Abstract
Six new sesquiterpene coumarin ethers, namely turcicanol A (1), turcicanol A acetate (2), turcicanol B (3), turcica ketone (4), 11′-dehydrokaratavicinol (5), and galbanaldehyde (6), and one new sulfur-containing compound, namely turcicasulphide (7), along with thirty-two known secondary metabolites were isolated from the root of the endemic species Ferula turcica Akalın, Miski, & Tuncay through a bioassay-guided isolation approach. The structures of the new compounds were elucidated by spectroscopic analysis and comparison with the literature. Cell growth inhibition of colon cancer cell lines (COLO205 and HCT116) and kidney cancer cell lines (UO31 and A498) was used to guide isolation. Seventeen of the compounds showed significant activity against the cell lines.
Keywords: Apiaceae, Ferula turcica, sesquiterpene coumarin ethers, sulfur-containing compounds, cytotoxic activity, colon cancer, kidney cancer
1. Introduction
Cancers are rapidly increasing in incidence worldwide and, in total, are the second most important cause of death worldwide. According to research by the World Health Organization (WHO), cancer was the cause of death of 10 million people in 2020 [1]. Cancer is still an incurable disease; thus, there is a need to find new molecules in this field. Türkiye is one of the leading countries in its herbal richness, biodiversity, and ethnobotanical knowledge [2,3]. An important source that inspires researchers in the discovery of pharmaceuticals for the treatment of many diseases is the use of botanical resources. According to studies, 81% of cancer drugs approved between 1940 and 2014 are compounds of natural origin [4].
The genus Ferula is one of the largest genera of the Apiaceae family and ranks third in the world and first in Asia, with approximately 185 species [5]. About 26 species of Ferula grow in Türkiye (Iran-Turan region), 16 of which are endemic, and they are commonly referred to as “Çaksir” or “Çasir” [6,7]. Ferula turcica Akalın, Miski, & Tuncay is a new species defined as a member of the section Merwia in Türkiye [6]. The use of gum-like resins (oleo–gum–resin) obtained from Ferula species for the treatment of several diseases, including cancer, for thousands of years has been recorded in various sources, including Dioscorides’ De Materia Medica and Avicenna’s The Canon of Medicine [8,9,10,11]. The compounds identified in Ferula species and frequently encountered in gum–resin drugs obtained from Ferula species are mostly sesquiterpene esters [12,13,14], sesquiterpene coumarin ethers [15,16], and sulfur-containing substances [17,18].
Studies with sesquiterpene coumarin ethers have shown that these secondary metabolites have cytotoxic activity; they induce apoptosis in Jurkat-derived apoptotic cells and contribute to tumor suppression by inhibiting macrophage secretion and facilitating beneficial phenotypes [19,20,21]. Due to the high affinity of sesquiterpene coumarins such as conferone toward the -p-glycoprotein (Pgp) transporter, conferone has a synergistic effect on the cytotoxic activity of cancer drugs, such as vinblastine, whose effectiveness is reduced in the treatment of cancer [22]. Therefore, sesquiterpene coumarins constitute an important group for promising new drug discovery in the field of cancer.
In this study, the dichloromethane extract of Ferula turcica roots belonging to the Merwia section of the Ferula species in Türkiye was investigated for its cytotoxic secondary metabolites.
2. Results
The dichloromethane and methanol extracts of the roots of Ferula turcica were tested against COLO205 (colon), HCT116 (colon), UO31 (kidney), and A498 (kidney) cancer cell lines. Comparison of the cytotoxic activity of dichloromethane and methanol extracts of the roots of F. turcica showed that the cytotoxic constituents were mainly concentrated in the dichloromethane extract (Table 1). The dichloromethane extract of the roots of F. turcica was subjected to Sephadex LH-20 fractionation, followed by preparative HPLC with reverse-phase C18 columns to yield 7 novel (Figure 1) and 30 known compounds.
Table 1.
Cytotoxic activities of Ferula turcica root extracts.
| Extracts | IC50 (µg/mL) | |||
|---|---|---|---|---|
| COLO 205 | HCT116 | A498 | UO31 | |
| Dichloromethane extract | 6.8 | 16.8 | 20 | 13.9 |
| Methanol extract | >100 | >100 | >100 | >100 |
Figure 1.
New compounds isolated from Ferula turcica roots.
2.1. Characterization of Cytotoxic Compounds
Six new sesquiterpene coumarin ethers, namely turcicanol A (1), turcicanol A acetate (2), turcicanol B (3), turcica ketone (4), 11′-dehydrokaratavicinol (5), and galbanaldehyde (6), and a new sulfur-containing compound, namely turcicasulphide (7), were isolated from the dichloromethane extract of the roots of Ferula turcica (Figure 1).
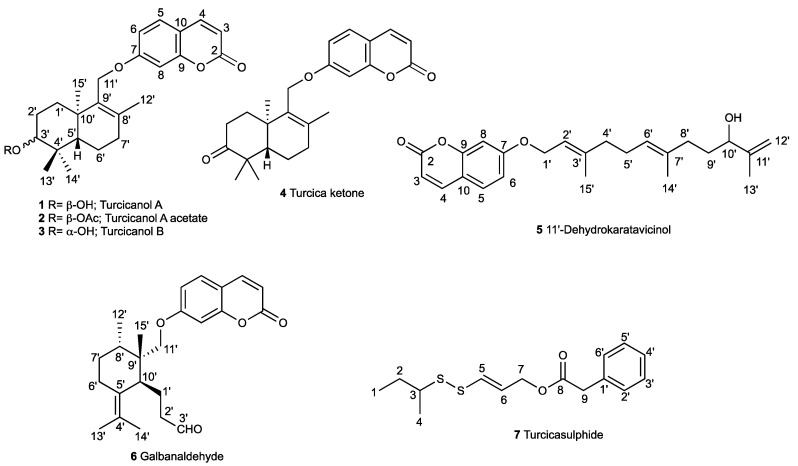
Turcicanol (1) was isolated as an amorphous white powder. The (+)-HRESIMS of 1 showed a [M + H]+ molecular ion peak at m/z 383.2219, suggesting a molecular formula of C24H30O4 for 1 with ten degrees of unsaturation. The 1H-NMR spectrum of 1 was closely similar to that of conferol (8) (Supplementary Materials Figure S67); thus, this compound should be an unsaturated bi-cyclic drimane sesquiterpene ether of umbelliferone. The most significant difference between the 1H-NMR spectra of conferol (8) and turcicanol A (1) was the lack of ABX signals of the H-11′a and H-11′b protons located at δH 4.02 and 4.17 ppm (each 1H, dd). The 1H-NMR spectrum of 1 displayed two AB-type doublets at δH 4.40 and 4.55 ppm (each 1H) (see Table 2); such difference strongly suggests that the double bond of 1 was located between C-8′ and C-9′, and the H–9′ proton of conferol (8) was not present. The 13C-NMR, 2D-COSY, HSQC, and HMBC spectra (Supplementary Materials, Figures S5–S8 and Figure 2a) confirmed the proposed structure of 1 as turcicanol A (Figure 1). The NOE correlations observed in the 2D-NOESY spectrum of 1 (Supplementary Materials, Figure S9 and Figure 2b) clearly confirmed the relative stereochemistry of turcicanol A as depicted in the formula 1 (Figure 1). Thus, turcicanol A (1) is a C-8′–C-9′ double-bond isomer of conferol (8).
Table 2.
1H NMR and 13C NMR shifts of compounds 1–4 (in CDCl3, δ in ppm, and J in Hz).
| Position | Turcicanol A (1) | Turcicanol A Acetate (2) | Turcicanol B (3) | Turcica Ketone (4) | ||||
|---|---|---|---|---|---|---|---|---|
| 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | |
| 2 | - | 161.5 | - | 161.5 | - | 161.4 | - | 161.4 |
| 3 | 6.25; d; 9.4; 1H | 113.0 | 6.24; d; 9.5; 1H | 113.1 | 6.25; d; 9.4; 1H | 113.1 | 6.25; d; 9.4; 1H | 113.2 |
| 4 | 7.64; d; 9.4; 1H | 143.5 | 7.64; d; 9.5; 1H | 143.6 | 7.64; d; 9.4; 1H | 143.6 | 7.64; d; 9.4; 1H | 143.5 |
| 5 | 7.36; d; 8.9; 1H | 128.8 | 7.37; d; 8.5; 1H | 128.8 | 7.37; d; 7.5; 1H | 128.7 | 7.37; d; 9.2; 1H | 129.0 |
| 6 | 6.87; dd; 2.3; 8.9; 1H | 113.4 | 6.89; dd; 2.2; 8.5; 1H | 113.2 | 6.87; dd; 2.3; 7.5; 1H | 113.3 | 6.85; dd; 2.4; 9.2; 1H | 113.2 |
| 7 | - | 162.9 | - | 162.5 | - | 162.5 | - | 162.3 |
| 8 | 6.87; d; 2.3; 1H | 101.4 | 6.87; d; 2.2; 1H | 101.6 | 6.86; d; 2.3; 1H | 101.5 | 6.86; d; 2.4; 1H | 101.5 |
| 9 | - | 156.2 | - | 156.0 | - | 156.0 | - | 156.0 |
| 10 | - | 112.5 | - | 112.5 | - | 112.6 | - | 112.7 |
| 1′α | 1.80; td; 3.7; 13.4; 1H | 29.4 | 1.45; dd; 3.1; 9.6; 1H | 30.0 | 1.70; m; 1H ** | 34.5 | 1.91; dd; 6.3; 8.5; 2H | 34.7 |
| 1′β | 1.43; dt; 3.7; 13.4; 1H | 1.67; m; 1H * | 1.49; td; 2.4; 13.1; 1H | |||||
| 2′α | 1.96; tt; 3.3; 14.3; 1H | 25.7 | 1.90; tddd; 1.7; 5.3; 14.1; 1H | 23.3 | 1.70; m; 2H ** | 27.8 | 2.51; m; 2H * | 34.2 |
| 2′β | 1.62; dq; 3.3; 15.3; 1H | 1.64; m; 1H * | ||||||
| 3′ | 3.46; t; 2.8; 1H | 75.8 | 4.69; t; 2.4; 1H | 77.7 | 3.29; dd; 4.5; 11.7; 1H | 78.9 | - | 217.0 |
| 4′ | - | 37.7 | - | 36.9 | - | 38.9 | - | 47.2 |
| 5′ | 1.68; m; 1H * | 44.8 | 1.7; dd; 1.7; 10.7; 1H | 45.9 | 1.24; dd; 2; 12.3; 1H | 50.7 | 1.83; dd; 2.4; 12.4; 1H | 50.8 |
| 6′α | 1.54; qd; 6.5; 12.6; 1H | 18.5 | 1.53; m; 1H * | 18.4 | 1.54; m; 1H * | 18.7 | 1.61; ddd; 6.5; 11.1; 12.7; 1H | 19.9 |
| 6′β | 1.64; m; 1H * | 1.66; m; 1H * | 1.75; dd; 6.8; 13.5; 1H | 1.67; ddt; 2.4; 6.5; 8.6; 1H | ||||
| 7′α | 2.16; dtd; 6.8; 18.2; 2H | 33.8 | 2.16; td; 6.4; 18.5; 1H | 33.6 | 2.17; d; 6.8; 2H | 34.0 | 2.21; m; 2H * | 33.6 |
| 7′β | 2.20; d; 7.6; 1H | |||||||
| 8′ | - | 135.8 | - | 136.0 | - | 136.2 | - | 136.7 |
| 9′ | - | 135.3 | 2.93; t; 5.1; 1H | 135.2 | - | 135.1 | - | 133.7 |
| 10′ | - | 37.8 | - | 37.8 | - | 37.9 | - | 37.67 |
| 11′a | 4.40; d; 9.9; 1H | 64.6 | 4.56; d; 9.9; 1H | 64.7 | 4.38; d; 9.9; 1H | 64.8 | 4.41; d; 10.1; 1H | 64.7 |
| 11′b | 4.55; d; 9.9; 1H | 4.40; d; 9.9; 1H | 4.54; d; 9.9; 1H | 4.55; d; 10.1; 1H | ||||
| 12′ | 1.68; s; 3H | 19.6 | 1.71; s; 3H | 19.7 | 1.69; s; 3H | 19.5 | 1.72; s; 3H | 19.7 |
| 13′ | 0.88; s; 3H | 22.1 | 0.90; s; 3H | 27.7 | 0.83; s; 3H | 15.7 | 1.08; s; 3H | 21.1 |
| 14′ | 1.00; s; 3H | 28.2 | 0.94; s; 3H | 21.7 | 1.04; s; 3H | 28.2 | 1.13; s; 3H | 27.0 |
| 15′ | 1.04; s; 3H | 20.8 | 1.05; s; 3H | 20.7 | 1.03; s; 3H | 20.9 | 1.11; s; 3H | 20.6 |
| CH3–(OAc) | - | - | 2.07; s; 3H | 21.5 | - | - | - | - |
| C=O (OAc) | - | - | - | 171.1 | - | - | - | - |
| Position | 11′-Dehydrokaratavicinol (5) | Galbanaldehyde (6) | Turcicasulphide (7) | |||||
| 1 H-NMR | 13 C-NMR | 1 H-NMR | 13 C-NMR | 1 H-NMR | 13 C-NMR | |||
| 1 | - | - | - | - | 0.97; t; 7.4; 3H | 11.7 | ||
| 2a | - | 161.5 | - | 161.2 | 1.51; m; 1H * | 28.2 | ||
| 2b | 1.68; td; 6.5; 13.2; 1H | |||||||
| 3 | 6.25; d; 9.5; 1H | 113.0 | 6.24; d; 9.4; 1H | 113.0 | 2.79; h; 6.7; 1H | 48.1 | ||
| 4 | 7.64; d; 9.5; 1H | 143.7 | 7.63; d; 9.4; 1H | 143.2 | 1.28; d; 6.9; 3H | 20.2 | ||
| 5 | 7.35; d; 8.6; 1H | 128.8 | 7.34; d; 8.6; 1H | 128.7 | 6.31; dt; 1.3; 14.8; 1H | 133.1 | ||
| 6 | 6.85; dd; 2.4; 8.6; 1H | 113.4 | 6.81; dd; 2.4; 8.6; 1H | 113.3 | 5.96; dt; 6.5; 14.8; 1H | 122.5 | ||
| 7 | - | 162.2 | - | 162.8 | 4.6; dd; 1.3; 6.5; 2H | 64.4 | ||
| 8 | 6.82; d; 2.4 | 101.6 | 6.75; d; 2.4; 1H | 101.2 | - | 171.3 | ||
| 9 | - | 155.8 | - | 156.0 | 3.63; s; 2H | 41.5 | ||
| 10 | - | 112.5 | - | 112.4 | - | - | ||
| 1′ | 4.60; d; 6.5; 2H | 65.4 | 1.84; m; 2H** | 19.6 | - | 134.0 | ||
| 2′ | 5.46; td; 1.2; 6.5; 1H | 118.6 | 2.29; t; 7.6; 2H | 42.3 | 7.29; m; 1H * | 129.4 | ||
| 3′ | - | 142.2 | 9.74; t; 1.5; 1H | 203.5 | 7.32; m; 1H ** | 128.3 | ||
| 4′ | 2.1; dd; 4.6; 11.6; 2H | 39.6 | - | 126.5 | 7.28; m; 1H * | 127.3 | ||
| 5′ | 2.15; q; 6.7; 2H | 26.2 | - | 129.7 | 7.32; m; 1H ** | 128.7 | ||
| 6′α | 5.14; t; 6.7; 1H | 124.1 | 1.86; m; 1H ** | 24.6 | 7.29; m; 1H * | 129.4 | ||
| 6′β | 2.5; dt; 3.1; 14.3; 1H | |||||||
| 7′α | - | 135.4 | 1.2; dd; 4.6; 13.5; 1H | 32.0 | - | - | ||
| 7′β | 1.6; m; 1H * | |||||||
| 8′a | 1.99; ddd; 6.4; 9.1; 14.4; 1H | 35.8 | 1.9; m; 1H ** | 34.8 | - | - | ||
| 8′b | 2.03; ddd; 6.22; 9.1; 15.1; 1H | - | - | |||||
| 9′ | 1.63; m; 2H * | 33.1 | - | 40.8 | - | - | ||
| 10′ | 4.03; dd; 5.4; 7.5; 1H | 75.6 | 2.92; dd; 4.2; 12; 1H | 42.7 | - | - | ||
| 11′a | - | 147.7 | 3.7; d; 8.2; 1H | 71.8 | - | - | ||
| 11′b | 3.87; d; 8.2; 1H | |||||||
| 12′a | 4.83; brs; 1H | 111.2 | 0.91; d; 6.7; 3H | 16.1 | - | - | ||
| 12′b | 4.93; brs; 1H | |||||||
| 13′ | 1.72; s; 3H | 17.8 | 1.43; s; 3H | 20.4 | - | - | ||
| 14′ | 1.61; s; 3H | 16.1 | 1.61; s; 3H | 20.4 | - | - | ||
| 15′ | 1.75; s; 3H | 16.9 | 1.16; s; 3H | 22.6 | ||||
*, ** Partially overlapped signals.
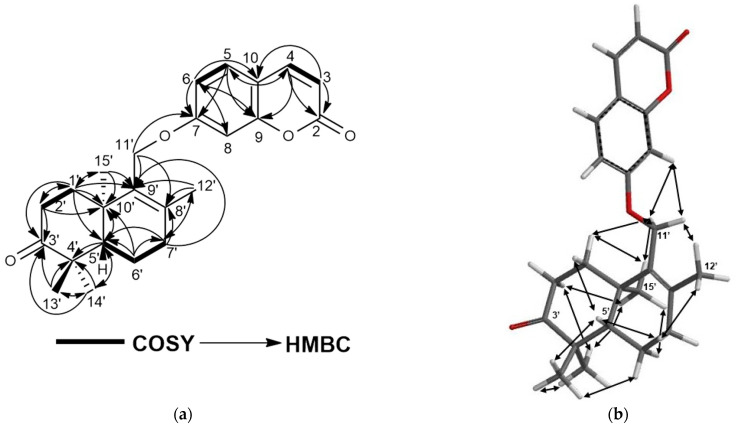
Figure 2.
(a) COSY and HMBC correlations of turcicanol A (1); (b) NOE correlations of turcicanol A (1).
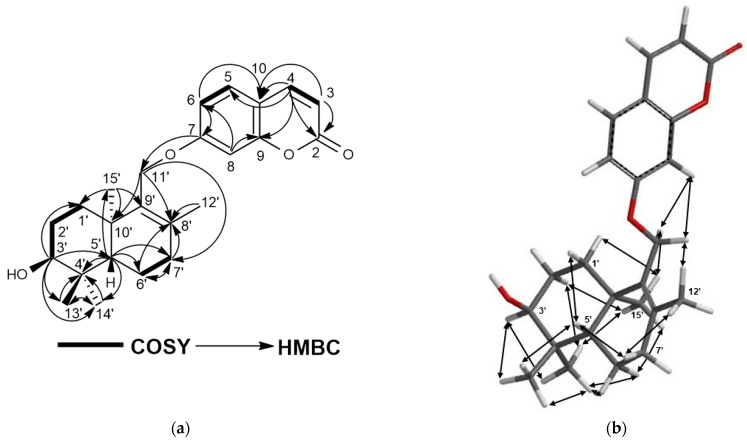
Turcicanol A acetate (2) was isolated as an amorphous white powder. The [M + H]+ molecular ion peak observed at m/z 425.2325 indicated a C26H32O5 molecular formula for 2 with 11 degrees of unsaturation. The 1H-NMR spectrum of 2 was similar to that of turcicanol A (1) with the exception of the ca. 1.5 ppm downfield shift of the H-3′ signal to δ 4.69 ppm, and the presence of a methyl singlet at δH 2.07 ppm clearly suggested the presence of an acetoxy group in 2. The HMBC correlation from H-3′ (δH 4.96) to the ester carbonyl at δC 171.1 established the acetyl group at position 3′ of 2. The 13C-NMR, 2D-COSY, HSQC, and HMBC spectra (Supplementary Materials, Figures S14–S17 and Figure 3a and Table 2) confirmed the proposed structure of 2 as turcicanol A acetate. In addition, the NOE correlations observed in the 2D-NOESY spectrum of 2 (Supplementary Materials, Figure S18 and Figure 3b) clearly confirmed the relative stereochemistry of turcicanol A acetate as depicted in Figure 1.
Figure 3.
(a) COSY and HMBC correlations of turcicanol A acetate (2); (b) NOE correlations of turcicanol A acetate (2).
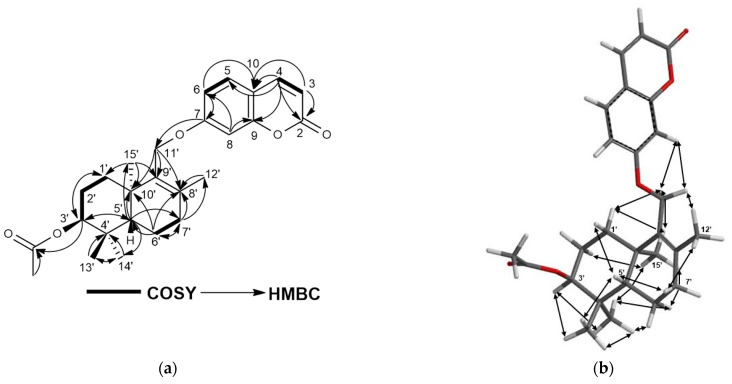
Turcicanol B (3) was isolated as an amorphous white powder. The (+)-HRESIMS of turcicanol B (3) showed an [M + H]+ molecular ion peak at m/z 383.2225, which indicated a C24H30O4 molecular formula for 3 with ten degrees of unsaturation. The 1H-NMR spectrum of 3 was similar to that of turcicanol A (1). The only difference was the shift of the H–3′ proton signal to δH 3.29 ppm (see Table 2) from δH 3.49 ppm. The 1H-NMR spectrum of turcicanol B (3) showed a hydroxyl geminal H-3′ proton as a dd (J = 4.5, 11.7 Hz), suggesting an axial orientation; thus, 3′-OH of turcicanol B (3) should be equatorial (i.e., α-OH) (Table 2). The 13C-NMR, 2D-COSY, HSQC, and HMBC spectra (Supplementary Materials, Figures S23–S26 and Figure 4a and Table 2) indicated the proposed structure for 3 as turcicanol B. The key NOE correlations observed in the 2D-NOESY spectrum of 3 (Supplementary Materials, Figure S27 and Figure 4b) confirmed that the relative configuration of turcicanol B was as depicted in the formula 3 (Figure 1).
Figure 4.
(a) COSY and HMBC correlations of turcicanol B (3); (b) NOE correlations of turcicanol B (3).
Compound 4 was isolated as an amorphous white powder. The [M + H]+ molecular ion of 4 was observed at m/z 381.2064 in the (+)-HRESIMS spectrum, indicating a C24H28O4 molecular formula for 4 with 11 degrees of unsaturation. The 1H-NMR and 13C-NMR spectra of 4 were similar to those of turcicanol A (1) and B (3) except for the lack of an H-3′ hydroxy geminal proton signal and the presence of a quaternary carbonyl signal at δC 217 ppm in the 13C-NMR spectrum of 4. The carbonyl signal in the 13C-NMR spectrum of 4 clearly showed correlation with the H–1′, H–2′, H–13′, and H–14′ protons in the 2D-HMBC spectrum of 4, confirming the presence of the carbonyl group at the C-3′ position. Thus, the structure of 4 is the keto form of turcicanol A (1) and turcicanol B (3) (Table 2). The 13C-NMR, 2D-COSY, HSQC, and HMBC spectra (Supplementary Materials, Figures S32–S35 and Figure 5a and Table 2) further corroborated the proposed structure of 4 as turcica ketone. Also, the strong anisotropic shift of the de-shielded H-2′ protons (0.6–0.8 ppm) strongly suggested the presence of a keto group at the C-3′ position (Table 2). Furthermore, the NOE correlations observed in the 2D-NOESY spectrum of 4 (Supplementary Materials, Figure S36 and Figure 5b) clearly confirmed the relative configuration of turcica ketone as shown in formula 4 (Figure 1).
Figure 5.
(a) COSY and HMBC correlations of turcica ketone (4); (b) NOE correlations of turcica ketone (4).
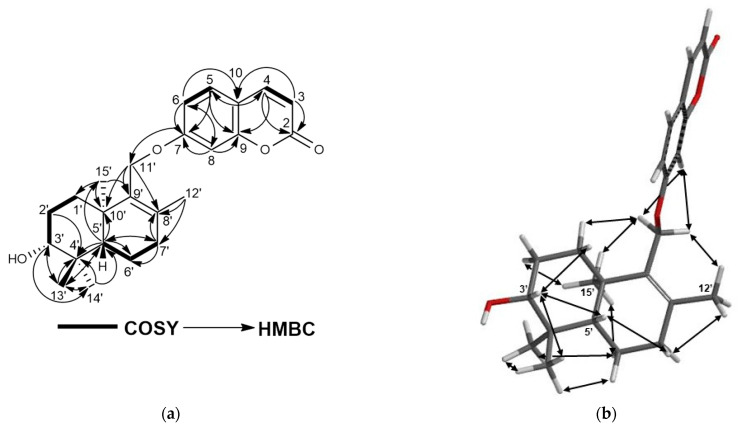
Compound 5 was isolated as an amorphous white powder. The (+)-HRESIMS spectrum of 5 displayed a [M + Na]+ molecular ion peak at m/z 405.2037, indicating a C24H30O4 molecular formula for 5 with 10 degrees of unsaturation. The 1H-NMR spectra of karatavicinol (32) (Supplementary Materials Figure S91) and compound 5 are similar except for the presence of two methylene proton singlets at δH 4.83 and δH 4.93 ppm in the 1H NMR of 5 (brs 1H for each) and the lack of hydroxyl adjacent to the C12′ and C13′ methyl signals of karatavicinol in 5 suggested that a double bond between C-11′ and C-12′ was present in 5. Furthermore, due to the allylic positioning of the C-10′ hydroxyl group, the chemical shift of the oxygenated methine proton at C-10′ in the 1H-NMR spectrum of 5 was shifted downfield ca. 0.7 ppm to δ 4.05 ppm (Table 2). The 13C-NMR, 2D-COSY, HSQC, and HMBC spectra (Supplementary Materials, Figures S41–S44 and Figure 6a and Table 2) confirmed the proposed structure of 5 as 11′-dehydrokaratavicinol. The key NOE correlations observed in the 2D-NOESY spectrum of 5 (Supplementary Materials, Figure S45 and Figure 6b) clearly confirmed the geometries of the double bonds of 11′-dehydrokaratavicinol as shown in formula 5 (Figure 1).
Figure 6.
(a) COSY and HMBC correlations of 11′-dehydrokaratavicinol (5); (b) NOE correlations of 11′-dehydrokaratavicinol (5).
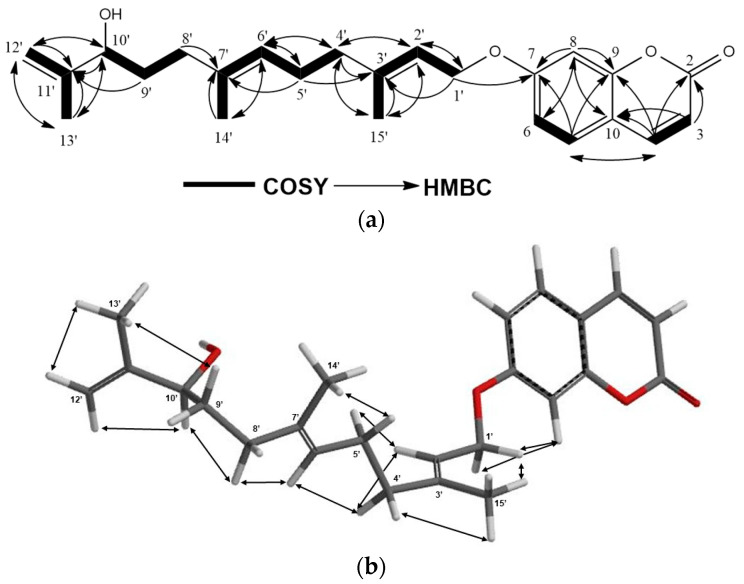
Compound 6 was isolated as an amorphous white powder. The [M + H]+ molecular ion of compound 6 at m/z 383.2233 indicated a molecular formula of C24H30O4 for 6 with 10 degrees of unsaturation. The 1H-NMR spectrum of 6 was very similar to that of galbanic acid (27) (Supplementary Materials, Figure S86) with the exception of the H-3′ signal appearing at δ 9.74 ppm as a narrow triplet, suggesting the presence of an aldehyde group at the C-3′ position (Table 2). The 13C-NMR, 2D-COSY, HSQC, and HMBC spectra (Supplementary Materials, Figures S50–S53 and Figure 7a and Table 2) confirmed the proposed structure of compound 6 as galbanaldehyde. The NOE correlations observed in the 2D-NOESY spectrum of 6 (Supplementary Materials, Figure S54 and Figure 7b) confirmed the relative configuration of galbanaldehyde as depicted in the formula 6 (Figure 1), which is identical to that of galbanic acid (27) [23,24,25].
Figure 7.
(a) COSY and HMBC correlations of galbanaldehyde (6); (b) NOE correlations of galbanaldehyde (6).
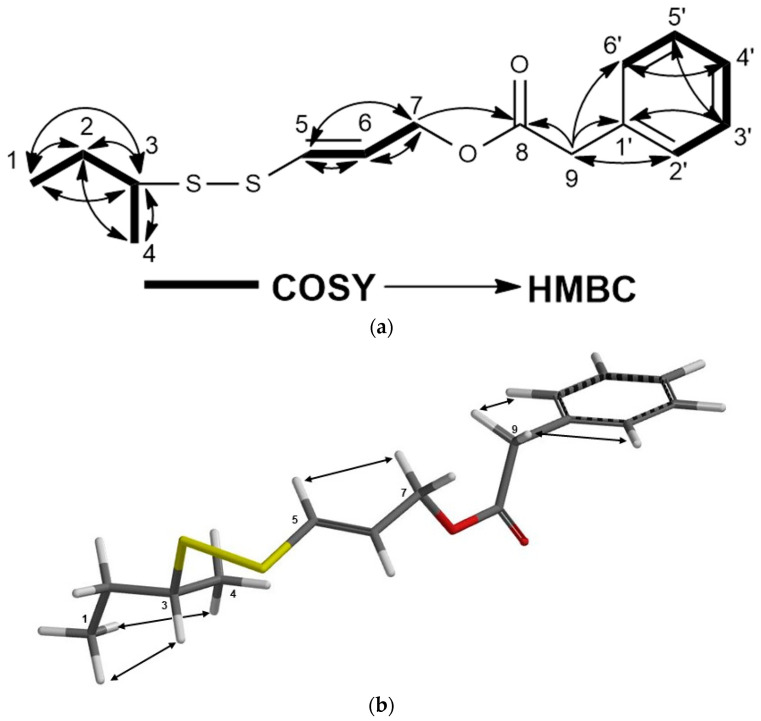
Compound 7 was isolated as colorless oil. The (+)-HRESIMS spectrum of compound 7 exhibited an [M + Na]+ molecular ion at m/z 319.0791, suggesting C15H20O2S2 as a molecular formula for turcicasulphide (7) with 6 degrees of unsaturation. The 1H-NMR spectra of persicasulphide C (39) (Supplementary Materials, Figure S98) and compound 7 were very similar to each other with the exception of presence of two doublets and one triplet in the aromatic region of 1HNMR, corresponding to the mono-substitute benzene ring in 7. The presence of aromatic and benzylic proton signals at δH 7.32, 7.29, 7.28, and 3.62 ppm suggested that a benzyl group is present in the molecule. Also, δH 4.6 (H-7) and δC 64.4 (C-7) indicated that C-7 is oxygenated. The HMBC correlations from δH 4.6 (H-7) and δH 3.62 (H-9) to δC 171.3 (C-8) connected the benzyl group to the position 7 of 7 through an ester linkage. This indicated that the 3-hydroxyisovalerate ester of persicasulphide C (39) was replaced with a benzyl ester in turcicasulphide (7) (Table 2). The 13C-NMR, 2D-COSY, HSQC, and HMBC spectra (Supplementary Materials, Figures S59–S62 and Figure 8a and Table 2) confirmed the proposed structure for 7 as turcicasulphide. The J coupling constant of 14.8 Hz between H-5 and H-6 indicated that these protons are trans to each other. The NOE correlations observed in the 2D-NOESY spectrum of 7 (Supplementary Materials, Figure S63 and Figure 8b) clearly confirmed the relative configuration of position 3 as shown in formula 7 (Figure 1).
Figure 8.
(a) COSY and HMBC correlations of turcicasulphide (7); (b) NOE correlations of turcicasulphide (7).
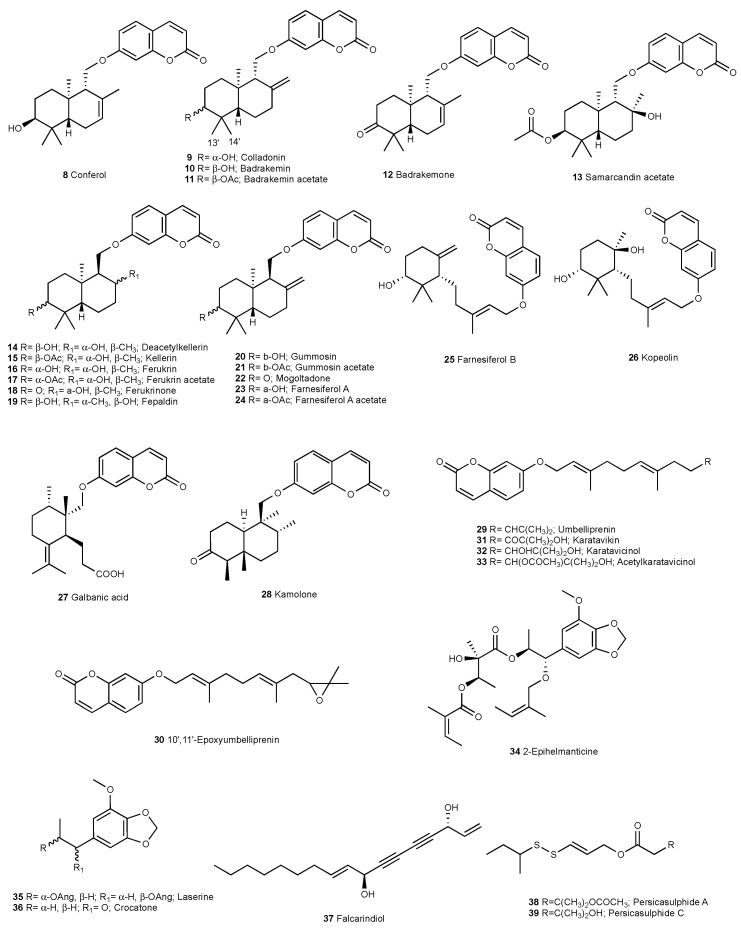
The known compounds conferol (8) [26], colladonin (9) [27], badrakemin (10) [27], badrakemin acetate (11) [28], badrakemone (12) [26], samarcandin acetate (13) [26], deacetylkellerin (14) [26], kellerin (15) [26], ferukrin (16) [26], ferukrin acetate (17) [26], ferukrinone (18) [29], fepaldin (19) [30,31], gummosin (20) [26], gummosin acetate (21) [29], mogoltadone (22) [26], farnesiferol A (23) [26], farnesiferol A acetate (24) [26], farnesiferol B (25) [32], kopeolin (26) [33], galbanic acid (27) [34], kamolone (28) [35], umbelliprenin (29) [36], 10′,11′-epoxyumbelliprenin (30) [37], karatavikin (31) [38], karatavicinol (32) [39], 10′-acetylkaratavicinol (33) [40], 2-epihelmanticine (34) [41], laserine (35) [42], crocatone (36) [43], falcarindiol (37) [44], persicasulphide A (38) [45], and persicasulphide C (39) [45] (Figure 9) were identified by the comparison of their spectroscopic data with that of the literature data.
Figure 9.
Known compounds isolated from Ferula turcica roots.
Most of the sesquiterpene coumarins isolated from the roots of Ferula turcica were bicyclic drimane sesquiterpene ethers of umbelliferone. The C-11′ hydroxymethylene group of the drimane sesquiterpene forms an ether linkage between the 7-OH of umbelliferone and the drimane moiety. The orientation of the C-11′ hydroxymethylene group of the drimane moiety was determined as equatorial in compounds 8–13 and axial in compounds 14–24 by NOE correlations observed in the 2D-NOESY spectra of those compounds. As it was shown by X-ray crystallography of the R-MTPA ester derivative of samarcandin and chemical transformations [26], stereochemistries of the other methyl groups of compounds 8–24 were identical, and their absolute configurations should be as depicted in formulas 8–24. Thus, the absolute configurations of the biogenetically related turcicanol derivatives should be as shown in formulas 1–4.
2.2. Cytotoxic Activity
The pure compounds of Ferula turcica were tested against colon cancer cell lines (COLO 205 and HCT 116) and kidney cell lines (UO31 and A498). The results are given in Table 3.
Table 3.
IC50 values of Ferula turcica compounds.
| Compound | IC50 (µM) | ||||
|---|---|---|---|---|---|
| Colo205 | HCT116 | A498 | UO31 | ||
| 1 | Turcicanol A | >50 | 41.1 | >50 | >50 |
| 2 | Turcicanol A acetate | >50 | >50 | >50 | >50 |
| 3 | Turcicanol B | >50 | >50 | >50 | 38.3 |
| 4 | Turcica ketone | 37.3 | 37.1 | >50 | 32.2 |
| 5 | 11′-Dehydrokaratavicinol | >50 | >50 | >50 | >50 |
| 6 | Galbanaldehyde | >50 | 48.8 | >50 | >50 |
| 7 | Turcicasulphide | >50 | >50 | >50 | >50 |
| 8 | Conferol | 16.9 | 36.2 | >50 | >50 |
| 9 | Colladonin | 35.9 | 47.4 | >50 | 33.1 |
| 10 | Badrakemin | >50 | >50 | >50 | >50 |
| 11 | Badrakemin acetate | >50 | 46.1 | >50 | 44.9 |
| 12 | Badrakemone | >50 | >50 | >50 | >50 |
| 13 | Samarcandin acetate | >50 | >50 | >50 | >50 |
| 14 | Deacetylkellerin | >50 | >50 | >50 | >50 |
| 15 | Kellerin | >50 | >50 | >50 | >50 |
| 16 | Ferukrin | >50 | >50 | >50 | >50 |
| 17 | Ferukrin acetate | >50 | >50 | >50 | >50 |
| 18 | Ferukrinone | >50 | >50 | >50 | >50 |
| 19 | Fepaldin | >50 | >50 | >50 | >50 |
| 20 | Gummosin | 12.7 | 18 | >50 | 19.7 |
| 21 | Gummosin acetate | >50 | 32.2 | 43.3 | 31.2 |
| 22 | Mogoltadone | 46.9 | >50 | >50 | >50 |
| 23 | Farnesiferol A | 35.7 | >50 | >50 | 45.3 |
| 24 | Farnesiferol A acetate | >50 | >50 | >50 | >50 |
| 25 | Farnesiferol B | 42.3 | >50 | >50 | >50 |
| 26 | Kopeolin | >50 | >50 | >50 | >50 |
| 27 | Galbanic acid | >50 | >50 | >50 | >50 |
| 28 | Kamolone | >50 | 43.5 | >50 | >50 |
| 29 | Umbelliprenin | 49.5 | >50 | >50 | >50 |
| 30 | 10′,11′-Epoxyumbelliprenin | 44.4 | >50 | >50 | >50 |
| 31 | Karatavikin | >50 | >50 | >50 | >50 |
| 32 | Karatavicinol | >50 | >50 | >50 | 34.4 |
| 33 | 10′-Acetylkaratavicinol | >50 | >50 | >50 | >50 |
| 34 | 2-Epihelmanticine | >50 | >50 | >50 | >50 |
| 35 | Laserine | >50 | >50 | >50 | >50 |
| 36 | Crocatone | >50 | >50 | >50 | >50 |
| 37 | Falcarindiol | >50 | >50 | >50 | >50 |
| 38 | Persicasulphide A | 49.9 | 15.8 | >50 | 42.5 |
| 39 | Persicasulphide C | 19.6 | >50 | >50 | 25.1 |
According to the cytotoxicity studies, the acetylation of the hydroxyl C-10 position in the sulfur-bearing compounds (compounds 38, 39) preserved the cytotoxic activity in the UO31 and COLO205 cell lines and slightly increased the activity in HCT116; however, the loss of hydroxyl at the C-10 and substitution of a benzene ring led to the loss of cytotoxic activity (see compound 7) in all cell lines. The cytotoxic activity was observed in colladonin (9) but not in badrakemin (10), whose hydroxyl substitution at the C-3′ was in the β position. In addition, the cytotoxic activity in gummosin (20), which is the C-9′ epimer of badrakemin (10), and colladonin (9) increased the cytotoxic activity with axial stereochemistry. The acetate derivatives of badrakemin (10) and gummosin (20) as well as the cytotoxic activity of badrakemin acetate (11) increased slightly in HCT116 cell lines, and the activity of gummosin acetate (21) decreased in COLO205 cell lines, decreased in HCT116, increased in A498, and decreased in UO31 cell lines. As for the ketone derivatives, the oxidation products at C-3′ of badrakemin (10) and gummosin (20) as well as oxidation did not cause any increase in badrakemone (12); it caused a decrease in cytotoxic activity in mogoltadone (22). Conferol (8) showed cytotoxic activity in colon cancer cell lines. We noted that even with the double-bond shift to C-8′ and C-9′ positions, cytotoxic activity in turcicanol A (1) is still significant in HCT116 colon cancer cells, just as in its isomers conferol (8) and gummosin (20). It was determined that the acetylation of the compound (turcicanol A acetate 2) causes activity to be lost in these four cell lines. In turcicanol B (3), an increase in cytotoxic activity was observed in the UO31 kidney cell line. When the cytotoxic activity results of the pure compound turcica ketone (4), an isomer of badrakemone (12) and mogoltadone (22), were examined, the endocyclic double bond was more cytotoxic than the exocyclic double bond, as in badrakemone (12) and mogoltadone (22). The cytotoxic activity results of galbanic acid (27) and its aldehyde derivative (6) showed that the aldehyde form of C-3′ increased the cytotoxic activity on HCT116 colon cancer cell lines.
3. Discussion
The literature data show that sesquiterpene coumarins such as gummosin, badrakemin acetate, ferukrinone, deacetylkellerin, farnesiferol A, farnesiferol B, farnesiferol C, samarcandin, umbelliprenin, kellerin, and gummosin have significant cytotoxic effects on breast (MCF-7) and prostate (PC-3) cancer cell lines (cytotoxic activity 30 and 32.1 µg/mL, respectively) [46]. Tosun et al. examined the cytotoxic activities of pure compounds on kidney cancer cells (UO31 and A498), colon cancer cells (COLO205 and KM12), and Ewing sarcoma cancer cell lines (A673 and TC32) and determined that umbelliprenin (1.8 µM), karatavicinol (7.6 µM), badrakemone (11 µM), badrakemin (0.38 µM), and colladonin (0.75 µM) suppressed growth of the UO31 kidney cancer cell line, while badrakemin (9.1 µM) and colladonin (2.5 µM) showed cytotoxic activity in the KM12 colon cancer cell line [27]. In another study, galbanic acid, a sesquiterpene coumarin obtained from F. szowitsiana roots, and farnesiferol A isolated from Ferula persica roots were found to be effective on doxorubicin-resistant breast cancer (MCF-7/Adr) cell lines [47]. In a combination study with sesquiterpene coumarins and doxorubucin, it was determined that the cytotoxic activity of doxorubicin was increased against MCF-7/Adr resistant cell lines, and the best result was seen with the combination of doxorubucin + lehmferin; it was determined that the activity increases in resistant cells during the using of the combination (5.08 µM) in contrast to doxorubucin usage alone (21.41 µM) [48].
According to the IC50 values, seventeen compounds (1, 3, 4, 6, 15, 17, 19–23, 25, 28–30, 32, 38, and 39) showed cytotoxic activity in this study. The most effective compounds against cancer cell lines were determined as conferol (8), gummosin (20), and persicasulphide A (38) and C (39), which is in agreement with the literature data. While it is true that no clear structure–activity patterns emerged from the testing, the diversity of structures may have limited any such conclusions.
4. Materials and Methods
4.1. General Experimental Procedures
LC-MS analysis was performed with Agilent Technologies® 6130 Quadrupole LC/MS (Santa Clara, CA, USA). UV–vis spectra were obtained using Shimadzu® UV-1700 PharmaSpec (Kyoto, Japan). IR spectra were determined using Bruker® Alpha FT-IR (Billerica, MA, USA). NMR spectra of the compounds were acquired on a Bruker® Avance III spectrometer operating at 600 MHz for 1H and 150 MHz for 13C in deuterated chloroform (Billerica, MA, USA). HRESIMS analysis of compounds 1–6 were performed using Agilent® 6530 Accurate Mass Q-TOF (Santa Clara, CA, USA), while the HRESIMS data of turcicasulphide (7) were acquired on a Thermo Scientific-Q Exactive® (Waltham, MA, USA). Optical rotation data were acquired using a Rudolph Analytical Autopol V Plus® in dichloromethane (Hackettstown, NJ, USA). A Buchi rotary evaporator was used to evaporate the solvent of the extract (Buchi, Flawil, Switzerland). A Sephadex LH-20 (Sigma Chem. Co. 25–100 µm) (GE Healthcare, Chicago, IL, USA) column (5 × 100 cm) was used for the initial fractionation. A Gilson® PLC 2050 was used for the further purification of the compounds (Saint-Avé, France). Hexane, dichloromethane, methanol, and acetonitrile (Merck, Darmstadt, Germany) were used during the chromatographic analyses.
4.2. Plant Material
The plant root materials used in this study were collected from the shores of Tuz Lake in Konya (Yavşan Tuzlası) on 16 June 2015, while the plant was fruiting, and the voucher specimen was archived in ISTE (Istanbul University Faculty of Pharmacy Herbarium) with the number 116,464. The species was identified by Prof. Emine Akalın and Hüseyin Onur Tuncay [6].
4.3. Extraction and Isolation
The powdered roots (270 g) of Ferula turcica were extracted by maceration at room temperature with dichloromethane (2 × 1 L) for 1 h in a Soxhlet extractor. After maceration, the plant material was further subjected to continuous extraction with dichloromethane and then with methanol using continuous extraction in a Soxhlet extractor. The dichloromethane extracts obtained by maceration and continuous extraction were concentrated separately under reduced pressure in a rotary evaporator at 35 °C. Since the TLC comparison of the extracts obtained by maceration and continuous extraction with dichloromethane showed close similarity, they were combined to yield the dichloromethane extract, 12 g (yield 4.5%). The methanol extract was evaporated to obtain 11 g (yield 4.1%) [26]. The cytotoxic dichloromethane extract (8.6 g) was fractionated on a Sephadex LH-20 column (5 × 100 cm) using a hexane: dichloromethane: methanol (7:4.5:0.5) solvent system as an initial mobile system, and the mobile system was eluted until 7:1:4 with the same solvent order [26]. The secondary metabolite profile of each fraction was examined by TLC chromatography, and similar fractions were combined to yield 30 fractions (Supplementary Material, Figure S1). Cytotoxic fractions with approximately 200 mg of mass (i.e., FST 10–29) were further purified on a reverse-phase preparative HPLC with a gradient elusion at a flow rate of 9 mL/min for 1 h to obtain 60 fractions. Fractions with less mass (<20 mg) were subjected to reverse-phase semipreparative HPLC purification at a flow rate of 4 mL/min for 45 min to obtain 45 fractions (one-minute collection) or 90 fractions (half-a-minute collection). Chromatograms were observed at 200–600 nm, 210 nm, 254 nm, 280 nm, and 366 nm wavelengths during HPLC purification. A Luna 5 µ Phenomenex® (21.2 × 150 mm) and a Luna 5 µ Phenomenex® (10 × 250 mm) C18 columns were used for purification. Acetonitrile and water were used as the mobile phase. The mobile phase composition was modified according to the polarity of fractions [49]. Thirty-two known compounds, namely samarcandin acetate (13, 10 mg), deacetylkellerin (14, 25.9 mg), kellerin (15, 61.8 mg), ferukrin (16, 29.3 mg), ferukrin acetate (17, 3.8 mg), ferukrinone (18, 5.3 mg), fepaldin (19, 0.6 mg), colladonin (9, 1 mg), badrakemin (10, 1.6 mg), badrakemin acetate (11, 6.2 mg), badrakemone (12, 1.3 mg), conferol (8, 1.3 mg), gummosin (20, 42.8 mg), gummosin acetate (21, 30.6 mg), mogoltadone (22, 77 mg), farnesiferol A (23, 24.1 mg), farnesiferol A acetate (24, 1.8 mg), farnesiferol B (25, 1.7 mg), kopeolin (26, 0.7 mg), galbanic acid (27, 5.6 mg), kamolone (28, 1.1 mg), umbelliprenin (29, 29.1 mg), 10′,11′-epoxyumbelliprenin (30, 0.8 mg), karatavikin (31, 1 mg), karatavicinol (32, 0.3 mg), 10′-acetylkaratavicinol (33, 6.3 mg), 2-epihelmanticine (34, 8 mg), laserine (35, 12 mg), crocatone (36, 0.8 mg), falcarindiol (37, 1.5 mg), persicasulphide A (38, 64.6 mg), persicasulphide C (39, 10 mg), and seven new compounds, turcicanol A (1, 2.9 mg), turcicanol A acetate (2, 4.3 mg), turcicanol B (3, 0.4 mg), turcica ketone (4, 1.4 mg), 11′-dehydrokaratavicinol (5, 3 mg), galbanaldehyde (6, 4.7 mg), and turcicasulphide (7, 0.6 mg), were obtained from the dichloromethane extract of F. turcica. (See Supplementary Materials Figure S1 for the isolation chart.)
Turcicanol A (1, 2.9 mg): Amorphous white powder, [α: −39° (c, 0.076 mg/mL, CH2Cl2); UV (c, 0.012 mg/mL) (MeOH) λmax (log ε) nm: 203 (4.59), 218 (sh) (4.12), 295 (sh) (3.83) nm, 324 (4.10) nm. IR υmax (NaCl) cm−1: 3053, 2942, 2866, 2826, 1730, 1613, 1554, 1507, 1475, 1455, 1427, 1402, 1385, 1349, 1278, 1230, 1198, 1156, 1124, 1096, 1062, 995, 921, 892, 835, 758, 736, 702, 662, 634, 616, 595, 544, 518, 492, 472, 459, 435, 419 cm−1. 1H-NMR and 13C-NMR data are in Table 2; HRESIMS m/z [M + H]+ 383.2219 (calculated for C24H31O4: 383.2222).
Turcicanol A acetate (2, 4.3 mg): Amorphous white powder, [α: −31° (c, 0.089 mg/mL, CH2Cl2); UV (c, 0.015 mg/mL) (MeOH) λmax (log ε) nm: 204 (4.71) nm, 219 (sh) (4.23) nm, 295 (sh) (3.96), 324 (4.23) nm. IR υmax (NaCl) cm−1: 3078, 3055, 2943, 2875, 2831, 1732, 1612, 1555, 1507, 1455, 1428, 1402, 1375, 1350, 1276, 1248, 1198, 1183, 1157, 1123, 1096, 1047, 1015, 996, 971, 891, 836, 736, 702, 664, 634, 615, 543, 516, 480, 460, 417, 407 cm−1. 1H-NMR and 13C-NMR data are in Table 2; HRESIMS m/z [M + H]+ 425.2325 (calculated for C26H33O5: 425.2328).
Turcicanol B (3, 0.4 mg): Amorphous white powder, [α: −43° (c, 0.04 mg/mL, CH2Cl2); UV (c, 0.012 mg/mL) (MeOH) λmax (log ε) nm: 203 (4.46) nm, 220 (sh) (3.94), 296 (sh) (3.62), 324 (3.86) nm. IR υmax (NaCl) cm−1: 2956, 2922, 2869, 2847, 1733, 1611, 1554, 1507, 1460, 1402, 1377, 1350, 1277, 1299, 1197, 1156, 1123, 1097, 1036, 997, 892, 835, 735, 700, 615, 461, 444, 415 cm−1. 1H-NMR and 13C-NMR data are in Table 2; HRESIMS m/z [M + H]+ 383.2225 (calculated for C24H31O4: 383.2222).
Turcica ketone (4, 1.4 mg): Amorphous white powder, [α: −14° (c, 0.04 mg/mL, CH2Cl2); UV (c, 0.012 mg/mL) (MeOH) λmax (log ε) nm: 203 (4.50) nm, 217 (sh) (4.06), 295 (sh) (3.75) nm, 324 (4.00) nm. IR υmax (NaCl) cm−1: 3077, 3057, 2954, 2929, 2871, 1731, 1704, 1611, 1554, 1507, 1459, 1428, 1401, 1382, 1348, 1276, 1229, 1196, 1156, 1122, 996, 974, 891, 834, 745, 702, 634, 615, 593, 534, 494, 477, 456, 419 cm−1. 1H-NMR and 13C-NMR data are in Table 2; HRESIMS m/z [M + H]+ 381.2064 (calculated for C24H29O4: 381.2066).
11′-Dehydrokaratavicinol (5, 3 mg): Amorphous white powder, [α: −1.5° (c, 0.003 mg/mL, CH2Cl2); UV (c, 0.012 mg/mL) (MeOH) λmax (log ε) nm: 203 (4.58) nm, 215 (sh) (4.12), 299 (sh) (3.78), 324 (4.02) nm. IR υmax (NaCl) cm−1: 3072, 2933, 2856, 1732, 1613, 1555, 1507, 1446, 1402, 1350, 1278, 1231, 1199, 1157, 1127, 1096, 1060, 1000, 895, 835, 757, 683, 634, 616, 559, 516, 460, 418, 406 cm−1. 1H-NMR and 13C-NMR data are given in Table 2; HRESIMS m/z [M + Na]+ 405.2037 (calculated for C24H30O4Na: 405.2042).
Galbanaldehyde (6, 4.7 mg): Amorphous white powder, [α: −24° (c, 0.078 mg/mL, CH2Cl2); UV (c, 0.012 mg/mL) (MeOH) λmax (log ε) nm: 203 (4.80), 218 (sh) (4.29) nm, 295 (sh) (4.02), 324 (4.30) nm. IR υmax (NaCl) cm−1: 3081, 3053, 2962, 2923, 2879, 2656, 1719, 1731, 1729, 1613, 1555, 1509, 1454, 1428, 1302, 1351, 1279, 1231, 1199, 1156, 1122, 1096, 1011, 988, 891, 835, 751, 735, 703, 633, 616, 546, 533, 514, 459, 417 cm−1. 1H-NMR and 13C-NMR data are given in Table 2; HRESIMS m/z [M + H]+ 383.2233 (calculated for C24H31O4: 383.2222).
Turcicasulphide (7, 0.6 mg): Colorless oil, UV (c, 0.013 mg/mL) (MeOH) λmax (log ε) nm: 203 (4.52) nm, 234 (sh) (4.09), 322 (3.58) nm. IR υmax (NaCl) cm−1: 2962, 2925, 2870, 2857, 1736, 1611, 1494, 1451, 1373, 1338, 1276, 1241, 1144, 993, 972, 939, 835, 722, 702, 464 cm−1. 1H-NMR and 13C-NMR data are in Table 2; HRESIMS m/z [M + Na]+ 319.0791 (calculated for C15H20O2S2Na: 319.0802).
4.4. 2DAY (Colon 2) XTT Cytotoxic Activity Assay
The two-XTT bioactivity test is an in vitro colorimetric cytotoxic activity test developed by the NCI MTP Assay Development and Screening Section [50] and used for this study. Colon (COLO205, HCT116) and kidney (A498, UO31) cancer cell lines were used during the tests. RPMI-1640 (Roswell Park Memorial Institute, Buffalo, NY, USA) medium, 10% FBS (fetal bovine serum), 1% glutamine, and 1% penicillin/streptomycin solutions were used for cell growth and treatment. Transfers were performed under laminar air flow in a sterile environment. The suspension containing the cells was seeded into 96-well plates with a volume of 45 µL with 3.5 × 105 cells per well. Then, the plate was incubated at 37 °C and 5% CO2 for 24 h. The extract and pure compounds prepared in DMSO were added and incubated for another 48 h. After incubation, 10 µL of the tetrazolium salt XTT (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanolide) was applied to the cells. After 4 h of incubation, dead cells were not stained with formazan dye, while viable cells could be counted in the EnVision plate reader under UV light (450 nm and 650 nm). Sanguinarine chloride hydrate was used as a positive control in the experiment.
5. Conclusions
A dichloromethane extract of Ferula turcica root was studied for the first time. Seven new and thirty-two known compounds (1–39) were isolated from the dichloromethane extract using bioactivity-directed fractionation, and their cytotoxic activities were investigated against COLO205, HCT116, A498, and UO31 cancer cell lines. The structures of the new compounds were determined by spectroscopic techniques, and the spectral data of the compounds are presented for the first time. Some structure–activity relationships of the compounds for cytotoxic activities illuminate the effects of substitution, oxidation, acetylation, and double bond positions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28155733/s1, Figures S1–S108: LC-MS analysis of the dichloromethane extract of F. turcica, isolation chart of the dichloromethane extract of F. turcica, structures of pure compounds, the 1D and 2D NMR, HRESIMS, UV, and IR data of pure compounds 1–7 as well as the 1H-NMR spectra of compounds 8–39 and cytotoxic activity graphic of pure compounds 1–39.
Author Contributions
Conceptualization, M.M. and J.A.B.; methodology, F.M.E. and S.P.D.S.; software, F.M.E., S.P.D.S., E.G. and M.M.; validation, F.M.E., S.P.D.S., E.G., J.A.B. and M.M.; formal analysis, F.M.E., S.P.D.S., J.A.W., J.A.B. and M.M.; investigation, F.M.E., S.P.D.S., J.A.W., J.A.B. and M.M.; resources, F.M.E., S.P.D.S., J.A.B. and M.M.; data curation and writing—original draft preparation, F.M.E. and M.M.; writing—review and editing, F.M.E., S.P.D.S., J.A.B. and M.M.; visualization, F.M.E. and M.M.; supervision, M.M. and J.A.B.; project administration, F.M.E., S.P.D.S., J.A.B. and M.M.; funding acquisition, J.A.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was funded by the Scientific Research Projects Coordination Unit of Istanbul University. Project number: 30731. The authors kindly appreciate the Fulbright Commission for the funding to support this study. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (ZIABC011470), and with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cancer. World Health Organization (WHO) Health Topics. [(accessed on 15 June 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
- 2.Özhatay N., Koçyiğit M., Bona M. İstanbul’un Ballı Bitkileri “Çiçek Varsa Bal Var”. 1st ed. Türkmenler Matbaacılık Rek. San. Tic. Ltd. Şti. Company; İstanbul, Türkiye: 2010. pp. 18–22. [Google Scholar]
- 3.Zachos F.E., Habel J.C. Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas. 1st ed. Springer; Berlin/Heidelberg, Germany: 2011. p. 171. [Google Scholar]
- 4.Newman D.J., Cragg G.M. Natural Products As Sources of New Drugs From 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 5.Pimenov M.G., Leonov M.V. The Asian Umbelliferae Biodiversity Database (ASIUM) With Particular Reference To South-West Asian Taxa. Turk. J. Bot. 2004;28:139–145. [Google Scholar]
- 6.Tuncay H.O., Akalın E., Doğru-Koca A., Eruçar F.M., Miski M. Two New Ferula (Apiaceae) Species From Central Anatolia: Ferula turcica and Ferula latialata. Horticulturae. 2023;9:144. doi: 10.3390/horticulturae9020144. [DOI] [Google Scholar]
- 7.Baytop A. Türkçe Bitki Adları Sözlüğü. 1st ed. Türk Dil Kurumu Yayınları Company; Ankara, Türkiye: 1997. p. 578. [Google Scholar]
- 8.Gunther R.T. The Greek Herbal of Dioscorides. 3rd ed. Hafner Publishing Company; London, UK: New York, NY, USA: 1968. pp. 323, 328–332. [Google Scholar]
- 9.Eisenman S.W., Zaurov D.E., Struwe L. Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan. Springer; New York, NY, USA: Berlin/Heidelberg, Germany: Dordrecht, The Netherlands: London, UK: 2013. p. 10. [Google Scholar]
- 10.Abu-Irmailah B., Afifi F. Treatment with Medicinal Plants in Jordan. Dirasat. Med. Biol. Sci. 2000;27:53–74. [Google Scholar]
- 11.Iranshahy M., Iranshahi M. Traditional Uses, Phytochemistry and Pharmacology of Asafoetida (Ferula assa-foetida Oleo-gum-resin)—A review. J. Ethnopharmacol. 2011;134:1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 12.Saidkhodzhaev A.I. Sesquiterpene Derivatives of The Genus Ferula. Chem. Nat. Compd. 1979;15:379–404. doi: 10.1007/BF00565032. [DOI] [Google Scholar]
- 13.Miski M., Mabry T.J. Daucane Esters From Ferula communis subsp. communis. Phytochemistry. 1985;24:1735–1741. doi: 10.1016/S0031-9422(00)82543-1. [DOI] [Google Scholar]
- 14.Saidkhodzhaev A.I., Mamatkhanov A.U. Terpenoids of Plants of The Ferula Genus. I. Natural Carotane Derivatives. Chem. Nat. Compd. 1995;31:767–780. [Google Scholar]
- 15.Saidkhodzhaev A.I., Kadyrov A.S., Malikov V.M. Stereochemistry of Feshurin, Nevskin, and Colladocin. Chem. Nat. Compd. 1979;15:266–268. doi: 10.1007/BF00566071. [DOI] [Google Scholar]
- 16.Nazari Z.E., Iranshahi M. Biologically Active Sesquiterpene Coumarins From Ferula species. Phytother. Res. 2011;25:315–323. doi: 10.1002/ptr.3311. [DOI] [PubMed] [Google Scholar]
- 17.Iranshahi M., Amin G.R., Amini M., Shafiee A. Sulfur Containing Derivatives from Ferula persica var. latisecta. Phytochemistry. 2003;63:965–966. doi: 10.1016/S0031-9422(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 18.Iranshahi M., Yazdi M.C., Hassanzadeh-Khayyat M., Sahebkar A. Sulfur Containing Compounds In The Volatile Oil of Ferula latisecta Rech. f. & Aell. Leaves. J. Essent. Oil-Bear. Plants. 2009;12:64–68. [Google Scholar]
- 19.Barthomeuf C., Lim S., Iranshahi M., Chollet P. Umbelliprenin From Ferula szowitsiana Inhibits The Growth of Human M4Beu Metastatic Pigmented Malignant Melanoma Cells Through Cell-Cycle Arrest In G1 and Induction of Caspase-dependent Apoptosis. Phytomedicine. 2008;15:103–111. doi: 10.1016/j.phymed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Gholami O., Jeddi-Tehrani M., Iranshahi M., Zarnani A.H., Ziai S.A. Umbelliprenin From Ferula szowitsiana Activates Both Intrinsic and Extrinsic Pathways of Apoptosis In Jurkat T-CLL Cell Line. Iran. J. Pharm. Res. 2013;12:371–376. [PMC free article] [PubMed] [Google Scholar]
- 21.Bahrami M., Haji Molla Hoseini M., Rezaei M., Ziai S.A. Umbelliprenin Increases The M1/M2 Ratio of Macrophage Polarization and Improves the M1 Macrophage Activity in THP-1 Cells Cocultured with AGS Cells. Evid.-Based Complement. Altern. Med. 2021;2021:9927747. doi: 10.1155/2021/9927747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barthomeuf C., Demeule M., Grassi J., Saidkhodjaev A., Beliveau R. Conferone From Ferula schtschurowskiana Enhances Vinblastine Cytotoxicity in MDCK-MDR1 Cells by Competitively Inhibiting P-Glycoprotein Transport. Planta Med. 2006;72:634–639. doi: 10.1055/s-2006-931574. [DOI] [PubMed] [Google Scholar]
- 23.Bagirov V.Y., Sheichenko V.I., Veselovskaya N.V., Sklyar Y.E., Savina A.A., Kir’yalov I.A. Structure and Stereochemistry of Galbanic Acid. Chem. Nat. Compd. 1980;16:439–441. doi: 10.1007/BF00571032. [DOI] [Google Scholar]
- 24.Corbu A., Aquino M., Perez M., Gandara Z., Arseniyadis S. Natural and Unnatural A-seco Terpenes from Pulegone: Synthesis of Galbanic acid and Marneral Revisited. Eur. J. Org. Chem. 2009;36:6386–6392. doi: 10.1002/ejoc.200901021. [DOI] [Google Scholar]
- 25.Kasaian J., Iranshahy M., Iranshahi M. Synthesis, Biosynthesis and Biological Activities of Galbanic acid—A review. Pharm. Biol. 2014;52:524–531. doi: 10.3109/13880209.2013.846916. [DOI] [PubMed] [Google Scholar]
- 26.Eruçar F.M., Kuran F.K., Altıparmak Ülbegi G., Özbey S., Karavuş Ş.N., Arcan G.G., Yazıcı Tütüniş S., Tan N., Aksoy Sağırlı P., Miski M. Sesquiterpene Coumarin Ethers with Selective Cytotoxic Activities From The Roots of Ferula huber-morathii Peşmen (Apiaceae) and Unequivocal Determination of the Absolute Stereochemistry of Samarcandin. Pharmaceuticals. 2023;16:792. doi: 10.3390/ph16060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tosun F., Beutler J.A., Ransom T.T., Miski M. Anatolicin, A Highly Potent and Selective Cytotoxic Sesquiterpene Coumarin From The root Extract of Heptaptera anatolica. Molecules. 2019;24:1153. doi: 10.3390/molecules24061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savina A.A., Sklyar Y.E., Sheichenko V.I., Kir’yanova I.A., Fesenko D.A. Migration of The Exocyclic Double Bond In Terpenoid Coumarins of The Iresane Series. Chem. Nat. Compd. 1979;15:550–556. doi: 10.1007/BF00565922. [DOI] [Google Scholar]
- 29.Xing Y., Li N., Zhou D., Chen G., Jiao K., Wang W., Si Y., Hou Y. Sesquiterpene Coumarins From Ferula sinkiangensis Act As Neuroinflammation Inhibitors. Planta. Med. 2017;83:135–142. doi: 10.1055/s-0042-109271. [DOI] [PubMed] [Google Scholar]
- 30.Malikov V.M., Saidkhodzhaev A.I. Coumarins: Plants, Structures, Properties, Chapter II. Chem. Nat. Compd. 1998;34:517–548. [Google Scholar]
- 31.Tashkhodzhaev B., Turgunov K.K., Izotova L.Y., Kamoldinov K.S. Stereochemistry of Samarcandin-Type Sesquiterpenoid Coumarins. Crystal Structures of Feshurin and Nevskin. Chem. Nat. Compd. 2015;51:242–246. doi: 10.1007/s10600-015-1253-4. [DOI] [Google Scholar]
- 32.Yadav J.S., Satyanarayana K., Sreedhar P., Srihari P., Shaik T.B., Kalivendi S.V. Total Synthesis of (+/−)-Elegansidiol, (+/−)-Farnesiferol B, and (+/−)-Farnesiferol D. Bioorg. Med. Chem. Lett. 2010;20:3814–3817. doi: 10.1016/j.bmcl.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 33.Miquet S., Vanthuyne N., Brémond P., Audran G. Enantioselective Syntheses of The Proposed Structures of Kopeolin and Kopeolone. Chemistry. 2013;19:10632–10642. doi: 10.1002/chem.201300711. [DOI] [PubMed] [Google Scholar]
- 34.Lee S.G., Ryu S.Y., Ahn J.W. Reinvestigation of The Sturcture of Galbanic Acid by 2D NMR Techniques Including 2D INACEQUATE. Bull. Korean Chem. Soc. 1998;19:384–386. [Google Scholar]
- 35.Ermatov N.E., Ban’kovskii A.I., Perel’son M.E., Syrova G.P., Sheinker Y.N. Structure of Kamolone and Kamolol-New Coumarins From Ferula penninervis. Chem. Nat. Compd. 1969;5:68–71. doi: 10.1007/BF00633278. [DOI] [Google Scholar]
- 36.Iranshahi M., Amin G.R., Jalalizadeh H., Shafiee A. New Germacrane Derivative From Ferula persica. Pharm. Biol. 2003;41:431–433. doi: 10.1076/phbi.41.6.431.17834. [DOI] [Google Scholar]
- 37.Güvenalp Z., Özbek H., Yerdelen K., Yilmaz G., Kazaz C., Demirezer L.Ö. Cholinesterase Inhibition and Molecular Docking Studies of Sesquiterpene Coumarin Ethers From Heptaptera cilicica. R. Nat. Prod. 2017;11:462–467. doi: 10.25135/rnp.58.17.03.051. [DOI] [Google Scholar]
- 38.Kir’yalov N.P., Bagirov V.Y. Structure of Karatavicin. Chem. Nat. Compd. 1967;3:185–187. doi: 10.1007/BF00564110. [DOI] [Google Scholar]
- 39.Ahmed A.A. Sesquiterpene Coumarins and Sesquiterpenes From Ferula sinaica. Phytochemistry. 1999;50:109–112. doi: 10.1016/S0031-9422(98)00489-0. [DOI] [Google Scholar]
- 40.Lee C.L., Chiang L.C., Cheng L.H., Liaw C.C., Abd El-Razek M.H., Chang F.R., Wu Y.C. Influenza A (H1N1) Antiviral and Cytotoxic Agents from Ferula assa-foetida. J. Nat. Prod. 2009;72:1568–1572. doi: 10.1021/np900158f. [DOI] [PubMed] [Google Scholar]
- 41.Iranshahi M., Arfa P., Ramezani M., Jaafari M.R., Sadeghian H., Bassarello C., Piacente S., Cosimo R. Sesquiterpene Coumarins From Ferula szowitsiana and In Vitro Antileishmanial Activity of 7-Prenyloxycoumarins Against Promastigotes. Phytochemistry. 2007;68:554–561. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Yang R.L., Yan Z.H., Lu Y. Cytotoxic Phenylpropanoids From Carrot. J. Agric. Food. Chem. 2008;56:3024–3027. doi: 10.1021/jf7036517. [DOI] [PubMed] [Google Scholar]
- 43.Kadyrov A.S., Nikonov G.K. 3-Methoxy-4,5-methylenedioxypropiophenone—A new component of the roots of Ferula ugamica. Chem. Nat. Compd. 1973;9:95. doi: 10.1007/BF00580902. [DOI] [Google Scholar]
- 44.Lechner D., Stavri M., Oluwatuyi M., Pereda-Miranda R., Gibbons S. The Anti-staphylococcal Activity of Angelica dahurica (Bai Zhi) Phytochemistry. 2004;65:331–335. doi: 10.1016/j.phytochem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Iranshahi M., Noroozi S., Behravan J., Karimi G., Schneider B. Persicasulphide C, A New Sulphur-Containing Derivative From Ferula persica. Nat. Prod. Res. 2009;23:1584–1588. doi: 10.1080/14786410802393571. [DOI] [PubMed] [Google Scholar]
- 46.Iranshahy M., Farhadi F., Paknejad B., Zareian P., Iranshahi M., Karami M., Abtahi S.R. Gummosin, A Sesquiterpene Coumarin From Ferula assa-foetida Is Preferentially Cytotoxic To Human Breast and Prostate Cancer Cell Lines. Avicenna J. Phytomed. 2019;9:446–453. [PMC free article] [PubMed] [Google Scholar]
- 47.Hanafi-Bojd M.Y., Iranshahi M., Mosaffa F., Tehrani S.O., Kalalinia F., Behravan J. Farnesiferol A From Ferula persica and Galbanic acid from Ferula szowitsiana Inhibit P-Glycoprotein-mediated rhodamine efflux in breast cancer cell lines. Planta Med. 2011;77:1590–1593. doi: 10.1055/s-0030-1270987. [DOI] [PubMed] [Google Scholar]
- 48.Kasaian J., Mosaffa F., Behravan J., Masullo M., Piacente S., Ghandadi M., Iranshahi M. Reversal of P-glycoprotein-mediated Multidrug Resistance In MCF-7/Adr Cancer Cells by Sesquiterpene coumarins. Fitoterapia. 2015;103:149–154. doi: 10.1016/j.fitote.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 49.Amin A., Tuenter E., Cos P., Maes L., Exarchou V., Apers S., Pieters L. Antiprotozoal and Antiglycation Activities of Sesquiterpene Coumarins from Ferula narthex Exudate. Molecules. 2016;21:1287. doi: 10.3390/molecules21101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J.B., Vistica D., Warren J.T., Bokesch H.R., Kenney S., Boyd M.R. New Colorimetric Cytotoxicity Assay For Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.