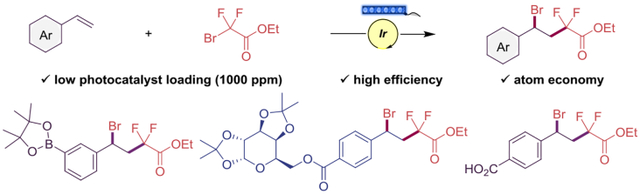
Abstract
An operationally simple and practical method is disclosed to achieve the difunctionalization of styrenes, generating fluorinated benzyl bromides via a photoinduced atom transfer radical addition (ATRA) process. The developed method is mild, atom-economical, cost-effective, employs very low photocatalyst loading (1000 ppm), and is highly compatible with a broad range of functional groups on styrene. The versatility of the fluorinated benzyl bromides is demonstrated through their derivatization to a variety of valuable compounds.
Graphical Abstract
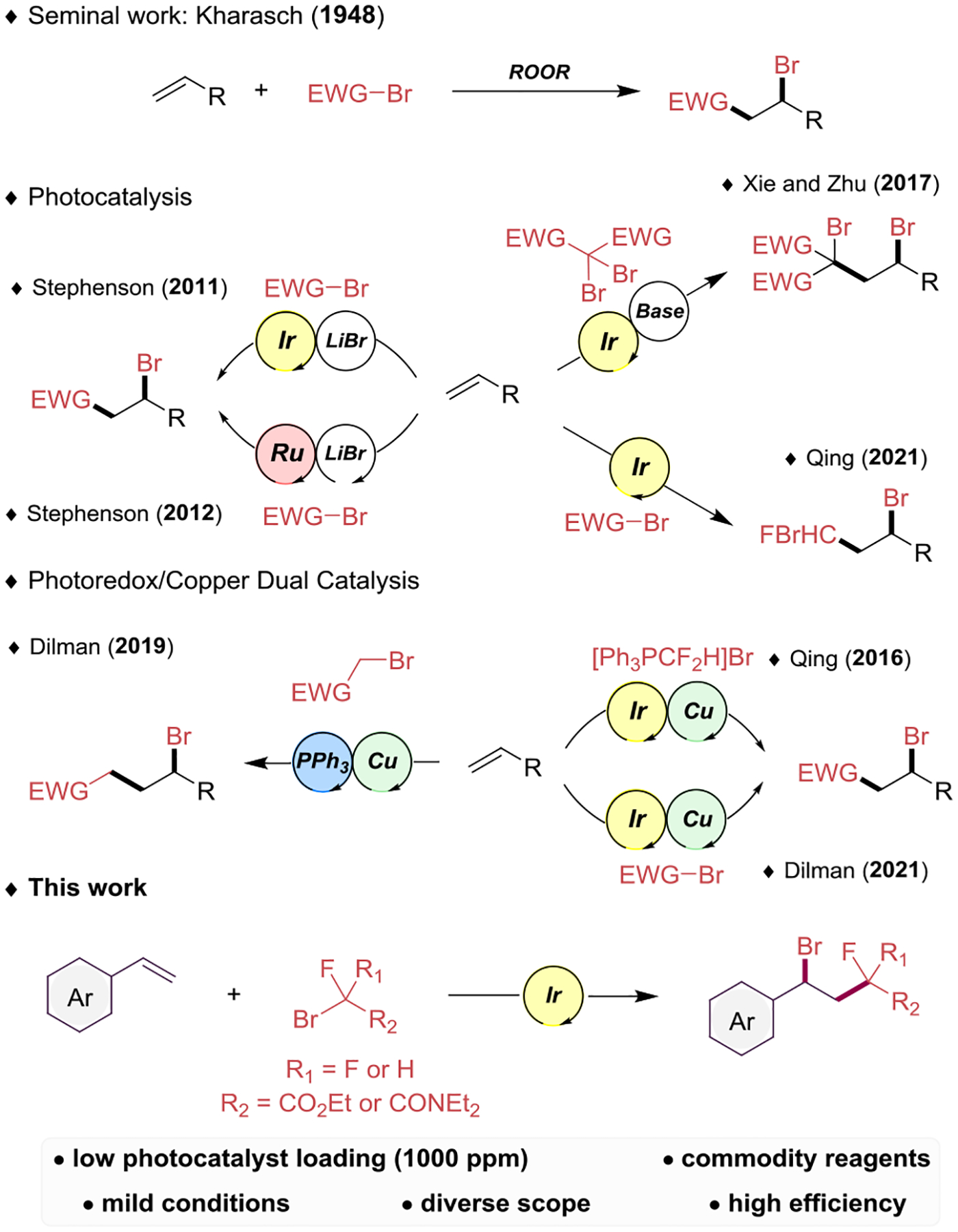
The introduction of two different functional groups across a double bond, known as alkene difunctionalization, is an attractive method from a synthetic point of view, especially if such difunctionalization occurs atom economically in a single step. Thus, 1,2-difunctionalization of alkenes has been broadly explored employing transition metal-catalyzed-,1 photoredox-,2 or hypervalent iodine reagents.3 The introduction of fluorinated moieties into a bioactive molecule can influence lipophilicity and binding selectivity, and enhance metabolic stability as well.4 The trifluoromethyl group is undoubtedly the most popular fluorinated functional group in medicinal chemistry,5 and thus its introduction to organic molecules has been widely explored.6 However, the difluoromethylene group is emerging as an equally interesting motif.7 Difunctionalizations assembling C–CF2R and C–X bonds in a single step have been developed by traditional methods,8 which typically require the use of hazardous chemicals and/or harsh conditions. Regarding the 1,2-difunctionalization of alkenes generating C–Br and C–CF2R bonds, several photoinduced methods9 have been reported in the last decade (Scheme 1). Stephenson’s group9a reported an atom transfer radical addition (ATRA) of haloalkanes and α-halocarbonyls to olefins employing an Ir photocatalyst and stochiometric LiBr. Later, the same group complemented the strategy using a Ru photocatalyst and substoichiometric LiBr.9b Further investigations involved the difunctionalization of alkenes using gem-dibromides9d or photoredox/copper dual catalysis approaches.9e,g Despite these precedents, the preparation of difluoromethylene-containing benzyl bromides still lacks a more versatile and milder method, thus suggesting the need for further exploration of reaction space.
Scheme 1.
Bromine Transfer Radical Addition Strategies
Benzyl bromides have served as classic electrophiles in synthetic organic chemistry.10 Regarding their application in synthesis, their use is not only limited to the classic nucleophilic substitution reaction - they have also been used for the preparation of aldehydes, ketones11 or triazoles.12 In addition, they have served as building blocks in biologically active molecules,13 as precursors in cross-coupling reactions promoted by transition metals,14 and they have found application in photoinduced reactions.15 Classically, the most popular preparations of benzyl bromides rely on the use of N-bromosuccinimide and a radical activator.16 The preparation from the corresponding benzylic alcohol using HBr or PBr3 is also popular. Other benzylic monobromination methods include the use of diatomic bromine,17 bromotrichloromethane,18 or NaBrO3,19 among others.20 However, these methods generally have limited functional group tolerance, the formed byproducts are challenging to remove from the desired product, and, typically, only moderate to good yields are obtained.
Given the interest in having fluorinated moieties in functionalized benzyl bromides, herein we report the development of a mild, rapid, and efficient visible light-mediated photocatalytic ATRA to styrenes using α-bromo-α-fluorocarbonyls (Scheme 1). As is demonstrated, the prepared fluorinated benzyl bromides serve as valuable intermediates in further transformations through their functional groups.
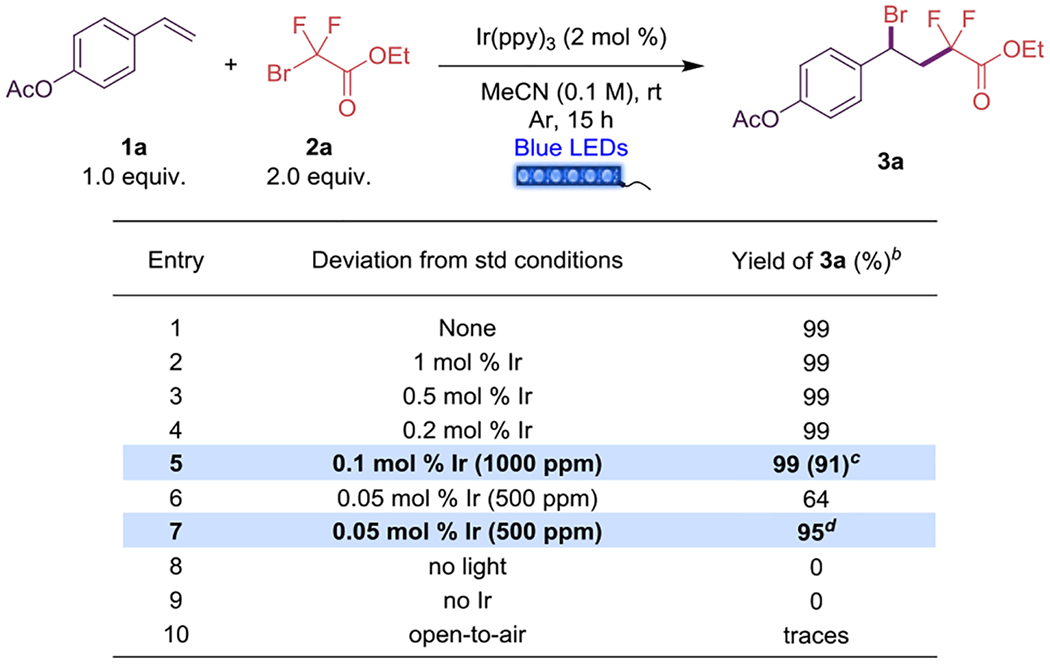
To initiate the studies, we selected 4-acetoxystyrene (1a) as a model substrate for the transformation, along with ethyl bromodifluoroacetate 2a. From the outset, full conversion of the styrene 1a to the desired fluorinated benzyl bromide (3a) was observed using Ir(ppy)3 as a photocatalyst in acetonitrile solvent after 15 h of blue (λmax = 455 nm) LED strip irradiation (Table 1, entry 1). With these conditions in hand, our next aim was to explore the minimum catalyst loading necessary to achieve full conversion of the starting materials. Thus, the photocatalyst loading was further decreased to 0.1 mol % (1000 ppm) without decreasing yield efficacy. When the reaction was scaled to 0.5 mmol, 24 h was needed to consume all the starting material 1a (Table 1, entry 5). Notably, and to the best of our knowledge, the use of only 1000 ppm of photocatalyst constitutes one of the lowest photocatalyst loadings used in a photoredox transformation.21 We further reduced the photocatalyst loading to 500 ppm (Table 1, entry 6) using the same standard conditions, and observed a 64% yield. Nevertheless, this yield was improved to 95% when the reaction was maintained for 36 h (Table 1, entry 7).
Table 1.
Optimization of Reaction Conditionsa
|
Reaction conditions: styrene 1a (0.1 mmol), 2a (0.2 mmol), Ir(ppy)3 (2 mol %) in MeCN (1.0 mL, 0.1 M), 15 h irradiation with blue LED strips (λmax = 456 nm).
Yields were determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard.
Isolated yield at 0.5 mmol scale and 24 h.
Reaction for 36 h. std: standard.
The photochemical nature of this transformation was confirmed when control experiments in the absence of light irradiation or the photocatalyst showed no conversion to product 3a (Table 1, entries 8 and 9). The atom-economy of this transformation and the use of volatile MeCN as the solvent makes straightforward isolation of 3a remarkably easy by simple filtration through a short pad of silica.
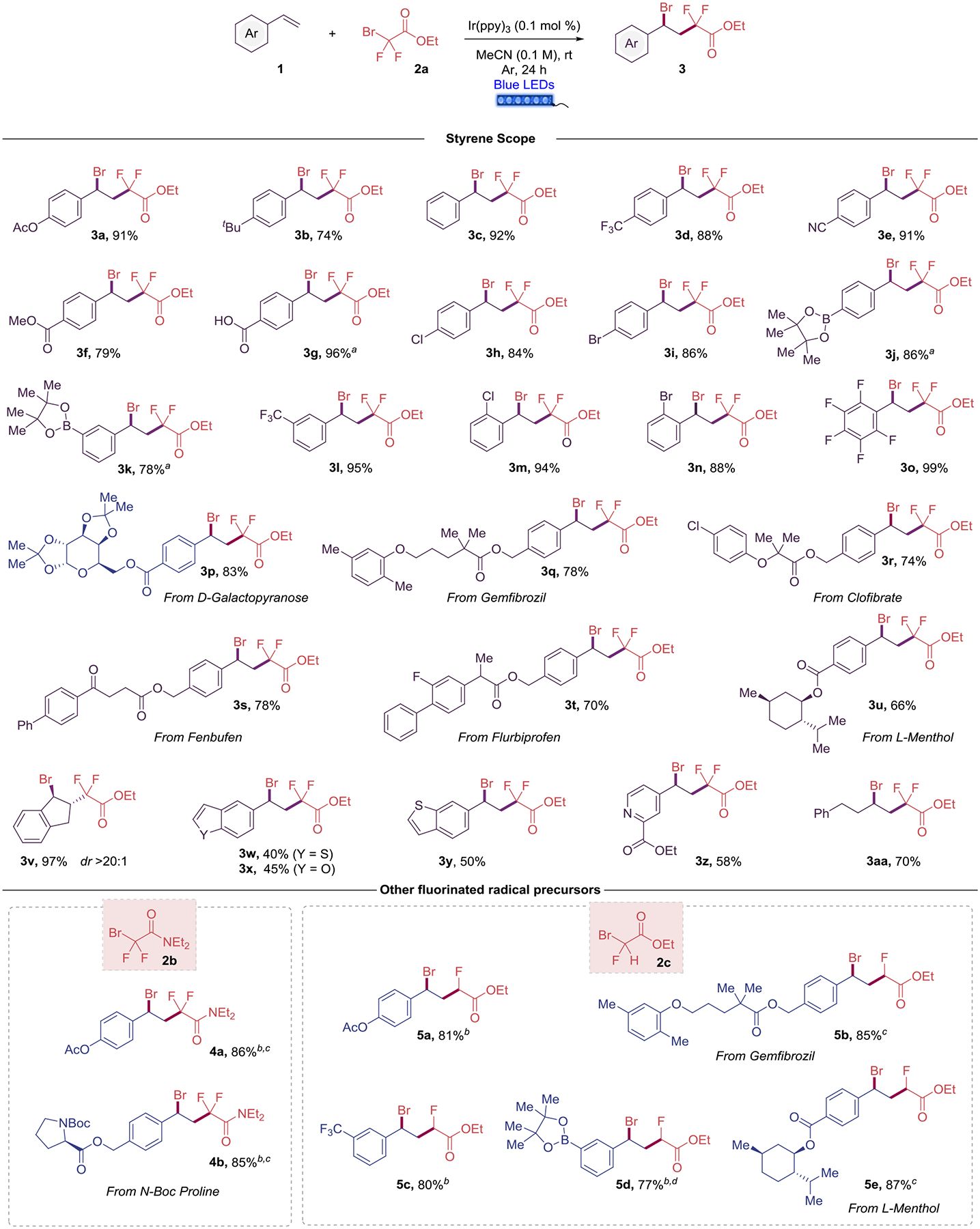
With the reaction conditions established, we first explored the scope of this transformation using different styrenes (Scheme 2).. In general, electron-rich, electron-neutral, and electron-poor styrenes were successfully difunctionalized. 4-Acetoxy- and 4-tert-butyl styrenes delivered the desired compounds (3a and 3b) in 91% and 74% yield, respectively. The presence of strong electron-withdrawing groups in the para position served as excellent substrates, providing trifluoromethyl- (3d), nitrile- (3e), ester- (3f), and pinacol boronate- (3j) containing fluorinated benzyl bromides in excellent yields. Of note, unprotected para-vinylbenzoic acid was also used to provide the desired 3g in 96% yield. A complete consumption of styrenes containing a carboxyl group and a boronic ester as substituents on the phenyl ring were achieved using 4 equiv of the radical precursor (3g, 3j-k). Additionally, both para chloro- (3h), and para-bromo- (3i) substituted derivatives were prepared in high yields.
Scheme 2.
Evaluation of Substrate Scope
General reaction conditions: styrene 1 (0.5 mmol), 2a (1.0 mmol), Ir(ppy)3 (0.1 mol %) in MeCN (5.0 mL, 0.1 M), 24 h irradiation with blue LED strips (λmax = 456 nm). a4 equiv (2.0 mmol) of 2a were used. b0.5 mol % Ir(ppy)3 was used. c1.1 equiv (0.55 mmol) of 2b was used. d4 equiv (2.0 mmol) of 2c were used. See Supporting Information for further details.
Furthermore, pinacol boronate (3k) and CF3 (3l) groups in the meta position delivered the fluorinated benzyl bromide in 78% and 95% yields, respectively. Moreover, the reactivity of this transformation was not affected by steric effects, as ortho halosubstituted styrenes also worked well with the optimized conditions (3m and 3n). Interestingly, the polyfluorinated benzyl bromide 3o was obtained in quantitative yield.
Further, the amenability of this difunctionalization reaction to late-stage complex molecule synthesis was examned using styrene-derived pharmaceuticals and biomolecules. Thus, bromine- and difluoroethyl acetate substituents were successfully installed in the styrene skeleton attached to the monosaccharide D-galactopyranose (3p), lipid-lowering agents gemfibrozil (3q) and clofibrate (3r), anti-inflammatories fenbufen (3s) and flurbiprofen (3t), and monoterpene alcohol L-menthol (3u). In these cases, the yields were good to excellent (66–92%).
Difunctionalization of indene provided 3v in high yield and diastereomeric ratio. Of note, heterocyclic-based styrenes were also accommodated under standard conditions (3w-z), although in lower yield because of competing oligomerization. Finally, 4-phenyl-1-butene was successfully difunctionalized as a non-activated alkene substrate in 70% yield.
Subsequently, we tested the reaction conditions using 2b and 2c as bromofluorinated radical precursors. When using the standard conditions with these two substrates, the conversion of the styrene 3a to the desired compounds (4a and 5a) were 60% and 50%, respectively. We observed full conversion of the styrene when we increased the amount of iridium photocatalyst to 0.5 mol %. Bromo N,N-diethyl-difluoroacetamide (2b) was successfully installed on p-acetoxy styrene (4a) and styrene-containing N-Boc protected proline amino acid (4b) in high yields (86 and 85%). Finally, monofluoro derivative 2c also served as a competent radical precursor for difunctionzalizaiton of styrenes. Thus, electron-rich (5a) and electron-poor styrenes (5c and 5d) were amenable substrates, as well as more complex styrenes (5b and 5e). Excellent yields were obtained in all tested cases. These brominated radical precursors (2a-c) have showcased they can serve as difunctional reagents22 also on styrenes in a sustainable manner. Of note, even though the method is rooted in the use of a transition metal-based photocatalyst, all the scope described herein (3a-3aa, 4a-b, 5a-e) has been performed utilizing less than 20 mg of Ir in total (which implies around $30 cost). This practical and selective fluorination protocol, based on the use of readily available starting materials and a cost-efficient photocatalyst, as well as the showcased functional group tolerance, makes it an attractive and general method for the assembly of fluoride-functionalized molecules.4d
To elucidate the reaction mechanism of this photoinduced reaction, photochemical quantum yield studies (Φ) were carried out (see Supporting Information). The experimental results reveal a Φ value of 1.8, thus indicating a feasible radical chain mechanism.23 Given previous reports9 and the mechanistic findings described herein, a plausible mechanism for this transformation is shown in Scheme 3A. The photoexcited state of the photocatalyst reduces the α-bromo-α-fluorocarbonyl (2) to the α-fluorocarbonyl radical (I), which enters a radical chain cycle. At this stage, the styrene (1) intercepts the α-fluorocarbonyl radical (I), generating the benzylic radical (II). This radical intermediate undergoes halogen-atom transfer (XAT)24 with 1, forming the final product (3) and regenerating the α-fluorocarbonyl radical (I). Although a mechanistic scenario in which the carbocation (III) can be generated via oxidation by the IrIV species and subsequently trapped by a bromide anion (radical/polar mechanism), it is an unlikely process with the described mechanistic findings depicted above.
Scheme 3.
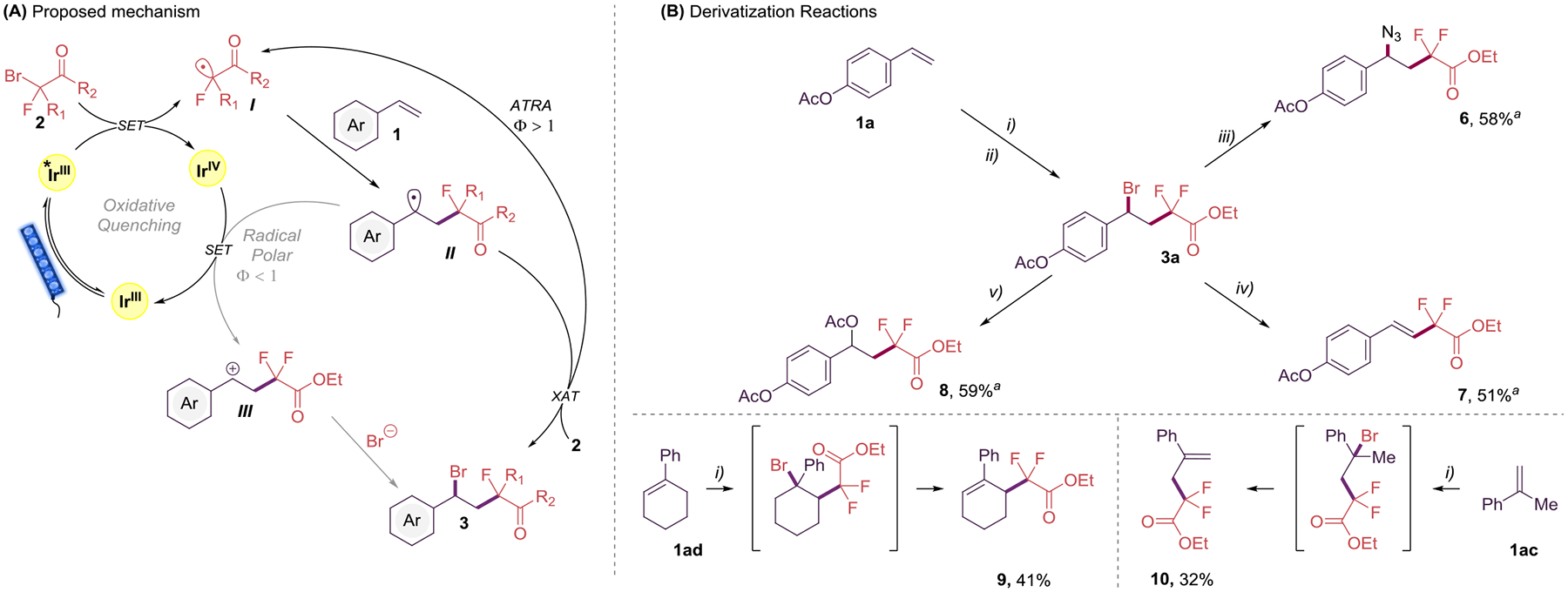
Proposed mechanism (A) and Derivatization Reactions (B)
(A) Proposed mechanism for the preparation of fluorinated benzyl bromides 3. (B) Derivatization reactions. Reaction conditions: i) 1 (0.5 mmol), 2a (1.0 mmol), Ir(ppy)3 (0.1 mol %) in MeCN (5.0 mL, 0.1 M), 24 h irradiation with blue LED strips (λmax = 456 nm). ii) Filtration through a short pad of silica. iii) NaN3 (0.75 mmol) in DMSO (5 mL, 0.1 M), overnight at rt. iv) EtONa (2.5 mmol) in EtOH (5 mL, 0.1 M), overnight at rt. v) AgOAc (0.5 mmol) in MeCN, 4 h at 50 °C. aIsolated yield from 1a.
Finally, to demonstrate the inherent synthetic value of the developed difunctionalization process, several derivatization experiments were undertaken (Scheme 3B). The crude benzyl bromide 3a formed via the standard condition was simply filtered through a short pad of silica and then directly employed with derivatization conditions to generate products such as an azide 6, useful for elaboration in click reactions. Further, treatment of 3a under basic conditions furnished the elimination product 7, and the reaction of 3a in the presence of AgOAc provided the formation of a new C–O bond (8). Lastly, when using -substituted styrenes 1ac or 1ad under the standard conditions, we observed the direct formation of the kinetically stable alkenes 9 and 10 in moderate yields.
In summary, a highly practical photoredox method for the formation of synthetically important fluorinated benzyl bromide products is reported. The process is not only mild and atom-economical, but also cost-effective as it requires only minimal loading of the photocatalyst. The method is vastly robust to accommodate a wide range of functional groups on styrene as well as medicinally relevant fluorinated groups. More importantly, the synthetic utility of the generated benzyl bromide was showcased by subjecting it to several derivatization conditions. Based on the mechanistic investigation and in accordance with experimentally determined quantum yield, we proposed a halogen-atom transfer (XAT) chain-mechanism for the developed process.
Supplementary Material
ACKNOWLEDGMENT
The authors are thankful to Dr. María Jesús Cabrera-Afonso (UPenn) and Dr. Longbo Li (UPenn) for the donation styrenes 1f, 1j, 1k and 1z. Weizhe Dong (UPenn) is also acknowledged for useful discussions. The authors are grateful for financial support provided by NIGMS (R35 GM 131680 to G.M. The NSF Major Research Instrumentation Program (award NSF CHE-1827457), the NIH supplement awards 3R01GM118510-03S1 and 3R01GM087605-06S1, as well as the Vagelos Institute for Energy Science and Technology supported the purchase of the NMRs used in this study. We thank Dr. Charles W. Ross, III (UPenn) for mass spectral data, and Kessil for the donation of lamps.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website. Preparation of starting materials, general procedures, characterization data for products [nuclear magnetic resonance (NMR), infrared (IR), and mass spectrometry (MS)], mechanistic studies, and NMR spectra (PDF).
The authors declare no competing financial interest
REFERENCES
- (1).Selected examples on transition-metal catalyzed alkene difunctionalization:; (a) Dhungana RK; KC S; Basnet P; Giri R Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec 2018, 18, 1314–1340. [DOI] [PubMed] [Google Scholar]; (b) KC S; Dhungana RK; Khanal N; Giri R Nickel-Catalyzed alpha-Carbonylalkylarylation of Vinylarenes: Expedient Access to gamma,gamma-Diarylcarbonyl and Aryltetralone Derivatives. Angew. Chem. Int. Ed 2020, 59, 8047–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang SY; Wang CH; Ye XH; Shi XD Intermolecular Alkene Difunctionalization via Gold-Catalyzed Oxyarylation. Angew. Chem. Int. Ed 2020, 59, 20470–20474. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Barve BD; Kuo YH; Li WT Pd-Catalyzed and ligand-enabled alkene difunctionalization via unactivated C-H bond functionalization. Chem. Commun 2021, 57, 12045–12057. [DOI] [PubMed] [Google Scholar]; (e) Jiang L; Sarró P; Teo WJ; Llop J Suero, M. G. Catalytic alkene skeletal modification for the construction of fluorinated tertiary stereocenters. Chem. Sci, 2022, 13, 4327–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Selected examples on photocatalyzed alkene difunctionalization:; (a) Campbell MW; Compton JS; Kelly CB; Molander GA Three-Component Olefin Dicarbofunctionalization Enabled by Nickel/Photoredox Dual Catalysis. J. Am. Chem. Soc, 2019, 141, 20069–20078. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sun S-Z; Duan Y; Mega RS; Somerville RJ; Martín R Site-Selective 1,2-Dicarbofunctionalization of Vinyl Boronates through Dual Catalysis. Angew Chem. Int. Ed 2020, 59, 4370–4374. [DOI] [PubMed] [Google Scholar]; (c) Cabrera-Afonso MJ; Sookezian A; Badir SO; El Khatib M; Molander GA Photoinduced 1,2-dicarbofunctionalization of alkenes with organotrifluoroborate nucleophiles via radical/polar crossover. Chem. Sci 2021, 12, 9189–9195. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xu S; Chen HR; Zhou ZJ; Kong WQ Three-Component Alkene Difunctionalization by Direct and Selective Activation of Aliphatic C-H Bonds. Angew. Chem. Int. Ed 2021, 60, 7405–7411. [DOI] [PubMed] [Google Scholar]; (e) Campbell MW; Yuan M; Polites VC; Gutierrez O; Molander GA Photochemical C–H Activation Enables Nickel-Catalyzed Olefin Dicarbofunctionalization. J. Am. Chem. Soc 2021, 143, 3901–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wei YL; Zhang H; Wu XX; Zhu C Alkene Difunctionalization Triggered by a Stabilized Allenyl Radical: Concomitant Installation of Two Unsaturated C-C Bonds. Angew Chem. Int. Ed 2021, 60, 20215–20219. [DOI] [PubMed] [Google Scholar]; (g) Patra T; Das M; Daniliuc CG; Glorius F Metal-free photosensitized oxyimination of unactivated alkenes with bifunctional oxime carbonates. Nat. Catal 2021, 4, 54–61. [Google Scholar]
- (3).Selected examples on hypervalent-mediated alkene difunctionalization:; (a) Lee JH; Choi S; Hong KB Alkene Difunctionalization Using Hypervalent Iodine Reagents: Progress and Developments in the Past Ten Years. Molecules 2019, 24, 2634. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang K; Wang H; Zheng JF; Yu L; Ding HF Hypervalent iodine mediated alkene difunctionalization of vinylphenols: diastereoselective synthesis of substituted indoles and indolizines. Chem. Commun 2015, 51, 6399–6402. [DOI] [PubMed] [Google Scholar]
- (4).(a) O’Hagan D Understanding organofluorine chemistry. An intro-duction to the C–F bond. Chem. Soc. Rev, 2008, 37, 308–319. [DOI] [PubMed] [Google Scholar]; (b) Purser S; Moore PR; Swallow S; Gouverneur V Fluorine in medicinal chemistry. Chem. Soc. Rev 2008, 37, 320–330. [DOI] [PubMed] [Google Scholar]; (c) Wang J; Sánchez-Roselló M; Aceña JL; del Pozo C; Sorochinsky AE; Fustero S; Soloshonok VA; Liu H Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001−2011). Chem. Rev 2014, 114, 2432–2506. [DOI] [PubMed] [Google Scholar]; (d) Liang T; Neumann CN; Ritter T Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem. Int. Ed 2013, 52, 8214–8264. [DOI] [PubMed] [Google Scholar]
- (5).(a) Francis F; Wuest F Advances in [18F]Trifluoromethylation Chemistry for PET Imaging. Molecules, 2021, 26, 6478. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bassetto M; Ferla S; Pertusati F Polyfluorinated groups in medicinal chemistry. Future Med. Chem 2015, 7, 527–546. [DOI] [PubMed] [Google Scholar]; (c) Yerein DE; Bonesi S Postigo, A. Fluorination Methods in Drug Discovery. Org. Biomol. Chem, 2016, 14, 8398–8427 [DOI] [PubMed] [Google Scholar]; (d) Tredwell M; Gouverneur V 1.5 Fluorine in Medicinal Chemistry: Importance of Chirality. Compr. Chirality, 2012, 1, 70–85. [Google Scholar]
- (6).Selected references on trifluoromethylation of organic molecules:; (a) Calvo R; Comas-Vives A; Togni A; Katayev D Taming Radical Intermediates for the Construction of Enantioenriched Trifluoromethylated Quaternary Carbon Centers. Angew. Chem. Int. Ed 2019, 58, 1447–1452. [DOI] [PubMed] [Google Scholar]; (b) Granados A; Rivilla I; Cossío F; Vallribera A Lanthanum-Catalyzed Enantioselective Trifluoromethylation by Using an Electrophilic Hypervalent Iodine Reagent. Chem. Eur. J 2019, 25, 8214–8218. [DOI] [PubMed] [Google Scholar]; (c) Liu X; Xu C; Wang M; Liu Q Trifluoromethyltrimethylsilane: Nucleophilic Trifluoromethylation and Beyond. Chem. Rev 2015, 115, 683–730. [DOI] [PubMed] [Google Scholar]; (d) Barata-Vallejo S; Lantaño B; Postigo A Recent Advances in Trifluoromethylation Reactions with Electrophilic Trifluoromethylating Reagents. Chem. Eur. J 2014, 20, 16806–16829. [DOI] [PubMed] [Google Scholar]
- (7).(a) Geri JB; Wolfe MMW; Szymczak NK The Difluoromethyl Group as a Masked Nucleophile: A Lewis Acid/Base Approach. J. Am. Chem. Soc 2018, 140, 9404–9408. [DOI] [PubMed] [Google Scholar]; (b) Zhu S-Q; Liu Y-L; Li H; Xu X-H; Qing F-L Direct and Regioselective C–H Oxidative Difluoromethylation of Heteroarenes. J. Am. Chem. Soc 2018, 140, 11613–11617. [DOI] [PubMed] [Google Scholar]; (c) Sessier CD; Rahm M; Becker S; Goldberg JM; Wang F; Lippard SJ CF2H, a Hydrogen Bond Donor. J. Am. Chem. Soc 2017, 139, 9325–9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Kharasch MS; Skell PS; Fisher P Reactions of Atoms and Free Radicals in Solution. XII. The Addition of Bromo Esters to Olefins. J. Am. Chem. Soc 1948, 70, 1055–1059. [Google Scholar]; (b) Curran DP; Bosch E; Kaplan J; Newcomb M, Rate Constants for Halogen Atom Transfer from Representative Alpha-Halocarbonyl Compounds to Primary Alkyl Radicals. J. Org. Chem 1989, 54, 1826–1831. [Google Scholar]; (c) Yorimitsu H; Nakamura T; Shinokubo H; Oshima K, Triethylborane-mediated atom transfer radical cyclization reaction in water. J. Org. Chem 1998, 63, 8604–8605. [Google Scholar]; (d) Renaud P; Ollivier C; Panchaud P, Radical carboazidation of alkenes: An efficient tool for the preparation of pyrrolidinone derivatives. Angew. Chem. Int. Ed 2002, 41, 3460–3462. [DOI] [PubMed] [Google Scholar]; (e) Ueda M; Miyabe H; Nishimura A; Miyata O; Takemoto Y; Naito T, Indium-mediated tandem radical addition-cyclization-trap reactions in aqueous media. Org. Lett 2003, 5, 3835–3838. [DOI] [PubMed] [Google Scholar]
- (9).(a) Nguyen JD; Tucker JW; Konieczynska MD; Stephenson CRJ Intermolecular Atom Transfer Radical Addition to Olefins Mediated by Oxidative Quenching of Photoredox Catalysts. J. Am. Chem. Soc 2011, 133, 4160–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wallentin CJ; Nguyen JD; Finkbeiner P; Stephenson CRJ Visible Light-Mediated Atom Transfer Radical Addition via Oxidative and Reductive Quenching of Photocatalysts. J. Am. Chem. Soc 2012, 134, 8875–8884. [DOI] [PubMed] [Google Scholar]; (c) Lin Q-Y; Ran Y; Xu X-H, Qing F-L Photoredox-Catalyzed Bromodifluoromethylation of Alkenes with (Difluoromethyl)triphenylphosphonium Bromide. Org. Lett 2016, 18, 2419–2422. [DOI] [PubMed] [Google Scholar]; (d) Cheng J; Cheng YX; Xie J; Zhu CJ, Photoredox Divergent 1,2-Difunctionalization of Alkenes with gem-Dibromides. Org. Lett 2017, 19, 6452–6455. [DOI] [PubMed] [Google Scholar]; (e) Fedorov OV; Scherbinina SI; Levin VV; Dilman AD, Light-Mediated Dual Phosphine-/Copper-Catalyzed Atom Transfer Radical Addition Reaction. J. Org. Chem 2019, 84, 11068–11079. [DOI] [PubMed] [Google Scholar]; (f) Zidan M; McCallum T; Swann R; Barriault L Formal Bromine Atom Transfer Radical Addition of Nonactivated Bromoalkanes Using Photoredox Gold Catalysis. Org. Lett 2020, 22, 8401–8406. [DOI] [PubMed] [Google Scholar]; (g) Kostromitin VS; Zemtsov AA; Kokorekin VA; Levin VV; Dilman AD Atom-transfer radical addition of fluoroalkyl bromides to alkenes via a photoredox/copper catalytic system. Chem. Commun 2021, 57, 5219–5222. [DOI] [PubMed] [Google Scholar]; (h) Chen F; Xu X-H; Qing F-L; Photoredox-Catalyzed Addition of Dibromofluoromethane to Alkenes: Direct Synthesis of 1-Bromo-1-fluoroalkanes. Org. Lett 2021, 23, 2364–2369. [DOI] [PubMed] [Google Scholar]
- (10).(a) Greene TW; Wuts PGM Protective Groups in Organic Synthesis, 2nd ed.; Wiley: New York, 1991. [Google Scholar]; (b) Protective Groups in Organic Chemistry; McOmie JFW, Ed.; Plenum: New York, 1973. [Google Scholar]; (c) Huo H; Shen X, Wang C; Zhang L; Röse P; Chen L-A; Harms K; Marsch M; Hilt G; Meggers E Asymmetric photoredox transition-metal catalysis activated by visible light. Nature, 2014, 515, 100–103. [DOI] [PubMed] [Google Scholar]; (d) Yao Z; Zhang M; Wu H; Yang L; Li R; Wang P Donor/Acceptor Indenoperylene Dye for Highly Efficient Organic Dye-Sensitized Solar Cells. J. Am. Chem. Soc 2015, 137, 3799–3802. [DOI] [PubMed] [Google Scholar]
- (11).Sharma RK; Yadav M; Monga Y; Gaur R; Adholeya A; Zboril R; Varma RS; Gawande MB, Silica-Based Magnetic Manganese Nanocatalyst - Applications in the Oxidation of Organic Halides and Alcohols. ACS. Sustain. Chem. Eng 2016, 4, 1123–1130. [Google Scholar]
- (12).Ming P; Liu X-L; Wei M-H; Sheng S-R Synthesis of 4-Vinyl-1H-1,2,3-triazoles on Solid Supports via Polystyrene-Bound But-3-ynyl Selenide. Heteroat. Chem 2016, 27, 184–189. [Google Scholar]
- (13).Sahn JJ, Granger BA; Martin SF Evolution of a strategy for preparing bioactive small molecules by sequential multicomponent assembly processes, cyclizations, and diversification. Org. Biomol. Chem, 2014, 12, 7659–7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Kaszás T; Tóth M; Langer P; Somsák L C-Glycosyl Styrene Type Compounds by Pd-Catalyzed Cross-Coupling Reactions of Anhydro-Aldose Tosylhydrazones with Benzyl Bromides. Adv. Synth. Catal 2019, 361, 105–117. [Google Scholar]; (b) Bzeih T; Zhang K; Khalaf A; Hachem A; Alami M, Hamze A One-Pot Reaction between N-Tosylhydrazones and 2-Nitrobenzyl Bromide: Route to NH-Free C2-Arylindoles. J. Org. Chem 2019, 84, 228–238. [DOI] [PubMed] [Google Scholar]; (c) Sun Z; He J; Li W; Li X; Feng Y; Liu Y; Liu P; Han S Pd-Catalyzed Regioselective Olefination of N-Tosylhydrazones with Benzyl Bromides. ChemistrySelect, 2020, 5, 7396–7399. [Google Scholar]
- (15).(a) Park G; Yi SY; Jung J; Cho EJ, You Y Mechanism and Applications of the Photoredox Catalytic Coupling of Benzyl Bromides. Chem. Eur. J 2016, 22,17790–17799. [DOI] [PubMed] [Google Scholar]; (b) Yu D; To W-P; Tong GSM, Wu L-L; Chang K-T; Du L, Phillips DL; Liu Y; Che C-M Luminescent tungsten(VI) complexes as photocatalysts for light-driven C–C and C–B bond formation reactions. Chem. Sci, 2020, 11, 6370–6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Djerassi C Brominations with N-Bromosuccinimide and Related Compounds. The Wohl-Ziegler Reaction. Chem. Rev 1948, 43, 271. [DOI] [PubMed] [Google Scholar]
- (17).Shaw H; Perlmutter HD; Gu C; Arco SD; Quibuyen TO Free-Radical Bromination of Selected Organic Compounds in Water. J. Org. Chem 1997, 62, 236. [DOI] [PubMed] [Google Scholar]
- (18).Huyser ES The Photochemically Induced Reactions of Bromotrichloromethane with Alkyl Aromatics. J. Am. Chem. Soc 1960, 82, 391. [Google Scholar]
- (19).Kikuchi D; Sakaguchi S; Ishii Y An Alternative Method for the Selective Bromination of Alkylbenzenes Using NaBrO3/NaHSO3 Reagent. J. Org. Chem 1998, 63, 6023. [DOI] [PubMed] [Google Scholar]
- (20).(a) Joseph KM; Larraza-Sanchez I Synthesis of benzyl bromides with hexabromoacetone: an alternative path to drug intermediates. Tetrahedron Lett. 2011, 52, 13–16. [Google Scholar]; (b) Ni S; El Remaily MAEAAA; Franzén J Carbocation Catalyzed Bromination of Alkyl Arenes, a Chemoselective sp3 vs. sp2 C-H functionalization Adv. Synth. Catal 2018, 360, 4197–4204. [Google Scholar]
- (21).Shaw MH; Twilton J; MacMillan DWC Photoredox Catalysis in Organic Chemistry. J. Org. Chem 2016, 81, 6898–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Huang H-M; Belloti P; Ma J; Dalton T; Glorius F Bifunctional reagents in organic synthesis. Nat. Rev. Chem, 2021, 5, 301–321. [DOI] [PubMed] [Google Scholar]
- (23).(a) Cismesia MA; Yoon TP Characterizing Chain Processes in Visible Light Photoredox Catalysis. Chem. Sci 2015, 6, 5426–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Buzzetti L; Crisenza GEM; Melchiorre P Mechanistic Studies in Photocatalysis. Angew. Chem., Int. Ed 2019, 58, 3730–3747. [DOI] [PubMed] [Google Scholar]
- (24).Juliá F; Constantin T; Leonori D Applications of Halogen-Atom Transfer (XAT) for the Generation of Carbon Radicals in Synthetic Photochemistry and Photocatalysis. Chem. Rev 2022, 122, 2292–2352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


