Abstract
Immunotherapy, particularly those based on immune checkpoint inhibitors (ICIs), has become a useful approach for many neoplastic diseases. Despite the improvements of ICIs in supporting tumour regression and prolonging survival, many patients do not respond or develop resistance to treatment. Thus, therapies that enhance antitumour immunity, such as anticancer vaccines, constitute a feasible and promising therapeutic strategy. Whole tumour cell (WTC) vaccines have been extensively tested in clinical studies as intact or genetically modified cells or tumour lysates, injected directly or loaded on DCs with distinct adjuvants. The essential requirements of WTC vaccines include the optimal delivery of a broad battery of tumour-associated antigens, the presence of tumour cell-derived molecular danger signals, and adequate adjuvants. These factors trigger an early and robust local innate inflammatory response that orchestrates an antigen-specific and proinflammatory adaptive antitumour response capable of controlling tumour growth by several mechanisms. In this review, the strengths and weaknesses of our own and others’ experiences in studying WTC vaccines are revised to discuss the essential elements required to increase anticancer vaccine effectiveness.

Subject terms: Immunization, Cancer immunotherapy
Background
Intensive efforts to develop alternative immunotherapeutic strategies against different malignancies, as monotherapy or combinatorial schemes, have increased in the last few years [1]. Approaches such as cytokine administration for nonspecific stimulation of antitumour immunity [2], adoptive cell transfer (ACT) of re-activated T cells or chimeric antigen (Ag) receptor (CAR)-T cells [3, 4], and active immunisations with therapeutic cancer vaccines [5], have been evaluated in numerous cancer patients to improve immune-mediated tumour regression. More recently, immunotherapies based on immune checkpoint inhibitors (ICIs) targeting inhibitory pathways, such as cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein-1 (PD-1) and its ligand (PD-L1), have shown remarkable success in promoting tumour regression and prolonging survival in patients with different types of cancer [6–8]. However, a significant number of patients do not respond or develop resistance to these interventions, motivating the elaboration of combinatorial therapies to augment both patients’ objective responses and survival times [1, 8].
A substantial aspect associated with patient response to ICIs is the occurrence of a natural proinflammatory antitumour immune response. On the one hand, it has been shown that the lack or shortage of tumour-infiltrating lymphocytes (TIL), characterising the so-called “cold tumours”, has been associated with resistance to ICIs. On the other hand, higher lymphocyte infiltration and interferon (IFN)-γ status in combination with a T cell inflamed phenotype corresponding to “hot tumours” constitute critical factors for effective anti-PD-1/PD-L1 therapies [9]. Therefore, the induction of adaptive immune cellular responses in patients bearing cold tumours is a desirable goal to increase the success of any immunological treatment. In this context, active immunotherapies, like the new generation of cancer vaccines, become an attractive option in combination with standard therapies [10].
An efficient systemic cancer vaccine-induced immune response must fulfil a series of conditions from the moment of immunisation until the development of Ag-specificity. Besides the crucial presence of relevant tumour Ags, potent adjuvants, and associated danger signals, it is required to elicit a response mediated by appropriate subtypes of dendritic cells (DCs) and functional activated effector T cells, that will promote an adequate immune cell infiltration and clearance of tumours.
Different strategies for cancer vaccine development have been explored, including those based on recombinant antigenic peptides, proteins, RNA/DNA, DCs loaded with tumour Ags, or whole tumour cell (WTC) vaccines, all with different outcomes [11–13]. Additional approaches include bacteria, like modified Bacille Calmenette-Guérin (BCG) expressing cytokines and cancer Ags, or modified viruses transduced with the granulocyte-macrophage colony-stimulating factor (GM-CSF) gene, for the treatment of prostate cancer or melanoma patients [14–16]. Moreover, the successful experience of mRNA vaccines during the COVID-19 pandemic has opened the door to new cancer vaccine formulations that are steadily advancing in clinical trials [17].
In general, results from cancer vaccine preclinical studies have successfully shown an effective induction of immunity against tumours, however, they have also shown limited clinical impact. WTC vaccines have demonstrated low objective response rates and limited improvements in patient survival, suggesting that these vaccines exhibit a suboptimal immunogenic potential [18, 19]. This apparent weak immunogenicity of WTC vaccines can be due to several causes, including: (i) absence of appropriate immunologic danger signals and adjuvants during early vaccine-induced inflammation, (ii) suboptimal activation of resident DCs, and (iii) inefficient delivery of relevant tumour Ags to adequate DC populations responsible for the cross-priming of protective CD8+ T cells [20–23]. Diverse experimental models using B16F10 melanoma cells or derived cell lysates as vaccines showed protective antitumour immune responses only if melanoma cells were genetically modified to express strong Ags [i.e., ovalbumin (OVA)] to release cytokines (i.e., GM-CSF), or when combined with ICIs [24–26]. Besides, DC vaccines loaded with tumour cell products have shown some impact on patients’ overall survival, although objective response rates rarely exceed 15% [22]. These limitations may be partly due to biological diversity among patient-derived cells that generate the vaccine, most of which are monocyte-derived DCs (MoDC) [18, 22].
In this review, based on the state of the arts and our own experience, we provide and critically discuss recent evidence for different aspects associated with optimal immune responses required for the design of effective WTC vaccines, such as their antigenicity, immunogenicity, and adjuvanticity, the role of adjuvants and acute inflammation, DC and T cell populations mediating WTC vaccines-triggered immune and antitumour responses, and the role of the tumour immune microenvironment (TME).
Whole tumour cell vaccines
WTC vaccines are a type of cancer immunotherapy that utilises entire or lysed tumour cells, either intact or modified, as a source of Ags and other immunogenic factors to stimulate an immune response against cancer. This approach delivers a vast repertoire of tumour Ags that stimulate strong and broad polyclonal CD8+ and CD4+ T cell responses. WTC vaccines can be prepared from autologous or allogeneic tumour cells. Autologous vaccines are fabricated using a patient’s tumour cells, ensuring that the vaccine contains specific Ags, including those derived from tumour-specific mutations, enhancing the immune response against the patient’s particular cancer (section “Tumour Ags elicited by WTC vaccines”). Thus, the risk of immune rejection or adverse reactions caused by autologous WTC vaccines is relatively low. Nevertheless, preparing autologous vaccines is a complex and time-consuming process and may not be suitable for patients requiring immediate treatment. Furthermore, the chronic exposition of dominant Ags derived from the autologous tumour can induce a state of tolerance that the autologous tumour vaccine may not revert.
On the other hand, allogeneic WTC vaccines are prepared using tumour cells obtained from a different individual or tumour cell lines. These vaccines can be manufactured in advance and are readily available for use, eliminating the need for a time-consuming and patient-specific preparation process. Additionally, these vaccines can be used as a generic source of shared tumour-associated and -specific Ags (TAAs and TSA, respectively) on patients where tumour sampling is not feasible. By using different tumour cell sources, these vaccines have the potential to target a more diverse array of Ags, enhancing the likelihood of immune recognition and response. However, allogeneic WTC vaccines carry a higher risk of immune rejection. The choice between autologous and allogeneic vaccines depends on factors such as the urgency of treatment, feasibility of patient-specific preparation, and the desired Ag coverage.
In recent years, WTC vaccine preparations have implemented novel strategies to increase tumour cells’ immunogenicity and adjuvanticity, including transgenic and immunogenic cell death (ICD)/cell stress induction (section “Cell stress improves WTC vaccines’ immunogenicity”). WTC vaccines could be categorised into two types, direct-use vaccines (type 1), corresponding to WTC (or their subproducts) directly injected into the patients (Fig. 1a), and Ag presenting cell (APC)-mediated vaccines (type 2), corresponding to autologous APC loaded with WTC or their subproducts (Fig. 1b). Then, tumour cells could be lysed, fractionated, irradiated, and administered directly into the patients in combination with adjuvants or used for ex vivo loading of autologous APCs. A list of representative clinical trials evaluating these different types of WTC vaccines is shown in Table 1.
Fig. 1. Whole tumour cell vaccine types.
a Type 1 WTC vaccines. After surgery, autologous tumour cells can be isolated from resected tumour tissue and used as a source of tumour Ags (1a) for WTC vaccine generation (personalised approach). Otherwise, WTC vaccines are produced from allogeneic tumour cell banks (1b) (generic approach). Immunogenic tumour cells are directly administered to the patients in combination with adjuvants (2). b Type 2 WTC vaccines. Immunogenic tumour cells (obtained as described in a) are used as a source of Ags and natural cancer cell-derived adjuvants (3) for autologous ex vivo-differentiated APC (4). Then, these cells are injected back into the patient in combination with adjuvants (5). Ag antigen, APC Ag presenting cells, DC dendritic cell, ICD immunogenic cell death, WTC whole tumour cell. The image was created with BioRender.
Table 1.
Clinical trials using whole tumour cell vaccines.
| Vaccine name | Vaccine type | Cancer target | Adjuvant used | NCT#; phase [ref](a,b) |
|---|---|---|---|---|
| Type 1 (direct use WTC vaccines) | ||||
| CancerVaxTM (Canvaxin) | Allogeneic irradiated tumour cells | Melanoma | BCG | NCT00052156; phase III [27] |
| GVAX® | Allogeneic transformed tumour cells | Prostate cancer | GM-CSF | NCT00089856; phase III [157]; NCT00133224; phase III [158] |
| GVAX® | Pancreatic cancer | GM-CSF | NCT01595321; phase Ia | |
| HyperAcute® (Algenpantucel-L) | Allogeneic transformed tumour cells | Pancreatic cancer | N.I./N.U. | NCT01072981; phase III [159]; NCT01836432; phase III [160] |
| HyperAcute® (Tergenpumatucel-L) | Allogeneic transformed tumour cells | Non-small lung cell cancer | N.I./N.U. | NCT01774578; phase III |
| OncoVax® | Sterile, live but non-dividing autologous tumour cells | Colon cancer | BCG | NCT02448173; phase III |
| MVX-ONCO-1 | Autologous genetically modified tumour cells | Head and neck squamous cell carcinoma | GM-CSF | NCT02999646; multicenter phase IIa |
| VigilTM(FANGTM) | Autologous genetically modified tumour cells | Colorectal carcinoma/melanoma | GM-CSF | NCT01505166; phase II [161]; NCT01453361; phase II [162] |
| SV-BR-1-GM | Allogeneic transformed irradiated tumour cells | Breast cancer | GM-CSF | NCT00095862; phase I/II [163] |
| GM-CSF-Secreting Breast Tumour | Allogeneic transformed irradiated tumour cells | Breast cancer | GM-CSF | NCT00847171; phase II |
| CpG-MCL vaccine | Autologous conditioned and irradiated tumour cells | Lymphoma | CpG 7909 (PF-3512676) | NCT00490529; phase I/II [129] |
| Melacine | Allogeneic tumour cell lysate | Melanoma | DETOX (MLPA) | NCT00002767; phase III |
| Vaccimel | Allogeneic tumour irradiated cells | Melanoma | BCG/GM-CSF | NCT01729663; phase II/III [164] |
| Type 2 (APC-WTC vaccines) | ||||
| DCVax®-L | Autologous DC loaded with autologous tumour cell lysate | Glioblastoma multiforme | N.I./N.U. | NCT00045968; phase III [126]a |
| DC vaccine | Autologous DC loaded with autologous whole tumour RNA | Uveal melanoma | N.I./N.U. | NCT01983748; phase III [165]a |
| DC vaccine | Autologous DC transfected with whole autologous tumour stem cell RNA | Glioblastoma multiforme | N.I./N.U. | NCT03548571; phase III [166]a |
| ADCTA | Autologous DC loaded with autologous tumour | Glioblastoma multiforme | N.I./N.U. | NCT04277221; phase III [167] |
| DC vaccine | Autologous DC loaded with autologous tumour cell lysates | Metastatic cancer with a high tumour mutation burden | N.I./N.U. | NCT03671720; early phase I |
| DENDR1 | DC loaded with autologous tumour cell lysates | Glioblastoma multiforme | N.I./N.U. | NCT04801147; phase I/IIb |
| OC-DC | Autologous DC loaded with autologous tumour lysate | Ovarian cancer | Montanide ISA-51 | NCT01312376; phase I |
| TAPCells | Autologous APC loaded with allogeneic tumour cell lysate | Melanoma; prostate cancer | KLH; CCH | Phase I/II [55, 56, 97] |
BCG Bacille Calmenette-Guérin, CCH Concholepas concholepas haemocyanin, GM-CSF granulocyte-macrophage colony-stimulating factor, KLH keyhole limpet haemocyanin, MLPA monophosphoryl lipid A, NCT national clinical trial, N.I./N.U. not informed/not used.
aStatus: active, not recruiting.
bStatus: recruiting.
Until now, the clinical effects of WTC vaccines have been limited and, in most cases, without achieving regression of tumours. For example, in a phase III study of postsurgical adjuvant therapy involving 496 stage IV melanoma patients, the efficacy of Canvaxin (type 1) was compared with a placebo. Canvaxin is composed of three irradiated whole melanoma cell lines plus BCG. The results showed that BCG/Canvaxin did not improve outcomes over BCG/placebo [27].
In a randomised phase II clinical trial in metastatic melanoma patients, the effectiveness of autologous DC vaccines loaded with autologous irradiated tumour cells (type 2) was compared with the effect of autologous irradiated tumour cell vaccines (type 1). The DC vaccine arm was associated with a doubling median overall survival compared with the type 1 vaccine (43.4 vs. 20.5 months) [28]. However, in this study, no tumour regression was observed. Additionally, this autologous tumour strategy is only feasible in patients with resectable tumours and depends on the success of establishing patients’ tumour cell lines.
This relatively scarce clinical effectiveness of WTC vaccines can be due to multiple causes leading to poor vaccine immunogenicity. As discussed later, our understanding of WTC vaccines’ mechanism of action has significantly increased in recent years. This new knowledge allows the evaluation of different strategies to improve the clinical power of WTC vaccines by providing appropriate immunologic danger signals and adjuvants during early vaccine-mediated inflammation and efficiently delivering relevant tumour Ags to DC populations responsible for cross-priming.
Adjuvants enhance early inflammation
Adjuvants are essential components of cancer vaccines and have been successfully used to trigger and modulate innate and adaptive immunity against tumours. Adjuvants can lengthen the half-life of vaccines, improve Ag delivery and uptake by APCs, induce activation/maturation of APCs and their migration to draining lymph nodes (dLN), stimulate the production of inflammatory cytokines, and promote caspase 1-mediated local inflammation and innate immune cells recruitment [29]. Despite the relevance of adjuvants in promoting vaccine success, regarding WTC vaccines, there has not been a systematic use of them, being absent in many formulations or poorly reported (Table 1). This omission has not favoured the optimisation of WTC vaccine design.
Regarding cancer vaccines, immunostimulant adjuvants are very relevant because they activate cell signalling pathways through pattern recognition receptors (PRRs), improving innate immune responses and releasing cytokines. Typical examples of this kind of adjuvants are poly‐I: C (polyinosinic: polycytidylic acid), which can activate TLR3, lipopolysaccharide (LPS), which activates TLR4, flagellin, which activates TLR5, imiquimod which targets TLR7 and CpG oligodeoxynucleotides which target TLR9 [29].
On the other hand, several studies are exploring the potential of TLR agonists as vaccine adjuvants [30]. For example, MLPA (a TLR4 agonist) is combined with the type 1 WTC vaccine Melacine [31].
Poly(ICLC) and poly(IC12U) are milder versions of poly(I:C), that in some settings can result in severe local or systemic toxicities [32]. These double-stranded RNA analogues have been tested as an adjuvant in DC-based immunotherapy in patients with advanced ovarian cancer [33, 34]. Imiquimod mimics single-stranded RNA, triggering signalling through MyD88. This adjuvant has been used to elicit an immune response in breast cancer, ovarian cancer, melanoma, and solid tumours [35]. Finally, CpG analogues have been shown to trigger a robust Th1 response in clinical trials, becoming ideal adjuvants for cancer vaccines [36]. Although, in general, the use of TLR agonists as adjuvants constitutes an efficient way to enhance the efficacy and potency of cancer vaccines, administering these adjuvants can result in local and systemic toxicities, varying from administration site redness to fever and nausea. Further, severe and rare immunological toxicity issues can potentially occur and require a thorough clinical evaluation of the adjuvant and the vaccine candidate [32, 33].
Another concern about TLR agonists as cancer vaccine adjuvants is that they can trigger some protumour effects in certain cancers. TLR3 and TLR9 are expressed in various types of cancer cells, where their expression is associated with the induction of vascular endothelial growth factor, matrix metalloproteinase-9, and other factors related to cell proliferation, migration, and metabolic reprogramming [37, 38]. In summary, the appropriate use of TLR agonists as vaccine adjuvants relies on balancing their immunostimulant properties and lower toxicity and the potential immunomodulatory role in certain cancers, which should be defined based on each type of vaccine and tumour target.
Recombinant cytokines have been tested in combination with WTC vaccines to enhance cell-mediated immune responses, including interleukin (IL)-2 [39] and GM-CSF [40], both with low impact. Although ectopic expression of GM-CSF in tumour cells augmented TAA presentation and secretion of proinflammatory cytokines by APC, clinical results in prostate and pancreatic cancer patients were unsatisfactory [40, 41].
Another example of an immunostimulatory adjuvant is BCG, the standard treatment for superficial non-muscular bladder cancer [42]. Some studies have explored the possibility of using BCG in combination with WTC vaccines as adjuvant therapies, such as Canvaxin, which includes BCG in its formulation. Although prolonged overall survival in early-phase clinical trials was reported [43], Canvaxin failed in phase III clinical trials, showing no statistical survival difference compared with the placebo [44]. Another WTC vaccine, Vaccimel, consisting of allogeneic tumour cells, BCG, and GM-CSF, showed sustainable long-term survival in stage II-III melanoma patients compared with IFN-α2b-treated patients [45].
One of the most potent and widely used natural adjuvants is the mixture of soluble triterpene glycosides purified from the soap bark tree (Quillaja Saponaria). The QS-21 fraction from this extract exhibits exceptional capacity to activate Th1 and CD8+ T cells. Indeed, phase I clinical trials using QS-21 conjugated with Keyhole Limpet Haemocyanin (KLH) as cancer vaccine adjuvants have been conducted in patients with melanoma [46], breast [47], prostate [48], ovary [49], and lung cancer [50], showing promising results.
Haemocyanins from mollusks, particularly KLH, are large metallated glycoproteins capable of activating the immune system of mammals, generating potent Th1 responses with beneficial clinical outcomes in cancer patients [51, 52]. It was described that the highly complex Concholepas concholepas haemocyanin (CCH) shows a structural stability that contributes to their potent immunostimulatory effects [53], strengthening innate and adaptive immunity in mammals and making it useful in combination with WTC vaccines [54, 55]. Haemocyanins have been used as carriers for tumour Ags, and adjuvants for type 2 WTC vaccines [52, 55, 56]. In the future, it is essential to consider using suitable adjuvants complementary to the properties of a new generation of WTC vaccines.
Tumour Ags elicited by WTC vaccines
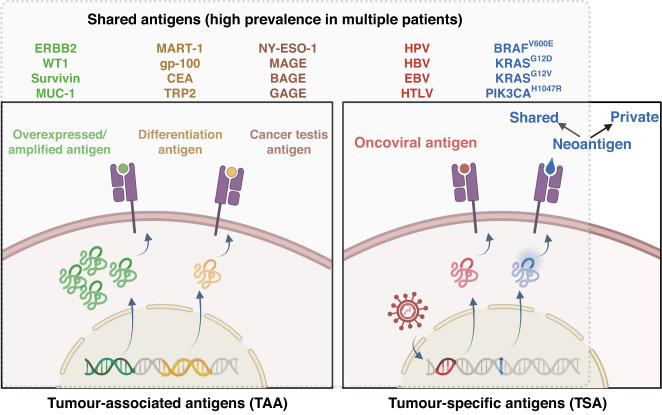
Depending on their origin and nature, tumour Ags can be classified into TAAs or TSAs (Fig. 2). Unlike TAAs, TSAs result mainly from genomic mutations occurring exclusively in tumour cells and exhibit entirely novel amino acid sequences [57]. These are also called tumour neoantigens and are excellent potential targets for personalised therapies and autologous WTC vaccines if those are present in the original tumour source [58, 59]. Moreover, conserved hotspot mutations that generate immunogenic T cell epitopes have been discovered in many cancer types [59–61], indicating that some neoantigens are shared among individuals. Therefore, responses against them can also be induced by allogeneic WTC vaccines. Oncovirus Ags also fall in this category because they are not expressed in healthy cells, making them highly tumour-specific. These Ags are also common in different patients, therefore, they can potentially be provided by allogeneic WTC vaccines. However, only 15% of cancers have a viral aetiopathology, limiting their clinical application [62].
Fig. 2. Types of tumour Ags present in allogeneic whole tumour cell vaccines.
Therapeutic vaccines (including WTC vaccines) target two general classes of tumour Ags, tumour-associated Ags (TAAs, including the overexpressed, differentiation, and cancer testis Ags) and tumour-specific Ags (TSAs, including the oncovirus Ags and neoantigens). Those Ags frequently observed (high prevalence) in tumours from different patients (even from different classes of cancers) are called shared Ags. Shared Ags are good target candidates for allogeneic WTC vaccines. Examples of shared tumour Ags used in WTC vaccine strategies are listed in different colours. ERBB2, epidermal growth factor receptor 2, WT1 Wilms’ tumour protein 1, RAGE-1 survivin, MUC-1 mucin-1, MART-1 melanoma antigen recognised by T cell, gp-100 glycoprotein 100, CEA carcinoembryonic antigen, TRP2 tyrosinase-related protein 2, NY-ESO-1 New York oesophageal squamous cell carcinoma-1, MAGE melanoma antigen gene, BAGE B melanoma antigen, GAGE G antigen, HPV human papillomavirus, HBV hepatitis B virus, EBV Epstein Barr virus, HTLV human T lymphotropic virus, BRAF v-raf murine sarcoma viral oncogene homologue B1, KRAS, Kirsten rat sarcoma virus, PIK3CA phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha. The figure was created with BioRender.
On the other hand, TAAs can derive from proteins overexpressed in tumour cells while also being present in non-malignant cells at low levels. TAAs include differentiation Ags and cancer testis Ags. Those fall into the category of shared Ags, as they can be expressed in tumours of patients with the same or even different cancer types [63], making them prospective WTC vaccine targets. However, this type of Ags shows significant variability in their immunogenicity, maybe due to the preferential depletion of high-affinity T cell receptors (TCRs) during central tolerance selection [64] or peripheral tolerance mechanisms induced at the TME [65]. Thus, several cancer cell conditioning strategies have emerged to break the tolerance of TAAs in WTC vaccines and increase their immunogenicity (section “Cell stress improves WTC vaccines’ immunogenicity”).
Tumours are heterogeneous tissues, so targeting a specific Ag can lead to selecting Ag-negative tumour cells, promoting tumour resistance to the administered treatment, and leading to therapy failure [66]. Therefore, targeting multiple Ags is a more competent procedure. In this context, WTC vaccines arise as an adequate and advantageous strategy in which target Ags do not need to be prospectively identified. A multiple-target strategy, such as WTC vaccines, contains many different MHC-I- and MHC-II-restricted Ags that can potentially induce adaptive immune responses. This approach allows the activation of not only CD8+ T cells but also tumour-specific CD4+ T cells, which have proven to be essential for an effective antitumour response [67]. WTC vaccines take advantage of the tumour’s unique molecular characteristics containing a wide range of specific Ags. These Ags can arise from various mechanisms, resulting in unique features that enhance the immunogenicity of the vaccine. Tumour-composing WTC vaccines often exhibit genetic abnormalities, including chromosomal rearrangements. These rearrangements can lead to the generation of novel fusion proteins, which are not present in normal cells serving as unique TAA. Other tumour cells may display aberrant splicing patterns, leading to the production of alternative protein isoforms. These isoforms can generate neoantigens that are not found in healthy tissues. Tumour cells often undergo abnormal post-translational modifications, such as phosphorylation, glycosylation, or acetylation, which can create neoepitopes capable of being recognised by the immune system as foreign [68]. Moreover, A to I RNA editing is a process where adenosine (A) in RNA is enzymatically deaminated to inosine (I), leading to altered protein sequences. Tumour cells can exhibit dysregulated A to I editing, resulting in modified protein structures and the generation of novel tumour-specific Ags [69]. Due to their Ag diversity, WTC vaccines enable the immune system to recognise and target tumour cells more effectively than conventional vaccines. However, using complete tumours makes identifying relevant TAAs and the molecular readout of T cell-mediated immune response challenging. The emergence of new technologies for mass monitoring at even the single-cell level surely will facilitate the monitoring and analysis of WTC vaccines elicited immune responses.
WTC vaccines overcome the limitation of personalised treatments, lowering costs and simplifying therapeutic procedures. The use of this multi-target strategy can reduce tumour sculpture. As multiple T cell pools are elicited, even if a particular Ag is lost in all cancer cells, a tumour attack can still occur mediated by other tumour-specific T cell clones in the induced repertoire [70]. Since multiple Ags can be provided, this strategy can potentially treat many patients with a polyvalent therapeutic vaccine based on the assumption that some could be shared in the entire repertoire of administered Ags [70].
Cell stress improves WTC vaccines’ immunogenicity
Besides being antigenically different from normal cells, cancer cells must present danger-associated molecular patterns (DAMPs), which act as natural adjuvants, stimulating the innate inflammatory response so that the immune system recognises them as foreign [71]. In this context, Matzinger et al. have shown that DCs can be activated by endogenous signals derived from stressed and necrotic malignant cells but not from healthy cells [72]. In parallel, it was demonstrated that supernatants or fragments from necrotic tumour cells, but not from the healthy counterpart, induce an adequate DC maturation [73], suggesting that tumour cells under stress are more prone to activate DCs in response to DAMPs in comparison with not-malignant cells.
Cells stressed by different agents mobilise calreticulin to the cell membrane generating eat-me signals recognised by phagocytic cells and Ag-presenting cells [71, 74]. Similarly, the nuclear high mobility group box-1 (HMGB1) protein is released through stress and during the ICD interacting with TLR4 and RAGE, both expressed in DCs, generating their activation and maturation [75, 76]. Other components, such as ATP and heat shock proteins (HSP) released under cell stress, can generate a proinflammatory environment capable of activating the innate immune response and thus generating an efficient adaptive response [71, 72]. These stress properties may serve for the ex vivo activation of Ag-presenting cells or the induction of in vivo inflammation when applied as WTC vaccines in conjunction with suitable adjuvants.
Since then, our knowledge of ICD and cell stress-induced adjuvanticity of tumour cells has considerably increased, impacting the design of therapeutic strategies (Fig. 3). Indeed, adjuvanticity of tumour cells can be induced by different approaches that trigger cell stress with or without the concomitant induction of ICD. These stressed/killed tumour cells could be used as a source of immunogenic Ags for WTC vaccines. Extensive reviews about ICD and adjuvanticity of tumour cells have been published [71, 77]. Therefore, only adjuvants associated with WTC vaccines will be discussed in this section.
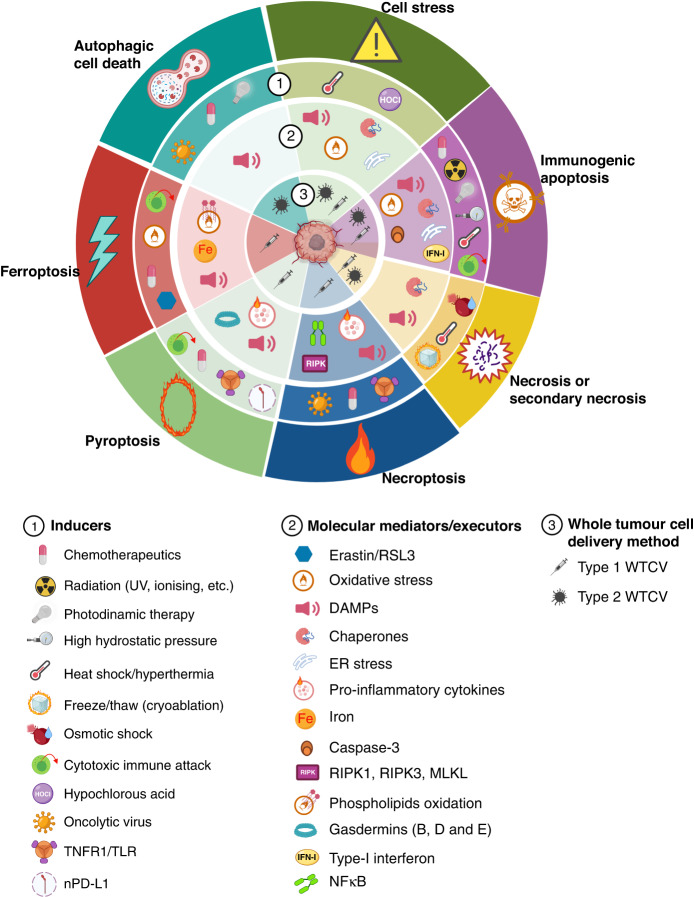
Fig. 3. Strategies to induce adjuvanticity and immunogenicity of tumour cells used as a source of Ags in whole tumour cell vaccines.
Several immunogenic cell death (ICD) modalities or cell stress inducers (level 1) have been used on tumour cells before preparing WTC vaccines to increase the induced antitumour immune response. The primary molecular mediators/executors for each stress or ICD modality are shown in level 2. Whether the immunogenic tumour cells generated with the different modalities were used directly or via loading autologous antigen-presenting cells (APC-WTC vaccines) is shown in level 3. For example, as indicated in dark green panels, stressors as heat shock or hypochlorous acid (HOCl) treatment promote adjuvanticity of tumour cells by inducing the generation of DAMPs [83, 85, 88–90], chaperone proteins overexpression [85, 152], and cellular oxidative and ER stress [85, 155]. Heat shock conditioned or HOCl-conditioned tumour cells have been used as type 1 [55, 90] or type 2 [86, 87] WTC vaccines. An additional example, depicted in purple panels, shown that immunogenic apoptosis could be induced in tumour cells by photodynamic therapy (PDT), using different photosensitisers. Cancer cells killed by PDT release DAMPs and are protective when as a type 1 WTC vaccine [156]. DAMP danger-associated molecular factor, ER endoplasmic reticulum, MLKL mixed lineage kinase domain-like, NFkB nuclear factor kappa B, nPD-L1 nuclear-programmed death-ligand 1, RIPK receptor-interacting serine/threonine-protein kinase, RSL3 RAS selective lethal 3, TLR toll-like receptor, TNFR1 tumour necrosis factor receptor 1. The figure was created with BioRender.
ICD types include immunogenic apoptosis, (secondary) necrosis, necroptosis, pyroptosis, ferroptosis, and autophagic cell death. All these types, except for accidental necrosis, drive the induction of different programmed cell death (PCD) pathways [77]. Different PCD pathways employ distinct molecular and cellular mediators to trigger inflammatory immune responses (Fig. 3).
During normal development and tissue homoeostasis, apoptosis is the main PCD pathway and seems immunologically neutral. However, apoptosis can also activate proinflammatory immune responses against tumours under specific conditions, such as endoplasmic reticulum or oxidative stress [78]. Furthermore, various chemotherapeutics can induce immunogenic apoptosis. Immunogenic apoptosis can also be provoked by radiation [79], photodynamic therapy [80], high hydrostatic pressures [81], and heat shock/hyperthermia [82, 83]. Moreover, it is known that cytotoxic T lymphocytes (CTLs) and natural killer cells induce tumour cell death by caspase-3-dependent ICD mechanisms [84, 85]. This evidence indicates that WTC vaccines from tumour cells killed by numerous immunogenic apoptosis-inducers show antitumour activities in several cancer models.
Other ICD types recently implicated in the adjuvanticity of tumour cells and WTC vaccine efficacy include necroptosis, pyroptosis, ferroptosis, and autophagy-mediated cell death. Necroptosis, a regulated form of necrosis, is defined by the activation of the receptor interacting serine/threonine kinase-3 (RIPK3) and the subsequent permeabilisation of the plasma membrane by the pseudokinase mixed lineage kinase domain-like (MLKL). Necroptotic tumour cells, generated by chemotherapeutic agents, oncolytic viruses, TNF-α, or TLR ligation, have induced potent antitumour immunity in different cancer models [86–89]. In addition, different stimuli, including chemotherapy, granzyme-mediated cytotoxicity, and nuclear PD-L1 expression, induce pyroptosis, promoting antitumour immunity [90–92]. Pyroptosis is a form of immunogenic cell death that can be induced by canonical caspase-1 inflammasomes or by different caspases (1, 4, 5, or 11) that cleavage the gasdermin family members B, D, and E with plasma membrane pore-forming activity. Recently, tumour cells dying for ferroptosis, an iron-dependent form of regulated cell death, were associated with the induction of antitumour immune effects, emerging its therapeutic use as a source of WTC vaccines [93–95]. However, ferroptotic cancer cells can also decrease phagocytosis and Ag cross-presentation by DC, thus, tumour evasion could be favoured in this setting [96].
WTC vaccines made from tumour cell lysates prepared with stressed cells also have shown immunogenicity and adjuvanticity in murine and human cancer models [55, 56, 97–100]. WTC vaccines prepared from hypochlorous acid (HOCl)-treated tumour cells showed increased immunogenicity compared to untreated control tumour cells [101, 102]. HOCl incubation induces an oxidative stress response in tumour cells, improving therapeutic outcomes mediated by polyfunctional vaccine-specific CD4+ T cell responses in ovarian cancer patients [101]. In this regard, we have reported that moderate heat shock stress induces the generation of DAMPs on tumour cells without provoking evident levels of cell death [97–100]. In a series of in vitro studies, we have demonstrated that heat-shock-conditioned melanoma cells can induce various stress signals, such as the release of ATP and HMGB1 and the translocation of calreticulin (a well-described “eat-me” signal). These signals are closely related to the immunogenicity of tumour cell lysates, promoting APC maturation and enhancing Ag cross-presentation [97–100]. Therefore, the induction of danger signals from the tumour cells before the lysis and irradiation steps and adequate immune stimulant adjuvants could surpass the low clinical efficacy of WTC vaccines.
Immunogenic autophagy and Ag spreading
Autophagy is a fundamental process in which damaged cytosolic proteins, and unnecessary organelles are sequestered in autophagosomes and delivered to lysosomes for clearance. Under normal conditions, autophagy suppresses inflammation, but proinflammatory autophagosomes can emerge under stressful situations, such as in tumours [103]. Importantly, tumour cell autophagosomes are efficient vehicles for tumour Ag cross-presentation by DCs [104]. Two main proteolytic pathways generate antigenic peptides. While lysosomes degrade long-lived proteins via autophagy [105], short-lived proteins, including defective ribosomal products (DRiPs), are ubiquitinated and degraded by proteasomes [104, 106]. These autophagosome-enriched DRiP-containing vesicles are known as DRibbles. DRibbles rapidly differentiate monocytes and DC progenitor cells into functional APCs via TLRs and nucleotide-binding oligomerisation domain-containing proteins, promoting inflammasome activation and Ag cross-presentation [106]. Tumour cell-derived DRibbles sequester a broad spectrum of Ags and DAMPs and are highly effective for priming Ag-specific T cells. Furthermore, DRibble’s autophagosome-based vaccines showed potent antitumour efficacy in murine models of lung cancer and melanoma [107].
Dying cancer cells or released cell components are engulfed by APCs leading to the generation of novel MHC-I and -II peptides that are transported to the cell surface to prime newly generated polyfunctional T cells with diverse specificities. When the cognate peptide is recognised within the context of MHC-complex, vaccine-induced or transferred Ag-specific T cells promote tumour cell lysis. Uptake and cross-presentation of Ags released during tumour cell destruction by T cells represent an essential initial step for epitope spreading in immunotherapy. Epitope spreading is characterised by the augmentation and diversification of the endogenous T cell response against antigenic epitopes that differ from the original target epitopes and do not cross-react with them [70, 108]. Cancer immunotherapy has convincingly demonstrated that the clinical outcome of tumour-specific T cells, either induced upon vaccination or adoptively transferred, can be increased by epitope-spreading mechanisms [109–111].
Independent research groups have recently demonstrated that CD8+ T cells induce ICD on tumour cells, generating spread immunity and a protective T cell response against endogenous tumour Ags [84, 85]. In one study, OVA-transfected MC38 colon cancer cells, exogenously pulsed to present the gp100 epitope, were killed in culture by mouse gp100-specific TCR transgenic CD8+ T cells. ICD markers, such as calreticulin exposure and soluble HMGB1, were found in the cocultures of tumour cells and cytotoxic effector cells [84]. Furthermore, immunisation of mice with the resulting destroyed cells induces epitope spreading, as observed by detecting OVA-specific T cells by MHC multimer staining and rejecting OVA+ EG7 lymphoma cells. These results indicate that allogeneic WTC vaccines can induce ICD and epitope spreading in the appropriate inflammatory context.
DC activation and tumour Ag cross-presentation
DCs play a fundamental role in orchestrating the immune response against tumours. DCs capture and process tumour Ags while recognising DAMPs released by dying tumour cells [71, 112]. This process induces DC maturation and migration to DNS. Once in the dLN, DCs present Ag peptides on MHC-II to CD4+ T cells, whereas those loaded on MHC-I can be recognised by CD8+ T cells, leading to their proliferation and activation. This Ag presentation process of internalised extracellular Ags in MHC-I is called cross-presentation and is crucial for optimal antitumour immune responses [113].
Phenotypically, DC are classified into four main subtypes: plasmacytoid DC (pDC), type 1 conventional DC (cDC1), type 2 cDC (cDC2), and MoDC. cDC1 are the most relevant for inducing antitumour-specific immune responses due to their superior Ag processing, cross-presentation/cross-dressing activities, and remarkable ability to prime Th1 responses [114–116]. cDC1 mediates tumour immunity through recruitment and maintenance of CD8+ T cell functions via secretion of IL-12 and reactivation of memory CD8+ T cell [117–119]. Indeed, several studies support the crucial role of cDC1 for immunogenic tumour rejection induced by ICIs and ACT immunotherapies [119]. Although human and mouse pDC and cDC2 show protective antitumour immune activities in some conditions [120, 121], they have been implicated in cancer-associated immunosuppression, mainly through stimulation of pro-tumoural Treg cells or poor T cell priming capacity [122, 123]. Finally, MoDC are rapidly recruited into inflammation sites and promote the activation of CD4+ T cells towards Th1, Th2, or Th17 phenotypes [124].
Despite ambiguous clinical results, DC vaccines are one of the most widely explored platforms due to their safety and minimal toxicity [21–23]. The best example is Sipuleucel-T, the first FDA-approved cell-based therapy for hormone-refractory prostate cancer patients [125]. In addition, DC-based vaccines are shown promising results in treating glioblastoma [126].
Accumulated evidence suggests that DCs, through their PRRs, interpret signals from peripheral physiological or pathological microenvironments, acquiring different functional capabilities [77, 79, 127]. Several endogenous factors, such as DAMPs, are translocated to the cell membrane or are released into the extracellular milieu by dying, stressed, or injured cells. Therefore, DAMPs can function as endogenous adjuvants for DC cells, warranting an immunogenic DC phenotype with strong T cell-activation capacity.
We and others have shown that human DCs loaded with allogeneic heat-shocked melanoma cells were more efficient at cross-priming human CD8+ T cells than DCs loaded with unheated, killed melanoma cells [97–99, 128]. Heat-shocked melanoma cells expressed enhanced amounts of heat shock protein-70 related to enhanced cross-priming activity [128]. Notably, heat shock promotes calreticulin plasma membrane translocation and release of HMGB1 by tumour cells associated with enhanced maturation of DCs and efficient Ag cross-presentation capacity, respectively [97–99]. It is crucial to overcome conventional DC-vaccine limitations to select optimal human tumour cell lines as a source for a generic TAA pool and their optimal processing and presentation. Starting from monocytes from the patient’s blood, loaded with a heat shock-conditioned melanoma lysate named TRIMEL, we prepared activated APCs [56, 97]. These cells, known as Tumour-Antigen-Presenting-Cells (TAPCells®), have a mature DC-like phenotype with increased potency to induce an antitumour immune response in patients [56, 97, 127]. TRIMEL displays a diverse antigenic repertoire and contains several factors, acting as danger signals suppliers for ex vivo [56, 97] and in vivo DC activation [100].
Evidence supports that tumour conditioning can generate cancer cell-derived lysates with proper configuration for ex vivo-generated antitumour-DCs [127–129]. TAPCells-based immunotherapy induced T cell-mediated immune responses and improved the long-term survival of stage IV melanoma patients [56, 97]. Significantly, 61% of tested patients (58 out of 94) showed a delayed-type hypersensitivity (DTH) reaction against TRIMEL, indicating the development of antitumour immunological memory that correlates with prolonged patient survival [56, 97]. In another phase I study, we could associate the induction of DTH against prostate cancer lines with the generation and activation of clinically relevant memory CD4+ and CD8+ T cells and a significant decrease in prostate-specific Ag serum levels [55].
An alternative to WTC-derived lysates is WTC-derived mRNA from WTC, which contains a vast antigenic pool. The main advantage of mRNA is its isolation and amplification simplicity. mRNA may act as a provider of danger signals to DC activation [130]. However, therapeutic applications of WTC-derived RNA vaccines are still limited, probably due to high intrinsic instability and safety concerns.
Although extensively used, moDCs may not be the best DC source for immunotherapy since they have been described to have decreased migratory and T cell activation capacity, probably due to the artificial differentiation by cytokines and extensive ex vivo culture periods. Naturally circulating DCs (nDCs) may be a potent alternative for moDCs, as the brief ex vivo exposure of nDCs might preserve the functional capabilities of the cells and prevent exhaustion. Direct isolation of CD1c (BDCA1)+ myeloid nDCs is now feasible and facilitates robust standardisation for multicenter trials and, eventually, standard care [131]. Several clinical trials with autologous nDC vaccination are performed in melanoma, prostate, and other solid tumour cancer patients using solely CD1c+ mDC loaded with peptides or combined with other immunotherapeutics [131]. The contribution of the diverse cell types to the activity of the vaccination product remains unclear. However, using nDC loaded with tumour cell lysates may be an exciting alternative for the future.
The tumour microenvironment
Immune responses against cancer shape the TME, including its cellular, metabolite, cytokine composition, and physicochemical characteristics. Certainly, tumours can escape the host immune defences by decreasing their intrinsic immunogenicity and inducing a specific immunosuppressive TME. According to the level of immune cell infiltration and capacity to trigger a robust immune response, tumours are classified as hot or cold. Hot tumours are immunogenic, inflamed, characterised by the presence of activated CTLs, high expression of T cell-attracting chemokines, show a type-I IFN transcriptional signature, and are efficiently rejected by the immune system. Conversely, cold tumours lack T cell infiltration, correlated with the absence of type-I IFN signature and poor chemokine production. The immune system ignores these tumours and responds poorly to immunotherapies [132].
TME CD4+ T cells can differentiate into distinct effector or Treg cell lineages. Upon Ag-specific stimulation, intra-tumour effector CD4+ Th1 cells release IL-2 and IFN-γ, promoting antitumour immunity [133]. On the contrary, TME Tregs inhibit effector T cell functions due to tumour-induced metabolic unbalance [134]. Moreover, intra-tumour cDC1 were crucial for in situ maintenance of effector functions of pre-activated CTLs [135]. cDC1 are required to initiate adaptive immunity against tumours in the dLN, for the CTLs life cycle in the TME, and for the generation and recall of memory to prevent relapse and metastases [136]. Accordingly, only tumours infiltrated by cDC1 and CTLs are spontaneously controlled in an experimental model of established immune memory [137].
The relevance of TILs highlights in their ability to predict the response to ICI treatments based on their location and density [138]. Indeed, patients with significant lymphocyte accumulation within the TME are more likely to respond [139]. Furthermore, increased TILs after ICI treatments have better prognostic and correlate with longer patient survival times [140].
The prognostic value of TIL induction in patients receiving therapeutic vaccines, including WTC, is an exciting challenge that should be further addressed, particularly in weakly immunogenic cold tumours. As mentioned before, ICI treatments may be less effective in cold tumours. Cancer vaccines can elicit Ag-specific T cell responses in such situations, leading to TME lymphocyte infiltration [141]. Although there remains a concern about increased adverse events related to autoimmune reactions to WTC vaccine-ICI combined therapy, this has not been observed in published reports [142].
Another challenge is related to the quality than quantity of lymphocytes in the TME. It is well-known that chronic Ag stimulation occurring in cancer results in CD8+ T cell miss-function [143]. Exhausted CD8+ T cells progressively showed decreased effector function and proliferative capacity. At an early stage, loss of IL-2 production and ex vivo killing capacity is observed [144, 145]. Then, at intermediate stages, the production of TNF-α is impaired, whereas, at advanced stages of exhaustion, IFN-γ and granzyme B production are also lost [145].
Exhausted T cells express high levels of inhibitory receptors, including PD-1, CTLA-4, and other modulatory receptors [146–149]. Importantly, exhausted T cell misfunction are partly caused by overexpression of PD-1. Various CD8+ TIL populations have been described based on their PD-1 expression levels: negative or low (PD-1N or PD-1lo), intermediate (PD-1mid), and high (PD-1hi) [150]. PD-1hi CD8+ T cells show an exhausted phenotype and lower production of proinflammatory cytokines, and they are associated with a worse prognosis in several types of cancer. In contrast, high levels of PD-1lo TILs indicate better clinical outcomes for cancer patients.
Although the consequences of ICI immunotherapy on the modulation of the TME constitute a crucial element in improving its effectiveness [151], reports of the TME after using WTC vaccines are very scarce. An example of the importance of vaccine effect on the TME appears evident in the study of our experimental WTC vaccine, TRIMELVax, in which the integrity of the components of the vaccine seems to be essential for an effective response and is reflected in the cellular composition of the TME post-immunisation [100]. In fact, multiparametric flow cytometry analysis of tumours and TdLN from vaccinated mice showed that both TRIMELVax and anti-PD-1 treatments induced an increased frequency of intratumour cDC1s without affecting the percentage of cDC2s [100]. With its better antitumour activity, TRIMELVax induced a higher tumour infiltration of CD3+, CD4+, and CD8+ T cells than anti-PD-1 monotherapy [100]. Regarding lymphoid compartments, unlike anti-PD-1 treatment, TRIMELVax, alone and in combination with anti-PD-1, showed a significatively enhanced intratumour CD8+/CD4+ T cell ratio. Regarding this, neither anti-PD-1 nor TRIMELVax induced significant changes in NK cell intratumour frequency. However, the B cell compartment and NK cell frequency were enriched in TdLNs from mice treated with TRIMELVax alone or combined with anti-PD-1, not with only the PD-1 blocker.
Moreover, the induction of suboptimal acute local inflammation generated by an incomplete vaccine is reflected by the accumulation of exhausted T lymphocytes in tumours. On the other hand, the complete WTC vaccine formulation generated abundant active effector cDC1 and CD8+ T cells [100].
Current challenges
Immunotherapy has proven to be a highly efficient strategy for metastatic oncological diseases in which traditional methods were initially unsuccessful, whether because significant adverse effects were manifested or because the standard treatment had suboptimal effects during the disease. Cell therapies such as CAR-T cells in haematopoietic cancers and ICIs in several solid tumours have positively impacted the survival of treated patients where standard therapies failed. In addition, the studies involving these novel therapies have revealed new mechanisms that regulate and control the interaction between the immune system and the tumour.
In general, uncontrolled growth of tumour cells is silent in the early stages of cancer, promoting a state of tolerance that generally translates into an immunosuppressive microenvironment that reduces the ability of the immune system to recognise and effectively eliminate these tumours. Thereby, it seems possible to transform cold tumours’ immunosuppressive microenvironments into hot tumours attracting T cells to the TME through different strategies, such as immunogenic tumour cell killing by certain chemotherapies, radiotherapy, oncolytic viruses, and other intratumoural therapeutic approaches. Vaccines constitute an integral immunotherapeutic approach since they can generate an active and systemic immune response against malignant neoantigens, resulting in immunological memory. Standard WTC vaccines were early attempts to mimic complex natural immune responses in a simple way. Nowadays, there is a much longer experience and a deeper understanding of the relationship between the main factors required for an optimal early induction and further development of a specific antitumour immune response and the clinical effectiveness of the WTC vaccines.
WTC vaccines obtained from a combination of allogeneic tumour cell lines are excellent providers of diverse TAAs. These include those shared neoantigens or hidden Ags that are not dominant but should be present in the autologous tumour. However, it is imperative to identify optimal tumour cell pretreatments with cell stress-inducing agents, immunogenic autophagy, or ICD. These are crucial for Ag processing and releasing associated DAMPs, enhancing Ag cross-presentation, and APC activation. In this regard, it is also relevant to use adequate adjuvants to provide essential inflammatory signals for the early activation of the innate immune system components, which are essential for the functional stability of these vaccines. Specific combinations of antigenic sources and adjuvants are crucial to improve the potency of vaccines without generating adverse effects.
Both WTC vaccine intrinsic composition and the presence of adjuvants enhance an early local inflammation that increases the recruitment of cDC1 and other relevant APCs. cDC1 migration to dLNs and TME is key to promoting TAA cross-presentation and activation of naïve T cells to induce Th lineage differentiation and further infiltration of effector Th1/Th17 TIL, therefore, immune monitoring during the treatment follow-up is also an important aspect to consider.
All factors considered in the immunisation product are directed to promote Ag spreading through tumour cell death, provoking the emergence of new T cell clones that will reduce the relative presence of exhausted lymphocytes and Treg cells at the TME.
Until now, WTC vaccines have not shown all their potential in the clinic, partly because of the manufacturing process’s challenges that impact their regulatory approval. Some of these critical points are related to biological quality controls, type of cell culture, cell viability, final dosage, cryopreservation, and administration [152]. Finally, other parameters, such as the scalability of the technology, prices of raw materials, sustainability, and product quality, must also be competitive with existing therapeutic approaches.
An important significant challenge in immunotherapy is tumour escape, where tumours develop mechanisms to evade immune surveillance. Tumour cells can employ several strategies to avoid immune recognition, including downregulating MHC molecules, upregulating immune checkpoint proteins, and promoting an immunosuppressive TME [153]. Overcoming tumour escape requires a multifaceted approach. Combined therapies that target multiple immune checkpoint molecules will enhance Ag presentation and modulate the TME inducing a more effective immune response. Developing personalised immunotherapeutic strategies tailored to individual tumour characteristics may also improve treatment efficacy. Understanding the complex interactions between the immune system and tumours is crucial to overcome the challenges posed by tumour escape and optimise immunotherapy’s effectiveness in combating cancer.
While WTC vaccines hold great promise for cancer immunotherapy, they face several obstacles that need to be addressed, including biological safety. WTC vaccines carry the risk of inducing autoimmune reactions or off-target immune responses. Since WTC vaccines contain a wide range of Ags, there is a potential for the immune system to target normal tissues expressing similar Ags mistakenly. Ensuring a safe immune profile of the vaccine is critical in clinical development. Remarkably, the variability in patient-derived tumour cells adds complexity to the manufacturing process. The regulatory landscape for such personalised therapies is evolving, and establishing standardised guidelines and protocols for safety, quality, reproducibility, and efficacy is essential to facilitate their development and approval.
Addressing these challenges requires multidisciplinary efforts involving immunologists, oncologists, geneticists, and other experts. Advances in understanding tumour biology, immune regulation, and personalised medicine are crucial in overcoming these obstacles and harnessing the full potential of WTC vaccines in cancer treatment.
Furthermore, it is important to consider the temporal schedule of WTC vaccines combined with other immunotherapies to potentiate an optimal immune response. Although combining immunotherapeutic strategies can improve the clinical response, it can also augment toxicity and adverse events. Not all approved drugs could be combined with immunotherapy. For example, the combination of vemurafenib with ipilimumab showed hepatotoxicity when they were co-administered [154].
Finally, clinical trials should consider the patient’s immune system status, as some patients in advanced disease stages or intensively treated with previous therapies cannot mount robust immune responses Therefore, intensive research into WTC vaccination’s immunological mechanism is still necessary.
Conclusions
The relationship between tumours and the immune system is regulated by a series of temporary and localised events that sculpt responses ranging from tolerance to inflammation capable of eradicating cancer. Among essential factors are innate immune receptors that recognise conserved DAMPs that trigger specific activation mechanisms for each threat. In the case of the antitumour response, antigen cross-presentation by particular DC subtypes is crucial. The activation of APC populations such as cDC1 is mediated by an inflammatory environment that can be induced by molecules expressed in stressed/killed (ICD) tumour cells and by adequate adjuvants. In tumours, cancer cell metabolism, as autophagy, promotes the appearance of new Ags that encourage a renewal of antigenic diversity and may impact TME structure. In this context, a new generation of cancer WTC vaccines that consider the factors indicated above will have the capacity to induce effective long-term tumour-specific cellular immune responses that break tolerance and efficiently synergise with ICIs. This therapeutic strategy could be especially recommended for patients harbouring weak spontaneous T cell responses against their tumours, which fail to respond to ICI-based treatments. Ag diversity and the presence of immunostimulants, including adjuvants (exogenous and endogenous DAMPs), cytokines, and other agents promoting early local inflammation and APC activation, can improve the efficacy of WTC vaccines, therefore, it is necessary to continue exploring this type of strategies.
Acknowledgements
We thank Marisol Briones (Universidad de Chile) for the administrative and technical support.
Author contributions
Literature search and chapter writing (AP-B, MAG, IF, CP), critical review and corrections (MN, JPA, GN, CQ), figures, conceptualisation, and manuscript writing (AT, FS-O).
Data availability
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Amarilis Pérez-Baños, María Alejandra Gleisner.
Contributor Information
Andrés Tittarelli, Email: atittarelli@utem.cl.
Flavio Salazar-Onfray, Email: fsalazar@uchile.cl.
References
- 1.Meric-Bernstam F, Larkin J, Tabernero J, Bonini C. Enhancing antitumor efficacy with immunotherapy combinations. Lancet. 2021;392:1010–22. doi: 10.1016/S0140-6736(20)32598-8. [DOI] [PubMed] [Google Scholar]
- 2.Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375:2255–62. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hossian AKMN, Hackett CS, Brentjens RJ, Rafiq S. Multipurposing CARs: same engine, different vehicles. Mol Ther. 2022;30:1381–95. doi: 10.1016/j.ymthe.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16:219–33. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 6.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–55. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pires da Silva I, Ahmed T, Reijers ILM, Weppler AM, Betof Warner A, Patrinely JR, et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study. Lancet Oncol. 2021;22:836–47. doi: 10.1016/S1470-2045(21)00097-8. [DOI] [PubMed] [Google Scholar]
- 8.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donninger H, Li C, Eaton JW, Yaddanapudi K. Cancer vaccines: promising therapeutics or an unattainable dream. Vaccines. 2021;9:668. doi: 10.3390/vaccines9060668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–21. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvo Tardón M, Allard M, Dutoit V, Dietrich PY, Walker PR. Peptides as cancer vaccines. Curr Opin Pharmacol. 2019;47:20–6. doi: 10.1016/j.coph.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21:360–78. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 14.Mustafa AS. BCG as a vector for novel recombinant vaccines against infectious diseases and cancers. Vaccines. 2020;8:736. doi: 10.3390/vaccines8040736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raman SS, Hecht JR, Chan E. Talimogene laherparepvec: review of its mechanism of action and clinical efficacy and safety. Immunotherapy. 2019;11:705–23. doi: 10.2217/imt-2019-0033. [DOI] [PubMed] [Google Scholar]
- 16.Chesney JA, Ribas A, Long GV, Kirkwood JM, Dummer R, Puzanov I, et al. Randomized, double-blind, placebo-controlled, global phase III trial of talimogene laherparepvec combined with pembrolizumab for advanced melanoma. J Clin Oncol. 2023;41:528–40. doi: 10.1200/JCO.22.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christopoulos PF. The future of tumor vaccines in the post-COVID-19 era-current challenges. Immun Inflamm Dis. 2021;9:1795–7. doi: 10.1002/iid3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenberg L, Belmans J, Van Woensel M, Riva M, Van Gool SW. Exploiting the immunogenic potential of cancer cells for improved dendritic cell vaccines. Front Immunol. 2016;6:663. doi: 10.3389/fimmu.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang MN, Nicholson LT, Batich KA, Swartz AM, Kopin D, Wellford S, et al. Antigen-loaded monocyte administration induces potent therapeutic antitumor T-cell responses. J Clin Investig. 2020;130:774–88. doi: 10.1172/JCI128267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yewdall AW, Drutman SB, Jinwala F, Bahjat KS, Bhardwaj N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen-presenting cells. PLoS ONE. 2010;5:e11144. doi: 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38:577–93. doi: 10.1016/j.it.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15:28. doi: 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baghdadi M, Nagao H, Yoshiyama H, Akiba H, Yagita H, Dosaka-Akita H, et al. Combined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomas. Cancer Immunol Immunother. 2013;62:629–37. doi: 10.1007/s00262-012-1371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan X, Quezada SA, Sepúlveda MA, Sharma P, Allison JP. Engagement of the ICOS pathway markedly enhances the efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med. 2014;211:715–25. doi: 10.1084/jem.20130590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faries MB, Mozzillo N, Kashani-Sabet M, Thompson JF, Kelley MC, DeConti RC, et al. Long-term survival after complete surgical resection and adjuvant immunotherapy for distant melanoma metastases. Ann Surg Oncol. 2017;24:3991–4000. doi: 10.1245/s10434-017-6072-3. [DOI] [PubMed] [Google Scholar]
- 28.Dillman RO, Cornforth AN, Nistor GI, McClay EF, Amatruda TT, Depriest C. Randomized phase II trial of autologous dendritic cell vaccines versus autologous tumor cell vaccines in metastatic melanoma: 5-year follow up and additional analyses. J Immunother Cancer. 2018;6:19. doi: 10.1186/s40425-018-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulendran BS, Arunachalam P, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20:454–75. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nijen Twilhaar MK, Czentner L, Bouma RG, Olesek K, Grabowska J, Wang AZ, et al. Incorporation of Toll-like receptor ligands and inflammasome stimuli in GM3 liposomes to induce dendritic cell maturation and T cell responses. Front Immunol. 2022;13:842241. doi: 10.3389/fimmu.2022.842241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sondak VK, Sosman JA. Results of clinical trials with an allogenic melanoma tumor cell lysate vaccine: Melacine. Semin Cancer Biol. 2003;13:409–15. doi: 10.1016/j.semcancer.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf. 2015;38:1059–74. doi: 10.1007/s40264-015-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Facciolà A, Visalli G, Laganà A, Di Pietro A. An overview of vaccine adjuvants: current evidence and future perspectives. Vaccines. 2022;10:819. doi: 10.3390/vaccines10050819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iribarren K, Bloy N, Buqué A, Cremer I, Eggermont A, Fridman WH, et al. Trial watch: immunostimulation with Toll-like receptor agonists in cancer therapy. Oncoimmunology. 2015;5:e1088631. doi: 10.1080/2162402X.2015.1088631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang CL, Kandalaft LE. In vivo cancer vaccination: which dendritic cells to target and how? Cancer Treat Rev. 2018;71:88–101. doi: 10.1016/j.ctrv.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin MJ, Svensson-Arvelund J, Lubitz GS, Marabelle A, Melero I, Brown BD, et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer. 2022;3:911–26. doi: 10.1038/s43018-022-00418-6. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka J, Sugimoto K, Shiraki K, Tameda M, Kusagawa S, Nojiri K, et al. Functional cell surface expression of toll-like receptor 9 promotes cell proliferation and survival in human hepatocellular carcinomas. Int J Oncol. 2010;37:805–14. [PubMed] [Google Scholar]
- 38.Matijevic Glavan T, Cipak Gasparovic A, Vérillaud B, Busson P, Pavelic J. Toll-like receptor 3 stimulation triggers metabolic reprogramming in pharyngeal cancer cell line through Myc, MAPK, and HIF. Mol Carcinog. 2017;56:1214–26. doi: 10.1002/mc.22584. [DOI] [PubMed] [Google Scholar]
- 39.Escobar A, López M, Serrano A, Ramírez M, Pérez C, Aguirre A, et al. Dendritic cell immunizations alone or combined with low doses of interleukin-2 induce specific immune responses in melanoma patients. Clin Exp Immunol. 2005;142:555–68. doi: 10.1111/j.1365-2249.2005.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comiskey MC, Dallas MC, Drake CG. Immunotherapy in prostate cancer: teaching an old dog new tricks. Curr Oncol Rep. 2018;20:75. doi: 10.1007/s11912-018-0712-z. [DOI] [PubMed] [Google Scholar]
- 42.Larsen ES, Joensen UN, Poulsen AM, Goletti D, Johansen IS. Bacillus Calmette-Guérin immunotherapy for bladder cancer: a review of immunological aspects, clinical effects and BCG infections. APMIS. 2020;128:92–103. doi: 10.1111/apm.13011. [DOI] [PubMed] [Google Scholar]
- 43.Hsueh EC, Essner R, Foshag LJ, Ollila DW, Gammon G, O’Day SJ, et al. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J Clin Oncol. 2002;20:4549–54. doi: 10.1200/JCO.2002.01.151. [DOI] [PubMed] [Google Scholar]
- 44.Motl SE. Technology evaluation: Canvaxin, John Wayne Cancer Institute/CancerVax. Curr Opin Mol Ther. 2004;6:104–11. [PubMed] [Google Scholar]
- 45.Mordoh A, Aris M, Carri I, Bravo AI, Podaza E, Pardo JCT, et al. An update of cutaneous melanoma patients treated in adjuvancy with the allogeneic melanoma vaccine VACCIMEL and presentation of a selected case report with in-transit metastases. Front Immunol. 2022;13:842555. doi: 10.3389/fimmu.2022.842555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ragupathi G, Livingston PO, Hood C, Gathuru J, Krown SE, Chapman PB, et al. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin Cancer Res. 2003;9:5214–20. [PubMed] [Google Scholar]
- 47.Gilewski TA, Ragupathi G, Dickler M, Powell S, Bhuta S, Panageas K, et al. Immunization of high-risk breast cancer patients with clustered sTn-KLH conjugate plus the immunologic adjuvant QS-21. Clin Cancer Res. 2007;13:2977–85. doi: 10.1158/1078-0432.CCR-06-2189. [DOI] [PubMed] [Google Scholar]
- 48.Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–8. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 49.Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 2007;13:4170–7. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 50.Krug LM, Ragupathi G, Ng KK, Hood C, Jennings HJ, Guo Z, et al. Vaccination of small cell lung cancer patients with polysialic acid or N-propionylated polysialic acid conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10:916–23. doi: 10.1158/1078-0432.CCR-03-0101. [DOI] [PubMed] [Google Scholar]
- 51.Zanjani NT, Saksena MM, Dehghani F, Cunningham AL. From ocean to bedside: the therapeutic potential of molluscan hemocyanins. Curr Med Chem. 2018;25:2292–303. doi: 10.2174/0929867324666170502124227. [DOI] [PubMed] [Google Scholar]
- 52.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328–32. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 53.De Ioannes P, Moltedo B, Oliva H, Pacheco R, Faunes F, De Ioannes AE, Becker MI. Hemocyanin of the molluscan Concholepas concholepas exhibits an unusual heterodecameric array of subunits. J Biol Chem. 2004;279:26134–42. doi: 10.1074/jbc.M400903200. [DOI] [PubMed] [Google Scholar]
- 54.Villar J, Salazar ML, Jiménez JM, Campo MD, Manubens A, Gleisner MA, et al. C-type lectin receptors MR and DC-SIGN are involved in recognition of hemocyanins, shaping their immunostimulatory effects on human dendritic cells. Eur J Immunol. 2021;51:1715–31. doi: 10.1002/eji.202149225. [DOI] [PubMed] [Google Scholar]
- 55.Reyes D, Salazar L, Espinoza E, Pereda C, Castellón E, Valdevenito R, et al. Tumor cell lysate-loaded dendritic cell vaccine induces biochemical and memory immune response in castration-resistant prostate cancer patients. Br J Cancer. 2013;109:1488–97. doi: 10.1038/bjc.2013.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.López MN, Pereda C, Segal G, Muñoz L, Aguilera R, González FE, et al. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T cells. J Clin Oncol. 2009;27:945–52. doi: 10.1200/JCO.2008.18.0794. [DOI] [PubMed] [Google Scholar]
- 57.Leko V, Rosenberg SA. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell. 2020;38:454–72. doi: 10.1016/j.ccell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harari A, Graciotti M, Bassani-Sternberg M, Kandalaft LE. Antitumour dendritic cell vaccination in a priming and boosting approach. Nat Rev Drug Discov. 2020;19:635–52. doi: 10.1038/s41573-020-0074-8. [DOI] [PubMed] [Google Scholar]
- 59.Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18:215–29. doi: 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klebanoff CA, Wolchok JD. Shared cancer neoantigens: making private matters public. J Exp Med. 2018;215:5–7. doi: 10.1084/jem.20172188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao W, Wu J, Chen S, Zhou Z. Shared neoantigens: ideal targets for off-the-shelf cancer immunotherapy. Pharmacogenomics. 2020;21:637–45. doi: 10.2217/pgs-2019-0184. [DOI] [PubMed] [Google Scholar]
- 62.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–16. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 63.Wculek SK, Amores-Iniesta J, Conde-Garrosa R, Khouili SC, Melero I, Sancho D. Effective cancer immunotherapy by natural mouse conventional type-1 dendritic cells bearing dead tumor antigen. J Immunother Cancer. 2019;7:100. doi: 10.1186/s40425-019-0565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stone JD, Harris DT, Kranz DM. TCR affinity for p/MHC formed by tumor antigens that are self-proteins: impact on efficacy and toxicity. Curr Opin Immunol. 2015;33:16–22. doi: 10.1016/j.coi.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nüssing S, Trapani JA, Parish IA. Revisiting T cell tolerance as a checkpoint target for cancer immunotherapy. Front Immunol. 2020;11:589641. doi: 10.3389/fimmu.2020.589641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kravtsov DS, Erbe AK, Sondel PM, Rakhmilevich AL. Roles of CD4+ T cells as mediators of antitumor immunity. Front Immunol. 2022;13:972021. doi: 10.3389/fimmu.2022.972021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbieri I, Kouzarides T. Role of RNA modifications in cancer. Nat Rev Cancer. 2020;20:303–22. doi: 10.1038/s41568-020-0253-2. [DOI] [PubMed] [Google Scholar]
- 69.Amweg A, Tusup M, Cheng P, Picardi E, Dummer R, Levesque MP, et al. The A to I editing landscape in melanoma and its relation to clinical outcome. RNA Biol. 2022;19:996–1006. doi: 10.1080/15476286.2022.2110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gulley JL, Madan RA, Pachynski R, Mulders P, Sheikh NA, Trager J, et al. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J Natl Cancer Inst. 2017;109:djw261. doi: 10.1093/jnci/djw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23:487–500. doi: 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
- 72.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 73.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 75.Harris H, Andersson U, Pisetsky D. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 76.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2017;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 77.Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21:678–95. doi: 10.1038/s41580-020-0270-8. [DOI] [PubMed] [Google Scholar]
- 78.Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71:768–78. doi: 10.1158/0008-5472.CAN-10-2788. [DOI] [PubMed] [Google Scholar]
- 79.Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25:11–7. doi: 10.1016/j.semradonc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva IV, Vedunova MV, Krysko DV. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021;9:e001926. doi: 10.1136/jitc-2020-001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, Partlova S, et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer. 2014;135:1165–77. doi: 10.1002/ijc.28766. [DOI] [PubMed] [Google Scholar]
- 82.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 83.Podolska MJ, Shan X, Janko C, Boukherroub R, Gaipl US, Szunerits S, et al. Graphene-induced hyperthermia (GIHT) combined with radiotherapy fosters immunogenic cell death. Front Oncol. 2021;11:664615. doi: 10.3389/fonc.2021.664615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Minute L, Teijeira A, Sanchez-Paulete AR, Ochoa MC, Alvarez M, Otano I, et al. Cellular cytotoxicity is a form of immunogenic cell death. J Immunother Cancer. 2020;8:e000325. doi: 10.1136/jitc-2019-000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jaime-Sanchez P, Uranga-Murillo I, Aguilo N, Khouili SC, Arias MA, Sancho D, et al. Cell death induced by cytotoxic CD8+ T cells is immunogenic and primes caspase-3-dependent spread immunity against endogenous tumor antigens. J Immunother Cancer. 2020;8:e000528. doi: 10.1136/jitc-2020-000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, et al. RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells. Science. 2015;350:328–34. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De Koker S, Heyndrickx L, et al. Vaccination with necroptotic cancer cells induces efficient antitumor immunity. Cell Rep. 2016;15:274–87. doi: 10.1016/j.celrep.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 88.Snyder AG, Hubbard NW, Messmer MN, Kofman SB, Hagan CE, Orozco SL, et al. Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci Immunol. 2019;4:eaaw2004. doi: 10.1126/sciimmunol.aaw2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sprooten J, De Wijngaert P, Vanmeerbeerk I, Martin S, Vangheluwe P, Schlenner S, et al. Necroptosis in immuno-oncology and cancer immunotherapy. Cells. 2020;9:1823. doi: 10.3390/cells9081823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a mastermind. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 91.Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu JM, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumor necrosis. Nat Cell Biol. 2020;22:1264–75. doi: 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:eaaz7548. doi: 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]
- 93.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8+ T cells regulate tumor ferroptosis during cancer immunotherapy. Nature. 2019;569:270–4. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8:e001369. doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang D, Kepp O, Kroemer G. Ferroptosis becomes immunogenic: implications for anticancer treatments. Oncoimmunology. 2020;10:1862949. doi: 10.1080/2162402X.2020.1862949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wiernicki B, Maschalidi S, Pinney J, Adjemian S, Vanden Berghe T, Ravichandran KS, et al. Cancer cells dying from ferroptosis impede dendritic cell-mediated antitumor immunity. Nat Commun. 2022;13:3676. doi: 10.1038/s41467-022-31218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aguilera R, Saffie C, Tittarelli A, González FE, Ramírez M, Reyes D, et al. Heat-shock induction of tumor-derived danger signals mediates rapid monocyte differentiation into clinically effective dendritic cells. Clin Cancer Res. 2011;17:2474–83. doi: 10.1158/1078-0432.CCR-10-2384. [DOI] [PubMed] [Google Scholar]
- 98.Rojas-Sepúlveda D, Tittarelli A, Gleisner MA, Ávalos I, Pereda C, Gallegos I, et al. Tumor lysate-based vaccines: on the road to immunotherapy for gallbladder cancer. Cancer Immunol Immunother. 2018;67:1897–910. doi: 10.1007/s00262-018-2157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Flores I, Hevia D, Tittarelli A, Soto D, Rojas-Sepúlveda D, Pereda C, et al. Dendritic cells loaded with heat shock-conditioned ovarian epithelial carcinoma cell lysates elicit T cell-dependent antitumor immune responses in vitro. J Immunol Res. 2019;2019:9631515. doi: 10.1155/2019/9631515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gleisner MA, Pereda C, Tittarelli A, Navarrete M, Fuentes C, Ávalos I, et al. A heat-shocked melanoma cell lysate vaccine enhances tumor infiltration by prototypic effector T cells inhibiting tumor growth. J Immunother Cancer. 2020;8:e000999. doi: 10.1136/jitc-2020-000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res. 2013;19:4801–15. doi: 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou R, Huang WJ, Ma C, Zhou Y, Yao YQ, Wang YX, et al. HOCl oxidation-modified CT26 cell vaccine inhibits colon tumor growth in a mouse model. Asian Pac J Cancer Prev. 2012;13:4037–43. doi: 10.7314/APJCP.2012.13.8.4037. [DOI] [PubMed] [Google Scholar]
- 103.Maes H, Rubio N, Garg AD, Agostinis P. Autophagy: shaping the tumor microenvironment and therapeutic response. Trends Mol Med. 2013;19:428–46. doi: 10.1016/j.molmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Wang LX, Pang P, Twitty C, Fox BA, Aung S, et al. Cross-presentation of tumor-associated antigens through tumor-derived autophagosomes. Autophagy. 2009;5:576–7. doi: 10.4161/auto.5.4.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, et al. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–54. doi: 10.1016/S1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 106.Xing Y, Cao R, Hu HM. TLR and NLRP3 inflammasome-dependent innate immune responses to tumor-derived autophagosomes (DRibbles) Cell Death Dis. 2016;7:e2322. doi: 10.1038/cddis.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Y, Wang LX, Pang P, Cui Z, Aung S, Haley D, et al. Tumor-derived autophagosome vaccine: mechanism of cross-presentation and therapeutic efficacy. Clin Cancer Res. 2011;17:7047–57. doi: 10.1158/1078-0432.CCR-11-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ribas A, Timmerman JM, Butterfield LH, Economou JS. Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol. 2003;24:58–61. doi: 10.1016/S1471-4906(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 110.Menares E, Gálvez-Cancino F, Cáceres-Morgado P, Ghorani E, López E, Díaz X, et al. Tissue-resident memory CD8+ T cells amplify antitumor immunity by triggering antigen spreading through dendritic cells. Nat Commun. 2019;10:4401. doi: 10.1038/s41467-019-12319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brossart P. The role of antigen spreading in the efficacy of immunotherapies. Clin Cancer Res. 2020;26:4442–7. doi: 10.1158/1078-0432.CCR-20-0305. [DOI] [PubMed] [Google Scholar]
- 112.Kvedaraite E, Ginhoux F. Human dendritic cells in cancer. Sci Immunol. 2022;7:eabm9409. doi: 10.1126/sciimmunol.abm9409. [DOI] [PubMed] [Google Scholar]
- 113.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–69. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 114.Böttcher JP, Reis e Sousa C. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer. 2018;4:784–92. doi: 10.1016/j.trecan.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.MacNabb BW, Tumuluru S, Chen X, Godfrey J, Kasal DN, Yu J, et al. Dendritic cells can prime antitumor CD8+ T cell responses through major histocompatibility complex cross-dressing. Immunity. 2022;55:982.e8–97.e8. doi: 10.1016/j.immuni.2022.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Enamorado M, Iborra S, Priego E, Cueto FJ, Quintana JA, Martínez-Cano S, et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat Commun. 2017;8:16073. doi: 10.1038/ncomms16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoch T, Schulz D, Eling N, Gómez JM, Levesque MP, Bodenmiller B. Multiplexed imaging mass cytometry of the chemokine milieus in melanoma characterizes features of the response to immunotherapy. Sci Immunol. 2022;7:eabk1692. doi: 10.1126/sciimmunol.abk1692. [DOI] [PubMed] [Google Scholar]
- 118.Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity. 2018;49:1148.e7–61.e7. doi: 10.1016/j.immuni.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Balan S, Radford KJ, Bhardwaj N. Unexplored horizons of cDC1 in immunity and tolerance. Adv Immunol. 2020;148:49–91. doi: 10.1016/bs.ai.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 120.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–75. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 121.Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, et al. Unleashing type-2 dendritic cells to drive protective antitumor CD4+ T cell immunity. Cell. 2019;177:556.e16–71.e16. doi: 10.1016/j.cell.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aspord C, Leccia MT, Charles J, Plumas J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol Res. 2013;1:402–15. doi: 10.1158/2326-6066.CIR-13-0114-T. [DOI] [PubMed] [Google Scholar]
- 123.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, et al. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–68. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 124.Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38:336–48. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 125.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 126.Liau LM, Ashkan K, Brem S, Campian JL, Trusheim JE, Iwamoto FM, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9:112–21. doi: 10.1001/jamaoncol.2022.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.González FE, Gleisner A, Falcón-Beas F, Osorio F, López MN, Salazar-Onfray F. Tumor cell lysates as immunogenic sources for cancer vaccine design. Hum Vaccin Immunother. 2014;10:3261–9. doi: 10.4161/21645515.2014.982996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shi H, Cao T, Connolly JE, Monnet L, Bennett L, Chapel S, et al. Hyperthermia enhances CTL cross-priming. J Immunol. 2006;176:2134–41. doi: 10.4049/jimmunol.176.4.2134. [DOI] [PubMed] [Google Scholar]
- 129.Frank MJ, Khodadoust MS, Czerwinski DK, Haabeth OAW, Chu MP, Miklos DB, et al. Autologous tumor cell vaccine induces antitumor T cell immune responses in patients with mantle cell lymphoma: a phase I/II trial. J Exp Med. 2020;217:e20191712. doi: 10.1084/jem.20191712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dörrie J, Schaft N, Schuler G, Schuler-Thurner B. Therapeutic cancer vaccination with ex vivo RNA-transfected dendritic cells-an update. Pharmaceutics. 2020;12:92. doi: 10.3390/pharmaceutics12020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bol KF, Schreiber G, Rabold K, Wculek SK, Schwarze JK, Dzionek A, et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J Immunother Cancer. 2019;7:109. doi: 10.1186/s40425-019-0580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, et al. Immune cells within the tumor microenvironment: biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–33. doi: 10.1016/j.canlet.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 133.Dai E, Zhu Z, Wahid S, Qu Z, Storkus WJ, Guo ZS. Epigenetic modulation of antitumor immunity for improved cancer immunotherapy. Mol Cancer. 2021;20:171. doi: 10.1186/s12943-021-01464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumor-infiltrating regulatory T cells by lactic acid. Nature. 2021;591:645–51. doi: 10.1038/s41586-020-03045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T-cell immunity. Cancer Cell. 2014;26:638–52. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell. 2017;31:711.e4–23.e4. doi: 10.1016/j.ccell.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Verneau J, Sautés-Fridman C, Sun CM. Dendritic cells in the tumor microenvironment: prognostic and theranostic impact. Semin Immunol. 2020;48:101410. doi: 10.1016/j.smim.2020.101410. [DOI] [PubMed] [Google Scholar]
- 138.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 140.Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE. 2014;9:e87705. doi: 10.1371/journal.pone.0087705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berry J, Vreeland T, Trappey A, Hale D, Peace K, Tyler J, et al. Cancer vaccines in colon and rectal cancer over the last decade: lessons learned and future directions. Expert Rev Clin Immunol. 2017;13:235–45. doi: 10.1080/1744666X.2016.1226132. [DOI] [PubMed] [Google Scholar]
- 142.Naimi A, Mohammed RN, Raji A, Chupradit S, Yumashev AV, Suksatan W, et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs), the pros and cons. Cell Commun Signal. 2022;20:44. doi: 10.1186/s12964-022-00854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Apetoh L, Smyth MJ, Drake CG, Abastado JP, Apte RN, Ayyoub M, et al. Consensus nomenclature for CD8+ T cell phenotypes in cancer. Oncoimmunology. 2015;4:e998538. doi: 10.1080/2162402X.2014.998538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 145.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107:14733–8. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–96. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–42. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kansy BA, Concha-Benavente F, Srivastava RM, Jie HB, Shayan G, Lei Y, et al. PD-1 status in CD8+ T cells associates with survival and anti-PD-1 therapeutic outcomes in head and neck cancer. Cancer Res. 2017;77:6353–64. doi: 10.1158/0008-5472.CAN-16-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46:577–86. doi: 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aijaz A, Li M, Smith D, Khong D, LeBlon C, Fenton OS, et al. Biomanufacturing for clinically advanced cell therapies. Nat Biomed Eng. 2018;2:362–76. doi: 10.1038/s41551-018-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jian Y, Yang K, Sun X, Zhao J, Huang K, Aldanakh A, et al. Current advance of immune evasion mechanisms and emerging immunotherapies in renal cell carcinoma. Front Immunol. 2021;12:639636. doi: 10.3389/fimmu.2021.639636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–6. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 155.Adkins I, Sadilkova L, Hradilova N, Tomala J, Kovar M, Spisek R. Severe, but not mild heat-shock treatment induces immunogenic cell death in cancer cells. Oncoimmunology. 2017;6:e1311433. doi: 10.1080/2162402X.2017.1311433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Turubanova VD, Mishchenko TA, Balalaeva IV, Efimova I, Peskova NN, Klapshina LG, et al. Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death. Sci Rep. 2021;11:7205. doi: 10.1038/s41598-021-86354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vuky J, Corman JM, Porter C, Olgac S, Auerbach E, Dahl K. Phase II trial of neoadjuvant docetaxel and CG1940/CG8711 followed by radical prostatectomy in patients with high-risk clinically localized prostate cancer. Oncologist. 2013;18:687–8. doi: 10.1634/theoncologist.2011-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Michael BD, Syndikus I, Clark A, Baborie A. Diffuse primary leptomeningeal melanocytosis in a patient receiving a novel cancer cell vaccine for prostate cancer. BMJ Case Rep. 2010;2010:bcr11.2009.2495. doi: 10.1136/bcr.11.2009.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, et al. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2013;17:94–101. doi: 10.1007/s11605-012-2064-6. [DOI] [PubMed] [Google Scholar]
- 160.Hewitt DB, Nissen N, Hatoum H, Musher B, Seng J, Coveler AL, et al. A phase 3 randomized clinical trial of chemotherapy with or without algenpantucel-L (hyperacute-pancreas) immunotherapy in subjects with borderline resectable or locally advanced unresectable pancreatic cancer. Ann Surg. 2022;275:45–53. doi: 10.1097/SLA.0000000000004669. [DOI] [PubMed] [Google Scholar]
- 161.Barve V, Adams N, Stanbery L, Manning L, Horvath S, Wallraven G, et al. Case report: marked survival advantage of two colorectal cancer patients with liver metastases treated with vigil and FOLFOX-6. Vaccines. 2021;9:1201. doi: 10.3390/vaccines9101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Senzer N, Barve M, Kuhn J, Melnyk A, Beitsch P, Lazar M, et al. Phase I trial of “bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol Ther. 2012;20:679–86. doi: 10.1038/mt.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wiseman CL, Kharazi A, Sunkari VG, Galeas JL, Dozio V, Hashwah H, et al. Regression of breast cancer metastases following treatment with irradiated SV-BR-1-GM, a GM-CSF overexpressing breast cancer cell line: intellectual property and immune markers of response. Recent Pat Anticancer Drug Discov. 2022;18:224–40. doi: 10.2174/1574892817666220518123331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aris M, Bravo AI, Pampena MB, Blanco PA, Carri I, Koile D, et al. Changes in the TCRβ repertoire and tumor immune signature from a cutaneous melanoma patient immunized with the CSF-470 vaccine: a case report. Front Immunol. 2018;9:955. doi: 10.3389/fimmu.2018.00955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schuler-Thurner B, Bartz-Schmidt KU, Bornfeld N, Cursiefen C, Fuisting B, Grisanti S, et al. Immunotherapy of uveal melanoma: vaccination against cancer. Multicenter adjuvant phase 3 vaccination study using dendritic cells laden with tumor RNA for large newly diagnosed uveal melanoma. Ophthalmologe. 2015;112:1017–21. doi: 10.1007/s00347-015-0162-z. [DOI] [PubMed] [Google Scholar]
- 166.Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tønnesen P, Suso EM, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother. 2013;62:1499–509. doi: 10.1007/s00262-013-1453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chang CN, Huang YC, Yang DM, Kikuta K, Wei KJ, Kubota T, et al. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci. 2011;18:1048–54. doi: 10.1016/j.jocn.2010.11.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.