ABSTRACT
A growing amount of evidence has supported that gut microbiota plays a vital role in the reproductive endocrine system throughout a woman’s whole life, and gut microbial β-glucuronidase (gmGUS) is a key factor in regulating host estrogen metabolism. Moreover, estrogen levels also influence the composition as well as the diversity of gut microbiota. In normal condition, the gmGUS-estrogen crosstalk maintains body homeostasis of physiological estrogen level. Once this homeostasis is broken, the estrogen metabolism will be disturbed, resulting in estrogen-related diseases, such as gynecological cancers, menopausal syndrome, etc. together with gut microbial dysbiosis, which may accelerate these pathological processes. In this review, we highlight the regulatory role of gmGUS on the physical estrogen metabolism and estrogen-related diseases, summarize the present evidence of the interaction between gmGUS and estrogen metabolism, and unwrap the potential mechanisms behind them. Finally, gmGUS may become a potential biomarker for early diagnosis of estrogen-induced diseases. Regulating gmGUS activity or transplanting gmGUS-producing microbes shows promise for treating estrogen-related diseases.
KEYWORDS: Gut microbiota, gmGUS, estrobolome, estrogen, GUS gene, β-glucuronidase, breast cancer, menopausal syndrome, hormone replacement therapy, interaction
Introduction
As a sex hormone, estrogen plays an important role throughout a woman’s lifetime. It not only has the physiological effect of promoting reproductive organs and maintaining female secondary sexual characteristics, but also has obvious effects on the metabolic process, cardiovascular system and bone growth and maturation. Estrogen metabolism is the main process in the regulation of circulating estrogen levels. Abnormalities in estrogen metabolism can lead to disruption of estrogen levels in the body and estrogen-related diseases as a result.
The human gut microbiota is a mature endocrine organ that can play both local and long-distance roles involving metabolites, immunologic messengers, and hormonal intermediates.1,2 Since decades ago, people have found that gut microbiota plays a central regulatory role in estrogen metabolism.3 Emerging evidence suggests an interaction between estrogen metabolism and gut microbiota4, which means the estrogen level also influences the homeostasis of the gut microbiome. Estrobolome is the aggregate of enteric bacterial genes capable of metabolizing estrogens.5 The GUS gene of estrobolome encodes gut microbial β-glucuronidase (gmGUS), which is the functional member of the estrobolome.6 The gmGUS enzyme plays a main regulatory role in physiological estrogen metabolism as well as estrogen-mediated diseases7,8 and is an important mediator for gut microbiota – host interaction correlating gut microbiome and breast cancer.9 This review discusses the current research linking estrogen metabolism and gut microbiota, focusing on how both crosstalk through the gut microbial β-glucuronidase in estrogen-driven diseases. Furthermore, based on the gut microbial regulatory mechanism of estrogen metabolism, we propose several potential treatments for estrogen-driven diseases.
Physiological process of estrogen metabolism
Estrogen is primarily produced mainly in the ovaries, and others in adrenal glands, and adipose tissue. Estrogen consists of estrone (E1), estradiol (E2), and estriol (E3). E1 and E2 are mainly synthesized by the ovaries and E3 is the main metabolite of E1 and E2 degradation in the liver. There are both free and conjugated forms of estrogen circulating in the bloodstream, with the conjugated form predominating, but the biologically active form of estrogen in the blood is the former. E2 is the most biologically active estrogen in the female body and an important indicator in blood draw tests. E1 and E2 can be converted into each other. Usually, E2 is converted into E1, and E1 is excreted through a series of metabolic degradation, which exerts the lowest biological effect.
Estrogen remains inactive in circulation mostly by binding to plasma proteins, primarily sex-binding globulin (SHBG). Once it is deconjugated, the free-form estrogen will regain biological activity. By combining with estrogen receptors (ERs) all over the body,10 estrogen plays a vital role in regulating physiologic processes. Thus, stable estrogen levels in the female body are important for maintaining body homeostasis.
In addition to estrogen production, estrogen metabolism is also important for the homeostasis of estrogen levels. Estrogen metabolism occurs both in the liver and other target tissues like the breast, but mainly in the liver through a two-stage reaction. The first stage is mainly the hydroxylation hydrolysis reaction of cellular P450 enzymes. Maternal estrogens (E1 and E2) can be irreversibly hydroxylated at positions C-2, C-4, or C-16 of the steroid ring. The second stage is a methylation reaction, aldehyde condensation reaction, and sulfation reaction, in which the maternal estrogen and its metabolites can be further modified by binding to sulfate or glucuronic acid portions, and then discharged through urine or bile. The conjugation form of estrogen excreted in bile ultimately passes into the intestine. Here, it is deconjugated by bacterial species with gmGUS activity and subsequently reabsorbed through the gut mucosa and into the portal vein for enterohepatic recycling (Figure 1). Studies of women injected with radiolabeled estrogen showed that about 65% of E2,11 48% of E1,11 and 23% of E312 were recovered in bile, and about 10% to 15% of E1, E2 and E3 existed in the conjugated form in feces,13 which suggests that the majority of deconjugated estrogens are reabsorbed through deconjugation activity. If the ratio of reabsorption to excretion is disrupted, the estrogen level in the body may fluctuate significantly and further lead to estrogen-related diseases.
Figure 1.
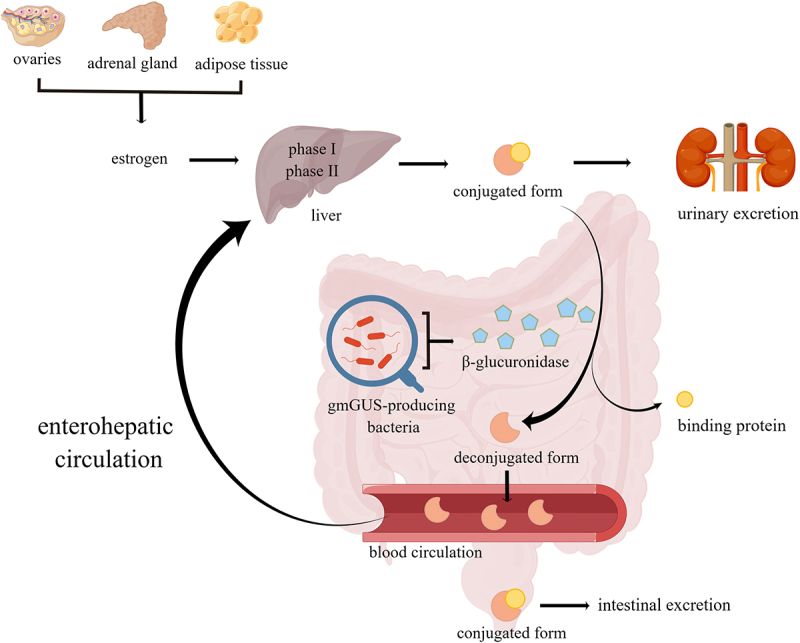
Estrogen produced by the ovaries, adrenal glands, and adipose tissue is metabolized in the liver within two phases to form a biologically inactive conjugated form, and the conjugated estrogen is deconjugated by β-glucuronidase encoded by gut microbiota and changes to a deconjugated form with biological activity, some of which is excreted in the stool and urine, while some of which is reabsorbed into the blood circulation and returned to the liver and this process is called enterohepatic recycling of estrogen.
Gut microbiota and estrogen
Plottel and Blaser have speculated that there is an important functional estrobolome, which is a collection of genes with the capability of metabolizing estrogen in the human body.5 In the intestinal tract, certain gut bacteria express certain genes from the estrobolome and produce the enzyme encoded by these genes. These enzymes can convert the conjugated estrogen into the free form and exert biological effects. Both the increased number of bacteria with estrobolome and the increased activity of these gene-encoding enzymes can accelerate the early dissociation and hydroxylation of estrogens in the intestine so that the free estrogens could increase significantly in enterohepatic circulation and maintain at a physiological level. Therefore, estrobolome is an important mediator in remaining body estrogen levels.
In the human estrobolome, the β-glucuronidase gene, also called the GUS gene, was first identified in Escherichia coli and other Enterobacteriaceae in 1934.7 In 1944, it was initially demonstrated that β-glucuronidase participated in physiological estrogen metabolism.14 The GUS gene encoding protein is a member of the glycoside hydrolase 2 (GH2) family, which includes β-glucuronidases, β-mannosidases, and β-galactosidases.15 Gut microbiota-derived β−glucuronidases and β−galactosidases participate in estrogen metabolism in the human body.16 The β−glucuronidase is involved in the disposition of many endogenous and exogenous compounds17 and it is the most studied enzyme in estrogenic metabolism and estrogen-driven diseases, especially in gynecological cancers.18 The β-galactosidase is also a kind of significant biomarker whose overexpression is closely associated with the progression of breast tumors.19 It has been summarized that 60 bacterial genera colonizing the human intestinal tract encode β-glucuronidase and/or β-galactosidase.20
Recently, Human Microbiome Project GI database has obtained an human intestinal β-glucuronidase atlas, which has identified about 279 GUS genes and 93.5% of them were taxonomically classified as Bacteroidetes (52%), Firmicutes (43%), Verrucomicrobia (1.5%) and Proteobacteria (0.5%).21,22 According to the differences in structural characteristics and biocatalytic properties, GUS genes are divided into seven categories, which are Loop 1 (L1), Mini-Loop 1 (mL1), Loop2 (L2), Mini-Loop 2 (mL2), Mini-Loop 1, 2 (mL1, 2), No Loop (NL) and No Coverage (NC) groups.21,23 The majority of intestinal GUS belongs to the category NL (57.3%), followed by mL1, L2, L1, mL2, NC, and mL1,2 in decreasing order.21 For GUS belonging to the mL1 and NL categories, the presence of the signal peptide is phylum-dependent: it is absent in Firmicutes and present in Bacteroidetes.24 For the gmGUS enzyme activity from human feces, there are 40 different bacterial strains have been screened, representing the dominant bacterial groups, and β-glucuronidase activity was found in some Firmicutes within clostridial clusters XIVa and IV.25 Based on degenerate PCR, a β-glucuronidase gene belonging to family 2 glycosyl hydrolases was detected in 10 of the 40 isolates.25 An in vitro analysis first explained that 35 GUS enzymes from the human microbiome reactivated the estrone-3-glucuronide and estradiol-17-glucuronide into estrone and estradiol from the perspective of molecular level.6
BG gene, another gene encoding bacterial β-D-glucuronidases (BG; E.C. 3.2.1.31), has been described by metagenomic analysis and are highly homologous to β-galactosidases based on their sequence.26 BGs and only a few of them have been investigated and annotated as β-D-glucuronidases in sequence databases.26 The function of gmGUS is regulated by GUS and BG genes, and both of which are well represented in Firmicutes, while only BG is found in Bacteroidetes.27 It was found that 96% of the amplified GUS sequences were Firmicutes, with three operational taxonomic units being particularly enriched, while 59% of the amplified BG sequences belonged to Bacteroidetes and 41% to Firmicutes.27 The functional test showed the different GUS enzyme activities between the GUS gene and the BG gene, and the GUS gene showed the primary response.27 Series observations have indicated the involvement of bacterial BGs in several pathologies.26 For example, lower BG activity in the feces of Crohn’s disease patients compared to healthy subjects.28 Study has found that a high BG activity is considered as a prognosis marker for colon cancer.29 However, few studies explore the interaction between the BG gene and estrogen-related disease, thus the review doesn’t cover this part.
In addition to bacterial β-glucosidases involved in the deconjugation of conjugated estrogen in the intestine, gut bacteria can also perform various reductive, oxidative, and hydrolytic reactions on estrogens.30 Early evidence has shown that the antibiotic reduced the reductive metabolism of estrogens in the gut, which reduced the transformation of E1 to E2 and increased E1/E2 and E1+E2/E3 ratios in feces.31 Recent research demonstrated that the gut-microbiome (Klebsiella aerogenes)-expressed 3β-hydroxysteroid dehydrogenases degraded estradiol and was associated with depression in premenopausal females.32 Thus, it is likely that the gut microbiota is involved in other physiological processes for degrading estradiol in addition to deconjugation, and this needs more evidence.
Interaction between estrogen and gut microbiota
Based on current evidence, we believe that there exists an interaction between estrogen and gut microbiota. The gut microbiota is not only involved in maintaining the balance of estrogen metabolism, but can also be disrupted and exhibit specific changes in estrogen-driven diseases and menopause-related diseases (Table 1).
Table 1.
Specific changes in gut microbiota associated with different estrogen-related diseases and menopause.
| Disease/model | Test sample | Related bacteria | Specific changes | Reference |
|---|---|---|---|---|
| Breast cancer | Human Blood |
Citrobacter*, Bacteroides*, Bifidobacterium* |
Were 10–100 times higher in cancer patients than in healthy controls; | 16 |
| Breast cancer | Human nipple aspirate fluid | Alistipes* | Was the most relatively abundant bacteria genus; | 33 |
| Breast cancer | Human feces | Bacteroides* | Was closely related to cancer: every 1% increase in its relative abundance increased the incidence rate of breast cancer by 5%; | 34 |
| Breast cancer | Human feces |
Romboutsia,* Coprococcus 2* |
Were strongest negative correlation with breast cancer: each 1% increase reduced the incidence rate of breast cancer by 91% and 55%, respectively; | 34 |
| Breast cancer | Human feces | Faecalibacterium* | Was present in almost 100% of controls and only 90% of both case groups, and was most strongly, inversely associated with odds of breast cancer and nonmalignant breast disease; | 34 |
| Breast cancer | Human feces | Christensenellaceae R-7 group, Dorea*, [Eubacterium] coprostanoligenes group*, Pseudobutyrivibrio, Lachnospira |
Were inversely associated with odds of breast cancer; | 34 |
| Breast cancer | Human feces |
Flavonifractor, Ruminococcaceae* |
Were positively associated with breast cancer; | 34 |
| Ovarian cancer | Human feces | Akkermansia (the unique genus in Verrucomicrobia phylumas) | Was remarkably reduced in the ovarian cancer and loss of the Akkermansia genus was a featuring characteristic of patients with ovarian cancer; | 35 |
| Ovarian cancer | Mice feces |
Bacteroides*, Lactobacillus* |
The abundance of Bacteroides was increased but that of Lactobacillus was decreased in ovarian cancer mice; | 36 |
| Endometrial cancer | Human feces |
Anaerostipes caccae, Ruminococcus sp.N15.MGS–57*, Prevotella sp.DJF_LS1653* |
Enriched in endometrial cancer and being associated with metabolites C16:1 and C20:2; | 37 |
| Endometrial cancer | Human feces |
Prevotella*, Bacteroides* |
Were dominated in endometrial cancer patients; | 38 |
| Endometriosis | Mice feces | Firmicutes*, Bacteroidetes* |
Firmicutes/Bacteroidetes ratio was elevated in mice with endometriosis; | 39 |
| Endometriosis | Mice feces | Bifidobacterium* | Increased in mice with endometriosis; | 39 |
| Endometriosis | Mice feces | Bacteroidetes*, Firmicutes* |
Higher abundance of Bacteroidetes and lower abundance of Firmicutes in the guts than mice without EMS; | 40 |
| Postmenopausalwomen | Human feces |
Bacteroides sp. strain Ga6A1*, Prevotella marshii*, Sutterella wadsworthensis |
Enriched in postmenopause; | 41 |
| Postmenopausalwomen | Human feces |
Escherichia coli-Shigella spp.*, Oscillibacter sp. strain KLE1745, Akkermansia muciniphila, Clostridium lactatifermentans*, Parabacteroides johnsonii*, Veillonella seminalis |
Depleted in postmenopause; | 41 |
| OVX mice (menopause) |
Mice feces | Lactobacillaceae*, Ruminococcaceae*, Streptococcaceae* |
Was related to fecal β-glucuronidase activity; | 42 |
| Postmenopausalwomen | Human feces | Lachnospiraceae, Ruminococcaceae* |
Were associated with estrogens and estrogen metabolites; | 43 |
| Premenopausal women | Human feces | Bacteroides* | Was inversely related to the ratio of estrogen metabolites to maternal estrogens; | 44 |
| Reproductive, premenopausal,postmenopausal women | Human feces | Clostridium* | As a predominance in all groups; | 45 |
| Postmenopausal women | Human feces | Clostridium* | Lower levels of beta-estradiol in the presence of higher copy numbers of Clostridium; | 45 |
| Postmenopausal women | Human feces | Non-Clostridiales*, three genera in the Ruminococcaceae* |
Were strongly associated with non-ovarian estrogen level | 46 |
| Menopausal syndrome | Human feces | Aggregatibacter segnis, Bifidobacterium animalis*, Acinetobacter guillouiae | Had a positive correlation with E2 and decreased in menopausal syndrome; | 47 |
| Menopausal syndrome | Human feces |
Ruminococcus torques*, Blautia obeum*, Butyricicoccus* pullicaecorum* |
Were positively correlated with Kupperman index scores; | 47 |
| Menopausal syndrome | Human feces | Lactobacillus delbrueckii* | Was inversely correlated with Kupperman index scores; | 47 |
| Menopausal hot flash | Human feces | Ruminococcus torques* | Was positively related to hot flash symptom score; | 47 |
| Menopausal hot flash | Human feces | Clostridium cocleatum* | Was negatively related to hot flash symptom score; | 47 |
| Menopausal hot flash | Human feces | Bifidobacterium*, Lactobacillus* | Showed a significant decrease; | 48 |
| Menopausal hot flash | Human feces |
Klebsiella*, Clostridiodes difficile* |
Showed an increase; | 48 |
| Premenopausal depression | Human feces | Klebsiella aerogenes* | Was higher in premenopausal women with depression than in those without depression | 32 |
| Perimenopausal insomnia | Human feces |
Roseburia faecis*, Ruminococcus*, Prevotella copri*, Fusicatenibacter saccharivorans, Blautia obeum* |
Showed an increase in the abundance; | 49 |
| Perimenopausal insomnia | Human feces |
Bacteroides*, Bacteroidetes*, Faecalibacterium prausnitzii* |
Showed a decrease in the abundance; | 49 |
| Osteoporosis | Mice feces |
Ruminococcus flavefaciens*, Clostridium*, Coprococcus*, Robinsoniella |
Were positively correlated with osteoclastic indicators | 50 |
| Osteoporosis | Mice feces |
Bacteroides*, Butyrivibrio* |
Were negatively correlated with loss of bone mass | 50 |
*Current gmGUS-encoding species have been known.
Gut microbiota dysbiosis in estrogen-driven diseases
Emerging evidence is describing the relationship between gut microbiota and estrogen-driven diseases. The gut microbiota plays a vital role in the regulation of estrogen levels, modulation of the inflammatory response, and interference with carbohydrate metabolism.51 Based on these pathogenic mechanisms, the dysbiosis of gut microbiota results in sorts of gynecological diseases and the elevated gmGUS activity has been observed in malignant tumors of the breast, ovary, and gastrointestinal tract.52,53 Here, we mainly focus on how gut microbial β-glucuronidases participate in modulating the level of systemic estrogen in different estrogen-driven diseases.
Breast cancer
Breast cancer is the second leading cause of cancer-related death among women worldwide and is one of the most common diagnoses. The dysbiosis of gut microbiota has been found involved in this estrogen-driven disease.54,55 Aided by the hyperactive gmGUS deglucuronidation activity, high systemic estrogens and a high ratio of circulating estrogen metabolites and parent estrogen are considered as strong risk factors for postmenopausal ER+ breast cancer.56 In postmenopausal women, it has been proved that reduction of the ratio of estrogen metabolites to parental compounds and the reduction of fecal microbiota diversity are associated with an increased risk of breast cancer.34,46 Lower gut microbiota diversity may be related to a higher relative abundance of gmGUS-producing species, which causes an increasing amount or activity of β-glucuronidases that accelerate the glucuronide-estrogen conjugates to break down, then estrogens revert to free form and are reabsorbed into the blood through the enterohepatic circulation, leading the body’s concentration of unconjugated estrogens to increase and in turn mechanistically linked to accelerate the development of hormone receptor-positive breast cancers.8,57,58
Emerging evidence is supporting this hypothesis. It has been early noticed that the difference between the plasma β−glucuronidase enzyme levels of the women with and without gynecological cancers was highly significant and serial observations demonstrated a rising titer of β−glucuronidase as well as its enzyme activity were corresponding to deterioration process in clinical state.58,59 Then, people found that some species of gut microbiota that produce gmGUS were abundant in breast cancer patients.60 A recent study compared the microbiota producing β−glucuronidase and/or β−galactosidase between breast cancer patients and healthy controls, and found the predominant bacteria in the breast cancer group were Citrobacter, Bacteroides, and Bifidobacterium, which are β−glucuronidase and β−galactosidase producing species whose abundance were 10 to 100 times higher in cancer patients than in healthy controls.16 In addition, the first study characterized the microbiome of breast cancer survivors’ nipple aspirate fluid(NAF), such as the fact that Alistipes, which encoded β-glucuronidase and β-galactosidase, was the relatively most abundant genus of bacteria in the NAF of women with breast cancer.33 A study found 45 species differed significantly between postmenopausal breast cancer women and premenopausal controls through shotgun metagenomic analysis.61
A study found that Bacteroides is most closely related to cancer, and every 1% increase in its relative abundance increased the incidence rate of breast cancer by 5%; while Romboutsia and Coprococcus 2 showed the strongest negative correlation with breast cancer, and each 1% increase in their relative abundance reduced the incidence rate of breast cancer by 91% and 55%, respectively.34 Bacteroides belongs to gmGUS-producing genera, thus we can explain the result caused by it, however, Coprococcus produces β-galactosidase that also may accelerate the reabsorption of estrogen into the circulation,20 but it makes a protective effect in this study. Higher-resolution sequencing methods are therefore needed for further characterization of the role of different gut microbial taxa in estrogen-induced diseases like breast cancer.
Ovarian cancer
The dysfunctions of estrogen levels and abnormal estrogen synthesis and metabolism may lead to ovarian cancer(OC),62 and a higher level of circulating E2 causes a higher risk of ovarian cancer.63 The progression of ovarian cancer was also related to changes in the composition of gut microbiota. A recent study has found some specific intestinal microbial composition (including Enterobacteriaceae that encodes β-glucuronidase) and microbial metabolites mediating the crosstalk between the gut microbiota and OC.64 A significant difference in β-diversity showed the difference between the gut microbiota of patients with OC and the benign controls.35 It has been showed obviously that the OC development accelerates after fecal microbiota transplantation (FMT) from patients with OC into OC-carrying mice.35 The administration of naringenin suppresses epithelial ovarian cancer by improving the composition of the microbiota in animals with ovarian tumor and significantly increased the abundances of Alistipes and Lactobacillus 36 (βglucuronidase producing genera), which may be inconsistent with our understanding that elevated β glucuronidase abundance is a detrimental factor in gynecological cancers.33
At present, two main mechanisms have been put forward to explain the possible link between the ovarian cancer development and the estrogen level: 1) the gut microbiome disturbs the enterohepatic circulation; 2) the gut microbiome interferes with the secretion of β-glucuronidases.65 At present, more evidence is needed to elucidate whether it is possible to suppress the development of ovarian cancer by manipulating βglucuronidase activity and how it works.
Endometrial cancer
A study explored the relationship between changes in gut microbiome and changes in the abundance of circulating metabolites. It showed that the metabolites C16:1 and C20:2 enriched in endometrial cancer (EC) were associated with certain βgalactosidase-encoding species which are dominated in EC patients, such as Firmicutes phylum members Anaerostipes caccae, Ruminococcus sp. N15.MGS–57 and Prevotella sp. DJF_LS16.37 The species above can activate the conjugated estrogens and elevate the level of active estrogen in the body circulation, thus accelerating the development of EC. Another study found the anorectal microbiome was dominated by βgalactosidase-encoding species, either Prevotella or Bacteroides, in patients with endometrioid or serous endometrial cancer.38 This is a preliminary hint that the occurrence of EC may be related to elevated GUS gene expression or increasing population composition of certain species that produce GUS enzymes.
Endometriosis
Endometriosis (EMS) is a frequent estrogen-driven disease among women at reproductive age, which is a kind of endometriotic lesions (ie, endometrial glands and stroma) outside the uterus.66 Studies have implied a bidirectional interaction between the gut microbiota and EMS,67 and gut microbiota may participate in several specific pathogenesis mechanisms of EMS, such as estrogen metabolism, immune inflammation, and tumor characteristics, etc.68 The hyper-estrogen has been implicated as an essential causative factor in EMS,69,70 and the estrobolome has been regarded as one of the key factors of EMS by dysregulating estrogen availability in endometriotic women through gut microbial β-glucuronidases.8,71 When estrobolome activity is impaired, gut microbiota dysbiosis increases the circulating estrogen, which may have a direct effect on stimulating the growth and cyclic bleeding of endometriotic lesions.66 A study has found that Firmicutes/Bacteroidetes ratio was elevated in mice with EMS, and the Bifidobacterium, a commonly used probiotic that encodes β-galactosidases,20 was also increased, indicating that EMS induces gut microbiota alterations,6,39,72 and the increasing level of the enzymes can improve the circulating estrogen which may directly accelerate endometriotic lesions.6,72 Therefore, inhibiting estrogen production through gut microbiota is one of the main targets of the available and emerging drugs. Antibiotic therapy like metronidazole, which targets Bacteroides genus, could reduce EMS progression in mice, but the lesion growth was restored after oral gavage of feces from mice with EMS, which further verifies that the gut bacteria promote endometriotic lesion progression.40 Taken together, we hypothesize that there is a bidirectional interaction between host and gut microbiota and that studies should be conducted to further clarify their potential relationship.
The alteration of gut microbial composition and diversity in menopausal periods
Not only does the gut microbiota regulates the circulating estrogen, but estrogens can also influence the diversity and composition of the gut microbiome. Nowadays, a growing body of literature indicates the change in estrogen level influences gut microbiota during different physiological periods, and each stage of a woman’s life has different hormonal states that drive the overall physiology of both the host microbe and the commensal microbe.73 During the period of menopause, ovarian follicle activity permanently terminates with a lack of menstrual cycle for over a year, which means the endpoint of natural reproductive ability in women.74 After menopause, a lower level of serum estrogen drives gut microbiota composition and quantity change significantly. Here, we mainly talk about the alteration of gut microbiota during menopause and also the menopause-related diseases in these peroids.
Change of gut microbiota during menopause
Some researchers think changes in estrogen status preceded changes in the gut microbiome because diets that are rich in isoflavones or other phytoestrogens provide a source for “health beneficial” organisms in the gut microbiome, and induce a change in microbiome composition and function.75,76 A finding found that the gut microbiota species in ovariectomized (OVX) ApoE-/- mice had the lowest abundances, but the diversity and the composition of gut microbiota returned to a level close to those of HFD-fed ApoE-/- mice or even normally fed C57BL/6 mice after estrogen supplementation.77 This study directly proved that gut microbial diversity and composition are influenced by estrogen concentrations.78 A study observed that postmenopausal women had lower diversity of gut microbiota with lower abundance of microbial β-glucuronidases than the premenopausal,41 like Bifidobacterium animalis abundance decreased.47 Besides, estrogen receptors also influence the composition and diversity of gut microbiota. Absence of intestinal estrogen receptor beta (ERβ) reduced the microbiota diversity in mice which had colitis-induced colorectal cancer79, and the loss of estrogen-related receptor alpha (ERRα) also led to a decrease in microbial α-diversity and depletion of healthy gut microbial constituents.80
Similar phenomena were observed in postmenopausal women. Metagenome functional analysis revealed significant differences in the composition of gut microbiota between premenopausal women and premenopausal men, but not postmenopausal; in addition, it showed a masculinization of the gut microbial characteristics after menopause.81,82 These facts suggest that estrogen plays a regulatory role in the composition of women’s gut microbiota, and premenopausal composition of gut microbiota may be related to maintaining the reproductive functions and normal secondary sexual characteristics of women. What’s more, the genus Bacteroides is inversely related to the ratio of estrogen metabolites to maternal estrogens in postmenopausal women and this association is independent of age and BMI.44 This suggests that changes in the menopause may play an independent role in inducing gut microbial disorders.
Gut microbial dysbiosis affected by estrogen consumption will in turn further affect the metabolism of estrogen in menopausal women. The alteration of the diversity of gut microbes encoding β-glucuronidase and their enzyme activity has been observed in menopausal women, which influence the enterohepatic circulation of estrogen. It has been proved that long-term estrogen supplementation directly decreased β-glucuronidase activity in the fecal microbiome and was positively related to the abundance of Lactobacillaceae in women’s feces.42 A Brazilian cohort study also showed there was a significant change of the genus Clostridium in menopausal women’s fecal microbiota following the change of hormone and metabolism, which may influence the estrogen metabolism due to the capability of producing β-glucuronidase.45 Another study indicated that gmGUS activity in postmenopausal women could influence non-ovarian estrogen level, which was strongly associated with fecal non-Clostridiales and three genera of the Ruminococcaceae family46 that are all gmGUS-producing gut microbes20. A study also found that the fecal β-glucuronidase activity was negatively correlated with fecal conjugated and deconjugated estrogen,46 revealing the important role of β-glucuronidases in excretion of estrogens in postmenopausal women. A study has found the correlation between fecal β-glucuronidase activity and the relative abundance of the Lactobacillaceae, Ruminococcaceae and Streptococcaceae in the fecal microbiota42 which all encode β-galactosidases,20 and another study also showed estrogen-metabolizing properties of fecal genera from families Lachnospiraceae and Ruminococcaceae in postmenopausal women.43 These observations demonstrated that the dysbiosis of these gut bacteria families producing β-glucuronidase and/or β-galactosidase may further influence estrogen levels in menopausal periods because of their capability of regulating estrogeneic metabolism based on the GUS enzyme activity.
Change of gut microbiota in menopause-related diseases
The menopausal syndrome includes a series of specific symptoms, the most common symptoms of which are vasomotor symptom (VMS),83 genitourinary syndrome (GSM),84 insomnia,85 and emotional disturbance (depression, anxiety or irritation).86 With the depletion of estrogen, menopausal syndrome happens to women and can last for decades. These symptoms can be accompanied by characteristic intestinal microbial disorders and may get worse because dysbiosis of the estrobolome may cause less estrogen reabsorption and accelerate the depletion of estrogen. It has been proved that the complement of estrogen maintains the gut microbial diversity in estrogen-deficient rats, and healthy ecological environment of gut microbiota may be helpful to prevent menopausal syndrome (MPS).87,88
A study has found the gut microbiota dysbiosis in MPS, showing a deficiency of the abundance of Aggregatibacter segnis, Bifidobacterium animalis and Acinetobacter guillouiae(all enriched in menopausal healthy women) which had a positive correlation with the level of E2.47 The domestic modified Kupperman index(KI) scores was positively correlated with Ruminococcus torques, Blautia obeum and Butyricicoccus pullicaecorum, while inversely correlated with Lactobacillus delbrueckii.47 Different menopausal symptoms are related to different intestinal bacterial disorders. The hot flash (HF) symptom scores were positively related to Ruminococcus torques, while negatively related to Clostridium cocleatum.47 Women with menopausal vasomotor disorders showed a significant decrease in the main representatives of Bifidobacterium and Lactobacillus, and an increase in the number of Klebsiella and Clostridiodes difficile48 that are all gmGUS-producing species. In recent years, the role of gut microbiota in the “gut-brain axis” has been uncovered, and alterations of gut microbial diversity is closely related to mood disorders.89 Many clinical studies have indicated that menopausal decline in circulating ovarian hormone levels were associated with increased emotional disturbances, including symptoms of postmenopausal depression (PMD) and postmenopausal anxiety (PMA).90 In OVX mice, treatment with progesterone improved PMD and anxiety behaviors through changes in gut microbiota composition, particularly via increasing the Lactobacillus spp. population.91 In human feces, a recent study found that the prevalence of Klebsiella aerogenes was higher in PMD than in those without depression.32 Perimenopausal insomnia (PI) has been proven linked with the dysbiosis of gut microbiota, which showed an increase in the abundance of Roseburia faecis, Ruminococcus, Prevotella copri, Fusicatenibacter saccharivorans, and Blautia obeum, while a decrease in the abundance of Bacteroides, fecal Bacteroidetes, and Faecalibacterium prausnitzii. 49
Furthermore, gut microbial alterations are also related to postmenopausal diseases, such as the pathogenesis of osteoporosis and GSM caused by steroid deficiency. A study has found Ruminococcus flavefaciens showed the largest difference in gut microbiota abundance and was also positively correlated with osteoclastic indicators and the estrobolome.50 In another study, researchers transplant feces from female mice with intact ovaries into OVX mice, and it was found that the atrophy of the vaginal epithelium was significantly alleviated together with changes in the gut microbiota.92
Currently, there are few studies on the association between menopausal symptoms and gut microbiota, and we need more evidence to clarify the role of gut microbiota in menopausal symptoms and postmenopausal-related diseases. Taken together, current understanding may reveal a potential gmGUS-based regulation of human estrogen metabolic homeostasis (Figure 2).
Figure 2.
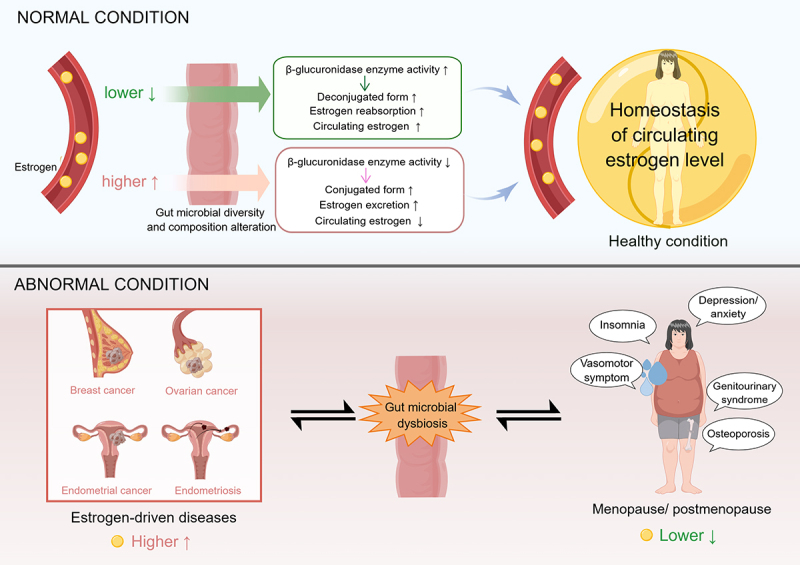
Higher systemic estrogens affect gut microbiome diversity and composition, thereby reducing β-glucuronidase availability and increasing estrogen excretion; when estrogen levels reduce, it also reshapes the gut microbiome, thereby increasing β-glucuronidase activity, promoting estrogen reabsorption and maintaining systemic homeostasis of estrogen levels. Thus, abnormalities in either of them may break this homeostasis and cause the occurrence of estrogen-driven diseases.
Clinical application base on gut microbial β-glucuronidase
Biomarker for estrogen-driven cancers
Estrogen-driven cancers (breast, endometrial, and ovarian cancers, etc.) are among the leading causes of female morbidity and mortality worldwide, and their common risk factor is the exposure to endogenous and exogenous estrogens. Early diagnosis and treatment is crucial for the prognosis of these patients’ life. At present, tissue biopsy is still the gold standard for accurate diagnosis of breast cancer, but it is not suitable for routine clinical use.93 Therefore, it is urgent to develop a simple and feasible diagnosis and monitoring method for breast cancer.94 In recent years, human gut microbiota has been found playing a potential role in predicting individual breast cancer risk, prognosis and clinical efficacy,95 which may explain the individual phenotypic variation in estrogen-driven cancer development and treatment efficacy. Human gut microbiome enriched with enzymes such as β-glucuronidase could play a major role in the deconjugation of both xenobiotics and estrogens, and elevate the risk of estrogen-driven cancers. An early observation has found a significantly positive correlation between β-glucuronidase activity and development stage of gynecological cancers.59 The gene expression studies found that βglucuronidase was one of the best reference genes in ovarian cancer.96 Therefore, we can assume that the close association of the β-glucuronidases with gynecological cancers is mainly due to the regulation of estrogen metabolism by β-glucuronidase activity. Assessing the metabolism of estrogen in the enterohepatic pathway may play a predictive role in the early diagnosis of the diseases, and β-glucuronidase as a mediator may be the key monitoring point. Therefore, the gmGUS can be a complementary biomarker for estrogen-driven cancers.97 Besides, the characterization of specific gut microbial composition enriched by gmGUS-producing genera can also be studied for the prevention of these diseases.
Nowadays, steroid metabolomics of breast cancer has great potential in digging up key metabolic pathways related to canceration,93 and testing enzyme activity of β-glucuronidase in human excrement may improve the diagnostic sensitivity of gynecological cancers. A recent study has summarized the latest sensing strategies to detect the β-glucuronidase activity,98 which may make this potential biomarker test comes true in the future.
Therapeutic target on estrogen-driven diseases
Over the past few decades, increasing evidence has proved that gmGUS acts as an important mediator of microbiota–host interactions that not only correlate the gut microbiome with the estrogen-driven diseases, but also participate in the maintenance of health and the treatment of disease through the metabolism of glucuronate-containing carbohydrates and drugs.17 On the one hand, the gmGUS as the active component derived from estrobolome may be amenable to control by using targeted small-molecule inhibitors6 to fight against estrogen-induced diseases; on the other hand, gmGUS can be utilized to improve the body’s bioavailability of hormone agents due to its biotransformation effect for women with estrogen deficiency. In addition, changing the composition and diversity of gut microbiota encoding β-glucuronidase may be a two-way regulatory approach for increasing or decreasing estrogen levels in the case of different diseases (Figure 3).
Figure 3.
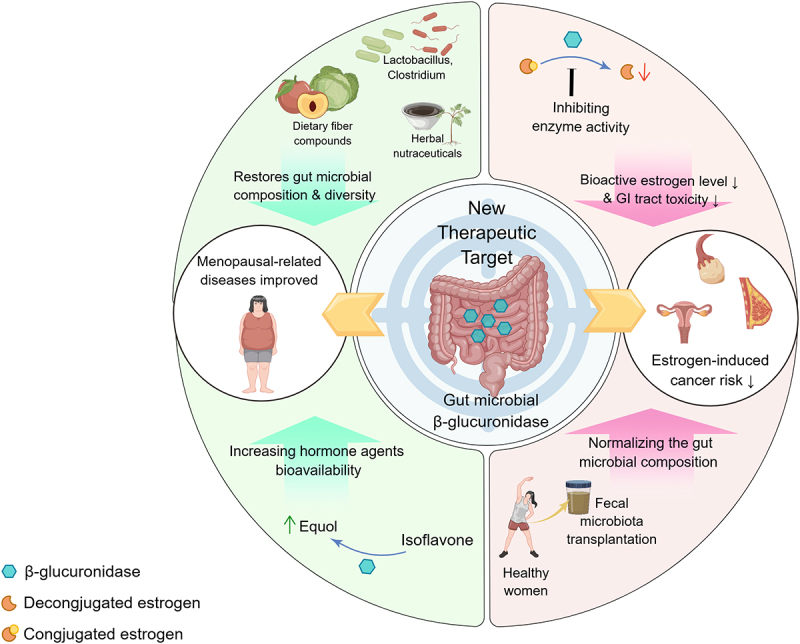
Gut β-glucuronidase is emerging as a new therapeutic target in the improvement of menopausal disorders such as menopausal syndrome, as well as in the prevention and treatment of gynecological cancers. As for improving menopausal symptoms, the composition and diversity of the gut microbiota can be normalized through the intake of dietary fiber, herbal nutrients, and beneficial gut microbiota, and by increasing the bioavailability of estrogenic agents, such as soy isoflavones, which can be converted into efficiently absorbed estradiol. In the prevention and treatment of gynecological cancers, by inhibiting gmGUS enzyme activity and reducing the level of biologically active estrogens, and reducing the intestinal side effects of anti-cancer drugs, which exerts a synergistic effect and suppress the progression of gynecological cancers.
Inhibiting β-glucuronidase enzyme activity
The gmGUS inhibitor has emerged as a new approach to managing diseases and medication therapy.99 A recent project explored the structural properties of gastrointestinal microbiome-encoded GUS enzymes (GUSOME) repertoire in healthy women and breast cancer patients and the researchers found that manipulating at the probiotics level showed potential of reducing breast cancer risk through inhibiting reactivation of estrobolome-associated protein.100
The effective β-glucuronidase inhibitor D-glucaro-1,4-lactone (1,4-GL) may exert an anti-cancer effect partly through influencing steroidogenesis,101 and bacterial-specific gmGUS inhibitor TCH-3511 can effectively prevent carcinogen-induced microbial dysbiosis.102 It has been reported that probiotics and prebiotics decrease estrogen-related cancer risk by suppressing β-glucuronidase activity in the intestine. For example, lactulose and oligofructose-enriched inulin could significantly decrease β-glucuronidase activity,103 suggesting the feasibility of improving the efficacy and safety of long-term estrogen administration in postmenopausal women and breast cancer patients by manipulating the activity of β-glucuronidase.42 Recent work established that inhibitors targeted toward gmGUS modulated the function of gut microbiota without adversely influencing the host metabolic system,104 which indicated its security in the clinical application.
In addition, the intestinal side effects of anticancer drugs are expected to be offset by inhibiting β-glucuronidase activity. For example, irinotecan (CPT-11) is an essential anti-cancer drug treating many cancers including gynecological cancers, like endometrial cancer,105 but its effectiveness is severely limited due to the GI toxicity caused by gmGUS enzymes.106,107 The gmGUS has been regarded as a possible predictive biomarker of irinotecan-induced diarrhea severity.108 A study has found that inhibiting gmGUS activity with using anti-cancer drugs alleviated GI toxicity without affecting the serum pharmacokinetics of the drug or its metabolites;109 in addition, it also maintained gut microbiota balance instead of causing cancer-related dysbiosis.102 For example, TCH-3562 is a specific gmGUS inhibitor that can alleviate irinotecan-induced diarrhea without impairing anti-cancer efficacy in vivo.110 The gmGUS activity increased by irinotecan can be blocked by the gmGUS inhibitor (gmGUSi).106 Probiotics and dietary fibers like apple pectin can prevent irinotecan-induced GI side-effects by inhibiting gut β-d-glucuronidase activity, and the latter further enhances the cytotoxic and proapoptotic effect of irinotecan.111,112 We suppose that the concomitant use of gmGUSi with anti-cancer drugs like irinotecan may potentially become a new therapy pattern in treating gynecological cancers because the gmGUSi can not only reduce the intestinal toxicity but also inhibit gmGUS’s estrogen reactivation potential, exerting synergistic anti-tumor effects against gynecological cancers.109
The substrate-dependent inhibitors of gmGUS, like piperazine- and piperidine-containing drugs, are widely used to treat a variety of diseases, including depression, infections, and cancers by the same mechanism, which selectively inhibits GUS activity.113 Altering the structure of active sites that participate in deglucuronidation by inducing gene mutations at specific points or deleting conserved protein motifs may destabilize protein structure, thus inhibiting its potential of estrogen reactivation.100 The binding affinities of β-glucuronidase for target molecules for a particular ligand, like estrogen, could be adjusted by using structure-guided mutations.114
Identifying the structural properties of the gmGUS enzyme found in normal and breast cancer patients might provide multiple directions for enzyme modification.100 A recent study helped us to better understand the structural and functional complexity of the gmGUS.115 It has been found that small changes in inhibitor structure can alter gmGUS active-site conformation,109 which shows the operability of inhibiting gmGUS enzyme activity. Besides, the inhibitory effects of 36 kinds of nature flavonoids toward β-glucuronidase enzyme activity through interacting with amino acid site,116 and the flavonoids in Mulberry bark displayed a strong inhibition of E. coli β-glucuronidase activity, suggesting it might be a promising dietary supplement for relieving gmGUS-mediated gut toxicity.117
Whether it is based on gene or protein-level regulation, studying the factors influencing gmGUS enzyme activity may also improve our knowledge of the effects of gut microbiota on estrogen reactivation.
Improving body’s bioavailability of hormone agents
As we know, different gut microbiome responds to different endogenous and exogenous glucuronide substrates, resulting in different bioavailability of these substrates.114 The microbial species with the capability of encoding GUS enzyme may play an important role in improving the bioavailability of estrogenic agents.
Hormone replacement therapy (HRT) is regarded as the first choice of women with menopausal syndrome, however, the percentage of clinical use is lower than expectation,118,119 mainly because of the potential risk of breast and uterine cancer.119 The gut microbial enzyme activity may affect an individual’s response to HRT,42 and combining probiotics with HRT makes it possible to manipulate gmGUS activity by increasing the half-life of bioactive estrogens while reducing the risk of estrogen-driven diseases, such as breast cancer.4,42
Phytoestrogens are a class of plant-produced polyphenolic compounds that are similar to 17β-estradiol and bind preferentially to the ERs, exerting estrogen-like effects with weak affinity and improving menopausal symptoms. Thus, phytoestrogens are becoming promising therapeutic molecules in improving the health of menopausal women. Many phytoestrogens exert potential health benefits and bioactivity via gut microbial biotransformation.120 For example, isoflavone is a phytoestrogen found mainly in soy and soy-derived products, and its bioavailability requires initial hydrolysis of the sugar moiety by gmGUS for uptake to the peripheral circulation.121,122 It derives equol with strong estrogenic activity, but only a fraction of the human population produces it.123 Some gmGUS producing species like Collinsella, Faecalibacterium, and members of the Clostridium clusters IV and XIVa were related to the equol production.124,125
Studies have proved that gmGUS assists phytoestrogen in exerting estrogenic effects and has prospects of maintaining homeostasis of intestinal microbial and estrogen metabolism. Feeding mice with L. rhamnosus-fermented soymilk that enriched Bacteroides and Lactobacillus bacterial taxa including certain β-glucuronidase-producing genera could keep the balance of gut microbiota and maintain the isoflavone metabolism under normal conditions when antibiotics were concomitantly used.20,126 Berberine relieved OVX-induced anxiety-like behaviors by enriching the equol-producing gut microbiota, and the majority of which are β-glucuronidase producing species, such as Bacteroides, Bifidobacterium, and Lactobacillus.90 Furthermore, a study found that tumor tissues contained a large amount of β-glucosidase, which could convert the genistein β-glucuronide conjugate into genistein aglycone and inhibit the induction of apoptosis in tumor tissues.127 Phytoestrogens also participate in controlling inflammatory metabolic disorders, and a recent study found that the dietary phytoestrogen secoisolariciresinol diglucoside had an anti-allergic property after being biotransformed by gmGUS.128
Overall, the gmGUS is essential for the bioactivity of phytoestrogens and plays an important role in biotransforming phytoestrogens into active compounds that can perform estrogenic or other biofunctions. For women who lack the gut microbial ability to produce estrogenic metabolite, supplying active metabolites in vitro by using the selected intestinal bacteria may be a solution. Thus, in order to explore possible theoretical knowledge for the developing functional probiotics, we can screen and isolate gut microbes capable of biotransforming phytoestrogens by β-glucosidase activity and characterize how these microbes influence the response of phytoestrogen metabolism.120 A study has found that fecal cultures from women with an equol producer phenotype produced equol when fermented with isoflavones in vitro,125 so transplanting certain fecal microbiota from equol producer may help improve body’s bioavailability of phytoestrogens. Further studies are needed to clarify which kind of gut microbial composition will improve the bioavailability of these hormonal agents.
Changing composition and diversity of gut microbiota encoding β-glucuronidase
Recent findings indicate that an intact microbiome acts as a tumor suppressor against epithelial ovarian cancer and transplanting cecal microbiota derived from normal mice prolonged survival, because it improved the disturbed composition of gut microbiota.64 Therefore, normalizing or balancing the gut microbial composition can also be used as a treatment strategy against gynecological malignancies.129 It may be easier to manipulate at the level of probiotics instead of genes in developing drugs against estrogen-induced diseases.100 For example, the gut microbiota may be manipulated to produce lower-affinity estrogens, thereby maintaining its physiological function but reducing the risk of estrogen-driven diseases such as breast cancer.4
Some researchers found that Enterobacteriaceae family of Proteobacteria, including Escherichia, Salmonella, Klebsiella, Shigella, and Yersinia pathobionts appear to uniquely respond to glucuronidated ligands, and they speculated that the GUS operon increase GUS enzyme activity and provide the ability for some Enterobacteriaceae to utilize intestinal endophytes and allogenic glucuronides as nutrients.114 Therefore, increasing the relative abundance of GUS operon-containing Enterobacteriaceae family in the gut may become a potential approach via increasing estrogen level against menopausal diseases. Microorganisms with a significantly reduced relative abundance in OVX group were considered as potential probiotic candidates, such as Lactobacillus, Clostridium, and of the potential candidates, Lactobacillus intestinalis YT2 restores the gut microbiota in OVX rats and improves menopausal symptoms.130 Increasing evidence shows that dietary fiber compounds decrease the risk of both pre- and post-menopausal breast cancer by reducing tumor promotion via altering the composition of the gut microbiota that influences E2 metabolism.131–133 The long-term consumption of a synbiotics formulated with Lactobacillus fermentum (probiotic) and β-glucan from cauliflower mushroom (prebiotic) could improve the gut microbiota in estrogen-deficient rats and delay the progression of menopausal symptoms.134 Consuming the prebiotics chitosan and citrus pectin can improve menopausal symptoms by influencing the diversity and composition of gut microbiota.135 Recent study also found herbal nutraceuticals like berberine ameliorated the ovariectomy-induced anxiety of SPF rats by modulating gut microbiota.90
In conclusion, the growing evidence shows that gut microbiome is a vital factor in occurring estrogen-related diseases. Future research will further identify specific characteristics of gut microbiome for developing novel approaches for the estrogen-driven diseases’ risk assessment, prevention and treatment.
Conclusion and future perspectives
There is still a big gap in the mechanism of interaction between gut microbiota and estrogen affecting women’s health. By summarizing the present evidence, we assume that, under physiological conditions, there may be a two-way regulatory effect of gmGUS on maintaining the body’s estrogen homeostasis as depicted in Figure 2, which requires further studies to confirm. For example, a study should be designed to explore the relationship between estrogen levels in different states of physiological fluctuation (such as different stages of the menstrual cycle) and the fecal gmGUS’s bioactivity in healthy women. Under the pathological conditions, female gut microbiota enriched with gmGUS-producing microbes may play an important role in developing estrogen-driven diseases by participating in the circulation or activation of endogenous and exogenous estrogen-like molecules. The present evidence shows that estrobolome participates in estrogen metabolism, among which GUS gene has been studied, but it has not been determined which of the various bacterial groups encoding GUS gene are the core bacterial groups that play a regulatory role, and the current evidence is relatively scattered.
We can only find some bacterial groups that are closely related to estrogen-related diseases and symptoms. However, it is still uncertain whether the role of these bacteria in these physiological and pathological processes is the whole picture, whether the regulation of the uncoupling process of estrogen is the core mechanism for these bacteria to play a role, or whether there are other undiscovered mechanisms at the same time, which need to be further explored. Therefore, it is necessary to characterize the main gmGUS producing species involved in specific physical, pathological or pharmacological processes and gmGUS enzymes from different bacterial families, which may improve our understanding of the role of intestinal microbiota in estrogen metabolism.
With further research into the relationship between the gut microbiota in vivo and the development of gynecological malignancies, it is possible to predict the early stages of these cancers by characterizing the disease-specific associated gut microbiota and its derived β-glucuronidase as a microbial biomarker, which could help us to achieve individualized cancer prevention and treatment. Although there are few clinical reports of gut microbiota-based treatments for gynecological cancers and menopause-related conditions, the fact that gut microbiota plays a role in these conditions cannot be denied. At present, based on our current understanding, gut microbiota can at least play an adjuvant therapeutic role, and it has been suggested that combining probiotics with estrogenic agents can improve the effectiveness and safety of current HRT.4 Even as an adjuvant, early use by patients should be advocated, before the prevention of dysbiosis in the gut microbial composition and function. Additionally, a study has indicated that inhibiting local enzyme is less important than inhibiting total-body estrogen synthesis as a treatment for ER+ breast cancer,136 and current evidence suggests that gmGUS is primarily involved in the regulation of circulating estrogen levels.8 Thus, regulating gmGUS activity may be a better choice for treating these estrogen-induced diseases. However, since intestinal microbiota provides immune and digestive benefits for cancer patients, it is urgent to explore how to maximize the therapeutic value while ensuring the safety of the body,137 and develop gmGUS drug design platform by more precise targeting of druggable sites in the future. Last but not least, the sulfonation and hydroxylation may also play roles in estrogen metabolism,6 thus, more studies are required to prove whether these metabolism steps are related to gut microbiota and evaluate their effect on gut estrogen metabolism compared with glucuronidation.
Funding Statement
This work was supported by Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine. (Grant No. ZYYCXTD-D-202001).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethics approval
This study does not involve human participants.
Author contributors
S.W,H and Q.Y,D are co-responsible for the collection, collation, and writing of the original manuscript. L.H,Z designed and revised the manuscript. W,Z, M.J,K and J,M are responsible for the concept development, revision, and review of the manuscript. All authors contributed to the article and approved the submitted version.
References
- 1.Guarner F, Malagelada JR.. Gut flora in health and disease. Lancet. 2003;361(9356):512–22. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 2.de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–1032. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksson H, Gustafsson JÅ, Sjövall J. Steroids in germfree and conventional rats. European Journal of Biochemistry 1969;9:286–290. [DOI] [PubMed] [Google Scholar]
- 4.Chen KL, Madak-Erdogan Z. Estrogen and microbiota crosstalk: Should we pay attention? Trends Endocrinol Metab. 2016;27(11):752–755. doi: 10.1016/j.tem.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host & Microbe. 2011;10(4):324–335. doi: 10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ervin SM, Li H, Lim L, Roberts LR, Liang X, Mani S, Redinbo MR. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem. 2019;294(49):18586–18599. doi: 10.1074/jbc.RA119.010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masamune H. Biochemical studies on carbohydrates: IV. On an enzyme which catalyses the hydrolysis of biosynthetic osides of glucuronic acid. J Biochem. 1934;19(2):353–375. doi: 10.1093/oxfordjournals.jbchem.a125337. [DOI] [Google Scholar]
- 8.Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Sui Y, Wu J, Chen J. The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front Cell Dev Biol. 2021;9:631552. doi: 10.3389/fcell.2021.631552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. Tissue distribution of Estrogen Receptors Alpha (ER-alpha) and Beta (ER-alpha) mRNA in the midgestational human fetus. J Clin Endocrinol Metab. 1997;82(10):3509–3512. doi: 10.1210/jc.82.10.3509. [DOI] [PubMed] [Google Scholar]
- 11.Sandberg AA, Slaunwhite WR Jr.. Studies on phenolic steroids in human subjects. II. The metabolic fate and hepato-biliary-enteric circulation of C14-estrone and C14-estradiol in women. J Clin Invest. 1957;36(8):1266–1278. doi: 10.1172/JCI103524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandberg AA, Slaunwhite WR Jr.. Studies on phenolic steroids in human subjects. vii. metabolic fate of estriol and its glucuronide. J Clin Invest. 1965;44(4):694–702. doi: 10.1172/JCI105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adlercreutz H, Järvenpää P. Assay of estrogens in human feces. J Steroid Biochem. 1982;17(6):639–645. doi: 10.1016/0022-4731(82)90565-9. [DOI] [PubMed] [Google Scholar]
- 14.Fishman WH, Fishman LW. THE ELEVATION of UTERINE β-GLUCURONIDASE ACTIVITY by ESTROGENIC HORMONES. J Biol Chem. 1944;152(2):487–488. doi: 10.1016/S0021-9258(18)72081-4. [DOI] [Google Scholar]
- 15.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(D1):D490–5. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An J, Kwon H, Lim W, Moon B-I. Staphylococcus aureus-derived extracellular vesicles enhance the efficacy of endocrine therapy in breast cancer cells. J Clin Med. 2022;11(7):11. doi: 10.3390/jcm11072030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao S, Sun R, Singh R, Yu so S, Chan CTY, Savidge T, Hu M. The role of gut microbial β-glucuronidase in drug disposition and development. Drug Discov Today. 2022;27(10):103316. doi: 10.1016/j.drudis.2022.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sui Y, Wu J, Chen J. The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front In Cell And Dev Biol. 2021;9. doi: 10.3389/fcell.2021.631552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q, Zhou QH, Li W, Ren T-B, Zhang X-B, Yuan L. Evolving an ultra-sensitive near-infrared β-galactosidase fluorescent probe for breast cancer imaging and surgical resection navigation. ACS Sens. 2022;7(12):3829–3837. doi: 10.1021/acssensors.2c01752. [DOI] [PubMed] [Google Scholar]
- 20.Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst. 2016;108(8). doi: 10.1093/jnci/djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollet RM, D’Agostino EH, Walton WG, Xu Y, Little MS, Biernat KA, Pellock SJ, Patterson LM, Creekmore BC, Isenberg HN, et al. An atlas of β-glucuronidases in the human intestinal microbiome. Structure. 2017;25(7):967–977.e5. doi: 10.1016/j.str.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creekmore BC, Gray JH, Walton WG, Biernat KA, Little MS, Xu Y, Liu J, Gharaibeh RZ, Redinbo MR. Mouse gut microbiome-encoded β-glucuronidases identified using metagenome analysis guided by protein structure. mSystems. 2019;4(4):4. doi: 10.1128/mSystems.00452-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, Venkatesh M, Jobin C, Yeh L-A, Mani S, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330(6005):831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Candeliere F, Raimondi S, Ranieri R, Musmeci E, Zambon A, Amaretti A, Rossi M. β-glucuronidase pattern predicted from gut metagenomes indicates potentially diversified pharmacomicrobiomics. Front Microbiol. 2022;13:826994. doi: 10.3389/fmicb.2022.826994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol. 2008;66(3):487–495. doi: 10.1111/j.1574-6941.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 26.Gloux K, Berteau O, El Oumami H, Béguet F, Leclerc M, Doré J. A metagenomic β-glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proc Natl Acad Sci USA. 2011;108(supplement_1):4539–4546. doi: 10.1073/pnas.1000066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntosh FM, Maison N, Holtrop G, Young P, Stevens VJ, Ince J, Johnstone AM, Lobley GE, Flint HJ, Louis P, et al. Phylogenetic distribution of genes encoding β-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ Microbiol. 2012;14(8):1876–1887. doi: 10.1111/j.1462-2920.2012.02711.x. [DOI] [PubMed] [Google Scholar]
- 28.Carrette O, Favier C, Mizon C, Neut, C., Cortot, A., Colombel, J F., Mizon, J.. Bacterial enzymes used for colon-specific drug delivery are decreased in active Crohn’s disease. Digest Dis Sci. 1995;40(12):2641–2646. doi: 10.1007/BF02220454. [DOI] [PubMed] [Google Scholar]
- 29.Geier MS, Butler RN, Howarth GS. Probiotics, prebiotics and synbiotics: a role in chemoprevention for colorectal cancer? Cancer Biol Ther. 2006;5(10):1265–1269. doi: 10.4161/cbt.5.10.3296. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi P, Goldin B, Boutin E, Gorbach SL. Metabolism of androgens and estrogens by human fecal microorganisms. J Steroid Biochem. 1978;9(8):795–801. doi: 10.1016/0022-4731(78)90203-0. [DOI] [PubMed] [Google Scholar]
- 31.Adlercreutz H, Pulkkinen MO, Hämäläinen EK, Korpela JT. Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J Steroid Biochem. 1984;20(1):217–229. doi: 10.1016/0022-4731(84)90208-5. [DOI] [PubMed] [Google Scholar]
- 32.Li D, Sun T, Tong Y, Le J, Yao Q, Tao J, Liu H, Jiao W, Mei Y, Chen J, et al. Gut-microbiome-expressed 3β-hydroxysteroid dehydrogenase degrades estradiol and is linked to depression in premenopausal females. Cell Metab. 2023;35(4):685–694.e5. doi: 10.1016/j.cmet.2023.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Chan AA, Bashir M, Rivas MN, Duvall K, Sieling PA, Pieber TR, Vaishampayan PA, Love SM, Lee DJ. Characterization of the microbiome of nipple aspirate fluid of breast cancer survivors. Sci Rep. 2016;6(1):28061. doi: 10.1038/srep28061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byrd DA, Vogtmann E, Wu Z, Han Y, Wan Y, Clegg‐Lamptey J-N, Yarney J, Wiafe‐Addai B, Wiafe S, Awuah B, et al. Associations of fecal microbial profiles with breast cancer and nonmalignant breast disease in the Ghana Breast Health Study. Int J Cancer. 2021;148(11):2712–2723. doi: 10.1002/ijc.33473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Qin X, Hu D, Huang J, Guo E, Xiao R, Li W, Sun C, Chen G. Akkermansia supplementation reverses the tumor-promoting effect of the fecal microbiota transplantation in ovarian cancer. Cell Rep. 2022;41(13):111890. doi: 10.1016/j.celrep.2022.111890. [DOI] [PubMed] [Google Scholar]
- 36.Lin C, Zeng Z, Lin Y, Wang P, Cao D, Xie K, Luo Y, Yang H, Yang J, Wang W, et al. Naringenin suppresses epithelial ovarian cancer by inhibiting proliferation and modulating gut microbiota. Phytomedicine. 2022;106:154401. doi: 10.1016/j.phymed.2022.154401. [DOI] [PubMed] [Google Scholar]
- 37.Zhao SS, Chen L, Yang J, Wu Z-H, Wang X-Y, Zhang Q, Liu W-J, Liu H-X. Altered gut microbial profile accompanied by abnormal fatty acid metabolism activity exacerbates endometrial cancer progression. Microbiol Spectr. 2022;10(6):e0261222. doi: 10.1128/spectrum.02612-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gressel GM, Usyk M, Frimer M, Kuo DYS, Burk RD. Characterization of the endometrial, cervicovaginal and anorectal microbiota in post-menopausal women with endometrioid and serous endometrial cancers. PLoS One. 2021;16(11):e0259188. doi: 10.1371/journal.pone.0259188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan M, Li D, Zhang Z, Sun H, An M, Wang G. Endometriosis induces gut microbiota alterations in mice. Hum Reprod. 2018;33(4):607–616. doi: 10.1093/humrep/dex372. [DOI] [PubMed] [Google Scholar]
- 40.Chadchan SB, Cheng M, Parnell LA, Yin Y, Schriefer A, Mysorekar IU, Kommagani R. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: a potential role for gut microbiota. Hum Reprod. 2019;34(6):1106–1116. doi: 10.1093/humrep/dez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters BA, Lin J, Qi Q, Usyk M, Isasi CR, Mossavar-Rahmani Y, Derby CA, Santoro N, Perreira KM, Daviglus ML, et al. Menopause is associated with an altered gut microbiome and estrobolome, with implications for adverse cardiometabolic risk in the hispanic community health study/study of latinos. mSystems. 2022;7(3):e0027322. doi: 10.1128/msystems.00273-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen KLA, Liu X, Zhao YC, Hieronymi K, Rossi G, Auvil LS, Welge M, Bushell C, Smith RL, Carlson KE, et al. Long-term administration of conjugated estrogen and bazedoxifene decreased murine fecal β-glucuronidase activity without impacting overall microbiome community. Sci Rep. 2018;8(1):8166. doi: 10.1038/s41598-018-26506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Z, Pfeiffer RM, Byrd DA, Wan Y, Ansong D, Clegg-Lamptey J-N, Wiafe-Addai B, Edusei L, Adjei E, Titiloye N, et al. Associations of circulating estrogens and estrogen metabolites with fecal and oral microbiome in postmenopausal women in the ghana breast health study. Microbiol Spectr. 2023:e0157223. doi: 10.1128/spectrum.01572-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, Goedert JJ. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;99(12):4632–4640. doi: 10.1210/jc.2014-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.da Silva TCA, Dos Santos Gonçalves JA, Souza L, Lima AA, Guerra-Sá R. The correlation of the fecal microbiome with the biochemical profile during menopause: a Brazilian cohort study. BMC Womens Health. 2022;22(1):499. doi: 10.1186/s12905-022-02063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, Gajer P, Ravel J, Goedert JJ. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10(1):253. doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Zhou Y, Mao T, Huang Y, Liang J, Zhu M, Yao P, Zong Y, Lang J, Zhang Y, et al. The relationship between menopausal syndrome and gut microbes. BMC Womens Health. 2022;22(1):437. doi: 10.1186/s12905-022-02029-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavlovska OM, Pavlovska KM, Heryak SM, Khmil SV, Khmil MS. Vasomotor menopausal disorders as a possible result of dysfunction of the microbiota-intestine-brain axis. J Med Life. 2022;15(2):234–240. doi: 10.25122/jml-2021-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X, Xiao H, Zeng Y, Huang L, Ji K, Deng D, Yang W, Liu L. Tianwang buxin granules influence the intestinal flora in perimenopausal insomnia. Biomed Res Int. 2021;2021:1–9. doi: 10.1155/2021/9979511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma S, Qin J, Hao Y, Shi Y, Fu L. Structural and functional changes of gut microbiota in ovariectomized rats and their correlations with altered bone mass. Aging (Albany NY). 2020;12(11):10736–10753. doi: 10.18632/aging.103290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borella F, Carosso AR, Cosma S, Preti M, Collemi G, Cassoni P, Bertero L, Benedetto C. Gut microbiota and gynecological cancers: A summary of pathogenetic mechanisms and future directions. ACS Infect Dis. 2021;7(5):987–1009. doi: 10.1021/acsinfecdis.0c00839. [DOI] [PubMed] [Google Scholar]
- 52.Fishman WH. B-Glucuronidase activity of the blood and tissues of obstetrical and surgical patients. Science. 1947;105(2738):646–647. doi: 10.1126/science.105.2738.646. [DOI] [PubMed] [Google Scholar]
- 53.Fishman WH, Anlyan AJ. Comparison of the β-glucuronidase activity of normal, tumor, and lymph node tissues of surgical patients. Science. 1947;106(2742):66–67. doi: 10.1126/science.106.2742.66. [DOI] [PubMed] [Google Scholar]
- 54.Hill MJ, Goddard P, Williams RE. Gut bacteria and aetiology of cancer of the breast. Lancet. 1971;298(7722):472–473. doi: 10.1016/S0140-6736(71)92634-1. [DOI] [PubMed] [Google Scholar]
- 55.Gorbach SL. Estrogens, breast cancer, and intestinal flora. Rev Infect Dis. 1984;6(Suppl 1):S85–90. doi: 10.1093/clinids/6.Supplement_1.S85. [DOI] [PubMed] [Google Scholar]
- 56.Awolade P, Cele N, Kerru N, Gummidi L, Oluwakemi E, Singh P. Therapeutic significance of β-glucuronidase activity and its inhibitors: A review. Eur J Med Chem. 2020;187:111921. doi: 10.1016/j.ejmech.2019.111921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg L, Bethea TN, Viscidi E, Hong C-C, Troester MA, Bandera EV, Haiman CA, Kolonel LN, Olshan AF, Ambrosone CB, et al. Postmenopausal female hormone use and estrogen receptor–positive and –negative breast cancer in African American Women. J Natl Cancer Inst. 2016;108(4):djv361. doi: 10.1093/jnci/djv361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitaker BL. Plasma β-glucuronidase levels in breast cancer. Br J Cancer. 1960;14(3):471–477. doi: 10.1038/bjc.1960.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beratis NG, Kaperonis A, Eliopoulou MI, Kourounis G, Tzingounis VA. Increased activity of lysosomal enzymes in the peritoneal fluid of patients with gynecologic cancers and pelvic inflammatory disease. J Cancer Res Clin Oncol. 2005;131(6):371–376. doi: 10.1007/s00432-004-0649-5. [DOI] [PubMed] [Google Scholar]
- 60.Laborda-Illanes A, Sanchez-Alcoholado L, Dominguez-Recio ME, Jimenez-Rodriguez B, Lavado R, Comino-Méndez I, Alba E, Queipo-Ortuño MI. Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers Basel. 2020;12(9):12. doi: 10.3390/cancers12092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu J, Liao M, Yao Z, Liang W, Li Q, Liu J, Yang H, Ji Y, Wei W, Tan A, et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 2018;6(1):136. doi: 10.1186/s40168-018-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He S, Li H, Yu Z, Zhang F, Liang S, Liu H, Chen H, Lü M. The gut microbiome and sex hormone-related diseases. Front Microbiol. 2021;12:711137. doi: 10.3389/fmicb.2021.711137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beral V, Gaitskell K, Hermon C. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. 2015;385:1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chambers LM, Esakov Rhoades EL, Bharti R, Braley C, Tewari S, Trestan L, Alali Z, Bayik D, Lathia JD, Sangwan N, et al. Disruption of the gut microbiota confers cisplatin resistance in epithelial ovarian cancer. Cancer Res. 2022;82(24):4654–4669. doi: 10.1158/0008-5472.CAN-22-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng H, Wang Z, Cui L, Wen Y, Chen X, Gong F, Yi H. Opportunities and challenges of the human microbiome in ovarian cancer. Front Oncol. 2020;10:163. doi: 10.3389/fonc.2020.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laschke MW, Menger MD. The gut microbiota: a puppet master in the pathogenesis of endometriosis? Am J Obstet Gynecol. 2016;215(1):.e68.1–.e68.4. doi: 10.1016/j.ajog.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 67.Talwar C, Singh V, Kommagani R. The gut microbiota: a double-edged sword in endometriosis†. Biol Reprod. 2022;107:881–901. doi: 10.1093/biolre/ioac147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin R, Tian G, Liu J, Cao L. The gut microbiota and endometriosis: From pathogenesis to diagnosis and treatment. Front Cell Infect Microbiol. 2022;12:1069557. doi: 10.3389/fcimb.2022.1069557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galvankar M, Singh N, Modi D. Estrogen is essential but not sufficient to induce endometriosis. J Biosci. 2017;42(2):251–263. doi: 10.1007/s12038-017-9687-4. [DOI] [PubMed] [Google Scholar]
- 70.Clemenza S, Vannuccini S, Ruotolo A, Capezzuoli T, Petraglia F. Advances in targeting estrogen synthesis and receptors in patients with endometriosis. Expert Opin Investig Drugs. 2022;31(11):1227–1238. doi: 10.1080/13543784.2022.2152325. [DOI] [PubMed] [Google Scholar]
- 71.Jiang I, Yong PJ, Allaire C, Bedaiwy MA. Intricate connections between the microbiota and endometriosis. Int J Mol Sci. 2021;22(11):22. doi: 10.3390/ijms22115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brandelli A, Passos EP. Glycosidases in the peritoneal fluid from infertile women with and without endometriosis. Clin Biochem. 1998;31(3):181–186. doi: 10.1016/S0009-9120(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 73.Graham ME, Herbert WG, Song SD, Raman HN, Zhu JE, Gonzalez PE, Walther-António MRS, Tetel MJ. Gut and vaginal microbiomes on steroids: implications for women’s health. Trends Endocrinol Metab. 2021;32(8):554–565. doi: 10.1016/j.tem.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update. 2007;13(6):559–565. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- 75.Schreurs MPH, de Vos van Steenwijk PJ, Romano A, Dieleman S, Werner HMJ. How the gut microbiome links to menopause and obesity, with possible implications for endometrial cancer development. JCM. 2021;10(13):10. doi: 10.3390/jcm10132916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Menon R, Watson SE, Thomas LN, Allred CD, Dabney A, Azcarate-Peril MA, Sturino JM. Diet complexity and estrogen receptor β status affect the composition of the murine intestinal microbiota. Appl Environ Microbiol. 2013;79(18):5763–5773. doi: 10.1128/AEM.01182-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meng Q, Ma M, Zhang W, Bi Y, Cheng P, Yu X, Fu Y, Chao Y, Ji T, Li J, et al. The gut microbiota during the progression of atherosclerosis in the perimenopausal period shows specific compositional changes and significant correlations with circulating lipid metabolites. Gut Microbes. 2021;13(1):1–27. doi: 10.1080/19490976.2021.1880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Insenser M, Murri M, Del Campo R, Martínez-García MÁ, Fernández-Durán E, Escobar-Morreale HF. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab. 2018;103(7):2552–2562. doi: 10.1210/jc.2017-02799. [DOI] [PubMed] [Google Scholar]
- 79.Song CH, Kim N, Nam RH, Choi SI, Lee H-N, Surh Y-J. 17β-Estradiol supplementation changes gut microbiota diversity in intact and colorectal cancer-induced ICR male mice. Sci Rep. 2020;10(1):12283. doi: 10.1038/s41598-020-69112-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tran A, Scholtes C, Songane M, Champagne C, Galarneau L, Levasseur M-P, Fodil N, Dufour CR, Giguère V, Saleh M, et al. Estrogen-related receptor alpha (ERRα) is a key regulator of intestinal homeostasis and protects against colitis. Sci Rep. 2021;11(1):15073. doi: 10.1038/s41598-021-94499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mayneris-Perxachs J, Arnoriaga-Rodríguez M, Luque-Córdoba D, Priego-Capote F, Pérez-Brocal V, Moya A, Burokas A, Maldonado R, Fernández-Real J-M. Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: influences of obesity and menopausal status. Microbiome. 2020;8(1):136. doi: 10.1186/s40168-020-00913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de la Cuesta-Zuluaga J, Kelley ST, Chen Y, Escobar JS, Mueller NT, Ley RE, McDonald D, Huang S, Swafford AD, Knight R, et al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems. 2019;4(4):4. doi: 10.1128/mSystems.00261-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaccaro CM, Capozzi A, Ettore G, Bernorio R, Cagnacci A, Gambacciani M, Coletta V, Maffei S, Nappi RE, Scambia G, et al. What women think about menopause: An Italian survey. Maturitas. 2021;147:47–52. doi: 10.1016/j.maturitas.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 84.Mili N, Paschou SA, Armeni A, Georgopoulos N, Goulis DG, Lambrinoudaki I. Genitourinary syndrome of menopause: a systematic review on prevalence and treatment. Menopause. 2021;28(6):706–716. doi: 10.1097/GME.0000000000001752. [DOI] [PubMed] [Google Scholar]
- 85.Luo M, Li J, Tang R, Li HJ, Liu B, Peng Y, Wang Y, Liu G, Lin S, Chen R, et al. Insomnia symptoms in relation to menopause among middle-aged Chinese women: Findings from a longitudinal cohort study. Maturitas. 2020;141:1–8. doi: 10.1016/j.maturitas.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 86.Zeng LN, Yang Y, Feng Y, Cui X, Wang R, Hall BJ, Ungvari GS, Chen L, Xiang Y-T. The prevalence of depression in menopausal women in China: A meta-analysis of observational studies. J Affect Disord. 2019;256:337–343. doi: 10.1016/j.jad.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 87.Park S, Kim DS, Kang ES, Kim DB, Kang S. Low-dose brain estrogen prevents menopausal syndrome while maintaining the diversity of the gut microbiomes in estrogen-deficient rats. Am J Physiol Endocrinol Metab. 2018;315(1):E99–e109. doi: 10.1152/ajpendo.00005.2018. [DOI] [PubMed] [Google Scholar]
- 88.Guadamuro L, Delgado S, Redruello B, Flórez AB, Suárez A, Martínez-Camblor P, Mayo B. Equol status and changes in fecal microbiota in menopausal women receiving long-term treatment for menopause symptoms with a soy-isoflavone concentrate. Front Microbiol. 2015;6:777. doi: 10.3389/fmicb.2015.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mörkl S, Butler MI, Lackner S. Advances in the gut microbiome and mood disorders. Curr Opin Psychiatry. 2022;36(1):1–7. doi: 10.1097/YCO.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 90.Fang Y, Zhang J, Zhu S, He M, Ma S, Jia Q, Sun Q, Song L, Wang Y, Duan L, et al. Berberine ameliorates ovariectomy-induced anxiety-like behaviors by enrichment in equol generating gut microbiota. Pharmacol Res. 2021;165:105439. doi: 10.1016/j.phrs.2021.105439. [DOI] [PubMed] [Google Scholar]
- 91.Sovijit WN, Sovijit WE, Pu S, Usuda K, Inoue R, Watanabe G, Yamaguchi H, Nagaoka K. Ovarian progesterone suppresses depression and anxiety-like behaviors by increasing the Lactobacillus population of gut microbiota in ovariectomized mice. Neurosci Res (NY). 2021;168:76–82. doi: 10.1016/j.neures.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 92.Huang J, Shan W, Li F, Wang Z, Cheng J, Lu F, Guo E, Beejadhursing R, Xiao R, Liu C, et al. Fecal microbiota transplantation mitigates vaginal atrophy in ovariectomized mice. Aging (Albany NY). 2021;13(5):7589–7607. doi: 10.18632/aging.202627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valko-Rokytovská M, Očenáš P, Salayová A, Kostecká Z. Breast cancer: Targeting of steroid hormones in cancerogenesis and diagnostics. Int J Mol Sci. 2021;22(11):22. doi: 10.3390/ijms22115878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tan B, Zhang Y, Zhang T, He J, Luo X, Bian X, Wu J, Zou C, Wang Y, Fu L, et al. Identifying potential serum biomarkers of breast cancer through targeted free fatty acid profiles screening based on a GC–MS platform. Biomed Chromatogr. 2020;34(10):e4922. doi: 10.1002/bmc.4922. [DOI] [PubMed] [Google Scholar]
- 95.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 96.Ofinran O, Bose U, Hay D, Abdul S, Tufatelli C, Khan R. Selection of suitable reference genes for gene expression studies in normal human ovarian tissues, borderline ovarian tumours and ovarian cancer. Mol Med Rep. 2016;14(6):5725–5731. doi: 10.3892/mmr.2016.5933. [DOI] [PubMed] [Google Scholar]
- 97.Jin Y, Tian X, Jin L, Cui Y, Liu T, Yu Z, Huo X, Cui J, Sun C, Wang C, et al. Highly specific near-infrared fluorescent probe for the real-time detection of β-glucuronidase in various living cells and animals. Anal Chem. 2018;90(5):3276–3283. doi: 10.1021/acs.analchem.7b04813. [DOI] [PubMed] [Google Scholar]
- 98.Li T, Li G, Su Z, Liu J, Wang P. Recent advances of sensing strategies for the detection of β-glucuronidase activity. Anal Bioanal Chem. 2022;414(9):2935–2951. doi: 10.1007/s00216-022-03921-y. [DOI] [PubMed] [Google Scholar]
- 99.Wang P, Jia Y, Wu R, Chen Z, Yan R. Human gut bacterial β-glucuronidase inhibition: An emerging approach to manage medication therapy. Biochem Pharmacol. 2021;190:114566. doi: 10.1016/j.bcp.2021.114566. [DOI] [PubMed] [Google Scholar]
- 100.Muccee F, Ghazanfar S, Ajmal W, Al-Zahrani M. In-silico characterization of estrogen reactivating β-glucuronidase enzyme in git associated microbiota of normal human and breast cancer patients. Genes (Basel). 2022;13(9):1545. doi: 10.3390/genes13091545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walaszek Z, Szemraj J, Narog M, Adams AK, Kilgore J, Sherman U, Hanausek M. Metabolism, uptake, and excretion of a D-glucaric acid salt and its potential use in cancer prevention. Cancer Detect Prev. 1997;21:178–190. [PubMed] [Google Scholar]
- 102.Cheng KW, Tseng CH, Chen IJ, Huang B-C, Liu H-J, Ho K-W, Lin W-W, Chuang C-H, Huang M-Y, Leu Y-L, et al. Inhibition of gut microbial β-glucuronidase effectively prevents carcinogen-induced microbial dysbiosis and intestinal tumorigenesis. Pharmacol Res. 2022;177:106115. doi: 10.1016/j.phrs.2022.106115. [DOI] [PubMed] [Google Scholar]
- 103.De Preter V, Raemen H, Cloetens L, Houben E, Rutgeerts P, Verbeke K. Effect of dietary intervention with different pre- and probiotics on intestinal bacterial enzyme activities. Eur J Clin Nutr. 2008;62(2):225–231. doi: 10.1038/sj.ejcn.1602706. [DOI] [PubMed] [Google Scholar]
- 104.Letertre MPM, Bhatt AP, Harvey M, Nicholson JK, Wilson ID, Redinbo MR, Swann JR. Characterizing the metabolic effects of the selective inhibition of gut microbial β-glucuronidases in mice. Sci Rep. 2022;12(1):17435. doi: 10.1038/s41598-022-21518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishio S, Shimokawa M, Tasaki K, Nasu H, Yoshimitsu T, Matsukuma K, Terada A, Tsuda N, Kawano K, Ushijima K, et al. A phase II trial of irinotecan in patients with advanced or recurrent endometrial cancer and correlation with biomarker analysis. Gynecol Oncol. 2018;150(3):432–437. doi: 10.1016/j.ygyno.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 106.Bhatt AP, Pellock SJ, Biernat KA, Walton WG, Wallace BD, Creekmore BC, Letertre MM, Swann JR, Wilson ID, Roques JR, et al. Targeted inhibition of gut bacterial β-glucuronidase activity enhances anticancer drug efficacy. Proc Natl Acad Sci USA. 2020;117(13):7374–7381. doi: 10.1073/pnas.1918095117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, Nagai E, Yokoi T, Kamataki T. Involvement of beta-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res. 1996;56:3752–3757. [PubMed] [Google Scholar]
- 108.Chamseddine AN, Ducreux M, Armand JP, Paoletti X, Satar T, Paci A, Mir O. Intestinal bacterial β-glucuronidase as a possible predictive biomarker of irinotecan-induced diarrhea severity. Pharmacology & Therapeutics. 2019;199:1–15. doi: 10.1016/j.pharmthera.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 109.Wallace BD, Roberts AB, Pollet RM, Ingle J, Biernat K, Pellock S, Venkatesh M, Guthrie L, O’Neal S, Robinson S, et al. Structure and inhibition of microbiome β-glucuronidases essential to the alleviation of cancer drug toxicity. Chem Biol. 2015;22(9):1238–1249. doi: 10.1016/j.chembiol.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng KW, Tseng CH, Tzeng CC, Leu Y-L, Cheng T-C, Wang J-Y, Chang J-M, Lu Y-C, Cheng C-M, Chen I-J, et al. Pharmacological inhibition of bacterial β-glucuronidase prevents irinotecan-induced diarrhea without impairing its antitumor efficacy in vivo. Pharmacol Res. 2019;139:41–49. doi: 10.1016/j.phrs.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 111.Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, Miskovska V, Bystricky B, Beniak J, Medvecova L, et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement Ther Med. 2015;23(3):356–362. doi: 10.1016/j.ctim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 112.Palko-Łabuz A, Maksymowicz J, Sobieszczańska B, Wikiera A, Skonieczna M, Wesołowska O, Środa-Pomianek K. Newly obtained apple pectin as an adjunct to irinotecan therapy of colorectal cancer reducing E. coli adherence and β-glucuronidase activity. Cancers Basel. 2021;13(12):2952. doi: 10.3390/cancers13122952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pellock SJ, Creekmore BC, Walton WG, Mehta N, Biernat KA, Cesmat AP, Ariyarathna Y, Dunn ZD, Li B, Jin J, et al. Gut microbial β-glucuronidase inhibition via catalytic cycle interception. ACS Cent Sci. 2018;4(7):868–879. doi: 10.1021/acscentsci.8b00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Little MS, Pellock SJ, Walton WG, Tripathy A, Redinbo MR. Structural basis for the regulation of β-glucuronidase expression by human gut Enterobacteriaceae. Proc Natl Acad Sci USA. 2018;115(2):E152–e161. doi: 10.1073/pnas.1716241115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang P, Wu R, Jia Y, Tang P, Wei B, Zhang Q, Wang VYF, Yan R. Inhibition and structure-activity relationship of dietary flavones against three Loop 1-type human gut microbial β-glucuronidases. Int J Biol Macromol. 2022;220:1532–1544. doi: 10.1016/j.ijbiomac.2022.09.018. [DOI] [PubMed] [Google Scholar]
- 116.Sun CP, Yan JK, Yi J, Zhang X-Y, Yu Z-L, Huo X-K, Liang J-H, Ning J, Feng L, Wang C, et al. The study of inhibitory effect of natural flavonoids toward β-glucuronidase and interaction of flavonoids with β-glucuronidase. Int J Biol Macromol. 2020;143:349–358. doi: 10.1016/j.ijbiomac.2019.12.057. [DOI] [PubMed] [Google Scholar]
- 117.Bai Y, Chen L, Cao YF, Hou X-D, Jia S-N, Zhou Q, He Y-Q, Hou J. Beta-glucuronidase inhibition by constituents of Mulberry Bark. Planta Med. 2021;87(08):631–641. doi: 10.1055/a-1402-6431. [DOI] [PubMed] [Google Scholar]
- 118.Marlatt KL, Beyl RA, Redman LM. A qualitative assessment of health behaviors and experiences during menopause: A cross-sectional, observational study. Maturitas. 2018;116:36–42. doi: 10.1016/j.maturitas.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chu K, Song Y, Chatooah ND, Weng Q, Ying Q, Ma L, Qu F, Zhou J. The use and discontinuation of hormone replacement therapy in women in South China. Climacteric. 2018;21(1):47–52. doi: 10.1080/13697137.2017.1397622. [DOI] [PubMed] [Google Scholar]
- 120.Seyed Hameed AS, Rawat PS, Meng X, Liu W. Biotransformation of dietary phytoestrogens by gut microbes: A review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol Adv. 2020;43:107576. doi: 10.1016/j.biotechadv.2020.107576. [DOI] [PubMed] [Google Scholar]
- 121.Rowland I, Faughnan M, Hoey L, Wähälä K, Williamson G, Cassidy A. Bioavailability of phyto-oestrogens. Br J Nutr. 2003;89(Suppl S1):S45–58. doi: 10.1079/BJN2002796. [DOI] [PubMed] [Google Scholar]
- 122.Setchell KD, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr. 2002;76(2):447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 123.Mayo B, Vázquez L, Flórez AB. Equol: A bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients. 2019;11(9):11. doi: 10.3390/nu11092231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu C, He D, Yu A, Deng Y, Wang L, Song Z. Correlation analysis between gut microbiota characteristics and melasma. Front Microbiol. 2022;13:1051653. doi: 10.3389/fmicb.2022.1051653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guadamuro L, Dohrmann AB, Tebbe CC, Mayo B, Delgado S. Bacterial communities and metabolic activity of faecal cultures from equol producer and non-producer menopausal women under treatment with soy isoflavones. BMC Microbiol. 2017;17(1):93. doi: 10.1186/s12866-017-1001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dai S, Pan M, El-Nezami HS, Wan JMF, Wang MF, Habimana O, Lee JCY, Louie JCY, Shah NP. Effects of lactic acid bacteria-fermented soymilk on isoflavone metabolites and short-chain fatty acids excretion and their modulating effects on gut microbiota. J Food Sci. 2019;84(7):1854–1863. doi: 10.1111/1750-3841.14661. [DOI] [PubMed] [Google Scholar]
- 127.Yuan L, Wagatsuma C, Yoshida M, Miura T, Mukoda T, Fujii H, Sun B, Kim J-H, Surh Y-J. Inhibition of human breast cancer growth by GCP™ (genistein combined polysaccharide) in xenogeneic athymic mice: involvement of genistein biotransformation by β-glucuronidase from tumor tissues. Mutat Res. 2003;523-524:55–62. doi: 10.1016/S0027-5107(02)00321-4. [DOI] [PubMed] [Google Scholar]
- 128.Sawane K, Nagatake T, Hosomi K, Kunisawa J. Anti-allergic property of dietary phytoestrogen secoisolariciresinol diglucoside through microbial and β-glucuronidase-mediated metabolism. J Nutr Biochem. 2023;112:109219. doi: 10.1016/j.jnutbio.2022.109219. [DOI] [PubMed] [Google Scholar]
- 129.Łaniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol. 2020;17(4):232–250. doi: 10.1038/s41585-020-0286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lim EY, Song EJ, Kim JG, Jung SY, Lee S-Y, Shin HS, Nam Y-D, Kim YT. Lactobacillus intestinalis YT2 restores the gut microbiota and improves menopausal symptoms in ovariectomized rats. Benef Microbes. 2021;12(5):503–516. doi: 10.3920/BM2020.0217. [DOI] [PubMed] [Google Scholar]
- 131.Farvid MS, Spence ND, Holmes MD, Barnett JB. Fiber consumption and breast cancer incidence: A systematic review and meta-analysis of prospective studies. Cancer. 2020;126(13):3061–3075. doi: 10.1002/cncr.32816. [DOI] [PubMed] [Google Scholar]
- 132.Zengul AG, Demark-Wahnefried W, Barnes S, Morrow CD, Bertrand B, Berryhill TF, Frugé AD. Associations between dietary fiber, the fecal microbiota and estrogen metabolism in postmenopausal women with breast cancer. Nutr Cancer. 2021;73(7):1108–1117. doi: 10.1080/01635581.2020.1784444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arts CJ, de Bie AT, van den Berg H, van ’t Veer P, Bunnik GSJ, Thijssen JHH. Influence of wheat bran on NMU-induced mammary tumor development, plasma estrogen levels and estrogen excretion in female rats. J Steroid Biochem Molecular Biol. 1991;39(2):193–202. doi: 10.1016/0960-0760(91)90063-B. [DOI] [PubMed] [Google Scholar]
- 134.Jeong SY, Kang S, Hua CS, Ting Z, Park S. Synbiotic effects of β-glucans from cauliflower mushroom and Lactobacillus fermentum on metabolic changes and gut microbiome in estrogen-deficient rats. Genes Nutr. 2017;12(1):31. doi: 10.1186/s12263-017-0585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu X, Kim MJ, Yang HJ, Park S. Chitosan alleviated menopausal symptoms and modulated the gut microbiota in estrogen-deficient rats. Eur J Nutr. 2021;60(4):1907–1919. doi: 10.1007/s00394-020-02382-2. [DOI] [PubMed] [Google Scholar]
- 136.Lønning PE, Haynes BP, Straume AH, Dunbier A, Helle H, Knappskog S, Dowsett M. Exploring breast cancer estrogen disposition: the basis for endocrine manipulation. Clin Cancer Res. 2011;17(15):4948–4958. doi: 10.1158/1078-0432.CCR-11-0043. [DOI] [PubMed] [Google Scholar]
- 137.Challa AP, Hu X, Zhang YQ, Hymes J, Wallace BD, Karavadhi S, Sun H, Patnaik S, Hall MD, Shen M, et al. Virtual screening for the discovery of microbiome β-glucuronidase inhibitors to alleviate cancer drug toxicity. J Chem Inf Model. 2022;62(7):1783–1793. doi: 10.1021/acs.jcim.1c01414. [DOI] [PMC free article] [PubMed] [Google Scholar]


