s medium (Gibco BRL) supplemented with 10% fetal bovine serum with virus- and prion-free certification (Moregate Biotech).
For characterization of the cultured cells, the surface markers expressed by these cells were assessed by flow cytometry (FACS Calibur; Becton, Dickinson and Co), as addressed in our earlier study. 27 Individual cell suspensions from each donor were stained with fluorescent-conjugated mouse anti-human monoclonal antibody for CD13, CD34, and CD44 (BD Pharmingen) to calculate the percentage-positive cells with these markers just before generation of the TECs. In all cases, the resulting cells met the International Society for Cellular Therapy criteria for MSCs based on the results of flow cytometry (CD13, 97.5% ± 2.9%; CD34, 0.6% ± 0.5%; CD44, 97.7% ± 1.9%). 10,18,20
According to our earlier studies, 3,4 synovial MSCs were cultured at a density of 4.0 × 105 cells/cm2 in a growth medium containing 0.2 mM ascorbate-2-phosphate to develop a TEC that matched the lesion size. For implantation of an autologous TEC, 4 to 6 weeks after obtaining the synovial biopsy, the cartilage defects were exposed by mini-arthrotomy under general anesthesia. The TEC was washed extensively with saline solution to remove residual culture medium and then implanted into the defect site without the use of sutures or fixation glue; it adhered immediately to the surface of the cartilage defect. To avoid potential complications, an air tourniquet was not used during the surgical procedure. The mean surgical time was 63.4 ± 10.7 minutes (range, 48-76 minutes). All patients had the knee immobilized for 2 weeks with a brace and then started on range of motion exercises. Partial weightbearing was started at 6 weeks, and full weightbearing was allowed at 8 weeks. Return to sports and/or high-impact activities was allowed after 10 to 12 months.
Outcome Assessments
The patients were evaluated clinically with the visual analog scale (VAS) for pain, Lysholm score, Tegner score, and Knee injury and Osteoarthritis Outcome Score (KOOS). In addition, patients underwent proton density-weighted MRI with a 3.0-T magnetic resonance scanner at 2 and 5 years after implantation for evaluation of the morphologic and compositional characteristics of the repair site, and as a quantitative assessment of repair quality, the MOCART (magnetic resonance observation of cartilage repair tissue) Version 2.0 Knee Score was calculated. 23 Finally, we measured the thickness at the center of the repaired cartilage tissue. Figure 1 provides an overview of the study and outcome assessment procedure.
Figure 1.
Diagram showing overall study procedure. MRI, magnetic resonance imaging; MSC, mesenchymal stromal cell; PE, physical examination; Pre-op, preoperatively; PROMs, patient-reported outcome measures; TEC, tissue-engineered construct.
Study Endpoints
The study endpoints were described in our earlier study. 27 The primary outcome of this clinical study was the safety of the procedure. Safety was assessed after implantation of the TEC, and all adverse events - both local (eg, effusion, swelling, infection) and systemic (eg, fever, allergic reaction) - were monitored carefully according to the International Conference on Harmonization-E6 good clinical practice guidelines. 15 Secondary outcomes related to the efficacy of the procedure. All data analysis was performed in a blinded manner by independent researchers of the research project team.
Statistical Analysis
Sample distribution was tested by the Shapiro-Wilk test. An analysis of variance with repeated measures was carried out to compare changes in patient-reported outcomes, the MOCART score, and the thickness of repair cartilage at different follow-up times and followed by a post hoc test with Wilcoxon signed-rank test. Data were analyzed using JMP 15 (SAS Institute) with a significance set at P < .05.
Results
Patient Characteristics and Safety Assessments
Table 1 presents the characteristics of the 5 study patients. As addressed in our earlier report, 27 joint pain, effusion, and swelling were observed in the early stages after surgery, but all symptoms were completely improved by 4 weeks. No serious adverse events such as postoperative infections were observed up to 5 years after TEC implantation. In addition, all patients were routinely followed over 5 years postoperatively and did not require additional treatment during this observational period.
Table 1.
Characteristics of Patients Participating in This Study a
| Patient | Age, y | Sex | Side | BMI, kg/m2 | Location | Size, cm2 |
ICRS Grade | FTA, deg | Time Since Symptoms, mo | Trauma History | Previous Surgery on Ipsilateral Knee |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | M | L | 24.3 | Groove | 1.5 | 4 | 177 | 3 | Yes | ACLR, revision ACLR |
| 2 | 28 | M | L | 21.4 | LFC | 2.7 | 4 | 175 | 4 | Yes | ACLR, LMR |
| 3 | 37 | M | R | 26.1 | LFC | 3.0 | 3-4 | 178 | 11 | No | None |
| 4 | 36 | F | L | 27 | MFC | 2.0 | 3-4 | 175 | 3 | Yes | ACLR, drilling (MFC) |
| 5 | 46 | M | R | 25.9 | MFC | 3.0 | 4 | 178 | 6 | No | None |
a ACLR, anterior cruciate ligament reconstruction; BMI, body mass index; F, female; FTA, femorotibial angle; ICRS, International Cartilage Regeneration and Joint Preservation Society; L, left; LFC, lateral femoral condyle; LMR, lateral meniscus repair; M, male; MFC, medial femoral condyle; R, right.
Patient-Reported Outcome Measures
All clinical scores were significantly improved from the preoperative evaluation to the 2- and 5-year follow-ups, and the results were stable over time with no significant differences detected between 2 and 5 years (Figure 2). The VAS scores showed significant pain relief between preoperatively and 2 years postoperatively; such relief was stably maintained up to 5 years postoperatively (Figure 2A). The mean Lysholm score (Figure 2C) improved significantly from 51.0 ± 21.1 (preoperative evaluation) to 95.6 ± 6.8 (2-year follow-up) and 93.4 ± 6.7 (5-year follow-up), and the mean Tegner score (Figure 2B) improved from 2.2 ± 1.6 (preoperative evaluation) to 5.6 ± 1.9 (2-year follow-up) and 6.0 ± 1.4 (5-year follow-up). Scores on all 5 KOOS subscales were improved significantly by 2 years postoperatively and remained high up to 5 years (Figure 2D-H). On the other hand, the scores on the KOOS-Sports and Recreational Activities and KOOS-Quality of Life subscales in patient 5 decreased slightly from 2 to 5 years postoperatively, but these 5-year scores were still significantly higher than the preoperative value.
Figure 2.
Patient-reported outcome measures from preoperatively to 5-year follow-up: (A) VAS for pain; (B) Tegner; (C) Lysholm; and (D-H) KOOS subscales Symptoms, Pain, ADL, Sports & Rec, and QOL. Clinical improvements from baseline to 2 years were maintained stably up to 5 years. The × within the box indicates the mean, the horizontal line indicates the median, the top and bottom of the box indicate the interquartile range, and the whiskers indicate the range. Statistically significant differences: *versus preoperatively and #versus 24 weeks postoperatively (P < .05). ADL, activities of daily living; KOOS, Knee injury and Osteoarthritis Outcome Score; pre, preoperatively; QOL, quality of life; Sports & Rec, Sports and Recreational Activities; VAS, visual analog scale.
MRI Assessments
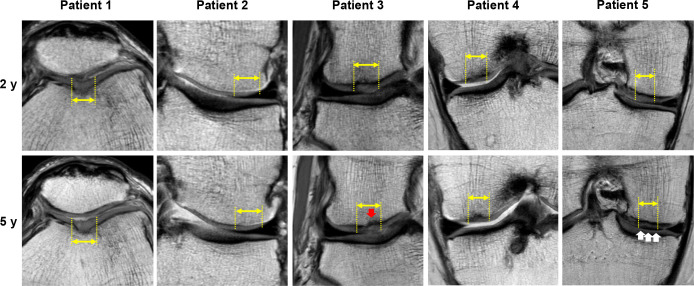
The MRI evaluation showed cartilage defects filled with newly generated tissues, and the repair tissue exhibited good tissue integration with adjacent host cartilage (Figure 3). The cartilage thickness and surface smoothness of the repair cartilage and the integration to adjacent cartilage were maintained up to 5 years postoperatively in all cases. Detailed observation in cases 1 and 2 showed that the quality of repair tissue and subchondral bone was maintained up to 5 years. In case 3, the repair tissue became more inhomogeneous and with a small bony defect in the subchondral bone area observed at 5 years. These findings suggest some decline in the quality of repair tissue and subchondral bone. In case 4, the repair cartilage exhibited inhomogeneous characteristics and slight subchondral bone edema was observed around the repair tissue at 2 years; however, such findings had not detectably changed at the 5-year timepoint. In case 5, the signal intensity of the repair tissue became close to the subchondral bone plate, suggesting some decline in the quality of the repair cartilage, while the quality of the subchondral bone was maintained at 5 years.
Figure 3.
Proton density-weighted MRI scans of the injured cartilage sites (yellow double arrows and dotted lines) at 2 and 5 years postoperatively for all study patients. The red arrow in patient 3 indicates a bony defect of the subchondral bone area. The white arrows in patient 5 indicate the abnormal signal intensity of the repaired cartilage. MRI, magnetic resonance imaging.
The MOCART 2.0 Knee Scores indicated that the quality of the repair tissue remained high at 5 years, although the total points decreased slightly from 2 to 5 years postoperatively (91 ± 10 vs 82 ± 13; P = .25) (Table 2). In a detailed evaluation of the subcategories, the scores for “structure of the repair tissue,” “signal intensity of the repair tissue,” “bony defect or bony overgrowth,” and “subchondral changes” worsened slightly from 2 to 5 years postoperatively, but no statistically significant differences were detected. Other than those aspects, the scores remained high at 5 years postimplantation of the TEC.
Table 2.
MOCART 2.0 Knee Scores at 2- and 5-Year Follow-up a
| MOCART Category | 2-y Follow-up | 5-y Follow-up | P |
|---|---|---|---|
| Volume fill of cartilage defect | 20 ± 0 | 20 ± 0 | >.99 |
| Integration into adjacent cartilage | 15 ± 0 | 15 ± 0 | >.99 |
| Surface of repair tissue | 9 ± 2 | 9 ± 2 | >.99 |
| Structure of repair tissue | 8 ± 4 | 4 ± 5 | .25 |
| Signal intensity of repair tissue | 12 ± 3 | 9 ± 5 | .5 |
| Bony defect or bony overgrowth | 10 ± 0 | 9 ± 2 | .5 |
| Subchondral changes | 17 ± 4 | 16 ± 5 | .5 |
| Total points | 91 ± 10 | 82 ± 13 | .25 |
a Data are shown as mean ± SD. MOCART, magnetic resonance observation of cartilage repair tissue.
The thickness in the center of the repaired cartilage was maintained in all cases, and there were no significant differences detected between 2 and 5 years postoperatively (Table 3).
Table 3.
Thickness of Repair Cartilage
| Cartilage Thickness, mm | ||
|---|---|---|
| Patient No. | 2-y Follow-up | 5-y Follow-up |
| 1 | 2.26 | 2.32 |
| 2 | 1.68 | 1.66 |
| 3 | 2.43 | 2.33 |
| 4 | 1.95 | 1.82 |
| 5 | 2.83 | 2.78 |
Discussion
We previously reported 2-year follow-up data on the safety and efficacy of an autologous TEC generated from synovial MSCs to mediate cartilage repair in a first-in-human clinical study. 27 The current evaluation of data at 5 years after the TEC implantation showed sustained efficacy across the full follow-up period, as demonstrated by stable Lysholm, Tegner, and KOOS values, as well as secure defect filling on MRI in all patients.
As addressed in our earlier studies, 24,25,27 there are several advantages to utilize the TEC in cartilage repair. The TEC is generated through the simple and rapid scaffold-free manufacturing process with synovium-derived MSCs, as compared with other cartilage tissue-engineering approaches. 6,12,19,22,28 Also, the TEC develops without any exogenous scaffold and thus, implantation of the TEC would have minimal risk of potential side effects induced by artificial or extrinsic biological materials contained in a scaffold. Moreover, the TEC is a soft spherical body with plasticity and adhesiveness to the cartilaginous matrix. 3,4 Such material properties would be advantageous, as they enable the ready matching to the needed shape and size for the repair of a chondral defect and allow for the rapid sutureless implantation by minimally invasive surgery. Thus, such TEC approaches could provide a novel treatment option with high chondrogenic capacity, safety, and lower cost. 27
An evaluation of the treatment effect showed that statistically significant improvement of patient-reported outcome measures was maintained over the 5 years in VAS pain score, Lysholm, Tegner, and all subcategories of the KOOS. Interestingly, the score for all subcategories of the KOOS assessment in all cases except for case 5 remained high at 5 years postoperatively. In addition, the safety assessment did not show any serious adverse events or clinical failures. Regarding the morphological evaluation on MRI, the repair cartilage thickness and surface smoothness of the repair cartilage and the integration to adjacent cartilage, these characteristics were maintained up to 5 years postimplantation of the TEC. Also, the MOCART 2.0 Knee Scores indicated that the quality of the repair tissue remained high at 5 years, although the total points decreased slightly from 2 to 5 years postoperatively. On the other hand, detailed observation showed that the quality of repair tissue and subchondral bone in cases 1, 2, and 4 was maintained up to 5 years, while the repair tissue exhibited some deterioration in cases 3 and 5 (Figure 3).
Of note, the structure of the repair tissue did not exhibit detectable improvement from 2 to 5 years for all cases. Previously, we reported that histology of the repair tissue at 48 weeks postimplantation yielded details not detected by MRI scan 26 ; additional detail was not observed after 5 years. A possible reason for some deterioration of repair tissue from 2 to 5 years postoperatively would be the presence of fibrocartilage-like tissue repair. As reported in our earlier study, 26 histological analysis of the biopsy specimens obtained at 48 weeks postoperatively showed that the repair tissue was mixed with hyaline cartilage-like and fibrocartilage-like tissues in some cases of the same cohort as evaluated in the present study. Thus, details of the histological analysis at 48 weeks might have predicted the mid- and long-term prognosis. As another possible explanation for these findings, it is possible that the lamina splendens was not re-established on the repair tissue, and this may have impacted the retention of proteoglycans and other molecules within the repair tissue over time. 13 The failure to reconstitute the lamina splendens after implantation of a TEC was also observed previously in a porcine model, 2 so the present findings are not unique to humans; however, this issue can likely be addressed in future studies.
Moreover, cartilage injury occurred without any significant history of trauma in cases 3 and 5 (Table 1), possibly due to chronic focal repetitive stresses leading to the development of the cartilage lesions in these 2 patients. It is likely that the cartilage and subchondral bone of the site of tissue damage in these cases might be the result of some pathological conditions such as development of early osteoarthritis before implantation of the TEC. 14,16 In addition, since the patients in cases 3 and 5 underwent the TEC implantation surgeries 6 months or more after knee chondral injury (Table 1), such delayed surgery (a more chronic condition) might affect clinical outcomes and the quality of the repair cartilage, as reported in the previous studies. 9,29 In any case, of note is that the slight deterioration of repair tissue did not immediately affect the scores of patient-reported outcome measures, and thus longer follow-up will be required to draw firmer conclusions. Taken together, the clinical benefit of the TEC implantation was proven to be durable with safety and efficacy for at least 5 years after surgery.
Midterm MRI findings have been reported for several other cell-based studies for cartilage repair. In a 5-year outcome of MRI results, Ebert et al 11 assessed 41 patients (53 grafts) after matrix-induced autologous chondrocyte implantation (MACI) to the knee and showed 67% of MACI grafts demonstrated complete infill, whereas 89% demonstrated good-to-excellent filling of the chondral defect. Similarly, Marlovits et al 17 performed a prospective evaluation of the MACI procedure in 21 patients with chondral defects of the knee. On MRI assessment, the MOCART scores significantly improved from baseline to year 5 (from 52.9 to 75.8). After 5 years, complete filling (83%) and integration (82%) of the graft were seen in the majority of patients. However, subchondral bone edema was still present in 47% of the patients at 5 years.
Anderson et al 1 treated 29 patients with symptomatic full-thickness cartilage lesions of the distal femoral condyle with NeoCart implant and observed them over a mean of 52.0 ± 15.5 months (median, 60 months). MOCART scores indicated significant improvement in cartilage quality from 3 to 24 months, with stabilization from 24 to 60 months. The MOCART parameters demonstrated defect fill (81% complete fill at 36 months vs 73% complete fill at 60 months), integration to the border zone (58% complete at 24 months), integration with bone (96% integrated at 12 months), repair surface quality (50% intact at 48 months), and tissue homogeneity (68% homogeneous at 24 months). However, the subchondral bone demonstrated edema, granulation, cysts, or sclerosis in 80% of patients across all points beyond 12 months.
Yoon et al 30 enrolled 7 patients with symptomatic, full-thickness cartilage lesions in this first-in-human study and implanted a costal chondrocyte-derived 3-D pellet to full-thickness cartilage defects in the knee. Significant improvements were seen in MOCART scores from preoperative baseline to the 5-year follow-up (from 28.33 to 83.33). Two patients had complete defect filling on MRI evaluation at 1 year. Moreover, at 5 years postoperatively, complete defect filling was observed in 4 patients, and hypertrophy or incomplete defect filling was observed in 2 patients. In the present study, the repair tissue exhibited complete defect filling without any hypertrophy and with good tissue integration to adjacent host cartilage, and these findings were maintained up to 5 years postoperatively in all cases (100%). Also, subchondral bone edema was observed at 5 years in only case 4 (20%).
Taken together, these results suggest that the TEC-based procedure could provide a superior midterm treatment option for articular cartilage defects in the knee, compared with other cell-based therapies. On the other hand, long-term follow-up will be necessary to confirm whether the TEC-induced repair tissue has the durability required to maintain long-term patient quality of life with minimal risk for accelerated development of osteoarthritis later in life.
Limitations
A potential limitation of this first-in-human study was the enrollment restriction for only 5 patients. Furthermore, this study did not include a control group. Therefore, we cannot exclude the possibility of a placebo effect on the improvement of subjective clinical scores. However, the consistent improvement in the clinical scores as well as evidence for the consistent structural repair of the defects on MRI over 5 years postoperatively strongly suggests that this limitation did not appear to influence the major conclusions of the present study. To address this limitation, a randomized controlled trial (phase III clinical trial) with more patient enrollment to further evaluate the TEC approach versus microfracture is currently being performed. Implementation and completion of the ongoing randomized controlled trial will further define the significance of this unique MSC-based therapy over another currently available treatment option. Subsequent regulatory approval of the TEC product for the repair of chondral defects could potentially mitigate the risk of subsequent osteoarthritis development in this patient population.
Conclusion
The present results highlight the efficacy and feasibility of the TEC-based procedure, showing good clinical outcomes with stable results at midterm follow-up. In addition, the MRI findings remained stable over time and secure defect filling with good integration was confirmed, although the quality of the repair tissue declined slightly in some cases compared with earlier assessments. Thus, autologous scaffold-free TEC derived from synovial MSCs could be used for cartilage repair via a sutureless and simple implantation procedure. On the other hand, a longer follow-up is required to evaluate any changes in the quality of the repair tissue.
Footnotes
Final revision submitted March 13, 2023; accepted April 24, 2023.
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was supported by a Health and Labor Sciences Research grant from the Ministry of Health, Labour and Welfare of Japan, a grant from the New Energy and Industrial Technology Development Organization, Japan, and a Grant-in-Aid for Scientific Research, Japan Society for the Promotion of Science. D.A.H. was supported by an Alberta Innovates Health Solutions Osteoarthritis Team Grant and the AHS Strategic Clinical Network Program. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Osaka University Graduate School of Medicine (approval No. HM1201).
References
- 1. Anderson DE, Williams RJ III, DeBerardino TM, et al. Magnetic resonance imaging characterization and clinical outcomes after neocart surgical therapy as a primary reparative treatment for knee cartilage injuries. Am J Sports Med. 2017;45(4):875–883. [DOI] [PubMed] [Google Scholar]
- 2. Ando W, Fujie H, Moriguchi Y, et al. Detection of abnormalities in the superficial zone of cartilage repaired using a tissue engineered construct derived from synovial stem cells. Eur Cell Mater. 2012;24:292–307. [DOI] [PubMed] [Google Scholar]
- 3. Ando W, Tateishi K, Hart DA, et al. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials. 2007;28(36):5462–5470. [DOI] [PubMed] [Google Scholar]
- 4. Ando W, Tateishi K, Katakai D, et al. In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: biological and mechanical properties and further chondrogenic potential. Tissue Eng Part A. 2008;14(12):2041–2049. [DOI] [PubMed] [Google Scholar]
- 5. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 6. Brittberg M, Recker D, Ilgenfritz J, Saris DBF. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018;46(6):1343–1351. [DOI] [PubMed] [Google Scholar]
- 7. Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;(402):21–37. [DOI] [PubMed] [Google Scholar]
- 8. De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. [DOI] [PubMed] [Google Scholar]
- 9. Dhollander A, Verdonk P, Tirico LEP, Gomoll AH. Treatment of failed cartilage repair: state of the art. J ISAKOS. 2016;1(6):338–346. [Google Scholar]
- 10. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 11. Ebert JR, Robertson WB, Woodhouse J, et al. Clinical and magnetic resonance imaging-based outcomes to 5 years after matrix-induced autologous chondrocyte implantation to address articular cartilage defects in the knee. Am J Sports Med. 2011;39(4):753–763. [DOI] [PubMed] [Google Scholar]
- 12. Gomoll AH, Ambra LF, Phan A, Mastrocola M, Shah N. Cell-seeded autologous chondrocyte implantation: a simplified implantation technique that maintains high clinical outcomes. Am J Sports Med. 2017;45(5):1028–1036. [DOI] [PubMed] [Google Scholar]
- 13. Grenier S, Bhargava MM, Torzilli PA. An in vitro model for the pathological degradation of articular cartilage in osteoarthritis. J Biomech. 2014;47(3):645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Im GI. The concept of early osteoarthritis and its significance in regenerative medicine. Tissue Eng Regen Med. 2022;19(3):431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Guideline for Good Clinical Practice E6. https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice-scientific-guideline
- 16. Jansen MP, Mastbergen SC. Joint distraction for osteoarthritis: clinical evidence and molecular mechanisms. Nat Rev Rheumatol. 2022;18(1):35–46. [DOI] [PubMed] [Google Scholar]
- 17. Marlovits S, Aldrian S, Wondrasch B, et al. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am J Sports Med. 2012;40(10):2273–2280. [DOI] [PubMed] [Google Scholar]
- 18. Muñiz C, Teodosio C, Mayado A, et al. Ex vivo identification and characterization of a population of CD13(high) CD105(+) CD45(-) mesenchymal stem cells in human bone marrow. Stem Cell Res Ther. 2015;6(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2–12. [DOI] [PubMed] [Google Scholar]
- 20. Ramos TL, Sánchez-Abarca LI, Muntión S, et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. [DOI] [PubMed] [Google Scholar]
- 22. Saris DB, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36(2):235–246. [DOI] [PubMed] [Google Scholar]
- 23. Schreiner MM, Raudner M, Marlovits S, et al. The MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) 2.0 Knee Score and Atlas. Cartilage. 2019:13(1)(suppl):571S–587S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shimomura K, Ando W, Moriguchi Y, et al. Next generation mesenchymal stem cell (MSC)-based cartilage repair using scaffold-free tissue engineered constructs generated with synovial mesenchymal stem cells. Cartilage. 2015;6(suppl 2):13S–29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimomura K, Ando W, Tateishi K, et al. The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials. 2010;31(31):8004–8011. [DOI] [PubMed] [Google Scholar]
- 26. Shimomura K, Hamada H, Hart DA, et al. Histological analysis of cartilage defects repaired with an autologous human stem cell construct 48 weeks postimplantation reveals structural details not detected by t2-mapping MRI. Cartilage. 2021;13(1)(suppl):694S–706S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimomura K, Yasui Y, Koizumi K, et al. First-in-human pilot study of implantation of a scaffold-free tissue-engineered construct generated from autologous synovial mesenchymal stem cells for repair of knee chondral lesions. Am J Sports Med. 2018;46(10):2384–2393. [DOI] [PubMed] [Google Scholar]
- 28. Siebold R, Suezer F, Schmitt B, Trattnig S, Essig M. Good clinical and MRI outcome after arthroscopic autologous chondrocyte implantation for cartilage repair in the knee. Knee Surg Sports Traumatol Arthrosc. 2018;26(3):831–839. [DOI] [PubMed] [Google Scholar]
- 29. Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39(12):2566–2574. [DOI] [PubMed] [Google Scholar]
- 30. Yoon KH, Park JY, Lee JY, Lee E, Lee J, Kim SG. Costal chondrocyte-derived pellet-type autologous chondrocyte implantation for treatment of articular cartilage defect. Am J Sports Med. 2020;48(5):1236–1245. [DOI] [PubMed] [Google Scholar]