Abstract
Classical psychedelics represent a subgroup of serotonergic psychoactive substances characterized by their distinct subjective effects on the human psyche. Another unique attribute of this drug class is that such effects become less apparent after repeated exposure within a short time span. The classification of psychedelics as a subgroup within the serotonergic drug family and the tolerance to their effects are replicated by the murine head twitch response (HTR) behavioral paradigm. Here, we aimed to assess tolerance and cross-tolerance to HTR elicited by psychedelic and nonpsychedelic serotonin 2A receptor (5-HT2AR) agonists in mice. We show that repeated (4 days) administration of the psychedelic 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) induced a progressive decrease in HTR behavior. Tolerance to DOI-induced HTR was also observed 24 h after a single administration of this psychedelic. Pretreatment with the 5-HT2AR antagonist M100907 reduced not only the acute manifestation of DOI-induced HTR, but also the development of tolerance to HTR. Additionally, cross-tolerance became apparent between the psychedelics DOI and lysergic acid diethylamide (LSD), whereas repeated administration of the nonpsychedelic 5-HT2AR agonist lisuride did not affect the ability of these two psychedelics to induce HTR. At the molecular level, DOI administration led to down-regulation of 5-HT2AR density in mouse frontal cortex membrane preparations. However, development of tolerance to the effect of DOI on HTR remained unchanged in β-arrestin-2 knockout mice. Together, these data suggest that tolerance to HTR induced by psychedelics involves activation of the 5-HT2AR, is not observable upon repeated administration of nonpsychedelic 5-HT2AR agonists, and occurs via a signaling mechanism independent of β-arrestin-2.
Keywords: Tolerance, psychedelics, classical hallucinogens, serotonin 2A receptor, 5-HT2AR, head-twitch response, lysergic acid diethylamide, lisuride
Graphical Abstract
INTRODUCTION
Classical psychedelics, such as psilocybin, mescaline, and lysergic acid diethylamide (LSD), elicit a distinctive characteristic array of changes in affect, perception, and cognitive processes in humans.1,2 However, upon repeated administration within a short period of time, tolerance to the hallucinogenic effects of this family of drugs manifests through the gradual accumulation of resistance to their own action.3–5 Like other psychoactive compounds, such as opioids and cannabinoids, the development of tolerance to the hallucinogenic effects of psychedelics suggests the necessary occurrence of compensatory homeostatic adaptations following repeated drug administration.6 Additionally, cross-tolerance to classical psychedelics, which refers to the generalization of tolerance between distinct chemical entities, has also been documented for different representatives of this drug class including mescaline, psilocybin, and LSD.7,8
Besides their hallucinogenic properties,9,10 recent clinical studies have also revealed that psychedelics can aid in bringing alleviation to patients suffering from severe psychiatric conditions, such as depression and substance use disorders.11,12 Unlike traditional antidepressants,13 psychedelics appear to produce fast-acting and sustained therapeutically relevant effects after a single administration.14 This clinical observation has also been corroborated in rodent models suggesting that a single administration of psychedelics such as 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI),15 psilocybin,16,17 N,N-dimethyltryptamine (DMT),18 and LSD19 produces prolonged synaptic plasticity and behavioral alterations that predict antidepressant-like activity. Interestingly, like tolerance, the maximal expression of the potentially beneficial effects of psychedelics become apparent after the acute hallucinogenic effects attributable to the first exposure to the drug have resolved. Understanding the basic mechanisms behind this process of tolerance to psychedelics may therefore yield significant insights into different aspects of the psychedelic experience aftermath.
Although their pharmacology is relatively complex, it has been demonstrated that most of the hallucinogenic properties of classical psychedelics are mediated via the serotonin 2A receptor (5-HT2AR)20,21—a G-protein-coupled receptor (GPCR) that signals principally through Gq/11-protein-dependent mechanisms.22 The desensitization of a GPCR response can be described as the signal attenuation that follows the stimulation by receptor agonists.23 Arrestin proteins, including β-arrestin-2 (βarr2), were first thought to only regulate GPCR desensitization and internalization,24 but more recent findings also suggest that arrestins play a role in multiple signaling pathways in the cell.25,26
The head-twitch response (HTR) paradigm in rodents is a classifier of psychedelic action with a broad operative range in the dose and magnitude of effect domains amenable for the study of psychedelics’ dynamics in animal models.20,21,27 This behavioral proxy of human hallucinogenic potential is induced by psychedelics including phenethylamines such as DOI, tryptamines such as psilocybin, and ergolines such as LSD, but not by nonpsychedelic 5-HT2AR agonists such as lisuride. Using HTR as a behavioral model of psychedelic action, previous studies demonstrate that several psychedelics recapitulate the tolerance28,29 and cross-tolerance30 phenomena observed in human subjective effects.
Here, we extend these observations by examining tolerance and cross-tolerance to HTR elicited by the phenethylamine and ergoline psychedelics DOI and LSD, respectively. To better understand the pharmacological properties of 5-HT2AR agonists, we studied the effect of repeated administration of lisuride on DOI- and LSD-induced HTR. Mechanistically, we put forward that the development of tolerance to HTR elicited by DOI can be prevented by administration of the 5-HT2AR antagonist M100907. Given the potential role of βarr2 is some of the acute responses to psychedelics,31,32 we also evaluated tolerance to DOI-induced HTR in βarr2 knockout mice and controls.
RESULTS AND DISCUSSION
Single Dose of DOI Down-regulates 5-HT2AR in the Mouse Frontal Cortex.
DOI is a phenethylamine psychedelic commonly used as a research tool in the study of 5-HT2AR agonism on phenotypes that include HTR,33 synaptic plasticity,15 and inflammation-related processes,34 among others. Previous findings showed that repeated administration of psychedelics such as DOI and LSD down-regulated 5-HT2AR density in rat brain samples,35–38 an observation that was also reported 24 h after a single dose of LSD.39 In agreement with these studies, our findings indicate that a single exposure to DOI resulted in decreased density of 5-HT2ARs in the mouse frontal cortex, a brain region previously involved in the unique effects of psychedelics.20,40 Thus, [3H]ketanserin binding was significantly reduced in membrane preparations from frontal cortex samples of mice that had received DOI (2 mg/kg) 24 h prior, as compared to vehicle (Figure 1A) (Student’s t test, t10 = 3.64, p < 0.01).
Figure 1.
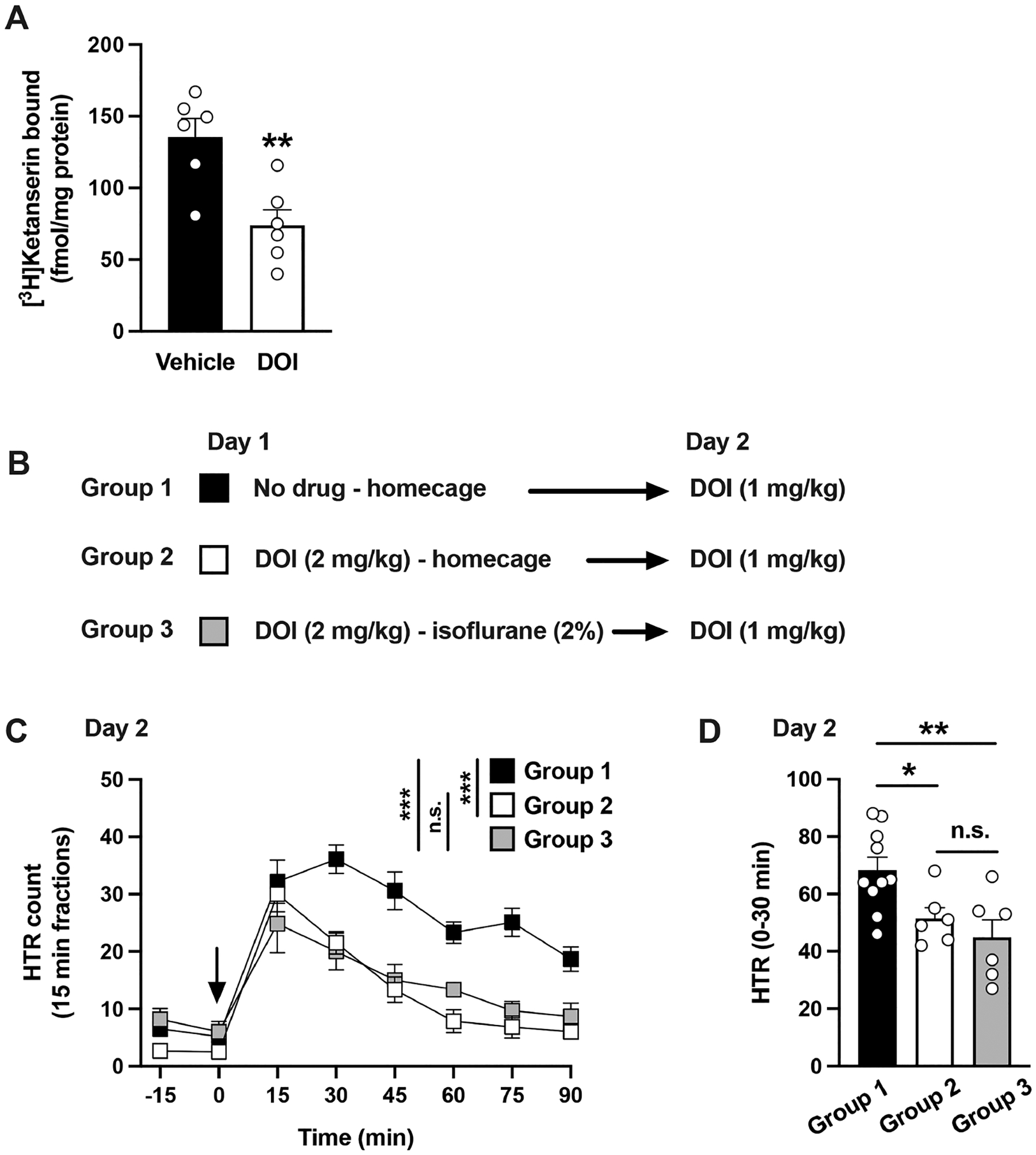
(A) [3H]Ketanserin binding in mouse frontal cortex samples. Mice received a single dose (i.p.) of DOI (2 mg/kg) or vehicle, and samples were collected 24 h after treatment (n = 6 mice per group). (B−D) Effect of anesthesia on the development of tolerance to HTR. Experimental design depicting the different treatment groups and days (n = 6−10 mice per group) (B). Time course of HTR on day 2 (C). Total HTR during the first 30 min following DOI administration on day 2 (D). Student’s t test (A), and two-way repeated measures ANOVA (C) and one-way ANOVA (D) followed by Bonferroni’s posthoc test; *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Arrow indicates time of injection (C). Data show mean ± standard error of the mean.
DOI Induces Tolerance Both in Freely Moving and Anesthetized Mice.
Considering the motor nature of HTR manifestation, and the high frequency of events elicited by DOI as compared to other psychedelics,20 we aimed to determine whether the reduction in HTR counts upon re-exposure to the drug may be due to phenomena associated with muscular stiffness or fatigue.
To test such hypothesis, animals were administered DOI (2 mg/kg) (day 1) and kept in their homecage or under isoflurane anesthesia with supportive care for 2 h. Animals under isoflurane anesthesia showed no motor function besides respiratory movements (data not shown). On the following day (day 2), all animals—including a reference group that was not subjected to any intervention on day 1—received DOI (1 mg/kg) (Figure 1B).
The development of tolerance to the action of DOI on day 2 became apparent in animals treated with DOI the previous day. Two-way ANOVA revealed that the treatment paradigm administered on day 1 statistically affected the HTRs elicited by DOI on day 2 (Figure 1C) (time, F[6,135] = 30.75, p < 0.001; day 1 treatment, F[2,135] = 49.40, p < 0.001). Posthoc analysis showed that HTRs elicited by DOI in either group (i.e., homecage and isoflurane) exposed to DOI on day 1 were different compared to those of the animals that were not subjected to any intervention on day 1 (Figure 1C) (DOI and homecage vs no intervention, p < 0.001; DOI and isoflurane vs no intervention, p < 0.001). Moreover, posthoc analysis did not show any differences in HTR counts on day 2 between animals that had received DOI (2 mg/kg) the previous day; regardless of whether they were in their homecage or under anesthesia for 2h (Figure 1C) (p > 0.05).
Such differences in HTR elicited by DOI on day 2 were also observed when the peak effect of DOI on HTR during the first 30 min postinjection were analyzed by one-way ANOVA (Figure 1D) (F[2,19] = 6.59, p < 0.01).
Consistent with our previous results showing that anesthesia during the peak effect of DOI does not delay the decay of its effect on HTR,41 these results highlight how the reduced manifestation of HTR upon re-exposure to the drug is not due to motor-related limitations emerging from the previous exposure to DOI.
DOI-Induced Tolerance Can Be Blocked by a 5-HT2AR Antagonist.
The involvement of the 5-HT2AR in mediating HTR induced by DOI has been demonstrated pharmacologically with antagonists42 and in genetically modified 5-HT2AR knockout animals.20,41 Following this lead, we aimed to explore whether HTR blockade with a 5-HT2AR antagonist would impact the development of tolerance.
On day 1, mice received vehicle or 1 mg/kg of the relatively selective 5-HT2AR antagonist M10090743 prior to the administration of DOI (1 mg/kg). After this, on day 2, all animals received the same dose of DOI (1 mg/kg) (Figure 2A). Two-way ANOVA revealed an effect of M100907 on DOI-induced HTR (Figure 2B,C) (time, F[6,112] = 19.24, p < 0.001; drug, F[3,112] = 72.54, p < 0.001). As expected,42 posthoc analysis revealed that the antagonist produced a significant reduction of DOI-induced HTR on day 1 (Figure 2B) (p < 0.001). As before with 2 mg/kg DOI (see Figure 1, above), posthoc analysis showed that the mice that had received 1 mg/kg of DOI on day 1 developed tolerance to HTR by DOI on day 2 (Figure 2B,C) (p < 0.001).
Figure 2.
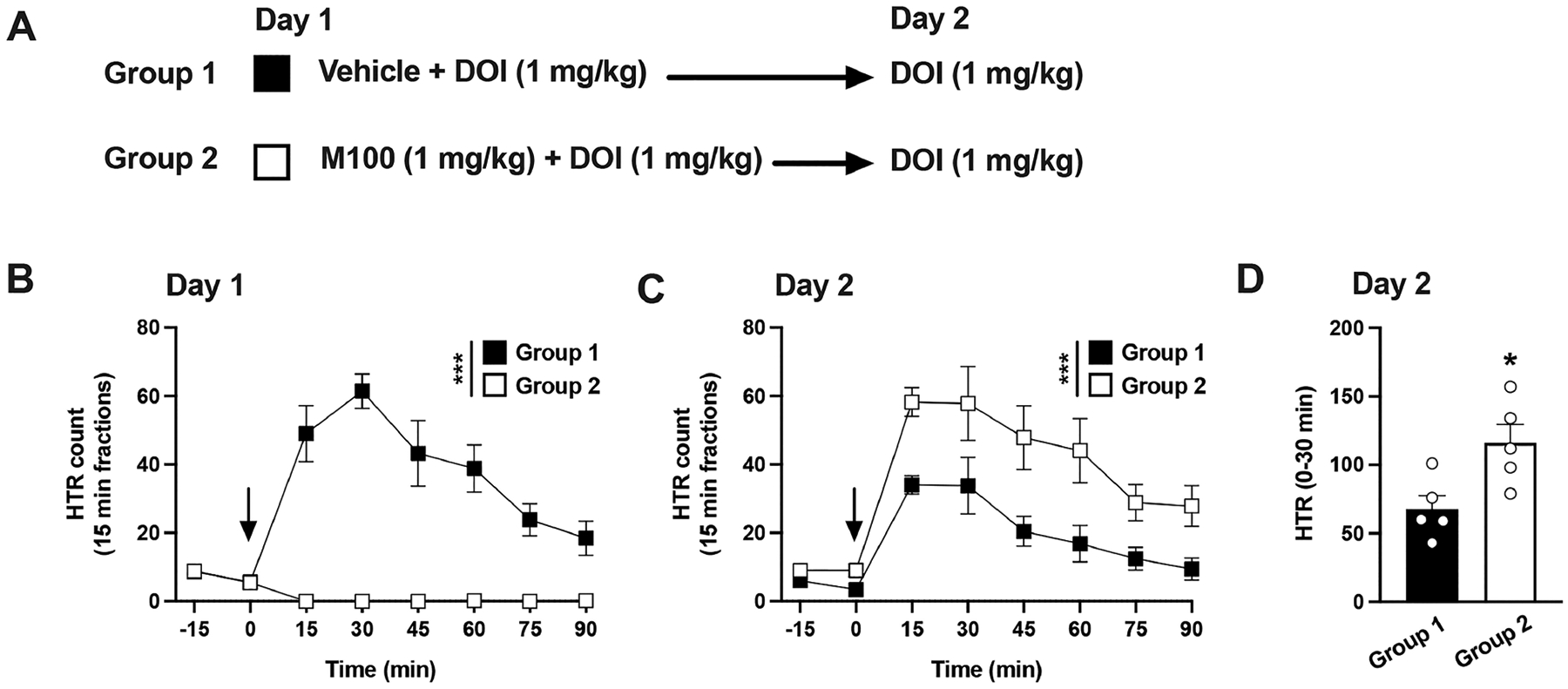
(A−D) Effect of M100907 on tolerance to HTR induced by DOI. Experimental design depicting the different treatment groups and days (n = 5 mice per group) (A). Time course of HTR on day 1 (B). Time course of HTR on day 2 (C). Total HTR during the first 30 min following DOI administration on day 2 (D). Student’s t test (D) and two-way repeated measures ANOVA (B,C) followed by Bonferroni’s posthoc test; *p < 0.05, ***p < 0.001. Arrows indicate time of injection (B,C). Data show mean ± standard error of the mean.
Interestingly, posthoc analysis indicated that the animals that had received both M100907 and DOI the day before showed greater HTR in response to DOI on day 2 than the comparison group on day 2 (Figure 2C) (p < 0.001). This was further corroborated during the collapsed HTR counts over the peak of effect on the first 30 min following DOI administration on day 2 (Figure 2D) (Student’s t test, t8 = 2.87, p < 0.05).
On a separate cohort of mice, we aimed to compare two different doses of M100907 on the blockade of DOI-induced HTR and the development of tolerance (Supplementary Figure 1A). On day 1, animals were treated with 2 mg/kg DOI only, 2 mg/kg DOI in combination with either 0.1 mg/kg or 1 mg/kg of M100907, or vehicle. To get a glimpse on the dynamics of the blockade, we monitored HTR responses for 3h in each of these experimental conditions (Supplementary Figure 1B). Two-way ANOVA analysis showed that a significant treatment effect emerged on day 1 (time, F[12,325] = 26.20, p < 0.001; drug, F[3,325] = 513.2, p < 0.001). Further inspection of the temporal dynamics revealed that M100907 (1 mg/kg) produced a complete blockade of DOI peak effect and throughout most of the HTR decay curve (see Supplementary Table 1 for posthoc analysis). M100907 (0.1 mg/kg), however, produced a biphasic response. First, an apparent complete blockade of DOI-induced HTR was observed, followed by a recovery of HTR counts undistinguishable from those induced by DOI alone during the decay stage (see Supplementary Table 1 for posthoc analysis).
On day 2, all animals were treated with DOI (2 mg/kg), and the HTR counts corresponding to the peak effect of DOI collapsed (30 min following drug administration) (Supplementary Figure 1A). As above on day 1, one-way ANOVA analysis revealed a significant treatment effect on day 2 (Supplementary Figure 1C) (F[3,26] = 9.59, p < 0.001). As expected (see Figure 1), posthoc analysis showed tolerance to HTR by DOI upon preexposure to DOI (Supplementary Figure 1C) (DOI on the previous day vs vehicle on the previous day, p < 0.001). Similarly, as shown above (see Figure 1), posthoc analysis showed that M100907 (1 mg/kg) prevented the development of tolerance (Supplementary Figure 1C) (M100907 + DOI on the previous day vs DOI alone on the previous day, p < 0.01; M100907 + DOI on the previous day vs vehicle on the previous day, p > 0.05). However, M100907 (0.1 mg/kg) only prevented partially the development of tolerance to the action of DOI (Supplementary Figure 1C) (M100907 + DOI on the previous day vs DOI alone on the previous day, p = 0.09; M100907 + DOI on the previous day vs vehicle on the previous day, p = 0.08).
Tolerance to DOI Following a Single Exposure Can Be Surmounted by Dose.
Daily administration of DOI produces a progressive development of tolerance to HTR that is unsurmountable by higher doses after 4 days of exposure.30 In light of this, we aimed to determine whether tolerance to DOI-induced HTR was surmountable with a higher dose of DOI administered 24 h after the first injection (Figure 3A).
Figure 3.
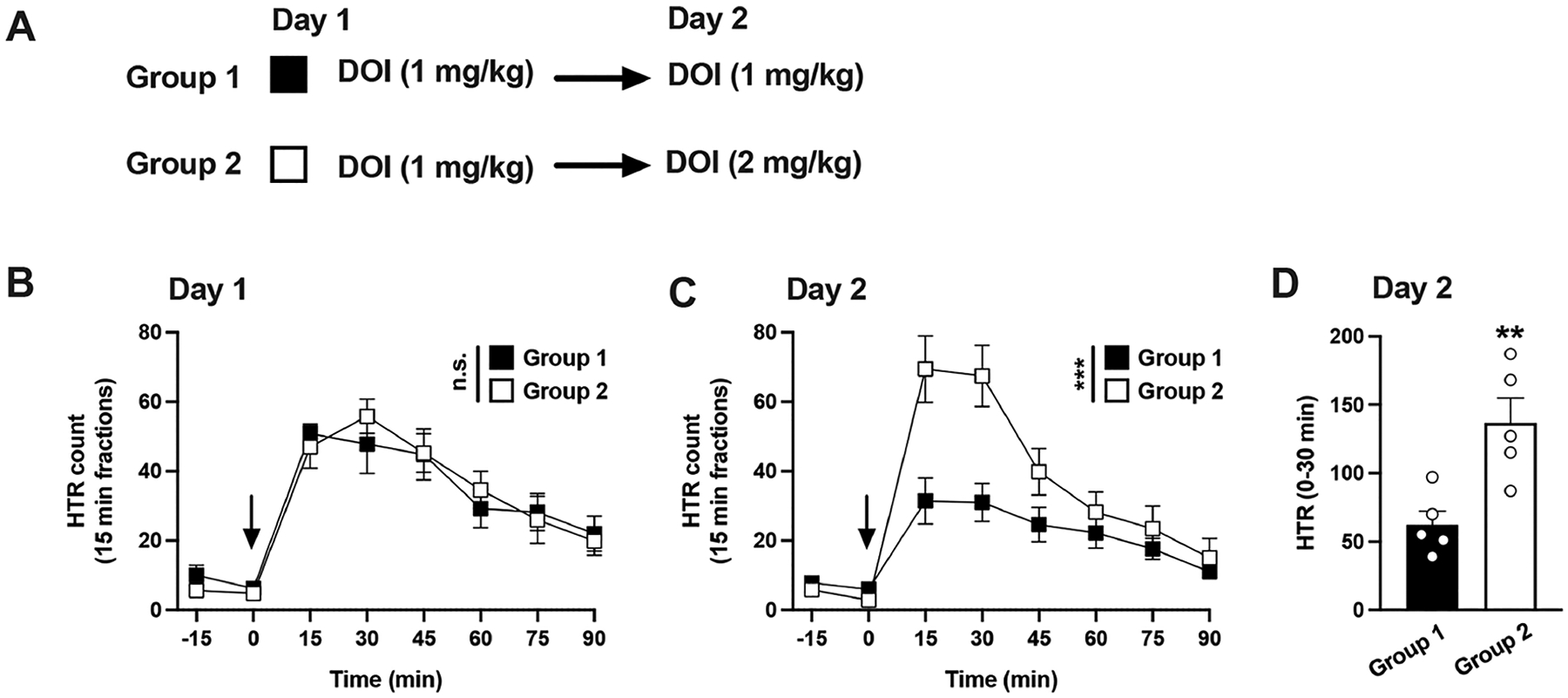
(A−D) Tolerance to HTR induced by DOI is surmountable by dose. Experimental design depicting the different treatment groups and days (n = 5 mice per group) (A). Time course of HTR on day 1 (B). Time course of HTR on day 2 (C). Total HTR during the first 30 min following DOI administration on day 2 (D). Student’s t test (D) and two-way repeated measures ANOVA (B,C) followed by Bonferroni’s posthoc test; **p < 0.01, ***p < 0.001, n.s., not significant. Arrows indicate time of injection (B,C). Data show mean ± standard error of the mean.
Two groups of animals received DOI (1 mg/kg) on day 1, and as expected, two-way ANOVA showed comparable responses (Figure 3B) (time, F[6,56] = 17.65, p < 0.001; group of mice, F[1,56] = 0.003, p > 0.05).
On day 2, the animals received a second dose of DOI (1 mg/kg or 2 mg/kg). As expected (see Figure 2), two-way ANOVA revealed an effect of repeated DOI administration (days 1 and 2) on HTR (Figure 3B,C) (time, F[6,112] = 34.33, p < 0.001; DOI treatment, F[3,122] = 10.18, p < 0.001). Additionally, posthoc analysis indicated that the HTR observed in animals that received on day 2 double the dose of DOI received on day 1 (2 mg/kg) was significantly increased compared to the animals that received the same dose (1 mg/kg) both days (Figure 3C) (p < 0.001).
Such differences were particularly noticeable during the peak effect of DOI. Thus, the sum of HTR during the first 30 min following administration of DOI on day 2 showed a significant effect (Figure 3D) (Student’s t test, t8 = 3.60, p < 0.01). These results suggest that tolerance to DOI effect on HTR following a single exposure to the drug can be surmounted by a higher dose.
DOI-Induced Tolerance Is Independent of βarr2.
As mentioned above, arrestins are crucial mediators of GPCR desensitization and clearance from the cytoplasmic membrane.26 For this reason, we aimed to evaluate whether expression of βarr2 was required for the development of tolerance upon repeated exposure to DOI. Both wild type (WT) and βarr2 knockout (β2KO) mice received daily DOI (1 mg/kg) injections for 4 consecutive days.
Two-way ANOVA with the time courses revealed the development of tolerance in both genotypes (Figure 4A–D) (WT: time, F[6,140] = 42.19, p < 0.001; day of treatment, F[3,140] = 48.99, p < 0.001) (β2KO: time, F[6,140] = 18.94, p < 0.001; day of treatment, F[3,140] = 21.88, p < 0.001).
Figure 4.
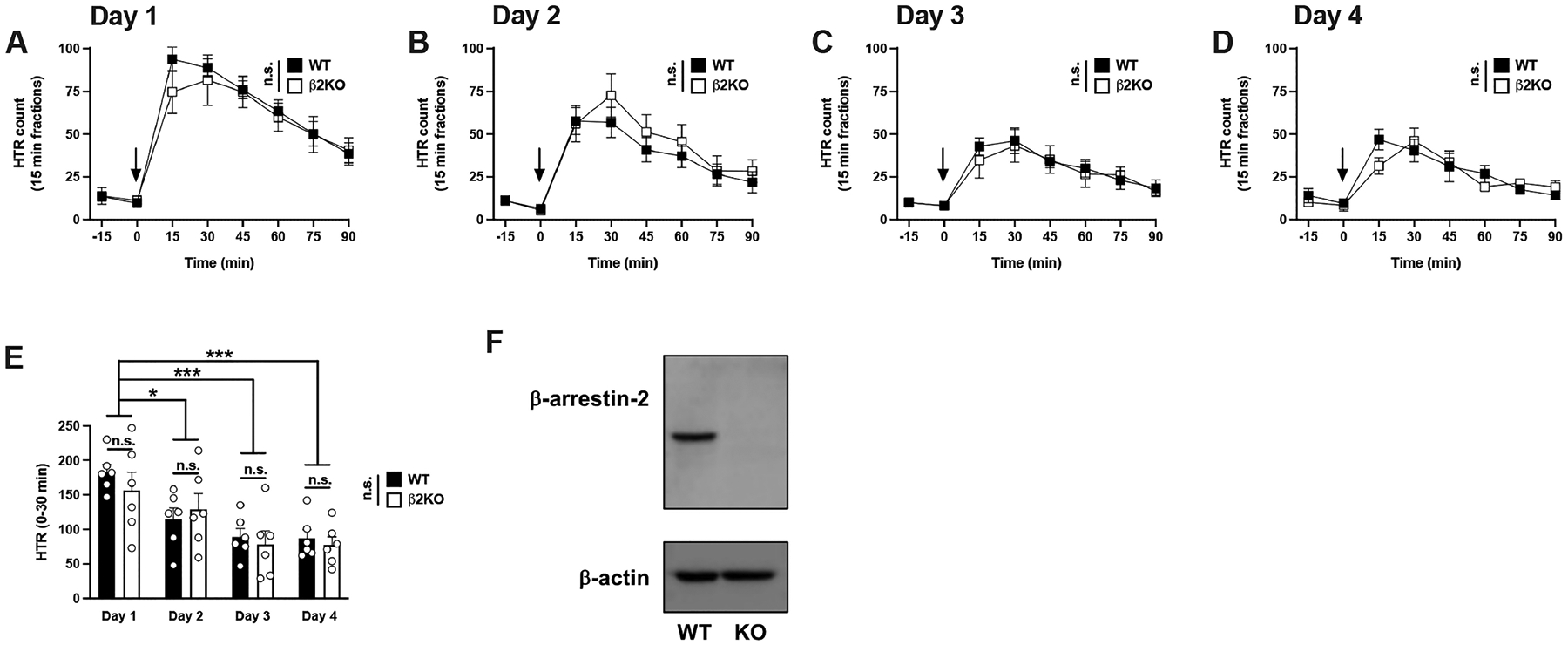
(A−E) Tolerance to HTR induced by DOI is independent of βarr2. Time courses of HTR elicited by DOI in WT and β2KO mice on days 1−4 (n = 6 mice per group) (A−D). Total HTR during the first 30 min following DOI administration in WT and β2KO mice on days 1−4 (E). Representative immunoblot image in frontal cortex samples of WT and β2KO mice (F). Two-way repeated measures ANOVA (A−D) and two-way ANOVA (E) followed by Bonferroni’s posthoc test; *p < 0.05, ***p < 0.001, n.s., not significant. Arrows indicate time of injection (A−D). Data show mean ± standard error of the mean.
As previously shown with this particular psychedelic,31 two-way ANOVA corroborated that the mouse genotype did not result in different HTR counts on day 1 upon a single exposure to DOI (Figure 4A) (time, F[6,70] = 21.83, p < 0.001; genotype, F[1,70] = 0.83, p > 0.05). Differences between genotypes in DOI-induced HTR were also absent throughout the entire course of repeated treatment (Figure 4B; day 2, time, F[6,70] = 12.57, p < 0.001; genotype, F[1,70] = 1.79, p > 0.05) (Figure 4C; day 3, time, F[6,70] = 8.28, p < 0.001; genotype, F[1,70] = 0.25, p > 0.05) (Figure 4D; day 4, time, F[6,70] = 12.49, p < 0.001; genotype, F[1,70] = 0.16, p > 0.05).
Ultimately, two-way ANOVA of peak HTR counts (30 min after DOI administration) showed that both groups of animals were undistinguishable in the development of tolerance to the effect of DOI on HTR (Figure 4E) (day of treatment, F[3,40] = 11.02, p < 0.001; genotype, F[1,40] = 0.43, p > 0.05).
The absence of βarr2 immunoreactivity in the knockout group was confirmed by immunoblot assays in frontal cortex samples (Figure 4F and Supplementary Figure 2) and quantitative PCR (data not shown).
Together, these results suggest that expression of βarr2 is not required for tolerance to HTR induced by repeated administration to the phenethylamine psychedelic DOI.
Repeated LSD Produces Cross-Tolerance to DOI.
Cross-tolerance between psychedelics has been documented both in human subjects and animal models, including HTR behavior.30 Particularly, it was reported that repeated administration to the phenethylamine psychedelic DOI reduced HTR induced by various doses of the phenethylamine 2C-T7 (2,5-dimethoxy-4-propylthiophenethylamine) or the tryptamine DPT (N,N-dipropyltryptamine).30 In light of our results, we aimed to investigate whether the tolerance built up following repeated exposure to the ergoline psychedelic LSD was transferred to DOI.
First, we tested the development of tolerance to HTR with LSD. Mice were administered LSD (0.2 mg/kg) every day for 4 days, and LSD-induced HTR was tested on day 1 and day 4 (Figure 5A,B). Two-way ANOVA showed that previous administration of repeated LSD significantly affected HTR (Figure 5B) (time, F[4,90] = 54.06, p < 0.001; day of treatment, F[1,90] = 24.58, p < 0.001). A similar result was obtained with peak effects of LSD (30 min after drug administration): HTR on day 1 was significantly different from HTR on day 4 (Student’s t test, t18 = 4.14, p < 0.001) (Figure 5C).
Figure 5.
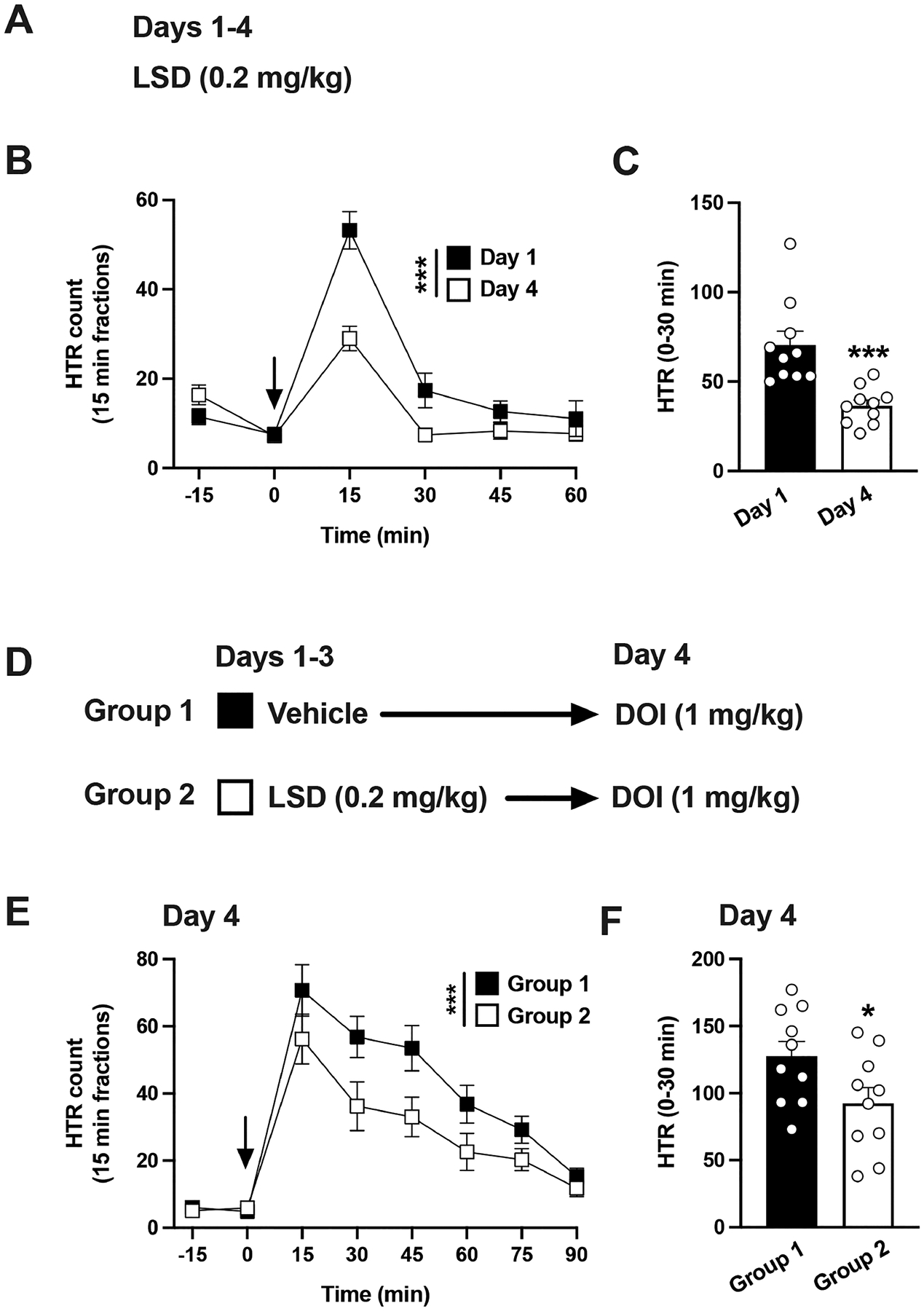
(A−C) Tolerance to HTR induced by LSD. Experimental design depicting the different treatment groups and days (n = 10 mice per group) (A). Time course of HTR on days 1 and 4 (B). Total HTR during the first 30 min following LSD administration on days 1 and 4 (C). (D−F) Cross-tolerance to HTR between LSD and DOI. Experimental design depicting the different treatment groups and days (n = 10 mice per group) (D). Time course of HTR on day 4 (E). Total HTR during the first 30 min following DOI administration on day 4 (F). Student’s t test (C,F) and two-way repeated measures ANOVA (B,E) followed by Bonferroni’s posthoc test; *p < 0.05, ***p < 0.001. Arrows indicate time of injection (B,E). Data show mean ± standard error of the mean.
For cross-tolerance experiments, mice that had received vehicle or LSD (0.2 mg/kg) daily for 3 days were challenged with a single dose of DOI (1 mg/kg) on day 4 and HTR quantified during this session (Figure 5D). Two-way ANOVA showed that previous administration of repeated LSD vs vehicle significantly affected DOI-induced HTR (Figure 5E) (time, F[6,126] = 28.97, p < 0.001; LSD administration, F[1,126] = 16.78, p < 0.001). Similarly, HTRs were statistically different when peak effects of DOI (30 min after drug administration) were tested in mice previously exposed to LSD as compared to vehicle (Figure 5F) (Student’s t test, t18 = 2.17, p < 0.05).
Overall, the results indicate that repeated exposure to LSD produced cross-tolerance to HTR induced by DOI.
Repeated Lisuride Does Not Produce Cross-Tolerance to LSD.
All classical psychedelics are 5-HT2AR agonists, but structural congeners of LSD, such as lisuride and ergotamine, lack comparable hallucinogenic properties and are considered nonpsychedelic 5-HT2AR agonists.2 For this reason, we also evaluated the effect of pre-exposure to lisuride on LSD-induced HTR.
HTR was recorded in two separate groups of mice for 5 days. During days 1−4, the mice were administered LSD (0.2 mg/kg) (group 1) or lisuride (0.4 mg/kg) (group 2) daily for 4 days. On day 5, all animals received the same dose of LSD (0.2 mg/kg) regardless of the pretreatment condition (Figure 6A). Using this model, we first compared the evolution of HTR following repeated administration of LSD vs lisuride. Two-way ANOVA (days 1−5) with the time courses revealed a treatment effect (Supplementary Figure 3) (group 1—repeated LSD followed by a single administration of LSD: time, F[2,74] = 19.35, p < 0.001; day of treatment, F[4,74] = 8.36, p < 0.001; group 2—repeated lisuride followed by a single administration of LSD: time, F[2,80] = 2.58, p = 0.07; day of treatment, F[4,80] = 9.28, p < 0.001).
Figure 6.
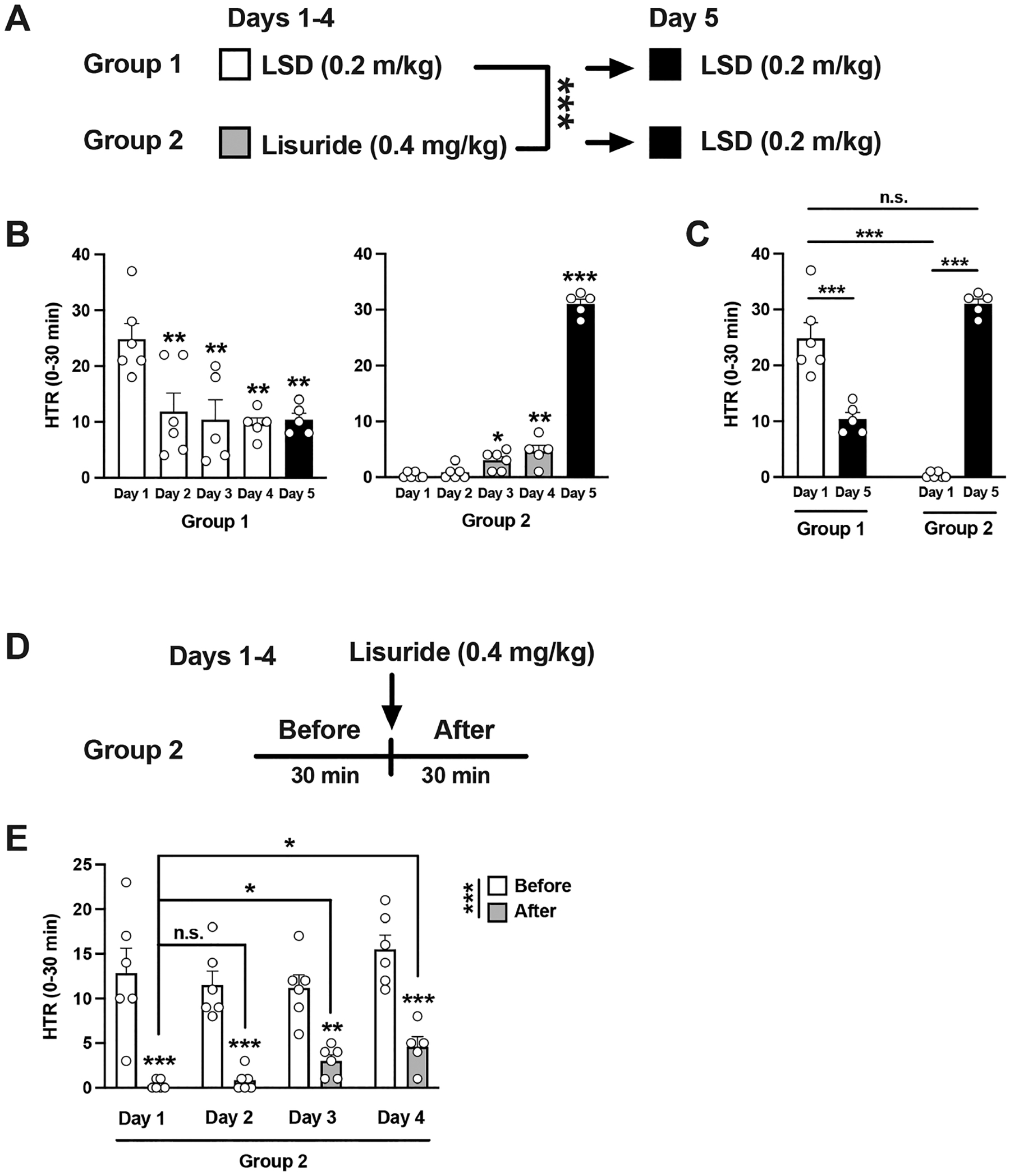
(A−C) Lack of cross-tolerance to HTR between lisuride and DOI. Experimental design depicting the different treatment groups on days 1−5 (n = 5−6 mice per group) (A). Total HTR during the first 30 min following drug administration on days 1−5 (B). Total HTR during the first 30 min following drug administration on days 1 and 5 (C). (D,E) Tolerance to the repressive effect on HTR by lisuride. Experimental design depicting the different treatment groups on days 1−4 (n = 5−6 mice per group) (D). Total HTR 30 min before and after lisuride administration on days 1−4 (E). One-way ANOVA (B) and two-way ANOVA (A,C,E) followed by Bonferroni’s posthoc test; *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Data show mean ± standard error of the mean.
Two-way ANOVA (days 1−4) of peak HTR counts (30 min after LSD or lisuride administration) also showed a strong treatment effect on HTR behavior (Figure 6A) (day of treatment, F[3,37] = 4.22, p < 0.05; LSD vs lisuride, F[1,37] = 66.00, p < 0.001).
Additionally, when these two groups were analyzed separately (one-way ANOVA—days 1−5—Figure 6B), there was a statistically significant day effect (group 1—repeated LSD followed by a single administration of LSD, F[4, 22] = 6.00, p < 0.01; group 2—repeated lisuride followed by a single administration of LSD, F[4, 23] = 317.4, p < 0.001). These data corroborate tolerance to LSD-induced HTR elicited by daily LSD administration (see Figure 5A–C).
More importantly, when comparing HTR on day 1 and day 5, two-way ANOVA showed differences between group 1 and group 2 (Figure 6C) (F[1,18] = 23.15, p < 0.001). Posthoc analysis also indicated that, as expected, HTR on day 1 was statistically different in mice injected with LSD vs lisuride (Figure 6C) (p < 0.001). Additionally, posthoc analysis showed tolerance development to HTR elicited by daily administration of LSD (day 1 vs day 5 in group 1) (Figure 6C) (p < 0.001), as well as an effect of LSD on HTR in lisuride-treated mice (day 1 vs day 5 in group 2) (Figure 6C) (p < 0.001). Lastly, posthoc analysis indicated that LSD-induced HTR in the cohort of mice pre-exposed to lisuride (day 5 in group 2) was undistinguishable from LSD-induced HTR on day 1 (day 1 in group 1) (Figure 6C) (p > 0.05). Together, these data suggest that whereas repeated LSD administration reduces LSD-induced HTR, cross-tolerance to HTR was not evident when lisuride-treated mice were tested with LSD.
Intriguingly, these findings also suggest that the repressive effect of lisuride on spontaneous HTR was slightly but significantly reduced upon repeated lisuride administration (Figure 6B—group 2—days 1−4). To gain further insights on this effect, we compared HTR behavior before and after lisuride administration throughout days 1−4 within the same data set (Figure 6D). Two-way ANOVA revealed a statistically significant difference between the spontaneous HTR (30 min before lisuride administration) and the HTR response observed 30 min after lisuride administration (Figure 6E) (F[1,39] = 101.8, p < 0.001), as well as a trend for a day effect (F[3,39] = 2.70, p = 0.058). Posthoc analysis also showed that lisuride decreased spontaneous HTR (Figure 6E) (HTR before vs after lisuride: day 1, p < 0.001; day 2, p < 0.001; day 3, p < 0.01; day 4, p < 0.001). Additionally, posthoc analysis indicated that the repressive effect of lisuride on spontaneous HTR was diminished upon repeated administration of the nonpsychedelic 5-HT2AR agonist (Figure 6E) (HTR after lisuride: day 1 vs day 2, p > 0.05; day 1 vs day 3, p < 0.05; day 1 vs day 4, p < 0.05).
Repeated Lisuride Does Not Produce Cross-Tolerance to DOI.
Because of the lack of effect of repeated lisuride administration on LSD-induced HTR, we also evaluated whether repeated exposure to a higher dose of lisuride could affect DOI-induced HTR. Mice were treated once a day for 4 days with lisuride (2 mg/kg) or vehicle, after which animals were tested for DOI-induced HTR on day 5 (Figure 7A). The lower dose of DOI (0.5 mg/kg) was chosen to allow for a wider range for the potential modulation of DOI-induced HTR after repeated lisuride administration. Nevertheless, two-way ANOVA indicated that lisuride administration on days 1−4 did not affect DOI-induced HTR on day 5 (Figure 7B) (time, F[6,56] = 15.33, p < 0.001; vehicle vs lisuride, F[1,56] = 0.03, p > 0.05). Similarly, HTRs were unaffected when peak effects of DOI (30 min after drug administration) were tested in mice previously exposed to lisuride compared to vehicle (Figure 7C) (Student’s t test, t8 = 0.04, p > 0.05).
Figure 7.
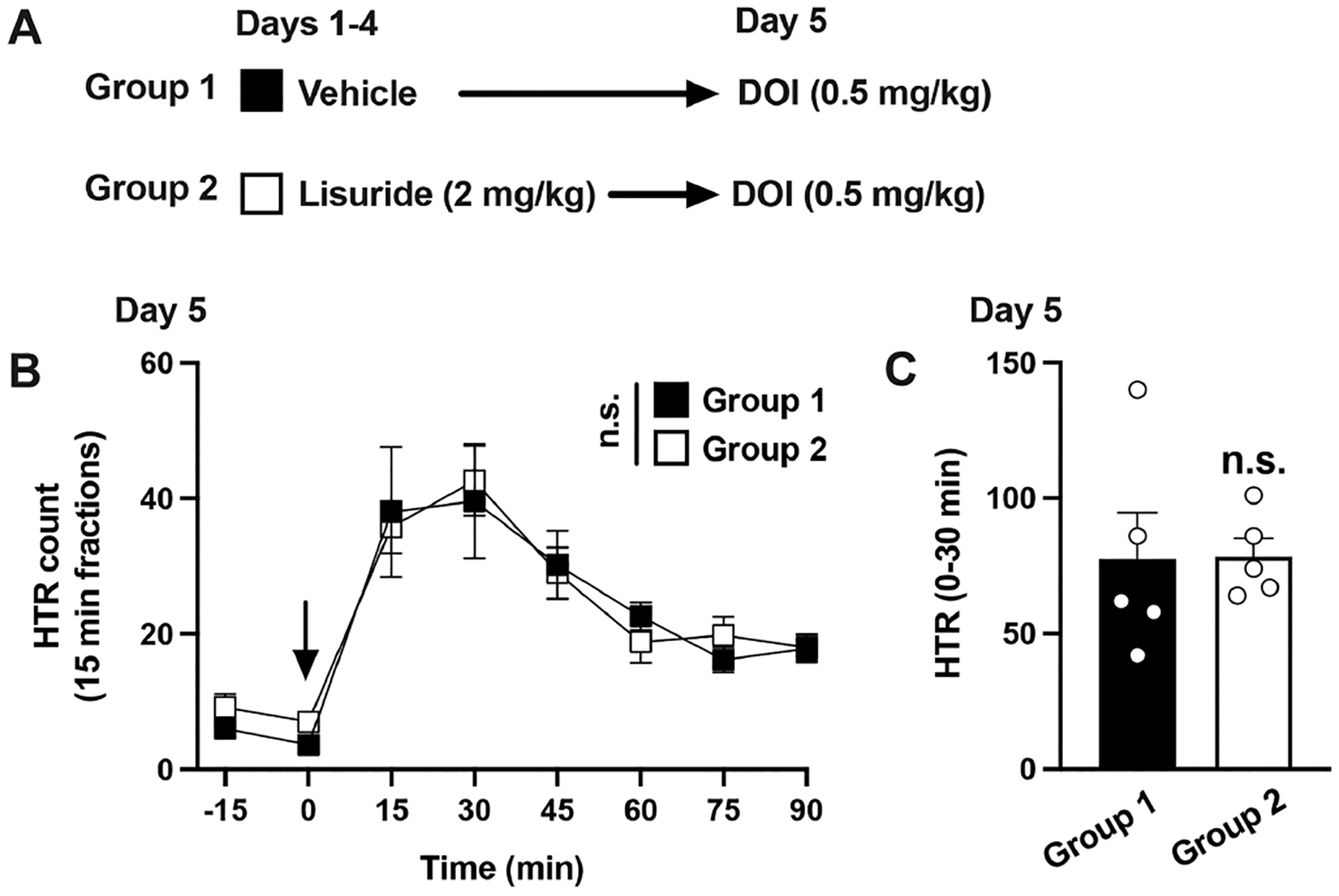
Lack of cross-tolerance to HTR between lisuride and DOI. Experimental design depicting the different treatment groups and days (n = 5 mice per group) (A). Time course of HTR on day 5 (B). Total HTR during the first 30 min following DOI administration on day 5 (C). Student’s t test (C) and two-way repeated measures ANOVA (B) followed by Bonferroni’s posthoc test. n.s., not significant. Arrow indicates time of injection (B). Data show mean ± standard error of the mean.
Psychedelics produce in both human subjects and animal models an ample repertoire of manifestations some of which are distinct for this drug class. One such preclinical model is the exacerbation of HTR in mice. This behavior, albeit not present in humans, serves as a predictor model of classical psychedelic activity.21,27 The profound effects of psychedelics on perception, cognition and sensory processes, among others are diminished by the development of tolerance that occurs upon repeated exposure. As we and others28–30 have shown, this feature is also recapitulated by the HTR model in mice, which provides a quantifiable variable to study the resistance to psychedelic action upon re-exposure.
Previous observations clearly showed that the development of tolerance to the behavioral action of psychedelics occurs concurrently with down-regulation of 5-HT2AR in the cortex of animal models,35–38 and can be transferred across and within structural classes.7,8 In the present study, we show that a single administration of a behaviorally active dose of the psychedelic drug DOI was sufficient to reduce 5-HT2AR density in the mouse frontal cortex, which corroborates previous observations with psilocybin and LSD in pig44 and rat39 brain samples, respectively, collected 1 day postinjection. This model of a single DOI administration also led to tolerance to HTR induced by the same psychedelic 5-HT2AR agonist. We also report that pretreatment with a single dose of the relatively selective 5-HT2AR antagonist M100907 (1 mg/kg) (also known as volinanserin43) precluded tolerance to HTR elicited by DOI. The lower dose of M100907 (0.1 mg/kg) was only partially effective at blocking the hours-long effect of DOI on HTR and the development of tolerance the day after. This corroborates our recent findings characterizing the pharmacodynamic (dose−response and time-course studies) properties of M100907 on DOI-induced HTR in mice45—we reported that low doses of M100907 (e.g., 0.032 mg/kg) were able to completely abolish LSD-induced HTR for up to 90 min, whereas during these time-course studies, DOI-induced HTR was similar in the vehicle + DOI and volinanserin + DOI groups at the 75 and 90 min time points. Our results in the present study hint toward a magnitude-matching effect on the ability of M100907 to antagonize the acute HTR to DOI and the posterior development of tolerance. However, considering the polypharmacology of this ligand,43 we cannot fully exclude the possibility that the effects of this serotonergic antagonist preventing tolerance to HTR induced by DOI are mediated via additional GPCR targets.
Antipsychotic medications such as clozapine also behave as 5-HT2AR antagonists/inverse agonists.46 Unlike atypical antipsychotics, some of which paradoxically drive endocytosis and down-regulation of 5-HT2AR in vitro47 and in vivo in the mouse frontal cortex,48 we previously showed that overnight exposure to M100907 (10 μM) augments cell surface subcellular localization of the 5-HT2AR in mammalian HEK293 cells.49 Further studies will be required to evaluate whether the effects of M100907 on tolerance to DOI-induced HTR can be extrapolated to alternative 5-HT2AR antagonists with approved clinical use such as clozapine, pimavanserin, or risperidone and whether such reduction of tolerance to HTR can be generalized to other psychedelics or alternative behavior models of psychedelic activity. It has also been previously reported that tolerance to HTR elicited by repeated administration of DOI (1 mg/kg) on days 1−3 was not surmounted by higher DOI doses (0.3−10 mg/kg) on day 4.30 Our data here suggest that tolerance to HTR induced by a single DOI exposure (1 mg/kg) can be surmounted by an increase in dose (2 mg/kg). This difference may be attributable to the length of agonist exposure, and further investigation will be necessary to test the extent to which adjunctive treatment with M100907 prevents tolerance to HTR elicited upon repeated administration of DOI.
Our results show that repeated (4 days) treatment with LSD or DOI reduces by ~50% the HTR induced by the same psychedelic. A similar phenotype (i.e., partial reduction of HTR) has been reported after repeated administration of classical psychedelics such as LSD, 1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane (DOB), and N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenylethylamine (25CN-NBOH).28,29 This, however, contrasts with previous observations in human subjects suggesting that daily administration of psychoactive doses vanishes the hallucinogenic properties of psychedelics such as LSD and mescaline.3–5 A potential explanation for these interspecies discrepancies could rely on dosage, length of the treatment, pharmacokinetic properties, or density of the 5-HT2AR in the frontal cortex.
Similarly, it is worth mentioning that there was substantial variability in the frequency of HTRs across individual experiments with an identical dose of the same psychedelic (see Figure 1C group 1 vs Figure 2B group 1, as an example). A number of intervening factors may underlie this interexperimental variability. The HTR experiments presented in this article span for several years and were conducted in separate locations using different HTR approaches (i.e., magnetic ear tags42 vs neodymium magnet on the skull surface41) and with unrelated cohorts of male C57BL/6 (Jackson) and B6J/B6N (in-house) mice. As mentioned in the Methods section, LSD was obtained from two different commercial sources. Although the effect of age on HTR is not the focus of this study, we have previously reported that HTR declines with age,41 which suggests that aging may be one of the independent factors affecting interexperimental variation throughout. Our previous reports in which all assays were conducted under almost identical experimental parameters ultimately resulted in more homogeneous HTR counts.45,50 However, it is important to remark that all the statistical methods for each experiment presented here correspond to groups of mice selected randomly from cohorts with identical age, strain (C57BL/6 and B6J/B6N), residence time in the vivarium, exposure to the testing room, and washout period between experiments. These cohorts were tested on the same or consecutive days, and with the same HTR reporter system. Consequently, a divergence in HTR counts for independent experiments becomes apparent, but we would like to note that the conclusions are drawn only from homogeneous groups of animals in which genotype and/or treatment were the only experimental variables to consider.
Unlike the human psychedelic experience, mice rely on the motor system to bring the direct effect of psychedelics on the 5-HT2AR into a characteristic side-to-side movement of the head. This interspecies difference in the manifestation of psychedelic action puts forward some considerations. For instance, as a motor event, the high frequency of HTR events triggered by psychedelics could lead to fatigue in the muscles involved in this behavioral response. However, we demonstrate that upon blunting skeletal muscle function via isoflurane anesthesia, the development of tolerance to HTR induced by DOI remained unchanged. Our previous findings suggest that clearance of both DOI41 and M10090751 occurs within hours following intraperitoneal administration, which precludes the possibility of drug carryover after repeated daily administration. Taken together, this supports that the development of tolerance to this classical psychedelic and its prevention by M100907 are linked to local phenomena in the brain neurobiology as opposed to a downstream involvement of the motor system.
Our data here also show that whereas repeated administration of the well-known psychedelic drug LSD resulted in a reduction of DOI-induced HTR, this pattern of cross-tolerance was not induced by previous exposure to the nonpsychedelic 5-HT2AR agonist lisuride. There is evidence that lisuride binds to the 5-HT2AR and activates 5-HT2AR-dependent signaling pathways with a similar efficacy as the psychedelic LSD,20,52 yet it is clearly devoid of any psychedelic effects in human subjects and does not induce HTR in mice. We also show that the number of spontaneous HTR were slightly but significantly reduced by a single lisuride administration, a phenotype that had been suggested in previous studies,53 and that this repressive effect on spontaneous HTR is reduced upon repeated lisuride administration. Additionally, repeated lisuride administration was unable to develop tolerance to LSD- or DOI-induced HTR. Further investigation will be necessary to unravel the molecular target and neural circuit mechanisms responsible for the discordant effects of lisuride vs LSD and DOI on the development of tolerance. However, the narrow comparison established by our current data suggests that cross-tolerance to HTR might be restricted to psychedelic 5-HT2AR agonists.
Psychedelics exert characteristic acute effects in both human subjects and animal models; however, it is the imprinting effects of these drugs that fueled the renewed interest of the scientific community on their potential postacute therapeutic action.14 Mounting evidence in animal models demonstrates that the adaptive plasticity effects responsible for the different potential therapeutic uses of psychedelics might not be restricted to the human species.15–19 Given the chronology of events (i.e., active psychedelic experience preceding the peak of the therapeutic benefit), one open question at the molecular level is whether both phenomena are directly tied to the acute action of the drug, emerge as a compensatory mechanism on the aftermath of exposure to the drug, or both. The development of tolerance supports the hypothesis that the brain undergoes adaptations responsible for such resistance to the drug’s action after the initial exposure−regardless of whether re-exposure to the drug occurs or not. Although the tolerance period is undoubtedly shorter in duration compared to the span of the therapeutic benefit observed in human subjects and rodent models, their co-occurrence in time suggests that the emergence of the latter occurs over the neurobiological adaptations that mediate reduced responsiveness to psychedelic drug action. Arguably, such adaptations involved in the development of tolerance could modulate the expression of therapeutic outcomes or even share cellular substrates. For example, lasting changes in synaptic plasticity linked to the therapeutic-like action of psychedelics emerge shortly after exposure; in parallel, some signature early expression genes linked to 5-HT2AR stimulation by psychedelics appeared transcriptionally active.15 Lasting adaptations in synaptic homeostasis have been linked to the therapeutic potential of psychedelics,14 which ultimately suggests that different postacute effects could share a common mechanistic substrate and impact one another. Since recent observations indicate that repeated LSD reverses stress-induced anxiety-like behavior and cortical synaptic plasticity deficits in mice,54 it will be interesting to expand this concept in future studies to characterize the function of these genes in processes related to synaptic plasticity, as well as the role of psychedelic-induced down-regulation of frontal cortex 5-HT2AR density in these long-lasting therapeutic effects.
In an attempt to pinpoint the molecular drivers responsible for the development of tolerance to HTR induced by psychedelics and concurrent down-regulation of 5-HT2AR, we evaluated the potential involvement of βarr2 recruitment. Arrestins form a complex with phosphorylated GPCRs that lead to the clearing of the receptor from the cell membrane among other downstream actions.26 The 5-HT2AR recruits βarr2 upon stimulation by agonist ligands, and this interaction has been shown to be involved in the HTR elicited by the 5-HT precursor 5-hydroxytryptophan (5-HTP)31 and the psychedelic LSD in mice.32 In our studies, the absence of βarr2 did not impact the effect of DOI on HTR or the tolerance to HTR elicited by repeated DOI administration. Recruitment of alternative arrestins as a compensatory mechanism in a full knockout model like the one we employed could be argued. However, it was previously shown that deletion of βarr2 did not lead to compensatory overexpression of βarr1.55 Beyond βarr1, our β2KO and WT mice counterparts were generated from homozygote breeding from ancestral heterozygote breeders. It is then plausible that our phenotypes following acute and repeated exposure to the psychedelic DOI are the result of adaptations to offset the absence of βarr2 throughout several generations. Further highlighting the complexity inherent to experimental models of arrestin deletion, GPCR and arrestin pharmacology testing outside the serotonergic receptor system has proven to be challenging. Thus, the concept of biased βarr2-dependent agonism had led to disparate outcomes in equivalent models of respiratory depression associated with μ-opioid receptor stimulation.56,57 As it pertains to psychedelics and their actions on 5-HT2AR, the development of tolerance to HTR associated with 5-HT2AR stimulation appears to be in agreement with previous in vitro studies suggesting that down-regulation of 5-HT2AR can occur via mechanisms independent of βarr2 yet dynamin-dependent.58
CONCLUSIONS
Psychedelics produce tolerance and cross-tolerance in human subjects, a phenotype that we reproduced in the rodent HTR model. We demonstrate that, in this behavioral model, the development of tolerance to psychedelic-induced HTR necessarily requires the activation of 5-HT2AR. Furthermore, such tolerance to HTR appears to discriminate psychedelic from nonpsychedelic 5-HT2AR agonists such as lisuride. Given the mounting interest in classical psychedelics as a potential treatment for mental health disorders, tolerance to their action opens an avenue into the exploration of biological adaptations that unfold following repeated exposures.
METHODS
Animals.
Adult (8−20 weeks old) C57BL/6 male mice (Jackson Laboratory), housed in groups of 3−4 animals per cage, had ad libitum access to food and water and were kept under a 12 h light/dark cycle (lights on 6 a.m. to 6 p.m.) in a temperature- and humiditycontrolled facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. The animal use protocol was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Experiments involving β2KO were performed in wild type and β2KO bred in-house from homozygote breeders (~81% B6J and ~19% B6N) received as a gift from Dr. Hamid Akbarali (Virginia Commonwealth University).59 Mouse genotypes were determined by standard PCR of tail DNA. Additionally, the genotypes were confirmed by immunoblots (see below) and RT-qPCR (Transnetyx) (data not shown).
Drugs.
(±)-1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane ((±)-DOI) hydrochloride was purchased from MilliporeSigma. (R)-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperinemethanol (M100907, also known as volinanserin) was obtained from the NIDA Drug Supply Program, and lisuride (N′-[(8α)-9,10-didehydro-6-methylergolin-8-yl]-N,N-diethylurea maleate) was purchased from Tocris. LSD was obtained from two different sources: Lipomed (Figure 5) and Sigma-Aldrich (Figure 6 and Supplementary Figure 3) as a free base. All drugs were administered in saline (0.9% NaCl) as vehicle. In the case of M100907, an equimolecular amount of HCl was added to form the hydrochloride salt in situ. LSD and lisuride stock solutions were made in DMSO and further diluted in saline prior to use. In the case of LSD and lisuride, the vehicle contained an equivalent volume of DMSO (<1% final volume). All drugs were administered intraperitonally (i.p.) at a volume of 5 μL/g.
Quantification of Head-Twitch Responses.
Both magnet implants and magnetic ear tags were employed as HTR reporters.41,42 For experiments assessing tolerance and cross-tolerance between LSD and lisuride (Figure 6), animals were surgically implanted a neodymium magnet (390 mg) on the skull surface as previously reported. All other experiments involving HTR were performed in mice with magnetic ear tags designed for automated HTR detection. Briefly, neodymium magnets bearing-ear tags (~50 mg) were placed bilaterally through the pinna antihelix under ketamine/xylazine anesthesia (120 and 12 mg/kg, respectively) or isoflurane (2%). All animals were housed in groups of 3−5 per cage and allowed to recover for a week prior to testing.
During testing sessions, animals were transferred from their homecage to the testing chamber where they were allowed to habituate to the environment. After 30 min, the corresponding drug or drugs were administered, in the latter case with 5 min between injections. Data acquisition in the magnetometer was performed as previously described.41,42 Some experiments comprised several consecutive days of testing sessions. After an experiment was completed, at least 1 week washout period was allowed between experiments.
Experiments involving use of isoflurane were performed following the same anesthesia protocol described above in addition to supportive care (heating pad, frequent monitoring of respiratory frequency and lack of reflexes, ointment to avoid eye dryness).
Data were processed using a previously described signal analysis protocol.41,42 To refine HTR detection, the signal was also processed using a deep learning-based protocol based on scalograms.60 Mismatches between both detection methods were inspected visually without clues relative to the timestamp of the event or treatment group as previously described.41,42
[3H]Ketanserin Binding.
Mice were sacrificed by cervical dislocation, and their frontal cortex (bregma 1.90−1.40 mm) was harvested as previously described48 and stored at −80 °C until further processing. The brain tissue samples were homogenized using a Teflon glass grinder in 5 mL of binding buffer (50 mM Tris-HCl; pH 7.4) supplemented with 0.25 M sucrose. The volume was made up to 10 mL with binding buffer, and the crude homogenate was centrifuged at 3000 rpm for 5 min at 4 °C. The supernatant was centrifuged at 18,000 rpm for 10 min at 4 °C, and the resultant pellet (P2) was washed with 10 mL of binding buffer and recentrifuged at 18,000 rpm for 15 min. Protein concentration was determined using the BioRad protein estimation assay.
Radioligand binding assays were carried out by incubating membrane preparations in binding buffer containing 5 nM [3H]-ketanserin (PerkinElmer Life and Analytical Sciences) at 37 °C for 60 min. The final volume in each well was 200 μL. Methysergide (10 μM, Tocris Bioscience) was used to determine nonspecific binding. The free ligand was separated from bound ligand by rapid filtration under vacuum through GF/C glass fiber filters using a MicroBeta Filtermat-96 harvester (PerkinElmer). These filters were then rinsed with ice-cold incubation buffer, dried at 65 °C for 1 h, and counted for radioactivity using a MicroBeta2 detector (PerkinElmer).
Immunoblot Assays.
An electric homogenizer was used to homogenize the prefrontal cortex sample (50 ups/downs) in 1 mL Tris-HCl 1× (50 mM Tris-HCl, 0.25 mM sucrose, pH 7.4). Western blot was conducted using 64 μg of total protein extract from mouse frontal cortex samples. Samples were mixed with 5 μL 5× Laemmli buffer and heated for 5 min at 97 °C. Proteins were resolved by 12% SDS-PAGE and transferred to nitrocellulose membranes by electroblotting. Nitrocellulose blots were washed with TBST (150 mM NaCl, 50 mM Tris-Cl, and 0.1% Tween 20, pH 7.6) before incubating the membrane in blocking buffer (TBST, 2.5% nonfat dry milk, 0.5% BSA) for 1 h at room temperature. Nitrocellulose blots were then incubated (overnight at 4 °C) with anti-β-arrestin-2 antibody (H-9) (mouse) (diluted 1:1000; Santa Cruz Biotechnology; sc-13140). Following six washes with TBST, blots were incubated with antimouse IgG horseradish peroxidase conjugated secondary Ab (1:5000; ACCURATE Chemical Science Corp.) After incubation, blots were washed six more times with TBST for 30 min and developed using enhanced chemiluminescence detection (SuperSignal West Pico PLUS Chemiluminescent Substrate; Thermo Fischer Scientific) in accordance with manufacture instructions. Immunoreactive bands were analyzed using ChemiDoc Imaging System and Image Lab (BioRad Laboratories).
Statistical Analysis.
Animals were randomly allocated into the different experimental groups. Data points were excluded based on previously established criterion and were set to ±2 standard deviation from the group mean. Statistical significance of experiments involving two or more groups and two or more treatments was assessed by two-way ANOVA followed by Bonferroni’s posthoc test. Statistical significance of experiments involving time courses and two or more treatments/genotypes was assessed by repeated-measures two-way ANOVA followed by Bonferroni’s posthoc test. Statistical significance involving three or more groups was assessed by one-way ANOVA followed by Bonferroni’s posthoc test. Statistical significance of experiments involving two groups was assessed by Student’s t test. Statistical analysis was performed with GraphPad Prism software version 9 (La Jolla, CA). The level of significance was set at p = 0.05. All values in the figure legends represent mean ± standard error of the mean.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Hamid Akbarali (Virginia Commonwealth University) for the donation of β-arrestin-2 knockout mice. This work was supported in part by the National Institutes of Health (NIH) grants R01MH084894 (J.G.-M.), R01MH111940 (J.G.-M.), and T32MH020030 (M.d.l.F.R.).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.2c00170.
Effect of M100907 on tolerance to HTR induced by DOI; immunoblot image in frontal cortex samples of WT and β2KO; time courses of HTR elicited by LSD and/or lisuride; posthoc analysis corresponding to supplementary Figure 1B (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acschemneuro.2c00170
The authors declare the following competing financial interest(s): J.G.-M. has a sponsored research contract with NeuRistic, and M.d.l.F.R. has a consulting agreement with Noetic Fund. The remaining authors declare no conflict of interests.
Contributor Information
Mario de la Fuente Revenga, Department of Physiology & Biophysics, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States; Virginia Institute of Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, Virginia 23298, United States.
Alaina M. Jaster, Department of Physiology & Biophysics and Department of Pharmacology & Toxicology, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States
John McGinn, Department of Physiology & Biophysics, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States.
Gabriella Silva, Department of Physiology & Biophysics, Virginia Commonwealth University School of Medicine,Richmond, Virginia 23298, United States;.
Somdatta Saha, Department of Physiology & Biophysics, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States.
Javier González-Maeso, Department of Physiology & Biophysics, Virginia Commonwealth University School of Medicine, Richmond, Virginia 23298, United States;.
REFERENCES
- (1).Glennon RA Classical hallucinogens: an introductory overview. NIDA Res. Monogr 1994, 146, 4–32. [PubMed] [Google Scholar]
- (2).Nichols DE Psychedelics. Pharmacol Rev. 2016, 68, 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cholden LS; Kurland A; Savage C Clinical reactions and tolerance to LSD in chronic schizophrenia. J. Nerv Ment Dis 1955, 122, 211–221. [DOI] [PubMed] [Google Scholar]
- (4).Abramson HA; Sklarofsky B; Baron MO; Fremont-Smith N Production of tolerance to psychosis-producing doses of lysergic acid diethylamide. Science 1957, 126, 1020. [DOI] [PubMed] [Google Scholar]
- (5).Abramson HA; Jarvik ME; Gorin MH; Hirsch MW Lysergic Acid Diethylamide (LSD-25): XVII. Tolerance Development and its Relationship to a Theory of Psychosis. Journal of psychology 1956, 41, 81–105. [Google Scholar]
- (6).Freedman DX; Aghajanian GK; Ornitz EM; Rosner BS Patterns of tolerance to lysergic acid diethylamide and mescaline in rats. Science 1958, 127, 1173–1174. [DOI] [PubMed] [Google Scholar]
- (7).Balestrieri A; Fontanari D Acquired and crossed tolerance to mescaline, LSD-25, and BOL-148. AMA Arch Gen Psychiatry 1959, 1, 279–282. [DOI] [PubMed] [Google Scholar]
- (8).Isbell H; Wolbach AB; Wikler A; Miner EJ Cross tolerance between LSD and psilocybin. Psychopharmacologia 1961, 2, 147–159. [DOI] [PubMed] [Google Scholar]
- (9).Vollenweider FX; Vollenweider-Scherpenhuyzen MF; Babler A; Vogel H; Hell D Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998, 9, 3897–3902. [DOI] [PubMed] [Google Scholar]
- (10).Schmid Y; et al. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol. Psychiatry 2015, 78, 544–553. [DOI] [PubMed] [Google Scholar]
- (11).Carhart-Harris R; et al. Trial of Psilocybin versus Escitalopram for Depression. N Engl J. Med 2021, 384, 1402–1411. [DOI] [PubMed] [Google Scholar]
- (12).Davis AK; Barrett FS; May DG; Cosimano MP; Sepeda ND; Johnson MW; Finan PH; Griffiths RR Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Krishnan V; Nestler EJ The molecular neurobiology of depression. Nature 2008, 455, 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Jaster AM; de la Fuente Revenga M; Gonzalez-Maeso J Molecular targets of psychedelic-induced plasticity. J. Neurochem 2022, 162, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).de la Fuente Revenga M; et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep 2021, 37, 109836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hesselgrave N; Troppoli TA; Wulff AB; Cole AB; Thompson SM Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc. Natl. Acad. Sci. U. S. A 2021, 118, e2022489118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Shao LX; Liao C; Gregg I; Davoudian PA; Savalia NK; Delagarza K; Kwan AC Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021, 109, 2535–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Ly C; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep 2018, 23, 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hibicke M; Landry AN; Kramer HM; Talman ZK; Nichols CD Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem. Neurosci 2020, 11, 864–871. [DOI] [PubMed] [Google Scholar]
- (20).Gonzalez-Maeso J; et al. Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 2007, 53, 439–452. [DOI] [PubMed] [Google Scholar]
- (21).Hanks JB; Gonzalez-Maeso J Animal models of serotonergic psychedelics. ACS Chem. Neurosci 2013, 4, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).McCorvy JD; Roth BL Structure and function of serotonin G protein-coupled receptors. Pharmacol Ther 2015, 150, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ferguson SS Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev 2001, 53, 1–24. [PubMed] [Google Scholar]
- (24).Benovic JL; Kuhn H; Weyand I; Codina J; Caron MG; Lefkowitz RJ Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc. Natl. Acad. Sci. U. S. A 1987, 84, 8879–8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Luttrell LM; et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 1999, 283, 655–661. [DOI] [PubMed] [Google Scholar]
- (26).Lefkowitz RJ; Shenoy SK Transduction of receptor signals by beta-arrestins. Science 2005, 308, 512–517. [DOI] [PubMed] [Google Scholar]
- (27).Halberstadt AL; Chatha M; Klein AK; Wallach J; Brandt SD Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 2020, 167, 107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Buchborn T; Lyons T; Knopfel T Tolerance and Tachyphylaxis to Head Twitches Induced by the 5-HT2A Agonist 25CN-NBOH in Mice. Front Pharmacol 2018, 9, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Buchborn T; Schroder H; Dieterich DC; Grecksch G; Hollt V Tolerance to LSD and DOB induced shaking behaviour: differential adaptations of frontocortical 5-HT(2A) and glutamate receptor binding sites. Behav Brain Res. 2015, 281, 62–68. [DOI] [PubMed] [Google Scholar]
- (30).Smith DA; Bailey JM; Williams D; Fantegrossi WE Tolerance and cross-tolerance to head twitch behavior elicited by phenethylamine- and tryptamine-derived hallucinogens in mice. J. Pharmacol Exp Ther 2014, 351, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Schmid CL; Raehal KM; Bohn LM Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Rodriguiz RM; Nadkarni V; Means CR; Pogorelov VM; Chiu YT; Roth BL; Wetsel WC LSD-stimulated behaviors in mice require beta-arrestin 2 but not beta-arrestin 1. Sci. Rep 2021, 11, 17690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Canal CE; Morgan D Head-twitch response in rodents induced by the hallucinogen 2, 5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Testing and Analysis 2012, 4, 556–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Nau F Jr.; Miller J; Saravia J; Ahlert T; Yu B; Happel KI; Cormier SA; Nichols CD Serotonin 5-HT(2) receptor activation prevents allergic asthma in a mouse model. Am. J. Physiol Lung Cell Mol. Physiol 2015, 308, L191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Gresch PJ; Smith RL; Barrett RJ; Sanders-Bush E Behavioral tolerance to lysergic acid diethylamide is associated with reduced serotonin-2A receptor signaling in rat cortex. Neuropsychopharmacology 2005, 30, 1693–1702. [DOI] [PubMed] [Google Scholar]
- (36).Buckholtz NS; Freedman DX; Middaugh LD Daily LSD administration selectively decreases serotonin2 receptor binding in rat brain. Eur. J. Pharmacol 1985, 109, 421–425. [DOI] [PubMed] [Google Scholar]
- (37).Buckholtz NS; Zhou DF; Freedman DX; Potter WZ Lysergic acid diethylamide (LSD) administration selectively downregulates serotonin2 receptors in rat brain. Neuropsychopharmacology 1990, 3, 137–148. [PubMed] [Google Scholar]
- (38).McKenna DJ; Nazarali AJ; Himeno A; Saavedra JM Chronic treatment with (±)DOI, a psychotomimetic 5-HT2 agonist, downregulates 5-HT2 receptors in rat brain. Neuropsychopharmacology 1989, 2, 81–87. [DOI] [PubMed] [Google Scholar]
- (39).Buckholtz NS; Zhou DF; Freedman DX Serotonin2 agonist administration down-regulates rat brain serotonin2 receptors. Life Sci. 1988, 42, 2439–2445. [DOI] [PubMed] [Google Scholar]
- (40).Vollenweider FX; Leenders KL; Scharfetter C; Maguire P; Stadelmann O; Angst J Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 1997, 16, 357–372. [DOI] [PubMed] [Google Scholar]
- (41).de la Fuente Revenga M; Shin JM; Vohra HZ; Hideshima KS; Schneck M; Poklis JL; Gonzalez-Maeso J Fully automated head-twitch detection system for the study of 5-HT2A receptor pharmacology in vivo. Sci. Rep 2019, 9, 14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).de la Fuente Revenga M; Vohra HZ; Gonzalez-Maeso J Automated quantification of head-twitch response in mice via ear tag reporter coupled with biphasic detection. J. Neurosci Methods 2020, 334, 108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Casey AB; Cui M; Booth RG; Canal CE ″Selective″ serotonin 5-HT2A receptor antagonists. Biochem. Pharmacol 2022, 200, 115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Raval NR; Johansen A; Donovan LL; Ros NF; Ozenne B; Hansen HD; Knudsen GM A Single Dose of Psilocybin Increases Synaptic Density and Decreases 5-HT2A Receptor Density in the Pig Brain. Int. J. Mol. Sci 2021, 22, 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Jaster AM; Elder H; Marsh SA; de la Fuente Revenga M; Negus SS; Gonzalez-Maeso J Effects of the 5-HT2A receptor antagonist volinanserin on head-twitch response and intracranial selfstimulation depression induced by different structural classes of psychedelics in rodents. Psychopharmacology (Berl) 2022, 239, 1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Meltzer HY Update on typical and atypical antipsychotic drugs. Annu. Rev. Med 2013, 64, 393–406. [DOI] [PubMed] [Google Scholar]
- (47).Raote I; Bhattacharyya S; Panicker MM Functional Selectivity in Serotonin Receptor 2A (5-HT2A) Endocytosis, Recycling, and Phosphorylation. Mol. Pharmacol 2013, 83, 42–50. [DOI] [PubMed] [Google Scholar]
- (48).Moreno JL; Holloway T; Umali A; Rayannavar V; Sealfon SC; Gonzalez-Maeso J Persistent effects of chronic clozapine on the cellular and behavioral responses to LSD in mice. Psychopharmacology (Berl) 2013, 225, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Toneatti R; Shin JM; Shah UH; Mayer CR; Saunders JM; Fribourg M; Arsenovic PT; Janssen WG; Sealfon SC; Lopez-Gimenez JF; Benson DL; Conway DE; Gonzalez-Maeso J Interclass GPCR heteromerization affects localization and trafficking. Sci. Signal 2020, 13, eaaw3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).de la Fuente Revenga M; Shah UH; Nassehi N; Jaster AM; Hemanth P; Sierra S; Dukat M; Gonzalez-Maeso J Psychedelic-like Properties of Quipazine and Its Structural Analogues in Mice. ACS Chem. Neurosci 2021, 12, 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Sierra S; et al. Sex-specific role for serotonin 5-HT2A receptor in modulation of opioid-induced antinociception and reward in mice. Neuropharmacology 2022, 209, 108988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Cao D; et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 2022, 375, 403–411. [DOI] [PubMed] [Google Scholar]
- (53).Halberstadt AL; Geyer MA Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl) 2013, 227, 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).De Gregorio D; Inserra A; Enns JP; Markopoulos A; Pileggi M; El Rahimy Y; Lopez-Canul M; Comai S; Gobbi G Repeated lysergic acid diethylamide (LSD) reverses stress-induced anxiety-like behavior, cortical synaptogenesis deficits and serotonergic neurotransmission decline. Neuropsychopharmacology 2022, 47, 1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Fang Y; et al. Opposing functions of beta-arrestin 1 and 2 in Parkinson’s disease via microglia inflammation and Nprl3. Cell Death Differ. 2021, 28, 1822–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Raehal KM; Walker JK; Bohn LM Morphine side effects in beta-arrestin 2 knockout mice. J. Pharmacol Exp Ther 2005, 314, 1195–1201. [DOI] [PubMed] [Google Scholar]
- (57).Kliewer A; Gillis A; Hill R; Schmiedel F; Bailey C; Kelly E; Henderson G; Christie MJ; Schulz S Morphine-induced respiratory depression is independent of beta-arrestin2 signalling. Br. J. Pharmacol 2020, 177, 2923–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Bhatnagar A; Willins DL; Gray JA; Woods J; Benovic JL; Roth BL The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J. Biol. Chem 2001, 276, 8269–8277. [DOI] [PubMed] [Google Scholar]
- (59).Muchhala KH; Jacob JC; Dewey WL; Akbarali HI Role of beta-arrestin-2 in short- and long-term opioid tolerance in the dorsal root ganglia. Eur. J. Pharmacol 2021, 899, 174007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Halberstadt AL Automated detection of the head-twitch response using wavelet scalograms and a deep convolutional neural network. Sci. Rep 2020, 10, 8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


