Abstract
Asthma is a complex heterogeneous disease caused by gene–environment interactions. Although numerous genome-wide association studies have been conducted, these interactions have not been systemically investigated. We sought to identify genetic factors associated with the asthma phenotype in 66,857 subjects from the Health Examination Study, Cardiovascular Disease Association Study, and Korea Association Resource Study cohorts. We investigated asthma-associated gene–environment (smoking status) interactions at the level of single nucleotide polymorphisms, genes, and gene sets. We identified two potentially novel (SETDB1 and ZNF8) and five previously reported (DM4C, DOCK8, MMP20, MYL7, and ADCY9) genes associated with increased asthma risk. Numerous gene ontology processes, including regulation of T cell differentiation in the thymus (GO:0033081), were significantly enriched for asthma risk. Functional annotation analysis confirmed the causal relationship between five genes (two potentially novel and three previously reported genes) and asthma through genome-wide functional prediction scores (combined annotation-dependent depletion, deleterious annotation of genetic variants using neural networks, and RegulomeDB). Our findings elucidate the genetic architecture of asthma and improve the understanding of its biological mechanisms. However, further studies are necessary for developing preventive treatments based on environmental factors and understanding the immune system mechanisms that contribute to the etiology of asthma.
Keywords: asthma, gene–environment interactions, Korean Genome and Epidemiology Study, genome-wide association study, smoking status
1. Introduction
Asthma is one of the most prevalent chronic respiratory diseases, with a worldwide burden associated with loss of life and overall morbidity [1,2]. It affected more than 330 million people in 2019 [3,4] and results in approximately 400,000 deaths annually [3,5,6]. The general symptoms of asthma include wheezing, coughing, chest tightness or pain, and shortness of breath [7]. Asthma can lead to respiratory distress, obstructive sleep apnea, psychological disorders, seizures, and other complications [8,9,10].
Asthma is a complex disease caused by a combination of multiple genetic and environmental factors [11,12]. In terms of genetic factors, heritability estimates range between 35 and 95% [13], with those based on twin studies ranging between 50 and 90% [14,15]. The first genome-wide association study (GWAS) for asthma was published in 2007 [16] and showed that genetic variants on chromosome 17q21.1 were associated in cis with transcript levels of ORMDL3. Numerous GWASs for asthma followed, and more than 140 susceptibility loci have been identified [17,18,19,20,21,22,23,24,25]. The identified variants in common risk alleles describe only a small proportion of asthma heritability [17,18,19]. The “missing heritability” is due to rare structural variants, such as copy number variants and insertion–deletion mutations (INDELs) [26,27]. However, exome sequencing revealed that the significance of rare variants in the development of asthma remains unknown [28]. Furthermore, several studies on asthma did not provide strong evidence that structural variants have a role in asthma susceptibility [29,30,31]. Further, the “missing heritability” is due to an overestimation of the role of genetics in asthma development [26,27,32]. Regarding the findings of twin studies, monozygotic twins have been found not only to be genetically identical when compared with dizygotic twins but also to have an environment identical to that of dizygotic twins [33]. Lastly, the “missing heritability” could be due to gene–gene and gene–environment interactions [27]. Systems biology applications that account for highly interconnected molecular networks have been proposed to better understand gene–gene interactions; however, the missing heritability of complex diseases still remains poorly understood. In contrast, gene–environment interaction studies have steadily progressed, and several environmental causes of asthma and allergic diseases have been identified [12,34,35,36,37,38]. Smoking status is a major environmental factor associated with the development of asthma [36,37]. Cigarettes contain nicotine and more than 4000 compounds that bind to and chemically alter or damage DNA [39]. For instance, genetic variants in 17q21 and 20p13-p12 were found to be significantly associated with asthma in patients exposed to smoking [40,41,42,43]. Furthermore, several GWASs have investigated the interactions between single nucleotide polymorphisms (SNPs) and smoking status [44,45,46]. GWAS have made considerable efforts in better understanding missing heritability. However, due to the small number of replicates, insufficient biological plausibility, and lack of statistical power, missing heritability remains poorly understood [47].
Therefore, to further understand the genetic architecture of asthma, we aimed to discover the effects of smoking status on asthma by analyzing genetic information from the Korean Genome and Epidemiology Study (KoGES) [48]. We evaluated gene–smoking interactions using SNP, gene, and gene-set analyses. To prioritize regulatory variants from the GWAS loci, we implemented linkage disequilibrium analyses and publicly available functional annotation tools: combined annotation-dependent depletion (CADD) [49], deleterious annotation of genetic variants using neural networks (DANN) [50], and RegulomeDB [51]. The study expands our knowledge of the genetic contribution to the development of asthma, thereby providing insight into asthma susceptibility and disease mechanisms.
2. Results
2.1. General Characteristics of Study Participants
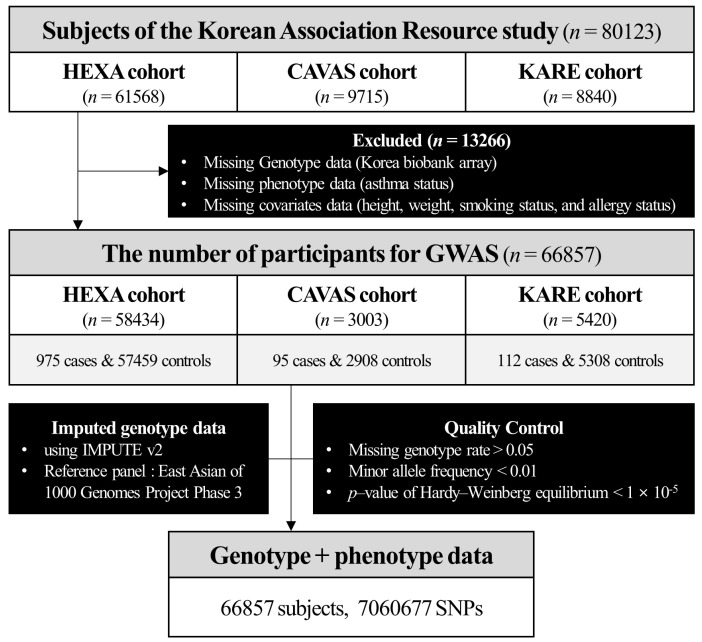
As shown in Table 1, a total of 66,857 participants were enrolled in the current study. The general characteristics of the participants for asthma analysis were described by the defined groups “case” and “control” in the Health Examination Study (HEXA), Cardiovascular Disease Association Study (CAVAS), and Korea Association Resource Study (KARE) cohorts of the KoGES consortium. The HEXA cohort included 975 cases with a mean age of 55.4 ± standard deviation (SD) 8.4 years and 57,479 controls with a mean age of 53.8 ± 8.0 years. The CAVAS cohort included 95 cases and 2908 controls with a mean age of 57.9 ± 7.8 and 55.4 ± 7.8 years, respectively. The KARE cohort included 112 cases and 5308 controls with a mean age of 53.3 ± 7.9 and 51.5 ± 8.5 years, respectively. Age was significantly associated with asthma in all three cohorts (p < 0.005, t-test). The average body mass index (BMI) values of patients with asthma (cases) and controls were 24.3 ± 3.2 and 23.9 ± 2.9 kg/m2 in the HEXA cohort (p = 0.0002, t-test), 25.5 ± 3.4 and 24.5 ± 3.0 kg/m2 in the CAVAS cohort (p = 0.0002, t-test), and 25.0 ± 3.5 and 24.6 ± 3.0 kg/m2 in the KARE cohort (p = 0.1536, t-test), respectively. The male-to-female ratio (SEX) of cases and controls was 29.0% and 71.0% in the HEXA cohort, 38.9% and 61.1% in the CAVAS cohort, and 34.8% and 65.2% in the KARE cohort, respectively. The SEX was significantly associated with asthma in the HEXA and KARE cohorts. The allergy (ALLER) status was significantly associated with asthma in all three cohorts (p < 0.0001, chi-square test). To investigate the effect of smoking status on asthma, we performed an association analysis, using a logistic regression model adjusted for age, sex, BMI, and ALLER status. A significant association between smoking status and asthma was found in both the HEXA (p = 0.0003, logistic regression) and KARE cohorts (p = 0.0129, logistic regression). However, such an association was not found in the CAVAS cohort (p = 0.6627, logistic regression).
Table 1.
General characteristics of participants from the HEXA, CAVAS, and KARE cohorts.
| 9 | HEXA | p-Value | CAVAS | p-Value | KARE | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Case (n = 975) |
Control (n = 57,459) |
Case (n = 95) |
Control (n = 2908) |
Case (n = 112) |
Control (n = 5308) |
||||
| SEX | <0.0001 | 0.8325 | 0.0048 | ||||||
| Male | 283 (29.0%) |
19,924 (34.7%) |
37 (38.9%) |
1164 (40.0%) |
39 (34.8%) |
2563 (48.3%) |
|||
| Female | 692 (71.0%) |
37,535 (65.3%) |
58 (61.1%) |
1744 (60.0%) |
73 (65.2%) |
2745 (51.7%) |
|||
| AGE (years) a | 55.4 ± 8.4 | 53.8 ± 8.0 | <0.0001 | 57.9 ±7.8 | 55.4 ± 7.8 | 0.0025 | 53.3 ± 7.9 | 51.5 ± 8.5 | 0.0026 |
| BMI (kg/m2) b | 24.3 ± 3.2 | 23.9 ± 2.9 | 0.0002 | 25.5 ±3.4 | 24.5 ± 3.0 | 0.0002 | 25.0 ± 3.5 | 24.6 ± 3.0 | 0.1536 |
| ALLER status c | <0.0001 | <0.0001 | <0.0001 | ||||||
| Non-ALLER | 727 (74.6%) |
53,642 (93.4%) |
74 (77.9%) |
2695 (92.7%) |
86 (76.8%) |
5015 (94.5%) |
|||
| ALLER | 248 (25.4%) |
3817 (6.6%) |
21 (22.1%) |
213 (7.3%) |
26 (23.2%) |
293 (5.5%) |
|||
| Smoking status d, e | 0.0003 | 0.6627 | 0.0129 | ||||||
| Non-smokers | 721 (73.9%) |
42,070 (73.2%) |
72 (75.8%) |
2123 (73.0%) |
71 (63.4%) |
3173 (59.8%) |
|||
| Smokers | 254 (26.1%) |
15,389 (26.8%) |
23 (24.2%) |
785 (27.0%) |
41 (37.6%) |
2135 (40.2%) |
|||
a Means ± standard deviation (SD); b body mass index (BMI); c allergy status (ALLER); d smoking status (Non-smoker: never smoker/Smoker: former smoker or current smoker); e the association between smoking status and asthma was analyzed by a logistic regression model adjusted for age, sex, BMI, and ALLER status.
2.2. GWAS Results
We performed gene–environment (smoking status) interaction analysis (GWAS) to identify the genetic factors (7,060,677 SNPs from 66,857 subjects from the HEXA, CAVAS, and KARE cohorts) associated with asthma, which were analyzed after genotype quality control (Figure 1). The p-values for the gene–environmental interactions factor were determined using the PLINK software (v1.90) [52], and the “--interaction” option. In Figure S1a, the quantile–quantile (Q–Q) plot showed no inflation of p-values. The lambda GC (λGC, genomic control) [53] in our analysis was 0.98. Figure S1b shows the Manhattan plot of the p-values in the GWAS gene–environment interaction analysis for asthma, where the horizontal red line denotes the threshold for a 0.05 genome-wide significance level by a Bonferroni correction of 7.08 × 10–9. Table 2 summarizes the top 40 SNPs with p-values less than the nominal significance level of 1.00 × 10–5. Notably, the most significant SNP–smoking status interacting signal was rs77079226 (OR = 3.083, 95% CI 1.985–4.788, p = 5.37 × 10−7), located in TMEM74 on chromosome 8. This study anticipated a strong interaction between rs77079226 and smoking status in asthma, indicating the deleterious effect of rs77079226(C). Conversely, in the case of rs2292731 (OR = 0.637, 95% CI 0.523–0.775, p = 6.68 × 10−6), we found an protective interaction effect between rs2292731 and smoking status on the risk of asthma. Furthermore, KDM4C [54,55,56,57] and MMP20 [58,59,60] were previously known to be associated with asthma.
Figure 1.
Schematic representation of the sample selection and study design.
Table 2.
SNPs most significantly associated with smoking and the development of asthma (p ≤ 1.0 × 10–5).
| CHR a | rsID b | Alt c | MAF d | OR | 95% CI | STAT | p-Value | Location | Nearest Gene |
|---|---|---|---|---|---|---|---|---|---|
| 8 | rs77079226 | C | 0.034 | 3.083 | 1.985–4.788 | 5.012 | 5.37 × 10–7 | Downstream transcript | TMEM74 |
| 10 | rs17153428 | A | 0.297 | 1.618 | 1.329–1.970 | 4.791 | 1.66 × 10–6 | Intron | RP11-383C5.4 |
| 22 | rs4823536 | G | 0.103 | 2.013 | 1.511–2.682 | 4.781 | 1.74 × 10–6 | Intergenic | MIR3201 |
| 2 | rs7563259 | A | 0.382 | 1.566 | 1.299–1.889 | 4.692 | 2.70 × 10–6 | Intergenic | RNU5E-7P |
| 10 | rs74743572 | G | 0.292 | 1.605 | 1.317–1.957 | 4.689 | 2.74 × 10–6 | Intron | RP11-383C5.4 |
| 12 | rs17837082 | A | 0.124 | 1.882 | 1.444–2.453 | 4.679 | 2.88 × 10–6 | Intron | RP11-121E16.1 |
| 2 | rs7602146 | G | 0.382 | 1.563 | 1.296–1.886 | 4.673 | 2.97 × 10–6 | Intergenic | RNU5E-7P |
| 5 | rs1469393 | T | 0.031 | 3.020 | 1.899–4.802 | 4.671 | 3.00 × 10–6 | Upstream transcript | FAM81B |
| 2 | rs6725714 | G | 0.382 | 1.563 | 1.296–1.885 | 4.670 | 3.01 × 10–6 | Intergenic | RNU5E-7P |
| 10 | rs79219793 | G | 0.334 | 1.576 | 1.301–1.910 | 4.652 | 3.29 × 10–6 | Intron | RP11-383C5.4 |
| 2 | rs10929394 | C | 0.383 | 1.557 | 1.291–1.877 | 4.630 | 3.67 × 10–6 | Intergenic | RNU5E-7P |
| 10 | rs57227860 | G | 0.464 | 0.644 | 0.533–0.778 | –4.568 | 4.93 × 10–6 | Upstream transcript | CDH23 |
| 19 | rs8104061 | A | 0.174 | 1.704 | 1.355–2.143 | 4.561 | 5.10 × 10–6 | Intron | ZNF8 |
| 9 | rs2765969 | T | 0.337 | 1.552 | 1.285–1.875 | 4.557 | 5.18 × 10–6 | Intergenic | RPL4P5 |
| 19 | rs112884174 | C | 0.174 | 1.703 | 1.355–2.141 | 4.557 | 5.19 × 10–6 | Intron | ZNF8 |
| 19 | rs10421600 | A | 0.174 | 1.702 | 1.354–2.140 | 4.552 | 5.30 × 10–6 | Intron | ZNF8 |
| 19 | rs10420807 | C | 0.174 | 1.702 | 1.354–2.140 | 4.552 | 5.30 × 10–6 | Intron | ZNF8 |
| 19 | rs7253055 | A | 0.174 | 1.700 | 1.352–2.138 | 4.543 | 5.56 × 10–6 | Intron | ZNF8 |
| 3 | rs144605956 | G | 0.016 | 5.094 | 2.522–10.29 | 4.539 | 5.64 × 10–6 | Intergenic | RP11-654C22.2 |
| 12 | rs74497942 | A | 0.131 | 1.836 | 1.412–2.387 | 4.538 | 5.68 × 10–6 | Intron | RP11-121E16.1 |
| 9 | rs2986259 | C | 0.350 | 1.543 | 1.278–1.862 | 4.508 | 6.55 × 10–6 | Intergenic | KDM4C |
| 11 | rs2292731 | T | 0.385 | 0.637 | 0.523–0.775 | –4.504 | 6.68 × 10–6 | Intergenic | MMP20 |
| 19 | rs57831195 | C | 0.070 | 2.130 | 1.532–2.961 | 4.498 | 6.85 × 10–6 | Intergenic | RFPL4A |
| 12 | rs61432389 | C | 0.131 | 1.820 | 1.402–2.362 | 4.495 | 6.97 × 10–6 | Intron | RP11-121E16.1 |
| 2 | rs6754791 | T | 0.381 | 1.536 | 1.273–1.853 | 4.486 | 7.26 × 10–6 | Intergenic | RNU5E-7P |
| 2 | rs6726202 | G | 0.381 | 1.536 | 1.273–1.852 | 4.484 | 7.32 × 10–6 | Intergenic | RNU5E-7P |
| 2 | rs4669006 | A | 0.374 | 1.538 | 1.274–1.857 | 4.480 | 7.46 × 10–6 | Intergenic | RNU5E-7P |
| 12 | rs17837077 | A | 0.130 | 1.823 | 1.402–2.371 | 4.477 | 7.56 × 10–6 | Intron | RP11-121E16.1 |
| 2 | rs4566338 | C | 0.372 | 1.536 | 1.273–1.855 | 4.469 | 7.86 × 10–6 | Intergenic | RNU5E-7P |
| 1 | rs34207591 | G | 0.090 | 1.990 | 1.471–2.692 | 4.466 | 7.97 × 10–6 | Upstream transcript | SETDB1 |
| 1 | rs71624514 | T | 0.090 | 1.990 | 1.471–2.692 | 4.466 | 7.97 × 10–6 | Upstream transcript | SETDB1 |
| 12 | rs7488720 | G | 0.131 | 1.815 | 1.397–2.359 | 4.461 | 8.17 × 10–6 | Intron | RP11-121E16.1 |
| 12 | rs75289086 | C | 0.133 | 1.805 | 1.392–2.341 | 4.452 | 8.52 × 10–6 | Intron | RP11-121E16.1 |
| 9 | rs2997570 | T | 0.335 | 1.537 | 1.272–1.858 | 4.446 | 8.76 × 10–6 | Intergenic | KDM4C |
| 12 | rs78145337 | C | 0.130 | 1.812 | 1.394–2.356 | 4.436 | 9.16 × 10–6 | Intron | RP11-121E16.1 |
| 10 | rs17153422 | C | 0.286 | 1.568 | 1.285–1.912 | 4.431 | 9.40 × 10–6 | Intron | RP11-383C5.3 |
| 9 | rs2997577 | A | 0.343 | 1.532 | 1.268–1.850 | 4.428 | 9.49 × 10–6 | Intergenic | KDM4C |
| 16 | rs12928443 | A | 0.187 | 1.677 | 1.334–2.109 | 4.426 | 9.58 × 10–6 | Intergenic | CTD-2535I10.1 |
| 12 | rs111721834 | C | 0.131 | 1.805 | 1.389–2.345 | 4.419 | 9.90 × 10–6 | Intron | RP11-121E16.1 |
| 9 | rs2997572 | T | 0.336 | 1.533 | 1.268–1.853 | 4.418 | 9.96 × 10–6 | Intergenic | KDM4C |
a Chromosome; b hg19, dbSNP150 version; c alternative allele; d minor allele frequency.
2.3. Gene Analysis and Gene-Set Analysis
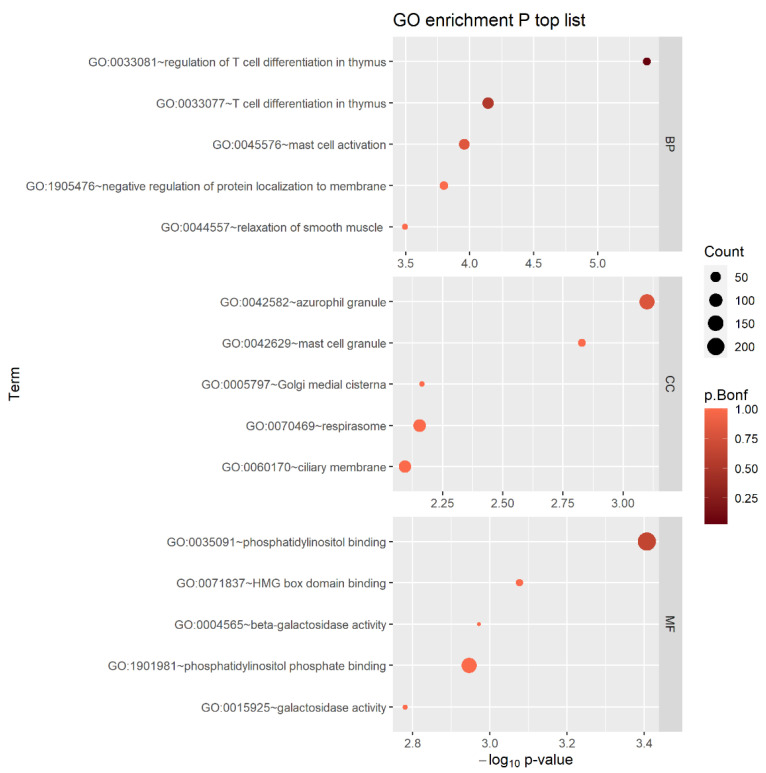
Using multi-marker analysis of genomic annotation (MAGMA) v1.08 [61] with the SNP-wise mean model integrated into the Functional Mapping and Annotation (FUMA) v1.4.0 platform (https://fuma.ctglab.nl/ (accessed on 1 June 2023)) [62], all SNPs were mapped to 18,792 genes. Figure S2 shows the Q−Q plot, which confirmed that there was no inflation in the test statistics (λGC = 0.98), and the Manhattan gene analysis plot. The glycerophosphodiester phosphodiesterase domain containing 1 (GDPD1) gene was found to have the lowest p-value, 5.75 × 10−5; however, the p-value was not statistically significant (p-value < 2.66 × 10−6 (0.05/18792)). The top 20 genes with a p-value < 1.00 × 10−3 are listed in Table S1. Among these, ADCY9 [63,64], MYL7 [65], and DOCK8 [66,67,68] have been previously reported to be associated with asthma. Gene-set analysis was performed using MAGMA integrated into FUMA, and the gene-set p-values were computed using the gene-based p-value for gene ontology (GO) terms, obtained from the Molecular Signatures Database (MsigDB) v7.0 (Gene Set Enrichment Analysis, UC San Diego, https://www.gsea-msigdb.org/gsea/msigdb/index.jsp (accessed on 1 June 2023)) [69,70,71]. In total, 9988 gene sets from the GO database (7343 GO biological processes (BPs), 1001 GO cellular components (CCs), and 1644 GO molecular functions (MFs)) were tested. The most significant GO process, which satisfied the Bonferroni-corrected significance level of α = 5.01 × 10−6 (p = 4.12 × 10−6), was the regulation of T cell differentiation in thymus (GO:0033081). The thymus is important to the immune system and contributes to the etiology of asthma [72,73,74]. The top five gene sets for each of the three GO sub-ontologies are shown in Figure 2. Among the 15 identified gene sets, the following were previously reported to be associated with asthma: BP—T cell differentiation in thymus (GO:0033077) [74]; BP—mast cell activation (GO:0045576) [75,76]; BP—relaxation of smooth muscle (GO:0044557) [77]; CC—azurophil granule (GO:0042582) [78,79]; CC—mast cell granule (GO:0042629) [80]; CC—ciliary membrane (GO:0060170) [81]; MF—beta galactosidase activity (GO:0004565) [82]; and MF—galactosidase activity (GO:0015925) [82].
Figure 2.
Bubble plot showing GO enrichment analysis (three sub-ontologies and top five enriched GO terms). Dot color corresponds to the Bonferroni-adjusted p-values and dot size to the number of genes mapped to the GO terms.
The regulation of T cell differentiation in the thymus (GO:0033081) is associated with nicotine as a main component of cigarettes via smoking [83,84,85]. In previous studies, nicotine exposure was found to affect the thymus, an important organ of the immune system, and to interfere with epithelial cell adhesion and growth [83,84]. Nicotine leads to T cell downregulation and maturation and to T helper (Th)1/Th2 imbalance and reduced immunity [83,84,86,87,88]. Nicotine also activates the nicotinic acetylcholine receptor (nAChR) and modulates thymocyte development [83]. T cell nicotinic responses were demonstrated to occur through the α7 subunit [84]. α7 nAChR activation triggers extracellular Ca2+ influx associated with upregulated Fas expression [89]. In a previous study, α7 nAChR was activated, and its levels increased concomitantly with caspase expression and apoptosis rate [90]. Moreover, α7 nAChR was shown to mediate nicotine pro-apoptotic effects on thymocytes by upregulating Fas expression and the Fas-mediated apoptotic pathway [91].
2.4. Functional Annotation
We applied follow-up bioinformatics analyses to two novel genes (SETDB1 and ZNF8), identified in this study, and five previously reported genes (KDM4C, DOCK8, MMP20, MYL7, and ADCY9) using genome-wide functional prediction scores (CADD, DANN, and RegulomeDB). For the SETDB1 gene, three variants had CADD scores > 10 or DANN scores > 0.7 and were associated with four transcription factor (TF)-binding sites. These TFs were previously reported to be associated with asthma (Table 3 and Table S2) [92,93,94,95,96,97]. In the ZNF8 gene, nine variants were associated with eight TF-binding sites (Table S2), and four variants had DANN scores > 0.7 (deleterious, Table 3). Among the eight TFs, EZH2 and POLR2A have been associated with the pathogenesis of asthma [98,99]. Two KDM4C variants, ten DOCK8 variants, one MMP20 variant, two MYL7 variants, and eight ADCY9 variants had CADD, DANN, or RegulomeDB rank scores >10.0, >0.7, or ≤3, respectively (Table S2). Among these, six variants were associated with TF-binding sites, and 31 TFs were associated with the pathogenesis of asthma (Table 3). However, we did not find significant results for KDM4C and MYL7.
Table 3.
Thirteen SNPs with asthma-related transcription factors identified using functional annotation.
| CHR | SNP | CADD a | DANN b | Transcription Factors c | Rank Score d | Gene |
|---|---|---|---|---|---|---|
| 1 | rs139189121 | 11.940 | 0.792 | AR [92] | 5 | SETDB1 |
| 1 | rs75406390 | 12.280 | 0.836 | AR [92] | 5 | SETDB1 |
| 1 | rs59024312 | 8.982 | 0.833 | FOXA1 [93], GATA3 [94,95], GATA6 [96,97] | 4 | SETDB1 |
| 19 | rs8104061 | 0.751 | 0.718 | EZH2 [98] | 2b | ZNF8 |
| 19 | rs112884174 | 3.736 | 0.812 | EZH2 [98] | 4 | ZNF8 |
| 19 | rs260498 | 10.310 | 0.895 | POLR2A [99] | 4 | ZNF8 |
| 19 | rs11671486 | 11.590 | 0.886 | POLR2A [99] | 1f | ZNF8 |
| 11 | rs2245803 | 17.260 | 0.985 | RXRA [100,101], FOXP1 [102], NFIL3 [103,104], RARA [105], SMAD4 [106] | 3a | MMP20 |
| 16 | rs2532008 | 0.206 | 0.558 | RXRA [100,101], ZNF341 [107,108], PRDM10 [109,110] | 2b | ADCY9 |
| 16 | rs2532007 | 0.350 | 0.382 | ATF3 [111,112], BCL6 [113,114], EGR1 [115,116], ELF3 [117], EP300 [118,119], FOXA1 [93], FOXA2 [94,95], FOXA3 [94,120], HDAC2 [121,122], IRF2 [123], JUND [124,125], KAT8 [126,127], NFIL3 [103,104], NFYC [128], PRDM1 [129,130], RAD21 [131,132], RARA [105], RXRA [100,101], RXRB [133], SMAD4 [106], SP1 [134], THRB [135,136], YY1 [137,138] ZEB2 [139], and USF1 [140,141] | 2b | ADCY9 |
| 16 | rs384067 | 2.631 | 0.677 | CTCF [142,143], CREB1 [144,145] | 2b | ADCY9 |
| 9 | rs10814023 | 16.070 | 0.540 | POLR2A [99], CTCF [142,143] | 3a | DOCK8 |
| 9 | rs10758219 | 13.540 | 0.851 | POLR2A [99], CTCF [142,143] | 3a | DOCK8 |
a The CADD PHRED score 10 means risk of top rank 10%, 20 means risk of top rank 1%; b the DANN score ranges from 0 to 1, where 1 represents the highest possibility for pathogenicity; c asthma-related transcription factor that controls the rate of transcription of genetic information from DNA to messenger RNA by binding to a specific DNA sequence; d scores range from 1 to 7, with lower values indicating that the variant is more likely to be located in the functional region.
3. Discussion
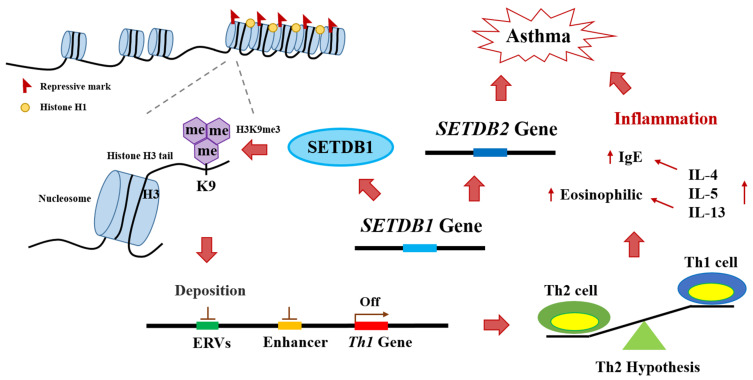
In this study, we conducted a gene–smoking interaction analysis to identify candidate genetic markers associated with asthma. We combined data from Korean multi-cohorts, (HEXA, CAVAS, and KARE) and identified two novel genes. We further contributed to understanding the functional associations of five previously identified asthma-associated genes. Using follow-up bioinformatics analyses, we also confirmed that two novel genes (SETDB1 and ZNF8) and three previously reported genes (DOCK8, MMP20, and ADCY9) are potential asthma-associated genes. The SETDB1 encodes a histone lysine N-methyl transferase; this important enzyme, involved in histone modification, is related to the Th1 gene downregulation mechanism in asthma [72,73]. As shown in Figure 3, SETDB1 causes epigenetic modifications through methylation of histone H3, which surrounds the DNA on the chromosome [72,146]. When histone H3 lysine 9 (H3K9) is methylated, deposition occurs in endogenous retroviruses and enhancer regions, which are necessary for Th1 gene expression [72,73]. Downregulation of the Th1 gene leads to overexpression of the Th2 gene, which increases the number of Th2 cells, causing Th1/Th2 cell imbalance [73]. Th2 cells secrete IL-4 and IL-5 and increase IgE levels and eosinophil numbers, which can trigger inflammation, leading to asthma [146,147,148]. Moreover, SETDB1 can induce macrophage recruitment by promoting AKT/mTOR-dependent CSF-1 induction and secretion [149]. Increased macrophage levels due to overexpression of SETDB1 can cause inflammation by causing an increase in the pro-inflammatory cytokine IL-8 [150,151,152]. High expression of IL-4 by Th2 cell upregulation hyperstimulates M0 macrophages to differentiate into M2 macrophages [153]. Upregulated M2 macrophages overexpress monocyte chemotactic protein-1 (MCP-1), IL-8, IL-13, etc., which can increase the risk of inflammation and asthma onset [154,155,156]. In addition, SETDB1 interacts with the asthma-related gene SETDB2 [157]; the latter antagonizes the KDM4C gene to control H3K9 methylation [157], which affects the expression of ORMDL3, a well-known asthma-related gene [55,158]. Therefore, SETDB2 dysregulation is associated with genomic instability and increased H3K9 methylation [157], leading to Th1/Th2 cell imbalance and asthma onset [73].
Figure 3.
SETDB1 plays an important role in asthma induction through histone 3 lysine 9 (H3K9) methylation. ERVs: Endogenous retroviruses.
Furthermore, several studies have shown that nicotine from smoking can interact with SETDB1 and induce asthma by causing Th1/Th2 cell imbalance [83,84,85,159,160]. In particular, these facts need to be investigated in conjunction with late-onset asthma (LOA) disease studies [161]. The phenotype of LOA is divided into two types according to the presence or absence of eosinophilic inflammation, Th2 and non–Th2-related LOA [162,163]. Th2-related LOA has been reported to be associated with sinusitis, nasal polyps, and sometimes aspirin-exacerbated respiratory disease (AERD) [164]. This type has recently been defined as uncontrolled asthma, often with severe symptoms from the onset [165]. Non-Th2-related LOA has been reported to be associated with gender, obesity, smoking, age, and corticosteroids and requires other treatment strategies, such as diet, macrolides, or smoking cessation [161,162]. In this study, nicotine exposure through smoking in adulthood degraded T cell regulation and maturation, induced Th1/Th2 imbalance and reduced immunity [83,84,86,87,88], and upregulated Fas expression and the Fas-mediated apoptosis pathway through α7 nAChR activation [91]. It has been suggested that non-Th2-related LOA can develop into severe Th2-related LOA. Further, this study suggested that the upregulation of SETDB1 by genetic variants, such as rs139189121, rs75406390, and rs59024312 (Table 3), can accelerate Th2-related LOA during nicotine exposure from smoking. rs139189121, rs75406390, and rs59024312 are genic upstream transcript variants (Table S2) located between exon 3 and exon 4 of SETDB1, which could influence SETDB1 expression activity and transcription of the SETDB1 protein, resulting in a different asthma prognosis. Fine-mapping analysis revealed that rs139189121, rs75406390, and rs59024312 were related to an increased risk of asthma when exposed to smoking (Table S2), and it was confirmed through RegulomeDB chip-seq data (hg19). As a result, they could be bonded asthma-related proteins, such as AR [92], FOXA1 [93], GATA3 [94,95], and GATA6 [96,97] (Table 3). Therefore, further biological investigation is needed to elucidate whether rs139189121, rs75406390, and rs59024312 can affect the expression of SETDB1 and asthma prognosis.
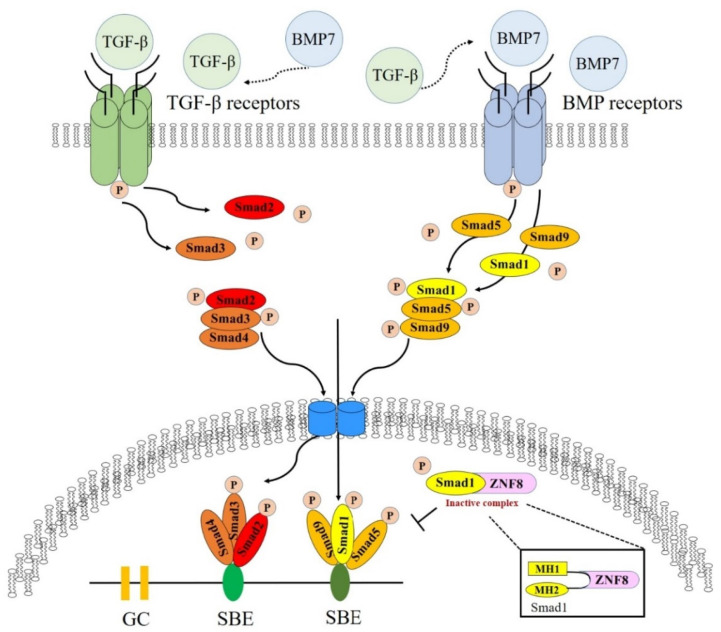
Another novel gene identified in our study is ZNF8 (Figure 4). ZNF8 encodes the zinc finger protein 8, an important protein that interacts with and binds to MH1 and MH2 domains of the Smad1 protein and is related to Smad1 suppression [166]. The main symptoms of asthma include reversible airway constriction, airway hyper-responsiveness, and chronic airway inflammation [167,168]. These symptoms are a complex process involving damage to the epithelial layer, hypertrophy and hyperplasia of the smooth muscles, and subepithelial fibrosis [169,170]. The key feature of subepithelial fibrosis is that human bronchial fibroblasts, derived from patients with asthma, show a characteristic transforming growth factor (TGF)-β1-induced fibroblast-to-myofibroblast transition (FMT) [166,170]. Antifibrotic TGF-β/Smad1 pathway activation is downregulated in fibroblasts; however, profibrotic TGF-β/Smad2/3 pathways are upregulated in patients with asthma [170]. Furthermore, the TGF-β/Smad1 and TGF-β/Smad2/3 pathways are antagonistic mechanisms [166,168,170]. Moreover, high expression of TGF-β1 is associated with eosinophil counts and asthma severity [171]. In asthmatic lungs, inflammatory cells express and secrete TGF-β1, which leads to an increase in eosinophils [171,172,173]. In particular, it has been reported that TGF-β1 is increased in chronic asthma and that eosinophils are the main source of asthma [172]. Thus, the ZNF8 protein, transcribed from ZNF8, interacts with Smad1, binds to the MH1 and MH2 domains of the Smad1 protein, and suppresses antifibrotic function [166]. Noteworthily, nicotine exposure from smoking increased KDM4C expression through epigenetic mechanisms [174]. These results suggest that the two novel genes (SETDB1 and ZNF8) may play important roles in the etiology of asthma.
Figure 4.
The ZNF8 gene plays an important role in asthma induction by Smad1 interaction.
One of the main strengths of this study is that it utilizes an older cohort sample to provide a better LOA phenotype. In particular, among the two novel genes associated with asthma in this study, the association of SETDB1 with LOA could be newly suggested through our analysis pipeline. Furthermore, this study focused on analyzing East Asian subjects, who have not been thoroughly investigated, unlike different ethnic groups. In this regard, we evaluated gene–smoking interactions using GWAS and gene analyses, revealing that genes, such as DOCK8, MMP20, and ADCY9 were suggested by previous studies. Additionally, we conducted gene-set analysis using GO processes, finding that the regulation of T cell differentiation in the thymus (GO:0033081) is related to the pathogenesis of asthma.
Our study had several limitations. First, our sample size was relatively small compared to that of recent asthma GWAS cohorts, such as the Trans-National Asthma Genetic Consortium (TAGC) [175] and the UK Biobank cohort [176]. Additional asthma risk variants were identified as the sample size increased. Second, GWAS is subject to unbalanced binary traits; that is, there are fewer case samples than control samples. The imbalance could produce large type-I error rates in logistic regression results [177,178,179], and the significance of true causal variants may be overlooked [180,181]. Further machine learning approaches, such as the synthetic minority oversampling technique (SMOTE) [182] and cost-sensitive learning [183], are required to address for the imbalance-associated errors. Third, the lack of independent replication datasets did not allow us to validate all the top signal GWAS loci, genes, and pathways for asthma. Therefore, replication studies on asthma using Korean or East Asian cohorts are needed as follow-up studies. Fourth, bioinformatics analysis identified certain genetic factors and pathways related to asthma pathogenesis; however, the underlying biological mechanisms of these factors require further investigation, such as rare variants or tissue and cell-type enrichment analyses. Finally, GWAS functional analysis did not provide sufficient biological insight into the two novel genes associated with asthma. Further in vitro and vivo experimental studies related to the role of the two novel genes are needed to identify individuals at risk of asthma.
4. Materials and Methods
4.1. Study Participants
Epidemiologic and genetic data were collected by the KoGES consortium [48] and included three population-based study cohorts: HEXA (n = 61,568), CAVAS (n = 9715), and KARE (n = 8840). The KoGES consortium was a long-term follow-up cohort study conducted, in the Korean population, from 2001 to 2010. It was designed to investigate and assess genetic and environmental factors as correlates to the incidence of chronic diseases, such as type 2 diabetes, hypertension, and cancer [48]. The current study excluded subjects without Korean Biobank Array genotype data and those with missing epidemiologic information (SEX, AGE, BMI, ALLER, SMOKE, and asthma diagnosis). After applying the exclusion criteria, 66,887 participants were included in the study (HEXA, n = 58,434; CAVAS, n = 3003; KARE, n = 5420; Figure 1). Patients with asthma were defined based on their answer to the asthma history question, with participants being categorized as “case” or “control” if their answer was “yes” or “no,” respectively. The allergy status was defined based on the participants’ answer to the allergy history (rhinitis, atopy, allergic conjunctivitis, food allergy, etc.) question, with participants being categorized as “ALLER” or “non-ALLER” if their answer was “yes” or “no,” respectively. The smoking status was defined based on the participants’ answer to the smoking history question, with participants being accordingly categorized as “never-smokers,” “former-smokers,” or “current-smokers.” However, in this study, we gathered former-smokers and current-smokers in a single group (smokers).
All participants provided written informed consent to participate in the study. The participants’ genetic information was produced using the Korean chip through the Korea Biobank Array Project of the National Institutes of Health, Korea Centers for Disease Control and Prevention [184,185]. This study was approved by the Institutional Review Board of Hanyang University (IRB no. HYUIRB-202210-013).
4.2. Quality Control
Genomic DNA was genotyped using the Korean Biobank Array (Korean Chip, KORV1.1, Thermo Fisher Scientific, Waltham, MA, USA), designed by the Center for Genome Science (Korea National Institute of Health (KNIH)), based on the platform of the UK Biobank Axiom array and manufactured by Affymetrix [184,185]. SNP imputation was performed using the IMPUTE v2 software [186] with East Asian (Chinese and Japanese, 286 samples) 1000 Genomes Project Phase 3 data as a reference panel. Then, the PLINK software (version 1.90, Boston, MA, USA) [52] was used for SNP loci quality control. We excluded SNPs with missing genotype call ratio > 0.05, minor allele frequency (MAF) < 0.01, and Hardy–Weinberg equilibrium (HWE) p-value ≤ 10−5. After imputation and quality control, 7,060,677 SNPs were selected for association analysis.
4.3. Genome-Wide Association Analysis
Genome-wide association analysis was conducted using a logistic regression model, with the PLINK-G × E option, to test the interaction between genetic variants and environmental factors (smoking status). Single-SNP interaction effects were assessed using the following model:
| (1) |
where β0, β1, β2, and β3 are the intercept value, effect sizes of the SNP, environmental factor, and interactions, respectively, and β4, β5, β6, and β7 denote coefficients of the covariate variables SEX, AGE, BMI, and ALLER, respectively. For the ASTH phenotype, the case group was denoted as 1, and the normal group was denoted as 0. The SNP was used as a marker to indicate the genetic information of each sample, and the genotype was assumed to be an additive genetic model (AA = 0, Aa = 1, and aa = 2, where “A” and “a” indicate the major and minor alleles, respectively). ALLER was categorized as either non-ALLER (coding = 0) or ALLER (coding = 1). SMOKE was classified into two groups: non-smokers (coding = 0) and smokers (former or current smokers; coding = 1). Moreover, as three integrated cohorts were analyzed in this study, we used covariates to adjust potential population stratifications in each cohort using dummy variables (HEXA: group 1 = 0, group 2 = 0; CAVAS: group 1 = 1, group 2 = 0; and KARE: group 1 = 0, group 2 = 1). Additionally, we conducted principal component (PC) analysis (PCA), which is the most widely used method to adjust for population stratification in GWASs [187]. We used the top 10 PC score that was calculated using the PLINK software (v1.90) and the “-- pca 10” option [52]. β8, β9, and β9–β19 denote the effect size of Groups 1, Groups 2, and PC1–PC10 values, respectively. To identify SNPs that interact with smoking status, we considered the following null hypothesis:
| (2) |
The p-values for the gene–environmental interaction factor, β12, that passed the Bonferroni correction threshold of 7.08 × 10–9 (0.05/7,060,677) were considered statistically significant.
4.4. Gene Analysis and Gene-Set Analysis
Gene and gene-set analyses were implemented in FUMA (v1.4.0) [62]. It used the input GWAS summary statistics to compute the p-value of gene-based and gene-set p-values, using the multi-MAGMA (v1.08) tool [61]. The MAGMA tool, with the multiple linear PC regression model [188], was used to identify multiple variant combined effects by incorporating linkage disequilibrium (LD) information among multiple variants within extended +/− 250 kb downstream or upstream of the gene [61]. We performed a MAGMA gene-based analysis using a reference panel from Phase 3 of the 1000 Genome Project East Asian data. For the gene-set analysis, we conducted the GENE2FUNC process to identify the putative biological mechanisms of the prioritized genes. The gene-set p-value was computed using a gene-based p-value for 9988 GO terms obtained from MsigDB (v7.0). Bonferroni correction was used to correct for multiple testing in both the gene and gene-set analyses.
4.5. Functional Annotation
Fine-mapping analysis was performed to prioritize candidate causal variants in the genomic regions surrounding the significant GWAS hits. For each GWAS hit, a SNP set consisting of genetic variants belonging to the same gene was selected and mapped to a significant SNP. Then, the LD among SNPs was computed using the Haploview software (v4.2) [189] to view the correlation structure. This is a commonly used functional annotation process considering LD to find the causal variant for the top SNP signals from the GWAS [190,191,192]. The most commonly used measures of LD are D′ and r2. D′ represents a statistic for estimating recombination differences, whereas r2 is the squared value of the correlation of the allelic states of two loci in the same gamete. Within the LD block containing a significant SNP, we extracted a list of adjacent SNPs with D′ > 0.9 or r2 > 0.8. Finally, functional annotation studies were performed to examine the relationship between asthma and the fine-mapping loci.
To annotate SNPs at our candidate loci, we conducted variant annotation (ANNOVAR) [193] (table_annovar.pl) using the hg19/GRCh37 reference genome and Ensembl gene (build 106). For intergenic SNPs, the gene boundaries overlap such that a single variant can be annotated to multiple genes. Therefore, the intergenic SNPs were mapped to the two closest upstream and downstream genes. All candidate SNPs were annotated using CADD (https://cadd.gs.washington.edu/ (accessed on 1 June 2023)), DANN (https://cbcl.ics.uci.edu/public_data/DANN/ (accessed on 1 June 2023)), and RegulomeDB scores (www.regulomedb.org/ (accessed on 1 June 2023)) to predict potential pathogenicity. For both coding and non-coding variants, CADD used more than 60 functional annotations to calculate deleterious scores. The raw CADD scores were then converted into PHRED-scaled scores ranging from 1 to 99, with higher values indicating more deleterious cases. For example, a PHRED-scaled score of 10 or greater indicates a raw CADD score in the top 10% of all possible reference genome SNPs. We also used a DANN to identify potentially functional SNPs using the VarSome prediction tool (v11.3.3, https://varsome.com (accessed on 1 June 2023)) [194]. The DANN score is a functional prediction scoring methodology with an output value in the range of 0 and 1, where higher values are more likely to indicate deleterious cases. RegulomeDB comprises datasets from the ENCODE Project Consortium, Gene Expression Omnibus, and other sources, totaling 962 datasets as well as published literature. RegulomeDB provides a SNP score that ranges from 1 to 6: the lower the SNP score, the stronger the regulatory association. The comparative characteristics of the RegulomeDB score categories are summarized in Table 4 [51].
Table 4.
Summary of the RegulomeDB category descriptions.
| RegulomeDB Category | Category Description |
|---|---|
| Likely to affect binding and linked to expression of a gene target | |
| 1a | eQTL a + TF b binding + matched TF motif + matched DNase c footprint + DNase peak |
| 1b | eQTL + TF binding + any motif + DNase footprint + DNase peak |
| 1c | eQTL + TF binding + matched TF motif + DNase peak |
| 1d | eQTL + TF binding + any motif + DNase peak |
| 1e | eQTL + TF binding + matched TF motif |
| 1f | eQTL + TF binding/DNase peak |
| Likely to affect binding | |
| 2a | TF binding + matched TF motif + matched DNase footprint + DNase peak |
| 2b | TF binding + any motif + DNase footprint + DNase peak |
| 2c | TF binding + matched TF motif + DNase peak |
| Less likely to affect binding | |
| 3a | TF binding + any motif + DNase peak |
| 3b | TF binding + matched TF motif |
| Minimal binding evidence | |
| 4 | TF binding + DNase peak |
| 5 | TF binding or DNase peak |
| 6 | Motif hit |
a Expression quantitative trait locus (eQTL); b transcription factor (TF); c deoxyribonuclease (DNase).
5. Conclusions
This study demonstrated that two potentially novel genes (SETDB1 and ZNF8) are associated with the development of asthma, based on gene–smoking interaction analyses using multiple cohorts of the KoGES consortium. Furthermore, the causal relationship between five genes (two potentially novel genes and three previously reported genes) and asthma was confirmed through follow-up bioinformatics analyses. Moreover, gene-set analysis revealed that the regulation of T cell differentiation in the thymus (GO:0033081) is significantly associated with the pathogenesis of asthma. Therefore, our findings indicate the importance of multi-levels analysis (GWAS loci, genes, pathways, and functional annotation) in gene–environment interaction studies, which may provide insight and an improved understanding of these complex traits. Our results reveal potential biological mechanisms that are involved in the pathogenesis of asthma. The results contribute to a greater understanding of asthma etiology and provide insights for further investigation.
Acknowledgments
We would like to thank all reviewers for their insightful suggestions to improve this article.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241512266/s1.
Author Contributions
Conceptualization, J.C. and S.C.; methodology, J.C. and S.C.; software, J.C. and S.C.; investigation, J.C.; writing—original draft preparation, J.C.; writing—review and editing, J.C. and S.C.; visualization, J.C. and S.C.; supervision, S.C.; funding acquisition, S.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Hanyang University (protocol code HYUIRB-202210-013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the HEXA, CAVAS, and KARE studies.
Data Availability Statement
The HEXA, CAVAS, and KARE Korean Chip (KORV1.1) datasets are a part of KoGES and are available upon approval of the genome center at the Korea National Institute of Health (https://is.kdca.go.kr/ (accessed on 1 June 2023)).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2018R1C1B6008277 and 2022R1F1A1072274); the Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korean government (MSIT) (No. RS-2022-00155885, Artificial Intelligence Convergence Innovation Human Resources Development [Hanyang University ERICA]); the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2019M3E5D3073365); and the National Biobank of Korea, the Korea Disease Control and Prevention Agency, Republic of Korea (No. NBK-2020-106).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Asher I., Pearce N. Global burden of asthma among children. Int. J. Tuberc. Lung Dis. 2014;18:1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 2.Mizgerd J.P. Lung infection--a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Chronic Respiratory Disease Collaborators Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enilari O., Sinha S. The Global Impact of Asthma in Adult Populations. Ann. Glob. Health. 2019;85:2. doi: 10.5334/aogh.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H., Ryu J., Nam E., Chung S.J., Yeo Y., Park D.W., Park T.S., Moon J.-Y., Kim T.-H., Sohn J.W., et al. Increased mortality in patients with corticosteroid-dependent asthma: A nationwide population-based study. Eur. Respir. J. 2019;54:1900804. doi: 10.1183/13993003.00804-2019. [DOI] [PubMed] [Google Scholar]
- 6.Seth D., Saini S., Poowuttikul P. Pediatric Inner-City Asthma. Pediatr. Clin. N. Am. 2019;66:967–979. doi: 10.1016/j.pcl.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Simpson C.B., Amin M.R. Chronic cough: State-of-the-art review. Otolaryngol. Head Neck Surg. 2006;134:693–700. doi: 10.1016/j.otohns.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Rank M.A., Shah N.D. Multiple Chronic Conditions and Asthma: Implications for Practice and Research. J. Allergy Clin. Immunol. Pr. 2014;2:518–524. doi: 10.1016/j.jaip.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Boulet L.-P. Influence of comorbid conditions on asthma. Eur. Respir. J. 2009;33:897–906. doi: 10.1183/09031936.00121308. [DOI] [PubMed] [Google Scholar]
- 10.Kauppi P., Linna M., Jantunen J., Martikainen J.E., Haahtela T., Pelkonen A., Mäkelä M. Chronic Comorbidities Contribute to the Burden and Costs of Persistent Asthma. Mediat. Inflamm. 2015;2015:819194. doi: 10.1155/2015/819194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 12.Ober C., Vercelli D. Gene-environment interactions in human disease: Nuisance or opportunity? Trends Genet. 2011;27:107–115. doi: 10.1016/j.tig.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ober C., Yao T.-C. The genetics of asthma and allergic disease: A 21st century perspective. Immunol. Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomsen S.F., Van Der Sluis S., Kyvik K.O., Skytthe A., Backer V. Estimates of asthma heritability in a large twin sample. Clin. Exp. Allergy. 2010;40:1054–1061. doi: 10.1111/j.1365-2222.2010.03525.x. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Pacheco N., Pino-Yanes M., Flores C. Genomic Predictors of Asthma Phenotypes and Treatment Response. Front. Pediatr. 2019;7:6. doi: 10.3389/fped.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moffatt M.F., Kabesch M., Liang L., Dixon A.L., Strachan D., Heath S., Depner M., von Berg A., Bufe A., Rietschel E., et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 17.Willis-Owen S.A., Cookson W.O., Moffatt M.F. The Genetics and Genomics of Asthma. Annu. Rev. Genom. Hum. Genet. 2018;19:223–246. doi: 10.1146/annurev-genom-083117-021651. [DOI] [PubMed] [Google Scholar]
- 18.Vicente C.T., Revez J.A., Ferreira M.A.R. Lessons from ten years of genome-wide association studies of asthma. Clin. Transl. Immunol. 2017;6:e165. doi: 10.1038/cti.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K.W., Ober C. Lessons Learned from GWAS of Asthma. Allergy Asthma Immunol. Res. 2019;11:170–187. doi: 10.4168/aair.2019.11.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrine N., Portelli M.A., John C., Artigas M.S., Bennett N., Hall R., Lewis J., Henry A.P., Billington C.K., Ahmad A., et al. Moderate-to-severe asthma in individuals of European ancestry: A genome-wide association study. Lancet Respir. Med. 2019;7:20–34. doi: 10.1016/S2213-2600(18)30389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daya M., Rafaels N., Brunetti T.M., Chavan S., Levin A.M., Shetty A., Gignoux C.R., Boorgula M.P., Wojcik G., Campbell M., et al. Association study in African-admixed populations across the Americas recapitulates asthma risk loci in non-African populations. Nat. Commun. 2019;10:880. doi: 10.1038/s41467-019-08469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira M.A., Mathur R., Vonk J.M., Szwajda A., Brumpton B., Granell R., Brew B.K., Ullemar V., Lu Y., Jiang Y., et al. Genetic Architectures of Childhood- and Adult-Onset Asthma Are Partly Distinct. Am. J. Hum. Genet. 2019;104:665–684. doi: 10.1016/j.ajhg.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pividori M., Schoettler N., Nicolae D.L., Ober C., Im H.K. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: Genome-wide and transcriptome-wide studies. Lancet Respir. Med. 2019;7:509–522. doi: 10.1016/S2213-2600(19)30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson A., Rask-Andersen M., Karlsson T., Ek W.E. Genome-wide association analysis of 350 000 Caucasians from the UK Biobank identifies novel loci for asthma, hay fever and eczema. Hum. Mol. Genet. 2019;28:4022–4041. doi: 10.1093/hmg/ddz175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olafsdottir T.A., Theodors F., Bjarnadottir K., Bjornsdottir U.S., Agustsdottir A.B., Stefansson O.A., Ivarsdottir E.V., Sigurdsson J.K., Benonisdottir S., Eyjolfsson G.I., et al. Eighty-eight variants highlight the role of T cell regulation and airway remodeling in asthma pathogenesis. Nat. Commun. 2020;11:393. doi: 10.1038/s41467-019-14144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maher B. Personal genomes: The case of the missing heritability. Nature. 2008;456:18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- 27.Manolio T.A., Collins F.S., Cox N.J., Goldstein D.B., Hindorff L.A., Hunter D.J., McCarthy M.I., Ramos E.M., Cardon L.R., Chakravarti A., et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igartua C., Myers R.A., Mathias R.A., Pino-Yanes M., Eng C., Graves P.E., Levin A.M., Del-Rio-Navarro B.E., Jackson D.J., Livne O.E., et al. Ethnic-specific associations of rare and low-frequency DNA sequence variants with asthma. Nat. Commun. 2015;6:5965. doi: 10.1038/ncomms6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ionita-Laza I., Perry G.H., Raby B.A., Klanderman B., Lee C., Laird N.M., Weiss S.T., Lange C. On the analysis of copy-number variations in genome-wide association studies: A translation of the family-based association test. Genet. Epidemiol. 2008;32:273–284. doi: 10.1002/gepi.20302. [DOI] [PubMed] [Google Scholar]
- 30.Walsh K.M., Bracken M.B., Murk W.K., Hoh J., Dewan A.T. Association between reduced copy-number at T-cell receptor gamma (TCRgamma) and childhood allergic asthma: A possible role for somatic mosaicism. Mutat. Res. 2010;690:89–94. doi: 10.1016/j.mrfmmm.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper G.M., Zerr T., Kidd J.M., Eichler E.E., Nickerson D.A. Systematic assessment of copy number variant detection via genome-wide SNP genotyping. Nat. Genet. 2008;40:1199–1203. doi: 10.1038/ng.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dominiczak A.F., McBride M.W. Genetics of common polygenic stroke. Nat. Genet. 2003;35:116–117. doi: 10.1038/ng1003-116. [DOI] [PubMed] [Google Scholar]
- 33.Heng H.H.Q. Missing heritability and stochastic genome alterations. Nat. Rev. Genet. 2010;11:812. doi: 10.1038/nrg2809-c3. [DOI] [PubMed] [Google Scholar]
- 34.Rava M., Smit L.A., Nadif R. Gene–environment interactions in the study of asthma in the postgenomewide association studies era. Curr. Opin. Allergy Clin. Immunol. 2015;15:70–78. doi: 10.1097/ACI.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 35.Taylor B., Mannino D., Brown C., Crocker D., Twum-Baah N., Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63:14–20. doi: 10.1136/thx.2007.082784. [DOI] [PubMed] [Google Scholar]
- 36.Curjuric I., Imboden M., Nadif R., Kumar A., Schindler C., Haun M., Kronenberg F., Künzli N., Phuleria H., Postma D.S., et al. Different Genes Interact with Particulate Matter and Tobacco Smoke Exposure in Affecting Lung Function Decline in the General Population. PLoS ONE. 2012;7:e40175. doi: 10.1371/journal.pone.0040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedman L., Bjerg A., Sundberg S., Forsberg B., Rönmark E. Both environmental tobacco smoke and personal smoking is related to asthma and wheeze in teenagers. Thorax. 2010;66:20–25. doi: 10.1136/thx.2010.143800. [DOI] [PubMed] [Google Scholar]
- 38.Guibas G.V., Mathioudakis A.G., Tsoumani M., Tsabouri S. Relationship of Allergy with Asthma: There Are More Than the Allergy “Eggs” in the Asthma “Basket”. Front. Pediatr. 2017;5:92. doi: 10.3389/fped.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodgman A., Smith C.J., Perfetti T.A. The composition of cigarette smoke: A retrospective, with emphasis on polycyclic components. Hum. Exp. Toxicol. 2000;19:573–595. doi: 10.1191/096032700701546514. [DOI] [PubMed] [Google Scholar]
- 40.Çalışkan M., Bochkov Y.A., Kreiner-Møller E., Bønnelykke K., Stein M.M., Du G., Bisgaard H., Jackson D.J., Gern J.E., Lemanske R.F., et al. Rhinovirus Wheezing Illness and Genetic Risk of Childhood-Onset Asthma. N. Engl. J. Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smit L.A.M., Bouzigon E., Pin I., Siroux V., Monier F., Aschard H., Bousquet J., Gormand F., Just J., Le Moual N., et al. 17q21 variants modify the association between early respiratory infections and asthma. Eur. Respir. J. 2009;36:57–64. doi: 10.1183/09031936.00154509. [DOI] [PubMed] [Google Scholar]
- 42.Bukvic B.K., Blekic M., Simpson A., Marinho S., Curtin J.A., Hankinson J., Aberle N., Custovic A. Asthma severity, polymorphisms in 20p13 and their interaction with tobacco smoke exposure. Pediatr. Allergy Immunol. 2013;24:10–18. doi: 10.1111/pai.12019. [DOI] [PubMed] [Google Scholar]
- 43.Van Eerdewegh P., Little R.D., Dupuis J., Del Mastro R.G., Falls K., Simon J., Torrey D., Pandit S., McKenny J., Braunschweiger K., et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418:426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 44.Hancock D.B., Artigas M.S., Gharib S.A., Henry A., Manichaikul A., Ramasamy A., Loth D.W., Imboden M., Koch B., McArdle W.L., et al. Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function. PLOS Genet. 2012;8:e1003098. doi: 10.1371/journal.pgen.1003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyake Y., Tanaka K., Arakawa M. IL3rs40401 Polymorphism and Interaction with Smoking in Risk of Asthma in Japanese Women: The Kyushu Okinawa Maternal and Child Health Study. Scand. J. Immunol. 2014;79:410–414. doi: 10.1111/sji.12171. [DOI] [PubMed] [Google Scholar]
- 46.Wu J., Hankinson J., Kopec-Harding K., Custovic A., Simpson A. Interaction between glutathione S-transferase variants, maternal smoking and childhood wheezing changes with age. Pediatr. Allergy Immunol. 2013;24:501–508. doi: 10.1111/pai.12086. [DOI] [PubMed] [Google Scholar]
- 47.Dempfle A., Scherag A., Hein R., Beckmann L., Chang-Claude J., Schafer H. Gene-environment interactions for complex traits: Definitions, methodological requirements and challenges. Eur. J. Hum. Genet. 2008;16:1164–1172. doi: 10.1038/ejhg.2008.106. [DOI] [PubMed] [Google Scholar]
- 48.Kim Y., Han B.G., KoGES Group Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017;46:e20. doi: 10.1093/ije/dyv316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rentzsch P., Witten D., Cooper G.M., Shendure J., Kircher M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2018;47:D886–D894. doi: 10.1093/nar/gky1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quang D., Chen Y., Xie X. DANN: A deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics. 2015;31:761–763. doi: 10.1093/bioinformatics/btu703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyle A.P., Hong E.L., Hariharan M., Cheng Y., Schaub M.A., Kasowski M., Karczewski K.J., Park J., Hitz B.C., Weng S., et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22:1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devlin B., Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341X.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 54.D’antona S., Castiglioni I., Porro D., Cava C. Consequences of exposure to pollutants on respiratory health: From genetic correlations to causal relationships. PLoS ONE. 2022;17:e0277235. doi: 10.1371/journal.pone.0277235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kidd C.D.A., Thompson P.J., Barrett L., Baltic S. Histone Modifications and Asthma. The Interface of the Epigenetic and Genetic Landscapes. Am. J. Respir. Cell Mol. Biol. 2016;54:3–12. doi: 10.1165/rcmb.2015-0050TR. [DOI] [PubMed] [Google Scholar]
- 56.Moffatt M.F., Gut I.G., Demenais F., Strachan D.P., Bouzigon E., Heath S., von Mutius E., Farrall M., Lathrop M., Cookson W., et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soini Y., Kosma V.-M., Pirinen R. KDM4A, KDM4B and KDM4C in non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015;8:12922–12928. [PMC free article] [PubMed] [Google Scholar]
- 58.Greenlee K.J., Werb Z., Kheradmand F., Hardy E., Hardy-Sosa A., Fernandez-Patron C., Sun W., Hu Q., Ji W., Wright G., et al. Matrix Metalloproteinases in Lung: Multiple, Multifarious, and Multifaceted. Physiol. Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X., Zhao D., Shan Y., Cui W., Xie Q., Jiang J., Peng W., Zhang C., Duan C. Development and validation of a novel immune-related prognostic signature in lung squamous cell carcinoma patients. Sci. Rep. 2022;12:20737. doi: 10.1038/s41598-022-23140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mok P.L., Anandasayanam A.N.K., David H.M.O., Tong J., Farhana A., Khan M.S.A., Sivaprakasam G., Koh A.E.-H., Alzahrani B. Lung development, repair and cancer: A study on the role of MMP20 gene in adenocarcinoma. PLoS ONE. 2021;16:e0250552. doi: 10.1371/journal.pone.0250552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Leeuw C.A., Mooij J.M., Heskes T., Posthuma D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLoS Comput. Biol. 2015;11:e1004219. doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe K., Taskesen E., van Bochoven A., Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017;8:1826. doi: 10.1038/s41467-017-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee N., Yoon H.-Y., Park J.-Y., Kim Y.-J., Hwang H.-S., Yee J., Gwak H.-S. Association between ADCY9 Gene Polymorphisms and Ritodrine Treatment Outcomes in Patients with Preterm Labor. Pharmaceutics. 2021;13:1653. doi: 10.3390/pharmaceutics13101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teixeira H.M., Alcantara-Neves N.M., Barreto M., Figueiredo C.A., Costa R.S. Adenylyl cyclase type 9 gene polymorphisms are associated with asthma and allergy in Brazilian children. Mol. Immunol. 2017;82:137–145. doi: 10.1016/j.molimm.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Ferreira C.M., Chen J.L., Li J., Shimomura K., Yang X., Lussier Y.A., Pinto L.H., Solway J. Genetic Interactions between Chromosomes 11 and 18 Contribute to Airway Hyperresponsiveness in Mice. PLoS ONE. 2012;7:e29579. doi: 10.1371/journal.pone.0029579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang J., Qin T., Zhang L., Liu Q., Wu J., Dai R., Zhou L., Zhao Q., Luo X., Wang H., et al. IL-21 Rescues the Defect of IL-10-Producing Regulatory B Cells and Improves Allergic Asthma in DOCK8 Deficient Mice. Front. Immunol. 2021;12:695596. doi: 10.3389/fimmu.2021.695596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freeman A.F., Olivier K.N. Hyper-IgE Syndromes and the Lung. Clin. Chest Med. 2016;37:557–567. doi: 10.1016/j.ccm.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu J., Zhang S., Qin T., Jiang J., Liu Q., Zhang L., Zhao X., Dai J. IL-21 alleviates allergic asthma in DOCK8-knockout mice. Biochem. Biophys. Res. Commun. 2018;501:92–99. doi: 10.1016/j.bbrc.2018.04.179. [DOI] [PubMed] [Google Scholar]
- 69.Liberzon A. A Description of the Molecular Signatures Database (MSigDB) Web Site. Methods Mol. Biol. 2014;1150:153–160. doi: 10.1007/978-1-4939-0512-6_9. [DOI] [PubMed] [Google Scholar]
- 70.Liberzon A., Birger C., Thorvaldsdottir H., Ghandi M., Mesirov J.P., Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liberzon A., Subramanian A., Pinchback R., Thorvaldsdóttir H., Tamayo P., Mesirov J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicetto D., Zaret K.S. Role of H3K9me3 heterochromatin in cell identity establishment and maintenance. Curr. Opin. Genet. Dev. 2019;55:1–10. doi: 10.1016/j.gde.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barnes P.J. Th2 cytokines and asthma: An introduction. Respir. Res. 2001;2:64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguyen M.L.T., Jones S.A., Prier J.E., Russ B.E. Transcriptional Enhancers in the Regulation of T Cell Differentiation. Front. Immunol. 2015;6:462. doi: 10.3389/fimmu.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brightling C.E., Bradding P., Pavord I.D., Wardlaw A.J. New insights into the role of the mast cell in asthma. Clin. Exp. Allergy. 2003;33:550–556. doi: 10.1046/j.1365-2222.2003.01636.x. [DOI] [PubMed] [Google Scholar]
- 76.Casale T.B., Marom Z. Mast cells and asthma. The role of mast cell mediators in the pathogenesis of allergic asthma. Ann. Allergy. 1983;51:2–6. [PubMed] [Google Scholar]
- 77.Stephens N.L., Jiang H. Relaxation of smooth muscle. Can. J. Physiol. Pharmacol. 1994;72:1345–1350. doi: 10.1139/y94-194. [DOI] [PubMed] [Google Scholar]
- 78.Rawat K., Syeda S., Shrivastava A. Neutrophil-derived granule cargoes: Paving the way for tumor growth and progression. Cancer Metastasis Rev. 2021;40:221–244. doi: 10.1007/s10555-020-09951-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lauredo I.T., Forteza R.M., Botvinnikova Y., Abraham W.M., Magnen M., Gueugnon F., Petit-Courty A., Baranek T., Sizaret D., Brewah Y.A., et al. Leukocytic cell sources of airway tissue kallikrein. Am. J. Physiol. Cell. Mol. Physiol. 2004;286:L734–L740. doi: 10.1152/ajplung.00129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chai O.H., Han E.-H., Lee H.-K., Song C.H. Mast cells play a key role in Th2 cytokine-dependent asthma model through production of adhesion molecules by liberation of TNF-α. Exp. Mol. Med. 2011;43:35–43. doi: 10.3858/emm.2011.43.1.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shoemark A., Dixon M., Beales P.L., Hogg C.L. Bardet Biedl syndrome: Motile ciliary phenotype. Chest. 2015;147:764–770. doi: 10.1378/chest.13-2913. [DOI] [PubMed] [Google Scholar]
- 82.Tanizaki Y., Hosokawa M., Akagi K., Matsuka Y., Shioda Y., Sato T., Tada S., Takahashi K., Kimura I. Function of blood monocytes in bronchial asthma--numerical changes and beta-galactosidase activity. Nihon Kyobu Shikkan Gakkai Zasshi. 1984;22:208–213. [PubMed] [Google Scholar]
- 83.Chen T., Yan Y.E., Liu S., Liu H.X., Yan H.Y., Hou L.F., Qu W., Ping J. Increased Fetal Thymocytes Apoptosis Contributes to Prenatal Nicotine Exposure-induced Th1/Th2 Imbalance in Male Offspring Mice. Sci. Rep. 2016;6:39013. doi: 10.1038/srep39013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Middlebrook A.J., Martina C., Chang Y., Lukas R.J., DeLuca D. Effects of Nicotine Exposure on T Cell Development in Fetal Thymus Organ Culture: Arrest of T Cell Maturation. J. Immunol. 2002;169:2915–2924. doi: 10.4049/jimmunol.169.6.2915. [DOI] [PubMed] [Google Scholar]
- 85.Qu W., Zhao W.-H., Wen X., Yan H.-Y., Liu H.-X., Hou L.-F., Ping J. Prenatal nicotine exposure induces thymic hypoplasia in mice offspring from neonatal to adulthood. Toxicol. Lett. 2018;304:30–38. doi: 10.1016/j.toxlet.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 86.Agarwal A., Verma S., Burra U., Murthy N.S., Mohanty N.K., Saxena S. Flow Cytometric analysis of Th1 and Th2 cytokines in PBMCs as a parameter of immunological dysfunction in patients of Superficial Transitional cell carcinoma of bladder. Cancer Immunol. Immunother. 2005;55:734–743. doi: 10.1007/s00262-005-0045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karpuzoglu-Sahin E., Hissong B.D., Ansar Ahmed S. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J. Reprod. Immunol. 2001;52:113–127. doi: 10.1016/S0165-0378(01)00117-6. [DOI] [PubMed] [Google Scholar]
- 88.Stromnes I.M., Cerretti L.M., Liggitt D., Harris R.A., Goverman J.M. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat. Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guerra-Álvarez M., Moreno-Ortega A.J., Navarro E., Fernández-Morales J.C., Egea J., López M.G., Cano-Abad M.F. Positive allosteric modulation of alpha-7 nicotinic receptors promotes cell death by inducing Ca2+ release from the endoplasmic reticulum. J. Neurochem. 2015;133:309–319. doi: 10.1111/jnc.13049. [DOI] [PubMed] [Google Scholar]
- 90.Vivekanandarajah A., Chan Y.L., Chen H., Machaalani R. Prenatal cigarette smoke exposure effects on apoptotic and nicotinic acetylcholine receptor expression in the infant mouse brainstem. Neurotoxicology. 2016;53:53–63. doi: 10.1016/j.neuro.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 91.Liu H.-X., Liu S., Qu W., Yang H.-Y., Wen X., Chen T., Hou L.-F., Ping J. α7 nAChR mediated Fas demethylation contributes to prenatal nicotine exposure-induced programmed thymocyte apoptosis in mice. Oncotarget. 2017;8:93741–93756. doi: 10.18632/oncotarget.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McManus J.M., Gaston B., Zein J., Sharifi N. Association Between Asthma and Reduced Androgen Receptor Expression in Airways. J. Endocr. Soc. 2022;6:bvac047. doi: 10.1210/jendso/bvac047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nie X., Wei J., Hao Y., Tao J., Li Y., Liu M., Xu B., Li B. Consistent Biomarkers and Related Pathogenesis Underlying Asthma Revealed by Systems Biology Approach. Int. J. Mol. Sci. 2019;20:4037. doi: 10.3390/ijms20164037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park S.-W., Verhaeghe C., Nguyenvu L.T., Barbeau R., Eisley C.J., Nakagami Y., Huang X., Woodruff P.G., Fahy J.V., Erle D.J. Distinct Roles of FOXA2 and FOXA3 in Allergic Airway Disease and Asthma. Am. J. Respir. Crit. Care Med. 2009;180:603–610. doi: 10.1164/rccm.200811-1768OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu L., An L., Ran D., Lizarraga R., Bondy C., Zhou X., Harper R.W., Liao S.-Y., Chen Y. The Club Cell Marker SCGB1A1 Downstream of FOXA2 is Reduced in Asthma. Am. J. Respir. Cell Mol. Biol. 2019;60:695–704. doi: 10.1165/rcmb.2018-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caramori G., Lim S., Ito K., Tomita K., Oates T., Jazrawi E., Chung K., Barnes P., Adcock I. Expression of GATA family of transcription factors in T-cells, monocytes and bronchial biopsies. Eur. Respir. J. 2001;18:466–473. doi: 10.1183/09031936.01.00040701. [DOI] [PubMed] [Google Scholar]
- 97.Fang P., Shi H.Y., Wu X.M., Zhang Y.H., Zhong Y.J., Deng W.J., Zhang Y.P., Xie M. Targeted inhibition of GATA-6 attenuates airway inflammation and remodeling by regulating caveolin-1 through TLR2/MyD88/NF-κB in murine model of asthma. Mol. Immunol. 2016;75:144–150. doi: 10.1016/j.molimm.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 98.Tumes D., Hirahara K., Papadopoulos M., Shinoda K., Onodera A., Kumagai J., Yip K.H., Pant H., Kokubo K., Kiuchi M., et al. Ezh2 controls development of natural killer T cells, which cause spontaneous asthma-like pathology. J. Allergy Clin. Immunol. 2019;144:549–560.e10. doi: 10.1016/j.jaci.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 99.Kozmus C.E., Potočnik U. Reference genes for real-time qPCR in leukocytes from asthmatic patients before and after anti-asthma treatment. Gene. 2015;570:71–77. doi: 10.1016/j.gene.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Jolliffe D.A., Kilpin K., MacLaughlin B.D., Greiller C.L., Hooper R.L., Barnes N.C., Timms P.M., Rajakulasingam R.K., Bhowmik A., Choudhury A.B., et al. Prevalence, determinants and clinical correlates of vitamin D deficiency in adults with inhaled corticosteroid-treated asthma in London, UK. J. Steroid Biochem. Mol. Biol. 2018;175:88–96. doi: 10.1016/j.jsbmb.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 101.Diez D., Goto S., Fahy J.V., Erle D.J., Woodruff P.G., Wheelock M., Wheelock C.E. Network analysis identifies a putative role for the PPAR and type 1 interferon pathways in glucocorticoid actions in asthmatics. BMC Med. Genom. 2012;5:27. doi: 10.1186/1755-8794-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pniewska E., Sokolowska M., Kupryś-Lipińska I., Kacprzak D., Kuna P., Pawliczak R. Exacerbating Factors Induce Different Gene Expression Profiles in Peripheral Blood Mononuclear Cells from Asthmatics, Patients with Chronic Obstructive Pulmonary Disease and Healthy Subjects. Int. Arch. Allergy Immunol. 2014;165:229–243. doi: 10.1159/000370067. [DOI] [PubMed] [Google Scholar]
- 103.Pazdrak K., Moon Y., Straub C., Stafford S., Kurosky A. Eosinophil resistance to glucocorticoid-induced apoptosis is mediated by the transcription factor NFIL3. Apoptosis. 2016;21:421–431. doi: 10.1007/s10495-016-1226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rothman P.B. The transcriptional regulator NFIL3 controls IgE production. Trans. Am. Clin. Clim. Assoc. 2010;121:156–171. [PMC free article] [PubMed] [Google Scholar]
- 105.Nowak J.K., Dworacka M., Gubaj N., Dossimov A., Dossimov Z., Walkowiak J. Expression profiling of ileal mucosa in asthma reveals upregulation of innate immunity and genes characteristic of Paneth and goblet cells. Allergy Asthma Clin. Immunol. 2021;17:82. doi: 10.1186/s13223-021-00584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song Y., Wang Z., Jiang J., Piao Y., Li L., Xu C., Piao H., Li L., Yan G. DEK-targeting aptamer DTA-64 attenuates bronchial EMT-mediated airway remodelling by suppressing TGF-β1/Smad, MAPK and PI3K signalling pathway in asthma. J. Cell Mol. Med. 2020;24:13739–13750. doi: 10.1111/jcmm.15942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin L., Wang Y., Sun B., Liu L., Ying W., Wang W., Zhou Q., Hou J., Yao H., Hu L., et al. The clinical, immunological and genetic features of 12 Chinese patients with STAT3 mutations. Allergy Asthma Clin. Immunol. 2020;16:65. doi: 10.1186/s13223-020-00462-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frey-Jakobs S., Hartberger J.M., Fliegauf M., Bossen C., Wehmeyer M.L., Neubauer J.C., Bulashevska A., Proietti M., Fröbel P., Nöltner C., et al. ZNF341 controls STAT3 expression and thereby immunocompetence. Sci. Immunol. 2018;3:eaat4941. doi: 10.1126/sciimmunol.aat4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schlag K., Steinhilber D., Karas M., Sorg B.L. Analysis of proximal ALOX5 promoter binding proteins by quantitative proteomics. FEBS J. 2020;287:4481–4499. doi: 10.1111/febs.15259. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y., Huang Y., Chen W.-X., Xu Z.-M. sj-xlsx-1-imr-10.1177_03000605211029521—Supplemental material for Identification of key genes in allergic rhinitis by bioinformatics analysis. J. Int. Med. Res. 2021;49:3000605211029521. doi: 10.1177/03000605211029521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jargosch M., Kröger S., Gralinska E., Klotz U., Fang Z., Chen W., Leser U., Selbig J., Groth D., Baumgrass R. Data integration for identification of important transcription factors of STAT6-mediated cell fate decisions. Genet. Mol. Res. 2016;15:4238. doi: 10.4238/gmr.15028493. [DOI] [PubMed] [Google Scholar]
- 112.Gilchrist M., Henderson W.R., Clark A.E., Simmons R.M., Ye X., Smith K.D., Aderem A. Activating transcription factor 3 is a negative regulator of allergic pulmonary inflammation. J. Exp. Med. 2008;205:2349–2357. doi: 10.1084/jem.20072254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ogasawara T., Hatano M., Satake H., Ikari J., Taniguchi T., Tsuruoka N., Watanabe-Takano H., Fujimura L., Sakamoto A., Hirata H., et al. Development of chronic allergic responses by dampening Bcl6-mediated suppressor activity in memory T helper 2 cells. Proc. Natl. Acad. Sci. USA. 2017;114:E741–E750. doi: 10.1073/pnas.1613528114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.He K., Hettinga A., Kale S.L., Hu S., Xie M.M., Dent A.L., Ray A., Poholek A.C. Blimp-1 is essential for allergen-induced asthma and Th2 cell development in the lung. J. Exp. Med. 2020;217:e20190742. doi: 10.1084/jem.20190742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Golebski K., Luiten S., van Egmond D., de Groot E., Röschmann K.I., Fokkens W.J., van Drunen C.M. High degree of overlap between responses to a virus and to the house dust mite allergen in airway epithelial cells. PLoS ONE. 2014;9:e87768. doi: 10.1371/journal.pone.0087768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang Y., Yan Q., Liu C.-X., Peng C.-W., Zheng W.-J., Zhuang H.-F., Huang H.-T., Liu Q., Liao H.-L., Zhan S.-F., et al. Insights into potential mechanisms of asthma patients with COVID-19: A study based on the gene expression profiling of bronchoalveolar lavage fluid. Comput. Biol. Med. 2022;146:105601. doi: 10.1016/j.compbiomed.2022.105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu J., Duan R., Cao H., Field D., Newnham C.M., Koehler D.R., Zamel N., Pritchard M.A., Hertzog P., Post M., et al. Regulation of epithelium-specific Ets-like factors ESE-1 and ESE-3 in airway epithelial cells: Potential roles in airway inflammation. Cell Res. 2008;18:649–663. doi: 10.1038/cr.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee S.-Y., Won H.-K., Kim B.-K., Kim S.-H., Chang Y.-S., Cho S.-H., Kelly H.W., Tantisira K.G., Park H.-W. Identification of a key gene module associated with glucocorticoid- induced derangement in bone mineral density in patients with asthma. Sci. Rep. 2019;9:20133. doi: 10.1038/s41598-019-56656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gunawardhana L.P., Gibson P.G., Simpson J.L., Powell H., Baines K.J. Activity and expression of histone acetylases and deacetylases in inflammatory phenotypes of asthma. Clin. Exp. Allergy. 2014;44:47–57. doi: 10.1111/cea.12168. [DOI] [PubMed] [Google Scholar]
- 120.Chen G., Korfhagen T.R., Karp C.L., Impey S., Xu Y., Randell S.H., Kitzmiller J., Maeda Y., Haitchi H.M., Sridharan A., et al. Foxa3 Induces Goblet Cell Metaplasia and Inhibits Innate Antiviral Immunity. Am. J. Respir. Crit. Care Med. 2014;189:301–313. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xia M., Xu H., Dai W., Zhu C., Wu L., Yan S., Ge X., Zhou W., Chen C., Dai Y. The role of HDAC2 in cigarette smoke–induced airway inflammation in a murine model of asthma and the effect of intervention with roxithromycin. J. Asthma. 2018;55:337–344. doi: 10.1080/02770903.2017.1337788. [DOI] [PubMed] [Google Scholar]
- 122.Mishra R., Chaturvedi R., Hashim Z., Nath A., Khan A., Gupta M., Singh H., Agarwal V. Role of P-gp and HDAC2 and their Reciprocal Relationship in Uncontrolled Asthma. Curr. Pharm. Biotechnol. 2021;22:408–413. doi: 10.2174/1389201021666200529104042. [DOI] [PubMed] [Google Scholar]
- 123.Kumar A., Das S., Agrawal A., Mukhopadhyay I., Ghosh B. Genetic association of key Th1/Th2 pathway candidate genes, IRF2, IL6, IFNGR2, STAT4 and IL4RA, with atopic asthma in the Indian population. J. Hum. Genet. 2015;60:443–448. doi: 10.1038/jhg.2015.45. [DOI] [PubMed] [Google Scholar]
- 124.Verma M., Michalec L., Sripada A., McKay J., Sirohi K., Verma D., Sheth D., Martin R., Dyjack N., Seibold M.A., et al. The molecular and epigenetic mechanisms of innate lymphoid cell (ILC) memory and its relevance for asthma. J. Exp. Med. 2021;218:e20201354. doi: 10.1084/jem.20201354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Díaz L.A.C., Ortega A.G., Álvarez R.D.C.C., Félix J.S.V., de Oca E.P.M. Regulatory SNP rs5743417 impairs constitutive expression of human β-defensin 1 and has high frequency in Africans and Afro-Americans. Int. J. Immunogenet. 2020;47:332–341. doi: 10.1111/iji.12475. [DOI] [PubMed] [Google Scholar]
- 126.Bosch T.v.D., Leus N.G., Wapenaar H., Boichenko A., Hermans J., Bischoff R., Haisma H.J., Dekker F.J. A 6-alkylsalicylate histone acetyltransferase inhibitor inhibits histone acetylation and pro-inflammatory gene expression in murine precision-cut lung slices. Pulm. Pharmacol. Ther. 2017;44:88–95. doi: 10.1016/j.pupt.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Y., Du J., Liu X., Wang L., Han Y., Huang C., Liang R., Zheng F., Shi G., Li B. MG149 inhibits histone acetyltransferase KAT8-mediated IL-33 acetylation to alleviate allergic airway inflammation and airway hyperresponsiveness. Signal Transduct. Target. Ther. 2021;6:321. doi: 10.1038/s41392-021-00667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van der Plaat D.A., Vonk J.M., Lahousse L., de Jong K., Faiz A., Nedeljkovic I., Amin N., van Diemen C.C., Brusselle G.G., Bossé Y., et al. Limited overlap in significant hits between genome-wide association studies on two airflow obstruction definitions in the same population. BMC Pulm. Med. 2019;19:58. doi: 10.1186/s12890-019-0811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fox A., Harland K.L., Kedzierska K., Kelso A. Exposure of Human CD8+ T Cells to Type-2 Cytokines Impairs Division and Differentiation and Induces Limited Polarization. Front. Immunol. 2018;9:1141. doi: 10.3389/fimmu.2018.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang S.-R., Hu R.-D., Ma M., You X., Cui H., He Y., Xu D., Zhao Z.-B., Selmi C., Gershwin M.E., et al. FoxO1 suppresses IL-10 producing B cell differentiation via negatively regulating Blimp-1 expression and contributes to allergic asthma progression. Mucosal Immunol. 2022;15:459–470. doi: 10.1038/s41385-022-00504-z. [DOI] [PubMed] [Google Scholar]
- 131.Zhang X., Myers J.M.B., Burleson J., Ulm A., Bryan K.S., Chen X., Weirauch M.T., Baker T.A., Kovacic M.S.B., Ji H. Nasal DNA methylation is associated with childhood asthma. Epigenomics. 2018;10:629–641. doi: 10.2217/epi-2017-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aneas I., Decker D.C., Howard C.L., Sobreira D.R., Sakabe N.J., Blaine K.M., Stein M.M., Hrusch C.L., Montefiori L.E., Tena J., et al. Asthma-associated genetic variants induce IL33 differential expression through an enhancer-blocking regulatory region. Nat. Commun. 2021;12:6115. doi: 10.1038/s41467-021-26347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Amo G., Martí M., García-Menaya J.M., Cordobés C., Cornejo-García J.A., Blanca-López N., Canto G., Doña I., Blanca M., Torres M.J., et al. Identification of Novel Biomarkers for Drug Hypersensitivity After Sequencing of the Promoter Area in 16 Genes of the Vitamin D Pathway and the High-Affinity IgE Receptor. Front. Genet. 2019;10:582. doi: 10.3389/fgene.2019.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mougey E., Lang J.E., Allayee H., Teague W.G., Dozor A.J., Wise R.A., Lima J.J. ALOX5 Polymorphism associates with increased leukotriene production and reduced lung function and asthma control in children with poorly controlled asthma. Clin. Exp. Allergy. 2013;43:512–520. doi: 10.1111/cea.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scaparrotta A., Franzago M., Marcovecchio M.L., Di Pillo S., Chiarelli F., Mohn A., Stuppia L. Role of THRB, ARG1, and ADRB2 Genetic Variants on Bronchodilators Response in Asthmatic Children. J. Aerosol. Med. Pulm. Drug Deliv. 2019;32:164–173. doi: 10.1089/jamp.2018.1493. [DOI] [PubMed] [Google Scholar]
- 136.García-Menaya J.M., Cordobés-Durán C., García-Martín E., Agúndez J.A.G. Pharmacogenetic Factors Affecting Asthma Treatment Response. Potential Implications for Drug Therapy. Front. Pharmacol. 2019;10:520. doi: 10.3389/fphar.2019.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pulleyn L.J., Newton R., Adcock I.M., Barnes P.J. TGFbeta1 allele association with asthma severity. Hum. Genet. 2001;109:623–627. doi: 10.1007/s00439-001-0617-y. [DOI] [PubMed] [Google Scholar]
- 138.Hwang S.S., Kim Y.U., Lee S., Jang S.W., Kim M.K., Koh B.H., Lee W., Kim J., Souabni A., Busslinger M., et al. Transcription factor YY1 is essential for regulation of the Th2 cytokine locus and for Th2 cell differentiation. Proc. Natl. Acad. Sci. USA. 2013;110:276–281. doi: 10.1073/pnas.1214682110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee M., Lim S., Kim Y.S., Khalmuratova R., Shin S.-H., Kim I., Kim H.-J., Kim D.-Y., Rhee C.-S., Park J.-W., et al. DEP-induced ZEB2 promotes nasal polyp formation via epithelial-to-mesenchymal transition. J. Allergy Clin. Immunol. 2022;149:340–357. doi: 10.1016/j.jaci.2021.04.024. [DOI] [PubMed] [Google Scholar]
- 140.Sakai H., Hirahara M., Chiba Y., Misawa M. Antigen challenge influences various transcription factors of rat bronchus: Protein/DNA array study. Int. Immunopharmacol. 2011;11:1133–1136. doi: 10.1016/j.intimp.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 141.Popa S.C., Shin J.A. The Intrinsically Disordered Loop in the USF1 bHLHZ Domain Modulates Its DNA-Binding Sequence Specificity in Hereditary Asthma. J. Phys. Chem. B. 2019;123:9862–9871. doi: 10.1021/acs.jpcb.9b06719. [DOI] [PubMed] [Google Scholar]
- 142.Sun X., Shen W., Li Z., Zhang W. CCCTC-binding factor transcriptionally regulates Galectin-7 and activates the JNK/STAT3 axis to aggravate bronchial epithelial cell injury. Pediatr. Pulmonol. 2022;57:90–99. doi: 10.1002/ppul.25726. [DOI] [PubMed] [Google Scholar]
- 143.Schmiedel B.J., Seumois G., Samaniego-Castruita D., Cayford J., Schulten V., Chavez L., Ay F., Sette A., Peters B., Vijayanand P. 17q21 asthma-risk variants switch CTCF binding and regulate IL-2 production by T cells. Nat. Commun. 2016;7:13426. doi: 10.1038/ncomms13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu Y., Lu Y., Zou F., Fan X., Li X., Zhang H., Chen H., Sun X., Liu Y. PTEN participates in airway remodeling of asthma by regulating CD38/Ca(2+)/CREB signaling. Aging. 2020;12:16326–16340. doi: 10.18632/aging.103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bartel S., Schulz N., Alessandrini F., Schamberger A.C., Pagel P., Theis F.J., Milger K., Noessner E., Stick S.M., Kicic A., et al. Pulmonary microRNA profiles identify involvement of Creb1 and Sec14l3 in bronchial epithelial changes in allergic asthma. Sci. Rep. 2017;7:srep46026. doi: 10.1038/srep46026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Adoue V., Binet B., Malbec A., Fourquet J., Romagnoli P., van Meerwijk J., Amigorena S., Joffre O.P. The Histone Methyltransferase SETDB1 Controls T Helper Cell Lineage Integrity by Repressing Endogenous Retroviruses. Immunity. 2019;50:629–644.e8. doi: 10.1016/j.immuni.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 147.Barnes P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018;18:454–466. doi: 10.1038/s41577-018-0006-6. [DOI] [PubMed] [Google Scholar]
- 148.Yuan Y.L., Zhang X., Liu L., Wang G., Chen-Yu Hsu A., Huang D., Wang G., Oliver B.G. Total IgE Variability Is Associated with Future Asthma Exacerbations: A 1-Year Prospective Cohort Study. J. Allergy Clin. Immunol. Pr. 2021;9:2812–2824. doi: 10.1016/j.jaip.2021.04.065. [DOI] [PubMed] [Google Scholar]
- 149.Han S., Zhen W., Guo T., Zou J., Li F. SETDB1 promotes glioblastoma growth via CSF-1-dependent macrophage recruitment by activating the AKT/mTOR signaling pathway. J. Exp. Clin. Cancer Res. 2020;39:218. doi: 10.1186/s13046-020-01730-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 150.Rot A. Endothelial cell binding of NAP-1/IL-8: Role in neutrophil emigration. Immunol. Today. 1992;13:291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- 151.Koch A.E., Kunkel S.L., Pearce W.H., Shah M.R., Parikh D., Evanoff H.L., Haines G.K., Burdick M.D., Strieter R.M. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am. J. Pathol. 1993;142:1423–1431. [PMC free article] [PubMed] [Google Scholar]
- 152.Ribeiro R.A., Flores C.A., Cunha F.Q., Ferreira S.H. IL-8 causes in vivo neutrophil migration by a cell-dependent mechanism. Immunology. 1991;73:472–477. [PMC free article] [PubMed] [Google Scholar]
- 153.Muñoz J., Akhavan N.S., Mullins A.P., Arjmandi B.H. Macrophage Polarization and Osteoporosis: A Review. Nutrients. 2020;12:2999. doi: 10.3390/nu12102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chung K.F. Cytokines in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 2001;34:50s–59s. doi: 10.1183/09031936.01.00229701. [DOI] [PubMed] [Google Scholar]
- 155.Wills-Karp M., Luyimbazi J., Xu X., Schofield B., Neben T.Y., Karp C.L., Donaldson D.D. Interleukin-13: Central Mediator of Allergic Asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 156.Rose C.E., Jr., Sung S.S., Fu S.M. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation. 2003;10:273–288. doi: 10.1080/mic.10.3-4.273.288. [DOI] [PubMed] [Google Scholar]
- 157.Torrano J., Al Emran A., Hammerlindl H., Schaider H. Emerging roles of H3K9me3, SETDB1 and SETDB2 in therapy-induced cellular reprogramming. Clin. Epigenetics. 2019;11:43. doi: 10.1186/s13148-019-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Verlaan D.J., Berlivet S., Hunninghake G.M., Madore A.M., Larivière M., Moussette S., Grundberg E., Kwan T., Ouimet M., Ge B., et al. Allele-specific chromatin remodeling in the ZPBP2/GSDMB/ORMDL3 locus associated with the risk of asthma and autoimmune disease. Am. J. Hum. Genet. 2009;85:377–393. doi: 10.1016/j.ajhg.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chase K.A., Sharma R.P. Nicotine induces chromatin remodelling through decreases in the methyltransferases GLP, G9a, Setdb1 and levels of H3K9me2. Int. J. Neuropsychopharmacol. 2013;16:1129–1138. doi: 10.1017/S1461145712001101. [DOI] [PubMed] [Google Scholar]
- 160.Lafuente-Sanchis A., Zúñiga Á., Galbis J.M., Cremades A., Estors M., Martínez-Hernández N.J., Carretero J. Prognostic value of ERCC1, RRM1, BRCA1 and SETDB1 in early stage of non-small cell lung cancer. Clin. Transl. Oncol. 2016;18:798–804. doi: 10.1007/s12094-015-1440-6. [DOI] [PubMed] [Google Scholar]
- 161.Hirano T., Matsunaga K. Late-onset asthma: Current perspectives. J. Asthma Allergy. 2018;11:19–27. doi: 10.2147/JAA.S125948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Baptist A.P., Ross J.A., Clark N.M. Older adults with asthma: Does age of asthma onset make a difference? J. Asthma. 2013;50:836–841. doi: 10.3109/02770903.2013.816967. [DOI] [PubMed] [Google Scholar]
- 163.Moore W.C., Meyers D.A., Wenzel S.E., Teague W.G., Li H., Li X., D’Agostino R., Jr., Castro M., Curran-Everett D., Fitzpatrick A.M., et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu W., Bleecker E., Moore W., Busse W.W., Castro M., Chung K.F., Calhoun W.J., Erzurum S., Gaston B., Israel E., et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J. Allergy Clin. Immunol. 2014;133:1280–1288. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McDonald V.M., Gibson P.G. Exacerbations of severe asthma. Clin. Exp. Allergy. 2012;42:670–677. doi: 10.1111/j.1365-2222.2012.03981.x. [DOI] [PubMed] [Google Scholar]
- 166.Jiao K., Zhou Y., Hogan B.L.M. Identification of mZnf8, a Mouse Krüpel-Like Transcriptional Repressor, as a Novel Nuclear Interaction Partner of Smad1. Mol. Cell. Biol. 2002;22:7633–7644. doi: 10.1128/MCB.22.21.7633-7644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Baldwin L., Roche W.R. Does remodelling of the airway wall precede asthma? Paediatr. Respir. Rev. 2002;3:315–320. doi: 10.1016/S1526054202002610. [DOI] [PubMed] [Google Scholar]
- 168.Grainge C.L., Lau L.C., Ward J.A., Dulay V., Lahiff G., Wilson S., Holgate S., Davies D.E., Howarth P.H. Effect of bronchoconstriction on airway remodeling in asthma. N. Engl. J. Med. 2011;364:2006–2015. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 169.Tagaya E., Tamaoki J. Mechanisms of Airway Remodeling in Asthma. Allergol. Int. 2007;56:331–340. doi: 10.2332/allergolint.R-07-152. [DOI] [PubMed] [Google Scholar]
- 170.Wnuk D., Paw M., Ryczek K., Bochenek G., Sładek K., Madeja Z., Michalik M. Enhanced asthma-related fibroblast to myofibroblast transition is the result of profibrotic TGF-β/Smad2/3 pathway intensification and antifibrotic TGF-β/Smad1/5/(8)9 pathway impairment. Sci. Rep. 2020;10:16492. doi: 10.1038/s41598-020-73473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Minshall E.M., Leung D.Y., Martin R.J., Song Y.L., Cameron L., Ernst P., Hamid Q. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am. J. Respir. Cell Mol. Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 172.Halwani R., Al-Muhsen S., Al-Jahdali H., Hamid Q. Role of transforming growth factor-β in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 173.Vignola A.M., Chanez P., Chiappara G., Merendino A., Pace E., Rizzo A., la Rocca A.M., Bellia V., Bonsignore G., Bousquet J. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Pt 1Am. J. Respir. Crit. Care Med. 1997;156:591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 174.Raez-Villanueva S., Debnath A., Hardy D.B., Holloway A.C. Prenatal nicotine exposure leads to decreased histone H3 lysine 9 (H3K9) methylation and increased p66shc expression in the neonatal pancreas. J. Dev. Orig. Health Dis. 2021;13:156–160. doi: 10.1017/S2040174421000283. [DOI] [PubMed] [Google Scholar]
- 175.Demenais F., Margaritte-Jeannin P., Barnes K.C., Cookson W.O.C., Altmüller J., Ang W., Barr R.G., Beaty T.H., Becker A.B., Beilby J., et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat. Genet. 2018;50:42–53. doi: 10.1038/s41588-017-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., Downey P., Elliott P., Green J., Landray M., et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Owen A.B. Infinitely imbalanced logistic regression. J. Mach. Learn. Res. 2007;8:761–773. [Google Scholar]
- 178.Cepeda M.S., Boston R., Farrar J.T., Strom B.L. Comparison of Logistic Regression versus Propensity Score When the Number of Events Is Low and There Are Multiple Confounders. Am. J. Epidemiol. 2003;158:280–287. doi: 10.1093/aje/kwg115. [DOI] [PubMed] [Google Scholar]
- 179.King G., Zeng L. Logistic regression in rare events data. Political Anal. 2001;9:137–163. doi: 10.1093/oxfordjournals.pan.a004868. [DOI] [Google Scholar]
- 180.He H.B., Garcia E.A. Learning from Imbalanced Data. IEEE Trans. Knowl. Data Eng. 2009;21:1263–1284. [Google Scholar]
- 181.Soda P. A multi-objective optimisation approach for class imbalance learning. Pattern Recognit. 2011;44:1801–1810. doi: 10.1016/j.patcog.2011.01.015. [DOI] [Google Scholar]
- 182.Chawla N.V., Bowyer K.W., Hall L.O., Kegelmeyer W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002;16:321–357. doi: 10.1613/jair.953. [DOI] [Google Scholar]
- 183.Elkan C. The Foundations of Cost-Sensitive Learning, International Joint Conference on Artificial Intelligence. Lawrence Erlbaum Associates Ltd.; Mahwah, NJ, USA: 2001. pp. 973–978. [Google Scholar]
- 184.Lee J.-E., Kim J.-H., Hong E.-J., Yoo H.S., Nam H.-Y., Park O. National Biobank of Korea: Quality control Programs of Collected-human Biospecimens. Osong Public Health Res. Perspect. 2012;3:185–189. doi: 10.1016/j.phrp.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moon S., Kim Y.J., Han S., Hwang M.Y., Shin D.M., Park M.Y., Lu Y., Yoon K., Jang H.-M., Kim Y.K., et al. The Korea Biobank Array: Design and Identification of Coding Variants Associated with Blood Biochemical Traits. Sci. Rep. 2019;9:1382. doi: 10.1038/s41598-018-37832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Howie B.N., Donnelly P., Marchini J. A Flexible and Accurate Genotype Imputation Method for the Next Generation of Genome-Wide Association Studies. PLOS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Solovieva E., Sakai H. PSReliP: An integrated pipeline for analysis and visualization of population structure and relatedness based on genome-wide genetic variant data. BMC Bioinform. 2023;24:135. doi: 10.1186/s12859-023-05169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Massy W.F. Principal Components Regression in Exploratory Statistical Research. J. Am. Stat. Assoc. 1965;60:234–256. doi: 10.1080/01621459.1965.10480787. [DOI] [Google Scholar]
- 189.Barrett J.C., Fry B., Maller J., Daly M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2004;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 190.Hu Y., Bien S.A., Nishimura K.K., Haessler J., Hodonsky C.J., Baldassari A.R., Highland H.M., Wang Z., Preuss M., Sitlani C.M., et al. Multi-ethnic genome-wide association analyses of white blood cell and platelet traits in the Population Architecture using Genomics and Epidemiology (PAGE) study. BMC Genom. 2021;22:432. doi: 10.1186/s12864-021-07745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Masoodi T.A., Banaganapalli B., Vaidyanathan V., Talluri V.R., Shaik N.A. Computational Analysis of Breast Cancer GWAS Loci Identifies the Putative Deleterious Effect of STXBP4 and ZNF404 Gene Variants. J. Cell. Biochem. 2017;118:4296–4307. doi: 10.1002/jcb.26080. [DOI] [PubMed] [Google Scholar]
- 192.Schaid D.J., Chen W., Larson N.B. From genome-wide associations to candidate causal variants by statistical fine-mapping. Nat. Rev. Genet. 2018;19:491–504. doi: 10.1038/s41576-018-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang K., Li M., Hakonarson H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kopanos C., Tsiolkas V., Kouris A., Chapple C.E., Aguilera M.A., Meyer R., Massouras A. VarSome: The human genomic variant search engine. Bioinformatics. 2019;35:1978–1980. doi: 10.1093/bioinformatics/bty897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The HEXA, CAVAS, and KARE Korean Chip (KORV1.1) datasets are a part of KoGES and are available upon approval of the genome center at the Korea National Institute of Health (https://is.kdca.go.kr/ (accessed on 1 June 2023)).