Abstract
Cardiovascular diseases (CVDs) remain the leading cause of morbidity and mortality worldwide. Recently, accumulating evidence has revealed hepatic mediators, termed as liver-derived secretory factors (LDSFs), play an important role in regulating CVDs such as atherosclerosis, coronary artery disease, thrombosis, myocardial infarction, heart failure, metabolic cardiomyopathy, arterial hypertension, and pulmonary hypertension. LDSFs presented here consisted of microbial metabolite, extracellular vesicles, proteins, and microRNA, they are primarily or exclusively synthesized and released by the liver, and have been shown to exert pleiotropic actions on cardiovascular system. LDSFs mainly target vascular endothelial cell, vascular smooth muscle cells, cardiomyocytes, fibroblasts, macrophages and platelets, and further modulate endothelial nitric oxide synthase/nitric oxide, endothelial function, energy metabolism, inflammation, oxidative stress, and dystrophic calcification. Although some LDSFs are known to be detrimental/beneficial, controversial findings were also reported for many. Therefore, more studies are required to further explore the causal relationships between LDSFs and CVDs and uncover the exact mechanisms, which is expected to extend our understanding of the crosstalk between the liver and cardiovascular system and identify potential therapeutic targets. Furthermore, in the case of patients with liver disease, awareness should be given to the implications of these abnormalities in the cardiovascular system. These studies also underline the importance of early recognition and intervention of liver abnormalities in the practice of cardiovascular care, and a multidisciplinary approach combining hepatologists and cardiologists would be more preferable for such patients.
Keywords: Liver, Cardiovascular system, Metabolite, Extracellular vesicle, Hepatokine
Graphical abstract
Introduction
Although remarkable advances have been made in clinical and basic research fields, cardiovascular diseases (CVDs) remain the leading cause of morbidity and mortality, and impose a significant global health care burden. The prevalence of CVDs cases nearly doubled from 1990 to 2019, with more than 500 million cases being reported in 2019. And the number of CVDs deaths has steadily increased over the same period, reaching 18.6 million in 2019.1 The liver is the largest internal organ and plays a vital role in various physiological and pathophysiological processes by providing essential metabolic, exocrine, and endocrine functions.2 Studies have revealed extensive crosstalk networks within the liver tissue3 and between the liver and other organs/tissues such as gut4 and skeletal muscle.5 The complex interactions between the liver and cardiovascular system have been studied extensively,6 especially in the context of metabolic disorder.7 An example is nonalcoholic fatty liver disease (NAFLD), which has become a serious public health problem affecting up to one-third of the world’s adult population and shown to be significantly associated with a greater risk of CVDs. These in turn, contribute to increased mortality among patients with NAFLD.7 Not only that, a growing body of evidence has revealed an essential role of liver-derived secretory factors (LDSFs) in regulating cardiovascular physiology and diseases. In this review, we summarize the current molecular evidence linking the liver to cardiovascular system with a focus on LDSFs, which may extend our understanding of the crosstalk between the liver and cardiovascular system and highlight the importance of these emerging novel mediators as potential biomarkers and therapeutic targets.
Trimethylamine N-oxide
Over the years, the gut microbiota and its metabolites have attracted increasing attention owing to their crucial roles in cardiovascular health and diseases by interacting with the host.8 Of these, trimethylamine N-oxide (TMAO) is an important and well-studied metabolite. It was reported that dietary phosphatidylcholine, choline and L-carnitine are metabolized by intestinal microbiota into trimethylamine and then further oxidized into TMAO by flavin monooxygenases in the liver.9,10 Many clinical investigations have shown that circulating TMAO is an independent risk factor for CVDs such as atherosclerosis, thrombosis, acute coronary syndromes, heart failure (HF) and myocardial infarction (MI).9–14 Mechanistically, TMAO was able to accelerate the progression of atherosclerosis by inducing vascular endothelial cells (VECs) pyroptosis through SDHB/ROS pathway,15 contribute to endothelial dysfunction by increasing HMGB1 expression and disrupting cell-cell junction proteins,16 promote vascular calcification by inducing osteogenic differentiation of vascular smooth muscle cells (VSMCs) via NLRP3 inflammasome and NF-κB pathway,17 and exacerbate cardiac function and cardiac fibrosis after MI by promoting the transition of fibroblasts into myofibroblasts through TGF-βRI/Smad2 axis.18 Moreover, it was found that TMAO levels were significantly correlated with the activity of tissue factor in patients with ST-elevation MI, and the activation of NF-κB signaling was necessary for TMAO-mediated tissue factor expression.19 Additionally, in the middle-aged/older groups, TMAO was markedly elevated and associated with impaired brachial artery flow-mediated dilation, which could be reversed in mice supplemented with TMAO.20 Similarly, circulating TMAO levels were found to increase with aging, and further study demonstrated that TMAO accelerated VECs senescence and vascular aging by reducing SIRT1 expression and enhancing oxidative stress,21 which caused aging-associated endothelial insufficiency by impairing endothelial nitric oxide synthase (eNOS) and enhancing the production of inflammatory cytokine and superoxide.22 Increased inflammation and oxidative stress were also responsible for TMAO-induced inhibition of angiogenesis and perfusion recover after hindlimb ischemia.23
Although there are many studies devoted to revealing the effects of TMAO on CVDs, little information is available about its influence on the liver. It was showed that TMAO at the physiological concentration is able to promote metabolic dysfunction by directly binding the hepatic PERK and thus activating the unfolded protein response. The authors suggested that hepatic TMAO-PERK pathway may represent a therapeutic target for this disorder.24 In addition, TMAO has been reported to affect the miRNA composition and function of the exosomes secreted from hepatocytes.25,26 These studies provided evidence that, although TMAO is generated in the liver, this metabolite can in turn act on the hepatocytes and exert systemic influence. However, liver may differ in its generative capacity and responsiveness for TMAO, particularly across disease state, and future studies should take this into account.
Extracellular vesicles
Extracellular vesicles (EVs) are membrane-bound vesicles secreted by almost all types of cells and include microparticles/microvesicles, exosomes, and apoptotic bodies depending on size, biogenesis, and cargo. EVs have been shown to have a key role in mediating intercellular communication by carrying a variety of bioactive molecules, surface receptors, and genetic information.27 Evidence of the cardiovascular effects of liver-secreted EVs is growing. A study reported that circulating hepatocyte-derived microparticles were found in patients with cirrhosis but not in healthy controls, and that they contributed to impairment of vasoconstrictor responses and reduction of blood pressure (BP).28 Hepatic EVs derived from mice with NAFLD were shown to augment coronary microvascular permeability by transferring novel-miR-7 and regulating the LAMP1/cathepsin B/NLRP3 inflammasome pathway.29 Jiang and colleagues30 found that EVs isolated from palmitic acid-treated hepatocytes can promote inflammation and atherogenesis by delivering miR-1 to VECs, thereby inhibiting KLF4 expression and activating NF-κB signaling. In addition, increased arginase activity was detected in the EVs produced by hepatocytes challenged with hepatotoxicant, and was responsible for EVs-induced impairment of endothelium-dependent relaxation.31 Also, among patients with low-coronary flow reserve, miR-224-5p levels were found to be remarkably increased in the plasma EVs that supposed to be released by the liver and negatively correlated with coronary flow reserve. EVs isolated from a liver cell line stimulated with TNF-α enhanced ICAM-1 expression in VECs.32 Additionally, liver-secreted exosomal miR-122 was found to contribute to the development of metabolic cardiomyopathy by inhibiting Arl-2 and affecting cardiac mitochondrial function.33 Hepatocyte-derived exosomal miR-194 was reported to be involved in hepatopulmonary syndrome by targeting pulmonary microvascular endothelial cells and promoting cell proliferation, migration, and tube formation as well as in vivo angiogenesis.34 Similarly, exosomes produced by TMAO-activated hepatocytes promoted the expression of inflammatory markers, impaired endothelial function and inhibited angiogenesis, which may have been related to the enriched miRNAs in the exosomes, such as miR-302d-3p, miR-302b-3p, miR-302a-3p, and miR-103-3p.25,26 Not only that, exosome-based therapy may be a promising approach for CVDs treatment in clinical practice. It was demonstrated that overexpression of Ldlr mRNA in the donor AML12 cells contributed to secretion of exosomes carried Ldlr mRNA, which increased the production of LDLR protein in the liver and reduced the number and size of atherosclerotic plaques in Ldlr−/− mice.35 The above studies provide evidence that EVs may not only be biomarkers of liver damage, but also effectors of the cardiovascular system. Knowledge of the cargo transported by the EVs appears to be decisive for understanding their biological function and molecular mechanism. Based on that, EVs-modifying therapies are emerging as potential treatments for CVDs.
Hepatokines
Hepatokines are a diverse family of cytokines secreted by the liver, and have been shown to exert autocrine, paracrine, and endocrine functions in metabolic disorders.36 Accumulating evidence shows that many of them are important to CVDs.
Adropin
Adropin is a peptide hormone secreted primarily by the liver and has been shown to have a significant role in regulating glucose and lipid homeostasis.37 Notably, the impact of adropin on cardiovascular physiology and disease has recently gained increasing attention. Adropin levels were found to be significantly lower in obese adolescents and adults and markedly increased following aerobic exercise, and its concentration had negative correlation with arterial stiffness and abdominal visceral fat and positive correlation with plasma nitrite/nitrate content, cardiorespiratory fitness as well as vascular reactive hyperemia indexes.38,39 Among individuals with type 2 diabetes mellitus (DM) and metabolic syndrome, the blood concentration of adropin was significantly declined and inversely associated with the coronary angiographic severity,40 and even lower values were found in the endothelial dysfunction group and had a positive correlation with the flow-mediated dilatation values.41,42 Additionally, serum adropin was reduced in patients with coronary artery disease (CHD),43 and lower levels were associated with hyperhomocysteinemia and more severe coronary disease44 and poor coronary collateral circulation.45 Also, in patients underwent drug-eluting stent implantation, serum adropin concentrations were significantly decreased among the in-stent restenosis (ISR) group, and its levels were inversely correlated with the neointimal volume in both groups.46 Moreover, plasma adropin concentrations were significantly decreased in hypertensive patients compared with normotensive subjects,47,48 and showed a negative correlation with endothelin-1, an indicator for endothelial dysfunction.48 However, another study reported opposite results regarding the association between adropin and hypertension.49 And in obese children, no correlation was observed between serum adropin levels and BP variables.50
Mechanically, adropin has been confirmed to regulate mitochondrial energy metabolism through GPR19-p44/42-PDK4 pathway,51 activate cardiac insulin signaling and improve cardiac efficiency,52 enhance cardiac glucose oxidation under high fat diet conditions53 and improve diastolic function by alleviating myocardial fibrosis in diabetic cardiomyopathy rats.54 Although short-term administration of adropin may fail to exert a protective effect on cardiac function in obese animals.55 Besides, adropin could promote eNOS activation and perfusion recovery after hindlimb ischemia by upregulating VEGFR2,56 suppress proliferation and phenotypic modulation of VSMCs induced by angiotensin II via AMPK/ACC signaling,46 and attenuate vascular calcification by repressing VSMCs osteogenic differentiation through JAK2/STAT3 signaling,57 as well as inhibit TNF-α-induced THP1 monocyte adhesion to VECs, prevent macrophages from polarizing into a pro-inflammatory phenotype and reduce the formation of atherosclerotic lesions in apoE−/− mice.58 Additionally, exposure to cell-free hemoglobin resulted in decreased expressions of adropin and increased paracellular permeability of VECs, and treatment with adropin was able to protect against the hyperpermeability and suppress macrophage trans-endothelial migration.59
In contrast, another study demonstrated that adropin levels were increased in serum from patients with Kawasaki disease and even higher in those with coronary artery lesions, and showed positive correlation with inflammatory markers and D-dimer.60 And among patients with HF, circulating adropin levels were significantly increased with the progressive deterioration in cardiac function,61 which could be effectively decreased by HF treatment.62
Fibroblast growth factor 21
Fibroblast growth factor 21 (FGF21), a peptide hormone synthesized primarily in the liver, adipose tissue, pancreas, and heart, has been found to exert pleiotropic functions, depending upon which organ is implicated.63 Liver was believed to be the major endocrine source of plasma FGF21 during bacterial inflammation, and elevated FGF21 was required for survival by contributing to the maintenance of thermogenesis and cardiac function.64 And the releases of FGF21 from hepatic cells and adipocytes were showed to be increased in mice after myocardial ischemia/reperfusion (I/R) injury, thus reducing cell death and MI as well as improving myocardial function through FGFR1/β-Klotho-PI3K-Akt1-BAD signaling.65 Also, Pan et al.66 demonstrated that liver may be the primary site for the production of circulating FGF21 in angiotensin II-induced hypertension, and increased expression of FGF21 could counteract angiotensin II-induced hypertension and vascular dysfunction by enhancing the generation of angiotensin-converting enzyme 2. FGF21 deficiency resulted in aggravation of atherosclerosis and premature death in apoE−/− mice, and FGF21 supplement could attenuate vascular inflammation and atherosclerotic plaque formation.67 Similar results were found in a study of atherosclerotic rats, in which FGF21 was able to alleviate inflammation and oxidative stress by activating the Nrf1-ARE pathway.68 FGF21 has also been reported to protect against diabetic cardiomyopathy in part through the activation of the AMPK-PON1 signaling69 and cardiac hypertrophy and fibrosis during hypertension,70 as well as suppress lipid- or diabetes-stimulated cardiac apoptosis via ERK1/2-p38 MAPK-AMPK pathway.71 Additionally, FGF21 could alleviate doxorubicin-induced cardiac insults by inhibiting oxidative stress, inflammation, and apoptosis via SIRT1/LKB1/AMPK pathway.72 However, it should be noted that, in the mouse model of myocardial hypertrophy, FGF21 levels were increased in cardiac tissue but remained unchanged in the circulation, suggesting that FGF21 may inhibit cardiac hypertrophy predominantly through its autocrine effects.73 Indeed, there is evidence suggesting that FGF21 can be expressed and secreted by the heart following cardiac damages such as cardiac hypertrophy, oxidative stress and MI, and exerts its diverse cardioprotective functions in an autocrine manner.74 Therefore, tissue-specific knock-out of FGF21 is necessary to elucidate its autocrine, paracrine, and endocrine effects, which may vary depending on the context.
Selenoprotein P
Selenoprotein P is a transport protein that is mainly synthesized and released by the liver, and plays an essential role in delivering selenium from the liver to other tissues.75 The population with lowest plasma concentrations of selenoprotein P have a higher risk of cardiovascular morbidity and mortality,76 and among patients with CVDs, circulating selenoprotein P levels were significantly lower in individuals with metabolic syndrome.77 And another study has suggested that plasma selenoprotein P can bind to proteoglycans on the vascular endothelium and form a protective layer against oxidants.78 Further, selenoprotein P was showed to protect low density lipids (LDLs) from oxidation79 and prevent tert-butylhydroperoxide-induced oxidative injury and loss of cellular membrane integrity by restoring the enzymatic activity of glutathione peroxidase in human endothelial cells.80 In addition, selenoprotein P was reported to exert a protective effect in cardiac fibrosis.81 However, the influence of selenoprotein P on the cardiovascular system reminds controversial, with opposed results in different studies. Elevation of selenoprotein P was observed in patients with HF, and its levels were associated with adverse cardiac outcomes.82 And inhibition of selenoprotein P protected the heart from I/R injury by activating RISK pathway.83 It was also found that the serum concentrations of selenoprotein P were significantly increased in patients with pulmonary hypertension and its levels were able to predict all-cause death and lung transplantation. Furthermore, the absolute changes in selenoprotein P after initial therapy were correlated with the hemodynamic changes and prognosis.84 Of note, however, it was demonstrated that selenoprotein P produced by pulmonary artery smooth muscle cells, but not by the liver, promoted the development of pulmonary arterial hypertension.85 Thus, further research is needed to clarify the sources of selenoprotein P and its roles in the pathogenesis of CVDs.
Fetuin-A
Fetuin-A is a multifunctional glycoprotein secreted by the liver, and has been shown to be an important inhibitor of mitral annular calcification in persons with CHD and without severe kidney disease86 and valvular calcification in patients with end-stage renal disease.87 In patients on dialysis, low serum fetuin-A was reported to increase the risk of all-cause and cardiovascular mortality.88 Data from patients with type 2 DM and without renal dysfunction further suggested that fetuin-A may suppresses the calcification of atherosclerotic plaques independently of the dialysis conditions.89 Moreover, fetuin-A-deficient mice spontaneously developed significant myocardial calcification, characterized by myocardial stiffness, cardiac remodeling and fibrosis, and diastolic dysfunction.90 In addition to acting as a calcification inhibitor, however, it may also act as an atherogenic factor. In a case-cohort study, significantly increased risks of MI and ischemic stroke were found in subjects with higher plasma fetuin-A levels.91 Fetuin-A also influenced the expression of proinflammatory and angiogenic proteins associated with atherosclerosis.92 These inconsistent behaviors raise the important questions about the potential protective or exacerbating role of fetuin-A in CVDs, which may be complicated by its multiple functionalities, and more research are therefore definitely needed to elucidate these aspects.
Fetuin-B
Fetuin-B, a liver-derived secretory protein, has been reported to have an adverse effect on the cardiovascular system. It was found that serum fetuin-B concentrations were independently associated with the presence of CHD and acute coronary syndromes,93 and urinary fetuin-B levels were higher in individuals with cardiovascular risk factors than healthy subjects.94 A recent study showed that plasma fetuin-B levels were significantly elevated in patients with ISR compared with non-ISR patients and healthy controls.95 Also, circulating fetuin-B levels were increased in patients with acute MI, and also that fetuin-B was regulate the migration of monocytes and macrophages, levels of vascular plaque-stabilizing factors, and increased atherosclerotic plaque rupture in mice.96 A subsequent study revealed that fetuin-B contributed to plaque rupture by inducing the expression of PAI-1 and MMP-2 in VSMCs through TGF-βR/Smad signaling.97 Increased levels of fetuin-B also led to the inhibition of cardiac insulin-induced signaling and thus exacerbating myocardial I/R injury.98
α1-microglobulin
α1-microglobulin is a glycoprotein synthesized and secreted mainly by the liver and serves as an indicator of renal tubular dysfunction.99 There is accumulating evidence linking α1-microglobulin to CVDs. In a retrospective analysis of patients with ST-elevation MI, urinary α1-microglobulin at admission was showed to be an independent predictor of in-hospital mortality.100 In patients with acute HF, urinary α1-microglobulin concentrations at admission were associated with all-cause mortality independent of glomerular function and provided additional prognostic value.101 In nondiabetic patients with chronic kidney disease, urinary α1-microglobulin levels were also found to be associated with CVDs events and mortality.102 In addition, despite increased urinary excretion of α1-microglobulin has been reported in patients undergoing myocardial revascularization surgery with cardiopulmonary bypass,103 and the increases were greater with longer duration of cardiopulmonary bypass,104 it was shown that preoperative but not postoperative urinary α1-microglobulin levels were positively associated with acute kidney injury, progressive chronic kidney disease, and all-cause mortality after cardiac surgery.105 However, the role of α1-microglobulin on CVDs is still controversial. Hakuno et al.106 demonstrated that α1-microglobulin can promote macrophage infiltration and inflammation and impair fibrotic repair after MI in mice. Nevertheless, it was shown to suppress oxidation of LDL, hemoglobin and lipids isolated from atherosclerotic plaques, and protect the endothelial cells from oxidative damage.107,108 More studies are needed to confirm the causal relationships between α1-microglobulin and CVDs.
MicroRNA-122
MicroRNAs (miRNAs) are a class of endogenous small noncoding RNA molecules that are evolutionarily conserved and have a critical role in regulating gene expression at the posttranscriptional level.109 It was found that plasma miRNAs are not only diagnostic biomarkers but also potential therapeutic targets for CVDs.110 MiR-122 is predominantly generated in the liver and constantly released into the circulation111 and has been shown to be significantly elevated in patients with acute HF.112 In a cohort study of population who experienced sudden cardiac arrest due to ventricular fibrillation, miR-122 levels were found to be higher in participants who died in hospital or survived to discharge compared with those who died in the field.113 However, the plasma levels of miR-122-5p at admission did not correlate to shock at admission or all-cause mortality among patients admitted due to out-of-hospital cardiac arrest.114 By contrast, circulating miR-122 was found to predict all-cause and cardiovascular mortality in patients with chronic systolic HF, and also improve current risk stratification.115 However, the roles and underlying mechanisms of circulating miR-122 in these pathophysiological processes remain to be elucidated. Because a significant increase in plasma miR-122 has been observed after liver injury,116 it is unclear whether the elevated miR-122 in the circulation have regulatory roles in the pathogenesis of CVDs or are simply biomarkers of hepatic damages. And the findings presented by Wang et al. might shed some light on this.33 In addition, based on its high content in the liver, miR-122 has been used to increase the specificity of adeno-associated virus-mediated cardiac gene transfer, while minimizing liver exposure to the vectors.117
Others
Very recently, coagulation factor XI was found to be capable of protecting against cardiac diastolic dysfunction by suppressing inflammation and fibrosis through cleaving BMP7 precursor and activating the BMP7-SMAD1/5 pathway.118 The effects of plasma protein factor XII (FXII) on vascular function have previously been thoroughly reviewed by Mailer and colleagues.119 FXII is synthesized by the liver and released as an inactive zymogen into the circulation, and plays an important role in promoting endothelial dysfunction, vascular inflammation, and atherosclerosis. By using Drosophila oenocytes as a hepatocyte model, Huang et al. found that peroxisomal import was impaired in aged oenocytes, thus promoting the release of upd3, an IL-6-like proinflammatory cytokine, from oenocytes and inducing cardiac arrhythmia.120
Conclusion
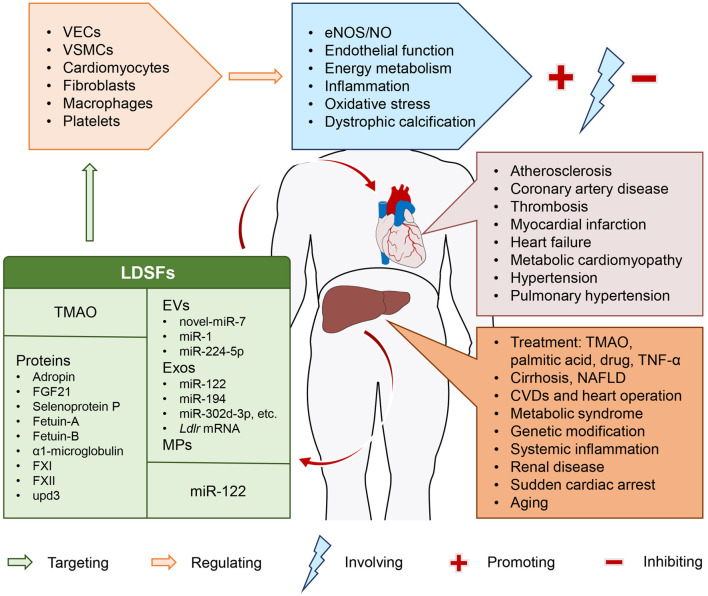
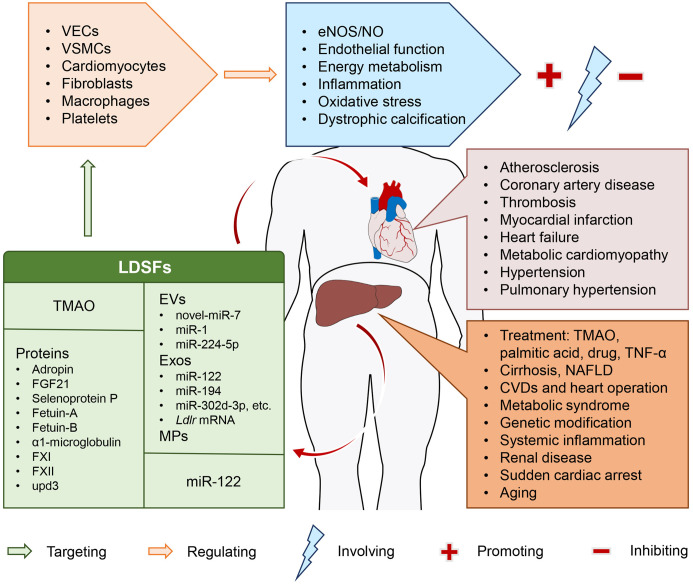
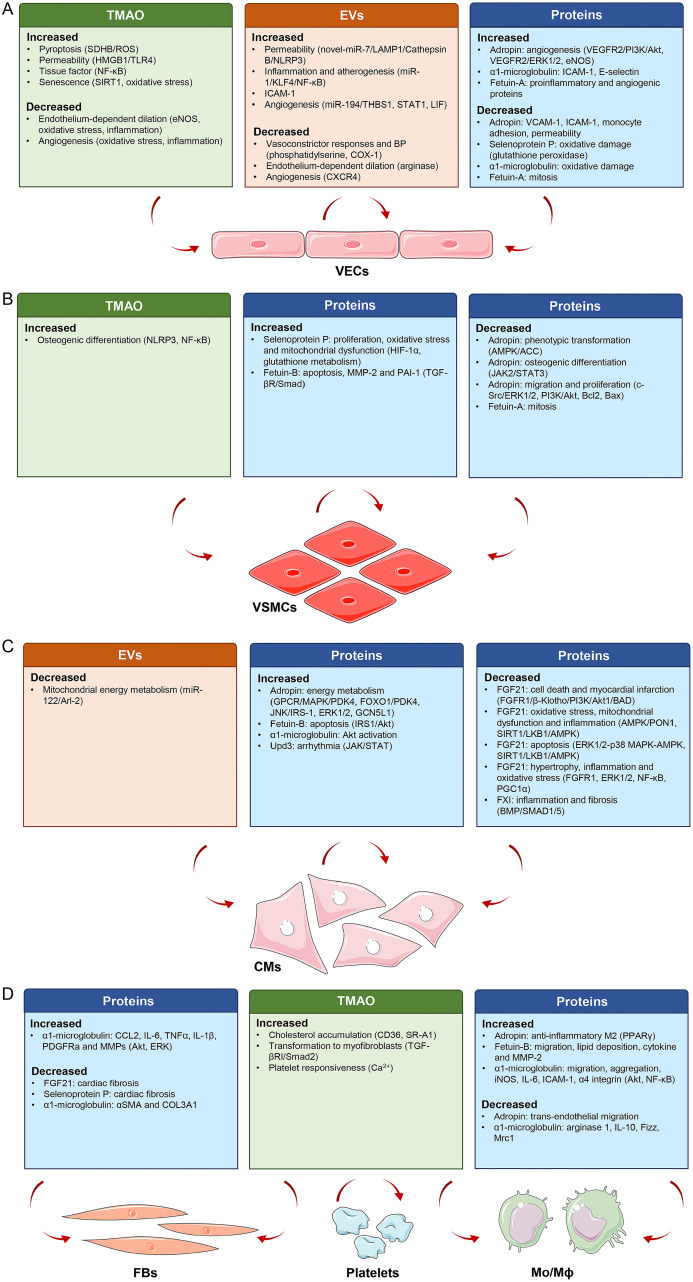
LDSFs presented here are a group of hepatic mediators that exclusively or mainly produced and released by the liver, including TMAO, EVs, proteins and miR-122, and are thought to exert their pleiotropic actions on cardiovascular system through an endocrine manner (Fig. 1). Accumulating evidence highlights the importance of these LDSFs in CVDs, such as atherosclerosis, CHD, thrombosis, MI, HF, metabolic cardiomyopathy, arterial hypertension, and pulmonary hypertension. These LDSFs primarily act on VECs, VSMCs, cardiomyocytes, fibroblasts, macrophages and platelets, and the predominant underlying mechanisms involve the regulation of eNOS/NO, endothelial function, energy metabolism, inflammation, oxidative stress, and dystrophic calcification (Fig. 2). Some LDSFs, including TMAO, EVs, fetuin-B, FXII and upd3 have been proven to be detrimental, and some, including adropin, FGF21 and factor XI, are protective. The activity of others, including selenoprotein P, fetuin-A, α1-microglobulin and miR-122, is not clear. A variety of factors may be responsible. First, the composition of the investigated populations was not the same across studies. Second, source of these LDSFs was not restricted to the liver, they can have their origins from other organs/tissues. The situation is complicated under pathophysiological conditions. Third, each LDSF may influence multiple targets within the cardiovascular system and show pleiotropic effects through different molecular mechanisms. Therefore, more studies are required to further identify the causal relationships between LDSFs and CVDs and elucidate the exact mechanisms, which may reveal novel molecular targets for the prevention and treatment of CVDs. Furthermore, in the case of patients with liver disease, awareness should be given to the implications of these abnormalities in the cardiovascular system. These studies also underline the importance of early recognition and intervention of liver abnormalities in the practice of cardiovascular care, and a multidisciplinary approach combining hepatologists and cardiologists would be more preferable for such patients. There are, however, some limitations of this review. First, the liver and the heart can crosstalk and affect each other to contribute to various diseases, and the underlying pathways are diverse. Here, we just addressed the unidirectional impact of liver on heart with a focus on LDSFs. Secondly, the definition of LDSFs might have been too broad, and there is a possibility that some important factors or literature may be missed. Thirdly, many of the studies included were observational, and only provided evidence of association, not cause. Further investigations are required to determine the roles and precise mechanisms of some LDSFs such as α1-microglobulin and miR-122. Finally, the functional states of the liver may determine the activities of LDSFs, but its histopathological and biochemical alterations were not clearly reported in many studies.
Fig. 1. Representative scheme of the release of liver-derived secretory factors in different physiological and pathophysiological states and their roles in cardiovascular disease.
Parts of the figure were obtained from Servier Medical Art (https://smart.servier.com/). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License. CVDs, cardiovascular diseases; EVs, extracellular vesicles; Exos, exosomes; MPs, microparticles; NAFLD, nonalcoholic fatty liver disease; TMAO, trimethylamine N-oxide; VECs, vascular endothelial cells; VSMCs, vascular smooth muscle cells.
Fig. 2. Effects of liver-derived secretory factors on targeted cells and the underlying mechanisms.
These factors mainly act on VECs, VSMCs, CMs, FBs, Mo/Mϕ and platelets, and the effects and mechanisms presented in the studies are summarized. Parts of the figure were obtained from Servier Medical Art (https://smart.servier.com/). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License. CMs, cardiomyocytes; EVs, extracellular vesicles; FBs, fibroblasts; Mo/Mϕ, monocytes/macrophages; TMAO, trimethylamine N-oxide; VECs, vascular endothelial cells; VSMCs, vascular smooth muscle cells.
Abbreviations
- ACC
acetyl-CoA carboxylase
- Akt
protein kinase B
- AMI
acute myocardial infarction
- AMPK
AMP-activated protein kinase
- apoE−/−
Apolipoprotein E knockout
- ARE
antioxidant response elements
- Arl-2
ADP-ribosylation factor-like 2
- BAD
BCL2 associated agonist of cell death
- BMP7
bone morphogenetic protein 7
- BP
blood pressure
- CHD
coronary artery disease
- CVDs
cardiovascular diseases
- DM
diabetes mellitus
- eNOS
endothelial nitric oxide synthase
- ERK1/2
extracellular signal-regulated kinase 1/2
- EVs
extracellular vesicles
- FGF21
fibroblast growth factor 21
- FGFR1
fibroblast growth factor receptor 1
- FXII
factor XII
- upd3
unpaired 3
- GPR19
G protein-coupled receptor 19
- HF
heart failure
- HMGB1
high mobility group box 1
- ICAM-1
intercellular adhesion molecule 1
- I/R
ischemia/reperfusion
- ISR
in-stent restenosis
- JAK2
Janus kinase 2
- KLF4
KLF transcription factor 4
- LAMP1
lysosomal associated membrane protein 1
- LDL
low-density lipoprotein
- Ldlr
low-density lipoprotein receptor
- Ldlr−/−
low-density lipoprotein receptor knockout
- LDSFs
liver-derived secretory factors
- LKB1
liver kinase B1
- MAPK
mitogen-activated protein kinases
- MI
myocardial infarction
- MMP-2
matrix metallopeptidase 2
- NAFLD
non-alcoholic fatty liver disease
- NF-κB
nuclear factor-kappaB
- NLRP3
NLR family pyrin domain containing 3
- Nrf2
transcription factor NF-E2-related 2
- PAI-1
plasminogen activator inhibitor-1
- PDK4
pyruvate dehydrogenase kinase 4
- PERK
protein kinase R-like endoplasmic reticulum kinase
- PI3K
phosphoinositide 3-kinase
- RISK
reperfusion injury salvage kinase
- PON1
paraoxonase 1
- ROS
reactive oxygen species
- SDHB
succinate dehydrogenase complex subunit B
- SIRT1
sirtuin 1
- SMAD2
SMAD family member 2
- STAT3
signal transducer and activator of transcription 3
- TGF-βRI
transforming growth factor-beta receptor type 1
- TMAO
trimethylamine N-oxide
- TNF-α
tumor necrosis factor-alpha
- VECs
vascular endothelial cells
- VEGFR2
vascular endothelial growth factor receptor-2
- VSMCs
vascular smooth muscle cells
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JS, Ward WO, Knapp G, Ren H, Vallanat B, Abbott B, et al. Transcriptional ontogeny of the developing liver. BMC Genomics. 2012;13:33. doi: 10.1186/1471-2164-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Haan W, Dheedene W, Apelt K, Décombas-Deschamps S, Vinckier S, Verhulst S, et al. Endothelial Zeb2 preserves the hepatic angioarchitecture and protects against liver fibrosis. Cardiovasc Res. 2022;118(5):1262–1275. doi: 10.1093/cvr/cvab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji Y, Yin Y, Sun L, Zhang W. The Molecular and Mechanistic Insights Based on Gut-Liver Axis: Nutritional Target for Non-Alcoholic Fatty Liver Disease (NAFLD) Improvement. Int J Mol Sci. 2020;21(9):3066. doi: 10.3390/ijms21093066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seo JA, Kang MC, Yang WM, Hwang WM, Kim SS, Hong SH, et al. Apolipoprotein J is a hepatokine regulating muscle glucose metabolism and insulin sensitivity. Nat Commun. 2020;11(1):2024. doi: 10.1038/s41467-020-15963-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Møller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J. 2013;34(36):2804–2811. doi: 10.1093/eurheartj/eht246. [DOI] [PubMed] [Google Scholar]
- 7.Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–1705. doi: 10.1136/gutjnl-2020-320622. [DOI] [PubMed] [Google Scholar]
- 8.Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol. 2018;16(3):171–181. doi: 10.1038/nrmicro.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Räber L, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38(11):814–824. doi: 10.1093/eurheartj/ehw582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64(18):1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu P, Chen J, Chen J, Tao J, Wu S, Xu G, et al. Trimethylamine N-oxide promotes apoE(-/-) mice atherosclerosis by inducing vascular endothelial cell pyroptosis via the SDHB/ROS pathway. J Cell Physiol. 2020;235(10):6582–6591. doi: 10.1002/jcp.29518. [DOI] [PubMed] [Google Scholar]
- 16.Singh GB, Zhang Y, Boini KM, Koka S. High Mobility Group Box 1 Mediates TMAO-Induced Endothelial Dysfunction. Int J Mol Sci. 2019;20(14):3570. doi: 10.3390/ijms20143570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Li Y, Yang P, Liu X, Lu L, Chen Y, et al. Trimethylamine-N-Oxide Promotes Vascular Calcification Through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-κB (Nuclear Factor κB) Signals. Arterioscler Thromb Vasc Biol. 2020;40(3):751–765. doi: 10.1161/ATVBAHA.119.313414. [DOI] [PubMed] [Google Scholar]
- 18.Yang W, Zhang S, Zhu J, Jiang H, Jia D, Ou T, et al. Gut microbe-derived metabolite trimethylamine N-oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J Mol Cell Cardiol. 2019;134:119–130. doi: 10.1016/j.yjmcc.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Cheng X, Qiu X, Liu Y, Yuan C, Yang X. Trimethylamine N-oxide promotes tissue factor expression and activity in vascular endothelial cells: A new link between trimethylamine N-oxide and atherosclerotic thrombosis. Thromb Res. 2019;177:110–116. doi: 10.1016/j.thromres.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 20.Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, et al. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension. 2020;76(1):101–112. doi: 10.1161/HYPERTENSIONAHA.120.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ke Y, Li D, Zhao M, Liu C, Liu J, Zeng A, et al. Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic Biol Med. 2018;116:88–100. doi: 10.1016/j.freeradbiomed.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Li T, Chen Y, Gua C, Li X. Elevated Circulating Trimethylamine N-Oxide Levels Contribute to Endothelial Dysfunction in Aged Rats through Vascular Inflammation and Oxidative Stress. Front Physiol. 2017;8:350. doi: 10.3389/fphys.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Jin Y, Wang N, Yuan M, Lin T, Lu W, et al. Trimethylamine N-oxide impairs perfusion recovery after hindlimb ischemia. Biochem Biophys Res Commun. 2020;530(1):95–99. doi: 10.1016/j.bbrc.2020.06.093. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Henderson A, Petriello MC, Romano KA, Gearing M, Miao J, et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019;30(6):1141–1151.e5. doi: 10.1016/j.cmet.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Shao Y, Tu J, Sun J, Dong B, Wang Z, et al. TMAO-Activated Hepatocyte-Derived Exosomes Impair Angiogenesis via Repressing CXCR4. Front Cell Dev Biol. 2021;9:804049. doi: 10.3389/fcell.2021.804049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Shao Y, Tu J, Sun J, Li L, Tao J, et al. Trimethylamine-N-oxide-stimulated hepatocyte-derived exosomes promote inflammation and endothelial dysfunction through nuclear factor-kappa B signaling. Ann Transl Med. 2021;9(22):1670. doi: 10.21037/atm-21-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126(4):1139–1143. doi: 10.1172/JCI87316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rautou PE, Bresson J, Sainte-Marie Y, Vion AC, Paradis V, Renard JM, et al. Abnormal plasma microparticles impair vasoconstrictor responses in patients with cirrhosis. Gastroenterology. 2012;143(1):166–76.e6. doi: 10.1053/j.gastro.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Zuo R, Ye LF, Huang Y, Song ZQ, Wang L, Zhi H, et al. Hepatic small extracellular vesicles promote microvascular endothelial hyperpermeability during NAFLD via novel-miRNA-7. J Nanobiotechnology. 2021;19(1):396. doi: 10.1186/s12951-021-01137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang F, Chen Q, Wang W, Ling Y, Yan Y, Xia P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J Hepatol. 2020;72(1):156–166. doi: 10.1016/j.jhep.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Royo F, Moreno L, Mleczko J, Palomo L, Gonzalez E, Cabrera D, et al. Hepatocyte-secreted extracellular vesicles modify blood metabolome and endothelial function by an arginase-dependent mechanism. Sci Rep. 2017;7:42798. doi: 10.1038/srep42798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James K, Bryl-Gorecka P, Olde B, Gidlof O, Torngren K, Erlinge D. Increased expression of miR-224-5p in circulating extracellular vesicles of patients with reduced coronary flow reserve. BMC Cardiovasc Disord. 2022;22(1):321. doi: 10.1186/s12872-022-02756-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Jin P, Liu J, Xie X. Exosomal microRNA-122 mediates obesity-related cardiomyopathy through suppressing mitochondrial ADP-ribosylation factor-like 2. Clin Sci (Lond) 2019;133(17):1871–1881. doi: 10.1042/CS20190558. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Han Y, Li Y, Chen B, Bai X, Belguise K, et al. Hepatocyte-derived exosomal MiR-194 activates PMVECs and promotes angiogenesis in hepatopulmonary syndrome. Cell Death Dis. 2019;10(11):853. doi: 10.1038/s41419-019-2087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Zhao P, Zhang Y, Wang J, Wang C, Liu Y, et al. Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model. Theranostics. 2021;11(6):2953–2965. doi: 10.7150/thno.49874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13(9):509–520. doi: 10.1038/nrendo.2017.56. [DOI] [PubMed] [Google Scholar]
- 37.Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8(6):468–481. doi: 10.1016/j.cmet.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Jiang L, Yang YJ, Ge RK, Zhou M, Hu H, et al. Aerobic exercise improves endothelial function and serum adropin levels in obese adolescents independent of body weight loss. Sci Rep. 2017;7(1):17717. doi: 10.1038/s41598-017-18086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujie S, Hasegawa N, Kurihara T, Sanada K, Hamaoka T, Iemitsu M. Association between aerobic exercise training effects of serum adropin level, arterial stiffness, and adiposity in obese elderly adults. Appl Physiol Nutr Metab. 2017;42(1):8–14. doi: 10.1139/apnm-2016-0310. [DOI] [PubMed] [Google Scholar]
- 40.Wu L, Fang J, Chen L, Zhao Z, Luo Y, Lin C, et al. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin Chem Lab Med. 2014;52(5):751–758. doi: 10.1515/cclm-2013-0844. [DOI] [PubMed] [Google Scholar]
- 41.Oruc CU, Akpinar YE, Dervisoglu E, Amikishiyev S, Salmaslıoglu A, Gurdol F, et al. Low concentrations of adropin are associated with endothelial dysfunction as assessed by flow-mediated dilatation in patients with metabolic syndrome. Clin Chem Lab Med. 2017;55(1):139–144. doi: 10.1515/cclm-2016-0329. [DOI] [PubMed] [Google Scholar]
- 42.Topuz M, Celik A, Aslantas T, Demir AK, Aydin S, Aydin S. Plasma adropin levels predict endothelial dysfunction like flow-mediated dilatation in patients with type 2 diabetes mellitus. J Investig Med. 2013;61(8):1161–1164. doi: 10.2310/JIM.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 43.Zhao LP, Xu WT, Wang L, You T, Chan SP, Zhao X, et al. Serum adropin level in patients with stable coronary artery disease. Heart Lung Circ. 2015;24(10):975–979. doi: 10.1016/j.hlc.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Zhao LP, You T, Chan SP, Chen JC, Xu WT. Adropin is associated with hyperhomocysteine and coronary atherosclerosis. Exp Ther Med. 2016;11(3):1065–1070. doi: 10.3892/etm.2015.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akkaya H, Güntürk EE, Akkaya F, Karabıyık U, Güntürk İ, Yılmaz S. Assessment of the Relationship Between the Adropin Levels and the Coronary Collateral Circulation in Patients wıth Chronic Coronary Syndrome. Arq Bras Cardiol. 2022;119(3):402–410. doi: 10.36660/abc.20210573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Zhao LP, Chen YQ, Chang XS, Xiong H, Zhang DM, et al. Adropin inhibits the phenotypic modulation and proliferation of vascular smooth muscle cells during neointimal hyperplasia by activating the AMPK/ACC signaling pathway. Exp Ther Med. 2021;21(6):560. doi: 10.3892/etm.2021.9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gulen B, Eken C, Kucukdagli OT, Serinken M, Kocyigit A, Kılıc E, et al. Adropin levels and target organ damage secondary to high blood pressure in the ED. Am J Emerg Med. 2016;34(11):2061–2064. doi: 10.1016/j.ajem.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Gu X, Li H, Zhu X, Gu H, Chen J, Wang L, et al. Inverse Correlation Between Plasma Adropin and ET-1 Levels in Essential Hypertension: A Cross-Sectional Study. Medicine (Baltimore) 2015;94(40):e1712. doi: 10.1097/MD.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Çelik HT, Akkaya N, Erdamar H, Gok S, Kazanci F, Demircelik B, et al. The Effects of Valsartan and Amlodipine on the Levels of Irisin, Adropin, and Perilipin. Clin Lab. 2015;61(12):1889–1895. doi: 10.7754/clin.lab.2015.150420. [DOI] [PubMed] [Google Scholar]
- 50.Altincik A, Sayin O. Evaluation of the relationship between serum adropin levels and blood pressure in obese children. J Pediatr Endocrinol Metab. 2015;28(9-10):1095–1100. doi: 10.1515/jpem-2015-0051. [DOI] [PubMed] [Google Scholar]
- 51.Thapa D, Stoner MW, Zhang M, Xie B, Manning JR, Guimaraes D, et al. Adropin regulates pyruvate dehydrogenase in cardiac cells via a novel GPCR-MAPK-PDK4 signaling pathway. Redox Biol. 2018;18:25–32. doi: 10.1016/j.redox.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Altamimi TR, Gao S, Karwi QG, Fukushima A, Rawat S, Wagg CS, et al. Adropin regulates cardiac energy metabolism and improves cardiac function and efficiency. Metabolism. 2019;98:37–48. doi: 10.1016/j.metabol.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Thapa D, Xie B, Zhang M, Stoner MW, Manning JR, Huckestein BR, et al. Adropin treatment restores cardiac glucose oxidation in pre-diabetic obese mice. J Mol Cell Cardiol. 2019;129:174–178. doi: 10.1016/j.yjmcc.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu M, Ai J, Shuai Z, Tang K, Li Z, Huang Y. Adropin Alleviates Myocardial Fibrosis in Diabetic Cardiomyopathy Rats: A Preliminary Study. Front Cardiovasc Med. 2021;8:688586. doi: 10.3389/fcvm.2021.688586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thapa D, Xie B, Mushala BAS, Zhang M, Manning JR, Bugga P, et al. Diet-induced obese mice are resistant to improvements in cardiac function resulting from short-term adropin treatment. Curr Res Physiol. 2022;5:55–62. doi: 10.1016/j.crphys.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta M, et al. Adropin is a novel regulator of endothelial function. Circulation. 2010;122(11 Suppl):S185–S192. doi: 10.1161/CIRCULATIONAHA.109.931782. [DOI] [PubMed] [Google Scholar]
- 57.Wang L, Jin F, Wang P, Hou S, Jin T, Chang X, et al. Adropin Inhibits Vascular Smooth Muscle Cell Osteogenic Differentiation to Alleviate Vascular Calcification via the JAK2/STAT3 Signaling Pathway. Biomed Res Int. 2022;2022:9122264. doi: 10.1155/2022/9122264. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Sato K, Yamashita T, Shirai R, Shibata K, Okano T, Yamaguchi M, et al. Adropin Contributes to Anti-Atherosclerosis by Suppressing Monocyte-Endothelial Cell Adhesion and Smooth Muscle Cell Proliferation. Int J Mol Sci. 2018;19(5):1293. doi: 10.3390/ijms19051293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dodd WS, Patel D, Lucke-Wold B, Hosaka K, Chalouhi N, Hoh BL. Adropin decreases endothelial monolayer permeability after cell-free hemoglobin exposure and reduces MCP-1-induced macrophage transmigration. Biochem Biophys Res Commun. 2021;582:105–110. doi: 10.1016/j.bbrc.2021.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang M, Pei Q, Zhang J, Weng H, Jing F, Yi Q. Association between adropin and coronary artery lesions in children with Kawasaki disease. Eur J Pediatr. 2021;180(7):2253–2259. doi: 10.1007/s00431-021-03977-5. [DOI] [PubMed] [Google Scholar]
- 61.Lian W, Gu X, Qin Y, Zheng X. Elevated plasma levels of adropin in heart failure patients. Intern Med. 2011;50(15):1523–1527. doi: 10.2169/internalmedicine.50.5163. [DOI] [PubMed] [Google Scholar]
- 62.Xu W, Qian L, Yuan X, Lu Y. Combined effects of hydralazine and nitrate on serum biochemistry and left ventricular remodeling in chronic heart failure patients. Pak J Pharm Sci. 2021;34(1 Special):381–386. doi: 10.36721/pjps.2021.34.1.Sp.381-386.1. [DOI] [PubMed] [Google Scholar]
- 63.Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 64.Huen SC, Wang A, Feola K, Desrouleaux R, Luan HH, Hogg R, et al. Hepatic FGF21 preserves thermoregulation and cardiovascular function during bacterial inflammation. J Exp Med. 2021;218(10):e20202151. doi: 10.1084/jem.20202151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC, et al. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci Rep. 2013;3:2767. doi: 10.1038/srep02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pan X, Shao Y, Wu F, Wang Y, Xiong R, Zheng J, et al. FGF21 Prevents Angiotensin II-Induced Hypertension and Vascular Dysfunction by Activation of ACE2/Angiotensin-(1-7) Axis in Mice. Cell Metab. 2018;27(6):1323–1337.e5. doi: 10.1016/j.cmet.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. 2015;131(21):1861–1871. doi: 10.1161/CIRCULATIONAHA.115.015308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jia H, Cheng J, Zhou Q, Peng J, Pan Y, Han H. Fibroblast growth factor 21 attenuates inflammation and oxidative stress in atherosclerotic rat via enhancing the Nrf1-ARE signaling pathway. Int J Clin Exp Pathol. 2018;11(3):1308–1317. [PMC free article] [PubMed] [Google Scholar]
- 69.Wu F, Wang B, Zhang S, Shi L, Wang Y, Xiong R, et al. FGF21 ameliorates diabetic cardiomyopathy by activating the AMPK-paraoxonase 1 signaling axis in mice. Clin Sci (Lond) 2017;131(15):1877–1893. doi: 10.1042/CS20170271. [DOI] [PubMed] [Google Scholar]
- 70.Ferrer-Curriu G, Redondo-Angulo I, Guitart-Mampel M, Ruperez C, Mas-Stachurska A, Sitges M, et al. Fibroblast growth factor-21 protects against fibrosis in hypertensive heart disease. J Pathol. 2019;248(1):30–40. doi: 10.1002/path.5226. [DOI] [PubMed] [Google Scholar]
- 71.Zhang C, Huang Z, Gu J, Yan X, Lu X, Zhou S, et al. Fibroblast growth factor 21 protects the heart from apoptosis in a diabetic mouse model via extracellular signal-regulated kinase 1/2-dependent signalling pathway. Diabetologia. 2015;58(8):1937–1948. doi: 10.1007/s00125-015-3630-8. [DOI] [PubMed] [Google Scholar]
- 72.Wang S, Wang Y, Zhang Z, Liu Q, Gu J. Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis. 2017;8(8):e3018. doi: 10.1038/cddis.2017.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013;4:2019. doi: 10.1038/ncomms3019. [DOI] [PubMed] [Google Scholar]
- 74.Planavila A, Redondo-Angulo I, Villarroya F. FGF21 and Cardiac Physiopathology. Front Endocrinol (Lausanne) 2015;6:133. doi: 10.3389/fendo.2015.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burk RF, Hill KE. Regulation of Selenium Metabolism and Transport. Annu Rev Nutr. 2015;35:109–134. doi: 10.1146/annurev-nutr-071714-034250. [DOI] [PubMed] [Google Scholar]
- 76.Schomburg L, Orho-Melander M, Struck J, Bergmann A, Melander O. Selenoprotein-P Deficiency Predicts Cardiovascular Disease and Death. Nutrients. 2019;11(8):1852. doi: 10.3390/nu11081852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gharipour M, Sadeghi M, Salehi M, Behmanesh M, Khosravi E, Dianatkhah M, et al. Association of expression of selenoprotein P in mRNA and protein levels with metabolic syndrome in subjects with cardiovascular disease: Results of the Selenegene study. J Gene Med. 2017;19(3):e2945. doi: 10.1002/jgm.2945. [DOI] [PubMed] [Google Scholar]
- 78.Arteel GE, Franken S, Kappler J, Sies H. Binding of selenoprotein P to heparin: characterization with surface plasmon resonance. Biol Chem. 2000;381(3):265–268. doi: 10.1515/BC.2000.034. [DOI] [PubMed] [Google Scholar]
- 79.Traulsen H, Steinbrenner H, Buchczyk DP, Klotz LO, Sies H. Selenoprotein P protects low-density lipoprotein against oxidation. Free Radic Res. 2004;38(2):123–128. doi: 10.1080/10715760320001634852. [DOI] [PubMed] [Google Scholar]
- 80.Steinbrenner H, Bilgic E, Alili L, Sies H, Brenneisen P. Selenoprotein P protects endothelial cells from oxidative damage by stimulation of glutathione peroxidase expression and activity. Free Radic Res. 2006;40(9):936–943. doi: 10.1080/10715760600806248. [DOI] [PubMed] [Google Scholar]
- 81.Schimmel K, Jung M, Foinquinos A, José GS, Beaumont J, Bock K, et al. Natural Compound Library Screening Identifies New Molecules for the Treatment of Cardiac Fibrosis and Diastolic Dysfunction. Circulation. 2020;141(9):751–767. doi: 10.1161/CIRCULATIONAHA.119.042559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takeishi R, Misaka T, Ichijo Y, Ishibashi S, Matsuda M, Yamadera Y, et al. Increases in Hepatokine Selenoprotein P Levels Are Associated With Hepatic Hypoperfusion and Predict Adverse Prognosis in Patients With Heart Failure. J Am Heart Assoc. 2022;11(11):e024901. doi: 10.1161/JAHA.121.024901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chadani H, Usui S, Inoue O, Kusayama T, Takashima SI, Kato T, et al. Endogenous Selenoprotein P, a Liver-Derived Secretory Protein, Mediates Myocardial Ischemia/Reperfusion Injury in Mice. Int J Mol Sci. 2018;19(3):878. doi: 10.3390/ijms19030878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kikuchi N, Satoh K, Satoh T, Yaoita N, Siddique MAH, Omura J, et al. Diagnostic and Prognostic Significance of Serum Levels of SeP (Selenoprotein P) in Patients With Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2019;39(12):2553–2562. doi: 10.1161/ATVBAHA.119.313267. [DOI] [PubMed] [Google Scholar]
- 85.Kikuchi N, Satoh K, Kurosawa R, Yaoita N, Elias-Al-Mamun M, Siddique MAH, et al. Selenoprotein P Promotes the Development of Pulmonary Arterial Hypertension: Possible Novel Therapeutic Target. Circulation. 2018;138(6):600–623. doi: 10.1161/CIRCULATIONAHA.117.033113. [DOI] [PubMed] [Google Scholar]
- 86.Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA. Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115(19):2533–2539. doi: 10.1161/CIRCULATIONAHA.106.682450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang AY, Woo J, Lam CW, Wang M, Chan IH, Gao P, et al. Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant. 2005;20(8):1676–1685. doi: 10.1093/ndt/gfh891. [DOI] [PubMed] [Google Scholar]
- 88.Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Böhm R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361(9360):827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 89.Emoto M, Mori K, Lee E, Kawano N, Yamazaki Y, Tsuchikura S, et al. Fetuin-A and atherosclerotic calcified plaque in patients with type 2 diabetes mellitus. Metabolism. 2010;59(6):873–878. doi: 10.1016/j.metabol.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Merx MW, Schäfer C, Westenfeld R, Brandenburg V, Hidajat S, Weber C, et al. Myocardial stiffness, cardiac remodeling, and diastolic dysfunction in calcification-prone fetuin-A-deficient mice. J Am Soc Nephrol. 2005;16(11):3357–3364. doi: 10.1681/ASN.2005040365. [DOI] [PubMed] [Google Scholar]
- 91.Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, et al. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118(24):2555–2562. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- 92.Siegel-Axel DI, Ullrich S, Stefan N, Rittig K, Gerst F, Klingler C, et al. Fetuin-A influences vascular cell growth and production of proinflammatory and angiogenic proteins by human perivascular fat cells. Diabetologia. 2014;57(5):1057–1066. doi: 10.1007/s00125-014-3177-0. [DOI] [PubMed] [Google Scholar]
- 93.Zhu K, Wang Y, Shu P, Zhou Q, Zhu J, Zhou W, et al. Increased serum levels of fetuin B in patients with coronary artery disease. Endocrine. 2017;58(1):97–105. doi: 10.1007/s12020-017-1387-1. [DOI] [PubMed] [Google Scholar]
- 94.Martínez PJ, Baldán-Martín M, López JA, Martín-Lorenzo M, Santiago-Hernández A, Agudiez M, et al. Identification of six cardiovascular risk biomarkers in the young population: A promising tool for early prevention. Atherosclerosis. 2019;282:67–74. doi: 10.1016/j.atherosclerosis.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 95.Hou J, Deng Q, Liu S, Qiu X, Deng X, Zhong W, et al. Plasma Proteome Profiling of Patients With In-stent Restenosis by Tandem Mass Tag-Based Quantitative Proteomics Approach. Front Cardiovasc Med. 2022;9:793405. doi: 10.3389/fcvm.2022.793405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jung SH, Won KJ, Lee KP, Kim HJ, Seo EH, Lee HM, et al. The serum protein fetuin-B is involved in the development of acute myocardial infarction. Clin Sci (Lond) 2015;129(1):27–38. doi: 10.1042/CS20140462. [DOI] [PubMed] [Google Scholar]
- 97.Jung SH, Lee D, Jin H, Lee HM, Ko HM, Lee KJ, et al. Fetuin-B regulates vascular plaque rupture via TGF-β receptor-mediated Smad pathway in vascular smooth muscle cells. Pflugers Arch. 2020;472(5):571–581. doi: 10.1007/s00424-020-02385-2. [DOI] [PubMed] [Google Scholar]
- 98.Xing W, Tan Y, Li K, Tian P, Tian F, Zhang H. Upregulated hepatokine fetuin B aggravates myocardial ischemia/reperfusion injury through inhibiting insulin signaling in diabetic mice. J Mol Cell Cardiol. 2021;151:163–172. doi: 10.1016/j.yjmcc.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 99.Yu H, Yanagisawa Y, Forbes MA, Cooper EH, Crockson RA, MacLennan IC. Alpha-1-microglobulin: an indicator protein for renal tubular function. J Clin Pathol. 1983;36(3):253–259. doi: 10.1136/jcp.36.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui H, Zhang X, Ding X, Zhou L, Liang S, Qiu H, et al. Urinary Alpha1-Microglobulin: A New Predictor for In-Hospital Mortality in Patients with ST-Segment Elevation Myocardial Infarction. Med Sci Monit. 2021;27:e927958. doi: 10.12659/MSM.927958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ishiwata S, Matsue Y, Nakamura Y, Dotare T, Sunayama T, Suda S, et al. Clinical and prognostic values of urinary alpha1-microglobulin as a tubular marker in acute heart failure. Int J Cardiol. 2021;338:115–120. doi: 10.1016/j.ijcard.2021.06.041. [DOI] [PubMed] [Google Scholar]
- 102.Garimella PS, Lee AK, Ambrosius WT, Bhatt U, Cheung AK, Chonchol M, et al. Markers of kidney tubule function and risk of cardiovascular disease events and mortality in the SPRINT trial. Eur Heart J. 2019;40(42):3486–3493. doi: 10.1093/eurheartj/ehz392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Faulí A, Gomar C, Campistol JM, Alvarez L, Manig AM, Matute P. Pattern of renal dysfunction associated with myocardial revascularization surgery and cardiopulmonary bypass. Eur J Anaesthesiol. 2003;20(6):443–450. doi: 10.1017/s0265021503000693. [DOI] [PubMed] [Google Scholar]
- 104.Boldt J, Brenner T, Lehmann A, Suttner SW, Kumle B, Isgro F. Is kidney function altered by the duration of cardiopulmonary bypass? Ann Thorac Surg. 2003;75(3):906–912. doi: 10.1016/s0003-4975(02)04559-9. [DOI] [PubMed] [Google Scholar]
- 105.Amatruda JG, Estrella MM, Garg AX, Thiessen-Philbrook H, McArthur E, Coca SG, et al. Urine Alpha-1-Microglobulin Levels and Acute Kidney Injury, Mortality, and Cardiovascular Events following Cardiac Surgery. Am J Nephrol. 2021;52(8):673–683. doi: 10.1159/000518240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hakuno D, Kimura M, Ito S, Satoh J, Nakashima Y, Horie T, et al. Hepatokine α1-Microglobulin Signaling Exacerbates Inflammation and Disturbs Fibrotic Repair in Mouse Myocardial Infarction. Sci Rep. 2018;8(1):16749. doi: 10.1038/s41598-018-35194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cederlund M, Deronic A, Pallon J, Sørensen OE, Åkerström B. A1M/α1-microglobulin is proteolytically activated by myeloperoxidase, binds its heme group and inhibits low density lipoprotein oxidation. Front Physiol. 2015;6:11. doi: 10.3389/fphys.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pethő D, Gáll T, Hendrik Z, Nagy A, Beke L, Gergely AP, et al. Ferryl Hemoglobin and Heme Induce A(1)-Microglobulin in Hemorrhaged Atherosclerotic Lesions with Inhibitory Function against Hemoglobin and Lipid Oxidation. Int J Mol Sci. 2021;22(13):6668. doi: 10.3390/ijms22136668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maegdefessel L. The emerging role of microRNAs in cardiovascular disease. J Intern Med. 2014;276(6):633–644. doi: 10.1111/joim.12298. [DOI] [PubMed] [Google Scholar]
- 110.Zhou SS, Jin JP, Wang JQ, Zhang ZG, Freedman JH, Zheng Y, et al. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018;39(7):1073–1084. doi: 10.1038/aps.2018.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Willeit P, Skroblin P, Kiechl S, Fernández-Hernando C, Mayr M. Liver microRNAs: potential mediators and biomarkers for metabolic and cardiovascular disease? Eur Heart J. 2016;37(43):3260–3266. doi: 10.1093/eurheartj/ehw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet. 2010;3(6):499–506. doi: 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed] [Google Scholar]
- 113.Wander PL, Enquobahrie DA, Pritchard CC, McKnight B, Rice K, Christiansen M, et al. Circulating microRNAs and sudden cardiac arrest outcomes. Resuscitation. 2016;106:96–101. doi: 10.1016/j.resuscitation.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gilje P, Frydland M, Bro-Jeppesen J, Dankiewicz J, Friberg H, Rundgren M, et al. The association between plasma miR-122-5p release pattern at admission and all-cause mortality or shock after out-of-hospital cardiac arrest. Biomarkers. 2019;24(1):29–35. doi: 10.1080/1354750X.2018.1499804. [DOI] [PubMed] [Google Scholar]
- 115.Stojkovic S, Koller L, Sulzgruber P, Hülsmann M, Huber K, Mayr M, et al. Liver-specific microRNA-122 as prognostic biomarker in patients with chronic systolic heart failure. Int J Cardiol. 2020;303:80–85. doi: 10.1016/j.ijcard.2019.11.090. [DOI] [PubMed] [Google Scholar]
- 116.Vliegenthart AD, Shaffer JM, Clarke JI, Peeters LE, Caporali A, Bateman DN, et al. Comprehensive microRNA profiling in acetaminophen toxicity identifies novel circulating biomarkers for human liver and kidney injury. Sci Rep. 2015;5:15501. doi: 10.1038/srep15501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Geisler A, Jungmann A, Kurreck J, Poller W, Katus HA, Vetter R, et al. microRNA122-regulated transgene expression increases specificity of cardiac gene transfer upon intravenous delivery of AAV9 vectors. Gene Ther. 2011;18(2):199–209. doi: 10.1038/gt.2010.141. [DOI] [PubMed] [Google Scholar]
- 118.Cao Y, Wang Y, Zhou Z, Pan C, Jiang L, Zhou Z, et al. Liver-heart cross-talk mediated by coagulation factor XI protects against heart failure. Science. 2022;377(6613):1399–1406. doi: 10.1126/science.abn0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mailer RK, Rangaswamy C, Konrath S, Emsley J, Renné T. An update on factor XII-driven vascular inflammation. Biochim Biophys Acta Mol Cell Res. 2022;1869(1):119166. doi: 10.1016/j.bbamcr.2021.119166. [DOI] [PubMed] [Google Scholar]
- 120.Huang K, Miao T, Chang K, Kim J, Kang P, Jiang Q, et al. Impaired peroxisomal import in Drosophila oenocytes causes cardiac dysfunction by inducing upd3 as a peroxikine. Nat Commun. 2020;11(1):2943. doi: 10.1038/s41467-020-16781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]