Abstract
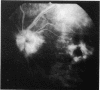
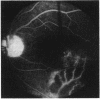
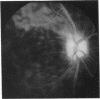
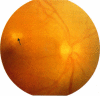
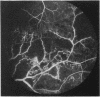
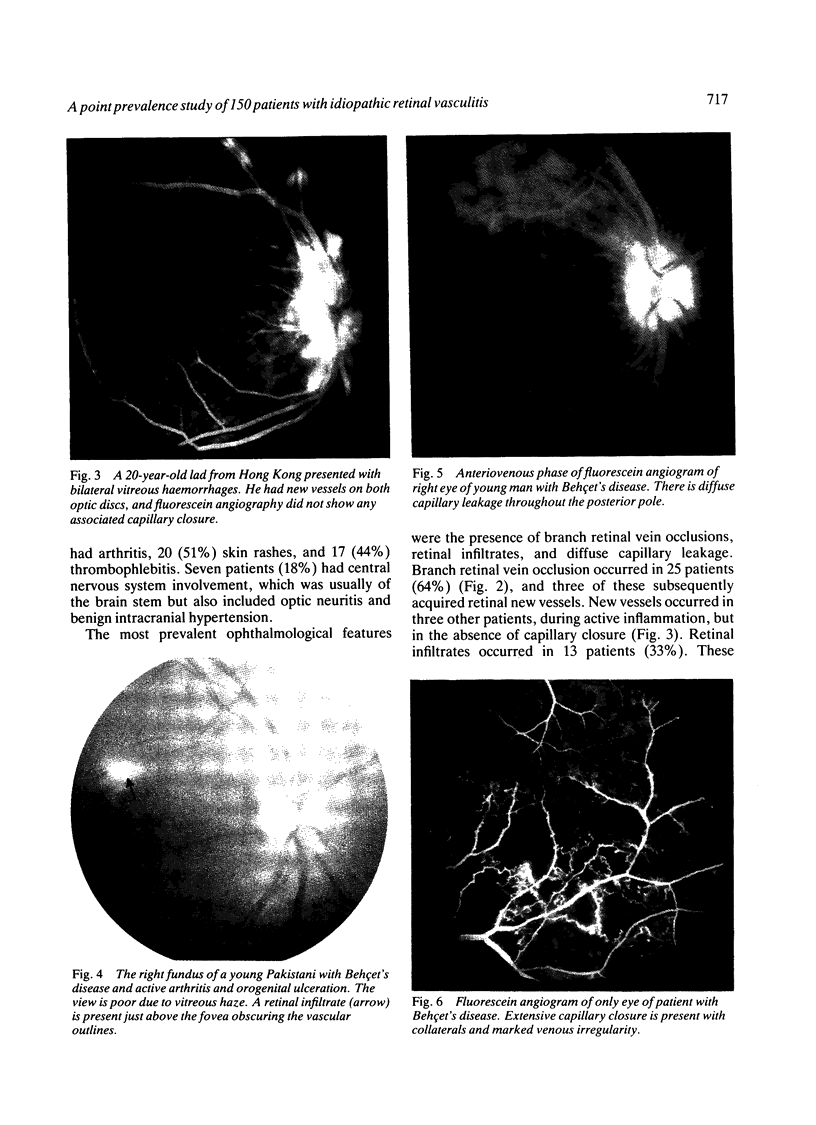
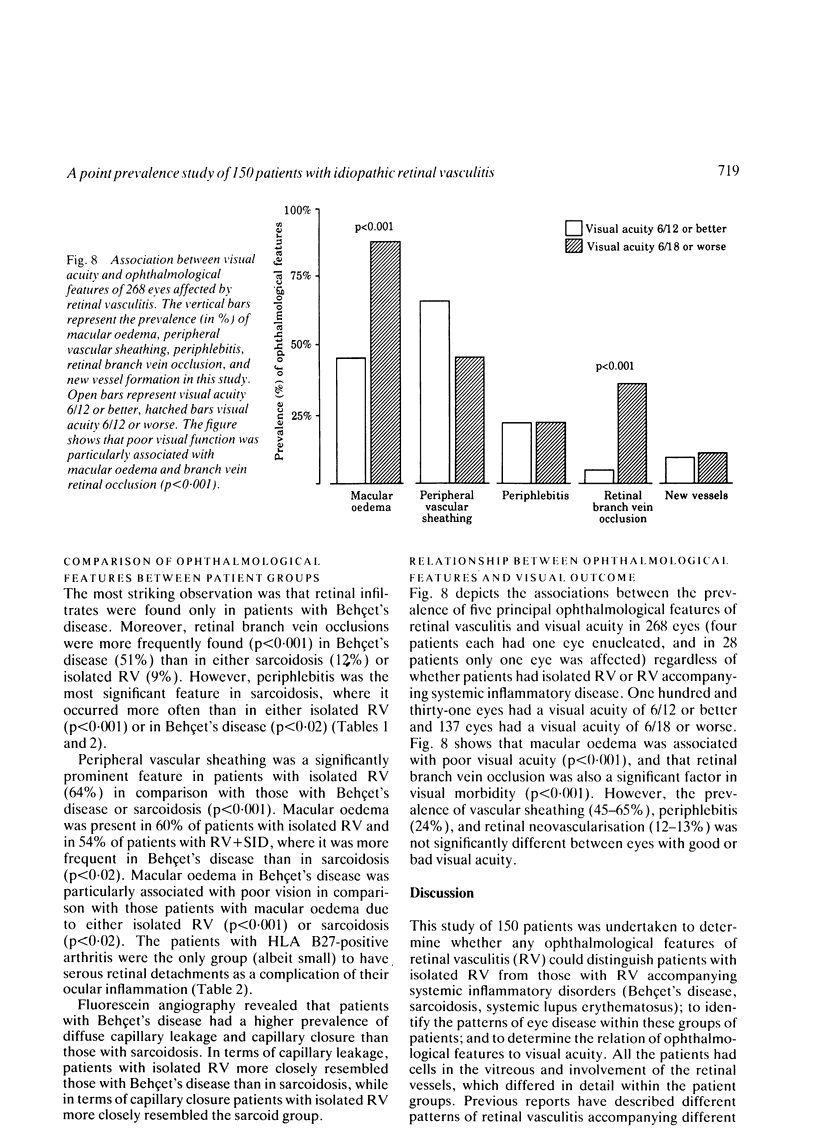
This paper describes the ophthalmological features of 150 patients with idiopathic retinal vasculitis, 67 of whom had isolated retinal vasculitis (RV) and 83 had RV associated with systemic inflammatory disease (RV + SID). The diagnosis of retinal vasculitis was made by ophthalmoscopy and fluorescein angiography, and patients with any identifiable cause (infection, ischaemia, or malignancy) were excluded from the study. Patients with isolated RV tended to have peripheral vascular sheathing, macular oedema, and diffuse capillary leakage. Those with RV accompanying Behçet's disease often had branch vein retinal occlusions and retinal infiltrates together with macular oedema and diffuse capillary leakage; the retinal infiltrates were pathognomonic for Behçet's disease. In sarcoidosis the retina typically showed features of periphlebitis associated with focal vascular leakage. Patients with uveomeningitis, multiple sclerosis, arthritis, or systemic vasculitis showed diffuse retinal capillary leakage associated with a mixture of the other features. Poor visual function was particularly associated with macular oedema and branch vein retinal occlusion, while the retina appeared to 'withstand' the impact of vascular sheathing, periphlebitis, or neovascularisation alone. Within the limitations of a point prevalence study it was concluded that different patterns of retinal vasculitis occur in different systemic inflammatory diseases, and that in isolated retinal vasculitis there is a particular association between peripheral vascular sheathing, macular oedema, and diffuse capillary leakage. In Part 2 we describe the results of examining the sera of these patients for the presence of antiretinal antibodies and circulating immune complexes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Culbertson W. W., Blumenkranz M. S., Pepose J. S., Stewart J. A., Curtin V. T. Varicella zoster virus is a cause of the acute retinal necrosis syndrome. Ophthalmology. 1986 May;93(5):559–569. doi: 10.1016/s0161-6420(86)33701-1. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C., Kasp-Grochowska E., Graham E., Sanders M. D., Faure J. P., de Kozak Y., van Tuyen V. Anti-retinal autoimmunity and circulating immune complexes in patients with retinal vasculitis. Lancet. 1982 Oct 9;2(8302):787–792. doi: 10.1016/s0140-6736(82)92679-4. [DOI] [PubMed] [Google Scholar]
- Egbert P. R., Pollard R. B., Gallagher J. G., Merigan T. C. Cytomegalovirus retinitis in immunosuppressed hosts. II. Ocular manifestations. Ann Intern Med. 1980 Nov;93(5):664–670. doi: 10.7326/0003-4819-93-5-664. [DOI] [PubMed] [Google Scholar]
- Engell T. Neurological disease activity in multiple sclerosis patients with periphlebitis retinae. Acta Neurol Scand. 1986 Feb;73(2):168–172. doi: 10.1111/j.1600-0404.1986.tb03259.x. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Haynes B., Katz P. The spectrum of vasculitis: clinical, pathologic, immunologic and therapeutic considerations. Ann Intern Med. 1978 Nov;89(5 Pt 1):660–676. doi: 10.7326/0003-4819-89-5-660. [DOI] [PubMed] [Google Scholar]
- Gass J. D., Olson C. L. Sarcoidosis with optic nerve and retinal involvement. Arch Ophthalmol. 1976 Jun;94(6):945–950. doi: 10.1001/archopht.1976.03910030475008. [DOI] [PubMed] [Google Scholar]
- Graham E. M., Spalton D. J., Barnard R. O., Garner A., Russell R. W. Cerebral and retinal vascular changes in systemic lupus erythematosus. Ophthalmology. 1985 Mar;92(3):444–448. doi: 10.1016/s0161-6420(85)34018-6. [DOI] [PubMed] [Google Scholar]
- Graham E. Intraocular involvement of T and B cell lymphomas. Eye (Lond) 1987;1(Pt 6):691–698. doi: 10.1038/eye.1987.113. [DOI] [PubMed] [Google Scholar]
- Griffin J. R., Pettit T. H., Fishman L. S., Foos R. Y. Blood-borne Candida endophthalmitis. A clinical and pathologic study of 21 cases. Arch Ophthalmol. 1973 Jun;89(6):450–456. doi: 10.1001/archopht.1973.01000040452002. [DOI] [PubMed] [Google Scholar]
- Hayreh S. S., Rojas P., Podhajsky P., Montague P., Woolson R. F. Ocular neovascularization with retinal vascular occlusion-III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983 May;90(5):488–506. doi: 10.1016/s0161-6420(83)34542-5. [DOI] [PubMed] [Google Scholar]
- Michelson J. B., Chisari F. V. Behçet's disease. Surv Ophthalmol. 1982 Jan-Feb;26(4):190–203. doi: 10.1016/0039-6257(82)90079-0. [DOI] [PubMed] [Google Scholar]
- Perkins E. S. Ocular toxoplasmosis. Br J Ophthalmol. 1973 Jan;57(1):1–17. doi: 10.1136/bjo.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. D., Shilling J. S. Retinal, choroidal, and optic disc involvement in sarcoidosis. Trans Ophthalmol Soc U K. 1976 Apr;96(1):140–144. [PubMed] [Google Scholar]
- Sarkies N. J., Shilling J. S., Russell R. W. Fluorescein angiography in carotid disease. Trans Ophthalmol Soc U K. 1986;105(Pt 4):489–493. [PubMed] [Google Scholar]
- Spalton D. J., Graham E. M., Page N. G., Sanders M. D. Ocular changes in limited forms of Wegener's granulomatosis. Br J Ophthalmol. 1981 Aug;65(8):553–563. doi: 10.1136/bjo.65.8.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Townes A. S., Robinson H., Kaplan S. B., Chandler R. W., Hanissian A. S., Masi A. T. Preliminary criteria for the classification of systemic lupus erythematosus (SLE). Evaluation in early diagnosed SLE and rheumatoid arthritis. Arthritis Rheum. 1974 Mar-Apr;17(2):184–188. doi: 10.1002/art.1780170212. [DOI] [PubMed] [Google Scholar]
- WELCH R. B., MAUMENEE A. E., WAHLEN H. E. Peripheral posterior segment inflammation, vitreous opacities, and edema of the posterior pole. Arch Ophthalmol. 1960 Oct;64:540–549. doi: 10.1001/archopht.1960.01840010542010. [DOI] [PubMed] [Google Scholar]
- Wekerle H. The lesion of acute experimental autoimmune encephalomyelitis. Isolation and membrane phenotypes of perivascular infiltrates from encephalitic rat brain white matter. Lab Invest. 1984 Aug;51(2):199–205. [PubMed] [Google Scholar]