Abstract
Simple Summary
Cancer cells alter their metabolisms to support growth, thus causing micro- and macronutrient changes in the metabolite profile that promote an immunosuppressive tumor microenvironment. Immune cells and stromal cells contribute to an immunosuppressive microenvironment by releasing metabolites, cytokines, and depositing extracellular matrix that prevents lymphocyte activation and recruitment and promotes T-cell exhaustion. In recent years, new dietary interventions such as calorie restriction and periodic fasting have been shown to have beneficial effects in preventing cancer and extending life expectancy in mouse models through modulation of immune system functions. In this review, we discuss how dietary interventions such as periodic fasting can manipulate tumor metabolism, prevent the formation of an immunosuppressive microenvironment, and enhance the efficacy of anti-tumor therapies.
Abstract
The remodeled cancer cell metabolism affects the tumor microenvironment and promotes an immunosuppressive state by changing the levels of macro- and micronutrients and by releasing hormones and cytokines that recruit immunosuppressive immune cells. Novel dietary interventions such as amino acid restriction and periodic fasting mimicking diets can prevent or dampen the formation of an immunosuppressive microenvironment by acting systemically on the release of hormones and growth factors, inhibiting the release of proinflammatory cytokines, and remodeling the tumor vasculature and extracellular matrix. Here, we discuss the latest research on the effects of these therapeutic interventions on immunometabolism and tumor immune response and future scenarios pertaining to how dietary interventions could contribute to cancer therapy.
Keywords: immune system, diets, metabolism
1. Introduction
The effectiveness of anti-tumor therapies is determined by cancer cell-intrinsic factors such as the genomic landscape and cell signaling, as well as by the connective tissue, vessels, fibroblasts, and immune cell infiltration that compose the tumor microenvironment (TME) and contribute to the generation of immunosuppressive environment and immune evasion [1,2,3].
Tumor cells determine TME cell composition through the release of chemokines and cytokines that attract certain cell populations and manipulate TME cell function and differentiation through direct cell–cell interactions or through the release of growth factors, cytokine, and metabolic byproducts.
In recent decades, cancer metabolism has emerged to play an essential role in shaping the TME composition and the properties and functions of its constituents [4]. Dietary interventions such as calorie restriction (CR), fasting, and fasting mimicking diets (FMD) have received much attention as several preclinical and clinical studies have shown their efficacy in modulating tumor metabolism, remodeling the tumor microenvironment, and even enhancing the antitumor immune response [5,6,7,8,9,10,11].
This review focuses on the current knowledge regarding cancer metabolism and how it will affect the TME. We will then examine how dietary interventions can affect cancer metabolism, reverse immunosuppressive TME, and improve immune response.
2. Immunosuppressive TME
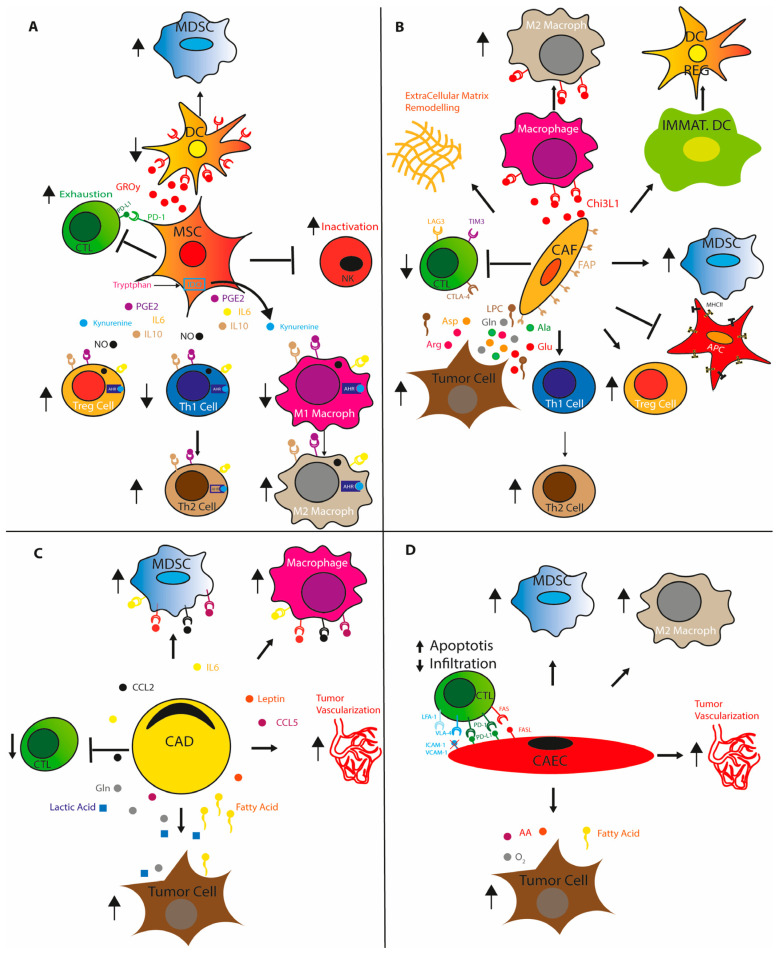
The TME contains mesenchymal stroma-/stem-like cells (MSCs), cancer-associated fibroblasts (CAFs), cancer-associated adipocytes (CAAs), cancer-associated endothelial cells (CAECs), immune cell populations, and extracellular matrix (ECM), which exert immunosuppressive activity and support tumor cell growth, survival, and spread [12,13,14] through the secretion of signaling molecules and extracellular matrix (ECM) components [15].
MSCs are a heterogeneous population of multipotent stromal cells capable of self-renewal and differentiation into various cell types which support the generation of immune regulatory cells with tolerogenic properties through the production of immunomodulatory factors [16]. Several chemokines secreted by MSC shape the immune cell composition in the TME by influencing leucocytes recruitment, macrophages polarization [17], collagen deposition [18], cancer cell invasion [19], and drug resistance development [20]. Chemokines drive leucocytes trafficking in TME and promote the selective recruitment of specific leucocytes based on their chemokine receptor expression. Thus, chemokines may have both an antitumor role by promoting the recruitment of NK cells, CD4+ Th1 cells, and CD8+ cytotoxic T lymphocytes (CTLs) into the tumor [21,22,23,24], and a protumor role by promoting the recruitment of immunosuppressive cells such as Tregs [25,26], tumor-associated macrophages (TAMs), and MDSCs [27,28,29]. GRO-y (growth-regulated oncogene) chemokine released from MSC drives monocyte-derived dendritic cells to differentiate into tolerogenic myeloid derived suppressor cells (MDSC) [30]. The secretion of nitric oxide (NO), PGE2, IL-6, IL-10, and kynurenine, generated from tryptophan by indoleamine 2,3-dioxygenase (IDO), induce a switch from the Th1 anti-tumor effector response to Th2 suppressive mode [16], expand immunosuppressive M2-polarized macrophages, tumor-associated macrophages (TAMs), and also Tregs populations [31,32]. Furthermore, the natural killer (NK) activity is impaired by MSC through downregulation of NKG2D-activating receptors expression [33]. MSCs expressing PD-1 by interacting with PD-L1/PDL2 ligand on tumor infiltrating lymphocytes (TILs) induce T cell anergy and exhaustion, thus allowing tumor progression [34].
CAFs are dysregulated fibroblasts which arise from differentiation of tumor stromal cells and are involved in extracellular matrix (ECM) remodeling and immune-surveillance evasion [35,36]. As the most abundant cell population of non-cancerous components in the TME of solid tumors [37], CAFs effectively contribute to the generation of immunosuppressive TME and tumor progression [38]. CAFs support tumor-promoting inflammation [39] by recruiting immune cells to the TME through the release of cytokines and chemokines. Secretion of chitinase-like protein 3 (Chi3L1) by CAFs increases macrophage infiltration, promotes macrophage switch to the M2 immunosuppressive phenotype, and limits the infiltration of cytotoxic CD8+ T cells into the breast TME [40]. The expression of fibroblast activation protein (FAP) on the surface of CAF promotes the recruitment of type M2 macrophages, MDSCs, and Tregs into the liver tumor bed and the switch from Th1 to Th2 immunity [41].
CAFs impair innate and adaptive immune responses by downregulating the expression of MHC class II (MHCII) molecules in antigen-presenting cells (APCs) [42], promoting the differentiation of immature dendritic cells (DCs) into regulatory DCs (DCregs) in hepatic carcinoma [43], and stimulating the expression of immunosuppressive proteins such as T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), CTLA-4, and LAG-3 in active T lymphocytes in pancreatic carcinoma [44].
CAFs contribute to liver tumor cell growth and migration through the secretion of various metabolites in the TME. Glutamine release in TME supports nucleotide synthesis and oxidative phosphorylation (OXPHOS) activity [41]. CAF-secreting alanine is converted to pyruvate, an essential source to fuel OXPHOS when glucose is in short supply in pancreatic tumor cells [45]. The secretion of aspartate and glutamate promotes the synthesis of nucleotides and glutathione, respectively, which is required to reduce oxidative stress in skin cancer cells [46]. The arginine released in the TME is instead utilized for the synthesis of nitric oxide (NO), which is essential to alleviate oxidative stress and promote glycolysis in ovarian and endometrial cancer cells [47]. Finally, CAFs promotepancreatic tumor growth both through the production of lysophosphatidylcholine, essential for the synthesis of cell membranes and of lysophosphatidic acid, a biomolecule with growth factor-like activity [48]; and the deposition of collagen, which is degraded and used by pancreatic cancer cells as a source for the production of proline, which is needed to support the tricarboxylic acid (TCA) cycle [49].
CADs are aberrant adipocytes that promote breast and prostate tumor cell proliferation, angiogenesis, dissemination, invasion and metastasis, and the generation of an immunosuppressive microenvironment through the release of adipokines such as leptin, adiponectin, interleukin (IL)-6, chemokine ligand 2 (CCL2), and chemokine ligand 5 (CCL5) [50,51,52]. Furthermore, tumor cells nourish themselves and support their growth by absorbing lactic acid, fatty acids, and glutamine secreted by CADs in TME [53]. Adipokines exert an immunomodulatory effect and remodel breast TME by favoring the infiltration of immunosuppressive cells such as monocytes, macrophages, and neutrophils and by inactivating the cytotoxic CD8 T lymphocyte [54,55,56].
CAECs support breast and melanoma tumor growth both by promoting tumor vasculature essential for nutrient and oxygen delivery and by favoring the recruitment of immunosuppressive rather than effector immune cells through the downregulation of adhesion molecules such as the ligands ICAM1 (intercellular adhesion molecule 1) and VCAM1 (vascular cell adhesion protein 1), which promote T cell extravasation into the TME by binding to LFA1 (antigen 1 associated with lymphocyte function) and VLA4 (very late antigen 4) on T cells [57]. Furthermore, CAEC expressing PD-L1 binds PD-1 and inhibits tumor infiltrating lymphocytes (TIL) activation, while the interaction of FASL, expressed on CAEC, with FAS, present on T cell, induces lymphocyte apoptosis [58,59]. Tumor vessels are dysfunctional, abnormal, and characterized by irregular blood flow, erratic branching, and high permeability/leakiness, which lead to an accumulation of metabolic waste in the TME that affects immune cell function [60] (Figure 1).
Figure 1.
Stromal cells contribute to generate an immunosuppressive tumor microenvironment. (A) Mesenchymal stem cells (MSC). (B) Cancer associated fibroblasts (CAFs). (C) Cancer associated adipocytes (CADs) and (D) Cancer associated endothelial cells (CAECs) promote immune suppressive cells recruitment, proliferation and differentiation by releasing chemokines, cytokines and metabolites in TME. Stromal cells induce T cells exhaustion by expressing immune checkpoint and prevents T cell recruitment by reshaping tumor vasculature. IL6, IL10: interleukin 6, 10; PGE2: prostaglandin E2; ID01: Indoleamine-pyrrole2,3-dioxygenase; AHR: Aryl Hydrocarbon Receptor; GRO-y: Growth-regulated oncogene-Y chemokine; Chi3L1: chitinase-like protein 3; Gin: glutamine; Glu: glutamate; Arg: arginine; Ala: alanine; Asp: aspartate; LPC: lysophosphatidylcholine; MDSCs: myeloid-derived suppressor cells; CTLs: cytotoxic T lymphocytes; DCs: dendritic cells; DCregs: regulatory DCs.
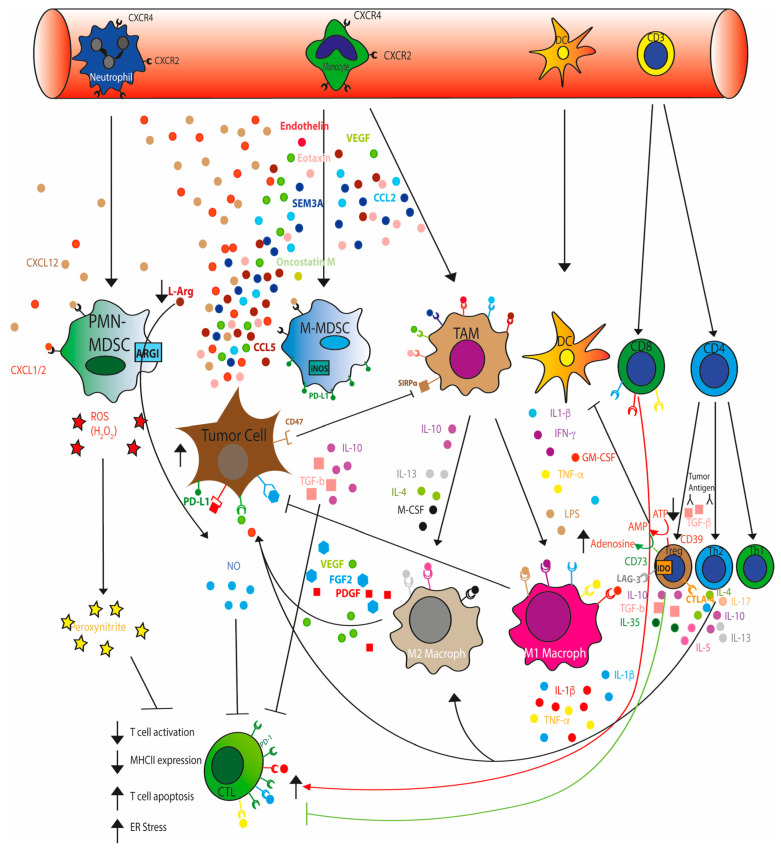
An immunosuppressive role in TME is exerted by both innate immune system cells (MDSC, TAM, γδ T cells) and by the adaptive system (Bregs, Tregs, Th2, γδ T cells).
MDSCs are immunosuppressive cells that originate from neutrophils or monocytes attracted to TME via the chemokine receptors CXCR2 and CXCR4 [30,34]. In fact, two populations of MDSCs are distinguished: polymorphonuclear (PMN) of neutrophilic origin and monocytic, which derive from monocytes (M). MDSC inhibit immune responses mediated by T cells, B cells, and natural killer (NK) cells through upregulation of signal transducer and activator of transcription 3 (STAT3) expression, induction of ER stress, expression of arginase1, and expression of S100A8/A9 in gastric cancer [61]. However, PMN-MDSCs exert immunosuppressive activity through the production of reactive oxygen species (ROS), peroxynitrite, arginase 1, and prostaglandin E2 (PGE2), whereas M-MDSCs preferentially utilize nitric oxide (NO), immunosuppressive cytokines such as IL-10 and TGFβ, and the expression of immune regulatory molecules such as PDL1 [62].
TAMs originate from bone marrow-derived monocytic precursors recruited in TME by migratory stimulating factors, such as VEGF, CCL2, CCL5, CSF-1, EMAP-II, endothelin-2, SEMA3A, oncostatin M, and eotaxin, released by breast, pancreas, lung, and glioma tumor cells [27,63,64,65,66,67]. According to TME stimuli, TAMs differentiate into M1 or M2 polarized macrophages [68]. IFN-γ-, lipopolysaccharide-, IL-1β-, TNF-, and/or GM-CSF-activated M1-like macrophages recognize and kill tumor cells via phagocytosis and the release of proinflammatory factors that stimulate the immune system. M2-like macrophages, activated by IL-4, IL-10, IL-13, and/or M-CSF, released by Th2, support breast, lung, colorectal, thymomas, and melanoma tumor growth by secreting growth factors (FGF2, PDGF, and VEGF) [69,70], immunosuppressive factors (iNOS, oxygen radicals or nitrogen species) [71,72,73], pro-angiogenic molecules (VEGF, FGF2, CXCL8, and IL-8), and proteases (MMP2, MMP7, and MMP9) [74,75,76].
However, an important role in tumorigenesis and tumor progression could also be played by tissue-specific macrophages responsible for maintaining tissue homeostasis. It has emerged from recent studies that tissue-resident macrophages differ mainly in their metabolism. In particular, OXPHOS-dependent macrophages are involved in the maintenance and handling of extracellular lipids and cholesterol. These OXPHOS-dependent macrophages acquire a proinflammatory phenotype in obese people and contribute to the generation of an inflammatory state which promotes immune evasion and tumor development [77].
Regulatory B cells arise from the differentiation of B cells, attracted to the tumor upon the release of chemoattractants, and suppress inflammatory responses through the secretion of immunomodulatory cytokines and cell-to-cell contact interactions. Secreted factors such as leukotriene B4 (breast cancer), glioma cell-derived placental growth factor (glioma), TNF-α (tumor cells), and IL-21 (T cells), as well as cell contact-dependent mechanisms (CD40-CD40L and PD-1-PD-L1 axis,), promote cell differentiation Bregs in the TME [78,79,80,81,82,83].
Inflammatory cues such as TLR ligands (CD40L, TLR9signals) and pro-inflammatory cytokines (IL-21, IL-1b, and IL-6; B cell-activating factor and A proliferation-inducing ligand) induce immune suppressor function of Bregs [84].
Bregs exert inhibitory activity mainly through the release of IL10, TGF-b, IL-35, and granzyme B (GzmB), but also mediate immunosuppressive function through the expression of PD-L1, FasL, and TIM-1. Tumors stimulate Bregs to secrete IL10 via CD40L signals or tumor-derived exosome [85,86,87,88]. IL10+ Bregs promote immune evasion by suppressing IFN-g release by effector CD8+ and CD4+ T cell and activated NK cells, by reducing GzmB synthesis in CD8+ T cells, by inhibiting the secretion of pro-inflammatory cytokine by CD4+ T cells, and by hampering CD20-mediated lymphoma clearance by preventing macrophage activation [85,87,89].
Bregs promote breast tumor progression and metastasis by producing and releasing TGF-b in TME. TGF-b convert naïve CD4+ T cells into Tregs and inhibit CD4+ and CD8+ T cell proliferation by inducing reactive oxygen species and NO production in MDSC [90,91].
Upon BCR and IL21 stimulation, Bregs produce GzmB which suppress CD4+T cell proliferation and Th1 and Th17 response by degrading z-chain [81]. Furthermore, Bregs support pancreas and gastric cancer by secreting IL35, which is a potent anti-inflammatory cytokine produced mainly by Tregs cells [92,93].
In pancreatic breast and cervical cancer, the engagement of PD1 with PD-L1, expressed on Bregs cells surface, induces CD4+ and CD8+ T cell suppression and exhaustion, IL-10-secreting type-1 regulatory CD4+T cells (Tr1) expansion, and reduced Th1/Th17 responses [94,95,96].
Bregs induce activated T cells anergy or apoptosis by expressing FasL on the cell surface. The interaction of FasL with Fas expressed by activated T cells leads to activation-induced cell death, a common apoptotic pathway [97,98]. Finally, Bregs promote tumor immune escape by expressing TIM-1 receptor, which is involved in regulating suppressive cytokines and ligands expression signaling pathways. The binging of TIM-1 with its ligand (TIM-4) expressed by myeloid cells leads B cells to produce IL10, TGF-b, and promote Treg and Tr1 generation, CD4+ and CD8+ T cell suppression, and inhibition of Th1 and Th17 differentiation [83,99,100].
Recently, it has been shown that Bregs mediate immunosuppressive function though the release of adenosine, IDO, progesterone-induced blocking factor 1, and heat shock protein-70 [101,102,103,104,105].
Treg and Th2 cells are derived from CD4 differentiation induced by tumor microenvironmental signals, such as tumor antigens, cytokines (such as TGF-β), and other soluble molecules [106], and have strong immunosuppressive, antitumor immunity inhibitory function [107,108,109].
Th2 cells contribute to fibrosarcoma and lung tumor progression by secreting IL-4, IL-5, IL-10, IL-13, and IL-17 [69,70,110,111] and promoting the recruitment of M2 macrophages and eosinophils via the expression of IL-5 and IL-13 [112]. Furthermore, Th2 cells promote tumor cell migration and invasion by releasing IL17, a pro-angiogenic factor, which induces vascular leakage [113] and increases MDSC infiltration.
Tregs promote tumor progression by impairing the functions of effector T cells (Teff), NK cells. and DCs by (1) secreting immunosuppressive cytokines, such as IL-10, TGF-β, and IL-35 [114]; (2) killing effector T cell through the release of granzymes and perforin; (3) regulating memory T cell quiescence through the expression of inhibitory receptor CTLA-4 [115]; (4) expressing CD39 and CD73 nuclease which increase the production of adenosine in TME, a molecule with antiproliferative effects [116]; and (5) hampering DC activation through the expression of activation gene 3 (LAG3), CTLA-4, and IDO [117].
γδ T cells represent a small sub-population poised at the border between the innate and adaptive immune system that can mediate an anti- or pro-tumor response based on the differentiation status. γδ Tcells producing effector cytokines IFNγ kill tumor cells via perforin and granzyme, whereas γδ T cells expressing IL17 support tumor growth and metastasis by recruiting other immunosuppressive immune cells, such as MDSC [118].
ECM is composed primarily of collagens, hyaluronan, fibronectin, elastin, and laminins, and constitutes 60% of tumor mass [119]. The tumor extracellular matrix is denser and stiffer than that of normal tissue [120], a characteristic that supports tumor growth by preventing drug diffusion and hindering the infiltration of T lymphocytes and NK cells into the tumor [121]. Dense ECM affects tumor immunosurveillance by collapsing lymph vessels and hindering migration of antigen-triggered DCs to draining lymph nodes and consequently antigen presentation and T-cell activation [122].
Tumor cells select and recruit TME cells through the release of hormones and cytokines and modulate their immunosuppressive activity through surface receptor–ligand interaction or secretion of hormones and metabolites.
Myeloid derived suppressor cells (MDSCs) and immunosuppressive regulatory T cells (Tregs) are recruited to the tumor region [123] by TGF-β1 (transforming growth factor beta-1) and granulocyte–macrophage colony-stimulating factor (CSF) secreted by tumor cells. CD47 expression on tumor cell membrane suppresses the antitumor activity of macrophages by inhibiting the phagocytosis through the interaction with SIRPα (signal regulatory protein α) [124]. The production of immunosuppressive metabolite kynurenine due to the upregulation of indoleamine-2,3-dioxygenase (IDO1) in tumor, tumor associated macrophages (TAMs), and monocytes prevents the activation of effector T cells, inhibits NK cell function, supports Treg activation, and promotes the expansion and activation of dendritic cells (DCs) and MDSCs [125].
Furthermore, tumors cells, MDSCs, monocytes, and TAMs promote immunosuppressing microenvironment by expressing the immune checkpoint PD-L1, which induces the T cells exhaustion and anergy by binding to PD1 [31,126] (Figure 2).
Figure 2.
Cancer cells shape the tumor microenvironment through secretion of soluble factors and recruitment of immunosuppressive cells. Neutrophils and monocytes migrate to TME attracted by chemokines such as CXCL12 and CXCL1, released by cancer cells, and differentiate into polymorphonuclear (PMN−) and mononuclear (M−) MDSCs. PMN-MDSCs suppress immune cells by producing ROS, peroxinitrite and prostaglandins and depleting TME of arginine by upregulating arginase I expression. M-MDSCs inhibit T cells, B cells and natural killer (NK) cells by producing nitric oxide (NO), immunosuppressive IL-10 and TGFβ and immune checkpoint molecules such as PDL1. Chemokine and growth factors (VEGF, CCL2, CCL5, CSF-1, EMAP-II, endothelin-2, SEMA3A, oncostatin M, and eotaxin), secreted by cancer cells, promote the migration of monocytes into the TME and their differentiation into tumor-associated macrophages (TAMs). TAMs differentiate into anti-tumor M1 macrophages upon stimulation with IFN-γ, lipopolysaccharide, IL-1β, TNF and/or GM-CSF, and into pro-tumor M2 macrophages upon stimulation with IL-4, IL-10, IL-13 and/or M-CSF. Tumor antigens, cytokines (such as TGF-B) promote CD4 differentiation in immunosuppressive Treg and T helper type 2 cells (Th2). Th2 cells secrete protumor cytokines IL-4, IL-5, IL-10, IL-13, and IL-17, recruits M2 macrophages by releasing IL-5 and IL-13 and promotes MDSCs infiltration by increasing vascular leakage through IL17 secretion. Tregs impair CTL, NK and dendritic cell function by secreting immunosuppressive cytokines such as IL-10, TGF-β and IL-35, expressing the immune checkpoint receptor (LAG3, CTLA4) the enzyme IDO, which converts tryptophan to kynurenine and CD39 and CD73 nucleases that convert ATP to adenosine.
Recent data indicate that NK cells and PGE2 play a fundamental role in reprogramming TME towards a cancer-promoting versus cancer-inhibitory condition which affects the anti-tumor immune response and the efficacy of immune therapy. The early intratumoral accumulation of natural killer (NK) cells producing interferon gamma (IFN-g), drives a broad polarization of myeloid cells toward an inflammatory profile conducive to effector T-cell infiltration. In contrast, tumor-secreted PGE2 in TME inhibits NK cells by binding to EP2 and EP4, the PGE2 receptors, and thus hinders NK-induced TME switch and promotes immune evasion of melanoma, breast, and colon cancer [76].
3. Cancer Metabolism
Cancer cells accumulate mutations in oncogenes and tumor suppressor genes leading to the constitutive or increased activation of signaling pathway which renders the tumor cells insensitive to or partially independent of growth factor stimuli and nutrient availability, leading to proliferation [127]. To support cell proliferation and meet the biosynthetic and bioenergetic requirements for growth, cancer cells require higher amounts of specific nutrients compared to healthy cells [128]. These high-nutrient demands change the metabolic landscape in the TME, and result in areas deprived of certain nutrients, including glucose and amino acids [129]. A nutrient-restricted TME impairs T cell differentiation and function while promoting the expansion and potentiating the suppressive capacity of immunosuppressive immune cells [130].
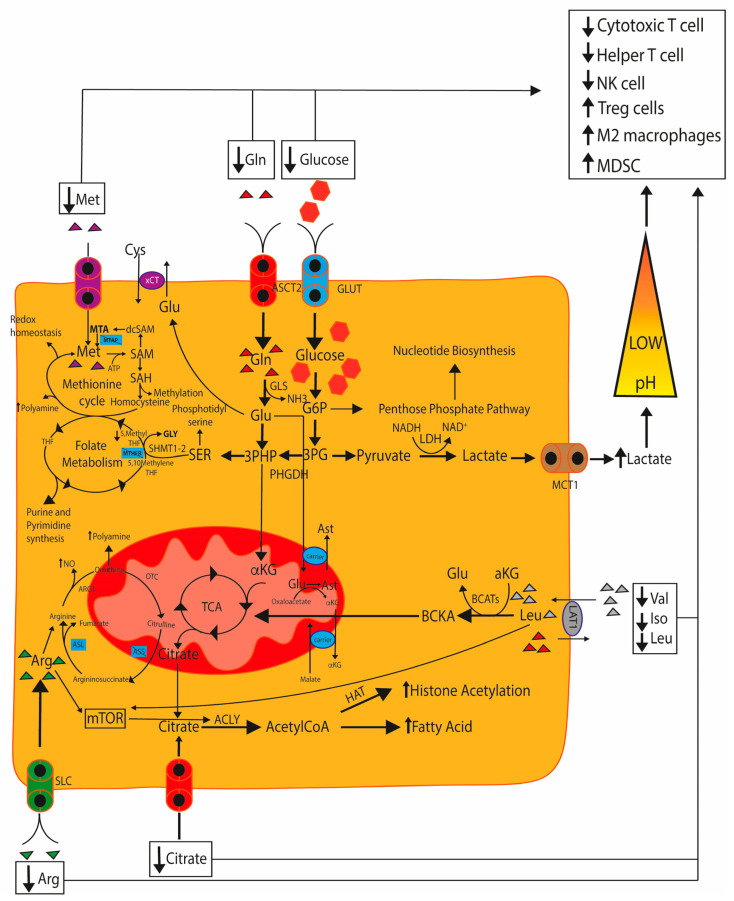
Most cancers prefer aerobic glycolysis over oxidative phosphorylation (OXPHOS) because even though it is a low-efficiency process for energy generation, it produces large amounts of glycolytic intermediates essential for the biosynthesis of biomass molecules and molecules involved in the cell’s redox state control [131,132].
Furthermore, aerobic glycolysis supports the biosynthesis of proteins, heme, and nucleotides by promoting the synthesis of serine from glucose through the activity of phosphoglycerate dehydrogenase (PHGDH) [133,134]. At the same time, PHDC, by generating 3-phosphohydroxypyruvate (3PHP), stimulates the conversion of glutamate to α-ketoglutarate (a-KG), which supplies the dietary tricarboxylic acid (TCA) cycle and allows pyruvate-derived citrate to be shuttled from the mitochondria to the cytosol where it is converted in oxaloacetate and acetyl-coA by ATP citrate lyase (ACLY) [135]. In the cytosol, acetyl-coA is mainly used for fatty acid synthesis [136,137].
The high metabolic demand of tumor cells reduces citrate concentrations in tumor cells, TME, blood, and urine of patients with multiple tumors [138].
High glucose consumption in the tumor leads to lactate secretion as pyruvate produced by aerobic glycolysis is converted to lactic acid by lactate dehydrogenase in order to sustain glycolysis through NAD+ regeneration [139]. Lactate accumulation in TME and lactate dehydrogenase (LDH) expression are associated with poor prognosis and survival in patients with various cancers, including breast cancer, gastrointestinal cancer, urogenital cancer, lung cancer, sarcoma, melanoma, myeloid malignancies, lymphoid malignancies, and other types of cancer [140]. High lactate concentrations cause acidification of TME leading to TIL depletion, infiltration of tolerogenic cells such as MDSCs, and, in turn, immune evasion and tumor progression [140,141].
After glucose, glutamine is the main source of energy for several types of cancers, so much so that some cancers are considered glutamine dependent. Glutaminase (GLS) catalyzes the breakdown of glutamine into ammonia and glutamate. Ammonia is utilized for the synthesis of purines and pyrimidines [142,143], whereas glutamate contributes to the production of ATP by entering the TCA cycle as α-ketoacids, to the synthesis of biomass in particular of lipids and other non-essential amino acids, and to the production of glutathione, an antioxidant essential for redox homeostasis [144,145].
Finally, glutamate contributes to aspartate synthesis, which is crucial for cancer cell proliferation, specifically for those with a severely dysfunctional electron transport chain (ETC), as it provides electrons for oxidative phosphorylation (OXPHOS) via the malate-aspartate shuttle (MAS) [146,147].
The dependence of tumors on glutamine also depends on the concentration of cystine. Cystine is essential for glutathione synthesis and thus redox homeostasis and is transported into the cell via the cystine–glutamate antiporter xCT/SLC7A11, which exchanges an extracellular cystine molecule for an intracellular glutamate molecule. This exchange causes a depletion of glutamate, which is produced through the glutaminolysis of glutamine [148].
Most cancer cells depend on methionine, an essential amino acid involved in glutathione and protein synthesis and DNA methylation. Methylthioadenosine phosphorylase (MTAP) [149] and methylenetetrahydrofolate reductase (MTHFR) defects result in methylthioadenosine accumulation, methyltetrahydrofolate deficiency, and impairment of the methionine salvage pathway, essential for the regeneration of methionine from methylthioadenosine (MTA) and for the production of polyamines critical for cell proliferation [150,151]. Deficiency of the cofactors cobalamin and folate, crucial for the production of tetrahydrofolate and the replacement of cysteine by homocysteine, not convertible in methionine due to defects in cysteine uptake, contribute to making tumor cells dependent on methionine [152,153].
Cancer cells are addicted to external arginine, as they are unable to synthesize it because they do not express arginine synthase 1 (ASS1), which, in combination with argininosuccinate lyase (ASL), synthesizes arginine in normal cells from citrulline and aspartate. In order to meet the high arginine demand, cancer cells overexpress several arginine transporters such as SLC6A14, SLC7A3, and SLC7A9.
Arginine is a non-essential amino acid involved in the synthesis of nitric oxide (NO) and polyamines [154,155], mTOR activation, and epigenetic remodeling. NO plays a pro-tumorigenic role by promoting angiogenesis, metastasis, and inhibiting apoptosis [156]. Arginine-activated mTOR is implicated in tumor cell growth and proliferation but also epigenetic reprogramming [157]; mTOR promotes acetyl-CoA synthesis through the activation of ATP citrate synthase (ACLY) and Acyl-coA synthetase short-chain family member 1–2 (ACSS 1–2) [158]. Increased acetyl-CoA promotes histone acetylation via the activation of histone acetyltransferases (HATs) [159].
Serine and glycine play important roles in tumor growth as they are involved in nucleotide synthesis and DNA methylation. Cancer cells can meet their need for serine by absorbing it from the extracellular matrix or synthesizing it de novo from glucose via the de novo serine synthesis (SSP) pathway [160]. De novo serine produced via the SSP pathway promotes and sustains the activation of glycolysis by binding to pyruvate kinase M2 (PKM2), the last rate-limiting enzyme of glycolysis [161,162]. The conversion of serine to glycine by serine hydroxymethyltransferase (SHMT1-2) generates 5,10-methylene-THF essential for purine and pyrimidine synthesis [163,164]. Furthermore, serine and glycine influence DNA/RNA methylation and de novo ATP synthesis in cancer cells through the one carbon pathway [165].
Leucine, isoleucine, and valine belong to the branched chain amino acids (BCAA) and are essential amino acids obtained only via food intake [166]. BCAA are transported via lat1(SLC7A5), a transporter that imports several essential amino acids (e.g., phenylalanine, leucine, isoleucine, tryptophan, histidine, and tyrosine) in exchange for intracellular histidine, tyrosine, and glutamines [167,168]. Lat1 is highly expressed in cancer cells as its transcription is regulated by oncogenes such as c-Myc [169], HIF2a [170], and NOTCH [171]. BCAAs are catabolized into glutamate and branched-chain α-ketoacids (BCKA), which are converted to TCA intermediates and provide acetyl-CoA and/or succinyl-CoA essential for energy production [172] or acetylation modification [173].
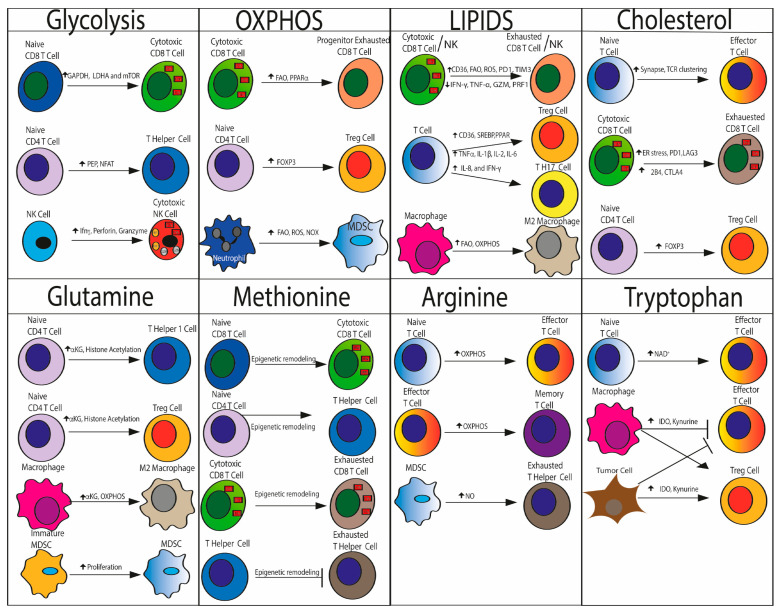
4. TME Metabolism
The different cell lines that make up TME have distinct metabolisms that may depend on cellular activation status and the availability of specific nutrients in the microenvironment. Highly proliferative CD4- and CD8-activated T cells, activated B cells, γδIFNγ cells, cancer cells, activated DCs, M1-like TAMs, and intratumoral myeloid cells rely heavily on glucose and glutaminolytic metabolism, preferentially using aerobic glycolysis over TCA-coupled OXPHOS for ATP production [174,175,176].
Glycolysis supports CD8 T cell and B cells’ effector function and interferon-gamma (IFNγ) production via glyceraldehyde 3-phosphate dehydrogenase (GAPDH), LDHA, and mTOR regulation [177,178,179,180,181], whereas glucose promotes CD4 activation and their antitumor activity by sustaining the production of the glycolytic intermediate phosphoenolpyruvate (PEP) which restores Ca2+—the nuclear factor of activated T cells (NFAT) signaling [138].
Since different cell lines require glucose to support their function, high glucose consumption can lead to glucose depletion in the TME. Glucose deficiency may induce a metabolic shift towards FA uptake and OXPHOS metabolism in CD8 T cells via PPAR-α signaling pathway activation [182] but may also make them metabolically inactive and exhausted [183]. In addition, glucose deprivation promotes differentiation of effector T cells into Treg cells by inducing Foxp3 expression and thus contributes to the establishment of an immunosuppressive environment [184].
Tumor-infiltrating MDSCs, unlike peripheral MDSCs that rely on glycolysis, exhibit an increased FAO and OXPHOS metabolism [185,186]. Under these TME conditions, neutrophils support the production of ROS by NADPH oxidase (NOX), which potently suppresses innate and adaptive immunity, adopting a metabolism based on mitochondrial oxidative metabolism and fatty acid oxidation (FAO) [187]. The cytotoxic activity of NK cells also depends on glucose metabolism; thus, the lack of glucose impairs the production and release of Ifng and cytotoxic perforins and granzymes [188] (Figure 3).
Figure 3.
Metabolic changes in TME regulates adaptive and innate immune system function. Glycolysis supports naïve CD8 and CD4 T cells and NK cell activation. Progenitor exhausted T cells, Tregs and MDDSC rely on oxidative phosphorylation (OXPHOS) and fatty acid oxidation (FAO) Increased concentration of FFAs within TME leads to CD8 T cell and NK exhaustion, renders T cell exhausted and anergic, and promotes the differentiation of CD4 T cells into Tregs and Th17 and skews macrophage polarization toward protumoral M2-like TAMs which mainly depend on FAO and OXPHOS for their metabolism and bioenergetic demands. Glutamine metabolism promotes CD4 T cell differentiation towards Th1 and Treg cells, TAM polarization towards the protumor M2 phenotype and MDSC expansion. Methionine metabolism regulate T cell activation and exhaustion by remodeling epigenetic landscape. Arginine enhances T cells survival and supports effector and memory T cells generation by modulates OXPHOS activity, whereas production of nitric oxide from arginine catabolism by MDSC-expressed arginase leads to Thelper cell dysfunction and anergy. Tryptophane is required for T lymphocyte effector functions as promotes NAD+ synthesis. Conversion of tryptophan to kynurenine via IDO1, expressed by tumors and macrophages, promotes Treg expansion and renders effector T cells exhausted. Cholesterol improves cytotoxic function by enhancing immunological synapse formation and maturation, and promoting TCR clustering and signaling. Instead, high cholesterol content in TME hampers T cell function as induces ER stress and expression of exhaustion markers, such as PD-1, LAG-3, TIM-3, 2B4 and CTLA-4. Finally cholesterol induces FOXP3 expression and promotes Treg differentiation. LDHA: lactate dehydrogenase A; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; PEP: phosphoenolpyruvate; NFAT-1: nuclear factor of activated T cells; lfnγ: interferon-γ; PRF1: perforin; GZM: granzyme B; α-KG: α-ketoglutarate; NO: nitric oxide; OXPHOS: oxidative phosphorylation; FAO: fatty acid oxidation; ROS: reactive oxygen species; NOX: NADPH oxidase isoforms.
High glucose consumption by myeloid cells, T cells, B cells, and tumor cells leads to an elevated lactic acid release which affects the function of various immune cells and reduce T cells antitumor efficacy [189,190].
Lactate accumulation reduces effector T cell proliferation and cytotoxicity by reducing IL-2 and IFN-γ production and lytic granule exocytosis through impairment of TCR-triggered phosphorylation of JNK, C-Jun, and p38, and inhibition of STAT5 and ERK activation and mTOR inhibition [141,191].
Low pH in TME, due to lactic acid release, inhibits monocyte and NK cell activation, dendritic cell differentiation, and promotes Th17 cell differentiation [192], MDSCs accumulation, and macrophages M2 polarization through arginase I [193,194,195,196]. Increased arginase I expression in macrophages due to acid lactic accumulation in TME leads to depletion of arginine, an essential amino acid for effector T cells activation and proliferation [197] (Figure 4).
Figure 4.
Cancer metabolism promotes immunosuppressive TME. Cancer cells mainly rely on glycolysis as it supports the biosynthesis of nucleotide, via penthose phosphate pathway and folate metabolism cycle, and molecules involved in controlling the redox state of the cell, via methionine cycle. LDH: lactate dehydrogenase; 3PG: 3-phosphoglyceric acid; 3PHP: 3-phospho-hydroxypyruvate; Glu: glutamate; α-KG: α-ketoglutarate; TCA: tricarboxylic acid cycle; ACLY: ATP citrate lyase; OXPHOS: oxidative phosphorylation; MAS: malate-aspartate shuttle; xCT: Cystine/Glutamate Antiporter; MTAP: methylthioadenosine phosphorylase; MTHFR: methylenetetrahydrofolate reductase; ASS1: arginine synthase 1; ASL: argininosuccinate lyase; Arg: arginine; SLC6A14, SLC7A3, SLC7A9: arginine transporters; NO: nitric oxide; ARG1: arginase 1; Lat1: branched amino acids transporter; BCKA: branched-chain α-ketoacids.
Finally, lactate promotes angiogenesis and immune evasion through the activation of G protein coupled receptor (GPR81) on immune cells and endothelial cells [198].
The reduction of citrate in TME due to high consumption by tumor cells influences the function and regulation of immune cells. The conversion of citrate to acetyl-CoA by ATP citrate lyase (ACLY) is essential for histone acetylation and thus for epigenetic reprogramming. Thus, citrate depletion affects epigenetic reprogramming, thereby impairing memory CD8+ T cell recall responses and CD4+ helper T cell (Th1 cell) proliferation and activation, while promoting Th17 viability at the expense of the Tregs [199,200] (Figure 4).
Since glucose is scarce in the tumor microenvironment, glutamine can become the primary energy source. Glutamine metabolism supports T and B cell activation and promotes CD4 T cell differentiation towards Th1 and Treg cells through the production of α-ketoglutarate essential for histone acetylation and epigenetic remodeling [201,202]. In addition, glutamine-derived α-ketoglutarate promotes MDSC expansion and TAM polarization towards the protumor M2 phenotype [203].
However, inhibition of glutamine metabolism promotes the immune response of effector T cells by switching metabolism towards glycolysis, slowing tumor growth by reducing both glycolysis and oxidative phosphorylation and increasing anti-tumor M1 polarized macrophages [204] (Figure 3)
Methionine metabolism is also critical for T cell function and differentiation as it regulates the epigenetic landscape through histone and DNA methylation [205]. Lack of methionine and S-adenosylmethionine due to the high consumption by cancer cells leads to CD4 and CD8 T cells dysfunction and exhaustion, inhibition of Th17 proliferation, and cytokine production [205,206].
Arginine is an amino acid valuable for the CD8+ T cells survival, antitumor response, and for the formation of memory T cells [207]. However, the high uptake of arginine by tumor cells, the catabolism of arginine by MDSC-expressed arginase [208,209], and the conversion of arginine to nitric oxide by macrophage-expressed nitric oxide synthetase [195,210] result in arginine depletion in the TME leading to T cell dysfunction and anergy.
Tryptophan and its metabolism also play an important role in generating a pro- or anti-tumorigenic microenvironment. Expression of IDO1 by tumor, Bregs, and TAM cells promotes the conversion of tryptophan to kynurenine, thus resulting in tryptophan deficiency in TME. Tryptophan is essential for T cell function, while kynurenine inhibits CD8 effector T cells by blocking mTOR signaling pathway and promoting Treg activation, MDSC expansion, and TAM M2 polarization [125,211,212]. Thus, tryptophan depletion and elevated kynurenine repress T cell responses and promote immune evasion [213].
At low concentrations, lipids have a beneficial effect on T cell proliferation and activation, while at high concentrations in TME, they promote immunosuppression and T lymphocyte apoptosis [214]. In a hypoglycemic environment, CD8 T cells adapt their metabolism by increasing the expression of genes such as PPAR-a and CD36 involved in fatty acid beta oxidation and uptake [203].
However, the accumulation of fatty acids increases oxidative phosphorylation metabolism in CD8 T cells and reduces the expression of very long chain acyl-CoA dehydrogenase (ACADVL), the first enzyme of fatty acid beta oxidation, which determines the accumulation of very long fatty acid, which leads to mitochondrial dysfunction, high reactive oxygen species (ROS) production, metabolic exhaustion, apoptosis, and ferroptosis [215,216,217]. FA uptake by CD36 induces PD-1 and TIM-3 expression, reduces IFN-γ and TNF-α production, and leads to CD8 terminal exhaustion [218].
In addition, fatty acids stimulate T lymphocytes to secrete pro-inflammatory cytokines, such as TNFα, IL-1β, IL-2, IL-6, IL-8, and IFN-γ, and promotes Treg differentiation, the generation of immunosuppressive and protumor TME [219,220]. Treg and Th17 cells, major immunosuppressive population, rely on FAO and OXPHOS metabolism to support their survival and immunosuppressive function [221]. To meet their high need for fatty acids, Tregs increase the expression of CD36 and SREBP, the major regulatory gene of de novo cholesterol and fatty acid synthesis, and induce PPAR-dependent lipid metabolism [13,222]. FA enrichment in TME leads TAM to shift their metabolism toward FAO and OXPHOS and promotes TAM polarization to immunosuppressive M2 phenotype [223]. FA affect NK cytotoxic activity, as high FA levels inhibit the protein complex mTORC1, essential for IFN-γ and granzyme B production [223].
In the breast, melanoma, colorectal tumor microenvironment dendritic cells, accumulate fatty acids because they overexpress the scavenger receptors (SR) involved in the intracellular transport of lipids. High levels of fatty acids impair the DC capacity to process and present antigen and their ability to stimulate and activate T cells [224].
Cholesterol is essential for T lymphocyte function as it enhances immunological synapse formation and maturation, promotes TCR clustering and signaling, and thus improves cytotoxic function [184,225]. However, high cholesterol content in TME impairs T cell function as it impairs lipid metabolism and induces ER stress leading to increased expression of exhaustion markers, such as PD-1, LAG-3, TIM-3, 2B4 and CTLA-4, on CD8+ T cells [226]. The accumulation of cholesterol esters, transported by CD36, leads to ferroptosis of CD8 T cells. Cholesterol oxidation by CYP27A1 generates oxysterol, which promotes tumor cell proliferation and the recruitment of immunosuppressive cells such as neutrophils and IL17-expressing γδ cells [226].
Finally, cholesterol induces FOXP3 expression and promotes Treg differentiation, thus supporting the generation of immunosuppressive TME [227] (Figure 3).
5. Fasting, Caloric Restriction, Metabolite Restriction, and Fasting Mimicking Diet (FMD)
Calorie restriction is a diet that involves a daily reduction (10–30%) of caloric intake for an extended period of time. Fasting, on the other hand, consists in complete abstinence from food, but not from water, for a limited period (from 16 h up to a few days) and is not easily applicable in the clinic as it is very restrictive and poorly tolerated by patients. For this reason, a new dietary intervention called “fasting-mimicking diet” has been developed, a 5-day food program which consists of a 50% reduction in calorie intake on the first day and a 90% reduction in the following 4 days. Unlike calorie restriction and fasting, FMD is easily tolerated by patients as it alternates a short period of severe calorie restriction with a normal calorie intake diet. The amino acids or metabolites restricted diet provides a normal caloric intake but lacks specific amino acids or metabolites essential for the regulation of signaling pathways, often altered in tumor cells. However, even these diets could have side effects such as causing serious weight loss in patients as is found in diets lacking essential amino acids.
6. Effects of Fasting and Metabolite Restriction on Cancer Cells
Food restriction induces systemic and local metabolic changes through the modulation of hormone and nutrient sensing pathways. The reduction of circulating glucose upon dietary restrictions leads initially to glycogenolysis in order to support gluconeogenesis; then, when glycogen storage is depleted, it leads to lipolysis and the release of glycerol and fatty acids essential for the production of ketone bodies, the main energy sources in the absence of external nutrients [8,228]. The organism responds to the macronutrients and particularly amino acid limitation by inhibiting the anabolic pathways through the downregulation of growth hormone (GH) and insulin growth factor (IGF1). The low level of IGF 1 and of amino acid and increased AMP/ATP ratio lead to the inhibition of PI3K and mTOR pathway and to the activation of autophagy, a process that provides energy and substrates by removing the damaged and redundant self-components [229,230].
In normal cells, the effects of CR on the reduction of growth factors is also associated with the upregulation of anti-stress response (NRF2 and antioxidant genes), which can include DNA repair and cause reduced inflammation trough the release of corticosteroids, ghrelin, and adiponectin and the inhibition of pro-inflammatory cytokine secretion [231,232].
The inhibition of the nutrient-sensing pathway (IGF1-PI3K-mTOR), the activation of the antistress response pathway, and the upregulation of genes involved in DNA repair induced by caloric restriction/fasting prompt normal but not cancer cells to slow down their proliferation and enter a quiescent state, an effect called “differential stress resistance” which protects normal but not malignant cells from the cytotoxicity of anti-tumor drugs. However, tumor cells that possess mutations in oncogenes (IGF-1R, Ras, AKT, and mTOR pathways) and tumor suppressor genes (p53, p16, and Rb) are not only insensitive to lack of nutrients and low levels of anabolic hormones and do not become protected during fasting condition, their uncontrolled proliferation and increased requirement for many metabolites can sensitizes them to anticancer therapy. This mechanism induced by fasting in tumor cells is termed differential stress sensitization, since only cancer cells (breast, glioma, neuroblastoma, melanoma) and not normal cells are sensitized to a wide variety of therapies ranging from chemotherapy to radiotherapy to hormone therapy (DSS) [233,234,235,236].
A key effect of fasting is the reduction of carbohydrates, which are the main energy source for many types of cancers. The scarcity of carbohydrates has a particular impact on the metabolism of tumor cells, which, prior to the restriction, adopt an increased glycolytic mode, called the Warburg effect, and which, in response to glucose restriction, attempt to shift from glycolysis to oxidative phosphorylation to generate more energy but also as the fatty acids and ketone bodies generated by lipolysis become a fuel source. However, glycolysis-dependent tumor cells (ovarian, breast, thyroid cancer) are unable to effectively adapt their metabolism to OXPHOS, so this change induces a slowdown in proliferation and an increase in oxidative stress and apoptosis [237,238,239].
The energy deficit induced by caloric restriction/fasting leads to an increase in NAD+, which activates histone deacetylase sirtuins. Sirtuins reshape the epigenetic landscape, causing an inhibition of glycolysis and thus reinforcing the inhibitory effect of calorie restriction on tumor glucose metabolism [240,241,242].
Cycles of fasting or fasting-mimicking diets (FMD), developed to simulate the effects of fasting and lasting 2–5 days, also sensitizes glycolysis-dependent cancer cells (TNBC) to glucose analogues (2DG) and affects cancer stem cell self-renewal [243]. Furthermore, the reduction of glucose and circulating IGF enhance the efficacy of cycline kinase inhibitors/hormone therapy combination and PI3 kinase inhibitors against ER-positive and triple-negative tumors, respectively, preventing drug resistance in ER+ breast cancers [244] and completely eradicating triple-negative breast tumors [243].
Fasting cycles could affect tumor growth by modulating the levels of essential (leucine, lysine, and methionine) and non-essential (serine, glycine, glutamine, and asparagine) amino acids [245].
For example, serine deprivation activates the serine synthesis pathway and inhibits glycolysis by promoting OXPHOS [165,246]. This metabolic switch increases ROS production, thus sensitizing colorectal tumors, lacking p53, to agents that increase ROS [247]. Fasting/FMD also alters the iron metabolism in KRAS-mutated tumor (pancreas, lung, colorectal) cells by reducing the levels of heme oxygenase and ferritin, thus increasing sensitization to high doses of vitamin C, which acts as a pro-oxidant agent [248].
Methionine restriction also inhibits tumor growth and increases the efficacy of chemotherapies [249,250] and reverses the drug resistance of RAS-driven colorectal cancer as it affects nucleotide metabolism and glutathione synthesis and reactivates the expression of silent tumor suppressor genes by reshaping the epigenetic and DNA methylation landscape [251].
Several cancer cells are glutamine-addicted because glutamine plays an essential role in cell metabolism as it supplements tricarboxylic acid (TCA) cycle and participates in the biosynthesis of nucleotides, glutathione (GSH), fatty acid, and other nonessential amino acids (alanine, proline, aspartate, asparagine, and arginine) [252]. In addition, glutamine support the production of α-KG, which is a cofactor for Jumonji C domain-containing histone demethylases (JmjC), and ten-eleven translocation (TET) family DNA demethylases [253,254]. Therefore, glutamine deprivation affects cancer growth by impairing the energy and nucleotide metabolism and redox homeostasis [255]. However, glutamine depletion leads to histone and DNA hypermethylation through the inhibition of JmjC and TET. These epigenetic changes promote cancer cells dedifferentiation and drug resistance [256].
Fasting mobilizes lipids from adipose tissue as beta-oxidation of fatty acids becomes the main energy source in glucose deficiency. Therefore, the high consumption of lipids at the cellular level allows for a reduction in plasma lipid levels [8]. This metabolic change affects the metabolism of cancer cells and their ability to adapt. In fact, tumor cells dependent on glycolysis can be more affected by this metabolic change as they are not able to effectively process the fatty acids that accumulate in the lipid droplets [257]. Furthermore, CR reduces the activity of the enzyme stearoyl-CoA desaturase (SCD), essential for maintaining the fluidity of the cytoplasmic membrane through the production of monounsaturated fatty acids (MUFA) [238].
On the contrary, the growth of tumor lines, whose metabolism relies on OXPHOS, is less affected by the effects of caloric restriction and fasting, as they support their growth through the beta oxidation of fatty acids, which are reduced in the tumor microenvironment [257].
Calorie restriction and fasting may exert an antitumor action through downregulation of the satiety hormone leptin and upregulation of adiponectin. Fasting blocks the progression of acute lymphocytic leukemia (ALL) through upregulation of the leptin receptor (LEPR). LEPR expression slows cell proliferation and promotes ALL differentiation by upregulating XBP1, involved in the unfolded protein response (UPR) pathway, and PRDM1, the tumor suppressor gene involved in T and B cell differentiation [258]. Calorie restriction has been shown to prevent the risk of radiation-induced myeloid leukemia in mice if performed both before and after irradiation [259]; however, it has no effect on multiple myeloma progression, although it is able to remodel the microenvironment of the bone marrow [260]. Therefore, the antitumor effects of CR and FMD are cancer specific and dependent on the genotypic and phenotypic characteristics of the tumors.
7. Effects of Fasting and Caloric Restriction on TME
Although the reduction of glucose and essential and non-essential amino acids has a detrimental effect on tumor growth, such restrictions also negatively impact the function of the immune system. Glucose reduction suppresses proliferation and activation of tumor infiltrating effector immune cells while promoting the formation of long term memory CD8+ T cells [261,262]. At the same time, glycine and serine restriction inhibit CD8 T cell activation [263], while low methionine levels impair CD4 T cell activation by reducing histone methylation at the levels of genes involved in T cell proliferation and activation [264].
Although calorie restriction/fasting reduces glucose and amino acids levels, such dietary interventions have been shown to have beneficial effects on the antitumor response by increasing the percentage of CD8+ cytotoxic T cells, memory T cells, and stem cell-like memory T cells and repressing Treg through the reduction of IGF1 levels, epigenetic reprogramming, and the production of ketone bodies [257,265,266,267,268,269,270,271].
More recently, fasting/FMD cycles were shown to promote the immune cell-dependent attack of different types of cancer cells (melanoma, breast, gastric cancers) [256,257,269,272,273].
This was attributed to the ability of fasting/FMD to reduce the Treg population and boost CD8 infiltration by downregulating the expression of heme oxygenase-1 (HO-1) and IGF-1 and by inducing autophagy in melanoma and breast cancer [271,272]. FMD potentiates the cytotoxic effect of chemotherapy and increases the immune infiltrate in breast and melanoma cancer by reducing tumor HO-1 expression, a potent immunomodulator involved in suppressing CD8+ T-cells infiltration and cytotoxic activity and promoting Treg accumulation [272]. Fasting sensitizes low immunogenic non-small-cell lung cancer lung tumors to anti-PD1 immunotherapy and even leads to complete tumor remission in preclinical studies by reversing or neutralizing the immune evasion mechanism through IGF-I signaling pathway downregulation [271].
Since IGF-1 cooperates with the receptor for advanced glycation end products (RAGE) in inducing metabolic-dependent inflammatory responses, and since RAGE activation amplifies IGF-1 signaling, CR and FMD could improve the antitumor immune response by modulating the RAGE signaling pathway. RAGE activation by advanced glycation end-products and 25 other ligands induces transcription of inflammatory cytokines and chemokines that contribute chronic inflammation in the tumor microenvironment promoting MDSC recruitment, TAM polarization from the proinflammatory M1 to the anti-inflammatory M2 phenotype, DC dysfunction, and T cell exhaustion. Furthermore, RAGE triggers the activation of the NLRP3 inflammasomes pathway, which favors immunosuppression by promoting the genesis and recruitment of MDSC or by inducing the differentiation of TAMs into a tolerogenic phenotype [274]. CR and FMD suppress inflammation and reduce oxidative damage in humans and in preclinical mouse models [232,275]. Furthermore, we show that FMD reduces the levels of inflammatory markers such as the NLRP3 inflammasome, leukotriene in the heart of mice bearing melanoma and lung cancer and treated with immune checkpoint inhibitors [205,276].
Fasting improves chemotherapy efficacy against fibrosarcomas, mammary carcinomas, and non-small cell lung cancers by boosting the T-cell-mediated immune response, which leads to Treg depletion through enhanced ATP release due to fasting-induced autophagy in the tumor [273].
The effects of calorie restriction and diet on immune system activation could also be mediated through the reduction of high mobility group box protein 1 (HMGB1) [277].
HMGB1 is a multifunctional redox sensitive protein secreted by innate immune system cells (macrophages and NK) in TME under stress or inflammatory conditions. Extracellular HMG1 can play both protumor and antitumor roles. Reduced HMGB1 promotes immune cell activation and chemotaxis by stimulating the synthesis and release of proinflammatory cytokines, such as TNFα and IL1,6, through activation of the receptor for advanced glycation end products (RAGE) and/or Toll-like receptor (TLR) signals 2, 4 [278]. On the other hand, oxidized HMGB1, mostly present in the interstitial fluid of TME, inhibits DC activation and renders them tolerogenic via RAGE activation [279].
In addition to modulating the immune response, HMGB1 inhibits tumor growth and promotes the death of some tumor forms (as colorectal cancer) by inducing a metabolic shift towards anaerobic glycolysis through the inhibition of PKM2, an enzyme involved in the conversion of phosphoenolpyruvate into pyruvate, essential for the fuel of TCA and oxidative phosphorylation [280].
It has recently been shown that inhibition of extracellular HMGB1 blocks the growth of breast tumors through activation of the adaptive immune system and improves efficacy of immune checkpoint therapy (PD-1) by reducing the percentage of Tregs and increasing the percentage of M1 macrophages and activated DCs [281].
Recently it is emerging that the effects of fasting mimicking diet and caloric restriction on the activation of the antitumor immune response could be mediated by the intestinal microbiota remodeling [282]. Several studies have shown that the efficacy of chemotherapy and immunotherapy depend on the abundance and presence of bacterial species in the intestinal microbiota capable of secreting short-chain fatty acids (SCFA: acetic acid, propionic acid, butyric acid) [283,284]. The release of butyric acid inhibits histone deacetylases (HDACs), prevents CD8 exhaustion, and promotes effector CD8 activation by increasing IL12Rb expression through upregulation of the transcriptional regulator ID2 [285]. Calorie restriction exerts the antitumor response against colon and breast cancer by promoting CD8 activation through the enrichment of the microbiota with SCFA producing microbial families (butyric acid and acetic acid) [282].
At the same time, the composition of the microbiota could also influence the activation of the immune system directly through the production of serotonin [286] and indirectly through the release of SCFA, which promotes the transcription of tryptophan hydroxylase 1 (TPH1), the rate-limiting enzyme of the serotonin biosynthetic pathway, and colon serotonin production by enterochromaffin cells [287]. Serotonin is synthesized from tryptophan and, in addition to its crucial role as a mediator between the gut and the brain, plays an important role in regulating the immune system and tumor progression.
In fact, serotonin mitogenic property favors the progression of cancer [288]. On the other hand, serotonin signaling stimulates T cell activation and proliferation, promotes DC maturation, supports B cell development, enhances NK cell cytotoxicity, and stimulates macrophage polarization towards the M2 phenotype, while inhibiting M1 macrophage polarization [289,290]. However, the conversion of tryptophan to serotonin or kynurenine leads to tryptophan depletion in TME and thus to T-cell exhaustion and immune evasion. Although serotonin plays contradictory roles in regulating the functions of different immune cells, serotonin may have a pro-tumorigenic effect and enhance tumor immune evasion by generating an anti-inflammatory microenvironment.
Furthermore fasting/FMD cycles reverse chemotherapy-induced immunosuppression by promoting hematopoietic stem cell self-renewal (HSC) [291] and enhances cancer immune surveillance by improving memory T cell survival [292]. Indeed, fasting/calorie restriction boosts secondary immune response as it promotes memory T cell migration into the bone marrow where they meet a favorable microenvironment favorable to their survival and protection against chemotherapy [10,291]. Furthermore, T cells subjected to fasting acquire stem features as the lack of nutrients leads to an increase in autophagy and mitochondrial metabolism which causes a reduction in metabolic cofactors, such as acetyl coenzyme A, essential for epigenetic remodeling and cell differentiation. At the same time, the reduction of methionine and its intermediates affects histone methylation and compromises the activation of signaling pathways involved in stemness suppression [293].
Fasting/FMD cycles promote the selective expansion of early/progenitor exhausted effector T cells at the expense of late exhausted effector T cells in breast cancer [257]. Early exhausted effector T cells represent a subpopulation of exhausted/dysfunctional T cells, which correlate with the success of immune checkpoint inhibition and increased patient survival [294,295]. This subpopulation expresses dysfunctional markers such as TOX and PD1, but also exhibit self-renewing capacity and features of memory T cells, associated with the expression of transcriptional regulator T cell factor 1 (TCF1) [296,297,298]. Immune checkpoint blockade reverses immunosuppression and boosts immune response by increasing early exhausted effector T cell proliferation and differentiation in effector T cells, essential for long-term maintenance of persistent T cell responses. mTOR inhibition expands early exhausted effector T cells ex vivo and enhances long term T cell response and the efficacy of immune checkpoint inhibition [299]. Therefore, the frequency of early exhausted T cells is considered to be a predictive biomarker for favorable clinical outcome of checkpoint therapy.
Early exhausted effector T cells display higher mitochondrial mass and better mitochondrial fitness compared with late exhausted effector T cells. These mitochondrial changes are associated with high OXPHOS capacity, essential for supporting the early exhausted effector T cells’ self-renewal [299]. Fasting/FMD promotes the selective expansion of early exhausted effector T cells at the expense of late exhausted effector T cells in the breast cancer model by shifting the metabolism from glycolysis to OXPHOS and increasing b-oxidation of fatty acids [257].
Fasting favors the accumulation and activation of gd T cells in breast tissue and strengthens the anti-tumor immune response of cytotoxic CD8 T cells by producing ketone bodies and inhibiting mTOR activity. γδ T cells, particularly the Vd1+ subtype, can orchestrate an effective anti-tumor response, as supported by the positive correlation of their presence with better prognosis in TNBC patients [300]. Notably, ketone bodies produced by a ketogenic diet have been shown to promote the expansion and activation of gd T cells in the visceral mass [301], while the mTOR inhibition impairs the development of ab T cells but promotes gd T cell generation in the thymus [243,302].
Short-term fasting attenuates monocyte metabolic and inflammatory activity and inhibits monocyte mobilization from the bone marrow through suppression of systemic CCL2 production, leading to a drastic reduction of circulating monocytes that could affect infiltration and the composition of the tumor microenvironment [303]. Furthermore, fasting/FMD cycles reduce the accumulation of immunosuppressive polymorphonuclear (PMN) MDSC in TME, thereby enhancing the response to immunotherapy in breast cancer [257]. Fasting could enhance the efficacy of immune-checkpoint blockade through the production of ketone body, 3-hydroxybutyrate, as demonstrated for ketogenic diet, by preventing the upregulation of PD-L1 on myeloid cells and by expanding CXCR3+ T cells [270].
Fasting/FMD cycles also could enhance the immune response of anticancer therapies by promoting tumor vessel normalization and a reduction in size, number, and density by repressing the secretion of pro-angiogenic factors such as VEGF, factor VIII, inter- leukin-6 [IL-6], TNF-α, and plasminogen activator inhibitor-1 [PAI- 1] in breast cancer [257,265,266,304]. In addition, CR and fasting reduces fibroblast collagen deposition in the TME and cancer fibrosis by inhibiting, respectively, mTOR and TGF-β signaling, which promotes the conversion of fibroblast in cancer associated fibroblast (CAF) [305,306,307,308].
The less dense and viscous stroma, generated by CR and fasting, reduces the secretion of pro-inflammatory and pro-fibrotic cytokines, allows the infiltration of immune cells into the TME, and consequently enhances the diffusion of the anticancer therapy, thus enhancing the efficacy of anticancer treatment in breast cancer model [257,309].
8. Conclusions
Tumor cells alter the tumor microenvironment and help to create an immunosuppressive TME by restricting nutrients availability and by releasing metabolites, chemokines, and growth factors that reprogram the normal cell function and metabolism in TME, promote the recruitment of immunosuppressive cells and their differentiation in immune cell subtypes with immunosuppressive activity, and inhibit the activation of cytotoxic T lymphocytes and NK cells.
To meet metabolic demands and sustain a continuous, high proliferation rate, cancer cells rely mainly on glycolysis and glutamine catabolism as they generate intermediate metabolites that supply the amino acids, lipids, and nucleotides synthesis pathway and contribute to the synthesis of molecules involved in redox homeostasis maintenance. High tumor metabolic rate leads to glucose, glutamine, and amino acids (methionine, arginine, tryptophane, leucine, serine) depletion and the secretion of immunosuppressant lactate in TME. To support proliferation and effector response, activated T and NK cells need environmental nutrients, including glucose and amino acids, essential to promote PI3K/AKT/mTOR anabolic pathway activation. Therefore, the scarcity of nutrients, high lactate concentration, and low pH in TME lead to cytotoxic T and NK cells exhaustion and promote the proliferation of immune cells with immunosuppressive activity as their metabolism is more suited to this environment.
At the same time, tumor cells release chemokines and growth factors that promote tumor angiogenesis, the recruitment of immunosuppressive cells, such as MDSCs and M2 macrophages, and collagen deposition via fibroblast, making the extracellular matrix dense and rigid and further impeding T lymphocyte infiltration. Therefore, TME becomes a complex milieu of immune and normal cells and acellular components which support angiogenesis, tumor progression, and immune evasion.
CR, fasting, and FMD may reverse the immunosuppressive TME by affecting the cancer and immune cells’ metabolism, regulating growth factors and inflammatory mediators, and reshaping the extracellular matrix. The reduction of glucose and glutamine induced by caloric restriction, fasting, and FMD mainly affects the survival and proliferation of tumor cells as they are unable to shift their metabolism from glycolysis to oxidative phosphorylation and adapt to the new restrictive conditions. Furthermore, CR-dependent downregulation of stearoyl-CoA desaturase (SCD) limits the synthesis of monounsaturated fatty acids, essential for cell membrane fluidity and synthesis.
The reduction of IGF-1 in response to caloric restriction inhibits the PI3K-AKT-mTOR pathway and thus reduces the proliferation of tumor cells, immunosuppressive Tregs, and collagen deposition in TME and promotes normalization of tumor vasculature.
The metabolic changes induced by caloric restriction in T lymphocytes reshape the epigenetic landscape by reducing the production of acetyl coenzyme A and the expression of immune checkpoint receptors such as PD1. These metabolic and epigenetic changes improve T cell persistence, multipotency, tumor clearance, and their responsiveness to immunotherapy. Overall, calorie restriction promotes several changes in tumor cells, the immune system, and the extracellular matrix that may sensitize tumor cells to immunotherapy and enhance the immune response. However, a more in-depth analysis and detailed characterization of the effects of caloric restriction on tumor cells and TME is needed in order to develop, in the future, new dietary interventions that may have a greater synergistic effect with targeted anticancer therapies, minimizing the adverse or counterproductive effects induced by caloric restriction.
Author Contributions
S.C. and V.D.L. conceived and designed this review and drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
V.D.L. holds intellectual property rights on clinical uses of FMD and equity interest in L-Nutra, a company that markets medical food.
Funding Statement
This research was funded by Associazione Italiana per la Ricerca sul Cancro (AIRC) grant number IG#17605 and IG#21820.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Elia I., Schmieder R., Christen S., Fendt S.M. Organ-Specific Cancer Metabolism and Its Potential for Therapy. Handb. Exp. Pharmacol. 2016;233:321–353. doi: 10.1007/164_2015_10. [DOI] [PubMed] [Google Scholar]
- 2.Mayers J.R., Torrence M.E., Danai L.V., Papagiannakopoulos T., Davidson S.M., Bauer M.R., Lau A.N., Ji B.W., Dixit P.D., Hosios A.M., et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science. 2016;353:1161–1165. doi: 10.1126/science.aaf5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuneva M.O., Fan T.W., Allen T.D., Higashi R.M., Ferraris D.V., Tsukamoto T., Mates J.M., Alonso F.J., Wang C., Seo Y., et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157–170. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlova N.N., Thompson C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofer S.J., Davinelli S., Bergmann M., Scapagnini G., Madeo F. Caloric Restriction Mimetics in Nutrition and Clinical Trials. Front. Nutr. 2021;8:717343. doi: 10.3389/fnut.2021.717343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriau E., Paillet J., Kroemer G., Pol J.G. Metabolic Reprogramming by Reduced Calorie Intake or Pharmacological Caloric Restriction Mimetics for Improved Cancer Immunotherapy. Cancers. 2021;13:1260. doi: 10.3390/cancers13061260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longo V.D., Anderson R.M. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell. 2022;185:1455–1470. doi: 10.1016/j.cell.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nencioni A., Caffa I., Cortellino S., Longo V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer. 2018;18:707–719. doi: 10.1038/s41568-018-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Cabo R., Mattson M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019;381:2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 10.Longo V.D., Cortellino S. Fasting, dietary restriction, and immunosenescence. J. Allergy Clin. Immunol. 2020;146:1002–1004. doi: 10.1016/j.jaci.2020.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Taylor S.R., Falcone J.N., Cantley L.C., Goncalves M.D. Developing dietary interventions as therapy for cancer. Nat. Rev. Cancer. 2022;22:452–466. doi: 10.1038/s41568-022-00485-y. [DOI] [PubMed] [Google Scholar]
- 12.Ribas A. Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discov. 2015;5:915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho P.C., Liu P.S. Metabolic communication in tumors: A new layer of immunoregulation for immune evasion. J. Immunother. Cancer. 2016;4:4. doi: 10.1186/s40425-016-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghesquiere B., Wong B.W., Kuchnio A., Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511:167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 15.Egeblad M., Nakasone E.S., Werb Z. Tumors as organs: Complex tissues that interface with the entire organism. Dev. Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hass R. Role of MSC in the Tumor Microenvironment. Cancers. 2020;12:2107. doi: 10.3390/cancers12082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kersten K., Coffelt S.B., Hoogstraat M., Verstegen N.J.M., Vrijland K., Ciampricotti M., Doornebal C.W., Hau C.S., Wellenstein M.D., Salvagno C., et al. Mammary tumor-derived CCL2 enhances pro-metastatic systemic inflammation through upregulation of IL1beta in tumor-associated macrophages. Oncoimmunology. 2017;6:e1334744. doi: 10.1080/2162402X.2017.1334744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brett E., Sauter M., Timmins E., Azimzadeh O., Rosemann M., Merl-Pham J., Hauck S.M., Nelson P.J., Becker K.F., Schunn I., et al. Oncogenic Linear Collagen VI of Invasive Breast Cancer Is Induced by CCL5. J. Clin. Med. 2020;9:991. doi: 10.3390/jcm9040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Zhang J., Sun X., Su Q., You C. Down-regulation of miR-29b in carcinoma associated fibroblasts promotes cell growth and metastasis of breast cancer. Oncotarget. 2017;8:39559–39570. doi: 10.18632/oncotarget.17136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Naour A., Prat M., Thibault B., Mevel R., Lemaitre L., Leray H., Joubert M.V., Coulson K., Golzio M., Lefevre L., et al. Tumor cells educate mesenchymal stromal cells to release chemoprotective and immunomodulatory factors. J. Mol. Cell Biol. 2020;12:202–215. doi: 10.1093/jmcb/mjz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendel M., Galani I.E., Suri-Payer E., Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008;68:8437–8445. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- 22.Hensbergen P.J., Wijnands P.G., Schreurs M.W., Scheper R.J., Willemze R., Tensen C.P. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8(+) T lymphocytes but not inhibition of angiogenesis. J. Immunother. 2005;28:343–351. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 23.Peng W., Liu C., Xu C., Lou Y., Chen J., Yang Y., Yagita H., Overwijk W.W., Lizee G., Radvanyi L., et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson A., Yang S.C., Huang M., Zhu L., Kar U.K., Batra R.K., Elashoff D., Strieter R.M., Dubinett S.M., Sharma S. IL-7 promotes CXCR3 ligand-dependent T cell antitumor reactivity in lung cancer. J. Immunol. 2009;182:6951–6958. doi: 10.4049/jimmunol.0803340. [DOI] [PubMed] [Google Scholar]
- 25.Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J.R., Zhang L., Burow M., et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 26.Facciabene A., Peng X., Hagemann I.S., Balint K., Barchetti A., Wang L.P., Gimotty P.A., Gilks C.B., Lal P., Zhang L., et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 27.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R., Kaiser E.A., Snyder L.A., Pollard J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Aldinucci D., Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediat. Inflamm. 2014;2014:292376. doi: 10.1155/2014/292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H.W., Chen H.Y., Wang L.T., Wang F.H., Fang L.W., Lai H.Y., Chen H.H., Lu J., Hung M.S., Cheng Y., et al. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J. Immunol. 2013;190:5065–5077. doi: 10.4049/jimmunol.1202775. [DOI] [PubMed] [Google Scholar]
- 31.Biswas S., Mandal G., Roy Chowdhury S., Purohit S., Payne K.K., Anadon C., Gupta A., Swanson P., Yu X., Conejo-Garcia J.R., et al. Exosomes Produced by Mesenchymal Stem Cells Drive Differentiation of Myeloid Cells into Immunosuppressive M2-Polarized Macrophages in Breast Cancer. J. Immunol. 2019;203:3447–3460. doi: 10.4049/jimmunol.1900692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandapathil M., Szczepanski M.J., Szajnik M., Ren J., Jackson E.K., Johnson J.T., Gorelik E., Lang S., Whiteside T.L. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J. Biol. Chem. 2010;285:27571–27580. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poggi A., Musso A., Dapino I., Zocchi M.R. Mechanisms of tumor escape from immune system: Role of mesenchymal stromal cells. Immunol. Lett. 2014;159:55–72. doi: 10.1016/j.imlet.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Najar M., Raicevic G., Fayyad-Kazan H., De Bruyn C., Bron D., Toungouz M., Lagneaux L. Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: The expression and impact of inflammatory priming. Stem Cell Rev. Rep. 2012;8:1188–1198. doi: 10.1007/s12015-012-9408-1. [DOI] [PubMed] [Google Scholar]
- 35.Winkler J., Abisoye-Ogunniyan A., Metcalf K.J., Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020;11:5120. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi H., Sakakura K., Kudo T., Toyoda M., Kaira K., Oyama T., Chikamatsu K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8:8633–8647. doi: 10.18632/oncotarget.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaish U., Jain T., Are A.C., Dudeja V. Cancer-Associated Fibroblasts in Pancreatic Ductal Adenocarcinoma: An Update on Heterogeneity and Therapeutic Targeting. Int. J. Mol. Sci. 2021;22:13408. doi: 10.3390/ijms222413408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi H., Enomoto A., Woods S.L., Burt A.D., Takahashi M., Worthley D.L. Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2019;16:282–295. doi: 10.1038/s41575-019-0115-0. [DOI] [PubMed] [Google Scholar]
- 39.Erez N., Truitt M., Olson P., Arron S.T., Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 40.Cohen N., Shani O., Raz Y., Sharon Y., Hoffman D., Abramovitz L., Erez N. Fibroblasts drive an immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase 3-like 1. Oncogene. 2017;36:4457–4468. doi: 10.1038/onc.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X., Lin Y., Shi Y., Li B., Liu W., Yin W., Dang Y., Chu Y., Fan J., He R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016;76:4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 42.Flavell R.A., Sanjabi S., Wrzesinski S.H., Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng J.T., Deng Y.N., Yi H.M., Wang G.Y., Fu B.S., Chen W.J., Liu W., Tai Y., Peng Y.W., Zhang Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. doi: 10.1038/oncsis.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorchs L., Fernandez Moro C., Bankhead P., Kern K.P., Sadeak I., Meng Q., Rangelova E., Kaipe H. Human Pancreatic Carcinoma-Associated Fibroblasts Promote Expression of Co-inhibitory Markers on CD4(+) and CD8(+) T-Cells. Front. Immunol. 2019;10:847. doi: 10.3389/fimmu.2019.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sousa C.M., Biancur D.E., Wang X., Halbrook C.J., Sherman M.H., Zhang L., Kremer D., Hwang R.F., Witkiewicz A.K., Ying H., et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479–483. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertero T., Oldham W.M., Grasset E.M., Bourget I., Boulter E., Pisano S., Hofman P., Bellvert F., Meneguzzi G., Bulavin D.V., et al. Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metab. 2019;29:124–140.e10. doi: 10.1016/j.cmet.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salimian Rizi B., Caneba C., Nowicka A., Nabiyar A.W., Liu X., Chen K., Klopp A., Nagrath D. Nitric oxide mediates metabolic coupling of omentum-derived adipose stroma to ovarian and endometrial cancer cells. Cancer Res. 2015;75:456–471. doi: 10.1158/0008-5472.CAN-14-1337. [DOI] [PubMed] [Google Scholar]
- 48.Auciello F.R., Bulusu V., Oon C., Tait-Mulder J., Berry M., Bhattacharyya S., Tumanov S., Allen-Petersen B.L., Link J., Kendsersky N.D., et al. A Stromal Lysolipid-Autotaxin Signaling Axis Promotes Pancreatic Tumor Progression. Cancer Discov. 2019;9:617–627. doi: 10.1158/2159-8290.CD-18-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olivares O., Mayers J.R., Gouirand V., Torrence M.E., Gicquel T., Borge L., Lac S., Roques J., Lavaut M.N., Berthezene P., et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat. Commun. 2017;8:16031. doi: 10.1038/ncomms16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu C., Dong S., Huang R., Chen X. Cancer-Associated Adipocytes and Breast Cancer: Intertwining in the Tumor Microenvironment and Challenges for Cancer Therapy. Cancers. 2023;15:726. doi: 10.3390/cancers15030726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundaram S., Yan L. High-fat Diet Enhances Mammary Tumorigenesis and Pulmonary Metastasis and Alters Inflammatory and Angiogenic Profiles in MMTV-PyMT Mice. AntiCancer Res. 2016;36:6279–6287. doi: 10.21873/anticanres.11223. [DOI] [PubMed] [Google Scholar]
- 52.Kim S., Yang X., Li Q., Wu M., Costyn L., Beharry Z., Bartlett M.G., Cai H. Myristoylation of Src kinase mediates Src-induced and high-fat diet-accelerated prostate tumor progression in mice. J. Biol. Chem. 2017;292:18422–18433. doi: 10.1074/jbc.M117.798827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Q., Li B., Li J., Sun S., Yuan J., Sun S. Cancer-associated adipocytes as immunomodulators in cancer. Biomark. Res. 2021;9:2. doi: 10.1186/s40364-020-00257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pu X., Chen D. Targeting Adipokines in Obesity-Related Tumors. Front. Oncol. 2021;11:685923. doi: 10.3389/fonc.2021.685923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ibrahim A.S., El-Shinawi M., Sabet S., Ibrahim S.A., Mohamed M.M. Role of adipose tissue-derived cytokines in the progression of inflammatory breast cancer in patients with obesity. Lipids Health Dis. 2022;21:67. doi: 10.1186/s12944-022-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang C., Yue C., Herrmann A., Song J., Egelston C., Wang T., Zhang Z., Li W., Lee H., Aftabizadeh M., et al. STAT3 Activation-Induced Fatty Acid Oxidation in CD8(+) T Effector Cells Is Critical for Obesity-Promoted Breast Tumor Growth. Cell Metab. 2020;31:148–161.e5. doi: 10.1016/j.cmet.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferjancic S., Gil-Bernabe A.M., Hill S.A., Allen P.D., Richardson P., Sparey T., Savory E., McGuffog J., Muschel R.J. VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary metastasis in mice. Blood. 2013;121:3289–3297. doi: 10.1182/blood-2012-08-449819. [DOI] [PubMed] [Google Scholar]
- 58.Pittet C.L., Newcombe J., Prat A., Arbour N. Human brain endothelial cells endeavor to immunoregulate CD8 T cells via PD-1 ligand expression in multiple sclerosis. J. Neuroinflamm. 2011;8:155. doi: 10.1186/1742-2094-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Motz G.T., Santoro S.P., Wang L.P., Garrabrant T., Lastra R.R., Hagemann I.S., Lal P., Feldman M.D., Benencia F., Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer. 2017;17:457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 61.Zhou X., Fang D., Liu H., Ou X., Zhang C., Zhao Z., Zhao S., Peng J., Cai S., He Y., et al. PMN-MDSCs accumulation induced by CXCL1 promotes CD8(+) T cells exhaustion in gastric cancer. Cancer Lett. 2022;532:215598. doi: 10.1016/j.canlet.2022.215598. [DOI] [PubMed] [Google Scholar]
- 62.Gabrilovich D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gordon S., Martinez F.O. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Poh A.R., Ernst M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018;8:49. doi: 10.3389/fonc.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leek R.D., Hunt N.C., Landers R.J., Lewis C.E., Royds J.A., Harris A.L. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J. Pathol. 2000;190:430–436. doi: 10.1002/(SICI)1096-9896(200003)190:4<430::AID-PATH538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 66.Pyonteck S.M., Akkari L., Schuhmacher A.J., Bowman R.L., Sevenich L., Quail D.F., Olson O.C., Quick M.L., Huse J.T., Teijeiro V., et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casazza A., Laoui D., Wenes M., Rizzolio S., Bassani N., Mambretti M., Deschoemaeker S., Van Ginderachter J.A., Tamagnone L., Mazzone M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 68.Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch. Pharm. Res. 2016;39:1588–1596. doi: 10.1007/s12272-016-0820-y. [DOI] [PubMed] [Google Scholar]
- 69.Kuwabara K., Ogawa S., Matsumoto M., Koga S., Clauss M., Pinsky D.J., Lyn P., Leavy J., Witte L., Joseph-Silverstein J., et al. Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc. Natl. Acad. Sci. USA. 1995;92:4606–4610. doi: 10.1073/pnas.92.10.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harmey J.H., Dimitriadis E., Kay E., Redmond H.P., Bouchier-Hayes D. Regulation of macrophage production of vascular endothelial growth factor (VEGF) by hypoxia and transforming growth factor beta-1. Ann. Surg. Oncol. 1998;5:271–278. doi: 10.1007/BF02303785. [DOI] [PubMed] [Google Scholar]
- 71.Movahedi K., Laoui D., Gysemans C., Baeten M., Stange G., Van den Bossche J., Mack M., Pipeleers D., In’t Veld P., De Baetselier P., et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 72.Molon B., Ugel S., Del Pozzo F., Soldani C., Zilio S., Avella D., De Palma A., Mauri P., Monegal A., Rescigno M., et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu T., Ramakrishnan R., Altiok S., Youn J.I., Cheng P., Celis E., Pisarev V., Sherman S., Sporn M.B., Gabrilovich D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J. Clin. Investig. 2011;121:4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Annamalai R.T., Turner P.A., Carson W.F.t., Levi B., Kunkel S., Stegemann J.P. Harnessing macrophage-mediated degradation of gelatin microspheres for spatiotemporal control of BMP2 release. Biomaterials. 2018;161:216–227. doi: 10.1016/j.biomaterials.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mantovani A., Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 2015;212:435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonavita E., Bromley C.P., Jonsson G., Pelly V.S., Sahoo S., Walwyn-Brown K., Mensurado S., Moeini A., Flanagan E., Bell C.R., et al. Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade. Immunity. 2020;53:1215–1229.e8. doi: 10.1016/j.immuni.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wculek S.K., Heras-Murillo I., Mastrangelo A., Mananes D., Galan M., Miguel V., Curtabbi A., Barbas C., Chandel N.S., Enriquez J.A., et al. Oxidative phosphorylation selectively orchestrates tissue macrophage homeostasis. Immunity. 2023;56:516–530.e9. doi: 10.1016/j.immuni.2023.01.011. [DOI] [PubMed] [Google Scholar]
- 78.Wejksza K., Lee-Chang C., Bodogai M., Bonzo J., Gonzalez F.J., Lehrmann E., Becker K., Biragyn A. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor alpha. J. Immunol. 2013;190:2575–2584. doi: 10.4049/jimmunol.1201920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han S., Feng S., Ren M., Ma E., Wang X., Xu L., Xu M. Glioma cell-derived placental growth factor induces regulatory B cells. Int. J. Biochem. Cell Biol. 2014;57:63–68. doi: 10.1016/j.biocel.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 80.Schioppa T., Moore R., Thompson R.G., Rosser E.C., Kulbe H., Nedospasov S., Mauri C., Coussens L.M., Balkwill F.R. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc. Natl. Acad. Sci. USA. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lindner S., Dahlke K., Sontheimer K., Hagn M., Kaltenmeier C., Barth T.F., Beyer T., Reister F., Fabricius D., Lotfi R., et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 2013;73:2468–2479. doi: 10.1158/0008-5472.CAN-12-3450. [DOI] [PubMed] [Google Scholar]
- 82.Zhou X., Su Y.X., Lao X.M., Liang Y.J., Liao G.Q. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol. 2016;53:27–35. doi: 10.1016/j.oraloncology.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Xiao S., Brooks C.R., Sobel R.A., Kuchroo V.K. Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. J. Immunol. 2015;194:1602–1608. doi: 10.4049/jimmunol.1402632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosser E.C., Mauri C. Regulatory B cells: Origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 85.Inoue S., Leitner W.W., Golding B., Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 86.Cai C., Zhang J., Li M., Wu Z.J., Song K.H., Zhan T.W., Wang L.H., Sun Y.H. Interleukin 10-expressing B cells inhibit tumor-infiltrating T cell function and correlate with T cell Tim-3 expression in renal cell carcinoma. Tumour Biol. 2016;37:8209–8218. doi: 10.1007/s13277-015-4687-1. [DOI] [PubMed] [Google Scholar]
- 87.Zhou M., Wen Z., Cheng F., Ma J., Li W., Ren H., Sheng Y., Dong H., Lu L., Hu H.M., et al. Tumor-released autophagosomes induce IL-10-producing B cells with suppressive activity on T lymphocytes via TLR2-MyD88-NF-kappaB signal pathway. Oncoimmunology. 2016;5:e1180485. doi: 10.1080/2162402X.2016.1180485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y.Q., Li P.C., Pan N., Gao R., Wen Z.F., Zhang T.Y., Huang F., Wu F.Y., Ou X.L., Zhang J.P., et al. Tumor-released autophagosomes induces CD4(+) T cell-mediated immunosuppression via a TLR2-IL-6 cascade. J. Immunother. Cancer. 2019;7:178. doi: 10.1186/s40425-019-0646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Horikawa M., Minard-Colin V., Matsushita T., Tedder T.F. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J. Clin. Investig. 2011;121:4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olkhanud P.B., Baatar D., Bodogai M., Hakim F., Gress R., Anderson R.L., Deng J., Xu M., Briest S., Biragyn A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bodogai M., Moritoh K., Lee-Chang C., Hollander C.M., Sherman-Baust C.A., Wersto R.P., Araki Y., Miyoshi I., Yang L., Trinchieri G., et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res. 2015;75:3456–3465. doi: 10.1158/0008-5472.CAN-14-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pylayeva-Gupta Y., Das S., Handler J.S., Hajdu C.H., Coffre M., Koralov S.B., Bar-Sagi D. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 2016;6:247–255. doi: 10.1158/2159-8290.CD-15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang K., Liu J., Li J. IL-35-producing B cells in gastric cancer patients. Medicine. 2018;97:e0710. doi: 10.1097/MD.0000000000010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen M., Wang J., Yu W., Zhang C., Liu M., Wang K., Yang L., Wei F., Wang S.E., Sun Q., et al. A novel MDSC-induced PD-1(−)PD-L1(+) B-cell subset in breast tumor microenvironment possesses immuno-suppressive properties. Oncoimmunology. 2018;7:e1413520. doi: 10.1080/2162402X.2017.1413520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang A., Dadaglio G., Oberkampf M., Di Carlo S., Peduto L., Laubreton D., Desrues B., Sun C.M., Montagutelli X., Leclerc C. B cells promote tumor progression in a mouse model of HPV-mediated cervical cancer. Int. J. Cancer. 2016;139:1358–1371. doi: 10.1002/ijc.30169. [DOI] [PubMed] [Google Scholar]
- 96.Zhao Y., Shen M., Feng Y., He R., Xu X., Xie Y., Shi X., Zhou M., Pan S., Wang M., et al. Regulatory B cells induced by pancreatic cancer cell-derived interleukin-18 promote immune tolerance via the PD-1/PD-L1 pathway. Oncotarget. 2018;9:14803–14814. doi: 10.18632/oncotarget.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tretter T., Venigalla R.K., Eckstein V., Saffrich R., Sertel S., Ho A.D., Lorenz H.M. Induction of CD4+ T-cell anergy and apoptosis by activated human B cells. Blood. 2008;112:4555–4564. doi: 10.1182/blood-2008-02-140087. [DOI] [PubMed] [Google Scholar]
- 98.Mabrouk I., Buart S., Hasmim M., Michiels C., Connault E., Opolon P., Chiocchia G., Levi-Strauss M., Chouaib S., Karray S. Prevention of autoimmunity and control of recall response to exogenous antigen by Fas death receptor ligand expression on T cells. Immunity. 2008;29:922–933. doi: 10.1016/j.immuni.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 99.Ding Q., Mohib K., Kuchroo V.K., Rothstein D.M. TIM-4 Identifies IFN-gamma-Expressing Proinflammatory B Effector 1 Cells That Promote Tumor and Allograft Rejection. J. Immunol. 2017;199:2585–2595. doi: 10.4049/jimmunol.1602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiao X., Lao X.M., Chen M.M., Liu R.X., Wei Y., Ouyang F.Z., Chen D.P., Zhao X.Y., Zhao Q., Li X.F., et al. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov. 2016;6:546–559. doi: 10.1158/2159-8290.CD-15-1408. [DOI] [PubMed] [Google Scholar]
- 101.Kaku H., Cheng K.F., Al-Abed Y., Rothstein T.L. A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J. Immunol. 2014;193:5904–5913. doi: 10.4049/jimmunol.1400336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saze Z., Schuler P.J., Hong C.S., Cheng D., Jackson E.K., Whiteside T.L. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122:9–18. doi: 10.1182/blood-2013-02-482406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nouel A., Pochard P., Simon Q., Segalen I., Le Meur Y., Pers J.O., Hillion S. B-Cells induce regulatory T cells through TGF-beta/IDO production in A CTLA-4 dependent manner. J. Autoimmun. 2015;59:53–60. doi: 10.1016/j.jaut.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 104.Huang B., Faucette A.N., Pawlitz M.D., Pei B., Goyert J.W., Zhou J.Z., El-Hage N.G., Deng J., Lin J., Yao F., et al. Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat. Med. 2017;23:128–135. doi: 10.1038/nm.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L., Fu Y., Yu B., Jiang X., Liu H., Liu J., Zha B., Chu Y. HSP70, a Novel Regulatory Molecule in B Cell-Mediated Suppression of Autoimmune Diseases. J. Mol. Biol. 2021;433:166634. doi: 10.1016/j.jmb.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 106.Wang L., Simons D.L., Lu X., Tu T.Y., Solomon S., Wang R., Rosario A., Avalos C., Schmolze D., Yim J., et al. Connecting blood and intratumoral T(reg) cell activity in predicting future relapse in breast cancer. Nat. Immunol. 2019;20:1220–1230. doi: 10.1038/s41590-019-0429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sakaguchi S., Mikami N., Wing J.B., Tanaka A., Ichiyama K., Ohkura N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020;38:541–566. doi: 10.1146/annurev-immunol-042718-041717. [DOI] [PubMed] [Google Scholar]
- 108.Toker A., Ohashi P.S. Expression of costimulatory and inhibitory receptors in FoxP3(+) regulatory T cells within the tumor microenvironment: Implications for combination immunotherapy approaches. Adv. Cancer Res. 2019;144:193–261. doi: 10.1016/bs.acr.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 109.Campbell C., Rudensky A. Roles of Regulatory T Cells in Tissue Pathophysiology and Metabolism. Cell Metab. 2020;31:18–25. doi: 10.1016/j.cmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Z., Jiang J., Wang Z., Zhang J., Xiao M., Wang C., Lu Y., Qin Z. Endogenous interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Res. 2008;68:8687–8694. doi: 10.1158/0008-5472.CAN-08-0449. [DOI] [PubMed] [Google Scholar]
- 111.Bankaitis K.V., Fingleton B. Targeting IL4/IL4R for the treatment of epithelial cancer metastasis. Clin. Exp. Metastasis. 2015;32:847–856. doi: 10.1007/s10585-015-9747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fu C., Jiang L., Hao S., Liu Z., Ding S., Zhang W., Yang X., Li S. Activation of the IL-4/STAT6 Signaling Pathway Promotes Lung Cancer Progression by Increasing M2 Myeloid Cells. Front. Immunol. 2019;10:2638. doi: 10.3389/fimmu.2019.02638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen K., Kolls J.K. Interluekin-17A (IL17A) Gene. 2017;614:8–14. doi: 10.1016/j.gene.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sullivan J.A., Tomita Y., Jankowska-Gan E., Lema D.A., Arvedson M.P., Nair A., Bracamonte-Baran W., Zhou Y., Meyer K.K., Zhong W., et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep. 2020;30:1039–1051.e5. doi: 10.1016/j.celrep.2019.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kalia V., Penny L.A., Yuzefpolskiy Y., Baumann F.M., Sarkar S. Quiescence of Memory CD8(+) T Cells Is Mediated by Regulatory T Cells through Inhibitory Receptor CTLA-4. Immunity. 2015;42:1116–1129. doi: 10.1016/j.immuni.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 116.Ohta A., Kini R., Ohta A., Subramanian M., Madasu M., Sitkovsky M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012;3:190. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ihara F., Sakurai D., Takami M., Kamata T., Kunii N., Yamasaki K., Iinuma T., Nakayama T., Motohashi S., Okamoto Y. Regulatory T cells induce CD4(−) NKT cell anergy and suppress NKT cell cytotoxic function. Cancer Immunol. Immunother. 2019;68:1935–1947. doi: 10.1007/s00262-019-02417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carmi Y., Rinott G., Dotan S., Elkabets M., Rider P., Voronov E., Apte R.N. Microenvironment-derived IL-1 and IL-17 interact in the control of lung metastasis. J. Immunol. 2011;186:3462–3471. doi: 10.4049/jimmunol.1002901. [DOI] [PubMed] [Google Scholar]
- 119.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 120.Levental K.R., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J.T., Fong S.F., Csiszar K., Giaccia A., Weninger W., et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Butcher D.T., Alliston T., Weaver V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spill F., Reynolds D.S., Kamm R.D., Zaman M.H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 2016;40:41–48. doi: 10.1016/j.copbio.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shidal C., Singh N.P., Nagarkatti P., Nagarkatti M. MicroRNA-92 Expression in CD133(+) Melanoma Stem Cells Regulates Immunosuppression in the Tumor Microenvironment via Integrin-Dependent Activation of TGFbeta. Cancer Res. 2019;79:3622–3635. doi: 10.1158/0008-5472.CAN-18-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steinert G., Scholch S., Niemietz T., Iwata N., Garcia S.A., Behrens B., Voigt A., Kloor M., Benner A., Bork U., et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 2014;74:1694–1704. doi: 10.1158/0008-5472.CAN-13-1885. [DOI] [PubMed] [Google Scholar]
- 125.Fallarino F., Grohmann U., Hwang K.W., Orabona C., Vacca C., Bianchi R., Belladonna M.L., Fioretti M.C., Alegre M.L., Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 126.Moghadasi S., Elveny M., Rahman H.S., Suksatan W., Jalil A.T., Abdelbasset W.K., Yumashev A.V., Shariatzadeh S., Motavalli R., Behzad F., et al. A paradigm shift in cell-free approach: The emerging role of MSCs-derived exosomes in regenerative medicine. J. Transl. Med. 2021;19:302. doi: 10.1186/s12967-021-02980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kalaany N.Y., Sabatini D.M. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Keenan M.M., Chi J.T. Alternative fuels for cancer cells. Cancer J. 2015;21:49–55. doi: 10.1097/PPO.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cluntun A.A., Lukey M.J., Cerione R.A., Locasale J.W. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer. 2017;3:169–180. doi: 10.1016/j.trecan.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heintzman D.R., Fisher E.L., Rathmell J.C. Microenvironmental influences on T cell immunity in cancer and inflammation. Cell. Mol. Immunol. 2022;19:316–326. doi: 10.1038/s41423-021-00833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DeBerardinis R.J., Chandel N.S. We need to talk about the Warburg effect. Nat. Metab. 2020;2:127–129. doi: 10.1038/s42255-020-0172-2. [DOI] [PubMed] [Google Scholar]
- 132.Lunt S.Y., Vander Heiden M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 133.Reid M.A., Allen A.E., Liu S., Liberti M.V., Liu P., Liu X., Dai Z., Gao X., Wang Q., Liu Y., et al. Serine synthesis through PHGDH coordinates nucleotide levels by maintaining central carbon metabolism. Nat. Commun. 2018;9:5442. doi: 10.1038/s41467-018-07868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sullivan M.R., Mattaini K.R., Dennstedt E.A., Nguyen A.A., Sivanand S., Reilly M.F., Meeth K., Muir A., Darnell A.M., Bosenberg M.W., et al. Increased Serine Synthesis Provides an Advantage for Tumors Arising in Tissues Where Serine Levels Are Limiting. Cell Metab. 2019;29:1410–1421.e4. doi: 10.1016/j.cmet.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rohrig F., Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 136.Possemato R., Marks K.M., Shaul Y.D., Pacold M.E., Kim D., Birsoy K., Sethumadhavan S., Woo H.K., Jang H.G., Jha A.K., et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Comerford S.A., Huang Z., Du X., Wang Y., Cai L., Witkiewicz A.K., Walters H., Tantawy M.N., Fu A., Manning H.C., et al. Acetate dependence of tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ho P.C., Bihuniak J.D., Macintyre A.N., Staron M., Liu X., Amezquita R., Tsui Y.C., Cui G., Micevic G., Perales J.C., et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ganeshan K., Chawla A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 141.Mendler A.N., Hu B., Prinz P.U., Kreutz M., Gottfried E., Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int. J. Cancer. 2012;131:633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- 142.Newsholme P., Procopio J., Lima M.M., Pithon-Curi T.C., Curi R. Glutamine and glutamate--their central role in cell metabolism and function. Cell Biochem. Funct. 2003;21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 143.Ahluwalia G.S., Grem J.L., Hao Z., Cooney D.A. Metabolism and action of amino acid analog anti-cancer agents. Pharmacol. Ther. 1990;46:243–271. doi: 10.1016/0163-7258(90)90094-I. [DOI] [PubMed] [Google Scholar]
- 144.Diehn M., Cho R.W., Lobo N.A., Kalisky T., Dorie M.J., Kulp A.N., Qian D., Lam J.S., Ailles L.E., Wong M., et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bansal A., Simon M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018;217:2291–2298. doi: 10.1083/jcb.201804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alkan H.F., Walter K.E., Luengo A., Madreiter-Sokolowski C.T., Stryeck S., Lau A.N., Al-Zoughbi W., Lewis C.A., Thomas C.J., Hoefler G., et al. Cytosolic Aspartate Availability Determines Cell Survival When Glutamine Is Limiting. Cell Metab. 2018;28:706–720.e6. doi: 10.1016/j.cmet.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Choi B.H., Coloff J.L. The Diverse Functions of Non-Essential Amino Acids in Cancer. Cancers. 2019;11:675. doi: 10.3390/cancers11050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Muir A., Danai L.V., Gui D.Y., Waingarten C.Y., Lewis C.A., Vander Heiden M.G. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. eLife. 2017;6:e27713. doi: 10.7554/eLife.27713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Konteatis Z., Travins J., Gross S., Marjon K., Barnett A., Mandley E., Nicolay B., Nagaraja R., Chen Y., Sun Y., et al. Discovery of AG-270, a First-in-Class Oral MAT2A Inhibitor for the Treatment of Tumors with Homozygous MTAP Deletion. J. Med. Chem. 2021;64:4430–4449. doi: 10.1021/acs.jmedchem.0c01895. [DOI] [PubMed] [Google Scholar]
- 150.Albers E. Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5′-methylthioadenosine. IUBMB Life. 2009;61:1132–1142. doi: 10.1002/iub.278. [DOI] [PubMed] [Google Scholar]
- 151.Kusano T., Berberich T., Tateda C., Takahashi Y. Polyamines: Essential factors for growth and survival. Planta. 2008;228:367–381. doi: 10.1007/s00425-008-0772-7. [DOI] [PubMed] [Google Scholar]
- 152.Cellarier E., Durando X., Vasson M.P., Farges M.C., Demiden A., Maurizis J.C., Madelmont J.C., Chollet P. Methionine dependency and cancer treatment. Cancer Treat. Rev. 2003;29:489–499. doi: 10.1016/S0305-7372(03)00118-X. [DOI] [PubMed] [Google Scholar]
- 153.Chaturvedi S., Hoffman R.M., Bertino J.R. Exploiting methionine restriction for cancer treatment. Biochem. Pharmacol. 2018;154:170–173. doi: 10.1016/j.bcp.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 154.Palmer R.M., Ashton D.S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 155.Keshet R., Erez A. Arginine and the metabolic regulation of nitric oxide synthesis in cancer. Dis. Model. Mech. 2018;11:dmm033332. doi: 10.1242/dmm.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lala P.K., Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 157.Saxton R.A., Chantranupong L., Knockenhauer K.E., Schwartz T.U., Sabatini D.M. Mechanism of arginine sensing by CASTOR1 upstream of mTORC1. Nature. 2016;536:229–233. doi: 10.1038/nature19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Porstmann T., Santos C.R., Griffiths B., Cully M., Wu M., Leevers S., Griffiths J.R., Chung Y.L., Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li X., Egervari G., Wang Y., Berger S.L., Lu Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 2018;19:563–578. doi: 10.1038/s41580-018-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ou Y., Wang S.J., Jiang L., Zheng B., Gu W. p53 Protein-mediated regulation of phosphoglycerate dehydrogenase (PHGDH) is crucial for the apoptotic response upon serine starvation. J. Biol. Chem. 2015;290:457–466. doi: 10.1074/jbc.M114.616359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chaneton B., Hillmann P., Zheng L., Martin A.C.L., Maddocks O.D.K., Chokkathukalam A., Coyle J.E., Jankevics A., Holding F.P., Vousden K.H., et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ye J., Mancuso A., Tong X., Ward P.S., Fan J., Rabinowitz J.D., Thompson C.B. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc. Natl. Acad. Sci. USA. 2012;109:6904–6909. doi: 10.1073/pnas.1204176109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Labuschagne C.F., van den Broek N.J., Mackay G.M., Vousden K.H., Maddocks O.D. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 2014;7:1248–1258. doi: 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 164.Pacold M.E., Brimacombe K.R., Chan S.H., Rohde J.M., Lewis C.A., Swier L.J., Possemato R., Chen W.W., Sullivan L.B., Fiske B.P., et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 2016;12:452–458. doi: 10.1038/nchembio.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maddocks O.D., Labuschagne C.F., Adams P.D., Vousden K.H. Serine Metabolism Supports the Methionine Cycle and DNA/RNA Methylation through De Novo ATP Synthesis in Cancer Cells. Mol. Cell. 2016;61:210–221. doi: 10.1016/j.molcel.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neinast M.D., Jang C., Hui S., Murashige D.S., Chu Q., Morscher R.J., Li X., Zhan L., White E., Anthony T.G., et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids. Cell Metab. 2019;29:417–429.e4. doi: 10.1016/j.cmet.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Puris E., Gynther M., Auriola S., Huttunen K.M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020;37:88. doi: 10.1007/s11095-020-02826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yue M., Jiang J., Gao P., Liu H., Qing G. Oncogenic MYC Activates a Feedforward Regulatory Loop Promoting Essential Amino Acid Metabolism and Tumorigenesis. Cell Rep. 2017;21:3819–3832. doi: 10.1016/j.celrep.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 170.Elorza A., Soro-Arnaiz I., Melendez-Rodriguez F., Rodriguez-Vaello V., Marsboom G., de Carcer G., Acosta-Iborra B., Albacete-Albacete L., Ordonez A., Serrano-Oviedo L., et al. HIF2alpha acts as an mTORC1 activator through the amino acid carrier SLC7A5. Mol. Cell. 2012;48:681–691. doi: 10.1016/j.molcel.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 171.Grzes K.M., Swamy M., Hukelmann J.L., Emslie E., Sinclair L.V., Cantrell D.A. Control of amino acid transport coordinates metabolic reprogramming in T-cell malignancy. Leukemia. 2017;31:2771–2779. doi: 10.1038/leu.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.She P., Reid T.M., Bronson S.K., Vary T.C., Hajnal A., Lynch C.J., Hutson S.M. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Campbell S.L., Wellen K.E. Metabolic Signaling to the Nucleus in Cancer. Mol. Cell. 2018;71:398–408. doi: 10.1016/j.molcel.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 174.Pearce E.L., Poffenberger M.C., Chang C.H., Jones R.G. Fueling immunity: Insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pearce E.J., Everts B. Dendritic cell metabolism. Nat. Rev. Immunol. 2015;15:18–29. doi: 10.1038/nri3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Arner E.N., Rathmell J.C. Metabolic programming and immune suppression in the tumor microenvironment. Cancer Cell. 2023;41:421–433. doi: 10.1016/j.ccell.2023.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Phan A.T., Doedens A.L., Palazon A., Tyrakis P.A., Cheung K.P., Johnson R.S., Goldrath A.W. Constitutive Glycolytic Metabolism Supports CD8(+) T Cell Effector Memory Differentiation during Viral Infection. Immunity. 2016;45:1024–1037. doi: 10.1016/j.immuni.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kumagai S., Koyama S., Itahashi K., Tanegashima T., Lin Y.T., Togashi Y., Kamada T., Irie T., Okumura G., Kono H., et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40:201–218.e9. doi: 10.1016/j.ccell.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 179.Xu K., Yin N., Peng M., Stamatiades E.G., Shyu A., Li P., Zhang X., Do M.H., Wang Z., Capistrano K.J., et al. Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science. 2021;371:405–410. doi: 10.1126/science.abb2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guo D., Tong Y., Jiang X., Meng Y., Jiang H., Du L., Wu Q., Li S., Luo S., Li M., et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IkappaBalpha. Cell Metab. 2022;34:1312–1324.e6. doi: 10.1016/j.cmet.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 181.Hermans D., Gautam S., Garcia-Canaveras J.C., Gromer D., Mitra S., Spolski R., Li P., Christensen S., Nguyen R., Lin J.X., et al. Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8(+) T cell stemness and antitumor immunity. Proc. Natl. Acad. Sci. USA. 2020;117:6047–6055. doi: 10.1073/pnas.1920413117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chowdhury P.S., Chamoto K., Kumar A., Honjo T. PPAR-Induced Fatty Acid Oxidation in T Cells Increases the Number of Tumor-Reactive CD8(+) T Cells and Facilitates Anti-PD-1 Therapy. Cancer Immunol. Res. 2018;6:1375–1387. doi: 10.1158/2326-6066.CIR-18-0095. [DOI] [PubMed] [Google Scholar]
- 183.Voss K., Larsen S.E., Snow A.L. Metabolic reprogramming and apoptosis sensitivity: Defining the contours of a T cell response. Cancer Lett. 2017;408:190–196. doi: 10.1016/j.canlet.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Macintyre A.N., Gerriets V.A., Nichols A.G., Michalek R.D., Rudolph M.C., Deoliveira D., Anderson S.M., Abel E.D., Chen B.J., Hale L.P., et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20:61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Al-Khami A.A., Rodriguez P.C., Ochoa A.C. Metabolic reprogramming of myeloid-derived suppressor cells (MDSC) in cancer. Oncoimmunology. 2016;5:e1200771. doi: 10.1080/2162402X.2016.1200771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hossain D.M., Panda A.K., Chakrabarty S., Bhattacharjee P., Kajal K., Mohanty S., Sarkar I., Sarkar D.K., Kar S.K., Sa G. MEK inhibition prevents tumour-shed transforming growth factor-beta-induced T-regulatory cell augmentation in tumour milieu. Immunology. 2015;144:561–573. doi: 10.1111/imm.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rapoport B.L., Steel H.C., Theron A.J., Smit T., Anderson R. Role of the Neutrophil in the Pathogenesis of Advanced Cancer and Impaired Responsiveness to Therapy. Molecules. 2020;25:1618. doi: 10.3390/molecules25071618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cham C.M., Driessens G., O’Keefe J.P., Gajewski T.F. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur. J. Immunol. 2008;38:2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Perez-Tomas R., Perez-Guillen I. Lactate in the Tumor Microenvironment: An Essential Molecule in Cancer Progression and Treatment. Cancers. 2020;12:3244. doi: 10.3390/cancers12113244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Z.H., Peng W.B., Zhang P., Yang X.P., Zhou Q. Lactate in the tumour microenvironment: From immune modulation to therapy. EBioMedicine. 2021;73:103627. doi: 10.1016/j.ebiom.2021.103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Erra Diaz F., Ochoa V., Merlotti A., Dantas E., Mazzitelli I., Gonzalez Polo V., Sabatte J., Amigorena S., Segura E., Geffner J. Extracellular Acidosis and mTOR Inhibition Drive the Differentiation of Human Monocyte-Derived Dendritic Cells. Cell Rep. 2020;31:107613. doi: 10.1016/j.celrep.2020.107613. [DOI] [PubMed] [Google Scholar]
- 192.Brand A., Singer K., Koehl G.E., Kolitzus M., Schoenhammer G., Thiel A., Matos C., Bruss C., Klobuch S., Peter K., et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 193.Gottfried E., Kunz-Schughart L.A., Ebner S., Mueller-Klieser W., Hoves S., Andreesen R., Mackensen A., Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 194.Nasi A., Fekete T., Krishnamurthy A., Snowden S., Rajnavolgyi E., Catrina A.I., Wheelock C.E., Vivar N., Rethi B. Dendritic cell reprogramming by endogenously produced lactic acid. J. Immunol. 2013;191:3090–3099. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- 195.Colegio O.R., Chu N.Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V., Cyrus N., Brokowski C.E., Eisenbarth S.C., Phillips G.M., et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Husain Z., Huang Y., Seth P., Sukhatme V.P. Tumor-derived lactate modifies antitumor immune response: Effect on myeloid-derived suppressor cells and NK cells. J. Immunol. 2013;191:1486–1495. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 197.Munder M., Engelhardt M., Knies D., Medenhoff S., Wabnitz G., Luckner-Minden C., Feldmeyer N., Voss R.H., Kropf P., Muller I., et al. Cytotoxicity of tumor antigen specific human T cells is unimpaired by arginine depletion. PLoS ONE. 2013;8:e63521. doi: 10.1371/journal.pone.0063521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brown T.P., Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 2020;206:107451. doi: 10.1016/j.pharmthera.2019.107451. [DOI] [PubMed] [Google Scholar]
- 199.Bricker D.K., Taylor E.B., Schell J.C., Orsak T., Boutron A., Chen Y.C., Cox J.E., Cardon C.M., Van Vranken J.G., Dephoure N., et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Herzig S., Raemy E., Montessuit S., Veuthey J.L., Zamboni N., Westermann B., Kunji E.R., Martinou J.C. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 201.Johnson M.O., Wolf M.M., Madden M.Z., Andrejeva G., Sugiura A., Contreras D.C., Maseda D., Liberti M.V., Paz K., Kishton R.J., et al. Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell. 2018;175:1780–1795.e19. doi: 10.1016/j.cell.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Best S.A., Gubser P.M., Sethumadhavan S., Kersbergen A., Negron Abril Y.L., Goldford J., Sellers K., Abeysekera W., Garnham A.L., McDonald J.A., et al. Glutaminase inhibition impairs CD8 T cell activation in STK11-/Lkb1-deficient lung cancer. Cell Metab. 2022;34:874–887.e6. doi: 10.1016/j.cmet.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 203.Wang H., Franco F., Tsui Y.C., Xie X., Trefny M.P., Zappasodi R., Mohmood S.R., Fernandez-Garcia J., Tsai C.H., Schulze I., et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 2020;21:298–308. doi: 10.1038/s41590-019-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Leone R.D., Zhao L., Englert J.M., Sun I.M., Oh M.H., Sun I.H., Arwood M.L., Bettencourt I.A., Patel C.H., Wen J., et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hung M.H., Lee J.S., Ma C., Diggs L.P., Heinrich S., Chang C.W., Ma L., Forgues M., Budhu A., Chaisaingmongkol J., et al. Tumor methionine metabolism drives T-cell exhaustion in hepatocellular carcinoma. Nat. Commun. 2021;12:1455. doi: 10.1038/s41467-021-21804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhao T., Lum J.J. Methionine cycle-dependent regulation of T cells in cancer immunity. Front. Oncol. 2022;12:969563. doi: 10.3389/fonc.2022.969563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Geiger R., Rieckmann J.C., Wolf T., Basso C., Feng Y., Fuhrer T., Kogadeeva M., Picotti P., Meissner F., Mann M., et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell. 2016;167:829–842.e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fletcher M., Ramirez M.E., Sierra R.A., Raber P., Thevenot P., Al-Khami A.A., Sanchez-Pino D., Hernandez C., Wyczechowska D.D., Ochoa A.C., et al. l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Res. 2015;75:275–283. doi: 10.1158/0008-5472.CAN-14-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Raber P., Ochoa A.C., Rodriguez P.C. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: Mechanisms of T cell suppression and therapeutic perspectives. Immunol. Investig. 2012;41:614–634. doi: 10.3109/08820139.2012.680634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Carmona-Fontaine C., Deforet M., Akkari L., Thompson C.B., Joyce J.A., Xavier J.B. Metabolic origins of spatial organization in the tumor microenvironment. Proc. Natl. Acad. Sci. USA. 2017;114:2934–2939. doi: 10.1073/pnas.1700600114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Frumento G., Rotondo R., Tonetti M., Damonte G., Benatti U., Ferrara G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Munn D.H., Sharma M.D., Baban B., Harding H.P., Zhang Y., Ron D., Mellor A.L. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 213.Peyraud F., Guegan J.P., Bodet D., Cousin S., Bessede A., Italiano A. Targeting Tryptophan Catabolism in Cancer Immunotherapy Era: Challenges and Perspectives. Front. Immunol. 2022;13:807271. doi: 10.3389/fimmu.2022.807271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vasseur S., Guillaumond F. Lipids in cancer: A global view of the contribution of lipid pathways to metastatic formation and treatment resistance. Oncogenesis. 2022;11:46. doi: 10.1038/s41389-022-00420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Manzo T., Prentice B.M., Anderson K.G., Raman A., Schalck A., Codreanu G.S., Nava Lauson C.B., Tiberti S., Raimondi A., Jones M.A., et al. Accumulation of long-chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells. J. Exp. Med. 2020;217:e20191920. doi: 10.1084/jem.20191920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zurier R.B., Rossetti R.G., Seiler C.M., Laposata M. Human peripheral blood T lymphocyte proliferation after activation of the T cell receptor: Effects of unsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids. 1999;60:371–375. doi: 10.1016/S0952-3278(99)80015-5. [DOI] [PubMed] [Google Scholar]
- 217.Lima T.M., Kanunfre C.C., Pompeia C., Verlengia R., Curi R. Ranking the toxicity of fatty acids on Jurkat and Raji cells by flow cytometric analysis. Toxicol. Vitr. 2002;16:741–747. doi: 10.1016/S0887-2333(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 218.Xu S., Chaudhary O., Rodriguez-Morales P., Sun X., Chen D., Zappasodi R., Xu Z., Pinto A.F.M., Williams A., Schulze I., et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity. 2021;54:1561–1577.e7. doi: 10.1016/j.immuni.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fernanda Cury-Boaventura M., Cristine Kanunfre C., Gorjao R., Martins de Lima T., Curi R. Mechanisms involved in Jurkat cell death induced by oleic and linoleic acids. Clin. Nutr. 2006;25:1004–1014. doi: 10.1016/j.clnu.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 220.Stentz F.B., Kitabchi A.E. Palmitic acid-induced activation of human T-lymphocytes and aortic endothelial cells with production of insulin receptors, reactive oxygen species, cytokines, and lipid peroxidation. Biochem. Biophys. Res. Commun. 2006;346:721–726. doi: 10.1016/j.bbrc.2006.05.159. [DOI] [PubMed] [Google Scholar]
- 221.Berod L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K., Sandouk A., Hesse C., Castro C.N., Bahre H., et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014;20:1327–1333. doi: 10.1038/nm.3704. [DOI] [PubMed] [Google Scholar]
- 222.Beier U.H., Angelin A., Akimova T., Wang L., Liu Y., Xiao H., Koike M.A., Hancock S.A., Bhatti T.R., Han R., et al. Essential role of mitochondrial energy metabolism in Foxp3(+) T-regulatory cell function and allograft survival. FASEB J. 2015;29:2315–2326. doi: 10.1096/fj.14-268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Su P., Wang Q., Bi E., Ma X., Liu L., Yang M., Qian J., Yi Q. Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages. Cancer Res. 2020;80:1438–1450. doi: 10.1158/0008-5472.CAN-19-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Herber D.L., Cao W., Nefedova Y., Novitskiy S.V., Nagaraj S., Tyurin V.A., Corzo A., Cho H.I., Celis E., Lennox B., et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pfrieger F.W. Role of cholesterol in synapse formation and function. Biochim. Biophys. Acta. 2003;1610:271–280. doi: 10.1016/S0005-2736(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 226.Ma X., Bi E., Lu Y., Su P., Huang C., Liu L., Wang Q., Yang M., Kalady M.F., Qian J., et al. Cholesterol Induces CD8(+) T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019;30:143–156.e5. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mailer R.K.W., Gistera A., Polyzos K.A., Ketelhuth D.F.J., Hansson G.K. Hypercholesterolemia Enhances T Cell Receptor Signaling and Increases the Regulatory T Cell Population. Sci. Rep. 2017;7:15655. doi: 10.1038/s41598-017-15546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee C., Longo V.D. Fasting vs dietary restriction in cellular protection and cancer treatment: From model organisms to patients. Oncogene. 2011;30:3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- 229.Bagherniya M., Butler A.E., Barreto G.E., Sahebkar A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res. Rev. 2018;47:183–197. doi: 10.1016/j.arr.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 230.Longo V.D., Fontana L. Calorie restriction and cancer prevention: Metabolic and molecular mechanisms. Trends Pharmacol. Sci. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cangemi A., Fanale D., Rinaldi G., Bazan V., Galvano A., Perez A., Barraco N., Massihnia D., Castiglia M., Vieni S., et al. Dietary restriction: Could it be considered as speed bump on tumor progression road? Tumour Biol. 2016;37:7109–7118. doi: 10.1007/s13277-016-5044-8. [DOI] [PubMed] [Google Scholar]
- 232.Longo V.D., Mattson M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.De Groot S., Pijl H., van der Hoeven J.J.M., Kroep J.R. Effects of short-term fasting on cancer treatment. J. Exp. Clin. Cancer Res. 2019;38:209. doi: 10.1186/s13046-019-1189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Raffaghello L., Lee C., Safdie F.M., Wei M., Madia F., Bianchi G., Longo V.D. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. USA. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lee C., Safdie F.M., Raffaghello L., Wei M., Madia F., Parrella E., Hwang D., Cohen P., Bianchi G., Longo V.D. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lee C., Raffaghello L., Brandhorst S., Safdie F.M., Bianchi G., Martin-Montalvo A., Pistoia V., Wei M., Hwang S., Merlino A., et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 2012;4:124ra127. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Phadngam S., Castiglioni A., Ferraresi A., Morani F., Follo C., Isidoro C. PTEN dephosphorylates AKT to prevent the expression of GLUT1 on plasmamembrane and to limit glucose consumption in cancer cells. Oncotarget. 2016;7:84999–85020. doi: 10.18632/oncotarget.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lien E.C., Westermark A.M., Zhang Y., Yuan C., Li Z., Lau A.N., Sapp K.M., Wolpin B.M., Vander Heiden M.G. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature. 2021;599:302–307. doi: 10.1038/s41586-021-04049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Luo H., Chiang H.H., Louw M., Susanto A., Chen D. Nutrient Sensing and the Oxidative Stress Response. Trends Endocrinol. Metab. 2017;28:449–460. doi: 10.1016/j.tem.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yoshino J., Mills K.F., Yoon M.J., Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mai A., Cheng D., Bedford M.T., Valente S., Nebbioso A., Perrone A., Brosch G., Sbardella G., De Bellis F., Miceli M., et al. epigenetic multiple ligands: Mixed histone/protein methyltransferase, acetyltransferase, and class III deacetylase (sirtuin) inhibitors. J. Med. Chem. 2008;51:2279–2290. doi: 10.1021/jm701595q. [DOI] [PubMed] [Google Scholar]
- 242.Keppler B.R., Archer T.K. Chromatin-modifying enzymes as therapeutic targets—Part 1. Expert Opin. Ther. Targets. 2008;12:1301–1312. doi: 10.1517/14728222.12.10.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Salvadori G., Zanardi F., Iannelli F., Lobefaro R., Vernieri C., Longo V.D. Fasting-mimicking diet blocks triple-negative breast cancer and cancer stem cell escape. Cell Metab. 2021;33:2247–2259.e6. doi: 10.1016/j.cmet.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Caffa I., Spagnolo V., Vernieri C., Valdemarin F., Becherini P., Wei M., Brandhorst S., Zucal C., Driehuis E., Ferrando L., et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. 2020;583:620–624. doi: 10.1038/s41586-020-2502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fontana L., Adelaiye R.M., Rastelli A.L., Miles K.M., Ciamporcero E., Longo V.D., Nguyen H., Vessella R., Pili R. Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget. 2013;4:2451–2461. doi: 10.18632/oncotarget.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Amelio I., Melino G., Frezza C. Exploiting tumour addiction with a serine and glycine-free diet. Cell Death Differ. 2017;24:1311–1313. doi: 10.1038/cdd.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mates J.M., Sanchez-Jimenez F.M. Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int. J. Biochem. Cell Biol. 2000;32:157–170. doi: 10.1016/S1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 248.Di Tano M., Raucci F., Vernieri C., Caffa I., Buono R., Fanti M., Brandhorst S., Curigliano G., Nencioni A., de Braud F., et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat. Commun. 2020;11:2332. doi: 10.1038/s41467-020-16243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Komninou D., Leutzinger Y., Reddy B.S., Richie J.P., Jr. Methionine restriction inhibits colon carcinogenesis. Nutr. Cancer. 2006;54:202–208. doi: 10.1207/s15327914nc5402_6. [DOI] [PubMed] [Google Scholar]
- 250.Sinha R., Cooper T.K., Rogers C.J., Sinha I., Turbitt W.J., Calcagnotto A., Perrone C.E., Richie J.P., Jr. Dietary methionine restriction inhibits prostatic intraepithelial neoplasia in TRAMP mice. Prostate. 2014;74:1663–1673. doi: 10.1002/pros.22884. [DOI] [PubMed] [Google Scholar]
- 251.Gao X., Sanderson S.M., Dai Z., Reid M.A., Cooper D.E., Lu M., Richie J.P., Jr., Ciccarella A., Calcagnotto A., Mikhael P.G., et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572:397–401. doi: 10.1038/s41586-019-1437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yoo H.C., Yu Y.C., Sung Y., Han J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020;52:1496–1516. doi: 10.1038/s12276-020-00504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Schvartzman J.M., Thompson C.B., Finley L.W.S. Metabolic regulation of chromatin modifications and gene expression. J. Cell Biol. 2018;217:2247–2259. doi: 10.1083/jcb.201803061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Tischler J., Gruhn W.H., Reid J., Allgeyer E., Buettner F., Marr C., Theis F., Simons B.D., Wernisch L., Surani M.A. Metabolic regulation of pluripotency and germ cell fate through alpha-ketoglutarate. EMBO J. 2019;38:e99518. doi: 10.15252/embj.201899518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Qing G., Li B., Vu A., Skuli N., Walton Z.E., Liu X., Mayes P.A., Wise D.R., Thompson C.B., Maris J.M., et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell. 2012;22:631–644. doi: 10.1016/j.ccr.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pan M., Reid M.A., Lowman X.H., Kulkarni R.P., Tran T.Q., Liu X., Yang Y., Hernandez-Davies J.E., Rosales K.K., Li H., et al. Regional glutamine deficiency in tumours promotes dedifferentiation through inhibition of histone demethylation. Nat. Cell Biol. 2016;18:1090–1101. doi: 10.1038/ncb3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cortellino S., Raveane A., Chiodoni C., Delfanti G., Pisati F., Spagnolo V., Visco E., Fragale G., Ferrante F., Magni S., et al. Fasting renders immunotherapy effective against low-immunogenic breast cancer while reducing side effects. Cell Rep. 2022;40:111256. doi: 10.1016/j.celrep.2022.111256. [DOI] [PubMed] [Google Scholar]
- 258.Lu Z., Xie J., Wu G., Shen J., Collins R., Chen W., Kang X., Luo M., Zou Y., Huang L.J., et al. Fasting selectively blocks development of acute lymphoblastic leukemia via leptin-receptor upregulation. Nat. Med. 2017;23:79–90. doi: 10.1038/nm.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yoshida K., Inoue T., Nojima K., Hirabayashi Y., Sado T. Calorie restriction reduces the incidence of myeloid leukemia induced by a single whole-body radiation in C3H/He mice. Proc. Natl. Acad. Sci. USA. 1997;94:2615–2619. doi: 10.1073/pnas.94.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bradey A.L., Fitter S., Duggan J., Wilczek V., Williams C.M.D., Cheney E.A., Noll J.E., Tangseefa P., Panagopoulos V., Zannettino A.C.W. Calorie restriction has no effect on bone marrow tumour burden in a Vk*MYC transplant model of multiple myeloma. Sci. Rep. 2022;12:13128. doi: 10.1038/s41598-022-17403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kouidhi S., Ben Ayed F., Benammar Elgaaied A. Targeting Tumor Metabolism: A New Challenge to Improve Immunotherapy. Front. Immunol. 2018;9:353. doi: 10.3389/fimmu.2018.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sukumar M., Liu J., Ji Y., Subramanian M., Crompton J.G., Yu Z., Roychoudhuri R., Palmer D.C., Muranski P., Karoly E.D., et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Investig. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ma E.H., Bantug G., Griss T., Condotta S., Johnson R.M., Samborska B., Mainolfi N., Suri V., Guak H., Balmer M.L., et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017;25:345–357. doi: 10.1016/j.cmet.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 264.Roy D.G., Chen J., Mamane V., Ma E.H., Muhire B.M., Sheldon R.D., Shorstova T., Koning R., Johnson R.M., Esaulova E., et al. Methionine Metabolism Shapes T Helper Cell Responses through Regulation of Epigenetic Reprogramming. Cell Metab. 2020;31:250–266.e9. doi: 10.1016/j.cmet.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 265.O’Flanagan C.H., Smith L.A., McDonell S.B., Hursting S.D. When less may be more: Calorie restriction and response to cancer therapy. BMC Med. 2017;15:106. doi: 10.1186/s12916-017-0873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hursting S.D., Dunlap S.M., Ford N.A., Hursting M.J., Lashinger L.M. Calorie restriction and cancer prevention: A mechanistic perspective. Cancer Metab. 2013;1:10. doi: 10.1186/2049-3002-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Pomatto-Watson L.C.D., Bodogai M., Bosompra O., Kato J., Wong S., Carpenter M., Duregon E., Chowdhury D., Krishna P., Ng S., et al. Daily caloric restriction limits tumor growth more effectively than caloric cycling regardless of dietary composition. Nat. Commun. 2021;12:6201. doi: 10.1038/s41467-021-26431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Manukian G., Kivolowitz C., DeAngelis T., Shastri A.A., Savage J.E., Camphausen K., Rodeck U., Zarif J.C., Simone N.L. Caloric Restriction Impairs Regulatory T cells Within the Tumor Microenvironment After Radiation and Primes Effector T cells. Int. J. Radiat. Oncol. Biol. Phys. 2021;110:1341–1349. doi: 10.1016/j.ijrobp.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Vernieri C., Fuca G., Ligorio F., Huber V., Vingiani A., Iannelli F., Raimondi A., Rinchai D., Frige G., Belfiore A., et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discov. 2022;12:90–107. doi: 10.1158/2159-8290.CD-21-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ferrere G., Tidjani Alou M., Liu P., Goubet A.G., Fidelle M., Kepp O., Durand S., Iebba V., Fluckiger A., Daillere R., et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207. doi: 10.1172/jci.insight.145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ajona D., Ortiz-Espinosa S., Lozano T., Exposito F., Calvo A., Valencia K., Redrado M., Remirez A., Lecanda F., Alignani D., et al. Short-term starvation reduces IGF-1 levels to sensitize lung tumors to PD-1 immune checkpoint blockade. Nat. Cancer. 2020;1:75–85. doi: 10.1038/s43018-019-0007-9. [DOI] [PubMed] [Google Scholar]
- 272.Di Biase S., Lee C., Brandhorst S., Manes B., Buono R., Cheng C.W., Cacciottolo M., Martin-Montalvo A., de Cabo R., Wei M., et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell. 2016;30:136–146. doi: 10.1016/j.ccell.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Pietrocola F., Pol J., Vacchelli E., Rao S., Enot D.P., Baracco E.E., Levesque S., Castoldi F., Jacquelot N., Yamazaki T., et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell. 2016;30:147–160. doi: 10.1016/j.ccell.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Vella V., Lappano R., Bonavita E., Maggiolini M., Clarke R.B., Belfiore A., De Francesco E.M. Insulin/IGF Axis and the Receptor for Advanced Glycation End Products: Role in Meta-inflammation and Potential in Cancer Therapy. Endocr. Rev. 2023;44:693–723. doi: 10.1210/endrev/bnad005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wang X., Yang Q., Liao Q., Li M., Zhang P., Santos H.O., Kord-Varkaneh H., Abshirini M. Effects of intermittent fasting diets on plasma concentrations of inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Nutrition. 2020;79–80:110974. doi: 10.1016/j.nut.2020.110974. [DOI] [PubMed] [Google Scholar]
- 276.Cortellino S.Q.V., Delfanti G., Blaževitš O., Chiodoni C., Maurea N., Di Mauro A., Tatangelo F., Pisati F., Shmahala A., Lazzeri S., et al. Fasting mimicking diet delays cancer growth and reduces immune thearapy- associated cardiovascular and systemic side effects. Nat. Commun. 2023 doi: 10.1038/s41467-023-41066-3. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hasegawa A., Iwasaka H., Hagiwara S., Asai N., Nishida T., Noguchi T. Alternate day calorie restriction improves systemic inflammation in a mouse model of sepsis induced by cecal ligation and puncture. J. Surg. Res. 2012;174:136–141. doi: 10.1016/j.jss.2010.11.883. [DOI] [PubMed] [Google Scholar]
- 278.Venereau E., Casalgrandi M., Schiraldi M., Antoine D.J., Cattaneo A., De Marchis F., Liu J., Antonelli A., Preti A., Raeli L., et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kazama H., Ricci J.E., Herndon J.M., Hoppe G., Green D.R., Ferguson T.A. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gdynia G., Sauer S.W., Kopitz J., Fuchs D., Duglova K., Ruppert T., Miller M., Pahl J., Cerwenka A., Enders M., et al. The HMGB1 protein induces a metabolic type of tumour cell death by blocking aerobic respiration. Nat. Commun. 2016;7:10764. doi: 10.1038/ncomms10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hubert P., Roncarati P., Demoulin S., Pilard C., Ancion M., Reynders C., Lerho T., Bruyere D., Lebeau A., Radermecker C., et al. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J. Immunother. Cancer. 2021;9:e001966. doi: 10.1136/jitc-2020-001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mao Y.Q., Huang J.T., Zhang S.L., Kong C., Li Z.M., Jing H., Chen H.L., Kong C.Y., Huang S.H., Cai P.R., et al. The antitumour effects of caloric restriction are mediated by the gut microbiome. Nat. Metab. 2023;5:96–110. doi: 10.1038/s42255-022-00716-4. [DOI] [PubMed] [Google Scholar]
- 283.Vetizou M., Pitt J.M., Daillere R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duong C.P., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillere R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 285.He Y., Fu L., Li Y., Wang W., Gong M., Zhang J., Dong X., Huang J., Wang Q., Mackay C.R., et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021;33:988–1000.e1007. doi: 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 286.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Reigstad C.S., Salmonson C.E., Rainey J.F., III, Szurszewski J.H., Linden D.R., Sonnenburg J.L., Farrugia G., Kashyap P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Jiang S.H., Hu L.P., Wang X., Li J., Zhang Z.G. Neurotransmitters: Emerging targets in cancer. Oncogene. 2020;39:503–515. doi: 10.1038/s41388-019-1006-0. [DOI] [PubMed] [Google Scholar]
- 289.Wu H., Denna T.H., Storkersen J.N., Gerriets V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019;140:100–114. doi: 10.1016/j.phrs.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 290.Huh J.R., Veiga-Fernandes H. Neuroimmune circuits in inter-organ communication. Nat. Rev. Immunol. 2020;20:217–228. doi: 10.1038/s41577-019-0247-z. [DOI] [PubMed] [Google Scholar]
- 291.Cheng C.W., Adams G.B., Perin L., Wei M., Zhou X., Lam B.S., Da Sacco S., Mirisola M., Quinn D.I., Dorff T.B., et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Collins N., Han S.J., Enamorado M., Link V.M., Huang B., Moseman E.A., Kishton R.J., Shannon J.P., Dixit D., Schwab S.R., et al. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell. 2019;178:1088–1101.e15. doi: 10.1016/j.cell.2019.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Vodnala S.K., Eil R., Kishton R.J., Sukumar M., Yamamoto T.N., Ha N.H., Lee P.H., Shin M., Patel S.J., Yu Z., et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science. 2019;363:eaau0135. doi: 10.1126/science.aau0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Miller B.C., Sen D.R., Al Abosy R., Bi K., Virkud Y.V., LaFleur M.W., Yates K.B., Lako A., Felt K., Naik G.S., et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019;20:326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sade-Feldman M., Yizhak K., Bjorgaard S.L., Ray J.P., de Boer C.G., Jenkins R.W., Lieb D.J., Chen J.H., Frederick D.T., Barzily-Rokni M., et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell. 2018;175:998–1013.e20. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Utzschneider D.T., Charmoy M., Chennupati V., Pousse L., Ferreira D.P., Calderon-Copete S., Danilo M., Alfei F., Hofmann M., Wieland D., et al. T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity. 2016;45:415–427. doi: 10.1016/j.immuni.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 297.Im S.J., Hashimoto M., Gerner M.Y., Lee J., Kissick H.T., Burger M.C., Shan Q., Hale J.S., Lee J., Nasti T.H., et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wu T., Ji Y., Moseman E.A., Xu H.C., Manglani M., Kirby M., Anderson S.M., Handon R., Kenyon E., Elkahloun A., et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol. 2016;1:eaai8593. doi: 10.1126/sciimmunol.aai8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Gabriel S.S., Tsui C., Chisanga D., Weber F., Llano-Leon M., Gubser P.M., Bartholin L., Souza-Fonseca-Guimaraes F., Huntington N.D., Shi W., et al. Transforming growth factor-beta-regulated mTOR activity preserves cellular metabolism to maintain long-term T cell responses in chronic infection. Immunity. 2021;54:1698–1714.e5. doi: 10.1016/j.immuni.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 300.Silva-Santos B., Serre K., Norell H. gammadelta T cells in cancer. Nat. Rev. Immunol. 2015;15:683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 301.Goldberg E.L., Shchukina I., Asher J.L., Sidorov S., Artyomov M.N., Dixit V.D. Ketogenesis activates metabolically protective gammadelta T cells in visceral adipose tissue. Nat. Metab. 2020;2:50–61. doi: 10.1038/s42255-019-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Yang K., Blanco D.B., Chen X., Dash P., Neale G., Rosencrance C., Easton J., Chen W., Cheng C., Dhungana Y., et al. Metabolic signaling directs the reciprocal lineage decisions of alphabeta and gammadelta T cells. Sci. Immunol. 2018;3:eaas9818. doi: 10.1126/sciimmunol.aas9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Jordan S., Tung N., Casanova-Acebes M., Chang C., Cantoni C., Zhang D., Wirtz T.H., Naik S., Rose S.A., Brocker C.N., et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell. 2019;178:1102–1114.e17. doi: 10.1016/j.cell.2019.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mukherjee P., Abate L.E., Seyfried T.N. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin. Cancer Res. 2004;10:5622–5629. doi: 10.1158/1078-0432.CCR-04-0308. [DOI] [PubMed] [Google Scholar]
- 305.Hettiarachchi S.U., Li Y.H., Roy J., Zhang F., Puchulu-Campanella E., Lindeman S.D., Srinivasarao M., Tsoyi K., Liang X., Ayaub E.A., et al. Targeted inhibition of PI3 kinase/mTOR specifically in fibrotic lung fibroblasts suppresses pulmonary fibrosis in experimental models. Sci. Transl. Med. 2020;12:eaay3724. doi: 10.1126/scitranslmed.aay3724. [DOI] [PubMed] [Google Scholar]
- 306.Lane H.A., Wood J.M., McSheehy P.M., Allegrini P.R., Boulay A., Brueggen J., Littlewood-Evans A., Maira S.M., Martiny-Baron G., Schnell C.R., et al. mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin. Cancer Res. 2009;15:1612–1622. doi: 10.1158/1078-0432.CCR-08-2057. [DOI] [PubMed] [Google Scholar]
- 307.Du W., Gerald D., Perruzzi C.A., Rodriguez-Waitkus P., Enayati L., Krishnan B., Edmonds J., Hochman M.L., Lev D.C., Phung T.L. Vascular tumors have increased p70 S6-kinase activation and are inhibited by topical rapamycin. Lab. Investig. 2013;93:1115–1127. doi: 10.1038/labinvest.2013.98. [DOI] [PubMed] [Google Scholar]
- 308.Lastwika K.J., Wilson W., III, Li Q.K., Norris J., Xu H., Ghazarian S.R., Kitagawa H., Kawabata S., Taube J.M., Yao S., et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 309.Lv X.X., Liu S.S., Hu Z.W. Autophagy-inducing natural compounds: A treasure resource for developing therapeutics against tissue fibrosis. J. Asian Nat. Prod. Res. 2017;19:101–108. doi: 10.1080/10286020.2017.1279151. [DOI] [PubMed] [Google Scholar]