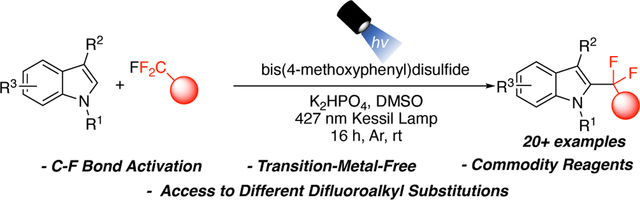
Abstract
An aryl disulfide-mediated C-F bond activation of the trifluoromethyl group to generate valuable gem-difluoroalkylindoles is described. This method relies on readily available commodity reagents under mild reaction conditions and represents the first transition metal-free redox-neutral C-F bond activation strategy. The reaction employs various substituted indoles and α-fluoro-substituted esters. Further, this mode of C-F activation was also amenable to the activation of trifluoromethylated arenes for the preparation of bis-benzylic gem-difluoromethylenes between indole and arene substructures, providing access to a unique chemical space.
Graphical Abstract
The incorporation of fluorine into organic molecules is well-known to alter chemical properties, including stability, reactivity, acidity, and conformational bias.1–3 Therefore, substantial efforts have been invested in incorporating these motifs into organic frameworks.1, 2, 4, 5 For example, over 50% of new agrochemicals introduced on the market from 1998–2020 contained at least one C-F bond,6 and over 40% of new small molecule drugs in 2018 and 2019 were fluorinated compounds.7
Another important class of pharmaceutically relevant motifs are indoles,8, 9 as they are among the most prevalent substructures in biologically active small-molecule drugs.10, 11 Consequently, the ability to incorporate fluorine onto indoles is of high interest and has been pursued in both academic and industrial laboratories.12 Specifically, the gem-difluoromethylene motif is an attractive bioisostere for common organic functional groups such as alcohols, amines, and ketones, and is known to improve biological properties while providing greater chemical stability.13, 14
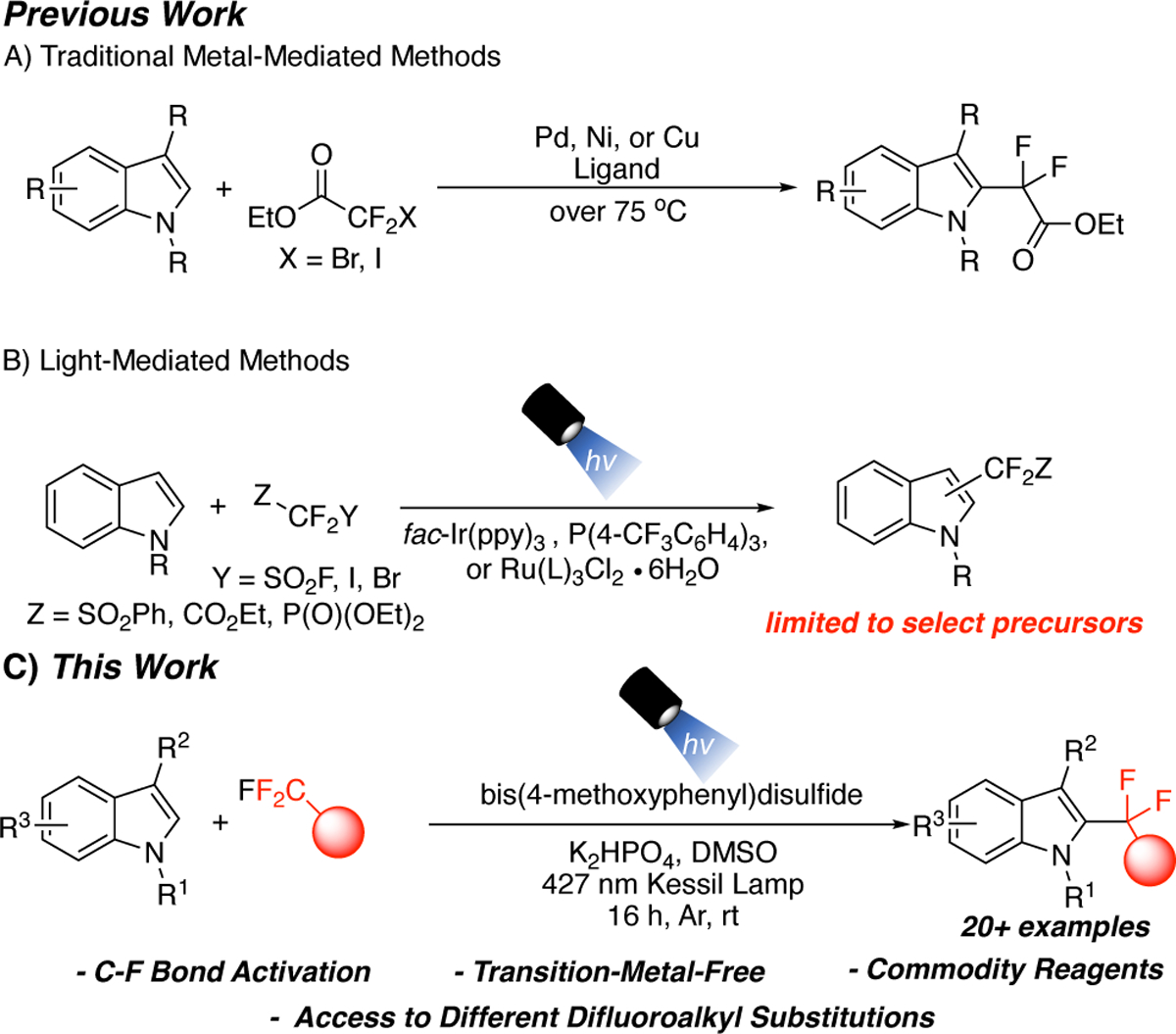
Early efforts on the synthesis of gem-difluoromethylated indoles relied on the use of transition metal-mediated or -catalyzed reactions (Scheme 1A). These methods require the use of catalytic Pd,15 Ni,16 or Cu17, 18 under elevated temperatures. Further, these methods make use of either the activated ethyl (iodo- or bromo)difluoroacetate precursor. Another report of a Rh-catalyzed method exists.19 However, this approach requires the use of a difluorovinyl precursor at elevated temperatures. More recently, light-induced methods for the preparation of difluoromethylene-containing indoles have appeared in the literature as an alternative (Scheme 1B). These methods used either an iridium,20, 21 ruthenium,22, 23 or a triphenylphosphine photocatalyst.24 However, these light-induced methods again relied on the use of activated, non-commodity reagents, and do not provide access to a large pool of available substrates. Thus, the ability to install the gem-difluoroalkyl group onto indoles with a more diverse precursor that did not require transition metals, harsh thermal conditions, or limited, active difluoro precursors under mild reaction conditions is an attractive goal.
Scheme 1.
Difluoroalkylation of Indoles
Our group has a long-standing interest in gem-difluoro functionalization of organic molecules.25–30 Recently, a photochemically mediated difluoroalkylation of olefins was developed utilizing a selective C-F bond activation method.31 The difluoro precursor was derived from the commodity feedstock ethyl trifluoroacetate, which is estimated to be 50–120 times cheaper than iodo- or bromodifluoroacetates.31 Therefore, we were interested in a C-F activation protocol that could be developed for installing the gem-difluoromethylene group onto indoles. This would overcome the reliance on more costly starting materials used in previous methods, potentially provide a mild, light-induced method, and most importantly, allow greater diversification because a wider scope of fluorinated substrates would be accessible. To perform this transformation, we were interested in the recent work by Shang’s group that utilized a light-induced strategy for C-F activation with aryl thiols (or disulfide).32 Shang’s work demonstrated that photoexcited aryl thiolates possess a low oxidation potential (−3.31 V vs. SCE), which is capable of reducing trifluoromethyl precursors such as ethyl trifluoroacetate (−2.0 V vs. SCE)31 to difluoroalkyl radicals for Giese type additions.32 Light-induced transformations utilizing various reactive sulfur species have become a topic of interest recently.33,34 Inspired by this work, we became interested in developing a C-F activation protocol for fluoroalkylation utilizing aryl thiol species for reactions other than Giese type additions. Therefore, we were intrigued to explore whether active difluoroalkyl radicals generated from aryl thiols would add to indoles in a reaction that did not require stoichiometric reductants as used in Giese type additions.31,35–38 This method would in turn provide the first transition metal-free redox-neutral C-F bond activation of ethyl trifluoroacetate and demonstrate the first transition metal-free C-F bond activation chemistry for fluoroalkylations beyond Giese type additions.
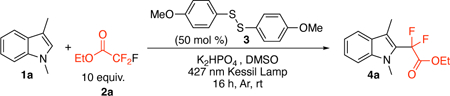
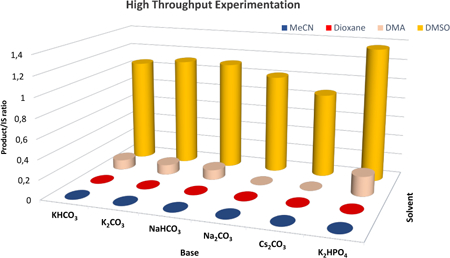
To explore the development of this proposed transformation, we used a microscale High-Throughput Experimentation (HTE) screening to assist in rapidly identifying conditions to enable the reaction described in Table 1 (bottom graph). Using readily available 1,3-dimethylindole (1a) as the model substrate for this optimization, we found the reaction with ethyl trifluoroacetate (2a), bis(4-methoxyphenyl) disulfide (3), and K2HPO4 in DMSO under LED array 445 nm light irradiation provided the greatest relative yield in the HTE screen. Transitioning the reaction to the bench scale provided a 63% yield with 0.5 equiv of 3 (entry 1), and excess 2a under 427 nm Kessil light irradiation. Control reactions demonstrated that the absence of light (entries 2), base (entry 3), or disulfide (entry 4) results in no product formation. The reaction takes place under open-to-air conditions (entry 5). However, the yield was diminished. Consistent with the HTE screen, the use of other bases or solvent resulted in decreased yields (see SI for additional optimizations). Further, the use of the 4-methoxybenzenethiol monomer instead of the dimer resulted in similar yields with the same sulfur loading (Table 1, entry 6 vs. 1). We elected to continue with 3 because of its commercial availability as an odorless solid that is easy to work with on the bench. Utilizing the traditional photocatalyst, Ir(ppy)3, resulted in no product detection (entry 7). Increasing 3 to 1 equiv led to an increase in yield to 72% (entry 8), and an isolated yield of 4a in 63% yield was achieved (0.5 mmol scale).
Table 1.
Optimization of the Reaction Conditionsa
| ||
|---|---|---|
| entry | deviation | 19F NMR yieldb (%) |
| 1 | none | 63 |
| 2 | no Kessil | n.d. |
| 3 | no base | n.d. |
| 4 | no 3 | n.d. |
| 5 | open to air | 40 |
| 6 | 4-methoxybenzenethiol | 61 |
| (100 mol %) | ||
| 7 | Ir(ppy)3 (5 mol %) | n.d. |
| 8 | 3 (100 mol %) | 72 (63)c |
| ||
Reaction conditions: indole 1a (0.1 mmol), 2a (1.0 mmol), K2HPO4 (0.2 mmol), 3 (0.05 mmol) in DMSO (1 mL), stirred 16 h under 427 nm Kessil light irradiation at rt using a fan.
Yields were determined by 19F NMR analysis using 2-bromo-5-(trifluoromethyl)pyridine as an internal standard.
Isolated yield.
With suitable conditions identified, the scope of the transformation was explored with various 3-substituted indoles (Scheme 2). Altering the substitution of the nitrogen with various alkyl groups (4a-4c) and the benzyl moiety (4d) was possible. The free, unprotected 3-methylindole (1e) was also successfully subjected to the reaction. However, utilizing indole or 2-methylindole resulted in low yields and a mixture of products by 19F NMR. The reaction accommodated various indoles containing free NH’s and N-methyl groups in good yields (4f-4h). Protected amine (4i) and alcohol (4j) substrates were also accommodated. Further, the natural product melatonin, bearing an amide and methoxy group, provided the corresponding difluorinated derivative in fair yields (4k). The reaction was successful when using amino acid derivative tryptophan (4l). Other heterocycles were explored in this transformation but were largely unsuccessful (see Supporting Information and vide infra). Considering the importance of scaling up the protocol for further applications, we performed the reaction of 1a on a gram scale and found a slight decrease in yield to 41%. However, we were able to recover some unreacted starting material in this case.
Scheme 2. Substrate Scope of Indolesa.
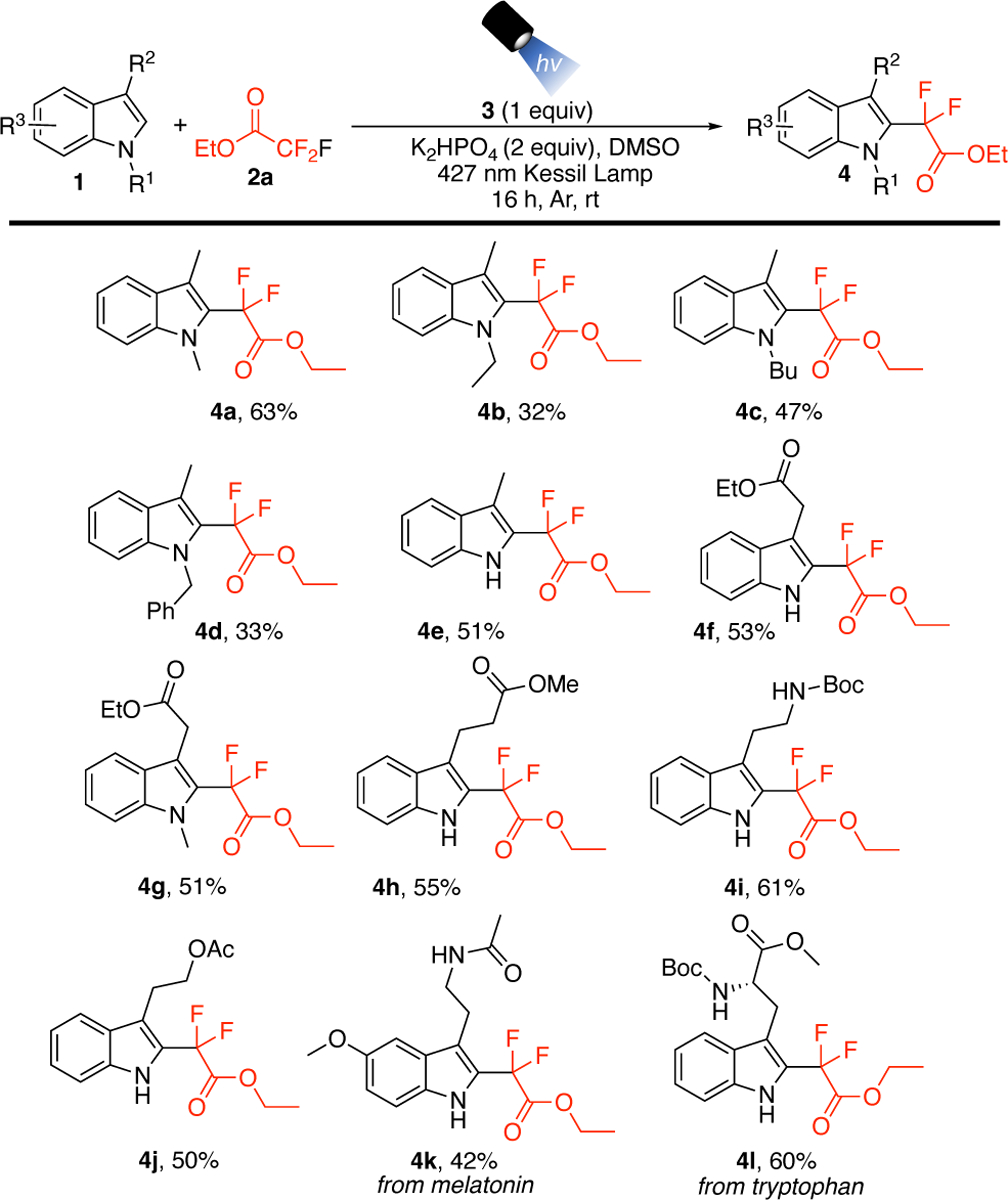
aReaction conditions: 1 (0.5 mmol), 2a (5 mmol), 3 (0.5 mmol), K2HPO4 (1.0 mmol) under Ar in DMSO (5 mL) and 427 nm Kessil lamp irradiation. Isolated yields.
Next, we evaluated the extension of the C-F activation protocol to other fluorinated starting materials (Scheme 3) Initial explorations utilized the isopropyl trifluoromethyl ester with 1a and 1b, in which cases fair yields were found for the generation of 5 and 6, indicating that varying the alkyl chain on the nitrogen is possible. To explore other fluorinated precursors, 1i was chosen as the model substrate to highlight the advantage of using this method for free, unprotected indoles and protected amines. Isopropyl trifluoromethyl acetate gave good yields with 1l, providing 7. Cyclohexane-containing ester provided 8, and the bulky adamantyl derivative 9 was synthesized in good yields. The ethyl pentafluoropropionate substrate selectively yielded 10, providing a product containing two unique fluorine containing functional units.
Scheme 3. Substrate Scope of Fluorinated Precursorsa.
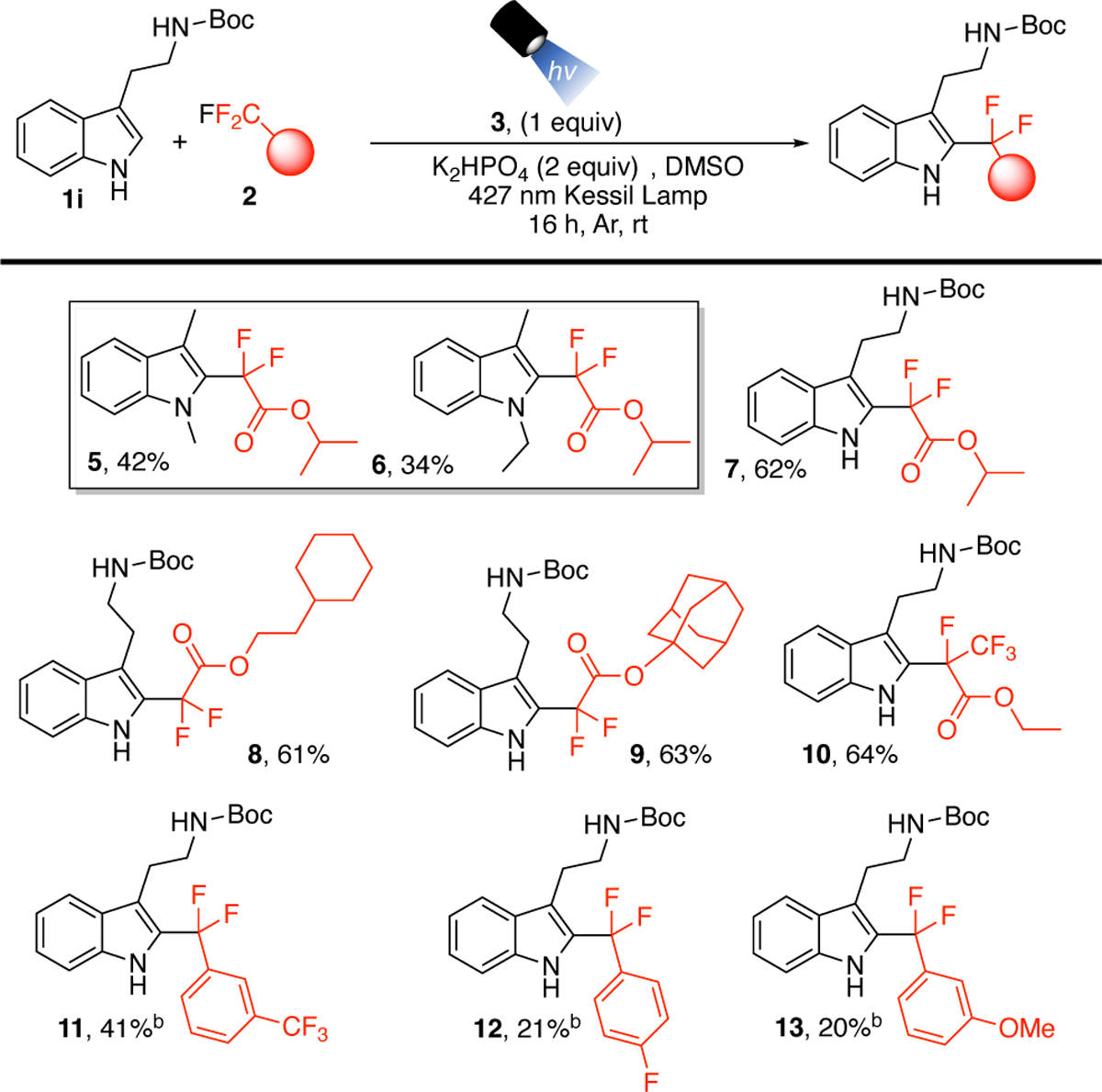
aReaction conditions: 1 (0.5 mmol), 2a (5 mmol), 3 (0.5 mmol), K2HPO4 (1.0 mmol) under Ar in DMSO (5 mL) and 427 nm Kessil lamp irradiation. Isolated yields. bSodium formate (0.75 mmol) was added.
Using conditions established for the trifluoroacetate, trifluoromethyl arenes were not suitable substrates for this protocol. The use of formate salts, however, has been shown to assist in C-F functionalization reactions in conjunction with reactive thiol species to generate aryl difluoromethyl radicals in Giese type additions. Consequently, we explored the addition of sodium formate in the reactions with arenes.32,36–38 Using this modified protocol allowed the synthesis of 11–13, generating a unique bis-benzylic gem-difluoromethylene motif between an arene and an indole. Prior methods to activate a C-F bond of trifluoromethyl arenes for coupling with other arenes required the use of Pd under thermal conditions, and was shown for trifluoromethyl arenes and aryl boronic acids.39 Therefore, this transition metal-free C-F bond activation strategy, allowing the installation of the bis-benzylic gem-difluoromethylene between an indole and arene (11–13), provides a simple, unprecedented protocol to access a unique chemical space and structural motif.
Attention was then turned to exploring the reaction mechanism and the nature of the radical species that were generated in situ. The reaction of 1a, 2a, and 3 with base in the presence of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) under standard reaction conditions inhibited the formation of product 4a, with detection of the thiol-TEMPO adduct 14 by GC-MS (Scheme 4A). Running the same reaction, replacing 3 with 4-methoxybenzenethiol, again inhibited product formation. However, in this case the difluorinated ester-TEMPO adduct 15 was detected by GC-MS (Scheme 4B). Therefore, based on these observations, we propose the reaction mechanism depicted in Scheme 3C. Upon irradiation with light, 3 undergoes homolytic cleavage of the disulfide (which was trapped by TEMPO in Scheme 3A), followed by hydrogen atom transfer (HAT) with solvent and deprotonation with a base to generate thiolate A.32 The thiolate can be photoexcited with light to generate the photoexcited thiolate species (B).32 Species B reduces 2a, generating C and D.32 Radical C undergoes a spin-centered shift (SCS),31,40 generating difluoroalkyl radical E (which was trapped by TEMPO as shown in Scheme 4B), which adds to the indole substrate, generating F. At this point, the thiyl radical D undergoes single electron transfer (SET) with F, regenerating thiolate A and cation G resulting from a radical/polar crossover. Deprotonation with the base then furnishes the product 4.
Scheme 4.
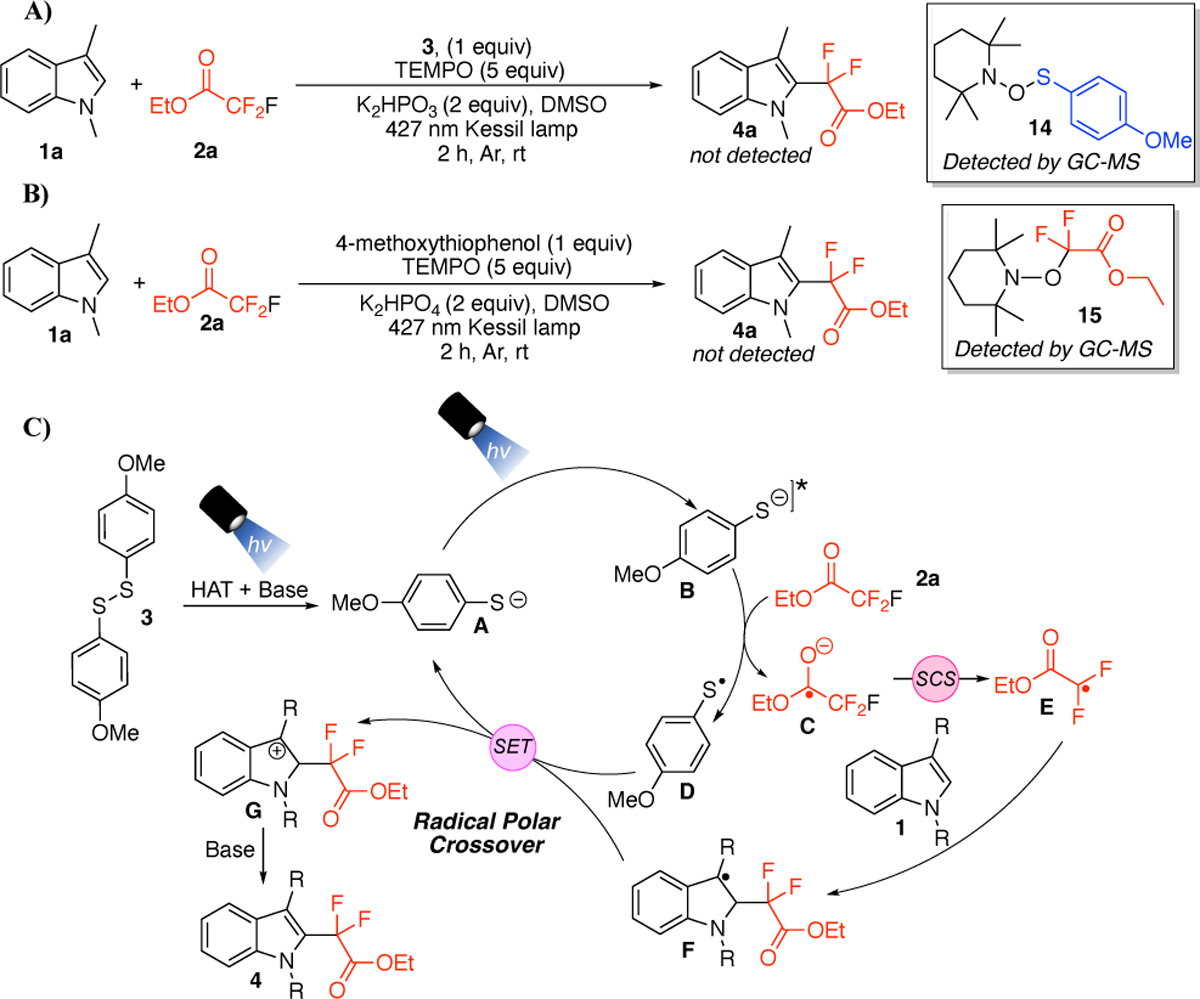
A and B) Radical Trapping Experiments with TEMPO, and C) Proposed Mechanism for gem-difluoroalkylation of indoles.
Utilizing catalytic amounts of 3 (see Supporting Information) led to a decrease in yields. In control experiments of 3 with 2a in the presence of a base, difluoroalkylated arylthiol products were formed under light irradiation (see Supporting Information). Thus, the electron rich disulfide/thiol undergoes side reactions with the radical species E, in turn requiring higher loadings of 3 for suitable formation of the desired gem-difluoroalkylation products. We believe this side reaction is why less electron-rich heterocycles were not amenable in this protocol, and this finding will be critical in future methods developments utilizing arylthiols or disulfides.
In summary, a new method for the gem-difluoroalkylation of indoles was described. The reaction represents the first transition metal-free, redox-neutral C-F bond activation of the inexpensive commodity feedstock chemical ethyl trifluoroacetate utilizing arylthiol or disulfide. This method is highlighted by utilizing a spin-centered shift C-F bond activation mechanism. The reaction is tolerant of a variety of functional groups, and further allows the installation of different fluorinated motifs and esters. Finally, the protocol was expanded to provide a unique bis-benzylic gem-difluoromethylene unit between two aromatic systems. This new synthetic method is simple, mild, and provides access to pharmaceutically relevant motifs, and was successfully scaled to the gram-scale level.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful for the financial support (RG2020) provided by Merck KGaA, Darmstadt, Germany, and NIGMS (R35 GM 131680 to G.A.M.). The NSF Major Research Instrumentation Program (award NSF CHE-1827457), the NIH supplement awards 3R01GM118510-03S1 and 3R01GM087605-06S1, as well as the Vagelos Institute for Energy Science and Technology, supported the purchase of the NMRs used in this study. The authors thank Dr. David M. Fialho (UPenn) for useful discussions. We thank Dr. Charles W. Ross, III (UPenn) for mass spectral data and Kessil for the donation of lamps used in this study.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
General procedures, characterization data for products (NMR, IR, MS), mechanistic studies details, gram scale reaction details and NMR spectra (PDF)
The authors declare no competing financial interest.
Data Availability Statement
The data underlying this study are available in the published article and its online supplementary material.
REFERENCES
- 1.Ni C; Hu J The unique fluorine effects in organic reactions: recent facts and insights into fluoroalkylations. Chem. Soc. Rev 2016, 45, 5441–5454. [DOI] [PubMed] [Google Scholar]
- 2.Purser S; Moore PR; Swallow S; Gouverneur V Fluorine in medicinal chemistry. Chem. Soc. Rev 2008, 37, 320–330. [DOI] [PubMed] [Google Scholar]
- 3.O’Hagan D Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev 2008, 37, 308–319. [DOI] [PubMed] [Google Scholar]
- 4.Barata-Vellejo S; Cooke MV; Postigo A Radical Fluoroalkylation Reactions. ACS Catal. 2018, 8, 7287–7307. [Google Scholar]
- 5.Wang J; Sánchez-Roselló M; Aceña JL; del Pozo C; Sorochinsky AE; Fustero S; Soloshonok VA; Liu H Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev 2014, 114, 2432–2506. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa Y; Tokunaga E; Kobayashi O; Hirai K; Shibata N Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue M; Sumii Y; Shibata N Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushik NK; Kaushik N; Attri P; Kumar N; Kim CH; Verma AK; Choi EH Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sravanthi TV; Manju SL Indoles — A promising scaffold for drug development. Eur. J. Pharm. Sci 2016, 91, 1–10. [DOI] [PubMed] [Google Scholar]
- 10.Heravi MM; Zadsirjan V Prescribed drugs containing nitrogen heterocycles: an overview. RSC Advances 2020, 10, 44247–44311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]
- 12.Nosova EV; Lipunova GN; Charushin VN; Chupakhin ON Fluorine-containing indoles: Synthesis and biological activity. J. Fluor. Chem 2018, 212, 51–106. [Google Scholar]
- 13.Erickson JA; McLoughlin JI Hydrogen Bond Donor Properties of the Difluoromethyl Group. J. Org. Chem 1995, 60, 1626–1631. [Google Scholar]
- 14.Meanwell NA Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem 2011, 54, 2529–2591. [DOI] [PubMed] [Google Scholar]
- 15.Shao C; Shi G; Zhang Y; Pan S; Guan X Palladium-Catalyzed C–H Ethoxycarbonyldifluoromethylation of Electron-Rich Heteroarenes. Org. Lett 2015, 17, 2652–2655. [DOI] [PubMed] [Google Scholar]
- 16.Soni V; Sharma DM; Punji B Nickel-Catalyzed Regioselective C(2)−H Difluoroalkylation of Indoles with Difluoroalkyl Bromides. Chem. Asian J 2018, 13, 2516–2521. [DOI] [PubMed] [Google Scholar]
- 17.He R-Y; Zeng H-T; Huang J-M Direct Fluoroalkylation of Indoles with Fluoroalkyl Halides Mediated by Copper. Eur. J. Org. Chen 2014, 2014, 4258–4263. [Google Scholar]
- 18.Wang X; Zhao S; Liu J; Zhu D; Guo M; Tang X; Wang G Copper-Catalyzed C–H Difluoroalkylations and Perfluoroalkylations of Alkenes and (Hetero)arenes. Org. Lett 2017, 19, 4187–4190. [DOI] [PubMed] [Google Scholar]
- 19.Zhu C; Song S; Zhou L; Wang D-X; Feng C; Loh TP Nonconventional difluoroalkylation of C(sp2)–H bonds through hydroarylation. Chem. Comm 2017, 53, 9482–9485. [DOI] [PubMed] [Google Scholar]
- 20.Yu W; Xu X-H; Qing F-L Photoredox Catalysis Mediated Application of Methyl Fluorosulfonyldifluoroacetate as the CF2CO2R Radical Source. Org. Lett 2016, 18, 5130–5133. [DOI] [PubMed] [Google Scholar]
- 21.Jung J; Kim E; You Y; Cho EJ Visible Light-Induced Aromatic Difluoroalkylation. Adv. Synth. Catal 2014, 356, 2741–2748. [Google Scholar]
- 22.Su Y-M; Hou Y; Yin F; Xu Y-M; Li Y; Zheng X; Wang X-S Visible Light-Mediated C–H Difluoromethylation of Electron-Rich Heteroarenes. Org. Lett 2014, 16, 2958–2961. [DOI] [PubMed] [Google Scholar]
- 23.Lin Q; Chu L; Qing F-L Direct Introduction of Ethoxycarbonyldifluoromethyl-Group to Heteroarenes with Ethyl Bromodifluoroacetate via Visible-Light Photocatalysis. Chin. J. Chem 2013, 31, 885–891. [Google Scholar]
- 24.Lu H; Wang D.-y.; Zhang A, Visible Light-Promoted Phosphine-Catalyzed Difluoroalkylation of Arenes and Heterocycles. J. Org. Chem 2020, 85, 942–951. [DOI] [PubMed] [Google Scholar]
- 25.Lang SB; Wiles RJ; Kelly CB; Molander GA Photoredox Generation of Carbon-Centered Radicals Enables the Construction of 1,1-Difluoroalkene Carbonyl Mimics. Angew. Chem. Int. Ed 2017, 56, 15073–15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelan JP; Lang SB; Sim J; Berritt S; Peat AJ; Billings K; Fan L; Molander GA Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis. J. Am. Chem. Soc 2019, 141, 3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiles RJ; Phelan JP; Molander GA Metal-free defluorinative arylation of trifluoromethyl alkenes via photoredox catalysis. Chem. Comm 2019, 55, 7599–7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levitre G; Granados A; Cabrera-Afonso MJ; Molander GA Synthesis of α-Fluorinated Areneacetates through Photoredox/Copper Dual Catalysis. Org. Lett 2022, 24, 3194–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granados A; Dhungana RK; Sharique M; Majhi J; Molander GA From Styrenes to Fluorinated Benzyl Bromides: A Photoinduced Difunctionalization via Atom Transfer Radical Addition. Org. Lett 2022, 24, 4750–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhungana RK; Granados A; Sharique M; Majhi J; Molander GA A three-component difunctionalization of N-alkenyl amides via organophotoredox radical-polar crossover. Chem. Comm 2022, 58, 9556–9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell MW; Polites VC; Patel S; Lipson JE; Majhi J; Molander GA Photochemical C–F Activation Enables Defluorinative Alkylation of Trifluoroacetates and -Acetamides. J. Am. Chem. Soc 2021, 143, 19648–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C; Li K; Shang R Arenethiolate as a Dual Function Catalyst for Photocatalytic Defluoroalkylation and Hydrodefluorination of Trifluoromethyls. ACS Catal. 2022, 12, 4103–4109. [Google Scholar]
- 33.Wang S; Wang H; König B Light Induced Single-Electron Transfer Processes involving Sulfur Anions as Catalysts. J. Am. Chem. Soc 2021, 143, 15530–15537. [DOI] [PubMed] [Google Scholar]
- 34.Li H; Liu Y; Chiba S Leveraging of Sulfur Anions in Photoinduced Molecular Transformations. JACS Au 2021, 1, 2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye J-H; Bellotti P; Heusel C; Glorius F Photoredox-Catalyzed Defluorinative Functionalizations of Polyfluorinated Aliphatic Amides and Esters. Angew Chem. Int. Ed 2022, 61, e202115456. [DOI] [PubMed] [Google Scholar]
- 36.Liu C; Shen N; Shang R Photocatalytic defluoroalkylation and hydrodefluorination of trifluoromethyls using o-phosphinophenolate. Nat. Commun 2022, 13, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogt DB; Seath CP; Wang H; Jui NT Selective C–F Functionalization of Unactivated Trifluoromethylarenes. J. Am. Chem. Soc 2019, 141, 13203–13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu P; Wang X-Y; Wang Z; Zhao J; Cao X-D; Xiong X-C; Yuan Y-C; Zhu S; Guo D; Zhu X Defluorinative Alkylation of Trifluoromethylbenzimidazole Enables by Spin-Center Shift: A Synergistic Photocatalysis/Thiol Catalysis Process with CO 2 ‒ Org. Lett 2022, 24, 4075–4080. [DOI] [PubMed] [Google Scholar]
- 39.Luo Y-C; Tong F-F; Zhang Y; He C-Y; Zhang X Visible-Light-Induced Palladium-Catalyzed Selective Defluoroarylation of Trifluoromethylarenes with Arylboronic Acids. J. Am. Chem. Soc 2021, 143, 13971–13979. [DOI] [PubMed] [Google Scholar]
- 40.Matsuo B; Granados A; Majhi J Sharique M; Levitre G; Molander GA 1,2-Radical Shifts in Photoinduced Synthetic Organic Transformations: A Guide to the Reactivity of Useful Radical Synthons. ACS Org. Inorg. Au 2022, 10.1021/acsorginorgau.2c00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its online supplementary material.


