Abstract
C57BL/6 (H-2b) mice generate type-specific cytolytic T-lymphocyte (CTL) responses to an immunodominant Kb-restricted epitope, KSPWFTTL located in the membrane-spanning domain of p15TM of AKR/Gross murine leukemia viruses (MuLV). AKR.H-2b congenic mice, although carrying the responder H-2b major histocompatibility complex (MHC) haplotype, are low responders or nonresponders for AKR/Gross MuLV-specific CTL, apparently due to the presence of inhibitory AKR.H-2b cells. Despite their expression of viral antigens and Kb, untreated viable AKR.H-2b spleen cells cause dramatic inhibition of the C57BL/6 (B6) antiviral CTL response to in vitro stimulation with AKR/Gross MuLV-induced tumor cells. This inhibition is specific (AKR.H-2b modulator spleen cells do not inhibit allogeneic MHC or minor histocompatibility antigen-specific CTL production), dependent on direct contact of AKR.H-2b cells in a dose-dependent manner with the responder cell population, and not due to soluble factors. Here, the mechanism of inhibition of the antiviral CTL response is shown to depend on Fas/Fas-ligand interactions, implying an apoptotic effect on B6 responder cells. Although B6.gld (FasL−) responders were as sensitive to inhibition by AKR.H-2b modulator cells as were B6 responders, B6.lpr (Fas−) responders were largely insensitive to inhibition, indicating that the responder cells needed to express Fas. A Fas-Ig fusion protein, when added to the in vitro CTL stimulation cultures, relieved the inhibition caused by the AKR.H-2b cells if the primed responders were from either B6 or B6.gld mice, indicating that the inhibitory AKR.H-2b cells express FasL. Because of the antigen specificity of the inhibition, these results collectively implicate a FasL/Fas interaction mechanism: viral antigen-positive AKR.H-2b cells expressing FasL inhibit antiviral T cells (“veto” them) when the AKR.H-2b cells are recognized. Consistent with this model, inhibition by AKR.H-2b modulator cells was MHC restricted, and resulted in approximately a 10- to 70-fold decrease in the in vitro expansion of pCTL/CTL. Both CD8+ CTL and CD4+ Th responder cells were susceptible to inhibition by FasL+ AKR.H-2b inhibitory cells as the basis for inhibition. The CTL response in the presence of inhibitory cells could be restored by several cytokines or agents that have been shown by others to interfere with activation-induced cell death (e.g., interleukin-2 [IL-2], IL-15, transforming growth factor β, lipopolysaccharide, 9-cis-retinoic acid) but not others (e.g., tumor necrosis factor alpha). These results raise the possibility that this type of inhibitory mechanism is generalized as a common strategy for retrovirus infected cells to evade immune T-cell recognition.
It is generally accepted that CD8+ CTL play a major role in host defense against virus infections and the development of neoplastically transformed cells. On the other hand, viruses, including retroviruses, and tumors have been selected to exhibit a number of “escape” mechanisms by which immune system surveillance by specific CTL is avoided. These escape mechanisms include both strategies to subvert recognition and lysis by effector CTL that have already been generated, such as FasL expression by certain tumor cells, and other mechanisms to inhibit the production of specific CTL. Animal models of virus infections and tumor development, including CTL responses to MuLV, have been particularly useful in the study of the generation of CTL responses and thus provide appropriate systems to examine virus or tumor escape at the level of induction of CTL.
We have previously demonstrated that mice of the H-2b haplotype, such as B6 mice, can elicit vigorous AKR/Gross MuLV type-specific CTL responses following in vivo priming and in vitro restimulation with AKR/Gross MuLV-positive, H-2b matched tumor cells (16). For these antiviral CTL, an immunodominant Kb-restricted epitope, KSPWFTTL, derived from the retroviral p15 TM envelope protein, has been identified (7, 19, 36, 46). The importance of this CTL epitope in immune system surveillance and clearance of AKR/Gross MuLV-infected cells has been demonstrated, in part through the use of the CTL-insusceptible, variant cl.18-5 clonal line (of the susceptible AKR.H-2b SL1 tumor), which, upon being pulsed with the KSPWFTTL peptide, became susceptible to lysis by antiviral CTL (19, 46). Also highlighting the importance of this intact CTL epitope, cells infected with retroviruses which have a substitution of arginine for the normal lysine at position 1 of this epitope, such as the B-ecotropic helper component of the LP-BM5 virus complex causing murine AIDS (8) and the Friend-Moloney-Rauscher family of viruses (36, 46), are not efficiently recognized by AKR/Gross MuLV-specific CTL.
AKR.H-2b mice are of the high-responder H-2b haplotype but are unable to generate anti-AKR/Gross MuLV/KSPWFTTL-specific CTL (17, 43). Unlike B6 mice, the AKR.H-2b strain carries and expresses the full complement of N-ecotropic AKR/Gross endogenous proviruses. The KSPWFTTL epitope has previously been shown to be presented by Kb on the surface on both AKR.H-2b T and B lymphocytes (15). Despite the expression of this immunodominant CTL epitope, AKR.H-2b mice contain normal numbers of antiretroviral pCTL, however, arguing against clonal deletion as the mechanism leading to nonresponsiveness (45). In contrast, in adoptive-transfer experiments with young responder congenic AKR.H-2b:Fv1b mice as recipients, donor AKR.H-2b CD4- and CD8-positive T cells, as well as B cells, were specifically inhibitory (31). Such cell transfers converted the recipient mice to an AKR/Gross MuLV-specific CTL nonresponsive status, without affecting minor H or allogeneic (H-2d)-specific CTL responsiveness. Moreover, these cell subsets of viable AKR.H-2b splenocytes, when added at the onset to in vitro restimulation cultures of AKR/Gross MuLV-primed B6 responder cells, specifically inhibited the B6 antiviral (but not minor H or allogeneic) CTL responses by a contact-dependent mechanism (32).
In the present study, we investigated the specific mechanism through which AKR.H-2b splenocytes inhibit the generation of AKR/Gross MuLV-specific CTL in vitro. Because of the fine specificity for antiviral CTL responses (31, 32), we questioned whether inhibition was occurring by T-cell-receptor-mediated recognition of viral Ag-positive AKR.H-2b cells by antiviral responder T cells, with involvement of subsequent FasL/Fas interactions. In this way, we sought insight into the mechanistic basis for AKR.H-2b inhibitory cell function and the antiviral CTL nonresponsiveness of AKR.H-2b mice.
MATERIALS AND METHODS
Abbreviations used in this report.
GCSA, Gross cell surface antigen; LDA, limiting-dilution analysis; MuLV, murine leukemia virus; FasL, Fas ligand; MLTC, mixed-lymphocyte tumor cell culture; cpm, counts per minute; Ig, immunoglobulin; B6, C57BL/6; B6.lpr, B6.MRL-Faslpr; B6.gld, B6.Smn.C3H-Faslgld; Ag, antigen; MAb, monoclonal antibody; AICD, activation-induced cell death; CTL, cytolytic T lymphocyte; pCTL, CTL precursor; minor H, minor histocompatibility antigen; MHC, major histocompatibility complex; LPS, lipopolysaccharide; RA, 9-cis-retinoic acid; IL, interleukin; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor β; APC, antigen-presenting cell; SIV, simian immunodeficiency virus; HIV, human immunodeficiency virus.
Mice.
The B6, B6.MRL-Faslpr (B6.lpr), B6.Smn.C3H-Faslgld (B6.gld), and AKR strains of mice were obtained from Jackson Laboratory, Bar Harbor, Maine, and were either inoculated or used as a source of splenic stimulator cells at 6 to 9 weeks of age. The AKR.H-2b congenic mouse strain was maintained through breeding of brother-sister pairs in the Animal Health Resource Facility, Dartmouth Medical School. Breeding pairs were originally provided by David Myers (Sloan Kettering Memorial Institute, New York, N.Y.).
Cell lines.
The E♂G2 (Gross virus-induced and GCSA+), and E♀K1 (AKR virus induced but GCSA−) tumors are of B6 (H-2b) strain origin. AKR.H-2b SL1 (SL1), a spontaneous GCSA+ tumor, was originally derived from the AKR.H-2b congenic mouse strain. B.GV, a Gross virus-induced GCSA+ tumor, was derived from a BALB.B (H-2b) mouse. These tumor cell lines have previously been described in detail (16). The following tumor cell lines were procured from the American Type Culture Collection, Rockville, Md.: P815, a methylcholanthrene-induced line derived from the DBA/2 (H-2d) strain; LB27.4 (H-2d/b), derived from the fusion of a BALB/c lymphoma line (H-2d) to T-cell-depleted spleen cells from a C57BL/10 (H-2b) mouse; and the highly NK-sensitive YAC-1. The AKR.79.6 cell line (26) is H-2k. The RF33.7 T-hybridoma cell line (33) was generously provided by Kenneth Rock (Dana Farber Cancer Institute, Boston, Mass.). Cell lines were maintained by thrice-weekly in vitro passage in RPMI 1640 (Gibco, Grand Island, N.Y.) supplemented with 5% fetal bovine serum, 5 × 10−5 M 2-mercaptoethanol, l-glutamine, and antibiotics.
Polyclonal CTL, CTL inhibition, responder cell fractionations, and Fas-Ig blocking.
51Cr-release assays were conducted as previously described (18) to measure CTL activity from bulk MLTC. Briefly, AKR/Gross MuLV-specific CTL were generated through in vivo inoculation of responder mice with 106 nonsyngeneic, H-2b-matched tumor cells. At 11 to 14 days postinoculation, 107 immune spleen cells were cultured in mixed lymphocyte tumor cell cultures (MLTC) with 2 × 105 irradiated E♂G2 or SL1 tumor stimulator cells or 2 × 106 splenic stimulator cells. Following 6 days of in vitro restimulation in MLTC medium containing RPMI 1640 supplemented with 5% fetal bovine serum, l-glutamine, and antibiotics, 104 radiolabeled tumor target cells were mixed with various numbers of effector cells (i.e., several effector-to-target-cell ratios), centrifuged, and incubated for 4 h at 37°C. At the end of this incubation, the cells were centrifuged again, and an aliquot of cell-free supernatant was removed for gamma counting and data reduction. The percent specific lysis against tumor cells is defined by the following formula: [(X-Y)/Z] × 100%, where X = cpm released by target cells incubated with effector cells, Y = cpm released by target cells incubated alone, and Z = cpm released by the freeze-thaw of target cells (approximately 80% of total cpm incorporated). In experiments designed to measure inhibition in the generation of AKR/Gross MuLV-specific CTL, 2 × 106 viable AKR.H-2b spleen cells were included in the MLTC. For reconstitution experiments, although the absolute number of responder B6 or B6.lpr CD4- and CD8-positive T cells remained essentially constant, the number of B cells ranged from 5 × 106 to 10 × 106. To deplete B6 or B6.lpr responder CD4- or CD8-positive T lymphocytes (prior to reconstitution with an equal number of CD4- or CD8-positive spleen cells from immune B6.lpr or B6 mice, respectively), spleen cells were incubated with rat IgM RL172 (anti-CD4) (kindly provided by R. Noelle, Dartmouth) or rat IgM 3.155 (anti-Lyt 2 [all alleles] derived from supernate of TIB 211 hybridoma cells) for 1 h at 4°C and then 107 splenocytes/ml were further incubated for 1 h at 37°C in rabbit complement (Cedarlane Laboratories, Westbury, N.Y.) diluted in Cedarlane cytotoxicity medium to obtain populations of responder lymphocytes which were enriched for either CD4−CD8+ or CD4+CD8− T lymphocytes, respectively. To confirm that CD4 or CD8 T-cell depletion had been accomplished, direct flow-cytometric analysis was performed on an aliquot of CD4 or CD8 T-cell-depleted cells by using a FACScan (Becton Dickinson) with fluorescein isothiocyanate-labeled anti-CD4 and anti-CD8 MAbs (Pharmingen, San Diego, Calif.). The efficiency of CD4 or CD8 T-cell depletion was in the range of 93 to 96% and 97 to 100% for experiments 1 and 2 (see Fig. 4), respectively. To block Fas/FasL interactions, a concentrated supernatant of a blocking Fas-Ig fusion protein (49) was derived as a secreted product of the NIH 3T3 Fas-Ig transfectant cell line, generously provided by Philip Leder (Howard Hughes Medical Institute, Boston, Mass.). As an independent means of verifying the presence of Fas-Ig (human IgG1 tail) in each supernatant preparation used, indirect flow-cytometric analysis was performed with RF33.7 T hybridoma cells (33), the kind gift of Kenneth Rock. The FasL+ RF33.7 cells were incubated with each Fas-Ig-containing supernatant preparation and then with fluorescein isothiocyanate-labeled F(ab′)2 goat anti-human IgG heavy-plus-light chains (Jackson ImmunoResearch Laboratories, West Grove, Pa.), and flow-cytometric analysis was performed. As a negative control for both flow-cytometric analysis and in vitro blocking of Fas/FasL interactions, a concentrated supernatant of cultured NIH 3T3 cells was used in parallel to Fas-Ig preparations.
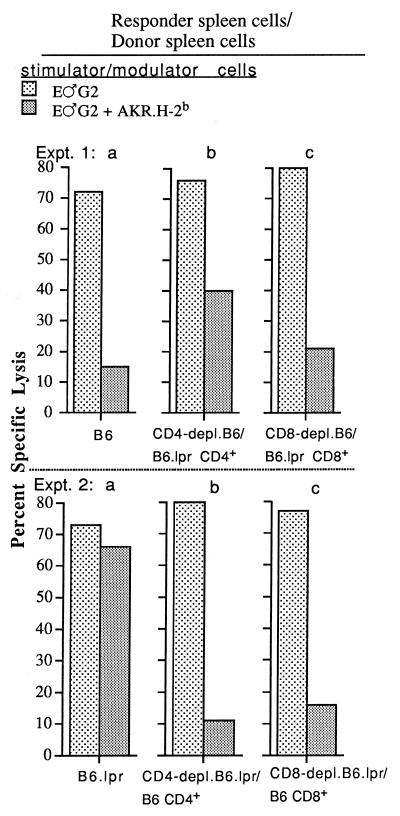
FIG. 4.
B6 and B6.lpr mice were immunized with 106 B.GV tumor cells. At 14 days later, B6 (experiment 1) or B6.lpr (experiment 2) responder spleen cell populations, which were either unfractionated or CD4 or CD8 depleted, were reconstituted to their original numbers with immune B6.lpr CD4+ or CD8+ (experiment 1)- or B6 CD4+ or CD8+ (experiment 2)-enriched spleen cells, respectively, and mixed with E♂G2 tumor stimulator cells without or with AKR.H-2b inhibitor cells, as indicated. The effector-to-target-cell ratios shown are 50:1 and 20:1 for experiments 1 and 2, respectively. The values for spontaneous release by E♂G2 tumor target cells ranged from 5.1 to 10.8%.
Reagents used to interfere with AKR.H-2b cell-mediated inhibition.
The reagents to block inhibition were added at the onset of the 6-day MLTC. Each blocking reagent was pretested over a range of concentrations to define the concentration which provided maximal blockade with minimal toxicity (see the legend to Fig. 2 for the optimal concentration of each inhibitor). LPS and RA were obtained from Sigma Chemical Co., St. Louis, Mo. Recombinant human IL-15 was obtained from Immunex Corp., Seattle, Wash., and recombinant murine TNF-α was obtained from Genentech Inc., South San Francisco, Calif. Recombinant human TGF-β was the kind gift of Bradley Arrick (Dartmouth).
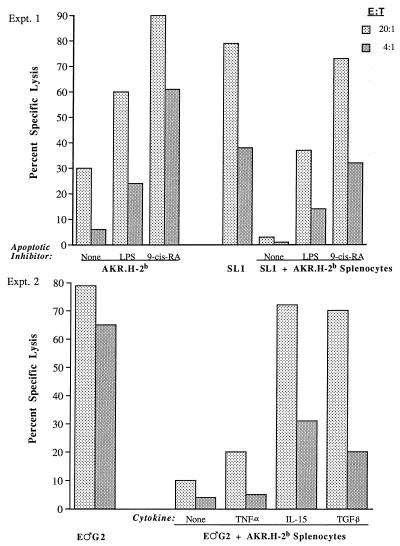
FIG. 2.
Inhibition of B6 antiviral CTL restimulation is partially blocked by the apoptosis-inhibiting agents LPS (10 μg/ml), RA (1 μg/ml), IL-15 (2 ng/ml), and TGF-β (100 pg/ml) but not by TNF-α (10 ng/ml), (see Materials and Methods for determination of these optimal doses). B6 mice were immunized with 106 B.GV tumor cells. At 11 (experiment 1) or 12 (experiment 2) days after immunization, B6 responder cells were mixed or not mixed with viable AKR.H-2b splenocytes, irradiated SL1 (experiment 1) or E♂G2 (experiment 2) tumor stimulator cells, and apoptosis-inhibiting agents, as indicated. Each blocking reagent was tested in at least two independent experiments, which yielded the same pattern of results. The values for spontaneous release by E♂G2 target cells for experiments 1 and 2 were 9.2 and 9.6%, respectively. E:T, effector-to-target-cell ratio.
Determination of pCTL frequencies.
The protocol for performing LDA has been described in detail previously (44, 45). Briefly, various numbers of responder cells, 5 × 106 irradiated B6 splenic feeder cells, and 105 irradiated E♂G2 tumor cells, as a source of viral Ag, were added to RPMI 1640 supplemented with a 1:20 dilution of rat T-stim culture supplement containing concanavalin A (Collaborative Biomedical Products, Bedford, Mass.), 100 mM methyl-α-d-mannopyranoside (Sigma), 5.4 U of IL-2 (Cetus Corp., Emeryville, Calif.) per well, 5 × 10−5 M 2-mercaptoethanol, and HEPES buffer in U-bottom 96-well cluster plates (Corning). At the end of 9 to 10 days in culture, cells from wells were split into three equal portions and tested on 3 × 103 51Cr-labeled target cells. E♂G2 tumor cells were used as targets to score pCTL/CTL specific for AKR/Gross virus Ag. E♀K1 (viral Ag−) and YAC-1 (NK sensitive) were used as negative control targets. Minimal estimates of pCTL/CTL frequencies were obtained by the Poisson distribution equation as the slope of the line relating the percent nonresponding wells (plotted on a logarithmic y axis) and the number of input spleen cells per well (plotted on a linear x axis). The slope of the regression line was determined with a computer and χ2 minimization analysis as described by Taswell (39). Software used for the determination of pCTL frequencies was kindly provided by Patrick Smith (Louisiana State University School of Medicine, Shreveport, La.). This analysis yields a minimal-frequency estimate (l/f), as well as a 95% confidence interval of the frequency estimate and a χ2 estimate of probability, with significance indicated by P > 0.05.
RESULTS
The secondary in vitro restimulation of AKR/Gross MuLV-specific CTL from B6 and B6.gld, but not B6.lpr, mice is inhibited by AKR.H-2b splenocytes.
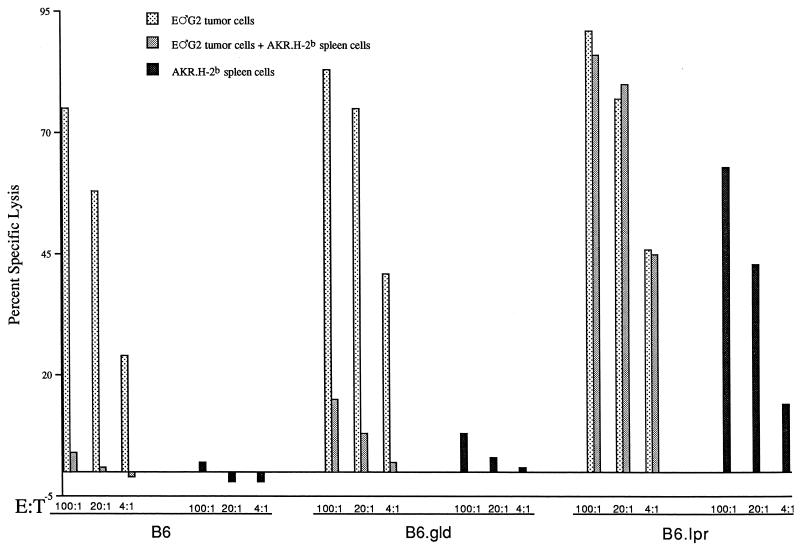
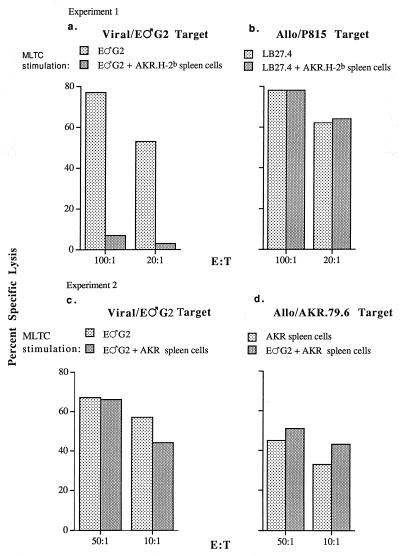
To determine whether FasL/Fas interactions might be involved in the down-modulation of the B6 antiviral CTL response by viable AKR.H-2b spleen cells as previously described (32), B6, B6.lpr (Fas−), and B6.gld (FasL−) mice were compared as responders for the generation of AKR/Gross MuLV-specific CTL. The 6- to 9-week-old B6, B6.gld, and B6.lpr mice were each capable of eliciting vigorous AKR/Gross MuLV-specific CTL responses (Fig. 1); this result was repeated in seven of seven experiments. Following in vivo priming with GCSA+ B.GV tumor cells and in vitro restimulation of primed spleen cells with irradiated GCSA+ E♂G2 (or AKR.H-2b SL1 [results not shown]) tumor cells in MLTC, the three responder strains produced comparably high levels of antiviral CTL activity. Significantly, viable (i.e., not irradiated or mitomycin C-treated) AKR/Gross MuLV Ag+ AKR.H-2b spleen cells were ineffective as in vitro stimulators for either B6 (32) or B6.gld immune spleen cells yet could serve as efficient stimulator cells for the generation of B6.lpr antiviral CTL (Fig. 1); this result was repeated in three of three experiments. These results demonstrated that the ability of viable AKR.H-2b spleen cells to serve as stimulatory APC for a secondary antiviral CTL response required that the primed responder cells be derived from a Fas-negative strain.
FIG. 1.
B6, B6.gld, and B6.lpr mice were immunized with 106 B.GV tumor cells. At 12 days later, 107 responder spleen cells were placed in MLTC with 2 × 105 E♂G2 tumor stimulator cells and/or 2 × 106 viable AKR.H-2b splenocytes, as indicated. At 6 days later, the cells were assayed for the ability to lyse 51Cr-labeled E♂G2 tumor target cells. The value for spontaneous release by target cells was 12.1%. E:T, effector-to-target-cell ratio.
To test more directly whether the AKR.H-2b spleen cells were playing a passive role as merely an inefficient APC (for responder cells from Fas+ mice) or might be playing an active role in inhibiting B6 and B6.gld AKR/Gross MuLV-specific CTL responses, “three-cell” (primed responder cell, irradiated E♂G2 tumor stimulator cell, and viable AKR.H-2b-spleen cell) in vitro cultures were set up. The vigorous antiviral CTL responses generated through E♂G2 tumor-cell restimulation of primed B6 (32) and primed B6.gld responder cells were dramatically inhibited by viable AKR.H-2b cells (Fig. 1). Again in clear contrast, addition of viable AKR.H-2b splenocytes to MLTC containing primed B6.lpr responder cells and E♂G2 tumor stimulator cells had little or no effect on CTL generation. This result was in keeping with the ability of viable AKR.H-2b spleen cells to function as stimulatory APC for B6.lpr antiviral CTL responses in the absence of E♂G2 tumor stimulator cells. Inhibition by AKR.H-2b cells in three-cell MLTC of B6 and B6.gld, but not B6.lpr, antiviral CTL (repeated in six of seven experiments) demonstrated that cells in the AKR/Gross MuLV-specific responder cell population must have the capacity to express Fas for the antiviral CTL response to be inhibited by viable AKR.H-2b splenocytes.
Inhibition of the generation of AKR/Gross MuLV-specific CTL by AKR.H-2b spleen cells is blocked by an Fas-Ig fusion protein.
The requirement for responder-cell Fas expression suggested that FasL/Fas interactions might be occurring between Fas+ B6 or B6.gld responder cells and FasL+ AKR.H-2b “inhibitory” cells. To test this possibility, in vitro experiments involving a blocking Fas-Ig fusion protein (49) in the three-cell experimental protocol were performed. While there was again dramatic inhibition of the AKR/Gross MuLV-specific CTL response when AKR.H-2b splenocytes were included in MLTC containing B6 or B6.gld primed responder cells and E♂G2 stimulator cells, preincubation of inhibitory AKR.H-2b splenocytes with Fas-Ig fusion protein largely or completely restored the generation of a vigorous AKR/Gross MuLV-specific CTL response (Table 1). Therefore, and importantly, because B6.gld responder mice are incapable of expressing FasL, functional FasL must be expressed solely on the AKR.H-2b spleen cells. Also of note, no increase in the generation of CTL activity was observed under control conditions where Fas-Ig was added to cultures containing only primed B6 responder cells plus E♂G2 stimulator cells (Table 1), indicating a lack of significant FasL/Fas-mediated inhibition independent of inhibitory AKR.H-2b spleen cells.
TABLE 1.
Preincubation of AKR.H-2b splenocytes with Fas-Ig fusion protein blocks inhibition of antiviral CTL from immune B6 and B6.gld responder micea
| Responder mouse strain | Secondary in vitro stimulation
|
Fas-Ig protein present | E:T ratiob | % Specific lysis
|
|||
|---|---|---|---|---|---|---|---|
| Control (E♀K1) | Viral
|
||||||
| Tumor cell | Splenocyte | SL1 | E♂G2 | ||||
| B6 (expt 1) | None | None | − | 50:1 | −2 | 1 | 3 |
| E♂G2 | None | − | 50:1 | −3 | 80 | 86 | |
| 10:1 | −3 | 72 | 91 | ||||
| 2:1 | −2 | 44 | 71 | ||||
| E♂G2 | AKR.H-2b | − | 50:1 | 7 | 24 | 26 | |
| None | None | + | 50:1 | −3 | 1 | 3 | |
| E♂G2 | None | + | 50:1 | −3 | 73 | 77 | |
| 10:1 | −1 | 75 | 79 | ||||
| 2:1 | −1 | 36 | 49 | ||||
| E♂G2 | AKR.H-2b | + | 50:1 | 3 | 64 | 77 | |
| 10:1 | 3 | 54 | 66 | ||||
| 2:1 | 0 | 24 | 30 | ||||
| B6.gld (expt 2) | None | None | − | 50:1 | −4 | 0 | −3 |
| E♂G2 | None | − | 50:1 | −3 | 53 | 55 | |
| 10:1 | −4 | 38 | 43 | ||||
| 2:1 | −4 | 11 | 15 | ||||
| E♂G2 | AKR.H-2b | − | 50:1 | −4 | 12 | 8 | |
| None | None | + | 50:1 | −2 | 4 | 1 | |
| E♂G2 | None | + | 50:1 | −4 | 65 | 55 | |
| 10:1 | −5 | 37 | 42 | ||||
| 2:1 | −5 | 13 | 16 | ||||
| E♂G2 | AKR.H-2b | + | 50:1 | −5 | 65 | 56 | |
| 10:1 | −5 | 35 | 39 | ||||
| 2:1 | −6 | 13 | 18 | ||||
B6 (experiment 1) or B6.gld (experiment 2) mice were immunized with 106 B.GV tumor cells 11 (experiment 1) or 13 (experiment 2) days before in vitro stimulation with 2 × 105 E♂G2 tumor cells without or with 2 × 106 AKR.H-2b splenocytes, as indicated. AKR.H-2b splenocytes were preincubated for 1 h at 37°C with concentrated supernatant containing Fas-Ig fusion protein, where indicated. At 6 days later, the cells were assayed for the ability to lyse 51Cr-labeled target cells. AKR.H-2b-mediated CTL inhibition was substantially blocked by Fas-Ig fusion protein in four of four and two of two experiments when B6 and B6.gld responder cells were used, respectively. The values for spontaneous release by target cells ranged from 7 to 9.4% and 9.1 to 23.7% for experiments 1 and 2, respectively.
E:T ratio, effector-to-target-cell ratio.
Exogenous IL-15, TGF-β, RA, or LPS, but not TNF-α, restores AKR/Gross MuLV CTL generation in the presence of inhibitory AKR.H-2b splenocytes.
Previously, we demonstrated that addition of IL-2 at the onset of in vitro restimulation cultures partially offsets the inhibition by AKR.H-2b splenocytes of B6 AKR/Gross MuLV-specific CTL responses (32). Here, we tested other reagents which have been described as being inhibitors of apoptosis to see if they might also “rescue” Fas-positive responder cells and thereby restore antiviral CTL responsiveness. Addition of LPS (41) or RA (48) to in vitro MLTC not only increased the ability of viable AKR.H-2b splenocytes to serve effectively as viral Ag-positive stimulator cells in the absence of tumor stimulator cells but also partially restored the generation of AKR/Gross MuLV-specific CTL lysis of E♂G2 target cells following addition to three-cell in vitro MLTC containing B6 responders, SL1 tumor stimulators, and AKR.H-2b inhibitory cells (Fig. 2, experiment 1). In Figure 2, experiment 2, two cytokines, IL-15 (which has a similar spectrum of activities to IL-2) and TGF-β, both of which are antiapoptotic (34, 51), but not TNF-α, which is proapoptotic (27), were also able to partially overcome the inhibitory effects of AKR.H-2b splenocytes and facilitate the restoration of an appreciable percentage of antiviral CTL production.
AKR.H-2b splenocytes inhibit AKR/Gross MuLV-specific pCTL/CTL expansion in vitro.
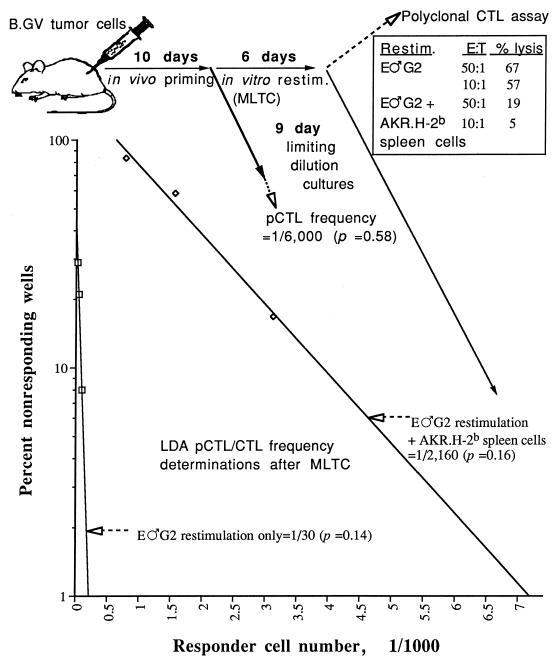
The above results taken together were consistent with an inhibitory mechanism in which FasL-bearing AKR.H-2b splenocytes inhibited the expansion of Fas-expressing AKR/Gross MuLV-specific responder pCTL/CTL. To determine the baseline pCTL frequency of AKR/Gross MuLV-specific pCTL, LDA of spleen cells taken directly from B.GV primed B6 mice were performed 10 to 14 days postinoculation. In keeping with our published data (45), we found that the pCTL frequency of immune spleen cells from B6 mice was about 1 in 6,000 (Fig. 3). If the LDA was instead conducted at the end of the standard in vitro MLTC period (after immune spleen cells were restimulated with E♂G2 tumor cells to generate bulk antiviral CTL), the antiviral pCTL/CTL frequency increased to approximately 1 in 30. This dramatic (approximately 200-fold) expansion in the number of AKR/Gross MuLV-specific pCTL/CTL correlated directly with the generation of polyclonal antiviral CTL activity (inset in Fig. 3). In sharp contrast, inclusion of AKR.H-2b spleen cells in three-cell MLTC resulted in a markedly decreased frequency of AKR/Gross MuLV-specific pCTL/CTL, to 1 in 2,160, a 72-fold reduction. This decrease in antiviral pCTL/CTL was consistent with the severe inhibition of the polyclonal AKR/Gross MuLV-specific CTL response by the AKR.H-2b spleen cells (inset in Fig. 3). In total, addition of viable AKR.H-2b splenocytes inhibited the in vitro generation of B6 AKR/Gross MuLV-specific pCTL/CTL by 10-fold in four of four experiments.
FIG. 3.
Minimal estimate of the frequency of B6 pCTL/CTL specific for syngeneic GCSA+ E♂G2 tumor cells. Solid arrows illustrate the kinetics of the treatment conditions of B6 spleen cells, which included in vivo priming (of B6 mice) with 106 B.GV tumor cells, in vitro restimulation of polyclonal spleen cells with E♂G2 tumor cells without or with AKR.H-2b spleen cells, and establishment of limiting-dilution cultures of immune spleen cells (taken directly from the immunized mouse or from MLTC wells obtained at the end point). Dashed arrows identify the results of 51Cr release assays, which include the pCTL/CTL frequency following in vivo priming of B6 mice, bulk CTL data following polyclonal restimulation (inset), and the pCTL/CTL frequency of primed and restimulated (restim.) B6 spleen cells. The estimates of probability as determined by χ2 minimization, where significance is indicated by P > 0.05, are shown in parentheses.
Both Fas-expressing B6 CD8- and CD4-positive responder spleen cells are targeted by FasL-expressing AKR.H-2b spleen cells.
To determine whether the dramatic decrease in polyclonal B6 AKR/Gross MuLV-specific CTL responsiveness (Fig. 1 and Table 1) and correspondingly diminished pCTL/CTL frequency (Fig. 3) were due to an AKR.H-2b cell-mediated direct inhibitory effect on responder B6 CD8+ pCTL/CTL and/or an indirect effect on responder CD4+ Th cells required for the CTL response, two related experimental approaches were used. In the first approach (Fig. 4, experiment 1), fractionated CD4+ or CD8+ T lymphocytes from immunized Fas-negative B6.lpr mice were mixed with CD4- or CD8-depleted primed B6 responder cells, respectively, at the in vitro MLTC stage. Consistent with the data of Fig. 1, the secondary AKR/Gross MuLV-specific CTL response of B6.lpr Fas− “donor” mice was not inhibitable by AKR.H-2b spleen cells (Fig. 4, experiment 2, panel a). This characteristic allowed us to limit the susceptibility to AKR.H-2b cell-mediated inhibition to either the CD8 or CD4 T-cell compartment. B6 responder cell populations, depleted of either CD4+ cells (experiment 1, panel b) or CD8+ T cells (experiment 1, panel c) and reconstituted to their original numbers with immune B6.lpr CD4+ or CD8+ spleen cells, respectively, generated antiviral CTL activity at levels essentially the same as that elicited by unfractionated B6 responder CTL. When the responder cell population contained either Fas+ B6 CD8+ (experiment 1, panel b) or Fas+ CD4+ (experiment 1, panel c) cells, along with the B6.lpr-derived Fas− counterpart T-cell subset, substantial inhibition (range, 47 to 74%) of generation of the antiviral CTL response was noted upon addition of AKR.H-2b spleen cells in a standard three-cell MLTC. These data suggested that FasL/Fas-mediated inhibition of either the responder CD8+ CTL or requisite CD4+ Th-cell populations could occur and form the basis for the AKR.H-2b spleen cell-mediated inhibition of the antiviral CTL response.
In the second approach, by reconstituting either CD4-depleted (experiment 2, panel b) or CD8-depleted (experiment 2, panel c) primed B6.lpr (Fas−) responder cell populations with CD4+ or CD8+ immune (Fas+) B6 spleen cells, respectively, the same conclusion was reached: the B6.lpr responder CTL response was converted to susceptibility to substantial (79 to 86%) inhibition by AKR.H-2b spleen cells when either the CD8+ or CD4+ T-cell subset originated from B6 mice. These two experimental approaches, each repeated once with essentially the same pattern of results, highlighted the importance of not only the CD8 CTL but also the CD4 Th-cell component in the generation of an optimal B6 AKR/Gross MuLV-specific CTL response and demonstrated that both Fas+ responder B6 CD4 and CD8 T-cell subsets are vulnerable to inhibition by FasL+ AKR.H-2b spleen cells.
Inhibition of B6 AKR/Gross MuLV-specific CTL responses is specific and MHC restricted.
Because of our previous demonstration of the fine antigen specificity of the inhibition mediated by viable AKR.H-2b spleen cells (i.e., only AKR/Gross MuLV, not allogeneic or minor H-specific CTL responses, were diminished), it seemed likely that only activated Fas+ AKR/Gross MuLV-specific responder T cells would be targeted by FasL and viral Ag-positive AKR.H-2b cells, upon MHC-restricted T-cell receptor recognition of the latter inhibitory cells by the responder T cells. Therefore, we tested whether the inhibitory function of AKR.H-2b cells was MHC restricted. To this end, we used, in parallel with AKR.H-2b congenic spleen cells, AKR (H-2k) splenocytes, which share the high-leukemic AKR genetic background, including Akv-type endogenous retrovirus expression and thus AKR/Gross MuLV Ag positivity. Under conditions where viable AKR.H-2b spleen cells caused a dramatic inhibition of the anti-AKR/Gross MuLV, but not an anti-allogeneic (H-2d), CTL response (Fig. 5a and b, respectively), as we have previously reported (32), viable AKR spleen cells were unable to substantially inhibit the antiviral CTL response (Fig. 5c). AKR spleen cells could serve effectively as stimulator cells for the generation of a B6 anti-allogeneic H-2k CTL response (Fig. 5d), however, indicating their capacity to be recognized as APC.
FIG. 5.
B6 mice were inoculated with 106 B.GV tumor cells. At 11 (experiment 1) or 12 (experiment 2) days later, 107 responder spleen cells were mixed with 2 × 105 E♂G2 or LB27.4 (H-2b/d) tumor stimulator cells, without or with the addition of 2 × 106 viable AKR.H-2b (experiment 1) or AKR (experiment 2) splenocytes as indicated. At 6 days later, the cells were assayed for the ability to lyse 51Cr-labeled target cells. The values for spontaneous release by target cells used in either experiment ranged from 4.7 to 15.5%. E:T, effector-to-target-cell ratio.
These results (confirmed in a second experiment) demonstrated that the interaction between the AKR.H-2b inhibitory cell and the lymphoid responder T cell was MHC (H-2b) restricted.
DISCUSSION
Unlike the prototypic high-responder B6 strain, AKR.H-2b congenic mice specifically fail to generate an antiviral CTL response to AKR/Gross MuLV (17, 43). As to the mechanism of this specific unresponsiveness, we showed preservation of pCTL frequencies (45) but determined that AKR.H-2b CD4+ and CD8+ T cells, as well as B cells, each can mediate inhibition of the AKR/Gross MuLV-specific CTL response in both in vitro (32) and in vivo (31) models, in a manner dependent on their viral Ag positivity (32). Here we extend these findings by showing that the AKR/Gross MuLV-specific CTL response of immune B6 (Fas+/FasL+), but not B6.lpr (Fas−), mice can be nearly completely inhibited by addition of AKR.H-2b splenocytes during the in vitro restimulation stage of CTL generation (Fig. 1). These and other data obtained through the use of a blocking Fas-Ig and several antiapoptotic reagents illustrate that cells in the virus-specific responder cell population must express Fas to be inhibited by FasL+ AKR.H-2b splenocytes. These results thus implicated FasL/Fas-mediated apoptosis as a mechanism of inhibition of the antiviral CTL response, although technical limitations did not allow us to assess the apoptotic cell death of antiviral T cells directly. The critical role of the viral Ag-positive AKR.H-2b spleen cells in this apparent apoptosis was clear; the related normal regulatory mechanism of AICD among responder cells did not seem likely. Evidence of the lack of FasL/Fas-mediated “fratricide” or “autologous suicide” by Fas- and FasL-expressing activated responder T cells included the following: (i) the levels of anti-AKR/Gross MuLV CTL activity generated were not consistently higher with B6.lpr and/or B6.gld then with B6 responders, and more incisively (ii) inclusion of the blocking Fas-Ig fusion protein in MLTC containing only responder B6 cells and E♂G2 stimulator cells did not lead to increased levels of CTL generation (Table 1). Rather, consistent with the strict Ag and MHC-restricted specificity of the inhibition (32), the inhibitory mechanism appeared to require viral Ag and FasL to be concomitantly expressed on AKR.H-2b cells, such that when the AKR.H-2b cells were recognized by Fas+ antiviral T cells, the latter were inhibited or “vetoed” (probably killed by Fas-dependent apoptosis) (11, 28). Although the observed dramatic decline in the numbers of AKR/Gross MuLV-specific pCTL/CTL was compatible with a FasL/Fas-mediated apoptosis of responder CD8+ CTL, both CD8+ pCTL/CTL and CD4+ Th cells were clearly shown to be susceptible to substantial inhibition by AKR.H-2b veto cells (Fig. 4). There was some evidence, however, that the extent of inhibition was not as complete when only the CD4 or CD8 T-cell compartment was Fas+ as was the inhibition when intact B6 responder cells were used, suggesting a cumulative inhibitory effect when both T-cell subsets are Fas+.
Veto cell-modulated decreases in antivirus-specific pCTL/CTL have been reported in other studies, such as that by Rammensee et al. (29), which demonstrated the capacity of veto cells to modulate a decrease in specific pCTL in vivo. Also, injection of anti-CD4 MAb causes Fas-mediated CD4+-T-cell depletion in vivo (42). Additional studies are required to determine whether the AKR/Gross MuLV-specific pCTL/CTL frequency of AKR.H-2b mice (45) decreases in vivo following activation coincident with upregulation of the expression of Fas and possibly FasL.
IL-2 (32), IL-15, and TGF-β (Fig. 2) greatly augmented but did not completely restore the generation of the B6 antiviral CTL response in the presence of AKR.H-2b inhibitory cells. Assuming that although both T-cell subsets can be targets for inhibition, some CD8+ T cells survive, these cytokines may function to replace the CD4+ Th1 helper function that is required for full CTL generation but lost when these Th1 cells are targeted for apoptosis by the veto cells. Indeed, some studies have suggested not only that CD4+ T cells are more susceptible than CD8+ T cells to FasL/Fas apoptosis but also that Th1 cells are more vulnerable to apoptosis than are Th2 cells (30, 50). Alternatively, these cytokines and the other agents able to partially restore CTL generation (LPS and RA) may provide direct antiapoptotic effects to the CD4+ Th1 and/or the CD8+ pCTL/CTL. For example, downmodulation in Bcl-2 expression may be blocked by exogenous IL-2 in vitro in our system, consistent with the study performed by Adachi et al. in which IL-2 rescued cells from apoptosis by promoting continued Bcl-2 expression (1).
Based on our in vitro experiments, however, the evidence is clearly suggestive that AKR.H-2b veto cells exhibit unidirectional apoptotic immunosuppression of the very virus-specific T lymphocytes required for viral clearance and thereby promote virus escape from immune system surveillance. On the other hand, Suzuki and Fink (38) recently investigated whether in addition to delivery of a negative apoptotic signal, FasL-bearing cells might receive positive “reverse signaling” through FasL/Fas ligation. In this latter study, it was determined that murine FasL expression was the initial signaling source for proliferation of activated B6 wild-type and B6.lpr CD8+ cell lines, that CD8+-T-cell proliferation was blocked by a soluble Fas-Ig fusion protein, and that CD4+ T cells were more susceptible to FasL/Fas-mediated apoptosis than were CD8+ CTL. Along these lines, further studies are needed to determine whether FasL+ AKR.H-2b T lymphocytes receive positive signals following their recognition by antiviral T lymphocytes.
The early literature on FasL/Fas-mediated apoptosis highlighted the importance of FasL expression on cells found in immunologically privileged sites, such as the eyes and testes (12). More recently, certain tumor cells have been shown to use a Fas-low/FasL-high phenotype as a means of escaping immune system surveillance: for example, human melanoma (21) and hepatocellular carcinoma (37) cells do not express significant levels of Fas but do express high levels of FasL. In each case, not only do the tumor cells escape CTL lysis but also they effectively deliver a FasL “death signal” to Fas-expressing, anti-tumor CTL when the CTL recognize class I-MHC presented tumor-specific Ag. Similarly, CD4+ T cells infected with the pj5 wild-type SIVmac32H clone have shown increased FasL expression which correlated with the death of SIV-specific CTL (47).
It has been proposed that the decrease in the number of CD4+ T lymphocytes in HIV-infected asymptomatic patients, which long has been considered a key indicator of subsequent disease progression, is due to an inappropriate induction of the apoptotic cell death program (2, 20). In a related study, Banda et al. (4) demonstrated that CD4 could be cross-linked with HIV glycoprotein gp120, resulting in apoptosis of the CD4+ cells. To determine if CD4 cross-linking might influence Fas expression, Desbarats et al. (9) used an MRL-lpr/lpr (Fas−) mouse model. In this study it was determined that cross-linking CD4 with anti-CD4 MAb correlated with an upregulation of Fas receptor on normal but not MRL-lpr/lpr spleen cells, consistent with apoptosis via the FasL/Fas-mediated pathway. Other studies with the HIV system have demonstrated that Fas-expressing CD8+, as well as CD4+, T cells can be directly targeted for apoptosis, although there is disagreement whether there is a correlation between apoptosis susceptibility and disease progression (10, 14, 25). There is also evidence at odds with a pathogenic role for FasL/Fas-mediated apoptosis in AIDS. For example, it has been reported that peripheral blood mononuclear cells from HIV-infected individuals do not express detectable FasL, in contrast to peripheral blood mononuclear cells from healthy control individuals (35). This abnormally low FasL expression was correlated with progression to a more advanced disease state.
The results of numerous studies of Fas and FasL expression on activated T-effector lymphocytes, following clearance of an invading pathogen or model antigen, have been taken to suggest that the then obsolete effector cells are deleted by AICD fratricide and/or autologous suicide as a means of maintaining normal homeostasis and preventing an accumulation of unneeded cells (see, e.g., references 3, 6, and 23). In addition, recent evidence has shown the importance of FasL expression on several other normal, non-T-lymphocyte cell types, including murine B cells (22), thymic epithelial and thymic dendritic cells (13), and human keratinocytes (5). Significantly, we have found that AKR.H-2b B cells consistently express similar or slightly higher densities of viral antigens compared to either the CD4 or CD8 subset of AKR.H-2b T cells (15, 32) and that these B cells can substantially inhibit AKR/Gross MuLV-specific CTL responses both in vivo and in vitro (31, 32). Therefore, we hypothesize that AKR.H-2b B cells, like AKR.H-2b CD4 and CD8 T cells, may function as FasL+ veto cells to induce apoptosis in responder T cells.
While this discussion has focused on the ability of retrovirus-infected cells to induce the apoptosis of specific immune T cells directed against viral epitopes, it is also worth considering strategies whereby virus-infected cells and tumors attempt to prevent their own apoptotic cell death. Several viruses, including adenovirus, baculovirus, cowpox virus, Epstein-Barr virus, African swine fever virus, herpesvirus, and papillomavirus, have been reported to contain potential mechanisms for escape from immune systems surveillance via FasL/Fas interactions by encoding antiapoptotic gene products (reviewed in references 35 and 40). In a recent study of human T-lymphocyte virus type 1, a retrovirus somewhat analogous to AKR/Gross MuLV that also causes T-cell lymphoma/leukemia, transgenic mice carrying the env-pX segment of human T-lymphocyte virus type 1 became more resistant to autoreactive T cells and to anti-Fas MAb-induced apoptosis than were their nontransgenic counterparts in an autoimmune arthropathy model (24). These findings demonstrated that the Tax segment of pX coded for an antiapoptotic gene product. Thus, by protecting virus-infected host cells from apoptosis, a virus may promote self-survival. Whether in addition to inducing apoptosis of responding T cells, AKR/Gross MuLV has sequences which may code for antiapoptotic gene products that spare infected cells has not been determined.
In summary, this report, to our knowledge, is the first to demonstrate that MuLV-infected cells may interfere with viral clearance by using the FasL/Fas apoptotic pathway to eliminate immune T lymphocytes via viral Ag-positive veto cells. With this study as a foundation, additional experiments must be performed to further probe the complex interactions between immune lymphocytes and AKR/Gross MuLV-infected cells, the challenging goal being to specifically block detrimental veto cell-mediated FasL/Fas-mediated apoptosis while maintaining AICD that is required for homeostasis.
ACKNOWLEDGMENTS
We thank Elizabeth Dziadik for technical assistance in performing experiments with LPS and RA. The NIH 3T3 Fas-Ig cell line was generously provided by Philip Leder (Howard Hughes Medical Institute, Boston, Mass.). The RF33.7 T-hybridoma cells were the kind gift of Kenneth Rock (Dana-Farber Cancer Institute, Boston, Mass.). TGF-β was the kind gift of Bradley Arrick (Dartmouth). Software used for the determination of precursor frequencies was kindly provided by Patrick Smith (Louisiana State University School of Medicine, Shreveport, La.). We also thank Kathy Green, Victor Kim, and Hillary White for helpful scientific discussions.
This work was supported by NIH grant CA69525. The DMS irradiation facilities and the Herbert C. Englert Flow Cytometer Facility, established by a grant from the Fannie E. Rippel Foundation, are partially supported by an NIH core grant of the Norris Cotton Cancer Center, CA23108.
REFERENCES
- 1.Adachi Y, Oyaizu N, Than S, McCloskey T W, Pahwa S. IL-2 rescues in vitro lymphocyte apoptosis in patients with HIV infection: correlation with its ability to block culture-induced down-modulation of Bcl2. J Immunol. 1996;157:4184–4193. [PubMed] [Google Scholar]
- 2.Ameisen J C, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 3.Anel A, Buferne M, Boyer C, Schmitt-Verhulst A M, Goldstein P. T cell receptor-induced Fas ligand expression in cytotoxic T lymphocyte clones is blocked by protein tyrosine kinase inhibitors and cyclosporin A. Eur J Immunol. 1994;24:2469–2476. doi: 10.1002/eji.1830241032. [DOI] [PubMed] [Google Scholar]
- 4.Banda N K, Bernier J, Kurahara D K, Kurrle R, Haigwood N, Sekaly R P, Finkel T H. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthou C, Michel L, Soulie A, Jean-Louis F, Flageul B, Dubertret L, Sigaux F, Zhang Y, Sasportes M. Acquisition of granzyme B and Fas ligand proteins by human keratinocytes contributes to epidermal cell defense. J Immunol. 1997;159:5293–5300. [PubMed] [Google Scholar]
- 6.Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboubi A, Echeverri F, Martin S J, Force W R, Lynch D H, Ware C F. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 7.Coppola M A, Green W R. Cytotoxic T lymphocyte responses to the envelope proteins of endogenous ecotropic and mink cytopathic focus-forming murine leukemia viruses in H-2b mice. Virology. 1994;202:500–505. doi: 10.1006/viro.1994.1370. [DOI] [PubMed] [Google Scholar]
- 8.Coppola M A, Lam T M, Strawbridge R R, Green W R. Recognition of ecotropic murine leukemia viruses by anti-AKR/Gross virus cytotoxic T lymphocytes: epitope variation in a CTL-resistant virus. J Gen Virol. 1995;76:635–643. doi: 10.1099/0022-1317-76-3-635. [DOI] [PubMed] [Google Scholar]
- 9.Desbarats J, Freed J H, Campbell P A, Newell M K. Fas (CD95) expression and death-mediating function are induced by CD4 cross-linking on CD4+ T cells. Proc Natl Acad Sci USA. 1996;93:11014–11018. doi: 10.1073/pnas.93.20.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P, Ameisen J C. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood. 1996;87:4959–4966. [PubMed] [Google Scholar]
- 11.Fink P J, Shimonkevitz R P, Bevan M J. Veto cells. Annu Rev Immunol. 1988;6:115–137. doi: 10.1146/annurev.iy.06.040188.000555. [DOI] [PubMed] [Google Scholar]
- 12.French L E, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French L E, Wilson A, Hahne M, Viard I, Tschopp J, MacDonald H R. Fas ligand expression is restricted to nonlymphoid thymic components in situ. J Immunol. 1997;159:2196–2202. [PubMed] [Google Scholar]
- 14.Gougeon M-L, Garcia S, Heeney J, Tschopp R, Lecoeur H, Guetard D, Rame V, Dauguet C, Montagnier L. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res Hum Retroviruses. 1993;9:553–563. doi: 10.1089/aid.1993.9.553. [DOI] [PubMed] [Google Scholar]
- 15.Green W R. Cell surface expression of cytotoxic T lymphocyte-defined, AKR/Gross leukemia virus-associated tumor antigens by normal AKR.H-2b splenic B cells. J Immunol. 1983;131:3078–3084. [PubMed] [Google Scholar]
- 16.Green W R. Genetic control of the induction of cytolytic T lymphocyte responses to AKR/Gross viral leukemias. I. H-2 encoded dominant gene control. J Immunol. 1984;132:2658–2664. [PubMed] [Google Scholar]
- 17.Green W R. Genetic control of the induction of cytolytic T lymphocyte responses to AKR/Gross viral leukemias. II. Negative control by the Fv-1 locus in AKR mice of responder H-2b haplotype. J Immunol. 1984;132:2665–2671. [PubMed] [Google Scholar]
- 18.Green W R, Nowinski R C, Henney C S. Specificity of cytolytic T cells directed against AKR/Gross virus-induced syngeneic leukemias: antibodies directed against H-2K, but not against viral proteins, inhibit lysis. J Immunol. 1980;125:647–655. [PubMed] [Google Scholar]
- 19.Green W R, Smith P M. Endogenous ecotropic and recombinant MCF mouse retroviral variation and escape from antiviral CTL. Semin Immunol. 1996;7:49–60. [Google Scholar]
- 20.Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen J C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahne H, Rimoldi D, Schroter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J-C, Tschopp J. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 22.Hahne M, Renno T, Schroeter M, Irmler M, French L, Bornand T, MacDonald H R, Tschopp J. Activated B cells express functional Fas ligand. Eur J Immunol. 1996;26:721–724. doi: 10.1002/eji.1830260332. [DOI] [PubMed] [Google Scholar]
- 23.Ju S-T, Panka D J, Cul H, Ettinger R, El-Khatib M, Sherr B H, Stanger B Z, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 24.Kishi S, Saijyo S, Arai M, Karasawa S, Ueda S, Kannagi M, Iwakura Y, Fujii M, Yonehara S. Resistance to Fas-mediated apoptosis of peripheral T cells in human T lymphocyte virus type I (HTLV-I) transgenic mice with autoimmune arthropathy. J Exp Med. 1997;186:57–64. doi: 10.1084/jem.186.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyaard L, Otto S A, Keet I P, Roos M T, Miedema F. Programmed cell death of T cells in human immunodeficiency virus infection. No correlation with progression to disease. J Clin Investig. 1994;93:982–988. doi: 10.1172/JCI117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen F L, Petermam G M. Neoplastic model for the differentiation of a subpopulation of lymphocytes bearing IgH-1-linked gene products. Immunol Rev. 1984;82:29–46. doi: 10.1111/j.1600-065x.1984.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 27.Oyaizu N, McCloskey T W, Than S, Hu R, Kalyanaraman V S, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-γ and tumor necrosis factor-α secretion. Blood. 1994;84:2622–2631. [PubMed] [Google Scholar]
- 28.Rammensee H G, Bevan M J, Fink P J. Antigen specific suppression of T cell responses—the veto concept. Immunol Today. 1985;6:41–43. doi: 10.1016/0167-5699(85)90044-1. [DOI] [PubMed] [Google Scholar]
- 29.Rammensee H G, Fink P J, Bevan M J. Functional clonal deletion of class I-specific cytotoxic T lymphocytes by veto cells that express antigen. J Immunol. 1984;133:2390–2396. [PubMed] [Google Scholar]
- 30.Ramsdell F, Seaman M S, Miller R E, Picha K S, Kennedy M K, Lynch D H. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation induced cell death. Int Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 31.Rich R F, Green W R. Nonresponsiveness of AKR.H-2b congenic mice for anti-AKR/Gross MuLV CTL responses: involvement of inhibitory cells as defined by adoptive transfer experiments. Cell Immunol. 1995;160:139–151. doi: 10.1016/0008-8749(95)80019-f. [DOI] [PubMed] [Google Scholar]
- 32.Rich R F, Green W R. AKR.H-2b lymphocytes inhibit the secondary in vitro cytotoxic T-lymphocyte response of primed responder cells to AKR/Gross murine leukemia virus-induced tumor cell stimulation. J Virol. 1996;70:402–414. doi: 10.1128/jvi.70.1.402-414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rock K L, Rothstein L, Gamble S. Generation of class I MHC-restricted T-T hybridomas. J Immunol. 1990;145:804–811. [PubMed] [Google Scholar]
- 34.Salmon M, Toeliner D S, Huissoon A P, Pilling D, Shamsadeen N, Hyde H, D’Angeac A D, Bacon P A, Emery P, Akbar A N. Inhibition of T cell apoptosis in the rheumatoid synovium. J Clin Investig. 1997;99:439–446. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieg S, Smith D, Yildirim Z, Kaplan D. Fas ligand deficiency in HIV disease. Proc Natl Acad Sci USA. 1997;94:5860–5865. doi: 10.1073/pnas.94.11.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sijts A J A M, Ossendorp F, Mengede E A M, van den Elsen P J, Melief C J M. Immunodominant mink cell focus-inducing murine leukemia virus (MuLV)-encoded CTL epitope, identified by its MHC class I-binding motif, explains MuLV-type specificity of MCF-directed T lymphocytes. J Immunol. 1994;152:106–116. [PubMed] [Google Scholar]
- 37.Strand S, Hofmann W J, Hug H, Muller M, Otto G, Strand D, Mariani S M, Stremmel W, Krammer P H, Galle P R. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells—a mechanism of immune evasion? Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki I, Fink P J. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med. 1998;187:123–128. doi: 10.1084/jem.187.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 40.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 41.Vella A T, McCormack J E, Linsley P S, Kappler J W, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z Q, Dudhane A, Orlikowsky T, Hoffmann M K, Clarke K, Li X, Darzynkiewicz Z. CD4 engagement induces Fas antigen-dependent apoptosis of T cells in vivo. Eur J Immunol. 1994;24:1549–1552. doi: 10.1002/eji.1830240714. [DOI] [PubMed] [Google Scholar]
- 43.Wegmann K W, Blank K J, Green W R. Induction of anti-MuLV cytotoxic T lymphocytes in the AKR.H-2b and AKR.H-2b:Fv-1b mouse strains. Cell Immunol. 1988;113:308–319. doi: 10.1016/0008-8749(88)90029-9. [DOI] [PubMed] [Google Scholar]
- 44.Wegmann K W, McMaster J S, Green W R. Mechanism of nonresponsiveness to AKR/Gross leukemia virus in AKR.H-2b:Fv-1b mice. An analysis of precursor cytotoxic T lymphocyte frequencies in young versus moderately aged mice. J Immunol. 1991;146:2469–2477. [PubMed] [Google Scholar]
- 45.Wegmann K W, Rich R F, Green W R. Generation of anti-AKR/Gross cytotoxic T-lymphocytes (CTL): an analysis of precursor CTL frequencies in the AKR.H-2b and C57BL/6 mouse strains. J Immunol. 1992;149:1593–1598. [PubMed] [Google Scholar]
- 46.White H D, Roeder D A, Green W R. An immunodominant Kb-restricted peptide from the p15E transmembrane protein of endogenous ecotropic murine leukemia virus (MuLV) AKR623 that restores susceptibility of a tumor line to anti-AKR/Gross MuLV cytotoxic T lymphocytes. J Virol. 1994;68:897–904. doi: 10.1128/jvi.68.2.897-904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X-N, Screaton G R, Gotch F M, Dong T, Tan R, Almond N, Walker B, Stebbings R, Kent K, Nagata S, Stott J E, McMichael A J. Evasion of cytotoxic T lymphocyte (CTL) responses by Nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186:7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, Vacchio M S, Ashwell J D. 9-cis-Retinoic acid inhibits activation-driven T-cell apoptosis: implications for retinoid X receptor involvement in thymocyte development. Proc Natl Acad Sci USA. 1993;90:6170–6174. doi: 10.1073/pnas.90.13.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zettlmeissl G, Gregsersen J-P, Duport J M, Mehdi S, Reiner G, Seed B. Expression and characterization of human CD4:immunoglobulin fusion proteins. DNA Cell Biol. 1990;9:347–353. doi: 10.1089/dna.1990.9.347. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Brunner T, Carter L, Dutton R W, Rogers P, Bradley L, Sato T, Reed J C, Green D, Swain S L. Unequal cell death in T helper (Th1) and Th2 effectors: Th1 but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;10:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Giangreco L, Broome H E, Dargan C M, Swain S L. Control of CD4 effector fate: transforming growth factor beta 1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. J Exp Med. 1995;182:699–709. doi: 10.1084/jem.182.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]