Abstract
Background:
Breast cancer incidence rates in women of Asian descent have been increasing in the United States (U.S.) and Asia.
Methods:
In a case-control study of Asian American women from the San Francisco Bay Area, we assessed associations with birthplace and migration-related characteristics and compared risk factors between Asian American and non-Hispanic White women by birthplace and birth cohort.
Results:
Birthplace and migration-related characteristics were associated with breast cancer risk only among women in the younger birth cohort (1951–1984) that comprised 355 cases diagnosed at age ≤55 years and 276 sister and population controls. Breast cancer risk was marginally increased among foreign-born women (OR=1.40, 95% CI=0.97–2.03) and two-fold among foreign-born Chinese women (OR=2.16, 95% CI=1.21–3.88). Two-fold increased risks were associated with migration at age ≥40 years and longer U.S. residence (≥30 years or ≥75% of life). The education level was high among both cases and controls. Differences in the prevalence of risk factors by birthplace and birth cohort suggest temporal changes in reproductive and lifestyle-related factors. The prevalence in risk factors was similar between foreign-born and U.S.-born women in the younger birth cohort, and did not fully explain the observed associations with birthplace and other migration characteristics.
Conclusions:
In contrast to studies from earlier decades, younger foreign-born Asian American women had a higher risk of breast cancer than U.S.-born Asian American women.
Impact:
It is important and urgent to understand what factors drive the increasing burden of breast cancer in women of Asian descent and implement effective prevention programs.
Keywords: Asian American, birthplace, birth cohort, breast cancer, risk factors
Introduction
Incidence rates of female breast cancer have historically been lower in Asian countries than in the United States (U.S.) (1). Over the last several decades, the incidence rates have increased rapidly in East and Southeast Asia (2,3), and in some East Asian countries incidence rates in younger birth cohorts are now higher than in U.S. White women (4). Rapidly rising incidence rates have also been observed in Asian American women in the U.S. (5–7) and California (8,9), and they have surpassed those of Hispanic and American Indian/Alaska Native women (10). Incidence rates vary by Asian ethnicity (7,8), and changes in incidence are distinct for specific Asian ethnic groups (5,7,9,11–13), likely reflecting their migration history and differences in their risk factor profiles.
In women migrating from low to high incidence countries, the incidence of breast cancer generally increases over successive generations, approaching the incidence rate of the population in the country of immigration (14–18). In 1973–1986, incidence rates in the U.S. were higher among U.S.-born Chinese and Japanese women compared to their foreign-born counterparts, whereas incidence rates were similar among U.S.-born and foreign-born Filipina women (19). In 1988–2004, incidence rates in California were higher among U.S.-born Chinese and Filipina women than their foreign-born counterparts, whereas no difference was seen between U.S.-born and foreign-born Japanese women (8). These cancer-registry based findings are consistent with a case-control study of Asian American women aged 20–55 years diagnosed from 1983–1987 that found a 60% higher risk of breast cancer among women born in the U.S. or other Western countries compared to their counterparts born in Asia (14). In contrast, a small case-control study recently reported 2- to 3-fold higher breast cancer risks among foreign-born Asian American women compared to their U.S.-born counterparts (20).
To further investigate the unexpected findings by Morey et al. (20), we conducted a case-control analysis in Asian American women enrolled in the Northern California Breast Cancer Family Registry (NC-BCFR) and examined associations with birthplace and migration characteristics overall and by birth cohort. Additionally, we compared risk factor profiles between Asian American and non-Hispanic White (NHW) women enrolled in NC-BCFR.
Materials and Methods
Study Sample
NC-BCFR enrolled women aged 18–64 years, newly diagnosed with breast cancer between 1995 and 2009, ascertained through the Greater Bay Area Cancer Registry (21,22). Eligible cases included those with a diagnosis before age 35 years, prior ovarian or childhood cancer, bilateral breast cancer with a first diagnosis before age 50 years, and/or a first-degree family history of breast, ovarian, or childhood cancer. Cases aged 35–64 years not meeting any of these criteria were randomly sampled (2.5% of NHWs and 33% of other racial and ethnic groups). Cases diagnosed with triple negative breast cancer from 2007–2009 were also eligible to enroll. Most Asian American cases were diagnosed from 1995–2003. Cases completed a detailed cancer family history questionnaire that enumerated all first-degree relatives and their cancer history. With the cases’ permission, adult relatives were invited to enroll in the study. Population controls were identified through random digit dialing and frequency-matched to cases diagnosed from 1995–1998 on race and ethnicity and 5-year age group, at a control-to-case ratio of 1 to 2. The study was approved by the Institutional Review Board of the Cancer Prevention Institute of California and Stanford University. Study participants provided written informed consent.
The present case-control study was based on 744 Asian American cases with a first primary invasive breast cancer and 462 Asian American controls [294 sisters, 83 unrelated NC-BCFR participants (i.e., sisters of ineligible cases), 85 population controls] aged <65 years at the baseline interview and never diagnosed with breast cancer. Participation rates were 71% for cases, 70% for sisters and unrelated participants, and 80% for population controls. For comparisons of risk factors, we also included 937 U.S.-born NHW controls aged <65 years at baseline interview (584 sisters, 353 population controls).
Data Collection
Participants completed a structured questionnaire administered by bilingual trained interviewers in English, Cantonese or Mandarin in home visits (cases and sisters who lived in the San Francisco Bay Area) or telephone interviews (all other sisters and population controls). The questionnaire asked about self-identified race and ethnicity, education, migration history (participant’s, parents’ and grandparents’ birthplace, year of U.S. immigration, duration of residence in the U.S.), reproductive history, weight, height, lifestyle factors, and medical history. Information on risk factors was collected up to the reference year (i.e., calendar year before diagnosis for casesor baseline interview for sisters and population controls). Moderate and strenuous recreational activities were assessed at ages 12–17, 18–24, 25–34, 35–44, 45–54, and ≥55 years. For each age interval and each type of activity (moderate, strenuous), duration of activity in hours/week (0.5, 1, 1.5, 2, 3, 4–6, 7–10, ≥11) and number of months/year (1–3, 4–6, 7–9, 10–12) were assessed (23). A 108-item food frequency questionnaire developed for the Multiethnic Cohort (24) was used to assess the frequency of usual consumption (never or hardly ever, once/month, 2–3 times/month, once/week, 2–3 times/week, 4–6 times/week, once/day, ≥2 times/day) and portion size (3 categories) during the reference year (25).
Analytic Variables
Analytic variables included birthplace (U.S.-born, foreign-born), generational status (U.S.-born second generation, U.S.-born first generation, foreign-born), age at migration (<10, 10–19, 20–29, 30–39, ≥40 years), duration of U.S. residence (<10, 10–19, 20–29, ≥30 years), percent of life in the U.S. (<25%, 25–49%, 50–74%, ≥75%), and language at interview (English, Chinese). Lifetime breast-feeding was calculated by summing across all live births the duration of breast-feeding reported as a categorical measure (0, <1, 1–5, 6–11, 12–24, ≥25 months), whereby the midpoint of each category was assigned (0.5 month was assigned to <1 month; 30 months was assigned to ≥25 months). Menopausal status was defined as premenopausal (still menstruating during the reference year, and under age 55 years), postmenopausal (periods had stopped prior to the reference year either naturally or due to surgery, medical treatment, or other reasons, or age ≥55 years), or unknown. Body mass index (BMI) was calculated as self-reported weight (kg) in the reference year divided by self-reported height (m) squared and was classified as <23, 23–27.4, or ≥27.5 kg/m2 using cut-points suggested for Asian populations (26). For recreational physical activity, hours/week were assigned the midpoint (15 hours were assigned to ≥11 hours/week), pro-rated by months/year, and weighted by the years in each age interval (up to age at diagnosis/interview). Lifetime average hours/week were calculated and converted into average lifetime metabolic equivalent (MET) hours/week by assigning MET values of 4.5 and 6.5, respectively, to moderate and strenuous activities (27). Met values were then combined to obtain average MET-hours/week of recreational activity.
Statistical Analysis
The analyses were based on 728 cases and 454 controls (287 sisters, 82 unrelated controls, 85 population controls) after excluding 24 individuals with missing covariate data. We fit logistic regression models and calculated odds ratios (OR) and 95% confidence intervals (CI) with cluster robust standard errors to account for potential correlations among sisters. We assessed associations with migration-related characteristics, adjusting for age at diagnosis (cases) or interview (controls), education (high school graduate or less, some college or technical school, college graduate or higher degree), lifetime breast-feeding (nulliparous, 0, ≤12, >12 months), oral contraceptive use (never, ever), and average lifetime recreational physical activity (tertiles of MET-hours among controls), and a composite BMI/menopausal status variable (premenopausal <23.0, 23.0–27.4, ≥27.5 kg/m2, postmenopausal). We did not adjust for breast cancer family history because all sisters and unrelated controls had a family history. We also performed analyses limiting the controls to sisters only or unrelated controls only. Given the possibility that risk factor profiles change over successive birth cohorts, we stratified the analyses by birth cohort (1931–1950, 1951–1984) and tested for heterogeneity by including interaction terms in the model. To assess differences in established risk factors and isoflavone and green tea intake, which have been associated with lower breast cancer risk among Asian American women (28–31), we compared the prevalence between Asian American and NHW controls, by birthplace and birth cohort. We tested for differences in risk factor prevalence using chi-square statistics or Fisher’s exact test.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request and with appropriate IRB approval.
Results
The majority of Asian American cases (80%) and controls (78%) self-identified as Chinese or Filipina, 9% of cases were diagnosed at age <35 years, and 14% had a first-degree family history of breast cancer (Table 1). High proportions of cases (57%) and controls (58%) had a college or higher degree. Compared to controls, cases were less likely to have histories of breast-feeding (68% vs. 77%), oral contraceptive use (47% vs. 57%), high physical activity (26% vs. 34%) and alcohol consumption (8% vs. 19%). Distributions of age at menarche, nulliparity, number of full-term pregnancies (FTP), age at first full-term pregnancy (FFTP), obesity, and histories of menopausal hormone use, benign breast disease or smoking did not differ between cases and controls. Among cases, we saw notable differences by birth cohort for some factors, with a greater prevalence of higher education (65% vs. 49%) and FFTP at age ≥30 years (45% vs. 28%) in the younger birth cohort.
Table 1.
Characteristics of study sample, by case-control status and birth cohort
| All women | Birth Cohort 1931–1950 Cases diagnosed at age 44–64 y |
Birth Cohort 1951–1984 Cases diagnosed at age 23–55 y |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases N=728 |
Controls 1 N=454 |
Chi square P value |
Cases N=373 |
Controls N=178 |
Chi square P value |
Cases N=355 |
Controls N=276 |
Chi square P value |
|||||||
| N | % | N | % | ||||||||||||
| Year of diagnosis | |||||||||||||||
| 1995–1999 | 269 | 37 | 85 | 19 | 149 | 40 | 40 | 22 | 120 | 34 | 45 | 16 | |||
| 2000–2004 | 432 | 59 | 308 | 68 | 217 | 58 | 116 | 65 | 215 | 61 | 192 | 70 | |||
| 2005–2009 | 27 | 4 | 51 | 11 | 7 | 2 | 20 | 11 | 20 | 6 | 31 | 11 | |||
| 2010–2011 | 0 | 0 | 10 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 8 | 3 | |||
| Age at diagnosis or interview (years) | |||||||||||||||
| <35 | 67 | 9 | 48 | 11 | 0 | 0 | 0 | 0 | 67 | 19 | 48 | 17 | |||
| 35–44 | 175 | 24 | 114 | 25 | 3 | 1 | 0 | 0 | 172 | 48 | 119 | 43 | |||
| 45–54 | 262 | 36 | 184 | 41 | 149 | 40 | 73 | 41 | 113 | 32 | 128 | 46 | |||
| 55–64 | 224 | 31 | 108 | 24 | 221 | 59 | 105 | 59 | 3 | 1 | 72 | 26 | |||
| Asian ethnicity | |||||||||||||||
| Chinese | 340 | 47 | 159 | 35 | 158 | 42 | 55 | 31 | 182 | 51 | 104 | 38 | |||
| Filipina | 242 | 33 | 196 | 43 | 140 | 38 | 75 | 42 | 102 | 29 | 121 | 44 | |||
| Japanese | 85 | 12 | 60 | 13 | 49 | 13 | 34 | 19 | 36 | 10 | 26 | 9 | |||
| Other Asian | 61 | 8 | 39 | 9 | 26 | 7 | 14 | 8 | 35 | 10 | 25 | 9 | |||
| First-degree family history of breast cancer 2 | |||||||||||||||
| Yes | 102 | 14 | 73 | 20 | 29 | 8 | |||||||||
| No | 626 | 86 | 300 | 80 | 326 | 92 | |||||||||
| Education | 0.56 | 0.03 | 0.09 | ||||||||||||
| High school or less | 121 | 17 | 65 | 14 | 83 | 22 | 25 | 14 | 38 | 11 | 40 | 14 | |||
| Some college or technical school | 192 | 26 | 125 | 28 | 107 | 29 | 46 | 26 | 85 | 24 | 79 | 29 | |||
| College graduate or higher degree | 415 | 57 | 264 | 58 | 183 | 49 | 107 | 60 | 232 | 65 | 157 | 57 | |||
| Personal history of benign breast disease | 0.84 | 0.82 | 0.87 | ||||||||||||
| No | 624 | 86 | 391 | 86 | 313 | 84 | 148 | 83 | 311 | 88 | 243 | 88 | |||
| Yes | 104 | 14 | 63 | 14 | 60 | 16 | 30 | 17 | 44 | 12 | 33 | 12 | |||
| Age at menarche (years) 3 | 0.74 | 0.08 | 0.50 | ||||||||||||
| <12 | 135 | 19 | 87 | 19 | 59 | 16 | 29 | 21 | 76 | 21 | 48 | 17 | |||
| 12 | 171 | 24 | 120 | 26 | 71 | 19 | 38 | 28 | 100 | 28 | 75 | 27 | |||
| 13 | 201 | 28 | 120 | 26 | 105 | 28 | 35 | 26 | 96 | 27 | 80 | 29 | |||
| ≥14 | 210 | 29 | 126 | 28 | 130 | 35 | 32 | 24 | 80 | 23 | 72 | 26 | |||
| Parity (number full-term pregnancies) | 0.91 | 0.96 | 0.10 | ||||||||||||
| Nulliparous | 191 | 26 | 118 | 26 | 77 | 21 | 35 | 20 | 114 | 32 | 83 | 30 | |||
| 1 | 128 | 18 | 72 | 16 | 50 | 13 | 25 | 14 | 78 | 22 | 47 | 17 | |||
| 2 | 246 | 34 | 154 | 34 | 126 | 34 | 62 | 35 | 120 | 34 | 92 | 33 | |||
| 3 | 110 | 15 | 76 | 17 | 77 | 21 | 33 | 19 | 33 | 9 | 43 | 16 | |||
| ≥4 | 53 | 7 | 34 | 7 | 43 | 12 | 23 | 13 | 10 | 3 | 11 | 4 | |||
| Age at first full-term pregnancy (years), parous women | 0.39 | 0.30 | 0.35 | ||||||||||||
| <25 | 141 | 26 | 93 | 28 | 101 | 34 | 50 | 35 | 40 | 17 | 43 | 22 | |||
| 25–29 | 204 | 38 | 129 | 38 | 112 | 38 | 54 | 38 | 92 | 38 | 75 | 39 | |||
| 30–34 | 126 | 23 | 85 | 25 | 54 | 18 | 32 | 22 | 72 | 30 | 53 | 27 | |||
| ≥35 | 66 | 12 | 29 | 9 | 29 | 10 | 7 | 5 | 37 | 15 | 22 | 11 | |||
| Lifetime breast-feeding duration (months), parous women | 0.01 | 0.17 | 0.10 | ||||||||||||
| Never | 171 | 32 | 78 | 23 | 109 | 37 | 40 | 28 | 62 | 26 | 38 | 20 | |||
| ≤12 | 255 | 47 | 168 | 50 | 121 | 41 | 64 | 45 | 134 | 56 | 104 | 54 | |||
| >12 | 111 | 21 | 90 | 27 | 66 | 22 | 39 | 27 | 45 | 19 | 51 | 26 | |||
| History of oral contraceptive use | <0.01 | <0.01 4 | 0.18 | ||||||||||||
| Never | 386 | 53 | 193 | 43 | 208 | 56 | 70 | 39 | 178 | 50 | 123 | 45 | |||
| Former | 311 | 43 | 242 | 53 | 163 | 44 | 107 | 60 | 148 | 42 | 135 | 49 | |||
| Current | 31 | 4 | 19 | 4 | 2 | 1 | 1 | 1 | 29 | 8 | 18 | 7 | |||
| Menopausal status | 0.05 | 0.51 | 0.32 | ||||||||||||
| Premenopausal | 395 | 54 | 273 | 60 | 76 | 20 | 32 | 18 | 319 | 90 | 241 | 87 | |||
| Postmenopausal | 333 | 46 | 181 | 40 | 297 | 80 | 146 | 82 | 36 | 10 | 35 | 13 | |||
| Menopausal hormone therapy (MHT) use, postmenopausal women 5 | 0.15 | 0.13 | 0.12 4 | ||||||||||||
| No MHT use | 169 | 51 | 83 | 46 | 142 | 48 | 58 | 40 | 27 | 75 | 25 | 71 | |||
| Former use | 90 | 27 | 45 | 25 | 83 | 28 | 41 | 28 | 7 | 19 | 4 | 11 | |||
| Current use | 71 | 21 | 53 | 29 | 70 | 24 | 47 | 32 | 1 | 3 | 6 | 17 | |||
| Body mass index (kg/m2) 6 premenopausal women | 0.11 | 0.32 | 0.14 | ||||||||||||
| <23 | 227 | 57 | 136 | 50 | 40 | 53 | 12 | 38 | 187 | 59 | 124 | 51 | |||
| 23.0–27.4 | 128 | 32 | 99 | 36 | 27 | 36 | 16 | 50 | 101 | 32 | 83 | 34 | |||
| ≥27.5 | 40 | 10 | 38 | 14 | 9 | 12 | 4 | 13 | 31 | 10 | 34 | 14 | |||
| Body mass index (kg/m2) 6 postmenopausal women | 0.74 | 0.97 | 0.38 | ||||||||||||
| <23 | 137 | 41 | 77 | 43 | 123 | 41 | 60 | 41 | 14 | 39 | 17 | 49 | |||
| 23.0–27.4 | 138 | 41 | 69 | 38 | 122 | 41 | 59 | 40 | 16 | 44 | 10 | 29 | |||
| ≥27.5 | 58 | 17 | 35 | 19 | 52 | 18 | 27 | 18 | 6 | 17 | 8 | 23 | |||
| Average lifetime recreational moderate or strenuous physical activity (MET-hours/ week) 7 | <0.01 | <0.01 | 0.07 | ||||||||||||
| <13.7 | 317 | 44 | 149 | 33 | 175 | 47 | 62 | 35 | 142 | 40 | 87 | 32 | |||
| 13.7–29.5 | 224 | 31 | 152 | 33 | 121 | 32 | 55 | 31 | 103 | 29 | 97 | 35 | |||
| ≥29.6 | 187 | 26 | 153 | 34 | 77 | 21 | 61 | 34 | 110 | 31 | 92 | 33 | |||
| History of alcohol consumption 8 | <0.01 | <0.01 | <0.01 | ||||||||||||
| Never | 667 | 92 | 370 | 81 | 346 | 93 | 142 | 80 | 321 | 90 | 228 | 83 | |||
| Former | 8 | 1 | 22 | 5 | 4 | 1 | 10 | 6 | 4 | 1 | 12 | 4 | |||
| Current | 50 | 7 | 62 | 14 | 22 | 6 | 26 | 15 | 28 | 8 | 36 | 13 | |||
| History of cigarette smoking 9 | 0.15 | 0.14 | 0.60 | ||||||||||||
| Never | 646 | 89 | 386 | 85 | 338 | 91 | 152 | 85 | 308 | 87 | 234 | 85 | |||
| Former | 50 | 7 | 41 | 9 | 24 | 6 | 20 | 11 | 26 | 7 | 21 | 8 | |||
| Current | 31 | 4 | 27 | 6 | 11 | 3 | 6 | 3 | 20 | 6 | 21 | 8 | |||
Includes 287 sister controls, 82 unrelated controls who are the sisters of ineligible cases, and 85 population controls.
Only shown for cases since all sister controls and unrelated controls had a family history of breast cancer.
Excludes 11 cases and 1 control with missing data on age at menarche.
Fisher’s exact test
Excludes 3 cases with missing data on menopausal hormone therapy use.
In reference year.
Between age 12 years and reference age.
Alcohol consumption was defined as ever consuming alcohol at least once a week for 6 months or longer. Excludes 3 cases with missing data on alcohol consumption.
Cigarette smoking was defined as ever smoking at least 1 cigarette a day for 3 months or longer. Excludes 1 case with missing data on cigarette smoking.
Migration-related characteristics were associated with breast cancer risk, with some attenuation of OR estimates after multivariable adjustment (Table 2). Compared to U.S.-born Asian American women, foreign-born women overall had a higher risk of breast cancer (OR=1.33, 95% CI=1.01–1.74). Risk was increased two-fold among foreign-born Chinese women (OR=1.94, 95% CI=1.25–3.00), but was not significantly elevated among Filipina or other Asian American women. Generational status was not associated with risk. There was a trend of increasing risk with older age at migration (Ptrend <0.01), with the highest risk associated with migration at age ≥40 years (OR=2.09, 95% CI=1.29–3.38). We found no effect modification of migration age by years of U.S. residence (Pinteraction=0.84). Risk was highest among immigrant women who lived in the U.S. for ≥30 years (OR=1.73, 95% CI=1.22–2.47), but there was no significant trend with increasing years of U.S. residence (Ptrend=0.20) or percent of life spent in the U.S. (Ptrend=0.08). Risk was increased three-fold among immigrant women who completed the interview in Chinese (OR=3.49, 95% CI=1.97–6.17).
Table 2.
Migration history, language at interview, and breast cancer risk among Asian American women
| Cases N=728 |
All controls 1 N=454 |
Age-adjusted OR (95% CI) |
All controls Multivariable- adjusted OR (95% CI) 2 |
Sister controls N=287 Multivariable- adjusted OR (95% CI) 2 |
Unrelated controls 3 N=167 Multivariable- adjusted OR (95% CI) 2 |
|||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Birthplace | ||||||||
| U.S.-born 4 | 153 | 21 | 135 | 30 | 1.0 | 1.0 | 1.0 | 1.0 |
| Foreign-born 5 | 575 | 79 | 319 | 70 | 1.60 (1.25–2.06) | 1.33 (1.01–1.74) | 1.48 (1.07–2.04) | 1.08 (0.66–1.77) |
| Asian ethnicity by birthplace | ||||||||
| Chinese | ||||||||
| U.S.-born | 67 | 20 | 57 | 36 | 1.0 | 1.0 | 1.0 | 1.0 |
| Foreign-born | 273 | 80 | 102 | 64 | 2.32 (1.54–3.49) | 1.94 (1.25–3.00) | 2.40 (1.46–3.94) | 1.22 (0.49–3.03) |
| Filipina | ||||||||
| U.S.-born | 14 | 6 | 22 | 11 | 1.0 | 1.0 | 1.0 | 1.0 |
| Foreign-born | 228 | 94 | 174 | 89 | 1.74 (0.89–3.41) | 1.50 (0.73–3.12) | 1.17 (0.54–2.52) | 2.07 (0.69–6.16) |
| Other Asians | ||||||||
| U.S.-born | 72 | 49 | 56 | 57 | 1.0 | 1.0 | 1.0 | 1.0 |
| Foreign-born | 74 | 51 | 43 | 43 | 1.31 (0.82–2.10) | 1.24 (0.69–2.22) | 1.72 (0.76–3.86) | 0.70 (0.30–1.65) |
| Generational status 6 | ||||||||
| U.S.-born 2nd generation 7 | 65 | 9 | 46 | 10 | 1.0 | 1.0 | 1.0 | 1.0 |
| U.S.-born 1st generation 8 | 88 | 12 | 88 | 19 | 0.73 (0.47–1.14) | 0.71 (0.45–1.12) | 0.61 (0.36–1.03) | 0.88 (0.40–1.96) |
| Foreign-born | 575 | 79 | 319 | 70 | 1.31 (0.91–1.89) | 1.06 (0.72–1.58) | 1.06 (0.67–1.70) | 1.00 (0.51–1.95) |
| Age at U.S. immigration (years) 9 | ||||||||
| U.S.-born 4 | 153 | 21 | 135 | 30 | 1.0 | 1.0 | 1.0 | 1.0 |
| <10 | 34 | 5 | 32 | 7 | 0.99 (0.59–1.68) | 0.98 (0.57–1.67) | 1.22 (0.62–2.40) | 0.71 (0.35–1.47) |
| 10–19 | 78 | 11 | 45 | 10 | 1.61 (1.09–2.37) | 1.43 (0.95–2.17) | 1.22 (0.78–1.93) | 2.18 (0.95–5.01) |
| 20–29 | 236 | 32 | 133 | 30 | 1.58 (1.15–2.17) | 1.32 (0.94–1.84) | 1.52 (1.01–2.27) | 1.01 (0.57–1.79) |
| 30–39 | 130 | 18 | 68 | 15 | 1.68 (1.17–2.42) | 1.34 (0.92–1.97) | 1.82 (1.13–2.93) | 0.82 (0.44–1.52) |
| 10–39 | 444 | 61 | 246 | 55 | 1.61 (1.24–2.10) | 1.35 (1.01–1.79) | 1.51 (1.08–2.12) | 1.09 (0.65–1.82) |
| ≥40 | 97 | 13 | 34 | 8 | 2.35 (1.51–3.66) | 2.09 (1.29–3.38) | 2.19 (1.25–3.82) | 1.88 (0.79–4.46) |
| Ptrend | <0.01 10 | <0.01 10 | <0.01 10 | 0.60 11 | ||||
| Duration of U.S. residence (years) 9 | ||||||||
| U.S.-born 4 | 153 | 21 | 135 | 30 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥30 | 175 | 24 | 74 | 17 | 1.96 (1.39–2.76) | 1.73 (1.22–2.47) | 1.83 (1.22–2.76) | 1.59 (0.84–3.01) |
| 20–29 | 155 | 21 | 113 | 25 | 1.22 (0.88–1.68) | 1.00 (0.71–1.41) | 1.16 (0.77–1.76) | 0.74 (0.41–1.31) |
| 10–19 | 163 | 22 | 77 | 17 | 1.98 (1.41–2.78) | 1.58 (1.09–2.28) | 1.87 (1.21–2.89) | 1.15 (0.60–2.20) |
| <10 | 82 | 11 | 48 | 11 | 1.59 (1.03–2.46) | 1.28 (0.80–2.05) | 1.40 (0.79–2.49) | 1.08 (0.52–2.27) |
| Ptrend | <0.01 | 0.20 | 0.10 | 0.96 | ||||
| Age at U.S. immigration (years) and duration of U.S. residence (years) 9 | ||||||||
| U.S.-born 4 | 153 | 21 | 135 | 30 | 1.0 | 1.0 | 1.0 | 1.0 |
| Age <30, ≥20 years | 266 | 37 | 160 | 36 | 1.45 (1.09–1.94) | 1.27 (0.94–1.71) | 1.43 (1.00–2.04) | 1.01 (0.60–1.70) |
| Age <30, <20 years | 82 | 11 | 50 | 11 | 1.74 (1.12–2.69) | 1.44 (0.91–2.28) | 1.31 (0.75–2.27) | 1.61 (0.73–3.57) |
| Age ≥30, ≥20 years | 64 | 9 | 27 | 6 | 1.84 (1.11–3.07) | 1.48 (0.88–2.50) | 1.67 (0.89–3.13) | 1.13 (0.48–2.62) |
| Age ≥30, <20 years | 163 | 22 | 75 | 17 | 1.90 (1.33–2.70) | 1.57 (1.08–2.30) | 2.04 (1.27–3.27) | 1.03 (0.55–1.91) |
| Pinteraction of age at immigration and years of residence | 0.71 | 0.84 | 0.80 | 0.37 | ||||
| % of life in the U.S. 9 | ||||||||
| U.S.-born 4 | 153 | 21 | 135 | 30 | 1.0 | 1.0 | 1.0 | 1.0 |
| 75–99 | 47 | 6 | 31 | 7 | 1.41 (0.88–2.26) | 1.38 (0.85–2.25) | 1.45 (0.81–2.61) | 1.32 (0.63–2.80) |
| 50–74 | 216 | 30 | 115 | 26 | 1.62 (1.18–2.23) | 1.37 (0.98–1.91) | 1.55 (1.04–2.30) | 1.07 (0.60–1.89) |
| 25–49 | 209 | 29 | 108 | 24 | 1.74 (1.27–2.39) | 1.40 (1.00–1.98) | 1.63 (1.07–2.48) | 1.04 (0.57–1.90) |
| <25 | 103 | 14 | 58 | 13 | 1.60 (1.08–2.37) | 1.33 (0.87–2.04) | 1.48 (0.89–2.47) | 1.09 (0.54–2.20) |
| Ptrend | <0.01 | 0.08 | 0.04 | 0.91 | ||||
| Language of interview | ||||||||
| U.S.-born 4 | 153 | 21 | 135 | 30 | 1.0 | 1.0 | 1.0 | 1.0 |
| English | 475 | 65 | 297 | 65 | 1.42 (1.10–1.84) | 1.21 (0.92–1.59) | 1.38 (1.00–1.91) | 0.95 (0.59–1.55) |
| Chinese | 100 | 14 | 22 | 5 | 3.94 (2.36–6.56) | 3.49 (1.97–6.17) | 2.76 (1.46–5.20) | 7.09 (2.22–22.7) |
Abbreviations: CI, confidence interval; OR, odds ratio.
Includes 287 sister controls, 82 unrelated controls who are the sisters of ineligible cases, and 85 population controls.
ORs were adjusted for continuous age at diagnosis (cases) or interview (sisters and population controls), education (high school graduate or less, some college or technical school, college graduate or higher degree), lifetime duration of breast-feeding (nulliparous, 0, ≤12, >12 months), oral contraceptive use (never, ever), 4-level BMI/menopausal status variable (premenopausal <23.0, 23.0–27.4, ≥27.5 kg/m2, postmenopausal), and average lifetime recreational physical activity (tertiles of MET-hours among controls).
Includes 167 controls who are unrelated to any case (82 unrelated controls who are the sisters of ineligible cases, and 85 population controls).
Includes 2 cases and 1 population control born in a western country other than the U.S. (i.e., Canada or Italy).
Includes cases/controls born in China (China, Hong Kong, Taiwan: 231/72), Philippines (246/185), Japan (23/10), Korea (4/2), India or Pakistan (19/17), Southeast Asia (45/29), Central American, South America or Africa (7/4).
Excludes 1 control with missing data on parents’ birthplace.
Study participants and both parents are U.S.-born.
Study participants are U.S.-born, 1 or 2 parents are foreign-born.
Excludes 7 controls with missing data on migration history.
Ptrend <0.01 for 6 categories (U.S.-born, <10, 10–19, 20–29, 30–39, ≥40 years) and Ptrend <0.01 for 4 categories (U.S.-born, <10, 10–39, ≥40 years).
Ptrend = 0.60 for 6 categories (U.S.-born, <10, 10–19, 20–29, 30–39, ≥40 years) and Ptrend = 0.25 for 4 categories (U.S.-born, <10, 10–39, ≥40 years).
While the proportion of foreign-born individuals was higher among cases than sisters (79% vs. 68%, P <0.01), the proportions were similar when comparing cases to unrelated controls (P=0.09) and population controls (P=0.60) (Supplemental Table 1). Comparing cases to sisters showed somewhat stronger associations with birthplace and other migration characteristics than when comparing cases to all controls (Table 2). Comparing cases to the smaller subset of unrelated controls, associations did not reach statistical significance.
Stratifying by birth cohort, associations with migration characteristics were confined to the younger cohort (cases diagnosed at age ≤55 years) (Table 3). Interactions between migration characteristics and birth cohort, however, were not statistically significant (all P ≥0.05). In the younger birth cohort, breast cancer risk was marginally increased among foreign-born women overall (OR=1.40, 95% CI=0.97–2.03), two-fold among Chinese women (OR=2.16, 95% CI=1.21–3.88), and suggestively among Filipina women (OR=2.06, 95% CI=0.80–5.35) relative to U.S.-born Asian American women. Two- to three-fold increased risks were associated with migration to the U.S. at age ≥40 years, residence in the U.S. for ≥30 years or for ≥75% of life, and interview completion in Chinese. Recent immigrants (<10 years or <25% of life in the U.S.) had a similar risk as U.S.-born Asian American women. In the older birth cohort, except for interview completion in Chinese, migration-related characteristics were not associated with breast cancer risk. We observed similar associations with birthplace when limiting the analysis to women of the same age in the two birth cohorts (i.e., 44–55 years), although power was limited to assess associations in this subset (birth cohort 1931–1950: OR=1.23, 95% CI=0.60–2.54; birth cohort 1951–1094: OR=1.57, 95% CI=0.83–2.95).
Table 3.
Migration history, language at interview, and breast cancer risk among Asian American women, by birth cohort
| Birth cohort 1931–1950 Cases diagnosed at age 44–64 y |
Birth cohort 1951–1984 Cases diagnosed at age 23–55 y |
|||||
|---|---|---|---|---|---|---|
| Cases N=373 |
Controls N=178 |
Multivariable- adjusted OR (95% CI) 1 |
Cases N=355 |
Controls N=276 |
Multivariable- adjusted OR (95% CI) 1 |
|
| Birthplace | ||||||
| U.S.-born | 80 | 56 | 1.0 | 73 | 79 | 1.0 |
| Foreign-born | 293 | 122 | 1.22 (0.80–1.86) | 282 | 197 | 1.40 (0.97–2.03) |
| Pheterogeneity by birth cohort = 0.76 | ||||||
| Asian ethnicity by birthplace | ||||||
| Chinese | ||||||
| U.S.-born | 33 | 22 | 1.0 | 34 | 35 | 1.0 |
| Foreign-born | 125 | 33 | 1.58 (0.75–3.35) | 148 | 69 | 2.16 (1.21–3.88) |
| Pheterogeneity by birth cohort = 0.59 | ||||||
| Filipina | ||||||
| U.S.-born | 5 | 2 | 1.0 | 9 | 20 | 1.0 |
| Foreign-born | 135 | 73 | 0.68 (0.12–3.92) | 93 | 101 | 2.06 (0.80–5.35) |
| Pheterogeneity by birth cohort = 0.26 | ||||||
| Age at U.S. immigration (years) | ||||||
| U.S.-born | 80 | 56 | 1.0 | 73 | 79 | 1.0 |
| <10 | 8 | 11 | 0.50 (0.18–1.39) | 26 | 21 | 1.31 (0.68–2.54) |
| 10–19 | 20 | 6 | 1.78 (0.69–4.60) | 58 | 39 | 1.46 (0.88–2.44) |
| 20–29 | 115 | 52 | 1.24 (0.74–2.07) | 121 | 81 | 1.41 (0.90–2.21) |
| 30–39 | 73 | 25 | 1.41 (0.79–2.54) | 57 | 43 | 1.29 (0.76–2.19) |
| 10–39 | 208 | 83 | 1.34 (0.84–2.12) | 236 | 163 | 1.39 (0.95–2.05) |
| ≥40 | 77 | 23 | 1.69 (0.89–3.23) | 20 | 11 | 2.26 (1.02–5.02) |
| Ptrend 2 | 0.06 | 0.04 | ||||
| Pheterogeneity 2 by birth cohort = 0.34 | ||||||
| Duration of U.S. residence (years) | ||||||
| U.S.-born | 80 | 56 | 1.0 | 73 | 79 | 1.0 |
| ≥30 | 125 | 50 | 1.45 (0.90–2.35) | 50 | 24 | 2.44 (1.34–4.41) |
| 20–29 | 77 | 43 | 0.88 (0.50–1.55) | 78 | 70 | 1.09 (0.69–1.74) |
| 10–19 | 61 | 16 | 1.68 (0.83–3.39) | 102 | 61 | 1.50 (0.94–2.39) |
| <10 | 30 | 8 | 1.78 (0.68–4.69) | 52 | 40 | 1.19 (0.69–2.04) |
| Ptrend | 0.25 | 0.52 | ||||
| Pheterogeneity by birth cohort = 0.14 | ||||||
| % of life in the U.S. | ||||||
| U.S.-born | 80 | 56 | 1.0 | 73 | 79 | 1.0 |
| 75–99 | 16 | 13 | 0.85 (0.42–1.72) | 31 | 18 | 1.90 (0.96–3.77) |
| 50–74 | 127 | 52 | 1.38 (0.83–2.29) | 89 | 63 | 1.39 (0.88–2.19) |
| 25–49 | 103 | 36 | 1.38 (0.80–2.36) | 106 | 72 | 1.36 (0.85–2.18) |
| <25 | 47 | 16 | 1.37 (0.65–2.87) | 56 | 42 | 1.32 (0.78–2.23) |
| Ptrend | 0.17 | 0.34 | ||||
| Pheterogeneity by birth cohort = 0.22 | ||||||
| Language of interview | ||||||
| U.S.-born | 80 | 56 | 1.0 | 73 | 79 | 1.0 |
| English | 237 | 114 | 1.12 (0.73–1.71) | 238 | 183 | 1.27 (0.87–1.86) |
| Chinese | 56 | 8 | 3.38 (1.32–8.64) | 44 | 14 | 3.95 (1.80–8.69) |
| Pheterogeneity by birth cohort = 0.75 | ||||||
Abbreviations: CI, confidence interval; OR, odds ratio.
ORs were adjusted for continuous age at diagnosis (cases) or interview (sisters and population controls), education (high school graduate or less, some college or technical school, college graduate or higher degree), lifetime duration of breast-feeding (nulliparous, 0, ≤12, >12 months), oral contraceptive use (never, ever), 4-level BMI/menopausal status variable (premenopausal <23.0, 23.0–27.4, ≥27.5 kg/m2, postmenopausal), and average lifetime recreational physical activity (tertiles of MET-hours among controls).
Based on categories U.S.-born, <10, 10–39, ≥40 years.
For several risk factors (oral contraceptive use, menopausal hormone use, obesity, histories of smoking and alcohol consumption), the prevalence was lowest among foreign-born Asian American controls, intermediate among U.S.-born Asian American controls, and greatest among U.S.-born NHW controls (Figure 1, Supplemental Table 2). Shorter or no breast-feeding was the only risk factor with a greater prevalence among foreign-born than U.S.-born Asian American controls. The prevalence of high intake of isoflavones and green or herbal tea did not differ by birthplace. Compared to NHW controls, U.S.-born Asian American controls had a greater prevalence of higher education, nulliparity, late FFTP, breast-feeding, and high intake of isoflavones and green or herbal tea. There was some variation in risk factor prevalence by Asian ethnicity. Notably, foreign-born Chinese controls had the highest prevalence of late FFTP (47%), short or no breast-feeding (82%), low physical activity (75%), and high isoflavone intake (80%).
Figure 1.
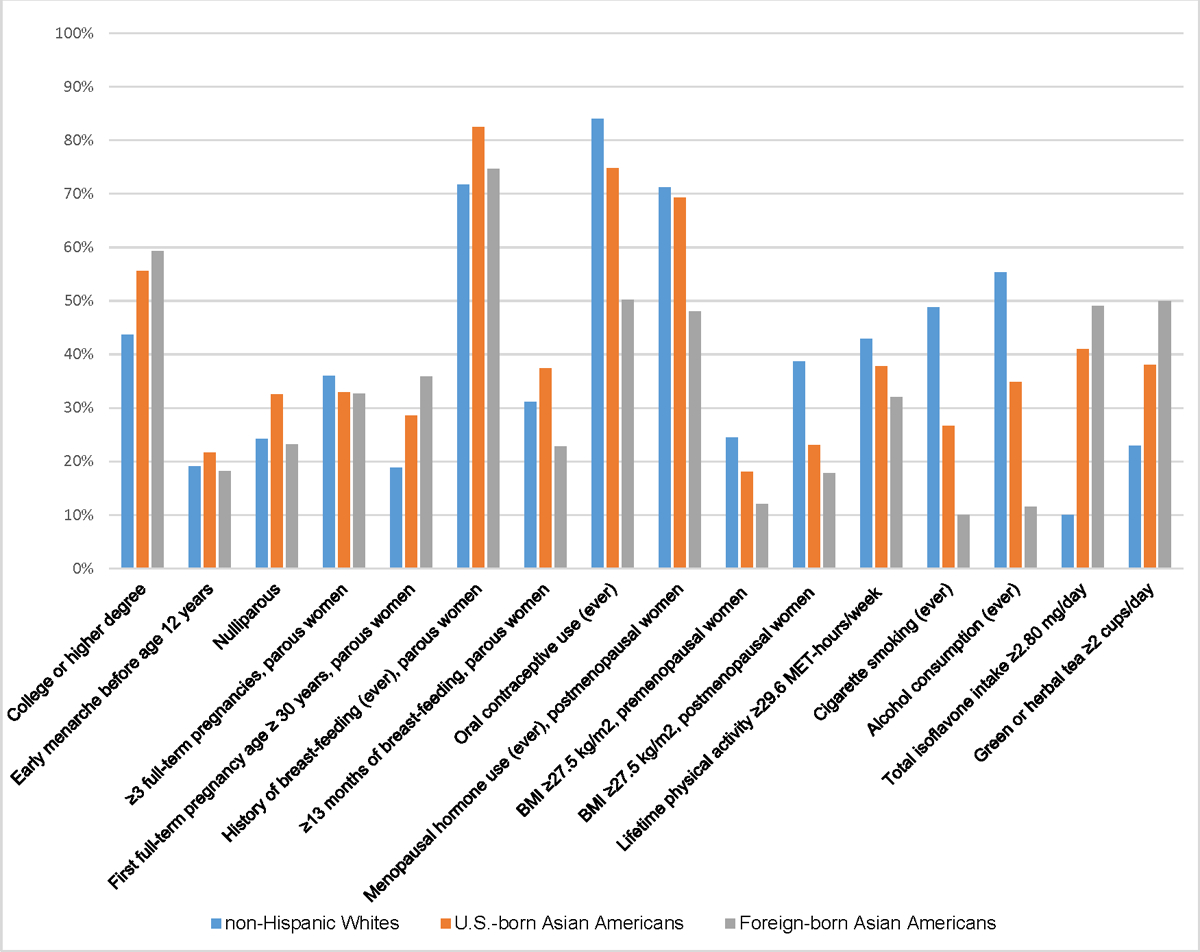
Prevalence of breast cancer risk factors among U.S. born non-Hispanic White controls and Asian American controls, by birthplace
Among foreign-born Asian American women, there were notable differences in the prevalence of some risk factors by birth cohort. Compared to the older birth cohort, foreign-born cases from the younger birth cohort had a greater prevalence of factors associated with increased breast cancer risk (high education, early menarche, nulliparity, low parity, late FFTP, oral contraceptive use, and history of smoking and alcohol consumption) (Figure 2, Supplemental Table 3). They also had a greater prevalence of breast-feeding and high physical activity, whereas high intake of isoflavones and green or herbal tea did not differ by birth cohort. Among foreign-born controls, only the prevalence of nulliparity and low parity was higher, and the prevalence of high green or herbal tea intake was marginally lower in the younger birth cohort compared to the older birth cohort (Figure 3, Supplemental Table 3).
Figure 2.
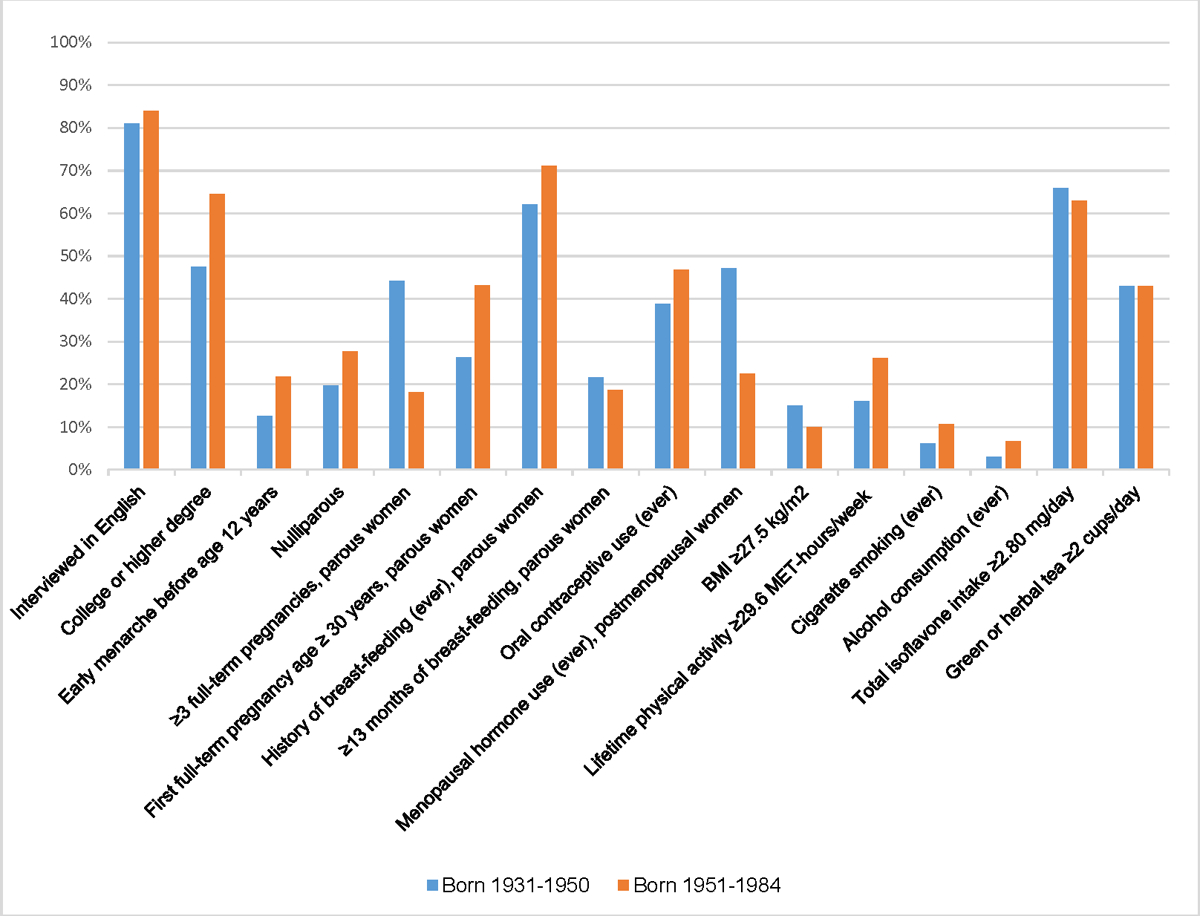
Prevalence of breast cancer risk factors among foreign-born Asian American cases, by birth cohort
Figure 3.
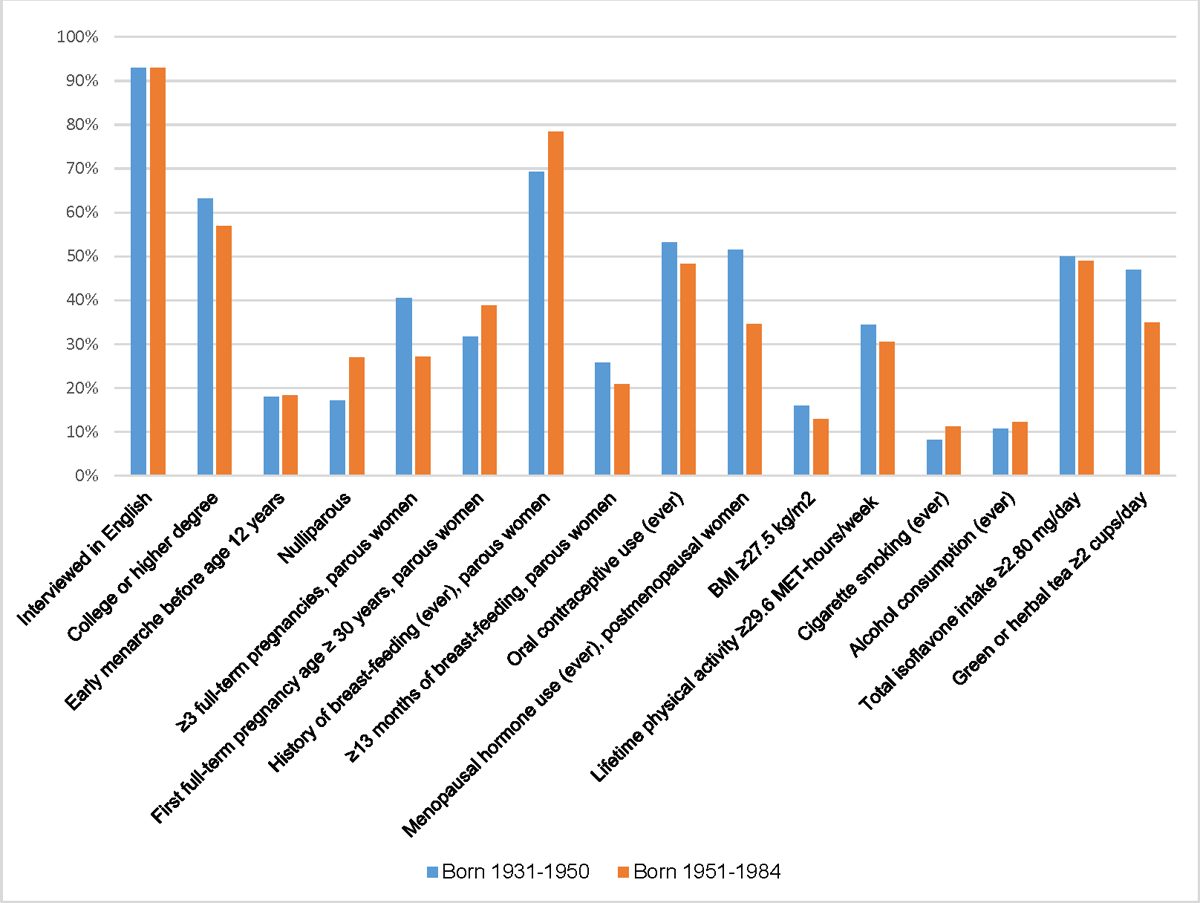
Prevalence of breast cancer risk factors among foreign-born Asian American controls, by birth cohort
Since the younger and older birth cohorts had somewhat different age distributions, we further limited the comparison of risk factor prevalence among foreign-born Asian American women to those aged 44–55 years, the age group common in both birth cohorts (Supplemental Table 4). Cases in the younger birth cohort had a greater prevalence of higher education (61% vs. 49%) and late FFTP (46% vs. 24%) and a lower prevalence of ≥3 FTP (20% vs. 36%) relative to cases in the older birth cohort.
Among Asian American cases in the younger birth cohort, high isoflavone intake was the only factor with a greater prevalence among foreign-born cases compared to U.S.-born cases. Other factors had a greater prevalence among U.S.-born cases (nulliparity, breast-feeding, oral contraceptive use, higher physical activity, histories of smoking and alcohol consumption) (Supplemental Figure 1, Supplemental Table 5). Among controls in the younger birth cohort, the prevalence of several factors (oral contraceptive use, physical activity, histories of smoking and alcohol consumption) was lowest among foreign-born Asian American controls, intermediate among U.S.-born Asian American controls, and highest among NHW controls (Supplemental Figure 2, Supplemental Table 5). Several factors (higher education, early menarche, late FFTP, high parity, breast-feeding, obesity, high green or herbal tea intake) had the same or similar distributions among foreign-born and U.S.-born Asian American controls. No or short breast-feeding and lower physical activity were the only risk factors with a greater prevalence among foreign-born than U.S.-born Asian American controls. Compared to NHW controls, high intake of total isoflavones was the only factor with a greater prevalence among U.S.-born Asian American controls.
Discussion
The present case-control analysis of Asian American women from the San Francisco Bay Area primarily comprised Chinese and Filipina women of whom the majority were foreign-born (79% of cases, 70% of controls) and had a college or higher degree (57% of cases, 58% of controls). Risk of breast cancer was higher among foreign-born than U.S.-born Asian American women, but only in the younger birth cohort (1951–1984), that comprised women aged ≤55 years. Relative to U.S.-born Asian American women, two-fold increased risks were associated with migration at age ≥40 years and with longer residence in the U.S. (≥30 years or ≥75% of life). Differences in the prevalence of risk factors by birthplace and birth cohort indicate that temporal changes have taken place.
The present findings differ from a California-based cancer registry analysis (8) and a case-control study conducted in California and Hawaii (14) that observed higher risks of breast cancer in U.S.-born compared to foreign-born Asian American women. In contrast, in a recent case-control study of Asian American women aged ≥20 years from the San Francisco Bay Area (132 cases diagnosed from 2005–2009, 438 controls), risk was increased two- to three-fold among foreign-born Asian American women compared to their U.S.-born counterparts (20). The association with foreign birthplace was more modest in our study and limited to the younger birth cohort. Longer residence in the U.S. (≥30 years or ≥75% of life) was associated with increased breast cancer risk and may be a proxy measure for acculturation and adoption of a more westernized reproductive and lifestyle factors.
Rising breast cancer incidence rates have been well-documented both in Asian (3,4) and Asian American (6,7,9–11,13) women, including U.S.-born Asian American women (8). Among select Asian countries (4) and Asian American ethnic groups (11), incidence rates, especially in young women, are approaching or surpassing those of U.S. NHW women (4,8,9,13,32). This has generally been attributed to changes in reproductive patterns (earlier menarche, higher nulliparity, fewer births, late first birth), lifestyle factors (higher obesity, taller height, lower physical activity, higher alcohol consumption, and dietary changes), and environmental exposures resulting from urbanization or westernization, both in the birth country and the country of immigration (2–4,6,10,11,33–35). Known reproductive and lifestyle risk factors, however, do not fully explain international differences in breast cancer incidence (36) or differences among Asian American women by birthplace (14), including in our study.
High proportions of Asian American cases (57%) and controls (58%) had a college or higher degree, with the highest percentages seen among U.S.-born (65%) and foreign-born (60%) Chinese controls, consistent with the high proportions of college graduates (61% of cases, 63% of controls) in the study by Morey et al. that was also conducted in the San Francisco Bay Area (20). In our study, even higher proportions were seen in the younger birth cohort (65% of foreign-born cases, 68% of U.S.-born cases). For comparison, the proportion of college-educated U.S.-born NHW controls enrolled in the NC-BCFR was 44%. The high educational level of Asian American women in the San Francisco Bay Area is likely not representative of all Asian American women in California and may have contributed to the higher breast cancer risk among foreign-born women in the younger birth cohort which is not consistent with the California-wide study by Gomez et al. (8).
Likely related to the observed high level of education, prevalence of a FFTP at age ≥30 years was high among foreign-born cases (43%) and controls (39%), similar to the findings by Morey et al. (41% and 43%) (20), but higher compared with NHW controls (28%). Prior studies have demonstrated that a longer interval between menarche and FFTP is associated with increased breast cancer risk in younger women (37–41). This time window has been identified as the most crucial factor in establishing future risk of breast cancer (42). More research is needed to understand what exposures affect breast cancer risk during that critical time window.
In the younger birth cohort, recent immigrants (<10 years or <25% of life in the U.S.) had a similar breast cancer risk as U.S.-born Asian American women. This finding raises the possibility that recent young immigrant women, many of whom had a high education, originated from more westernized regions in Asia and had acquired certain risk factors before they migrated to the U.S. Our study did not collect data on prior residential history. Immigrant women who migrated at age ≥40 years had a two-fold increased risk, suggesting that they likely had completed their childbearing in their birth country before migration and combined with a higher education, they may have had a less favorable risk factor profile by the time they migrated to the U.S.
When we compared risk factors profiles by birth cohort and birthplace, we saw that each case and control subgroup had unique combinations of factors associated with breast cancer risk. Among all controls combined and among controls in the younger birth cohort, we found that for lifestyle factors (oral contraceptive use, physical activity, obesity, histories of smoking and alcohol consumption), the prevalence was lowest among foreign-born Asian Americans, intermediate among U.S.-born Asian Americans, and highest among U.S-born NHWs. Such trends are consistent with a higher breast cancer risk among U.S.-born than foreign-born Asian American women (8,14). For hormonal factors, we observed that foreign-born Asian American cases and controls in the younger birth cohort had a higher prevalence of reproductive risk factors (nulliparity, low parity, late FFTP) compared to the older birth cohort. This pattern was also seen in the analysis limited to cases of the same age (44–55 years) in the two cohorts. These data suggest that temporal changes in reproductive and other risk factors have taken place, with a less favorable risk factor profile in the younger birth cohort. However, we observed few differences in the prevalence of established risk factors between foreign-born and U.S.-born Asian American women in the younger birth cohort and cannot fully explain the observed positive association with foreign birthplace.
It remains uncertain what specific factors explain the higher risk of breast cancer in foreign-born Asian American women relative to U.S.-born women observed in the younger birth cohort. The higher level of education among foreign-born cases may indicate an accumulation of other risk factors in younger women, including exposures during the period between menarche and FFTP. Our finding may be comparable to the higher incidence of breast cancer in young women of Asian descent compared to U.S. NHW women that has been previously observed (4,8,13,32). Our analyses showed that positive associations with foreign birthplace, longer duration of residence in the U.S., or migration at age ≥40 years remained after multivariable adjustment for established breast cancer risk factors, suggesting the importance of other influential factors. They could include environmental exposures, dietary changes, hormonal changes, and early-life exposures not considered in the present study. The impact of dietary changes could be mediated by circulating hormone concentrations (34,43). Dietary changes in childhood could also be important. For example, childhood soy intake has been associated with lower breast cancer risk in Asian populations (28). Among controls we saw few differences in isoflavone intake by birthplace or birth cohort. The NC-BCFR did not collect data on early-life exposures, thus we could not evaluate such associations. Since sisters growing up in the same family (either in Asia or in the U.S.) may have similar early-life exposures, a case-control study that uses sisters for comparison may not be the optimal design to examine associations with early-life factors and may bias results towards the null.
The present analysis has several strengths. Asian race and ethnicity was based on self-report which reduces potential ethnicity misclassification. We collected detailed data on migration history, unlike cancer registry-based studies that have derived birthplace from medical records or through statistical imputation (8). We collected comprehensive data on established breast cancer risk factors, birthplace, and birth cohort. Limitations include the limited Asian ethnic diversity and the small proportion of U.S.-born Asian American women, which limited our subgroup analyses. Green tea consumption was not collected as a single food item, but combined with herbal and other tea. It is possible that more educated women are more likely to migrate to the San Francisco Bay Area. Thus, our findings may not be generalizable to Asian American women in other regions. Finally, the finding of a three-fold increased breast cancer risk among immigrant women who completed the interview in Chinese vs. English compared to U.S.-born women was unexpected. This finding is difficult to interpret with small numbers of controls who completed the interview in Chinese.
In conclusion, in the younger birth cohort that comprised cases diagnosed at age ≤55 years, recent immigrants had a similar risk of breast cancer as U.S.-born Asian American women, but risk was increased two-fold among foreign-born women with long residence in the U.S. (≥30 years or ≥75% of life). Few risk factors had a greater prevalence in foreign-born compared to U.S.-born Asian American women. Thus, other factors must underlie the higher breast cancer risk in foreign-born Asian American women. Our findings warrant confirmation in larger studies that are more representative of all Asian American populations and include women across a wider range of educational and socioeconomic backgrounds. Given the rising breast cancer incidence rates in Asian and Asian American women, it is important and urgent to gain a deeper understanding of what factors drive the increasing burden of breast cancer and to implement effective prevention programs.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute (grant number U01 CA164920 to E. M. John). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the Breast Cancer Family Registry (BCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government or the BCFR.
Abbreviations
- CI
confidence interval
- ER
estrogen receptor status
- NC-BCFR
Northern California Breast Cancer Family Registry
- NHW
non-Hispanic White
- OR
odds ratio
- PR
progesterone receptor status
- U.S.
United States
Footnotes
Authors’ Disclosures
The authors declare no potential conflicts of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55(2):74–108. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res 2004;6(6):229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control 2010;21(11):1777–85. [DOI] [PubMed] [Google Scholar]
- 4.Sung H, Rosenberg PS, Chen WQ, Hartman M, Lim WY, Chia KS, et al. Female breast cancer incidence among Asian and Western populations: more similar than expected. J Natl Cancer Inst 2015;107(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control 2008;19(3):227–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez SL, Noone AM, Lichtensztajn DY, Scoppa S, Gibson JT, Liu L, et al. Cancer incidence trends among Asian American populations in the United States, 1990–2008. J Natl Cancer Inst 2013;105(15):1096–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuan AW, Davis Lynn BC, Chernyavskiy P, Yu M, Gomez SL, Gierach GL, et al. Breast Cancer Incidence Trends by Estrogen Receptor Status Among Asian American Ethnic Groups, 1990–2014. JNCI Cancer Spectr 2020;4(2):pkaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez SL, Quach T, Horn-Ross PL, Pham JT, Cockburn M, Chang ET, et al. Hidden breast cancer disparities in Asian women: disaggregating incidence rates by ethnicity and migrant status. Am J Public Health 2010;100 Suppl 1:S125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Zhang J, Wu AH, Pike MC, Deapen D. Invasive breast cancer incidence trends by detailed race/ethnicity and age. Int J Cancer 2012;130(2):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du XL, Song L. Breast cancer incidence trends in Asian women aged 20 or older as compared to other ethnic women in the United States from 2000 to 2018 by time period, age and tumor stage. Cancer Epidemiol 2022;76:102076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deapen D, Liu L, Perkins C, Bernstein L, Ross RK. Rapidly rising breast cancer incidence rates among Asian-American women. Int J Cancer 2002;99(5):747–50. [DOI] [PubMed] [Google Scholar]
- 12.Keegan TH, Gomez SL, Clarke CA, Chan JK, Glaser SL. Recent trends in breast cancer incidence among 6 Asian groups in the Greater Bay Area of Northern California. Int J Cancer 2007;120(6):1324–9. [DOI] [PubMed] [Google Scholar]
- 13.Gomez SL, Von Behren J, McKinley M, Clarke CA, Shariff-Marco S, Cheng I, et al. Breast cancer in Asian Americans in California, 1988–2013: increasing incidence trends and recent data on breast cancer subtypes. Breast Cancer Res Treat 2017;164(1):139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst 1993;85(22):1819–27. [DOI] [PubMed] [Google Scholar]
- 15.John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev 2005;14(12):2905–13. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DB, Karagas MR. Migrant Studies. In: Schottenfeld D, Fraumeni JF Jr., editors. Cancer Epidemiology and Prevention 2nd edition ed. New York, NY: Oxford University Press; 1996. p 236–54. [Google Scholar]
- 17.Parkin DM, Khlat M. Studies of cancer in migrants: rationale and methodology. Eur J Cancer 1996;32A(5):761–71. [DOI] [PubMed] [Google Scholar]
- 18.McCredie M Cancer epidemiology in migrant populations. Recent Results Cancer Res 1998;154:298–305. [DOI] [PubMed] [Google Scholar]
- 19.Stanford JL, Herrinton LJ, Schwartz SM, Weiss NS. Breast cancer incidence in Asian migrants to the United States and their descendants. Epidemiology 1995;6(2):181–3. [DOI] [PubMed] [Google Scholar]
- 20.Morey BN, Gee GC, von Ehrenstein OS, Shariff-Marco S, Canchola AJ, Yang J, et al. Higher Breast Cancer Risk Among Immigrant Asian American Women Than Among US-Born Asian American Women. Prev Chronic Dis 2019;16:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John EM, Sangaramoorthy M, Koo J, Whittemore AS, West DW. Enrollment and biospecimen collection in a multiethnic family cohort: the Northern California site of the Breast Cancer Family Registry. Cancer Causes Control 2019;30(4):395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT, et al. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res 2004;6(4):R375–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keegan TH, Milne RL, Andrulis IL, Chang ET, Sangaramoorthy M, Phillips KA, et al. Past recreational physical activity, body size, and all-cause mortality following breast cancer diagnosis: results from the Breast Cancer Family Registry. Breast Cancer Res Treat 2010;123(2):531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stram DO, Hankin JH, Wilkens LR, Pike MC, Monroe KR, Park S, et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol 2000;151(4):358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang FF, Haslam DE, Terry MB, Knight JA, Andrulis IL, Daly MB, et al. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: The Breast Cancer Family Registry. Cancer 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.W.H.O. Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(9 Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 28.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis 2002;23(9):1491–6. [DOI] [PubMed] [Google Scholar]
- 29.Wu AH, Ziegler RG, Horn-Ross PL, Nomura AM, West DW, Kolonel LN, et al. Tofu and risk of breast cancer in Asian-Americans. Cancer Epidemiol Biomarkers Prev 1996;5(11):901–6. [PubMed] [Google Scholar]
- 30.Wu AH, Ziegler RG, Nomura AM, West DW, Kolonel LN, Horn-Ross PL, et al. Soy intake and risk of breast cancer in Asians and Asian Americans. Am J Clin Nutr 1998;68(6 Suppl):1437S–43S. [DOI] [PubMed] [Google Scholar]
- 31.Wu AH, Yu MC, Tseng CC, Hankin J, Pike MC. Green tea and risk of breast cancer in Asian Americans. Int J Cancer 2003;106(4):574–9. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds P, Hurley S, Goldberg D, Quach T, Rull R, Von Behren J. An excess of breast cancer among young California-born Asian women. Ethn Dis 2011;21(2):196–201. [PubMed] [Google Scholar]
- 33.Nelson NJ. Migrant studies aid the search for factors linked to breast cancer risk. J Natl Cancer Inst 2006;98(7):436–8. [DOI] [PubMed] [Google Scholar]
- 34.Houghton LC, Ganmaa D, Rosenberg PS, Davaalkham D, Stanczyk FZ, Hoover RN, et al. Associations of Breast Cancer Risk Factors with Premenopausal Sex Hormones in Women with Very Low Breast Cancer Risk. Int J Environ Res Public Health 2016;13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Tse LA, Chan WC, Kwok CC, Leung SL, Wu C, et al. Disparities of time trends and birth cohort effects on invasive breast cancer incidence in Shanghai and Hong Kong pre- and post-menopausal women. BMC Cancer 2017;17(1):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoover RN. That recognised risk factors can explain past and present international differences in breast cancer incidence: misconceptions 5. Br J Cancer 2012;107(3):408–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li CI, Malone KE, Daling JR, Potter JD, Bernstein L, Marchbanks PA, et al. Timing of menarche and first full-term birth in relation to breast cancer risk. Am J Epidemiol 2008;167(2):230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warner ET, Colditz GA, Palmer JR, Partridge AH, Rosner BA, Tamimi RM. Reproductive factors and risk of premenopausal breast cancer by age at diagnosis: are there differences before and after age 40? Breast Cancer Res Treat 2013;142(1):165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritte R, Tikk K, Lukanova A, Tjonneland A, Olsen A, Overvad K, et al. Reproductive factors and risk of hormone receptor positive and negative breast cancer: a cohort study. BMC Cancer 2013;13:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaudet MM, Gierach GL, Carter BD, Luo J, Milne RL, Weiderpass E, et al. Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer Res 2018;78(20):6011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.John EM, Phipps AI, Hines LM, Koo J, Ingles SA, Baumgartner KB, et al. Menstrual and reproductive characteristics and breast cancer risk by hormone receptor status and ethnicity: The Breast Cancer Etiology in Minorities study. Int J Cancer 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev 1995;4(5):567–71. [PubMed] [Google Scholar]
- 43.Troisi R, Ganmaa D, dos Santos Silva I, Davaalkham D, Rosenberg PS, Rich-Edwards J, et al. The role of hormones in the differences in the incidence of breast cancer between Mongolia and the United Kingdom. PLoS One 2014;9(12):e114455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request and with appropriate IRB approval.


