Abstract
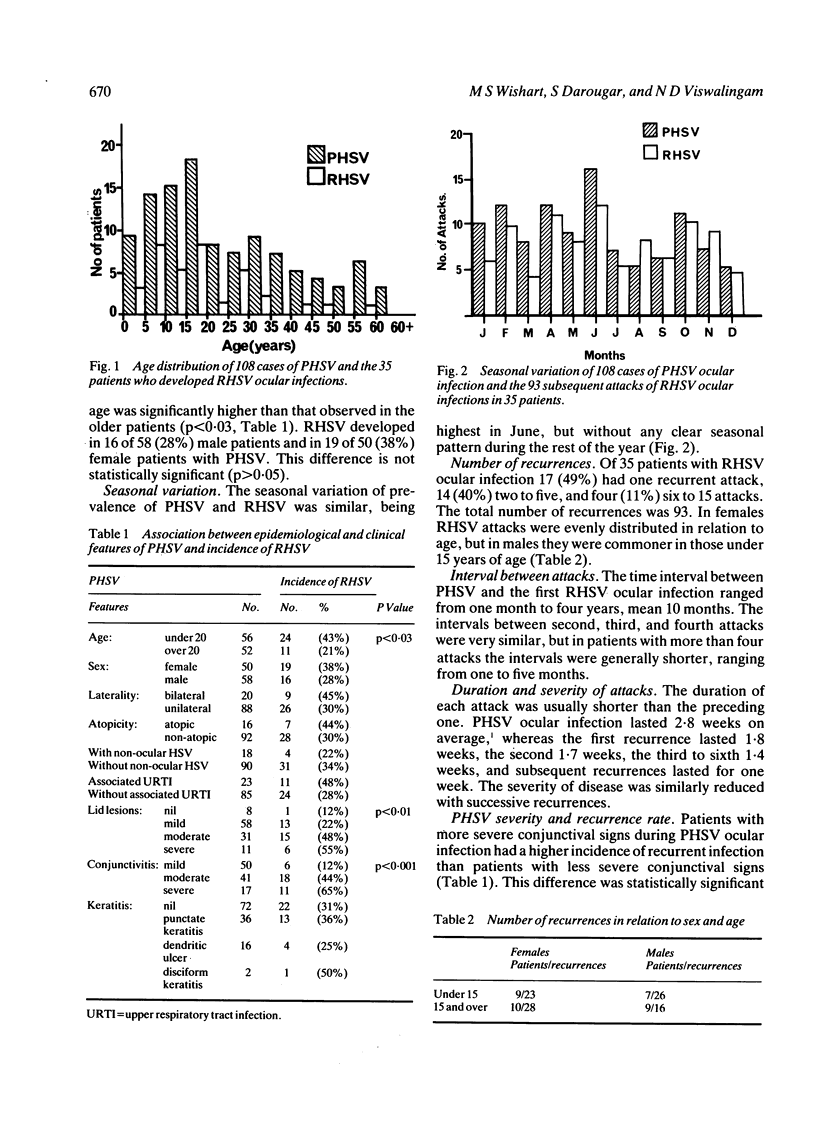
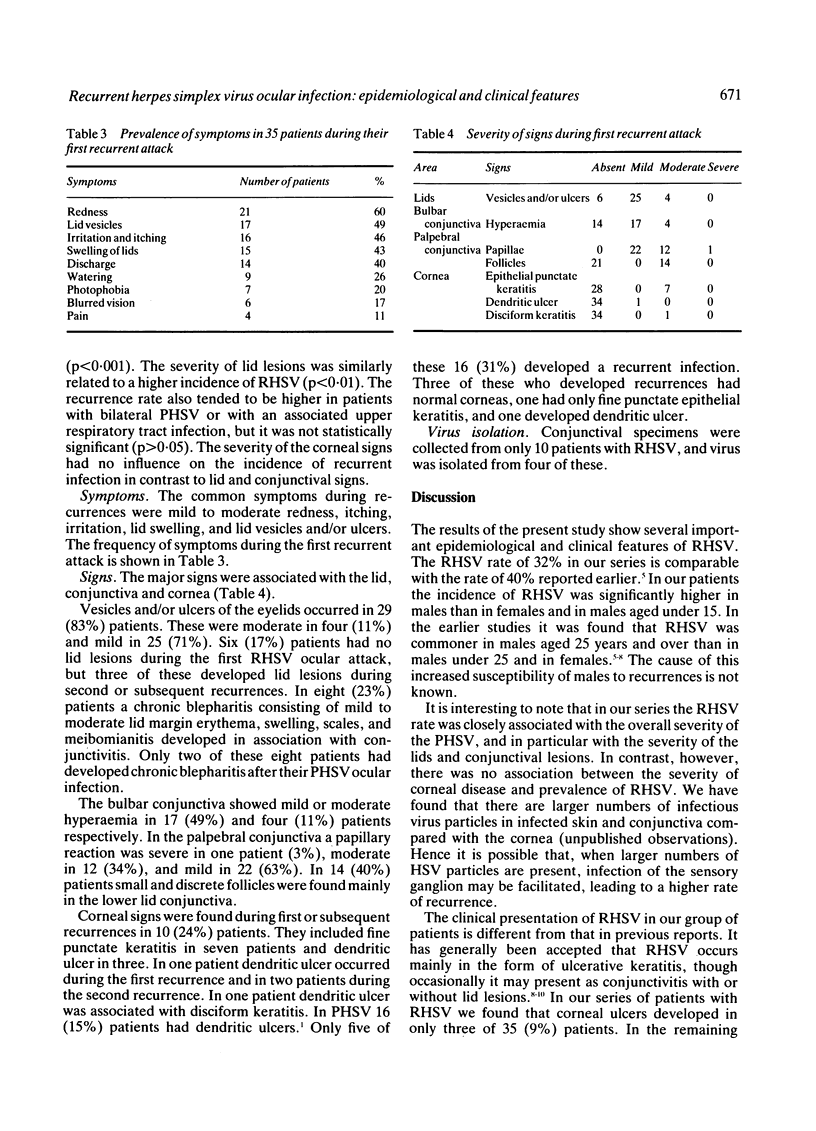
The epidemiological and clinical features of recurrent herpes simplex virus ocular infection (RHSV) were studied. Of 108 patients with primary herpes simplex virus ocular infection (PHSV) who were followed up for two to 15 years 35 (32%) suffered one or more recurrent attacks. The recurrence rate was significantly higher in patients under 20 years of age, but there was no significant difference between recurrence rates in males and females. Of 35 patients with RHSV 17 (49%) had one recurrent attack, 14 (40%) had between two and five, and four (11%) had between six and 15 attacks. The mean time interval between PHSV and the first four RHSV attacks was 10 months, and was shorter in subsequent attacks. The duration and severity of RHSV were reduced in successive recurrences. Patients with more severe conjunctivitis and lid lesions during PHSV ocular infection had a higher incidence of recurrent infection. The severity of the corneal signs in PHSV had no influence on the incidence of recurrent infection. Several clinical forms of RHSV were observed. Conjunctivitis associated with lid lesions was observed in 29 (83%) patients. In six (17%) patients the disease presented as an acute follicular conjunctivitis without characteristic lid or corneal lesions. Dendritic ulcer was found in three (9%) patients, and in one of them it was associated with a disciform keratitis. A chronic blepharoconjunctivitis developed in eight (23%) patients. The epidemiological and clinical features of RHSV were compared with those of PHSV.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darougar S., Wishart M. S., Viswalingam N. D. Epidemiological and clinical features of primary herpes simplex virus ocular infection. Br J Ophthalmol. 1985 Jan;69(1):2–6. doi: 10.1136/bjo.69.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- HOGAN M. J., KIMURA S. J., THYGESON P. Observations on herpetic keratitis and keratoconjunctivitis. AMA Arch Ophthalmol. 1956 Sep;56(3):375–388. doi: 10.1001/archopht.1956.00930040383006. [DOI] [PubMed] [Google Scholar]
- JONES B. The management of ocular herpes. Trans Ophthalmol Soc U K. 1959;79:425–437. [PubMed] [Google Scholar]
- LAIBSON P. R., LEOPOLD I. H. AN EVALUATION OF DOUBLE-BLIND IDU THERAPY IN 100 CASES OF HERPETIC KERATITIS. Trans Am Acad Ophthalmol Otolaryngol. 1964 Jan-Feb;68:22–34. [PubMed] [Google Scholar]
- LEOPOLD I. H., SERY T. W. EPIDEMIOLOGY OF HERPES SIMPLEX KERATITIS. Invest Ophthalmol. 1963 Oct;2:498–503. [PubMed] [Google Scholar]
- McSwiggan D. A., Darougar S., Rahman A. F., Gibson J. A. Comparison of the sensitivity of human embryo kidney cells, HeLa cells, and WI38 cells for the primary isolation of viruses from the eye. J Clin Pathol. 1975 May;28(5):410–413. doi: 10.1136/jcp.28.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus K. R., Coster D. J., Donovan H. C., Falcon M. G., Jones B. R. Prognostic indicators of herpetic keratitis. Analysis of a five-year observation period after corneal ulceration. Arch Ophthalmol. 1981 Sep;99(9):1578–1582. doi: 10.1001/archopht.1981.03930020452009. [DOI] [PubMed] [Google Scholar]


