Abstract
Newcastle disease (ND), avian influenza (AI, H5N8), and infectious bronchitis (IB) are important diseases in the poultry industry and cause significant losses. Vaccination is the most practical method for controlling infectious diseases. To reduce vaccination costs and several disorders in poultry farms, using herbal water supplements for immunomodulation with vaccination is critical to improving or preventing some conditions in the poultry industry. However, drinking water supplementation of ginger extract (GE)/propolis extract (PE) alone/in combination may increase broilers’ humoral and cellular immunity due to the immunomodulatory effects of ginger and propolis. This protocol aimed to see how GE/PE alone or in combination improved the immunity, immune organ gene expression, and histology of the immune organs of broilers for 35 d after vaccination against NDV, H5N8, IBV, and IBDV. The chicks were dispensed into 5 groups according to GE and/or PE with vaccination. The control group was offered normal drinking water without any supplements or vaccinations. The GE group was supplemented with ginger extract (1 mL/L drinking water) in the drinking water before and after vaccination for 2 and 3 d, respectively. The GE+PE group was supplemented with GE (0.5 mL/L drinking water) and PE (0.5 mL/L drinking water) in the drinking water before and after vaccination for 2 and 3 d, respectively. The PE group was supplemented with propolis extract (1 mL/L drinking water) in the drinking water before and after vaccination for 2 and 3 d, respectively. The fifth group was the vaccinated untreated group. This experiment showed the immunomodulatory properties of GE and/or PE against 3 common diseases, NDV, AI, and IB, in broiler chicken farms for 35 d applied to a vaccination program. Thus, ginger extract and propolis extract supplementation in drinking water increased antibody titer, INF, IL10, and IL2 and TLR3 gene expression in the bursa of Fabricius, thymus, and spleen, respectively, as well as cellular immunity as indicated by increased CD3, CD4, and CD8 in the bursa of Fabricius, thymus, and spleen, respectively, with normal lymphocytes in the medulla of the bursa, thymus, and spleen. In conclusion, propolis extracts alone or with GE improved all of the metrics mentioned above without harming the histology of the immune organs.
Key words: ginger extract, propolis extract, immunity, broiler, vaccine
INTRODUCTION
Poultry farms are attacked by many major contagious endemic viral diseases, including Newcastle disease (ND), avian influenza (AI), and infectious bronchitis (IB), which cause significant destructive economic effects and deaths in the poultry industry worldwide (Talebi et al., 2015). Moreover, humans in direct contact with poultry farms infected by AI virus (AIV) are susceptible to death (Li et al., 2019). On the other hand, vaccination is one of the most effective public health interventions for disease prevention. However, it has adverse side effects such as poor immune responses and causes incomplete sterilizing immunity (El-Dabae et al., 2018). Immunity is the body's defense mechanism against infections caused by microorganisms (Shams et al., 2019). Many herbal extracts have antiviral properties as they have immunomodulatory effects (Alagawany et al., 2019), which are mediated by increasing the production of pro-inflammatory cytokines like IL-6 and IL-12 (Trinchieri, 2003). Vaccination with an herbal adjuvant improves primary and memory adaptive responses while decreasing inflammatory responses (Sakure et al., 2008). Adjuvants have the most significant role in enhancing the efficacy of vaccines (Meunier et al., 2016).
Ginger (Zingiber officinal, Zingiberaceae) is widely used as an appetizer and medical treatment for certain diseases (Al-Amin et al., 2006). Ginger (GE) contains important protein compounds, including gingerol, gingerdiol, and gingerdione that have antioxidant activity and impact microbial activity by stimulating digestive enzymes in broilers (Barazesh et al., 2013). Ginger strongly enhances birds’ immune and digestive systems (Hanieh et al., 2010; Al-Shuwaili et al., 2015). Ginger has antimicrobial solid, antiparasitic, anticancer, anticholesteremic, vasodilator, and antidiabetic (Morakinyo et al., 2011) activities. Ginger is considered a natural growth promoter alternative to antibiotics, the most common artificial growth promoters (Demir et al., 2005). Several studies have found that ginger has analgesic, anti-inflammatory (Zhang et al., 2009), antipyretic, antiulcer, antiemetic, and cardiac relaxing properties and improves animal health and production (Barazesh et al., 2013).
Propolis (bee glue) is the natural resin bees gather from different living plants to create their nests (de Groot, 2013). Propolis (PE) contains several chemical compounds, including steroids, polyphenols (flavonoids, alcohols, phenolic aldehydes, and acids), and terpenoids (Rosen, 2007). Propolis has several biological activities, including antimicrobial, antiparasitic, antiprotozoan, anti-inflammatory, immunomodulatory, and antioxidant (Attia et al., 2017). Moreover, Castaldo and Capasso (2002) showed that propolis has a hepatoprotective effect, increases the body's defense against infections, and treats gastroduodenal ulcers in human medicine (Mutsaers et al., 2005; Attia et al., 2019b). Propolis has several biological importance in the poultry industry. It improves poultry performance by stimulating enzymes such as amylases, saccharases, and phosphatases, enhancing digestion and nutrient absorption. Propolis has been used as a dietary supplement in broiler farms; it can increase the helpful bacterial count while decreasing the pathogenic bacterial count in broiler intestines (Kačániová et al., 2013).
Therefore, this study aimed to determine the effects of GE and/or PE on immune system parameters (humoral or cellular response) to 3 common diseases such as NDV, AI, and IB in broiler chicken farms applied for a vaccination program, as well as their effect on the histologic architecture of the immune organs.
MATERIALS AND METHODS
Ethical Approval
The Faculty of Veterinary Medicine's Ethical Committee approved the current study for Live Bird Sampling at Alexandria University in Egypt (Permit #2022/014/11).
Chicks and Experimental Design
This experiment was conducted at the Faculty of Veterinary Medicine, Alexandria University, Egypt. A total of 200 one-day-old unsexed commercial Cobb broiler chicks were taken from a farm located in Alexandria city, Egypt, and distributed among 5 treatments by the ranking method (40 chicks, each with 4 replicates). The chickens were fed the same commercial diet (NASco, Cairo, Egypt) and were submitted to the following treatments in the drinking water free access: control group without supplementation (neither treatment nor vaccination), the ginger group supplemented with ginger (obtained from local market) extract (GE) at (1 mL/L drinking water), ginger plus propolis group (GE+PE) at (0.5 mL/L drinking water from each extract), propolis group supplemented with propolis extract (PE) at (1 mL/L drinking water) from 2 d before vaccination and 3 d after in the 3 treated groups, and the vaccinated group fed the commercial ration only without treatment in the drinking water. The doses and time of administration were chosen after performing the pilot test. The ginger and propolis extracts were gathered from the local market at Alexandria, identified by botanists at the faculty of Agriculture Alexandria University, then air-dried and ground into fine powder. At room temperature, 150 g of ginger powder was macerated in 200 mL of distilled water for 24 h, then filtered and concentrated in a rotary evaporator to obtain the final ginger aqueous extract (El-Bahr et al., 2022). About 200 g of propolis powder was soaked in 500 mL distilled water for 2 h at 70°C under the filtered and concentrated stirring to obtain the final propolis aqueous extract (de Moura et al., 2011).
Housing and Management
The chicks were kept in a clean, well-ventilated chamber that had been fumigated with formaldehyde gas created by combining 40% formalin with potassium permanganate powder. To provide the proper temperature for broiler chicks, the room was equipped with a gas heater and 2 electric bulbs, each rated at 200 watts. The experiment was conducted in a standard room with a 23-h light-to-1-h dark cycle. The chicks at 1-day-old were maintained at 32°C then the temperature was decreased linearly by 2°C per week with a relative humidity of 50% to 70% throughout the experiment. The floor of the room was partitioned into partitions. Each compartment was bedded with fresh, clean wheat straw, forming an 8-cm deep litter.
Vaccination Schedule
The birds in all groups were vaccinated on the 12th day of life with NDV vaccines via drinking water (Combivac C Vaccine, JoVac, Cairo, Egypt), as well as via injection (Mefluvac H5 Vaccine, Me-Vac, Cairo, Egypt); on the 16th day of life Nobilis GUMBORO D78®” (MSD Inc, Kenilworth, NJ) via drinking water; and on the 20th day of life (Lasota, SerVac, Abbasia, Egypt) via intraocular.
Sample Collection
To evaluate humoral immunity, on the morning of d 14, 24, and 35 of the experiment, 10 birds were randomly picked out from each of the 5 treatments after 12 h without feed. Blood samples (5.0 mL) were collected and taken from the wing vein into nonheparinized tubes. To reduce discomfort during blood sample collection, birds were given an intraperitoneal injection of sodium pentobarbital (50 mg/kg). Blood samples were incubated at 37°C for 2 h, subsequently centrifuged at (3,000 rpm for 20 min), and the serum was stored at −20°C for further assay. Meanwhile, to perform gene expression, flow cytometric analysis, and histopathology of the immune organs, the bird was slaughtered by cervical dislocation after bleeding at 35 d, and the whole tissue samples (bursa, thymus, and spleen) were immediately dissected, rinsed with chilled normal saline 0.9%, and divided into parts.
ELISA Test for Evaluation of AI H5N8, NDV, and IB
Commercial ELISA kits (Synbiotics Corp., San Diego, California) were used according to the manufacturer's instructions to evaluate humoral immune responses against AI H5N8, NDV, and IB in collected serum.
RNA Extraction and qRT-PCR
About 100 mg of the bursa, thymus, and spleen tissues were rinsed in sterilized phosphate buffer saline. After rinsing, the tissues were homogenized with a Teflon and pestle homogenizer in liquid nitrogen. The homogenates were then kept at −80°C until the RNA isolation step. Following the manufacturer's instructions, total RNA was extracted using TRIzol (Fermentas, K0731, Thermo Fisher Scientific, Waltham, MA). Using Revert Aid H Minus Reverse Transcriptase (Thermo Scientific, Fermentas, #EP0451) Reverse Transcription Kit, cDNA was produced from the isolated RNA. The reaction mixture included RNA and the master mix was placed at 42°C then inactivated at 70°C. The qRT-PCR for the target genes was performed using 2X Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific, # K0221). Table 1 lists each target and reference gene's PCR conditions and primer sequences. After recording the Ct values for the reference and target genes using the 2-ΔΔCt technique, the fold change of mRNA expression was computed (Livak and Schmittgen, 2001).
Table 1.
Primers for gene expression by RT-PCR.
| Gene | Direction | Primer sequence | Accession number | Annealing °C |
|---|---|---|---|---|
| B-Actin | Sense | CAACACAGTGCTGTCTGGTGGTA | X00182 | 58 |
| Antisense | ATCGTACTCCTGCTTGCTGATCC | |||
| IFNγ | Sense | TGGCGGCGGGAGGAAAAGTG | NM_001030558 | 60 |
| Antisense | CACCGTGCTCCAGCTCAGGC | |||
| IL-2 | Sense | GCAGGGCACGTTCAGGTGGG | NM_204153.1 | 58 |
| Antisense | GCCACACAGCCTGGCTCCCT | |||
| IL-10 | Sense | CGGGAGCTGAGGGTGAA | NM_001004414.2 | 62 |
| Antisense | GTGAAGAAGCGGTGACAGC | |||
|
TLR3 |
Sense | AAGGGGAAAATTTCTTAGCAGGT | FJ915472.1 | 60 |
| Antisense | AGGAAAGCAGTCTGCAAAGGT |
Abbreviations: IFN, Interferon; IL, Interleukin; TLR3, Toll-like receptor 3.
Flow Cytometric Analysis
Following Chen et al. (2014), the bursal, thymus, and spleen tissues (6 birds/group) were minced using enzymatic digestion or mechanical dissociation of the tissue. Then the minced tissues were filtered by mechanical filtration to prevent unwanted instrument clogs and obtain higher quality flow data. A total of 5 mL of complete Roswell Park Memorial Institute (RPMI) (Invitrogen, Burlington, Canada) media was added to tissue samples after they were washed with HBSS and strained through a 40 mm nylon cell strainer with the flat end of a 1 mL syringe (10% fetal bovine serum and 1% penicillin-streptomycin: Gibco, Grand Island, NY). A white buffy coat at the interface was obtained after harvesting and rinsing twice in the RPMI medium. After counting the cells with an automated cell counter (MOXI Z, Orflo, Ketchum, ID), the mononuclear cell suspension was planted at a density of 1 × 106 cells/well in RPMI medium. The next step was to transfer 100 L of the cell solution to a fresh tube and stain it with mouse antichicken antibodies, CD3, CD4, and CD8 (SouthernBiotech, Birmingham, AL) for 40 min at 25°C. Flow cytometry was used to analyze the stained cells after a single wash with PBS. The cells were then resuspended in 0.5 mL PBS (BD FACSCalibur). The flow rates in the cytometer are set, and the samples are run (McKinnon, 2018).
Histopathology of Different Lymphoid Organs (Spleen, Bursa, and Thymus)
At 35 d of broiler age, 3 birds from each replicate were collected randomly and slaughtered by cervical dislocation after anesthesia, and the (spleen, bursa, and thymus) were removed and divided into 2 segments for morphologic analysis. The samples were then dehydrated in ethyl alcohol in ascending grades, cleared with xylene, and embedded in melted paraffin wax. Paraffin blocks were made, and thin sections (3–7 µm thick) were prepared and mounted on egg albumin–glycerin-coated glass slides, dried, and stained with hematoxylin and eosin (H&E) for general inspection. This was done as outlined by Bancroft et al. (2013). The morphometric measurements were taken in a binocular microscope equipped with a clear Nikon camera and coupled with an image-analyzing system from Optika (Catanzarite et al., 2001). The data collected were analyzed statistically by analysis of variance.
Statistical Analysis
The data were all reported as means with standard deviations (SD). Duncan's multiple range test was used to produce individual comparisons, and 1-way analysis of variance (ANOVA) was used to determine statistical significance using SPSS 18.0 software, 2011 (SPSS Inc., Chicago, IL) Duncan's multiple range test. When P < 0.05, values were considered statistically significant.
RESULTS
Effect of Ginger GE and/or Propolis PE Extracts on Humeral Immunity
Effect of Ginger GE and/or Propolis PE Extracts on NDV Antibody Titer
The antibody titer of NDV was determined at 14, 24, and 35 d of the experiment. On the 14th day, the Ab titer in the GE, GE+PE, and vaccinated untreated groups increased insignificantly (P > 0.05) compared to the control and PE-treated groups. On the 24th day, the Ab titer in all groups showed an insignificant (P > 0.05) decrease compared to the control, except for the GE+PE-treated group. Finally, on the 35th day, Ab titer in all groups was significantly (P < 0.05) increased compared to the control, with the highest titer observed in the PE-treated group compared to other groups (Table 2).
Table 2.
NDV (genotype VII Ag) antibody titer (Log2).
| Group | Day 14 | Day 24 | Day 35 |
|---|---|---|---|
| Normal control group | 1.57 ± 0.29 | 1.71 ± 0.68 | 0.43 ± 0.29b |
| Ginger-treated group (GE) | 2.00 ± 0.62 | 1.43 ± 0.65 | 6.50 ± 0.62a |
| Ginger and propolis-treated group (GE+PE) | 2.29 ± 0.64 | 3.14 ± 0.55 | 5.71 ± 1.17a |
| Propolis-treated group (PE) | 1.71 ± 0.56 | 1.43 ± 0.29 | 7.71 ± 0.47a |
| Vaccinated with no treatment group | 2.14 ± 0.51 | 1.50 ± 0.50 | 7.00 ± 0.71a |
Means within the same column under the same category carry different superscripts a significantly different (P < 0.05).
Effect of Ginger and/or Propolis Extracts on AIV H5N8 Antibody Titer
The AI H5N8 antibody titer was determined after 14, 24, and 35 d of experimentation. On the 14th day, the AI H5N8 Ab titer in the GE and vaccinated untreated groups showed a significant (P < 0.05) increase compared to the other groups, with the highest value in the GE-treated group. On the 24th day, the AI H5N8 titer in the GE+PE and PE groups showed an insignificant increase compared to the control. Meanwhile, the vaccinated untreated group showed a significant decrease compared to the control. Finally, on the 35th day, the AI H5N8 titer in the GE+PE, PE, and vaccinated untreated groups was significantly increased compared to the control, with the highest titer observed in the propolis-treated group compared to other groups. On the other hand, ginger supplementation insignificantly increased AI H5N8 titer compared to the control (Table 3).
Table 3.
AIV H5N8 antibody titer.
| Group | Day 14 | Day 24 | Day 35 |
|---|---|---|---|
| Normal control group | 3.29 ± 0.18 b | 3.00 ± 0.11 a | 3.86 ± 0.63 c |
| Ginger-treated group (GE) | 4.14 ± 0.14 a | 2.71 ± 0.18 ab | 4.33 ± 0.33 c |
| Ginger and propolis-treated group (GE+PE) | 3.43 ± 0.20 b | 3.29 ± 0.42 a | 5.00 ± 0.44 b |
| Propolis-treated group (PE) | 3.71 ± 0.18 b | 3.43 ± 0.20 a | 6.00 ± 0.31 a |
| Vaccinated with no treatment group | 4.00 ± 0.44 a | 2.00 ± 0.71 b | 5.00 ± 0.71 b |
Means within the same column under the same category carry different superscripts a significantly different (P < 0.05).
Effect of Ginger and/or Propolis Extracts on IBV Antibody Titer (ELIZA)
The data in Table 4 of IB antibody titer were determined at 14, 24, and 35 d of the experiment. On the 14th day, the IB Ab titer in the GE and PE-treated groups showed an insignificant (P > 0.05) decrease compared to the control and vaccinated untreated groups. While the GE+PE-treated group was insignificantly (P > 0.05) increased compared to the other groups. On the 24th day, the IB titer in the GE showed a significant (P < 0.05) decrease compared to the control. In contrast, the PE, GE+PE, and vaccinated untreated groups showed no important (P > 0.05) variation compared to the control, with the highest value in the PE-treated group. Finally, on the 35th day, IB titer in all treated groups was insignificantly (P > 0.05) increased compared to the control, with the highest value in the GE+PE-treated group.
Table 4.
IBV antibody titer (ELIZA).
| Group | Day 14 | Day 24 | Day 35 |
|---|---|---|---|
| Normal control group | 13.0 ± 1.32 | 7.30 ± 0.68a | 7.15 ± 0.38 |
| Ginger-treated group (GE) | 11.9 ± 1.83 | 4.73 ± 0.22b | 10.2 ± 1.43 |
| Ginger and propolis-treated group (GE+PE) | 16.7 ± 3.01 | 6.05 ± 1.09a | 11.4 ± 4.44 |
| Propolis-treated group (PE) | 12.7 ± 1.96 | 8.43 ± 1.06a | 8.56 ± 0.75 |
| Vaccinated with no treatment group | 13.5 ± 1.62 | 8.34 ± 0.52a | 8.23 ± 2.04 |
Means within the same column under the same category carry different superscripts a significantly different (P < 0.05).
Effect of GE and/or PE Extracts on INF, IL10, and IL2, and TLR3 Genes Expressions in the Bursa of Fabricius, Thymus, and Spleen, Respectively
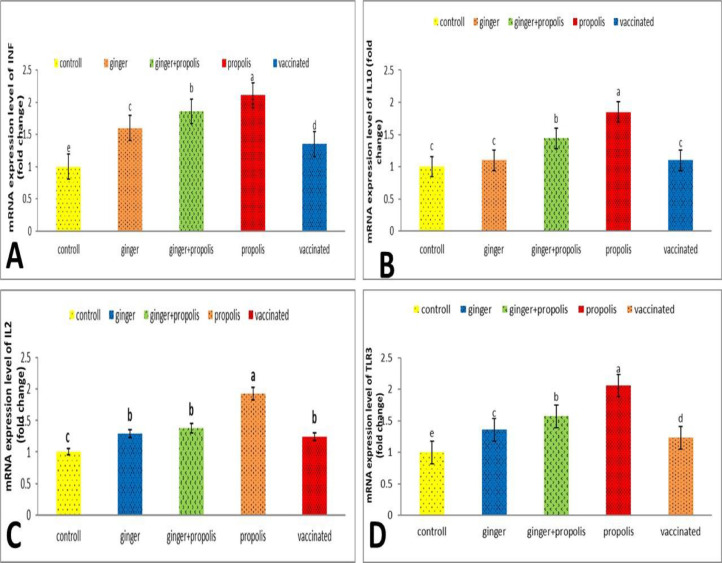
Figure 1A showed that the GE, PE, and their combination significantly upregulated the IFN mRNA gene expression. There is a significant (P ≤ 0.05) difference between 5 groups as the PE group had a considerably higher upregulated (P ≤ 0.05) INF gene expression level in the bursa than the GE+PE, GE, and vaccinated untreated groups, respectively, compared to the normal control group. On the other hand, our results showed no significant (P > 0.05) difference in GE-treated, vaccinated untreated, and control groups on IL10 gene expression. While the GE+PE-treated group significantly (P ≤ 0.05) upregulated the IL10 mRNA gene expression. Moreover, the PE-treated group showed the thymus's highest upregulated IL10 gene expression level (Figure 1B).
Figure 1.
Graphical presentation of real-time quantitative PCR analysis of the expression of INF, IL10, IL2, and TLR3 genes in chicken bursa, thymus, and spleen, respectively, of normal and viral vaccinated chickens fed on rations containing ginger extract and/or propolis extract. Columns carrying different letters are significantly different at P ≤ 0.05.
Moreover, compared to the vaccinated untreated value, there were no significant differences among the GE and GE+PE-treated groups in IL2 gene expression. Meanwhile, the PE-treated group showed the highest upregulated IL2 gene expression level in the spleen (Figure 1C). The data in Figure 1D, on the other hand, showed that the GE, GE+PE, and PE extract treated groups, as well as the vaccinated untreated group, showed a significant (P ≤ 0.05) increase in TLR3 gene expression compared to the control group, with the highest upregulated value observed in the PE-treated group compared to the other groups.
GE and/or PE Extracts on Cellular Immunity Parameters: T-Repertoire in Immune Organs
Table 5 shows the results of the effect of the GE and/or PE extracts on CD3, CD4, and CD8 percent in the bursa of Fabricius, thymus, and spleen, respectively. Our results revealed significant (P ≤ 0.05) differences between treated groups. Compared to control and vaccinated untreated values, the GE, PE, and GE+PE-treated groups showed a significant (P ≤ 0.05) increase in CD3 value. While in the GE+PE-treated group showed the highest significant (P ≤ 0.05) increased value in CD3 of the bursa compared with other groups. Moreover, control and vaccinated untreated groups showed the lowest CD3 value in the bursa.
Table 5.
Effect of ginger and/or propolis extracts on CD3, CD4, and CD8 percent in the bursa of Fabricius, thymus, and spleen, respectively.
| Parameters Group |
CD3 | CD4 | CD8 |
|---|---|---|---|
| Normal control group | 31.4 ± 1.57d | 24.5 ± 1.06e | 45.3 ± 1.05d |
| Ginger-treated group (GE) | 53.1 ± 1.75b | 60.6 ± 2.30c | 71.9 ± 1.19b |
| Ginger and propolis-treated group (GE+PE) | 81.4 ± 0.42a | 75.8 ± 1.26b | 83.7 ± 0.79a |
| Propolis-treated group (PE) | 47.7 ± 0.60c | 87.1 ± 1.57a | 73.3 ± 1.25b |
| Vaccinated with no treatment group | 31.8 ± 1.55d | 51.7 ± 2.30d | 57.1 ±0.94c |
Means within the same column carrying different symbols are significantly different. Data expressed as means ± standard errors of means (SEM, n = 3).
Effect of GE and/or PE extracts on CD4 percent in the thymus, the PE-treated group showed a significant increase in CD4 value to GE+PE and GE, respectively. Moreover, the control group showed the lowest CD4 value in the thymus. On the other hand, Table 5 showed the results of the effect of GE and/or PE extracts on CD8 percent in the spleen. Compared to control and vaccinated untreated values, GE, PE, and GE+PE-treated groups showed a significant (P ≤ 0.05) increase in CD8 value. While GE+PE-treated group showed the highest significant (P ≤ 0.05) increased value in CD8 of the spleen compared with other groups. Moreover, the control group showed the lowest CD8 value in the spleen.
Histopathologic Investigation
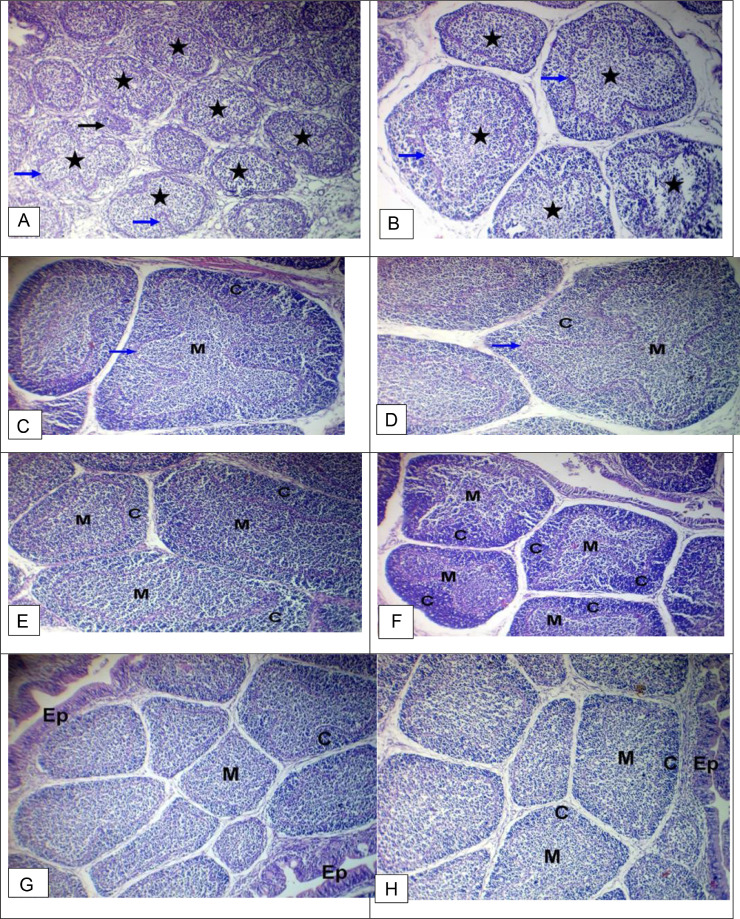
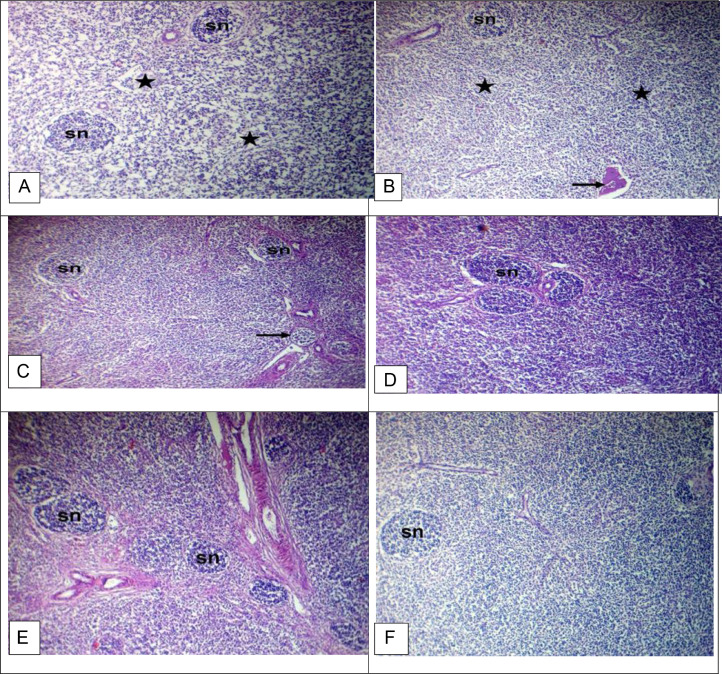
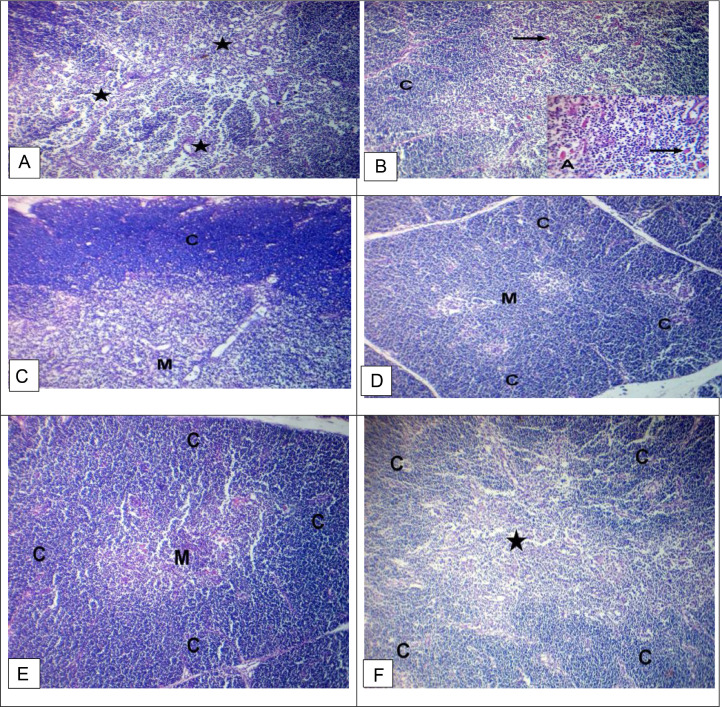
The bursa of Fabricius of a chicken was examined in the control group. It showed depletion of lymphocytes in the cortices and medullae with enfolding of the reticuloendothelial layer and sporadic atrophied lymphoid follicles with sporadic atrophied lymphoid follicles (Figures 2A and 2B). In contrast, the GE group showed mild depletion of lymphocytes in the cortices with enfolding of the reticuloendothelial layer (Figures 2C and 2D). The bursa of Fabricius of a chicken was examined in the GE+PE group, and PE-treated group showed relatively normal lymphocytes in the cortices and medullae. The bursa of Fabricius of a chicken was examined in the vaccinated group without treatment. It showed relatively normal lymphocytes in the cortices, medullae, and epithelium (Figure 2H; Table 6). The spleen of a chicken from the control group showed a depletion of lymphocytes with hemolyzed RBCs (Figures 3A and 3B). The spleen of a chicken from the GE group showed normal lymphocytes with sporadic atrophied splenic nodules. The spleen of a chicken from the GE+PE and PE-treated group showed normal lymphocytes in the white pulp and splenic nodules. The spleen of a chicken from the vaccinated group without treatment showed relatively normal lymphocytes in the white pulp and splenic nodules (Figure 3F; Table 6). The thymus of a chicken from the control group showed depletion of lymphocytes in the medullae with increased numbers of Hassall's corpuscles in the thymic medulla (Figure 4A). The thymus of a chicken from the control group showed hemorrhage and hemolyzed RBCs in the medullae with increased numbers of Hassall's corpuscles in the thymic medulla (Figure 4B). The thymus of a chicken from the GE, the GE+PE, and PE group showed a depletion of lymphocytes in the medullae with a normal cortex and normal. The thymus of a chicken from the vaccinated without treatment group showed depletion of lymphocytes in the medullae with a normal cortex (Figure 4F; Table 6).
Figure 2.
Light microscopy photomicrographs of H&E stained sections of broiler chicken (bursa) (×100): (A and B) bursa of Fabricius control group showing normal structure with depletion of lymphocytes in the cortices and medullae (stars) with enfolding of the reticuloendothelial layer (blue arrows) and sporadic atrophied lymphoid follicle (black arrow). (C and D) ginger-treated group GE group showing mild depletion of lymphocytes in the cortices (C) and medullae (M) with enfolding of the reticuloendothelial layer (blue arrow). (E and F) bursa of Fabricius of a chicken of ginger and propolis combination GE+PE group showing relatively normal lymphocytes in the cortices (C) and medullae (M). (G) Bursa of Fabricius of propolis-treated group showing relatively normal lymphocytes in the cortices (C), medullae (M), and epithelium (Ep). (H) The vaccinated without treatment group showed relatively normal lymphocytes in the cortices (C), medullae (M), and epithelium (Ep).
Table 6.
Scoring of histopathologic lesions of all experimental groups.
| Groups organs lesions |
G1 |
G2 |
G3 |
G4 |
G5 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bursa | Thymus | Spleen | Bursa | Thymus | Spleen | Bursa | Thymus | Spleen | Bursa | Thymus | Spleen | Bursa | Thymus | Spleen | |
| Depletion of lymphocytes | + | + | + | + | + | - | - | - | - | - | - | - | - | + | - |
| The enfolding of the reticuloendothelial layer | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - |
| Atrophied lymphoid follicle | + | - | - | + | - | + | - | - | - | - | - | - | - | - | - |
| Hassall's corpuscles in | - | +++ | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Hemolyzed RBCs | - | ++ | + | - | - | - | - | - | - | - | - | - | - | - | - |
| Hemorrhage | - | ++ | - | - | - | - | - | - | - | - | - | - | - | - | |
| Splenic nodule | - | - | ++ | - | - | + | - | - | + | - | - | + | - | - | + |
Lesion scoring: (-) absence of the lesion = 0%, (+) mild = 5%–25%, (++) moderate = 26%–50%, and (+++) severe >50% of the examined tissue sections.
Figure 3.
Light microscopy photomicrographs of H&E stained sections of broiler chicken (spleen) (×100): (A and B) Spleen of a chicken of control group showing depletion of lymphocytes in white pulp (stars) and splenic nodules (sn) with hemolyzed RBCs (arrow). (C) The ginger-treated chicken of the GE group spleen showed normal lymphocytes in white pulp and splenic nodules (sn) with sporadic atrophied splenic nodules (arrow). (D) The ginger and propolis combination-treated chicken spleen of the GE+PE group showed normal lymphocytes in white pulp and splenic nodules (sn). (E) The spleen of a chicken of the PE group shows normal lymphocytes in white pulp and splenic nodules (sn). (F) The spleen of a chicken of the vaccinated group without treatment showed relatively normal lymphocytes in white pulp and splenic nodules (sn).
Figure 4.
Light microscopy photomicrographs of H&E stained sections of broiler chicken (thymus) (×100): (A and B) thymus of normal control group showing depletion of lymphocytes in the medullae (stars) with hemorrhage and hemolyzed RBCs in the medullae (blue arrows) with increased numbers of Hassall's corpuscles in the thymic medulla (black arrow). (C) Thymus of ginger-treated group (GE) showing depletion of lymphocytes in the medullae (M) with the normal cortex (C). (D) Thymus of ginger and propolis combination group (GE+PE) showing relatively normal lymphocytes in the medullae (M) with normal cortex (C). (E) Thymus of propolis-treated group (PE) showing relatively normal lymphocytes in the medullae (M) with normal cortex (C). (F) The thymus of the vaccinated without treatment group showed depletion of lymphocytes in the medullae (stars) with normal cortex (C).
DISCUSSION
Newcastle disease, avian influenza (AIV H5N8), and IB virus are important diseases in the poultry industry and cause significant losses. Vaccination is the most practical method for controlling infectious diseases (Hadipour et al., 2011). To reduce vaccination costs and several disorders in poultry farms, using herbals as feed additives for immunomodulation with vaccination is a critical issue in improving or preventing some diseases in the poultry industry (Attia et al., 2015; Attia et al., 2019a; Eladl et al., 2020). Because of the above, the present experiment was carried out to exhibit the immunomodulatory properties of GE and/or PE to 3 common diseases such as NDV, AI, and IB, in broiler chicken farms that applied for a vaccination program. Ginger extract has several pharmacologic characteristics, such as antioxidant, anti-inflammatory, analgesic, gastrointestinal regulating agent, immunomodulatory, and antimicrobial properties (Attia and Hassan, 2018; Abd El-Hack, et al., 2020).
On the other hand, propolis is used as an antibacterial, antifungal, anti-inflammatory, antiviral, anesthetic, antioxidant (Vickers, 2017), antitumoral, antiprotozoal, anticancer (Sforcin, 2016), antihypertensive, anticarcinogenic, and antihepatotoxic in addition to possessing cytotoxic activity (Toreti et al., 2013). Results in Tables 2 to 4 showed that using ginger and/or propolis extracts improved antibody titers against NDV, avian influenza, and IBD vaccination with a special effect to propolis extract alone. These results agree with those of Attia et al. (2014) and Desoky and Abdulhamid (2016), who reported that propolis positively affected the immune status of the bird. These positive effects may be attributed to the immunomodulatory properties of propolis's chemical compounds, such as aromatic acids, alkaloids, flavonoids, polyphenols, and terpenoids, which activate macrophages, promote the production of antibodies, and boost the immune response. In addition, Hsiao et al. (2022) reported that adding propolis to broiler drinking water before and after vaccination improves the antibody titer response to infectious bronchitis virus at 28 d of age. This increase in the antibody production suggests that propolis stimulates chicken immune responses and might be utilized to boost antigen-specific antibody responses to vaccinations (Karpala et al., 2011).
In contrast to our study, only IBDV titer levels were similar in broilers fed ether extract propolis (Sahin and Ozturk, 2018). The disparity between the studies might be because broiler breeder hens had a longer vaccination program life expectancy than broilers. Furthermore, differential outcomes might be attributed to changes in propolis quality (kind, dosage, form, plant species, location, and season), experimental birds (species, age, gender, stress, heat, and management), and other parameters including propolis application timing and duration (Mahmoud et al., 2016). However, our results showed the positive effect of ginger extract alone and mixed with propolis. In agreement with our results, El-Deek (2003) and Hagmohamadi et al. (2020) reported that dietary supplementation of ginger to broilers improves the antibody against viral diseases.
Moreover, Taghdisi and Hejazi (2019) reported significant improvement in the antibody titer of the Ross breed broiler supplemented with ginger powder. According to Sahoo et al. (2019), adding ginger powder did not affect antibodies against ND titer. The cellular immunity of broilers supplemented with ginger is much greater than that of the control group, but there is no significant impact on humoral immunity (Sahoo et al., 2019). However, these differences may be attributed to the root of supplementation or the quality of the plant and the bird species used.
The Ginger extract and/or Propolis extract revealed significantly upregulated IFN and TLR3 gene expression levels in all treated groups, with the highest level observed in the propolis-treated group. On the other hand, propolis extract increased the upregulation of IL10 and IL2. These results confirmed the results of humoral immunity where elevated expression of IL-2 and interferon—since these cytokines drive antibody formation in birds, they are likely to be a factor in the increased quantities of antibodies generated following antigenic stimulation in propolis-treated birds (Wang et al., 2006). However, this result agreed with Hsiao et al. (2022), who reported that adding propolis to broiler drinking water before and after vaccination upregulates the IL10. Moreover, Wang et al. (2006) found that propolis increases expressions of IL2 and IFN in chickens. On the other hand, the expression of TLR3 was upregulated in the PE group. Many TLRs capable of detecting pathogen‐associated molecular patterns from various pathogens are expressed in endosomes, where they identify viral genomes during endocytosis, triggering type I IFN expression (Pichlmair, 2007). TLR3, primarily specified as a dsRNA virus sensor (Alexopoulou et al., 2001), activates dendritic cells following the phagocytosis of infected cells (Schulz et al., 2005) and has a function in preventing herpes simplex virus infection in the central nervous system (Reinert et al., 2012). The avian TLR3, despite being associated with upregulation of innate immune responses during virus infection, remains poorly characterized (Zhang et al., 2015). TLR3 induces the host's innate immune response to recognizing viral double-stranded RNA (dsRNA). TLR3 triggers downstream signaling, causing interferons and pro-inflammatory cytokine genes to be transcribed (Vickers, 2017). Karpala et al. (2011) found that activating the avian TLR3 leads to increased IFN- production. To our knowledge, there is no research on the effect of ginger on the TLR3, IL10, IL2, and interferon expressions in broilers.
This study showed increased CD3+ T-helper lymphocyte, CD4+ T-lymphocyte population levels, and CD8+ T-cytotoxic cell subpopulation levels in all treated groups compared with the control group. This increase indicates that immune activity in the peripheral system is improving (Correa et al., 2014). The mechanisms by which activated T-lymphocytes regulate the infection remain unknown. Positive cells of the major histocompatibility complex class II have increased in number. This might explain the increased percentages of CD3+ and CD4+ T-lymphocytes and higher CD8 levels, even in the early stages of infection (Cao et al., 2020). However, our results agree with those of (SI et al., 2021), who reported a significant increase in CD3+ in the broilers supplemented with propolis. Rifa'I (2017) said that CD8 T-cell development might be triggered by propolis administration in mice.
Moreover, Rifa'I and Widodo (2014) reported that propolis administration in the mouse changes CD4 and CD8 immune responses. Compared to the control group, the CD4+, and CD8+ T-lymphocyte levels are not significantly reduced in propolis therapy at all dosages in cell culture (Kusnul et al., 2017). Moreover, Draganova-Filipova et al. (2010) found that low doses of propolis unaffected CD4+ and CD8+ T-lymphocyte percentages. On the other side, Du et al. (2010) studied the effects of ginger extract in mice and cell culture, respectively, and found a significant increase in CD3+ and CD8+. In contrast, Lu et al. (2011) reported that ginger extract decreases the percentage of CD3 in mice. However, these differences in the results may be attributed to the method of extraction, root of administration, season of collection, and animal used in the experiment. For example Wagh (2013) reported that propolis samples’ biological and chemical activity varies due to their different geographical origins.
The histopathology results revealed the depletion of the lymphocytes in the control group in all immune organs but in the ginger and vaccinated untreated groups in the bursa and thymus, respectively. Meanwhile, GE+PE, PE, and ginger groups showed relatively normal lymphocytes in the medullae of the bursa, spleen, thymus, and spleen, respectively. These results are confirmed by the results of Mona et al. (2021), who reported that normal lymphocytes in the bursa, spleen, and thymus tissue of the propolis-supplemented broilers. Moreover, Taghdisi and Hejazi (2019) found normal lymphocytes in the splenic medulla of ginger-supplemented broilers. In contrast, Ahmed et al. (2016) reported depletion of the bursal lymphocyte in broilers supplemented with different doses of Chinese propolis. This difference may be attributed to the source of the propolis. However, the present experiment demonstrates the new importance of the addition of ginger extract (1 mm/L D.W)/propolis extract (1 mm/L D.W) alone/in combination (0.5 mm/L D.W from each extract) to the drinking water of broilers before and after vaccination. Applying for a vaccination program, it exhibited the immunomodulatory properties of GE and/or PE to 3 common diseases such as NDV, AI, and IB in broiler chicken farms for 35 d. Thus, in conclusion, drinking water supplementation of ginger extract (1 mL/L drinking water) or propolis (1 mL/L drinking water) extract or in a combination (0.5 mL for each/L drinking water) may increase the antibody titer, INF, IL10, and IL2 and TLR3 gene expression in the bursa of Fabricius, thymus, and spleen, respectively, and cellular immunity indicated by increased CD3, CD4, and CD8 in the bursa of Fabricius, thymus, and spleen, respectively. However, the effect of the propolis extract on humoral immunity and cellular immunity in the bursa, thymus, and spleen is of great importance.
Acknowledgments
ACKNOWLEDGMENTS
The Science, Technology & Innovation Funding Authority (STDF) provides open access funding in cooperation with The Egyptian Knowledge Bank (EKB). The authors are grateful to the deanship of scientific research at King Khalid University (R.G.P2/121/44).
Animal Welfare Statement: The ethical policies of the journal have been adhered to, the appropriate ethical approval has been received, and the research meets EU standards for the protection and use of animals for scientific purposes and/or feed legislation.
Author Contributions: All authors have read and approved the manuscript. A. A. E. F. D. worked the experiment. N. M. T. and M. A. L. designed the in vivo experiments. M. A. L. performed the data analysis. M. S., M. S. A., and E. M. A. reviewed the manuscript. S. E. F. wrote the manuscript.
DISCLOSURES
The authors declare that they have no competing interests.
REFERENCES
- Abd El-Hack M.E., Alagawany M., Shaheen H., Samak D., Othman S.I., Allam A.A., Taha A.E., Khafaga A.F., Arif M., Osman A. Ginger and its derivatives as promising alternatives to antibiotics in poultry feed. Animals. 2020;10:452. doi: 10.3390/ani10030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed O.B., Mahmoud U.T., Mahmoud M.A., El-Bab M.R.F. Histomorphological changes associated with different doses of Chinese propolis in the Bursa of fabricius of chickens. J. Adv. Vet. Res. 2016;6:1–6. [Google Scholar]
- Al-Amin Z.M., Thomson M., Al Qattan K., Peltonen-Shalaby R., Ali M. Anti diabetic and hypoglikemic properties of ginger (Zingiber officinale) in streptozocin induced diabetic rats. Br. J. Nutr. 2006;96:660–666. doi: 10.1079/bjn20061849. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L., Holt A.C., Medzhitov R. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Al-Shuwaili M.A., Ibrahim E., Al-Bayati M.Naqi. Effect of dietary herbal plants supplement in turkey diet on performance and some blood biochemical parameters. Glob. J. Biosci. Biotechnol. 2015;4:153–157. [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., Abd El-Hack M.E., Khafaga A.F., Taha A.E., Tiwari R., Yatoo M.I., Bhatt P., Marappan G. Use of licorice (Glycyrrhiza glabra) herb as a feed additive in poultry: current knowledge and prospects. Animals. 2019;9:536. doi: 10.3390/ani9080536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Y., Al-Khalaifah H., Ibrahim M., Abd Al-Hamid A., Al-Harthi M., El-Naggar A. Blood hematological and biochemical constituents, antioxidant enzymes, immunity and lymphoid organs of broiler chicks supplemented with propolis, bee pollen and mannan oligosaccharides continuously or intermittently. Poult. Sci. 2017;96:4182–4192. doi: 10.3382/ps/pex173. [DOI] [PubMed] [Google Scholar]
- Attia Y., Bovera F., El-Tahawy W., El-Hanoun A., Al-Harthi M., Habiba H. Productive and reproductive performance of rabbits does as affected by bee pollen and/or propolis, inulin and/or mannan-oligosaccharides. World Rabbit Sci. 2015;23:273–282. [Google Scholar]
- Attia Y.A., Abd Al-Hamid A., Ibrahim M.S., Al-Harthi M., Bovera F., Elnaggar A.S. Productive performance, biochemical and hematological traits of broiler chickens supplemented with propolis, bee pollen, and mannan oligosaccharides continuously or intermittently. Livest. Sci. 2014;164:87–95. [Google Scholar]
- Attia Y.A., Bovera F., Abd-Elhamid A.E.-H.E., Calabrò S., Mandour M.A., Al-Harthi M.A., Hassan S.S. Evaluation of the carryover effect of antibiotic, bee pollen and propolis on growth performance, carcass traits and splenic and hepatic histology of growing rabbits. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019;103:947–958. doi: 10.1111/jpn.13068. [DOI] [PubMed] [Google Scholar]
- Attia Y.A., Bovera F., Abd-Elhamid A.E.-H.E., Nagadi S.A., Mandour M.A., Hassan S.S. Bee pollen and propolis as dietary supplements for rabbit: effect on reproductive performance of does and on immunological response of does and their offspring. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019;103:959–968. doi: 10.1111/jpn.13069. [DOI] [PubMed] [Google Scholar]
- Attia Y.A.E., Hassan S.S. Response of broiler chickens to dietary supplementation of ginger (Zingiber officinale) continuously or intermittently in comparison with prebiotics. Egypt. Poult. Sci. J. 2018;37:523–543. [Google Scholar]
- Bancroft J.D., Layton C., Suvarna S.K. Bancroft’s Theory and Practice of Histological Techniques. Churchill Livingstone Elsevier; New York, USA: 2013. [Google Scholar]
- Barazesh H., Pour M.B., Salari S., Abadi T.M. The effect of ginger powder on performance, carcass characteristics and blood parameters of broilers. Int. J. Adv. Biol. Biomed. Res. 2013;1:1645–1651. [Google Scholar]
- Cao B., Chen X., Zhang L., Wei Q., Liu H., Feng W., Chen Y., Shang H. Elevated percentage of CD3+ T-cells and CD4+/CD8+ ratios in multiple system atrophy patients. Front Neurol. 2020;11:658. doi: 10.3389/fneur.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldo S., Capasso F. Propóleos, un antiguo remedio utilizado en la medicina moderna. Fitoterapia. 2002;73:S1–S6. doi: 10.1016/s0367-326x(02)00185-5. [DOI] [PubMed] [Google Scholar]
- Catanzarite V., Maida C., Thomas W., Mendoza A., Stanco L., Piacquadio K. Prenatal sonographic diagnosis of vasa previa: ultrasound findings and obstetric outcome in ten cases. Ultrasound Obstet. Gynecol. 2001;18:109–115. doi: 10.1046/j.1469-0705.2001.00448.x. [DOI] [PubMed] [Google Scholar]
- Chen K., Peng X., Fang J., Cui H., Zuo Z., Deng J., Chen Z., Geng Y., Lai W., Tang L. Effects of dietary selenium on histopathological changes and T cells of spleen in broilers exposed to aflatoxin B1. Int. J. Environ. Res. Public Health. 2014;11:1904–1913. doi: 10.3390/ijerph110201904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa B.L., Ornaghi A.P., Muller G.C., Engroff P., Lopes R.P., da Silva Filho I.G., Bosch J.A., Bonorino C., Bauer M.E. The inverted CD4: CD8 ratio is associated with cytomegalovirus, poor cognitive and functional states in older adults. Neuroimmunomodulation. 2014;21:206–212. doi: 10.1159/000356827. [DOI] [PubMed] [Google Scholar]
- de Groot A.C. Propolis: a review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis. 2013;24:263–282. doi: 10.1097/DER.0000000000000011. [DOI] [PubMed] [Google Scholar]
- Demir E., Sarica S., Ozcan M., Suicmez M. The use of natural feed additives as alternatives to an antibiotic growth promoter in broiler diets. Arch. Geflugelk. 2005;69:110–116. [Google Scholar]
- de Moura S.A., Negri G., Salatino A., Lima L.D., Dourado L.P., Mendes J.B., Andrade S.P., Ferreira M.A., Cara D.C. Aqueous extract of brazilian green propolis: primary components, evaluation of inflammation and wound healing by using subcutaneous implanted sponges. Evid. Based Complement. Alternat. Med. 2011;2011:748283. doi: 10.1093/ecam/nep112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoky W., Abdulhamid A. Effects of ginger and bee propolis on the performance, carcass characteristics and blood constituents of growing Japanese quail. Egypt. Poult. Sci. J. 2016;36:143–159. [Google Scholar]
- Draganova-Filipova M., Nikolova M., Mihova A., Peychev L., Sarafian V. A pilot study on the immunomodulatory effect of Bulgarian propolis. Biotechnol. Biotechnol. Equip. 2010;24:119–124. [Google Scholar]
- Du X., Pan H., Zhang C., Zhang H., Liu H., Chen Z., Zeng X. Zingiber officinale extract modulates γ-rays-induced immunosuppression in mice. J. Med. Plant Res. 2010;4:1647–1655. [Google Scholar]
- El-Bahr S.M., Elzoghby R.R., Alfattah M.A., Kandeel M., Hamouda A.F. Aqueous ginger (Zingiber officinale) extract ameliorates the harmful effects of high-dose lornoxicam in albino male rats. Biomed. Res. Int. 2022;2022:1–15. doi: 10.1155/2022/1546734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Dabae W.H., Hussein H.A., Rohaim M.A., El-Safty M.M., Ata N.S., Reda I.M. Saponin-adjuvanted vaccine protects chickens against velogenic Newcastle disease virus. Arch. Virol. 2018;163:2423–2432. doi: 10.1007/s00705-018-3917-4. [DOI] [PubMed] [Google Scholar]
- El-Deek A. Effect of anise (Pimpinella anisum), fennel (Foeniculum vulgare) and ginger (Zingiber officinale Roscoe) on growth performance, carcass criteria and meat quality of broilers. Arch. Geflugelk. 2003;67:92–96. [Google Scholar]
- Eladl A.H., Mosad S.M., El-Shafei R.A., Saleh R.M., Ali H.S., Badawy B.M., Elshal M.F. Immunostimulant effect of a mixed herbal extract on infectious bursal disease virus (IBDV) vaccinated chickens in the context of a co-infection model of avian influenza virus H9N2 and IBDV. Comp. Immunol. Microbiol. Infect. Dis. 2020;72 doi: 10.1016/j.cimid.2020.101505. [DOI] [PubMed] [Google Scholar]
- Hadipour M., Habibi G., Golchin P., Hadipourfard M., Shayanpour N. The role of avian influenza, Newcastle disease and infectious bronchitis viruses during the respiratory disease outbreak in commercial broiler farms of Iran. Int. J. Anim. Vet. Adv. 2011;3:69–72. [Google Scholar]
- Hagmohamadi M., Salarmoini M., Afsharmanesh M., Tavakkoli H. Effect of ginger powder in comparison with flavophospholipol antibiotic on growth performance, intestinal micro-flora and morphology, and immune response in broiler chickens. Anim. Sci. J. 2020;32:131–144. [Google Scholar]
- Hanieh H., Narabara K., Piao M., Gerile C., Abe A., Kondo Y. Modulatory effects of two levels of dietary Alliums on immune response and certain immunological variables, following immunization, in White Leghorn chickens. Anim. Sci. J. 2010;81:673–680. doi: 10.1111/j.1740-0929.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- Hsiao F.S.-H., Artdita C.A., Hua K.-F., Tsai C.-J., Chien Y.-H., Chen Y.-W., Cheng Y.-H., Yu Y.-H. Optimization of emulsification conditions on ethanol extract of Taiwanese green propolis using polysorbate and its immunomodulatory effects in broilers. Animals. 2022;12:446. doi: 10.3390/ani12040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kačániová M., Rovná K., Arpášová H., Hleba L., Petrová J., Haščík P., Čuboň J., Pavelková A., Chlebo R., Bobková A. The effects of bee pollen extracts on the broiler chicken's gastrointestinal microflora. Res. Vet. Sci. 2013;95:34–37. doi: 10.1016/j.rvsc.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Karpala A.J., Stewart C., McKay J., Lowenthal J.W., Bean A.G. Characterization of chicken Mda5 activity: regulation of IFN-β in the absence of RIG-I functionality. J. Immunol. 2011;186:5397–5405. doi: 10.4049/jimmunol.1003712. [DOI] [PubMed] [Google Scholar]
- Kusnul Z., Rahayu P., Rifai M., Widjajanto E. Immunomodulatory effect of propolis extract on granzyme expression in CD8+ and CD4+ CD25+ T Cells. Turk. J. Immunol. 2017;5:13–19. [Google Scholar]
- Li Y.-T., Linster M., Mendenhall I.H., Su Y.C., Smith G.J. Avian influenza viruses in humans: lessons from past outbreaks. Br. Med. Bull. 2019;132:81–95. doi: 10.1093/bmb/ldz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J., Guan S., Shen X., Qian W., Huang G., Deng X., Xie G. Immunosuppressive activity of 8-gingerol on immune responses in mice. Molecules. 2011;16:2636–2645. doi: 10.3390/molecules16032636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud U., Cheng H., Applegate T. Functions of propolis as a natural feed additive in poultry. Worlds Poult. Sci. J. 2016;72:37–48. [Google Scholar]
- McKinnon K.M. Flow cytometry: an overview. Curr. Protoc. Immunol. 2018;120:5.1.1–5.1.11. doi: 10.1002/cpim.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M., Chemaly M., Dory D. DNA vaccination of poultry: the current status in 2015. Vaccine. 2016;34:202–211. doi: 10.1016/j.vaccine.2015.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mona S.I., Naglaa A.A., Ismail H.M. Effect of propolis on the immune response and meat quality in experimentally escherichia coli infected broilers. Assiut Vet. Med. J. 2021;67:101–135. [Google Scholar]
- Morakinyo A., Akindele A., Ahmed Z. Modulation of antioxidant enzymes and inflammatory cytokines: possible mechanism of anti-diabetic effect of ginger extracts. Afr. J. Biomed. Res. 2011;14:195–202. [Google Scholar]
- Mutsaers M., Blitterswijk H.v., Leven L., Kerkvliet J. Bee products properties, processing, and marketing. Agromisa Foundation; Wageningen: 2005. pp. 34–35. [Google Scholar]
- Pichlmair A. Sousa, CRe Innate Recognition of Viruses. Immunity. 2007;27 doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Reinert L.S., Harder L., Holm C.K., Iversen M.B., Horan K.A., Dagnæs-Hansen F., Ulhøi B.P., Holm T.H., Mogensen T.H., Owens T. TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J. Clin. Invest. 2012;122:1368–1376. doi: 10.1172/JCI60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifa'I M. Proc. AIP Conference Proceedings. 2017. Studies on the therapeutic effect of propolis in streptozotocin-induced diabetic mice. [Google Scholar]
- Rifa'I M., Widodo N. Significance of propolis administration for homeostasis of CD4+ CD25+ immunoregulatory T cells controlling hyperglycemia. Springerplus. 2014;3:1–8. doi: 10.1186/2193-1801-3-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G. Holo-analysis of the efficacy of Bio-Mos® in broiler nutrition. Br. Poult. Sci. 2007;48:21–26. doi: 10.1080/00071660601050755. [DOI] [PubMed] [Google Scholar]
- Sahin H.A., Ozturk E. Effects of raw propolis or water and ethanol extracts of propolis on performance, immune system, and some blood parameters of broiler breeders. Rev. Brasil. Zoot. 2018;47:1–7. [Google Scholar]
- Sahoo N., Mishra S., Swain R., Acharya A., Pattnaik S., Sethy K., Sahoo L. Effect of turmeric and ginger supplementation on immunity, antioxidant, liver enzyme activity, gut bacterial load and histopathology of broilers. Indian J. Anim. Sci. 2019;9:774–779. [Google Scholar]
- Sakure S., Negi V.D., Mitra S.K., Nandakumar K.S., Chakravortty D. Vaccine with herbal adjuvant—a better cocktail to combat the infection. Vaccine. 2008;26:3387–3388. doi: 10.1016/j.vaccine.2008.01.060. [DOI] [PubMed] [Google Scholar]
- Schulz O., Diebold S.S., Chen M., Näslund T.I., Nolte M.A., Alexopoulou L., Azuma Y.-T., Flavell R.A., Liljeström P., Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- Sforcin J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016;30:894–905. doi: 10.1002/ptr.5605. [DOI] [PubMed] [Google Scholar]
- Shams G.A., Ibrahim H.A., Ismail H.A., Ezzat R.M. Some immunomodulating effects of ginger oil and thyme oil in rabbits. Benha Vet. Med. J. 2019;36:24–32. [Google Scholar]
- Taghdisi A., Hejazi S. The effect of Zingiber officinale on the spleen tissue and antibody titer of broiler chickens. J. Morphol. Sci. 2019;36:046–050. [Google Scholar]
- Talebi A., Amani A., Pourmahmod M., Saghaei P., Rezaie R. Proc. Veterinary Research Forum. 2015. Synbiotic enhances immune responses against infectious bronchitis, infectious bursal disease, Newcastle disease and avian influenza in broiler chickens. [PMC free article] [PubMed] [Google Scholar]
- Toreti V.C., Sato H.H., Pastore G.M., Park Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin, Evid. Based Complement. Altern. Med. 2013;2013:1–13. doi: 10.1155/2013/697390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Vickers N.J. Animal communication: when I'm calling you, will you answer too? Curr. Biol. 2017;27:R713–R715. doi: 10.1016/j.cub.2017.05.064. [DOI] [PubMed] [Google Scholar]
- Wagh V.D. Propolis: a wonder bees product and its pharmacological potentials. Adv. Pharmacol. Pharm. Sci. 2013;2013 doi: 10.1155/2013/308249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Li X., Xu L., Hu Y., Zhang B., Liu J. Immunologic synergism with IL-2 and effects of cCHMIs on mRNA expression of IL-2 and IFN-γ in chicken peripheral T lymphocyte. Vaccine. 2006;24:7109–7114. doi: 10.1016/j.vaccine.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Zhang G., Yang Z., Wang Y., Yang W., Jiang S., Gai G. Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poult. Sci. 2009;88:2159–2166. doi: 10.3382/ps.2009-00165. [DOI] [PubMed] [Google Scholar]
- Zhang M., Song K., Li C., Chen Z., Ding C., Liu G. Molecular cloning of Peking duck Toll-like receptor 3 (duTLR3) gene and its responses to reovirus infection. Virol. J. 2015;12:1–8. doi: 10.1186/s12985-015-0434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]