Abstract
An experimental paradigm with subjective and objective assessments was used to further explicate the role of positive emotion dysregulation on risky behavior. Participants were 151 community women currently experiencing intimate partner violence and using substances (Mage = 40.81, 43.0% white). Participants were randomly assigned to positive, negative, and neutral idiographic emotion inductions. Subjective (state self-report) and objective (high frequency heart rate variability [hfHRV], skin conductance response, and salivary cortisol) markers of emotion dysregulation were assessed, following which participants completed subjective (state urges for substances) and objective (Balloon Analogue Risk Task) measures of risky behavior. Results showed (a) greater self-reported state emotion dysregulation and lower hfHRV predicted more urges for substances in the positive (versus negative and neutral) emotion induction conditions; and (b) lower hfHRV predicted more behavioral risk-taking propensity in the positive (versus neutral) emotion induction condition. Findings provide additional support for the influence of positive emotion dysregulation on risky behavior.
Keywords: emotion dysregulation, risky behavior, substance use, experimental, heart rate variability, skin conductance response, cortisol
Risky behavior poses substantial threats to the health and well-being of individuals (Ben-Zur & Zeidner, 2009). Of particular clinical significance are risky behaviors that increase morbidity and mortality, such as substance use (Martins et al., 2015; Roerecke & Rehm, 2013). Modifiable, transdiagnostic, cognitive-affective vulnerability factors that are associated with engagement in risky behaviors have received growing attention (Sauer-Zavala et al., 2017). An important factor to consider in this regard is emotion dysregulation (Tull & Aldao, 2015).
Literature on emotion dysregulation and risky behavior has seen exponential growth in recent years (Weiss et al., 2015b). Theoretical frameworks propose that elevated levels of emotion dysregulation may increase subsequent risky behavior. Consistent with negative reinforcement models (e.g., Baker et al., 2004), risky behavior among individuals who experience emotion dysregulation may function to escape or avoid emotional states perceived as aversive. Alternatively, positive reinforcement models (e.g., Cooper et al., 2016) underscore the role of gratification associated with risky behavior in countering unpleasant emotional states. Another explanation is that increased attention toward acquiring rewarding experiences to neutralize distress in the context of emotion dysregulation may reduce an individual’s ability to inhibit risky behavior in emotionally salient contexts (Inzlicht & Schmeichel, 2012). Consistent with theory, cross-sectional studies have found retrospectively reported emotion dysregulation to be positively associated with risky behavior (Weiss et al., 2012), including substance use (Weiss et al., 2022a), HIV/sexual risk behaviors (Tull et al., 2012), aggression (Shorey et al., 2011), disordered eating (Lavender et al., 2014), and non-suicidal self-injury (Gratz & Tull, 2010). While fewer in number, traditional longitudinal investigations using retrospective reports have also documented a positive relation between earlier emotion dysregulation and later risky behavior (Racine & Wildes, 2015; Tull et al., 2015; Weiss et al., 2019d). Further, more recently, experience sampling methods have shown evidence for momentary relations between emotion dysregulation and risky behavior at the micro-longitudinal level (Weiss et al., in press-a; Weiss et al., in press-b). Finally, improvements in emotion dysregulation have been linked to reductions in risky behavior, assessed through both self-report questionnaire and behavioral measurement of risk-taking propensity task in the laboratory (Weiss et al., 2014). Collectively, literature provides robust support for a relation between emotion dysregulation and risky behavior.
One critically important limitation of the research on emotion dysregulation and risky behavior is that it has primarily captured dysregulation stemming from negative emotions. A fast-growing body of evidence has demonstrated that individuals may also exhibit dysregulation in the context of positive emotions, including non-accepting responses and impulsive or goal-incongruent behaviors (Weiss et al., 2015a). For instance, when presented with positive emotional stimuli, some individuals may experience competing negative cognitions (Frewen et al., 2012), such as the thought that they do not deserve to feel happy or that happiness is short-lived. This, in turn, may lead to attempts to reduce positive emotions (Roemer et al., 2001), including through risky behavior (Weiss et al., 2020; Weiss et al., 2021). In addition, some individuals may experience behavioral dyscontrol in the context of positive emotions, perhaps due to impairment in the ability to control or suppress an automatic response, found to be impaired in emotional contexts (Billieux et al., 2010) and also associated with risky behavior (Noël et al., 2001). In line with these above suggestions, retrospective reports of positive emotion dysregulation and substance use (Weiss et al., 2018b; Weiss et al., 2019c), HIV/sexual risk behavior (Weiss et al., 2019a), aggression (Simpson et al., in press), disordered eating (Tobar-Santamaria et al., 2021), and non-suicidal self-injury (Raudales et al., 2020; Raudales et al., 2021) are positively associated in cross-sectional examinations. While preliminary in nature, these findings signal that some individuals may experience dysregulation in the context of positive emotions, which, in turn, may increase engagement in risky behavior.
To advance literature in this area, the current study utilized an experimental paradigm with subjective and objective assessments to further explicate the influence of positive emotion dysregulation on risky behavior. Participants were randomly assigned to positive, negative, and neutral idiographic emotion induction conditions. Subjective (i.e., self-reported state emotion dysregulation) and objective (i.e., high frequency heart rate variability [hfHRV], skin conductance response [SCR], salivary cortisol) markers of emotion dysregulation were assessed, following which participants completed subjective (i.e., self-reported state urges for substances) and objective (i.e., Balloon Analogue Risk Task [BART]) measures of risky behavior. This design addresses important limitations of the existing research. First, investigations of positive emotion dysregulation—and largely of negative emotion dysregulation (for some exceptions, see Szasz et al., 2016; Szasz et al., 2012; Tull et al., 2018)—in relation to risky behavior have relied on cross-sectional designs, precluding determination of the causal and temporal association between emotion dysregulation and risky behavior in the context of positive emotions. Use of an experimental design will speak to the influence of emotion dysregulation elicited by positive (in comparison to negative and neutral) emotional stimuli on subsequent risky behavior. Second, studies that have examined the association of positive emotion dysregulation to risky behavior have relied on retrospective reports, subject to memory decay and distortion as well as heuristic (e.g., availability) biases, particularly relevant to the study of emotions (Shiffman et al., 2008). Assessment of state subjective experiences alongside objective markers will enhance the ecological validity of results, increasing their relevance for intervention development.
Given growing experimental research on risky behavior in the context of both negative and positive emotion states (Cyders et al., 2010; Um et al., 2022), this latter gap represents one of the major contributions of the current study. Research points to several psychophysiological markers of emotion. High-frequency heart rate variability (hfHRV)—the characteristic beat-to-beat modulation of heart rate by parasympathetic activation of the vagus nerve—is arguably the most well-validated psychophysiological index of emotion dysregulation (see Balzarotti et al., 2017; Holzman & Bridgett, 2017). According to the Theory of Neurovisceral Integration (Thayer & Lane, 2009), the relation of emotion dysregulation to hfHRV can be understood through the roles of central and autonomic nervous system structures on cardiac functioning (Appelhans & Luecken, 2006). Specifically, research posits that hfHRV captures the extent to which the brain is able to exhibit control over the periphery (Thayer et al., 2012), and, thus, is a parasympathetic index of emotion dysregulation. Consistent with theory, extant studies have linked subjective reports of emotion dysregulation to hfHRV; lower hfHRV is related to (1) greater negative emotion dysregulation (Visted et al., 2017; Williams et al., 2015) and (2) more positive emotion dysregulation at low state positive affect (Weiss et al., 2021). Although more strongly tied to emotional arousal (Hellhammer et al., 2009; Kreibig, 2010), skin conductance response (SCR) and salivary cortisol have also been identified as salient (sympathetic) biomarkers of emotion dysregulation. In particular, higher SCR has been associated with greater emotion dysregulation (Shepherd & Wild, 2014), including more nonacceptance of emotions following emotional stimuli (Salters-Pedneault et al., 2007), whereas lower (blunted) salivary cortisol has been associated with increased use of maladaptive emotion regulation strategies (Zoccola et al., 2008) and greater emotion dysregulation (Cărnuţă et al., 2015). Of particular importance to the current study, these psychophysiological markers of emotion have been linked to risky behavior (see Eddie et al., 2015; 2020), underscoring the potential utility of examining hfHRV, SCR, and salivary cortisol as objective indices of emotion in relation to risky behavior.
Of note, we examined these associations in a clinically relevant sample of community women who currently experience intimate partner violence (IPV) and use substances. IPV is a global health concern (Garcia-Moreno et al., 2006) that is highly prevalent among women, with one in three women reporting experiences of sexual violence, physical violence, and/or stalking by an intimate partner during their lifetime (Smith et al., 2018). Risky behavior is an especially devastating consequence of IPV (Peters et al., 2012), with evidence suggesting an increased pattern of risky behavior following IPV (Devries et al., 2014). While less understood, emotion dysregulation is elevated among women who experience IPV (Weiss et al., 2018a) and related to their risky behavior (Weiss et al., 2022b). Thus, examination of the role of positive emotion dysregulation on risky behavior in women who experience IPV is important.
For the current study, we expected that emotion induction condition would moderate the associations between emotion dysregulation and risky behavior, such that these relations would be stronger for participants in the positive versus neutral emotion induction conditions. A dearth of research has compared the contributions of positive and negative emotion dysregulation on risky behavior, thus no a priori hypotheses were made regarding the strength of these associations among participants in the positive versus negative emotion induction conditions.
Method
Participants
Recruitment materials were posted in community establishments throughout Providence, Rhode Island. Eligibility was determined through a phone screen. Participants were women who reported experiencing physical and/or sexual victimization in the past six months by their current male partner and having used any amount of alcohol or drugs during the past 30 days. Additional inclusion criteria were: (1) age 18 or older, (2) fluent in the English language, and (3) current involvement in a relationship of at least six months’ duration. Exclusion criteria were (a) current mania/psychosis, (b) current impairment in cognitive functioning, (c) current pregnancy, (d) color blindness, (e) cardiovascular disease, and (f) residence in a shelter or group home. Sample size to achieve power of .80 for moderation was determined based on a priori power analyses assuming a small-medium effect size (Cohen’s f2 = .09) and an alpha of .05 (N = 149). The final sample here included 151 women who participated in the baseline and experimental sessions.
Procedures
All procedures were reviewed and approved by the University of Rhode Island Institutional Review Board. The study entailed (a) a baseline session, (b) an experimental session, (c) 30 days of experience sampling using interactive voice technology, and (d) a follow-up session. The current study used data from the baseline and experimental sessions. To limit participant burden, these sessions were conducted on separate days. Individual interviews were conducted by female bachelors- or masters-level clinical psychology doctoral students in a private office to protect participants’ safety and confidentiality. We report how we determined our sample size, all data exclusions, all manipulations, and all measures in the study.
Baseline Session.
Participants provided informed consent, following which they were interviewed using a structured diagnostic assessment and completed self-report measures on a computer. Prior to the baseline session, participants were randomly assigned to one of three emotion induction conditions (negative, positive, or neutral). For participants in the negative and positive emotion induction conditions, a standardized protocol for developing idiographic emotion induction scripts was followed. The method for generating these idiographic emotion induction scripts was based on procedures developed by Lang and Cuthbert (1984) and reliably induces emotional responses in trauma-exposed samples (Tull et al., 2011; Tull et al., 2019).
To hold arousal constant, emotion inductions targeted excitement and anger, which are oppositely-valenced but high in arousal (Barrett, 1998). Specifically, participants were asked to recall a recent or vivid event during which they became “very excited” (positive condition) or “very angry” (negative condition) that did not involve substances or a traumatic experience. This portion of the session was audio recorded so that the interviewer could subsequently create a script using the participant’s own language. Participants were asked to picture the situation in their mind and attempt to remember, as vividly as possible, what the event entailed and their feelings at the time. Participants were then asked to describe the incident in as much detail as possible. The interviewer probed for key aspects of the event (e.g., time and place of the event, as well as emotions, thoughts, and bodily sensations experienced during the event).
Following the baseline session, a personalized script consisting of a series of autobiographical statements, appraisals, and emotional responses generated from the interview was recorded onto an audiotape. This script was approximately one minute in length and the narrator was consistent across all scripts (the principal investigator). All scripts were presented in a female voice with a neutral tone (to reduce reactivity given that the sample is characterized by experiences of IPV with a male partner). The script is designed to maximize emotional responses by depicting the events in a salient, emotion-focused form in second person, present tense.
Neutral scripts were also developed for this study. Consistent with Keane et al. (1998), the neutral script was standardized and consistent across participants. It provided a description of activities related to getting up in the morning (e.g., brushing teeth, getting dressed). The neutral script was also approximately one minute in length and similarly consisted of descriptions of morning events, as well as thoughts and feelings that a person may experience in response.
At the end of the baseline session, participants were instructed to abstain from alcohol and illicit drugs for a period of at least 24 hours prior to the experimental session to reduce risk for intoxication and acute withdrawal (Coffey et al., 2011). Participants were compensated $40 for completing the baseline session.
Experimental Session.
At the start of the experimental session (4–7 days after the baseline session), participants’ compliance with substance use restrictions was assessed. A urine drug screen was administered to test for metabolites of THC, cocaine, opiates, amphetamines, benzodiazepines, methamphetamine, oxycodone, propoxyphene, barbiturates, and MDMA, and to assess recent alcohol intoxication, expired air samples were analyzed. Participants who tested positive for illicit drugs or who had a breath alcohol concentration > .01 were rescheduled. Due to the long half-life of THC metabolites, participants who tested positive for THC, but reported no marijuana use in the past 24 hours, were allowed to participate in this session.
Participants were provided with instructions on how to place the electrodes for the electrocardiogram (beneath the right and left clavicle and the right ribcage) and electrodermal (medial phalanges of the middle and index finger of participant’s non-dominant hand) monitoring. Following this, a two-phase emotion induction paradigm was implemented. First, a neutral mood was induced by displaying colors, one after another, on a screen in front of the participants for five minutes. This procedure, called the “vanilla baseline procedure,” has been found to produce a more neutral mood (e.g., less anxiety) compared with an absence of activities (i.e., having the participant sit and do nothing for 5 minutes; Jennings et al., 1992). Next, participants listened to either the one-minute idiographic emotion induction script developed during the baseline session (negative and positive emotion induction conditions) or the standardized neutral emotion induction script (neutral emotion induction condition). Once the tape was finished, participants were instructed to close their eyes and imagine vividly the event taking place in real time for one minute. After emotion induction, participants completed measures of emotion dysregulation and then cravings, and subsequently were administered a behavioral measure of risk-taking propensity. A saliva sample was provided 20-minutes post-emotion induction. Participants were compensated $25 for completing the experimental session.
Measures
Diagnostic Measure
A computerized version of the SCID-5 was administered to establish current alcohol and drug use disorders (First & Williams, 2016). The SCID-5 is a gold standard semi-structured assessment instrument for psychiatric disorders. Inter-rater reliability of the SCID-5 found kappas of .84 and .94 respectively for alcohol and drug use disorders (Osório et al., 2019). SCID-5 interviews were conducted by clinical psychology doctoral students trained to reliability with the principal investigator, a licensed clinical psychologist in the state of Rhode Island. All diagnostic data were reviewed and confirmed by the principal investigator during weekly meetings with the diagnostic interviewers. In the case of ambiguous responses, data were discussed by the principal investigator and interviewer until a consensus was reached.
Subjective Measures
State emotions.
The Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) is a 20-item scale assessing state positive (10 items) and negative (10 items) emotions. Participants indicate how they feel, in the present moment, on a five-point scale from 0 (very slightly/not at all) to 4 (extremely), and respective items were summed to create positive and negative emotion subscales. The PANAS was administered pre- (Cronbach’s α = .90 and .91 for state positive and negative emotion subscales, respectively) and post-emotion induction (Cronbach’s α = .90 and .91 for state positive and negative emotion subscales, respectively).
State emotion dysregulation.
The Responses to Emotions Questionnaire (REQ; Campbell-Sills et al., 2006) is an eight-item scale assessing the degree to which individuals use emotion regulation strategies during emotion-eliciting tasks. The measure contains four items relevant to avoiding or changing emotional experience and four items relating to awareness and acceptance of emotional experience. Participants rate their degree of strategy use from 0 (not at all) to 10 (all the time). Consistent with Brake et al. (2016), three of the awareness/acceptance items were reverse scored and combined with the four avoidance items to create a composite emotion dysregulation score. One mindfulness item (“I didn’t mind feeling uncomfortable during the exercises”) was excluded because it is conceptually similar to distress (Brake et al., 2016). Cronbach’s α in the present study was .58 for the total scale score.
State urges for substances.
On two separate items, participants rate the strength of their urges for alcohol and drug use (0 = “no urges,” 9 = “very strong urges;” Chapman et al., 2009). These two items were averaged to create a total state substance use urges item.
Dissociation.
The Dissociative Tension Scale-4 (DES-4; Stiglmayr et al., 2009) is a four-item scale that assesses acute dissociative experiences. Participants rate their degree of dissociation from 0 (none) to 9 (very strong) and items are summed to create a total scale score. Cronbach’s α in the present study was .75 for the total scale score.
Objective Measures
High frequency heart rate variability (hfHRV).
Electrocardiogram (ECG) was acquired using the Biopac MP36RWSW. Sequences of heart beat-to-beat intervals (RRI) were recorded via ECG and exported into AcqKnowledge 4.3 software to be used for calculation of hfHRV. Heart rate, expressed as beats per minute, was derived by calculating the average number of R-spikes in the ECG signal occurring each minute during the recording period, and HRV was calculated from edited sequential RR intervals derived from the ECG signal. Frequency domain HRV indices were calculated using Fourier analysis (Taylor et al., 1998). Frequency domain indices of HRV provide information about how power distributed as a function of frequency (Malik, 1996). We calculated power spectral density (msec2/Hz) in the high frequency domain (hf: 0.15–0.4 Hz). For this study, average hfHRV was calculated during the emotion induction.
Skin conductance response (SCR).
Electrodermal activity (EDA) was acquired using the Biopac MP36RWSW and exported into AcqKnowledge 4.3 software to be used for calculation of SCR. EDA data were manually checked for artifacts and edited manually as needed. In the current study, mean SCR was calculated by averaging the number of SCRs (responses exceeding .05 μS) during the emotion induction.
Salivary cortisol.
Saliva samples were collected by instructing participants to pool saliva in their mouth, then transfer the saliva into a centrifuge tube with a Salivette. Approximately 0.5mL of saliva was collected, then sealed and stored in a freezer. All samples were assayed in duplicate for salivary cortisol offsite using a highly sensitive enzyme immunoassay. The test used 25 μL of saliva per determination, has a lower limit of sensitivity of 0.003 μg/dL, standard curve range from 0.012 μg/dL to 3.0 μg/dL, an average intra-assay coefficient of variation (CV) of 3.8%, and an average inter-assay CV of 5.1%. For the purpose of the current study, salivary cortisol was collected 20 minutes following the idiographic emotion induction script (cortisol peaks 20 minutes after an emotionally-evocative cue; Nicolson, 2007).
Risk-taking propensity.
The BART (Lejuez et al., 2002) requires participants to inflate a balloon model presented on a computer screen. Participants accrue money for each pump of the balloon in a temporary bank; however, the odds of the balloon “popping” increase with each pump of the balloon. When a balloon explodes, all money in the temporary bank is lost and the next uninflated balloon is displayed. At any point during each balloon trial, the participant can stop pumping the balloon and transfer the money from the temporary bank to the permanent bank. Thirty balloons (i.e., trials) are presented. At the start of this task, participants were told that they could earn up to $25. Immediately following this task, they were told that everyone was paid the same amount of money for the experimental session (i.e., $25), regardless of how well they did on this computer task. The BART has been shown to be positively correlated with measures of related constructs (e.g., sensation seeking, impulsivity), as well as actual involvement in risky behaviors (substance use and risky sexual behavior; Lejuez et al., 2002). Consistent with scoring guidelines, the average number of pumps excluding balloons that exploded was calculated, with higher scores representing greater risky behavior propensity.
Demographics
Participants completed a demographic questionnaire in which they self-described their age, racial/ethnic background, household income, and number of years of education.
Analytic Strategy
First, frequencies and descriptive data were calculated for demographics and clinical characteristics. Then, we examined bivariate correlations between demographic and outcome variables for consideration as possible covariates in regression models. Following this, we conducted analyses of variance (ANOVAs) to examine the effect of emotion induction condition (positive vs. negative vs. neutral) on risky behavior. Next, we conducted one-way repeated analyses of variance (rANOVAs) with eta-squared (η2) effect size estimates and Tukey’s post hoc tests as a manipulation check to confirm that the positive and negative emotion inductions resulted in respective increases in positive and negative emotions assessed by the PANAS. As an additional test of potency, ANOVAs assessed dissociation and emotional disengagement across the emotion induction conditions.
To test the primary study aims, moderation analyses were conducted using the PROCESS SPSS macro (Model 1; Hayes, 2018) to examine whether the strength of the relations between subjective and objective markers of both emotion dysregulation (i.e., REQ, hfHRV, SCR, salivary cortisol) and risky behavior (i.e., self-reported state urges for substances, BART) varied as a function of emotion induction condition (0 = positive, 1 = negative, 2 = neutral). The PROCESS procedures use ordinary least squares regression and bootstrapping methodology, which confers more statistical power than do standard approaches to statistical inference and does not rely on distributional assumptions. Bootstrapping was done with 5,000 random samples generated from the observed covariance matrix to estimate bias-corrected 95% confidence intervals (CIs) and significance values. Given the multicategorical nature of the moderator variable, indicator coding was utilized in the PROCESS macro analysis (Hayes & Montoya, 2017), resulting in two dummy coded variables (W1 [positive versus negative emotion induction condition] and W2 [positive versus neutral emotion induction condition]). Thus, each moderation model included W1, W2, emotion dysregulation, W1 X emotion dysregulation, and W2 X emotion dysregulation. Multicategorical moderation only provides a test of the comparison group to the other conditions, but not of the other conditions to each other. Since the goal of this study was to extend our understanding of positive emotion dysregulation specifically, the positive emotion induction condition was made the comparison group; thus, comparisons are not conducted between the negative and neutral emotion induction conditions. For significant interactions, following the methods described by Aiken and West (1991), we plotted regression slopes of differences in risky behavior for each idiographic emotion induction condition and examined whether the slopes of the regression lines differed significantly from zero. For each moderation analysis, we computed post-hoc power achieved given the observed model R2 effect size and sample size (considering missing data). For any analyses with lower than optimal post hoc power (< .8), we do not summarize findings in-text.
Results
Preliminary Analyses
Participant characteristics are summarized in Table 1. Women ranged in age from 19 to 65 years (M = 40.81, SD = 11.64). In terms of racial/ethnic background, 30.5% of participants (n = 46) were Black, 43.0% (n = 65) were white, 11.3% (n = 17) were Hispanic or Latina, 7.9% (n = 12) were American Indian/Alaska Native, and 7.3% (n = 11) were another or multiple racial/ethnic backgrounds. Most women were unemployed (n = 94; 62.3%) or were not in the labor force (e.g., homemaker; n = 21; 13.9%); 5.3% (n = 8) were employed full-time and 11.3% (n = 17) were employed part-time. Monthly household income ranged from $0 to $10,416.67 (M = $1278.03; SD = $1594.52) and mean level of education was 12.39 years (SD = 2.04). Most women were unmarried (n = 113; 74.8%); 7.9% (n = 12) were married and 8.6% (n = 13) were separated or divorced. Mean years in a relationship with their partner was 6.08, ranging from six months to 30 years (SD = 5.88). On average, women spent 5.80 (SD = 1.89) days per week with their partner. Most women had a current substance use disorder (n = 124; 70.1%), with 45.8% (n = 81) and 58.8% (n = 104) having current alcohol and drug use disorders, respectively. None of the participant characteristics were significantly related to the outcomes of interest (ps > .05).
Table 1.
Sample demographic characteristics and descriptive statistics
| Characteristic | M (SD) | Range | n (%) |
|---|---|---|---|
|
| |||
| Age | 40.81 (11.64) | 19 – 65 | |
| Race/Ethnicity | |||
| Black or African American | 46 (30.5%) | ||
| White | 65 (43.0%) | ||
| American Indian/Alaska Native | 12 (7.9%) | ||
| Hispanic or Latina | 17 (11.3%) | ||
| Not listed | 9 (6.0%) | ||
| Prefer not to respond | 2 (1.3%) | ||
| Years of Education Completed | 12.39 (2.04) | 6 – 18 | |
| Employment | |||
| Full time (35+ hours per week) | 8 (5.3%) | ||
| Part time (<35 hours per week) | 17 (11.3%) | ||
| Unemployed | 94 (62.3%) | ||
| Not in labor force (e.g., student, homemaker) | 21 (13.9%) | ||
| Prefer not to respond | 11 (7.3%) | ||
| Monthly Household Income | $1,278.03 ($1,594.52) | $0 – $10,416.67 | |
| Relationship Status | |||
| Married | 12 (7.9%) | ||
| Unmarried | 113 (74.8%) | ||
| Separated or divorced | 13 (8.6%) | ||
| Prefer not to respond | 13 (8.6%) | ||
| Relationship Length (in months) | 73.00 (70.54) | 6 – 360 | |
| Days Per Week with Partner | 5.80 (1.89) | 0 – 7 | |
| Alcohol Use Disorder | 81 (45.8%) | ||
| Drug Use Disorder | 104 (58.8%) | ||
| Substance Use Disorder | 124 (70.1%) | ||
| Pre-Induction PANAS Positive Emotion Subscale | 16.89 (9.81) | 0 – 40 | |
| Pre-Induction PANAS Negative Emotion Subscale | 17.36 (10.97) | 0 – 40 | |
| Post-Induction PANAS Positive Emotion Subscale | 6.48 (8.02) | 0 – 34 | |
| Post-Induction PANAS Negative Emotion Subscale | 7.25 (9.24) | 0 – 36 | |
| Responses to Emotions Questionnaire | 17.24 (9.46) | 0 – 44 | |
| High Frequency Heart Rate Variability | 6.59 (2.06) | 2.61 – 12.15 | |
| Skin Conductance Response | 3.41 (5.71) | 0 – 38 | |
| Salivary Cortisol | 0.19 (0.46) | 0.01 – 4.65 | |
| Urges to Use Substances | 1.65 (2.17) | 0 – 9 | |
| BART Scores | 20.28 (12.45) | 0 – 54.80 | |
Note. PANAS = Positive and Negative Affect Schedule
There were no significant differences across positive (M = 20.14, SD = 12.27), negative (M = 19.76, SD = 12.81), and neutral (M = 20.89, SD = 12.46) emotion induction conditions on the BART (F[2, 151] = 0.11, p = .89, η2 = .002). There were no significant differences across positive (M = 1.41, SD = 2.26), negative (M = 2.04, SD = 2.20), and neutral (M = 1.49, SD = 2.06) emotion induction conditions on substance use urges (F[2, 151] = 1.25, p = .29, η2 = .02).
Manipulation Check
Estimated marginal means depicting changes in positive and negative emotions (based on the PANAS) from pre- to post-induction across conditions are visually displayed in Figure 1. There were significant differences across idiographic emotion induction conditions from pre- to post-induction with respect to positive (F[2, 151] = 33.51, p < .001, η2 = .31) and negative (F[2, 151] = 16.86, p < .001, η2 = .18) emotions. Participants in the positive induction condition reported increased positive emotions compared to those in the negative (Mdiff = 9.90, SE = 1.31, p < .001) and neutral (Mdiff = 8.72, SE = 1.29, p < .001) induction conditions. Participants in the negative emotion induction condition reported increased negative emotions compared to those in the positive (Mdiff = 6.19, SE = 1.38, p < .001) and neutral (Mdiff = 7.09, SE = 1.31, p < .001) induction conditions. There was no difference in change in positive emotions between the negative and neutral induction conditions (Mdiff = 1.18, SE = 1.25, p = .61), nor was there a significant difference in change in negative emotions between the positive and neutral conditions (Mdiff = 0.90, SE = 1.35, p = .61).
Figure 1.
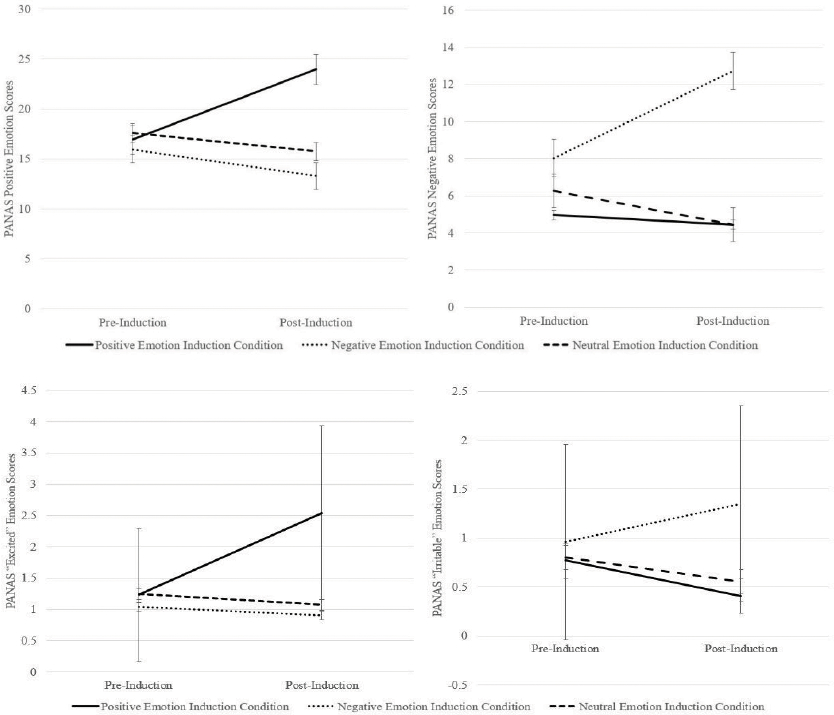
Estimated marginal means for PANAS positive and negative emotion scores pre- and post-idiographic emotion induction
Note. PANAS = Positive and Negative Affect Schedule; bars represent standard errors
Estimated marginal means depicting changes in scores on the “excited” and “irritable” PANAS emotion items—discrete emotions targeted by the emotion induction procedure—are visually displayed in Figure 1. There were significant differences across idiographic emotion induction conditions from pre- to post-induction with respect to scores on the “excited” (F[2, 151] = 30.69, p < .001, η2 = .29) and “irritable” (F[2, 150] = 8.89, p < .001, η2 = .11) emotion items. Participants in the positive induction condition reported increased scores on the “excited” emotion item compared to those in the negative (Mdiff = 1.54, SE = 0.22, p < .001) and neutral (Mdiff = 1.49, SE = 0.22, p < .001 .002) induction conditions. Participants in the negative induction condition reported increased scores on the “irritable” emotion item compared to those in the positive (Mdiff = 0.79, SE = 0.20, p < .001) and neutral (Mdiff = 0.66, SE = 0.19, p = .003) induction conditions. There was no difference in change in the “excited” emotion item between those in the negative and neutral induction conditions (Mdiff = −0.05, SE = 0.21, p = .97), nor was there a significant difference in change in the “irritable” emotion item between those in the positive and neutral induction conditions (Mdiff = −0.13, SE = 0.20, p = .79).
Differences in changes on each PANAS emotion item across idiographic emotion induction conditions from pre- to post-induction are presented in Supplemental Table 1. The above pattern of findings was replicated for most PANAS items in the direction that would be expected (e.g., PANAS positive emotions increased in the positive emotion induction condition but not in the negative or neutral emotion induction conditions). Supplemental Table 1 also indicates levels of dissociation and emotional disengagement during the emotion induction across idiographic emotion induction conditions to further test whether the conditions differed in their potency. No significant differences were found with respect to dissociation and all but one item of emotional disengagement that asked whether respondents engaged emotional suppression. Specifically, compared to the neutral emotion induction condition, those in the negative and positive emotion induction conditions reported greater efforts to suppress or hold back their emotional reactions. There was no significant difference on this item between the positive and negative emotion induction conditions.
Moderation Analyses
Substance Use Urges (Table 2)
Table 2.
Main and interactive effects of emotion dysregulation indices and emotion induction condition on urges to use substances and behavioral risk-taking propensity
| Overall Model |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Constructs | b | SE | t | p | 95% CI | F | df | p | R2 | Post Hoc Power |
|
| ||||||||||
| Urges to Use Substances | 7.31 | [5, 147] | <.001 | .20 | .99 | |||||
| Responses to Emotions Questionnaire | ||||||||||
| Responses to Emotions Questionnaire | 0.15 | 0.03 | 4.64 | <.001 | [0.08, 0.21] | |||||
| Negative Emotion Induction Conditiona | 2.03 | 0.77 | 2.63 | .01 | [0.50, 3.56] | |||||
| Neutral Emotion Induction Conditiona | 0.38 | 0.87 | 0.44 | .66 | [−1.34, 2.11] | |||||
| Responses to Emotions Questionnaire X Negative Emotion Induction Condition | −0.10 | 0.04 | −2.48 | .01 | [−0.18, −0.02] | |||||
| Responses to Emotions Questionnaire X Neutral Emotion Induction Condition | −0.05 | 0.05 | −1.02 | .31 | [−0.14, 0.04] | |||||
| High Frequency Heart Rate Variability | 4.63 | [5, 93] | .02 | .13 | .99 | |||||
| High Frequency Heart Rate Variability | −0.52 | 0.21 | −2.49 | .01 | [−0.93, −0.10] | |||||
| Negative Emotion Induction Conditiona | −4.28 | 1.94 | −2.21 | .03 | [−8.13, −0.43] | |||||
| Neutral Emotion Induction Conditiona | −2.80 | 1.77 | −1.58 | .12 | [−6.31, 0.72] | |||||
| High Frequency Heart Rate Variability X Negative Emotion Induction Condition | 0.76 | 0.28 | 2.72 | .01 | [0.21, 1.32] | |||||
| High Frequency Heart Rate Variability X Neutral Emotion Induction Condition | 0.37 | 0.27 | 1.38 | .17 | [−0.16, 0.90] | |||||
| Skin Conductance Response | 1.90 | [5, 134] | .10 | .07 | .99 | |||||
| Skin Conductance Response | −0.08 | 0.05 | −1.61 | .11 | [−0.18, 0.02] | |||||
| Negative Emotion Induction Conditiona | 0.79 | 0.56 | 1.41 | .16 | [−0.32, 1.89] | |||||
| Neutral Emotion Induction Conditiona | −0.58 | 0.50 | −1.16 | .25 | [−1.56, 0.41] | |||||
| Skin Conductance Response X Negative Emotion Induction Condition | −0.03 | 0.09 | −0.33 | .74 | [−0.21, 0.15] | |||||
| Skin Conductance Response X Neutral Emotion Induction Condition | 0.11 | 0.07 | 1.63 | .11 | [−0.02, 0.25] | |||||
| Salivary Cortisol | 1.37 | [5, 138] | .24 | .05 | .99 | |||||
| Salivary Cortisol | −0.35 | 0.49 | −0.73 | .47 | [−1.32, 0.61] | |||||
| Negative Emotion Induction Conditiona | 0.25 | 0.60 | 0.42 | .68 | [−0.94, 1.45] | |||||
| Neutral Emotion Induction Conditiona | −0.35 | 0.49 | −0.71 | .48 | [−1.31, 0.62] | |||||
| Salivary Cortisol X Negative Emotion Induction Condition | 2.05 | 2.62 | 0.78 | .43 | [−3.13, 7.23] | |||||
| Salivary Cortisol X Neutral Emotion Induction Condition | 1.77 | 0.88 | 2.00 | .05 | [0.02, 3.51] | |||||
| Balloon Analogue Risk Task | ||||||||||
| Responses to Emotions Questionnaire | 0.21 | [5, 146] | .96 | .01 | 0.34 | |||||
| Responses to Emotions Questionnaire | 0.14 | 0.20 | 0.68 | .50 | [−0.26, 0.53] | |||||
| Negative Emotion Induction Conditiona | −0.10 | 2.60 | −0.04 | .97 | [−5.24, 5.04] | |||||
| Neutral Emotion Induction Conditiona | 0.35 | 2.56 | 0.14 | .89 | [−4.71, 5.41] | |||||
| Responses to Emotions Questionnaire X Negative Emotion Induction Condition | −0.24 | 0.26 | −0.91 | .36 | [−0.75, 0.28] | |||||
| Responses to Emotions Questionnaire X Neutral Emotion Induction Condition | −0.07 | 0.29 | −0.26 | .80 | [−0.65, 0.50] | |||||
| High Frequency Heart Rate Variability | 1.83 | [5, 92] | .12 | .09 | 0.92 | |||||
| High Frequency Heart Rate Variability | −2.05 | 1.14 | −1.79 | .08 | [−4.32, 0.22] | |||||
| Negative Emotion Induction Conditiona | −3.57 | 3.13 | −1.14 | .26 | [−9.79, 2.65] | |||||
| Neutral Emotion Induction Conditiona | 2.94 | 2.86 | 1.03 | .31 | [−2.73, 8.61] | |||||
| High Frequency Heart Rate Variability X Negative Emotion Induction Condition | 2.16 | 1.54 | 1.41 | .16 | [−0.89, 5.21] | |||||
| High Frequency Heart Rate Variability X Neutral Emotion Induction Condition | 3.19 | 1.53 | 2.09 | .04 | [0.15, 6.23] | |||||
| Skin Conductance Response | 0.84 | [5, 134] | .53 | .03 | 0.66 | |||||
| Skin Conductance Response | 0.13 | 0.30 | 0.44 | .66 | [−0.46, 0.71] | |||||
| Negative Emotion Induction Conditiona | −0.09 | 2.57 | −0.03 | .97 | [−5.16, 4.99] | |||||
| Neutral Emotion Induction Conditiona | 1.66 | 2.56 | 0.65 | .52 | [3.40, 6.71] | |||||
| Skin Conductance Response X Negative Emotion Induction Condition | 0.22 | 0.53 | 0.42 | .68 | [−0.83, 1.26] | |||||
| Skin Conductance Response X Neutral Emotion Induction Condition | −0.57 | 0.40 | −1.42 | .16 | [−1.37, 0.22] | |||||
| Salivary Cortisol | 0.60 | [5, 139] | .70 | .02 | 0.52 | |||||
| Salivary Cortisol | −0.02 | 2.80 | −0.01 | .996 | [−5.56, 5.53] | |||||
| Negative Emotion Induction Conditiona | −1.00 | 2.71 | −0.37 | .71 | [−6.35, 4.35] | |||||
| Neutral Emotion Induction Conditiona | 0.53 | 2.60 | 0.21 | .84 | [−4.61, 5.68] | |||||
| Salivary Cortisol X Negative Emotion Induction Condition | −8.26 | 15.00 | −0.55 | .58 | [−37.92, 21.41] | |||||
| Salivary Cortisol X Neutral Emotion Induction Condition | 6.59 | 5.08 | 1.30 | .20 | [−3.45, 16.63] | |||||
Note.
Reference group is positive emotion induction condition; bolded typeface indicates significance at the level p < .05
Post hoc power for analyses examining substance use urges were found to be adequate (Power = 0.99). The interaction between state subjective emotion dysregulation and emotion induction condition was significant (ΔR2 = .03, F[2, 147] = 3.12, p = .046). Analysis of simple slopes (see Figure 2) revealed that the association between subjective emotion dysregulation and urges to use alcohol was significant and positive for those in the positive emotion induction condition (b = 0.15, SE = 0.03, t = 4.64, p < .001, 95%CI [0.08, 0.21]), but was not significant for those in the negative (b = 0.04, SE = 0.03, t = 1.69, p = .09, 95%CI [−0.01, 0.10]) or neutral (b = 0.10, SE = 0.03, t = 1.96, p = .05, 95%CI [0.03, 0.17]) emotion induction conditions.
Figure 2.
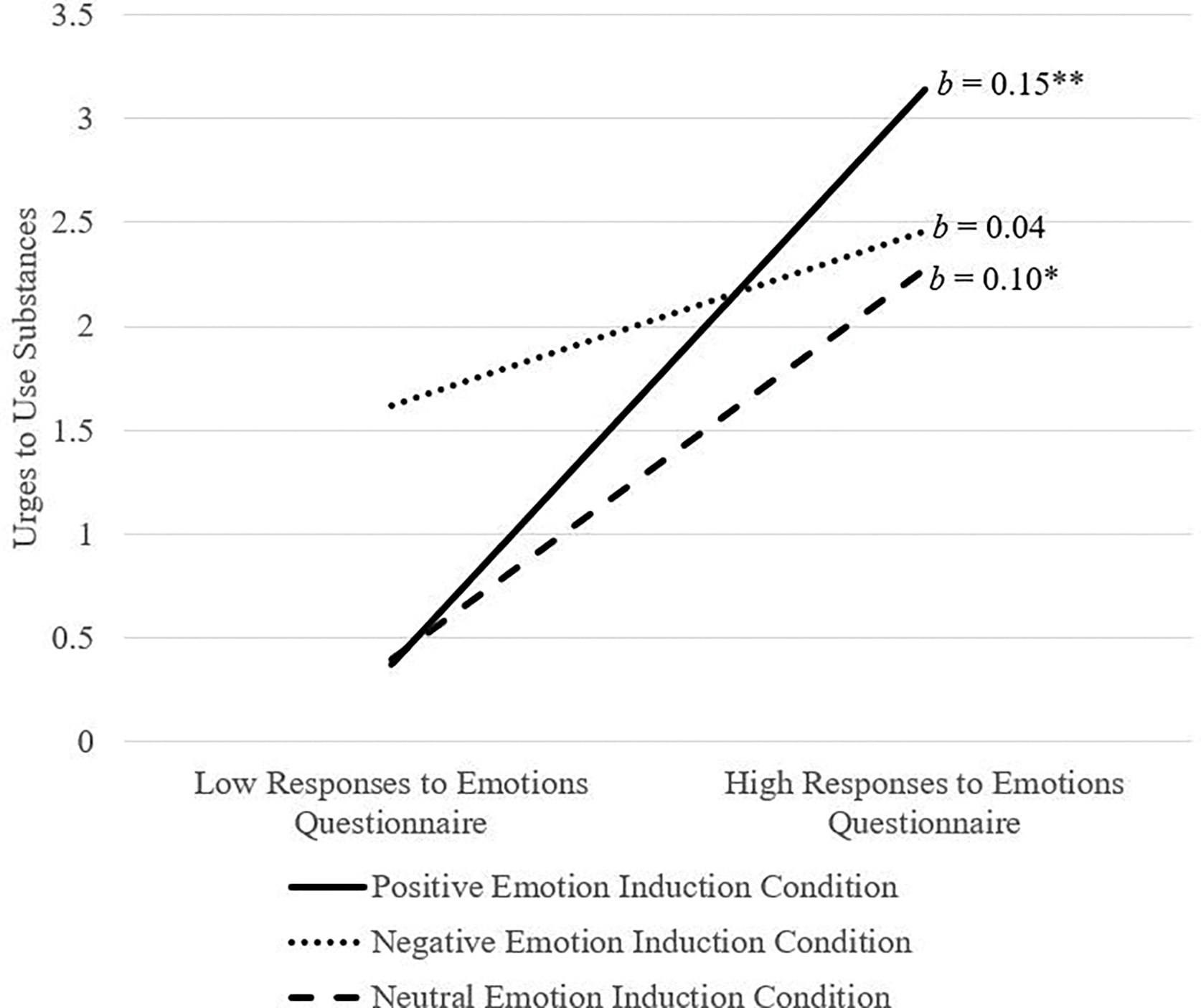
Emotion induction condition by Responses to Emotions Questionnaire predicting urges to use substances
Note. The Responses to Emotions Questionnaires was used as a continuous variable in the model but was plotted at high and low levels (i.e., 1 SD above and below the mean) for visualization purposes; *p < .01, **p < .001
The interaction of hfHRV and emotion induction condition was significant (ΔR2 = .07, F[2, 93] = 3.73, p = .03). Analysis of simple slopes (see Figure 3) revealed that the relation between hfHRV and urges to use alcohol was significant and negative for those in the positive emotion induction condition (b = −0.52, SE = 0.21, t = −2.49, p = .01, 95%CI [−0.93, −0.10]), but was not significant for those in the negative (b = 0.24, SE = 0.19, t = 1.30, p = .20, 95%CI [−0.13, 0.62]) or neutral (b = −0.15, SE = 0.17, t = −0.91, p = .37, 95%CI [−0.48, 0.18]) emotion induction conditions.
Figure 3.
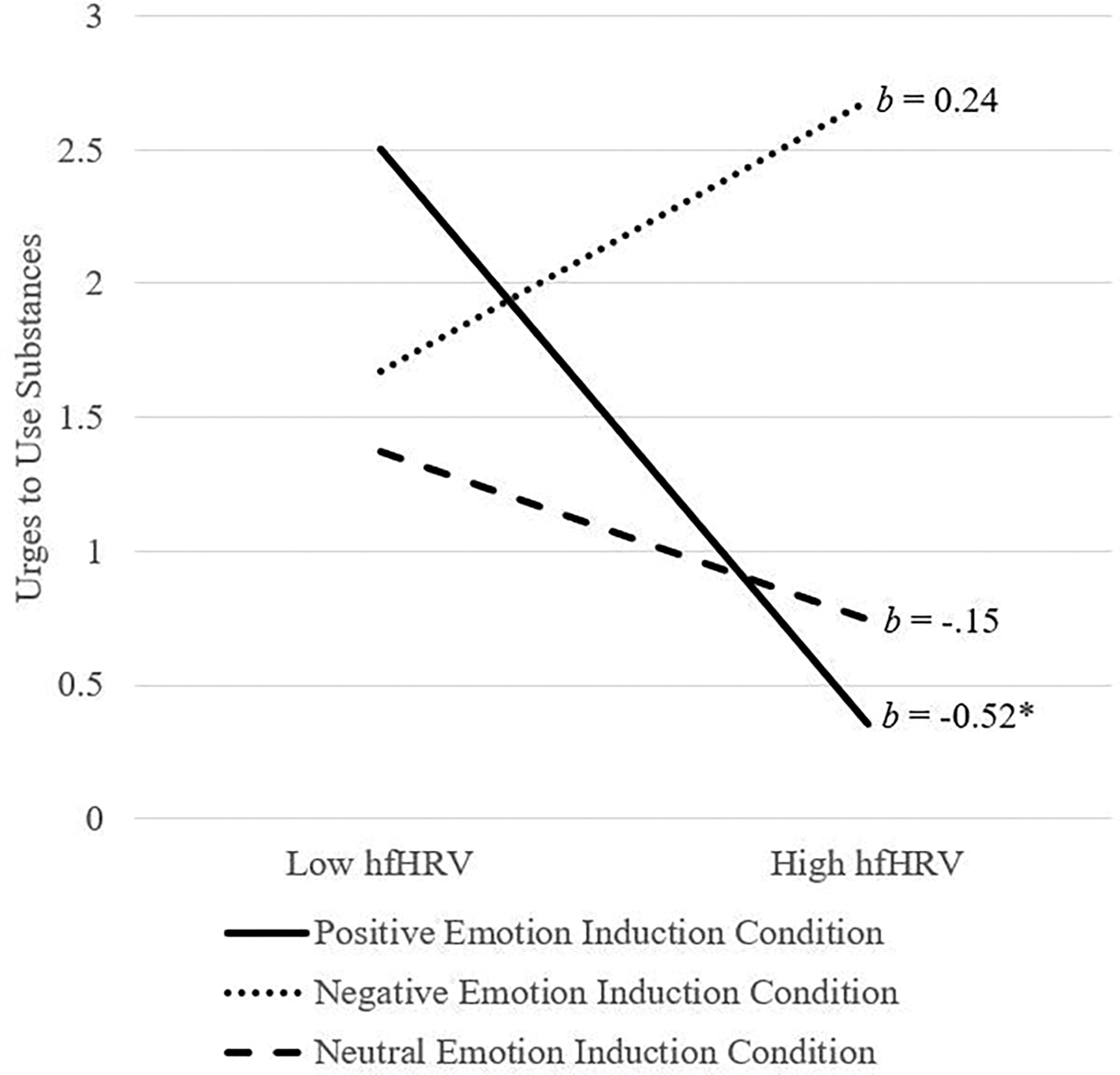
Emotion induction condition by hfHRV predicting urges to use substances
Note. hfHRV = high frequency heart rate variability; hfHRV was used as a continuous variable in the model but was plotted at high and low levels (i.e., 1 SD above and below the mean) for visualization purposes; *p < .05
The interactions of SCR (ΔR2 = .03, F[2, 134] = 1.91, p = .15) and salivary cortisol (ΔR2 = .03, F[2, 138] = 2.17, p = .12) with emotion induction condition were not significant, nor were lower-order interactions significant (bs = −0.11–2.05, ps = .05-.74).
BART (Table 2)
Post hoc power for analyses examining the main and interactive effects of hfHRV on BART outcome were adequate (Power = 0.92). The interaction of hfHRV and emotion induction condition was not significant (ΔR2 = .04, F[2, 92] = 2.22, p = .11). However, the lower-order interaction of hfHRV and positive (versus neutral) emotion induction condition was significant (b = 3.19, p = .04). Analysis of simple slopes (see Figure 4) revealed that the association between hfHRV and the BART was significant and negative for those in the positive emotion induction condition (b = −2.60, SE = 1.30, t = −2.00, p = .048, 95%CI [−5.17, −0.23]), but was not significant for those in the neutral (b = 0.85, SE = 1.07, t = −0.79, p = .43, 95%CI [−1.28, 2.98]) emotion induction condition (see Figure 6). The lower-order interaction of hfHRV and positive (versus negative) emotion induction condition was not significant (b = −3.57, p = .26).
Figure 4.
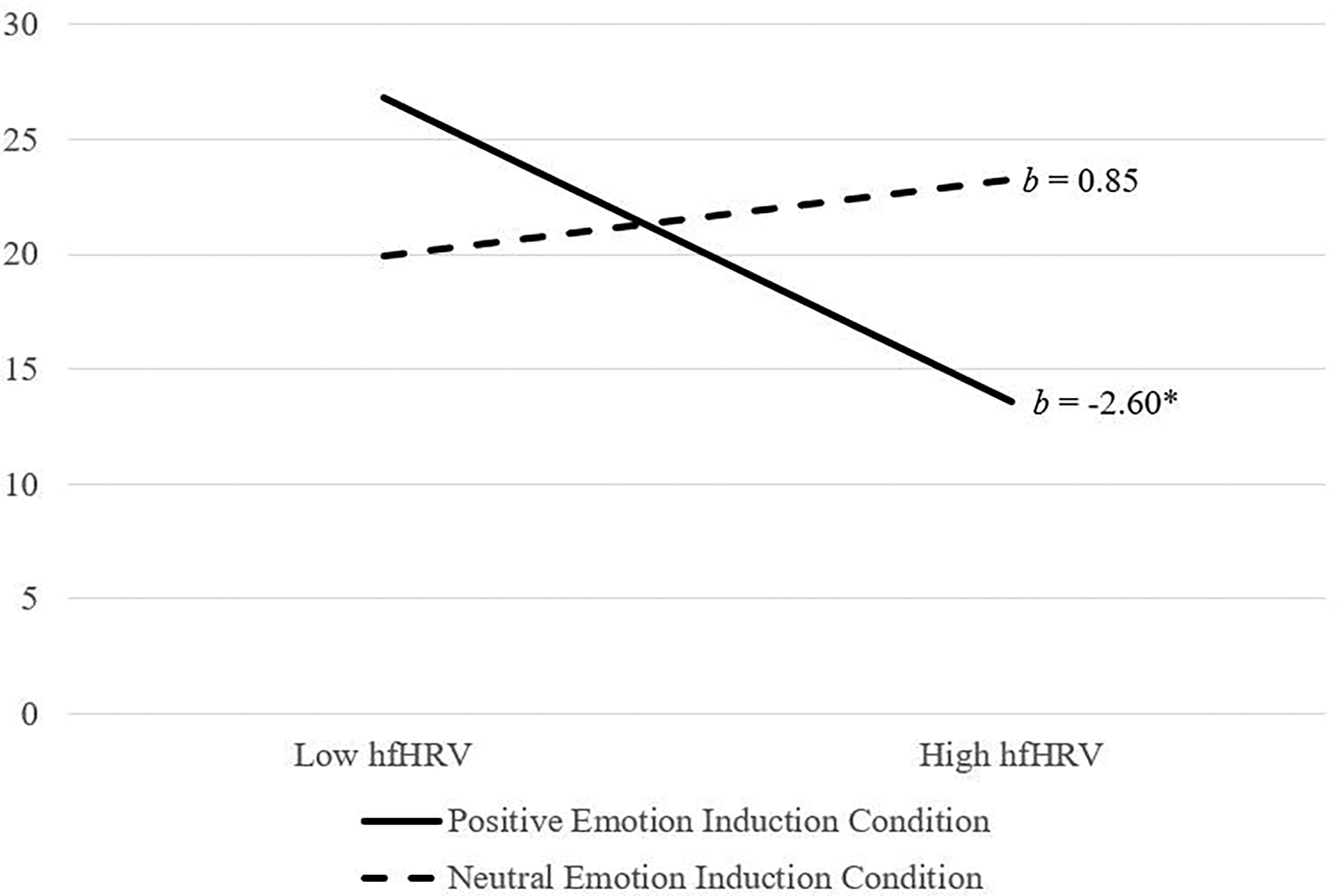
Emotion induction condition by hfHRV predicting BART scores
Note. hfHRV = high frequency heart rate variability; hfHRV was used as a continuous variable in the model but was plotted at high and low levels (i.e., 1 SD above and below the mean) for visualization purposes; p < .05
Post hoc power estimates for analyses examining the main and interactive effects of subjective emotion dysregulation, SCR, and salivary cortisol on BART were sub-optimal (Power = 0.34 to 0.66). Thus, we did not interpret these analyses.
Discussion
The goal of the current study was to advance understanding of the influence of positive emotion dysregulation on risky behavior. Addressing important limitations of extant research in this area, an experimental paradigm with subjective and objective assessments was utilized to assess the role of emotion dysregulation following positive, negative, and neutral idiographic emotion inductions on risky behavior in the laboratory. Results provided support for an association between subjective and objective markers of emotion dysregulation following positive emotion induction on state urges to use substances and behavioral risk-taking propensity. These findings provide experimental support for the role of positive emotion dysregulation on risky behavior, highlighting the importance of future research in this area to inform clinical decisions and practice.
Regarding the relation between subjective emotion dysregulation and risky behavior, our results provided support for an interaction between self-reported state emotion dysregulation and positive (versus negative and neutral) emotion induction condition on urges to use substances. Specifically, self-reported state emotion dysregulation was significantly and positively associated with urges to use substances for women in the positive—but not negative or neutral— emotion induction condition. This finding suggests that emotion dysregulation stemming from positive emotional stimuli may increase proximal risk for substance use. A growing body of cross-sectional evidence provides support for the role of positive emotion dysregulation in substance use (Weiss et al., 2018b; Weiss et al., 2020; Weiss et al., in press-c; Weiss et al., 2019b; Weiss et al., 2019c). Findings here extend this past work by using an experimental paradigm with random assignment to demonstrate that emotion dysregulation predicts subsequent urges to use substances. Future studies are needed to explore whether these results generalize to actual substance use in the laboratory (e.g., self-administered alcohol) in the context of positive emotions. Further, to address the question of ecological validity, research is needed to explore the association between positive emotion dysregulation and substance urges and use in the real world, such as through ecological momentary assessment (EMA) methods.
In terms of the relation between objective markers of emotion dysregulation and risky behavior, this study provided support for an interaction between hfHRV and positive (versus negative and neutral) emotion induction condition on urges to use substances, and positive (versus neutral) emotion induction condition on behavioral risk-taking propensity. Specifically, hfHRV was significantly and negatively associated with urges to use substances for individuals in the positive—but not negative or neutral—emotion induction condition, and behavioral risk-taking propensity for individuals in the positive—but not neutral—emotion induction condition. In the last decade, hfHRV—an index of parasympathetic nervous system activity—has gained growing attention as a sensitive biomarker of risky behavior. In particular, lower hfHRV—aligned with a reduced ability to regulate emotional responses to stress (Thayer & Lane, 2000)—has been associated with risky behavior (for reviews, see Cheng et al., 2019). Our findings extend this work by suggesting that hfHRV may serve as a particularly important risk factor for risky behavior in positively-valenced emotional contexts. To inform targeted assessment and intervention for reducing risky behavior, future empirical investigations are needed to better understand the role of hfHRV in risky behavior across diverse positive emotional contexts.
Of note, SCR and salivary cortisol were not found to be associated with risky behavior, either generally (main effects) or for select emotion induction conditions (interactive effects). These objective biomarkers of emotion dysregulation are distinguished from hfHRV in that they are indirect measures of sympathetic autonomic activity, and as such are highly tied to arousal (Hellhammer et al., 2009). Thus, while one possible explanation for our findings is that sympathetic biomarkers play a less salient role in risky behavior, future research should first consider the influence of high versus low arousal positive and negative emotional stimuli on the relations between both SCR and salivary cortisol and risky behavior. Given the nature of the current sample, another important avenue for future work would be examination of the impact of trauma history and posttraumatic stress symptoms on SCR and salivary cortisol in relation to risky behavior. Specifically, past studies have found sympathetic autonomic activity to be blunted amongst individuals with trauma (Carpenter et al., 2011) and posttraumatic stress symptoms (Metz et al., 2020), which, in turn, may distort observed relations with risky behavior.
It also warrants discussion that the strength of the associations between emotion dysregulation (i.e., state self-report and hfHRV) and risky behavior (i.e., self-report state urges for substances and BART) were significantly stronger for women in the positive versus negative emotion induction condition. While no hypotheses were made for these lower-order interactions, this finding is surprising given strong cross-sectional evidence for a relation between negative emotion dysregulation and risky behavior (Weiss et al., 2015b). However, laboratory investigations of the association between negative emotion dysregulation and risky behavior are limited, and those that do exist have produced mixed findings. For instance, Tull et al. (2018) found a non-significant relation between self-reported state emotion dysregulation and cravings for substances following idiographic trauma cue exposure. Nevertheless, as these results are unexpected, other possibilities should be considered. For instance, perhaps the measures of risky behavior used here may be more strongly tied to positive versus negative emotion dysregulation. Alternatively, risky behavior in negative emotional contexts may have been more strongly tied to some function (e.g., impulsivity) other than emotion dysregulation, as operationalized here. In any event, our findings highlight emotion dysregulation in positive emotional contexts as an antecedent for risky behavior, consistent with past research on positive urgency (Cyders & Smith, 2008) and celebratory drinking (Glindemann et al., 2007). Future investigations are needed to better understand the relative and unique contributions of emotion dysregulation to risky behavior across positive and negative emotion contexts.
Although not a primary aim of the current study, it is important to note that there were no significant differences in self-report state urges for substances and the BART across negative, positive, and neutral emotion induction conditions. These findings are partially consistent with a meta-analysis by Um et al. (2022), which found that negative emotion induction increased risk-taking and craving in the laboratory to a small degree, whereas positive emotion induction failed to elicit risk-taking or craving. Of note, however, are important differences between the current study and Um et al.’s (2022) meta-analytic review. First and foremost, the goal of the current study was to compare positive to negative and neutral emotion induction conditions; primary analyses did not include comparison of negative and neutral emotion induction conditions. Conversely, only studies comparing negative and positive to neutral emotion induction conditions were included in Um et al. (2022); negative and positive emotion induction conditions were not compared to one another. Also noteworthy is the fact that few studies reviewed in Um et al. (2022) that examined positive emotion inductions used autobiographic recall (craving: k = 1, N = 42; behavioral risk-taking: k = 2, N = 80), and none induced excitement or used a sample of community women identified by IPV. Further, the goals of the current study differed from Um et al. (2022). The current study explored the relation of emotion dysregulation to risky behavior following emotion induction, while Um et al. (2022) excluded studies that measured aspects of emotion beyond in-the-moment emotion ratings, such as dysregulation. Additionally, while the current study examined both subjective and objective metrics of emotion dysregulation, Um et al. (2022) excluded studies when emotion ratings were solely derived from physiological responses. Overall, our findings underscore the need for additional research to better evaluate methods for assessing emotion-based risk-taking and craving in the laboratory, including the mechanisms through which emotion inductions elicit risky behavior (e.g., emotion dysregulation) and the utility of objective metrics of emotion (e.g., HRV). It is particularly important that such research also include positive emotion inductions, as studies in this area are sparse and thus findings on positive emotion dependent risk-taking and craving should be interpreted with caution.
In evaluating the current findings, it is important to note that the emotion inductions were found to be successful and to not differ in potency. Specifically, the positive emotion induction resulted in significantly greater increases in overall positive emotions and excitement (the positive emotion targeted) as well as all other PANAS positive emotion items except attentive and alert compared to negative and neutral emotion inductions. Likewise, the negative emotion induction resulted in significantly greater increases in overall negative emotions and irritability (the negative emotion targeted) as well as all other PANAS negative emotion items except scared, nervous, and afraid compared to positive and neutral emotion inductions. Further, reports of dissociation and emotional disengagement did not differ across emotion inductions, with the exception of an item assessing emotional suppression, which was rated higher in the positive and negative versus neutral emotion inductions, but did not differ across positive and negative induction conditions. This latter finding aligns with growing evidence to suggest that positive emotions may also be perceived as aversive (Weiss et al., 2015a) and strategically withheld (Beblo et al., 2012; 2013). Research is needed to better understand the form and function of positive emotional suppression.
The current study has several limitations that warrant consideration when interpreting results. First, while not large, this study is a well-sized experimental study (N = 151), with target sample size being determined a priori. For the sake of transparency and consistent with recommendations for rigorous research practices, we also provide post-hoc power estimates in Table 2. Analyses with subjective cravings as the outcome (subjective emotion regulation, HRV, SCR, salivary cortisol) were all powered (≥.99). Only analyses with the behavioral measure of risk-taking propensity (BART) as the outcome were not powered in post-hoc analysis, with the exception of HRV, which was both powered and significant. Non-significant findings that lacked adequate statistical power should be interpreted with caution. Replication of these findings in larger samples is an important avenue for future research. Second, while use of a community sample of women experiencing IPV and using substances is a strength of the current study, findings cannot be assumed to generalize to non-IPV, non-substance-using populations. Research is needed to examine our results across a more diverse group of individuals who experience IPV (e.g., women recruited from shelters, men) and use substances (e.g., treatment-seeking). Similarly, this study was restricted to women in heterosexual relationships given evidence that dynamics in same-sex IPV relationships are unique (Johnson & Ferraro, 2000). Future research in this area with gender and sexual minority populations is necessary. Third, idiographic positive and negative emotion inductions were developed in relation to recent situations that elicited excitement and anger, respectively. These emotions were selected because they are oppositely-valenced but similarly characterized by high arousal (Barrett, 1998). Further, a focus on discrete emotions extends past laboratory studies in this area that have almost entirely relied on trauma scripts (Coffey et al., 2002; Saladin et al., 2003; Tull et al., 2011), despite evidence that non-trauma-specific emotions, such as excitement and anger, also increase engagement in risky behavior (Cyders & Smith, 2008; DeSteno et al., 2000). Future investigations would benefit from examination of the relation of emotion dysregulation to risky behavior following other emotion states, such as shame and sadness. Relatedly, while the idiographic negative emotion induction utilized here targeted anger, the PANAS, which is considered to be a gold standard measure of emotions, does not specifically assess anger, thus we had to use irritability as the closest proxy for anger. Lastly, not surprising given its checklist nature (Streiner, 2003), internal consistency for the REQ was low. Indeed, this measure assesses different strategies that may be implemented to regulate emotional experiences, and we would expect individuals to utilize some—but not all—of the strategies to regulate their emotions. Nonetheless, future work is needed to better understand the heterogeneity in emotion regulation strategy implementation as well as the influence of specific emotion regulation strategies on risky behavior—especially in positive emotional contexts.
Despite these limitations, findings of the current experimental study add to the growing body of literature on emotion dysregulation and risky behavior. Specifically, results demonstrated that both subjective and objective assessments of emotion dysregulation following positive emotion induction predicted urges to use substances and behavioral risk-taking propensity. As such, findings from the present study offer additional support for the potential benefits of targeting positive emotion dysregulation in the prevention and intervention of risky behavior. Psychological treatments that improve emotion dysregulation show promise for reducing risky behavior (for a review, see Gratz et al., 2015). Future investigations are needed to explore the extent to which these treatments target dysregulation from positive emotions as well as the effect of addressing positive emotion dysregulation in the treatment of risky behavior. As one example, interventions to reduce risky behavior may benefit from addressing nonacceptance of positive emotions, which, in turn, may encourage engagement with (versus suppression of) positive emotions. Mindfulness- and acceptance-based treatments may be efficacious in this regard by helping to facilitate a non-judgmental and non-evaluative stance toward positive emotional experiences (e.g., Mindfulness-based Relapse Prevention; Witkiewitz et al., 2005).
Supplementary Material
Acknowledgments
This research was supported by National Institute on Drug Abuse Grant K23 DA039327, awarded to the first author (NHW). NHW also acknowledges the support from the Center for Biomedical Research and Excellence (COBRE) on Opioids and Overdose funded by the National Institute on General Medical Sciences (P20 GM125507). Work on this paper by the fourth author (SRF) was supported by National Institute on Drug Abuse Grant F31 DA051167.
References
- Appelhans BM, & Luecken LJ (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. 10.1037/1089-2680.10.3.229 [DOI] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review, 111(1), 33–51. 10.1037/0033-295X.111.1.33 [DOI] [PubMed] [Google Scholar]
- Balzarotti S, Biassoni F, Colombo B, & Ciceri MR (2017). Cardiac vagal control as a marker of emotion regulation in health adults: a review. Biological Psychology, 130, 54–66. 10.1016/j.biopsycho.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Beblo T, Fernando S, Klocke S, Griepenstroh J, Aschenbrenner S, & Driessen M (2012). Increased suppression of negative and positive emotions in major depression. Journal of Affective Disorders, 141(2–3), 474–479. 10.1016/j.jad.2012.03.019 [DOI] [PubMed] [Google Scholar]
- Beblo, Fernando S, Kamper P, Griepenstroh J, Aschenbrenner S, Pastuszak A, Schlosser N, & Driessen M (2013). Increased attempts to suppress negative and positive emotions in borderline personality disorder. Psychiatry Research, 210(2), 505–509. 10.1016/j.psychres.2013.06.036 [DOI] [PubMed] [Google Scholar]
- Ben-Zur H, & Zeidner M (2009). Threat to life and risky behavior behaviors: A review of empirical findings and explanatory models. Personality and Social Psychology Review, 13, 109–128. 10.1177/1088868308330104 [DOI] [PubMed] [Google Scholar]
- Billieux J, Gay P, Rochat L, & Van der Linden M (2010). The role of urgency and its underlying psychological mechanisms in problematic behaviours. Behavior Research and Therapy, 48(11), 1085–1096. 10.1016/j.brat.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Brake CA, Sauer-Zavala S, Boswell JF, Gallagher MW, Farchione TJ, & Barlow DH (2016). Mindfulness-based exposure strategies as a transdiagnostic mechanism of change: an exploratory alternating treatment design. Behavior Therapy, 47(2), 225–238. 10.1016/j.beth.2015.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH, Brown TA, & Hofmann SG (2006). Acceptability and suppression of negative emotion in anxiety and mood disorders. Emotion, 6(4), 587–595. 10.1037/1528-3542.6.4.587 [DOI] [PubMed] [Google Scholar]
- Cărnuţă M, Crişan LG, Vulturar R, Opre A, & Miu AC (2015). Emotional nonacceptance links early life stress and blunted cortisol reactivity to social threat. Psychoneuroendocrinology, 51, 176–187. 10.1016/j.psyneuen.2014.09.026 [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, & Price LH (2011). Effect of childhood physical abuse on cortisol stress response. Psychopharmacology, 214, 367–375. 10.1007/s00213-010-2007-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AL, Rosenthal MZ, & Leung DW (2009). Emotion suppression in borderline personality disorder: An experience sampling study. Journal of Personality Disorders, 23(1), 29–47. 10.1521/pedi.2009.23.1.29 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Huang Y-C, & Huang W-L (2019). Heart rate variability as a potential biomarker for alcohol use disorders: A systematic review and meta-analysis. Drug and Alcohol Dependence, 204, 107502. 10.1016/j.drugalcdep.2019.05.030 [DOI] [PubMed] [Google Scholar]
- Coffey SF, Schumacher JA, Baschnagel JS, Hawk LW, & Holloman G (2011). Impulsivity and risky behavior in borderline personality disorder with and without substance use disorders. Personality Disorders: Theory, Research, and Treatment, 2(2), 128. 10.1037/a0020574 [DOI] [PubMed] [Google Scholar]
- Cooper ML, Kuntsche E, Levitt A, Barber LL, & Wolf S (2016). Motivational models of substance use: A review of theory and research on motives for using alcohol, marijuana, and tobacco. In Sher KJ (Ed.), The Oxford handbook of substance use and substance use disorders (pp. 375–421). Oxford University Press. [Google Scholar]
- Cyders MA, & Smith GT (2008). Emotion-based dispositions to rash action: Positive and negative urgency. Psychological Bulletin, 134(6), 807–828. 10.1037/a0013341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Zapolski TC, Combs JL, Settles RF, Fillmore MT, & Smith GT (2010). Experimental effect of positive urgency on negative outcomes from risk taking and on increased alcohol consumption. Psychology of Addictive Behaviors, 24(3), 367–375. 10.1037/a0019494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries KM, Child JC, Bacchus LJ, Mak J, Falder G, Graham K, Watts C, & Heise L (2014). Intimate partner violence victimization and alcohol consumption in women: A systematic review and meta-analysis. Addiction, 109(3), 379–391. 10.1111/add.12393 [DOI] [PubMed] [Google Scholar]
- Eddie D, Vaschillo E, Vaschillo B, & Lehrer P (2015). Heart rate variability biofeedback: Theoretical basis, delivery, and its potential for the treatment of substance use disorders. Addiction Research & Theory, 23(4), 266–272. 10.3109/16066359.2015.1011625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddie D, Bates ME, & Buckman JF (2020). Closing the brain-heart loop: Towards more holistic models of addiction and addiction recovery. Addiction Biology, e12958. 10.1111/adb.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, & Williams JBW (2016). SCID-5-CV: Structured Clinical Interview for DSM-5 Disorders: Clinician Version. American Psychiatric Association Publishing. [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, & Lanius RA (2012). Disturbances of emotional awareness and expression in posttraumatic stress disorder: Meta-mood, emotion regulation, mindfulness, and interference of emotional expressiveness. Psychological Trauma: Theory, Research, Practice, and Policy, 4(2), 152–161. 10.1037/a0023114 [DOI] [Google Scholar]
- Garcia-Moreno C, Jansen HAFM, Ellsberg M, Heise L, & Watts CH (2006). Prevalence of intimate partner violence: Findings from the WHO multi-country study on women’s health and domestic violence. The Lancet, 368(9543), 1260–1269. 10.1016/S0140-6736(06)69523-8 [DOI] [PubMed] [Google Scholar]
- Glindemann KE, Wiegand DM, & Geller ES (2007). Celebratory drinking and intoxication: a contextual influence on alcohol consumption. Environment and Behavior, 39(3), 352–366. 10.1177/001391650290949 [DOI] [Google Scholar]
- Gratz KL, & Tull MT (2010). The relationship between emotion dysregulation and deliberate self-harm among inpatients with substance use disorders. Cognitive Therapy & Research, 34(6), 544–553. 10.1007/s10608-009-9268-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Weiss NH, & Tull MT (2015). Examining emotion regulation as an outcome, mechanism, or target of psychological treatments. Current Opinion in Psychology, 3, 85–90. 10.1016/j.copsyc.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2018). Introduction to Mediation, Moderation and Conditional Process Analysis: A Regression-Based Approach (2nd ed.). The Guilford Press. [Google Scholar]
- Hayes AF, & Montoya AK (2017). A tutorial on testing, visualizing, and probing an interaction involving a multicategorical variable in linear regression analysis. Communication Methods and Measures, 11(1), 1–30. 10.1080/19312458.2016.1271116 [DOI] [Google Scholar]
- Hellhammer DH, Wüst S, & Kudielka BM (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology, 34(2), 163–171. 10.1016/j.psyneuen.2008.10.026 [DOI] [PubMed] [Google Scholar]
- Holzman JB, & Bridgett DJ (2017). Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neuroscience & Biobehavioral Reviews, 74, 233–255. 10.1016/j.neubiorev.2016.12.032 [DOI] [PubMed] [Google Scholar]
- Inzlicht M, & Schmeichel BJ (2012). What is ego depletion? Toward a mechanistic revision of the resource model of self-control. Perspectives on Psychological Science, 7(5), 450–463. 10.1177/1745691612454134 [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, & Johnson P (1992). Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology, 29(6), 742–750. 10.1111/j.1469-8986.1992.tb02052.x [DOI] [PubMed] [Google Scholar]
- Keane TM, Kolb LC, Kaloupek DG, Orr SP, Blanchard EB, Thomas RG, Hsieh FY, & Lavori PW (1998). Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: results from a Department of Veterans Affairs Cooperative Study. Journal of Consulting and Clinical Psychology, 66(6), 914–923. 10.1037/0022-00X.66.6.914 [DOI] [PubMed] [Google Scholar]
- Kreibig SD (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84(3), 394–421. 10.1016/j.biopsycho.2010.03.010 [DOI] [PubMed] [Google Scholar]
- Lang PJ, & Cuthbert BN (1984). Affective information processing and the assessment of anxiety. Journal of Behavioral Assessment, 6(4), 369–395. 10.1007/bf01321326 [DOI] [PubMed] [Google Scholar]
- Lavender JM, Wonderlich SA, Peterson CB, Crosby RD, Engel SG, Mitchell JE, Crow SJ, Smith TL, Klein MH, & Goldschmidt AB (2014). Dimensions of emotion dysregulation in bulimia nervosa. European Eating Disorders Review, 22(3), 212–216. 10.1002/erv.2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, & Brown RA (2002). Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART). Journal of Experimental Psychology: Applied, 8(2), 75–84. 10.1037/1076-898x.8.2.75 [DOI] [PubMed] [Google Scholar]
- Malik M (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use: Task force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Annals of Noninvasive Electrocardiology, 1(2), 151–181. 10.1111/j.1542-474x.1996.tb00275.x [DOI] [Google Scholar]
- Martins SS, Sampson L, Cerdá M, & Galea S (2015). Worldwide prevalence and trends in unintentional drug overdose: A systematic review of the literature. American Journal of Public Health, 105, e29–e49. 10.2105/ajph.2015.302843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz S, Duesenberg M, Hellmann-Regen J, Wolf OT, Roepke S, Otte C, & Wingenfeld K (2020). Blunted salivary cortisol response to psychosocial stress in women with posttraumatic stress disorder. Journal of Psychiatric Research, 130, 112–119. 10.1016/j.jpsychires.2020.07.014 [DOI] [PubMed] [Google Scholar]
- Nicolson NA (2007). Measurement of cortisol. In Luecken LJ & Gallo LC (Eds.), Handbook of physiological research methods in health psychology (pp. 37–74). Sage Publications. 10.4135/9781412976244.n3 [DOI] [Google Scholar]
- Noël X, Paternot J, Van der Linden M, Sferrazza R, Verhas M, Hanak C, Kornreich C, Martin P, De Mol J, & Pelc I (2001). Correlation between inhibition, working memory and delimited frontal area blood flow measured by 99MTC–bicisate spect in alcohol–dependent patients. Alcohol and Alcoholism, 36(6), 556–563. 10.1093/alcalc/36.6.556 [DOI] [PubMed] [Google Scholar]
- Peters EN, Khondkaryan E, & Sullivan TP (2012). Associations between expectancies of alcohol and drug use, severity of partner violence, and posttraumatic stress among women. Journal of Interpersonal Violence, 27(11), 2108–2127. 10.1177/0886260511432151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, & Wildes JE (2015). Dynamic longitudinal relations between emotion regulation difficulties and anorexia nervosa symptoms over the year following intensive treatment. Journal of Consulting and Clinical Psychology, 83(4), 785–795. 10.1037/ccp0000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudales AM, Darosh AG, Contractor AA, Schatten HT, Dixon-Gordon KL, & Weiss NH (2021). Positive emotion dysregulation identifies trauma-exposed community individuals at risk for suicide and nonsuicidal self-injury. The Journal of Nervous and Mental Disease, 209(6), 434–442. 10.1097/NMD.0000000000001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudales AM, Weiss NH, Goncharenko S, Forkus SR, & Contractor AA (2020). Posttraumatic stress disorder and deliberate self-harm among military veterans: Indirect effects through negative and positive emotion dysregulation. Psychological Trauma: Theory, Research, Practice, and Policy, 12(7), 707–715. 10.1037/tra0000962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer L, Litz BT, Orsillo SM, & Wagner AW (2001). A preliminary investigation of the role of strategic withholding of emotions in PTSD. Journal of Traumatic Stress, 14(1), 149–156. 10.1023/a:1007895817502 [DOI] [Google Scholar]
- Roerecke M, & Rehm J (2013). Alcohol use disorders and mortality: A systematic review and meta-analysis. Addiction, 108(9), 1562–1578. 10.1111/add.12231 [DOI] [PubMed] [Google Scholar]
- Salters-Pedneault K, Gentes E, & Roemer L (2007). The role of fear of emotion in distress, arousal, and cognitive interference following an emotional stimulus. Cognitive Behaviour Therapy, 36(1), 12–22. 10.1080/16506070600874281 [DOI] [PubMed] [Google Scholar]
- Sauer-Zavala S, Gutner CA, Farchione TJ, Boettcher HT, Bullis JR, & Barlow DH (2017). Current definitions of “transdiagnostic” in treatment development: A search for consensus. Behavior Therapy, 48(1), 128–138. 10.1016/j.beth.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Shepherd L, & Wild J (2014). Emotion regulation, physiological arousal and PTSD symptoms in trauma-exposed individuals. Journal of Behavior Therapy and Experimental Psychiatry, 45(3), 360–367. 10.1016/j.jbtep.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological momentary assessment. Annual Review of Clinical Psychology, 4, 1–32. 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- Shorey RC, Brasfield H, Febres J, & Stuart GL (2011). An examination of the association between difficulties with emotion regulation and dating violence perpetration. Journal of Aggression, Maltreatment & Trauma, 20(8), 870–885. 10.1080/10926771.2011.629342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson LE, Raudales AM, Reyes ME, Sullivan TP, & Weiss NH (in press). Intimate partner violence and posttraumatic stress symptoms: Indirect effects through negative and positive emotion dysregulation. Journal of Interpersonal Violence. 10.1177/08862605211006371 [DOI] [PubMed] [Google Scholar]
- Smith SG, Zhang X, Basile KC, Merrick MT, Wang J, Kresnow M, & Chen J (2018). The National Intimate Partner and Sexual Violence Survey (NISVS): 2015 Data Brief – Updated Release. Retrived from https://stacks.cdc.gov/view/cdc/60893 [DOI] [PMC free article] [PubMed]
- Stiglmayr C, Schmahl C, Bremner JD, Bohus M, & Ebner-Priemer U (2009). Development and psychometric characteristics of the DSS-4 as a short instrument to assess dissociative experience during neuropsychological experiments. Psychopathology, 42(6), 370–374. 10.1159/000236908 [DOI] [PubMed] [Google Scholar]
- Szasz PL, Hofmann SG, Heilman RM, & Curtiss J (2016). Effect of regulating anger and sadness on decision-making. Cognitive Behaviour Therapy, 45(6), 479–495. 10.1080/16506073.2016.1203354 [DOI] [PubMed] [Google Scholar]
- Szasz PL, Szentagotai A, & Hofmann SG (2012). Effects of emotion regulation strategies on smoking craving, attentional bias, and task persistence. Behavior Research and Therapy, 50(5), 333–340. 10.1016/j.brat.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, & Eckberg DL (1998). Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation, 98(6), 547–555. 10.1161/01.cir.98.6.547 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers III JJ, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36, 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. 10.1016/s0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33(2), 81–88. 10.1016/j.neubiorev.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Tobar-Santamaria A, Kiefer R, Godin J, Contractor AA, & Weiss NH (2021). Sexual victimization and disordered eating among community individuals: The influence of negative and positive emotion dysregulation. Eating Behaviors, 43, 101567. 10.1016/j.eatbeh.2021.101567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull MT, & Aldao A (2015). Editorial overview: New directions in the science of emotion regulation. Current Opinion in Psychology, 3, iv–x. 10.1016/j.copsyc.2015.03.009 [DOI] [Google Scholar]
- Tull MT, Berghoff CR, Wheeless LE, Cohen RT, & Gratz KL (2018). PTSD symptom severity and emotion regulation strategy use during trauma cue exposure among patients with substance use disorders: Associations with negative affect, craving, and cortisol reactivity. Behavior Therapy, 49(1), 57–70. 10.1016/j.beth.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull MT, Forbes CN, Weiss NH, & Gratz KL (2019). An investigation of the effect of trauma script exposure on risk-taking among patients with substance use disorders and posttraumatic stress disorder. Journal of Anxiety Disorders, 62, 77–85. 10.1016/j.janxdis.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Tull MT, McDermott MJ, Gratz KL, Coffey SF, & Lejuez CW (2011). Cocainerelated attentional bias following trauma cue exposure among cocaine dependent inpatients with and without post-traumatic stress disorder. Addiction, 106(1), 1810–1818. 10.1111/j.1360-0443.2011.03508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull MT, Weiss NH, Adams CE, & Gratz KL (2012). The contribution of emotion regulation difficulties to risky sexual behavior within a sample of patients in residential substance abuse treatment. Addictive Behaviors, 37(10), 1084–1092. 10.1016/j.addbeh.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um M, Revilla R, & Cyders MA (2022). A meta-analytic review of the effectiveness of mood inductions in eliciting emotion-based behavioral risk-taking and craving in the laboratory. Emotion. 10.1037/emo0001062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visted E, Sørensen L, Osnes B, Svendsen JL, Binder PE, & Schanche E (2017). The association between self-reported difficulties in emotion regulation and heart rate variability: the salient role of not accepting negative emotions. Frontiers in Psychology, 8, 328. 10.3389/fpsyg.2017.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Weiss NH, Brick LA, Forkus SR, Goldstein SC, Thomas ED, Schick MR, Barnett NP, Contractor AA, & Sullivan TP (in press-a). Modeling reciprocal relations between emotion dysregulation and alcohol use using dynamic structural equation modeling: A micro-longitudinal study. Alcoholism: Clinical and Experimental Research. 10.1111/acer.14881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Brick LA, Schick MR, Forkus SR, Raudales AM, Contractor AA, & Sullivan TP (in press-b). Posttraumatic stress disorder strengthens the daily associations between emotion dysregulation and substance use: A micro-longitudinal study of community women experiencing intimate partner violence. Addiction. 10.1111/add.15992 [DOI] [PubMed] [Google Scholar]
- Weiss NH, Darosh A, Contractor A, Forkus SR, Dixon-Gordon KL, & Sullivan TP (2018a). Heterogeneity in emotion regulation difficulties among women victims of domestic violence: A latent profile analysis. Journal of Affective Disorders, 239, 192–200. [DOI] [PubMed] [Google Scholar]
- Weiss NH, Forkus SR, Contractor A, Darosh A, Goncharenko S, & Dixon-Gordon KL (2019a). Do difficulties regulating positive emotions contribute to risky sexual behavior among trauma-exposed individuals?: A path analysis. Archives of Sexual Behavior, 48, 2075–2087. 10.1007/s10508-019-1410-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Forkus SR, Contractor AA, & Schick MR (2018b). Difficulties regulating positive emotions and alcohol and drug misuse: A path analysis. Addictive Behaviors, 84, 45–52. 10.1016/j.addbeh.2018.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Forkus SR, Raudales AM, Schick MR, & Contractor AA (2020). Alcohol misuse to down-regulate positive emotions: A cross-sectional multiple mediator analysis among US military veterans. Addictive Behaviors, 105, 106322. 10.1016/j.addbeh.2020.106322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Goncharenko S, Raudales AM, Forkus SR, & Contractor AA (in press-c). PTSD symptoms are associated with alcohol misuse through alcohol use to down-regulate positive emotions. Addictive Behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Gratz KL, & Lavender J (2015a). Factor structure and initial validation of a multidimensional measure of difficulties in the regulation of positive emotions: The DERS-Positive. Behavior Modification, 39(3), 431–453. 10.1177/0145445514566504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Kiefer R, Goncharenko S, Raudales AM, Forkus SR, Schick MR, & Contractor AA (2022a). Emotion regulation and substance use: A meta-analysis. Drug and Alcohol Dependence, 230, 109131. 10.1016/j.drugalcdep.2021.109131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Risi MM, Bold KW, Sullivan TP, & Dixon-Gordon KL (2019b). Daily relationship between positive affect and drinking to cope: The moderating role of difficulties regulating positive emotions. The American Journal of Drug and Alcohol Abuse, 45(2), 189–198. 10.1080/00952990.2018.1508470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Schick MR, Contractor AA, & Dixon-Gordon KL (2019c). Posttraumatic stress disorder and substance use: Identifying the underlying role of difficulties regulating positive emotions. Addictive Behaviors, 96, 119–126. 10.1016/j.addbeh.2019.04.029 [DOI] [PubMed] [Google Scholar]
- Weiss NH, Schick MR Waite EE, Haliczer LA, & Dixon-Gordon KL (2021). Assocaition of positive emotion dysregulation to resting heart rate variability: The influence of positive affect intensity. Personality and Individual Differences, 173, 110607. 10.1016/j.paid.2020.110607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Schick MR, Contractor AA, Reyes ME, Suazo NC, & Sullivan TP (2022b). Racial/ethnic differences in alcohol and drug misuse among IPV-victimized women: Exploring the role of difficulties regulating positive emotions. Journal of Interpersonal Violence, 37(5–6), 2826–2850. 10.1177/0886260520943735 [DOI] [PubMed] [Google Scholar]
- Weiss NH, Sullivan TP, & Tull MT (2015b). Explicating the role of emotion dysregulation in risky behaviors: A review and synthesis of the literature with directions for future research and clinical practice. Current Opinion in Psychology, 3, 22–29. 10.1016/j.copsyc.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Tull MT, & Gratz KL (2014). A preliminary experimental examination of the effect of emotion dysregulation and impulsivity on risky behaviors among women with sexual assault-related posttraumatic stress disorder. Behavior Modification, 38(6), 914–939. 10.1177/0145445514547957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Tull MT, Viana AG, Anestis MD, & Gratz KL (2012). Impulsive behaviors as an emotion regulation strategy: Examining associations between PTSD, emotion dysregulation, and impulsive behaviors among substance dependent inpatients. Journal of Anxiety Disorders, 26(3), 453–458. 10.1016/j.janxdis.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Walsh KL, DiLillo D, Messman-Moore T, & Gratz KL (2019d). A longitudinal examination of posttraumatic stress disorder symptoms and risky sexual behavior: Evaluating emotion dysregulation dimensions as mediators. Archives of Sexual Behavior, 48, 975–986. 10.1007/s10508-019-1392-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DP, Cash C, Rankin C, Bernardi A, Koenig J, & Thayer JF (2015). Resting heart rate variability predicts self-reported difficulties in emotion regulation: A focus on different facets of emotion regulation. Frontiers in Psychology, 6, 261. 10.3389/fpsyg.2015.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, & Zaldivar FP (2008). Rumination and cortisol responses to laboratory stressors. Psychosomatic Medicine, 70(6), 661–667. 10.1097/PSY.0b013e31817bbc77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


