Abstract
Background:
Psychosis is a defining feature of schizophrenia and highly prevalent in bipolar disorder. Notably, individuals suffering with these illnesses also have major disruptions in sleep and circadian rhythms, and disturbances to sleep and circadian rhythms can precipitate or exacerbate psychotic symptoms. Psychosis is associated with the striatum, though no study to date has directly measured molecular rhythms and determined how they are altered in the striatum of subjects with psychosis.
Methods:
Here, we perform RNA-sequencing and both differential expression and rhythmicity analyses to investigate diurnal alterations in gene expression in human postmortem striatal subregions (NAc, caudate, and putamen) in subjects with psychosis (n=36) relative to unaffected comparison subjects (n=36).
Results:
Across regions, we find differential expression of immune-related transcripts and a substantial loss of rhythmicity in core circadian clock genes in subjects with psychosis. In the nucleus accumbens (NAc), mitochondrial-related transcripts have decreased expression in psychosis subjects, but only in those who died at night. Additionally, we find a loss of rhythmicity in small nucleolar RNAs and a gain of rhythmicity in glutamatergic signaling in the NAc of psychosis subjects. Between region comparisons indicate that rhythmicity in the caudate and putamen is far more similar in subjects with psychosis than in matched comparison subjects.
Conclusions:
Together, these findings reveal differential and rhythmic gene expression differences across the striatum that may contribute to striatal dysfunction and psychosis in psychotic disorders.
Keywords: Human Postmortem, Circadian Rhythms, RNA-seq, Striatum, Schizophrenia, Psychosis
Introduction
Psychosis, an impairment in reality testing that manifests as hallucinations and/or delusions, is a defining feature of schizophrenia (SCZ) and highly prevalent in bipolar disorder (BD). This clinical feature is strongly associated with striatal dysfunction and altered dopaminergic transmission in the striatum (1). Further implicating this region, antipsychotic medications block dopamine D2/D3 receptors in the striatum (2, 3) and treatment response is directly correlated with D2/D3 binding of antipsychotics in the striatum, but not non-striatal regions which also express D2/D3 receptors (4).
In humans, the striatum is anatomically subdivided into three main regions that serve overlapping but distinct functions (1). The ventral striatum (primarily nucleus accumbens, NAc) is involved in reward processing, goal-directed behavior, and motivation (5). The dorsal striatum includes both the caudate nucleus, which is associated with cognition, anxiety, movement, and working memory, and the putamen, which is largely associated with repetitive behavior, motor learning, and motor control (6, 7).
Neuroimaging studies find that dopaminergic dysfunction in psychosis is greatest in nigrostriatal pathways, suggesting a prominent role for dorsal striatal regions in psychosis (1). These studies find the largest increase in dopaminergic function in the associative striatum, some increase in sensorimotor regions, and no significant differences in the limbic striatum (8–10). However, patients with psychosis also frequently experience impairments in motivation, mood, and reward which likely involves ventral striatal regions (11, 12).
Diurnal rhythms in several components of dopamine signaling have been observed in the striatum (13–16), and dopamine regulates the striatal expression of core circadian clock genes (17, 18). Thus, alterations in dopamine signaling may lead to disruptions in circadian rhythms and vice versa (19). Schizophrenia and BD are associated with major disruptions to the sleep/wake cycle, as well as rhythms in a variety of other physiological measures (20). Many transcripts in the brain show circadian rhythms in expression. These include not only core circadian genes directly involved in molecular clock function, but thousands of other “clock-controlled genes” that can be highly specific depending on the brain region (21, 22). In fact, over 80% of protein-coding transcripts have diurnal rhythmicity in expression in the non-human primate in at least one tissue (22). Previously, we performed a time of death (TOD) analysis to measure diurnal rhythms across the striatum in a cohort of non-psychiatric subjects to determine how gene expression normally fluctuates across striatal regions. We measured robust diurnal rhythms in all three striatal regions, including rhythms in core clock genes (25). However, we found distinct differences between regions, including an enrichment of rhythmic non-coding RNAs in the NAc, suggesting each region must be considered to understand the full scope of diurnal transcript regulation in the striatum (25). In the current study, we examined how transcript expression differs across striatal subregions in subjects who experienced psychosis relative to unaffected comparison subjects. We performed both differential expression and rhythmicity analyses to determine how transcripts are altered across the striatum in subjects with psychosis, and whether these alterations are due to differences in diurnal rhythmicity.
Methods and Materials
Detailed methods are provided in the Supplement.
Human Postmortem Brain Samples
NAc, caudate, and putamen samples were collected from subjects with psychosis [n=36; SCZ/schizoaffective disorder (n=28) and BD with psychosis (n=8)] and unaffected comparison subjects (n=36) obtained through the University of Pittsburgh Brain Tissue Donation Program and NIH NeuroBioBank (Datasets S1 and S2). We specifically created a psychosis cohort, which included subjects with either SCZ or BD with psychosis, as we were interested in this phenotype and evidence suggests these subjects have a high degree of overlap at the clinical, genetic, and transcriptomic levels (26–28).
Matched comparison cohort
An unbiased method was used to select the 36 unaffected comparison subjects from a group of 59 unaffected subjects described previously (25). This method permitted selection of subjects best matched to the 36 psychosis subjects by sex, race, age, TOD, and PMI.
Differential Expression Analyses
We previously found differential expression changes in the dlPFC that selectively occur in subjects with SCZ who died at night (24). Similar stratified analyses were applied to our data (see Supplement). Transcripts were considered DE if p<0.01 and log2FC≤−0.26 or ≥0.26 (FC±1.2 or 20% expression change) (31).
Rhythmicity Analyses
Circadian patterns of gene expression were detected using nonlinear regression based on individual subject TOD (24, 25, 32). Samples were ordered by their TOD and expression for each transcript was fitted to a sinusoidal curve. We also tested correlations between TOD and covariates (Dataset S3).
Pathway Enrichment
Ingenuity pathway analysis (IPA) software (Qiagen) was used to identify enriched biological pathways and upstream regulators.
Biological Process Enrichment
Biological process enrichment was performed using the web-based portal, Metascape (35), and visualized using Cytoscape (36).
Results
Diurnal differential expression between psychosis and comparison subjects
We measured differential expression between psychosis and comparison subjects in the NAc, caudate, and putamen using RNA-seq (Tables S1 and S2; Datasets S1 and S2). The number of differentially expressed (DE) transcripts differed between regions, with more in the putamen (n=672) than the caudate (n=296) and NAc (n=107; Table S3; Datasets S4–S6). An even distribution of transcripts were down- and up-regulated in the NAc [(54 (down) vs. 53 (up)], but more transcripts were down- than up-regulated in the caudate [180 (down) vs. 116 (up); Bonferroni-adjusted-p (BH-p)<0.01] and putamen [539 (down) vs. 133 (up); BH-p<0.001] (Fig. 1a; Fig. S1a; Dataset S6).
Figure 1.

Differential expression between psychosis and comparison subjects across the NAc, caudate, and putamen. (A) Volcano plots for differentially expressed (DE) transcripts in the NAc, caudate, and putamen, with −log10p-value on the y-axis and log2FC on the x-axis. Horizontal dashed lines represent a cutoff of p<0.01 and vertical dashed lines represent log2FC cutoffs of ≤−0.26 or ≥0.26. Transcripts meeting both cutoffs are represented by red circles. (B) Rank-Rank Hypergeometric Overlap (RRHO) plots showing a high degree of overlap in expression between striatal regions. Hot spots in the bottom left quadrants represent overlap in transcripts up-regulated in both regions (concordant) and hot spots in the upper right quadrants represent overlap in transcripts down-regulated in both regions (concordant). (C) Pathway enrichment (top 5 significant pathways) for meta-analyzed transcripts that are DE (both up- and down-regulated) in all three striatal regions. (D) Pathway enrichment for DE transcripts (both up- and down-regulated) in each individual striatal region (top 5 significant pathways for each region). The significance threshold for pathway enrichment is p<0.05 (-log10(p-value)>1.3 in plots).
Next, we determined the overlap in DE transcripts across regions using a threshold-free Rank-Rank Hypergeometric Overlap (RRHO) analysis (33, 34) (Fig. 1b) and found that DE transcripts were highly similar across regions, consistent with Venn diagram analyses (Fig. S2; Dataset S7). Since RRHO investigates p-value concordance across regions and does not capture effect size concordance, we also applied a two-way ANOVA linear model and found no significant interactions in differential expression (q<0.05) between brain region and disease, suggesting disease effects are mostly consistent across regions (Dataset S8). Given the high degree of similarity, we performed a meta-analysis combining differential expression data from all three regions (Datasets S9 and S10) and observed 106 transcripts that were DE. The majority of these transcripts were up-regulated and enriched for immune- and inflammation-related pathways, similar to individual region analyses (Fig. 1c–d; Dataset S11 and S12; upstream regulators in Fig. S3),
Previous findings from our group identified time-of-day-dependent differential expression in the dlPFC in SCZ subjects who died at night (24). Here, we performed similar stratified differential expression analyses by dividing subjects into those who died during the day or night. More DE transcripts were observed in the day in the caudate [346 (day) vs. 156 (night); BH-p<0.001] and putamen [708 (day) vs. 368(night); BH-p<0.001] and during the night in the NAc [100 (day) vs. 159 (night); BH-p<0.01] (Fig. 2; Table S3; Dataset S6). Notably, there is a low degree of overlap between transcripts DE during the day compared to night (Fig. S4; Dataset S7). Between region comparisons showed a high degree of overlap in DE transcripts across regions during the day, but much lower overlap at night (Figure S5; Dataset S7). There were more down- than up-regulated transcripts at night in the NAc [133 (down) vs. 26 (up); BH-p<0.001] and caudate [112 (down) vs. 44 (up); BH-p<0.001] (Fig. S1; Dataset S6). In the putamen, there were more down- than up-regulated transcripts during both the day [574 (down) vs. 134 (up); BH-p<0.001] and night [305 (down) vs. 63 (up); BH-p<0.001] (Fig. S1; Dataset S6). In the NAc, DE transcripts were significantly enriched for mitochondrial-related functions, but only in subjects who died at night (Fig. 2; Dataset S12). These mitochondrial-related transcripts were primarily down-regulated (Fig. S1; Dataset S13). In the caudate and putamen, day/night differences in immune- and inflammation-related signaling pathways were observed (Fig. 2; Dataset S12). Together, these findings suggest that while differential expression is largely similar across the striatum, differences emerge when TOD is taken into consideration.
Figure 2.

Differential expression between psychosis and comparison subjects who died during the day compared to night. The psychosis and matched comparison cohorts were split into subjects who died either during the day or during the night and differential expression analyses were performed for NAc (A), caudate (B), and putamen (C). Volcano plots (left) are shown for each region and time of day, with horizontal dashed lines representing a cutoff of p<0.01 and vertical dashed lines represent log2FC cutoffs of ≤−0.26 or ≥0.26. Transcripts meeting both cutoffs are represented by red circles.. Pathway enrichment (right; top 5 significant pathways) comparing transcripts that were DE only in subjects who died during the day compared to those that were only DE in subjects who died at night. Both up- and down-regulated transcripts were considered for pathway analyses. The significance threshold for pathway enrichment is p<0.05 (-log10(p-value)>1.3 in plots).
Loss of gene expression rhythms in psychosis subjects
Next, we determined if rhythmic changes in the striatum could be contributing to time-of-day-dependent differential expression. We detected many rhythmic transcripts across the striatum in comparison subjects (Fig. 3a; Table S4; Dataset S14), with more in the putamen compared to the NAc and caudate, similar to our previous larger cohort (25). Subjects with psychosis had far fewer rhythmic transcripts (Fig. 3a; Table S4), which were distinct from comparison subjects (Fig. 3b, Figs. S6 and S7).
Figure 3.

Comparisons of diurnal rhythms in gene expression between psychosis and comparison subjects. (A) Amplitude vs p-value plots for comparison (top) and psychosis (bottom) subjects for NAc (left), caudate (middle), and putamen (right). Blue dots represent transcripts that meet a threshold of p<0.01 (indicated by the black dashed line at −log10p=2). (B) RRHO plots show low degree of overlap in rhythmic transcripts between psychosis and comparison subjects in each striatal region.
Consistent with previous human postmortem studies (23–25, 32, 41), many of the core circadian clock genes were rhythmic in comparison subjects with consistent peak times across the striatum (Fig. 4; Dataset S15). In psychosis subjects, however, core clock genes lose rhythmicity (Fig. 4; Dataset S15). Previously, we observed that small nucleolar RNAs (snoRNAs), were the top rhythmic transcripts in the NAc of a larger cohort of unaffected subjects from which the comparison subjects in this study were selected (25). Consistent with these findings, biotype plots revealed that snoRNAs were enriched (10%; p<0.0001) among the top 100 rhythmic transcripts in the NAc of comparison subjects (Fig. 5a; Dataset S16), but not psychosis subjects (Fig. 5a; Fig. S8; Dataset S16). SnoRNAs were not rhythmic in the caudate or putamen. In the NAc, there was a significant loss of rhythmicity in snoRNAs in psychosis subjects (Fig. 5b; Table S5), which may have implications for translation or RNA splicing.
Figure 4.

Core circadian clock genes lose rhythmicity in psychosis subjects. Scatterplots are shown for the core clock genes, BMAL1 (A), PER2 (B), CRY1 (C), and Rev-Erbα (D) in the NAc (top), caudate (middle), and putamen (bottom) of comparison and psychosis subjects. In the scatterplots, each dot represents a subject with the x-axis indicating time of death on a ZT scale (−6 – 18h) and the y-axis indicating level of transcript expression. The red line is the fitted sinusoidal curve. Peak times and rhythmicity p-values are located above the plots. Loss of rhythmicity p-values are located below the psychosis plots.
Figure 5.

Rhythmic snoRNAs are enriched in the NAc of comparison subjects and lose rhythmicity in psychosis. (A) Biotype charts of the top 100 rhythmic transcripts in the NAc of comparison and psychosis subjects. (B) Scatterplots of the top four rhythmic snoRNAs in the NAc of comparison subjects (top) compared to psychosis subjects (bottom). In the scatterplots, each dot represents a subject with the x-axis indicating time of death on a ZT scale (−6 – 18h) and the y-axis indicating level of transcript expression. The red line is the fitted sinusoidal curve. Peak times and rhythmicity p-values are located above the plots. Loss of rhythmicity and amplitude p-values are located below the psychosis plots.
To more rigorously assess rhythm alterations in psychosis, we performed gain/loss of rhythmicity analyses. We identified subsets of transcripts that gain or lose rhythmicity in each region, with most losing rhythmicity in psychosis (Fig. 6a; Dataset S17). The top pathways that lose rhythmicity in the NAc of psychosis subjects are related to vesicular trafficking and inflammation (Fig. 6b; Dataset S18; see Fig. S9 and Dataset S19 for lists of the top rhythmic pathways). In the caudate and putamen, the top pathways that lose rhythmicity are related to cellular stress and mitochondrial function, respectively (Fig. 6b; Dataset 18), and core circadian clock proteins were among the top enriched upstream regulators (Fig. S10). Moreover, circadian-related biological processes also lose rhythmicity in the caudate and putamen (Fig. 6b; Dataset S20). Splicing-related processes lose rhythmicity in the NAc and putamen (Fig. 6b; Dataset S20).
Figure 6.

Gain and loss of rhythmicity analyses between psychosis and comparison subjects in the NAc, caudate, and putamen. (A) Table showing the number of transcripts that gain or lose rhythmicity in the NAc, caudate, and putamen. Pathway enrichment (left) for transcripts that lose (B) or gain (C) rhythmicity in each striatal region. The significance threshold for pathway is p<0.05 (-log10(p-value)>1.3 in plots). GO biological process enrichment (right) via Metascape for transcripts that lose (B) or gain (C) rhythmicity in each striatal region. Meta-analysis was used to compare process enrichment across striatal regions, which is shown in the Cytoscape network plots. The most statistically significant term within a cluster was chosen as the representative cluster name. The top 10 significant terms were chosen for visualization. The nodes are represented as pie charts, where the size of the pie is proportional to the total number of gene hits for that specific term. The pie charts are color-coded based on striatal region, with the size of the slice representing the percentage of transcripts enriched for each corresponding term.
Glutamate receptor signaling gains rhythmicity in the NAc of subjects with psychosis
More transcripts gain rhythmicity in the NAc compared to the caudate and putamen (Fig. 6a). These transcripts are enriched for calcium and glutamate receptor signaling (Fig. 6c; Datasets S18 and S20), which include the AMPA and NMDA glutamate receptor subunits (e.g., GRIA1 and GRIN2A) heavily implicated in SCZ (42).Together, while there is a major loss in circadian rhythm signaling across the striatum, glutamate receptor signaling gains rhythmicity in the NAc in subjects with psychosis, suggesting circadian reprogramming.
Differential expression may be driven by rhythmic changes unique to each striatal subregion
Next, we determined whether changes in rhythmicity were related to differential expression patterns. In the NAc, RRHO analyses showed a high degree of overlap between transcripts rhythmic in psychosis and DE at night (Fig. 7a). These transcripts were enriched for mitochondrial function, EIF2 signaling, and mTOR signaling, suggesting that rhythmicity of these transcripts contributes to their differential expression, similar to the cortex (Fig. 7a; Dataset S21) (24). In the caudate, there is minimal overlap between transcripts rhythmic in psychosis and DE during day or night, with enrichment for immune-related pathways at night (Fig. 7b; Dataset S21). In the putamen, there is overlap between transcripts rhythmic in psychosis and DE during the day, with enrichment for various immune-related pathways (Fig. 7c; Dataset S21).
Figure 7.
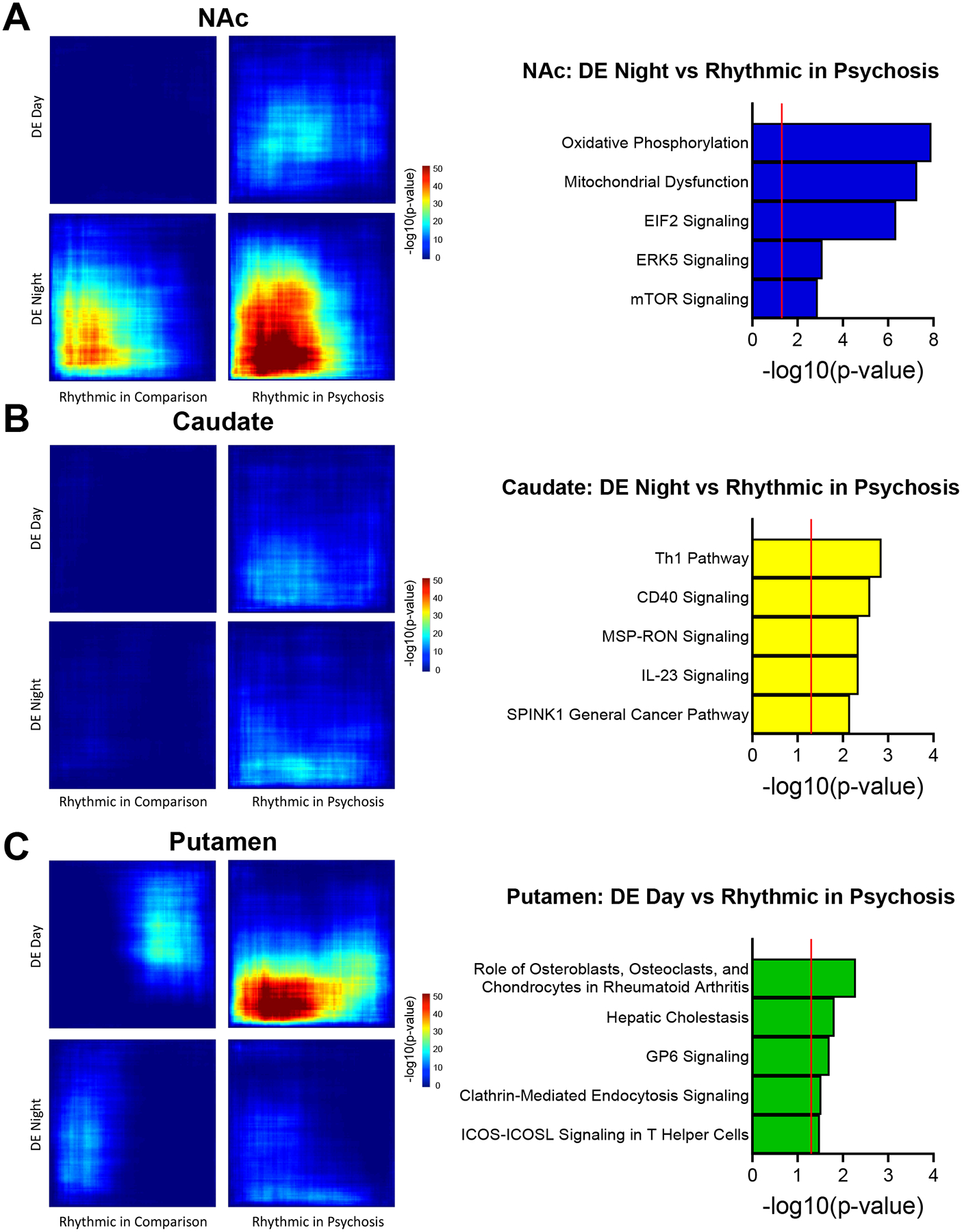
Overlap between transcripts that are rhythmic and differentially expressed during the day or night. RRHO plots (left) showing the overlap between transcripts rhythmic in comparison or psychosis subjects with transcripts differentially expressed during the day or night in the NAc (A), caudate (B), and putamen (C). (A) Pathway enrichment (right) for transcripts in the RRHO plot that are rhythmic in psychosis and DE at night in the NAc (bottom right panel). (B, C) Pathway enrichment (right) for transcripts in the RRHO plot that are rhythmic in psychosis and DE during the night in the caudate (B) and during the day in the putamen (C).
Overlap in rhythmicity between striatal regions
Lastly, we used RRHO analyses to determine the specificity of rhythmicity across striatal regions. In comparison subjects, there was little rhythmic overlap between the NAc and caudate, with higher overlap between the NAc and putamen and caudate and putamen (Fig. 8a; Fig. S11), consistent with previous findings (25). While there was little overlap between the NAc and caudate/putamen in psychosis subjects, there was a striking degree of overlap between the caudate and putamen, suggesting synchronization of rhythms across dorsal striatal regions in psychosis (Fig. 8a). Pathway analyses showed an enrichment in cAMP-mediated signaling and immune-related pathways among overlapping transcripts (Fig. 8b; Dataset S22).
Figure 8.

Overlap in rhythmic transcripts between striatal regions in psychosis and comparison subjects. (A) RRHO plots showing the overlap in rhythmic transcripts between NAc and caudate (left), NAc and putamen (middle), and caudate and putamen (right) for comparison (top) and psychosis (bottom) subjects. (B) Pathway enrichment for transcripts in the RRHO plot (bottom right in panel “A”) that are rhythmic in both caudate and putamen.
Discussion
Decades of research on antipsychotic medications and brain imaging analyses have implicated the striatum in psychosis. Here, we designed a postmortem cohort based on the clinical feature of psychosis, along with a matched comparison cohort, and performed deep sequencing separately in the NAc, caudate, and putamen from the same subjects. We first performed differential expression analyses comparing the psychosis and comparison groups within each region and identify many DE transcripts, with more in the putamen than the caudate and NAc. Notably, we find a high degree of similarity in differential expression across striatal regions. Meta-analyses combining data from all three regions reveal an up-regulation in immune- and inflammation-related transcripts, consistent with a previous microarray study (44). A notable example of a transcript similarly regulated across the striatum is FKBP5. Genetic association studies suggest that variations in FKBP5, a co-chaperone of the glucocorticoid receptor, confer risk for both BD and SCZ, likely via response to environmental stressors including adverse early life events (37, 38). Previous studies have also found higher expression of FKBP5 in the PFC in subjects with SCZ and BD, suggesting this may not be a region-specific phenomenon (39). Another transcript strongly up-regulated across all three regions is the long non-coding RNA, LINC02884. Little is known about its function, but the NHGRI-EBI Catalog of GWAS studies lists associations between SNPs in this transcript with “neuropsychological tests” and “trauma exposure measurement”. There are other transcripts with more specific changes. For example, tyrosine hydroxylase (TH), the rate limiting enzyme in dopamine synthesis (45), is strongly down-regulated in the caudate, unchanged in the NAc, and not detectable in the putamen.
Our previous work in the dlPFC found gene expression differences that selectively occurred in SCZ subjects who died at night (24). When we split subjects in the current study according to TOD, we find differences between ventral and dorsal striatal regions. Overall, the NAc has more DE transcripts in subjects who died at night. Most of these transcripts are down-regulated and enriched in mitochondrial-related pathways, consistent with the dlPFC findings (24). In contrast, the caudate and putamen have more DE transcripts in subjects who died during the day, with an enrichment for different immune- and inflammation-related pathways across day and night. Differences in timing of differential expression across ventral and dorsal striatal regions suggest specificity in circadian phase relationships in these processes. Indeed, we previously identified specific “clusters” of rhythmic transcripts that peak at different times of day between striatal regions(25). Future studies should compare the timing of gene expression patterns in the striatum with other important regions associated with psychosis (e.g., hippocampus, thalamus, prefrontal cortex, and midbrain regions) to determine how these changes are impacted at the network level (46–48).
Next, we further explored diurnal differences in expression between psychosis and comparison subjects by performing rhythmicity analyses and found a substantial loss of rhythmicity in psychosis subjects across all regions, including a loss of rhythmicity in core circadian clock genes. A unique group of transcripts that lose rhythmicity in the NAc are snoRNAs, which are not rhythmic in the caudate or putamen, but are the most highly rhythmic transcripts in the NAc of comparison subjects. SnoRNAs are known to chemically modify other RNAs, thereby regulating processes such as ribosomal biogenesis, RNA splicing, and RNA editing (49, 50). The loss of rhythmicity in snoRNAs from these initial, exploratory analyses suggests potential circadian dysregulation of these processes. One notable example of a snoRNA that loses rhythmicity in psychosis is SNORD115–1, which regulates the alternative splicing of the serotonin 5-HT2C receptor (51). The 5-HT2C receptor is implicated in antipsychotic treatment (52, 53) and RNA editing of this receptor is altered in SCZ (54). Interestingly, a previous RNA-seq study in human postmortem anterior cingulate cortex found differential expression of snoRNAs, including SNORD115, between female SCZ and comparison subjects, but not across diagnosis as a whole, suggesting sex-specific effects (55). Although we do not observe differential expression of snoRNAs, we were not sufficiently powered to examine sex effects. Future studies should explore sex differences in snoRNA expression in psychosis and the relationship between the loss of rhythmicity in SNORD115–1 and other snoRNAs and the splicing, editing, and function of 5-HT2C and other transcripts. Interestingly, in the putamen, we find a loss of rhythmicity in mitochondrial-related transcripts. This is notable since we previously found these transcripts are not rhythmic in control subjects in the dlPFC, but instead gain rhythmicity in SCZ (24).
Circadian reprograming, or change in the rhythmic transcriptome, is now known to occur in the context of many different diseases, as well as in response to environmental changes such as fat content in diet and in normal aging (24, 32, 56, 57). We find that circadian reprogramming primarily occurs in the NAc of psychosis subjects and consists of genes involved in glutamate receptor and calcium signaling. Notably, these pathways include AMPA and NMDA glutamate receptor subunits thought to play a role in SCZ (42). These findings suggest enhanced rhythmicity in glutamatergic signaling and perhaps glutamatergic input to the NAc in psychosis.
We next wanted to determine if changes in rhythmicity might be driving differential expression patterns. Similar to the dlPFC, we find high overlap between transcripts that are rhythmic in the NAc of psychosis subjects and DE in subjects who died at night. These transcripts are involved in mitochondrial function, EIF2 signaling, and mTOR signaling. Taken together, these data strongly suggest that in SCZ, and more broadly with psychosis, there is general down-regulation of mitochondrial-related transcripts within the PFC-NAc circuit that specifically occurs at night. Future studies are needed to understand the implications of this in terms of energy homeostasis and overall function of this circuit across the day/night cycle. Interestingly, previous postmortem studies have found that the number of mitochondria per synapse is decreased in striatal regions of treatment-responsive, but not treatment-resistant, SCZ subjects(58).
Finally, we determined the specificity of the rhythmic transcriptome across striatal regions in psychosis and comparison subjects. Surprisingly, we find an extremely robust overlap between rhythmic transcripts in the caudate and putamen of psychosis subjects, suggesting synchronization in daily rhythms across the dorsal striatum in these subjects. The pathways represented by this overlap are cAMP-mediated signaling and immune-related pathways. It is tempting to speculate that this shared rhythmicity is a result of enhanced rhythms in dopaminergic transmission as the dopamine receptors are G-protein coupled receptors which alter cAMP signaling (59, 60). In support of this, we find that the DRD3 receptor is up-regulated in the caudate and putamen, but not NAc, of subjects with psychosis and this is driven by an increase specifically during the day. This suggests a potential model of increased DRD3 signaling in day versus night in psychosis, leading to an enhanced diurnal rhythm of downstream signaling. This could be due to the disease process itself as psychosis is known to involve excess dopamine, particularly in dorsal striatal regions (1). However, this could also be explained by antipsychotic treatment as these medications target the dopamine D2/D3 receptors (61). While these medications typically have very long half-lives, leading to a steady state across the 24-hour cycle, antipsychotics are often taken at night, thus it is possible they synchronize rhythmicity in dopaminergic receptor signaling. However, we could not test this possibility in the current study given that most of the subjects with psychosis were on antipsychotic medication at TOD. It is important to note that chronic antipsychotic treatment in monkeys does not have a significant impact on differential expression, at least for metabolism-related genes in the cortex (62, 63). Moreover, a previous microarray study found robust up-regulation in inflammation-related transcripts in the associative striatum of SCZ subjects, an effect not observed in the rat striatum following chronic antipsychotic treatment (44). However, in the current study, we cannot exclude the possibility of an effect of medication on our results and future studies will be necessary to determine the effects of antipsychotic treatment on differential expression and molecular rhythms in the striatum. It is also possible that this synchronization is via a different G-protein coupled receptor. One such candidate is the serotonin, 5-HT2A receptor, which is significantly up-regulated in the putamen and trending in the caudate in the psychosis cohort. This effect is also driven by a strong up-regulation during the day. The 5-HT2A receptor is a well-known target of psychedelic drugs such as LSD and is thought to be involved in psychosis (64). Therefore, it is possible that enhanced rhythms in serotonin signaling via the 5-HT2A receptor serves to synchronize these two regions in subjects with psychosis.
The current study includes several design aspects which mitigate interpretative limitations typically associated with postmortem human brain research. First, all subjects are obtained from a community-based population that died suddenly, with limited or no agonal state, consistent with the excellent measures of tissue preservation. Second, the rigorous diagnostic procedures for all subjects ensure accurate, lifetime diagnoses (65), and exclusion of subjects older than 65 years of age avoids the potential confound of age-induced molecular rhythm changes (32). Third, the strict inclusion criteria of documented TOD within a 4-hour window supports the accuracy of our differential expression and molecular rhythm findings. Fourth, the inclusion of SCZ and BD subjects with psychosis supports the interpretation that findings reflect a common feature of psychosis which spans diagnostic groupings. These findings are consistent with a recent RNA-seq study in cortical regions, which found that similarities in gene expression differences across BD and SCZ diagnoses were due to the presence or absence of psychosis, and BD subjects with psychosis were more similar to SCZ subjects than BD subjects without psychosis (27). However, some potential limitations are not addressed by these design features. For example, sleep disturbances are common in SCZ and BD, and altered sleep/wake cycles can influence molecular rhythms (66–68). Whether the current findings reflect the effects of altered sleep/wake cycles, a component of disease pathology, or some combination is unknown. Future studies in animal models will be necessary to determine how sleep disruption impacts molecular rhythms in the striatum. We also cannot rule out the influence of other social zeitgebers (e.g., employment or marital status) on rhythmicity. However, a previous study found that patients with SCZ show severe disruptions in rest-activity patterns and melatonin rhythms compared to healthy, unemployed individuals (69), suggesting employment is not a significant contributor. Due to the limited sample size and nature of the study design, many of the analyses in our study are exploratory and use p-value cutoffs for determining statistical significance. In addition, the relatively small sample size of subjects who died at night compared to day could influence our differential expression findings. However, pathway enrichment for transcripts that are DE at night in the NAc are consistent with findings in the dlPFC, providing confidence in our results (24). Finally, 69% of the included subjects were male, and we recently published a study in which we found profound sex differences in rhythmic transcripts in cortical regions (41). Future studies designed to examine sex-specific effects of psychosis will be important.
In conclusion, we have identified gene expression differences in striatal subregions in subjects with psychosis, as well as changes in 24-hour rhythms in transcription that could underlie disease pathology and response to treatment. Some of these differences are specific to particular regions, such as the down-regulation in mitochondrial-related genes at night in the NAc, while others are common to all striatal regions including a loss of rhythmicity in core clock genes. We also find a surprisingly robust increase in concordance of rhythmic transcripts across dorsal striatal regions with psychosis, which involves an enrichment of cAMP signaling pathways. Future studies will determine the mechanisms through which these changes occur, which could include both genetic and epigenetic changes induced by environmental conditions. We can also use animal and cell culture models to begin to understand how a loss or gain of rhythmicity in these transcripts and regions impacts cellular function and behavior.
Supplementary Material
Acknowledgements and Disclosures
We thank John Enwright III and William MacDonald for helpful discussions. This project was funded by a NARSAD Independent Investigator Award to CAM (Brain and Behavior Foundation) and NIH grants MH111601 to CAM and GT, MH106460 to CAM, and MH120907 and MH128763 to KDK. Brain tissue for this study was provided by the University of Pittsburgh Brain Tissue Donation Program and the NIH NeuroBioBank. Most importantly, we would like to acknowledge and thank all the family members that donated tissue.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.McCutcheon RA, Abi-Dargham A, Howes OD (2019): Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 42:205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, et al. (2006): Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 20:389–409. [DOI] [PubMed] [Google Scholar]
- 3.Kaar SJ, Natesan S, McCutcheon R, Howes OD (2020): Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 172:107704. [DOI] [PubMed] [Google Scholar]
- 4.Agid O, Mamo D, Ginovart N, Vitcu I, Wilson AA, Zipursky RB, et al. (2007): Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response--a double-blind PET study in schizophrenia. Neuropsychopharmacology. 32:1209–1215. [DOI] [PubMed] [Google Scholar]
- 5.Haber SN, Knutson B (2010): The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi EY, Tanimura Y, Vage PR, Yates EH, Haber SN (2017): Convergence of prefrontal and parietal anatomical projections in a connectional hub in the striatum. Neuroimage. 146:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haber SN (2016): Corticostriatal circuitry. Dialogues Clin Neurosci. 18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. (2010): Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 67:231–239. [DOI] [PubMed] [Google Scholar]
- 9.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. (2009): Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 66:13–20. [DOI] [PubMed] [Google Scholar]
- 10.McCutcheon R, Beck K, Jauhar S, Howes OD (2018): Defining the Locus of Dopaminergic Dysfunction in Schizophrenia: A Meta-analysis and Test of the Mesolimbic Hypothesis. Schizophr Bull. 44:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duggirala SX, Schwartze M, Pinheiro AP, Kotz SA (2020): Interaction of emotion and cognitive control along the psychosis continuum: A critical review. Int J Psychophysiol. 147:156–175. [DOI] [PubMed] [Google Scholar]
- 12.Der-Avakian A, Markou A (2012): The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 35:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castaneda TR, de Prado BM, Prieto D, Mora F (2004): Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 36:177–185. [DOI] [PubMed] [Google Scholar]
- 14.Ferris MJ, Espana RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, et al. (2014): Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci U S A. 111:E2751–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozburn AR, Falcon E, Twaddle A, Nugent AL, Gillman AG, Spencer SM, et al. (2014): Direct Regulation of Diurnal Drd3 Expression and Cocaine Reward by NPAS2. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parekh PK, Becker-Krail D, Sundaravelu P, Ishigaki S, Okado H, Sobue G, et al. (2017): Altered GluA1 (Gria1) Function and Accumbal Synaptic Plasticity in the ClockDelta19 Model of Bipolar Mania. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imbesi M, Yildiz S, Dirim Arslan A, Sharma R, Manev H, Uz T (2009): Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience. 158:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, et al. (2010): Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 30:14046–14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashton A, Jagannath A (2020): Disrupted Sleep and Circadian Rhythms in Schizophrenia and Their Interaction With Dopamine Signaling. Front Neurosci. 14:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan RW, McClung CA (2019): Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 20:49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welz PS, Benitah SA (2020): Molecular Connections Between Circadian Clocks and Aging. J Mol Biol. 432:3661–3679. [DOI] [PubMed] [Google Scholar]
- 22.Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, et al. (2018): Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. (2013): Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A. 110:9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seney ML, Cahill K, Enwright JF 3rd, Logan RW, Huo Z, Zong W, et al. (2019): Diurnal rhythms in gene expression in the prefrontal cortex in schizophrenia. Nat Commun. 10:3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ketchesin KD, Zong W, Hildebrand MA, Seney ML, Cahill KM, Scott MR, et al. (2021): Diurnal rhythms across the human dorsal and ventral striatum. Proc Natl Acad Sci U S A. 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markota M, Coombes BJ, Larrabee BR, McElroy SL, Bond DJ, Veldic M, et al. (2018): Association of schizophrenia polygenic risk score with manic and depressive psychosis in bipolar disorder. Transl Psychiatry. 8:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enwright JF, Lewis DA (2021): Similarities in Cortical Transcriptome Alterations Between Schizophrenia and Bipolar Disorder Are Related to the Presence of Psychosis. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bipolar D, Schizophrenia Working Group of the Psychiatric Genomics Consortium. Electronic address drve, Bipolar D, Schizophrenia Working Group of the Psychiatric Genomics C (2018): Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes. Cell. 173:1705–1715 e1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Law CW, Chen Y, Shi W, Smyth GK (2014): voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. (2015): limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seney ML, Kim SM, Glausier JR, Hildebrand MA, Xue X, Zong W, et al. (2021): Transcriptional Alterations in Dorsolateral Prefrontal Cortex and Nucleus Accumbens Implicate Neuroinflammation and Synaptic Remodeling in Opioid Use Disorder. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CY, Logan RW, Ma T, Lewis DA, Tseng GC, Sibille E, et al. (2015): Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plaisier SB, Taschereau R, Wong JA, Graeber TG (2010): Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 38:e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cahill KM, Huo Z, Tseng GC, Logan RW, Seney ML (2018): Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Sci Rep. 8:9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. (2019): Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 10:1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. (2003): Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13:2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daskalakis NP, Binder EB (2015): Schizophrenia in the spectrum of gene-stress interactions: the FKBP5 example. Schizophr Bull. 41:323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matosin N, Halldorsdottir T, Binder EB (2018): Understanding the Molecular Mechanisms Underpinning Gene by Environment Interactions in Psychiatric Disorders: The FKBP5 Model. Biol Psychiatry. 83:821–830. [DOI] [PubMed] [Google Scholar]
- 39.Sinclair D, Fillman SG, Webster MJ, Weickert CS (2013): Dysregulation of glucocorticoid receptor co-factors FKBP5, BAG1 and PTGES3 in prefrontal cortex in psychotic illness. Sci Rep. 3:3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiTacchio L, Le HD, Vollmers C, Hatori M, Witcher M, Secombe J, et al. (2011): Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science. 333:1881–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logan RW, Xue X, Ketchesin KD, Hoffman G, Roussos P, Tseng G, et al. (2021): Sex Differences in Molecular Rhythms in the Human Cortex. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schizophrenia Working Group of the Psychiatric Genomics C (2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature. 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiihonen J, Koskuvi M, Lahteenvuo M, Trontti K, Ojansuu I, Vaurio O, et al. (2021): Molecular signaling pathways underlying schizophrenia. Schizophr Res. 232:33–41. [DOI] [PubMed] [Google Scholar]
- 44.Lanz TA, Reinhart V, Sheehan MJ, Rizzo SJS, Bove SE, James LC, et al. (2019): Postmortem transcriptional profiling reveals widespread increase in inflammation in schizophrenia: a comparison of prefrontal cortex, striatum, and hippocampus among matched tetrads of controls with subjects diagnosed with schizophrenia, bipolar or major depressive disorder. Transl Psychiatry. 9:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daubner SC, Le T, Wang S (2011): Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 508:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kesby JP, Eyles DW, McGrath JJ, Scott JG (2018): Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl Psychiatry. 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomes FV, Grace AA (2021): Beyond Dopamine Receptor Antagonism: New Targets for Schizophrenia Treatment and Prevention. Int J Mol Sci. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonnenschein SF, Gomes FV, Grace AA (2020): Dysregulation of Midbrain Dopamine System and the Pathophysiology of Schizophrenia. Front Psychiatry. 11:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupuis-Sandoval F, Poirier M, Scott MS (2015): The emerging landscape of small nucleolar RNAs in cell biology. Wiley Interdiscip Rev RNA. 6:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hombach S, Kretz M (2016): Non-coding RNAs: Classification, Biology and Functioning. Adv Exp Med Biol. 937:3–17. [DOI] [PubMed] [Google Scholar]
- 51.Kishore S, Stamm S (2006): The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 311:230–232. [DOI] [PubMed] [Google Scholar]
- 52.Castensson A, Aberg K, McCarthy S, Saetre P, Andersson B, Jazin E (2005): Serotonin receptor 2C (HTR2C) and schizophrenia: examination of possible medication and genetic influences on expression levels. Am J Med Genet B Neuropsychiatr Genet. 134B:84–89. [DOI] [PubMed] [Google Scholar]
- 53.Rosenzweig-Lipson S, Comery TA, Marquis KL, Gross J, Dunlop J (2012): 5-HT(2C) agonists as therapeutics for the treatment of schizophrenia. Handb Exp Pharmacol.147–165. [DOI] [PubMed] [Google Scholar]
- 54.Sodhi MS, Burnet PW, Makoff AJ, Kerwin RW, Harrison PJ (2001): RNA editing of the 5-HT(2C) receptor is reduced in schizophrenia. Mol Psychiatry. 6:373–379. [DOI] [PubMed] [Google Scholar]
- 55.Ragan C, Patel K, Edson J, Zhang ZH, Gratten J, Mowry B (2017): Small non-coding RNA expression from anterior cingulate cortex in schizophrenia shows sex specific regulation. Schizophr Res. 183:82–87. [DOI] [PubMed] [Google Scholar]
- 56.Xue X, Zong W, Glausier JR, Kim SM, Shelton MA, Phan BN, et al. (2022): Molecular rhythm alterations in prefrontal cortex and nucleus accumbens associated with opioid use disorder. Transl Psychiatry. 12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, et al. (2013): Reprogramming of the circadian clock by nutritional challenge. Cell. 155:1464–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Somerville SM, Lahti AC, Conley RR, Roberts RC (2011): Mitochondria in the striatum of subjects with schizophrenia: relationship to treatment response. Synapse. 65:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drago J, Padungchaichot P, Accili D, Fuchs S (1998): Dopamine receptors and dopamine transporter in brain function and addictive behaviors: insights from targeted mouse mutants. Dev Neurosci. 20:188–203. [DOI] [PubMed] [Google Scholar]
- 60.Missale C, Fiorentini C, Collo G, Spano P (2010): The neurobiology of dopamine receptors: evolution from the dual concept to heterodimer complexes. Journal of receptor and signal transduction research. 30:347–354. [DOI] [PubMed] [Google Scholar]
- 61.Meltzer HY (2017): New Trends in the Treatment of Schizophrenia. CNS Neurol Disord Drug Targets. 16:900–906. [DOI] [PubMed] [Google Scholar]
- 62.Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P (2002): Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci. 22:2718–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glausier JR, Enwright JF 3rd, Lewis DA (2020): Diagnosis- and Cell Type-Specific Mitochondrial Functional Pathway Signatures in Schizophrenia and Bipolar Disorder. Am J Psychiatry. 177:1140–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Gregorio D, Comai S, Posa L, Gobbi G (2016): d-Lysergic Acid Diethylamide (LSD) as a Model of Psychosis: Mechanism of Action and Pharmacology. Int J Mol Sci. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glausier JR, Kelly MA, Salem S, Chen K, Lewis DA (2020): Proxy measures of premortem cognitive aptitude in postmortem subjects with schizophrenia. Psychol Med. 50:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Husse J, Kiehn JT, Barclay JL, Naujokat N, Meyer-Kovac J, Lehnert H, et al. (2017): Tissue-Specific Dissociation of Diurnal Transcriptome Rhythms During Sleep Restriction in Mice. Sleep. 40. [DOI] [PubMed] [Google Scholar]
- 67.Archer SN, Oster H (2015): How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J Sleep Res. 24:476–493. [DOI] [PubMed] [Google Scholar]
- 68.Hor CN, Yeung J, Jan M, Emmenegger Y, Hubbard J, Xenarios I, et al. (2019): Sleep-wake-driven and circadian contributions to daily rhythms in gene expression and chromatin accessibility in the murine cortex. Proc Natl Acad Sci U S A. 116:25773–25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM (2012): Sleep and circadian rhythm disruption in schizophrenia. The British journal of psychiatry : the journal of mental science. 200:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


