Abstract
Background
Carbon-fibre (CF) plates are increasingly used for fracture fixation. This systematic review evaluated complications associated with CF plate fixation. It also compared outcomes of patients treated with CF plates versus metal plates, aiming to determine if CF plates offered comparable results. The study hypothesized that CF plates display similar complication rates and clinical outcomes as metal plates for fracture fixation.
Methods
The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The following databases were searched from database inception until June 2023: PubMed, MEDLINE, Embase, Web of Science, Cochrane Library, Emcare, Academic Search Premier and Google Scholar. Studies reporting on clinical and radiological outcomes of patients treated with CF plates for traumatic fractures and (impending) pathological fractures were included. Study quality was assessed, and complications were documented as number and percentage per anatomic region.
Results
A total of 27 studies of moderate to very low quality of evidence were included. Of these, 22 studies (800 patients, median follow-up 12 months) focused on traumatic fractures, and 5 studies (102 patients, median follow-up 12 months) on (impending) pathological fractures. A total of 11 studies (497 patients, median follow-up 16 months) compared CF plates with metal plates. Regarding traumatic fractures, the following complications were mostly reported: soft tissue complications (52 out of 391; 13%) for the humerus, structural complications (6 out of 291; 2%) for the distal radius, nonunion and structural complication (1 out of 34; 3%) for the femur, and infection (4 out of 104; 4%) for the ankle. For (impending) pathological fractures, the most frequently reported complications were infections (2 out of 14; 14%) for the humerus and structural complication (6 out of 86; 7%) for the femur/tibia. Comparative studies reported mixed results, although the majority (7 out of 11; 64%) reported no significant differences in clinical or radiological outcomes between patients treated with CF or metal plates.
Conclusion
This systematic review did not reveal a concerning number of complications related to CF plate fixation. Comparative studies showed no significant differences between CF plates and metal plates for traumatic fracture fixation. Therefore, CF plates appear to be a viable alternative to metal plates. However, high-quality randomized controlled trials (RCTs) with long-term follow-up are strongly recommended to provide additional evidence supporting the use of CF plates.
Level of evidence: III, systematic review.
Keywords: Fracture fixation, CFR PEEK, Carbon-fibre plates, Complications
Introduction
Carbon-fibre (CF) plates, reinforced with polyetheretherketone, have gained increasing interest due to potential advantages compared with metal plates. For instance, CF plates offer radiolucency, which enables better radiographic visualization of postoperative fracture reduction, bone healing and surveillance of tumour recurrence for oncological patients [1–4]. Furthermore, the absence of metallic artefacts allows for precise radiotherapy planning and accurate delivery after placement of CF implants [5–7]. Another advantage specific to CF plates may be reduced stress shielding, as their modulus of elasticity closely matches that of cortical bone; 13 gigapascal (GPa) for CF versus 12 GPa for cortical bone [8]. Additionally, in vitro studies on CF plates have demonstrated superior fatigue strength compared with current metal plates; this may potentially enhance bone healing and reduce the risk of complications [8, 9]. Finally, cold welding does not occur in CF plate constructs, which would facilitate easy implant removal [10].
Despite the increasing use of CF plates for fixating traumatic and (impending) pathological fractures, reported experience in the literature remains limited. Previous systematic reviews have primarily focused on comparative studies or specifically examined traumatic distal radius fracture fixation with CF plates [11–13]. In these studies, CF plates were considered as a valid alternative due to comparable results to metal plates [11–13]. However, cohort studies and case reports have identified several disadvantages associated with CF plates that were not mentioned in the aforementioned systematic reviews. Drawbacks include the inability to deform the plate, plate breakage without clear trauma and brittleness when plate breakage occurs [14–17]. Conducting a systematic review that includes all relevant existing evidence would provide a comprehensive overview and is crucial for assessing the safety and effectiveness of CF plates. Therefore, the aim of this systematic review was to evaluate complications associated with CF plate fixation for traumatic and (impending) fracture fixation. It also compared outcomes of patients treated with CF plates versus metal plates, aiming to determine if CF plates offered comparable results. Based on the aforementioned systematic reviews, this study hypothesized that CF plates display similar complication rates and clinical outcomes as metal plates for (pathological) fracture fixation.
Methods
Search strategy and study selection
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and followed a pre-registered PROSPERO protocol (CRD42021254603) [18]. A medical librarian assisted in developing the search strategy, which was based on the following population, intervention, comparison and outcome (PICO) algorithm: P = patients with traumatic or (impending) pathological fractures, I = CF plate fixation, C = no specific controls or patients treated with metal plates and O = radiological and/or clinical outcomes (including complications). Ultimately, the search was divided into two parts: (1) CF plates used for traumatic fractures and (2) CF plates used for (impending) pathological fractures. The search contained keywords related to “carbon-fiber” and “fracture” for traumatic fractures, and “carbon-fiber” and “bone tumor” for (impending) pathological fractures (Appendix 1). The following databases were reviewed from database inception up to June 2023: PubMed, MEDLINE, Embase, Web of Science, Cochrane Library, Emcare, Academic Search Premier and Google Scholar.
Eligibility criteria
Eligible study designs included randomized controlled trials (RCTs), cohort studies (with prospective and retrospective designs), case–control studies, cross-sectional studies and case reports. Studies were included if they involved patients with traumatic or (impending) pathological fractures fixated with CF plates. Excluded were meeting abstracts, reviews, editorials, commentaries, surveys, animal-only, in vitro, cadaver or biomechanical studies. No filters or other constraints were used in the database search.
Study selection
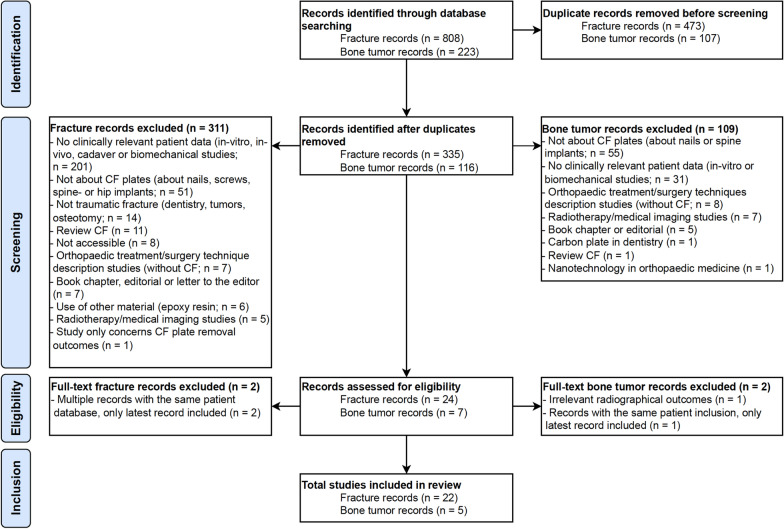
After the retrieval of eligible studies, duplicates were removed. Out of the initial pool of 808 traumatic fracture records and 223 oncologic (bone tumour) records, a total of 335 studies on trauma fractures and 116 studies on (impending) pathological fractures remained. Abstracts were obtained and evaluated. Preliminary screening of titles and abstracts led to the exclusion of 311 studies for trauma fractures and 109 studies for (impending) pathological fractures. Subsequently, the full text of 24 studies on trauma fractures were reviewed, and 2 of them were excluded because a more recent third study used the same patient database. Similarly, two of the seven studies concerning (impending) pathological fractures were excluded after full-text screening: one due to irrelevant outcome measurements and one because the same patient had been included in a more recent study (Fig. 1).
Fig. 1.
PRISMA flow diagram of the study. CF Carbon-fibre
Quality assessment
Methodological quality assessment varied based on the study design. According to the Cochrane Handbook guidelines, the Risk of Bias II (RoB 2) tool was applied for RCTs, the Risk of Bias in Nonrandomized Studies of Intervention (ROBINS-I) tool for non-RCTs, and the Joanna Briggs Institute Checklist for case reports [19–21]. With the aid of these tools, various forms of bias were evaluated, including confounding bias, selection bias, bias in classification of intervention, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcome and bias in selection of the reported results [19–21]. In addition, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was utilized to grade quality of evidence, which is important for assessing appropriateness and trustworthiness of recommendations done in the evaluated studies [22]. Within GRADE the following quality of evidence options are possible: high, moderate, low and very low. Randomized trials were initially rated high, observational studies low and other levels of evidence very low. However, high-quality evidence was downgraded if methodological flaws existed, and low-quality evidence could be upgraded when large effect sizes exist. Three reviewers (Z.R., A.W., S.D.) independently assessed the risk of bias for the included studies, discrepancies were discussed and the senior author (M.v.d.S.) was consulted in case of persistent disagreement.
Data extraction
A standard data extraction form was used to collect relevant data from the included studies. The extraction form captured study characteristics (authors, year of publication, country, setting, title, number of included patients and level of evidence), patient characteristics [age, sex, smoking, body mass index (BMI), ASA classification, comorbidities, indication for CF plate fixation and number of patients that received a CF plate], and outcomes (complications, union, clinical, radiological and patient reported outcomes, as well as the duration of follow-up) [23].
Data analysis
To summarize the findings in a quantitative form, complications were subdivided per anatomical region and presented separately for the upper and lower extremities, considering complications might depend on mechanical loading [24, 25]. Descriptive statistics were performed using SPSS v.24 (IBM Corp., Armonk, NY, USA). Demographics of all included studies were shown using medians for continuous variables, as demographic data contained outliers and skewed data due to the inclusion of case reports.
Results
Study characteristics
A total of 22 studies involving 800 patients with trauma fractures (median follow-up 12 months) and 5 studies involving 102 patients (median follow-up 12 months) with (impending) pathological fractures were included in the systematic review. Among them, 11 studies (497 patients, median follow-up 12 months), including three RCTs, compared CF plates with metal plates for trauma fractures (Table 1).
Table 1.
Demographics of all included studies (n = 27) for trauma fractures (n = 22), of which 11 were comparative, and (impending) pathological fractures (n = 5)
| Parameter | Median (range)/% (n) |
|---|---|
| Trauma fracture studies (n = 22; 800 patients) | |
| Number of patients | 31 (1–160) |
| Patient age in years | 58 (18–94) |
| Percentage of patient who were woman | 66% (393) |
| BMI in kg/m2* | 28 (16–44) |
| Follow-up in months* | 12 (1–48) |
| (Impending) pathological fracture studies (n = 5; 102 patients) | |
| Number of patients | 2 (1–96) |
| Patient age in years | 30 (2–77) |
| Percentage of patient who were woman | 61% (62) |
| BMI in kg/m2* | 24 (20–27) |
| Follow-up in months* | 12 (6–35) |
| Comparative studies (n = 11; 497 patients) | |
| Number of patients | 42 (22–87) |
| Patient age in years | 59 (18–89) |
| Percentage of patient who were woman | 64% (317) |
| BMI in kg/m2* | 27 (19–48) |
| Follow-up in months* | 16 (2–36) |
BMI body mass index; kg/m2 kilograms per square meter
*Not reported in all included studies
Study quality
The overall quality assessment score for RCTs, according to RoB 2 tool was “some concerns” for all included RCTs (n = 3; Table 2). The ROBINS-I criteria score for non-comparative studies ranged from low to moderate (n = 19; Table 3), and the mean score for case reports was 6 out of 8 (n = 5; Table 4). Following the GRADE approach, randomized trials were initially rated with a high certainty of evidence. However, due to the risk of bias of the included RCTs, scores were lowered in their certainty of evidence to moderate (Table 5). Observational studies and case reports were rated as with a low or very low certainty of evidence (Table 5). Consequently, recommendations of using CF plates for fixating fractures should be done with caution.
Table 2.
Risk of Bias II (RoB 2) tool for RCTs
| Fracture studies | Randomization process | Deviations from intended interventions | Missing outcome data | Measurements of the outcome | Selection of the reported results | Overall bias judgement |
|---|---|---|---|---|---|---|
| Perugia [36] | Low | Low | Low | Low | Some concerns | Some concerns |
| Ziegler [29] | Some concerns | Low | Some concerns | Low | Some concerns | Some concerns |
| Berger-Groch [37] | Low | Low | Low | Low | Some concerns | Some concerns |
Scoring: low risk, some concerns or high risk
Table 3.
Risk of Bias in Nonrandomized Studies of Intervention (ROBINS-I) tool for non-RCTs
| Study | Confounding | Selection of participants | Classification of interventions | Deviation from intended interventions | Missing data | Measurements of outcomes | Selection of reported results | Overall |
|---|---|---|---|---|---|---|---|---|
| Baker et al. [39] | NI | Low | Moderate | Low | Low | Moderate | Low | Moderate |
| Rotini et al. [16] | NI | Low | Moderate | Low | Moderate | Moderate | Low | Moderate |
| Maggio et al. [34] | NI | Low | Moderate | Low | Moderate | Moderate | Low | Moderate |
| Pinter et al. [45] | Moderate | Low | Moderate | Low | Moderate | Moderate | Low | Moderate |
| Allemann et al. [32] | NI | Moderate | Moderate | Low | Low | Moderate | Low | Moderate |
| Tarallo et al. [10] | NI | Low | Moderate | Low | Moderate | Moderate | Low | Moderate |
| Guzzini et al. [38] | NI | Moderate | Moderate | Low | Moderate | Moderate | Low | Moderate |
| Paracuollo et al. [35] | NI | Low | Moderate | Low | Low | Moderate | Low | Moderate |
| Caforio et al. [43] | NI | Moderate | Low | Low | Low | Moderate | Moderate | Moderate |
| Rijs et al. [47] | Moderate | Moderate | Moderate | Low | Low | Moderate | Low | Moderate |
| Schliemann et al. [28] | NI | Moderate | Moderate | Low | Low | Moderate | Low | Moderate |
| Guzzini et al. [44] | NI | Low | Low | Low | Moderate | Low | Low | Moderate |
| Katthagen et al. [26] | NI | Moderate | Low | Low | Low | Low | Low | Moderate |
| Mitchell et al. [42] | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Padolino et al. [27] | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Byun et al. [40] | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Hazra et al. [30] | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Behrendt et al. [33] | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Kimmeyer et al. [31] | Moderate | Low | Moderate | Low | Moderate | Moderate | Low | Moderate |
Table 4.
Joanna Briggs Institute Critical Appraisal Checklist for Case Reports (n = 5)
| JBI checklist questions | Fracture | Tumour | |||
|---|---|---|---|---|---|
| Mellon [41] | Laux [46] | Barnds [48] | Zoccali [50] | Yeung [49] | |
| 1. Were the patient’s demographic characteristics clearly described? | Yes | Yes | Yes | Yes | Yes |
| 2. Was the patient’s history clearly described and presented as a timeline? | No | No | Yes | No | Yes |
| 3. Was the current clinical condition of the patient on presentation clearly described? | Yes | Yes | Yes | Yes | Yes |
| 4. Were diagnostic tests or assessment methods and the results clearly described? | Yes | Yes | Yes | Yes | Yes |
| 5. Was the intervention(s) or treatment procedure(s) clearly described? | No | No | Yes | Yes | No |
| 6. Was the post-intervention clinical condition clearly described? | Yes | Yes | Yes | Yes | Yes |
| 7. Were adverse events (harms) or unanticipated events identified and described? | Yes | Yes | Yes | Yes | Yes |
| 8. Does the case report provide takeaway lessons? | Yes | Yes | No | No | No |
| Overall appraisal | Included | Included | Included | Included | Included |
Scoring: yes, no, unclear or not applicable. JBI Joanna Briggs Institute
Table 5.
Reported complications
| Trauma fracture fixation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Study design | Level of evidence | Quality of evidence (GRADE) | Number of patients* | Age** | Gender | Anatomic region of the plate | Indication | Follow-up (in months) | Complications |
| Dey Hazra [30] | RCS | III | Moderate | 65 (30 CF) | 61 | 22/30 female | Proximal humerus | Proximal humeral fracture | 32 |
Structural complication [CF (n = 1) versus titanium (n = 1)]; Soft tissue complication [CF (n = 2) versus titanium (n = 0)]; humeral head necrosis [CF (n = 0) versus titanium (n = 3)] |
| Katthagen [26] | PCS | III | Low | 42 (21 CF) | 67 | 14 out of 21 female | Proximal humerus | Proximal humeral fracture | 12 | Soft tissue complications [CF (n = 4) versus titanium n = 0)] |
| Kimmeyer [31] | RCS | III | Low | 98 | 66 | 74 out of 98 female | Proximal humerus | Proximal humeral fracture | 28 | Avascular necrosis (n = 12); head shaft malreduction (n = 12); soft tissue complications (n = 7); structural complications (n = 5); tuberosity malreduction (n = 5); malreduction of the fracture (n = 3); tuberosity resorption/dislocation (n = 2); secondary glenohumeral osteoarthritis (n = 2); infection (n = 1) |
| Padolino [27] | RCS | III | Low | 42 (21 CF) | 57 | 12 out of 21 female | Proximal humerus | Proximal humeral fracture | 31 | Structural complication [CF (n = 2) versus titanium (n = 0)]; humeral head necrosis [CF (n = 1) versus titanium (n = 1)]; tuberosity resorption [> 50%; CF (n = 3) versus titanium (n = 9)]; varus/valgus malalignment [CF (n = 2) versus titanium (n = 0)] |
| Rotini [16] | PCaS | III | Low | 160 | 64 | s1 19 out of 160 female | Proximal humerus | Proximal humeral fracture | 24 | Structural complication (n = 15); soft tissue complication (n = 39); humeral head necrosis (n = 13); reduction loss/tuberosity dislocation (n = 7); nonunion (n = 2) |
| Schliemann [28] | RCS | III | Low | 58 (29 CF) | 66 | 22 out of 29 female | Proximal humerus | Proximal humeral fracture | 24 | Humeral head necrosis [CF (n = 1) versus metal (n = 3)]; varus malalignment [CF (n = 4) versus metal (n = 7)] |
| Ziegler [29] | RCT | II | Moderate | 63 (32 CF) | 62 | 26 out of 32 female | Proximal humerus | Proximal humeral fracture | 6 | None |
| Allemann [32] | RCaS | IV | Low | 10 | 53 | 4 out of 10 female | Distal radius | Distal radius fracture | 12 | None |
| Behrendt [33] | PCS | III | Low | 26 (14 CF) | 57 | 11 out of 14 female | Distal radius | Distal radius fracture | 2 | None |
| Berger-Groch [37] | RCT | II | Moderate | 31 (16 CF) | 59 | 10 out of 16 female | Distal radius | Distal radius fracture | 36 | Soft tissue complications [CF (n = 1) versus titanium (n = 2)] |
| Di Maggio [34] | RCaS | IV | Low | 64 | 57 | 38 out of 64 female | Distal radius | Distal radius fracture | 12 | None |
| Paracuollo [35] | RCaS | IV | Low | 40 | 62 | 22 out of 40 female | Distal radius | Distal radius fracture | 12 | None |
| Perugia [36] | RCT | II | Moderate | 30 (15 CF) | 57 | 10 out of 15 female | Distal radius | Distal radius fracture | 16 | None |
| Tarallo [10] | RCaS | IV | Low | 110 | 58 | 77 out of 110 female | Distal radius | Distal radius fracture | 48 | Structural complication (n = 5); soft tissue complication (n = 3); infection (n = 1) |
| Guzzini [38] | PCaS | III | Low | 22 | 51 | 14 out of 22 female | Distal radius | Distal radius fracture | 12 | Soft tissue complication (n = 1) |
| Baker [39] | RCaS | IV | Low | 12 | 78 | Not reported | Proximal femur | THA periprosthetic fracture | Not reported | Nonunion (n = 1) |
| Byun [40] | RCS | III | Low | 31 (10 CF) | 50 | 3 out of 10 female | Distal femur | Distal femur fracture | 6 | None |
| Mellon [41] | CR | IV | Very low | 1 | 64 | 1 out of 1 female | Distal femur | Distal femur fracture | 1 | Structural complication (n = 1) |
| Mitchell [42] | RCS | III | Low | 22 (11 CF) | 72 | 8 out of 11 female | Distal femur | Distal femur fracture | 12 | Structural complications [CF (n = 0) versus stainless steel (n = 4)]; nonunion [CF (n = 1) versus stainless steel (n = 4)] |
| Caforio [43] | PCaS | IV | Low | 27 | 57 | 13 out of 27 female | Distal fibula + distal tibia | Ankle fracture | 4 | Soft tissue complication (n = 1) |
| Guzzini [44] | PCS | III | Low | 87 (47 CF) | 57 | 32 out of 46 female | Distal fibula + distal tibia | Ankle fracture | 24 | Infection [CF (n = 3) versus stainless steel (n = 4)] |
| Pinter [45] | RCaS | IV | Low | 30 | 47 | 18 out of 30 female | Distal fibula | Unstable lateral malleolus fracture | 20 | Soft tissue complication (n = 1); infection (n = 1); nonunion (n = 1) |
| (Impending) pathological fracture fixation | ||||||||||
| Laux [46] | CR | IV | Very low | 2 | 77 | 2 out of 2 male | Humerus and tibia | Pathological fracture and prophylactic plate after curettage | 6 and 8 | None |
| Zoccali [50] | CR | IV | Very low | 1 | 3 | 1 out of 1 female | Femur | Plate fixation after reconstruction | 12 | None |
| Yeung [49] | CR | IV | Very low | 2 | 60 | 2 out of 2 female | Femur | Plate fixation after reconstruction | 12 and 15 | None |
| Rijs [47] | RCaS | IV | Low | 96 | 43 | 59 out of 96 female | Femur (n = 67), tibia (n = 14), humerus (n = 13) and radius (n = 2) | (Impending) pathological fractures and plate fixation after reconstructions | 35 | Structural complication (n = 7); infection (n = 4); soft tissue complication (n = 1); tumour progression (n = 5); aseptic loosening (n = 1); nonunion (n = 2); angular deformation (n = 2) |
| Barnds [48] | CR | IV | Very low | 1 | 9 | 1 out of 1 male | Tibia | Plate fixation after reconstruction | 3 | Structural complication (n = 1) |
THA total hip arthroplasty; CF = carbon-fibre; RCS = retrospective cohort study; RCaS = retrospective case study; PCS prospective cohort study, PCaS prospective case study, CR case report(s), RCT randomized controlled trial
*Number of patients treated with carbon-fibre plates between brackets
**Mean or median age (as reported in the study)
Reported complications after CF plate fixation for trauma fractures
In the upper extremity, seven studies evaluated CF plate fixation after traumatic proximal humerus fractures, involving a total of 391 patients [16, 26–31]. The most frequently reported complications were soft tissue complications (n = 52; 13%), including impingement between plate and acromion (n = 18), rotator cuff lesions (n = 18), adhesive capsulitis/shoulder stiffness (n = 15) and an intra-articular bicep tendon rupture (n = 1). Avascular humeral head necrosis/collapse was also frequently reported (n = 27; 7%). In addition, structural complications were frequently observed (n = 23; 6%), which consisted of secondary screw perforation (n = 12), screws backing out (n = 5), plate breakages (n = 4) and malpositioning of the plate (n = 2). Furthermore, secondary loss of reduction or resorption (> 50%) of tuberosity (n = 17; 4%), varus/valgus malalignment (n = 6; 2%), head shaft malreduction (n = 12; 3%), malreduction of the fracture (n = 3; 1%), nonunions (n = 2; 1%), secondary glenohumeral osteoarthritis (n = 2; 1%) and an infection (n = 1; < 1%) were documented as unfavourable events. Eight studies reported on traumatic distal radius fractures, with a total of 291 patients [10, 32–38]. Complications for this group included structural complications (n = 6; 2%), soft tissue complications (n = 5; 2%) and an infection (n = 1; < 1%).
Regarding the lower extremity, four studies assessed traumatic femur fracture fixations with CF plates, encompassing a total of 34 patients [39–42]. Complications observed in this group included one nonunion (n = 1; 3%) and one structural complication (plate breakage, n = 1; 3%). Furthermore, three studies evaluated ankle fractures treated with CF plates [43–45], involving 104 patients in total. The most frequently reported complications included infections (n = 4; 4%), soft tissue complication (n = 2; 2%) and one nonunion (n = 1; 1%; Table 5).
Reported complications after CF plate fixation for (impending) pathological fractures
In the upper extremity, two studies evaluated pathological fractures involving 14 humerus and 2 distal radius CF plates [46, 47]. Most frequently reported humerus complications included infections (n = 2; 14%), a structural complication (traumatic plate breakage, n = 1; 7%) and a tumour progression (n = 1; 7%) for which the plate was removed. No complications were reported for the 2 distal radius CF plates.
Regarding the lower extremity, five studies encompassing a total of 86 patients investigated femoral and/or tibial (impending) pathological fractures [46–50]. Complications included structural failures (n = 6; 7%), consisting of plate breakages without clear trauma (n = 2), periprosthetic fractures (n = 2), screw breakage (n = 1) and screw backing out (n = 1). Additionally, documented complications consisted of tumour progressions (n = 5; 6%), infections (n = 4; 5%), nonunion (n = 3; 4%), aseptic loosening (n = 2; 3%), paediatric complications (valgus deformations treated with eight-plates, n = 2; 3%) and a soft tissue complication (wound dehiscence after radiotherapy treatment, n = 1; 2%; Table 5).
Studies comparing CF plates with metal plates
Eleven studies have compared CF plates with metal plates, all focusing on traumatic fractures [26–30, 33, 36, 37, 40, 42, 44]. Among these studies, three were RCTs, and the remaining eight were prospective (n = 4) or retrospective (n = 4) comparative studies. This study hypothesized that CF plates display similar complication rates and clinical outcomes as metal plates for fracture fixation.
In the upper extremity, five studies examined CF plates compared with metal plates for humerus fractures. Firstly, Dey Hazra et al. conducted a retrospective study comparing range of motion after 2 years after fixation using CF plates (n = 30) or titanium plates (n = 35) [30]. The CF group demonstrated significantly improved forward flexion, internal rotation and abduction compared with the titanium group, with similar patient reported outcomes. Secondly, Katthagen et al. prospectively enrolled 21 CF-treated patients and matched them with 21 titanium treated patients [26]. Although functional outcomes were comparable after 12 months, the titanium group required more revisions due to screw perforations (5 versus 0; p = 0.048). Thirdly, Schliemann et al. conducted a prospective study comparing clinical and radiographic results of CF-treated patients (n = 29) to those treated with metal locking plates (n = 29) [28]. After 2 years, patients treated with CF plates achieved significantly better Constant Murley and Oxford Shoulder scores (p = 0.038 and 0.029, respectively), with fewer cases with loss of reduction or varus deformity in the CF group. Fourthly, Padolino et al. conducted a retrospective study comparing clinical and radiographic outcomes of CF-treated patients (n = 21) to those treated with titanium plates (n = 21) [27]. Shoulder mobility, clinical and pain scores were similar in both patient groups after 2 years, while cortical thinning was significantly greater in the CF group (p = 0.0003). Besides, the metal group exhibited a significantly higher rate of tuberosity resorption (p = 0.040). Lastly, Ziegler et al. performed an RCT comparing CF plates (n = 32) with titanium plates (n = 31), but reported no clinical or radiological differences after 6 month’s follow-up [29]. For distal radius fractures, three comparative studies consistently demonstrated similar clinical and radiological outcomes during follow-up evaluations spanning 2 weeks to 3 years [33, 36, 37].
In the lower extremity, two studies evaluated CF and metal plates for distal femur fractures. Mitchell et al. compared CF plates (n = 11) with stainless steel plates (n = 11), observing a trend towards better outcomes in the CF plate group, including less nonunion, less structural failures and less reoperations (9% versus 36%; 0% versus 18%; and 9% versus 36%, respectively) [42]. Byun et al. also compared CF (n = 10) with stainless steel (n = 21), noting better callus formation at 3 months, although this effect diminished at 6 months [40]. Regarding ankle fractures, Guzzini et al. compared CF plates (n = 47) with stainless steel plates (n = 41), reporting no significant differences in terms of pain, radiographic and clinical outcomes at 6-, 12- and 24-month follow-up evaluations [44] (Table 5).
Discussion
As hypothesized, the findings of this systematic review indicate that utilization of CF plates for the fixation of traumatic, and (impending) pathological fractures is associated with a comparable incidence of complications and clinical outcomes to conventional metal plates. CF implants have gained increasing interest due to their potential advantages over metal implants. These advantages include radiolucency, which allows for improved visualization of bone healing and early detection of tumor recurrence, ensuring timely interventions if necessary. The absence of metallic artefacts on radiographic imaging enables more precise postoperative radiotherapy planning. Other advantages include reduced stress shielding which potentially leads to better bone quality, and the absence of cold welding, which facilitates easier removal [1–6, 8, 10]. The reported complication data can serve as a valuable benchmark for clinicians and patients, helping manage expectations during CF plate treatment. Although existing evidence suggests CF plates are a viable addition to the surgeons’ armamentarium, quality of current evidence is moderate to weak. Hence, recommendations of utilizing CF plates instead of conventional metal plates should be done with caution.
Adoption of CF plates as standard care for fracture fixation may face challenges due to the well-established use of conventional metal plates and the surgeons’ extensive training and experience with these conventional plates [51]. New technologies are often associated with a learning curve, as performance tends to improve over time [52, 53]. Nevertheless, the surgical procedure in terms of operation time and accuracy of implant position was similar in CF plates compared with metal plates [31, 33]. Moreover, comparable rates of reported complications suggest that implementation of CF plates does not necessitate additional training. Costs of innovations are another important factor for implementation. Although there is a lack of cost-effectiveness studies for CF plates, a recent study comparing CF nails with metal nails showed comparable cost profiles [54]. Yet, long-term evidence on safety and effectiveness needs to be further investigated before adaptation on a large scale is feasible. Rotini et al. and Tarallo et al. both described intraoperative plate breakages at an oval screw hole in the first generation of CF plates [10, 16]. This issue was not reported in more recent studies. Still, one of the drawbacks of CF is the inability to bend the plate to match the patient’s surface anatomy during surgery. Therefore, good preoperative planning is recommended when using these implants. Importantly, patients should be involved in the decision-making and evaluation of implant material, and other osteosynthesis methods, such as intramedullary nailing, should be considered before definitive treatment [55, 56].
Three systematic reviews have previously evaluated CF plates for trauma fracture fixation. Firstly, Saracco et al. included seven studies on distal radius fractures, and reported CF as a potential alternative to conventional metal plates [12]. Secondly, Theivendran et al. evaluated CF fixation in a broader population with small improvements in functional recovery of CF plates after humerus fractures, while there was insufficient evidence to support its widespread use [13]. Thirdly, Choloros et al. (9 studies, 361 patients) states that, considering their high union rates in extremity fracture fixation, CF seems to be a valid alternative to conventional metal plating [11]. Our systematic review (27 studies, 1297 patients), which also included pathological fractures, aligns with these previous results, and reported comparable material specific complications to their metal counterparts. However, high-quality RCTs with long-term follow-up are strongly recommended to provide additional evidence supporting the use of CF plates, their hypothesized advantages and possible contraindications.
Limitations
This systematic review has several limitations. First, its quality is inherently related to the quality of the included studies. Level I or II comparative studies were limited, which represents a major limitation. In general, Level III and IV studies are more prone to selection bias (related to patient selection and/or uncontrolled confounders). The moderate to weak outcomes of the risk of bias assessment and GRADE approach to rate quality of evidence reflected our methodological concerns. However, all studies were still included because we wanted to provide a thorough overview of all available literature. Second, the lack of high-quality studies comparing CF and metal plates was a notable limitation. Especially for (impending) pathological fractures, the absence of comparative studies is a drawback which invites future research. Third, due to the lack of homogenous (comparative) studies and heterogeneity in patient populations, cancer types and complications, a meta-analysis was not performed. Pooling results with data on different complications and types of trauma or cancers would yield results with limited clinical validity. Fourth, there was a lack of clarity between minor and major (complications requiring surgical) interventions, which also limited our reporting about complications. Lastly, most of the included studies only reported short- or midterm follow-up results, which hampers our ability to draw conclusions on the long-term safety and effectiveness of CF plates. Further research is needed to generate high-quality evidence on the long-term safety and effectiveness of CF plates compared with metal plates. Nevertheless, this review provides a comprehensive overview with a complete up-to-date summary on the complication profile of CF plates in traumatic and (impending) pathological fractures.
Conclusion
This systematic review hypothesized that CF plates display similar complication rates and clinical outcomes as metal plates for fracture fixation. Based on the available evidence, this systematic review concludes that CF plates are a viable alternative to metal plates for fracture fixation, without increased material-specific complications. However, more high-quality studies are needed to strengthen the evidence, especially for (impending) pathological fractures. In the meantime, the study’s complication data can serve as a valuable benchmark for clinicians and patients, helping manage expectations during CF plate treatment.
Acknowledgements
Not applicable.
Abbreviation
- CF
Carbon-fibre
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- BMI
Body mass index; measured in kg/m2; kilograms per square meter
- ASA
American Society of Anesthesiologists Classification
- RCT
Randomized controlled trial
- ROB 2
Risk of bias II tool
- ROBINS-I
Risk of Bias in Nonrandomized Studies of Intervention tool
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
Appendix 1
Search strategy PubMed, search strategies other databases and their results are available upon request.
Fracture; 132 results in PubMed from database inception up until 20 June 2023.
((“carbon fiber reinforced polyetheretherketon plate”[tw] OR “carbon fiber reinforced polyetheretherketon plates”[tw] OR “carbon fiber reinforced polyetheretherketon”[tw] OR “carbon fiber reinforced polyether ether ketone”[tw] OR “carbon fiber reinforced poly ether ether ketone”[tw] OR “Carbon fiber reinforced poly etheretherketone”[tw] OR “CFR PEEK plates”[tw] OR “CFR PEEK plate”[tw] OR “CFR PEEK”[tw] OR “CFR PEEK*”[tw] OR “CFRPEEK”[tw] OR “CFRPEEK*”[tw] OR “Carbon Fiber Reinforced PEEK”[tw] OR “carbon peek”[tw] OR “Carbon fiber plates”[tw] OR “Carbon fiber plate”[tw] OR “CF plates”[tw] OR “CF plate”[tw] OR “Carbon fiber implants””[tw] OR “Carbon fiber implant”[tw] OR “CF implants”[tw] OR “CF implant”[tw] OR ((“carbon fiber*”[tw] OR “carbonfiber*”[tw] OR “CFR”[tw]) AND (“polyetheretherketon*”[tw] OR “polyether ether keton*”[tw] OR “poly ether ether ketone”[tw] OR “poly etheretherketone”[tw] OR “PEEK”[tw])) OR ((“Carbon Fiber”[Mesh] OR “Carbon”[Mesh] OR “carbon fiber”[tw] OR “carbon fibers”[tw] OR “carbon fibre”[tw] OR “carbon fibres”[tw]) AND (“Bone Plates”[Mesh] OR “bone plate”[tw] OR “bone plates”[tw] OR “bone plating”[tw] OR “plate”[ti] OR “plates”[ti]))) AND (“Fractures, Bone”[Mesh] OR “Fractures”[tw] OR “Fracture”[tw] OR “Fractur*”[tw] OR “break”[tw] OR “breaks”[tw] OR “broken”[tw] OR “broke”[tw] OR “malunion*”[tw] OR “mal union*”[tw] OR “nonunion*”[tw] OR “non union*”[tw]) NOT (“Animals”[mesh] NOT “Humans”[mesh]) AND english[la]).
Bone tumor; 44 results in PubMed from database inception up until 20 June 2023.
((“carbon fiber reinforced polyetheretherketon plate”[tw] OR “carbon fiber reinforced polyetheretherketon plates”[tw] OR “carbon fiber reinforced polyetheretherketon”[tw] OR “carbon fiber reinforced polyether ether ketone”[tw] OR “carbon fiber reinforced poly ether ether ketone”[tw] OR “Carbon fiber reinforced poly etheretherketone”[tw] OR “CFR PEEK plates”[tw] OR “CFR PEEK plate”[tw] OR “CFR PEEK”[tw] OR “CFR PEEK*”[tw] OR “CFRPEEK”[tw] OR “CFRPEEK*”[tw] OR “Carbon Fiber Reinforced PEEK”[tw] OR “carbon peek”[tw] OR “Carbon fiber plates”[tw] OR “Carbon fiber plate”[tw] OR “CF plates”[tw] OR “CF plate”[tw] OR “Carbon fiber implants”[tw] OR “Carbon fiber implant”[tw] OR “CF implants”[tw] OR “CF implant”[tw] OR ((“carbon fiber*”[tw] OR “carbonfiber*”[tw] OR “CFR”[tw]) AND (“polyetheretherketon*”[tw] OR “polyether ether keton*”[tw] OR “poly ether ether ketone”[tw] OR “poly etheretherketone”[tw] OR “PEEK”[tw])) OR ((“Carbon Fiber”[Mesh] OR “Carbon”[Mesh] OR “carbon fiber”[tw] OR “carbon fibers”[tw] OR “carbon fibre”[tw] OR “carbon fibres”[tw]) AND (“Bone Plates”[Mesh] OR “bone plate”[tw] OR “bone plates”[tw] OR “bone plating”[tw] OR “plate”[ti] OR “plates”[ti]))) AND (“Bone Neoplasms”[Mesh] OR “Neoplasms, Bone Tissue”[Mesh] OR “Bone Neoplasm”[tw] OR “Bone Neoplasms”[tw] OR “Bone Malignancy”[tw] OR “Bone Malignancies”[tw] OR “Orthopaedic oncology”[tw] OR “Orthopedic oncology”[tw] OR “Orthopedic tumor”[tw] OR “Orthopedic tumors”[tw] OR “Orthopaedic tumor”[tw] OR “Orthopaedic tumors”[tw] OR “Orthopaedic tumour”[tw] OR “Orthopaedic tumours”[tw] OR “Bone tumor”[tw] OR “Bone tumors”[tw] OR “Bone tumour”[tw] OR “Bone tumours”[tw] OR “Bone cancer”[tw] OR “Bone cancers”[tw] OR “Adamantinoma”[tw] OR “Adamantinomas”[tw] OR “Osteochondroma”[tw] OR “Osteochondromas”[tw] OR “Giant cell tumor”[tw] OR “Giant cell tumors”[tw] OR “Giant cell tumour”[tw] OR “Giant cell tumours”[tw] OR “Osteoblastoma”[tw] OR “Osteoblastomas”[tw] OR “Ewing sarcoma”[tw] OR “Ewing sarcomas”[tw] OR “Ewings sarcomas”[tw] OR “Ewings sarcoma”[tw] OR “Ewing’s sarcomas”[tw] OR “Ewing’s sarcoma”[tw] OR “Soft tissue sarcoma”[tw] OR “Soft tissue sarcomas”[tw] OR “Osteosarcoma”[tw] OR “Osteosarcomas”[tw] OR “Femoral Neoplasm”[tw] OR “Femoral Neoplasms”[tw] OR “Femoral Tumor”[tw] OR “Femoral Tumors”[tw] OR “Femoral Tumour”[tw] OR “Femoral Tumours”[tw] OR “Jaw Cancer”[tw] OR “Jaw Malignancies”[tw] OR “Jaw Malignancy”[tw] OR “Jaw Neoplasm”[tw] OR “Jaw Neoplasms”[tw] OR “Jaw Tumor”[tw] OR “Jaw Tumors”[tw] OR “Jaw Tumour”[tw] OR “Jaw Tumours”[tw] OR “Mandibular Cancer”[tw] OR “Mandibular Malignancies”[tw] OR “Mandibular Malignancy”[tw] OR “Mandibular Neoplasm”[tw] OR “Mandibular Neoplasms”[tw] OR “Mandibular Tumor”[tw] OR “Mandibular Tumors”[tw] OR “Mandibular Tumour”[tw] OR “Mandibular Tumours”[tw] OR “Maxillary Cancer”[tw] OR “Maxillary Cancers”[tw] OR “Maxillary Malignancies”[tw] OR “Maxillary Malignancy”[tw] OR “Maxillary Neoplasm”[tw] OR “Maxillary Neoplasms”[tw] OR “Maxillary Tumor”[tw] OR “Maxillary Tumors”[tw] OR “Maxillary Tumour”[tw] OR “Maxillary Tumours”[tw] OR “Orbital Cancer”[tw] OR “Orbital Cancers”[tw] OR “Orbital Malignancies”[tw] OR “Orbital Malignancy”[tw] OR “Orbital Neoplasm”[tw] OR “Orbital Neoplasms”[tw] OR “Orbital Tumor”[tw] OR “Orbital Tumors”[tw] OR “Orbital Tumour”[tw] OR “Orbital Tumours”[tw] OR “Palatal Cancer”[tw] OR “Palatal Cancers”[tw] OR “Palatal Malignancies”[tw] OR “Palatal Neoplasm”[tw] OR “Palatal Neoplasms”[tw] OR “Palatal Tumor”[tw] OR “Palatal Tumors”[tw] OR “Palatal Tumour”[tw] OR “Palatal Tumours”[tw] OR “Skull Base Cancer”[tw] OR “Skull Base Cancers”[tw] OR “Skull Base Malignancies”[tw] OR “Skull Base Malignancy”[tw] OR “Skull Base Neoplasm”[tw] OR “Skull Base Neoplasms”[tw] OR “Skull Base Tumor”[tw] OR “Skull Base Tumors”[tw] OR “Skull Base Tumour”[tw] OR “Skull Base Tumours”[tw] OR “Skull Neoplasm”[tw] OR “Skull Neoplasms”[tw] OR “Skull Tumor”[tw] OR “Skull Tumors”[tw] OR “Skull Tumour”[tw] OR “Skull Tumours”[tw] OR “Spinal Cancer”[tw] OR “Spinal Malignancies”[tw] OR “Spinal Malignancy”[tw] OR “Spinal Neoplasm”[tw] OR “Spinal Neoplasms”[tw] OR “Spinal Tumor”[tw] OR “Spinal Tumors”[tw] OR “Spinal Tumour”[tw] OR “Spinal Tumours”[tw] OR “Spine Cancer”[tw] OR “Spine Cancers”[tw] OR “Spine Malignancy”[tw] OR “Spine Neoplasm”[tw] OR “Spine Neoplasms”[tw] OR “Spine Tumor”[tw] OR “Spine Tumors”[tw] OR “Spine Tumour”[tw] OR “Spine Tumours”[tw]) NOT (“Animals”[mesh] NOT “Humans”[mesh]) AND english[la]).
Author contributions
ZR, AW, SD and JWS: conceptualization, methodology and investigation. ZR and SD: formal analysis and data curation. ZR, AW and SD: conceptualization, project administration, validation, visualization and writing. OQG, SLC and MvdS: supervision and revision. All authors read and approved the final manuscript.
Funding
No funding was received.
Data availability
All data used for analysis in this study was public.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Disclosures SLC: Paid consultant ONKOS, paid consultant illuminOss, paid speaker Carbofix. All other authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zeger Rijs and Amber Weekhout contributed equally to this work.
References
- 1.Gaspar N, Hawkins DS, Dirksen U, et al. Ewing sarcoma: current management and future approaches through collaboration. J Clin Oncol. 2015;33:3036–3046. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 2.Baidya KP, Ramakrishna S, Rahman M, et al. Quantitative radiographic analysis of fiber reinforced polymer composites. J Biomater Appl. 2001;15:279–289. doi: 10.1106/BKLQ-E2YG-D2LA-RG3R. [DOI] [PubMed] [Google Scholar]
- 3.Feerick EM, Kennedy J, Mullett H, et al. Investigation of metallic and carbon fibre PEEK fracture fixation devices for three-part proximal humeral fractures. Med Eng Phys. 2013;35:712–722. doi: 10.1016/j.medengphy.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Hak DJ, Mauffrey C, Seligson D, et al. Use of carbon-fiber-reinforced composite implants in orthopedic surgery. Orthopedics. 2014;37:825–830. doi: 10.3928/01477447-20141124-05. [DOI] [PubMed] [Google Scholar]
- 5.Takayanagi A, Siddiqi I, Ghanchi H, et al. Radiolucent carbon fiber-reinforced implants for treatment of spinal tumors-clinical, radiographic, and dosimetric considerations. World Neurosurg. 2021;152:61–70. doi: 10.1016/j.wneu.2021.05.100. [DOI] [PubMed] [Google Scholar]
- 6.Tedesco G, Gasbarrini A, Bandiera S, et al. Composite PEEK/Carbon fiber implants can increase the effectiveness of radiotherapy in the management of spine tumors. J Spine Surg. 2017;3:323–329. doi: 10.21037/jss.2017.06.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Depauw N, Pursley J, Lozano-Calderon SA, et al. Evaluation of carbon fiber and titanium surgical implants for proton and photon therapy. Pract Radiat Oncol. 2023;13:256–262. doi: 10.1016/j.prro.2023.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Mugnai R, Tarallo L, Capra F, et al. Biomechanical comparison between stainless steel, titanium and carbon-fiber reinforced polyetheretherketone volar locking plates for distal radius fractures. Orthop Traumatol Surg Res. 2018;104:877–882. doi: 10.1016/j.otsr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Bagheri ZS, Tavakkoli Avval P, Bougherara H, et al. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136:091002. doi: 10.1115/1.4027669. [DOI] [PubMed] [Google Scholar]
- 10.Tarallo L, Giorgini A, Novi M, et al. Volar PEEK plate for distal radius fracture: analysis of adverse events. Eur J Orthop Surg Traumatol. 2020;30:1293–1298. doi: 10.1007/s00590-020-02701-7. [DOI] [PubMed] [Google Scholar]
- 11.Chloros GD, Prodromidis AD, Wilson J, et al. Fracture fixation in extremity trauma with carbon fiber-reinforced polyetheretherketone (CFR-PEEK) plates: evidence today. Eur J Trauma Emerg Surg. 2022;48:2387–2406. doi: 10.1007/s00068-021-01778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saracco M, Fulchignoni C, Velluto C, et al. Safety and reliability of carbon-peek plate for the treatment of distal radius fractures: a review of the literature. Orthop Rev. 2021;13:28362. doi: 10.52965/001c.28362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theivendran K, Arshad F, Hanif UK, et al. Carbon fibre reinforced PEEK versus traditional metallic implants for orthopaedic trauma surgery: a systematic review. J Clin Orthop Trauma. 2021;23:101674. doi: 10.1016/j.jcot.2021.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagheri ZS, El Sawi I, Schemitsch EH, et al. Biomechanical properties of an advanced new carbon/flax/epoxy composite material for bone plate applications. J Mech Behav Biomed Mater. 2013;20:398–406. doi: 10.1016/j.jmbbm.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Wilson WK, Morris RP, Ward AJ, et al. Torsional failure of carbon fiber composite plates versus stainless steel plates for comminuted distal fibula fractures. Foot Ankle Int. 2016;37:548–553. doi: 10.1177/1071100715625291. [DOI] [PubMed] [Google Scholar]
- 16.Rotini R, Cavaciocchi M, Fabbri D, et al. Proximal humeral fracture fixation: multicenter study with carbon fiber peek plate. Musculoskelet Surg. 2015;99(Suppl 1):S1–8. doi: 10.1007/s12306-015-0371-2. [DOI] [PubMed] [Google Scholar]
- 17.Goudriaan WA, Tordoir RL, Broekhuis D, et al. Early failure of a carbon-fiber-reinforced polyetheretherketone distal femur plate: a case report. JBJS Case Connect. 2020;10(e20):00041. doi: 10.2106/JBJS.CC.20.00041. [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute. TJB. JBI Critical Appraisal Checklist for Case Reports. 2017.
- 20.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle DJ, Hendrix JM, Garmon EH. American Society of Anesthesiologists Classification. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Joseph Maxwell Hendrix declares no relevant financial relationships with ineligible companies. Disclosure: Emily Garmon declares no relevant financial relationships with ineligible companies.: StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC.; 2023.
- 24.Sundfeldt M, Carlsson LV, Johansson CB, et al. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177–197. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- 25.Liverani E, Rogati G, Pagani S, et al. Mechanical interaction between additive-manufactured metal lattice structures and bone in compression: implications for stress shielding of orthopaedic implants. J Mech Behav Biomed Mater. 2021;121:104608. doi: 10.1016/j.jmbbm.2021.104608. [DOI] [PubMed] [Google Scholar]
- 26.Katthagen JC, Ellwein A, Lutz O, et al. Outcomes of proximal humeral fracture fixation with locked CFR-PEEK plating. Eur J Orthop Surg Traumatol. 2017;27:351–358. doi: 10.1007/s00590-016-1891-7. [DOI] [PubMed] [Google Scholar]
- 27.Padolino A, Porcellini G, Guollo B, et al. Comparison of CFR-PEEK and conventional titanium locking plates for proximal humeral fractures: a retrospective controlled study of patient outcomes. Musculoskelet Surg. 2018;102:49–56. doi: 10.1007/s12306-018-0562-8. [DOI] [PubMed] [Google Scholar]
- 28.Schliemann B, Hartensuer R, Koch T, et al. Treatment of proximal humerus fractures with a CFR-PEEK plate: 2-year results of a prospective study and comparison to fixation with a conventional locking plate. J Shoulder Elbow Surg. 2015;24:1282–1288. doi: 10.1016/j.jse.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler P, Maier S, Stöckle U, et al. The treatment of proximal humerus fracture using internal fixation with fixed-angle plates. Dtsch Arztebl Int. 2019;116:757–763. doi: 10.3238/arztebl.2019.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dey Hazra RO, Szewczyk K, Ellwein A, et al. Minimum 2-year results of the second-generation CFR-PEEK locking plate on the proximal humeral fracture. Eur J Orthop Surg Traumatol. 2022;33:1307–1314. doi: 10.1007/s00590-022-03298-9. [DOI] [PubMed] [Google Scholar]
- 31.Kimmeyer M, Schmalzl J, Rentschler V, et al. Functional results and unfavorable events after treatment of proximal humerus fractures using a new locking plate system. BMC Musculoskelet Disord. 2023;24:63. doi: 10.1186/s12891-023-06176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allemann F, Halvachizadeh S, Rauer T, et al. Clinical outcomes after carbon-plate osteosynthesis in patients with distal radius fractures. Patient Saf Surg. 2019;13:30. doi: 10.1186/s13037-019-0210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behrendt P, Kruse E, Klüter T, et al. Winkelstabile karbonverstärkte Polymerkompositplatte zur Versorgung einer distalen Radiusfraktur. Unfallchirurg. 2017;120:139–146. doi: 10.1007/s00113-015-0088-6. [DOI] [PubMed] [Google Scholar]
- 34.Di Maggio B, Sessa P, Mantelli P, et al. PEEK radiolucent plate for distal radius fractures: multicentre clinical results at 12 months follow up. Injury. 2017;48(Suppl 3):S34–s38. doi: 10.1016/S0020-1383(17)30655-1. [DOI] [PubMed] [Google Scholar]
- 35.Paracuollo M, Coscione AV, Coppola A, et al. Clinical and radiographic outcomes of distal radius fracture treatment with Carbon-Fiber-Reinforced- Polymer Volar Plates (CFRPEEK): analysis of 40 cases. Lo Scalpello J. 2022;36:185–190. doi: 10.36149/0390-5276-224. [DOI] [Google Scholar]
- 36.Perugia D, Guzzini M, Mazza D, et al. Comparison between Carbon-Peek volar locking plates and titanium volar locking plates in the treatment of distal radius fractures. Injury. 2017;48(Suppl 3):S24–s29. doi: 10.1016/S0020-1383(17)30653-8. [DOI] [PubMed] [Google Scholar]
- 37.Berger-Groch J, Stodtmeister AC, Petersen JP, et al. Palmar plating of distal radius fractures : 3-year follow-up with titanium and PEEK plates give similar outcomes. Acta Orthop Belg. 2021;87:521–527. doi: 10.52628/87.3.18. [DOI] [PubMed] [Google Scholar]
- 38.Guzzini M, Lupariello D, Lanzetti RM, et al. Preliminary experience with triangular CarboFix "Piccolo" Distal Radius Plate in wrist fractures. Clin Radiolog Results Acta Biomed. 2018;90:61–66. doi: 10.23750/abm.v90i1-S.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35:596–598. doi: 10.1016/j.injury.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Byun SE, Vintimilla DR, Bedeir YH, et al. Evaluation of callus formation in distal femur fractures after carbon fiber composite versus stainless steel plate fixation. Eur J Orthop Surg Traumatol. 2020;30:1103–1107. doi: 10.1007/s00590-020-02681-8. [DOI] [PubMed] [Google Scholar]
- 41.Mellon MB. Late recognition of an early catastrophic failure of a carbon fiber reinforced distal femoral plate: a case report. Trauma Case Rep. 2021;34:100493. doi: 10.1016/j.tcr.2021.100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell PM, Lee AK, Collinge CA, et al. Early comparative outcomes of carbon fiber-reinforced polymer plate in the fixation of distal femur fractures. J Orthop Trauma. 2018;32:386–390. doi: 10.1097/BOT.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 43.Caforio M, Perugia D, Colombo M, et al. Preliminary experience with Piccolo Composite™, a radiolucent distal fibula plate, in ankle fractures. Injury. 2014;45(Suppl 6):S36–38. doi: 10.1016/j.injury.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Guzzini M, Lanzetti RM, Lupariello D, et al. Comparison between carbon-peek plate and conventional stainless steal plate in ankle fractures. A prospective study of two years follow up. Injury. 2017;48:1249–1252. doi: 10.1016/j.injury.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 45.Pinter ZW, Smith KS, Hudson PW, et al. A retrospective case series of carbon fiber plate fixation of ankle fractures. Foot Ankle Spec. 2018;11:223–229. doi: 10.1177/1938640017718343. [DOI] [PubMed] [Google Scholar]
- 46.Laux CJ, Hodel SM, Farshad M, et al. Carbon fibre/polyether ether ketone (CF/PEEK) implants in orthopaedic oncology. World J Surg Oncol. 2018;16:241. doi: 10.1186/s12957-018-1545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rijs Z, Weekhout A, Lozano-Calderon SA, et al. Complications of patients with bone tumors treated with carbon-fiber plates: an international multicenter study. Sci Rep. 2022;12:18969. doi: 10.1038/s41598-022-23519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnds B, Johnson A, Rosenthal H, et al. Ipsilateral rotational double-barrel fibula autograft for limb salvage in a pediatric patient with lower extremity intramedullary osteosarcoma: a case report. Microsurgery. 2020;40:247–251. doi: 10.1002/micr.30487. [DOI] [PubMed] [Google Scholar]
- 49.Yeung CM, Bhashyam AR, Patel SS, et al. Carbon fiber implants in orthopaedic oncology. J Clin Med. 2022;11:4959. doi: 10.3390/jcm11174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zoccali C, Careri S, Attala D, et al. A new proximal femur reconstruction technique after bone tumor resection in a very small patient: an exemplificative case. Children. 2021;8:442. doi: 10.3390/children8060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uhthoff HK, Poitras P, Backman DS. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006;11:118–126. doi: 10.1007/s00776-005-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarpong NO, Herndon CL, Held MB, et al. What is the learning curve for new technologies in total joint arthroplasty? A Review Curr Rev Musculoskelet Med. 2020;13:675–679. doi: 10.1007/s12178-020-09671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramsay CR, Grant AM, Wallace SA, et al. Assessment of the learning curve in health technologies. A systematic review. Int J Technol Assess Health Care. 2000;16:1095–1108. doi: 10.1017/S0266462300103149. [DOI] [PubMed] [Google Scholar]
- 54.Herzog LN, Traven SA, Walton ZJ, et al. The use of carbon fiber implants for impending or existing pathologic fractures. J Orthop Trauma. 2022;36:e260–e264. doi: 10.1097/BOT.0000000000002320. [DOI] [PubMed] [Google Scholar]
- 55.de Mik SML, Stubenrouch FE, Balm R, et al. Systematic review of shared decision-making in surgery. Br J Surg. 2018;105:1721–1730. doi: 10.1002/bjs.11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woudstra K, Tummers M, Rovers MM, et al. Innovators’ views on involving users and patients in surgical device development: a qualitative interview study. BMJ Open. 2021;11:e050801. doi: 10.1136/bmjopen-2021-050801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for analysis in this study was public.