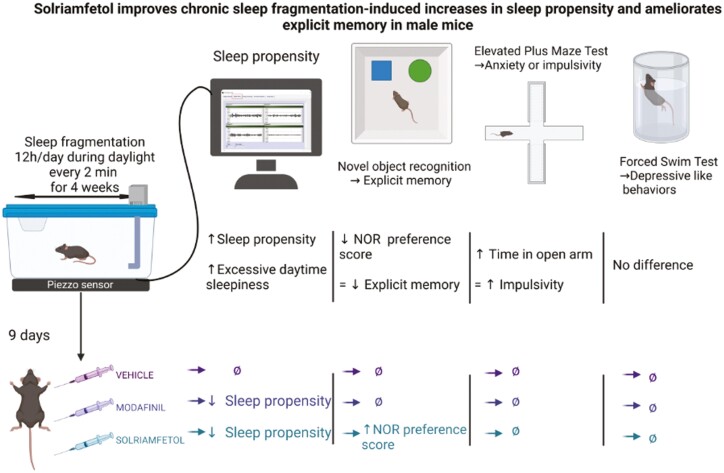
Abstract
Obstructive sleep apnea (OSA) is a highly prevalent condition characterized by episodes of partial or complete breath cessation during sleep that induces sleep fragmentation (SF). One of the frequent manifestations of OSA is the presence of excessive daytime sleepiness (EDS) associated with cognitive deficits. Solriamfetol (SOL) and modafinil (MOD) are wake-promoting agents commonly prescribed to improve wakefulness in OSA patients with EDS. This study aimed to assess the effects of SOL and MOD in a murine model of OSA characterized by periodic SF. Male C57Bl/6J mice were exposed to either control sleep (SC) or SF (mimicking OSA) during the light period (06:00 h to 18:00 h) for 4 weeks, which consistently induces sustained excessive sleepiness during the dark phase. Both groups were then randomly assigned to receive once-daily intraperitoneal injections of SOL (200 mg/kg), MOD (200 mg/kg), or vehicle for 1 week while continuing exposures to SF or SC. Sleep/wake activity and sleep propensity were assessed during the dark phase. Novel Object Recognition test, Elevated-Plus Maze Test, and Forced Swim Test were performed before and after treatment. SOL or MOD decreased sleep propensity in SF, but only SOL induced improvements in explicit memory, while MOD exhibited increased anxiety behaviors. Chronic SF, a major hallmark of OSA, induces EDS in young adult mice that is mitigated by both SOL and MOD. SOL, but not MOD, significantly improves SF-induced cognitive deficits. Increased anxiety behaviors are apparent in MOD-treated mice. Further studies aiming to elucidate the beneficial cognitive effects of SOL are warranted.
Keywords: solriamfetol, modafinil, obstructive sleep apnea, excessive daytime sleepiness, behavisor, cognition, explicit memory
Graphical Abstract
Graphical Abstract.
Statement of Significance.
Excessive sleepiness is a frequent if not universal manifestation of sleep fragmentation as occurs in obstructive sleep apnea and other sleep disorders. Novel wake-promoting agents have recently emerged, but their sleep, cognitive and behavioral effects have not been compared between them. Here, we show that solriamfetol and modafinil reduce sleepiness but induce different effects on cognitive and behavioral functions.
Introduction
Obstructive sleep apnea (OSA) affects 9%–38% of the general population and is associated with increased mortality and significant morbidities including cardiovascular and metabolic diseases, as well as cognitive and behavioral impairments [1–7]. OSA is characterized by increased upper airway resistance and collapsibility during sleep. Recurrent collapse of the airway induces both intermittent hypoxia (IH) and episodic arousals, leading to sleep fragmentation (SF) [8, 9]. Several human studies have documented significant changes in emotional and mood regulation, as well as in cognitive function in patients with OSA [10–15]. Although associated with excessive daytime sleepiness (EDS), such deficits may extend beyond the functional deficits imposed by the underlying EDS [13, 14, 16–19]. Classic EDS-induced impairments in daytime functioning included reduced attention, cognitive dysfunction, impaired performance of psychomotor tasks, reduced health-related quality of life, and increased risk of motor vehicular and workplace accidents [10, 16, 18–21]. Of note, EDS is a prominent and frequently presenting symptom of OSA and can persist in a large proportion of patients when treated with continuous positive airway pressure or other therapies [22–27].
To remediate the residual EDS, stimulants or wake-promoting drugs have been commonly used in patients with OSA [7]. Traditional stimulants based on amphetamines and methylphenidate have been extensively used, but even though they are effective, rebound hypersomnolence and several major side effects related to the drug are commonly present and limit their more extensive adoption [28, 29]. Consequently, more novel wake-promoting agents have been advocated either palliate EDS in untreated patients with OSA or as an adjuvant therapy in treated patients with OSA with residual EDS [30, 31]. Modafinil (MOD), a low-potency inhibitor of the dopamine transporter, was developed to increase wakefulness in the treatment of narcolepsy [32] and is extensively used to improve wakefulness in adult patients with excessive sleepiness (ES) associated with narcolepsy, OSA, or shiftwork disorder [1–10, 33, 34]. Some studies reported that MOD induced an improvement in working memory, cognitive control, and attention in healthy sleep-deprived volunteers, and in rodents [35]. The drug is also extensively used to treat residual EDS in patients with OSA [31, 36–41]. However, MOD use is fraught with some limitations in OSA whereby 49% of patients with EDS fail to respond to this treatment [42, 43]. Efforts to find alternative options resulted in the development of solriamfetol (SOL), a low-potency dopamine and norepinephrine reuptake inhibitor [44–46], which has now gone through several extensive clinical trials [26, 27, 46–53] and can be used either as initial therapy or as replacement therapy in patients who fail treatment or experience side effects with other wake-promoting agents or stimulants [26, 44, 45, 54–56]. However, there is only scarce information regarding head-to-head comparisons between MOD and SOL in a murine model of OSA.
In the context of OSA, although both SF and IH likely contribute in a dose-dependent manner to the cognitive deficits, it is virtually impossible to extricate their relative contributions in patients with OSA because SF and IH are concurrently present [57]. To overcome this problem, a mouse model of SF patterns mimicking the episodic arousals recorded in patients with OSA has been generated, and exhibit deficits across several standard behavioral tests in exposed animals [57–69]. Thus, the objectives of this study were primarily to assess effects of MOD and SOL on sleep and sleep propensity in mice exposed to chronic SF and as secondary outcomes evaluate the impact of such drugs on cognition (Novel Object Recognition test; NOR), depression (Forced Swim Test; FST), and anxiety (Elevated Plus Maze Test; EPMT).
Methods
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Missouri (IACUC 9720).
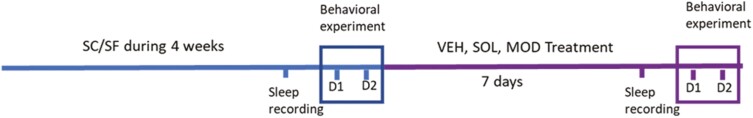
One hundred and thirty-five male C57BL/6 J mice (8-week-old) were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were housed in a controlled environment with 12 h light-dark cycles (06:00 h to 18:00 h) at constant temperature (24 ± 0.2°C) with ad libitum access to food (normal chow) and water. All animals were allowed to recover and fully acclimate within the animal care facility for at least 7 days upon arrival. Animals were then randomized into two different groups, namely SF or sleep control (SC). Each exposure group and corresponding controls were randomly assigned to receive SOL, MOD, or vehicle (VEH) at the beginning of the dark phase upon completion of the daily SF exposures conducted during daylight for 9 days.
SF
The SF device used to induce sleep disruption has been previously described [58, 60, 70–72]. Briefly, mice were housed in custom-designed cages containing the SF apparatus (Model 80391; Lafayette Instruments, Lafayette, IN). Sleep arousals were induced by a mechanical horizontal bar sweeping just above the cage floor from one side to the other side of a standard mouse laboratory cage, which was operated by a nearly silent motorized system. To apply SF patterns that mimic OSA, 2-minute intervals between each sweep (i.e. 30 events/h) were applied during the murine rest period (06:00 h to 18:00 h) for a total period of 5 weeks (4 weeks before treatment followed by 9 days during the drug treatment). Between behavioral tests, animals were maintained in the SF or SC conditions.
Treatment
Animals received once-daily intraperitoneal (i.p.) injections of SOL (200 mg/kg), MOD (200 mg/kg), or VEH (5 mL/kg) at the beginning of the dark phase (6:00 pm) for 9 days while continuing to undergo either the SF or SC exposure. MOD (TRC; Toronto, ON, Canada) was freshly dissolved in dimethyl sulfoxide (DMSO), 10%, Tween 20 15%, and phosphate buffered saline (PBS) 75% (V/V/V) [73]. SOL (provided by Jazz Pharmaceuticals; Palo Alto, CA) was dissolved in PBS. DMSO was purchased from ATCC (Manassas, VA), and Tween 20 and PBS from Thermo Fischer Scientific (Waltham, MA). PBS was used as control treatment (VEH). The treatments were injected at 18:00 h at the end of the SF and before the beginning of the dark (active) phase of the mice.
Behavioral testing
Sleep recordings.
Sleep/wake activity was monitored using a validated, computerized piezoelectric system (PiezoSleep; Signal Solutions, Lexington, KY)). This noninvasive system, automatically scores sleep and waking states in mice (SleepStat; Signal Solutions, Lexington, KY) [74–78]. Briefly, a piezoelectric film able to detect pressure variations is placed under the cage floor. For all sleeping postures of the mouse, pressure variations from breathing are detected. Sleep states are characterized by quasi-periodic signals with low variations in amplitude, whereas wakefulness and rest states are characterized by irregular transient and high amplitude pressure variations corresponding to body movements and weight shifting. Signal features sensitive to the differences between the sleep and wake states are extracted from short-time pressure signal segments, and classification is automatically performed every 2 s. Sleep/wake activity was recorded and scored for each treatment.
The system records pressure changes using a piezoelectric film, the duration and intensity of which are automatically scored by computer algorithms and classified as wake or sleep states. The Piezo system exhibits 90% accuracy compared to electroencephalogram/electromyogram-based sleep acquisition methodologies and scoring approaches [78] and have been previously validated against standard polysomnographic methods [9]. Data collected were binned over 5 min using a rolling average of percentage sleep, and by individual wake bout length from which the mean of bout lengths was calculated. Wake bouts were defined by any contiguous wake pattern that remained uninterrupted by sleep periods for more than 30 s. In addition, bout length counts were only initiated when a 30-second interval contained greater than 50% wake and were terminated when a 30-second interval contained less than 50% wake [79].
Behavioral tests.
Behavioral experiments were performed by operators who were blinded to the various treatments and conducted by three observers (Figure 1). The behavioral test battery consisted of the NOR test, EPMT, and FST. Throughout the duration of the experiments, the experimental set-ups were cleaned with ethanol 70% to prevent odor cues. All mazes were purchased at Maze Engineers (Cambridge, MA). EPMT and NOR were recorded from a vertical point of view with a video camera suspended above the experimental area. For the FST, a horizontal point of view was selected. All experiments were interfaced with a video tracking system (Noldus Ethovision XT16 Software, Leesburg, VA). Behavioral tests were conducted after 4 weeks of SF exposures and repeated after 1 week of SOL, MOD, or VEH treatment during the dark phase immediately upon completion of the daily SF exposures.
Figure 1.
Schematic representation of the experimental design. All experiments were conducted in the same order, but the order of the experimental groups was randomized.
NOR test.
The NOR test was based on the tendency for mice to explore novelty as described previously [64, 72, 80], and is used to measure explicit memory based on the preference for novelty. The task was performed in a blue opaque open-field plastic chamber (40L × 40W × 30H cm per chamber). For each trial, mice were placed at the center of the arena. Two habituation trials were conducted for each animal, which consisted of a 10-minute exploration period in an empty arena. On the second day, identically shaped blocks were used as familiar objects and different colors were used as unfamiliar objects. Each mouse was exposed to two tower blocks in the same color (Supplemental Figure 1A). Objects were placed 5 cm from the side walls in the center of the arena. The mice were allowed to freely explore the objects for 5 min. One hour later, one object was replaced by a novel object. Different objects in different colors, shapes, and sizes were used as novel objects (Supplemental Figure 1B) The mice were allowed to freely explore the objects for 5 min. Positive exploration by the mouse was defined as touching the object with the nose. The time spent exploring the objects was analyzed and quantified by the tracking software and was supervised by a blinded operator. The total exploration time for both objects was recorded. Results were reported as preference score using the following formula [72, 80, 81]:
The ratio between the time spent to explore new object and the familiar object was also analyzed.
To avoid bias, we used a set of different novel objects, with the new object being placed randomly either on the left or on the right side of the arena, and the choice of familiar versus novel object was also randomly allocated. The objects were different between the experiments before and after drug treatments to avoid any residual memory biases. Mice who did not explore objects were removed from the experiment. Animals were considered to have an explicit preference for novelty if their preference score was > 50%.
EPMT.
The EPMT is the most frequently utilized animal model for assessing anxiety-like behaviors [67, 82]. This task exploits the counterbalance between two innate rodent behaviors, the avoidance of open space exposure and the tendency to explore novel environments as described previously [72, 83]. Fearful mice spend less time in the open arms of EPMT, and conversely, when more time is spent in open arm this can be reflect a less fearing animal with hyperactivity or high impulsivity [63, 72, 83]. The apparatus consists of an elevated cross (56 cm above the floor) formed by two open arms and two closed arms radiating from a central platform to form a plus-sign. Animals were placed in the central area facing one open arm and allowed to explore the maze for 5 min.
FST.
The FST is a behavioral test used for evaluation of depressive-like states in rodent. The task was performed in transparent cylindrical containers with a depth of 15 cm of water at 25 ± 2°C as described previously [72]. Mice were individually placed and forced to swim in the cylinder for a total duration of 6 min. The immobility time, defined as the absence of escape-oriented behaviors, was scored for a total period of 4 min (the last 4 min) [60]. Each mouse was deemed as being immobile when it made only movements necessary to keep its head above water. Not moving was defined as the duration of time when the velocity of mouse motion decreased below 2 cm/s [84]. At the end of the experiment, mice were dried and placed under a red heat lamp until their fur was completely dry. Animals exhibiting panic behavior, that is, excessive swimming for the first few minutes and then not being able to keep their heads above water, were excluded from the experiments.
Statistical analysis
Statistical analysis was performed using Prism 9.2 for windows (GraphPad Software, San Diego, CA, www.graphpad.com). Two-way ANOVA with Sidak post hoc tests were used to compare treatments in SF and SC groups for sleep studies. Mixed effect model with Sidak and Tukey post hoc tests were used for unpaired and paired analyses. The data were expressed as mean ± SD. A two-tailed p-value < 0.05 was considered statistically significant.
Results
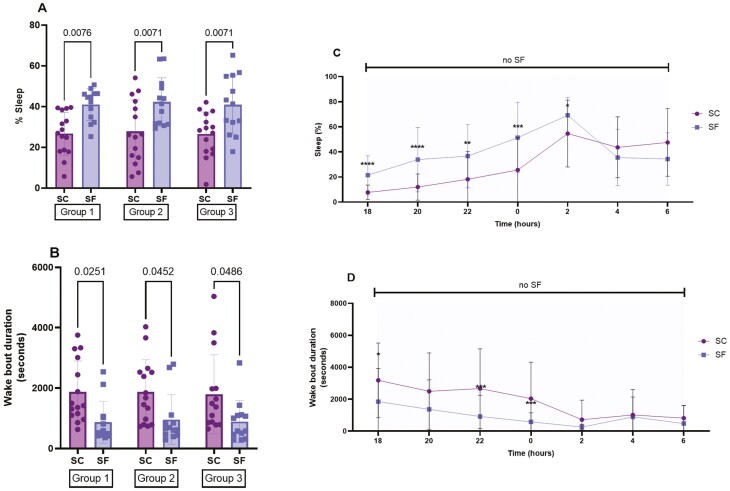
After 4 weeks, SF significantly increased sleep percentages during the dark period when compared to SC in all randomized groups (group 1: 40.9 ± 7.7% vs 26.6 ± 10.5%, p = 0.0076 − group 2: 42.3 ± 11.9 vs 27.8 ± 15.5, p = 0.0071 − group 3: 40.9 ± 14.4 vs 26.4 ± 10.9, p = 0.0071) (Figure 2A) and significantly reduced wake bout lengths in all randomized groups (group 1: SF: 869 ± 693 s vs SC: 1867 ± 1027 s, p = 0.0251 − group 2: SF: 959 ± 818 s vs SC: 1876 ± 959 s, p = 0.0452 − group 3: SF: 889 ± 695 s vs SC: 1795 ± 1310 s, p = 0.0486) (Figure 2B). The changes in bi-hourly sleep percentages and wake bout durations induced by SF indicated that ES occurred preferentially during the first half of the dark phase (Figure 2, C and D).
Figure 2.
Sleep patterns during the dark phase after 4 weeks of SF during the light phase. (A) Sleep percentage in the three randomized groups before injection of treatment. (B) Wake bout durations in the three groups before injection of treatment. (C and D) Bihourly sleep percentages and wake bout durations. SC corresponds to control mice not subjected to SF. Data are presented as mean ± SD, n = 13–15/group for A and B and n = 40–45/group for C and D, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
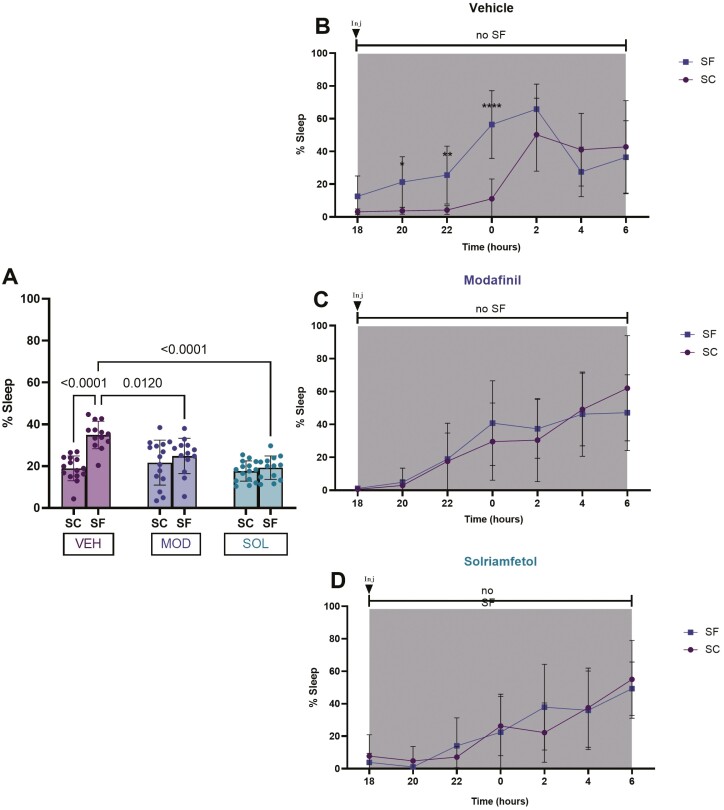
SF in VEH-treated mice significantly increased the percentage of time spent in sleep during the dark period when compared to SC (35 ± 7% vs 18.9 ± 7%, p < 0.0001). Sleep percentages during the dark phase were significantly reduced in mice treated either with SOL or MOD (SOL: 19 ± 6%, MOD: 24 ± 8%) when compared to VEH-treated animals (35 ± 7%, vs SOL p < 0.0001; vs MOD p = 0.012) (Figure 3A). Bi-hourly sleep percentages showed evidence of improved wakefulness after SOL or MOD treatments and further indicated that increased sleep propensity occurred mostly during the first half of the dark phase (Figure 3B–D).
Figure 3.
Sleep percentage during the dark (active) phase. Mice were subjected to 4 weeks of SF during the light (rest) phase of the illumination cycle and then treated with solriamfetol (200 mg/kg, i.p.), modafinil (200 mg/kg, i.p.), or vehicle (5 mL/kg, i.p.) for 9 days. SF was continued during the treatment. SC represent values of control mice not subjected to SF. (A) Sleep percentage during the dark phase. (B–D) Bihourly sleep percentages across the dark phase. Data are presented as mean ± SD (n = 12–15/experimental group), *p < 0.05, **p < 0.01, ****p < 0.0001.
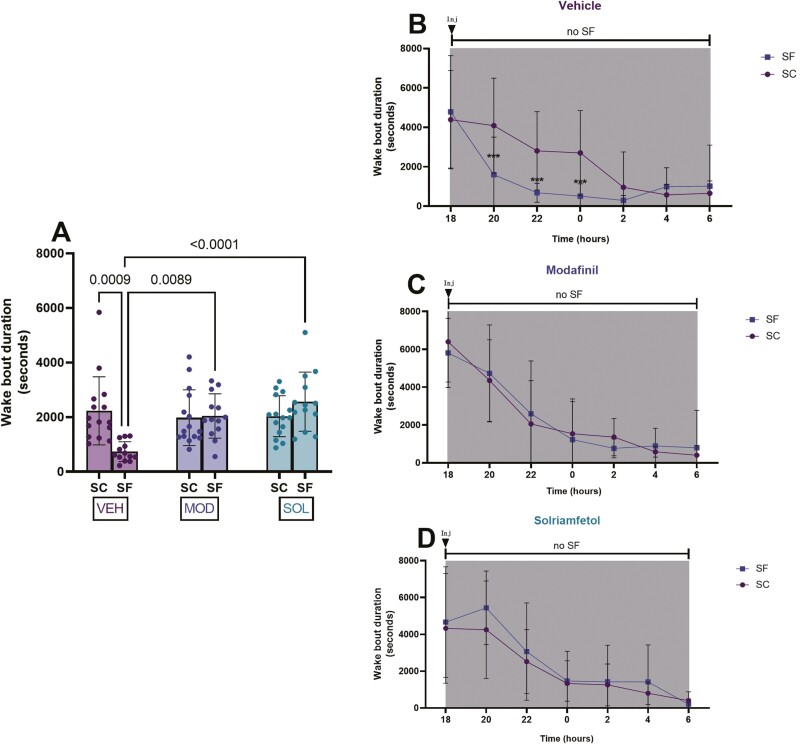
VEH-treated mice exposed to SF exhibited significantly reduced wake bout lengths (SF: 733 ± 364 s vs SC: 2227 ± 1246 s, p = 0.0009). Wake bout lengths were significantly increased in both treatment groups (SOL: 2564 ± 1080 s, MOD: 2041 ± 811 s) when compared to VEH-treated group values (733 ± 364 s, vs SOL p < 0.0001, vs MOD p = 0.0089) (Figure 4A). Bi-hourly wake bout durations showed evidence of improved wakefulness after both treatments and indicated that increases in sleep propensity occurred mostly during the first half of the dark phase (Figure 4B–D).
Figure 4.
Wake bout durations during the dark (active) phase. Mice were subjected to 4 weeks of SF during the light (rest) phase of the illumination cycle and then treated with solriamfetol (200 mg/kg, i.p,), modafinil (200 mg/kg, i.p.), or vehicle (5 mL/kg, i.p.) for 9 days. SF was continued during the treatment. SC reflect measures in control mice not subjected to SF. (A) Wake bout duration during the dark. (B–D) Bihourly wake bout durations during the dark phase. Data are presented as mean ± SD (n = 12–15/experimental group), *p < 0.05, **p < 0.01, ****p < 0.0001.
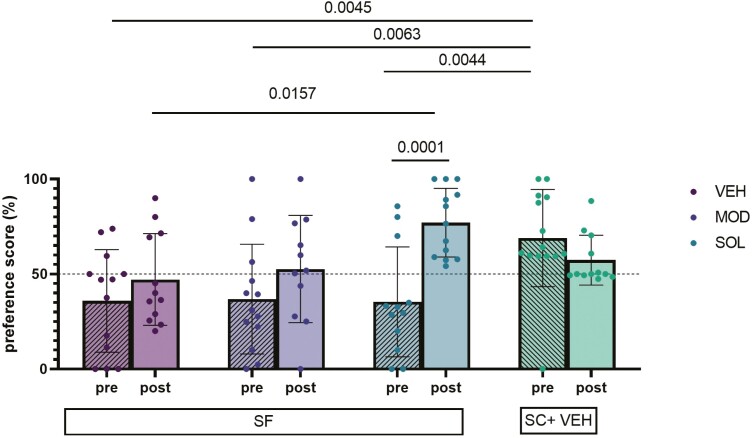
Before treatment, the NOR preference scores for all groups of mice exposed to SF were significantly lower when compared to SC (69 ± 26 % vs VEH: 36 ± 27, p = 0.0045; vs MOD: 37 ± 28%, p = 0.0063; vs SOL: 35 ± 28% p = 0.0044). Intriguingly, after treatment NOR preference scores were significant increased only for SOL-treated mice (77 ± 18%) when compared to NOR findings in the same mice before treatment (35 ± 28%, p = 0.0001). NOR preference scores for mice treated with SOL were also significantly higher than VEH-treated mice (47 ± 24%, p = 0.0157) (Figure 5). No differences in NOR scores emerged for MOD treatment group. We used also the ratio of time spent exploring the novel objects and the familiar objects to confirm our findings (Supplemental Data #2). All treatments in the SC condition induced no modifications in the NOR scores (Supplemental Data 3A).
Figure 5.
Preference score performance in the NOR test. Mice were subjected to 4 weeks of SF during the light (rest) phase of the illumination cycle and then treated with solriamfetol (200 mg/kg, i.p.), modafinil (200 mg/kg, i.p.), or vehicle (5 mL/kg, i.p.) for 9 days. SF was continued during the treatment. SC represent values of control mice not subjected to SF treated with VEH. The NOR test was conducted twice (before and after 1-week treatment) during the light phase. Mixed-effects analysis with Sidak and Tukey post hoc tests were used for unpaired and paired analyses. Data are presented as mean ± SD (n = 11–14/experimental group).
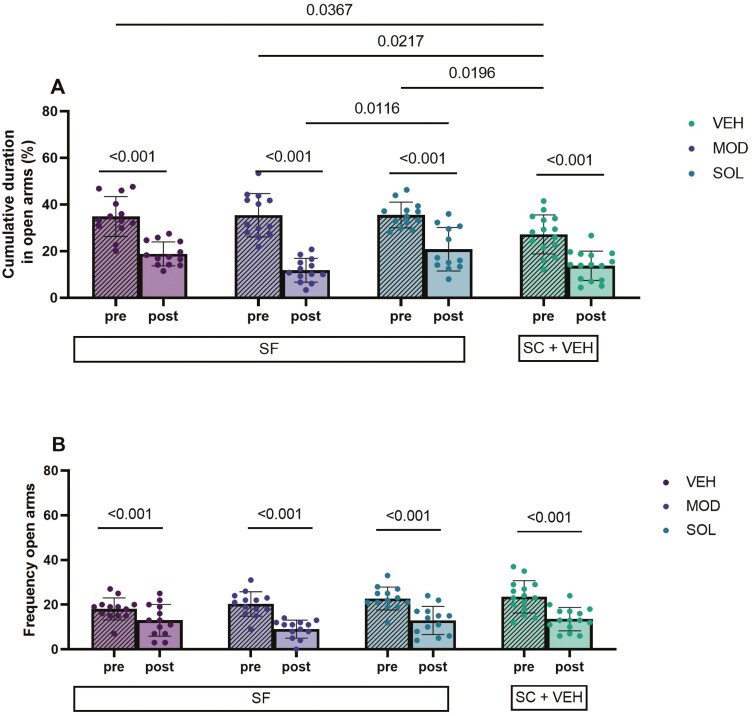
Before treatment, the time spent in open arms of the EPMT for all groups of mice exposed to SF was significantly higher when compared to SC (27 ± 8% vs VEH: 35 ± 9, p = 0.0367; vs MOD: 35 ± 9, p = 0.0217; vs SOL: 36 ± 5, p = 0.0196). The time spent in open arms was significantly reduced in all groups after treatment. However, animals subjected to SF spent more time in the open arms after SOL treatment in comparison with MOD-treated mice (20 ± 3% vs 12 ± 1%, p = 0.012) (Figure 6A).
Figure 6.
Cumulative durations (A) and frequencies (B) in the EPMT open arms. Mice were subjected to 4 weeks of sleep fragmentation (SF) during the light (rest) phase of the illumination cycle and then treated with solriamfetol (200 mg/kg, i.p.), modafinil (200 mg/kg, i.p.), or vehicle (5 mL/kg, i.p.) for 9 days. SF was continued during the treatment. SC represent values of control mice not subjected to SF treated with VEH. The EPMT was conducted twice (before and after treatment) during the light phase. Mixed-effects analysis with Sidak and Tukey post hoc tests were used for unpaired and paired analysis. Data are presented as mean ± SD (n = 12–15/experimental group).
Regarding the frequency in open arms, no differences emerged between treatments. All conditions exhibited a reduction of the frequency in open arms (Figure 6B, p < 0.001) and such findings are also observed in SC-exposed animals (Supplemental Data 3C, p < 0.001).
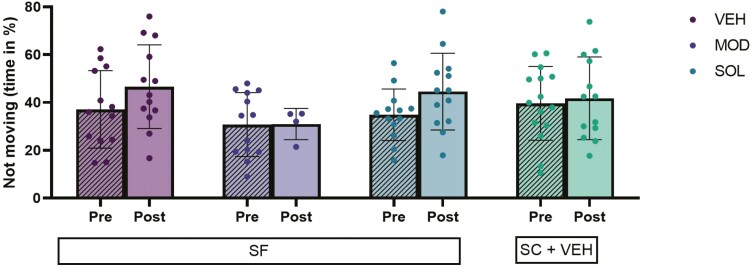
In the context of FST, neither SF exposures nor treatments were associated with significant differences in either duration of immobility when compared to SC or to the pretreatment responses (Figure 7). No differences emerged in SC mice treated with SOL, MOD, or VEH (Supplemental Data 3C). In animals exposed to SF treated with MOD we had to exclude several mice from the experiment due to the emergence of panic behaviors.
Figure 7.
Time spent immobile during the FST. Mice were subjected to 4 weeks of SF during the light (rest) phase of the illumination cycle and then treated with solriamfetol (200 mg/kg, i.p.), modafinil (200 mg/kg, i.p.), or vehicle (5 mL/kg, i.p.) for 9 days. SF was continued during the treatment. SC displays values of control mice not subjected to SF treated with VEH. The FST was conducted twice (before and after treatment) during the light phase. Mixed-effects analysis with Sidak and Tukey post hoc tests were used for unpaired and paired analysis. Data are presented as mean ± SD (n = 14–15/experimental group).
Discussion
This study shows and further corroborates the previous findings that chronic SF simulating the episodic arousals in the context of OSA induces substantial increases in sleep propensity during the dark phase in mice in the absence of sleep curtailment [58, 59, 70] Furthermore, and as previously reported [58, 59, 70], chronic SF also manifested as explicit memory declines and changes in behavioral anxiety. However, the major findings reported herein revolve around the relatively similar improvements in sleep propensity elicited by treatment with either SOL or MOD, but the discrepant effects of these two drugs on cognitive and behavioral functions.
The presence of EDS, which constitutes the most common symptom among patients with OSA, results not only in the inherent difficulty of remaining awake during the day, but also imposes additional deleterious consequences affecting multiple end-organ systems [58, 85–89], and more particularly is fraught with both cognitive and behavioral dysfunction, even after treatment is initiated and adherently implemented. Thus, improved interventions aimed at OSA-induced EDS and residual EDS that also improve the associated cognitive impairments is an important public health issue, particularly considering the overall aging trends in the population and the anticipated increase in the prevalence of OSA, indirectly resulting in an increase in the number of patients with cognitive decline [90–92].
The SF model implemented in our current study is a well-validated approach that recapitulates the recurring arousals experienced by patients with OSA, and also faithfully and reproducibly leads to the emergence of increased and long-lasting sleep propensity in mice. Furthermore, we now show that long-term SF also induces cognitive impairments in the context of the NOR test, suggesting the emergence of dysfunctional ability to generate explicit memories [72]. In parallel, increased impulsivity is also apparent in this rodent model [72], such that the current observations before initiation of any drug treatment intervention are congruent with previous findings involving implementation of several models of sleep disorders in rodents [58–60, 72, 93–100].
The reduction of percentages of time spent in sleep state and the reciprocal increases in the wake bout lengths after treatment with either MOD or SOD in SF-exposed mice strongly suggest that both drugs led to reductions in sleep propensity, which for the sake of convenience we will designate as EDS. The reductions in EDS were anticipated since they gave been previously demonstrated using alternative rodent models of EDS and have also been extensively explored in clinical trials [7, 37, 42, 44, 46, 53, 55, 101, 102]. Notwithstanding, although both SOL and MOD attenuated EDS, the differences were more pronounced after treatment with SOL, suggesting that SOL may have a bigger impact on improving wakefulness and reducing sleepiness than MOD, as previously mentioned in reviews and analysis of extant clinical studies [7, 28, 44, 52]. In contrast with such indirect comparisons, the present study performed a head-to-head comparison of SOL and MOD effects, and to the best of our knowledge, this is the first study to directly examine this issue. However, it remains unclear whether the nature and duration of the sleep perturbation leading to EDS, for example, sleep deprivation (SD) versus fragmentation versus IH plays a role in the magnitude of the EDS reversal or reduction response to either SOL or MOD, and whether specific and predictable subgroups of patients with OSA with EDS will more likely respond to one of the wake-promoting drugs compared to the other.
Numerous publications have shown improvements in quality of life as well as reduced risk of motor vehicular and workplace accidents in patients with OSA after treatment with wake-promoting agents [7, 26, 41, 44, 52, 54]. Additional studies also showed increases in attention span and improvements in working memory task performance, work productivity, reaction times, logical reasoning, mental addition exercises, and in overall vigilance when evaluated in healthy participants, healthy participants exposed to SD, and in patients with OSA [7, 26, 41, 103–110]. However, there is only a paucity of studies on cognitive functions after treatment with SOL or MOD in either patients with OSA or in rodents. We selected the NOR test because it can be repeated with due precautions without generating a substantial learning bias [80, 111], making the test a valid approach in pharmacological studies [64, 80]. Our a priori assumptions posited that the degree of improvement in NOR performance, if any, would be associated with parallel reductions in EDS induced by the drugs. Surprisingly, we found that SOL, but not MOD, significantly improved SF-induced NOR deficits despite the fact that both drugs reduced EDS when compared to VEH-treated mice exposed to chronic SF. As such, there were no discernible correlations between the degree of improvement in EDS and the changes in NOR performance. Of note, our findings, although not comparable to existing previous studies, contradict these studies which indicated that MOD ameliorates cognitive impairments induced by sleep disturbance [39, 40, 102, 112–114]. However, these publications explored the effects of MOD in rodents exposed to different sleep perturbations that incorporated substantial stress, for example, separation, REM SD, along with a variety of learning and memory test paradigms, such as NOR [39, 114], Morris Water Maze [40, 102, 112, 113], and T-Maze [113] after or during acute SD. Of note, other publications have shown beneficial effects of MOD on control rodents who have not been exposed to any sleep manipulation [104, 115–117]. However, neither SOL nor MOD elicited any behavioral changes in the control mice (Supplemental Data 2 and 3). This can be interpreted as indicative that the wake-promoting agents do not improve cognition in otherwise unaffected mice, but have an effect in fragmented mice. Based on aforementioned findings, more extensive evaluation of the beneficial effects of SOL on explicit memory performance and the underlying mechanisms of such improvements seem warranted.
Symptoms of both anxiety and depression are prevalent among patients with OSA [19, 118]. In the current study, SF promoted increases in impulsivity similar to those previously described in rodents exposed to a variety of models of sleep disorders [72, 99, 100, 119, 120]. Currently, no behavioral tests in rodents that putatively measure anxiety allow for clear separation of impulsivity from anxious behaviors [72, 99]. Notwithstanding, all conditions (treatment and controls) showed a decrease in the time spent in the open arms in the second iteration of the EPMT test, which is likely the result of the repetition of the test within one week from each other. Indeed, the EPMT exploits the counterbalance between two innate rodent behaviors, the inherent tendency of avoidance of open space exposures and the drive to explore novel environments [72, 83]. In the circumstance of our experimental design, the changes are not related to any novelty, and the effect in all groups is the same. However, SOL and MOD appear to exert opposite effects. SOL-treated mice spent more time in the open arms in comparison to the MOD-treated mice. Thus, we interpret these findings as reflecting some degree of increased anxiety induced by MOD with opposite effects of SOL in this regard. MOD has heterogeneous effects on anxiety in humans and anxiety-like behaviors in animal models [121–123]. However, although SOL and MOD differ from each other, they do not show significant differences with SF animals treated with VEH, suggesting that SOL may have an anxiolytic effect. Whereby some studies have shown anxiogenic effects in humans, while others have shown anxiolytic effects. It will be important to further examine whether such phenomena emerge in the context of clinical settings for SOL. In one of the trials, 7% reported anxiety as an adverse effect when treated with SOL [44].
During the FST, a test based on the natural tendency of rodents to escape from water [124] no evidence of depressive-like behaviors emerged following SF as previously described [72, 97] and no changes occurred after the various treatments. However, we observed, albeit inconsistently, that several animals treated with MOD had to be removed from the FST apparatus because their swimming patterns were “panic like” and they could not sustain their heads above water. None of the mice treated with SOL exhibited this behavior. In addition, some studies have indicated that MOD may have different antidepressant effects depending on the stress and concentration of the treatment [115, 125]. However, few publications on the effects of MOD during FST in rodents are available, and such studies concern either healthy or stressed animals. In these studies, decreases in the depressive like behaviors occurred in MOD-treated mice [126, 127]. Such effects of MOD did not occur in the present study. To our knowledge, there are no publications focused on the effects of SOL during FST.
Before we conclude, several limitations of the present study deserve mention. We treated animals with a single daily dose and examined only one duration of treatment (9 days). It is therefore necessary to expand such observations to multiple daily dosages and to variable durations of treatment to generate a more comprehensive therapeutic profile. Indeed, in human studies, the effects of wake-promoting agents may vary depending on the dose and duration of treatment [41, 44, 115]. Moreover, patients with OSA with residual ES may be difficult to treat and may need a combination of different drugs [7, 28, 42]. For obvious reasons related to the large number of mice that would be required, extending our experiments to include different dosages, varying duration, and combination of treatments was precluded. In addition, we evaluated only male mice who were also relatively young when exposed to SF. Consequently, the effects of such exposures in female, aging, or in very young mice remain unexplored.
Conclusion
Chronic SF, a major hallmark feature of OSA, induces EDS in young adult mice that is mitigated by SOL and MOD, with SOL displaying comparable and possibly improved efficacy to MOD as a wake-promoting agent, at least when EDS is prompted by SF. SF induces cognitive NOR impairments in mice along with reduced anxiety behaviors. SOL, but not MOD, significantly improves SF-induced NOR deficits but seems to have no effects on EPMT or FST performance in comparison to SF-VEH-treated mice.
Supplementary Material
Contributor Information
Clementine Puech, Child Health Research Institute, Department of Child Health, University of Missouri School of Medicine, Columbia, MO, USA.
Mohammad Badran, Child Health Research Institute, Department of Child Health, University of Missouri School of Medicine, Columbia, MO, USA.
Max B Barrow, Undergraduate Student Research Program, University of Missouri, Columbia, MO, USA.
Alexandra R Runion, Undergraduate Student Research Program, University of Missouri, Columbia, MO, USA.
David Gozal, Child Health Research Institute, Department of Child Health, University of Missouri School of Medicine, Columbia, MO, USA; Department of Medical Pharmacology and Physiology, University of Missouri School of Medicine, Columbia, MO, USA.
Funding
This study was supported by an investigator-initiated grant from Jazz Pharmaceuticals. DG is also supported in part by NIH grant AG061824, a Tier 2 grant, and a TRIUMPH grant from the University of Missouri, and by the Leda J. Sears Foundation.
Disclosure Statement
The sponsor has no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Financial disclosure: None to Disclose
Nonfinancial disclosure: None to Declare
References
- 1. Stranks EK, et al. The cognitive effects of obstructive sleep apnea: an updated meta-analysis. Arch Clin Neuropsychol. 2016;31(2):186–193. doi: 10.1093/arclin/acv087. [DOI] [PubMed] [Google Scholar]
- 2. Krysta K, et al. Cognitive deficits in adults with obstructive sleep apnea compared to children and adolescents. J Neural Transm. 2017;124(Suppl 1):187–201. doi: 10.1007/s00702-015-1501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lajoie AC, et al. Obstructive sleep apnea in neurodegenerative disorders: current evidence in support of benefit from sleep apnea treatment. J Clin Med. 2020;9(2):E297. doi: 10.3390/jcm9020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Senaratna CV, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 5. Gileles-Hillel A, et al. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat Rev Endocrinol. 2016;12(5):290–298. doi: 10.1038/nrendo.2016.22. [DOI] [PubMed] [Google Scholar]
- 6. Yeghiazarians Y, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the american heart association. Circulation. 2021;144(3):e56–e67. doi: 10.1161/CIR.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 7. Abad VC. Profile of solriamfetol in the management of excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea: focus on patient selection and perspectives. Nat Sci Sleep. 2021;13:75–91. doi: 10.2147/NSS.S245020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Almendros I, et al. Obesity, sleep apnea, and cancer. Int J Obes. 2020;44(8):1653–1667. doi: 10.1038/s41366-020-0549-z. [DOI] [PubMed] [Google Scholar]
- 9. Badran M, et al. Fecal microbiota transplantation from mice exposed to chronic intermittent hypoxia elicits sleep disturbances in naïve mice. Exp Neurol. 2020;334:113439. doi: 10.1016/j.expneurol.2020.113439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirsch Allen AJM, et al. The effect of OSA on work disability and work-related injuries. Chest. 2015;147(5):1422–1428. doi: 10.1378/chest.14-1949. [DOI] [PubMed] [Google Scholar]
- 11. Bucks RS, et al. Reviewing the relationship between OSA and cognition: where do we go from here? Respirol Carlton Vic. 2017;22(7):1253–1261. doi: 10.1111/resp.13140. [DOI] [PubMed] [Google Scholar]
- 12. Naegele B, et al. Cognitive executive dysfunction in patients with obstructive sleep apnea syndrome (OSAS) after CPAP treatment. Sleep. 1998;21(4):392–397. doi: 10.1093/sleep/21.4.392. [DOI] [PubMed] [Google Scholar]
- 13. Sforza E, et al. Sleep apnea syndrome and cognition. Front Neurol. 2012;3:87. doi: 10.3389/fneur.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vanek J, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–58. doi: 10.1016/j.sleep.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 15. Lal C, et al. The link between obstructive sleep apnea and neurocognitive impairment: an official American Thoracic Society workshop report. Ann Am Thorac Soc. 2022;19(8):1245–1256. doi: 10.1513/AnnalsATS.202205-380ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waldman LT, et al. Understanding the burden of illness of excessive daytime sleepiness associated with obstructive sleep apnea: a qualitative study. Health Qual Life Outcomes. 2020;18(1):128. doi: 10.1186/s12955-020-01382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stepnowsky C, et al. Comorbidities, health-related quality of life, and work productivity among people with obstructive sleep apnea with excessive sleepiness: findings from the 2016 US National Health and Wellness Survey. J Clin Sleep Med. 2019;15(2):235–243. doi: 10.5664/jcsm.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iannella G, et al. Quality of life and excessive daytime sleepiness in adults with obstructive sleep apnea who are treated with multilevel surgery or adherent to continuous positive airway pressure. J Clin Med. 2022;11(9):2375. doi: 10.3390/jcm11092375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garbarino S, et al. Association of anxiety and depression in obstructive sleep apnea patients: a systematic review and meta-analysis. Behav Sleep Med. 2020;18(1):35–57. doi: 10.1080/15402002.2018.1545649. [DOI] [PubMed] [Google Scholar]
- 20. Imen T, et al. Daytime sleepiness and quality of life in patients with obstructive sleep apnea. Eur Respir J. 2021;58(Suppl 65):PA382. doi: 10.1183/13993003.congress-2021.PA382. [DOI] [Google Scholar]
- 21. Sabil A, et al. Risk factors for sleepiness at the wheel and sleep-related car accidents among patients with obstructive sleep apnea: data from the French Pays de la Loire sleep cohort. Nat Sci Sleep. 2021;13:1737–1746. doi: 10.2147/NSS.S328774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pépin JL, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J. 2009;33(5):1062–1067. doi: 10.1183/09031936.00016808. [DOI] [PubMed] [Google Scholar]
- 23. Gasa M, et al.; Scientific Council of the Sleep Registry of the French Federation of Pneumology-FFP. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res. 2013;22(4):389–397. doi: 10.1111/jsr.12039. [DOI] [PubMed] [Google Scholar]
- 24. He K, et al. Sleep-disordered breathing and excessive daytime sleepiness. Sleep Med Clin. 2017;12(3):369–382. doi: 10.1016/j.jsmc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 25. Dongol EM, et al. Residual excessive sleepiness in patients with obstructive sleep apnea on treatment with continuous positive airway pressure. Curr Opin Pulm Med. 2016;22(6):589–594. doi: 10.1097/MCP.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 26. Weaver TE, et al. Long-term effects of solriamfetol on quality of life and work productivity in participants with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea. J Clin Sleep Med. 2021;17(10):1995–2007. doi: 10.5664/jcsm.9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Craig S, et al. Investigation and management of residual sleepiness in CPAP-treated patients with obstructive sleep apnoea: the European view. Eur Respir Rev. 2022;31(164):210230. doi: 10.1183/16000617.0230-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abad VC, et al. Solriamfetol for the treatment of daytime sleepiness in obstructive sleep apnea. Expert Rev Respir Med. 2018;12(12):1007–1019. doi: 10.1080/17476348.2018.1541742. [DOI] [PubMed] [Google Scholar]
- 29. Gandhi KD, et al. Excessive daytime sleepiness: a clinical review. Mayo Clin Proc. 2021;96(5):1288–1301. doi: 10.1016/j.mayocp.2020.08.033. [DOI] [PubMed] [Google Scholar]
- 30. Avellar ABCC, et al. Pharmacotherapy for residual excessive sleepiness and cognition in CPAP-treated patients with obstructive sleep apnea syndrome: a systematic review and meta-analysis. Sleep Med Rev. 2016;30:97–107. doi: 10.1016/j.smrv.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 31. Sukhal S, et al. Effect of wakefulness-promoting agents on sleepiness in patients with sleep apnea treated with CPAP: a meta-analysis. J Clin Sleep Med. 2015;11(10):1179–1186. doi: 10.5664/jcsm.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Madras BK, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319(2):561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 33. Maski K, et al. Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 1895;17(9):1945. doi: 10.5664/jcsm.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maski K, et al. Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 1881;17(9):1893. doi: 10.5664/jcsm.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sahakian BJ, et al. Pharmacological cognitive enhancement: treatment of neuropsychiatric disorders and lifestyle use by healthy people. Lancet Psychiatry. 2015;2(4):357–362. doi: 10.1016/S2215-0366(15)00004-8. [DOI] [PubMed] [Google Scholar]
- 36. Inoue Y, et al. Findings of the maintenance of wakefulness test and its relationship with response to modafinil therapy for residual excessive daytime sleepiness in obstructive sleep apnea patients adequately treated with nasal continuous positive airway pressure. Sleep Med. 2016;27-28:45–48. doi: 10.1016/j.sleep.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 37. Inoue Y, et al. Efficacy and safety of adjunctive modafinil treatment on residual excessive daytime sleepiness among nasal continuous positive airway pressure-treated Japanese patients with obstructive sleep apnea syndrome: a double-blind placebo-controlled study. J Clin Sleep Med. 2013;9(8):751–757. doi: 10.5664/jcsm.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chapman JL, et al. Residual daytime sleepiness in obstructive sleep apnea after continuous positive airway pressure optimization: causes and management. Sleep Med Clin. 2016;11(3):353–363. doi: 10.1016/j.jsmc.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 39. Garcia VA, et al. Modafinil ameliorates cognitive deficits induced by maternal separation and sleep deprivation. Behav Brain Res. 2013;253:274–279. doi: 10.1016/j.bbr.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 40. Xiong X, et al. Modafinil reduces neuronal pyroptosis and cognitive decline after sleep deprivation. Front Neurosci. 2022;16:816752. doi: 10.3389/fnins.2022.816752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Black JE, et al. Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep. 2005;28(4):464–471. doi: 10.1093/sleep/28.4.464. [DOI] [PubMed] [Google Scholar]
- 42. Pack AI, et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164(9):1675–1681. doi: 10.1164/ajrccm.164.9.2103032. [DOI] [PubMed] [Google Scholar]
- 43. Black JE, et al. The long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension study. J Clin Sleep Med. 2010;6(5):458–466. [PMC free article] [PubMed] [Google Scholar]
- 44. Schweitzer PK, et al.; TONES 3 Study Investigators. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3). a randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421–1431. doi: 10.1164/rccm.201806-1100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thorpy MJ. Recently approved and upcoming treatments for narcolepsy. CNS Drugs. 2020;34(1):9–27. doi: 10.1007/s40263-019-00689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Krystal AD, et al. Solriamfetol treatment of excessive daytime sleepiness in participants with narcolepsy or obstructive sleep apnea with a history of depression. J Psychiatr Res. 2022;155:202–210. doi: 10.1016/j.jpsychires.2022.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Singh H, et al. Solriamfetol titration and administration (START) in patients with obstructive sleep apnea: a retrospective chart review and hypothetical patient scenario. Adv Ther. 2022;39(9):4359–4373. doi: 10.1007/s12325-022-02249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosenberg R, et al. Incidence and duration of common early-onset adverse events in randomized controlled trials of solriamfetol for treatment of excessive daytime sleepiness in obstructive sleep apnea and narcolepsy. J Clin Sleep Med. 2022;18(1):235–244. doi: 10.5664/jcsm.9550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schweitzer PK, et al. Randomized controlled trial of solriamfetol for excessive daytime sleepiness in OSA: an analysis of subgroups adherent or nonadherent to OSA treatment. Chest. 2021;160(1):307–318. doi: 10.1016/j.chest.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iturburu A, et al. Solriamfetol for the use of narcolepsy: a systematic review. Cureus. 2022;14(5):e24937. doi: 10.7759/cureus.24937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vinckenbosch F, et al. Effects of solriamfetol on on-the-road driving performance in participants with excessive daytime sleepiness associated with obstructive sleep apnoea. Hum Psychopharmacol. 2022;37(6):e2845. doi: 10.1002/hup.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ronnebaum S, et al. Indirect treatment comparison of solriamfetol, modafinil, and armodafinil for excessive daytime sleepiness in obstructive sleep apnea. J Clin Sleep Med. 2021;17(12):2543–2555. doi: 10.5664/jcsm.9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang J, et al. Efficacy and safety of solriamfetol for excessive sleepiness in narcolepsy and obstructive sleep apnea: findings from randomized controlled trials. Sleep Med. 2021;79:40–47. doi: 10.1016/j.sleep.2020.12.039. [DOI] [PubMed] [Google Scholar]
- 54. Strollo PJ, et al.; Tones 4 Study Investigators. Solriamfetol for the treatment of excessive sleepiness in OSA: a placebo-controlled randomized withdrawal study. Chest. 2019;155(2):364–374. doi: 10.1016/j.chest.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 55. Malhotra A, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020;43(2). doi: 10.1093/sleep/zsz220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lal C, et al. Excessive daytime sleepiness in obstructive sleep apnea. mechanisms and clinical management. Ann Am Thorac Soc. 2021;18(5):757–768. doi: 10.1513/AnnalsATS.202006-696FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ward CP, et al. Spatial learning and memory deficits following exposure to 24 h of sleep fragmentation or intermittent hypoxia in a rat model of obstructive sleep apnea. Brain Res. 2009;1294:128–137. doi: 10.1016/j.brainres.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ramesh V, et al. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J Neuroinflammation. 2012;9:91. doi: 10.1186/1742-2094-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kaushal N, et al. TNF-α and temporal changes in sleep architecture in mice exposed to sleep fragmentation. PLoS One. 2012;7(9):e45610. doi: 10.1371/journal.pone.0045610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nair D, et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184(11):1305–1312. doi: 10.1164/rccm.201107-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Toth LA, et al. Animal models of sleep disorders. Comp Med. 2013;63(2):91–104. [PMC free article] [PubMed] [Google Scholar]
- 62. Tartar JL, et al. Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open field test of anxiety while increasing plasma corticosterone levels. Behav Brain Res. 2009;197(2):450–453. doi: 10.1016/j.bbr.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yaoita F, et al. Involvement of the hippocampal alpha2a-adrenoceptors in anxiety-related behaviors elicited by intermittent rem sleep deprivation-induced stress in mice. Biol Pharm Bull. 2020;43(8):1226–1234. doi: 10.1248/bpb.b20-00255. [DOI] [PubMed] [Google Scholar]
- 64. Leger M, et al. Object recognition test in mice. Nat Protoc. 2013;8(12):2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 65. Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215(2):244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 66. Gulinello M, et al. Rigor and reproducibility in rodent behavioral research. Neurobiol Learn Mem. 2019;165:106780. doi: 10.1016/j.nlm.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Walf AA, et al. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Can A, et al. The mouse forced swim test. J Vis Exp. 2012;(59):e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yankelevitch-Yahav R, et al. The forced swim test as a model of depressive-like behavior. J Vis Exp. 2015;(97):52587. doi: 10.3791/52587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kaushal N, et al. Human apolipoprotein E4 targeted replacement in mice reveals increased susceptibility to sleep disruption and intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2012;303(1):R19–R29. doi: 10.1152/ajpregu.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kaushal N, et al. Socially isolated mice exhibit a blunted homeostatic sleep response to acute sleep deprivation compared to socially paired mice. Brain Res. 2012;1454:65–79. doi: 10.1016/j.brainres.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Puech C, et al. Explicit memory, anxiety and depressive like behavior in mice exposed to chronic intermittent hypoxia, sleep fragmentation, or both during the daylight period. Neurobiol Sleep Circadian Rhythms. 2022;13:100084. doi: 10.1016/j.nbscr.2022.100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mereu M, et al. The unique psychostimulant profile of (±)-modafinil: investigation of behavioral and neurochemical effects in mice. Eur J Neurosci. 2017;45(1):167–174. doi: 10.1111/ejn.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Flores AE, et al. Pattern recognition of sleep in rodents using piezoelectric signals generated by gross body movements. IEEE Trans Biomed Eng. 2007;54(2):225–233. doi: 10.1109/TBME.2006.886938. [DOI] [PubMed] [Google Scholar]
- 75. Vanneau T, et al. Determination of the sleep-wake pattern and feasibility of NREM/REM discrimination using the non-invasive piezoelectric system in rats. J Sleep Res. 2021;30(6):e13373. doi: 10.1111/jsr.13373. [DOI] [PubMed] [Google Scholar]
- 76. Donohue KD, et al. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed Eng Online. 2008;7(1):14. doi: 10.1186/1475-925X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yaghouby F, et al. Noninvasive dissection of mouse sleep using a piezoelectric motion sensor. J Neurosci Methods. 2016;259:90–100. doi: 10.1016/j.jneumeth.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mang GM, et al. Evaluation of a piezoelectric system as an alternative to electroencephalogram/ electromyogram recordings in mouse sleep studies. Sleep. 2014;37(8):1383–1392. doi: 10.5665/sleep.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Paulose JK, et al. The effects of aging on sleep parameters in a healthy, melatonin-competent mouse model. Nat Sci Sleep. 2019;11:113–121. doi: 10.2147/NSS.S214423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Antunes M, et al. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13(2):93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hammond RS, et al. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82(1):26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 82. Sweis BM, et al. A modified beam-walking apparatus for assessment of anxiety in a rodent model of blast traumatic brain injury. Behav Brain Res. 2016;296:149–156. doi: 10.1016/j.bbr.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 83. Aduema W, et al. Using the elevated plus maze task in assessing anxiety and fear in Swiss white mice. J Complement Med Altern Healthc. 2018;6(1). doi: 10.19080/JCMAH.2018.06.555678. [DOI] [Google Scholar]
- 84. Crowley JJ, et al. Automated tests for measuring the effects of antidepressants in mice. Pharmacol Biochem Behav. 2004;78(2):269–274. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 85. Carreras A, et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep. 2014;37(11):1817–1824. doi: 10.5665/sleep.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hakim F, et al. Chronic sleep fragmentation during the sleep period induces hypothalamic endoplasmic reticulum stress and PTP1b-mediated leptin resistance in male mice. Sleep. 2015;38(1):31–40. doi: 10.5665/sleep.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hakim F, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74(5):1329–1337. doi: 10.1158/0008-5472.CAN-13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang Y, et al. Chronic sleep fragmentation promotes obesity in young adult mice. Obes Silver Spring Md. 2014;22(3):758–762. doi: 10.1002/oby.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang SXL, et al. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int J Obes. 2014;38(4):619–624. doi: 10.1038/ijo.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gozal D, et al. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. J Neurochem. 2003;86(6):1545–1552. doi: 10.1046/j.1471-4159.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- 91. Shieu MM, et al. The association between obstructive sleep apnea risk and cognitive disorders: a population-based study. J Clin Sleep Med. 2022;18(4):1177–1185. doi: 10.5664/jcsm.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93(12):1778–1794. doi: 10.1002/jnr.23634. [DOI] [PubMed] [Google Scholar]
- 93. Ramesh V, et al. Sleep fragmentation differentially modifies EEG delta power during slow wave sleep in socially isolated and paired mice. Sleep Sci. 2009;2(2):64–75. [Google Scholar]
- 94. Djonlagic I, et al. Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PLoS One. 2012;7(3):e34106. doi: 10.1371/journal.pone.0034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Palchykova S, et al. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem. 2006;85(3):263–271. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 96. Tartar JL, et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23(10):2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Xie Y, et al. Chronic sleep fragmentation shares similar pathogenesis with neurodegenerative diseases: endosome-autophagosome-lysosome pathway dysfunction and microglia-mediated neuroinflammation. CNS Neurosci Ther. 2020;26(2):215–227. doi: 10.1111/cns.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shahveisi K, et al. Novel object recognition memory in REM sleep-deprived rats: role of the cannabinoid CB1 receptor. Behav Brain Res. 2020;381:112311. doi: 10.1016/j.bbr.2019.112311. [DOI] [PubMed] [Google Scholar]
- 99. Pires GN, et al. Effects of experimental sleep deprivation on anxiety-like behavior in animal research: Systematic review and meta-analysis. Neurosci Biobehav Rev. 2016;68:575–589. doi: 10.1016/j.neubiorev.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 100. Suchecki D, et al. Hormonal and behavioural responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol. 2002;14(7):549–554. doi: 10.1046/j.1365-2826.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 101. Subedi R, et al. Efficacy and safety of solriamfetol for excessive daytime sleepiness in narcolepsy and obstructive sleep apnea: a systematic review and meta-analysis of clinical trials. Sleep Med. 2020;75:510–521. doi: 10.1016/j.sleep.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 102. Yan WW, et al. Effects of modafinil on behavioral learning and hippocampal synaptic transmission in rats. Int Neurourol J. 2015;19(4):220–227. doi: 10.5213/inj.2015.19.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fernandes HA, et al. Effects of post-training modafinil administration in a discriminative avoidance task in mice. Acta Neuropsychiatr. 2015;27(4):235–241. doi: 10.1017/neu.2015.16. [DOI] [PubMed] [Google Scholar]
- 104. Minzenberg MJ, et al. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33(7):1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 105. Sheng P, et al. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: a systematic review and meta-analysis. PLoS One. 2013;8(12):e81802e81802. doi: 10.1371/journal.pone.0081802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Weaver TE, et al. Effects of solriamfetol on quality-of-life measures from a 12-week phase 3 randomized controlled trial. Ann Am Thorac Soc. 2020;17(8):998–1007. doi: 10.1513/AnnalsATS.202002-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Baranski JV, et al. Effects of modafinil on cognitive and meta-cognitive performance. Hum Psychopharmacol. 2004;19(5):323–332. doi: 10.1002/hup.596. [DOI] [PubMed] [Google Scholar]
- 108. Wesensten NJ, et al. Performance and alertness effects of caffeine, dextroamphetamine, and modafinil during sleep deprivation. J Sleep Res. 2005;14(3):255–266. doi: 10.1111/j.1365-2869.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 109. Thorpy MJ, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019;85(3):359–370. doi: 10.1002/ana.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Thomas RJ, et al. Modafinil activates cortical and subcortical sites in the sleep-deprived state. Sleep. 2006;29(11):1471–1481. doi: 10.1093/sleep/29.11.1471. [DOI] [PubMed] [Google Scholar]
- 111. Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. 2017;(126):55718. doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cao Y, et al. Modafinil protects hippocampal neurons by suppressing excessive autophagy and apoptosis in mice with sleep deprivation. Br J Pharmacol. 2019;176(9):1282–1297. doi: 10.1111/bph.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Piérard C, et al. Modafinil restores memory performance and neural activity impaired by sleep deprivation in mice. Pharmacol Biochem Behav. 2007;88(1):55–63. doi: 10.1016/j.pbb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 114. Wadhwa M, et al. Caffeine and modafinil ameliorate the neuroinflammation and anxious behavior in rats during sleep deprivation by inhibiting the microglia activation. Front Cell Neurosci. 2018;12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Béracochéa D, et al. Enhancement of learning processes following an acute modafinil injection in mice. Pharmacol Biochem Behav. 2003;76(3–4):473–479. doi: 10.1016/j.pbb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 116. Ward CP, et al. Modafinil facilitates performance on a delayed nonmatching to position swim task in rats. Pharmacol Biochem Behav. 2004;78(4):735–741. doi: 10.1016/j.pbb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 117. Morgan RE, et al. Modafinil improves attention, inhibitory control, and reaction time in healthy, middle-aged rats. Pharmacol Biochem Behav. 2007;86(3):531–541. doi: 10.1016/j.pbb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 118. Akberzie W, et al. The prevalence of anxiety and depression symptoms in obstructive sleep apnea. Cureus. 2020;12(10):e1. doi: 10.7759/cureus.11203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pokk P, et al. The effects of the nitric oxide synthase inhibitors on the behaviour of small-platform-stressed mice in the plus-maze test. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(2):241–247. doi: 10.1016/s0278-5846(01)00261-5. [DOI] [PubMed] [Google Scholar]
- 120. Pires GN, et al. Effects of acute sleep deprivation on state anxiety levels: a systematic review and meta-analysis. Sleep Med. 2016;24:109–118. doi: 10.1016/j.sleep.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 121. Schwartz JRL, et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea: a 12-week, open-label study. Chest. 2003;124(6):2192–2199. doi: 10.1378/chest.124.6.2192. [DOI] [PubMed] [Google Scholar]
- 122. Rasetti R, et al. Modulatory effects of modafinil on neural circuits regulating emotion and cognition. Neuropsychopharmacology. 2010;35(10):2101–2109. doi: 10.1038/npp.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hofmann SG, et al. Cognitive enhancers for anxiety disorders. Pharmacol Biochem Behav. 2011;99(2):275–284. doi: 10.1016/j.pbb.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kraeuter AK, et al. The forced swim test for depression-like behavior in rodents. Methods Mol Biol. 2019;1916:75–80. doi: 10.1007/978-1-4939-8994-2_5. [DOI] [PubMed] [Google Scholar]
- 125. Mahmoudi J, et al. Antidepressant-like effect of modafinil in mice: evidence for the involvement of the dopaminergic neurotransmission. Pharmacol Rep. 2015;67(3):478–484. doi: 10.1016/j.pharep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 126. Omidi-Ardali H, et al. Nitric oxide mediates the antidepressant-like effect of modafinil in mouse forced swimming and tail suspension tests. J Basic Clin Physiol Pharmacol. 2020;32(2):25–31. doi: 10.1515/jbcpp-2020-0021. [DOI] [PubMed] [Google Scholar]
- 127. Regenthal R, et al. Depression-like deficits in rats improved by subchronic modafinil. Psychopharmacology. 2009;204(4):627–639. doi: 10.1007/s00213-009-1493-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.