Abstract
The accumulation of proteinaceous inclusions in the brain is a common feature among neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease (PD), and dementia with Lewy bodies (DLB). The main neuropathological hallmark of PD and DLB are inclusions, known as Lewy bodies (LBs), enriched not only in α-synuclein (aSyn), but also in lipid species, organelles, membranes, and even nucleic acids. Furthermore, several genetic risk factors for PD are mutations in genes involved in lipid metabolism, such as GBA1, VSP35, or PINK1. Thus, it is not surprising that mechanisms that have been implicated in PD, such as inflammation, altered intracellular and vesicular trafficking, mitochondrial dysfunction, and alterations in the protein degradation systems, may be also directly or indirectly connected through lipid homeostasis. In this review, we highlight and discuss the recent evidence that suggests lipid biology as important drivers of PD, and which require renovated attention by neuropathologists. Particularly, we address the implication of lipids in aSyn accumulation and in the spreading of aSyn pathology, in mitochondrial dysfunction, and in ER stress. Together, this suggests we should broaden the view of PD not only as a proteinopathy but also as a lipidopathy.
Keywords: Parkinson’s disease, Proteinopathy, Alpha-synuclein, Lipidopathy, Lipidostasis, Neurodegeneration
Introduction
The accumulation of proteinaceous inclusions in the brain is a common feature among neurodegenerative diseases. Proteins, often in the form of fibrillar amyloid structures, are the major components of those inclusions, and have been used to define them. Thus, neurodegenerative diseases are considered to be proteinopathies. Among these, Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and dementia with Lewy bodies (DLB) are the most prevalent [34], affecting millions of people worldwide.
The histopathological hallmark of PD and DLB are inclusions enriched in α-synuclein (aSyn), known as Lewy bodies (LBs) [100, 173]. Although often ignored, LB are not only composed of proteins, but also contain a core of lipid species [7, 68] and, as recent data suggest, organelles, membranes, and even nucleic acids [166]. Interestingly, aSyn has been demonstrated to interact directly with lipids and certain membranes enriched with certain type of fatty acids [99]. Strikingly, several genetic risk factors for PD are mutations in genes involved in lipid metabolism, such as GBA1, VSP35, or PINK1 [145]. Intriguingly, the consumption of certain fat in diets seems to have a significant impact in the development and progression of neurodegenerative diseases [79].
Although alterations in lipid metabolism and the balance of their species, known as lipid homeostasis (herein referred to as lipidostasis, in analogy to proteostasis), is deeply associated with neurodegeneration in PD (also reviewed in [5, 33, 39, 55, 59, 107]), the molecular mechanisms involved are still poorly understood. Nevertheless, integrated genome-wide association studies (GWAS) of PD show that several of the possible pathways implicated in PD are directly or indirectly connected with lipidostasis [103]. These include inflammation, altered intracellular and vesicular trafficking, mitochondrial dysfunction, and alterations in the protein degradation systems [41, 103]. The latter emerge from data showing that aSyn interacts with certain lipid species and that the accumulation of both occur in lysosomal storage diseases, for example Gaucher’s disease. Mutations in GBA1, the gene causing Gaucher’s disease, increase the risk for PD. Additionally, given that the brain is highly enriched in lipids, and these molecules can, for example, regulate neuronal membrane arrangement, function as secondary messengers, store energy and participate in neuronal signaling pathways [179], imbalances in lipidostasis might be key players in altered neuronal function and possible neurodegeneration. In this review, we discuss the recent evidence that suggests how lipid biology can play major roles in PD pathology, emphasizing the implications on the accumulation and spreading of aSyn pathology, on mitochondrial dysfunction, and on endoplasmic reticulum (ER) stress.
Parkinson’s disease
PD is the second most common neurodegenerative disease and the first most common synucleinopathy, typically affecting people over 65 years old. Over 10 million people worldwide live with PD and this number is increasing alongside with the increase in life expectancy. Resting tremor, dystonia, rigidity, bradykinesia, and postural instability are the characteristic features of PD [100]. These features result from the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SN). aSyn accumulation and LB formation are major components thought to trigger several cellular pathways that lead to this neuronal loss [171, 173]. Although aging is the most significant risk factor for PD [38, 88, 142], lipid balance and their metabolism are emerging as important factors for PD, and will be discussed in the present review.
The scenario complicates considering that mutations in various genes, such as LRRK2, PINK1, SNCA, DJ-1, VPS35, and GBA1, have been implicated in familial and sporadic forms of PD [42, 44, 70, 140]. In particular, an overproduction of aSyn protein caused by duplications, or triplications of the SNCA locus, or point mutations in the SNCA gene, are associated with familial forms of PD [106, 152, 171, 199]. Even though most of these genetic alterations are either rare or confer variable risk to develop PD, they provide mechanistic insight into the molecular pathways associated with disease, especially since several have also been found associated with sporadic PD. In this sense, the overproduction of wild-type (WT) or mutant forms of aSyn has additional toxic effects, which might be independent of aggregation, for example when in contact with different lipids and through interactions with organelle membranes [36, 49, 60, 99, 126, 162].
Although tremendous progress has been made over the past decades, the precise molecular mechanisms underlying neuronal death are still unclear. Particularly, those that involve the interplay between genetic and environmental risk factors.
Neuropathology of PD: protein and lipid deposition/alterations
The accumulation of aSyn in proteinaceus aggregates known as LBs and/or Lewy neurites (LNs) is one of the main neuropathological hallmarks of PD [100]. aSyn is a 14.5-kDa protein that is enriched in the presynaptic terminals of neurons [94, 125] and has been implicated as an important player in synaptic vesicle trafficking and dopamine release [1, 25, 50, 124, 198]. However, aSyn interacts with lipids and membranes and is present in various other tissues, including blood, where it likely performs other functions.aSyn aggregation is not limited to the SN, as aggregates can be found in other brain structures progressively many years before the symptomatology. Efforts have been made to classify the progression of the disease, based on the distribution of Lewy-pathology-in the brain [20–22].
Importantly, the morphology of LBs can vary depending on the brain structure where they occur, probably as a result of the stage the pathology, and likely representing a progressive process that is caught at a particular stage at the time of death. At the early stages of PD, aSyn staining starts as a diffuse-granular and pale cytoplasmic mark. As the pathology progresses, the staining becomes more intense and structures, referred to as Pale bodies, start to emerge. Finally, LBs appear, probably as a consequence of the peripheral condensation of the Pale bodies [190]. Given that as much as 90% of aSyn found in LB is phosphorylated at serine 129 [6, 65], it has been suggested that this form is involved in the initial stages of LB formation and PD pathology.
Initially, the LB structure was thought to consist of fibrillar aSyn [12, 22, 190] but it is becoming accepted that more components are involved in LB formation and maturation. The biochemical composition of LBs is highly complex and includes ~ 300 other proteins [115], ~ 90 of which have been confirmed by immunohistochemistry assays [190]. Furthermore, LBs were found to be enriched in lipids [68], membranous components that might come from vesicles, and fragmented organelles, as shown by several methods such as Fourier transform infrared micro-spectroscopy (FTIRM) [7], correlative light and electron microscopy (CLEM), stimulated emission depletion (STED)-based super-resolution microscopy, and laser-capture microdissection microscopy coupled to liquid chromatography-mass spectrometry (LC–MS) [166]. Particularly, this study identified that sphingomyelin and phosphatidylcholine are strongly enriched in these samples, further confirming that LBs are also composed of lipid species and membranes of organelles taken at some point from the cell. The latter study suggests that lipid species are tightly linked to LBs formation and/or maturation and might be associated to aSyn function, localization, and/or dynamics. Additionally, the fact that lipids are found in the core and are involved in LBs’ formation also suggests that lipidostasis impairment might be an important factor prior to protein deposition in PD.
Recently, metabolomics has opened a new door for potential biochemical biomarkers that may inform on the beginning of the disease, progression, or prognosis. Among these markers, lipid profiles or different species of fatty acids are emerging as potential ones based on evidence found in PD models and in patients [176, 180, 194, 201].
Lipidomic analyses of PD patient samples revealed alterations in 80 lipid species out of 200 that were analyzed in the visual cortex of PD patients in the Braak stage IV or V [32, 83, 165, 176]. The lipid species identified belong to the following major lipid families: sphingolipids (SL), glycerophospholipids and cholesterol. In the SL family, multiple species of sphingomyelin, ceramides, and gangliosides were found to be increased, while most lipids from the glycerophospholipid family were decreased. In this family, species of phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol decreased [32], while phosphatidylserine species increased [32]. Interestingly, the primary visual cortex is affected in advanced stages of PD, and, among the non-motor symptoms of PD, visual hallucinations are one of the most common features [63, 85]. Furthermore, this alteration in lipidostasis reflects neuronal dysfunction that compromises the circuitry and may precede neuronal death. This finding was consistent with those by another group that found phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol decreased in the SN of male PD patients [165]. Interestingly, modifying the concentration of certain lipid species, such as the synthesis of phosphatidylethanolamine, in PD models, leads to the accumulation of aSyn, to ER stress and mitochondrial dysfunction [193]. These reports further support the role of specific lipid species in PD, raising the possibility that some lipid species might be important players in the early or advanced neuropathological stages.
Studies in postmortem tissue and fibroblasts of PD patients revealed decreased levels of brain cholesterol, associated with a reduction in the expression of isopentenyl diphosphate isomerase and β-Hydroxy β-methylglutaryl-CoA reductase (HMG-CoA reductase), key enzymes in the biosynthesis of isoprenoids [136, 137]. Additionally, isotope-dilution mass-spectrometry analyses of the cholesterol metabolites 24S-hydroxycholesterol and 27-hydroxycholesterol in cerebrospinal fluid of PD patients revealed higher levels than in non-PD patients [16]. Strikingly, the levels of 24S-hydroxycholesterol correlate with disease duration [16]. Altogether, these and other studies suggest that lipidostasis imbalances likely play an important role in PD. While some may influence disease onset, others may act as markers of damage at later stages of the disease process.
Interestingly, altered SL metabolism and fatty acid biosynthesis have been detected in sebum of PD patients versus non-PD subjects [170]. Additionally, staining of postmortem brain sections from PD individuals using the lipid dye boron-dipyrromethene (BODIPY) showed that dopaminergic neurons in the SN accumulated lipids while astrocytes had a diminished lipid content [23]. This suggests lipidostasis is altered in different cell types in the brain and that this lipid alteration and accumulation seems to be specific for neurons.
PD risk is associated with deregulation of lipidostasis
Given that almost 50% of the brain’s dry weight are lipids [24], it would not be surprising that many neurodegenerative diseases, including PD, may be heavily influenced by imbalances in lipidostasis [48], as evidenced by several genetic studies that we will discuss throughout this review. Consistently, several genes associated with increased risk of PD are involved in lipid metabolism.
GWAS in different populations identified the GAK/DGKQ/IDUA region as one of the top three risk loci for PD [31, 119, 138, 145, 169]. This region harbors the gene that encodes for the enzyme diacylglycerol kinase theta (DGKQ) that catalyzes the regeneration of phosphatidylinositol from diacylglycerol. This finding is consistent with reduced levels of phosphatidylinositol that are found in PD patients [32]. Transcriptomic studies found that the gene ELOVL7, that encodes for a fatty acid elongase, is also associated with PD [102, 116]. Furthermore, in several PD models, aSyn inclusions and toxicity are reduced upon inhibition of stearoyl-CoA desaturase (SCD) [58, 92, 186]. This enzyme catalyzes the rate-limiting step in the formation of monounsaturated fatty acids, suggesting that some lipid metabolic pathways have a tight relation with aSyn accumulation. Although no clear mechanism on how these genes might be involved in PD pathogenesis have been uncovered, it is important to highlight that additional genetic risk factors that involve lipid metabolism are being identified.
Fatty acids are not only important as membrane components or energy sources, but also serve as donors for post-translational modifications (PTMs). A mechanism that is dependent on specific lipid species, in this case palmitic acid, is protein palmitoylation. Palmitoylation can regulate the localization and interaction between proteins with lipid membranes, and between proteins in the same lipid domains and organelles [75, 120]. In a recent study, the palmitome of PD patients was characterized, and identified an increase in the palmitoylation of several proteins that interact with PD-associated proteins (LRRK2, DJ-1, GBA1 and aSyn) when compared to control subjects. Additionally, these proteins were found to be part of pathways associated to inflammation, cytoskeletal architecture, and mitochondrial dysfunction [30]. This suggests that lipid overload, particularly palmitic acid, may lead to excessive protein palmitoylation that might affect interaction among proteins involved in neuronal dysfunction contributing to PD onset and progression.
Perhaps the strongest direct genetic connection is that linking GBA1 mutations with sporadic forms of PD. Glucocerebrosidase (GCase), the enzyme encoded by the GBA1 gene, regulates SL metabolism, further supporting the view that certain lipid species likely play a role in PD onset and progression. This is the topic of the next section.
GBA1 mutations and SL metabolism alterations as a risk factor for PD
GBA1 mutations are the most common genetic risk factor for PD, increasing the risk by approximately fivefold [35, 148, 168]. GCase resides in lysosomes and is an important regulator of SL metabolism. The catabolic reaction of GCase results in the hydrolysis of glucosylceramide into glucose and ceramide [72, 164] (Fig. 1a). Homozygous loss-of-function mutations lead to a lysosomal storage disease called Gaucher’s Disease (OMIM 606423). Gaucher’s Disease patients can be classified into five types (1, 2, 3, perinatal lethal, and cardiovascular) according to substrate accumulation and neuronal affections. Type 2 and 3 patients show a degree of neurodegeneration and neuropathic manifestations that resemble clinical features of PD (reviewed in [66]). Initially, this suggested that GCase deficiency degree could be an important mechanism involved in PD.
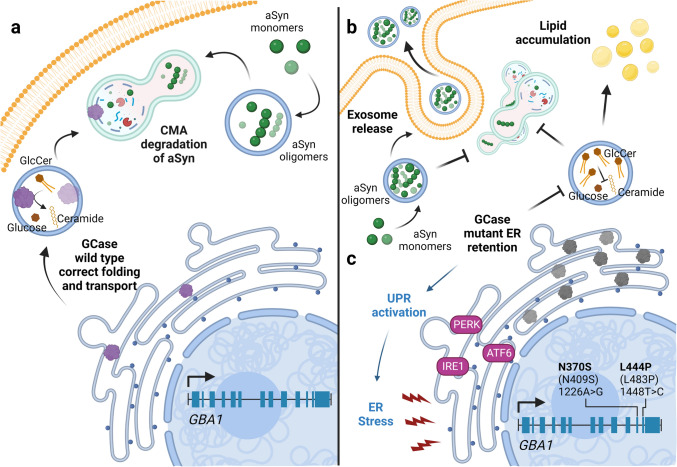
Fig. 1.
Putative loss- and gain-of-function effects of GCase mutations. a GBA1 encodes for GCase. Wild type enzyme (purple protein) is correctly folded and can be transported to the lysosomes (blue complete circles) where it hydrolyzes glucosylceramide (GlcCer) into glucose and ceramide. This contributes to the correct function of the autophagic system which, through the Chaperone Mediated Autophagy (CMA) pathway, is able to degrade proteins and prevent their accumulation, for example aSyn. b In the loss-of-function hypothesis due to GBA1 mutations, unfolded GCase cannot be transported to the lysosome, sphingolipid metabolism is compromised and GlcCer is accumulated. This also impairs the formation of autophagolysosomes, promoting the accumulation of aSyn oligomeric forms inside the cell. To reduce aSyn burden, changes in exosomal-mediated release of aSyn may take place. c In the gain-of-function hypothesis, the retention of mutant GCase in the ER activates the UPR response proteins (PERK, IRE1 and ATF6), generating ER stress which may, in turn, alter lipidostasis
Consistently, GBA1 heterozygote mutations (haploinsufficiency) are associated with increased PD risk. N370S, associated with mild risk, and L444P, associated with higher risk, are the most common ones [8, 52, 69, 130, 168]. Patients carrying GBA1 haploinsufficiency mirror sporadic PD patients to a large extent (reviewed in [161]). Nevertheless, the onset is approximately 5 years earlier, and there is a faster progression of motor and cognitive impairment when compared to sporadic PD patients [35, 52, 69, 168]. Additionally, the levels and activity of GCase are decreased in PD brains [71, 91, 132, 135, 155], leading to altered SL metabolism. Strikingly, there is a decrease in GCase activity in normal aging that reaches the levels found in PD patients, alongside with the accumulation of glucosylsphingosine in the SN [110, 155]. This suggests that alterations of the SL metabolism might be an important component of PD neuropathology, not only in carriers of GBA1 mutations but also for sporadic PD patients where age-associated reduction in GCase activity might contribute to the onset of the pathology.
The precise mechanisms by which mutant GCase mutations increase PD risk are still unclear. There is evidence supporting both loss- or gain-of-function hypotheses (reviewed in [98]). The loss-of-function is due to defects in the correct folding of the enzyme, which leads to disrupted transport of GCase to the lysosome and a concomitant accumulation in the ER [139, 156, 164] (Fig. 1b). This alteration in GCase localization leads to the accumulation of SLs, such as glucosylceramide and glucosylsphingosine [9, 77, 91]. Interestingly, some lipid species (sphingomyelin, ceramide and monohexosylceramides) have been found increased in the plasma of PD patients [77] and, importantly, their physiological role is not only structural but also of high importance for cellular processes like autophagy, senescence, and inflammation, among others [2, 19, 93]. In the proposed loss-of-function mechanism, the reduction in GCase enzymatic activity also affects the protein degradation systems through impairment in lysosomal function and recycling [123, 153], which leads to impaired aSyn clearance and, consequently, to its accumulation [139]. Moreover, the accumulation of glucosylceramide affects aSyn aggregation by stabilizing soluble aSyn oligomers and also by inducing aggregation [127, 149] (Fig. 1b). This creates a pathogenic loop that further disrupts GCase stability and folding, fueling additional aSyn accumulation. Interestingly, when GCase mutants are overexpressed or wild type GCase is inhibited by pharmacological strategies there is an increase in the release of exosomes that contain aSyn [29, 98, 146]. In contrast, overexpression of wild type GCase results in a decrease in exosome secretion [146]. This suggests that the reduced activity of GCase contributes to aSyn spreading pathology [11, 98, 110, 131] (Fig. 1b).
Although the loss-of-function hypothesis is valid and plausible, recent results from clinical trials suggest that therapeutic approaches that overexpress wild type GCase or try to correct its folding may not be completely suitable for PD patients, particularly since GBA1 mutations in PD patients are heterozygous (reviewed in [17]). An alternative is the gain-of-function hypothesis, whereby the retention of misfolded GCase in the ER would be responsible for lysosomal dysfunction, but through ER stress, and activation of the unfolded protein response (UPR) [61, 109] (Fig. 1c). Interestingly, the degree of GCase retention in the ER is influenced by the mutation, and this has been correlated with the severity of the pathology in Gaucher’s disease [156], and this is consistent with the reports that show that different mutations cause different risk degrees for PD.
Importantly, although there is evidence supporting loss- and gain-of-function hypotheses, one may be the result of the other. This is most likely the case in PD patients carrying GBA1 mutations [146] (Fig. 1b, c).
Further evidence linking SLs to PD involve other enzymes that participate in this particular type of lipid metabolism. Ceramides and sphingomyelin are increased in the brain of PD patients [32, 165]. The accumulation of these metabolites correlates with an increase in the expression of genes that encode enzymes involved in the biosynthetic pathway, such as Serine palmitoyl transferase long chain base subunit 2 (SPTLC2), degenerative spermatocyte homolog 1 lipid desaturase (DEGS), sphingomyelin synthase 1 (SGMS1), and UDP-galactosyltransferase 8A (UGT8A) [32]. Another study performed in the plasma and CSF of PD patients showed that several lipid species are altered, particularly those involved in the SL metabolism [176]. Nevertheless, since SL biology is highly complex, it will be important to explore further ramifications of the pathway in order to understand how they relate to PD [111].
The role of lipidostasis in aSyn pathology
Although progress has been made in identifying neuropathological markers of PD, the molecular and cellular mechanisms that lead to them are still unclear. Strikingly, lipid biology alterations seem to be an important player in most of the described mechanisms, particularly due to their pleiotropic functions in cellular physiology. Thus, it is important to understand how alterations in lipid species may directly affect key proteins in PD, such as aSyn, and also how such alterations impact organelles, such as mitochondria and ER, which are lipid-rich compartments that have been identified as important players in PD onset and progression.
In cells, aSyn is thought to exist primarily as a monomer [178] and, in some situations, as aggregation-resistant tetramers [13]. In pathological conditions it can be found as oligomers or fibrils. Structurally, aSyn is composed of three regions as folows: an N-terminal region that can fold into an amphipathic α-helical structure, and that binds to lipid membranes and vesicles; a central hydrophobic domain that can fold into β-sheets, the main domain responsible for its aggregation propensity; and an acidic and highly disordered C-terminal domain. Particularly, the N-terminal domain preferentially associates with glycosphingolipids (usually containing sulfate, phosphate, or sialic acid) in the membrane of synaptic vesicles [97]. This interaction is important to promote the formation of the SNARE complex between two membranes and the concomitant vesicle docking [121]. Additionally, this domain can interact with apolipoproteins, such as apolipoprotein E (ApoE), which have been implicated in increased risk for PD and DLB when the APOE4 allele is present [18, 56, 181]. This is mediated by an increase in the aggregation propensity of aSyn when APOE4 is present compared to other APOE isoforms [54]. It is interesting to highlight that most point mutations in the SNCA gene fall at the N-terminal domain of aSyn, the lipid interacting region, affecting the protein’s secondary structure and its lipid binding properties [28, 97, 150]. Evidence shows that aSyn interacts with lipids through several domains and that point mutations in these regions, or risk alleles involved in lipid metabolism, affect aSyn aggregation propensity.
The hydrophobic domain can also regulate the affinity of aSyn for lipid membranes [53, 67]. In this sense, it has been demonstrated a six-fold increase in the interaction between aSyn and the inner plasma membrane when gangliosides are enriched in this membrane [124]. Strikingly, a ~ 20% reduction in the levels of gangliosides is observed in PD patients [165]. This suggests that aSyn might lose some of its plasma membrane affinity, detaching and gaining aggregation properties. Additionally, it has been proposed that lipid arrangements in the membranes can induce conformational changes in aSyn amphipathic α-helical structure [62], further supporting the idea that aSyn structure and aggregation propensity could be modulated through membrane lipid composition. The evidence on how membrane lipid composition affects aSyn affinity highlights its relevance as a regulator of aSyn conformation.
Even though aSyn is traditionally seen as a presynaptic protein involved in vesicle trafficking, other functions, and interactions with membranes of other organelles, are emerging [64, 175].
Interplay between lipid droplets and aSyn aggregation
Sterol esters and triglycerides (neutral lipids) [96, 167, 200] can be stored in the core of highly dynamic organelles called lipid droplets (LD). These organelles are composed of a phospholipid monolayer, coating proteins (such as perilipins), and enzymes [143]. A protective role against lipotoxicity is attributed to LD, due to their storing capacity during periods of nutrient surplus where harmful lipid species might be consumed/synthesized [95, 141, 163].
In a diverse range of PD models, from yeast to human cell lines, overexpression of aSyn is accompanied by an accumulation of LD [74, 144, 172, 192]. Studies in primary cortical neurons demonstrated a tight connection between aSyn toxicity, lipids, and LD, where high concentrations of oleic acid were associated with increased aSyn inclusion formation. Furthermore, if LD biogenesis is prevented aSyn toxicity increases [58]. Interestingly, in cells exposed to fatty acids, aSyn translocates from the cytoplasm to the membrane of the LD [37]. Even overexpression of selected aSyn mutants, like the A53T, induce an increase in LD accumulation [160]. This suggests that there is a connection between the excess of free lipid species in the cytoplasm and aSyn inclusion formation and toxicity. This relationship is likely bidirectional, as lipids seem to be key contributors for aSyn toxicity and, in turn, physiological levels of aSyn maintain lipid homeostasis.
The role of lipidostasis in the life cycle of aSyn
The degradation and recycling of monomeric aSyn is thought to occur via chaperone-mediated autophagy, in the lysosome, and via the proteasome/ubiquitin system [109, 127, 128]. Once aggregates are formed, aSyn degradation takes place via macroautophagy. Several mechanisms are triggered to avoid further accumulation and toxicity, like the induction of heat shock proteins, such as HSP70, in order to stabilize soluble forms of aSyn [46, 104, 122].
Several studies suggest that GCase mutants lead to aSyn accumulation in lysosomes [43] and, as a consequence, to increased cellular release [45, 61, 151, 197], to avoid further aggregation. This mechanism contributes to the hypothesis of the prion-like spreading of aSyn pathology. This aggregation and spreading of aSyn is exacerbated in the presence of certain gangliosides, GM1 and GM3, which are also found in exosomes. This might be related to the reduced levels of gangliosides found in PD patients. Interestingly, phospholipase D1 can activate the autophagic flux, preventing the accumulation of aSyn and this enzyme is downregulated in patients with DLB [10]. This suggests that lipidostasis plays an important role in aSyn accumulation and release [4, 45, 177], saturating other neurons and disrupting their cellular machinery and function [61, 87] and contributing to the spreading of aSyn pathology.
Interestingly, aSyn accumulation has not only been reported in synucleopathies or in Gaucher’s disease. Mutations in genes that encode enzymes that are part of lipid metabolism in the lysosomes lead to diseases such as Fabry’s disease, Krabbe’s disease, and Niemann-Pick disease type C1. In these disorders, in addition to aSyn accumulation, there is also accumulation of certain SL species. Furthermore, these lysosomal storage diseases increase the risk for developing PD (reviewed in [80]). Again, this suggests that alterations in lipidostasis are associated with the accumulation of lipid-binding proteins, such as aSyn, and that such lipidic alterations might be important neuropathological alterations prior to the onset of proteinopathy.
Lipidostasis alterations as a key player in mitochondrial impairment and ER stress
Several PD genes, such as PINK1 and VPS13, establish a direct bridge between lipidostasis and mitochondria [47, 101, 114, 138, 185]. PINK1 is a mitochondrial serine/threonine kinase that, when accumulated in the outer membrane of the mitochondria, phosphorylates Parkin to induce mitophagy [105]. Several PD PINK1 deficient models display ceramide accumulation in mitochondria, negatively affecting the electron transport chain and reducing the β-oxidation rate (Fig. 2a) [133, 188]. These effects can be ameliorated when ceramide levels are lowered, or by induction of β-oxidation [188]. The lack of PINK1 is also associated with increased mitochondrial-ER contacts that cause abnormal lipid trafficking, leading to a depletion in phosphatidylserine from the ER (Fig. 2c) [189]. Furthermore, if fatty acid synthase is inhibited in PINK1 deficient models, the toxicity caused by excess in fatty acid synthesis is reduced considerably. Additionally, the inhibition of the fatty acid synthase also lowers palmitate levels and increases cardiolipin, rescuing the defects in complex I of the electron transport chain [184]. A study using a cohort of Spanish patients harboring heterozygous mutations of PINK1 revealed the presence of LBs in the brainstem and SN, and neuronal loss in the SN [159]. These features mirror those found in sporadic PD patients, suggesting that similar mechanisms might be behind the neuropathological features of PD and, again, highlighting the idea that alterations in PD-associated proteins may lead to a disruption in lipidostasis.
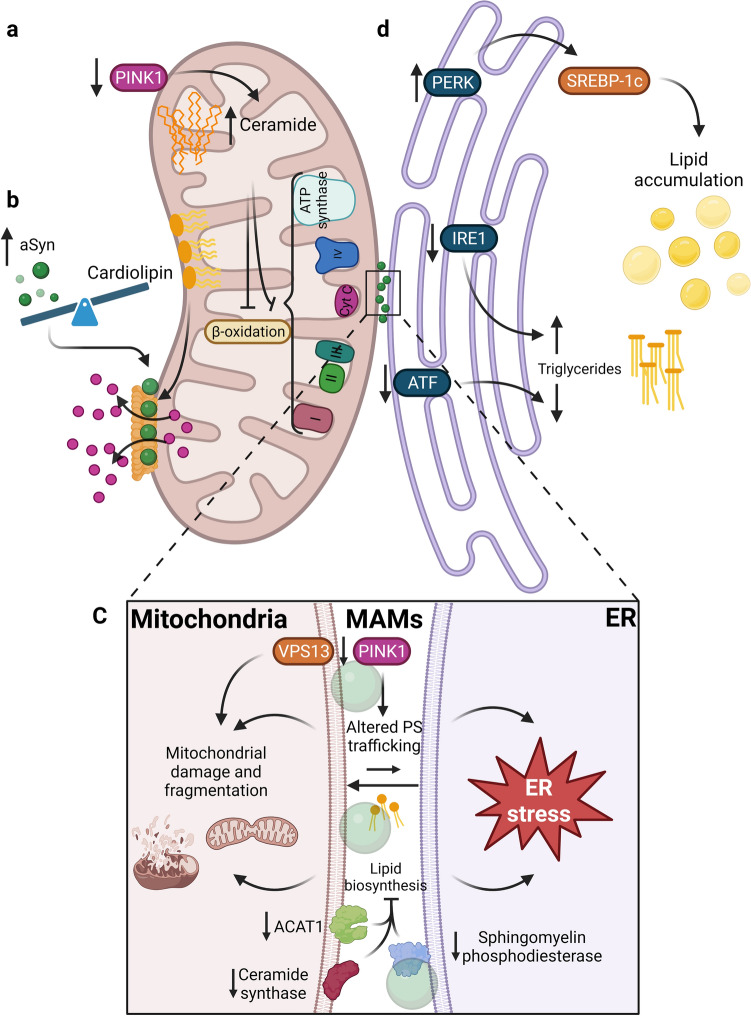
Fig. 2.
Mitochondrial dysfunction, ER stress and alterations in membrane contact sites (MAMs) are related to lipidostasis alterations. a Depletion or mutations in PINK1 are associated with increased ceramide levels, thereby altering beta-oxidation and the electron transport chain. b When the balance between aSyn and cardiolipin is altered, favoring the accumulation of aSyn, aSyn associates with the cardiolipin on the mitochondrial outer membrane inducing the formation of pores that release cytochrome c (Cyt c). c When proteins related to lipid metabolism, such as ACAT1, sphingomyelin phosphodiesterase, ceramide synthase or VPS13 are downregulated, processes such as lipid biosynthesis and phosphoserine trafficking are affected. Additionally, the reduction in the levels or mutations in PINK1 are also associated with altered phosphoserine trafficking between organelles. All of them lead to altered lipidostasis in the MAMs, contributing to mitochondrial dysfunction and ER stress. d When PERK is overexpressed, SREBP-1c is activated leading to lipid accumulation. A reduction in IRE1 and ATF leads to increased or diminished triglyceride content, respectively. Therefore, the UPR pathways in the ER can modulate and contribute to alterations in lipidostasis
Mitochondrial membranes have a high content of cardiolipin [129] and, due to this glycerophospholipid, the binding affinity of aSyn to neuronal mitochondria is enhanced [27, 147, 182]. One of the first effects observed due to this enhancement is the formation of ion-permeable pores that allow the release of cytochrome c (Fig. 2b) [73]. Nevertheless, cardiolipin is also important and beneficial for aSyn refolding, preventing aggregation in some studies [158]. This suggests aSyn might be involved in the loss of mitochondrial integrity in a mechanism that is dependent on the balance between aSyn and cardiolipin.
Mitochondria and the ER communicate through physical contacts known as mitochondria-associated membranes (MAMs), which are enriched with lipids and proteins that regulate processes such as lipid synthesis and trafficking, autophagy, the unfolded protein response (UPR), redox states, among others [157]. aSyn can associate with the MAMs since it preferentially binds to membrane domains with a high composition of acidic phospholipids. However, mutations in aSyn (A30P and A53T) decrease the association with to the MAMs, thereby impairing organelle function [76].
The VPS13 locus encodes 4 proteins (VPS13A, VPS13B, VPS13C, VPS13D) involved in the phospholipid exchange through the aqueous environment from one bilayer to another [183]. These lipid transfer proteins localize to different contact sites between organelles [26]. VPS13A, VPS13C, and VPS13D are localized at the MAMs (Fig. 2c), at the ER, and in the endolysosomal system [78, 108]. When their expression is altered lipid composition changes [82], contributing to altered organelle function (ER stress and mitochondrial dysfunction).
MAMs also play an important role in lipid homeostasis and LD biogenesis. The enzyme acyl-CoA cholesterol acyltransferase (ACAT1), which is in charge of the conversion of free cholesterol into cholesteryl esters, is enriched and has higher enzymatic activity in the MAMs than in the ER [154]. The same has been observed for enzymes important for ceramide biosynthesis, such as ceramide synthase and sphingomyelin phosphodiesterase [15, 195]. Inhibition of these enzymes leads to a relocation of the characteristic proteins of the MAMs [84], suggesting that lipid metabolism is important in maintaining these contact sites (Fig. 2c). Thus, alterations in lipidostasis causing dysfunction of the MAMs are associated with mitochondrial fragmentation [76], ER stress, and presumably even with LD biogenesis and maintenance [157]—dysfunction of all of these organelles have been observed in PD.
The ER plays a crucial role in lipid metabolism since this is the compartment where most of the lipids are synthetized, particularly membrane lipids and neutral lipids [57, 129]. Another role of the ER is to prevent the accumulation of lipids to avoid lipotoxicity [81, 174]. Additionally, the ER contains chaperones and proteins that respond to fluctuations in proteostasis, inducing a response known as the UPR in conditions of stress [40, 51]. This clearly suggests a close association between lipidostasis and proteostasis in the ER, and that impairments in either or both networks may be related to a variety of cellular problems, including those linked to neurodegeneration [89, 187]. First, a connection between aSyn and the UPR was established in a neuronal model derived from induced pluripotent stem cells obtained from a patient with a SNCA triplication. Neurons containing an increased aSyn load displayed an activation of IRE1/XBP1 compared to the isogenic cell line. Additionally, the presence of pIRE1α, pPERK, and pIF2a was found in neurons of PD patients that also contained LBs [86, 90], further confirming the activation of the UPR when neurons express increased levels of aSyn. Second, it is hypothesized that lipid perturbations may trigger ER stress and activate the UPR response through three known pathways: ATF6, IRE1, and PERK (Fig. 2d) [81]. Evidence supporting that alterations in lipidostasis are tightly linked with the UPR response has been obtained in non-neuronal tissues. For example, it was demonstrated that when Ire1α is deleted, an excess of triglycerides is detected in hepatocytes [191]. Furthermore, XBP, an important component of the IRE1 pathway, has also been demonstrated to be involved in lipogenesis [113]. Compared to the IRE1/XBP pathway, overexpression of PERK has been associated with overactivation of SREBP-1c, leading to lipid accumulation [112]. Interestingly, transgenic Atf4-/- mice show a minor accumulation of triglycerides compared to wild type mice when fed with either a high-carbohydrate or high-fructose diet [117, 196]. However, besides aSyn accumulation, alterations in lipidostasis may also trigger ER stress and further contribute to protein aggregation in neurons.
In total, and although additional studies will be necessary to firmly establish the role of lipidostasis in neurodegeneration, the findings above clearly demonstrate that several components of the lipid metabolic network are tightly linked to known PD-related proteins, suggesting that modulation of lipid species may constitute valid strategies for therapeutic intervention.
Concluding remarks
PD and related synucleinopathies have been traditionally classified as a proteinopathies due to an imbalance between protein synthesis and degradation systems that lead to misfolding and accumulation of aSyn, and to a concomitant neuronal dysfunction and death. However, a fresher view into genetic, epidemiological, and mechanistic data, has brought lipidostasis into the spotlight. This idea is also fueled by the limited success in clinical trials focusing on the traditional view of synucleinopathies purely as proteinopathies, which calls for critical reconsideration of the hypotheses being tested, in the hope that greater progress can be made in the coming years. In this context, lipidostasis alterations are an emerging and exciting area. Strong evidence suggests that membrane lipids are of high importance for aSyn biology/pathobiology, contributing to aSyn fibrilization and accumulation in laboratory models. Strikingly, aSyn-lipid interactions are likely an important component in LB formation and, possible also for spreading of pathology. In summary, lipids are emerging as major contributors and drivers of PD (Fig. 3) given the following:
Several genes involved in lipid metabolism have been identified as genetic risk factors for PD onset and progression.
Lipids are abundant components of LB.
aSyn structure and lipid binding is affected by the membrane composition.
Lipidostasis imbalances are linked to impaired organelle function, such as mitochondrial dysfunction and ER stress.
Alterations between aSyn-lipid interactions impact on organelle function.
aSyn accumulation alters lipid droplet homeostasis.
SLs and long-chain ceramides have been implicated in pro-inflammatory processes (reviewed in [3, 14, 19, 80, 118, 134]), consistent with the growing role of neuroinflammation and immune response in PD.
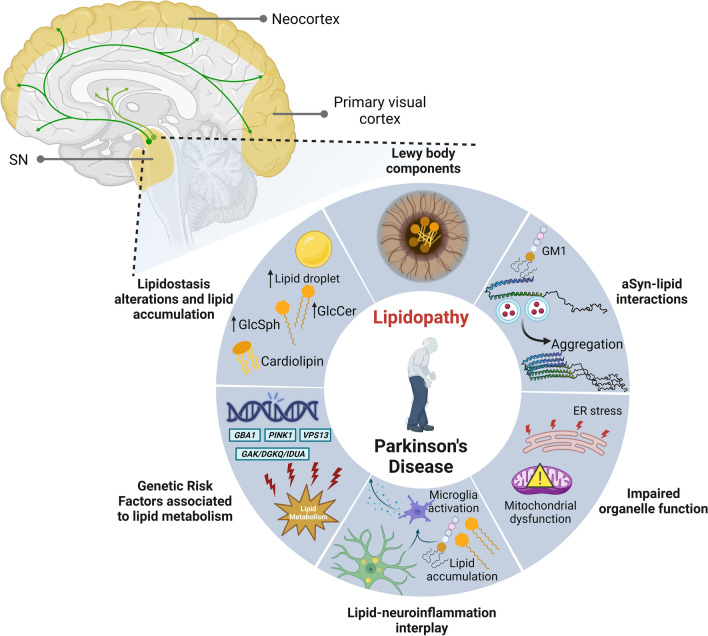
Fig. 3.
PD and synucleinopathies as lipidopathies. Alterations in lipidostasis have been observed in several brain regions (highlighted in yellow) that are also affected by the spreading of aSyn pathology (green pathways with arrows). Given that: (i) lipids/membranes are core components of LB; (ii) that aSyn structure and lipid-binding properties are affected by the proportion of lipids in organelles; (iii) that lipidostasis alterations are linked to impaired organelle function; (iv) that neuronal lipid accumulation and high concentration of lipids in the parenchyma are associated with microglial activation and neuroinflammation; (v) that several genes involved in lipid metabolism have been identified as genetic risk factors for PD and progression; and (vi) that there is a general alteration in lipidostasis leading to accumulation of particular lipid species, we posit that these diseases should be considered not only proteinopathies but also lipidopathies
In conclusion, since lipid imbalances are emerging as an important driver of neurodegeneration, we posit that a better understanding of how alterations of lipidostasis contribute to neuropathology in PD and in other synucleinopathies will open novel avenues for therapeutic intervention and, perhaps, also for the development of novel disease biomarkers.
Acknowledgements
TFO is supported by the Deutsche Forschungsgemeinschaft (DFG, German ResearchFoundation) under Germany’s Excellence Strategy—EXC 2067/1- 390729940“. All illustrations were created with BioRender.com.
Author contributions
MFL designed the review outline, did the literature search, wrote the manuscript, designed, and prepared illustrations. TFO designed the review outline, performed literature research, wrote, and proofread the manuscript. All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare no commercial or financial relationships that could be considered as conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/S0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 2.Alessenko AV, Albi E. Exploring sphingolipid implications in neurodegeneration. Front Neurol. 2020;11:437. doi: 10.3389/fneur.2020.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allende ML, Zhu H, Kono M, Hoachlander-Hobby LE, Huso VL, Proia RL. Genetic defects in the sphingolipid degradation pathway and their effects on microglia in neurodegenerative disease. Cell Signal. 2021 doi: 10.1016/J.CELLSIG.2020.109879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJA, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/J.NBD.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alza NP, Iglesias González PA, Conde MA, Uranga RM, Salvador GA. Lipids at the crossroad of α-synuclein function and dysfunction: biological and pathological implications. Front Cell Neurosci. 2019 doi: 10.3389/FNCEL.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JP, Walker DE, Goldstein JM, De Laat R, Banducci K, Caccavello RJ, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/JBC.M600933200. [DOI] [PubMed] [Google Scholar]
- 7.Araki K, Yagi N, Ikemoto Y, Yagi H, Choong CJ, Hayakawa H, et al. Synchrotron FTIR micro-spectroscopy for structural analysis of Lewy bodies in the brain of Parkinson’s disease patients. Sci Reports. 2015;51(5):1–8. doi: 10.1038/srep17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asselta R, Rimoldi V, Siri C, Cilia R, Guella I, Tesei S, et al. Glucocerebrosidase mutations in primary parkinsonism. Park Relat Disord. 2014;20:1215–1220. doi: 10.1016/J.PARKRELDIS.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avisar H, Guardia-Laguarta C, Area-Gomez E, Surface M, Chan AK, Alcalay RN, et al. Lipidomics prediction of Parkinson’s disease severity: a machine-learning analysis. J Parkinsons Dis. 2021;11:1141–1155. doi: 10.3233/JPD-202476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae EJ, Lee HJ, Jang YH, Michael S, Masliah E, Min DS, et al. Phospholipase D1 regulates autophagic flux and clearance of α-synuclein aggregates. Cell Death Differ. 2014;217(21):1132–1141. doi: 10.1038/cdd.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae EJ, Yang NY, Song M, Lee CS, Lee JS, Jung BC, et al. Glucocerebrosidase depletion enhances cell-to-cell transmission of α-synuclein. Nat Commun. 2014;51(5):1–11. doi: 10.1038/ncomms5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartels T. A traffic jam leads to Lewy bodies. Nat Neurosci. 2019;227(22):1043–1045. doi: 10.1038/s41593-019-0435-y. [DOI] [PubMed] [Google Scholar]
- 13.Bartels T, Choi JG, Selkoe DJG. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nat. 2011;4777362(477):107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belarbi K, Cuvelier E, Bonte MA, Desplanque M, Gressier B, Devos D, et al. Glycosphingolipids and neuroinflammation in Parkinson’s disease. Mol Neurodegener. 2020;151(15):1–16. doi: 10.1186/S13024-020-00408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Björkhem I, Lövgren-Sandblom A, Leoni V, Meaney S, Brodin L, Salveson L, et al. Oxysterols and Parkinson’s disease: evidence that levels of 24S-hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci Lett. 2013;555:102–105. doi: 10.1016/J.NEULET.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Blandini F, Cilia R, Cerri S, Pezzoli G, Schapira AHV, Mullin S, et al. Glucocerebrosidase mutations and synucleinopathies: toward a model of precision medicine. Mov Disord. 2018;34:9–21. doi: 10.1002/mds.27583. [DOI] [PubMed] [Google Scholar]
- 18.Blázquez L, Otaegui D, Sáenz A, Paisán-Ruiz C, Emparanza JI, Ruiz-Martinez J, et al. Apolipoprotein E epsilon4 allele in familial and sporadic Parkinson’s disease. Neurosci Lett. 2006;406:235–239. doi: 10.1016/J.NEULET.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 19.Bo RX, Li YY, Zhou TT, Chen NH, Yuan YH. The neuroinflammatory role of glucocerebrosidase in Parkinson’s disease. Neuropharmacology. 2022;207:108964. doi: 10.1016/j.neuropharm.2022.108964. [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Rüb U, Jansen Steur ENH, Del Tredici K, De Vos RAI. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64:1404–1410. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis. 2017;7:S73–S87. doi: 10.3233/JPD-179001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 23.Brekk OR, Honey JR, Lee S, Hallett PJ, Isacson O. Cell type-specific lipid storage changes in Parkinson’s disease patient brains are recapitulated by experimental glycolipid disturbance. Proc Natl Acad Sci U S A. 2020;117:27646–27654. doi: 10.1073/PNAS.2003021117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce KD, Zsombok A, Eckel RH. Lipid processing in the brain: a key regulator of systemic metabolism. Front Endocrinol (Lausanne) 2017;8:60. doi: 10.3389/FENDO.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burré J. The synaptic function of α-synuclein. J Parkinsons Dis. 2015;5:699–713. doi: 10.3233/JPD-150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai S, Wu Y, Guillen-Samander A, Hancock-Cerutti W, Liu J, De CP. In situ architecture of the lipid transport protein VPS13C at ER-lysosomes membrane contacts. Proc Natl Acad Sci U S A. 2022;119:29. doi: 10.1073/pnas2203769119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camilleri A, Zarb C, Caruana M, Ostermeier U, Ghio S, Högen T, et al. Mitochondrial membrane permeabilisation by amyloid aggregates and protection by polyphenols. Biochim Biophys Acta. 2013;1828:2532–2543. doi: 10.1016/J.BBAMEM.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Candelise N, Schmitz M, Thüne K, Cramm M, Rabano A, Zafar S, et al. Effect of the micro-environment on α-synuclein conversion and implication in seeded conversion assays. Transl Neurodegener. 2020;91(9):1–16. doi: 10.1186/S40035-019-0181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerri S, Ghezzi C, Ongari G, Croce S, Avenali M, Zangaglia R, et al. GBA mutations influence the release and pathological effects of small extracellular vesicles from fibroblasts of patients with Parkinson’s disease. Int J Mol Sci. 2021;22:2215. doi: 10.3390/IJMS22042215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cervilla-Martínez JF, Rodríguez-Gotor JJ, Wypijewski KJ, Fontán-Lozano Á, Wang T, Santamaría E, et al. Altered cortical palmitoylation induces widespread molecular disturbances in Parkinson’s disease. Int J Mol Sci. 2022;23:14018. doi: 10.3390/IJMS232214018/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YP, Song W, Huang R, Chen K, Zhao B, Li J, et al. GAK rs1564282 and DGKQ rs11248060 increase the risk for Parkinson’s disease in a Chinese population. J Clin Neurosci. 2013;20:880–883. doi: 10.1016/J.JOCN.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Cheng D, Jenner AM, Shui G, Cheong WF, Mitchell TW, Nealon JR, et al. Lipid pathway alterations in Parkinson’s disease primary visual cortex. PLoS ONE. 2011;6:e17299. doi: 10.1371/JOURNAL.PONE.0017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiurchiù V, Tiberi M, Matteocci A, Fazio F, Siffeti H, Saracini S, et al. Lipidomics of bioactive lipids in Alzheimer’s and Parkinson’s diseases: where are we? Int J Mol Sci. 2022 doi: 10.3390/IJMS23116235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciccocioppo F, Bologna G, Ercolino E, Pierdomenico L, Simeone P, Lanuti P, et al. Neurodegenerative diseases as proteinopathies-driven immune disorders. Neural Regen Res. 2020;15:850. doi: 10.4103/1673-5374.268971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, et al. Survival and dementia in GBA-associated Parkinson’s disease: the mutation matters. Ann Neurol. 2016;80:662–673. doi: 10.1002/ANA.24777. [DOI] [PubMed] [Google Scholar]
- 36.Cole NB, DiEuliis D, Leo P, Mitchell DC, Nussbaum RL. Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res. 2008;314:2076–2089. doi: 10.1016/J.YEXCR.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL. Lipid droplet binding and oligomerization properties of the parkinson’s disease protein α-synuclein. J Biol Chem. 2002;277:6344–6352. doi: 10.1074/JBC.M108414200. [DOI] [PubMed] [Google Scholar]
- 38.Coleman C, Martin I. Unraveling Parkinson’s disease neurodegeneration: does aging hold the clues? J Parkinsons Dis. 2022;12:1–18. doi: 10.3233/jpd-223363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper O, Hallett P, Isacson O. Upstream lipid and metabolic systems are potential causes of Alzheimer’s disease. Parkinson’s disease and dementias. FEBS J. 2022 doi: 10.1111/FEBS.16638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Costa CA, El MW, Duplan E, Checler F. The endoplasmic reticulum stress/unfolded protein response and their contributions to Parkinson’s disease physiopathology. Cells. 2020 doi: 10.3390/CELLS9112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Croisier E, Moran LB, Dexter DT, Pearce RKB, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: Relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:1–8. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cronin KD, Ge D, Manninger P, Linnertz C, Rossoshek A, Orrison BM, et al. Expansion of the Parkinson disease-associated SNCA-Rep1 allele upregulates human alpha-synuclein in transgenic mouse brain. Hum Mol Genet. 2009;18:3274–3285. doi: 10.1093/HMG/DDP265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuervo AM, Stafanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science (80-) 2004;305:1292–1295. doi: 10.1126/SCIENCE.1101738. [DOI] [PubMed] [Google Scholar]
- 44.Custodia A, Aramburu-n M, Correa-paz C, Posado-Fernandez A, Gómez-Larrauri A, Castillo J, et al. Ceramide metabolism and Parkinson ’ s disease—therapeutic targets. Biomolecules. 2021 doi: 10.3390/biom11070945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:1–18. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danzer KM, Ruf WP, Putcha P, Joyner D, Hashimoto T, Glabe C, et al. Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 2011;25:326–336. doi: 10.1096/FJ.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darvish H, Bravo P, Tafakhori A, Azcona LJ, Ranji-Burachaloo S, Johari AH, et al. Identification of a large homozygous VPS13C deletion in a patient with early-onset Parkinsonism. Mov Disord. 2018;33:1968–1970. doi: 10.1002/MDS.27516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawson G. Measuring brain lipids. Biochim Biophys Acta. 2015;1851:1026. doi: 10.1016/J.BBALIP.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dettmer U, Ramalingam N, von Saucken VE, Kim TE, Newman AJ, Terry-Kantor E, et al. Loss of native α-synuclein multimerization by strategically mutating its amphipathic helix causes abnormal vesicle interactions in neuronal cells. Hum Mol Genet. 2017;26:3466–3481. doi: 10.1093/HMG/DDX227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, et al. Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife. 2013 doi: 10.7554/ELIFE.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Domenico F, Lanzillotta C. The disturbance of protein synthesis/degradation homeostasis is a common trait of age-related neurodegenerative disorders. Adv Protein Chem Struct Biol. 2022;132:49–87. doi: 10.1016/BS.APCSB.2022.05.008. [DOI] [PubMed] [Google Scholar]
- 52.Duran R, Mencacci NE, Angeli AV, Shoai M, Deas E, Houlden H, et al. The glucocerobrosidase E326K variant predisposes to Parkinson’s disease, but does not cause Gaucher’s disease. Mov Disord. 2013;28:232–236. doi: 10.1002/MDS.25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eliezer D, Kutluay E, Bussell R, Browne G. Conformational properties of α-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/JMBI.2001.4538. [DOI] [PubMed] [Google Scholar]
- 54.Emamzadeh FN, Aojula H, McHugh PC, Allsop D. Effects of different isoforms of apoE on aggregation of the α-synuclein protein implicated in Parkinson’s disease. Neurosci Lett. 2016;618:146–151. doi: 10.1016/J.NEULET.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 55.Estes RE, Lin B, Khera A, Davis MY. Lipid metabolism influence on neurodegenerative disease progression: is the vehicle as important as the cargo? Front Mol Neurosci. 2021;14:321. doi: 10.3389/FNMOL.2021.788695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Factor SA, Kyle Steenland N, Higgins DS, Molho ES, Kay DM, Montimurro J, et al. Postural instability/gait disturbance in Parkinson’s disease has distinct subtypes: an exploratory analysis. J Neurol Neurosurg Psychiatry. 2011;82:564–568. doi: 10.1136/JNNP.2010.222042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fagone P, Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res. 2009 doi: 10.1194/JLR.R800049-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fanning S, Haque A, Imberdis T, Baru V, Barrasa MI, Nuber S, et al. Lipidomic analysis of α-synuclein neurotoxicity identifies stearoyl CoA desaturase as a target for Parkinson treatment. Mol Cell. 2019;73:1001–1014.e8. doi: 10.1016/J.MOLCEL.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fanning S, Selkoe D, Dettmer U. Vesicle trafficking and lipid metabolism in synucleinopathy. Acta Neuropathol. 2021;141:491–510. doi: 10.1007/S00401-020-02177-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng C, Flores M, Dhoj C, Garcia A, Belleca S, Abbas DA, et al. Observation of α-synuclein preformed fibrils interacting with SH-SY5Y neuroblastoma cell membranes using scanning ion conductance microscopy. ACS Chem Neurosci. 2022 doi: 10.1021/ACSCHEMNEURO.2C00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandes HJR, Hartfield EM, Christian HC, Emmanoulidou E, Zheng Y, Booth H, et al. ER stress and autophagic perturbations lead to elevated extracellular α-synuclein in GBA-N370S Parkinson’s iPSC-derived dopamine neurons. Stem Cell Reports. 2016;6:342–356. doi: 10.1016/J.STEMCR.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferreon ACM, Gambin Y, Lemke EA, Deniz AA. Interplay of α-synuclein binding and conformational switching probed by single-molecule fluorescence. Proc Natl Acad Sci U S A. 2009;106:5645–5650. doi: 10.1073/PNAS.0809232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forsaa EB, Larsen JP, Wentzel-Larsen T, Goetz CG, Stebbins GT, Aarsland D. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67:996–1001. doi: 10.1001/ARCHNEUROL.2010.166. [DOI] [PubMed] [Google Scholar]
- 64.Fortin DL, Troyer MD, Nakamura K, Kubo SI, Anthony MD, Edwards RH. Lipid Rafts Mediate the Synaptic Localization of α-Synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;42(4):160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 66.Furderer ML, Hertz E, Lopez GJ, Sidransky E. Neuropathological features of gaucher disease and gaucher disease with parkinsonism. Int J Mol Sci. 2022;23:5842. doi: 10.3390/IJMS23105842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fusco G, De Simone A, Gopinath T, Vostrikov V, Vendruscolo M, Dobson CM, et al. Direct observation of the three regions in α-synuclein that determine its membrane-bound behaviour. Nat Commun. 2014;51(5):1–8. doi: 10.1038/ncomms4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gai WP, Yuan HX, Li XQ, Power JTH, Blumbergs PC, Jensen PH. In Situ and in vitro study of colocalization and segregation of α-synuclein, ubiquitin, and lipids in lewy bodies. Exp Neurol. 2000;166:324–333. doi: 10.1006/EXNR.2000.7527. [DOI] [PubMed] [Google Scholar]
- 69.Gan-Or Z, Amshalom I, Kilarski LL, Bar-Shira A, Gana-Weisz M, Mirelman A, et al. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology. 2015;84:880–887. doi: 10.1212/WNL.0000000000001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ge P, Dawson VL, Dawson TM. PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson’s disease. Mol Neurodegener. 2020;151(15):1–18. doi: 10.1186/S13024-020-00367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gegg ME, Burke D, Heales SJR, Cooper JM, Hardy J, Wood NW, et al. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol. 2012;72:455–463. doi: 10.1002/ANA.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gegg ME, Menozzi E, Schapira AHV. Glucocerebrosidase-associated Parkinson disease: pathogenic mechanisms and potential drug treatments. Neurobiol Dis. 2022;166:105663. doi: 10.1016/j.nbd.2022.105663. [DOI] [PubMed] [Google Scholar]
- 73.Ghio S, Camilleri A, Caruana M, Ruf VC, Schmidt F, Leonov A, et al. Cardiolipin promotes pore-forming activity of alpha-synuclein oligomers in mitochondrial membranes. ACS Chem Neurosci. 2019;10:3815–3829. doi: 10.1021/ACSCHEMNEURO.9B00320. [DOI] [PubMed] [Google Scholar]
- 74.Girard V, Jollivet F, Knittelfelder O, Celle M, Arsac JN, Chatelain G, et al. Abnormal accumulation of lipid droplets in neurons induces the conversion of alpha-Synuclein to proteolytic resistant forms in a Drosophila model of Parkinson’s disease. PLoS Genet. 2021 doi: 10.1371/JOURNAL.PGEN.1009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greaves J, Chamberlain L. Palmitoylation-dependent protein sorting. J Cell Biol. 2007;176:249–254. doi: 10.1083/JCB.200610151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guardia-Laguarta C, Area-Gomez E, Rüb C, Liu Y, Magrané J, Becker D, Voos W, Schon EA, Przedborski S. α-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci. 2014;34:249–259. doi: 10.1523/JNEUROSCI.2507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guedes LC, Chan RB, Gomes MA, Conceição VA, Machado RB, Soares T, Xu Y, Gaspar P, Carriço JA, Alcalay RN, Ferreira JJ, Outeiro TF, Miltenberger-Miltenyi G. Serum lipid alterations in GBA-associated Parkinson’s disease. Park Relat Disord. 2017;44:58–65. doi: 10.1016/j.parkreldis.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 78.Guillén-Samander A, Leonzino M, Hanna MG, Tang N, Shen H, De Camilli P. VPS13D bridges the ER to mitochondria and peroxisomes via Miro. J Cell Biol. 2021 doi: 10.1083/JCB.202010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Habashy KJ, Ahmad F, Ibeh S, Mantash S, Kobeissy F, Issa H, et al. Western and ketogenic diets in neurological disorders: can you tell the difference? Nutr Rev. 2022;80:1927–1941. doi: 10.1093/NUTRIT/NUAC008. [DOI] [PubMed] [Google Scholar]
- 80.Hallett PJ, Engelender S, Isacson O. Lipid and immune abnormalities causing age-dependent neurodegeneration and Parkinson’s disease. J Neuroinflamm. 2019;161(16):1–15. doi: 10.1186/S12974-019-1532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han J, Kaufman RJ. The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res. 2016;57:1329–1338. doi: 10.1194/JLR.R067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hancock-Cerutti W, Wu Z, Xu P, Yadavalli N, Leonzino M, Tharkeshwar AK, et al. ER-lysosome lipid transfer protein VPS13C/PARK23 prevents aberrant mtDNA-dependent STING signaling. J Cell Biol. 2022 doi: 10.1083/JCB.202106046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hattingen E, Magerkurth J, Pilatus U, Mozer A, Seifried C, Steinmetz H, et al. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain. 2009;132:3285–3297. doi: 10.1093/BRAIN/AWP293. [DOI] [PubMed] [Google Scholar]
- 84.Hayashi T, Fujimoto M. Detergent-resistant microdomains determine the localization of σ-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol Pharmacol. 2010;77:517–528. doi: 10.1124/MOL.109.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/MDS.21956. [DOI] [PubMed] [Google Scholar]
- 86.Heman-Ackah SM, Manzano R, Hoozemans JJM, Scheper W, Flynn R, Haerty W, et al. Alpha-synuclein induces the unfolded protein response in Parkinson’s disease SNCA triplication iPSC-derived neurons. Hum Mol Genet. 2017;26:4441–4450. doi: 10.1093/HMG/DDX331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henriques A, Rouvière L, Giorla E, Farrugia C, El Waly B, Poindron P, et al. Alpha-synuclein: the spark that flames dopaminergic neurons, in vitro and in vivo evidence. Int J Mol Sci. 2022;23:9864. doi: 10.3390/IJMS23179864/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T. The incidence of Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2016;46:292–300. doi: 10.1159/000445751. [DOI] [PubMed] [Google Scholar]
- 89.Ho N, Xu C, Thibault G. From the unfolded protein response to metabolic diseases - lipids under the spotlight. J Cell Sci. 2018 doi: 10.1242/JCS.199307. [DOI] [PubMed] [Google Scholar]
- 90.Hoozemans JJM, van Haastert ES, Eikelenboom P, de Vos RAI, Rozemuller JM, Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun. 2007;354:707–711. doi: 10.1016/J.BBRC.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 91.Huebecker M, Moloney EB, Van Der Spoel AC, Priestman DA, Isacson O, Hallett PJ, et al. Reduced sphingolipid hydrolase activities, substrate accumulation and ganglioside decline in Parkinson’s disease. Mol Neurodegener. 2019;14:1–21. doi: 10.1186/s13024-019-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imberdis T, Negri J, Ramalingam N, Terry-Kantor E, Ho GPH, Fanning S, et al. Cell models of lipid-rich α-synuclein aggregation validate known modifiers of α-synuclein biology and identify stearoyl-CoA desaturase. Proc Natl Acad Sci U S A. 2019;116:20760–20769. doi: 10.1073/PNAS.1903216116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Indellicato R, Trinchera M. The link between gaucher disease and Parkinson’s disease sheds light on old and novel disorders of sphingolipid metabolism. Int J Mol Sci. 2019;20:3304. doi: 10.3390/IJMS20133304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, Rohan de Silva HA, et al. The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-X. [DOI] [PubMed] [Google Scholar]
- 95.Jarc E, Kump A, Malavašič P, Eichmann TO, Zimmermann R, Petan T. Lipid droplets induced by secreted phospholipase A2 and unsaturated fatty acids protect breast cancer cells from nutrient and lipotoxic stress. Biochim Biophys acta Mol cell Biol lipids. 2018;1863:247–265. doi: 10.1016/J.BBALIP.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 96.Jarc E, Petan T. Focus: organelles: lipid droplets and the management of cellular stress. Yale J Biol Med. 2019;92:435. [PMC free article] [PubMed] [Google Scholar]
- 97.Jo E, Fuller N, Rand RP, St George-Hyslop P, Fraser PE. Defective membrane interactions of familial Parkinson’s disease mutant A30P α-synuclein. J Mol Biol. 2002;315:799–807. doi: 10.1006/JMBI.2001.5269. [DOI] [PubMed] [Google Scholar]
- 98.Johnson PH, Weinreb NJ, Cloyd JC, Tuite PJ, Kartha RV. GBA1 mutations: Prospects for exosomal biomarkers in α-synuclein pathologies. Mol Genet Metab. 2020;129:35–46. doi: 10.1016/J.YMGME.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kachappilly N, Srivastava J, Swain BP, Thakur P. Interaction of alpha-synuclein with lipids. Methods Cell Biol. 2022;169:43–66. doi: 10.1016/BS.MCB.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 101.Kasten M, Hartmann C, Hampf J, Schaake S, Westenberger A, Vollstedt EJ, et al. Genotype-phenotype relations for the Parkinson’s disease genes parkin, PINK1, DJ1: MDSGene systematic review. Mov Disord. 2018;33:730–741. doi: 10.1002/MDS.27352. [DOI] [PubMed] [Google Scholar]
- 102.Keo A, Mahfouz A, Ingrassia AMT, Meneboo JP, Villenet C, Mutez E, et al. Transcriptomic signatures of brain regional vulnerability to Parkinson’s disease. Commun Biol. 2020;31(3):1–12. doi: 10.1038/s42003-020-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klemann CJHM, Martens GJM, Sharma M, Martens MB, Isacson O, Gasser T, et al. Poelmans G (2017) Integrated molecular landscape of Parkinson’s disease. npj Park Dis. 2017;31(3):1–7. doi: 10.1038/s41531-017-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 reduces α-synuclein aggregation and toxicity. J Biol Chem. 2004;279:25497–25502. doi: 10.1074/JBC.M400255200. [DOI] [PubMed] [Google Scholar]
- 105.Ko TK, Tan DJY. Is disrupted mitophagy a central player to Parkinson’s disease pathology? Cureus. 2023 doi: 10.7759/CUREUS.35458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/NG0298-106. [DOI] [PubMed] [Google Scholar]
- 107.Kubo SI. Membrane lipids as therapeutic targets for Parkinson’s disease: a possible link between Lewy pathology and membrane lipids. Expert Opin Ther Targets. 2016;20:1301–1310. doi: 10.1517/14728222.2016.1086340. [DOI] [PubMed] [Google Scholar]
- 108.Kumar N, Leonzino M, Hancock-Cerutti W, Horenkamp FA, Li PQ, Lees JA, et al. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol. 2018;217:3625–3639. doi: 10.1083/JCB.201807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuo SH, Tasset I, Cheng MM, Diaz A, Pan MK, Lieberman OJ, et al. Mutant glucocerebrosidase impairs α-synuclein degradation by blockade of chaperone-mediated autophagy. Sci Adv. 2022;8:6393. doi: 10.1126/SCIADV.ABM6393. [DOI] [PubMed] [Google Scholar]
- 110.Kurzawa-Akanbi M, Tammireddy S, Fabrik I, Gliaudelytė L, Doherty MK, Heap R, et al. Altered ceramide metabolism is a feature in the extracellular vesicle-mediated spread of alpha-synuclein in Lewy body disorders. Acta Neuropathol. 2021;142:961–984. doi: 10.1007/S00401-021-02367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lansbury P. The sphingolipids clearly play a role in parkinson’s disease, but nature has made it complicated. Mov Disord. 2022;37:1985–1989. doi: 10.1002/MDS.29204. [DOI] [PubMed] [Google Scholar]
- 112.Lauressergues E, Bert E, Duriez P, Hum D, Majd Z, Staels B, et al. Does endoplasmic reticulum stress participate in APD-induced hepatic metabolic dysregulation? Neuropharmacology. 2012;62:784–796. doi: 10.1016/J.NEUROPHARM.2011.08.048. [DOI] [PubMed] [Google Scholar]
- 113.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science (80-) 2008;320:1492–1496. doi: 10.1126/SCIENCE.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lesage S, Drouet V, Majounie E, Deramecourt V, Jacoupy M, Nicolas A, et al. Loss of VPS13C function in autosomal-recessive parkinsonism causes mitochondrial dysfunction and increases PINK1/parkin-dependent mitophagy. Am J Hum Genet. 2016;98:500–513. doi: 10.1016/j.ajhg.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leverenz JB, Umar I, Wang Q, Montine TJ, McMillan PJ, Tsuang DW, et al. Proteomic identification of novel proteins in cortical Lewy bodies. Brain Pathol. 2007;17:139–145. doi: 10.1111/J.1750-3639.2007.00048.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li G, Cui S, Du J, Liu J, Zhang P, Fu Y, et al. Association of GALC, ZNF184, IL1R2 and ELOVL7 With Parkinson’s Disease in Southern Chinese. Front Aging Neurosci. 2018;10:402. doi: 10.3389/FNAGI.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li H, Meng Q, Xiao F, Chen S, Du Y, Yu J, Wang C, Guo F. ATF4 deficiency protects mice from high-carbohydrate-diet-induced liver steatosis. Biochem J. 2011;438:283–289. doi: 10.1042/BJ20110263. [DOI] [PubMed] [Google Scholar]
- 118.Li P, Song C. Potential treatment of Parkinson’s disease with omega-3 polyunsaturated fatty acids. Nutr Neurosci. 2020;25:180–191. doi: 10.1080/1028415X.2020.1735143. [DOI] [PubMed] [Google Scholar]
- 119.Lim JL, Ng EY, Lim SY, Tan AH, Abdul-Aziz Z, Ibrahim KA, et al. Association study of MCCC1/LAMP3 and DGKQ variants with Parkinson’s disease in patients of Malay ancestry. Neurol Sci. 2021;42:4203–4207. doi: 10.1007/S10072-021-05056-X. [DOI] [PubMed] [Google Scholar]
- 120.Linder M, Deschenes R. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/NRM2084. [DOI] [PubMed] [Google Scholar]
- 121.Lou X, Kim J, Hawk BJ, Shin YK. α-Synuclein may cross-bridge v-SNARE and acidic phospholipids to facilitate SNARE-dependent vesicle docking. Biochem J. 2017;474:2039–2049. doi: 10.1042/BCJ20170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luk KC, Mills IP, Trojanowski JQ, Lee VMY. Interactions between Hsp70 and the hydrophobic core of α-synuclein inhibit fibril assembly. Biochemistry. 2008;47:12614–12625. doi: 10.1021/BI801475R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Magalhaes J, Gegg ME, Migdalska-Richards A, Doherty MK, Whitfield PD, Schapira AHV. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum Mol Genet. 2016;25:3432–3445. doi: 10.1093/HMG/DDW185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Man WK, Tahirbegi B, Vrettas MD, Preet S, Ying L, Vendruscolo M, et al. The docking of synaptic vesicles on the presynaptic membrane induced by α-synuclein is modulated by lipid composition. Nat Commun. 2021;121(12):1–10. doi: 10.1038/s41467-021-21027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masaracchia C, Hnida M, Gerhardt E, Lopes da Fonseca T, Villar-Pique A, Branco T, Stahlberg MA, et al. Membrane binding, internalization, and sorting of alpha-synuclein in the cell. Acta Neuropathol Commun. 2018;6:79. doi: 10.1186/S40478-018-0578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/J.CELL.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mazzulli JR, Zunke F, Tsunemi T, Toker NJ, Jeon S, Burbulla LF, et al. Activation of β-glucocerebrosidase reduces pathological α-synuclein and restores lysosomal function in Parkinson’s PATIENT Midbrain neurons. J Neurosci. 2016;36:7693. doi: 10.1523/JNEUROSCI.0628-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Migdalska-Richards A, Schapira AHV. The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem. 2016 doi: 10.1111/JNC.13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Migdalska-Richards A, Wegrzynowicz M, Harrison IF, Verona G, Bellotti V, Spillantini MG, et al. L444P Gba1 mutation increases formation and spread of α-synuclein deposits in mice injected with mouse α-synuclein pre-formed fibrils. PLoS ONE. 2020 doi: 10.1371/JOURNAL.PONE.0238075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moors TE, Paciotti S, Ingrassia A, Quadri M, Breedveld G, Tasegian A, Chiasserini D, et al. Characterization of brain lysosomal activities in GBA-related and sporadic Parkinson’s disease and dementia with lewy bodies. Mol Neurobiol. 2019;56:1344–1355. doi: 10.1007/s12035-018-1090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, Vanbrabant M, et al. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1:99–111. doi: 10.1002/EMMM.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Motyl JA, Strosznajder JB, Wencel A, Strosznajder RP. Recent insights into the interplay of alpha-synuclein and sphingolipid signaling in Parkinson’s disease. Int J Mol Sci. 2021;22:6277. doi: 10.3390/IJMS22126277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, et al. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain. 2014;137:834–848. doi: 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Musanti R, Parati E, Lamperti E, Ghiselli G. Decreased cholesterol biosynthesis in fibroblasts from patients with Parkinson disease. Biochem Med Metab Biol. 1993;49:133–142. doi: 10.1006/BMMB.1993.1016. [DOI] [PubMed] [Google Scholar]
- 137.Nakamura K, Mori F, Tanji K, Miki Y, Yamada M, Kakita A, et al. Isopentenyl diphosphate isomerase, a cholesterol synthesizing enzyme, is localized in Lewy bodies. Neuropathology. 2015;35:432–440. doi: 10.1111/NEUP.12204. [DOI] [PubMed] [Google Scholar]
- 138.Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;469(46):989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Navarro-Romero A, Fernandez-Gonzalez I, Riera J, Montpeyo M, Albert-Bayo M, Lopez-Royo T, et al. Lysosomal lipid alterations caused by glucocerebrosidase deficiency promote lysosomal dysfunction, chaperone-mediated-autophagy deficiency, and alpha-synuclein pathology. Npj Park Dis. 2022;81(8):1–15. doi: 10.1038/s41531-022-00397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nguyen APT, Tsika E, Kelly K, Levine N, Chen X, West AB, et al. Dopaminergic neurodegeneration induced by Parkinson’s disease-linked G2019S LRRK2 is dependent on kinase and GTPase activity. Proc Natl Acad Sci U S A. 2020;117:17296–17307. doi: 10.1073/pnas.1922184117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nguyen TB, Louie SM, Daniele JR, Tran Q, Dillin A, Zoncu R, et al. DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Dev Cell. 2017;42:9–21.e5. doi: 10.1016/J.DEVCEL.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Norouzkhani N, Karimi AG, Badami N, Jalalifar E, Mahmoudvand B, Ansari A, et al. From kitchen to clinic: pharmacotherapeutic potential of common spices in Indian cooking in age-related neurological disorders. Front Pharmacol. 2022;13:1–30. doi: 10.3389/fphar.2022.960037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2018;203(20):137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science (/0-) 2003;302:1772–1775. doi: 10.1126/SCIENCE.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, et al. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124:593–605. doi: 10.1007/S00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Papadopoulos VE, Nikolopoulou G, Antoniadou I, Karachaliou A, Arianoglou G, Emmanouilidou E, et al. Modulation of β-glucocerebrosidase increases α-synuclein secretion and exosome release in mouse models of Parkinson’s disease. Hum Mol Genet. 2018;27:1696–1710. doi: 10.1093/HMG/DDY075. [DOI] [PubMed] [Google Scholar]
- 147.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci. 2008;65:1272–1284. doi: 10.1007/S00018-008-7589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Parlar SC, Grenn FP, Kim JJ, Baluwendraat C, Gan-Or Z. Classification of GBA1 variants in Parkinson’s disease: the GBA1-PD browser. Mov Disord. 2023;38:489–495. doi: 10.1002/MDS.29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Paul A, Jacoby G, Laor Bar-Yosef D, Beck R, Gazit E, Segal D. Glucosylceramide associated with gaucher disease forms amyloid-like twisted ribbon fibrils that induce α-synuclein aggregation. ACS Nano. 2021;15:11854–11868. doi: 10.1021/ACSNANO.1C02957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human α-synuclein and Parkinson’s disease variants with phospholipids: structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/JBC.M004851200. [DOI] [PubMed] [Google Scholar]
- 151.Pinto-Costa R, Harbachova E, La Vitola P, Di Monte DA. Overexpression-Induced α-synuclein brain spreading. Neurotherapeutics. 2022 doi: 10.1007/S13311-022-01332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science (80-) 1997;276:2045–2047. doi: 10.1126/SCIENCE.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 153.Pradas E, Martinez-Vicente M. The consequences of GBA deficiency in the autophagy-lysosome system in Parkinson’s disease associated with GBA. Cells. 2023;12:191. doi: 10.3390/CELLS12010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Puglielli L, Konopka G, Pack-Chung E, Ingano LAMK, Berezovska O, Hyman BT, et al. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat Cell Biol. 2001;310(3):905–912. doi: 10.1038/ncb1001-905. [DOI] [PubMed] [Google Scholar]
- 155.Rocha EM, Smith GA, Park E, Cao H, Brown E, Hallett P, et al. Progressive decline of glucocerebrosidase in aging and Parkinson’s disease. Ann Clin Transl Neurol. 2015;2:433–438. doi: 10.1002/ACN3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14:2387–2398. doi: 10.1093/HMG/DDI240. [DOI] [PubMed] [Google Scholar]
- 157.Rose G, Crocco P, Fernandes T, Domingues MR, Moreira PI, Pereira CF. A perspective on the link between mitochondria-associated membranes (MAMs) and lipid droplets metabolism in neurodegenerative diseases. Biol. 2023;12:414. doi: 10.3390/BIOLOGY12030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ryan T, Bamm VV, Stykel MG, Coackley CL, Humphries KM, Jamieson-Williams R. Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat Commun. 2018 doi: 10.1038/S41467-018-03241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Samaranch L, Lorenzo-Betancor O, Arbelo JM, Ferrer I, Lorenzo E, Irigoyen J, et al. PINK1-linked parkinsonism is associated with Lewy body pathology. Brain. 2010;133:1128–1142. doi: 10.1093/BRAIN/AWQ051. [DOI] [PubMed] [Google Scholar]
- 160.Sánchez Campos S, Alza NP, Salvador GA. Lipid metabolism alterations in the neuronal response to A53T α-synuclein and Fe-induced injury. Arch Biochem Biophys. 2018;655:43–54. doi: 10.1016/J.ABB.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 161.Schneider SA, Hizli B, Alcalay RN. Emerging targeted therapeutics for genetic subtypes of Parkinsonism. Neurotherapeutics. 2020;17:1378–1392. doi: 10.1007/S13311-020-00920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sciacca MF, Lolicato F, Tempra C, Scollo F, Sahoo BR, Watson MD, et al. Lipid-chaperone hypothesis: a common molecular mechanism of membrane disruption by intrinsically disordered proteins. ACS Chem Neurosci. 2020;11:4336–4350. doi: 10.1021/ACSCHEMNEURO.0C00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Senkal CE, Salama MF, Snider AJ, Allopenna JJ, Rana NA, Koller A, et al. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab. 2017;25:686–697. doi: 10.1016/J.CMET.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Senkevich K, Rudakou U, Gan-Or Z. Genetic mechanism vs genetic subtypes: the example of GBA. Handb Clin Neurol. 2023;193:155–170. doi: 10.1016/B978-0-323-85555-6.00016-3. [DOI] [PubMed] [Google Scholar]
- 165.Seyfried TN, Choi H, Chevalier A, Hogan D, Akgoc Z, Schneider JS. Sex-related abnormalities in substantia Nigra lipids in Parkinson’s disease. ASN Neuro. 2018 doi: 10.1177/1759091418781889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shahmoradian SH, Lewis AJ, Genoud C, Hench J, Moors TE, Navarro PP, Castaño-Díez D, Schweighauser G, Graff-Meyer A, Goldie KN, Sütterlin R, et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci. 2019;22:1099–1109. doi: 10.1038/s41593-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 167.Shpilka T, Welter E, Borovsky N, Amar N, Mari M, Reggiori F, et al. Lipid droplets and their component triglycerides and steryl esters regulate autophagosome biogenesis. EMBO J. 2015;34:2117–2131. doi: 10.15252/EMBJ.201490315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Simón-Sánchez J, van Hilten JJ, van de Warrenburg B, Post B, Berendse HW, Arepalli S, et al. Genome-wide association study confirms extant PD risk loci among the Dutch. Eur J Hum Genet. 2011;196(19):655–661. doi: 10.1038/ejhg.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sinclair E, Trivedi DK, Sarkar D, Walton-Doyle C, Milne J, Kunath T, et al. Metabolomics of sebum reveals lipid dysregulation in Parkinson’s disease. Nat Commun. 2021;121(12):1–9. doi: 10.1038/s41467-021-21669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/SCIENCE.1090278. [DOI] [PubMed] [Google Scholar]
- 172.Smith LJ, Bolsinger MM, Chau K-Y, Gegg ME, Schapira AHV. The GBA variant E326K is associated with alpha-synuclein aggregation and lipid droplet accumulation in human cell lines. Hum Mol Genet. 2022 doi: 10.1093/HMG/DDAC233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nat. 1997;3886645(388):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 174.Stevenson J, Huang EY, Olzmann JA. Endoplasmic reticulum-associated degradation and lipid homeostasis. Annu Rev Nutr. 2016;36:511–542. doi: 10.1146/ANNUREV-NUTR-071715-051030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stöckl M, Fischer P, Wanker E, Herrmann A. α-Synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. J Mol Biol. 2008;375:1394–1404. doi: 10.1016/J.JMB.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 176.Stoessel D, Schulte C, Teixeira dos Santos MC, Scheller D, Rebollo-Mesa I, Deuschle C, et al. Promising metabolite profiles in the plasma and CSF of early clinical Parkinson’s disease. Front Aging Neurosci. 2018 doi: 10.3389/FNAGI.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stuendl A, Kunadt M, Kruse N, Bartels C, Moebius W, Danzer KM, et al. Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain. 2016;139:481–494. doi: 10.1093/BRAIN/AWV346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Theillet FX, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nat. 2016;5307588(530):45–50. doi: 10.1038/nature16531. [DOI] [PubMed] [Google Scholar]
- 179.Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST. Neuronal lipid metabolism: Multiple pathways driving functional outcomes in health and disease. Front Mol Neurosci. 2018;11:10. doi: 10.3389/FNMOL.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Trupp M, Jonsson P, Ohrfelt A, Zetterberg H, Obudulu O, Malm L, et al. Metabolite and peptide levels in plasma and CSF differentiating healthy controls from patients with newly diagnosed Parkinson’s disease. J Parkinsons Dis. 2014;4:549–560. doi: 10.3233/JPD-140389. [DOI] [PubMed] [Google Scholar]
- 181.Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, et al. APOE ϵ 4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70:223–228. doi: 10.1001/JAMANEUROL.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ugalde CL, Annesley SJ, Gordon SE, Mroczek K, Perugini MA, Lawson VA, et al. Misfolded α-synuclein causes hyperactive respiration without functional deficit in live neuroblastoma cells. DMM Dis Model Mech. 2020 doi: 10.1242/DMM.040899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ugur B, Hancock-Cerutti W, Leonzino M, De Camilli P. Role of VPS13, a protein with similarity to ATG2, in physiology and disease. Curr Opin Genet Dev. 2020;65:61–68. doi: 10.1016/J.GDE.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Valadas JS, Esposito G, Vandekerkhove D, Miskiewicz K, Deaulmerie L, Raitano S, et al. ER lipid defects in neuropeptidergic neurons impair sleep patterns in Parkinson’s disease. Neuron. 2018;98:1155–1169.e6. doi: 10.1016/J.NEURON.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 185.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MMK, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science (80-) 2004;304:1158–1160. doi: 10.1126/SCIENCE.1096284. [DOI] [PubMed] [Google Scholar]
- 186.Vincent BM, Tardiff DF, Piotrowski JS, Aron R, Lucas MC, Chung CY, et al. Inhibiting stearoyl-CoA desaturase ameliorates α-synuclein cytotoxicity. Cell Rep. 2018;25:2742–2754.e31. doi: 10.1016/J.CELREP.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 187.Volmer R, Ron D. Lipid-dependent regulation of the unfolded protein response. Curr Opin Cell Biol. 2015;33:67–73. doi: 10.1016/J.CEB.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vos M, Dulovic-Mahlow M, Mandik F, Frese L, Kanana Y, Diaw SH, et al. Ceramide accumulation induces mitophagy and impairs β-oxidation in PINK1 deficiency. Proc Natl Acad Sci U S A. 2021 doi: 10.1073/PNAS.2025347118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vos M, Geens A, Böhm C, Deaulmerie L, Swerts J, Rossi M, et al. Cardiolipin promotes electron transport between ubiquinone and complex I to rescue PINK1 deficiency. J Cell Biol. 2017;216:695–708. doi: 10.1083/JCB.201511044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, Takahashi H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol Neurobiol. 2013;47:495–508. doi: 10.1007/s12035-012-8280-y. [DOI] [PubMed] [Google Scholar]
- 191.Wang S, Chen Z, Lam V, Han J, Hassler J, Finck BN, et al. IRE1α-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab. 2012;16:473–486. doi: 10.1016/J.CMET.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang S, Horn PJ, Liou LC, Muggeridge MI, Zhang Z, Chapman KD, et al. A peroxisome biogenesis deficiency prevents the binding of alpha-synuclein to lipid droplets in lipid-loaded yeast. Biochem Biophys Res Commun. 2013;438:452–456. doi: 10.1016/J.BBRC.2013.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang S, Zhang S, Liou LC, Ren Q, Zhang Z, Caldwell GA, et al. Phosphatidylethanolamine deficiency disrupts α-synuclein homeostasis in yeast and worm models of Parkinson disease. Proc Natl Acad Sci U S A. 2014;111:E3976–E3985. doi: 10.1073/PNAS.1411694111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Willkommen D, Lucio M, Moritz F, Forcisi S, Kanawati B, Smirnov KS, et al. Metabolomic investigations in cerebrospinal fluid of Parkinson’s disease. PLoS ONE. 2018 doi: 10.1371/JOURNAL.PONE.0208752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu BX, Rajagopalan V, Roddy PL, Clarke CJ, Hannun YA. Identification and characterization of murine mitochondria-associated neutral sphingomyelinase (MA-nSMase), the mammalian sphingomyelin phosphodiesterase 5. J Biol Chem. 2010;285:17993–18002. doi: 10.1074/JBC.M110.102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xiao G, Zhang T, Yu S, Lee S, Calabuig-Navarro V, Yamauchi J, et al. ATF4 protein deficiency protects against high fructose-induced hypertriglyceridemia in mice. J Biol Chem. 2013;288:25350–25361. doi: 10.1074/JBC.M113.470526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xie YX, Naseri NN, Fels J, Kharel P, Na Y, Lane D, et al. Lysosomal exocytosis releases pathogenic α-synuclein species from neurons in synucleinopathy models. Nat Commun. 2022;131(13):1–16. doi: 10.1038/s41467-022-32625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yoo G, Shin YK, Lee NK. The role of α-synuclein in SNARE-mediated synaptic vesicle fusion. J Mol Biol. 2023;435:167775. doi: 10.1016/J.JMB.2022.167775. [DOI] [PubMed] [Google Scholar]
- 199.Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ANA.10795. [DOI] [PubMed] [Google Scholar]
- 200.Zhang W, Xu L, Zhu L, Liu Y, Yang S, Zhao M. Lipid Droplets, the Central Hub Integrating Cell Metabolism and the Immune System. Front Physiol. 2021;12:2155. doi: 10.3389/FPHYS.2021.746749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao H, Wang C, Zhao N, Li W, Yang Z, Liu X, et al. Potential biomarkers of Parkinson’s disease revealed by plasma metabolic profiling. J Chromatogr B. 2018;1081–1082:101–108. doi: 10.1016/J.JCHROMB.2018.01.025. [DOI] [PubMed] [Google Scholar]