PURPOSE
Small-cell lung cancer (SCLC) is an aggressive malignancy with limited treatments. Delta-like ligand 3 (DLL3) is aberrantly expressed in most SCLC. Tarlatamab (AMG 757), a bispecific T-cell engager molecule, binds both DLL3 and CD3 leading to T-cellb–mediated tumor lysis. Herein, we report phase I results of tarlatamab in patients with SCLC.
PATIENTS AND METHODS
This study evaluated tarlatamab in patients with relapsed/refractory SCLC. The primary end point was safety. Secondary end points included antitumor activity by modified RECIST 1.1, overall survival, and pharmacokinetics.
RESULTS
By July 19, 2022, 107 patients received tarlatamab in dose exploration (0.003 to 100 mg; n = 73) and expansion (100 mg; n = 34) cohorts. Median prior lines of anticancer therapy were 2 (range, 1-6); 49.5% received antiprogrammed death-1/programmed death ligand-1 therapy. Any-grade treatment-related adverse events occurred in 97 patients (90.7%) and grade b % 3 in 33 patients (30.8%). One patient (1%) had grade 5 pneumonitis. Cytokine release syndrome was the most common treatment-related adverse event, occurring in 56 patients (52%) including grade 3 in one patient (1%). Maximum tolerated dose was not reached. Objective response rate was 23.4% (95% CI, 15.7 to 32.5) including two complete and 23 partial responses. The median duration of response was 12.3 months (95% CI, 6.6 to 14.9). The disease control rate was 51.4% (95% CI, 41.5 to 61.2). The median progression-free survival and overall survival were 3.7 months (95% CI, 2.1 to 5.4) and 13.2 months (95% CI, 10.5 to not reached), respectively. Exploratory analysis suggests that selecting for increased DLL3 expression can result in increased clinical benefit.
CONCLUSION
In patients with heavily pretreated SCLC, tarlatamab demonstrated manageable safety with encouraging response durability. Further evaluation of this promising molecule is ongoing.
INTRODUCTION
Small-cell lung cancer (SCLC) is an aggressive lung cancer subtype with neuroendocrine differentiation diagnosed in more than 150,000 people worldwide each year.1,2 The 3-year survival rate for patients with extensive stage (ES) SCLC is 6%.3 The addition of immune checkpoint inhibitors atezolizumab or durvalumab to platinum and etoposide chemotherapy followed by maintenance therapy with checkpoint inhibitor alone as first-line treatment for SCLC has led to approximately 30% reduction in the risk of death and durable but modest survival gains for a small subset of patients with ES-SCLC.3,4 Available therapies are limited for the majority of patients with SCLC who relapse. Topotecan, the most widely used second-line agent globally, has limited efficacy and an unfavorable safety profile.5,6 Lurbinectedin, in 2020 became the first drug approval by the US Food and Drug Administration in over 20 years for second line, was conditionally approved on the basis of an objective response rate (ORR) of 35%; however, a randomized study failed to demonstrate OS benefit.7,8 No agent is specifically approved for third-line treatment of relapsed SCLC.
CONTEXT
Key Objective
Tarlatamab, a half-life extended bispecific T-cell engager molecule targeting delta-like ligand 3, is the first agent in this new therapeutic class to be evaluated clinically in patients with small-cell lung cancer (SCLC). An analysis of data from the large phase I study of tarlatamab in patients with relapsed/refractory SCLC was performed to assess safety and efficacy.
Knowledge Generated
With more than 100 patients treated in the phase I study, tarlatamab has shown tolerability and remarkable antitumor activity over a wide range of target doses in patients with heavily pretreated SCLC. A promising response rate, high median duration of response, and encouraging median overall survival, along with a reasonable safety profile, observed in this study form the basis for planned and ongoing later-stage studies.
Relevance (T.E. Stinchcombe)
-
Tarlatamab has a novel mechanism of action, and the activity in previously treated SCLC, a patient population with few therapeutic options, is promising. The results of additional studies are eagerly awaited.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
The notch signaling pathway is a regulator of neuroendocrine differentiation in SCLC.9,10 The inhibitory notch ligand delta-like ligand 3 (DLL3) is aberrantly expressed on the surface of up to 85% of SCLC cells and minimally expressed in normal tissues, making it a compelling therapeutic target.11-14 In vitro SCLC models have indicated a role for DLL3 in promoting tumor growth, migration, and invasion.15 The DLL3-targeted antibody-drug conjugate rovalpituzumab tesirine showed clinical antitumor activity in patients with SCLC.16
Tarlatamab, a half-life extended bispecific T-cell engager (HLE BiTE) molecule, binds both DLL3 on cancer cells and CD3 on T cells leading to T-cell–mediated tumor lysis. Tarlatamab promotes tumor regression in preclinical models of SCLC.17 Tarlatamab is the first DLL3-targeted immune therapy to be evaluated clinically in SCLC.
In this phase I trial, we evaluated the safety, pharmacokinetics (PK), and preliminary efficacy of tarlatamab in patients with SCLC.
PATIENTS AND METHODS
Study Design and Participants
DeLLphi-300 (ClinicalTrials.gov identifier: NCT03319940) is a phase I, multicountry, open-label, dose-escalation study evaluating tarlatamab monotherapy and combination with antiprogrammed cell death protein one (PD-1) therapy in patients with SCLC. The results reported here are limited to the monotherapy regimen including dose escalation and expansion cohorts. Eligible patients were age 18 years or older with histologically or cytologically confirmed SCLC who had progressed or recurred after at least one previous platinum-based regimen and, if standard of care, a programmed cell death ligand 1 (PD-L1) inhibitor in addition to chemotherapy. Patients had an Eastern Cooperative Oncology Group performance status of two or less and at least two measurable lesions defined per modified response evaluation criteria in solid tumors (RECIST), version 1.1 and if present, brain metastases that were clinically and radiologically stable after treatment. Key exclusion criteria were untreated brain metastases and severe or recurrent immune-mediated adverse events (AEs) or infusion-related reactions while on prior immunotherapy. Full eligibility criteria are included in the study Protocol (online only).
The protocol and amendments were approved by the institutional review board or ethics committee at each participating site. The trial was conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. All patients provided written informed consent.
Procedures
The planned tarlatamab dose levels were 0.003, 0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, and 100 mg by intravenous (IV) infusion administered every 2 weeks. The first four dose levels were planned as single-patient cohorts as AEs were expected to be low. Tarlatamab administration was continued until disease progression, unacceptable side effects, or consent withdrawal.
Outcomes
The primary end point was safety including dose-limiting toxicities (DLTs; defined as tarlatamab-related toxicity within 28 days after the first dose and meeting protocol criteria), AEs during the treatment period (treatment-emergent AEs [TEAEs]), and TEAEs possibly related to tarlatamab per investigator review (treatment-related AEs). Secondary end points included PK, antitumor activity including objective response per modified RECIST 1.118-20 by investigator assessment, duration of response (DOR), time to response, progression-free survival (PFS), and overall survival (OS). Exploratory end points assessed included antitarlatamab antibody formation and relationship between baseline target protein expression and clinical benefit (Data Supplement [online only]).
Statistical Analysis
This analysis included patients enrolled in escalation and expansion cohorts. Data cutoff was July 19, 2022. Sample size was based on clinical considerations and standard dose-escalation design. A two-parameter Bayesian Logistic Regression Model guided dose exploration.21 The Sponsor, in consultation with investigators, reviewed the Bayesian Logistic Regression Model recommended dose level and cumulative data by cohort before dose escalation decisions. AEs and DLTs were evaluated continually. On the basis of the overall benefit-risk profile of 100 mg, this dose was further evaluated as the expansion dose. Descriptive statistics are provided for selected demographics, safety, PK, pharmacodynamics, and biomarker data. Exploratory analysis was performed to evaluate the relationship between baseline expression of DLL3 and clinical benefit (Data Supplement). Kaplan-Meier methods were used to estimate the median and percentiles for time to event end points with 95% CI calculated using the Brookmeyer and Crowley22 method.
RESULTS
Patients
Between December 26, 2017, and April 28, 2022, 107 patients received tarlatamab in dose escalation (0.003-100 mg; n = 73) and expansion (100 mg; n = 34) cohorts (Data Supplement). Step dosing was used starting with the 3-mg cohort (using 1 mg as the run-in dose, followed by target dose on day 8, day 15, and every 2 weeks thereafter) because of observed cytokine release syndrome (CRS) in prior cohorts.
Baseline characteristics are summarized in Table 1 (additional in Data Supplement). The median age was 63 years (range, 32-80). Eastern Cooperative Oncology Group performance status was 0-1 in 99% of patients. More than 70% of patients had b % 2 lines of prior therapy, 25% were platinum refractory, and 50% had prior PD-1/PD-L1 inhibitor.
TABLE 1.
Patient Demographics and Baseline Characteristics
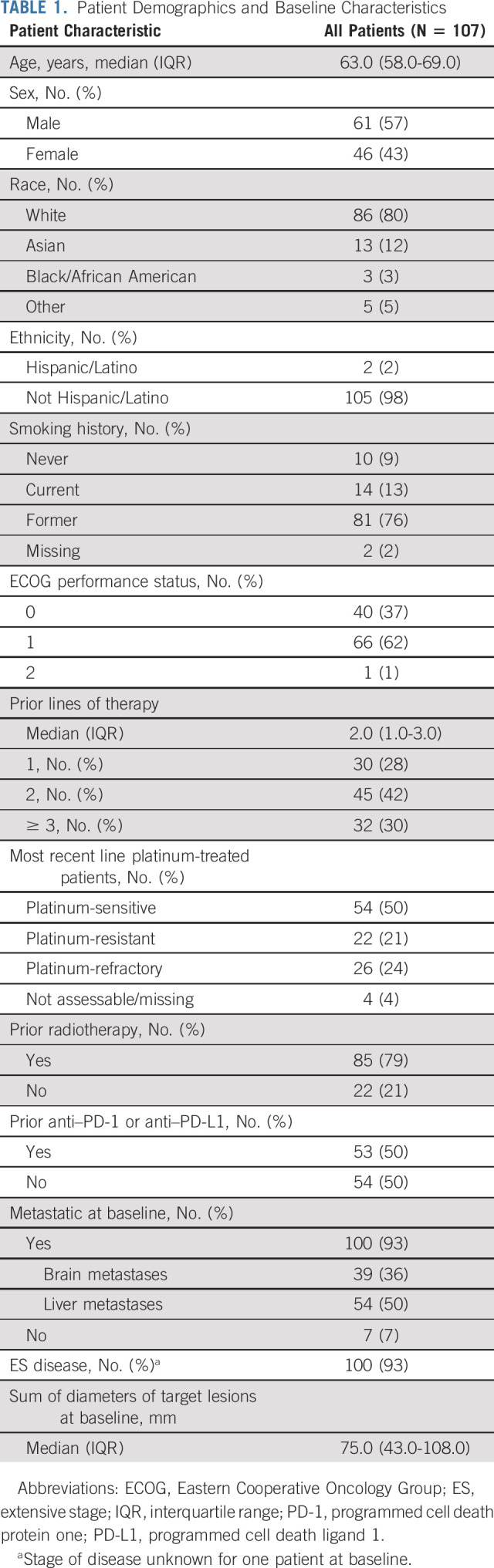
The median follow-up was 8.7 months (range, 0.2-31.8). Treatment was discontinued in 92 patients (86%), most commonly for disease progression (n = 77 [72%]). At data cutoff, 47 patients (43.9%) had ended study because of death. The median number of treatment cycles started was 3 (interquartile range [IQR], 1-8), and the median number of tarlatamab doses received was 6 (IQR, 3-16).
Retrospective DLL3 immunohistochemistry analysis showed that DLL3 was expressed (b % 1%) in 85 of 90 (94%) evaluable patients; the median H-score was 186 (range, 0-300), and the median tumor cell positivity was 95% (range, 0%-100%).
Safety and Tolerability
DLTs occurred in six patients including pneumonitis (n = 1 [last prior dose, 0.3 mg]), increased alanine aminotransferase (n = 1 [1 mg]), CRS (n = 1 [1 mg]), encephalopathy (n = 1 [10 mg]), chills, pyrexia, and neutropenia (n = 1 each [100 mg]). A maximum tolerated dose was not reached; the highest protocol-planned dose (100 mg) was evaluated in the expansion cohort. Four patients (3.7%) discontinued tarlatamab because of treatment-related AEs of encephalopathy (n = 1), immune effector cell-associated neurotoxicity (ICANS; n = 1), and pneumonitis (n = 2). A single G5 pneumonitis event was recorded in a 70-year old man with a history of prior carboplatin/etoposide chemotherapy, chronic obstructive pulmonary disease, and radiation to the lung and pleural nodules. The event onset was cycle 1 day 18, 3 days after the second tarlatamab treatment (both doses 0.3 mg), and was confounded by clinically significant disease progression at the time of pneumonitis requiring urgent palliative radiation to the lung and to a soft tissue mass in the thoracic spine causing spinal cord compression. The cause of death was attributed by the investigator to disease progression and pneumonitis. An additional G3 and three additional G2 TEAEs of pneumonitis were observed (5 of 107 [4.7%] overall incidence of pneumonitis). Among patients with G2 pneumonitis, one patient ended treatment because of neurotoxicity (not pneumonitis), one patient had resolution of pneumonitis before discontinuation for PD, and one patient resumed treatment without dose change.
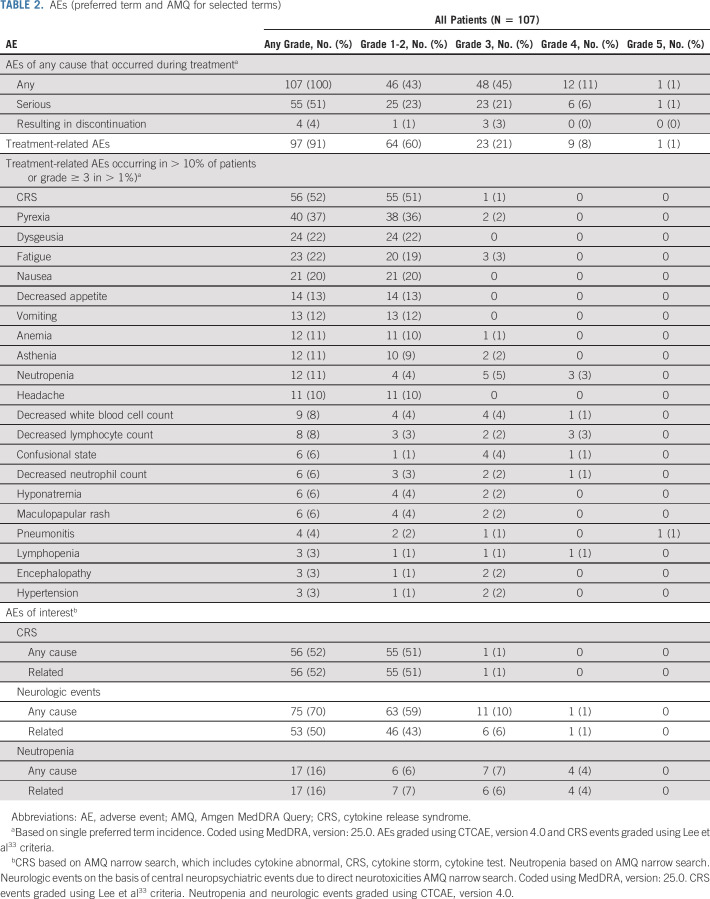
TEAEs of any cause/grade occurred in 107 patients (100%; Table 2 and Data Supplement). The most common were CRS (56 patients [52.3%]), pyrexia (43 [40.2%]), and constipation (33 [30.8%]). Grade b % 3 AEs occurred in 61 patients (57.0%) with the most common being neutropenia (8.4%; Data Supplement). Serious AEs occurred in 55 patients (51.4%; Data Supplement). TEAEs led to dose reductions in nine patients (8.4%) with 4 (3.7%) having CRS-related reductions (Data Supplement). Dose interruption occurred in 20 patients (18.7%; Data Supplement). Any-grade and grade b % 3 treatment-related AEs occurred in 97 (90.7%) and 33 (30.8%) patients, respectively (Data Supplement).
TABLE 2.
AEs (preferred term and AMQ for selected terms)
CRS, neutropenia, and neurologic events were monitored as events of interest on the basis of preclinical, clinical, and mechanistic data with tarlatamab, other BiTE molecules, and other T-cell–associated therapies. Amgen MedDRA Query narrow searches were performed to supplement standard system organ class single preferred term safety reporting (defined in Data Supplement and summarized in Table 2). Measures to ameliorate the potential for CRS included prophylactic corticosteroids (cycle 1 only) and IV hydration. Grade b % 2 treatment-emergent CRS was reported in 15 patients (14.0%) and grade 3 CRS in one patient (0.9%); no grade 4 or 5 CRS has been reported. In patients where time of onset of CRS as well as date of onset of CRS was recorded (n = 47), the median time to onset of CRS after the last dose was 17.5 hours. CRS was transient (median duration, 3 days [IQR, 2-4 days]) and resolved in all cases. Eight patients (7.5%) received tocilizumab for CRS. CRS was largely confined to cycle 1. A total of five patients (4.7%) had CRS in cycle 2 or later; four had prior CRS in cycle 1 while one first experienced CRS in cycle 2. Treatment-emergent neurologic AEs of any grade occurred in 75 patients (70.1%) and were mostly grade 1: dysgeusia (29.0%), headache (19.6%), and dizziness (10.3%) were most common. Grade b % 3 treatment-emergent neurologic events occurred in 12 patients (11.2%) including confusional state (4.7%), delirium (1.9%), and encephalopathy (1.9%). One patient had a grade 4 neurologic event (confusion), and none had grade 5. All grade b % 3 neurologic AEs resolved, with one patient discontinuing tarlatamab because of G3 encephalopathy and two other patients continuing treatment at reduced doses. G2 ICANS was the other neurologic cause leading to discontinuation in one patient. First onset of any grade neurological event was mostly within the first 30 days of treatment (median, 9 days [IQR, 2-29 days]) with a median duration of 5 days (IQR, 2-15 days). Grade b % 3 neutropenia occurred in 11 patients (10.3%). Any-grade neutropenia first onset occurred at a median of 30 days (IQR, 21-31 days) after first tarlatamab administration, and the median duration was 7 days (IQR, 4-13); overall, 10 patients (9.3%) received G-CSF. Febrile neutropenia occurred in one patient and was not considered treatment related.
Efficacy
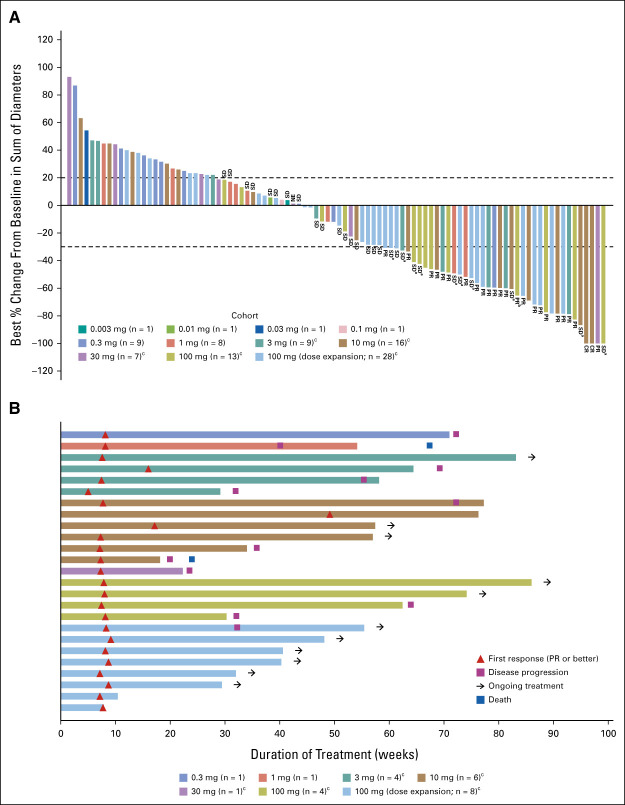
Confirmed ORR was 23.4% (95% CI, 15.7 to 32.5) including two complete and 23 partial responses by modified RECIST 1.1 per investigator assessment (Table 3). Figure 1A illustrates best percentage change from baseline in sum of diameters for patients with evaluable postbaseline assessments (n = 94). The disease control rate was 51.4% (95% CI, 41.5 to 61.2). Responses were seen starting with the 0.3 mg dose and generally higher rates of response were observed at doses of 3 mg and above. At least 30% tumor shrinkage in target lesions at postbaseline assessment was observed in 39 patients (36.4%). Among confirmed responders, the median time to response was 1.8 months (range, 1.2-7.4) and the median DOR was 12.3 months (95% CI, 6.6 to 14.9; Fig 1B). The longest DOR was 14.9 months, and 11 patients (44% of responders) had ongoing response at data cutoff. The median PFS was 3.7 months (95% CI, 2.1 to 5.4), and the median OS was 13.2 months (95% CI, 10.5 to NE; Fig 2), respectively. Of 77 progressive disease events, eight were in the brain (10.4%). Twenty-eight patients (26.2%) received subsequent anticancer therapies after tarlatamab. Representative computed tomography scans from patients receiving tarlatamab are provided (Data Supplement). An exploratory post hoc analysis demonstrates the sensitivity and specificity of enriching for responders (using confirmed OR) when total DLL3 expression is retrospectively considered for patient selection (Fig 3). Clinical benefit is observed across a range of thresholds.
TABLE 3.
Tumor Response to Tarlatamab According to Investigator Assessment
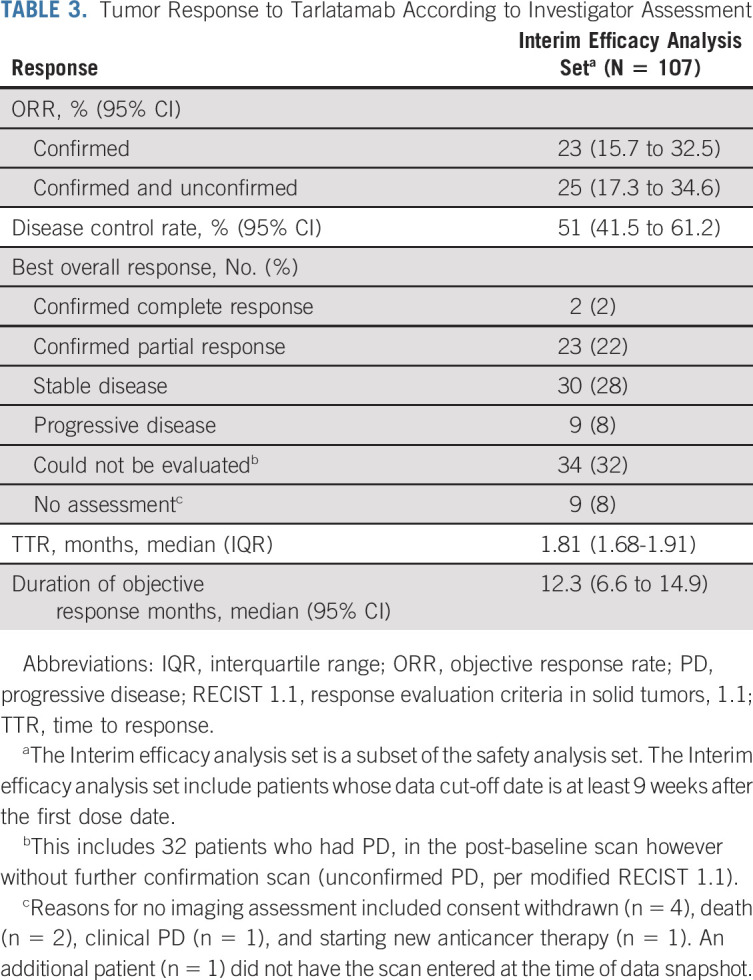
FIG 1.
Response to tarlatamab. (A) Best percent change from baseline in tumor burden (defined by the sum of the longest diameters of all target lesions) in 94 patients whose data cutoff date is at least 9 weeks after the first dose date and for whom postbaseline tumor data were available. aSD, patients had an initial response but did not have confirmation of response on the subsequent scan and bPR, patients had an initial PR and still have potential for future confirmative scans. One confirmed patient in the 100 mg expansion cohort had missing sum of diameters for lesion measurement and was not included in the plot. cStep dosing (ie, 1 mg run-in dose) was used in these cohorts. (B) TTR, the duration of treatment, and patient status as of the data cutoff date according to dose of tarlatamab for all patients with confirmed response (n = 25). CR, complete response; NE, not evaluable; PR, partial response; SD, stable disease; TTR, time to response.
FIG 2.
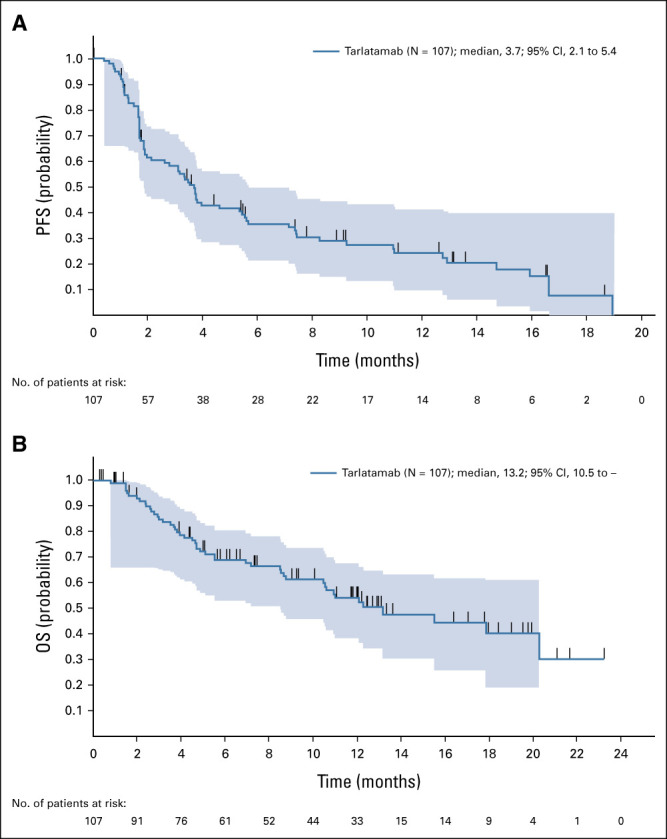
Efficacy of tarlatamab in patients with SCLC. (A) Kaplan-Meier curve of PFS for patients whose data cutoff date is at least 9 weeks after the first dose date (N = 107). (B) Kaplan-Meier curve of OS for patients whose data cutoff date is at least 9 weeks after the first dose date (N = 107). OS, overall survival; PFS, progression-free survival; SCLC, small-cell lung cancer.
FIG 3.
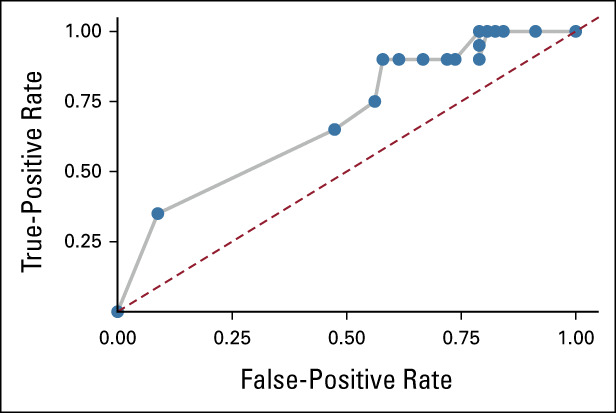
ROC curve of objective response enrichment by DLL3 expression. ROC curve reflecting true-positive rate and false-positive rate of enrichment of objective response when FIH patients (1-100 mg cohorts) are retrospectively examined for selection using DLL3 expression assessed with the Ventana SP347 assay. ROC analysis included 77 patients enrolled in the 1-100 mg cohorts for whom pretreatment DLL3 expression readouts were available. DLL3, delta-like ligand 3; FIH, first-in-human; ROC, receiver operating characteristic.
Clinical PK
As of April 15, 2022, preliminary pharmacokinetic data from dose escalation and expansion cohorts were available for 101 patients. Briefly, tarlatamab exhibited approximate dose proportional increase in serum exposures. Approximate steady state in serum tarlatamab exposures were achieved within 4 weeks of every other week target regimen initiation, with minimal accumulation. The mean (B1 standard deviation) terminal elimination half-life estimated at steady state across the evaluated target dose range was approximately 5.7 (B1 2.2) days, which is consistent with the intended half-life extension of the HLE platform relative to non-HLE BiTEs.23
Immunogenicity
Among patients with available samples, 10 of 97 (10.3%) developed anti-tarlatamab antibodies after tarlatamab administration. Two of 99 patients (2.0%) had preexisting antibodies at baseline. There is no apparent antidrug antibody impact on tarlatamab exposures or the safety profile in these patients.
Pharmacodynamics
The pharmacodynamic response after first dose of tarlatamab infusion was characterized by initial T-cell redistribution (Data Supplement [Figs 3A and 3B, online only]), T-cell activation (Data Supplement [Fig 3C]), and transient IFN-gamma elevation (Data Supplement [Fig 3D]). For step-dose cohorts, pharmacodynamic responses were greatest after initial administration of 1-mg step dose and not exceeded with target dose administration.
DISCUSSION
Tarlatamab demonstrated a manageable safety profile across a wide dose range through the expansion dose of 100 mg and was associated with encouraging response rates in a heavily pretreated population of patients with SCLC. Confirmed responses were durable, and OS seems promising. Across all doses (N = 107), tarlatamab was discontinued in only 4 (3.7%) patients. A maximum tolerated dose was not reached; the highest dose (100 mg) was further evaluated in dose expansion.
CRS is expected based on the mechanism of action of tarlatamab. CRS was the most frequent TEAE observed (56%) but was generally low-grade, transient, and typically occurred in the first cycle. CRS was reversible and generally managed with antipyretics, IV fluids, and steroids; tocilizumab was used to treat CRS in eight of 107 patients (7.5%). Neutropenia was a risk associated with tarlatamab observed in this study and was unexpected based on preclinical data; the mechanism is not understood. The study protocol was updated accordingly for specific monitoring and management. Further evaluation of neutropenia will be relevant to trials of tarlatamab use in combination with other marrow-suppressing therapies. Neurologic evaluation was conducted as part of frequent clinical evaluation to assess study patients for CRS and/or neurologic AEs due to the known association with immune effector cell therapies. Most neurologic AEs were mild and self-limiting without need for treatment discontinuation or dose reduction, though 12 patients (11.2%) had grade b % 3 neurologic AEs. Neurologic AEs led to tarlatamab discontinuation in two patients (encephalopathy, ICANS). Careful evaluation of neurologic AEs is ongoing to better characterize these events and identify risk factors or interventions that might specifically improve management.
Few approved therapies exist for SCLC after first line. A phase II study of lurbinectedin in second-line SCLC found an ORR of 35% and a median DOR of 5.3 months.7 In a randomized study of topotecan versus combination chemotherapy in recurrent SCLC, topotecan ORR was 24% and the median DOR was 3.3 months.5 The prior conditional approval by US Food and Drug Administration of nivolumab and pembrolizumab for third-line or later SCLC was based on response rates of 12% and 19%, respectively, with durable responses seen at b % 12 months in > 60% of responding patients.24 These approvals were subsequently withdrawn as survival benefit was not demonstrated.25 The ORR of 23% and median DOR of 12.3 months for tarlatamab compares well with other therapies, especially considering that more than 70% of patients had at least two prior lines of therapy. Fifty percent of patients in this study had received prior PD-1/PD-L1 therapy, representative of current practice in first-line SCLC. Despite the relatively short median PFS (3.7 months) seen with tarlatamab, the median OS (13.2 months) is relatively high and compares favorably with 9.3-month median OS reported previously with lurbinectedin or approximately 6-month OS with topotecan, although the value of comparisons is limited by differences in study design and patient populations.5,7 The promising OS benefit may reflect the long durability of response seen thus far in those who respond to tarlatamab but further follow up is needed in larger randomized studies. Another explanation could be that OS benefit derived from post-tarlatamab treatment, although this is less likely a major factor because only 26.2% of patients received such treatment in this heavily pretreated cohort. Identifying factors predictive of response and/or toxicity (eg, prior therapies) is an ongoing effort. An exploratory analysis suggests that increased DLL3 expression trends with higher magnitude of clinical benefit.
Limitations of this study include moderate median follow-up time (8.7 months) and selected patient population required for a first-in-human trial. Interpretation of preliminary efficacy is limited in a single-arm, dose-ranging study. Efficacy data from several different dose levels was pooled for some analyses; enrollment to the expansion cohort is ongoing.
The first DLL3-targeted agent to enter the clinic was the antibody-drug conjugate rovalpituzumab tesirine (Rova-T).11 During development, data showed strong enrichment in clinical benefit in patients with relatively high DLL3 expression; however, despite implementing DLL3 expression as an eligibility or stratification factor in later phase studies, Rova-T failed to show a survival benefit versus standard of care in two phase III studies and was discontinued.16,26,27 Rova-T toxicity in preclinical and especially clinical studies turned out to be a significant problem with this construct and was more consistent with general effects of the pyrrolobenzodiazepine payload rather than the DLL3 target.16,26,28 In contrast, tarlatamab has exhibited tolerability and efficacy over a broad range of doses in a heavily pretreated population confirming the value of this target. Other DLL3-specific therapies undergoing clinical evaluation include the T-cell engaging molecules HPN328 and BI 764532 and the chimeric antigen receptor T-cell therapy AMG 119.29-32
These results demonstrate promising activity in patients with high unmet medical need and have led to ongoing investigations of tarlatamab as monotherapy in SCLC and other neuroendocrine cancers (phase II study in patients with third-line SCLC [NCT05060016]; phase Ib study in neuroendocrine prostate cancer [NCT04702737]) and in combination in earlier lines of therapy (phase I study of first-line ES-SCLC tarlatamab in combination with carboplatin, etoposide, and PD-L1 inhibitor [NCT05361395]).
ACKNOWLEDGMENT
We thank the patients for participating in this trial and their families. Study and technical support was provided by Alicia Zhang, Beate Sable, Chia-Hsin Ju, Yanchen Zhou, Xi Chen, Marie-Anne Damiette Smit, and Marichu Endraca. Medical writing support was provided by Eugene J. Gillespie, PhD, Jacqueline Sayyah, PhD (both Amgen), Lisa R. Denny, PhD, and Maryann T. Travaglini, PharmD (ICON, Blue Bell, PA). Graphics support was provided by Robert Dawson (Cactus Communications on behalf of Amgen Inc).
Consortium list is provided in Appendix Table A1 (online only).
APPENDIX
TABLE A1.
Study Sites and Primary Investigators
Luis Paz-Ares
Leadership: Genomica, ALTUM Sequencing
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, BeiGene, GlaxoSmithKline, Janssen, Medscape, Regeneron
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), Kura Oncology (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, SERVIER, Sanofi, Roche, Amgen, Merck
Stephane Champiat
Honoraria: Amgen, AstraZeneca, Bristol Myers Squibb, MSD, Novartis, Roche, Fresenius Kabi, Eisai Europe, Genmab, Janssen, Merck KGaA, Merck Serono
Consulting or Advisory Role: Alderaan Biotechnology, Amgen, AstraZeneca, Avacta Life Sciences, Celanese, Domain Therapeutics, Ellipses Pharma, Genmab, Immunicom, Nanobiotix, Oncovita, Pierre Fabre, Seattle Genetics, Tatum Bioscience, Tollys SAS, UltraHuman8, NextCure
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Janssen-Cilag (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Sanofi (Inst), AbbVie (Inst), Adaptimmune (Inst), Aduro Biotech (Inst), Agios (Inst), Amgen (Inst), arGEN-X BVBA (Inst), Arno Therapeutics (Inst), Astex Pharmaceuticals (Inst), AstraZeneca (Inst), Bayer (Inst), BB Biotech Ventures (Inst), BeiGene (Inst), BioAlliance Pharma (Inst), BioNTech (Inst), Blueprint Medicines (Inst), Boehringer Ingelheim (Inst), Boston Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Cephalon (Inst), Chugai Pharma (Inst), Clovis Oncology (Inst), Cullinan Oncology (Inst), Daiichi Sankyo (Inst), Debiopharm Group (Inst), Eisai (Inst), Lilly (Inst), Exelixis (Inst), FORMA Therapeutics (Inst), GamaMabs Pharma (Inst), Genentech (Inst), Gilead Sciences (Inst), GlaxoSmithKline (Inst), Glenmark (Inst), H3 Biomedicine (Inst), Roche (Inst), Incyte (Inst), Innate Pharma (Inst), Pierre Fabre (Inst), SERVIER (Inst), Janssen-Cilag (Inst), Kura Oncology (Inst), Kyowa Hakko Kirin (Inst), Loxo (Inst), Lytix Biopharma (Inst), MedImmune (Inst), Menarini (Inst), Merck KGaA (Inst), Merck Sharp & Dohme (Inst), Merrimack (Inst), Merus (Inst), Millennium (Inst), Molecular Partners (Inst), Nanobiotix (Inst), Nektar (Inst), Nerviano Medical Sciences (Inst), Novartis (Inst), Octimet (Inst), Oncoethix (Inst), OncoMed (Inst), Oncopeptides (Inst), Onyx (Inst), Orion (Inst), Oryzon Genomics (Inst), Ose Pharma (Inst), Pfizer (Inst), PharmaMar (Inst), Philogen (Inst), Pierre Fabre (Inst), Plexxikon (Inst), RigonTEC (Inst), Sanofi/Aventis (Inst), Sierra Oncology (Inst), Sotio (Inst), Syros Pharmaceuticals (Inst), Taiho Pharmaceutical (Inst), Tesaro (Inst), Tioma Therapeutics (Inst), Wyeth (Inst), Xencor (Inst), Y's Therapeutics (Inst), Cytovation, Eisai/H3 Biomedicine, ImCheck therapeutics, Molecular Partners, MSD, OSE Immunotherapeutics, Pierre Fabre, Sanofi, Sotio, Transgene, Boehringer Ingelheim, AbbVie, Amgen, Adlai Nortye (Inst), AVEO (Inst), Basilea Pharmaceutical (Inst), BBB Technologies (Inst), Bicycle Therapeutics (Inst), CASI Pharmaceuticals (Inst), CellCentric (Inst), CureVac (Inst), Faron Pharmaceuticals (Inst), ITeos Therapeutics (Inst), Relay Therapeutics (Inst), Seattle Genetics (Inst), Transgene (Inst), Turning Point Therapeutics (Inst), GlaxoSmithKline (Inst)
Patents, Royalties, Other Intellectual Property: T-cell immunogens derived from anti-viral proteins and methods of using same WO2010039223A2
Travel, Accommodations, Expenses: MSD, AstraZeneca, Amgen, Bristol Myers Squibb, Merck, OSE Immunotherapeutics, Roche, Sotio
Other Relationship: AstraZeneca (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Johnson & Johnson (Inst), Lilly (Inst), MedImmune (Inst), Merck (Inst), Pfizer (Inst), Roche (Inst), GlaxoSmithKline (Inst), TherAGuiX (Inst)
W. Victoria Lai
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Consulting or Advisory Role: G1 Therapeutics, AstraZeneca, Jazz Pharmaceuticals, Merck, AstraZeneca/MedImmune
Research Funding: Daiichi Sankyo (Inst), Amgen (Inst), AbbVie (Inst), BMS (Inst), Merck (Inst)
Hiroki Izumi
Honoraria: Takeda, Merck, Ono Pharmaceutical, MSD, Chugai/Roche, Bristol Myers Squibb Japan
Consulting or Advisory Role: Amgen
Research Funding: AstraZeneca (Inst), Amgen (Inst), Takeda (Inst), Eisai/MSD (Inst), Ono Pharmaceutical (Inst)
Ramaswamy Govindan
Consulting or Advisory Role: Merck, Inivata
Michael Boyer
Honoraria: AstraZeneca, CancerAid
Consulting or Advisory Role: Merck Sharp & Dohme (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Janssen (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Genentech/Roche (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Ascentage Pharma (Inst), Novartis (Inst), Janssen (Inst), Merck Serono (Inst), Imugene (Inst), Nektar (Inst)
Horst-Dieter Hummel
Honoraria: Amgen, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Amgen (Inst), Revolution Medicines (Inst), Merck (Inst), Bristol Myers Squibb/Pfizer (Inst), AstraZeneca (Inst), Johnson & Johnson/Janssen
Consulting or Advisory Role: Amgen, Boehringer Ingelheim
Travel, Accommodations, Expenses: Amgen, Boehringer Ingelheim
Hossein Borghaei
Stock and Other Ownership Interests: Sonnet, Rgenix, Nucleai
Honoraria: Bristol Myers Squibb, Celgene, Axiom Biotechnologies, Pfizer, Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Lilly, Celgene, Genentech, Pfizer, Boehringer Ingelheim, EMD Serono, Novartis, Merck, AstraZeneca, Genmab, Regeneron, Cantargia AB, BioNTech, AbbVie, PharmaMar, Takeda, Amgen, HUYA Bioscience International, Sonnet, Rgenix, Beigene, Jazz Pharmaceuticals, Mirati Therapeutics, Guardant Health, Janssen Oncology, ITeos Therapeutics, Natera, Oncocyte, Da Volterra
Research Funding: Millennium (Inst), Merck (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Lilly, Clovis Oncology, Celgene, Genentech, Novartis, Merck, Amgen, EMD Serono
Other Relationship: University of Pennsylvania, Takeda, Incyte, Novartis
Melissa L. Johnson
Consulting or Advisory Role: Genentech/Roche (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Merck (Inst), Sanofi (Inst), Mirati Therapeutics (Inst), Ribon Therapeutics (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Gritstone Bio (Inst), Janssen Oncology (Inst), Lilly (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Axelia Oncology (Inst), Black Diamond Therapeutics (Inst), CytomX Therapeutics (Inst), EcoR1 Capital (Inst), Editas Medicine (Inst), Genmab (Inst), IDEAYA Biosciences (Inst), ITeos Therapeutics (Inst), Oncorus (Inst), Regeneron (Inst), Turning Point Therapeutics (Inst), Astellas Pharma (Inst), Checkpoint Therapeutics (Inst), Genocea Biosciences (Inst), Molecular Axiom (Inst), Novartis (Inst), Revolution Medicines (Inst), Takeda (Inst), VBL Therapeutics (Inst)
Research Funding: EMD Serono (Inst), Kadmon (Inst), Janssen (Inst), Mirati Therapeutics (Inst), Genmab (Inst), Pfizer (Inst), AstraZeneca (Inst), Stem CentRx (Inst), Novartis (Inst), Array BioPharma (Inst), Regeneron (Inst), Merck (Inst), Hengrui Pharmaceutical (Inst), Lycera (Inst), BeiGene (Inst), Tarveda Therapeutics (Inst), Loxo (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), Sanofi (Inst), CytomX Therapeutics (Inst), Dynavax Technologies (Inst), Corvus Pharmaceuticals (Inst), Incyte (Inst), Genocea Biosciences (Inst), Gritstone Bio (Inst), Amgen (Inst), Genentech/Roche (Inst), Adaptimmune (Inst), Syndax (Inst), Neovia Oncology (Inst), Acerta Pharma (Inst), Takeda (Inst), Shattuck Labs (Inst), GlaxoSmithKline (Inst), Apexigen (Inst), Atreca (Inst), OncoMed (Inst), Lilly (Inst), Immunocore (Inst), University of Michigan (Inst), TCR2 Therapeutics (Inst), Arcus Biosciences (Inst), Ribon Therapeutics (Inst), BerGenBio (Inst), Calithera Biosciences (Inst), Tmunity Therapeutics, Inc (Inst), Seven and Eight Biopharmaceuticals (Inst), Rubius Therapeutics (Inst), Curis (Inst), Silicon Therapeutics (Inst), Dracen (Inst), PMV Pharma (Inst), Artios (Inst), BioAtla (Inst), Elicio Therapeutics (Inst), Erasca, Inc (Inst), Harpoon (Inst), Helsinn Healthcare (Inst), Hutchison MediPharma (Inst), IDEAYA Biosciences (Inst), IGM Biosciences (Inst), Memorial Sloan-Kettering Cancer Center (Inst), NeoImmuneTech (Inst), Numab (Inst), RasCal (Inst), Relay Therapeutics (Inst), Revolution Medicines (Inst), Tempest Therapeutics (Inst), Tizona Therapeutics, Inc (Inst), Turning Point Therapeutics (Inst), Vyriad (Inst), Y-mAbs Therapeutics (Inst), Exelixis (Inst), Fate Therapeutics (Inst), Merus (Inst), Black Diamond Therapeutics (Inst), Kartos Therapeutics (Inst), Carisma Therapeutics (Inst), Rain Therapeutics (Inst), Nuvalent, Inc (Inst), Palleon Pharmaceuticals (Inst), IMPAC Medical Systems (Inst), EQRx (Inst)
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Genentech, Incyte, Merck, Pfizer, Sanofi
Neeltje Steeghs
Consulting or Advisory Role: Boehringer Ingelheim (Inst), Ellipses Pharma (Inst), Cogent Biosciences (Inst), Luszana (Inst)
Research Funding: AstraZeneca/MedImmune (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Roche (Inst), Boehringer Ingelheim (Inst), Blueprint Medicines (Inst), Deciphera (Inst), Genentech (Inst), Merck Sharp & Dohme (Inst), Amgen (Inst), Merus (Inst), Incyte (Inst), AbbVie (Inst), Actuate Therapeutics (Inst), Sanofi (Inst), Cytovation (Inst), InteRNA (Inst), Array BioPharma (Inst), Cantargia AB (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Ascendis Pharma (Inst), BridgeBio Pharma (Inst), CellCentric (Inst), Crescendo Biologics (Inst), Lilly (Inst), Exelixis (Inst), Janssen (Inst), Merck KGaA (Inst), Molecular Partners (Inst), Numab (Inst), Seattle Genetics (Inst), Cogent Biosciences (Inst), Kinnate Biopharma (Inst), Kling Biotherapeutics (Inst), Navire (Inst), Luszana (Inst), Relay Therapeutics (Inst), Revolution Medicin (Inst), Dragonfly Therapeutics (Inst)
Fiona Blackhall
Honoraria: Medivation, AstraZeneca, Pfizer, Bayer, Amgen, Takeda
Consulting or Advisory Role: Medivation, Cell Medica, Amgen, Blueprint Medicines, Pfizer, AbbVie, Janssen, AstraZeneca, Amgen
Speakers' Bureau: Takeda, AstraZeneca
Research Funding: AstraZeneca (Inst), Boehringer Ingelheim (Inst), Novartis (Inst), Amgen (Inst), Pfizer (Inst), Bristol Myers Squibb Foundation (Inst), AbbVie (Inst), Blueprint Medicines, Celgene, PharmaMar (Inst), Merck (Inst), Mirati Therapeutics (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim
Afshin Dowlati
Consulting or Advisory Role: AbbVie/Stemcentrx, AstraZeneca, Bristol Myers Squibb, Ipsen, Merck
Research Funding: Lilly/ImClone (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), Takeda (Inst), Mirati Therapeutics (Inst), AbbVie/Stemcentrx (Inst), Bayer (Inst), Seattle Genetics (Inst), Ipsen (Inst), Pionyr (Inst), Coordination Pharmaceuticals (Inst), Astellas Pharma (Inst), Bicycle Therapeutics (Inst), Gilead Sciences (Inst)
Noemi Reguart
Consulting or Advisory Role: Pfizer, MSD Oncology, BMS, Boehringer Ingelheim, AstraZeneca, Roche, Takeda, Amgen, Lilly, Sanofi, Janssen
Speakers' Bureau: AstraZeneca, Roche, Roche Molecular Diagnostics, MSD, Boehringer Ingelheim, Amgen, Novartis, Merck Serono, Takeda, Sanofi
Research Funding: Pfizer, Novartis
Travel, Accommodations, Expenses: MSD, Roche
Tatsuya Yoshida
Honoraria: AstraZeneca, MSD Oncology, Ono Pharmaceutical, Chugai/Roche, Lilly Japan, Nippon Boehringer Ingelheim, Bristol Myers Squibb Japan, Novartis, Archer, Takeda, Pfizer
Consulting or Advisory Role: Lilly Japan, Chugai/Roche, Novartis, Boehringer Ingelheim, AstraZeneca
Research Funding: Chugai/Roche (Inst), AstraZeneca (Inst), AbbVie (Inst), Amgen (Inst), MSD (Inst), Bristol Myers Squibb (Inst), Ono Pharmaceutical (Inst), Takeda (Inst), Daiichi Sankyo (Inst), Chugai/Roche (Inst), Novartis (Inst)
Kai He
Consulting or Advisory Role: Perthera, Mirati Therapeutics, Bristol Myers Squibb, Iovance Biotherapeutics, Geneplus, Lyell Immunopharma, AstraZeneca
Research Funding: Bristol Myers Squibb (Inst), Mirati Therapeutics (Inst), Adaptimmune (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), Amgen (Inst), Iovance Biotherapeutics (Inst), AbbVie (Inst), Oncoc4 (Inst)
Shirish M. Gadgeel
Honoraria: Merck
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Bristol Myers Squibb, Takeda, Daichii-Sanyko, Novartis, Blueprint Medicines, Lilly, Pfizer, Janssen Oncology, Mirati Therapeutics, Merck, Esai Pharma, Gilead Sciences, GlaxoSmithKline
Research Funding: Merck, Genentech/Roche (Inst), Merck (Inst), Blueprint Medicines (Inst), Astellas Pharma (Inst), Daiichi Sankyo (Inst), I-Mab (Inst), Nektar (Inst), AstraZeneca (Inst), Pfizer (Inst), Amgen (Inst), Turning Point Therapeutics (Inst), Regeneron (Inst), Mirati Therapeutics (Inst), Nektar (Inst), Janssen Oncology (Inst), BioMed Valley Discoveries (Inst), Ymabs Therapeutics Inc (Inst), Calithera Biosciences (Inst), InventisBio (Inst), Daichii Sanyko (Inst), Dragonfly Therapeutics (Inst), eFFECTOR Therapeutics (Inst), Elevation Oncology (Inst), Erasca, Inc (Inst), Helsinn Therapeutics (Inst), Incyte (Inst), Numab (Inst), Verastem (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Mirati Therapeutics
Other Relationship: AstraZeneca
Enriqueta Felip
Consulting or Advisory Role: Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Lilly, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Pfizer, Sanofi, Takeda, Peptomyc, Daiichi Sankyo Europe GmbH, F. Hoffmann LaRoche, Merck Sharp & Dohme
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Lilly, Medscape, Merck Sharp & Dohme, PeerVoice, Pfizer, Takeda, Amgen, F. Hoffmann LaRoche, Janssen, Medical Trends, Merck Serono, Sanofi, TouchONCOLOGY
Research Funding: Merck (Inst), Merck KGaA (Inst)
Other Relationship: GRIFOLS
Yiran Zhang
Employment: Gilead Sciences, Amgen
Stock and Other Ownership Interests: Amgen
Amrita Pati
Employment: Amgen
Stock and Other Ownership Interests: Amgen, Roche Sequencing Solutions, AbbVie, Regeneron, Gilead Sciences, BioNTech SE
Patents, Royalties, Other Intellectual Property: ClaMS—A computational application that classified Metagenome sequences. Receive royalty for above application SW through Lawrence Berkeley National Labs
Mukul Minocha
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Sujoy Mukherjee
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Amanda Goldrick
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Dirk Nagorsen
Employment: Amgen
Leadership: Amgen
Stock and Other Ownership Interests: Amgen
Patents, Royalties, Other Intellectual Property: Inventor on patents related to the use of CD3 bispecifics
Nooshin Hashemi Sadraei
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Travel, Accommodations, Expenses: Amgen
Taofeek K. Owonikoko
Stock and Other Ownership Interests: CAMBIUM MEDICAL TECHNOLOGIES, Taobob LLC, GenCart, Coherus Biosciences
Consulting or Advisory Role: Novartis, Celgene, AbbVie, Eisai, G1 Therapeutics, Takeda, Bristol Myers Squibb, MedImmune, BerGenBio, Lilly, Amgen, AstraZeneca, PharmaMar, Boehringer Ingelheim, EMD Serono, Xcovery, Bayer, Merck, Jazz Pharmaceuticals, Zentalis, Wells Fargo, Ipsen, Eisai, Roche/Genentech, Janssen, Exelixis, BeiGene, Triptych Health Partners, Daichi, Xcovery, Coherus Biosciences
Speakers' Bureau: AbbVie
Research Funding: Novartis (Inst), Astellas Pharma (Inst), Bayer (Inst), Regeneron (Inst), AstraZeneca/MedImmune (Inst), AbbVie (Inst), G1 Therapeutics (Inst), Bristol Myers Squibb (Inst), Corvus Pharmaceuticals, United Therapeutics (Inst), Amgen (Inst), Loxo/Lilly (Inst), Fujifilm (Inst), Pfizer (Inst), Aeglea Biotherapeutics (Inst), Incyte (Inst), Merck (Inst), Oncorus (Inst), Ipsen (Inst), GlaxoSmithKline (Inst), Calithera Biosciences (Inst), Eisai (Inst), WindMIL (Inst), Turning Point Therapeutics (Inst), Roche/Genentech (Inst), Mersana (Inst), Meryx Pharmaceuticals (Inst), Boehringer Ingelheim (Inst)
Patents, Royalties, Other Intellectual Property: OVERCOMING ACQUIRED RESISTANCE TO CHEMOTHERAPY TREATMENTS THROUGH SUPPRESSION OF STAT3 (Inst), SELECTIVE CHEMOTHERAPY TREATMENTS AND DIAGNOSTIC METHODS RELATED THERETO (Inst), DR4 Modulation and its Implications in EGFR-Target Cancer Therapy Ref:18089 PROV (CSP) United States Patent Application No. 62/670210 June 26, 2018 (Co-Inventor; Inst), Soluble FAS ligand as a biomarker of recurrence in thyroid cancer; provisional patent 61/727519 (Inventor; Inst)
Other Relationship: Roche/Genentech, EMD Serono, Novartis
Uncompensated Relationships: Reflexion Medical
Open Payments Link: https://openpaymentsdata.cms.gov/physician/253457
No other potential conflicts of interest were reported.
See accompanying editorial on page 2877
SUPPORT
This study was designed by the sponsor (Amgen, Inc) and study investigators. Data were collected by the investigators and their site personnel. The authors and sponsor did data analyses and data interpretation. The sponsor managed the study database, supplied the study drug, and provided editorial assistance.
CLINICAL TRIAL INFORMATION
NCT03319940 (DeLLphi-300)
L.P.-A. and S.C. contributed equally to this work and share lead authorship.
DATA SHARING STATEMENT
There is a plan to share data. This may include deidentified individual patient data for variables necessary to address the specific research question in an approved data sharing request, also related data dictionaries, study protocol, statistical analysis plan, informed consent form, and/or clinical study report.
Data sharing requests relating to data in this manuscript will be considered after the publication date and (1) this product and indication (or other new use) have been granted marketing authorization in both the United States and Europe, or (2) clinical development discontinues and the data will not be submitted to regulatory authorities. There is no end date for eligibility to submit a data sharing request for these data.
Qualified researchers may submit a request containing the research objectives, the Amgen product(s) and Amgen study/studies in scope, end points/outcomes of interest, statistical analysis plan, data requirements, publication plan, and qualifications of the researcher(s).
In general, Amgen does not grant external requests for individual patient data for the purpose of reevaluating safety and efficacy issues already addressed in the product labeling. A committee of internal advisors review requests. If not approved, requests may be further arbitrated by a Data Sharing Independent Review Panel. Requests that pose a potential conflict of interest or an actual or potential competitive risk may be declined at Amgen's sole discretion and without further arbitration.
Upon approval, information necessary to address the research question will be provided under the terms of a data sharing agreement. This may include anonymized individual patient data and/or available supporting documents, containing fragments of analysis code where provided in analysis specifications.
Further details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request.
AUTHOR CONTRIBUTIONS
Conception and design: Nooshin Hashemi Sadraei
Provision of study materials or patients: Luis Paz-Ares, Stephane Champiat, W. Victoria Lai, Hiroki Izumi, Ramaswamy Govindan, Michael Boyer, Horst-Dieter Hummel, Hossein Borghaei, Melissa L. Johnson, Neeltje Steeghs, Fiona Blackhall, Afshin Dowlati, Noemi Reguart, Tatsuya Yoshida, Kai He, Shirish M. Gadgeel, Enriqueta Felip, Taofeek K. Owonikoko
Collection and assembly of data: Luis Paz-Ares, Stephane Champiat, W. Victoria Lai, Hiroki Izumi, Ramaswamy Govindan, Michael Boyer, Horst-Dieter Hummel, Hossein Borghaei, Melissa L. Johnson, Neeltje Steeghs, Fiona Blackhall, Afshin Dowlati, Noemi Reguart, Tatsuya Yoshida, Kai He, Shirish M. Gadgeel, Enriqueta Felip, Yiran Zhang, Taofeek K. Owonikoko
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Luis Paz-Ares
Leadership: Genomica, ALTUM Sequencing
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, BeiGene, GlaxoSmithKline, Janssen, Medscape, Regeneron
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), Kura Oncology (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, SERVIER, Sanofi, Roche, Amgen, Merck
Stephane Champiat
Honoraria: Amgen, AstraZeneca, Bristol Myers Squibb, MSD, Novartis, Roche, Fresenius Kabi, Eisai Europe, Genmab, Janssen, Merck KGaA, Merck Serono
Consulting or Advisory Role: Alderaan Biotechnology, Amgen, AstraZeneca, Avacta Life Sciences, Celanese, Domain Therapeutics, Ellipses Pharma, Genmab, Immunicom, Nanobiotix, Oncovita, Pierre Fabre, Seattle Genetics, Tatum Bioscience, Tollys SAS, UltraHuman8, NextCure
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Janssen-Cilag (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Sanofi (Inst), AbbVie (Inst), Adaptimmune (Inst), Aduro Biotech (Inst), Agios (Inst), Amgen (Inst), arGEN-X BVBA (Inst), Arno Therapeutics (Inst), Astex Pharmaceuticals (Inst), AstraZeneca (Inst), Bayer (Inst), BB Biotech Ventures (Inst), BeiGene (Inst), BioAlliance Pharma (Inst), BioNTech (Inst), Blueprint Medicines (Inst), Boehringer Ingelheim (Inst), Boston Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Cephalon (Inst), Chugai Pharma (Inst), Clovis Oncology (Inst), Cullinan Oncology (Inst), Daiichi Sankyo (Inst), Debiopharm Group (Inst), Eisai (Inst), Lilly (Inst), Exelixis (Inst), FORMA Therapeutics (Inst), GamaMabs Pharma (Inst), Genentech (Inst), Gilead Sciences (Inst), GlaxoSmithKline (Inst), Glenmark (Inst), H3 Biomedicine (Inst), Roche (Inst), Incyte (Inst), Innate Pharma (Inst), Pierre Fabre (Inst), SERVIER (Inst), Janssen-Cilag (Inst), Kura Oncology (Inst), Kyowa Hakko Kirin (Inst), Loxo (Inst), Lytix Biopharma (Inst), MedImmune (Inst), Menarini (Inst), Merck KGaA (Inst), Merck Sharp & Dohme (Inst), Merrimack (Inst), Merus (Inst), Millennium (Inst), Molecular Partners (Inst), Nanobiotix (Inst), Nektar (Inst), Nerviano Medical Sciences (Inst), Novartis (Inst), Octimet (Inst), Oncoethix (Inst), OncoMed (Inst), Oncopeptides (Inst), Onyx (Inst), Orion (Inst), Oryzon Genomics (Inst), Ose Pharma (Inst), Pfizer (Inst), PharmaMar (Inst), Philogen (Inst), Pierre Fabre (Inst), Plexxikon (Inst), RigonTEC (Inst), Sanofi/Aventis (Inst), Sierra Oncology (Inst), Sotio (Inst), Syros Pharmaceuticals (Inst), Taiho Pharmaceutical (Inst), Tesaro (Inst), Tioma Therapeutics (Inst), Wyeth (Inst), Xencor (Inst), Y's Therapeutics (Inst), Cytovation, Eisai/H3 Biomedicine, ImCheck therapeutics, Molecular Partners, MSD, OSE Immunotherapeutics, Pierre Fabre, Sanofi, Sotio, Transgene, Boehringer Ingelheim, AbbVie, Amgen, Adlai Nortye (Inst), AVEO (Inst), Basilea Pharmaceutical (Inst), BBB Technologies (Inst), Bicycle Therapeutics (Inst), CASI Pharmaceuticals (Inst), CellCentric (Inst), CureVac (Inst), Faron Pharmaceuticals (Inst), ITeos Therapeutics (Inst), Relay Therapeutics (Inst), Seattle Genetics (Inst), Transgene (Inst), Turning Point Therapeutics (Inst), GlaxoSmithKline (Inst)
Patents, Royalties, Other Intellectual Property: T-cell immunogens derived from anti-viral proteins and methods of using same WO2010039223A2
Travel, Accommodations, Expenses: MSD, AstraZeneca, Amgen, Bristol Myers Squibb, Merck, OSE Immunotherapeutics, Roche, Sotio
Other Relationship: AstraZeneca (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Johnson & Johnson (Inst), Lilly (Inst), MedImmune (Inst), Merck (Inst), Pfizer (Inst), Roche (Inst), GlaxoSmithKline (Inst), TherAGuiX (Inst)
W. Victoria Lai
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Consulting or Advisory Role: G1 Therapeutics, AstraZeneca, Jazz Pharmaceuticals, Merck, AstraZeneca/MedImmune
Research Funding: Daiichi Sankyo (Inst), Amgen (Inst), AbbVie (Inst), BMS (Inst), Merck (Inst)
Hiroki Izumi
Honoraria: Takeda, Merck, Ono Pharmaceutical, MSD, Chugai/Roche, Bristol Myers Squibb Japan
Consulting or Advisory Role: Amgen
Research Funding: AstraZeneca (Inst), Amgen (Inst), Takeda (Inst), Eisai/MSD (Inst), Ono Pharmaceutical (Inst)
Ramaswamy Govindan
Consulting or Advisory Role: Merck, Inivata
Michael Boyer
Honoraria: AstraZeneca, CancerAid
Consulting or Advisory Role: Merck Sharp & Dohme (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Janssen (Inst)
Research Funding: Merck Sharp & Dohme (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Genentech/Roche (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Ascentage Pharma (Inst), Novartis (Inst), Janssen (Inst), Merck Serono (Inst), Imugene (Inst), Nektar (Inst)
Horst-Dieter Hummel
Honoraria: Amgen, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Amgen (Inst), Revolution Medicines (Inst), Merck (Inst), Bristol Myers Squibb/Pfizer (Inst), AstraZeneca (Inst), Johnson & Johnson/Janssen
Consulting or Advisory Role: Amgen, Boehringer Ingelheim
Travel, Accommodations, Expenses: Amgen, Boehringer Ingelheim
Hossein Borghaei
Stock and Other Ownership Interests: Sonnet, Rgenix, Nucleai
Honoraria: Bristol Myers Squibb, Celgene, Axiom Biotechnologies, Pfizer, Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Lilly, Celgene, Genentech, Pfizer, Boehringer Ingelheim, EMD Serono, Novartis, Merck, AstraZeneca, Genmab, Regeneron, Cantargia AB, BioNTech, AbbVie, PharmaMar, Takeda, Amgen, HUYA Bioscience International, Sonnet, Rgenix, Beigene, Jazz Pharmaceuticals, Mirati Therapeutics, Guardant Health, Janssen Oncology, ITeos Therapeutics, Natera, Oncocyte, Da Volterra
Research Funding: Millennium (Inst), Merck (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Lilly, Clovis Oncology, Celgene, Genentech, Novartis, Merck, Amgen, EMD Serono
Other Relationship: University of Pennsylvania, Takeda, Incyte, Novartis
Melissa L. Johnson
Consulting or Advisory Role: Genentech/Roche (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Merck (Inst), Sanofi (Inst), Mirati Therapeutics (Inst), Ribon Therapeutics (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Gritstone Bio (Inst), Janssen Oncology (Inst), Lilly (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Axelia Oncology (Inst), Black Diamond Therapeutics (Inst), CytomX Therapeutics (Inst), EcoR1 Capital (Inst), Editas Medicine (Inst), Genmab (Inst), IDEAYA Biosciences (Inst), ITeos Therapeutics (Inst), Oncorus (Inst), Regeneron (Inst), Turning Point Therapeutics (Inst), Astellas Pharma (Inst), Checkpoint Therapeutics (Inst), Genocea Biosciences (Inst), Molecular Axiom (Inst), Novartis (Inst), Revolution Medicines (Inst), Takeda (Inst), VBL Therapeutics (Inst)
Research Funding: EMD Serono (Inst), Kadmon (Inst), Janssen (Inst), Mirati Therapeutics (Inst), Genmab (Inst), Pfizer (Inst), AstraZeneca (Inst), Stem CentRx (Inst), Novartis (Inst), Array BioPharma (Inst), Regeneron (Inst), Merck (Inst), Hengrui Pharmaceutical (Inst), Lycera (Inst), BeiGene (Inst), Tarveda Therapeutics (Inst), Loxo (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), Sanofi (Inst), CytomX Therapeutics (Inst), Dynavax Technologies (Inst), Corvus Pharmaceuticals (Inst), Incyte (Inst), Genocea Biosciences (Inst), Gritstone Bio (Inst), Amgen (Inst), Genentech/Roche (Inst), Adaptimmune (Inst), Syndax (Inst), Neovia Oncology (Inst), Acerta Pharma (Inst), Takeda (Inst), Shattuck Labs (Inst), GlaxoSmithKline (Inst), Apexigen (Inst), Atreca (Inst), OncoMed (Inst), Lilly (Inst), Immunocore (Inst), University of Michigan (Inst), TCR2 Therapeutics (Inst), Arcus Biosciences (Inst), Ribon Therapeutics (Inst), BerGenBio (Inst), Calithera Biosciences (Inst), Tmunity Therapeutics, Inc (Inst), Seven and Eight Biopharmaceuticals (Inst), Rubius Therapeutics (Inst), Curis (Inst), Silicon Therapeutics (Inst), Dracen (Inst), PMV Pharma (Inst), Artios (Inst), BioAtla (Inst), Elicio Therapeutics (Inst), Erasca, Inc (Inst), Harpoon (Inst), Helsinn Healthcare (Inst), Hutchison MediPharma (Inst), IDEAYA Biosciences (Inst), IGM Biosciences (Inst), Memorial Sloan-Kettering Cancer Center (Inst), NeoImmuneTech (Inst), Numab (Inst), RasCal (Inst), Relay Therapeutics (Inst), Revolution Medicines (Inst), Tempest Therapeutics (Inst), Tizona Therapeutics, Inc (Inst), Turning Point Therapeutics (Inst), Vyriad (Inst), Y-mAbs Therapeutics (Inst), Exelixis (Inst), Fate Therapeutics (Inst), Merus (Inst), Black Diamond Therapeutics (Inst), Kartos Therapeutics (Inst), Carisma Therapeutics (Inst), Rain Therapeutics (Inst), Nuvalent, Inc (Inst), Palleon Pharmaceuticals (Inst), IMPAC Medical Systems (Inst), EQRx (Inst)
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Genentech, Incyte, Merck, Pfizer, Sanofi
Neeltje Steeghs
Consulting or Advisory Role: Boehringer Ingelheim (Inst), Ellipses Pharma (Inst), Cogent Biosciences (Inst), Luszana (Inst)
Research Funding: AstraZeneca/MedImmune (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Roche (Inst), Boehringer Ingelheim (Inst), Blueprint Medicines (Inst), Deciphera (Inst), Genentech (Inst), Merck Sharp & Dohme (Inst), Amgen (Inst), Merus (Inst), Incyte (Inst), AbbVie (Inst), Actuate Therapeutics (Inst), Sanofi (Inst), Cytovation (Inst), InteRNA (Inst), Array BioPharma (Inst), Cantargia AB (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Ascendis Pharma (Inst), BridgeBio Pharma (Inst), CellCentric (Inst), Crescendo Biologics (Inst), Lilly (Inst), Exelixis (Inst), Janssen (Inst), Merck KGaA (Inst), Molecular Partners (Inst), Numab (Inst), Seattle Genetics (Inst), Cogent Biosciences (Inst), Kinnate Biopharma (Inst), Kling Biotherapeutics (Inst), Navire (Inst), Luszana (Inst), Relay Therapeutics (Inst), Revolution Medicin (Inst), Dragonfly Therapeutics (Inst)
Fiona Blackhall
Honoraria: Medivation, AstraZeneca, Pfizer, Bayer, Amgen, Takeda
Consulting or Advisory Role: Medivation, Cell Medica, Amgen, Blueprint Medicines, Pfizer, AbbVie, Janssen, AstraZeneca, Amgen
Speakers' Bureau: Takeda, AstraZeneca
Research Funding: AstraZeneca (Inst), Boehringer Ingelheim (Inst), Novartis (Inst), Amgen (Inst), Pfizer (Inst), Bristol Myers Squibb Foundation (Inst), AbbVie (Inst), Blueprint Medicines, Celgene, PharmaMar (Inst), Merck (Inst), Mirati Therapeutics (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim
Afshin Dowlati
Consulting or Advisory Role: AbbVie/Stemcentrx, AstraZeneca, Bristol Myers Squibb, Ipsen, Merck
Research Funding: Lilly/ImClone (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), Takeda (Inst), Mirati Therapeutics (Inst), AbbVie/Stemcentrx (Inst), Bayer (Inst), Seattle Genetics (Inst), Ipsen (Inst), Pionyr (Inst), Coordination Pharmaceuticals (Inst), Astellas Pharma (Inst), Bicycle Therapeutics (Inst), Gilead Sciences (Inst)
Noemi Reguart
Consulting or Advisory Role: Pfizer, MSD Oncology, BMS, Boehringer Ingelheim, AstraZeneca, Roche, Takeda, Amgen, Lilly, Sanofi, Janssen
Speakers' Bureau: AstraZeneca, Roche, Roche Molecular Diagnostics, MSD, Boehringer Ingelheim, Amgen, Novartis, Merck Serono, Takeda, Sanofi
Research Funding: Pfizer, Novartis
Travel, Accommodations, Expenses: MSD, Roche
Tatsuya Yoshida
Honoraria: AstraZeneca, MSD Oncology, Ono Pharmaceutical, Chugai/Roche, Lilly Japan, Nippon Boehringer Ingelheim, Bristol Myers Squibb Japan, Novartis, Archer, Takeda, Pfizer
Consulting or Advisory Role: Lilly Japan, Chugai/Roche, Novartis, Boehringer Ingelheim, AstraZeneca
Research Funding: Chugai/Roche (Inst), AstraZeneca (Inst), AbbVie (Inst), Amgen (Inst), MSD (Inst), Bristol Myers Squibb (Inst), Ono Pharmaceutical (Inst), Takeda (Inst), Daiichi Sankyo (Inst), Chugai/Roche (Inst), Novartis (Inst)
Kai He
Consulting or Advisory Role: Perthera, Mirati Therapeutics, Bristol Myers Squibb, Iovance Biotherapeutics, Geneplus, Lyell Immunopharma, AstraZeneca
Research Funding: Bristol Myers Squibb (Inst), Mirati Therapeutics (Inst), Adaptimmune (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), Amgen (Inst), Iovance Biotherapeutics (Inst), AbbVie (Inst), Oncoc4 (Inst)
Shirish M. Gadgeel
Honoraria: Merck
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Bristol Myers Squibb, Takeda, Daichii-Sanyko, Novartis, Blueprint Medicines, Lilly, Pfizer, Janssen Oncology, Mirati Therapeutics, Merck, Esai Pharma, Gilead Sciences, GlaxoSmithKline
Research Funding: Merck, Genentech/Roche (Inst), Merck (Inst), Blueprint Medicines (Inst), Astellas Pharma (Inst), Daiichi Sankyo (Inst), I-Mab (Inst), Nektar (Inst), AstraZeneca (Inst), Pfizer (Inst), Amgen (Inst), Turning Point Therapeutics (Inst), Regeneron (Inst), Mirati Therapeutics (Inst), Nektar (Inst), Janssen Oncology (Inst), BioMed Valley Discoveries (Inst), Ymabs Therapeutics Inc (Inst), Calithera Biosciences (Inst), InventisBio (Inst), Daichii Sanyko (Inst), Dragonfly Therapeutics (Inst), eFFECTOR Therapeutics (Inst), Elevation Oncology (Inst), Erasca, Inc (Inst), Helsinn Therapeutics (Inst), Incyte (Inst), Numab (Inst), Verastem (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Mirati Therapeutics
Other Relationship: AstraZeneca
Enriqueta Felip
Consulting or Advisory Role: Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Lilly, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Pfizer, Sanofi, Takeda, Peptomyc, Daiichi Sankyo Europe GmbH, F. Hoffmann LaRoche, Merck Sharp & Dohme
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Lilly, Medscape, Merck Sharp & Dohme, PeerVoice, Pfizer, Takeda, Amgen, F. Hoffmann LaRoche, Janssen, Medical Trends, Merck Serono, Sanofi, TouchONCOLOGY
Research Funding: Merck (Inst), Merck KGaA (Inst)
Other Relationship: GRIFOLS
Yiran Zhang
Employment: Gilead Sciences, Amgen
Stock and Other Ownership Interests: Amgen
Amrita Pati
Employment: Amgen
Stock and Other Ownership Interests: Amgen, Roche Sequencing Solutions, AbbVie, Regeneron, Gilead Sciences, BioNTech SE
Patents, Royalties, Other Intellectual Property: ClaMS—A computational application that classified Metagenome sequences. Receive royalty for above application SW through Lawrence Berkeley National Labs
Mukul Minocha
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Sujoy Mukherjee
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Amanda Goldrick
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Dirk Nagorsen
Employment: Amgen
Leadership: Amgen
Stock and Other Ownership Interests: Amgen
Patents, Royalties, Other Intellectual Property: Inventor on patents related to the use of CD3 bispecifics
Nooshin Hashemi Sadraei
Employment: Amgen
Stock and Other Ownership Interests: Amgen
Travel, Accommodations, Expenses: Amgen
Taofeek K. Owonikoko
Stock and Other Ownership Interests: CAMBIUM MEDICAL TECHNOLOGIES, Taobob LLC, GenCart, Coherus Biosciences
Consulting or Advisory Role: Novartis, Celgene, AbbVie, Eisai, G1 Therapeutics, Takeda, Bristol Myers Squibb, MedImmune, BerGenBio, Lilly, Amgen, AstraZeneca, PharmaMar, Boehringer Ingelheim, EMD Serono, Xcovery, Bayer, Merck, Jazz Pharmaceuticals, Zentalis, Wells Fargo, Ipsen, Eisai, Roche/Genentech, Janssen, Exelixis, BeiGene, Triptych Health Partners, Daichi, Xcovery, Coherus Biosciences
Speakers' Bureau: AbbVie
Research Funding: Novartis (Inst), Astellas Pharma (Inst), Bayer (Inst), Regeneron (Inst), AstraZeneca/MedImmune (Inst), AbbVie (Inst), G1 Therapeutics (Inst), Bristol Myers Squibb (Inst), Corvus Pharmaceuticals, United Therapeutics (Inst), Amgen (Inst), Loxo/Lilly (Inst), Fujifilm (Inst), Pfizer (Inst), Aeglea Biotherapeutics (Inst), Incyte (Inst), Merck (Inst), Oncorus (Inst), Ipsen (Inst), GlaxoSmithKline (Inst), Calithera Biosciences (Inst), Eisai (Inst), WindMIL (Inst), Turning Point Therapeutics (Inst), Roche/Genentech (Inst), Mersana (Inst), Meryx Pharmaceuticals (Inst), Boehringer Ingelheim (Inst)
Patents, Royalties, Other Intellectual Property: OVERCOMING ACQUIRED RESISTANCE TO CHEMOTHERAPY TREATMENTS THROUGH SUPPRESSION OF STAT3 (Inst), SELECTIVE CHEMOTHERAPY TREATMENTS AND DIAGNOSTIC METHODS RELATED THERETO (Inst), DR4 Modulation and its Implications in EGFR-Target Cancer Therapy Ref:18089 PROV (CSP) United States Patent Application No. 62/670210 June 26, 2018 (Co-Inventor; Inst), Soluble FAS ligand as a biomarker of recurrence in thyroid cancer; provisional patent 61/727519 (Inventor; Inst)
Other Relationship: Roche/Genentech, EMD Serono, Novartis
Uncompensated Relationships: Reflexion Medical
Open Payments Link: https://openpaymentsdata.cms.gov/physician/253457
No other potential conflicts of interest were reported.
REFERENCES
- 1.Wang S, Tang J, Sun T, et al. : Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep 7:1339, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Forjaz G, Mooradian MJ, et al. : The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 383:640-649, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paz-Ares L, Chen Y, Reinmuth N, et al. : Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 7:100408, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu SV, Reck M, Mansfield AS, et al. : Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 39:619-630, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Pawel J, Schiller JH, Shepherd FA, et al. : Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 17:658-667, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Petrelli F, Ghidini A, Luciani A: Topotecan or other agents as second-line therapy for relapsed small-cell lung cancer: A meta-analysis of randomized studies. Mol Clin Oncol 15:218, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trigo J, Subbiah V, Besse B, et al. : Lurbinectedin as second-line treatment for patients with small-cell lung cancer: A single-arm, open-label, phase 2 basket trial. Lancet Oncol 21:645-654, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Baena J, Modrego A, Zeaiter A, et al. : Lurbinectedin in the treatment of relapsed small cell lung cancer. Future Oncol 17:2279-2289, 2021 [DOI] [PubMed] [Google Scholar]
- 9.George J, Lim JS, Jang SJ, et al. : Comprehensive genomic profiles of small cell lung cancer. Nature 524:47-53, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim JS, Ibaseta A, Fischer MM, et al. : Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 545:360-364, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders LR, Bankovich AJ, Anderson WC, et al. : A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 7:302ra136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka K, Isse K, Fujihira T, et al. : Prevalence of Delta-like protein 3 expression in patients with small cell lung cancer. Lung Cancer 115:116-120, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Huang RSP, Holmes BF, Powell C, et al. : Delta-like protein 3 prevalence in small cell lung cancer and DLL3 (SP347) assay characteristics. Arch Pathol Lab Med 143:1373-1377, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Rojo F, Corassa M, Mavroudis D, et al. : International real-world study of DLL3 expression in patients with small cell lung cancer. Lung Cancer 147:237-243, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Furuta M, Kikuchi H, Shoji T, et al. : DLL3 regulates the migration and invasion of small cell lung cancer by modulating Snail. Cancer Sci 110:1599-1608, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudin CM, Pietanza MC, Bauer TM, et al. : Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 18:42-51, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giffin MJ, Cooke K, Lobenhofer EK, et al. : AMG 757, a half-life extended, DLL3-targeted bispecific T-cell engager, shows high potency and sensitivity in preclinical models of small-cell lung cancer. Clin Cancer Res 27:1526-1537, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Nishino M, Giobbie-Hurder A, Gargano M, et al. : Developing a common language for tumor response to immunotherapy: Immune-related response criteria using unidimensional measurements. Clin Cancer Res 19:3936-3943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Wolchok JD, Hoos A, O'Day S, et al. : Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res 15:7412-7420, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Neuenschwander B, Branson M, Gsponer T: Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med 27:2420-2439, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Brookmeyer R, Crowley J: A confidence interval for the median survival time. Biometrics 38:29-41, 1982 [Google Scholar]
- 23.Zhu M, Wu B, Brandl C, et al. : Blinatumomab, a bispecific T-cell engager (BiTE((R))) for CD-19 targeted cancer immunotherapy: Clinical pharmacology and its implications. Clin Pharmacokinet 55:1271-1288, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Beaver JA, Pazdur R: “Dangling” accelerated approvals in oncology. N Engl J Med 384:e68, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Lemery S, Pazdur R: Approvals in 2021: Dangling accelerated approvals, drug dosing, new approvals and beyond. Nat Rev Clin Oncol 19:217-218, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackhall F, Jao K, Greillier L, et al. : Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: Results from the phase 3 TAHOE study. J Thorac Oncol 16:1547-1558, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Johnson ML, Zvirbule Z, Laktionov K, et al. : Rovalpituzumab tesirine as a maintenance therapy after first-line platinum-based chemotherapy in patients with extensive-stage-SCLC: Results from the phase 3 MERU study. J Thorac Oncol 16:1570-1581, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Morgensztern D, Besse B, Greillier L, et al. : Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: Results from the phase II TRINITY study. Clin Cancer Res 25:6958-6966, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hipp S, Voynov V, Drobits-Handl B, et al. : A bispecific DLL3/CD3 IgG-like T-cell engaging antibody induces antitumor responses in small cell lung cancer. Clin Cancer Res 26:5258-5268, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Wermke M, Felip E, Gambardella V, et al. : A phase I, open-label, dose-escalation trial of BI 764532, a DLL3/CD3 bispecific antibody, in patients (pts) with small cell lung carcinoma (SCLC) or other neuroendocrine neoplasms expressing DLL3. J Clin Oncol 39:TPS8588, 2021 [Google Scholar]
- 31.Aaron WH, Austin R, Barath M, et al. : Abstract C033: HPN328: An anti-DLL3 T cell engager for treatment of small cell lung cancer. Mol Cancer Ther 18:C033, 2019 [Google Scholar]
- 32.Owen DH, Giffin MJ, Bailis JM, et al. : DLL3: An emerging target in small cell lung cancer. J Hematol Oncol 12:61, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DW, Gardner R, Porter DL, et al. : Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188-195, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is a plan to share data. This may include deidentified individual patient data for variables necessary to address the specific research question in an approved data sharing request, also related data dictionaries, study protocol, statistical analysis plan, informed consent form, and/or clinical study report.
Data sharing requests relating to data in this manuscript will be considered after the publication date and (1) this product and indication (or other new use) have been granted marketing authorization in both the United States and Europe, or (2) clinical development discontinues and the data will not be submitted to regulatory authorities. There is no end date for eligibility to submit a data sharing request for these data.
Qualified researchers may submit a request containing the research objectives, the Amgen product(s) and Amgen study/studies in scope, end points/outcomes of interest, statistical analysis plan, data requirements, publication plan, and qualifications of the researcher(s).
In general, Amgen does not grant external requests for individual patient data for the purpose of reevaluating safety and efficacy issues already addressed in the product labeling. A committee of internal advisors review requests. If not approved, requests may be further arbitrated by a Data Sharing Independent Review Panel. Requests that pose a potential conflict of interest or an actual or potential competitive risk may be declined at Amgen's sole discretion and without further arbitration.
Upon approval, information necessary to address the research question will be provided under the terms of a data sharing agreement. This may include anonymized individual patient data and/or available supporting documents, containing fragments of analysis code where provided in analysis specifications.
Further details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request.