Abstract
Background
Hyperemesis gravidarum is a severe form of nausea and vomiting in pregnancy affecting 0.3% to 1.0% of pregnancies, and is one of the most common indications for hospitalization during pregnancy. While a previous Cochrane review examined interventions for nausea and vomiting in pregnancy, there has not yet been a review examining the interventions for the more severe condition of hyperemesis gravidarum.
Objectives
To assess the effectiveness and safety, of all interventions for hyperemesis gravidarum in pregnancy up to 20 weeks' gestation.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register and the Cochrane Complementary Medicine Field's Trials Register (20 December 2015) and reference lists of retrieved studies.
Selection criteria
Randomized controlled trials of any intervention for hyperemesis gravidarum. Quasi‐randomized trials and trials using a cross‐over design were not eligible for inclusion.
We excluded trials on nausea and vomiting of pregnancy that were not specifically studying the more severe condition of hyperemesis gravidarum.
Data collection and analysis
Two review authors independently reviewed the eligibility of trials, extracted data and evaluated the risk of bias. Data were checked for accuracy.
Main results
Twenty‐five trials (involving 2052 women) met the inclusion criteria but the majority of 18 different comparisons described in the review include data from single studies with small numbers of participants. The comparisons covered a range of interventions including acupressure/acupuncture, outpatient care, intravenous fluids, and various pharmaceutical interventions. The methodological quality of included studies was mixed. For selected important comparisons and outcomes, we graded the quality of the evidence and created 'Summary of findings' tables. For most outcomes the evidence was graded as low or very low quality mainly due to the imprecision of effect estimates. Comparisons included in the 'Summary of findings' tables are described below, the remaining comparisons are described in detail in the main text.
No primary outcome data were available when acupuncture was compared with placebo, There was no clear evidence of differences between groups for anxiodepressive symptoms (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.73 to 1.40; one study, 36 women, very low‐quality evidence), spontaneous abortion (RR 0.48, 95% CI 0.05 to 5.03; one study, 57 women, low‐quality evidence), preterm birth (RR 0.12, 95% CI 0.01 to 2.26; one study, 36 women, low‐quality evidence), or perinatal death (RR 0.57, 95% CI 0.04 to 8.30; one study, 36 women, low‐quality evidence).
There was insufficient evidence to identify clear differences between acupuncture and metoclopramide in a study with 81 participants regarding reduction/cessation in nausea or vomiting (RR 1.40, 95% CI 0.79 to 2.49 and RR 1.51, 95% CI 0.92 to 2.48, respectively; very low‐quality evidence).
In a study with 92 participants, women taking vitamin B6 had a slightly longer hospital stay compared with placebo (mean difference (MD) 0.80 days, 95% CI 0.08 to 1.52, moderate‐quality evidence). There was insufficient evidence to demonstrate a difference in other outcomes including mean number of episodes of emesis (MD 0.50, 95% CI ‐0.40 to 1.40, low‐quality evidence) or side effects.
A comparison between metoclopramide and ondansetron identified no clear difference in the severity of nausea or vomiting (MD 1.70, 95% CI ‐0.15 to 3.55, and MD ‐0.10, 95% CI ‐1.63 to 1.43; one study, 83 women, respectively, very low‐quality evidence). However, more women taking metoclopramide complained of drowsiness and dry mouth (RR 2.40, 95% CI 1.23 to 4.69, and RR 2.38, 95% CI 1.10 to 5.11, respectively; moderate‐quality evidence). There were no clear differences between groups for other side effects.
In a single study with 146 participants comparing metoclopramide with promethazine, more women taking promethazine reported drowsiness, dizziness, and dystonia (RR 0.70, 95% CI 0.56 to 0.87, RR 0.48, 95% CI 0.34 to 0.69, and RR 0.31, 95% CI 0.11 to 0.90, respectively, moderate‐quality evidence). There were no clear differences between groups for other important outcomes including quality of life and other side effects.
In a single trial with 30 women, those receiving ondansetron had no difference in duration of hospital admission compared to those receiving promethazine (MD 0.00, 95% CI ‐1.39 to 1.39, very low‐quality evidence), although there was increased sedation with promethazine (RR 0.06, 95% CI 0.00 to 0.94, low‐quality evidence) .
Regarding corticosteroids, in a study with 110 participants there was no difference in days of hospital admission compared to placebo (MD ‐0.30, 95% CI ‐0.70 to 0.10; very low‐quality evidence), but there was a decreased readmission rate (RR 0.69, 95% CI 0.50 to 0.94; four studies, 269 women). For other important outcomes including pregnancy complications, spontaneous abortion, stillbirth and congenital abnormalities, there was insufficient evidence to identify differences between groups (very low‐quality evidence for all outcomes). In other single studies there were no clear differences between groups for preterm birth or side effects (very low‐quality evidence).
For hydrocortisone compared with metoclopramide, no data were available for primary outcomes and there was no difference in the readmission rate (RR 0.08, 95% CI 0.00 to 1.28;one study, 40 women).
In a study with 80 women, compared to promethazine, those receiving prednisolone had increased nausea at 48 hours (RR 2.00, 95% CI 1.08 to 3.72; low‐quality evidence), but not at 17 days (RR 0.81, 95% CI 0.58 to 1.15, very low‐quality evidence). There was no clear difference in the number of episodes of emesis or subjective improvement in nausea/vomiting. There was insufficient evidence to identify differences between groups for stillbirth and neonatal death and preterm birth.
Authors' conclusions
On the basis of this review, there is little high‐quality and consistent evidence supporting any one intervention, which should be taken into account when making management decisions. There was also very limited reporting on the economic impact of hyperemesis gravidarum and the impact that interventions may have.
The limitations in interpreting the results of the included studies highlights the importance of consistency in the definition of hyperemesis gravidarum, the use of validated outcome measures, and the need for larger placebo‐controlled trials.
Keywords: Female, Humans, Pregnancy, Acupuncture Therapy, Acupuncture Therapy/methods, Adrenal Cortex Hormones, Adrenal Cortex Hormones/adverse effects, Adrenal Cortex Hormones/therapeutic use, Antiemetics, Antiemetics/adverse effects, Antiemetics/therapeutic use, Hydrocortisone, Hydrocortisone/therapeutic use, Hyperemesis Gravidarum, Hyperemesis Gravidarum/therapy, Metoclopramide, Metoclopramide/adverse effects, Metoclopramide/therapeutic use, Ondansetron, Ondansetron/adverse effects, Ondansetron/therapeutic use, Placebo Effect, Prednisolone, Prednisolone/adverse effects, Prednisolone/therapeutic use, Promethazine, Promethazine/therapeutic use, Pyridoxine, Pyridoxine/adverse effects, Pyridoxine/therapeutic use
Plain language summary
Interventions for treating severe nausea and vomiting during pregnancy (hyperemesis gravidarum)
What is the issue and why is it important?
Although severe nausea and vomiting in pregnancy (hyperemesis gravidarum) rarely causes death, it is an important cause of ill health with emotional, physical, and economic consequences. Women may need hospital treatment and may not be able to work and it occasionally causes pregnancy complications and adverse outcomes for babies such as low birthweight. Many pharmaceutical, complementary, and alternative therapies are available and the objective of this review was to examine the effectiveness and safety of interventions for hyperemesis gravidarum.
What evidence did we find?
Twenty‐five trials (involving 2052 women) were included examining 18 different comparisons covering a range of interventions including acupressure/acupuncture, outpatient care, intravenous fluids, and various commonly used anti‐sickness drugs. The quality of included studies was mixed and for most outcomes findings were from single studies with low numbers of women taking part and the evidence was assessed as being of low or very low quality. We have described findings for selected important comparisons below, the remaining comparisons are described in detail in the main text.
There was no clear evidence of differences between acupuncture and placebo for symptoms of anxiety or depression, spontaneous abortion, preterm birth or perinatal death.
There was insufficient evidence to identify clear differences between acupuncture and metoclopramide (an anti‐nausea medication) for reduction or cessation in nausea or vomiting.
Women taking vitamin B6 had a slightly longer hospital stay compared with placebo but there was no clear evidence of differences in other outcomes including the average number of episodes of vomiting, hospital readmission rate, or side effects.
A comparison between two anti‐nausea medications, metoclopramide and ondansetron, identified no clear difference in the severity of nausea or vomiting, but more women taking metoclopramide complained of drowsiness and dry mouth. In a study comparing metoclopramide with promethazine, more women taking promethazine reported drowsiness and dizziness but there were no clear differences between groups for other important outcomes including quality of life and other side effects. In a study looking at ondansetron versus promethazine women spent similar lengths of time in hospital but there was increased sedation with promethazine.
Regarding corticosteroids, there was no difference in days of hospital admission compared to placebo, but there was a decreased readmission rate. For other important outcomes including pregnancy complications, spontaneous abortion, stillbirth and congenital abnormalities, preterm birth and side effects, there was insufficient evidence to identify differences between groups.
In a study comparing hydrocortisone (a corticosteroid) with metoclopramide, no data were available for primary outcomes, but there was no difference in hospital readmission rate.
In a study comparing promethazine and prednisolone (a corticosteroid) those receiving prednisolone had increased nausea at 48 hours but not at 17 days. There was no clear difference in the number of episodes of vomiting. There was insufficient evidence to identify differences between groups for stillbirth and neonatal death and preterm birth
What does this mean?
Given that there was little evidence to support the superiority of one intervention over another in the treatment of hyperemesis, larger controlled trials are needed on these therapies. More research should be done comparing the side effects and safety, as well as the economic costs and benefits of these interventions to aid in the selection of the optimal treatment.
Reporting on adverse maternal and infant outcomes was limited and we did not find any studies on dietary or other lifestyle interventions.
Summary of findings
Summary of findings for the main comparison. Acupuncture versus placebo.
| Acupuncture versus placebo [Note: This table relates to the first comparison described in the abstract rather than the 'main' or most important comparison presented in the review] | ||||||
| Patient or population: pregnant women with hyperemesis gravidarum Setting: Studies in Croatia (1) and UK (1) Intervention: Acupuncture and acupressure Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Acupuncture and acupressure | |||||
| Quality of life: anxiodepressive symptomatology | Study population | RR 1.01 (0.73 to 1.40) | 36 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Absolute effects calculated from results of single study. | |
| 800 per 1000 | 808 per 1000 (584 to 1000) | |||||
| Spontaneous abortion | Study population | RR 0.48 (0.05 to 5.03) | 57 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | Absolute effects calculated from results of single study. | |
| 71 per 1000 | 34 per 1000 (4 to 359) | |||||
| Preterm birth less than 37 weeks | Study population | RR 0.12 (0.01 to 2.26) | 36 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | Absolute effects calculated from results of single study. | |
| 154 per 1000 | 18 per 1000 (2 to 348) | |||||
| Stillbirth and neonatal death | Study population | RR 0.57 (0.04 to 8.30) | 36 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | Absolute effects calculated from results of single study. | |
| 77 per 1000 | 44 per 1000 (3 to 638) | |||||
| 1. Severity, reduction or cessation in nausea/vomiting 2. Number of episodes of emesis 3. Days of hospital admission 4. Intervention side effects |
Not reported | Not estimable | Studies included in this comparison did not report these review outcomes | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Single study with design limitations contributing data
2 Small sample size and wide 95% CIs crossing the line of no effect
3 Small sample size, low event rate and wide 95% CIs crossing the line of no effect
Summary of findings 2. Acupuncture versus metoclopramide.
| Acupuncture versus metoclopramide | ||||||
| Patient or population: pregnant women with hyperemesis gravidarum Setting: Study in Italy Intervention: Acupuncture Comparison: Metoclopramide | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with metoclopramide | Risk with Acupuncture | |||||
| Reduction or cessation in nausea | Study population | RR 1.40 (0.79 to 2.49) | 81 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Absolute effects calculated from results of single study. | |
| 316 per 1000 | 442 per 1000 (249 to 786) | |||||
| Reduction or cessation in vomiting | Study population | RR 1.51 (0.92 to 2.48) | 81 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Absolute effects calculated from results of single study. | |
| 368 per 1000 | 556 per 1000 (339 to 914) | |||||
| 1. Number of episodes of emesis 2. Days of hospital admission 3. Intervention side effects 4. Quality of life outcomes 5. Pregnancy complications 6. Adverse fetal/neonatal outcomes |
Not reported | Not estimable | The study included in this comparison did not report these review outcomes. | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Single study with design limitations contributing data
2 Small sample size and wide 95% CIs crossing the line of no effect
Summary of findings 3. Pyridoxine versus placebo.
| Pyridoxine versus placebo | ||||||
| Patient or population: pregnant women with hyperemesis gravidarum Setting: Study in Malaysia Intervention: Pyridoxine Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Pyridoxine | |||||
| Number of episodes of emesis | Mean episodes 1.4 | Mean episodes 1.9 | The mean number of episodes of emesis in the intervention group was 0.5 more (0.4 fewer to 1.4 more) | 66 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | The absolute effects were from a single study |
| Days of hospital admission | The mean days of hospital admission was 3.1 | The mean days of hospital admission was 3.9. | The mean days of hospital admission in the intervention group was 0.8 days more (0.08 more to 1.52 more) | 92 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | The absolute effects were from a single study |
| Interventions side effects: dizziness | Study population | RR 1.67 (0.85 to 3.26) | 66 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | The absolute effects were calculated from a single study | |
| 273 per 1000 | 455 per 1000 (232 to 889) | |||||
| Interventions side effects: headaches | Study population | RR 1.33 (0.52 to 3.42) | 66 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | The absolute effects were calculated from a single study | |
| 182 per 1000 | 242 per 1000 (95 to 622) | |||||
| Interventions side effects: diarrhoea | Study population | RR 3.00 (0.13 to 71.07) | 66 (1 RCT) | 3 | The absolute effects were calculated from a single study | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Interventions side effects: palpitations | Study population | RR 1.00 (0.22 to 4.60) | 66 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | The absolute effects were calculated from a single study | |
| 91 per 1000 | 91 per 1000 (20 to 418) | |||||
| Interventions side effects: dry mouth | Study population | RR 0.82 (0.49 to 1.38) | 66 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | The absolute effects were calculated from a single study | |
| 515 per 1000 | 422 per 1000 (252 to 711) | |||||
| 1. Severity, reduction or cessation in nausea/vomiting 2. Quality of life outcomes 3. Pregnancy complications 4. Adverse fetal/neonatal outcomes |
Not reported | Not estimable | The studies included in this comparison did not include these review outcomes | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Small sample size and wide 95% CIs crossing the line of no effect
2 Estimate based on single study with small sample size
3 Small sample size, low event rate and wide 95% CIs crossing the line of no effect
Summary of findings 4. Metoclopramide versus ondansetron.
| Metoclopramide versus ondansetron | ||||||
| Patient or population: pregnant women with hyperemesis gravidarum Setting: Studies in Iran (1) and Malaysia (1) Intervention: Metoclopramide Comparison: Ondansetron | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with ondansetron | Risk with Metoclopramide | |||||
| Severity of nausea | The mean severity score with ondansetron was 3.4 | The mean severity score with metoclopramide was 5.1 | The mean severity of nausea in the intervention group was 1.7 more (0.15 less to 3.55 more) | 83 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Absolute effects from single study |
| Severity of vomiting | The mean severity of vomiting score with ondansetron was 4.8 | The mean severity score with metoclopramide was 4.7. | The mean severity of vomiting in the intervention group was 0.1 less (1.63 less to 1.43 more) | 83 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Absolute effects from single study |
| Quality of life | The mean quality of life score with ondansetron was 8.7. | The mean quality of life score with metoclopramide was 8.3. | The mean quality of life in the intervention group was 0.4 less (0.83 less to 0.03 more) | 160 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | Absolute effects from single study |
| Intervention side effects: felt drowsy | Study population | RR 2.40 (1.23 to 4.69) | 160 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | Absolute effects calculated from single study | |
| 125 per 1000 | 300 per 1000 (154 to 586) | |||||
| Intervention side effects: unable to sleep | Study population | RR 1.29 (0.50 to 3.28) | 160 (1 RCT) | ⊕⊕⊝⊝ LOW 2 | Absolute effects calculated from single study | |
| 88 per 1000 | 113 per 1000 (44 to 287) | |||||
| Intervention side effects: dry mouth | Study population | RR 2.38 (1.10 to 5.11) | 160 (1 RCT) | ⊕⊕⊕⊝ MODERATE 3 | Absolute effects calculated from single study | |
| 100 per 1000 | 238 per 1000 (110 to 511) | |||||
| Intervention side effects: felt dizzy | Study population | RR 2.33 (0.94 to 5.77) | 160 (1 RCT) | ⊕⊕⊝⊝ LOW 2 | Absolute effects calculated from single study | |
| 75 per 1000 | 175 per 1000 (71 to 433) | |||||
| 1. Severity, reduction or cessation in nausea/vomiting 2.Days of hospital admission 3. Pregnancy complications 4. Adverse fetal/neonatal outcomes |
Not reported | Not estimable | The studies included in this comparison did not report these review outcomes | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Single study with design limitations contributing data
2 Small sample size and wide 95% CIs crossing the line of no effect
3 Estimate based on single study with small sample size
Summary of findings 5. Metoclopramide versus promethazine.
| Metoclopramide versus promethazine | ||||||
| Patient or population: Pregnant women with hyperemesis gravidarum Setting: Study in Malaysia Intervention: Metoclopramide Comparison: Promethazine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with promethazine | Risk with Metoclopramide | |||||
| Quality of life | The mean quality of life score was 7.1 | The mean quality of life score was 7.6 | The mean quality of life score in the intervention group was 0.5 more (0.22 less to 1.22 more) | 149 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Absolute effects from single study. |
| Intervention side effects: drowsy | Study population | RR 0.70 (0.56 to 0.87) | 143 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | Absolute effects calculated from single study. | |
| 836 per 1000 | 585 per 1000 (468 to 727) | |||||
| Intervention side effects: unable to sleep | Study population | RR 0.78 (0.40 to 1.53) | 143 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Absolute effects calculated from single study. | |
| 219 per 1000 | 171 per 1000 (88 to 335) | |||||
| Intervention side effects: dry mouth | Study population | RR 0.91 (0.62 to 1.34) | 143 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Absolute effects calculated from single study. | |
| 438 per 1000 | 399 per 1000 (272 to 587) | |||||
| Intervention side effects: felt dizzy | Study population | RR 0.48 (0.34 to 0.69) | 143 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | Absolute effects calculated from single study. | |
| 712 per 1000 | 342 per 1000 (242 to 492) | |||||
| Intervention side effects: diarrhea | Study population | RR 1.39 (0.32 to 5.99) | 143 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | Absolute effects calculated from single study. | |
| 41 per 1000 | 57 per 1000 (13 to 246) | |||||
| Intervention side effects: headache | Study population | RR 0.81 (0.47 to 1.38) | 143 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Absolute effects calculated from single study. | |
| 301 per 1000 | 244 per 1000 (142 to 416) | |||||
| 1. Severity, reduction or cessation in nausea/vomiting 2. Number of episodes of emesis 3. Days of hospital admission 4. Pregnancy complications 5. Adverse fetal/neonatal outcomes |
Not reported | Not estimable | The study included in this comparison did not report these review outcomes. | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Small sample size and wide 95% CIs crossing the line of no effect
2 Estimate based on single study with small sample size
3 Small sample size, low event rate and wide 95% CIs crossing the line of no effect
Summary of findings 6. Ondansetron versus promethazine.
| Ondansetron versus promethazine | ||||||
| Patient or population: Pregnant women with hyperemesis gravidarum Setting: Study in the USA Intervention: Ondansetron Comparison: Promethazine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with promethazine | Risk with Ondansetron | |||||
| Days of hospital admission | The mean days of hospital admission was 4.47 with promethazine | The mean days of hospital admission was 4.47 days with ondansetron | The mean difference in days of hospital admission in the intervention group was 0 (1.39 fewer to 1.39 more) | 30 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Absolute effect from one study |
| Intervention side effect: sedation | Study population | RR 0.06 (0.00 to 0.94) | 30 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | Absolute effects calculated from one study | |
| 533 per 1000 | 32 per 1000 (0 to 501) | |||||
| 1. Severity, reduction or cessation in nausea/vomiting 2. Number of episodes of emesis 3. Quality of life outcomes 4. Pregnancy complications 5. Adverse fetal/neonatal outcomes |
Not reported | Not estimable | The study included in this comparison did not report these review outcomes | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Single study with design limitations contributing data
2 Small sample size and wide 95% CIs crossing the line of no effect
3 Estimated based on single study with small sample size
Summary of findings 7. Corticosteroids versus promethazine.
| Corticosteroids versus promethazine | ||||||
| Patient or population: Pregnant women with hyperemesis gravidarum Setting: Studies in the USA (1) and Iran (1) Intervention: Corticosteroids Comparison: Promethazine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with promethazine | Risk with Corticosteroids | |||||
| Severe nausea 48 hours | Study population | RR 2.00 (1.08 to 3.72) | 80 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | Absolute estimates calculated from single study | |
| 250 per 1000 | 500 per 1000 (270 to 930) | |||||
| Severe nausea 17th day | Study population | RR 0.81 (0.58 to 1.15) | 78 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | Absolute estimates calculated from single study | |
| 692 per 1000 | 561 per 1000 (402 to 796) | |||||
| Episodes of vomiting 48 hours | Study population | RR 3.00 (0.33 to 27.63) | 80 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | Absolute estimates calculated from single study | |
| 25 per 1000 | 75 per 1000 (8 to 691) | |||||
| Episodes of vomiting 17th day | Study population | RR 1.00 (0.21 to 4.65) | 78 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | Absolute estimates calculated from single study | |
| 77 per 1000 | 77 per 1000 (16 to 358) | |||||
| Intervention side effects: drowsiness 48hrs and 3‐10 days | Study population | RR 0.08 (0.00 to 1.32) | 80 (1 RCT) | ⊕⊕⊝⊝ LOW 1 5 | Absolute estimates calculated from single stud | |
| 150 per 1000 | 12 per 1000 (0 to 198) | |||||
| Stillbirth and neonatal death | RR 3.00 (0.13 to 69.52) | 40 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | Low event rate. Absolute estimate not calculated | ||
| Preterm birth | RR 3.00 (0.13 to 69.52) | 40 (1 RCT) | ⊕⊕⊝⊝ LOW 4 | Low event rate. Absolute estimate not calculated | ||
| 1. Days of hospital admission 2. Quality of life outcomes 3. Pregnancy complications |
Not reported | Not estimable | The studies included in this comparison did not report these review outcomes | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Single study with design limitations contributing data
2 Estimate based on single study with small sample size
3 Small sample size and wide 95% CIs crossing the line of no effect
4 Small sample size, low event rate and wide 95% CIs crossing the line of no effect
5 Estimate based on single study with small sample size and low event rate
Summary of findings 8. Corticosteroids versus placebo.
| Corticosteroids versus placebo | ||||||
| Patient or population: Pregnant women with hyperemesis gravidarum Setting: Studies in the USA (2) and UK (1) Intervention: Corticosteroids Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Corticosteroids | |||||
| Days of hospital admission | The mean days of hospital admission was 2.2 days | The mean days of hospital admission was 1.9 days | The mean days of hospital admission in the intervention group was 0.3 days fewer (0.7 fewer to 0.1 more) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Absolute effects from a single study |
| Pregnancy complications | Study population | RR 0.61 (0.26 to 1.47) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | Absolute effects calculated from a single study | |
| 204 per 1000 | 124 per 1000 (53 to 299) | |||||
| Spontaneous abortion | Study population | RR 0.64 (0.11 to 3.70) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | Absolute effects calculated from a single study | |
| 56 per 1000 | 36 per 1000 (6 to 206) | |||||
| Stillbirth and neonatal death | Study population | RR 0.70 (0.15 to 3.34) | 134 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | Absolute effects calculated from a single study | |
| 45 per 1000 | 32 per 1000 (7 to 152) | |||||
| Congenital abnormalities | Study population | RR 0.32 (0.01 to 7.73) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | Absolute effects calculated from a single study | |
| 19 per 1000 | 6 per 1000 (0 to 143) | |||||
| Intervention side effects | Study population | RR 0.79 (0.06 to 11.20) | 25 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | Absolute effects calculated from a single study | |
| 91 per 1000 | 72 per 1000 (5 to 1000) | |||||
| Preterm birth | Study population | RR 1.01 (0.31 to 3.28) | 134 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 4 5 | Absolute effects based on inconsistent findings in 2 studies | |
| 121 per 1000 | 122 per 1000 (38 to 398) | |||||
| 1. Severity, reduction or cessation in nausea/vomiting 2. Number of episodes of emesis 3. Quality of life outcomes |
Not reported | Not estimable | The studies included in this comparison did not report these review outcomes | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Single study with design limitations contributing data
2 Small sample size and wide 95% CIs crossing the line of no effect
3 Small sample size, low event rate and wide 95% CIs crossing the line of no effect
4 Data from studies with design limitations
5 Inconsistent effect in the two studies contributing data
Background
Description of the condition
While nausea and vomiting in early pregnancy are very common, affecting approximately 80% of pregnancies, hyperemesis gravidarum is a severe form affecting 0.3% to 1.0% of pregnancies (Gadsby 1993; Niebyl 2010). The definition of hyperemesis gravidarum varies but generally includes intractable nausea/vomiting, signs of dehydration such as ketonuria, high urine specific gravity, electrolyte imbalances, and weight loss of at least 5% of pre‐pregnancy weight, excluding other diagnoses (ACOG 2004; Mella 2011). The onset is generally in the first trimester at six to eight weeks, peaking by 12 weeks, with most women having resolution of symptoms by 20 weeks' gestation (Jarvis 2011). The lack of standard criteria has implications for inclusion criteria and outcome measurements of controlled studies. For example, requirement of at least 5% weight loss is not always used as an inclusion criteria in studies of interventions for hyperemesis gravidarum, but one study found that the efficacy of corticosteroids may vary depending on this criterion (Moran 2002).
It is important to exclude other causes of severe nausea and vomiting before arriving at the diagnosis of hyperemesis gravidarum. Other causes include gastrointestinal (GI) etiologies such as infection, gastritis, cholecystitis, hepatitis, appendicitis, and pancreatitis. Neurological causes include migraines or other central nervous system diseases. Genitourinary etiologies include urinary tract infection/pyelonephritis. Metabolic or endocrine disturbances include hypercalcemia, Addisons's disease, thyrotoxicosis. Psychological disorders include the spectrum of eating disorders. Finally, other pregnancy‐associated conditions such as molar pregnancy must also be excluded (Ismail 2007; Mella 2011).
The epidemiology of hyperemesis gravidarum is generally young women, primiparous, non‐smokers, and non‐Caucasian (Bailit 2005; Klebanoff 1985; Niebyl 2010). Other risk factors include prior history of hyperemesis, pre‐existing diabetes, hyperthyroid disorder, depression or psychiatric illness, asthma, and GI disorders (Fell 2006). Fetal abnormalities such as triploidy and hydrops have also been associated with hyperemesis (Kelly 2009). The etiology of hyperemesis gravidarum is poorly understood, although it is generally thought to be associated with hormonal changes associated with pregnancy. Postulated mechanisms include human chorionic gonadotropin stimulating secretory processes in the upper GI tract and/or stimulation of the thyroid stimulating hormone receptor. Estrogen levels have also been positively associated with nausea and vomiting in pregnancy, perhaps through delayed GI motility and gastric emptying. Physiological stimulation of the thyroid gland in early pregnancy causes a transient thyrotoxicosis that may lead to hyperemesis. Several studies have found a significant increase in Helicobacter pylori (H. pylori) infection among women with hyperemesis, although whether this is a cause, risk factor, or consequence of hyperemesis is not well established (Ismail 2007; Kelly 2009).
Hyperemesis gravidarum has both maternal and fetal complications. Although hyperemesis gravidarum is rarely a source of mortality, it is a significant source of morbidity. It is the most common indication for hospitalization in early pregnancy, and the second most common indication for hospitalization in pregnancy (ACOG 2004). Malnutrition and vitamin deficiencies may lead to anemia and peripheral neuropathies, or more serious, but rare, complications such as Wernicke's encephalopathy and central pontine myelinolysis. Prolonged vomiting may lead to esophageal trauma such as Mallory‐Weiss tears. Nausea and vomiting in early pregnancy are associated with psychiatric morbidity. Although a causal relationship is uncertain, the severity of nausea and vomiting has been correlated with somatic symptoms, social dysfunction, anxiety, insomnia, and severe depression (Ismail 2007; Kramer 2013; Mella 2011; Swallow 2004). There may also be significant psychosocial morbidity associated with hyperemesis. Multiple studies have demonstrated an association with decreased psychosocial well‐being, depression, and anxiety (ACOG 2004; Munch 2011; Poursharif 2008). The physical and psychological/social burden of hyperemesis gravidarum has also been associated with termination of pregnancy (ACOG 2004; Poursharif 2007). Fetal complications include preterm birth (delivery less than 37 weeks' gestation), low birthweight (generally less than 2.5 kg), and small‐for‐gestational age (less than the 10th percentile of expected weight for gestational age). There does not appear to be an increased risk of spontaneous abortion (usually defined as less than 20 weeks), stillbirth (death of a fetus >= 20 weeks' gestation or greater than 500 g), or neonatal death (death of a baby born live within 28 days of birth) (Bailit 2005; Dodds 2006). The socioeconomic costs of hyperemesis are also significant, stemming from individual expense in paying for treatment, lost job productivity from time off work, and high healthcare costs related to provision of services and hospital admissions. One study found that the cost of hyperemesis was about $200,000,000 (USD) per year for the United States (Bailit 2005). Studies in Canada have estimated that severe nausea and vomiting in pregnancy result in as many as 14 hospitalizations/1000 births, and has a cost of $653/woman/week (CAD) (Neutel 2000; Piwko 2007).
Description of the interventions and how they might work
A range of interventions are commonly used for the treatment of hyperemesis gravidarum. These include dietary and lifestyle modifications, complementary therapies (i.e. acupuncture, herbal remedies), pharmaceutical therapies including a variety of classes of antiemetics and corticosteroids, and enteral/parenteral nutrition. The goals of therapy are generally to reduce nausea and vomiting, minimize hospitalization, prevent progression of symptom severity, and improve quality of life. Prior studies examining intervention efficacy have used subjective measures of nausea/vomiting such as visual analogue scales (Sullivan 1996; Tan 2009) and the Rhodes Index of Nausea, Vomiting, and Retching (Rhodes 1984; Rhodes 1999; Rosen 2003; Shin 2007), quantitative measures such as days of hospital admission and readmission rates, and quality of life measures such as the General Health Questionnaire (Swallow 2004), and the Edinburgh Postpartum Depression Screen (Bown 2008; Cox 1987; Kramer 2013). Secondary outcomes often include adverse maternal and fetal outcomes. It can be difficult to extrapolate safety data from trials designed to examine efficacy because they may not be powered to detect such outcomes, and it is difficult to determine whether certain outcomes, such as preterm delivery, are related to the intervention or the condition of hyperemesis. However, given that some adverse outcomes, such as congenital abnormalities, are not associated with hyperemesis, data on some specific outcomes may be used to draw conclusions on safety.
Non‐pharmacological interventions
Dietary and lifestyle modifications
Dietary modifications include recommendations to have small and frequent meals, avoid spicy or fatty foods, and drink fluids regularly. Lifestyle modifications include avoiding noxious sensory stimuli, eating crackers in the morning after waking, and increasing rest. Although these are common recommendations, there are few published studies evaluating the efficacy of these changes for prevention or treatment of nausea/vomiting of pregnancy (ACOG 2004; Arsenault 2002; Matthews 2015).
Complementary therapies
There are a number of non‐pharmacological therapies that have been used for the treatment of nausea and vomiting in pregnancy and hyperemesis gravidarum. Acupressure and electrical stimulation wrist bands have been associated with benefit for nausea/vomiting of early pregnancy, although the evidence is mixed and limited (Heazell 2006; Ismail 2007; Matthews 2015; Mella 2011; Rosen 2003; Shin 2007). Acupuncture has also been shown to have some benefit in the treatment of nausea and vomiting in pregnancy although again, the evidence is limited (ACOG 2004; Carlsson 2000; Mella 2011). A Cochrane review evaluating its efficacy in nausea and vomiting in early pregnancy found one study that demonstrated an improvement in severity of nausea and vomiting (Matthews 2015). These methods are based on traditional Chinese medicine that specifies a point PC6 5 cm proximal to the wrist crease that is associated with decreasing nausea. Acupuncture and other stimulation at this point has been suggested to reduce opioid‐related post operative nausea as well as chemotherapy‐associated nausea (Carlsson 2000).
Ginger
Ginger is another commonly recommended non‐pharmacological intervention for the treatment of nausea and vomiting in pregnancy. The active ingredient in ginger responsible for its therapeutic effect is not well understood but it has long been used as a herbal medicine in Asian culture for the treatment of nausea and vomiting in pregnancy. Several randomized controlled trials have demonstrated a benefit of ginger in nausea and vomiting of pregnancy without any demonstrable adverse pregnancy outcomes (Arsenault 2002; Matthews 2015; Mella 2011).
Intravenous fluids/enteral nutrition/parenteral nutrition
Hyperemesis gravidarum is commonly characterized by metabolic and electrolyte disturbance requiring hospital admission, with the initial therapy frequently being intravenous rehydration/repletion of electrolytes. (ACOG 2004).
Both enteral and parenteral nutrition are used in refractory hyperemesis gravidarum. Complications of enteral nutrition can include infection, bleeding, tube dislodgement, preterm labor, and discomfort for the woman (ACOG 2004; Saha 2009). Parenteral nutrition is associated with a high incidence of complications including infection, thrombosis, and mechanical failure, and therefore is recommended only in the failure of medical management and enteral nutrition (ACOG 2004; Holmgren 2008).
Pharmacological interventions
A number of different classes of pharmaceutical agents have been evaluated for the treatment of hyperemesis gravidarum.
Vitamin B6
Vitamin B6 or pyridoxine is commonly used as a first line treatment for nausea and vomiting in pregnancy. It is a water soluble vitamin used as a cofactor in a wide array of metabolic processes and in the synthesis of nucleic acids and some neurotransmitters. Used on its own, it is associated with a decrease in nausea but not in vomiting (Mella 2011). Vitamin B6 has not been shown to cause increased risk in major or minor congenital malformations (Arsenault 2002; Mazzotta 2000).
Antihistamines
Antihistamines may act through different mechanisms. Doxylamine is a H‐1 receptor antagonist that had been used frequently in combination with B6. When the combination B6 and doxylamine was available in the United States, there was an association with decreased admissions for hyperemesis, however it was removed from the market secondary to safety concerns that were later unfounded (ACOG 2004; Ismail 2007). The combination of doxylamine/B6 has been found to be both safe, with no evidence of teratogenicity, and effective in the treatment of nausea and vomiting in pregnancy (Arsenault 2002; Mazzotta 2000; Mella 2011).
H1‐receptor antagonists such as doxylamine, hydroxyzine, and diphenhydramine are thought centrally to reduce vestibular symptoms. There is one randomized controlled trial showing that diphenhydrinate is as effective as ginger in the treatment of nausea and vomiting of pregnancy (Pongrojpaw 2007).
H2‐receptor antagonists such as famotidine and ranitidine act peripherally in reducing reflux, which may help with reducing symptoms of nausea and vomiting, although this has not been well studied either.
A meta‐analysis of antihistamines showed no increased risk of congenital malformations, risk of miscarriage, or preterm delivery (Gill 2009; Mella 2011).
Dopamine antagonists
Dopamine‐2 antagonists such as metoclopramide stimulate GI motility and have been shown to be effective in decreasing vomiting. Limited studies have demonstrated its safety in pregnancy (Arsenault 2002; Mella 2011). Phenothiazines, such as promethazine, are dopamine 2‐receptor antagonists that act centrally to suppress the chemoreceptor trigger zone (CTZ) that is responsible for stimulating vomiting. These have been shown to be safe in pregnancy with regards to teratogenicity (Arsenault 2002).
Benzodiazepines
Benzodiazepines such as diazepam are thought to be helpful in the condition of hyperemesis gravidarum, presumably through alleviating psychosomatic symptoms such as anxiety. However, the safety of these medications in pregnancy is still controversial with some studies demonstrating a positive association between neonatal exposure to diazepam and prematurity and low birth weight (Mella 2011; Tasci 2009).
Serotonin antagonists
Serotonin antagonists such as ondansetron also act centrally to suppress the CTZ. Safety data are limited, animal studies and small case studies have not demonstrated any teratogenic effect (Mazzotta 2000). Recently, a large retrospective cohort study in Denmark found no association between ondansetron and adverse fetal outcomes (Pasternak 2013). Despite the limited safety and efficacy data, its efficacy in treating chemotherapy‐associated nausea/vomiting has led to increased use of this medication (ACOG 2004).
Corticosteroids
Corticosteroids are often used as a last resort for treatment of refractory hyperemesis. They have been used for the treatment of chemotherapy‐associated nausea and are postulated to modify the CTZ. However, their use in early pregnancy has been associated with oral cleft malformations, so it is generally reserved as a last resort intervention (ACOG 2004; Arsenault 2002; Ismail 2007; Mazzotta 2000).
Why it is important to do this review
Although a recent Cochrane review examined the efficacy and safety of many of these interventions for nausea/vomiting of early pregnancy (Matthews 2015), there has not yet been a review assessing interventions for the more severe condition of hyperemesis gravidarum.
Objectives
To assess the effectiveness as well as maternal and fetal safety of all published interventions for hyperemesis gravidarum.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials of any intervention for hyperemesis gravidarum. We included randomized controlled trials reported in abstract, provided that there was sufficient information in the abstract or available from the author to allow us to assess eligibility and risk of bias. We excluded quasi‐randomized trials and trials using a cross‐over design. Multi‐armed trials were included and pair‐wise comparison were conducted separately.
Types of participants
Pregnant women with a normal intrauterine pregnancy up to 20 weeks' gestation diagnosed with hyperemesis gravidarum according to the definition of the trials.
Types of interventions
We included all published interventions for hyperemesis gravidarum. Each intervention (i.e. acupressure or ondansetron) was analyzed separately versus placebo or no treatment, and versus other interventions. Compound interventions (i.e. ondansetron and metoclopramide) were treated as single unique interventions.
Types of outcome measures
For the sake of comparison, some outcome measures for this review align with the outcome measures used in the previous Cochrane review on interventions for nausea and vomiting in early pregnancy (Matthews 2015). The time frame for follow‐up of outcome measures, including maternal and neonatal safety data, was defined by individual trials. The outcomes below are slightly different from what was initially published in the protocol for this review. Severity of nausea/vomiting was added as a primary outcome because it was found that this was often what was reported in the included studies. Similarly, rather than reporting the number of women requiring additional antiemetics, the outcome "number of antiemetics required" was used instead as this was more often reported.
Primary outcomes
Intervention efficacy
Severity, reduction, or cessation in nausea/vomiting
Number of episodes of emesis
Days of hospital admission
Secondary outcomes
Intervention efficacy
Hospital readmission
Number of women requiring additional antiemetics
Need for enteral or parenteral nutrition
Adverse maternal outcomes
Pregnancy complications (i.e. antepartum hemorrhage, pre‐eclampsia, gestational hypertension)
Weight loss
Adverse fetal/neonatal outcomes
Spontaneous abortion
Stillbirth and neonatal death
Congenital abnormalities
Low birthweight
Preterm birth
Quality of life
Quality of life outcomes including emotional, psychological, and physical well‐being
Intervention side effects
Decision to terminate the pregnancy
Economic costs
Direct financial costs to women
Productivity costs
Healthcare system costs
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s (PCG) Trials Register by contacting the Trials Search Co‐ordinator (20 December 2015).
The Register is a database containing over 20,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the PCG Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane PCG Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific PCG review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
In addition, we contacted the Cochrane Complementary Medicine Field to search their Trials Register (20 September 2014) and checked again via The Cochrane Register of Studies (CRSO) (20 December 2015) (see: Appendix 1).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors (RB and SB) independently assessed for inclusion all the potential studies we identify as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author (AK).
Data extraction and management
We designed a form to extract data. For eligible studies, three review authors (RB, SB, GS) extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a fourth author (AK). We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (RB and SB) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving an additional assessor (AK).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings.
Assessment of the quality of the evidence using the GRADE approach
For this update the quality of the evidence has been assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes.
Severity, reduction or cessation in nausea/vomiting
Number of episodes of emesis
Days of hospital admission
Intervention side effects
Quality of life outcomes including emotional, psychological, and physical well‐being
Pregnancy complications (i.e. antepartum hemorrhage, pre‐eclampsia, gestational hypertension)
Adverse fetal/neonatal outcomes (i.e. spontaneous abortion, stillbirth and neonatal death, congenital abnormalities, low birthweight, preterm birth)
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create 'Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Where reported, the above seven outcomes have been set out in 'Summary of findings' tables. Other important outcomes such as hospital readmission, pregnancy termination and the use of additional antiemetics have been described in full in the results section.
A broad range of interventions for hyperemesis gravidarum were examined in the included trials and so to summarize findings, we selected those non‐pharmacological and pharmacological comparisons that we considered to be most clinically relevant. Findings for nine different comparisons have been set out in the 'Summary of findings' tables.
Acupuncture versus placebo
Acupuncture versus metoclopramide
Pyridoxine versus placebo
Metoclopramide versus ondansetron
Hydrocortisone versus metoclopramide
Metoclopramide versus promethazine
Ondansetron versus promethazine
Corticosteroids versus promethazine
Corticosteroids versus placebo
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials and the standardized mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomized trials
We planned to include cluster‐randomized trials in the analyses along with individually‐randomized trials, however all included studies were individually‐randomized trials.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis, however because most trials had a unique comparison and we were unable to group most included studies, therefore a sensitivity analysis was not performed.
For all outcomes, we will carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, we presented the results as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
The majority of our analyses are based on data from single studies with small numbers of participants. If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. In future updates, if more trials are included, we will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses:
women with weight loss of at least 5% of pre‐pregnancy weight versus women with weight loss of less than 5% of pre‐pregnancy weight;
singleton gestation versus twin gestations;
primiparous versus multiparous.
We planned to use the primary outcomes in subgroup analysis.
Severity, reduction, or cessation in nausea/vomiting. We will examine outcomes measured by all commonly used instruments to assess nausea and vomiting.
Number of episodes of emesis.
Days of hospital admission.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014). However there were insufficient data to conduct a subgroup analysis.
Sensitivity analysis
We planned to perform sensitivity analysis in trials found to have a high or unclear risk of attrition bias, and high or unclear risk of other biases. However, because most trials were unique comparisons we did not carry out a sensitivity analysis.
Results
Description of studies
Results of the search
See: Figure 1).
1.
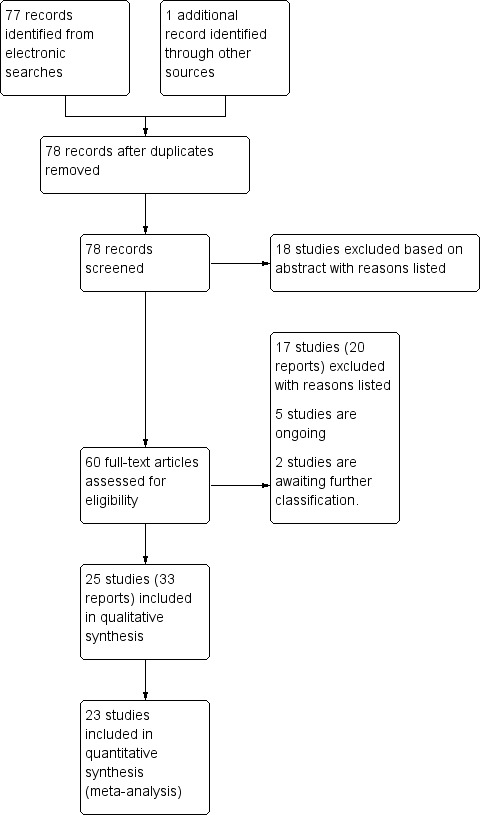
Study flow diagram.
The search strategy identified 78 total reports representing 67 distinct studies (some studies were resulted in more than one publication). Of these 67 studies, 25 met inclusion criteria for the review, 35 were excluded, two are awaiting translation (and are listed in Characteristics of studies awaiting classification) and five studies are ongoing (see Characteristics of ongoing studies).
Included studies
Twenty‐five studies (involving 2052 women) met inclusion criteria. The included studies examined a range of interventions.
Non‐pharmacological interventions
Acupuncture or acupressure at the P6 (or Neigun) point was examined in seven studies; in five studies it was compared with placebo (Habek 2004; Heazell 2006; Mamo 1995; Miller 2001; Shin 2007); in one study it was compared with metoclopramide (Neri 2005); and in one study it was compared with Western medicine (intravenous fluids, electrolyte repletion and phenobarbital) and Chinese medicine (Mao 2010). One study compared the efficacy of progressive muscle relaxation with pharmacotherapy versus pharmacotherapy alone (Gawande 2011). Pharmacotherapy in this case consisted of the progressive use of doxylamine succinate, ondansetron, metoclopramide, and promethazine. One study evaluated intravenous hydration with dextrose saline versus normal saline (Tan 2013). One study compared midwife‐led outpatient care versus routine care with inpatient admission (McParlin 2008). One study examined holistic assessment with individualized care plan and support and advice from nurses versus standard care (Fletcher 2015). There were no randomized controlled trials on hyperemesis gravidarum that examined other dietary or lifestyle modifications, or the use of ginger.
Pharmacological interventions
All studies recruited women with hyperemesis gravidarum or severe nausea and vomiting of pregnancy, as defined by the authors. The spectrum of severe symptoms necessary for inclusion in these studies varied but included failure of outpatient therapy, need for inpatient admission, ketonuria, weight loss, electrolyte imbalance. The gestational age of pregnancy at which women were recruited was generally in the first and second trimester (less than 20 weeks), although one study included women up to 30 weeks, however none of the women recruited were beyond 12 weeks (Shin 2007).
Most studies collected data on the severity, reduction, or cessation in nausea and vomiting. However, pooling data was complicated by variations in reporting and the individual time frames used. Most studies used a 10‐point visual analogue scale (VAS) for the severity of nausea and/or vomiting, where a higher number represented more severe symptoms (Abas 2014; Ditto 1999; Kashifard 2013; Nelson‐Piercy 2001; Sullivan 1996; Tan 2009; Tan 2010; Tan 2013; Ziaei 2004). Other studies used individualized measures such as a scale of zero to two for nausea/vomiting/food intake/functioning (Neri 2005), and a “severity” and “relief” scoring system developed by the authors of Ylikorkala 1979. Other scales such as the “Hyperemesis Impact of Symptoms Questionnaire” (Fletcher 2015), the “Pregnancy Unique Quantification of Emesis and Vomiting” (McParlin 2008), and the “Rhodes Index of Nausea, Vomiting, and Retching” (Miller 2001; Shin 2007) were also used. A number of studies reported on the number of episodes of vomiting (Abas 2014; Bondok 2006; Kashifard 2013; Tan 2009; Tan 2010; Tan 2013; Ziaei 2004). Other measures of nausea and vomiting that we did not analyze included recurrence of vomiting (Duggar 2001), number of antiemetics required and days required to achieve no vomiting (Gawande 2011), lack of need for medication (Habek 2004), number of antiemetic doses and need for additional antiemetics (Heazell 2006), need for antiemetic medication (Mamo 1995), and therapy failure defined by persistent vomiting (more than five times/day), inability to tolerate liquids by mouth, or the impression that the woman was not better (Safari 1998). In this review we chose to describe outcomes relating to women’s nausea and vomiting at the time points reported by the study. In addition to the severity of nausea/vomiting and number of episodes of emesis, our primary outcomes also included the number of days of hospital admission, which was reported in several studies (Abas 2014; Ditto 1999; Heazell 2006; McParlin 2008; Nelson‐Piercy 2001; Sullivan 1996; Tan 2009; Tan 2010; Tan 2013; Yost 2003).
Our secondary outcomes included intervention efficacy, which included the number of women requiring additional antiemetics, hospital readmission rate, and the need for enteral or parenteral nutrition. The number of women requiring additional antiemetics was reported in a few studies (Ditto 1999; Habek 2004; Nelson‐Piercy 2001; Safari 1998). Hospital readmission was reported by several studies (Bondok 2006; Ditto 1999; Duggar 2001; Nelson‐Piercy 2001; Safari 1998; Tabatabaii 2008; Tan 2009; Ylikorkala 1979; Yost 2003). Only Bondok 2006 reported on the need for enteral or parenteral nutrition.
Other secondary maternal and neonatal outcomes included adverse pregnancy outcomes, pregnancy complications, and maternal weight loss. A number of studies reported on adverse pregnancy outcomes and complications (Ditto 1999, Nelson‐Piercy 2001, Heazell 2006, McParlin 2008, Safari 1998, Yost 2003). A few studies reported change in weight (Nelson‐Piercy 2001; Sullivan 1996; Tan 2009; Ylikorkala 1979). Another secondary outcome was quality of life, which included measures of quality of life, intervention side effects, and the decision to terminate the pregnancy. A few studies that evaluated quality of a life with a variety of measures including a Clinical Global Improvement score (Gawande 2011), Anxiodepressive Symptom Index (Habek 2004), pregnancy‐unique quantification of emesis and nausea (PUQE) score (McParlin 2008), well‐being rating (Nelson‐Piercy 2001; Tan 2009; Tan 2010; Tan 2013), well‐being verbal numeric rating scale (VNRS) (Abas 2014), and Short Form (36) Health Survery (McParlin 2008). Several studies reported on the number of women who decided to terminate the pregnancy (Ditto 1999; Gawande 2011; Heazell 2006; McParlin 2008; Nelson‐Piercy 2001; Safari 1998). Several studies also reported on intervention side effects (Abas 2014; Duggar 2001; Kashifard 2013; Sullivan 1996; Tan 2009; Tan 2010; Tan 2013; Ziaei 2004). The final secondary outcome was economic costs, which were only reported in one study (McParlin 2008).
Studies awaiting further assessment and ongoing studies
There are two studies awaiting further classification. Translations were not available for He 2009 or Eftekhari 2013.
There are five ongoing studies. Cyna 2008 is a randomized controlled trial examining the efficacy of hypnosis on hyperemesis and is still recruiting. One randomized controlled trial (Mehrolhasani 2012) evaluated demitron versus promethazine in the treatment of hyperemesis gravidarum; according to the trial registry, recruitment has been completed, but no results were found. We have contacted the authors for information. Guttuso 2014 is a randomized trial comparing gabapentin and metoclopramide that is currently recruiting. Mitchell‐Jones 2014 is a randomized controlled trial comparing inpatient versus outpatient management of severe nausea and vomiting of pregnancy that is also currently recruiting. Finally, Koren 2014 is an ongoing multicenter randomized controlled trial evaluating the safety and efficacy of doxylamine succinate and pyridoxine hydrochloride (trade name Diclegis) in nausea and vomiting of pregnancy; the inclusion criteria does not specify hyperemesis gravidarum but there is no exclusion of severe nausea and vomiting so the final study results may include a subgroup of women with hyperemesis.
Excluded studies
After assessment of study eligibility, we excluded 35 studies. The main reasons for exclusion were that the study was on nausea and vomiting of pregnancy and not hyperemesis gravidarum (19 studies), or the study used a cross‐over design (five studies). Two studies were quasi‐randomized, and in one study it was unclear whether the study was randomized or quasi‐randomized. Two studies were not randomized controlled trials. Three studies were not reports on trials. Finally, three studies were on prophylactic treatment for prevention rather than treatment of the condition and as such were excluded.
Risk of bias in included studies
Sequence generation (selection bias)
In 10 of the included studies, the methods used to randomize women were not described or were unclear (Duggar 2001; Gawande 2011; Habek 2004; Mamo 1995; Mao 2010; McParlin 2008; Miller 2001; Sullivan 1996; Tabatabaii 2008; Ylikorkala 1979). The remainder of the studies were assessed to have adequate randomization methods. Eight studies used computer‐generated randomization list (Bondok 2006; Fletcher 2015; Kashifard 2013; Nelson‐Piercy 2001; Neri 2005; Safari 1998; Tan 2013; Yost 2003). Two studies used a random number table (Ditto 1999; Ziaei 2004). Only one study used coin toss (Shin 2007) as a method of randomization. Three studies used block randomization (Abas 2014; Tan 2009; Tan 2010), and one study used external randomization services (Heazell 2006).
Allocation
Fourteen of the included studies were unclear on allocation concealment (Ditto 1999; Duggar 2001; Habek 2004; Kashifard 2013; Mamo 1995; Mao 2010; McParlin 2008; Miller 2001;Shin 2007; Sullivan 1996; Tabatabaii 2008; Ylikorkala 1979; Yost 2003; Ziaei 2004). One study was deemed to have a high risk of bias in allocation concealment ‐ Gawande 2011 reported treating obstetricians were blinded to whether the women received a muscle relaxation session, however there was no report on concealment of allocation, and the control group received no placebo intervention. The remaining studies were judged to have adequate allocation concealment. Four studies used a code that was held by a third party: Bondok 2006 described using a withheld code and identical appearing interventions. Nelson‐Piercy 2001 used sequentially numbered trial packs with the copy of the allocation schedule held by the pharmacy, Neri 2005 used a code that was held under the control of a midwife, Safari 1998 used envelopes that were prepared in advance by a third party, although the envelopes were not specified to be opaque. Four studies described using opaque, sealed envelopes (Abas 2014; Tan 2009; Tan 2010; Tan 2013). One study described drawing ticket from an opaque bag (Heazell 2006).One study reported random allocation done remotely via telephone (Fletcher 2015).
Blinding
Blinding of participants was unclear or not described in 12 studies (Ditto 1999; Duggar 2001; Kashifard 2013; Mamo 1995; Mao 2010; McParlin 2008; Miller 2001; Safari 1998; Tabatabaii 2008; Tan 2009; Ylikorkala 1979; Ziaei 2004). There was a high risk of bias in three studies. Gawande 2011, as mentioned previously, had a control group of women called into the office but they received no kind of placebo muscle relaxation intervention. Similarly, in Fletcher 2015 participants were not blinded. Neri 2005 had a comparison group but no placebo intervention for acupuncture or acupressure. The remainder of the studies were deemed to have adequate participant blinding with masking of participants and similar appearing interventions.
Blinding of outcome assessors was unclear or not described in seventeen studies (Ditto 1999; Duggar 2001; Gawande 2011; Heazell 2006; Mamo 1995; Mao 2010; McParlin 2008; Miller 2001; Neri 2005; Shin 2007; Sullivan 1996; Tabatabaii 2008; Tan 2010; Tan 2013; Ylikorkala 1979; Yost 2003; Ziaei 2004). Fletcher 2015 primary outcomes were self reported and the participants were not blinded, thus was deemed to have a high risk of bias. The remainder of the studies were deemed to have adequately described blinding of investigators.
Incomplete outcome data
Attrition was either unclear or not reported in 13 studies (Bondok 2006; Ditto 1999; Duggar 2001; Habek 2004; Kashifard 2013; Mamo 1995; McParlin 2008; Miller 2001; Neri 2005; Shin 2007; Sullivan 1996; Tabatabaii 2008; Tan 2009). The remainder of the studies were deemed to have a low risk of attrition bias, with a low rate of attrition, accompanying reasons provided, and similar numbers lost to follow‐up in each comparison group.
Selective reporting
The risk of reporting bias was unclear or not described in six studies, five of which were abstracts in which there was not enough information to adequately judge the presence of bias (Duggar 2001; Mamo 1995; McParlin 2008; Miller 2001; Tabatabaii 2008). Mao 2010 reported outcomes as described in the methods, however it was unclear how the outcomes were measured, whether standard tools were used or not, as such, we considered the risk of reporting bias to be unclear. The remainder of the studies were deemed to be low risk of reporting bias with expected outcomes reported, or outcomes specified as being predetermined.
Other potential sources of bias
Most studies did not have enough information to adequately assess the presence other forms of bias and as such were deemed to have an unclear risk of bias. There was a high risk of bias in one study. Nelson‐Piercy 2001 reported that their trial was prematurely ended due to a combination of factors including departure of key staff members and "the erroneous belief that steroids had such a dramatic beneficial effect that continued randomization was not justified".
Figure 2 and Figure 3 provides a summary of our 'Risk of bias' assessment.
2.
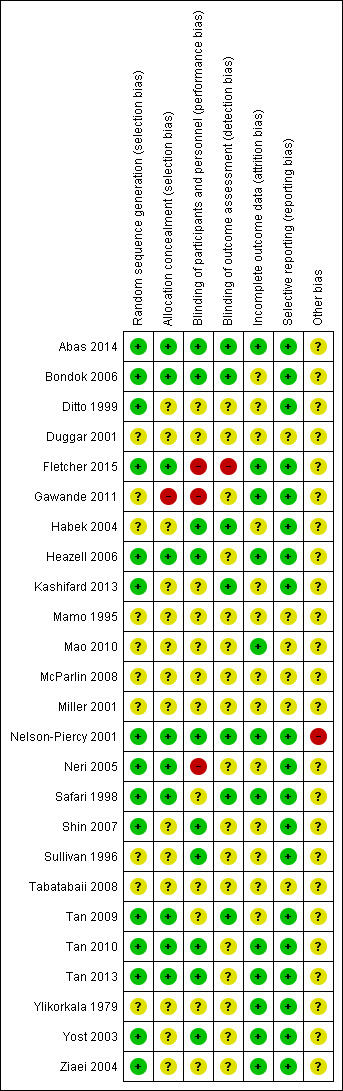
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
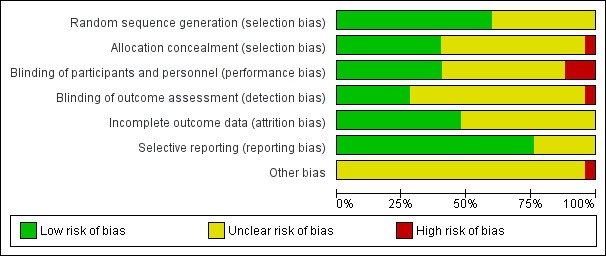
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
We included 25 studies (involving 2052 women) in this review but the majority of our analyses are based on data from single studies with small numbers of participants.
Acupunture/Acupressure
Acupuncture and acupressure versus placebo
Three studies (involving 182 women) compared P6 acupressure or acupuncture versus placebo and were included in the analysis.
Two additional studies were in abstract form only and did not have data that could be entered into the analysis. Miller 2001 compared nerve stimulation with a watch‐like device at P6 versus placebo and reported lower symptoms in the intervention group, without specific data reported. Mamo 1995 compared acupressure Sea‐band applied to each wrist versus control with no acupressure and reported more women required additional antiemetics than in the control group, again without specific data reported.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
With regards to this primary outcome, only one study (Shin 2007) reported a decreased mean nausea score (using Rhodes index of nausea vomiting or retching), however, no standard deviation was reported so this could not be entered into our data and analysis tables.
Number of episodes of emesis
None of the studies reported on the number of episodes of emesis.
Days of hospital admission
None of the studies reported on the number days of hospital admission.
Secondary outcomes
Regarding the secondary outcomes. The number of women requiring additional antiemetics was lower in the acupuncture/acupressure group compared to placebo (risk ratio (RR) 0.20, 95% confidence interval (CI) 0.08 to 0.50, one study (Habek 2004), 36 women (Analysis 1.1)). However, there was no difference between the treatment group and placebo control with regard to spontaneous abortion (RR 0.48, 95% CI 0.05 to 5.03, one study (Heazell 2006), 57 women (Analysis 1.2), low‐quality evidence), preterm birth less than 37 weeks (RR 0.12, 95% CI 0.01 to 2.26, one study (Heazell 2006), 36 women (Analysis 1.3), low‐quality evidence), stillbirth or neonatal death (RR 0.57, 95% CI 0.04 to 8.30, one study (Heazell 2006), 36 women (Analysis 1.4), low‐quality evidence), decision to terminate the pregnancy (RR 0.72, 95% CI 0.18 to 2.95 (Heazell 2006), 57 women (Analysis 1.5)), or anxiodepressive symptomology (RR 1.01, 95% CI 0.73 to 1.40, one study (Habek 2004), 36 women (Analysis 1.6), very low‐quality evidence). (Findings for this comparison are set out in Table 1.)
1.1. Analysis.
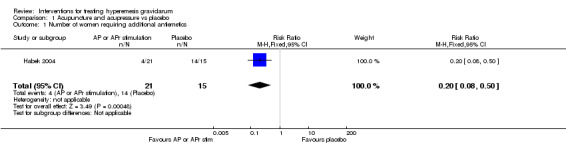
Comparison 1 Acupuncture and acupressure vs placebo, Outcome 1 Number of women requiring additional antiemetics.
1.2. Analysis.
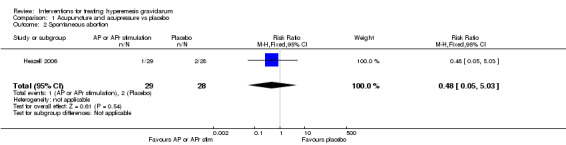
Comparison 1 Acupuncture and acupressure vs placebo, Outcome 2 Spontaneous abortion.
1.3. Analysis.
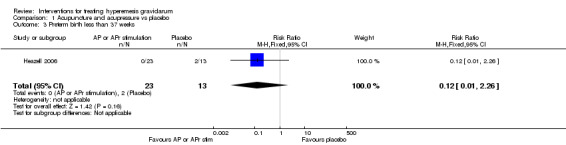
Comparison 1 Acupuncture and acupressure vs placebo, Outcome 3 Preterm birth less than 37 weeks.
1.4. Analysis.
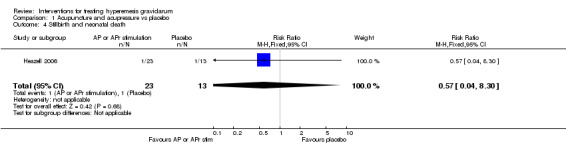
Comparison 1 Acupuncture and acupressure vs placebo, Outcome 4 Stillbirth and neonatal death.
1.5. Analysis.
Comparison 1 Acupuncture and acupressure vs placebo, Outcome 5 Decision to terminate the pregnancy.
1.6. Analysis.
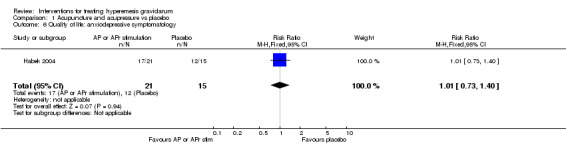
Comparison 1 Acupuncture and acupressure vs placebo, Outcome 6 Quality of life: anxiodepressive symptomatology.
Acupuncture versus metoclopramide
One study (Neri 2005, involving 81 women), evaluated the efficacy of acupuncture twice weekly versus metoclopramide infusion in the treatment of hyperemesis gravidarum. Findings for this comparison are set out in Table 2.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Regarding the primary outcome, after the cessation of the last treatment, the rate of women who experienced a reduction of nausea in the acupuncture group was no different from the metoclopramide group (RR 1.40, 95% CI 0.79 to 2.49 (Analysis 2.1), very low‐quality evidence) neither was the rate of women who experienced a reduction in vomiting (RR 1.51, 95% CI 0.92 to 2.48 (Analysis 2.2), very low‐quality evidence).
2.1. Analysis.
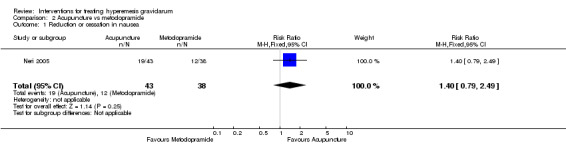
Comparison 2 Acupuncture vs metoclopramide, Outcome 1 Reduction or cessation in nausea.
2.2. Analysis.
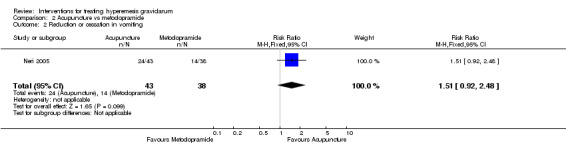
Comparison 2 Acupuncture vs metoclopramide, Outcome 2 Reduction or cessation in vomiting.
Number of episodes of emesis
There were no data available on number of episodes of emesis.
Days of hospital admission
There were no data available on the number of days of hospital admission.
Secondary outcomes
There were no data available on any of this review's secondary outcomes.
Acupuncture versus Western medicine (phenobarbital)
There was one study (Mao 2010), involving 90 women that evaluated acupuncture versus Western medicine. Both groups received fluid hydration and electrolyte repletion, Acupuncture was performed at BL11, ST37, PC6, SP4, RN12, and ST36, as determined by symptoms. The Western medicine group received 30 mg phenobarbital three times a day.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Results were reported as Complete Recovery: cessation of nausea and vomiting, normal appetite; Obvious Effects: reduction by at least 50% in frequency of nausea and vomiting, appetite improved; Effects Showed: reduction by 25% to 50% in nausea and vomiting, somewhat improved appetite; and Ineffective: reduction less than 25% in nausea/vomiting, no improvement in appetite. Total effectiveness rate was defined as the number of women with either Complete Recovery, Obvious Effects, or Effects Showed. At the end of one week of treatment, there were significantly more women with complete recovery and less women with ineffective therapy in the acupuncture versus the phenobarbital group (RR 6.75, 95% CI 2.69 to 16.94 (Analysis 3.1) and (RR 0.06, 95% CI 0.01 to 0.44 (Analysis 3.4)). Acupuncture was more likely to have any effectiveness (total effective rate) compared to phenobarbital (RR 2.07, 95% CI 1.40 to 3.05, (Analysis 3.5)).
3.1. Analysis.
Comparison 3 Acupunture vs Western medicine (Phenobarbital), Outcome 1 Complete recovery.
3.4. Analysis.
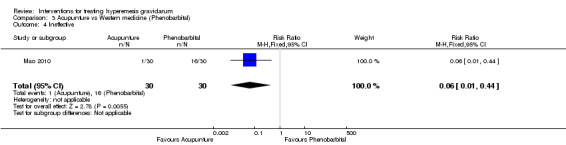
Comparison 3 Acupunture vs Western medicine (Phenobarbital), Outcome 4 Ineffective.
3.5. Analysis.
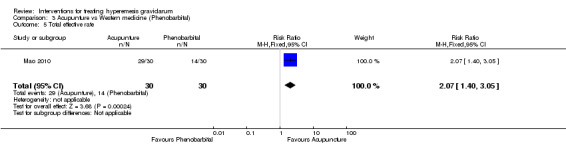
Comparison 3 Acupunture vs Western medicine (Phenobarbital), Outcome 5 Total effective rate.
Number of episodes of emesis
There were no data available on number of episodes of emesis.
Days of hospital admission
There were no data available on the number of days of hospital admission.
Secondary outcomes
There were no data available on any of this review's secondary outcomes.
Acupuncture versus Chinese medicine
There was one study (Mao 2010, involving 90 women) that evaluated acupuncture versus Chinese medicine. Both groups received fluid hydration and electrolyte repletion, Acupuncture was performed at BL11, ST37, PC6, SP4, RN12, and ST36, as determined by symptoms. Women in the Chinese medicine group received a selection of traditional Chinese medication according to the dialectical classification.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Results were reported as Complete Recovery: cessation of nausea and vomiting, normal appetite; Obvious Effects: reduction by at least 50% in frequency of nausea and vomiting, appetite improved; Effects Showed: reduction by 25% to 50% in nausea and vomiting, somewhat improved appetite; and 'Ineffective': reduction less than 25% in nausea/vomiting, no improvement in appetite. Total effectiveness rate was defined as the number of women with either Complete Recovery, Obvious Effects, or Effects Showed. At the end of one week of treatment, there were significantly more women with complete recovery and less women with ineffective therapy in the acupuncture versus Chinese medicine group (RR 9.00, 95% CI 3.06 to 26.51(Analysis 4.1) and (RR 0.08, 95% CI 0.01 to 0.60 (Analysis 4.4)). Acupuncture was more likely to have any effectiveness (total effective rate) than Chinese medicine (RR 1.61, 95% CI 1.19 to 2.17, (Analysis 4.5))
4.1. Analysis.
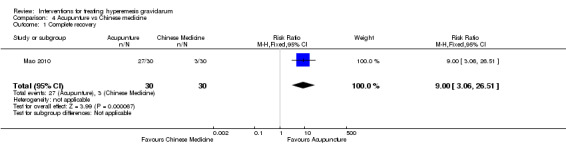
Comparison 4 Acupunture vs Chinese medicine, Outcome 1 Complete recovery.
4.4. Analysis.
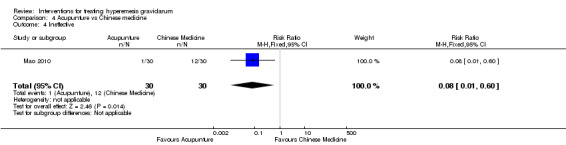
Comparison 4 Acupunture vs Chinese medicine, Outcome 4 Ineffective.
4.5. Analysis.
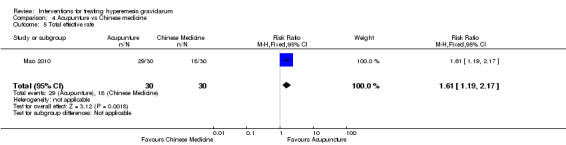
Comparison 4 Acupunture vs Chinese medicine, Outcome 5 Total effective rate.
Number of episodes of emesis
There were no data available on number of episodes of emesis.
Days of hospital admission
There were no data available on the number of days of hospital admission.
Secondary outcomes
There were no data available on any of this review's secondary outcomes.
Chinese medicine versus Western medicine (phenobarbital)
There was one study (Mao 2010, involving 90 women) that evaluated Chinese medicine versus Western medicine. The Chinese medicine group received a selection of traditional Chinese medication according to the dialectical classification and the "Western Medicine" group received phenobarbital 30 mg orally three times daily.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Results were reported as Complete Recovery: cessation of nausea and vomiting, normal appetite; Obvious Effects: reduction by at least 50% in frequency of nausea and vomiting, appetite improved; Effects Showed: reduction by 25% to 50% in nausea and vomiting, somewhat improved appetite; and Ineffective: reduction less than 25% in nausea/vomiting, no improvement in appetite. Total effectiveness rate was defined as the number of women with either Complete Recovery, Obvious Effects, or Effects Showed. At the end of one week of treatment, there was no significant difference between the two groups in number of women with either complete recovery (RR 0.75, 95% CI 0.18 to 3.07 (Analysis 5.1)) or any effectiveness (total effective rate) (RR 1.29, 95% CI 0.79 to 2.08, (Analysis 5.5)).
5.1. Analysis.
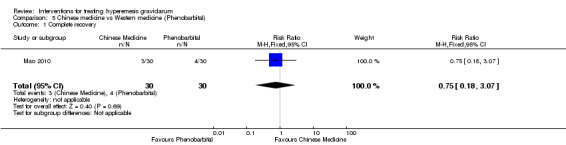
Comparison 5 Chinese medicine vs Western medicine (Phenobarbital), Outcome 1 Complete recovery.
5.5. Analysis.
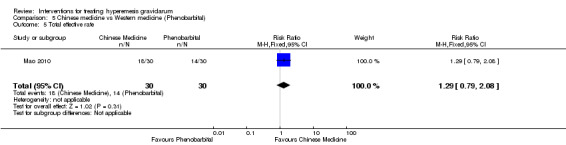
Comparison 5 Chinese medicine vs Western medicine (Phenobarbital), Outcome 5 Total effective rate.
Number of episodes of emesis
There were no data available on number of episodes of emesis.
Days of hospital admission
There were no data available on the number of days of hospital admission.
Secondary outcomes
There were no data available on any of this review's secondary outcomes.
Progressive muscle relaxation and pharmacotherapy versus pharmacotherapy
One study (Gawande 2011, involving 30 women) compared progressive muscle relaxation with pharmacotherapy versus pharmacotherapy alone. Pharmacotherapy included the progressive use of doxylamine succinate, ondansetron, metoclopramide, and promethazine.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Regarding the primary outcome, Gawande 2011 reported a Clinical Global Improvement Score, a significant improvement in muscle relaxation with pharmacotherapy compared to pharmacotherapy alone (mean difference (MD) ‐0.54 points, 95% CI ‐1.04 to ‐0.04 (Analysis 6.1)).
6.1. Analysis.
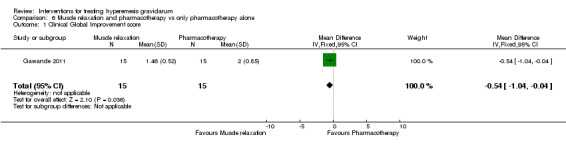
Comparison 6 Muscle relaxation and pharmacotherapy vs only pharmacotherapy alone, Outcome 1 Clinical Global Improvement score.
Number of episodes of emesis
None of the studies reported on the number of episodes of emesis.
Days of hospital admission
None of the studies reported on the number of days of hospital admission.
Secondary outcomes
Regarding secondary outcomes, there were no women in either group who decided to terminate the pregnancy.
No data were reported for any of this review's other secondary outcomes.
Midwife‐led outpatient care versus routine care
We found one study (involving 53 women) that examined midwife‐led outpatient care versus routine care with admission (McParlin 2008). Data were obtained from communication with the authors.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
There was no clear differences in the mean PUQE (pregnancy‐unique quantification of emesis and nausea) score between the group of women who received midwife‐led outpatient care and women who received routine care with admission (MD ‐0.70 points, 95% CI ‐3.17 to 1.77 (Analysis 7.1)).
7.1. Analysis.
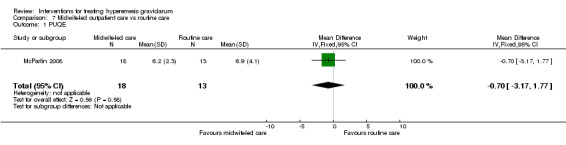
Comparison 7 Midwife‐led outpatient care vs routine care, Outcome 1 PUQE.
Number of episodes of emesis
No data were reported for this outcome.
Days of hospital admission
Women who received midwife‐led care remained in the hospital for fewer hours (MD ‐33.20 hours, 95% CI ‐46.91 to ‐19.49 (Analysis 7.2).
7.2. Analysis.
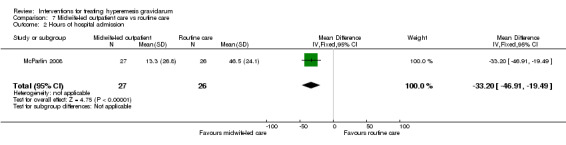
Comparison 7 Midwife‐led outpatient care vs routine care, Outcome 2 Hours of hospital admission.
Secondary outcomes
Regarding secondary outcomes, there was no clear difference in the rate of women who decided to terminate the pregnancy (RR 2.89, 95% CI 0.12 to 67.96 (Analysis 7.3)). There was also no clear difference in spontaneous miscarriage (RR 0.96, 95% CI 0.15 to 6.34 (Analysis 7.4)), or in the rate ofsmall‐for‐gestational‐age infants (RR 1.44, 95% CI 0.26 to 7.96) (Analysis 7.5)).In terms of economic costs there was also no evidence of a difference between groups in relation to the rate of women who lost time from paid employment (RR 1.04, 95% CI 0.28 to 3.87 (Analysis 7.6)).
7.3. Analysis.
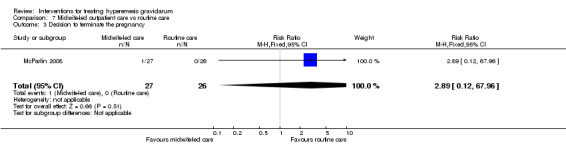
Comparison 7 Midwife‐led outpatient care vs routine care, Outcome 3 Decision to terminate the pregnancy.
7.4. Analysis.
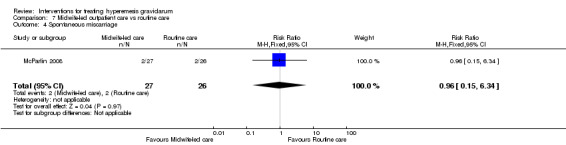
Comparison 7 Midwife‐led outpatient care vs routine care, Outcome 4 Spontaneous miscarriage.
7.5. Analysis.
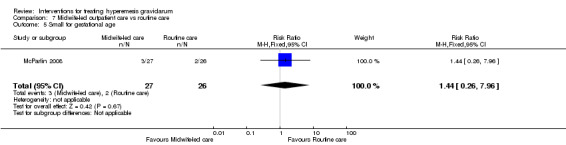
Comparison 7 Midwife‐led outpatient care vs routine care, Outcome 5 Small for gestational age.
7.6. Analysis.
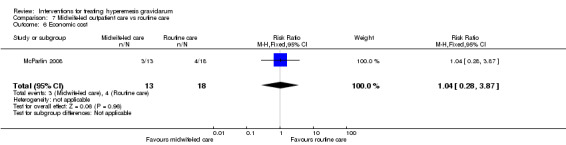
Comparison 7 Midwife‐led outpatient care vs routine care, Outcome 6 Economic cost.
Data were not available for any other secondary outcomes in this review.
Holistic assessment with standard care versus standard care
One study (Fletcher 2015, involving 273 women) compared holistic assessment with an individualized care plan in addition to standard medical care versus standard medical care alone. The holistic assessment involved a Hyperemeis impact of symptoms (HIS) questionnaire (Power 2009) that was used to tailor a care package comprising practical and supportive care. Standard care was intravenous rehydration and antiemetic therapy.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
There was no significant difference in the severity of nausea and vomiting as measured by PUQE (MD ‐0.20, 95% CI ‐1.10 to 0.70, (Analysis 8.1)).
8.1. Analysis.
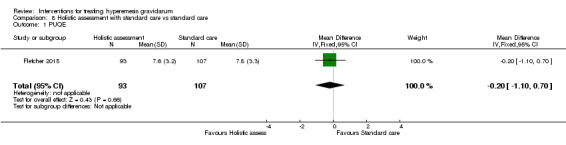
Comparison 8 Holistic assessment with standard care vs standard care, Outcome 1 PUQE.
Number of episodes of emesis
None of the studies reported on the number of episodes of emesis.
Days of hospital admission
Fletcher 2015 reported days of hospital admission for the two groups but did not include a standard deviation so this data could not be entered for analysis. Per the authors' report, there was a significantly shorter length of stay in the holistic assessment group (4.97 days versus 6.14 days, P = 0.05).
Secondary outcomes
Fletcher 2015 reported on quality of life by assessing both social functioning and client satisfaction and found no clear difference between holistic assessment with standard care versus standard care alone (MD 2.00, 95% CI ‐6.70 to 10.70 (Analysis 8.2)); (MD ‐0.50, 95% CI ‐1.90 to 0.90 (Analysis 8.3)).
8.2. Analysis.
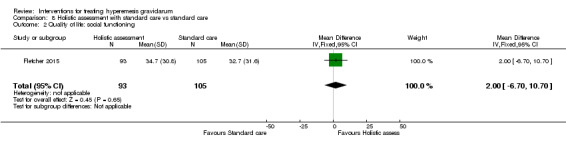
Comparison 8 Holistic assessment with standard care vs standard care, Outcome 2 Quality of life: social functioning.
8.3. Analysis.
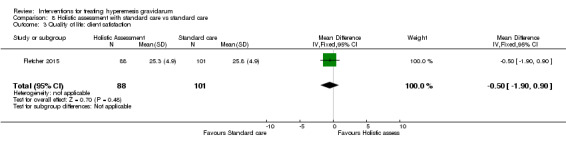
Comparison 8 Holistic assessment with standard care vs standard care, Outcome 3 Quality of life: client satisfaction.
Fletcher 2015 reported on economic costs by looking at both productivity costs from days lost at work and health system costs but did not report standard deviations so this data could not be entered for analysis. Per the authors report the total healthcare costs were higher in the standard care arm (£1360.50 versus 1185.90), however the holistic assessment group was associated with more days lost from work (£1930.50 versus £1468.80), thus the holistic assessment group had a higher societal cost than the standard care alone group (£3174.90 versus £2977.50).
Dextrose saline versus normal saline
One study (Tan 2013, involving 203 women) randomized women to either rehydration with dextrose saline versus normal saline.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Regarding the primary outcome, improvement in nausea/vomiting and number of episodes of emesis was reported in median (interquartile range), so that data could not be used for analysis in the RevMan tables. Tan 2013 reported a reduction in the median nausea visual numerical rating scale score at eight and 16 hours favoring dextrose saline, however this difference dissipated at 24 hours.
Number of episodes of emesis
No data were reported for this outcome.
Days of hospital admission
There was no difference identified in the length of hospital stay between the two groups (MD ‐5.00 hours, 95% CI ‐10.78 to 0.78 (Analysis 9.1)).
9.1. Analysis.
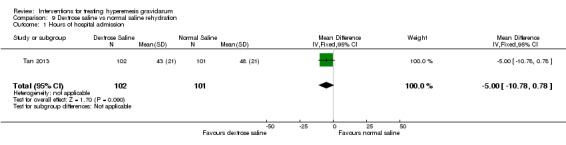
Comparison 9 Dextrose saline vs normal saline rehydration, Outcome 1 Hours of hospital admission.
Secondary outcomes
Regarding secondary outcomes, quality of life was reported, but as a median so the data could not be used for analysis in our RevMan tables, however the authors reported no significant difference in median well‐being score at 24 hours.
There were no data available on any other secondary outcomes.
Vitamin B6
Pyridoxine versus placebo
One study (Tan 2009, involving 94 women) randomized women to receive pyridoxine 20 mg orally three times a day versus placebo, in addition to all women receiving standard care with intravenous rehydration, metoclopramide, and oral thiamine. Interventions were continued for two weeks, outcomes examined a the one‐ and two‐week mark, results reported here are at the one‐week mark due to significant attrition by two weeks. Findings for this comparison are set out in Table 3.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Tan 2009, reported nausea score as a median rather than a mean score ‐ so the data could not be used for the RevMan tables, however the trialist reported no significant difference in nausea scores.
Number of episodes of emesis
There was no strong evidence of a difference in the daily mean vomiting episodes (MD 0.50 vomiting episodes, 95% CI ‐0.40 to 1.40, 66 women (Analysis 10.1), low‐quality evidence)
10.1. Analysis.
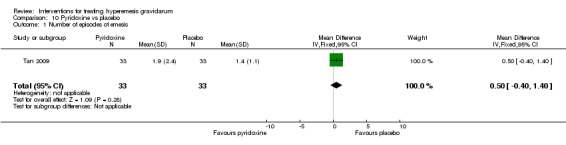
Comparison 10 Pyridoxine vs placebo, Outcome 1 Number of episodes of emesis.
Days of hospital admission
There was a slightly longer hospital stay associated with B6 compared with placebo (MD 0.80 day, 95% CI 0.08 to 1.52, 92 women (Analysis 10.2, moderate‐quality evidence).
10.2. Analysis.
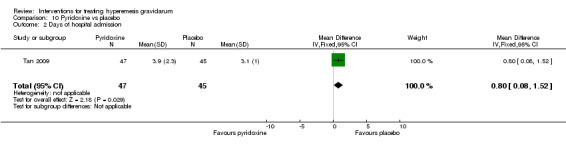
Comparison 10 Pyridoxine vs placebo, Outcome 2 Days of hospital admission.
Secondary outcomes
Regarding the secondary outcomes, there was no clear difference in hospital readmission (RR 1.78, 95% CI 0.85 to 3.71, 78 women (Analysis 10.3) or in weight loss after one week (MD 0.00 kg, 95% CI ‐0.93 to 0.93) (Analysis 10.4). Quality of life was reported as a median and therefore could not be included in the analysis, however Tan 2009 reports no difference between groups in well‐being score. Tan 2009 did report on intervention side effects, and there was no differences in the rate of dizziness (RR 1.67, 95% CI 0.85 to 3.26, 66 women, low‐quality evidence (Analysis 10.5)), headaches, (RR 1.33, 95% CI 0.52 to 3.42, 66 women, low‐quality evidence (Analysis 10.6)), diarrhea (RR 3.00, 95% CI 0.13 to 71.07, 66 women (Analysis 10.7)), palpitations (RR 1.00, 95% CI 0.22 to 4.60, 66 women, low‐quality evidence (Analysis 10.8)) and dry mouth (RR 0.82, 95% CI 0.49 to 1.38, 66 women, low‐quality evidence (Analysis 10.9)) in the pyridoxine group compared to placebo after one week of treatment. There were also no cases of rash or photosensitivity in either group.
10.3. Analysis.
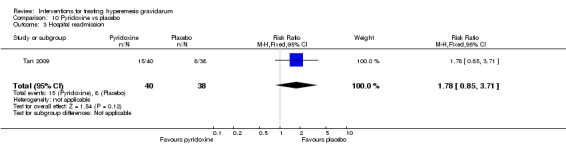
Comparison 10 Pyridoxine vs placebo, Outcome 3 Hospital readmission.
10.4. Analysis.
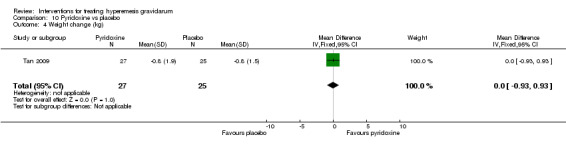
Comparison 10 Pyridoxine vs placebo, Outcome 4 Weight change (kg).
10.5. Analysis.
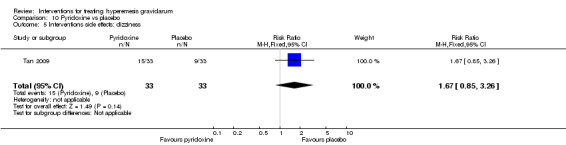
Comparison 10 Pyridoxine vs placebo, Outcome 5 Interventions side effects: dizziness.
10.6. Analysis.
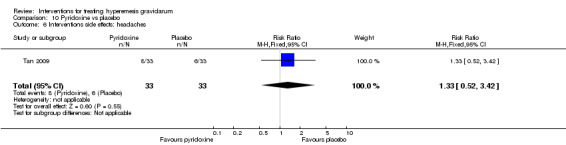
Comparison 10 Pyridoxine vs placebo, Outcome 6 Interventions side effects: headaches.
10.7. Analysis.
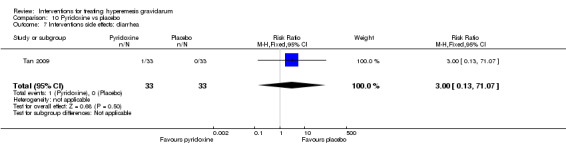
Comparison 10 Pyridoxine vs placebo, Outcome 7 Interventions side effects: diarrhea.
10.8. Analysis.
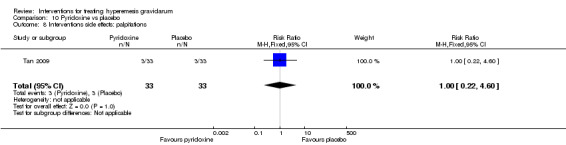
Comparison 10 Pyridoxine vs placebo, Outcome 8 Interventions side effects: palpitations.
10.9. Analysis.
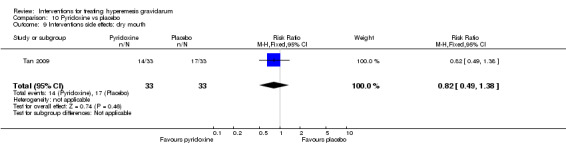
Comparison 10 Pyridoxine vs placebo, Outcome 9 Interventions side effects: dry mouth.
There were no data available on any other secondary outcomes.
Antihistamines
No studies that examined antihistamines were identified.
Dopamine antagonists
Metoclopramide versus acupuncture
One study (Neri 2005) evaluated the efficacy of acupuncture twice weekly versus metoclopramide infusion in the treatment of hyperemesis gravidarum ‐ the results have already been reported under the comparison of acupuncture versus metoclopramide (see above and Comparison 2 in Data and analyses).
Metoclopramide versus ondansetron
There were two studies (involving 243 women) that compared metoclopramide with ondansetron (Abas 2014; Kashifard 2013). Abas 2014 used 10 mg intravenous metoclopramide every eight hours for four doses versus 4 mg ondansetron intravenous every eight hours for four doses, while Kashifard 2013 used oral medications in the same doses for two weeks and assessed severity of nausea and vomiting during the treatment period and two days one week after completion of therapy. Results for this comparison have been set out in Table 4.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Regarding this primary outcome, Abas 2014 reported nausea score as a median so it could not be analyzed in combination with the other study (Kashifard 2013), but Abas 2014 reports no significant difference between groups. Kashifard 2013 reported no significant difference between the metoclopramide and ondansetron groups in terms of the severity of nausea (MD 1.70 point, 95% CI ‐0.15 to 3.55, one study, 83 women (Analysis 11.1), very low‐quality evidence), or in the severity of vomiting according to a 10‐point VAS rating score on the second day one week after completion of therapy (MD ‐0.10 points, 95% CI ‐1.63 to 1.43, one study, 83 women (Analysis 11.2), very low‐quality evidence).
11.1. Analysis.
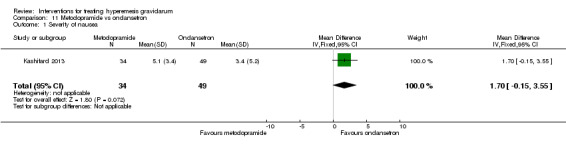
Comparison 11 Metoclopramide vs ondansetron, Outcome 1 Severity of nausea.
11.2. Analysis.
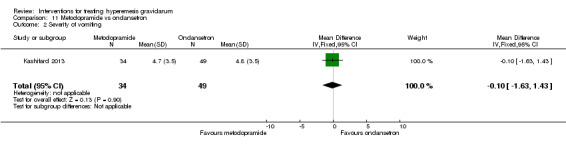
Comparison 11 Metoclopramide vs ondansetron, Outcome 2 Severity of vomiting.
Number of episodes of emesis
No data were reported for this outcome.
Days of hospital admission
No data were reported for this outcome.
Secondary outcomes
Regarding secondary outcomes, Abas 2014 provided data (from 160 women) in relation to intervention side effects. The number of women who felt drowsy (RR 2.40, 95% CI 1.23 to 4.69 (Analysis 11.3) moderate‐quality evidence), and who had a dry mouth (RR 2.38, 95% CI 1.10 to 5.11 (Analysis 11.4), moderate‐quality evidence) was higher in the metoclopramide group compared to the group of women who received ondansetron. There were no clear differences in the rate of women unable to sleep (RR 1.29, 95% CI 0.50 to 3.28 (Analysis 11.5), felt dizzy (RR 2.33, 95% CI 0.94 to 5.77, low‐quality evidence (Analysis 11.6)), had diarrhea (RR 9.00, 95% CI 0.49 to 164.46 (Analysis 11.7)), had headache (RR 1.22, 95% CI 0.54 to 2.79 (Analysis 11.8)), experienced palpitations (RR 2.50, 95% CI 0.50 to 12.51 (Analysis 11.9)), or noticed skin rash (RR 1.00, 95% CI 0.06 to 15.71 (Analysis 11.10)); no cases of dystonia in both groups were reported (Analysis 11.11). Kashifard 2013 reported no side effects in either the metoclopramide or the ondansetron group, although the side effects examined were not specified. In addition, Abas 2014 reported no difference in the well‐being VNRS score about quality of life outcome (MD ‐0.40 points, 95% CI ‐0.83 to 0.03, one study 160 women, moderate‐quality evidence (Analysis 11.12)).
11.3. Analysis.
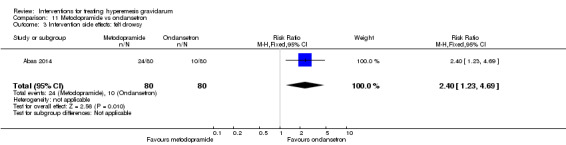
Comparison 11 Metoclopramide vs ondansetron, Outcome 3 Intervention side effects: felt drowsy.
11.4. Analysis.
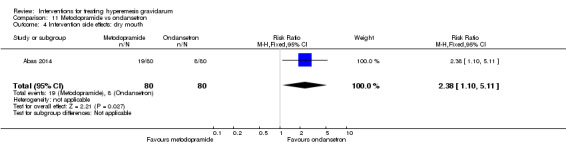
Comparison 11 Metoclopramide vs ondansetron, Outcome 4 Intervention side effects: dry mouth.
11.5. Analysis.
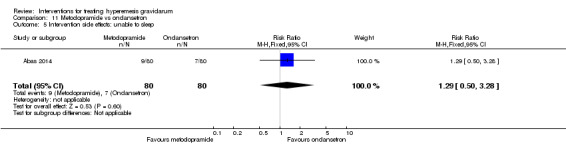
Comparison 11 Metoclopramide vs ondansetron, Outcome 5 Intervention side effects: unable to sleep.
11.6. Analysis.
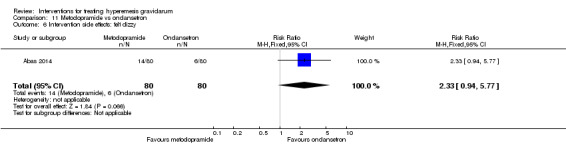
Comparison 11 Metoclopramide vs ondansetron, Outcome 6 Intervention side effects: felt dizzy.
11.7. Analysis.
Comparison 11 Metoclopramide vs ondansetron, Outcome 7 Intervention side effects: diarrhea.
11.8. Analysis.
Comparison 11 Metoclopramide vs ondansetron, Outcome 8 Intervention side effects: headache.
11.9. Analysis.
Comparison 11 Metoclopramide vs ondansetron, Outcome 9 Intervention side effects: palpitations.
11.10. Analysis.
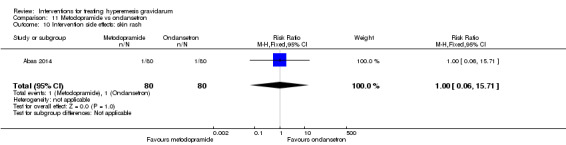
Comparison 11 Metoclopramide vs ondansetron, Outcome 10 Intervention side effects: skin rash.
11.11. Analysis.
Comparison 11 Metoclopramide vs ondansetron, Outcome 11 Intervention side effects: dystonia.
11.12. Analysis.
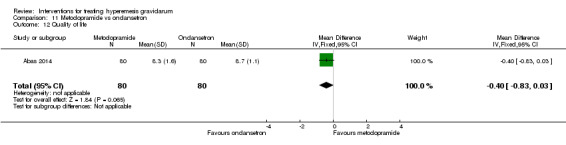
Comparison 11 Metoclopramide vs ondansetron, Outcome 12 Quality of life.
There were no data available on any other secondary outcomes.
Hydrocortisone versus metoclopramide
There was one study (Bondok 2006, involving 40 women) that compared women receiving 300 mg intravenous hydrocortisone daily for three days, tapered over the week, versus 10 mg of metoclopramide intravenously three times daily for one week. We had intended to produce a 'Summary of findings' table for this comparison but none of the pre‐specified outcomes were reported.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
There were no data on the subjective severity, reduction, or cessation of nausea.
Number of episodes of emesis
Mean number of daily episodes of emesis were reported by Bondok 2006 as significantly decreased in the hydrocortisone group, although the actual numbers were not available to be included into the analysis.
Days of hospital admission
No data were reported for this outcome.
Secondary outcomes
Regarding secondary outcomes, there was no difference in the rate of hospital readmission between the metoclopramide and hydrocortisone groups (RR 0.08, 95% CI 0.00 to 1.28 (Analysis 12.1) moderate‐quality evidence). Similarly, there was no clear difference in the number of women requiring enteral or parenteral nutrition between the two groups (RR 0.33, 95% CI 0.01 to 7.72 (Analysis 12.2)).
12.1. Analysis.
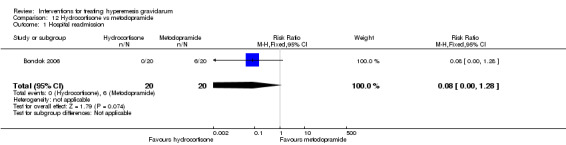
Comparison 12 Hydrocortisone vs metoclopramide, Outcome 1 Hospital readmission.
12.2. Analysis.
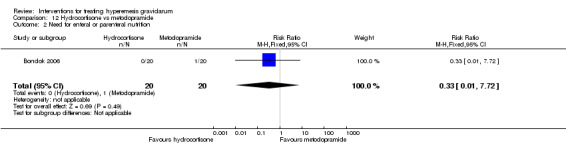
Comparison 12 Hydrocortisone vs metoclopramide, Outcome 2 Need for enteral or parenteral nutrition.
No other secondary outcomes were reported.
Metoclopramide versus promethazine
One study (Tan 2010) compared 10 mg intravenous metoclopramide versus 25 mg intravenous promethazine given eight hourly for 24 hours. (SeeTable 5.)
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Nausea score was reported by Tan 2010 as a median so data could not be included in our analysis, but the trialist reported no significant difference in nausea score between groups.
Number of episodes of emesis
The number of vomiting episodes were reported by Tan 2010 as a median so these data could not be included in our analysis, but the trialist reported no significant difference in the number of vomiting episodes between groups.
Days of hospital admission
No data were reported for this outcome.
Secondary outcomes
In relation to quality of life, the mean well‐being VNRS score was similar in the metoclopramide group and the promethazine groups (MD 0.50 points, 95% CI ‐0.22 to 1.22, low‐quality evidence, (Analysis 13.1)). Tan 2010, provided data on the intervention side effects ‐ there was no strong evidence showing any differences in the number of women unable to sleep (RR 0.78, 95% CI 0.40 to 1.53, (Analysis 13.2)), had a dry mouth (RR 0.91, 95% CI 0.62 to 1.34 (Analysis 13.3)), had diarrhea (RR 1.39, 95% CI 0.32 to 5.99 (Analysis 13.4)), had headache (RR 0.81, 95% CI 0.47 to 1.38 (Analysis 13.5)) (low‐quality evidence for the aforementioned side effects), experienced palpitations (RR 0.61, 95% CI 0.25 to 1.46 (Analysis 13.6)), and noticed skin rash (RR 1.39, 95% CI 0.32 to 5.99 (Analysis 13.7) . However, the number of women who felt drowsy (RR 0.70, 95% CI 0.56 to 0.87, moderate‐quality evidence (Analysis 13.8), the number of women who felt dizzy (RR 0.48, 95% CI 0.34 to 0.69, moderate‐quality evidence (Analysis 13.9)) and the number of women who experienced dystonia (RR 0.31, 95% CI 0.11 to 0.90 (Analysis 13.10)) was lower in the metoclopramide group compared to the group of women who received promethazine.
13.1. Analysis.
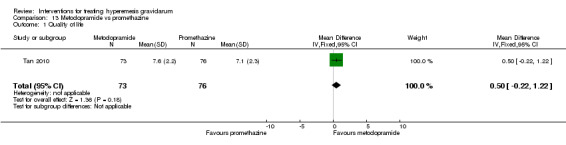
Comparison 13 Metoclopramide vs promethazine, Outcome 1 Quality of life.
13.2. Analysis.
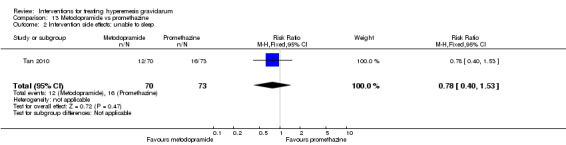
Comparison 13 Metoclopramide vs promethazine, Outcome 2 Intervention side effects: unable to sleep.
13.3. Analysis.
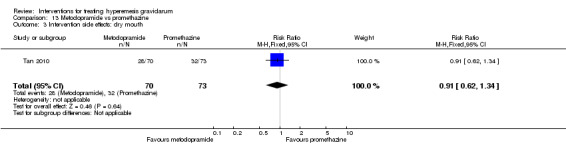
Comparison 13 Metoclopramide vs promethazine, Outcome 3 Intervention side effects: dry mouth.
13.4. Analysis.
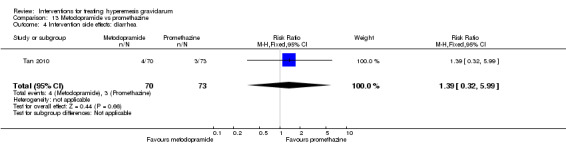
Comparison 13 Metoclopramide vs promethazine, Outcome 4 Intervention side effects: diarrhea.
13.5. Analysis.
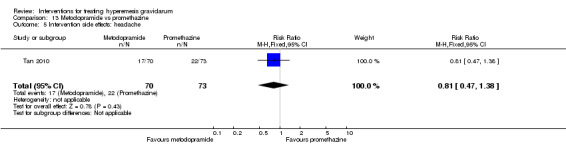
Comparison 13 Metoclopramide vs promethazine, Outcome 5 Intervention side effects: headache.
13.6. Analysis.
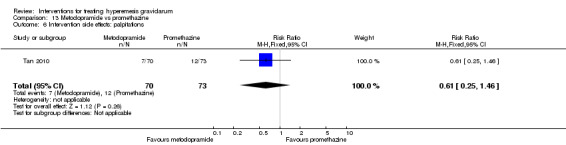
Comparison 13 Metoclopramide vs promethazine, Outcome 6 Intervention side effects: palpitations.
13.7. Analysis.
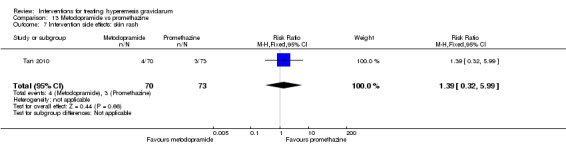
Comparison 13 Metoclopramide vs promethazine, Outcome 7 Intervention side effects: skin rash.
13.8. Analysis.
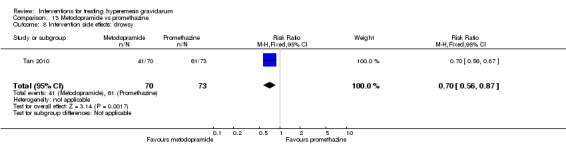
Comparison 13 Metoclopramide vs promethazine, Outcome 8 Intervention side effects: drowsy.
13.9. Analysis.
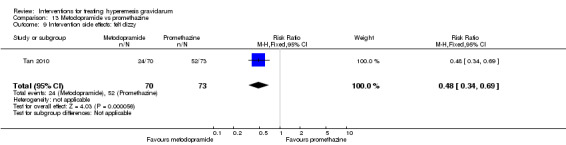
Comparison 13 Metoclopramide vs promethazine, Outcome 9 Intervention side effects: felt dizzy.
13.10. Analysis.
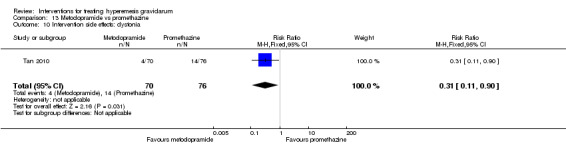
Comparison 13 Metoclopramide vs promethazine, Outcome 10 Intervention side effects: dystonia.
No other secondary outcomes were reported.
Benzodiazepines
Parenteral fluid with diazepam versus without diazepam
There was one study (Ditto 1999, involving 50 women) that evaluated the treatment of hyperemesis gravidarum with or without 10 mg intravenous diazepam twice daily while admitted, and with 5 mg oral diazepam twice daily versus placebo on discharge.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
The trial authors did not report a MD in nausea score, but did report a significantly decreased number of women with severe nausea in the diazepam group.
Number of episodes of emesis
Ditto 1999 did not report the specific number of episodes of emesis but reported a similar number of women with decreased emesis in both groups.
Days of hospital admission
The mean hospital stay was shorter in the diazepam group compared to placebo (MD ‐1.10, 95% CI ‐2.07 to ‐0.13 (Analysis 14.1)).
14.1. Analysis.
Comparison 14 Parenteral fluid with diazepam vs parenteral fluid without diazepam, Outcome 1 Days of hospital admission.
Secondary outcomes
Regarding secondary outcomes, there were no clear differences in hospital readmission (RR 0.17, 95% CI 0.02 to 1.29 (Analysis 14.2)), or the number of women requiring additional antiemetic drugs (RR 0.50, 95% CI 0.05 to 5.17 (Analysis 14.3)) between the group of women who received parenteral fluid with diazepam group and those who received parenteral fluid without diazepam. Similarly, there was no differences identified between the two groups in terms of the number of women who had congenital abnormalities (the trialist authors reported no congenital abnormalities in either group, Analysis 14.4), the incidence of preterm delivery (RR 0.50, 95% CI 0.05 to 5.17 (Analysis 14.5)), or in the rate of women who decided to terminate the pregnancy due to the hyperemesis (RR 3.00, 95% CI 0.13 to 70.30) (Analysis 14.6).
14.2. Analysis.
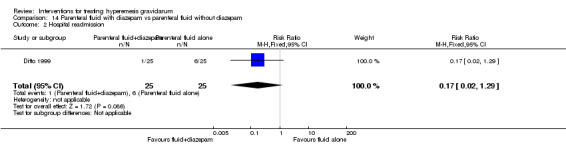
Comparison 14 Parenteral fluid with diazepam vs parenteral fluid without diazepam, Outcome 2 Hospital readmission.
14.3. Analysis.
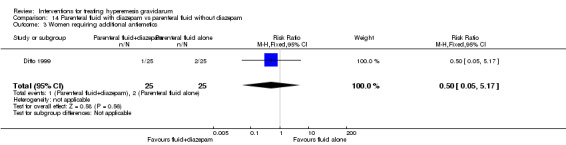
Comparison 14 Parenteral fluid with diazepam vs parenteral fluid without diazepam, Outcome 3 Women requiring additional antiemetics.
14.4. Analysis.
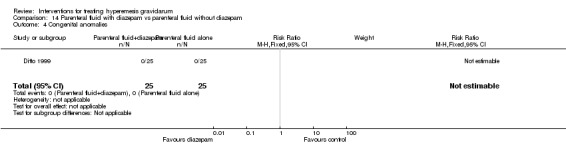
Comparison 14 Parenteral fluid with diazepam vs parenteral fluid without diazepam, Outcome 4 Congenital anomalies.
14.5. Analysis.
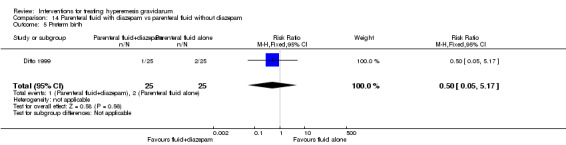
Comparison 14 Parenteral fluid with diazepam vs parenteral fluid without diazepam, Outcome 5 Preterm birth.
14.6. Analysis.
Comparison 14 Parenteral fluid with diazepam vs parenteral fluid without diazepam, Outcome 6 Decision to terminate the pregnancy.
No other secondary outcome data were available for analysis.
Serotonin antagonist
Ondansetron versus promethazine
One study (Sullivan 1996, involving 30 women) randomized women to receive either 10 mg intravenous ondansetron or 50 mg intravenous promethazine for one dose then every eight hours as needed. (SeeTable 6.)
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Specific subjective nausea scores were not reported by Sullivan 1996 and could not be entered into our RevMan tables. However, the trialist reported no significant difference in the severity of nausea between the two groups.
Number of episodes of emesis
There were no data available on number of episodes of emesis.
Days of hospital admission
There was no difference between the ondansetron and promethazine groups in terms of the number of days of hospital admission (MD 0.00 days, 95% CI ‐1.39 to 1.39 (Analysis 15.1), very low‐quality evidence).
15.1. Analysis.
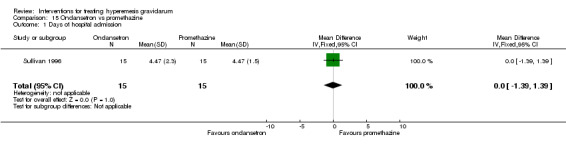
Comparison 15 Ondansetron vs promethazine, Outcome 1 Days of hospital admission.
Secondary outcomes
Regarding secondary outcomes, the rate of sedation (adverse effect) was decreased with ondansetron (RR 0.06, 95% CI 0.00 to 0.94 (Analysis 15.2), low‐quality evidence), no other side effects were observed.
15.2. Analysis.
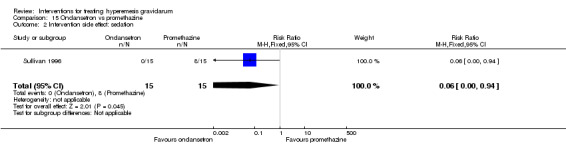
Comparison 15 Ondansetron vs promethazine, Outcome 2 Intervention side effect: sedation.
No other secondary outcomes were reported.
Ondansetron versus metoclopramide (two studies with 243 women)
Two studies (involving 243 women) compared ondansetron with metoclopramide ‐ the results have already been reported under the comparison of metoclopramide versus ondansetron (see above and Comparison 11 in Data and analyses).
Phenothiazines
Promethazine versus metoclopramide
One study (Tan 2010, involving 149 women) compared 10 mg intravenous metoclopramide to 25 mg intravenous promethazine given every 8 hours for 24 hours ‐ the results have already been reported under the comparison of metoclopramide versus promethazine (see above and Comparison 13 in Data and analyses).
Ondansetron versus promethazine
One study (Sullivan 1996, involving 30 women) randomized women to receive either 10 mg intravenous ondansetron or 50 mg intravenous promethazine for one dose then every eight hours as needed. The results have already been reported under the comparison of promethazine versus ondansetron (see above and Comparison 15 in Data and analyses).
Corticosteroids versus promethazine (two studies with 120 women)
Two studies (involving a total of 120 women) were involved in this comparison. One study (Safari 1998) evaluated oral methylprednisolone 16 mg three times daily versus oral promethazine 25 mg three times daily, while another one (Ziaei 2004) compared 5 mg oral prednisolone with 75 mg oral promethazine daily for 10 days. (SeeTable 7.)
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
In one study (Ziaei 2004), the number of women with severe nausea at 48 hours was higher in the prednisolone group compared to the promethazine group (RR 2.00, 95% CI 1.08 to 3.72 (Analysis 16.1), low‐quality evidence) and at day 17 was not significantly different between groups (RR 0.81, 95% CI 0.58 to 1.15 (Analysis 16.2), very low‐quality evidence). We did not find any difference in the number of episodes of vomiting at 48 hours (RR 3.00, 95% CI 0.33 to 27.63 (Analysis 16.3)) and at 17 days (RR 1.00, 95% CI 0.21 to 4.65 (Analysis 16.4), very low‐quality evidence).
16.1. Analysis.
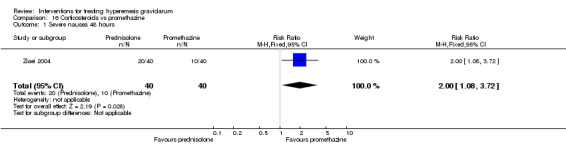
Comparison 16 Corticosteroids vs promethazine, Outcome 1 Severe nausea 48 hours.
16.2. Analysis.
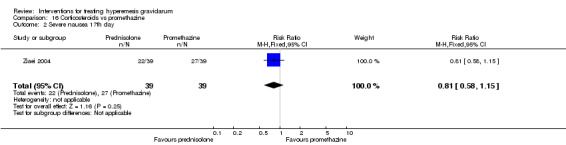
Comparison 16 Corticosteroids vs promethazine, Outcome 2 Severe nausea 17th day.
16.3. Analysis.
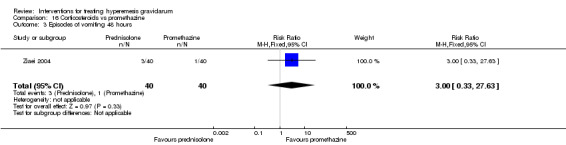
Comparison 16 Corticosteroids vs promethazine, Outcome 3 Episodes of vomiting 48 hours.
16.4. Analysis.
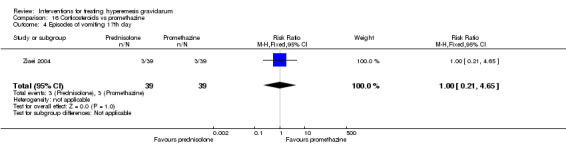
Comparison 16 Corticosteroids vs promethazine, Outcome 4 Episodes of vomiting 17th day.
In another study, Safari 1998 reported on therapy failure as defined by persistence of vomiting more than five times/day, inability to tolerate liquids, and the women’s impression that they were not better, and there was no difference between groups (RR 1.50, 95% CI 0.28 to 8.04 (Analysis 16.5)).
16.5. Analysis.
Comparison 16 Corticosteroids vs promethazine, Outcome 5 Therapy failure in 2 days.
Number of episodes of emesis
Ziaei (Ziaei 2004) reported increased number of episodes of emesis in the prednisolone group at 48 hours, but no difference at day 17; however data were reported as a median so were not able to be analyzed.
Days of hospital admission
There were no data available on the number of days of hospital admission.
Secondary outcomes
Regarding secondary outcomes, there was no strong evidence of differences in the rate of hospital readmission (RR 0.09, 95% CI 0.01 to 1.53 (Safari 1998, 34 women) (Analysis 16.6)), in the number of women requiring additional antiemetics (RR 1.50, 95% CI 0.28 to 8.04 (Safari 1998, 40 women) (Analysis 16.7)), or in the rate of stillbirth/neonatal death (RR 3.00, 95% CI 0.13 to 69.52 (Safari 1998, 40 women, low‐quality evidence) (Analysis 16.8), in the rate of preterm birth (RR 3.00, 95% CI 0.13 to 69.52 (Safari 1998, 40 women, low‐quality evidence) (Analysis 16.9)), or in the rate of women who decided to terminate the pregnancy (RR 3.00, 95% CI 0.13 to 69.52 (Safari 1998, 40 women) (Analysis 16.10)). In terms of side effects, there was no difference in the rate of women who felt abdominal pain during the first 48 hours (RR 0.33, 95% CI 0.07 to 1.55 (Ziaei 2004, 80 women) (Analysis 16.11)), and between the third and 10th day (RR 0.11, 95% CI 0.10 to 2.00 (Ziaei 2004, 80 women) (Analysis 16.12)). The rate of drowsiness was also not substantially different (RR 0.08, 95% CI 0.00 to 1.32 (Ziaei 2004, 80 women), low‐quality evidence (Analysis 16.13)). Regarding quality of life, the number of women who reported becoming well or partially well by 48 hours was lower in the prednisolone group compared to promethazine (RR 0.67, 95% CI 0.47 to 0.95 (Analysis 16.14)), while no difference was identified in the number of women who reported becoming well or partially well by 17 days (RR 1.67, 95% CI 0.95 to 2.92 (Analysis 16.15)) (Ziaei 2004).
16.6. Analysis.
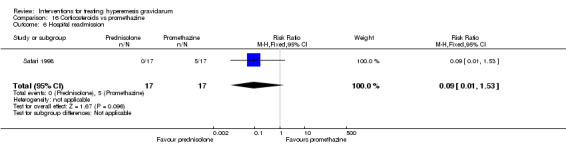
Comparison 16 Corticosteroids vs promethazine, Outcome 6 Hospital readmission.
16.7. Analysis.
Comparison 16 Corticosteroids vs promethazine, Outcome 7 Number of women requiring additional antiemetics.
16.8. Analysis.
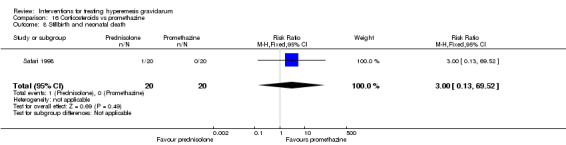
Comparison 16 Corticosteroids vs promethazine, Outcome 8 Stillbirth and neonatal death.
16.9. Analysis.
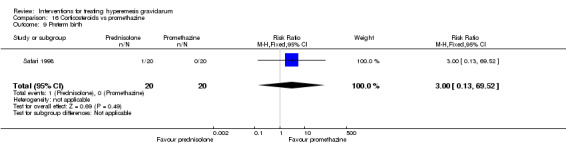
Comparison 16 Corticosteroids vs promethazine, Outcome 9 Preterm birth.
16.10. Analysis.
Comparison 16 Corticosteroids vs promethazine, Outcome 10 Decision to terminate the pregnancy.
16.11. Analysis.
Comparison 16 Corticosteroids vs promethazine, Outcome 11 Intevention side effects: abdominal pain 48 hours.
16.12. Analysis.
Comparison 16 Corticosteroids vs promethazine, Outcome 12 Intervention side effects: abdominal pain 3‐10 days.
16.13. Analysis.
Comparison 16 Corticosteroids vs promethazine, Outcome 13 Intervention side effects: drowsiness 48 hours and 3‐10 days.
16.14. Analysis.
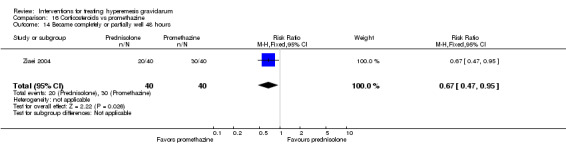
Comparison 16 Corticosteroids vs promethazine, Outcome 14 Became completely or partially well 48 hours.
16.15. Analysis.
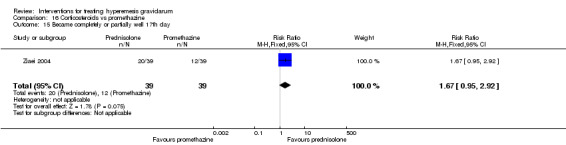
Comparison 16 Corticosteroids vs promethazine, Outcome 15 Became completely or partially well 17th day.
No other secondary outcomes were reported.
Steroid hormones
Corticosteroids versus placebo
There were four studies (involving 271 women) that evaluated the efficacy of steroids versus placebo in hyperemesis gravidarum (Duggar 2001; Nelson‐Piercy 2001; Tabatabaii 2008; Yost 2003). The women in Duggar 2001 received oral methylprednisone 12 tablets of 4 mg methylprednisone daily for three days followed by a 10‐day taper. The women in Nelson‐Piercy 2001 received 20 mg of oral prednisolone every 12 hours for one week; they also received additional antiemetics as deemed necessary by the providers. The women in Tabatabaii 2008 and Yost 2003 received 125 mg of intravenous methylprednisolone followed by an oral prednisone taper; in the former study the women also received B6, in the latter study the women also received metoclopramide and promethazine as standard of care. Results for this comparison are set out in Table 8.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
Nelson‐Piercy 2001 reported a non‐significant reduction in severity of nausea in the steroid versus placebo group, however this was reported as a median and could not be included into the analysis.
Number of episodes of emesis
There were no data available on number of episodes of emesis.
Days of hospital admission
Days of hospital admission were available from Yost 2003, there was no clear difference (MD ‐0.30 day, 95% CI ‐0.70 to 0.10, very low‐quality evidence) in the number of days of hospital admission between groups (Analysis 17.1).
17.1. Analysis.
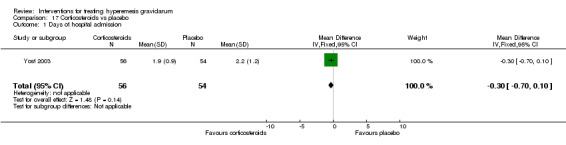
Comparison 17 Corticosteroids vs placebo, Outcome 1 Days of hospital admission.
Secondary outcomes
Regarding secondary outcomes, the rate of hospital readmission was lower in the steroid hormone group compared to the placebo group of women (RR 0.69, 95% CI 0.50 to 0.94, four studies, 269 women (Analysis 17.2)) (Duggar 2001; Nelson‐Piercy 2001; Tabatabaii 2008; Yost 2003). There was no difference in the rate of pregnancy complications (pregnancy hypertension or gestational diabetes (RR 0.61, 95% CI 0.26 to 1.47, very low‐quality evidence (Analysis 17.3)) based on data reported in Yost 2003 (110 women). There was no significant difference in the rate of spontaneous abortion (RR 0.64, 95% CI 0.11 to 3.70 (Yost 2003, 110 women, very low‐quality evidence) (Analysis 17.4)). There was no difference in the rate of stillbirth or neonatal death (RR 0.70, 95% CI 0.09 to 5.29, two studies, 134 women, very low‐quality evidence) (Analysis 17.5)). Only one study (Yost 2003) reported on congenital abnormalities, and there was no difference between groups (RR 0.32, 95% CI 0.01 to 7.73, one study 110 women, very low‐quality evidence (Analysis 17.6)). One study (Yost 2003) reported on low birthweight (RR 1.35, 95% CI 0.46 to 4.00, 110 women (Analysis 17.7), very low‐quality evidence) and another study (Nelson‐Piercy 2001) reported on small‐for‐gestational‐age infants (RR 1.00, 95% CI 0.07 to 14.21, 24 women (Analysis 17.8)) and there was no significant difference between groups for either outcome. One study (Yost 2003) reported on preterm birth less than 36 weeks and Nelson‐Piercy 2001 reported on preterm birth less than 37 weeks, when we combined these data using a random‐effects analysis (due to substantial statistical heterogeneity) there was no difference between groups (average RR 1.01, 95% CI 0.31 to 3.28; two studies, 134 women, Tau² = 0.27, I² = 37% (Analysis 17.9), very low‐quality evidence). Duggar 2001 reported intervention side effects (specifics side effects not reported) and found no difference in the rate of side effects (RR 0.79, 95% CI 0.06 to 11.20, 25 women, very low‐quality evidence (Analysis 17.10)). One study (Nelson‐Piercy 2001) reported on the number of women requiring additional antiemetics and there was no clear difference between groups for this outcome (RR 0.56, 95% CI 0.26 to 1.17, 24 women (Analysis 17.11)) and there was also no difference in the number of women who decided to terminate the pregnancy (RR 0.33, 95% CI 0.01 to 7.45, (Nelson‐Piercy 2001) 24 women (Analysis 17.12)).
17.2. Analysis.
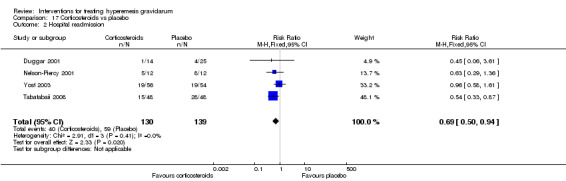
Comparison 17 Corticosteroids vs placebo, Outcome 2 Hospital readmission.
17.3. Analysis.
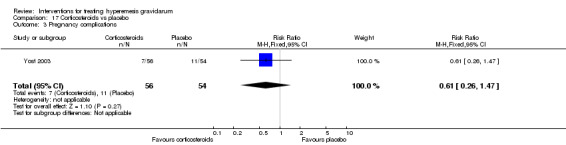
Comparison 17 Corticosteroids vs placebo, Outcome 3 Pregnancy complications.
17.4. Analysis.
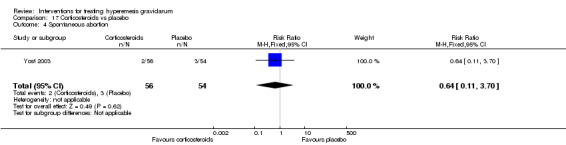
Comparison 17 Corticosteroids vs placebo, Outcome 4 Spontaneous abortion.
17.5. Analysis.
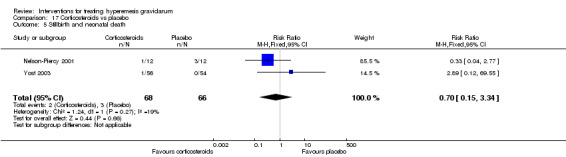
Comparison 17 Corticosteroids vs placebo, Outcome 5 Stillbirth and neonatal death.
17.6. Analysis.
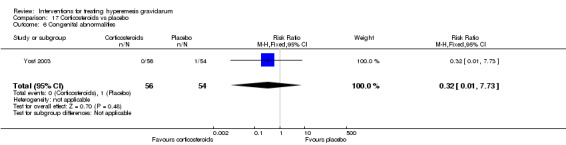
Comparison 17 Corticosteroids vs placebo, Outcome 6 Congenital abnormalities.
17.7. Analysis.
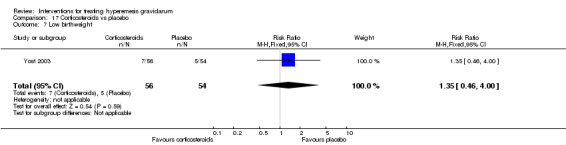
Comparison 17 Corticosteroids vs placebo, Outcome 7 Low birthweight.
17.8. Analysis.
Comparison 17 Corticosteroids vs placebo, Outcome 8 Small‐for‐gestational age.
17.9. Analysis.
Comparison 17 Corticosteroids vs placebo, Outcome 9 Preterm birth.
17.10. Analysis.
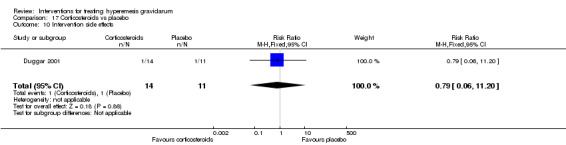
Comparison 17 Corticosteroids vs placebo, Outcome 10 Intervention side effects.
17.11. Analysis.
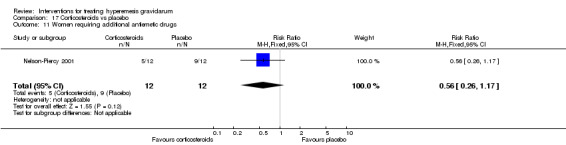
Comparison 17 Corticosteroids vs placebo, Outcome 11 Women requiring additional antiemetic drugs.
17.12. Analysis.
Comparison 17 Corticosteroids vs placebo, Outcome 12 Decision to terminate the pregnancy.
There were no data available on any other secondary outcome.
Corticosteroids versus promethazine (two studies with 120 women)
Two studies (involving a total of 120 women) were involved in this comparison. One study (Safari 1998) evaluated oral methylprednisolone 16 mg three times daily versus oral promethazine 25 mg three times daily, while another one (Ziaei 2004) compared 5 mg oral prednisolone with 75 mg oral promethazine daily for 10 days. The results have already been reported under the comparison of corticosteroids versus promethazine above (see above and Comparison 14 in Data and analyses).
Hydrocortisone versus metoclopramide (one study with 40 women)
One study (Bondok 2006, involving 40 women) compared women receiving 300 mg intravenous hydrocortisone daily for three days, tapered over the week, versus 10 mg of intravenous metoclopramide three times daily for one week. The results have already been reported under the comparison of hydrocortisone versus metoclopramide (see above and Comparison 12 in Data and analyses).
Adrenocorticotropic hormone (ACTH) versus placebo
One study (Ylikorkala 1979, involving 32 women) randomized women to 0.5 mg intramuscular ACTH versus placebo for four days. There were no data available on number of episodes of emesis or days of hospital admission.
Primary outcomes
Severity, reduction, or cessation in nausea/vomiting
In terms of mean relief score, there was no difference between the group of women who received intramuscular ACTH and the group of women who received a placebo (MD 0.60 points, 95% CI ‐1.65 to 2.85 (Analysis 18.1)).
18.1. Analysis.
Comparison 18 ACTH vs placebo, Outcome 1 Reduction or cessation in nausea/vomiting.
Number of episodes of emesis
There were no data available on number of episodes of emesis.
Days of hospital admission
There were no data available on the number of days of hospital admission.
Secondary outcomes
Regarding secondary outcomes, the group of women who received intramuscular ACTH had a higher mean weight gain than the women in the placebo group (MD 1.0 kg, 95% CI 0.34 to 1.66 (Analysis 18.2)). There was no difference between groups in terms of the rate of hospital readmission (RR 1.00, 95% CI 0.16 to 6.25 (Analysis 18.3)), rate of spontaneous abortion (RR 1.00, 95% CI 0.07 to 14.64 (Analysis 18.4), or rate of preterm birth (RR 3.00, 95% CI 0.13 to 68.57 (Analysis 18.5)).
18.2. Analysis.
Comparison 18 ACTH vs placebo, Outcome 2 Weight gain (kg).
18.3. Analysis.
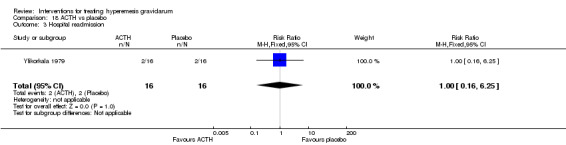
Comparison 18 ACTH vs placebo, Outcome 3 Hospital readmission.
18.4. Analysis.
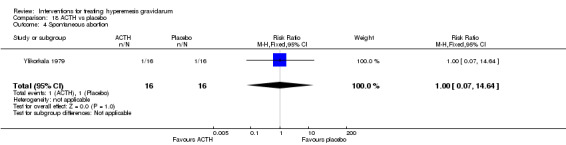
Comparison 18 ACTH vs placebo, Outcome 4 Spontaneous abortion.
18.5. Analysis.
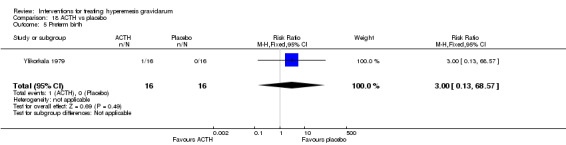
Comparison 18 ACTH vs placebo, Outcome 5 Preterm birth.
Data on other secondary outcomes were not available.
Subgroup analysis
Subgroup analysis on primary outcomes was planned to be performed on the following subgroups: singleton versus multiple gestation, prime versus multiparous, and women with over 5% weight loss versus women without less than 5% weight loss. Unfortunately, there were insufficient data to carry out these subgroup analyses. No studies that included multiple gestations or multiparous women reported data separately for those groups. Eight studies included only singleton gestations, but they were all also the only study in their comparison group (Bondok 2006; Ditto 1999; Gawande 2011; Neri 2005; Sullivan 1996; Tan 2009; Tan 2010; Tan 2013). Both Kashifard 2013, which included only singletons, and Abas 2014, which included multiple gestations, compared metoclopramide and ondansetron, however Kashifard 2013 did not have data available on primary outcomes for analysis. Three studies included weight loss over 5% as an inclusion criteria (Bondok 2006; Ditto 1999; Neri 2005), one study required women to have more than 2.25 kg weight loss (Sullivan 1996), and one study required women to have had weight loss of at least 3 kg (Kashifard 2013), but again they were the only studies in their comparison group or did not have data on primary outcomes available for analysis.
Discussion
Summary of main results
This review included 25 studies (involving 2052 women), but the majority of our analyses are based on data from single studies with small numbers of participants. The included studies covered a range of interventions (both pharmacological and non‐pharmacological, such as acupressure/acupuncture, outpatient care, intravenous fluids, and various pharmaceutical interventions) for treating hyperemesis gravidarum. There were no studies of dietary or other lifestyle interventions. However, the majority of interventions were evaluated in small, often unique, trials, making strong clinical recommendations impossible.
Acupuncture/acupressure was associated with fewer women requiring additional antiemetics compared to placebo. There was no clear difference in miscarriage, preterm birth, stillbirth or neonatal death, decision to terminate the pregnancy, or anxiodepressive symptoms compared to placebo. There was no difference in the rate of women who experienced a reduction in nausea or vomiting compared to metoclopramide. Acupuncture was associated with greater improvement in nausea/vomiting compared to phenobarbital and Chinese medicine.
Chinese medicine compared to phenobarbital had no difference in improvement in nausea and vomiting.
There were no studies solely on dietary or lifestyle modification, although, midwife‐led outpatient care was associated with fewer hours of hospital admission than routine inpatient admission with no difference in pregnancy‐unique quantification of emesis and nausea (PUQE) score, decision to terminate the pregnancy, miscarriage, small‐for‐gestational age infants, or time off work when compared with routine care.
Tailored care based on a holistic assessment in addition to standard care compared to standard care alone did not demonstrate any difference in improvement of nausea and vomiting or quality of life.
There was greater degree of subjective improvement found with muscle relaxation therapy and pharmacotherapy compared to pharmacotherapy alone with no clear difference in decision to terminate the pregnancy.
There was no difference in duration of hospital stay with dextrose saline fluids versus normal saline for rehydration.
Vitamin B6 showed a slightly longer hospital admission compared with placebo, but no difference in the number of episodes of emesis. There was also no difference found in the rate of hospital readmission, weight loss, or medication side effects compared to placebo.
Parenteral fluid with diazepam compared to parenteral fluid alone had a decreased mean duration of hospital admission, but there was no difference in the hospital readmission rate, number of women requiring additional antiemetics, number of women choosing to terminate the pregnancy, or in the rate of preterm delivery. Neither group had any cases of congenital anomalies.
Metoclopramide compared to ondansetron had similar nausea and vomiting severity, but increased rate of drowsiness and dry mouth. Compared to hydrocortisone, metoclopramide had similar rates of hospital readmission and need for enteral/parenteral nutrition. Compared to promethazine, there was no difference in quality of life measures, but there were decreased rates of drowsiness, dizziness and dystonia with metoclopramide.
Ondansetron compared to promethazine had no clear difference in days of hospital admission but decreased rate of sedation, and compared to metoclopramide, had a lower rate of drowsiness and dry mouth with no difference in nausea/vomiting severity.
Promethazine compared to metoclopramide had similar mean well‐being outcomes with increased rate of drowsiness, dizziness and dystonia. Promethazine compared with ondansetron showed no difference in days of hospital admission but had an increased rate of sedation. Promethazine compared with corticosteroids had improved well‐being scores and improved nausea at 48 hours, but not by day 17. Compared with corticosteroids, there was no difference episodes of emesis, in the number of women who had persistent vomiting, inability to tolerate oral intake, or participant perceived improvement. There was also no significant difference in readmission rate, women requiring additional antiemetics, rate of stillbirth, neonatal death, preterm delivery, decision to terminate the pregnancy, or medication side effects.
Corticosteroids compared to placebo had a lower rate of hospital readmission, but otherwise demonstrated no difference in days of hospital admission, medication side effects, number of women requiring additional antiemetics, decision to terminate the pregnancy or adverse pregnancy outcomes including hypertension, diabetes, stillbirth or neonatal death, congenital anomalies, and low birthweight. Compared to promethazine, steroids were associated with lower well‐being, and had more severe nausea at 48 hours, although this did not persist by day 17. There was also no significant difference in readmission rate, women requiring additional antiemetics, rate of stillbirth, neonatal death, preterm delivery, decision to terminate the pregnancy, or medication side effects. Compared to metoclopramide, treatment with corticosteroids demonstrated no difference in hospital readmission.
Treatment with ACTH compared to placebo had no strong evidence of difference in mean relief score, but did demonstrate increased mean weight gain. There was also no clear difference found in rate of readmission, miscarriage, or preterm delivery.
Overall completeness and applicability of evidence
We attempted to be as inclusive as possible in the search strategy and have included studies in languages other than English. Nonetheless, the studies reported are predominantly from European and North American journals, which may limit the external validity of these result.
Interpreting and comparing the findings of the studies included was difficult because of the variation in the reporting of the subjective outcome of severity of nausea and vomiting, thus the meta‐analysis component of this review is limited. In addition, even within a comparison, often dosages or route of administration varied between studies, we treated them as equivalent which is not necessarily clinically true.
Limited data were available regarding adverse maternal and neonatal outcomes, thus the lack of report on adverse events or the lack of statistical significance does not necessarily mean no harm is present. Larger studies on individual interventions need to be examined to determine the safety of these many interventions.
There was also very limited reporting on the economic impact of hyperemesis gravidarum and the impact on this economic burden that interventions may have. Although studies often reported an overall well‐being score, this does not necessarily equate with ability to return to work.
Quality of the evidence
We were unable to pool findings for most interventions reviewed here because of heterogeneity in intervention and comparison groups and in outcomes measured, thus most results presented are from individual studies. Additionally, inclusion and exclusion criteria varied between studies. Studies were included on the basis of their own definition of hyperemesis gravidarum, which may or may not have included objective criteria like weight loss. The methodological quality of the included studies varied. A number of studies were published in abstract form only, and not enough methodological detail was provided to appropriately assess any type of risk of bias. For studies published in full, the quality was generally good with adequate quality in randomization, outcome reporting, and limited attrition. However, for a number of studies were there was not enough information to appropriately assess blinding of either participants or investigators. Additionally, no study reported on the quality of their blinding, thus it is possible that, for example, sham acupuncture was not believable. Appropriate blinding is especially important in this condition because the primary outcomes are often subjective and self‐reported. There was one study on outpatient versus inpatient management where blinding was inherently impossible. Another limitation is that studies with a placebo comparison often included standard care for both groups which involved one or a combination of other antiemetic medications, which varied by study, and the details of which were not reported. Finally, there was heterogeneity in the way in which outcomes were measured. Most studies used a 10‐point visual analogue scale, without providing support of its validation in nausea and vomiting of pregnancy. Other studies used the Rhodes Index of Nausea, Vomiting, and Retching, which was originally developed to measure symptoms of chemotherapy, but has been validated in pregnancy (Rhodes 1984; Zhou 2001). Other studies used pregnancy specific questionnaires, either of the author's creation, without support of validation, or the more commonly used PUQE, which has been validated (Koren 2005).
For important outcomes and comparisons we graded the quality of the evidence using the GRADE approach. When outcomes were reported, at best the evidence was graded as being of moderate quality, while for most outcomes the evidence was assessed as being of low or very low quality. The main reasons for downgrading the evidence were design limitations in the studies contributing data, but most importantly the imprecision of effect estimates. For most outcomes, single studies with relatively small sample sizes contributed data. Studies were mainly under‐powered to identify differences between comparison groups and most effect estimates had wide 95% CIs crossing the line of no effect.
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8.
Potential biases in the review process
There is potential for bias in every step of the review process. We attempted to limit the bias by having at least two review authors independently carry out the evaluation of studies for inclusion, data extraction, and quality assessment, with any discrepancies being resolved by a third review author. However, such an assessment is still by nature subjective and a different team of review authors may have had a different assessment.
Agreements and disagreements with other studies or reviews
There are several other reviews and overviews on hyperemesis gravidarum, with varying degrees of support from the literature (Eliakim 2000; Goodwin 1998; Ismail 2007; Jarvis 2011; Maltepe 2013; McCarthy 2014a; Philip 2003; Sonkusare 2008). Goodwin 1998 mentions randomized trials on both nausea and vomiting of pregnancy as well as hyperemesis gravidarum and acknowledges the limited support from trials of various interventions. The authors do mention studies reviewed here (Sullivan 1996; Ylikorkala 1979), as well as a study we excluded based on cross‐over design, on the effect of ginger (Fischer‐Rasmussen 1991). Their review also supports the use of steroids in reducing the need for hospitalization, although does not include the more recent studies reviewed here.
Eliakim and colleagues (Eliakim 2000) mention a few interventions in their review, but with limited data supporting their conclusion. In contrast to our review, they mention benefit of vitamin B6, although the study they cite is for the treatment of nausea and vomiting in pregnancy, rather than hyperemesis gravidarum. They mention beneficial results for a number of antiemetics including Bendectin, meclizine, metoclopramide, promethazine, hydroxyzine, trimethobenzamine, thielpyrazin, mepryramine, dimenhydrinate, droperidol, diphenhydramine, ondansetron, methylprednisolone, and ginger, but cites other reviews on nausea and vomiting of pregnancy as their sources rather than specific studies.
Philip 2003 provides a review that includes randomized trials, most of which we have also included, as well as retrospective studies. The authors have similarly found no benefit with ACTH (Ylikorkala 1979), no benefit of ondansetron over promethazine (Sullivan 1996), and possible benefit of corticosteroids (Duggar 2001; Safari 1998). They also conclude no benefit with vitamin B6. In contrast to our review, they found no benefit from P6 acupressure where we have found that there may be some benefit compared to placebo.
A review by Ismail and colleagues (Ismail 2007) similarly concludes limited benefit acupuncture/acupressure; they recommend use of antiemetics, but do not provide data to support their efficacy in hyperemesis gravidarum, and, in contrast to our review, found no benefit with corticosteroids, while we did find a decreased rate of hospitalization compared to placebo. Sonkusare 2008 examines a number of different interventions, however includes trials on nausea and vomiting of pregnancy along with hyperemesis gravidarum, and is not limited to randomized controlled trials. In contrast to our review, they found benefit with vitamin B6 and ginger. They found no benefit with corticosteroids, based on only one study, also included in this review (Yost 2003), and finally, they concluded there was benefit with diazepam, nerve stimulation, erythromycin, and cannabis (Sonkusare 2008).
The review by Jarvis in 2011 (Jarvis 2011) mentions trials for both nausea and vomiting and pregnancy as well as hyperemesis, and is not limited to randomized controlled trials. The authors similarly found little benefit of one antiemetic over another, describe similar side‐effect profiles to our review, although they conclude drowsiness is most common with phenothiazines, which is not something supported by evidence in our review. They also recommend normal saline over dextrose saline, for the risk of Wernicke's encephalopathy, although our review found no difference between the two. They also found that corticosteroids may reduce hospital admissions. Maltepe 2013 provides a review primarily on nausea and vomiting of pregnancy with some mention of antiemetics for hyperemesis gravidarum, without specific recommendations or conclusions on their benefit. McCarthy 2014a provides a brief overview on treatment for hyperemesis gravidarum, citing, however, the previously published Cochrane review on nausea and vomiting of pregnancy, which specifically excludes hyperemesis gravidarum (Matthews 2015). They similarly conclude limited evidence regarding the benefit of one antiemetic over another.
Authors' conclusions
Implications for practice.
Although there have been a number of reviews on the management of hyperemesis gravidarum, as described above, they often have limited evidence to support their conclusions, and combine interventions for nausea and vomiting of pregnancy with the more severe condition of hyperemesis gravidarum.
On the basis of this review, there is little high‐quality and consistent evidence supporting any one intervention, which should be taken into account when making management decisions. Additionally, in evaluating various interventions, we have combined various forms of a specific therapy for the purpose of the meta‐analysis, such as intravenous and oral forms and different dosages, so we cannot provide guidelines on specific doses or routes of the antiemetics examined here.
Implications for research.
The difficulty in interpreting the results of this review highlights the importance of having a specific definition of hyperemesis gravidarum for use in trials, conducting randomized controlled trials in comparing interventions, and using validated instruments for the measurement of severity of nausea and vomiting. There should be an agreed‐upon set of clearly‐defined and measurable outcomes in trials of interventions for hyperemesis gravidarum, so that outcomes of trials can be combined in future meta‐analyses.
The vast majority of the trials evaluating interventions for hyperemesis gravidarum were evaluated in only small, often unique, trials, so almost all interventions deserve to be evaluated further in much larger, well‐designed trials. We found little data on the use of ginger, antihistamines, and dietary and lifestyle modifications in the treatment of hyperemesis gravidarum, which is an area of further research. There was only one study on outpatient‐led care that found reduced hospital stay compared with routine care, which certainly warrants further study. There were a number of studies comparing the commonly used antiemetics metoclopramide, ondansetron, and promethazine, although data on primary outcomes were limited and the main differences found were in side‐effect profiles. There were also a number of studies on corticosteroids, but the heterogeneity in inclusion criteria, specific medications, and comparison groups in these studies makes it difficult to draw conclusions from our results. Placebo‐controlled trials on steroids included other antiemetics in both groups as standard of care, which varied by study, but the finding of decreased hospital readmission rate warrants further study.
Finally, given that there was little evidence to support the superiority of one intervention over another in the treatment of hyperemesis, more research should be done comparing the side‐effect profiles and safety, as well as the economic costs and benefits of these interventions to aid in the selection of the optimal regimen.
What's new
| Date | Event | Description |
|---|---|---|
| 19 May 2016 | Amended | We have edited the plain language title to include a plain language description of hyperemesis gravidarum ‐ severe nausea and vomiting during pregnancy. |
Acknowledgements
Thanks to Susan Wieland, Information Specialist from the Cochrane Complementary Medicine Field for searching their Trials Register.
Therese Dowswell (Cochrane Pregnancy and Childbirth Group) assisted with grading the evidence and producing the 'Summary of findings' tables.
Farhad Shokraneh, Youngmee Hahn and Pei Ling Haussecker for translation of non‐English language trial papers.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Cochrane Complementary and Alternative Therapies Field Register search
Searched by the Information Specialist (20 September 2014) and then via The Cochrane Register of Studies (CRSO) (20 December 2015)
(pregnan* OR antenatal OR prenatal) AND (nause* OR sickness OR vomit* OR emesis OR hyperemisis OR antiemetic)
Data and analyses
Comparison 1. Acupuncture and acupressure vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women requiring additional antiemetics | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.08, 0.50] |
| 2 Spontaneous abortion | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.03] |
| 3 Preterm birth less than 37 weeks | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.26] |
| 4 Stillbirth and neonatal death | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.04, 8.30] |
| 5 Decision to terminate the pregnancy | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.18, 2.95] |
| 6 Quality of life: anxiodepressive symptomatology | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.40] |
Comparison 2. Acupuncture vs metoclopramide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction or cessation in nausea | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.79, 2.49] |
| 2 Reduction or cessation in vomiting | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.92, 2.48] |
Comparison 3. Acupunture vs Western medicine (Phenobarbital).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete recovery | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.75 [2.69, 16.94] |
| 2 Obvious effects | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.52] |
| 3 Effects showed | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.98] |
| 4 Ineffective | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.44] |
| 5 Total effective rate | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.40, 3.05] |
3.2. Analysis.
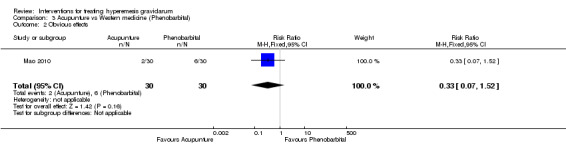
Comparison 3 Acupunture vs Western medicine (Phenobarbital), Outcome 2 Obvious effects.
3.3. Analysis.
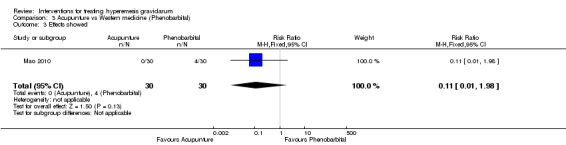
Comparison 3 Acupunture vs Western medicine (Phenobarbital), Outcome 3 Effects showed.
Comparison 4. Acupunture vs Chinese medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete recovery | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [3.06, 26.51] |
| 2 Obvious effects | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.84] |
| 3 Effects showed | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.57] |
| 4 Ineffective | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.60] |
| 5 Total effective rate | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.19, 2.17] |
4.2. Analysis.
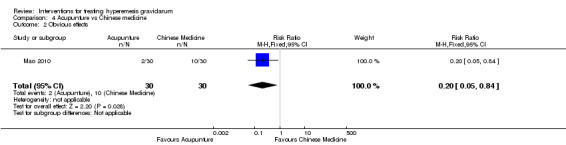
Comparison 4 Acupunture vs Chinese medicine, Outcome 2 Obvious effects.
4.3. Analysis.
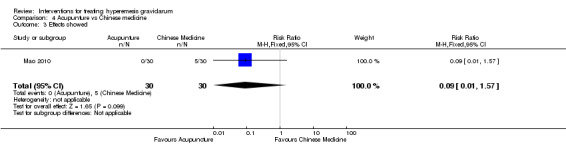
Comparison 4 Acupunture vs Chinese medicine, Outcome 3 Effects showed.
Comparison 5. Chinese medicine vs Western medicine (Phenobarbital).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete recovery | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.07] |
| 2 Obvious effects | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.69, 4.00] |
| 3 Effects showed | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.37, 4.21] |
| 4 Ineffective | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.43, 1.30] |
| 5 Total effective rate | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.79, 2.08] |
5.2. Analysis.
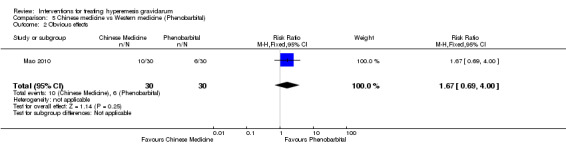
Comparison 5 Chinese medicine vs Western medicine (Phenobarbital), Outcome 2 Obvious effects.
5.3. Analysis.
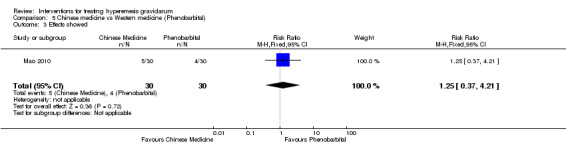
Comparison 5 Chinese medicine vs Western medicine (Phenobarbital), Outcome 3 Effects showed.
5.4. Analysis.
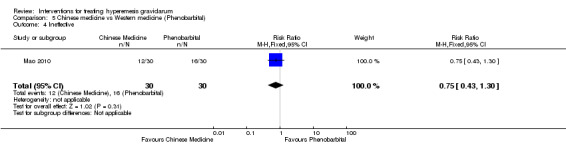
Comparison 5 Chinese medicine vs Western medicine (Phenobarbital), Outcome 4 Ineffective.
Comparison 6. Muscle relaxation and pharmacotherapy vs only pharmacotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Global Improvement score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.04, ‐0.04] |
Comparison 7. Midwife‐led outpatient care vs routine care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PUQE | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐3.17, 1.77] |
| 2 Hours of hospital admission | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐33.2 [‐46.91, ‐19.49] |
| 3 Decision to terminate the pregnancy | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 67.96] |
| 4 Spontaneous miscarriage | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.15, 6.34] |
| 5 Small for gestational age | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.26, 7.96] |
| 6 Economic cost | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.28, 3.87] |
Comparison 8. Holistic assessment with standard care vs standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PUQE | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.10, 0.70] |
| 2 Quality of life: social functioning | 1 | 198 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐6.70, 10.70] |
| 3 Quality of life: client satisfaction | 1 | 189 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.90, 0.90] |
Comparison 9. Dextrose saline vs normal saline rehydration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hours of hospital admission | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐10.78, 0.78] |
Comparison 10. Pyridoxine vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of episodes of emesis | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.40, 1.40] |
| 2 Days of hospital admission | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.08, 1.52] |
| 3 Hospital readmission | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.85, 3.71] |
| 4 Weight change (kg) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.93, 0.93] |
| 5 Interventions side effects: dizziness | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.85, 3.26] |
| 6 Interventions side effects: headaches | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.52, 3.42] |
| 7 Interventions side effects: diarrhea | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.07] |
| 8 Interventions side effects: palpitations | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.22, 4.60] |
| 9 Interventions side effects: dry mouth | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.38] |
Comparison 11. Metoclopramide vs ondansetron.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity of nausea | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐0.15, 3.55] |
| 2 Severity of vomiting | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.63, 1.43] |
| 3 Intervention side effects: felt drowsy | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [1.23, 4.69] |
| 4 Intervention side effects: dry mouth | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.10, 5.11] |
| 5 Intervention side effects: unable to sleep | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.28] |
| 6 Intervention side effects: felt dizzy | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.94, 5.77] |
| 7 Intervention side effects: diarrhea | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.49, 164.46] |
| 8 Intervention side effects: headache | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.54, 2.79] |
| 9 Intervention side effects: palpitations | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.50, 12.51] |
| 10 Intervention side effects: skin rash | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.71] |
| 11 Intervention side effects: dystonia | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Quality of life | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.83, 0.03] |
Comparison 12. Hydrocortisone vs metoclopramide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital readmission | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.28] |
| 2 Need for enteral or parenteral nutrition | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
Comparison 13. Metoclopramide vs promethazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.22, 1.22] |
| 2 Intervention side effects: unable to sleep | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.40, 1.53] |
| 3 Intervention side effects: dry mouth | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.34] |
| 4 Intervention side effects: diarrhea | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.32, 5.99] |
| 5 Intervention side effects: headache | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.47, 1.38] |
| 6 Intervention side effects: palpitations | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.25, 1.46] |
| 7 Intervention side effects: skin rash | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.32, 5.99] |
| 8 Intervention side effects: drowsy | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.56, 0.87] |
| 9 Intervention side effects: felt dizzy | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.69] |
| 10 Intervention side effects: dystonia | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.90] |
Comparison 14. Parenteral fluid with diazepam vs parenteral fluid without diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days of hospital admission | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.07, ‐0.13] |
| 2 Hospital readmission | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.29] |
| 3 Women requiring additional antiemetics | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
| 4 Congenital anomalies | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Preterm birth | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
| 6 Decision to terminate the pregnancy | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
Comparison 15. Ondansetron vs promethazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days of hospital admission | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.39, 1.39] |
| 2 Intervention side effect: sedation | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.94] |
Comparison 16. Corticosteroids vs promethazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe nausea 48 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.08, 3.72] |
| 2 Severe nausea 17th day | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.15] |
| 3 Episodes of vomiting 48 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 4 Episodes of vomiting 17th day | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.65] |
| 5 Therapy failure in 2 days | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.28, 8.04] |
| 6 Hospital readmission | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.53] |
| 7 Number of women requiring additional antiemetics | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.28, 8.04] |
| 8 Stillbirth and neonatal death | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 9 Preterm birth | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 10 Decision to terminate the pregnancy | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 11 Intevention side effects: abdominal pain 48 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.55] |
| 12 Intervention side effects: abdominal pain 3‐10 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.00] |
| 13 Intervention side effects: drowsiness 48 hours and 3‐10 days | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.32] |
| 14 Became completely or partially well 48 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.47, 0.95] |
| 15 Became completely or partially well 17th day | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.92] |
Comparison 17. Corticosteroids vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days of hospital admission | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.70, 0.10] |
| 2 Hospital readmission | 4 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.50, 0.94] |
| 3 Pregnancy complications | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.26, 1.47] |
| 4 Spontaneous abortion | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.70] |
| 5 Stillbirth and neonatal death | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.15, 3.34] |
| 6 Congenital abnormalities | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.73] |
| 7 Low birthweight | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.46, 4.00] |
| 8 Small‐for‐gestational age | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.21] |
| 9 Preterm birth | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.31, 3.28] |
| 10 Intervention side effects | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.06, 11.20] |
| 11 Women requiring additional antiemetic drugs | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.26, 1.17] |
| 12 Decision to terminate the pregnancy | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.45] |
Comparison 18. ACTH vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction or cessation in nausea/vomiting | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.65, 2.85] |
| 2 Weight gain (kg) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.34, 1.66] |
| 3 Hospital readmission | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.25] |
| 4 Spontaneous abortion | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 5 Preterm birth | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abas 2014.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women with singleton gestations of 16 weeks or less with clinical diagnosis of HG with clinical dehydration and ketonuria (2+ or greater) hospitalized for the first time with this diagnosis. 80 women randomized to interventions and 80 to controls. | |
| Interventions | 4 mg ondansetron IV infused over 10 minutes every 8 hours for 4 doses versus 10 mg metoclopramide IV infused over 10 minutes every 8 hours for 4 doses. | |
| Outcomes | Vomiting episodes, well‐being (10‐point visual numeric rating scale), nausea intensity (10‐point visual numeric rating scale), ketonuria all at 24 hours, treatment curtailment, open‐label use of IV metoclopramide after the study, length of hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated that randomization was carried out in blocks of 4 or 8 with sequence generated by computer. |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed, opaque envelopes were used to allocate treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 1 author prepared and labeled the solutions. Study drug packs were identical. Labeling of drugs was swapped periodically to prevent inadvertent elucidation of allocation from adverse effects. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Person self‐reported number of emesis episodes and recorded nausea in a diary. Maintenance of masking was high and person unlikely to be aware of treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of women lost to follow‐up was low, and accompanying reasons are reported. 4/80 and 3/80 from each group did not complete the trial drug. |
| Selective reporting (reporting bias) | Low risk | Methods state that predetermined outcomes were used and reported, all expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Bondok 2006.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women at less than 16 weeks' gestation with diagnosis of intractable HG (severe persistent vomiting, ketonuria, and weight loss > 5% of prepregnancy weight) necessitation ICU admission. 20 women randomized to interventions and 20 to controls. | |
| Interventions | 300 mg IV hydrocortisone daily with taper versus 10 mg metoclopramide IV every 8 hours. | |
| Outcomes | Number of vomiting episodes, readmission to ICU. | |
| Notes | 1 participant required TPN. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization conducted with a computer‐generated randomized list. |
| Allocation concealment (selection bias) | Low risk | The code was held and syringes used for both groups were identical in appearance. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study is described as a double‐blind study. It is stated that the personnel administering the drugs was masked to treatment. The description of the administration schedule is a little ambiguous but it seems that people in each group received a 10 mL injection every 8 hours. The syringes were identical in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses recording the number of episodes of emesis were blinded to the treatment, main investigators were also blinded to which participants were in which group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether anyone randomized to treatment withdrew from treatment or was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Ditto 1999.
| Methods | Randomized controlled trial. | |
| Participants | Women with HG at less than 16 weeks' gestation, defined by persistent nausea and vomiting for 1 week with at least 1 of the following: weight loss > 5% since beginning of symptoms, ketonuria (3% increase), serum potassium less than 3.4 mEq/L. 25 women randomized to interventions and 25 to controls. | |
| Interventions | IV saline, glucose, multivitamins with 10 mg IV twice daily and PO 5 mg bid diazepam on discharge versus IV saline, glucose, multivitamins without diazepam. | |
| Outcomes | Severity of nausea (VAS 0‐10), number of episodes of vomiting, hospital admission length, number of readmissions, pregnancy outcome, neonatal outcome. lack of improvement defined by persistent (> 5 x/day) vomiting. | |
| Notes | Lack of improvement defined as persistent (> 5 x/day) vomiting. Primary outcome reported as number of participants with improvement in figure form, specific numbers not reported. No response from authors in request for additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used to allocate participants. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the personnel assessing outcomes was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether anyone randomized to treatment withdrew from treatment or was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Duggar 2001.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women with admission diagnosis of HG. 14 women randomized to interventions and 11 to controls. | |
| Interventions | Methylprednisone PO versus placebo. | |
| Outcomes | Recurrence of vomiting after randomization, readmission, medication tolerance. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study is described as randomized, but details on method of randomization not available. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods of allocation concealment not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as double‐blind and it is stated that treatment in the placebo group "looked like" that in the methylprednisolone group. However, it is not stated who was masked to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the personnel assessing outcomes was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of people randomized to each group not reported. It is unclear whether anyone withdrew from treatment or was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | As a conference abstract, insufficient information reported to determine presence of selective reporting. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Fletcher 2015.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women at least 16 years old diagnosed with HG defined as need for admission with nausea and vomiting early in pregnancy, admitted within the previous 24 hours, excluded women if nausea/vomiting commenced after 14 weeks. | |
| Interventions | Intervention comprised creation of a bespoke treatment plan for each patient based on their response to the Hyperemesis Impact of Symptoms questionnaire. Scale based on 10 questions, scoring 0‐3. A score of 2 or more indicates woman needs support in that area. Treatment plan included practical and supportive care (dietary advice, practical advice on symptom management and advice on psychological impact of symptoms). These Women also received standard care (IV hydration plus antiemetic therapy). Comparison group received standard care alone. | |
| Outcomes | Womens' health status assessed using SF‐36 and EQ‐5D. PUQE score recorded. Client Satisfaction Questionnaire used to evaluate satisfaction with health care. Hospital readmission rate. Cost‐effectiveness. Primary outcome is social functioning, as assessed using the SF‐36. |
|
| Notes | Data for analysis taken from the 2‐week time point because this point had the highest response rate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated that randomization was carried out remotely by the York Trials Unit, using computer‐generated simple allocation. |
| Allocation concealment (selection bias) | Low risk | Allocation randomized remotely via telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. Unclear whether research personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded. Unclear whether research personnel were blinded. Outcomes were reported by participants, thus the fact that they were not blinded may influence outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of women lost to follow‐up was low, and accompanying reasons are reported. The proportion of women lost to follow‐up from each group was similar. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes, given the objective of the trial, are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Gawande 2011.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women at less than 12 weeks' gestation with HG defined by severe vomiting, dehydration, acidosis and hypokalemia. 15 women randomized to interventions and 15 to controls. | |
| Interventions | Progressive muscle relaxation daily for 2 weeks and pharmacotherapy versus pharmacotherapy alone. | |
| Outcomes | Number of antiemetics required, number of days to achieve complete response (no vomiting 24 hours), number of participants with recurrence after complete response, clinical global improvement at the end of 2 weeks (CGI score). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomized, but details on the method of randomization are not reported. |
| Allocation concealment (selection bias) | High risk | Treatment group received progressive muscle relaxation sessions, placebo group were called to psychiatric OPD but received no intervention. Treating obstetricians were reported to be blinded, details not specified, risk of compromise given that participants were not blinded. Methods used to conceal allocation not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treating obstetricians were reportedly blinded but participants were not, participants were called to psychiatric OPD sessions but did not receive a placebo intervention, so there is a high risk treating providers would still be able to determine group allocation by speaking with participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is stated that the "observer" was masked to treatment. It is unclear whether the observer is the person assessing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analyzed on all people randomized. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Habek 2004.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women with HG. 22 women randomized to interventions and 15 to controls. | |
| Interventions | 2 active interventions: 1: bilateral manual acupuncture of Pc6 acupoints (30 mins a day for 7 days); 2: bilateral acupressure of Pc6 acupoints (self‐applied for 30 mins when feeling nausea throughout the day). 2 placebo groups: 1: superficial acupuncture; 2: superficial acupressure. |
|
| Outcomes | Resolution of symptoms of nausea and vomiting, and lack or need for medication for treatment of symptoms. | |
| Notes | Outcome criteria were defined as disappearance of symptoms and no requirement for additional medication. However, efficacy was based on participant report and independent evaluation of the participant's clinical condition. No further details reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomized, but details on the method of randomization are not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as a double‐blind study. It is stated that the women and the clinician assessing therapeutic efficacy were masked to treatment. Sham treatment used to mask key personnel to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The clinician assessing therapeutic efficacy was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether anyone randomized to treatment withdrew from treatment or was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Heazell 2006.
| Methods | Randomized controlled trial. | |
| Participants | Women with nausea and vomiting on their first inpatient admission and between 5 and 14 weeks' gestation. Women also had to have at least 2+ of ketonuria on urinalysis, an inability to tolerate oral fluids and a requirement for antiemetic medication. 40 women randomized to interventions and 40 to controls. | |
| Interventions | Acupressure at the P6 meridian point (wristbands worn for 8 hours a day) versus placebo acupressure at a point on the dorsal aspect of the forearm (wristbands worn for 8 hours a day). | |
| Outcomes | Days of hospital admission, number of participants requiring 4 or more days, requirement of additional antiemetic treatment, amount of IV fluids required within 24 hours, and number of additional antiemetics required. Pregnancy outcome (SAB, TAB, PTD, IUFD, term delivery, congenital anomalies). | |
| Notes | No response from authors for request for additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated that women were randomly allocated to either the treatment or placebo group by an independent remote researcher with no prior knowledge of the participant. |
| Allocation concealment (selection bias) | Low risk | It is stated that a ticket that indicated either placebo or treatment group was drawn from an opaque bag. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to their assignment, identical bead was placed at an acupressure versus alternate forearm site. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the personnel assessing outcomes was masked to treatment. However, the outcomes assessed are predominantly objective outcomes (e.g., number of days of hospital stay) and at a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of women lost to follow‐up was low, and accompanying reasons are reported. The proportion of women lost to follow‐up from each group was similar. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Kashifard 2013.
| Methods | Randomized controlled trial. | |
| Participants | 18‐35 years, gestational age less than 16 weeks, vomiting 3 times a day with weight loss more than 3 kg and ketonuria. 34 women randomized to interventions and 49 to controls. | |
| Interventions | 10 mg metoclopramide PO 3 times daily versus ondansetron 4 mg PO 3 times daily. | |
| Outcomes | Severity of nausea (VAS 1‐10), number of vomiting episodes within 2 weeks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated that the randomized list was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as a double‐blind study, with investigators and participants masked to treatment. It is unclear whether the treatments were of similar appearance and, thus, whether masking could have been compromised. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators reported to be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether anyone randomized to treatment withdrew from treatment or was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Mamo 1995.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women in first trimester with severe pregnancy vomiting. 19 women randomized to interventions and 19 to controls. | |
| Interventions | Sea‐band acupressure versus placebo. | |
| Outcomes | Need for antiemetic medication, need for hospitalization. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomized, but details on the method of randomization are not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported on level of masking. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the personnel assessing outcomes was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether anyone randomized to treatment withdrew from treatment or was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | As a conference abstract, insufficient information reported to determine presence of selective reporting. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Mao 2010.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women age 20‐36 years with frequent vomiting, inability to tolerate food, dehydration, electrolyte abnormalities, diagnosed with HG by the Obstetrics and Gynaecology department; based on definition in a medical textbook. Study included women with gestation up to 12 weeks, although this was not a specified inclusion criterion. | |
| Interventions | Acupuncuncture ‐ standard care (hydration, electrolytes) plus acupuncture at BL11, ST37, PC6, SP4, RN12, ST36; OR Western medicine ‐ 30 mg luminal (phenobarbital) orally 3 times daily in addition to standard care; OR Chinese medicine based according to dialectical classification. | |
| Outcomes | Ketone bodies, CO2‐CP decline, electrolyte imbalance and severity of nausea and vomiting. Severity of nausea and vomiting defined as: Complete Recovery: Nausea and vomiting ceased and normal appetite returned. Obvious Effects: The frequency of nausea and vomiting reduced by over 50% and the appetite has increased. Effects Showed: The frequency of nausea and vomiting was reduced by 25%‐50% and the appetite has some slight increase. Ineffectiveness: Frequent vomiting continued, the reduction of vomiting frequency was below 25% and there was no change in appetite. |
|
| Notes | Data for analysis taken from day 7 time point as that was the completion of therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomized, but details on the method of randomization are not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details on level of masking unclear. Possibly an open‐label trial as it is likely to be difficult to mask the intervention (acupuncture versus oral medicine). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details on level of masking unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It is not stated whether anyone randomized to treatment withdrew from treatment or was lost to follow‐up. However, results are reported based on all women analyzed (total 30 women in each group at the 2 time points assessed). |
| Selective reporting (reporting bias) | Unclear risk | Although outcomes captured were described and absolute event rates for some clinical outcomes are reported, methods to measure said outcomes were not specified, so it is unclear whether key or expected outcomes were measured appropriately and reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
McParlin 2008.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women with severe nausea and vomiting in pregnancy. 27 women randomized to interventions and 26 to controls. | |
| Interventions | Outpatient care: rapid IV rehydration (3 liters over 6 hours), and IV cyclizine, followed by discharge home with oral cyclizine, and an advice leaflet. Participants also received ongoing midwifery support through 2 follow‐up telephone calls versus inpatient admission and routine care. | |
| Outcomes | Physical symptoms evaluated by the pregnancy unique quantification of emesis and vomiting score on admission and at 7 days. Quality of life measured on days 1 and 7 using the SF36.v2 score. Readmission rate and admission time. | |
| Notes | Abstract only. Outcomes were not pre‐specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomized, but details on the method of randomization are not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details on method used to conceal allocation not available, given nature of intervention not possible to blind those who participated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details on method used to conceal allocation not available, given nature of intervention not possible to blind those who participated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the personnel assessing outcomes was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Itis unclear whether anyone randomized to treatment withdrew from treatment or was lost to follow‐up. There was only 69% protocol adherence in the intervention group; effect on outcome unclear. |
| Selective reporting (reporting bias) | Unclear risk | As a conference abstract, insufficient information reported to determine presence of selective reporting. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Miller 2001.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women at 6‐12 weeks' gestation with severe nausea and vomiting. 45 women randomized to interventions and 28 to controls. | |
| Interventions | Nerve stimulation therapy (with a watch‐like device) over the volar aspect of the wrist at the P6 point (Reliefband) versus placebo. | |
| Outcomes | Rhodes index of nausea, vomiting, and retching and 1, 2, and 3 weeks. Medication use, weight gain, urinary ketones. | |
| Notes | Abstract only, specific data not reported, unable to contact author for additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomized, but details on method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details on level of masking unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details on level of masking unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Conference abstract only, unclear whether any person was lost to follow‐up or withdrew from treatment. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, unclear whether all outcomes reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Nelson‐Piercy 2001.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women with severe or prolonged HG, with onset of symptoms before 12 weeks' gestation. Women were also dependent on IV fluids for at least 1 week (first admission for HG) or for 24 hours (second or subsequent admission for HG), were receiving regular treatment with at least 1 antiemetic, had ketonuria on admission, no infection (as evidence by mid‐stream urine sample), normal random blood glucose (unless known diabetic), vomiting at least twice a day, or nausea so severe they were unable to eat or drink, and were receiving thiamine. 12 women randomized to interventions and 13 to controls. | |
| Interventions | Prednisolone 20 mg orally every 12 hours for 1 week. If after 72 hours, a woman was still vomiting and was dependent on IV fluid, the regimen was changed to an IV equivalent versus placebo at same dosing regimen as prednisolone (either oral tablet or saline). | |
| Outcomes | Frequency of vomiting (vomiting score measured on a scale from 0 to 4), dependence on IV fluids after 1 week of treatment, length of hospital stay, duration of IV fluid therapy after randomization, severity of nausea (measured on a scale from 0 to 10), need for antiemetics, presence of ptyalism, well‐being rating (measured on a scale from 0 to 10), intake of oral fluids and food, change in thyroid function tests, change in liver function tests, weight gain. Pregnancy outcomes: birthweight, preterm delivery, gestational age of delivery, birthweight < 5th percentile, stillbirth, multiple gestation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated that women were randomly allocated individually using a computer‐generated allocation schedule. |
| Allocation concealment (selection bias) | Low risk | It is stated that each center was allocated sequentially numbered trial packs held in the pharmacy, and each pharmacy held a copy of the allocation schedule. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is stated that the clinicians (assessors), nurses, midwives and participants were blinded to the study medication. The prednisolone tablets were identical in appearance to the placebo tablets. The local pharmacists were blinded until the need for IV therapy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The clinician assessing outcomes was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 woman withdrew from the study due to pregnancy termination on day 1. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | High risk | Per the authors' report, this study was prematurely halted due to "a combination of different factors in different centers, including the departure of key members of staff, and the erroneous belief that steroids had had such a dramatic beneficial effect that continued randomization of women was not justified". |
Neri 2005.
| Methods | Randomized controlled trial. | |
| Participants | Women with HG and who had a singleton pregnancy, were at less than 12 weeks' gestation, and had a diagnosis of HG based on the commonly accepted criteria of nausea and vomiting leading to clinical symptoms of dehydration and weight loss > 5%. 43 women randomized to interventions and 38 to controls. | |
| Interventions | Acupuncture (includes stimulation at 5 acupoints) twice a week for 2 weeks. Women were also advised to wear a device giving acupressure at the Pc6 point (worn for 6‐8 hours per day) versus metoclopramide infusion (20 mg/500 mL saline infused over 60 mins) twice a week for 2 weeks. Oral treatment was supplemented with vitamin B12 (30 mg/day). | |
| Outcomes | Number of participants with improved intensity of nausea, improved episodes of vomiting, improved rate of food intake, daily functioning. Pregnancy outcome (gestational age at delivery, birthweight, rate of cesarean section). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated that a computer‐generated random list was used to allocate women to treatment group: odd and even numbers formed the basis of allocation to treatment. |
| Allocation concealment (selection bias) | Low risk | Allocation code held under the control of a midwife. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo used, participants not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the personnel assessing outcomes was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Results are based on 81 out of 88 women randomized. The number of women lost from the metoclopramide group was considerably higher than that from the acupressure group (6 women versus 1 woman, respectively). 1 woman withdrew from acupuncture group due to perceived inefficacy. 4 women refused to take metoclopramide, and 2 had spontaneous abortions at 10 weeks. The influence of this imbalance on estimate of effect is unclear. |
| Selective reporting (reporting bias) | Low risk | Although the protocol of the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Safari 1998.
| Methods | Randomized controlled trial. | |
| Participants | Women with an intrauterine pregnancy of gestation of 16 weeks or less and diagnosis of HG (persistent vomiting, large ketonuria, and weight loss). If nausea and vomiting did not resolve after IV hydration, or if a woman had been previously admitted to hospital for hyperemesis, they were offered participation in the study. 20 women randomized to interventions and 20 to controls. | |
| Interventions | Methylprednisolone 16 mg orally 3 times a day for 3 days, followed by a tapering regimen (halving of dose every 3 days) to none during the course of 2 weeks versus promethazine 25 mg tablets 3 times a day for 2 weeks. | |
| Outcomes | Improvement of symptoms within 2 weeks of starting therapy. Lack of improvement was defined as persistent vomiting (> 5 times a day), inability to tolerate liquids by mouth, or participant's impression that she was not better, readmission to hospital. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated that a computer‐generated random list was used to allocate women to treatment group. |
| Allocation concealment (selection bias) | Low risk | It is stated that "Envelopes containing the study assignment were prepared in advance and sequentially labeled by a third party not involved in the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The primary investigators, attending physicians, and the participants were masked to treatment. However, Nurses dispensing the medication were able to observe the difference in the shape of the pills but were not informed which pills corresponded to which medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Attending physician was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of women lost to follow‐up was low, with the same number of women lost from each group (3 women, 6 women in total). |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine the presence of other forms of bias. |
Shin 2007.
| Methods | Randomized controlled trial. | |
| Participants | Women diagnosed with HG, defined as consistent nausea and vomiting, electrolyte imbalance, more than 5% loss of weight, dehydration, positive ketonuria, and increased urine specific gravity. Women were also aged 20 to 40 years and at gestation of 5 to 30 weeks. Women were receiving only conventional IV fluid therapy. Women had no other complications of pregnancy. 23 women randomized to interventions and 22 to controls. | |
| Interventions | Acupressure at the P6 meridian point. Pressure was applied for 7 seconds with 2‐second pauses, 3 times daily before breakfast, lunch and dinner. Each session lasted 10 minutes versus placebo acupressure (as for acupressure but pressure applied at a bony part around the radial pulse) versus control (no treatment other than conventional IV therapy). | |
| Outcomes | Degree of nausea and vomiting (measured using a modified version of the Rhodes Index; score between 6 and 30), ketonuria. | |
| Notes | Insufficient reporting to determine the presence of other forms of bias. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by coin toss. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study is described as double‐blind. The nurses administering treatment were not aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported to be double‐blind, however unclear whether or how the person assessing outcomes was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether anyone randomized to treatment withdrew from treatment or was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine the presence of other forms of bias. |
Sullivan 1996.
| Methods | Randomized controlled trial. | |
| Participants | Women with severe HG during the first and second trimesters of pregnancy that had not been previously treated by IV medication or hospitalization. Women also had to have 2 of: at least a 5‐pound weight loss compared with the initial prenatal visit or previous record; ketonuria > 80 mg/dL in a random urine specimen; hypokalemia (potasium < 3.0 mEq/dL) or hyponatremia (sodium < 134 mEq/dL requiring IV replacement; positive test result for serum acetone; or more than 2 visits to the obstetric emergency department requiring IV hydration or promethazine suppositories. 15 women randomized to interventions and 15 to controls. | |
| Interventions | Ondansetron 10 mg IV every 8 hours (infused over 30 minutes) versus promethazine 50 mg IV every 8 hours (infused over 30 minutes). | |
| Outcomes | Severity of nausea (assessed on a VAS; 10 cm scale), duration of hospital stay, treatment failure, daily weight gain, antiemetic usage, and adverse effects. | |
| Notes | Unclear how many women were randomized. Seems to be 30, but states that 30 were evaluable, which suggests more women were randomized than analyzed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomized, but details on method of randomization are not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details on method used to conceal allocation not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study is described as double‐blind, but it is unclear who was masked to treatment. Although it is unclear who was masked to treatment, maintenance of masking seems adequate: the infusion solution was marked as "hyperemesis study drug" and the infusion bag covered with a plain brown bag. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether personnel assessing outcomes was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether anyone randomized to treatment withdrew from treatment or was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Tabatabaii 2008.
| Methods | Randomized controlled trial. | |
| Participants | Pregnant women with HG in first half of pregnancy. 48 women randomized to interventions and 48 to controls. | |
| Interventions | Methylprednisolone (125 mg) IV infusion followed by an oral prednisone taper (40 mg for 1 day, 20 mg for 3 days, 10 mg for 3 days, 5 mg for 7 days) versus placebo. Both groups also received 100 mg vitamin B6 daily. | |
| Outcomes | Number of women requiring rehospitalization for HG. | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomized, but details on the method of randomization are not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is described as double‐blind but details on who was masked, or how masking was maintained (other than that the placebo infusion was identical in appearance to the methylprednisolone infusion), are not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether personnel assessing outcomes were masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether anyone randomized to treatment withdrew from treatment or was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | As a conference abstract, insufficient information reported to determine the presence of selective reporting. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Tan 2009.
| Methods | Randomized controlled trial. | |
| Participants | Women with singleton gestation at less than 20 weeks and with presumed HG (severe nausea and vomiting during pregnancy with clinical features warranting hospitalization). Women were experiencing their first hospital admission for HG and were enrolled within 12 hours of admission. 47 women randomized to interventions and 45 to controls. | |
| Interventions | Oral pyridoxine (20 mg 3 times daily from admission to 2 weeks after hospital discharge) versus placebo. | |
| Outcomes | Readmission rate for HG in the 2 weeks after hospital discharge, daily vomiting episodes at home by diary, and nausea score at enrollment, hospital discharge and week 1 and 2 reviews, adverse effects. Other outcomes were admission to discharge interval, compliance, body weight, ketonuria, dry retching episodes by diary and an overall well‐being score using a 10‐point VAS (higher score denotes greater well‐being). Nausea evaluated with a 10‐point VAS (higher score denotes more severe nausea). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was randomly generated in blocks of 10. Randomization was carried out by opening the next available envelope. |
| Allocation concealment (selection bias) | Low risk | Envelopes were sealed and opaque. Treatment allocation was not revealed to the participants or providers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treatment allocation was not revealed to the participants or the providers. At hospital discharge, women were supplied their allocated medication in identical packaging. Pyridoxine tablets were white. Placebo tablets were white "tic tacs", which have a mint flavor. It is unclear whether tic tacs are sufficiently similar in appearance or taste to pyridoxine tablets to maintain the masking of treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It is not stated whether the personnel assessing outcomes was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of women lost to follow‐up was low with similar rates of attrition between groups at week 1, < 20%, similar rate of attrition between groups at week 2, > 20%. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine the presence of other forms of bias. |
Tan 2010.
| Methods | Randomized controlled trial. | |
| Participants | Women with singleton gestation at 16 weeks or less and with presumed HG (dehydration and detectable ketonuria by urine dipstick test). Women were experiencing their first hospital admission for HG. 73 women randomized to interventions and 76 to controls. | |
| Interventions | Metoclopramide 10 mg IV (infused over 1 to 2 mins) Treatment given just after randomization and again at 8, 16 and 24 hours versus Promethazine 25 mg IV (infused over 1 to 2 mins) Treatment given just after randomization and again at 8, 16 and 24 hours |
|
| Outcomes | Vomiting episodes, severity of nausea (as measured using a 10‐point VAS), well‐being (as measured using a 10‐point VAS), ketonuria, treatment curtailment, total doses of IV antiemetic during admission, interval of admission, time needed for IV rehydration, adverse effects. | |
| Notes | Contact authors regarding the numbers for each group for primary outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation sequence was computer‐generated in random blocks of 4 or 8. Women were assigned randomly by the sequential opening of numbered, sealed, opaque envelopes stating “Drug A” or “Drug B”. |
| Allocation concealment (selection bias) | Low risk | Envelopes were sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant allocation was concealed and study drugs were in identical vials. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the personnel assessing the outcomes was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 6 out of 149 did not complete symptom profile questionnaire, the proportion of women lost from each group was similar. |
| Selective reporting (reporting bias) | Low risk | Preset primary and secondary outcomes. |
| Other bias | Unclear risk | Insufficient reporting to determine the presence of other forms of bias. |
Tan 2013.
| Methods | Randomized controlled trial. | |
| Participants | Women aged 18 years or older, with singleton gestation at 16 weeks or less and with presumed HG (intractable nausea and vomiting of pregnancy with dehydration and starvation clinically judged to require hospitalization for IV rehydration and antiemetic drug administration). Women also had ketonuria by urine dipstick of at least 1+ on admission, plasma glucose 110 mg/dL or less, and sodium 125 mmol/L or greater. Women were experiencing their first hospital admission for HG and were enrolled within 2 hours of admission to the ward. 111 women randomized to interventions and 111 to controls. |
|
| Interventions | 5% dextrose–0.9% saline (IV infusion at a rate 125 mL/h over 24 hours) versus 0.9% saline (IV infusion at a rate 125 mL/h over 24 hours). All women also received oral thiamine daily plus IV antiemetic. |
|
| Outcomes | Severity of nausea (as measured using a 10‐point VAS), well‐being (as measured using a 10‐point VAS), ketonuria, frequency of vomiting, hyponatremia (135 mmol/L or less), hypokalemia (3.5 mmol/L or less), hypochloremia (99 mmol/L or less), hyperglycemia (8 mmol/L or greater), duration of IV antiemetic and IV rehydration during admission, interval of admission, time to oral intake. | |
| Notes | Women already under IV rehydration therapy were not eligible for enrollment. All women also received a multivitamin containing thiamine and IV antiemetic. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated that the allocation sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Envelopes were sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was double‐blind. Participants and healthcare providers were masked to treatment. IV solutions were prepared in 500 mL containers with the manufacturer’s label removed and the container relabeled as solution A or B. The solutions and containers were identical in appearance, with the exception of labels (A or B). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the personnel assessing outcomes were masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of women lost to follow‐up was low and similar between groups. 2/111 and 1/111 in each group withdrew, 7/111 and 9/111 in each group excluded for pre‐specified criteria. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias. |
Ylikorkala 1979.
| Methods | Randomized controlled trial. | |
| Participants | Women admitted to hospital because of HG and whose vomiting did not stop or decrease significantly during their first 2 days in hospital. 16 women randomized to interventions and 16 to controls. | |
| Interventions | Synthetic ACTH (tetracosactid) 0.5 mg IM on 4 consecutive days versus placebo IM on 4 consecutive days. | |
| Outcomes | Symptom severity, daily number of vomiting attacks, weight gain/loss, serum cortisol and urine steroids. | |
| Notes | Symptom severity was evaluated using a scoring system designed by the authors. Scoring system differed across symptoms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is described as randomized, but details on method of randomization are not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is described as double‐blind, however details on who was masked to the treatment are not reported. It is stated that treatments were numbered and similar in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is stated that nurses counted the daily number of episodes of emesis. It is unclear whether the nurses were masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No‐one withdrew from the study. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Insufficient reporting to determine the presence of other forms of bias. |
Yost 2003.
| Methods | Randomized controlled trial. | |
| Participants | Women at less than 20 weeks' gestation with HG, and who had not responded to outpatient therapy and who had 3+ or 4+ dipstick urinary ketones (evidence of dehydration). 64 women randomized to interventions and 62 to controls. | |
| Interventions | Methylprednisolone 125 mg IV, followed by a tapering regimen of oral prednisone (40 mg for 1 day, 20 mg for 3 days, 10 mg for 3 days, and 5 mg for 7 days). Standard of care also included IV fluids, metoclopramide, and promethazine versus placebo IV followed by oral placebo tablets (tapering regimen). Standard of care also included IV fluids, metoclopramide, and promethazine. |
|
| Outcomes | Number of ER visits, hospital readmission, number of hospital admissions, hospital length of stay, total hospital days for all admissions in pregnancy, pregnancy outcomes (SAB, gestational diabetes, pregnancy hypertension, preterm delivery < 26 weeks, cesarean delivery), neonatal outcomes (gender, anomalies, birthweight, IUGR, stillbirth, neonatal death). | |
| Notes | Study was under powered to detect a difference between groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated that randomization was performed by computer‐generated blocks of 20. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation are not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women were reported to be blinded and the intervention and placebo were identical in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the personnel assessing outcomes was masked to the treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A similar number of women, < 15%, was lost to follow‐up in each group, accompanying reasons are reported. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Based on the reported power calculations, the study may have been underpowered to identify a statistically significant difference, unclear what effect this may have had. Insufficient reporting to determine presence of other forms of bias. |
Ziaei 2004.
| Methods | Randomized controlled trial. | |
| Participants | Women at between 6 and 12 weeks' gestation and vomiting more than 3 times per day during the last 72 hours or ketonuria that did not respond to dietary manipulation and caused weight loss. 39 women randomized to interventions and 39 to controls. | |
| Interventions | Prednisolone 5 mg/day orally in the morning for 10 days versus promethazine 25 mg 3 times daily (oral) for 10 days. | |
| Outcomes | Severity of nausea (VAS 10‐point scale), number of vomiting episodes per day, response to treatment, adverse effects (abdominal pain, drowsiness). Participants were also asked to rate how ill they felt (became completely or partially well, no change or became worse). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated that randomization was carried out using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Details on methods used to conceal allocation not available.. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is stated that the main investigators did not which participants were placed in each group. It is unclear whether the participants were masked to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether the personnel assessing outcomes was masked to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of women lost to follow‐up was low, and accompanying reasons are reported. The proportion of women lost to follow‐up was similar in each group, 1/40 women in each group were lost to follow‐up on the 17th day. |
| Selective reporting (reporting bias) | Low risk | Although the protocol for the trial is not available, expected clinical outcomes are reported. |
| Other bias | Unclear risk | Based on the reported power calculations, the study may have been underpowered to identify a statistically significant difference, unclear what effect this may have had. Insufficient reporting to determine presence of other forms of bias. |
ACTH: adrenocorticotropic hormone CGI: clinical global improvement ER: emergency room HG: hyperemesis gravidarum ICU: intensive care unit IM: intramuscular IUFD: intrauterine fetal demise IUGR: intrauterine growth restriction IV: intravenous OPD: outpatient department PO: oral administration PTD: preterm delivery PUQE: pregnancy‐unique quantification of emesis and nausea SAB: spontaneous abortion SF‐36v2: Short form 36, version 2 TAB: therapeutic/elective abortion TPN: total parenteral nutrition VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamczak 2007 | Not a study on hyperemesis gravidarum. |
| Carlsson 2000 | Cross‐over design. |
| Dehkordi 2013 | Study excluded participants with severe nausea and vomiting, not a study on hyperemesis gravidarum. |
| Erez 1971 | Not a study on hyperemesis gravidarum. |
| Ferruti 1982 | Not a study on hyperemesis gravidarum. |
| Fischer‐Rasmussen 1991 | Cross‐over design. |
| Ghahiri 2011 | Not randomized. Not a study on hyperemesis gravidarum. |
| Gordon 2013 | Letter to editor, not a study. |
| Kadan 2009 | Cross‐over design. Not a study on hyperemesis gravidarum. |
| Koren 2006 | Study on pre‐emptive treatment or prophylaxis, not for treatment of diagnosed hyperemesis gravidarum. |
| Koren 2010 | Not a study on hyperemesis gravidarum. |
| Koren 2013 | Study on pre‐emptive treatment or prophylaxis, not for treatment of diagnosed hyperemesis gravidarum. |
| Koren 2015 | Not a study specifically on hyperemesis gravidarum. |
| Lask 1953 | Not clearly a study on hyperemesis gravidarum. |
| Ling 1994 | Quasi‐randomized. |
| Liu 1994 | Not clear whether study was randomized or quasi‐randomized |
| Madegard‐Linh 2004 | Cross‐over design. |
| Magee 1996 | Case report, not randomized controlled trial |
| Maina 2012 | Cross‐over design. |
| Maina 2014 | Cross‐over design. |
| Maltepe 2012 | Study on pre‐emptive treatment or prophylaxis, not for treatment of diagnosed hyperemesis gravidarum. |
| Matok 2013 | Not a randomized controlled trial |
| Matok 2014 | Not a randomized controlled trial |
| McCarthy 2014b | Not a study on hyperemesis gravidarum. |
| Nguyen 2008 | Not a study on hyperemesis gravidarum. |
| Oliveira 2014 | Not a study on hyperemesis gravidarum. |
| Ozgoli 2009 | Not a study on hyperemesis gravidarum. |
| Ozgoli 2011 | Not a study on hyperemesis gravidarum. |
| Price 1964 | Not a study on hyperemesis gravidarum. |
| Rad 2010 | Not a study on hyperemesis gravidarum. |
| Rosen 2003 | Not a study on hyperemesis gravidarum. |
| Shin 2005 | Quasi‐randomized. |
| Weiner 1990 | Letter, not a trial. |
| Wibowo 2012 | Not a study on hyperemesis gravidarum. |
| Willetts 2003 | Not a study on hyperemesis gravidarum. |
Characteristics of studies awaiting assessment [ordered by study ID]
Eftekhari 2013.
| Methods | Randomized controlled trial |
| Participants | Pregnant women under 20 weeks gestation diagnosed with hyperemesis gravidarum. |
| Interventions | Ondansetron and promethazine. |
| Outcomes | Treatment response and side effects, further details of outcomes not able to be determined. |
| Notes | Full translation not available. |
He 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Translation not available. |
Characteristics of ongoing studies [ordered by study ID]
Cyna 2008.
| Trial name or title | Hypnosis for nausea and vomiting in early pregnancy: a randomized controlled trial |
| Methods | Randomized controlled trial. |
| Participants | Pregnant women suffering from nausea and vomiting of pregnancy. |
| Interventions | Usual care (supportive and pharmacological medication and/or intravenous fluids as required) plus audio CD on hypnosis lasting 1/2 hour for 7 consecutive days versus usual care. |
| Outcomes | Suffering associated with nausea and vomiting as measured by a 5‐point Likert scale, incidence of nausea in previous 24 hours, incidence of vomiting in previous 24 hours, anxiety as measured by Spielberger, number of days off work. |
| Starting date | February 2007. |
| Contact information | Dr. A. M. Cyna: allan.cyna@cywhs.sa.gov.au |
| Notes | Still recruiting, results not yet published, authors contacted for further information. |
Guttuso 2014.
| Trial name or title | Comparison of gabapentin and metoclopramide for treating hyperemesis gravidarum |
| Methods | Randomized controlled trial. |
| Participants | Women 18years or older at less than 16 weeks gestation who have required at least 2 administrations of IV hydration 1 week apart, or daily emesis for the last 14 days and 1 administration of IV hydration, with at least 1 of the following: 3‐4+ ketonuria, serum potassium < 3.4 mmol, or > 5% weight loss from initial antenatal weight, having failed therapy with at least 1 antiemetic, excluding other medical problems that could contribute to symptoms, with a PUQE score of >=12 for the 24‐hour baseline. |
| Interventions | Gapabentin versus metoclopramide. |
| Outcomes | Mean per cent change from baseline to study endpoint in daily PUQE score. Mean per cent change from baseline to study endpoint in individual PUQE score, daily oral nutrition scores, days of hospital admission, NVPQOL questionnaire, and relief score; need for repeat IV hydration or hospital admission for hyperemesis; per cent of participants choosing to continue with experimental therapy, per cent of participants downgrading from an answer of 3‐5 at Baseline to 1‐2 at study endpoint, maternal side effects and pregnancy outcomes, mean per cent change in laboratory values, mean per cent change from baseline to days 26‐28 in daily PUQE and NVPQOL scores, mean satisfaction questionnaire scores day 28, per cent of participants downgrading from an answer of 3‐5 at baseline to 1‐2 at day 28 on the HGPTC questionnaire. |
| Starting date | June 2014. |
| Contact information | Thomas Guttuso, Jr MD: tguttuso@buffalo.edu |
| Notes | Currently recruiting. |
Koren 2014.
| Trial name or title | A multicenter trial of the efficacy and safety of Diclegis® for nausea and vomiting of pregnancy in pregnant adolescents |
| Methods | Randomized controlled trial. |
| Participants | Pregnant women ages 12‐17 between 7‐14 weeks' gestation suffering from nausea and vomiting of pregnancy with a PUQE score >= 6. |
| Interventions | Diclegis 2 tablets at bedtime, increasing to 4 tablets as needed, for 14 days, versus placebo. |
| Outcomes | Nausea and vomiting of pregnancy severity from baseline to day 15 using PUQE score and Global Assessment of Well‐being scores, severity and occurrences of maternal adverse events. |
| Starting date | February 2014. |
| Contact information | Gideon Koren,MD‐ Hospital for Sick Children, 555 University Avenue, Toronto ON Canada, M5G‐1X8 |
| Notes | Currently recruiting. |
Mehrolhasani 2012.
| Trial name or title | Comparison of Demitron and promethazine in treatment of hyperemesis gravidarum. |
| Methods | Randomized controlled trial. |
| Participants | Pregnant women with gestation of 20 weeks or less, suffering from dehydration due to nausea and vomiting. |
| Interventions | Demitron 8 mg intramuscular every eight hours for 48 hours versus promethazine 25 mg intramuscular every eight hours for 48 hours. |
| Outcomes | Nausea and vomiting at 48 hours determined by a questionnaire, adverse drug reactions. |
| Starting date | April 2011. |
| Contact information | Yasamin Mehrolhasani(MD)‐ yasamin_m@yahoo.com |
| Notes | Recruitment completed, authors contacted for results. |
Mitchell‐Jones 2014.
| Trial name or title | Hyperemesis in Pregnancy (HIP) Trial: Inpatient versus outpatient management of severe nausea and vomiting in pregnancy |
| Methods | Randomized controlled trial. |
| Participants | Pregnant women < 20 weeks gestation with symptoms of hyperemesis gravidarum and at least 1+ ketonuria, excluding women with another medical condition that may induce nausea and vomiting, diabetes. Potassium < 3.2, sodium < 130, or abnormal liver or thyroid function tests. |
| Interventions | Rapid outpatient rehydration versus inpatient standard care. |
| Outcomes | Daily PUQE score, eating and drinking score, well‐being score, weight, and blood test results, repeat attendance and admissions, weight change at 7 days, number of days of IV fluids needed, number of women still taking antiemetics 1 week after discharge, costs of treatment. |
| Starting date | 1/3/2014 |
| Contact information | Nicola Mitchell‐Jones Chelsea and Westminster Hospital 369 Fulham Road London SW10 9NH United Kingdom nicola.mitchell‐jones@chelwest.nhs.uk |
| Notes | Currently recruiting. |
HG: hyperemesis gravidarum HGPTC: Hyperemesis Gravidarum Pregnancy Termination Consideration IV: intravenous NVPQOL: Health‐Related Quality of Life for Nausea and Vomiting during Pregnancy PUQE: pregnancy‐unique quantification of emesis and nausea
Differences between protocol and review
We edited one of our primary outcomes from 'Reduction or cessation in nausea/vomiting' to 'Severity, reduction or cessation in nausea/vomiting' because it was found that this was often what was reported.
We edited one of our secondary outcomes from 'Number of antiemetics required' to 'Number of women requiring additional antiemetics,' again because this outcome was more often reported than number of antiemetics.
Contributions of authors
Rupsa C Boelig: contact person and guarantor of review, drafted initial protocol and final review, reviewed articles for inclusion, conducted data extraction and quality analysis.
Vincenzo Berghella: helped to initiate this review, edited protocol, provided both methodological and clinical perspective.
Anthony J Kelly: helped to initiate this review, provided both methodological and clinical perspective, reviewed discrepancies in articles of inclusion, data extraction, and quality analysis.
Steven J Edwards: provided methodological perspective.
Samantha J Barton: provided methodological perspective, reviewed articles for inclusion, conducted data extraction and quality analysis.
Gabriele Saccone: conducted data analysis/meta‐analysis and constructed accompanying figures and tables.
Declarations of interest
Rupsa C Boelig: none known.
Samantha J Barton: none known.
Gabriele Saccone: none known.
Anthony J Kelly:none known.
Steve J Edwards: none known.
Vincenzo Berghella:none known.
Edited (no change to conclusions)
References
References to studies included in this review
Abas 2014 {published data only}
- Abas MN, Tan PC, Azmi N, Omar SZ. Ondansetron compared with metoclopramide for hyperemesis gravidarum. Obstetrics & Gynecology 2014;123(6):1272‐9. [DOI] [PubMed] [Google Scholar]
- Omar SZ. Ondansetron versus metoclopramide for hyperemesis gravidarum. ISRCTN Registry (http://www.isrctn.com/) [accessed 28 October 2015] 2012.
Bondok 2006 {published data only}
- Bondok RS, Sharnouby NM, Eid HE, Abd Elmaksoud AM. Pulsed steroid therapy is an effective treatment for intractable hyperemesis gravidarum. Critical Care Medicine 2006;34(11):2781‐3. [DOI] [PubMed] [Google Scholar]
Ditto 1999 {published data only}
- Ditto A, Morgante G, la‐Marca A, De‐Leo V. Evaluation of treatment of hyperemesis gravidarum using parenteral fluid with or without diazepam. Gynecologic and Obstetric Investigation 1999;48:232‐6. [DOI] [PubMed] [Google Scholar]
Duggar 2001 {published data only}
- Duggar CR, Carlan SJ. The efficacy of methylprednisolone in the treatment of hyperemesis gravidarum: a randomized double‐blind controlled study [abstract]. Obstetrics & Gynecology 2001;97(4 Suppl):45S. [DOI] [PubMed] [Google Scholar]
Fletcher 2015 {published data only}
- Fletcher SJ, Waterman H, Nelson L, Carter LA, Chuang LH, Roberts C, et al. The effectiveness and cost‐effectiveness of a holistic assessment and individualised package of care of women with hyperemesis gravidarum: randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):552‐3. [Google Scholar]
- Fletcher SJ, Waterman H, Nelson L, Carter LA, Dwyer L, Roberts C, et al. Holistic assessment of women with hyperemesis gravidarum: a randomised controlled trial. International Journal of Nursing Studies 2015;52(11):1669‐77. [DOI] [PubMed] [Google Scholar]
Gawande 2011 {published data only}
- Gawande S, Vaidya M, Tadke R, Kirpekar V, Bhave S. Progressive muscle relaxation in hyperemesis gravidarum. Journal of SAFOG 2011;3(1):28‐32. [Google Scholar]
Habek 2004 {published data only}
- Habek D, Barbir A, Habek JC, Janculiak D, Bobic‐Vukovic M. Success of acupuncture and acupressure of the pc 6 acupoint in the treatment of hyperemesis gravidarum. Forschende Komplementarmedizin und Klassische Naturheilkunde 2004;11(1):20‐3. [DOI] [PubMed] [Google Scholar]
Heazell 2006 {published data only}
- Heazell A, Thorneycroft J, Walton V, Etherington I. Acupressure for the in‐patient treatment of nausea and vomiting in early pregnancy: a randomized control trial. American Journal of Obstetrics and Gynecology 2006;194(3):815‐20. [DOI] [PubMed] [Google Scholar]
Kashifard 2013 {published data only}
- Kashifard M, Basirat Z, Kashifard M, Golsorkhtabar‐Amiri M, Moghaddamnia A. Ondansetrone or metoclopromide? Which is more effective in severe nausea and vomiting of pregnancy? A randomized trial double‐blind study. Clinical and Experimental Obstetrics and Gynecology 2013;40(1):127‐30. [PubMed] [Google Scholar]
- Pasha NG. A comparison of ondansetron vs. metoclopramide for treatment of nausea and vomiting in pregnancy. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
Mamo 1995 {published data only}
- Mamo J, Mamo D, Pace M, Felice D. Evaluation of sea‐band acupressure device for early pregnancy nausea and vomiting. 27th British Congress of Obstetrics And Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:283.
Mao 2010 {published data only}
- Mao ZN, Liang CE. Observation on the therapeutic effects of acupuncture on hyperemesis gravidarum. International Journal of Clinical Acupuncture 2010;19(2):60‐5. [Google Scholar]
- Mao ZN, Liang CE. Observation on therapeutic effect of acupuncture on hyperemesis gravidarum. Zhongguo Zhenjiu//Chinese Acupuncture & Moxibustion 2009;29(12):973‐6. [PubMed] [Google Scholar]
McParlin 2008 {published data only}
- McParlin C, Carrick‐Sen D, Steen IN, Taylor P, Robson SC. Hyperemesis in pregnancy study: a randomised controlled trial of midwife‐led "outpatient" care. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa9. [Google Scholar]
- Robson SC. Hyperemesis in pregnancy study (ongoing trial). Current Controlled Trials (www.controlled‐trials.com) (accessed 15 February 2007) 2007.
Miller 2001 {published data only}
- Veciana M, Stewart L, Miller H, Slotnick R, Rebarber A, Rosen T. Multicenter randomized controlled trial of nerve stimulation therapy for the relief of nausea and vomiting in pregnancy [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S182. [DOI] [PubMed] [Google Scholar]
- Miller H, Veciana M, Stewart L, Rebarber A, Rosen T, Slotnick R. A multicenter randomized controlled trial of nerve stimulation therapy: subanalysis of severe nausea and vomiting symptoms [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S188. [Google Scholar]
Nelson‐Piercy 2001 {published data only}
- Nelson‐Piercy C, Fayers P, Swiet M. Randomised, double‐blind, placebo‐controlled trial of corticosteroids for the treatment of hyperemesis gravidarum. BJOG: an international journal of obstetrics and gynaecology 2001;108:9‐15. [DOI] [PubMed] [Google Scholar]
Neri 2005 {published data only}
- Neri I, Allais G, Schiapparelli P, Blasi I, Benedetto C, Facchinetti F. Acupuncture versus pharmacological approach to reduce hyperemesis gravidarum discomfort. Minerva Ginecologica 2005;57(4):471‐5. [PubMed] [Google Scholar]
Safari 1998 {published data only}
- Safari HR, Fassett MJ, Souter IC, Alsulyman OM, Goodwin TM. The efficacy of methylprednisolone in the treatment of hyperemesis gravidarum: a randomized, double‐blind, controlled study. American Journal of Obstetrics and Gynecology 1998;179(4):921‐4. [DOI] [PubMed] [Google Scholar]
- Safari HR, Fassett MJ, Souter IC, Goodwin TM. Randomized double‐blind trial of methylprednisolone versus promethazine in the treatment of hyperemesis gravidarum. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S60. [DOI] [PubMed] [Google Scholar]
Shin 2007 {published data only}
- Shin HS, Song YA, Seo S. Effect of Nei‐Guan point (P6) acupressure on ketonuria levels, nausea and vomiting in women with hyperemesis gravidarum. Journal of Advanced Nursing 2007;59(5):510‐9. [DOI] [PubMed] [Google Scholar]
Sullivan 1996 {published data only}
- Sullivan CA, Johnson CA, Roach H, Martin RW, Stewart DK, Morrison JC. A pilot study of intravenous ondansetron for hyperemesis gravidarum. American Journal of Obstetrics and Gynecology 1996;174(5):1565‐8. [DOI] [PubMed] [Google Scholar]
- Sullivan CA, Johnson CA, Roach H, Martin RW, Stewart DK, Morrison JC. A prospective, randomized, double‐blind comparison of the serotonin antagonist ondansetron to a standardized regimen of promethazine for hyperemesis gravidarum. A preliminary investigation. American Journal of Obstetrics and Gynecology 1995;172:299. [Google Scholar]
Tabatabaii 2008 {published data only}
- Tabatabaii A, Sekhavat L, Mojibian M. A randomized, placebo‐controlled trial of corticosteroids for hyperemesis gravidarum. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):225. [Google Scholar]
Tan 2009 {published data only}
- Tan PC, Yow CM, Omar SZ. A placebo‐controlled trial of oral pyridoxine in hyperemesis gravidarum. Gynecologic and Obstetric Investigation 2009;67(3):151‐7. [DOI] [PubMed] [Google Scholar]
Tan 2010 {published data only}
- Tan PC, Khine PP, Vallikkannu N, Omar SZ. Promethazine compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstetrics & Gynecology 2010;115(5):975‐81. [DOI] [PubMed] [Google Scholar]
Tan 2013 {published data only}
- Tan PC, Norazilah MJ, Omar SZ. Dextrose saline compared with normal saline rehydration of hyperemesis gravidarum: a randomized controlled trial. Obstetrics and Gynecology 2013;121(2 Pt 1):291‐8. [DOI] [PubMed] [Google Scholar]
Ylikorkala 1979 {published data only}
- Ylikorkala O, Kauppila A, Ollanketo ML. Intramuscular ACTH and placebo in the treatment of hyperemesis gravidarum. Acta Obstetricia et Gynecologica Scandinavica 1979;58:453‐5. [DOI] [PubMed] [Google Scholar]
Yost 2003 {published data only}
- Yost NP, McIntire DD, Wians FH, Ramin SM, Balko JA, Leveno KH. A randomized, placebo‐controlled trial of corticosteroids for hyperemesis due to pregnancy. Obstetrics & Gynecology 2003;102(6):1250‐4. [DOI] [PubMed] [Google Scholar]
Ziaei 2004 {published data only}
- Ziaei S, Hosseiney FS, Faghihzadeh S. The efficacy low dose of prednisolone in the treatment of hyperemesis gravidarum. Acta Obstetricia et Gynecologica Scandinavica 2004;83:272‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adamczak 2007 {published data only}
- Adamczak J, Kasdaglis J, Rinehart B, Antebi Y, Wolf E, Terrone D. A prospective randomized trial of solumedrol dose pack vs. phenergan for the treatment of symptomatic nausea and vomiting in pregnancy. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S88, Abstract no: 277. [Google Scholar]
Carlsson 2000 {published data only}
- Carlsson CP, Axemo P, Bodin A, Carstensen H, Ehrenroth B, Madegard‐Lind I, et al. Manual acupuncture reduces hyperemesis gravidarum: a placebo‐controlled, randomized, single‐blind, crossover study. Journal of Pain & Symptom Management 2000;20(4):273‐9. [DOI] [PubMed] [Google Scholar]
Dehkordi 2013 {published data only}
- Dehkordi EJ. Comparison of "Cydonia Oblonga" fruit product with B6 on nausea and vomiting in pregnancy. IRCT: Iranian Register of Clinical Trials 2013.
Erez 1971 {published data only}
- Erez S, Schifrin BS, Dirim O. Double‐blind evaluation of hydroxyzine as an antiemetic in pregnancy. Journal of Reproductive Medicine 1971;7:57‐9. [PubMed] [Google Scholar]
Ferruti 1982 {published data only}
- Ferruti MM, Speranza R. Use of the combination of adrenal cortex extract, pyridoxal‐5‐phosphate, cyanocobalamin and ascorbic acid in the treatment of hypocorticism in pregnancy [Italian] [Sulla utilita dell'associazione di estratto corticosurrenale, piridossal‐5‐fosfato, cianocobalamina e acido ascorbico nel trattamento degli stati di ipocorticalismo in gravidanza.]. Minerva Ginecologica 1982;34:619‐23. [PubMed] [Google Scholar]
Fischer‐Rasmussen 1991 {published data only}
- Fischer‐Rasmussen W, Kjaer SK, Dahl C, Asping U. Ginger treatment of hyperemesis gravidarum. European Journal of Obstetrics & Gynecology and Reproductive Biology 1991;38(1):19‐24. [DOI] [PubMed] [Google Scholar]
Ghahiri 2011 {published data only}
- Ghahiri AA, Abdi F, Mastoo R, Ghasemi M. The effect of ondansetron and metoclopramide in nausea and vomiting of pregnancy. Journal of Isfahan Medical School 2011; Vol. 29, issue 131.
Gordon 2013 {published data only}
- Gordon GH. Dextrose saline compared with normal saline rehydration of hyperemesis gravidarum: a randomized controlled trial. Obstetrics and Gynecology 2013;121(6):1360. [DOI] [PubMed] [Google Scholar]
Kadan 2009 {published data only}
- Kadan Y. Does thiamine help vomiting and nausea in pregnancy?. ClinicalTrials.gov (http://clinicaltrials.gov) (accessed 31 July 2009) 2009.
Koren 2006 {published data only}
- Koren G. Pre‐emptive treatment of severe nausea and vomiting of pregnancy (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006) 2006.
Koren 2010 {published data only}
- Koren G, Clark S, Hankins GD, Caritis SN, Miodovnik M, Umans JG, et al. Effectiveness of delayed‐release doxylamine and pyridoxine for nausea and vomiting of pregnancy: a randomized placebo controlled trial. American Journal of Obstetrics and Gynecology 2010;203(6):571.e1‐7. [DOI] [PubMed] [Google Scholar]
Koren 2013 {published data only}
- Koren G, Maltepe C. Preemptive Diclectin therapy for the management of nausea and vomiting of pregnancy and hyperemesis gravidarum. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S20. [Google Scholar]
Koren 2015 {published data only}
- Koren G, Clark S, Hankins GD, Caritis SN, Umans JG, Miodovnik M, et al. Maternal safety of the delayed‐release doxylamine and pyridoxine combination for nausea and vomiting of pregnancy; a randomized placebo controlled trial. BMC Pregnancy and Childbirth 2015;15(1):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lask 1953 {published data only}
- Lask S. Treatment of nausea and vomiting of pregnancy with antihistamines. British Medical Journal 1953;1:652‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ling 1994 {published data only}
- Ling KM, Dalton M. Progesterone and hyperemesis. British Journal of Family Planning 1994;19(Suppl):9‐10. [Google Scholar]
Liu 1994 {published data only}
- Liu RF, Chen XQ. Treatment of severe pregnant vomiting with mainly self‐made Zengye Ding'ou Tang in 68 cases. Chinese Journal of Integrated Traditional and Western Medicine 1994;14(11):692‐3. [Google Scholar]
Madegard‐Linh 2004 {published data only}
Magee 1996 {published data only}
- Magee LA, Redman CW. An N‐of‐1 trial for treatment of hyperemesis gravidarum. British Journal of Obstetrics and Gynaecology 1996;103(5):478‐80. [DOI] [PubMed] [Google Scholar]
Maina 2012 {published data only}
- Maina A. Transdermal clonidine in the treatment of severe hyperemesis gravidarum (clonemesi). ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 May 2013] 2012.
Maina 2014 {published data only}
- Maina A, Arrotta M, Cicogna L, Donvito V, Mischinelli M, Todros T, et al. Transdermal clonidine in the treatment of severe hyperemesis. A pilot randomised control trial: CLONEMESI. BJOG: an International Journal of Obstetrics and Gynaecology 2014;121(12):1556‐63. [DOI] [PubMed] [Google Scholar]
Maltepe 2012 {published data only}
- Maltepe C, Koren G. Pre‐emptive diclectin treatment for nausea and vomiting of pregnancy: Results of a randomized controlled trial. Birth Defects Research Part A ‐ Clinical and Molecular Teratology 2012;94(5):327. [Google Scholar]
Matok 2013 {published data only}
- Matok I, Umans J, Feghali MN, Clark S, Caritis S, Miodovnik M, et al. Characteristics of women with nausea and vomiting of pregnancy who chose to continue compassionate use of placebo after a randomised trial. Journal of Obstetrics & Gynaecology 2013;33(6):557‐60. [DOI] [PubMed] [Google Scholar]
Matok 2014 {published data only}
- Matok I, Clark S, Caritis S, Miodovnik M, Umans G, Hankins G, et al. Studying the antiemetic effect of vitamin B6 for morning sickness: Pyridoxine and pyridoxal are prodrugs. Journal of Clinical Pharmacology 2014;54(12):1429‐33. [DOI] [PubMed] [Google Scholar]
McCarthy 2014b {published data only}
- Higgins JR. Day care versus inpatient management of nausea and vomiting of pregnancy ‐ D.I.M. trial. Current Controlled Trials (http://controlled‐trials.com/) (accessed 5 January 2009) 2009.
- Higgins JR. Randomized controlled trial of day care versus inpatient management of nausea and vomiting of pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 May 2013) 2008.
- McCarthy FP, Murphy A, Khashan AS, McElroy B, Spillane N, Marchocki Z, et al. Day care compared with inpatient management of nausea and vomiting of pregnancy: a randomized controlled trial. Obstetrics & Gynecology 2014;124(4):743‐8. [DOI] [PubMed] [Google Scholar]
Nguyen 2008 {published data only}
- Nguyen H. The efficacy of diclectin for nausea and vomiting of pregnancy. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008) 2008.
Oliveira 2014 {published data only}
- Capp S, Oliveira L, Carstairs S, You W. Ondansetron versus doxylamine/pyridoxine for treatment of nausea and vomiting in pregnancy: a prospective randomized double‐blind trial. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S39. [Google Scholar]
- Oliveira LG, Capp SM, You WB, Riffenburgh RH, Carstairs SD. Ondansetron compared with Doxylamine and Pyridoxine for treatment of nausea in pregnancy: a randomized controlled trial. Obstetrics and Gynecology 2014;124(4):735‐42. [DOI] [PubMed] [Google Scholar]
Ozgoli 2009 {published data only}
- Ozgoli G, Goli M, Simbar M. Effects of ginger capsules on pregnancy, nausea, and vomiting. Journal of Alternative and Complementary Medicine 2009;15(3):243‐6. [DOI] [PubMed] [Google Scholar]
Ozgoli 2011 {published data only}
- Ozgoli G. Study of Cardamom powder effect on the severity of nausea and vomiting in pregnant women referred to health centers in Chalus city 1389‐90. IRCT Iranian Registry of Clinical Trials (www.irct.ir) [accessed 1 March 2014] 2011.
Price 1964 {published data only}
- Price JJ, Barry MC. A double blind study of fluphenazine with pyridoxine. Pennsylvania Medical Journal 1964;67:37‐40. [PubMed] [Google Scholar]
Rad 2010 {published data only}
- Rad MN. Evaluation of the influence of KID 21 (youmen) point acupressure on nausea and vomiting of pregnancy. IRCT Iranian Registry of Clinical Trials (www.irct.ir) [accessed 6 December 2010] 2010.
Rosen 2003 {published data only}
- Rosen T, Veciana M, Miller HS, Stewart L, Rebarber A, Slotnick RN. A randomized controlled trial of nerve stimulation for relief of nausea and vomiting in pregnancy. Obstetrics & Gynecology 2003;102(1):129‐35. [DOI] [PubMed] [Google Scholar]
Shin 2005 {published data only}
- Shin HS, Song YA. The effect of P6 acupressure for symptom control in pregnant women having hyperemesis gravidarum. Journal of Korean Academy of Nursing 2005;35(3):593‐601. [DOI] [PubMed] [Google Scholar]
Weiner 1990 {published data only}
- Weiner CP. Trial to evaluate the efficacy of promethazine vs metoclopramine in the treatment of hyperemesis gravidarum. Personal communication 1990.
Wibowo 2012 {published data only}
- Wibowo N, Purwosunu Y, Sekizawa A, Farina A, Tambunan V, Bardosono S. Vitamin B(6) supplementation in pregnant women with nausea and vomiting. International Journal of Gynecology and Obstetrics 2012;116(3):206‐10. [DOI] [PubMed] [Google Scholar]
Willetts 2003 {published data only}
- Willetts KE, Ekangaki A, Eden JA. Effect of a ginger extract on pregnancy‐induced nausea: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43:139‐44. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Eftekhari 2013 {published data only}
- Eftekhari N, Mehralhasani Y. A comparison of ondansetron and promethasin in treating hyperemesis gravidarum. Journal of Kerman University of Medical Sciences 2013;20(4):354‐65. [Google Scholar]
He 2009 {published data only}
- He XL, Zhong G, He Y. Clinical observation on treatment of hyperemesis gravidarum by integrative Chinese and Western medicine and its influence on serum motilin. Chinese Journal of Integrated Traditional and Western Medicine 2009;29(10):872‐4. [PubMed] [Google Scholar]
References to ongoing studies
Cyna 2008 {published data only}
- Cyna A. The effects of antenatal hypnosis on suffering associated with hyperemesis in early pregnancy. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 19 February 2008) 2008.
Guttuso 2014 {published data only}
- Guttuso T. Comparison of gabapentin and metoclopramide for treating hyperemesis gravidarum. ClinicalTrials.gov (http://clinicaltrials.gov) [accessed 28 October 2015] 2014.
Koren 2014 {published data only}
- Koren G, Hankins G. A multicenter trial of the efficacy and safety of Diclegis® for nausea and vomiting of pregnancy in pregnant adolescents. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 5 February 2014] 2014.
Mehrolhasani 2012 {published data only}
- Mehrolhasani Y. Comparison of Demitron and promethazin in treatment of hyperemesis gravidarum. Iranian Registry of Clinical Trials (accessed 22 July 2013) 2012.
Mitchell‐Jones 2014 {published data only}
- Mitchell‐Jones N. Hyperemesis In Pregnancy (HIP) Trial: Inpatient versus outpatient management of severe nausea and vomiting in pregnancy. ISRCTN Registry (http://www.isrctn.com/) [accessed 28 October 2015] 2014.
Additional references
ACOG 2004
- American College of Obstetrics and Gynecology. Nausea and Vomiting of Pregnancy. ACOG Practice Bulletin April 2004:1‐12. [PubMed]
Arsenault 2002
- Arsenault M, Lane CA. The management of nausea and vomiting of pregnancy. Journal of Obsetrics and Gynaecology Canada 2002;24(10):817‐23. [PubMed] [Google Scholar]
Bailit 2005
- Bailit JL. Hyperemesis gravidarum: epidemiologic findings from a large cohort. American Journal of Obstetrics and Gynecology 2005;193:811‐4. [DOI] [PubMed] [Google Scholar]
Bown 2008
- Bowen A, Bowen R, Maslany G, Muhajarine N. Anxiety in a socially high‐risk sample of pregnant women in Canada. Canadian Journal of Psychiatry 2008;53:435‐40. [DOI] [PubMed] [Google Scholar]
Cox 1987
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10‐item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry 1987;150:782‐6. [DOI] [PubMed] [Google Scholar]
Dodds 2006
- Dodds L, Fell DB, Joseph KS, Allen VM, Butler B. Outcomes of pregnancies complicated by hyperemesis gravidarum. Obstetrics and Gynecology 2006;107:285‐92. [DOI] [PubMed] [Google Scholar]
Eliakim 2000
- Eliakim R, Abulafia O, Sherer DM. Hyperemesis gravidarum: a current review. American Journal of Perinatology 2000;17(4):207‐18. [DOI] [PubMed] [Google Scholar]
Fell 2006
- Fell DB, Dodds L, Joseph KS, Allen VM, Butler B. Risk factors for hyperemesis gravidarum requiring hospital admission during pregnancy. Obstetrics and Gynecology 2006;107:277‐84. [DOI] [PubMed] [Google Scholar]
Gadsby 1993
- Gadsby R, Barni‐Adshead AM, Jagger C. A prospective study of nausea and vomiting during pregnancy. British Journal of General Practice 1993;43:245‐8. [PMC free article] [PubMed] [Google Scholar]
Gill 2009
- Gill SK, O'Brien L, Koren G. The safety of histamine 2 blockers in pregnancy: a meta‐analysis. Digestive Diseases and Sciences 2009;54(9):1835‐8. [DOI] [PubMed] [Google Scholar]
Goodwin 1998
- Goodwin TM. Hyperemesis gravidarum. Clinical Obstetrics and Gynecology 1998;41:597‐605. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holmgren 2008
- Holmgren C, Aagaard‐Tillery KM, Silver RM, Porter TF, Varner M. Hyperemesis in pregnancy: an evaluation of treatment strategies with maternal and neonatal outcomes. American Journal of Obstetrics and Gynecology 2008;198(1):56.e1‐4. [DOI] [PubMed] [Google Scholar]
Ismail 2007
- Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Practice & Research Clinical Gastroenterology 2007;21(5):755‐69. [DOI] [PubMed] [Google Scholar]
Jarvis 2011
- Jarvis S, Nelson‐Piercy C. Management of nausea and vomiting in pregnancy. BMJ 2011;342:1407‐12. [DOI] [PubMed] [Google Scholar]
Kelly 2009
- Kelly TF, Savides TJ. Gastrointestinal disease in pregnancy. In: Creasy RK, Resnik R, Iams RD editor(s). Creasy and Resnik’s Maternal‐Fetal Medicine: Principles and Practice. 6th Edition. Philadelphia: Saunders, 2009:1041‐57. [Google Scholar]
Klebanoff 1985
- Klebanoff MA, Koslow PA, Keslow R, Rhoads GG. Epidemiology of vomiting in early pregnancy. Obstetrics and Gynecology 1985;66:612‐6. [PubMed] [Google Scholar]
Koren 2005
- Koren G, Piwko C, Ahn E, Boskovic R, Maltepe C, Einarson A. Validation studies of the Pregnancy Unique Quantification of Emesis (PUQE) scores. Journal of Obstetrics and Gynecology 2005;25(3):241‐4. [DOI] [PubMed] [Google Scholar]
Kramer 2013
- Kramer J, Bowen A, Stewart N, Muhajarine N. Nausea and vomiting of pregnancy: prevalence, severity and relation to psychosocial health. American Journal of Maternal/Child Nursing 2013;38(1):21‐7. [DOI] [PubMed] [Google Scholar]
Maltepe 2013
- Maltepe C, Koren G. The management of nausea and vomiting of pregnancy and hyperemesis gravidarum‐ a 2013 update. Journal of Population Therapeutics and Clinical Pharmacology 2013;20(2):e184‐e192. [PubMed] [Google Scholar]
Matthews 2015
- Matthews A, Haas DM, O'Mathuna DP, Dowswell T. Interventions for nausea and vomiting in early pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD007575.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazzotta 2000
- Mazzotta P, Magee LA. A risk‐benefit assessment of pharmacological and non‐pharmacological treatments for nausea and vomiting of pregnancy. Drugs 2000;50(4):781‐800. [DOI] [PubMed] [Google Scholar]
McCarthy 2014a
- McCarthy FP, Lutomski JE, Greene RA. Hyperemesis gravidarum: current perspectives. International Journal of Women's Health 2014;6:719‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mella 2011
- Mella MT. Nausea/vomiting of pregnancy and hyperemesis gravidarum. In: Berghella V editor(s). Maternal‐Fetal Evidence Based Guidelines. 2. New York City, New York USA: Informa Healthcare, 2011:72‐80. [Google Scholar]
Moran 2002
- Moran P, Taylor R. Management of hyperemesis gravidarum: the importance of weight loss as a criterion for steroid therapy. Quarterly Journal of Medicine 2002;95:153‐8. [DOI] [PubMed] [Google Scholar]
Munch 2011
- Munch S, Korst LM, Hernandez GD, Romero R, Goodwin TM. Health‐related quality of life in women with nausea and vomiting of pregnancy: the importance of psychosocial context. Journal of Perinatology 2011;31(1):10‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neutel 2000
- Neutel CI. Variation in rates of hospitalization for excessive vomiting in pregnancy by Benedectin/Dicletin use in Canada. In: Koren G, Basahi R editor(s). Nausea and Vomiting of Pregnancy: State of the Art 2000. Toronto: Motherisk, 2000:54‐9. [Google Scholar]
Niebyl 2010
- Niebyl JR. Nausea and vomiting in pregnancy. New England Journal of Medicine 2010;363(16):1544‐50. [DOI] [PubMed] [Google Scholar]
Pasternak 2013
- Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. New England Journal of Medicine 2013;368(9):814‐23. [DOI] [PubMed] [Google Scholar]
Philip 2003
- Philip B. Hyperemesis gravidarum: a literature review. Wisconsin Medical Journal 2003;102(3):46‐51. [PubMed] [Google Scholar]
Piwko 2007
- Piwko C, Ungar WJ, Einarson TR, Wolpin J, Koren G. The weekly cost of nausea and vomiting of pregnancy for women calling the Toronto Motherisk Program. Current Medical Research and Opinion 2007;23(4):833‐40. [DOI] [PubMed] [Google Scholar]
Pongrojpaw 2007
- Pongrojpaw D, Somprasit C, Chanthasenanont A. A randomized comparison of ginger and dimenhydrinate in the treatment of nausea and vomiting in pregnancy. Journal of the Medical Association of Thailand 2007;90(9):1703‐9. [PubMed] [Google Scholar]
Poursharif 2007
- Poursharif B, Korst LM, MacGibbon KW, Fejzo JS, Romero R, Goodwin TM. Elective pregnancy termination in a large cohort of women with hyperemesis gravidarum. Contraception 2007;76:451‐5. [DOI] [PubMed] [Google Scholar]
Poursharif 2008
- Poursharif B, Korst LM, Fejzo MS, MacGibbon KW, Romero R, Goodwin TM. The psychosocial burden of hyperemesis gravidarum. Journal of Perinatology 2008;28:176‐81. [DOI] [PubMed] [Google Scholar]
Power 2009
- Power Z, Kitchener H, Campbell M, Waterman H. The Hyperemesis Impact of Symptoms Questionaire: development and validation of a clinical tool. International Journal of Nursing Studies 2009;47(1):67‐77. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 1984
- Rhodes VA, Watson PM, Johnson MH. Development of reliable and valid measures of nausea and vomiting. Cancer Nursing 1984;7:33‐41. [PubMed] [Google Scholar]
Rhodes 1999
- Rhodes VA, McDaniel RW. The Index of Nausea, Vomiting,and Retching: a new format of the Index of Nausea and Vomiting. Oncology Nursing Forum 1999;26(5):889‐94. [PubMed] [Google Scholar]
Saha 2009
- Saha S, Loranger D, Pricolo V, Degli‐Esposti S. Feeding jejunostomy for the treatment of severe hyperemesis gravidarum: a case series. Journal of Parenteral and Enteral Nutrition 2009;33(5):529‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonkusare 2008
- Sonkusare S. Hyperemesis gravidarum: a review. Medical Journal of Malaysia 2008;63(3):272‐7. [PubMed] [Google Scholar]
Swallow 2004
- Swallow BL, Lindow SW, Masson EA, Hay DM. Psychological health in early pregnancy: relationship with nausea and vomiting. Journal of Obstetrics and Gynaecology 2004;24(1):28‐32. [DOI] [PubMed] [Google Scholar]
Tasci 2009
- Tasci Y, Demir B, Dilbaz S, Haberal A. Use of diazepam for hyperemesis gravidarum. Journal of Maternal‐Fetal and Neonatal Medicine 2009;22:353‐6. [DOI] [PubMed] [Google Scholar]
Zhou 2001
- Zhou Q, O'Brien B, Soeken K. Rhodes Index of Nausea and Vomiting‐ Form 2 in pregnant women. A confirmatory factor analysis. Nursing Research 2001;50(4):251‐7. [DOI] [PubMed] [Google Scholar]


