Abstract
A viral mRNA of the late kinetic class expressed by murine cytomegalovirus (MCMV) contains an open reading frame (ORF) whose predicted protein, designated MCK-1, has homology to β chemokines (M. R. MacDonald, X.-Y. Li, and H. W. Virgin IV, J. Virol. 71:1671–1678, 1997). The present study analyzed further the structure of the transcript in infected fibroblast cells. A splicing event removed the MCK-1 stop codon, bringing a downstream ORF into frame with the chemokine homolog and demonstrating that the MCK-1 ORF was an exon of a larger gene. The predicted 31.4-kDa protein, designated MCK-2, contains a putative amino-terminal signal sequence and a β chemokine domain, followed by a carboxyl-terminal domain without significant homology to known proteins. Quantitative analysis of mRNA forms in MCMV-infected fibroblast cells at late times after infection indicated that the viral chemokine RNA was predominantly spliced. There was no evidence for expression of RNA encoding either MCK-1 or MCK-2 at immediate early or early times after infection with MCMV. Monoclonal antibodies generated against bacterially expressed MCK-2 recognized multiple proteins in the range of ∼30 to ∼45 kDa in Western blot analysis of MCK-2 expressed in transfected COS cells. The monoclonal antibodies immunoprecipitated a similar group of proteins in transfected COS cells metabolically labeled with radioactive cysteine. Radiolabelled protein of apparent higher molecular mass was immunoprecipitated from culture medium overlying the transfected cells, suggesting that posttranslationally modified MCK-2 can be secreted. Two proteins with apparent molecular mass suggestive of posttranslational modification were detected by Western blot analysis of cells harvested at late times after infection with MCMV. These studies show that MCMV encodes and expresses a β chemokine homolog with a novel predicted structure.
Human cytomegalovirus (HCMV) infection of immunocompromised people or neonates can cause significant morbidity and mortality, while infection of normal hosts typically causes asymptomatic, although lifelong, infection (18, 45, 47, 54). The ability of the virus to reactivate from a latent state and cause acute infection is a significant clinical problem in the setting of solid-organ transplantation (45). Understanding the interactions between HCMV and the host’s innate and adaptive immune response to viral infection is critical to development of preventative or therapeutic interventions. Infection of mice with the related betaherpesvirus murine cytomegalovirus (MCMV) has been used as a model for infection of humans with cytomegalovirus (reviewed in reference 50), since the strict host specificity of the betaherpesviruses precludes the use of HCMV in a small-animal model.
We have previously noted that MCMV contains an open reading frame (ORF) with significant homology to murine and human β chemokines (32). The mammalian chemokines are a group of small, inducible, secreted inflammatory cytokines with a common structural motif. The members of this family were originally identified by biochemical purification of secreted inflammatory factors, but family members have been discovered by identification of induction-specific or cell-type-specific cDNA sequences. Recently, analysis of the sequences obtained from genome sequencing projects has led to the identification of a large number of chemokines and receptors. Interleukin-8 is the best characterized of the mammalian chemokines, and determination of its crystal structure (7) has allowed the modeling of the structures of other family members. Within the secondary structure of the chemokines, four conserved cysteine residues are involved in covalent sulfhydryl linkages, with the first conserved cysteine bonding to the third and the second bonding to the fourth. Based on the placement of the conserved cysteine residues, the family members were originally divided into two classes: the α (or CXC) chemokines, which have an intervening amino acid residue between the first and second cysteine residues, and the β (or CC) chemokines, in which the first two conserved cysteine residues are adjacent. Chemokines of the α class attract and activate primarily, although not exclusively, neutrophils and fibroblasts, while β chemokines act primarily on monocytes and lymphocytes.
Recent identification of two chemokines with distinct structures has led to the addition of two new groups. γ (or C) chemokines, represented by the lymphocyte attractant lymphotactin and SCM-1, contain only two of the four conserved cysteine residues (28, 56). The δ (or CX3C) chemokine group contains three intervening amino acids between the first and second conserved cysteine residues. The only known δ class member, fractalkine (8) (also named neurotactin [41]), is predicted to be tethered by a mucin-like stalk to the plasma membrane and contains a short cytoplasmic tail. Whether the newer classes of chemokines will be found to have additional members remains to be seen.
To date, all identified receptors for the chemokine molecules belong to the class of G-protein-coupled seven-transmembrane domain receptors. Binding of chemokine results in pertussis toxin-sensitive increases in cytoplasmic free calcium through the actions of phospholipase C and inositol triphosphate. Diacylglycerol is also formed, with resultant activation of protein kinase C, leading to a variety of cellular responses (reviewed in references 9 and 37). Receptors which have been identified can be divided both by sequence and function into several subgroups, with α chemokines signaling through one group and β chemokines signaling through the other. Multiple receptor family members within each subgroup have recently been identified, with receptors exhibiting distinct but overlapping ligand specificity (reviewed in references 5 and 33). Receptors for the newer C and CX3C chemokine classes have recently been identified (25, 55).
Both a chemokine homolog and G-protein-coupled receptor homologs have been noted in MCMV, providing the opportunity to address, by the use of mutant viruses in the mouse model, the role of these possible immune modulating proteins on pathogenesis in vivo. MCMV contains ORFs predicting two possible G-protein-coupled receptors (44), and recombinant virus lacking one (M33) was found to have severely attenuated growth in the salivary glands of infected mice (17). We noted that the predicted protein of MCMV ORF HJ1 (50) was highly homologous to β chemokines (32). After the complete MCMV genome sequence was determined (44), this ORF, which lies within the HindIII J genomic fragment, was designated ORF m131. Our previous studies showed that a 0.9-kb RNA was expressed in infected fibroblasts and macrophages as a member of the late kinetic class of viral transcripts. The RNA was polyadenylated, suggesting that it was transported to the cytoplasm for translation. Although the ORF’s predicted protein contains a 5′ extension showing no homology to chemokines, mapping of the 5′ end of the RNA indicated that the fourth ORF methionine was the site of translation initiation. Thus, the 81-amino-acid predicted protein, designated MCK-1, was entirely homologous to β chemokine family members and included a predicted 21-amino-acid signal peptide which would target the protein for secretion. To facilitate generation of recombinant MCMV lacking the gene product, we further studied the structure and expression of the 0.9-kb mRNA and have determined that the RNA is spliced, with removal of an intron just 3′ to the MCK-1 ORF. The splicing event removes the MCK-1 stop codon and predicts the generation of a novel protein, designated MCK-2, with an amino-terminal domain with β chemokine homology. We then proved, using a panel of newly isolated monoclonal antibodies, that MCMV-infected cells express MCK-2 and that at least one form of the protein can be secreted. The unique predicted structure of this viral chemokine homolog is, to our knowledge, unlike that of any previously described β chemokine and opens up the possibility of a variety of biological actions which might contribute to MCMV pathogenesis.
MATERIALS AND METHODS
Cells, cell culture, and viral infection.
Murine NIH 3T12 fibroblast cells (ATCC CCL 164) were grown as previously described (24) in Dulbecco’s modified Eagle medium (DMEM) supplemented with 2 mM glutamine (Biofluids, Rockville, Md.), 10 mM HEPES (Biofluids), and 10% fetal bovine serum (FBS; HyClone, Logan, Utah). COS 1 (ATCC CRL-1650; simian virus 40 African green monkey kidney cells) were grown in DMEM supplemented with 1 mM sodium pyruvate and 10% FBS. Hybridoma cell lines were maintained in Iscove’s modified Dulbecco’s medium (BioWhittaker Inc., Walkersville, Md.) supplemented with 4 mM glutamine, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, and 10% FBS. Tissue culture-passaged MCMV stocks (strain Smith MSGV; ATCC VR-194) were prepared and titered as previously described (24). Infection of NIH 3T12 cells with MCMV was carried out in a small volume of medium for 1 h at 37°C, with the addition of fresh medium after removal of the inoculum. Protein synthesis or DNA replication was inhibited by the inclusion of cycloheximide at 50 μg/ml or phosphonoacetic acid at 250 μg/ml, respectively, in the medium as previously described (27, 32). Cells were enriched for MCMV-encoded transcripts of the immediate-early (IE), early (E), or late (L) kinetic class by infection with MCMV for 6 h in the presence of cycloheximide, 24 h in the presence of phosphonoacetic acid, or 24 h in the absence of inhibitors, respectively.
Construction of an MCK-2 bacterial expression plasmid.
Plasmid pET-MCK-2-Sig/His for bacterial expression of MCK-2 was generated in plasmid pET-30a(+) (Novagen, Madison, Wis.) by using standard techniques (46). All plasmids containing viral DNA derived by PCR were sequenced to ensure that undesired mutations had not arisen. PCR primers 1 and 2 (Table 1) were used to amplify plasmid pmt21MCK-2-cStop (see “Construction of eukaryotic expression plasmids” below) by 35-cycle PCR (95°C, 55°C, and 72°C, 1 min each), using the Expand High Fidelity PCR system (Boehringer Mannheim, Indianapolis, Ind.) as recommended by the manufacturer. Primer 1 contains an NdeI site for cloning and encodes an initiator methionine and amino acids predicted to be at the amino terminus of MCK-2 after signal peptidase cleavage between amino acids 21 and 22. Primer 2 is homologous to the carboxyl terminus of MCK-2 and encodes a His epitope tag and XhoI site. The resultant PCR product was digested with NdeI and XhoI and cloned into similarly digested pET-30a(+) to generate pET-MCK-2-Sig/His. Under appropriate expression conditions in the presence of T7 RNA polymerase, this plasmid should generate a 265-amino-acid 30.1-kDa protein consisting of an initiator methionine, amino acids 22 to 280 of MCK-2, followed by the His epitope tag for purification.
TABLE 1.
Oligonucleotide primers used
| Oligo-nucleotide | Sequence |
|---|---|
| 1 | GGAATTCCATATGGACCTCCGCGAGCCATG |
| 2 | CCGCCTCGAGCTAGTGATGGTGATGGTGTTCATCGGACAGTCGTTGTA |
| 3 | CCGCTCGAGAATTCACCATGGGAACGCTCCTCGTG |
| 4 | GCCGCTCGAGTCACCTGACCAGACACAAGAG |
| 5 | GCCGCTCGAGTTATTCATCGGACAGTCGTTGTA |
| 6 | GTGTTAGTGTATTCGGCGCTCTGTACCGCG |
| 7 | GTCGTGTCTACCGTCGCGGACCTCCG |
| 8 | GATAGAGTGTGTCTGTGAGATCAC |
| 9 | GTGCGGACGAGAGGTGGTTTTCAC |
| 10 | TTAGCGCACACCCTCATCCCAGAG |
| 11 | GGCGTGTTGTATGATGGTGCGACGGGG |
| 12 | GGGAAGGTGTAGATCTTGACGTCGGGG |
| 13 | CGAGCTGGTACGCGATGTCGACGGCGC |
| 14 | GGTGTCAGTGCGTATCGCCCTCGAAACCG |
Construction of eukaryotic expression plasmids.
Plasmids for expression of MCK-1 and MCK-2 in COS 1 cells under the control of the adenovirus major late promoter were generated in plasmid pmt21 (generously provided by Barrett Rollins, Harvard University, Boston, Mass.), which has been used for expression of the human β chemokine monocyte chemoattractant protein 1 (MCP-1) in COS cells (57). PCR primers 3 and 4 (Table 1) were used to amplify DNA from plasmid pHDJLsma-bam. Primer 3 contains an EcoRI site for cloning followed by nucleotides homologous to the amino terminus of MCK-1. Primer 4 contains nucleotides homologous to the carboxyl terminus of MCK-1, a stop codon, and an XhoI site for cloning. The PCR product was digested with EcoRI and XhoI and cloned into EcoRI and XhoI-digested pmt21 to generate plasmid pmt21MCK-cStop.
For analysis of MCK-2 biosynthesis, plasmid pmt21MCK-cStop was modified to express the carboxyl-terminally extended form. Primer 5 (Table 1) was designed to contain an XhoI site for cloning and nucleotides homologous to the authentic carboxyl terminus of MCK-2. Primer 5 and the T7 promoter primer (Promega, Madison, Wis.) were used for PCR amplification using plasmid pBSMCK3′RACE (see “RACE” below) as the template. Plasmid pBSMCK3′RACE contains the carboxyl-terminal portion of the MCK-2 cDNA and includes the splice junction. The PCR product was digested with NotI and XhoI, and this portion was cloned into pmt21MCK-cStop from which the NotI-to-XhoI region encoding the carboxyl-terminal portion of MCK-1 had been removed. Thus, the MCK-1 expression plasmid was converted into a plasmid (pmt21MCK-2-cStop) for expression of authentic MCK-2.
RACE.
Total cellular RNA was isolated from cell monolayers (RNAzol B; TelTest, Inc.), and polyadenylated species were purified by oligo(dT) column chromatography (mRNA purification kit; Pharmacia Biotech) as previously described (32). Rapid amplification of cDNA ends (RACE) analysis was carried out by using a Marathon cDNA amplification kit (Clontech, Palo Alto, Calif.) as recommended by the manufacturer. Template RNA for cDNA generation was 1 μg of polyadenylated RNA isolated 5 days after infection (multiplicity of infection of 0.05) of NIH 3T12 fibroblast cells with MCMV. After first- and second-strand cDNA synthesis and ligation of the Marathon adaptor to the cDNA ends, separate 5′ and 3′ PCR amplifications were carried out. For amplification of the 5′ end of the cDNA, primer 6 (Table 1) and the Adaptor primer supplied with the kit were used. For amplification of the 3′ end of the cDNA, primer 7 (Table 1) and the Adaptor primer were used. After gel purification, the PCR products were digested with NotI and cloned into NotI phosphatase-treated pBluescript II KS. Inserts were sequenced by using T3 and T7 promoter primers as well as MCK-specific primers 8, 9, and 10. One 3′ RACE reaction clone was chosen as plasmid pBSMCK3′RACE and contains the region of MCK-2 encoding cDNA from the NotI site through the stop codon, the AAUAAA polyadenylation signal, followed by 35 nucleotides (nt) and a poly(A) tail.
RPA.
Polyadenylated RNA (1 μg) from MCMV-infected cells, or control RNA generated by transcription in vitro, was precipitated with yeast RNA (Boehringer Mannheim) to equal 20 μg per sample. The RNA samples were resuspended in 30 μl of hybridization solution containing 2 × 105 cpm of 32P-labelled RNA probe and were subjected to RNase protection analysis (RPA) as described elsewhere (46), and an aliquot was size separated by denaturing (urea) 4% polyacrylamide gel electrophoresis (4% PAGE). The dried gel was subjected to autoradiography and phosphorimage analysis (STORM840 and ImageQuant software; Molecular Dynamics).
32P-labelled RNA probe antisense to the unspliced MCK-encoding mRNA (see Fig. 3A) was generated by bacteriophage SP6 RNA polymerase (Epicentre Technologies, Madison, Wis.) in vitro transcription of NotI-linearized plasmid pHDJLbr (32) according to the manufacturer’s recommendations and was purified by denaturing (urea) 5% PAGE.
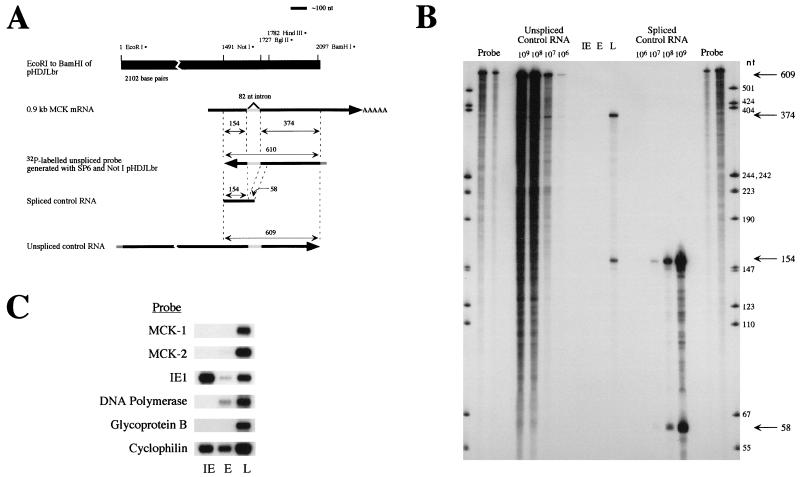
FIG. 3.
RPA of mRNA from MCMV-infected fibroblasts. (A) Schematic representation of the RPA. The solid black bar shows the MCMV genomic DNA present in plasmid pHDJLbr, with pertinent restriction sites indicated. The arrow below shows the MCMV chemokine-encoding mRNA, with the location of the 82-nt intron shaded. Antisense 32P-labelled probe shown by the leftward arrow was generated by using NotI-digested pHDJLbr as the template. Portions of the probe expected to be protected from digestion after hybridization to mRNA lacking or containing the 82-nt intron are indicated by the double-headed arrows above the probe. RNAs generated in vitro for use as controls for spliced and unspliced mRNA in the RPA are shown below the probe, with expected sizes of protected fragments indicated by double-headed arrows. (B) Results of the RPA. Control or sample RNA was hybridized with 2.5 × 105 cpm of radiolabelled probe and subjected to RPA. Protected fragments were analyzed by denaturing gel electrophoresis, and an autoradiograph is shown. Migration of 32P-labelled HpaII-digested pGEM1 size standards is shown in the outer lanes, with sizes indicated. Lanes labelled “Probe” contain two dilutions of probe which was not subjected to the RPA assay. Protected products from control unspliced or spliced RNA were loaded into the indicated lanes. Migration of the expected 609-nt (unspliced) and 154- and 58-nt (spliced) protected fragments are indicated by the arrows on the right. IE, E, and L indicated lanes containing protected products after RPA using RNA of the indicated kinetic class. No protected fragments were seen with IE or E RNA, even after prolonged exposures. Protected fragments of the expected sizes indicative of spliced RNA (154 and 374 nt) were obtained after RPA using L RNA and are indicated by the arrows to the right. A faint band of ∼610 nt was barely detectable after prolonged exposure. Similar results were obtained in three RPA experiments using two independently isolated preparations of RNA. (C) Confirmation of RNA integrity by Northern blot analysis. Samples of the RNA used in the RPA were subjected to Northern blot analysis, and the blot was probed sequentially with the indicated probes. Controls included analysis of known immediate early (IE1), early (DNA polymerase), and late (glycoprotein B) viral transcripts, as well as a cellular (cyclophilin) transcript.
Both spliced and unspliced control RNAs were generated by transcription in vitro as described above in the presence of unlabelled ribonucleotides (0.5 mM each). Ten microcuries of [5-3H]UTP (21 Ci/mmol; Amersham Life Science Inc., Arlington Heights, Ill.) was included in the reaction; after DNase digestion, the percent incorporation was determined by scintillation spectrophotometry of [3H]RNA which remained bound to DE81 (Whatman International Ltd., Maidstone, England) chromatography paper (46). After extraction and precipitation, the control RNAs were resuspended at a concentration of 1010 molecules per μl in 10 mM Tris (pH 8)–1 mM EDTA (TE) and were serially diluted in TE. Aliquots from the dilutions containing 109, 108, 107, and 106 molecules were precipitated with 20 μg of yeast RNA and were subjected to RPA as described above. Template DNA for generation of unspliced (intron present) control RNA was BamHI-linearized plasmid pHDJLbr (Fig. 3A). For generation of spliced (intron absent) control RNA, template plasmid pBSMCK-Not/HindIII-spliced was constructed from plasmid pBSMCK3′RACE by digestion with HindIII followed by religation, which removes portions of the cDNA 3′ to the HindIII site but leaves the splice junction. HindIII-linearized pBSMCK-Not/HindIII-spliced was used as the template in the transcription reaction (Fig. 3A).
Northern analysis.
Polyadenylated RNA isolated from cells infected with MCMV was size separated by formaldehyde denaturing 1.5% agarose gel electrophoresis, transferred to a nylon membrane, and hybridized with random-prime-labelled DNA probes for MCK-1-, IE1-, and cyclophilin-encoding RNAs as previously described (32, 43). The probe for RNA encoding MCK-2 consisted of the BamHI-to-BglII fragment of plasmid pHDJLbr (see Fig. 3A) as described previously (32) for analysis of the presence of sequences 3′ to the MCK-1 ORF. Probes for RNA encoding the MCMV proteins DNA polymerase and glycoprotein B were generated from PCR products obtained from template MCMV HindIII D-fragment DNA in plasmid pACYC184 (generously provided by Deborah Spector), using primer pairs (Table 1) 11-12 and 13-14, respectively.
Generation and purification of protein for immunization.
Plasmid pET-MCK-2-Sig/His (see above) was transformed into Escherichia coli BL21(DE3), and expression of MCK-2 was induced by the addition of 1 mM isopropylthiogalactoside. His epitope-tagged MCK-2 lacking the putative signal peptide was purified by affinity chromatography on Ni-nitilotriacetic acid agarose (Qiagen Inc., Santa Clarita, Calif.) under denaturing conditions essentially as recommended by the manufacturer. Fractions were screened for the presence of recombinant protein by sodium dodecyl sulfate (SDS)–12.5% PAGE (see below) followed by Coomassie blue staining. Recombinant MCK-2 was refolded by dialysis against buffer containing 8 M urea, 10% glycerol, 3 mM cysteine, 100 mM sodium phosphate, 10 mM Tris, 150 mM NaCl, and 0.02% polyoxyethylene-20-sorbitan monolaurate (Tween 20), with stepwise reductions in urea concentration. The protein precipitated out of solution between 4 and 2 M urea, and this insoluble protein was used as the immunogen for the generation of anti-MCK-2 polyclonal and monoclonal antibodies.
Generation of polyclonal anti-MCK-2 antiserum.
Rabbits and guinea pigs were immunized and boosted with bacterially expressed recombinant His epitope-tagged MCK-2 (lacking the signal peptide) in Freund’s adjuvant according to the Cocalico Biologicals, Inc. (Reamstown, Pa.) standard protocol. Test bleeds of the immunized animals were screened by Western blotting for the presence of antibodies recognizing the bacterially expressed immunogen and MCK-2 expressed in eukaryotic cells (see below). One rabbit (WU1073) produced antibodies of sufficient titer for experimental use.
Generation and Screening of anti-MCK-2 monoclonal antibody.
BALB/c mice were immunized by subcutaneous injection with 25 μg of bacterially expressed His epitope-tagged MCK-2 (lacking the putative signal peptide) emulsified in complete Freund’s adjuvant. Booster doses (25 to 40 μg) in incomplete Freund’s adjuvant were given at 4 and 9 weeks. At 11 weeks the mice received 50 μg of MCK-2 given by intraperitoneal injection, and 4 days later spleens were harvested and fused to generate hybridoma cell lines. Spleen cell fusion, selection, and subcloning were carried out by the Hybridoma Center at Washington University (49). Supernatants from hybridoma clones were screened by enzyme-linked immunosorbent assay as described previously (53). Monoclonal antibodies were also screened by Western blotting for their ability to recognize MCK-1 and MCK-2 expressed in eukaryotic cells. Six clones (1H9, 2H9, 3F8, 5A5, 6A2, and 11D7) were chosen and subcloned twice.
Eukaryotic expression of MCK-1 and MCK-2.
COS 1 cells were plated at 2 × 106 cells per 10-cm-diameter tissue culture dish and were transfected the following day with 12 μg of vector plasmid pmt21 (control), plasmid pmt21MCK-cStop (MCK-1 expression), or pmt21MCK-2-cStop (MCK-2 expression), using 30 μl of LipofectAmine Reagent (Life Technologies, Gaithersburg, Md.) in 3 ml of DMEM without additives. After incubation for 3 to 4 h, the transfection solution was replaced with medium. After 24 to 48 h, the medium was replaced with serum-free medium, and the cells were incubated for an additional 1 to 2 days before either lysing directly for Western analysis or metabolic labelling for analysis by immunoprecipitation. For Western analysis, the cells were washed with phosphate-buffered saline (PBS) and were lysed directly in 2× Laemmli sample buffer (30). Samples were forced several times through a 26-gauge needle and stored at −70°C. Metabolic labelling was carried out on cells which had been washed and preincubated in cysteine-free medium. For labelling, cells were incubated for 30 min in 2 ml of cysteine-free, serum-free medium supplemented with 1 μCi of l-[35S]cysteine (>800 Ci/mmol; ICN Pharmaceuticals Inc., Costa Mesa, Calif.). After being washed once, the cells were incubated for various times in 10 ml of normal culture medium containing 15 μg of unlabelled cysteine per ml. For analysis of MCK-2 released into the medium, the medium was cleared of cellular debris by centrifugation (300 × g) for 5 min at 4°C, and the supernatant fluid was stored at −70°C. For analysis of cell-associated MCK-2, cells were scraped into ice cold PBS, collected by centrifugation (300 × g) for 5 min at 4°C, and stored at −70°C until immunoprecipitation analysis.
SDS-PAGE.
Cell lysates or immunoprecipitated proteins were analyzed by a modification (3) of the method of Laemmli (30), using either 20% polyacrylamide gels containing 50 mM NaCl or 12.5% polyacrylamide gels. Stacking gels were 5% polyacrylamide. Gels either were immediately subjected to electrophoretic transfer for Western analysis or were fixed, incubated in Amplify fluorographic reagent (Amersham Life Science), and dried prior to autoradiography. Rainbow molecular weight markers (Amersham Life Science) were used as size standards.
Western blot analysis.
Proteins were transferred from the gel by semidry electrophoresis onto Hybond ECL (enhanced chemiluminescence) nitrocellulose membranes (Amersham Life Science), and the blots were incubated overnight at 4°C in blocking buffer (10% goat serum [Life Technologies], 5% nonfat dry milk, and 0.05% Tween 20 in PBS). Primary antibody consisted of either hybridoma culture medium which had been cleared of cells by centrifugation at 300 × g or polyclonal antiserum diluted 1:250 in blocking buffer. Peroxidase-conjugated secondary antibody consisted of goat anti-mouse immunoglobulin G diluted 1:3,000 or 1:4,000 (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) or donkey anti-rabbit immunoglobulin diluted 1:2,000 (Amersham Life Science). Immunoreactive proteins were detected by use of the luminol-based chemiluminescence reaction (ECL Western blotting detection reagents; Amersham Life Science) as recommended by the manufacturer.
Immunoprecipitation.
MCK-2-expressing COS 1 cells which had been radiolabelled were lysed by incubation in 1 ml of radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris [pH 8]) containing protease inhibitors (1 mM iodoacetamide, 1 mM phenylmethylsulfonyl fluoride, 0.015 U of aprotinin per ml) for 30 min on ice. For analysis of proteins secreted into the medium, an equal volume of RIPA buffer containing protease inhibitors was added. All samples were cleared of insoluble material by centrifugation at 10,000 × g for 10 min at 4°C. Nonspecific absorbing materials were removed by incubation with normal rabbit serum and a 10% suspension of Staphylococcus aureus Cowan II bacteria (Pansorbin cells; Calbiochem-Novabiochem Corporation, La Jolla, Calif.). After centrifugation, an aliquot of the supernatant fluid corresponding to 1/20 of the original sample was incubated for 1 h on ice with 100 μl of hybridoma culture supernatant. The immune complexes were collected by incubation with protein A-Sepharose (Sigma, St. Louis, Mo.) in RIPA buffer containing protease inhibitors. After centrifugation, the pellets were washed extensively and then resuspended in 50 μl of 2× Laemmli sample buffer. After heating and centrifugation, a 20-μl aliquot of the supernatant fluid was analyzed by SDS-PAGE and fluorography.
Endoglycosidase digestion.
Cells were lysed directly in 0.5% SDS containing 1% 2-mercaptoethanol and were forced several times through a 26-gauge needle. Medium was cleared of cellular debris as described above (see “Eukaryotic expression of MCK-1 and MCK-2”), concentrated in a Centriprep 10 concentrator (Amicon, Inc., Beverly, Mass.), and adjusted to contain 0.5% SDS and 1% 2-mercaptoethanol. After boiling, aliquots of the soluble material were treated with peptide:N-glycosidase F (PNGase F; New England Biolabs, Inc., Beverly, Mass.) as recommended by the manufacturer.
RESULTS
A spliced mRNA encoding the MCMV chemokine homolog predicts a protein of novel primary structure.
We have previously characterized the expression and mapped the 5′ end of an MCMV-encoded mRNA which contains sequences encoding a chemokine homolog, MCK-1 (32). To confirm the structure of the 5′ end of the message and to determine if any splicing of the mRNA occurs in infected cells, 5′ and 3′ RACE were carried out. An adaptor-ligated cDNA library was generated by oligo(dT) priming of polyadenylated mRNA isolated from fibroblast cells which had been infected with MCMV. Specific PCR amplification of the 5′ end of the MCK-1 cDNA was carried out with a primer complementary to the adaptor in combination with an MCK-1-specific primer (MCK 5′ primer; oligonucleotide 6 [Table 1]) and is shown schematically in Fig. 1A. Analysis of the PCR products by agarose gel electrophoresis revealed one major band of approximately 200 bp, which was digested with NotI and cloned. Sequencing of 16 independent clones revealed that the 5′ end of the cDNA ranged from 10 nt upstream to 28 nt downstream from nucleotides encoding the previously identified initiator methionine (data not shown). Given that generation of the cDNA involved the creation of blunt ends with T4 DNA polymerase prior to ligation of the adaptors, these data are consistent with the previous identification (using primer extension and S1 nuclease digestion) of the 5′ end of the message as being ∼20 nt upstream of the methionine-encoding nucleotides (32). In addition, comparison of the clone sequences with the MCMV genomic sequence (44) indicated that no splicing occurred between the 5′ end of the message and the NotI site used in cloning.
FIG. 1.
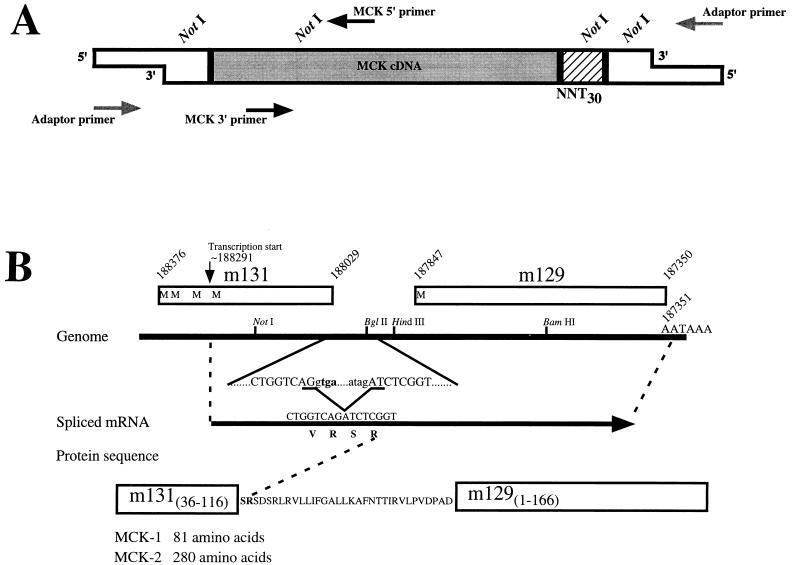
RACE analysis of mRNA encoding the MCMV chemokine homolog. (A) Schematic representation of the RACE strategy. The MCK cDNA to which adaptors had been ligated was amplified by PCR using the indicated primers. The adaptor sequences are shown by open boxes, while the MCK cDNA is shaded. The hatched area represents the oligonucleotide sequences used for priming cDNA synthesis, with NNT30 representing the oligo(dT) portion with the two degenerate nucleotides at the 3′ end of the primer. Approximate locations of the sites where primers used in PCR bind are indicated with arrows, with the direction of the arrow indicating the direction of elongation. 5′ RACE used the MCK 5′ primer (Table 1, primer 6) and the commercially available Adaptor primer, while 3′ RACE used the MCK 3′ primer (Table 1, primer 7) and the Adaptor primer. The locations of NotI sites used in cloning are indicated above the diagram. (B) Schematic representation of RACE results. The region of MCMV genomic DNA surrounding the chemokine homolog is indicated by the solid line, with the location of relevant restriction and AATAAA polyadenylation sites noted. Open boxes with numbers above indicate the locations and genome coordinates (44) of ORFs m131, located in genome fragment HindIII-J, and m129, located in genome fragment HindIII-L, with possible initiator methionines indicated by the letter M. The location and genome coordinate of the 5′ end of the mRNA, as previously mapped (32), is indicated by the arrow above. The nucleotide sequence surrounding the BglII site is shown below the genomic representation, with the location of an 82-nt intron identified by the RACE analysis indicated in lowercase letters. The MCK-1 stop codon (tga) is shown in bold. The resultant mRNA is indicated by the arrow, with the nucleotide and protein sequences shown above and below, respectively. At the bottom, the amino acid sequence which is predicted from the spliced mRNA is shown in the single-letter code. Regions derived from previously identified ORFs are depicted with boxes. The amino acid lengths of proteins predicted to be derived from unspliced (MCK-1) and spliced (MCK-2) mRNAs are indicated.
Analysis of the MCMV genomic sequence (44) downstream of the MCK-1-encoding ORF m131 suggests that the next ORF, designated m129, may be contained within the m131 mRNA, since the first AAUAAA polyadenylation signal is located 3′ to the m129 ORF (Fig. 1B). Our previous analysis (32) by Northern blotting revealed no evidence for a separate mRNA that would encode the protein encoded by m129. To determine the structure of the 3′ end of the MCK-1 mRNA, RACE analysis was performed by PCR amplification of cDNA using the adaptor-specific primer and MCK 3′ primer (oligonucleotide 7 [Table 1]) as indicated schematically in Fig. 1A. A major product of ∼0.9 kb was generated and, after digestion with NotI, cloned.
Comparison of the sequence of 11 independent clones with the MCMV genomic sequence revealed that all 11 cDNA clones lacked an 82-nt genomic sequence located between ORFs m131 (MCK-1) and m129. Inspection of the MCMV genomic sequence reveals splice donor and acceptor sites flanking the 82-nt intron, which are depicted in Fig. 1B. Removal of the 82-nt intron removes the MCK-1 stop codon, and Fig. 1B indicates the sequence of the resultant mRNA and the predicted protein which it would encode. The predicted protein, which we have designated MCK-2, includes amino acids 36 to 116 of ORF m131, 33 amino acids encoded by sequences 5′ to ORF m129, and amino acids 1 to 166 of ORF m129 (44). The MCK-2 protein is depicted schematically in Fig. 2. Interestingly, the splicing event generates an mRNA which predicts a protein which includes an amino-terminal domain with chemokine homology (amino acids 36 to 116 of ORF m131, previously identified as MCK-1) fused to additional sequences, including the entire m129 ORF. Other than the region with homology to mammalian β chemokines, there is no obvious homology to other protein sequences present in the database. The predicted protein contains a putative signal peptide which would target the protein to the endoplasmic reticulum, five potential sites for asparagine-linked glycosylation, possible sites for O-linked glycosylation, as well as possible sites for phosphorylation. In addition, an 18-amino-acid stretch of predominantly hydrophobic amino acids, which might function as a transmembrane sequence, is located downstream of the domain with chemokine homology.
FIG. 2.
Schematic representation of the MCK-2 protein. The predicted amino acid sequence of MCK-2 is indicated in the single-letter code. Amino acid residues derived from ORF m131 are shaded light gray (top); residues derived from ORF m129 are shaded dark gray (bottom). Amino acids in the putative signal peptide are shown in italics, and conserved cysteine residues characteristic of β chemokines are indicated with asterisks. Potential sites for asparagine-linked glycosylation are boxed, and a stretch of predominantly hydrophobic amino acids is underlined.
MCK-2, not MCK-1, mRNA is expressed at late times after infection of fibroblasts with MCMV.
The location of the 82-nt intron, just downstream of the MCK-1 ORF, suggests that both MCK-1 and MCK-2 protein could be generated, from unspliced and spliced mRNAs, respectively. In our RACE analysis we detected only spliced mRNA, possibly due to failure to generate cDNA from unspliced mRNA or amplify the unspliced cDNA during PCR. Levels of spliced and unspliced MCK mRNA were determined by RPA using samples of polyadenylated RNA isolated from cells treated to enrich for viral transcripts of the IE, E, and L kinetic classes. The reagents used for RPA and the predicted results are shown schematically in Fig. 3A. The procedure generated a 32P-labelled RNA antisense to the MCK mRNA which when hybridized to unspliced message would have 610 nt protected from RNase digestion, while hybridization to spliced MCK mRNA would protect 154- and 374-nt portions of the probe. Transcription in vitro generated positive control unspliced and spliced RNAs which after hybridization would protect 609-nt fragments and 154- and 58-nt fragments, respectively, from RNase digestion. The results of the RPA analysis of mRNA from cells enriched for IE, E, and L viral transcripts are shown in Fig. 3B. No MCK-1 or MCK-2 message was detected in the IE or E viral transcripts. In mRNA from cells enriched for viral transcripts of the L kinetic class, spliced message, which would predict production of MCK-2, was detected. No significant amount of message encoding MCK-1 (unspliced) was detected, although a faint band was barely detectable upon prolonged exposures (not shown). Quantitation by phosphorimage analysis revealed approximately 700-fold more spliced than unspliced message, suggesting that in an infected fibroblast, the predominant form of the protein produced is MCK-2 and that little or no MCK-1 is generated. The integrity of the RNA used for RPA is shown in Fig. 3C.
MCK-2 is posttranslationally modified when expressed in eukaryotic cells.
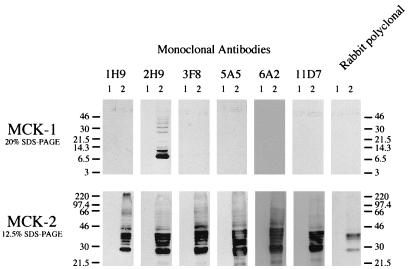
To assess the specificity of antibody reagents and to characterize the expression of MCK-2 in eukaryotic cells, MCK-2 was expressed under the control of the adenovirus major late promoter after transient transfection of COS 1 cells. The bottom panel of Fig. 4 shows MCK-2 expressed in COS 1 cells detected by antibodies generated against bacterially expressed MCK-2. The six monoclonal antibodies as well as the rabbit polyclonal antiserum did not react with proteins present in cells transfected with vector plasmid alone (lane 1) but reacted with a protein migrating in SDS-PAGE consistent with the predicted molecular mass (29.3 kDa) of MCK-2 from which the putative 21-amino-acid signal peptide has been removed (lane 2). In addition, however, multiple immunoreactive forms migrating with increased apparent molecular mass were seen. These findings are consistent with posttranslational modification of MCK-2. After treatment of lysates of cells transfected with the MCK-2 expression plasmid with PNGase F, the immunoreactive species migrated as a single band (see Fig. 7), suggesting that the protein was posttranslationally modified by the addition of asparagine-linked glycans. That MCK-2 contained the domain with chemokine homology was confirmed by the binding of one of the monoclonal antibodies (2H9) which is able to recognize both MCK-1 (the chemokine domain [Fig. 4, top panel]) and MCK-2 expressed by transient transfection of COS 1 cells.
FIG. 4.
Western blot analysis of MCK-2 expression in transfected COS 1 cells. COS 1 cells were transfected with vector alone (lane 1) or expression plasmids (lane 2) for MCK-1 (top) or MCK-2 (bottom). Samples were separated by SDS-PAGE using the indicated percentage acrylamide gels, and Western blot analysis was performed with a panel of anti-MCK-2 monoclonal antibodies as well as polyclonal anti-MCK-2 antiserum. 1H9, 2H9, 3F8, 5A5, 6A2, and 11D7 indicate individual monoclonal antibodies. Rabbit polyclonal indicates the use of rabbit serum from an animal immunized with bacterially expressed MCK-2. Sizes are indicated in kilodaltons.
FIG. 7.
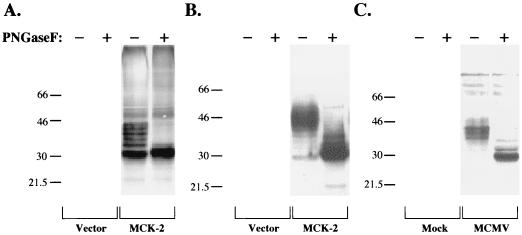
Treatment of MCK-2 with PNGase F. Cell lysates or medium overlying cells were incubated with PNGase F, and the digested proteins were subjected to SDS–12.5% PAGE and Western analysis using anti-MCK-2 monoclonal antibody 5A5. COS 1 cells (A) or the overlying medium (B) were harvested after transfection of the cells with plasmid pmt21 (Vector) or the MCK-2 expression construct pmt21MCK-2-cStop (MCK-2), as indicated. The proteins were incubated with (+) or without (−) PNGase F prior to Western analysis. (C) NIH 3T12 fibroblast cells were mock or MCMV infected as indicated; after 72 h, the cells were harvested and lysed, and proteins were incubated with (+) or without (−) PNGase F. Sizes are indicated in kilodaltons.
MCK-2 can be secreted.
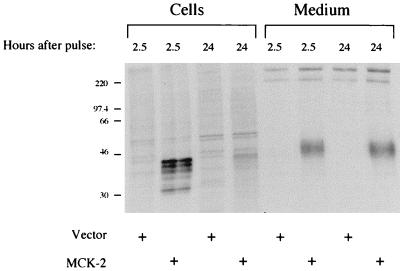
That the MCK-2 primary sequence contains a possible signal peptide and that the protein when expressed in COS 1 cells is posttranslationally modified by the addition of N-linked glycans suggests that the protein traverses through the endoplasmic reticulum during biosynthesis and may well be secreted. To address this, we carried out pulse-chase analysis of MCK-2 expression in transiently transfected COS 1 cells which had been metabolically labelled with 35S-labelled cysteine. Figure 5 shows the labelled proteins from transfected COS 1 cells and the overlying medium which were immunoprecipitated with anti-MCK-2 monoclonal antibody 3F8. As in the Western blot analysis, a group of immunoreactive proteins was detected in cells early after the pulse-labelling. With time these proteins disappeared, consistent with progression through the secretory pathway, or degradation. A posttranslationally modified form of MCK-2 which migrated with even higher apparent molecular mass was detected in the medium, suggesting that additional posttranslational modifications had occurred. Similar results were obtained in assays using several of the anti-MCK-2 monoclonal antibodies (not shown). The protein is not likely a cytoplasmic protein present in the medium due to cellular breakdown, since the cell-associated forms have lower apparent molecular masses. While this protein is most likely secreted, since similar amounts are seen at 2.5 and 24 h after the pulse-label, during which time the cell associated forms disappear, it is not possible to conclude a direct precursor-product relationship between the cell-associated and secreted forms. Western analysis using anti-MCK-2 antiserum confirms these results since concentrates of medium overlying transfected COS 1 cells revealed a protein of similar size (not shown). Additionally, the protein is not likely present in the medium in a membrane-associated form, since after centrifugation at 100,000 × g it remains in the supernatant fraction (not shown). Together these data support the hypothesis that MCK-2 is extensively posttranslationally modified and secreted from the cell.
FIG. 5.
Immunoprecipitation of cell-associated and secreted MCK-2 from metabolically labelled COS 1 cells. COS 1 cells were transfected with vector plasmid pmt21 or the MCK-2 expression plasmid pmt21MCK-2-cStop and metabolically labelled for 30 min with [35S]cysteine. The cells were then incubated for the indicated times, cells and medium overlying the cells were harvested, and proteins were immunoprecipitated with anti-MCK-2 monoclonal antibody 3F8. The samples were subjected to SDS–12.5% PAGE and fluorography, and an autoradiograph of the gel is shown. Similar results were obtained with anti-MCK-2 monoclonal antibodies 2H9, 5A5, and 6A2 (not shown). Sizes are indicated in kilodaltons.
MCK-2 protein is expressed in MCMV-infected fibroblasts.
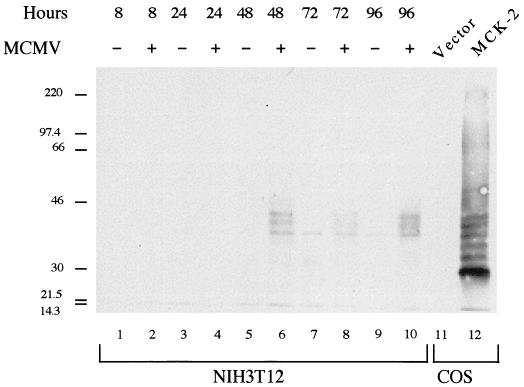
To confirm that MCK-2 is expressed in the context of viral infection, Western blot analysis was carried out on mock- or MCMV-infected fibroblast cells. Figure 6 shows that MCK-2 was detected at late times after infection with MCMV. We detected two specific immunoreactive species (lanes 6, 8, and 10) which migrated in SDS-PAGE with characteristics suggestive of posttranslational modification. The identity of a third immunoreactive protein detected at late times in both mock- and MCMV-infected cells (lanes 6 to 10) is unknown. While in this experiment MCK-2 was detected first at 48 h after infection with MCMV, in a similar experiment the proteins were first detected 24 h after infection, with maximal levels seen at 72 h after infection (not shown). Consistent with our analysis of mRNA expression (Fig. 3), MCK-1 was undetectable (not shown) in infected fibroblast cells by both Western blot and immunoprecipitation analyses using monoclonal antibody 2H9, which recognizes MCK-1 (Fig. 4).
FIG. 6.
Expression of MCK-2 in fibroblasts infected with MCMV. NIH 3T12 fibroblast cells were mock infected or infected with MCMV at multiplicity of infection of 5, and cells were harvested at the indicated times after infection. An aliquot of each sample was subjected to SDS–12.5% PAGE and Western analysis using anti-MCK-2 monoclonal antibody 5A5. Lanes 1 through 10 contain NIH 3T12 cell samples. Lanes 11 and 12 show for comparison proteins present in COS 1 cells transfected with vector pmt21 or the MCK-2 expression construct pmt21MCK-2-cStop, respectively. Sizes are indicated in kilodaltons.
MCK-2 is posttranslationally modified by the addition of asparagine-linked glycans.
MCK-2 expressed in transfected COS 1 cells and after infection of fibroblasts with MCMV was treated with PNGase F, and the results are shown in Fig. 7. After PNGase F treatment, the multiple MCK-2 immunoreactive forms migrated predominantly as a single band with an apparent molecular mass of approximately 30 kDa, indicating that the MCK-2 forms with apparent higher molecular mass contain asparagine-linked glycans.
DISCUSSION
In this report we show the following important points. First, the previously described (44) MCMV ORF (m131) which encodes the β chemokine homolog MCK-1, whose mRNA transcriptional start site was previously mapped (32), is expressed from a spliced mRNA encoding a chemokine homolog, designated MCK-2, of novel predicted structure. Second, the 3′ exon of this mRNA contains amino acid-encoding sequences 5′ to the previously identified m129 ORF (44) and includes the entire m129 ORF in frame. Third, while the genomic structure of the MCK-1/MCK-2 locus presents the opportunity to express both MCK-1 and MCK-2 proteins from unspliced and spliced mRNAs, respectively, in infected fibroblasts, only the MCK-2-encoding mRNA is abundantly expressed. Fourth, using a newly derived panel of monoclonal antibodies, we detected MCK-2 protein in MCMV-infected cells. Finally, expression of MCK-2 in COS 1 cells demonstrates that a posttranslationally modified form of MCK-2 which contains N-linked glycans is released into the medium overlying the cells.
DNA viruses and the mammalian chemokine network.
Data emerging over the past few years indicate that influencing or subverting the mammalian chemokine pathway may be a common mechanism by which DNA viruses gain a pathogenic advantage. Poxviruses have been shown to secrete chemokine binding proteins which function in the context of viral infection to limit the number of inflammatory cells present at the site of infection (21, 31). In addition, several poxvirus family members contain possible chemokine receptor homologs identified by sequence analysis (14, 35). The genome of molluscum contagiosum virus contains an ORF with homology to β chemokines (48), and the expressed protein antagonizes the biological activity of a variety of both α and β chemokines (16).
Herpesvirus family members appear to interact in several ways with the mammalian chemokine pathway. Epstein-Barr virus, human herpesvirus (HHV-6), and HHV-7 have been shown to upregulate expression of host chemokine receptors upon infection (11, 23), and HHV-6 (20) and HHV-7 (39) each contain ORFs predicting possible G-protein-coupled receptors. The HHV-6 U12 ORF product binds several β chemokines with resultant mobilization of calcium and may play a role in pathogenesis through β chemokine-mediated signalling (26). Herpesvirus saimiri, HHV-8, and murine gammaherpesvirus 68, all members of the gammaherpesvirus family, contain genes encoding α chemokine receptor homologs (2, 15, 22, 52). Interestingly, the herpesvirus saimiri receptor homolog binds interleukin-8 and other α chemokines and signals, as assessed by changes in intracellular calcium concentrations (1), while the HHV-8 receptor homolog appears to be constitutively active, conferring a growth advantage as well as tumorigenicity to cells in which it is expressed (4, 6). HHV-8 also encodes several β chemokine homologs (36, 40) which appear to be functional, as assessed by their ability to block human immunodeficiency virus infection mediated by cellular chemokine receptors (36), to antagonize host chemokine action through a broad spectrum of α and β chemokine receptors (29), and to signal through a known CC chemokine receptor (13). HCMV also contains an ORF (US28) which both in the context of viral infection and when expressed is able to signal upon addition of β chemokines (10, 19, 38, 51) and is able to function as a chemokine coreceptor for human immunodeficiency virus infection (42). The protein may also function to alter the chemokine environment by binding and sequestration of β chemokines (12). HCMV contains two other G-protein-coupled receptor homologs, one of which (UL33) has been determined to be a virion protein and to be nonessential for growth in tissue culture cells (34).
We initially noted the homology between ORF m131 (44) of MCMV and mammalian β chemokines, and we characterized the 5′ end and expression of viral mRNA encoding the chemokine homolog, which we designated MCK-1 (32). We initiated further analysis of the RNA structure so that an appropriate strategy for generation of mutant virus specifically lacking MCK-1 could be developed. In this report we show the surprising results that the MCK-1 mRNA is spliced and that the sequence of the predicted protein, MCK-2, is unique among known viral and mammalian β chemokines. While ORFs encoding possible chemokine homologs have been noted in genomic sequences of other DNA viruses (20, 36, 40, 48), this work clearly shows that the possibility should be considered that the sequences encoding the ORFs represent exons of larger genes which encode novel proteins. Determination of the sequence of the virus-encoded RNA and/or protein made during infection is critical for the appropriate interpretation of the possible roles of ORF products in viral pathogenesis.
Precedent for β chemokines of novel predicted structure.
We have deduced the primary amino acid sequence of what is likely a novel member of the β chemokine subfamily of chemotactic cytokines. The protein contains an amino-terminal putative signal sequence, followed by a domain with high homology to β chemokines which contains one potential site for asparagine-linked glycosylation. The chemokine domain is followed by a stretch of hydrophobic amino acids separating the amino-terminal portion from the carboxyl-terminal domain, which lacks obvious homology to known or predicted protein sequences in the database. Recently an unusual membrane-anchored chemokine, fractalkine (8, 41), a δ (or CX3C) chemokine, was described as containing (i) a chemokine domain on a mucin-like stalk and (ii) a short cytoplasmic tail. Interestingly, the δ family member may exist as a soluble as well as a membrane-bound form and has been found to be expressed in activated endothelial cells. Given the ability of the soluble form to promote chemotaxis of monocytes and T cells and the full-length form to promote cellular adhesion, it has been postulated to control leukocyte trafficking at the endothelium (8). The molecule also has been found to be expressed by activated brain microglial cells and to be chemotactic for neutrophils and T lymphocytes, and it may play a role in inflammatory processes in the brain (41). One might postulate that the central hydrophobic stretch of MCK-2 can function as a stop transfer sequence to generate a type I membrane protein with the chemokine domain exposed on the cell surface and the carboxyl-terminal domain functioning as a cytoplasmic tail. Similar to functions proposed for fractalkine, MCK-2 might facilitate cellular adhesion or control leukocyte trafficking at the site of MCMV infection. In addition, the cytoplasmic tail suggests that the possibility of signalling through MCK-2 should be considered.
Although these are intriguing considerations, our studies here indicate that at least one form of posttranslationally modified MCK-2 is secreted from COS 1 cells (Fig. 5). It is possible that this represents a portion of the MCK-2 molecule being shed into the culture medium such as has been seen after expression of recombinant fractalkine (8). However, the apparent molecular mass by SDS-PAGE of secreted MCK-2 suggests extensive posttranslational modification. Our studies show that both intracellular and secreted forms contain multiple N-linked glycans. Since only one of the five possible sites for N-linked glycan addition is located within the chemokine domain, it seems likely that the carboxyl-terminal portion of MCK-2 is present within the secreted form. In addition, the secreted form is recognized by monoclonal antibodies generated against MCK-2 which do not recognize epitopes in the chemokine domain and thus recognize epitopes within the carboxyl-terminal portion of the molecule. Future studies will address directly whether MCK-2 exists in a membrane-bound form.
Finally, we have shown that this virally encoded novel homolog of the β chemokine subfamily of chemotactic cytokines is expressed within cells infected with MCMV. Thus, MCK-2 has the potential to contribute to pathogenesis, possibly through agonist or antagonist action at host (or viral) chemokine receptors. The ability to study viral pathogenesis in a small-animal model provides an opportunity to begin to understand the role of the mammalian chemokine network in host defense and the role of viral chemokine and chemokine receptor homologs in pathogenesis. Knowledge gained on the role of host chemokines and the MCMV chemokine homolog in pathogenesis is likely to be helpful in understanding the pathogenesis of viruses, including HCMV, for which there are no small-animal models. The unusual nature of the MCMV-encoded β chemokine homolog suggests that the molecule may exhibit biological properties beyond agonism or antagonism at chemokine receptors which might contribute to pathogenesis. While these studies indicate that in infected fibroblasts it is MCK-2, not MCK-1, which is expressed, it is possible that cell- or tissue-specific splicing results in differential expression of MCK-1 and MCK-2 within an infected animal. Further studies including analysis of the pathogenesis of mutant MCMV lacking MCK-2, as well as further characterization of MCK-1 and MCK-2 expression in vivo, are needed to address the possible role(s) of the MCMV-encoded chemokine homolog in pathogenesis.
ACKNOWLEDGMENTS
We thank Barrett Rollins for providing plasmid pmt21, Deborah Spector for MCMV HindIII-D in pACYC184, Brett Lindenbach for helpful suggestions concerning RPA analysis, and Kathy Sheehan for providing advice in generating monoclonal antibodies.
This work was supported by Public Health Service grants K08 AI-01418 (M.R.M.) and R01 AI-39616 (H.W.V.) from the National Institutes of Health.
REFERENCES
- 1.Ahuja S K, Murphy P M. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J Biol Chem. 1993;268:20691–20694. [PubMed] [Google Scholar]
- 2.Albrecht J-C, Nicholas J, Biller D, Cameron K R, Biesinger B, Newman C, Wittmann S, Craxton M A, Coleman H, Fleckenstein B, Honess R W. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson C W, Baum P R, Gesteland R F. Processing of adenovirus 2-induced proteins. J Virol. 1973;12:241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn M C, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 5.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 6.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Cesarman E, Gerhengorn M C, Mesri E A. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin E T, Weber I T, St. Charles R, Xuan J, Appella E, Yamada M, Matsushima K, Edwards B F P, Clore G M, Gronenborn A M, Wlodawer A. Crystal structure of interleukin 8: symbiosis of NMR and crystallography. Proc Natl Acad Sci USA. 1991;88:502–506. doi: 10.1073/pnas.88.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greaves D R, Zlotnik A, Schall T J. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Baruch A, Michiel D F, Oppenheim J J. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–11706. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- 10.Billstrom M A, Johnson G L, Avdi N J, Worthen G S. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J Virol. 1998;72:5535–5544. doi: 10.1128/jvi.72.7.5535-5544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol. 1997;67:2209–2220. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodaghi B, Jones T R, Zipeto D, Vita C, Sun L, Laurent L, Arenzana-Seisdedos F, Virelizier J L, Michelson S. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J Exp Med. 1998;188:855–866. doi: 10.1084/jem.188.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boshoff C, Endo Y, Collins P D, Takeuchi Y, Reeves J D, Schweickart V L, Siani M A, Sasaki T, Williams T J, Gray P W, Moore P S, Chang Y, Weiss R A. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 14.Cao J X, Gershon P D, Black D N. Sequence analysis of HindIII Q2 fragment of Capripoxvirus reveals a putative gene encoding a G-protein-coupled chemokine receptor homologue. Virology. 1995;209:207–212. doi: 10.1006/viro.1995.1244. [DOI] [PubMed] [Google Scholar]
- 15.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, Knowles D M. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damon I, Murphy P M, Moss B. Broad spectrum chemokine antagonistic activity of a human poxvirus chemokine homolog. Proc Natl Acad Sci USA. 1998;95:6403–6407. doi: 10.1073/pnas.95.11.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis-Poynter N J, Lynch D M, Vally H, Shellam G R, Rawlinson W D, Barrell B G, Farrell H E. Identification and characterization of a G protein-coupled receptor homolog encoded by murine cytomegalovirus. J Virol. 1997;71:1521–1529. doi: 10.1128/jvi.71.2.1521-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobbins J G, Stewart J A, Demmler G J. Surveillance of congenital cytomegalovirus disease, 1990–1991. Morbid Mortal Weekly Rep. 1992;41:35–44. [PubMed] [Google Scholar]
- 19.Gao J L, Murphy P M. Human cytomegalovirus open reading frame US28 encodes a functional β chemokine receptor. J Biol Chem. 1994;269:28539–28542. [PubMed] [Google Scholar]
- 20.Gompels U A, Nicholas J, Lawrence G, Jones M, Thomson B J, Martin M E D, Efstathiou S, Craxton M, Macaulay H A. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology. 1995;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 21.Graham K A, Lalani A S, Macen J L, Ness T L, Barry M, Liu L, Lucas A, Clark-Lewis I, Moyer R W, McFadden G. The T1/35kDa family of poxvirus-secreted proteins bind chemokines and modulate leukocyte influx into virus-infected tissues. Virology. 1997;229:12–24. doi: 10.1006/viro.1996.8423. [DOI] [PubMed] [Google Scholar]
- 22.Guo H, Browning P, Nicholas J, Hayward G S, Tschachler E, Jiang Y, Sadowska M, Raffeld M, Colombini S, Gallo R C, Reitz M S., Jr Characterization of a chemokine receptor-related gene in human herpesvirus 8 and its expression in Kaposi’s sarcoma. Virology. 1997;228:371–378. doi: 10.1006/viro.1996.8386. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa H, Utsunomiya Y, Yakukawa M, Yanagisawa K, Fujita S. Induction of G protein-coupled peptide receptor EBI 1 by human herpesvirus 6 and 7 infection in CD4+ T cells. J Virol. 1994;68:5326–5329. doi: 10.1128/jvi.68.8.5326-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heise M T, Virgin H W., IV The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infection. J Virol. 1995;69:904–909. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall T J, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 26.Isegawa Y, Ping Z, Nakano K, Sugimoto N, Yamanishi K. Human herpesvirus 6 open reading frame U12 encodes a functional β-chemokine receptor. J Virol. 1998;72:6104–6112. doi: 10.1128/jvi.72.7.6104-6112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keil G M, Ebeling-Keil A, Koszinowski U H. Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate early times after infection. J Virol. 1984;50:784–795. doi: 10.1128/jvi.50.3.784-795.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelner G S, Kennedy J, Bacon K B, Kleyensteuber S, Largaespada D A, Jenkins N A, Copeland N G, Bazan J F, Moore K W, Schall T J, Zlotnik A. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 29.Kledal T N, Rosenkilde M M, Coulin F, Simmons G, Johnsen A H, Alouani S, Power C A, Luttichau H R, Gerstoft J, Clapham P R, Clark-Lewis I, Wells T N C, Schwartz T W. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Lalani A S, Graham K, Mossman K, Rajarathnam K, Clark-Lewis I, Kelvin D, McFadden G. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J Virol. 1997;71:4356–4363. doi: 10.1128/jvi.71.6.4356-4363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald M R, Li X-Y, Virgin H W., IV Late expression of a β chemokine homolog by murine cytomegalovirus. J Virol. 1997;71:1671–1678. doi: 10.1128/jvi.71.2.1671-1678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackay C R. Chemokines: what chemokine is that? Curr Biol. 1997;7:R384–R386. doi: 10.1016/s0960-9822(06)00181-3. [DOI] [PubMed] [Google Scholar]
- 34.Margulies B J, Browne H, Gibson W. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology. 1996;225:111–125. doi: 10.1006/viro.1996.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massung R F, Jayarama V, Moyer R W. DNA Sequence analysis of conserved and unique regions of swinepox virus: identification of genetic elements supporting phenotypic observations including a novel G protein-coupled receptor homologue. Virology. 1993;197:511–528. doi: 10.1006/viro.1993.1625. [DOI] [PubMed] [Google Scholar]
- 36.Moore P S, Boshoff C, Weiss R A, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 37.Murphy P M. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 38.Neote K, DiGregorio D, Mak J Y, Horuk R, Schall T J. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell. 1993;72:415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- 39.Nicholas J. Determination and analysis of the complete nucleotide sequence of human herpesvirus 7. J Virol. 1996;70:5975–5989. doi: 10.1128/jvi.70.9.5975-5989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholas J, Ruvolo V R, Burns W H, Sandford G, Wan X, Ciufo D, Hendrickson S B, Guo H, Hayward G S, Reitz M S. Kaposi’s sarcoma-associated human herpesvirus-8 encodes homologs of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 41.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo J, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos J C, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 42.Pleskoff P, Treboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 43.Pollock J L, Virgin H W., IV Latency, without persistence, of murine cytomegalovirus in spleen and kidney. J Virol. 1995;69:1762–1768. doi: 10.1128/jvi.69.3.1762-1768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rawlinson W D, Farrell H E, Barrell B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubin R H. Impact of cytomegalovirus infection on organ transplant recipients. Rev Infect Dis. 1990;12(Suppl. 7):S754–S766. doi: 10.1093/clinids/12.supplement_7.s754. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 47.Schooley R T. Cytomegalovirus in the setting of infection with human immunodeficiency virus. Rev Infect Dis. 1990;12(Suppl. 7):S811–S819. doi: 10.1093/clinids/12.supplement_7.s811. [DOI] [PubMed] [Google Scholar]
- 48.Senkevich T G, Bugert J J, Sisler J R, Koonin E V, Darai G, Moss B. Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes. Science. 1996;273:813–816. doi: 10.1126/science.273.5276.813. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan K C, Calderon J, Schreiber R D. Generation and characterization of monoclonal antibodies specific for the human IFN-gamma receptor. J Immunol. 1988;140:4231–4237. [PubMed] [Google Scholar]
- 50.Vieira J, Farrell H E, Rawlinson W D, Mocarski E S. Genes in the HindIII J fragment of the murine cytomegalovirus genome are dispensable for growth in cultured cells: insertional mutagenesis with a lacZ/gpt cassette. J Virol. 1994;68:4837–4846. doi: 10.1128/jvi.68.8.4837-4846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vieira J, Schall T J, Corey L, Geballe A P. Functional analysis of the human cytomegalovirus US28 gene by insertion mutagenesis with the green fluorescent protein gene. J Virol. 1998;72:8158–8165. doi: 10.1128/jvi.72.10.8158-8165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virgin H W, IV, Latreille P, Wamsley P, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Virgin H W, IV, Mann M A, Fields B N, Tyler K L. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J Virol. 1991;65:6772–6781. doi: 10.1128/jvi.65.12.6772-6781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winston D J, Ho W G, Champlin R E. Cytomegalovirus infections after allogeneic bone marrow transplantation. Rev Infect Dis. 1990;12(Suppl. 7):S776–S792. doi: 10.1093/clinids/12.supplement_7.s776. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida T, Imai T, Kakizaki M, Nishimura M, Takagi S, Yoshie O. Identification of single C motif-1/lymphotactin receptor XCR1. J Biol Chem. 1998;273:16551–16554. doi: 10.1074/jbc.273.26.16551. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida T, Imai T, Kakizaki M, Nishimura M, Yoshie O. Molecular cloning of a novel C or gamma type chemokine, SCM-1. FEBS Lett. 1995;360:155–159. doi: 10.1016/0014-5793(95)00093-o. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y J, Rutledge B J, Rollins B J. Structure/activity analysis of human monocyte chemoattractant protein-1 (MCP-1) by mutagenesis. J Biol Chem. 1994;269:15918–15924. [PubMed] [Google Scholar]