Abstract
Women frequently experience sleep disturbances, particularly nighttime awakenings, as they transition menopause and enter post-menopause. Sleep is essential for optimal functioning and health. Persistent and distressing sleep disturbances across menopause can negatively impact daytime functioning, productivity, and increase risk for mental and physical health conditions. While multiple factors can disturb sleep, two unique factors in the context of menopause are vasomotor symptoms and the changing reproductive hormone environment. Vasomotor symptoms are associated with sleep disturbances and contribute significantly to awakenings and amount of time spent awake during the night. Even after accounting for vasomotor and depressive symptoms, lower estradiol and higher follicle stimulating hormone levels, indicative of menopause, are associated with sleep disturbance, particularly awakenings, suggesting that the hormone environment may directly affect sleep. Management strategies for clinically-significant menopausal sleep disturbances include cognitive behavioral therapy for insomnia, which is effective and durable in treating menopausal insomnia. Hormone therapy alleviates sleep disturbances, particularly in the presence of disruptive vasomotor symptoms. Sleep disturbances have a significant impact on women’s functioning and health and there is a need for further research of the underlying mechanisms to advance effective preventative and treatment strategies that ensure optimal health and wellbeing of midlife women.
Keywords: menopause, climacteric, subjective sleep quality, sleep architecture, polysomnography, vasomotor symptoms, hot flashes, depressive symptoms
INTRODUCTION
Menopause is an important transition point from a reproductive to a nonreproductive stage of life encompassed within the normal aging process for women. Menopause occurs at a median age of 51.4 years,1 but can range between 40 and 58 years of age. The transition to menopause and post-menopause can be associated with the emergence of several symptoms, including sleep disturbances, which have a major impact on longterm health, healthcare utilization, quality of life, and work productivity. Sleep disturbance can arise due to multiple factors, including underlying physical and mental health conditions, medications, personal life stressors, socioeconomic factors, as well as factors specifically related to menopause (e.g. vasomotor symptoms). For some women, sleep disturbances that are already present before menopause can become exacerbated during this time. Surgical menopause can be associated with more severe sleep disturbances than natural menopause, as reviewed elsewhere.3 Here, I provide an overview of sleep disturbance in the natural menopausal transition, focusing on its association with vasomotor symptoms and the underlying changes in female reproductive hormones. I also highlight current treatment options. Sleep disturbance significantly affects functioning and health in the context of menopause and there is a need for further research of the underlying mechanisms to advance effective preventative and treatment strategies that ensure optimal health and wellbeing of midlife women.
THE MENOPAUSAL TRANSITION
Natural menopause is defined as the time of the final menstrual period, confirmed to be present after 12 months of amenorrhea, resulting from depletion of ovarian follicles. There are complex changes in the central nervous and endocrine systems across menopause, such that it is not defined by one event but rather as a transitionarly phase encompassing several years before and after the final menstrual period.4 The menopausal transition is defined by Stages of Reproductive Aging Workshop (STRAW6, 7) criteria. The early menopausal transition is marked by a persistent difference of 7 days or more in the length of consecutive menstrual cycles and the late menopausal transition is marked by the occurrence of amenorrhea of ≥60 days, increased variability in cycle length, and follicle stimulating hormone (FSH) levels ≥25 IU/l.7 Early postmenopause describes the first two years after menopause, with increasing FSH and decreasing estradiol levels, until hormone levels finally stabilize.7 While hormonal changes roughly map onto the STRAW stages, changes are non-linear over time8 and ovulatory menstrual cycles can still occur,9 adding to the inter-individual variability in hormonal patterns across the menopause transition.10,11 Vasomotor symptoms (hot flashes, night sweats) typically emerge in the late reproductive stage or early menopausal transition and peak in the late menopausal transition and first 2 years after menopause.12 Peri-menopause encompasses the menopausal transition and the first year after the final menstrual period.
SLEEP HEALTH
Sleep is essential for overall health and emotional well-being.13,14 Sleep health is a multidimensional construct that promotes physical and mental well-being15 and is comprised of six key dimensions, including regularity (getting in and out of bed at similar times each day), subjective satisfaction (feeling satisfied with one’s sleep), alertness during waking hours (ability to stay awake during the day without dozing), appropriate timing (sleeping between 2:00 a.m. and 4:00 a.m.), high efficiency (being awake for less than 30 minutes each night after trying to fall asleep), and sleep duration (obtaining between 6 and 8 hours of sleep per night).15 Better sleep health is associated with better physical and mental health outcomes as well as better quality of life,16 with a wide range of positive effects, including strengthening of immunity,17 optimal brain and cognitive functioning,18 and well-regulated energy metabolism19 (Figure 1). Conversely, sleep disturbance implies poor sleep health and negatively affects various physiological systems, and is associated with adverse health outcomes including cardiovascular disease,20 obesity & diabetes,21 all-cause mortality,22 and psychological disorders such as depression.23
Figure 1:
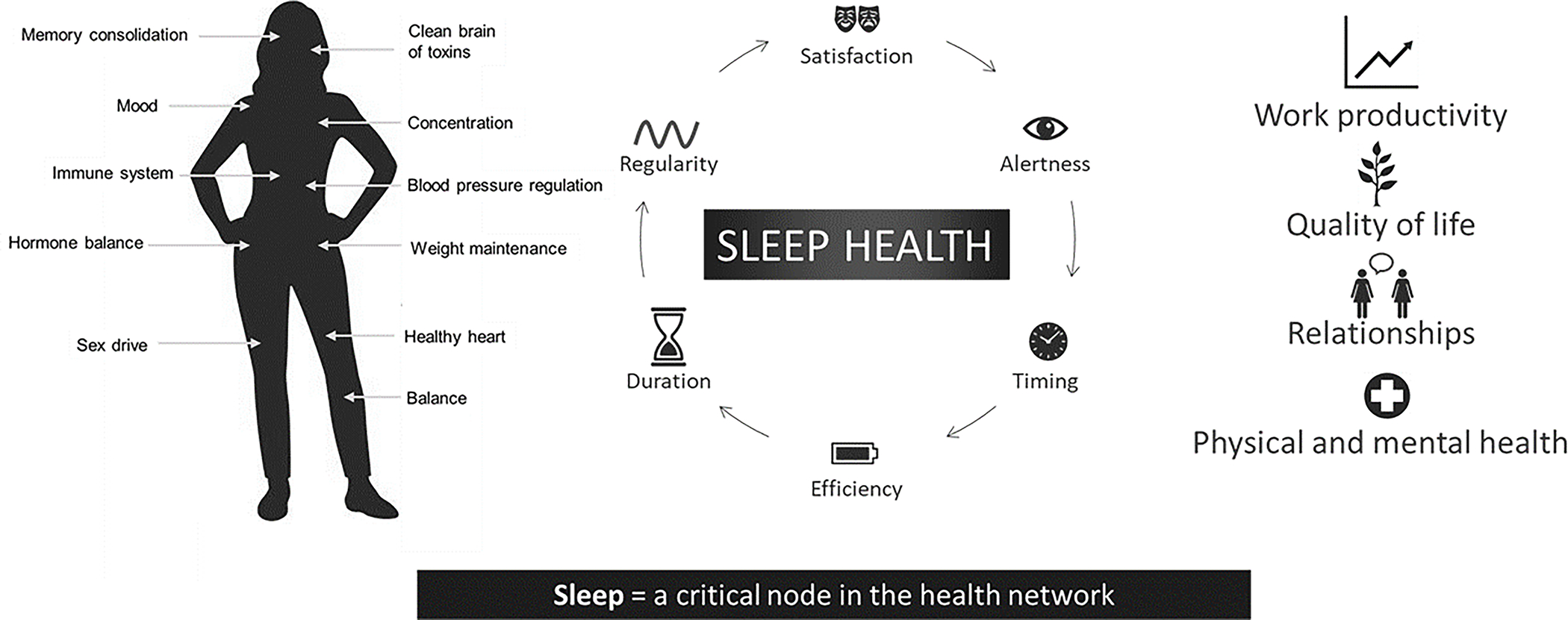
Schematic showing the multiple dimensions of sleep health as defined in: Buysse DJ. Sleep health: can we define it? Does it matter? Sleep 2014;37:9–17. Sleep health is essential for multiple functions, including weight maintenance, cardiovascular health, cognitive function, and mood. When sleep is disturbed, multiple systems are affected.
Sleep can be assessed using several methodological approaches, including surveys and daily diaries, wearables and research-grade actigraphy, and polysomnography (PSG). PSG allows for the objective assessment of sleep and quantification of sleep stages including rapid eye movement (REM) sleep and slow wave sleep (SWS) by measuring brain activity (electroencephalography, EEG), eye activity, and muscle activity. These data are continuous and detailed, thus informing mechanisms and physiology of sleep. However, assessments using questionnaires and sleep diaries are still essential in providing valuable information regarding an individual’s perception of their sleep,24 which drives treatment seeking behavior. Use of wearables and actigraphy can also provide important longitudinal data about patterns of sleep-wake activity over weeks or months. Combinations of PSG, self-report, and wearable technology/actigraphy assessments can provide complementary information about sleep in the context of menopause. Assessment of the presence of sleep disorders including obstructive sleep apnea (OSA) and restless legs syndrome requires additional clinical evaluation.
SLEEP QUALITY DURING THE MENOPAUSAL TRANSITION
Between 40–60% of women report having sleep disturbances during the menopausal transition and postmenopause26–31 This greater prevalence of sleep disturbance in the menopausal transition relative to pre-menopause is evident from several cross-sectional and longitudinal studies (reviewed in 32) even after controlling for age and across different populations.33 Sleep disturbance is a common symptom for which women seek care in the menopausal transition,34 and is linked with functional impairment, poor quality of life, and increased healthcare use.35,36 Sleep disturbance in midlife women is associated with risk of unemployment and $2.2 billion/year in lost productivity.37 It also has long-term negative effects on mental health, with increased risk for persistent depression,38 and poor physical health, notably poor cardiovascular health39: Shorter sleep duration, poorer sleep quality, and greater severity of insomnia in postmenopausal women are all associated with worse cardiovascular health as assessed with the American Heart Association Life’s Simple 7 scores.40
The most common sleep-related complaint is nighttime awakenings. Longitudinal data from several studies, including the Study on Women’s Health Across the Nation (SWAN), have confirmed that women transitioning from premenopause through the menopausal transition have higher odds of reporting waking up several times compared to women who have not yet transitioned, after adjusting for demographics and health-related factors.28,41–43 40% of women in the late menopausal transition report waking several times, and this percentage remains stable into post-menopause.44 Sleep disturbance in the menopausal transition could be a consequence of several factors,3 including aging-related factors, psychosocial stress and socioeconomic factors, mental and physical health comorbidities, as well as menopause-related factors of vasomotor symptoms28,30 and reproductive hormone changes (decrease in estradiol and increase in FSH).28
Despite the overwhelming evidence that women, on average, report more sleep disturbances as they transition menopause, PSG studies that have compared groups of women at different life stages have not necessarily found evidence of objective sleep disturbance. Some reported no differences in sleep architecture between pre- and post-menopausal women,45–47 others found more deep SWS in peri- and post-menopausal women than pre-menopausal women,48–50 or differences only in the sleep EEG (more high frequency beta EEG activity, suggesting greater cortical hyperarousal) in late-perimenopausal and postmenopausal women compared with pre-menopausal and early peri-menopausal women.45 In their longitudinal study of changes in PSG measures across the menopausal transition, Lampio and colleagues49 reported that women had less total sleep time and more wakefulness after sleep onset (WASO) 6 years after a baseline visit after adjusting for vasomotor symptoms, BMI, and mood. These changes in sleep were linked with advancing age rather than increased FSH levels. A possible explanation for disparate findings between subjective and objective sleep measures could be the high inter-individual variability in the extent of sleep disturbances along with variability in hormone trajectories, duration of the menopausal transition, and life experiences all of which can interact to affect sleep and dilute any potential group differences in the smaller samples of women typically studied with PSG. Indeed, an analysis of trajectories for waking up several times at least 3 nights per week reported by women in the SWAN study across their natural final menstrual period showed that there were four clusters, with group 1 (37.9%) having low prevalence, group 2 (28.4%) having moderate prevalence, group 3 (15.3%) having increasing prevalence, and group 4 (18.4%) having a high prevalence of problems waking several times across the final menstrual period.51 Thus, not all women have the same sleep experience as they transition menopause, with some already having moderate-high sleep disturbance before the approach to the final menstrual period.
One quarter of women experience severe sleep disturbances in the context of the menopausal transition that impact daytime functioning, qualifying them for a diagnosis of insomnia.32 Sleep disturbance in women with menopausal insomnia is not isolated to self-reported symptoms since they also have more PSG-measured WASO, poorer sleep efficiency, and a short sleep duration compared to good sleeper controls, also in the menopausal transition.52 One factor that contributed to their PSG-measured sleep disturbances was nocturnal hot flashes, which matches the literature linking chronic insomnia symptoms with more severe hot flashes.30
RELATIONSHIP BETWEEN VASOMOTOR SYMPTOMS AND SLEEP DISTURBANCE
Vasomotor symptoms affect up to 80% of women during the menopausal transition and into post-menopause.53 Data from SWAN show a median duration for vasomotor symptoms of 7.4 years,54 although there is wide individual variability in timing of symptom onset, persistence, and daily frequency.55,56 A hot flash is a sensation of heat, sweating, flashing, anxiety, and chills lasting 3–10 min57 and is characterized by peripheral vasodilation and sweating to increase hot loss, which are components of the classic heat dissipation response.58 Hot flashes emerge as estrogen levels decline but their mechanism is more complex, with evidence implicating involvement of central noradrenergic activity53,59,60 and hypothalamic kisspeptin, neurokinin B and dynorphin (KNDy) neuron activity.61 KNDy neurons project to structures critical for body temperature regulation, and their effects on temperature regulation are sensitive to estrogen.62 This discovery is leading to the development of potential non-hormonal pharmacological treatments that antagonize neurokinin B/neurokinin-3 receptor (NK3R) signaling pathways for the treatment of vasomotor symptoms.63
Self-reported vasomotor symptoms are consistently associated with poorer self-reported sleep quality and chronic insomnia.3 Longitudinal SWAN data show that women with moderate-severe hot flashes are almost three times more likely to report sleep disturbance (frequent nocturnal awakenings) compared to women without hot flashes.44 Also, effective treatment of vasomotor symptoms with hormone therapy reduces sleep disturbance.26,31,64,34
Associations between vasomotor symptoms and objective sleep disturbance (PSG or actigraphy) are less consistent.26 Several studies have not found an association between vasomotor symptoms and overall objective sleep quality.46,50,65–68 Another study that examined hot flash events and PSG awakenings showed a time of night effect: physiological hot flashes (measured with sternal skin conductance) were more likely to precede PSG awakenings in the first half of the night but awakenings were more likely to precede hot flashes in the second half of the night.69 Others, including our own work have found that the majorit of nocturnal physiological hot flash events are linked with PSG awakenings, regardless of time of night, and/or with more WASO.70–72 For example, we found that 69% of nocturnal hot flashes were associated with an awakening/arousal (Figure 2) and wake-time associated with hot flashes contributed an average of 27% to total wakefulness after sleep onset.71 Similarly, in an experimental model of new-onset hot flashes in young pre-menopausal women treated with a gonadotropin-releasing hormone agonist that simulates menopause, 66% of objectively measured vasomotor symptoms were associated with an awakening.72 The strong overlap in timing between many hot flash events and awakenings suggests there may be a common mechanism within the central nervous system in response to estrogen withdrawal, although further work is needed to investigate this possibility.
Figure 2.
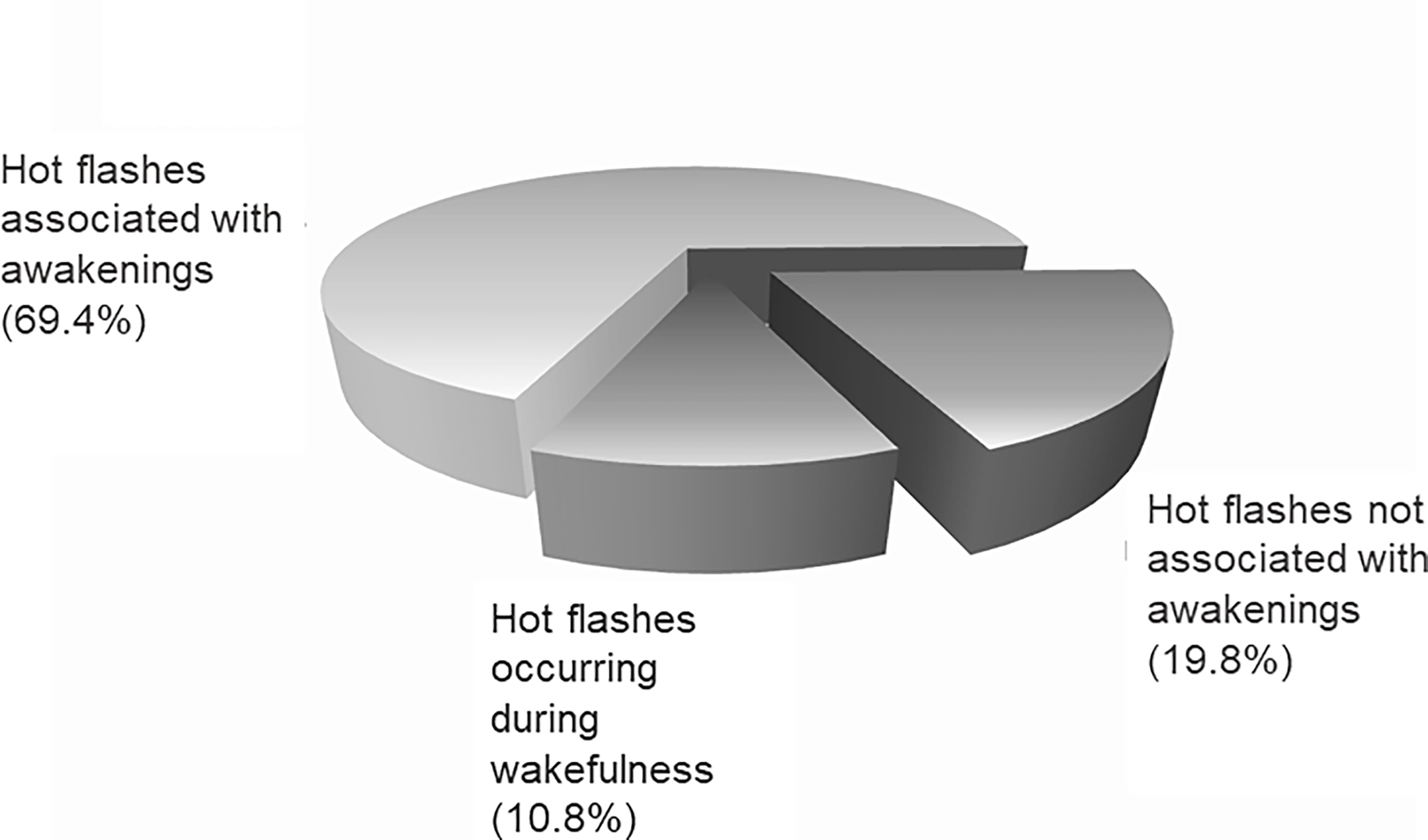
In a sample of 34 perimenopausal women with physiological hot flashes detected from sternal skin conductance measures during overnight polysomnography recordings (n = 222 hot flash events), an awakening occurred within a 3-min window around the onset of the hot flash in 69.4% of events. Sleep was undisturbed in 19.8% of hot flash events, and the remainder (10.8%) occurred when the woman was already awake. Data taken from de Zambotti M, Colrain IM, Javitz HS, Baker FC. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014 Dec;102(6):1708–15.
Taken together, nocturnal hot flashes play a key role in sleep disturbance, although there is substantial individual variability and not all women who have menopause-related sleep problems complain of hot flashes26 and not all hot flashes trigger an awakening. It is therefore important to consider other factors that could be associated with sleep disturbances, including the direct effect of reproductive hormone changes.
RELATIONSHIP BETWEEN REPRODUCTIVE HORMONES AND SLEEP DISTURBANCE
Work in animal models shows that ovarian hormones, including estradiol, regulate female sleep.73 However, the underlying neurobiological substrates, mechanisms, and pathways remain unknown,74 and further work is needed to define the neural circuits involved,75 considering circadian timing and reproductive stage, especially given some of the paradoxical effects76 of estradiol on sleep-wake behavior in rodents versus women. Estradiol is a neuroactive steroid and there are estrogen receptors in sleep and arousal-regulating nuclei, including the preoptic area of the hypothalamus, suprachiasmatic nucleus, and locus coeruleus.74 Estradiol (and other hormones of the hypothalamic pituitary ovarian axis), therefore, may influence sleep-wake regulation directly, for example by influencing adenosinergic actions in the preoptic area (a sleep-promoting nucleus) to affect sleep homeostasis76 or by influencing arousal systems in the lateral hypothalamus (rich in hypocretin-releasing neurons, which promote wakefulness),74 or the locus coeruleus (primary site of norepinephrine, involved in arousal).74 Estradiol could also act indirectly to influence sleep via other systems, including thermosensory and thermoregulatory sites in the hypothalamus, which overlap with sleep-active sites.77
Knowledge about the effects of reproductive hormones on sleep in midlife women comes from observational and interventional studies, including randomized controlled trials of hormone therapy, however findings are mixed and many studies have had limitations in sample size, infrequent hormone sampling, inadequate control of covariates, and varied in assessment tools of sleep (self-report, PSG).75 The longitudinal SWAN study that addressed many limitations, found that decreasing estradiol and increasing FSH levels across the menopausal transition were associated with higher odds of reported frequent awakenings28 and that a greater rate of change in FSH was associated with poorer sleep quality.78 Also, the 13-year prospective Melbourne Women’s Midlife Health Project found that a steeper declining slope in estradiol was associated with more severe sleep problems.8 In contrast, the SWAN PSG sub-study found that a more rapid rate of change in FSH was associated with higher amount of SWS and longer total sleep time during follow-up,78 suggesting there may be varied relationships between sleep continuity and sleep architecture measures and female reproductive hormone changes. A cross-sectional study of a combined sample of reproductive age and still cycling women in the menopausal transition found that higher FSH concentrations were associated with more PSG-measured WASO, after adjusting for age and BMI.79 Recently, Coborn and colleagues advanced understanding of relationships between reproductive hormone changes and sleep by tracking daily sleep assessments and weekly hormone levels across 8-weeks in perimenopausal women.80 They showed an association between more self-reported nightly awakenings and lower estradiol (especially when in the post-menopausal range) and higher FSH levels, independent of vasomotor or depressive symptoms.80 These data suggest that the hormonal milieu of the menopausal transition is associated with sleep discontinuity, in particular.80
MANAGEMENT OF SLEEP DISTURBANCE
Sleep disturbance in women transitioning menopause and post-menopause is common, however, it is not universal and varies between women in chronicity and severity. Some women may have a pre-existing sleep problem, which could be exacerbated as they transition and enter post-menopause. Greater awareness and public education about sleep disturbances that may arise in the context of menopause as well as management options is needed for women to be fully informed. Healthcare providers should inquire about sleep, recognizing that it is a window to health and that improving sleep can have multiple longterm positive benefits for daytime functioning, mood, and physical health. Sleep can be evaluated with a sleep history assessment81 or sleep diary,82 and women should be questioned about the timing of sleep disturbances in relation to changes in bleeding patterns and menopausal symptoms like hot flashes and night sweats. In cases where sleep disturbance is distressing with a negative effect on functioning and quality of life, treatment options should be considered. Women should be screened for underlying medical conditions and primary sleep disorders, which increase in prevalence in women after menopause.3 Medical conditions as well as use of medications become more common with advancing age and may lead to sleep disturbances.83 Post-menopausal women have a more than 3-fold increased risk of severe obstructive sleep apnea compared with pre-menopausal women, after adjusting for confounding factors including age, BMI, and smoking.84 Apneas and periodic limb movements disrupt sleep and were shown to be the best predictors for poorer PSG-defined sleep quality in peri- and post-menopausal women reporting sleep disturbances.85 Continuous positive airway pressure is the treatment of choice for obstructive sleep apnea, and healthy lifestyle changes (e.g. weight loss and exercise) should also be implemented. Patients with comorbid sleep-disordered breathing and insomnia may benefit from concomitant cognitive behavioral therapy for insomnia (CBT-I).86
There is a growing number of options for managing menopausal sleep disturbances including cognitive-behavioral therapy for insomnia, hormonal and non-hormonal pharmacological medication as well as non-pharmacological and self-management strategies, as reviewed in detail elsewhere.26,32,35,87 CBT-I, comprised of behavioral, cognitive, and educational components, is the primary intervention for patients with chronic insomnia,88 and is superior to sleep medication alone in the long-term.89 CBT-I has been shown in randomized controlled trials to be effective in treating insomnia in peri- and/or post-menopausal women.90,91 In the MsFlash study, 8-weeks of CBT-I delivered via telephone led to a greater reduction in insomnia symptoms and also reduced sleep-related interference from hot flashes compared with a menopause education control condition, with improvements maintained at 6 months post-treatment.91 Another controlled trial of CBT-I delivered face-to-face by a specialist to women diagnosed with insomnia disorder that either developed or worsened within 6 months of menopause also showed high efficacy relative to control (sleep hygiene education).90 Women treated with CBT-I showed large reductions in insomnia symptoms, increases in total sleep time, and high remission rates (>50%) that were maintained 6 months later. A single component of CBT-I (sleep restriction therapy for 2 weeks) was also highly effective in alleviating insomnia symptoms, although CBT-I had better long-term outcomes.90 In this same group, CBT-I and sleep restriction therapy also reduced depressive symptoms, dysfunctional beliefs about sleep, and presleep somatic hyperarousal, and improved daytime function, quality of life, and work performance, with stronger effects for CBT-I.92,93 Taken together, these results are supportive for the use of CBT-I for successfully treating menopausal insomnia and concomitant subclinical depressive symptoms.
Hormone therapy (estrogen/progestin or estrogen alone for women after a hysterectomy) is the most effective treatment for menopausal symptoms such as severe vasomotor symptoms, however, its risk profile differs between women and current guidance recommends that treatment be individualized to maximize benefits and minimize risks.94 Several studies have evaluated the effect of hormone therapy on sleep. While some studies found no benefit, the majority of good quality randomized controlled trials found that hormone therapy reduced sleep disturbances.75 Hormone therapy may be particularly beneficial for women with sleep disturbances associated with nocturnal hot flashes, although a recent controlled trial showed that transdermal estrogen therapy plus intermittent progesterone reduced sleep disturbances over a 12-month treatment period, independent of vasomotor symptom bother and depressive symptoms, in perimenopausal women.95 Non-hormonal pharmacological options for treating hot flashes and associated insomnia symptoms are available when hormone therapy is contra-indicated or not preferred, including low-dose selective serotonin/serotonin norepinephrine reuptake inhibitors, although the side-effect profiles of these medications need to be considered before use.3
CONCLUSION
Sleep disturbances, particularly difficulties maintaining sleep, frequently arise or worsen as women transition menopause and enter post-menopause. When these sleep disturbances are distressing and prolonged, they can negatively affect daytime functioning, quality of life, productivity, and longterm physical and mental health. Vasomotor symptoms are a key factor that disturb sleep in peri- and post-menopausal women, contributing specifically to sleep-maintenance difficulties. The changes in the hormone milieu across the menopausal transition, with declining estradiol and increasing FSH, are also directly linked with sleep disturbances, even after accounting for vasomotor and depressive symptoms. Beyond the disruptive effects to sleep continuity of these menopause-specific factors, sleep disturbances can arise from primary sleep disorders, mood disturbance, medical conditions, medications, or life stressors. Women should be informed about sleep disturbances in the context of menopause as well as management options when symptoms are distressing. These sleep disturbances vary in severity, chronicity, and etiology and treatment options need to be tailored to the individual.
DISCLOSURE STATEMENT
This work is supported by National Institutes of Health, Grant RF1AG061355. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Baker holds stocks and consults for Lisa Health. She also is a consultant for Bayer Consumer Care. Dr. Baker has received research funding from Noctrix Health, Inc., Verily Life Sciences LLC.
Footnotes
This review was presented as a paper at the 18th IMS World Congress, Lisbon, Portugal in October 2022.
REFERENCES
- 1.Santoro N The menopausal transition. Am J Med. 2005;118 Suppl 12B:8–13. [DOI] [PubMed] [Google Scholar]
- 2.WHO WHO. Menopause. 2022. Accessed 30 November, 2022.
- 3.Baker FC, Lampio L, Saaresranta T, Polo-Kantola P. Sleep and Sleep Disorders in the Menopausal Transition. Sleep Med Clin. 2018;13(3):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobo RA, Kelsey J, Marcus R. Menopause: biology and pathobiology. New York: Academic Press; 2000. [Google Scholar]
- 5.Roberts H, Hickey M. Managing the menopause: An update. Maturitas. 2016;86:53–58. [DOI] [PubMed] [Google Scholar]
- 6.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril. 2001;76(5):874–878. [DOI] [PubMed] [Google Scholar]
- 7.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennerstein L, Lehert P, Burger HG, Guthrie JR. New findings from non-linear longitudinal modelling of menopausal hormone changes. Hum Reprod Update. 2007;13(6):551–557. [DOI] [PubMed] [Google Scholar]
- 9.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92(8):3060–3067. [DOI] [PubMed] [Google Scholar]
- 10.El Khoudary SR, Thurston RC. Cardiovascular Implications of the Menopause Transition: Endogenous Sex Hormones and Vasomotor Symptoms. Obstet Gynecol Clin North Am. 2018;45(4):641–661. [DOI] [PubMed] [Google Scholar]
- 11.Tepper PG, Randolph JF Jr., McConnell DS, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). J Clin Endocrinol Metab. 2012;97(8):2872–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacon JL. The Menopausal Transition. Obstet Gynecol Clin North Am. 2017;44(2):285–296. [DOI] [PubMed] [Google Scholar]
- 13.Chow CM. Sleep and Wellbeing, Now and in the Future. Int J Environ Res Public Health. 2020;17(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119(1–3):56–64. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalmases M, Benitez ID, Mas A, et al. Assessing sleep health in a European population: Results of the Catalan Health Survey 2015. PLoS One. 2018;13(4):e0194495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zielinski MR, Krueger JM. Sleep and innate immunity. Front Biosci (Schol Ed). 2011;3(2):632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eugene AR, Masiak J. The Neuroprotective Aspects of Sleep. MEDtube Sci. 2015;3(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- 19.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett. 2008;582(1):142–151. [DOI] [PubMed] [Google Scholar]
- 20.Brindle RC, Yu L, Buysse DJ, Hall MH. Empirical derivation of cutoff values for the sleep health metric and its relationship to cardiometabolic morbidity: results from the Midlife in the United States (MIDUS) study. Sleep. 2019;42(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grandner MA, Patel NP, Perlis ML, et al. Obesity, diabetes, and exercise associated with sleep-related complaints in the American population. Z Gesundh Wiss. 2011;19(5):463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai L, Zhang H, Zhang D. Sleep Duration and Depression among Adults: A Meta-Analysis of Prospective Studies. Depress Anxiety. 2015;32(9):664–670. [DOI] [PubMed] [Google Scholar]
- 24.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6(3):179–188. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Sleep Medicine AAoSM. International classification of sleep disorders, Diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 26.Joffe H, Massler A, Sharkey KM. Evaluation and management of sleep disturbance during the menopause transition. Seminars in reproductive medicine. 2010;28(5):404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10(1):19–28. [DOI] [PubMed] [Google Scholar]
- 28.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–990. [PMC free article] [PubMed] [Google Scholar]
- 29.Nowakowski S, Meliska CJ, Martinez LF, Parry BL. Sleep and menopause. Curr Neurol Neurosci Rep. 2009;9(2):165–172. [DOI] [PubMed] [Google Scholar]
- 30.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166(12):1262–1268. [DOI] [PubMed] [Google Scholar]
- 31.Polo-Kantola P Sleep problems in midlife and beyond. Maturitas. 2011;68(3):224–232. [DOI] [PubMed] [Google Scholar]
- 32.Baker FC, de Zambotti M, Colrain IM, Bei B. Sleep problems during the menopausal transition: prevalence, impact, and management challenges. Nat Sci Sleep. 2018;10:73–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Q, Lang CP. Examining the relationship between subjective sleep disturbance and menopause: a systematic review and meta-analysis. Menopause. 2014. [DOI] [PubMed] [Google Scholar]
- 34.Pinkerton JV, Abraham L, Bushmakin AG, Cappelleri JC, Komm BS. Relationship between changes in vasomotor symptoms and changes in menopause-specific quality of life and sleep parameters. Menopause. 2016. [DOI] [PubMed] [Google Scholar]
- 35.Attarian H, Hachul H, Guttuso T, Phillips B. Treatment of chronic insomnia disorder in menopause: evaluation of literature. Menopause. 2015;22(6):674–684. [DOI] [PubMed] [Google Scholar]
- 36.Kloss JD, Twedy K, Gilrain K. Psychological factors associated with sleep disturbance in menopausal women. Behav Sleep Med. 2004;2(4):177–190. [DOI] [PubMed] [Google Scholar]
- 37.Kagan R, Shiozawa A, Epstein AJ, Espinosa R. Impact of sleep disturbances on employment and work productivity among midlife women in the US SWAN database: a brief report. Menopause. 2021;28(10):1176–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bromberger JT, Kravitz HM, Youk A, Schott LL, Joffe H. Patterns of depressive disorders across 13 years and their determinants among midlife women: SWAN mental health study. J Affect Disord. 2016;206:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Khoudary SR, Aggarwal B, Beckie TM, et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation. 2020;142(25):e506–e532. [DOI] [PubMed] [Google Scholar]
- 40.Makarem N, St-Onge MP, Liao M, Lloyd-Jones DM, Aggarwal B. Association of sleep characteristics with cardiovascular health among women and differences by race/ethnicity and menopausal status: findings from the American Heart Association Go Red for Women Strategically Focused Research Network. Sleep Health. 2019;5(5):501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berecki-Gisolf J, Begum N, Dobson AJ. Symptoms reported by women in midlife: menopausal transition or aging? Menopause. 2009;16(5):1021–1029. [DOI] [PubMed] [Google Scholar]
- 42.Tom SE, Kuh D, Guralnik JM, Mishra GD. Self-reported sleep difficulty during the menopausal transition: results from a prospective cohort study. Menopause. 2010;17(6):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33(4):539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kravitz HM, Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am. 2011;38(3):567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell IG, Bromberger JT, Buysse DJ, et al. Evaluation of the association of menopausal status with delta and beta EEG activity during sleep. Sleep. 2011;34(11):1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freedman RR, Roehrs TA. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril. 2004;82(1):138–144. [DOI] [PubMed] [Google Scholar]
- 47.Kalleinen N, Polo-Kantola P, Himanen SL, et al. Sleep and the menopause - do postmenopausal women experience worse sleep than premenopausal women? Menopause Int. 2008;14(3):97–104. [DOI] [PubMed] [Google Scholar]
- 48.Hachul H, Frange C, Bezerra AG, et al. The effect of menopause on objective sleep parameters: data from an epidemiologic study in Sao Paulo, Brazil. Maturitas. 2015;80(2):170–178. [DOI] [PubMed] [Google Scholar]
- 49.Lampio L, Polo-Kantola P, Himanen SL, et al. Sleep During Menopausal Transition: A 6-Year Follow-Up. Sleep. 2017;40(7). [DOI] [PubMed] [Google Scholar]
- 50.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26(6):667–672. [DOI] [PubMed] [Google Scholar]
- 51.Kravitz HM, Janssen I, Bromberger JT, et al. Sleep Trajectories Before and After the Final Menstrual Period in The Study of Women’s Health Across the Nation (SWAN). Curr Sleep Med Rep. 2017;3(3):235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baker F, Willoughby AR, Sassoon S, Colrain IM, de Zambotti M. Insomnia in women approaching menopause: beyond perception. Psychoneuroendocrinology. 2015;60:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Archer DF, Sturdee DW, Baber R, et al. Menopausal hot flushes and night sweats: where are we now? Climacteric. 2011;14(5):515–528. [DOI] [PubMed] [Google Scholar]
- 54.Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA internal medicine. 2015;175(4):531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mishra GD, Kuh D. Health symptoms during midlife in relation to menopausal transition: British prospective cohort study. BMJ (Clinical research ed. 2012;344:e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tepper PG, Brooks MM, Randolph JF Jr., et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23(10):1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kronenberg F Menopausal hot flashes: a review of physiology and biosociocultural perspective on methods of assessment. J Nutr. 2010;140(7):1380S–1385S. [DOI] [PubMed] [Google Scholar]
- 58.Freedman RR. Hot flashes: behavioral treatments, mechanisms, and relation to sleep. Am J Med. 2005;118 Suppl 12B:124–130. [DOI] [PubMed] [Google Scholar]
- 59.Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Seminars in reproductive medicine. 2005;23(2):117–125. [DOI] [PubMed] [Google Scholar]
- 60.Freedman RR. Menopausal hot flashes: mechanisms, endocrinology, treatment. The Journal of steroid biochemistry and molecular biology. 2014;142:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A. 2012;109(48):19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Modi M, Dhillo WS. Neurokinin 3 Receptor Antagonism: A Novel Treatment for Menopausal Hot Flushes. Neuroendocrinology. 2019;109(3):242–248. [DOI] [PubMed] [Google Scholar]
- 64.Moe KE. Hot flashes and sleep in women. Sleep Med Rev. 2004;8(6):487–497. [DOI] [PubMed] [Google Scholar]
- 65.Shaver J, Giblin E, Lentz M, Lee K. Sleep patterns and stability in perimenopausal women. Sleep. 1988;11(6):556–561. [DOI] [PubMed] [Google Scholar]
- 66.Polo-Kantola P, Erkkola R, Irjala K, Pullinen S, Virtanen I, Polo O. Effect of short-term transdermal estrogen replacement therapy on sleep: a randomized, double-blind crossover trial in postmenopausal women. Fertil Steril. 1999;71(5):873–880. [DOI] [PubMed] [Google Scholar]
- 67.Sharkey KM, Bearpark HM, Acebo C, Millman RP, Cavallo A, Carskadon MA. Effects of menopausal status on sleep in midlife women. Behav Sleep Med. 2003;1(2):69–80. [DOI] [PubMed] [Google Scholar]
- 68.Thurston RC, Santoro N, Matthews KA. Are vasomotor symptoms associated with sleep characteristics among symptomatic midlife women? Comparisons of self-report and objective measures. Menopause. 2012;19:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13(4):576–583. [DOI] [PubMed] [Google Scholar]
- 70.Bianchi MT, Kim S, Galvan T, White DP, Joffe H. Nocturnal Hot Flashes: Relationship to Objective Awakenings and Sleep Stage Transitions. J Clin Sleep Med. 2016;12(7):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Zambotti M, Colrain IM, Javitz HS, Baker FC. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014;102(6):1708–1715 e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joffe H, Crawford S, Economou N, et al. A Gonadotropin-Releasing Hormone Agonist Model Demonstrates that Nocturnal Hot Flashes Interrupt Objective Sleep. Sleep. 2013;36(12):1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown AMC, Gervais NJ. Role of Ovarian Hormones in the Modulation of Sleep in Females Across the Adult Lifespan. Endocrinology. 2020;161(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dorsey A, de Lecea L, Jennings KJ. Neurobiological and Hormonal Mechanisms Regulating Women’s Sleep. Front Neurosci. 2020;14:625397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haufe A, Baker FC, Leeners B. The role of ovarian hormones in the pathophysiology of perimenopausal sleep disturbances: A systematic review. Sleep Med Rev. 2022;66:101710. [DOI] [PubMed] [Google Scholar]
- 76.Smith PC, Phillips DJ, Pocivavsek A, et al. Estradiol influences adenosinergic signaling and nonrapid eye movement sleep need in adult female rats. Sleep. 2022;45(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szymusiak R Body temperature and sleep. Handb Clin Neurol. 2018;156:341–351. [DOI] [PubMed] [Google Scholar]
- 78.Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31(10):1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 79.de Zambotti M, Colrain IM, Baker FC. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. 2015;100(4):1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coborn J, de Wit A, Crawford S, et al. Disruption of Sleep Continuity During the Perimenopause: Associations with Female Reproductive Hormone Profiles. J Clin Endocrinol Metab. 2022;107(10):e4144–e4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. New York: Kluwer cademic/Plenum Publishers; 2003. [Google Scholar]
- 82.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tom SE, Kuh D, Guralnik JM, Mishra GD. Patterns in trouble sleeping among women at mid-life: results from a British prospective cohort study. J Epidemiol Community Health. 2009;63(12):974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181–1185. [DOI] [PubMed] [Google Scholar]
- 85.Freedman RR, Roehrs TA. Sleep disturbance in menopause. Menopause. 2007;14(5):826–829. [DOI] [PubMed] [Google Scholar]
- 86.Lack L, Sweetman A. Diagnosis and Treatment of Insomnia Comorbid with Obstructive Sleep Apnea. Sleep Med Clin. 2016;11(3):379–388. [DOI] [PubMed] [Google Scholar]
- 87.Caretto M, Giannini A, Simoncini T. An integrated approach to diagnosing and managing sleep disorders in menopausal women. Maturitas. 2019;128:1–3. [DOI] [PubMed] [Google Scholar]
- 88.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD, “Clinical Guidelines Committee of the American College of Physicians”,. Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 89.Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13(3):205–214. [DOI] [PubMed] [Google Scholar]
- 90.Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McCurry SM, Guthrie KA, Morin CM, et al. Telephone-Based Cognitive Behavioral Therapy for Insomnia in Perimenopausal and Postmenopausal Women With Vasomotor Symptoms: A MsFLASH Randomized Clinical Trial. JAMA internal medicine. 2016;176(7):913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kalmbach DA, Cheng P, Arnedt JT, et al. Treating insomnia improves depression, maladaptive thinking, and hyperarousal in postmenopausal women: comparing cognitive-behavioral therapy for insomnia (CBTI), sleep restriction therapy, and sleep hygiene education. Sleep Med. 2019;55:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kalmbach DA, Cheng P, Arnedt JT, et al. Improving Daytime Functioning, Work Performance, and Quality of Life in Postmenopausal Women With Insomnia: Comparing Cognitive Behavioral Therapy for Insomnia, Sleep Restriction Therapy, and Sleep Hygiene Education. J Clin Sleep Med. 2019;15(7):999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.The Hormone Therapy Position Statement of The North American Menopause Society” Advisory P. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29(7):767–794. [DOI] [PubMed] [Google Scholar]
- 95.Geiger PJ, Eisenlohr-Moul T, Gordon JL, Rubinow DR, Girdler SS. Effects of perimenopausal transdermal estradiol on self-reported sleep, independent of its effect on vasomotor symptom bother and depressive symptoms. Menopause. 2019;26(11):1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]


