Video
Dilation balloon-occlusion technique for EUS-guided gastrojejunostomy.
Background
EUS-guided gastrojejunostomy (EUS-GJ) offers an alternative treatment to luminal stenting and surgical gastrojejunostomy for the treatment of gastric outlet obstruction, particularly in cases secondary to malignancy.1,2 The basic premise of performing an EUS-GJ entails using a lumen-apposing metal stent (LAMS) to create a connection between the stomach and small bowel (typically jejunum), thereby creating a bypass by which food and liquid can enter the unobstructed small bowel. Recent retrospective data have demonstrated equivalent, if not higher, clinical success rates with EUS-GJ in comparison to duodenal stents with significantly lower stent dysfunction rates.3,4 Nevertheless, adverse events remain a significant concern with this procedure, especially given the typically frail patient population who receive this treatment. While a variety of techniques exist for performing EUS-GJ, such as the antegrade direct and downstream methods and the EUS balloon-occluded GJ bypass (EPASS) method commonly performed in Asia, creating a stable stent insertion site under good visualization remains a challenge and typically requires many different instruments such as the balloon-occlusion catheter in the EPASS method, which is not available in the United States.5 We therefore describe a simple modification to facilitate performing EUS-GJ using commonly available instruments.
Endoscopic Methods
Preparation
We obtained informed consent from the patient and administered 1 dose of prophylactic antibiotics at the start of the procedure. Under general anesthesia, the patient was placed in the prone position typically used in ERCP. We premixed a 250-mL solution consisting of 50% sterile water and 50% contrast, plus half a vial of methylene blue.
Technique
Step 1
As demonstrated in Video 1 (available online at www.videogie.org), a standard gastroscope was used to identify the site of narrowing (Fig. 1). Once the site of narrowing was identified, a long-angled 0.035-inch guidewire (Hydra Jagwire; Boston Scientific, Marlborough, Mass, USA) was advanced under endoscopic and fluoroscopic guidance into the jejunum with ideally several loops formed. The length of the stenosis was typically determined by either contrast injection or pulling back with an inflated extraction balloon that had been advanced over the guidewire past the stenosis. The endoscope was then removed from the patient while performing an exchange process to keep the guidewire in the jejunum.
Figure 1.
Site of malignant obstruction in the first portion of the duodenum.
Step 2
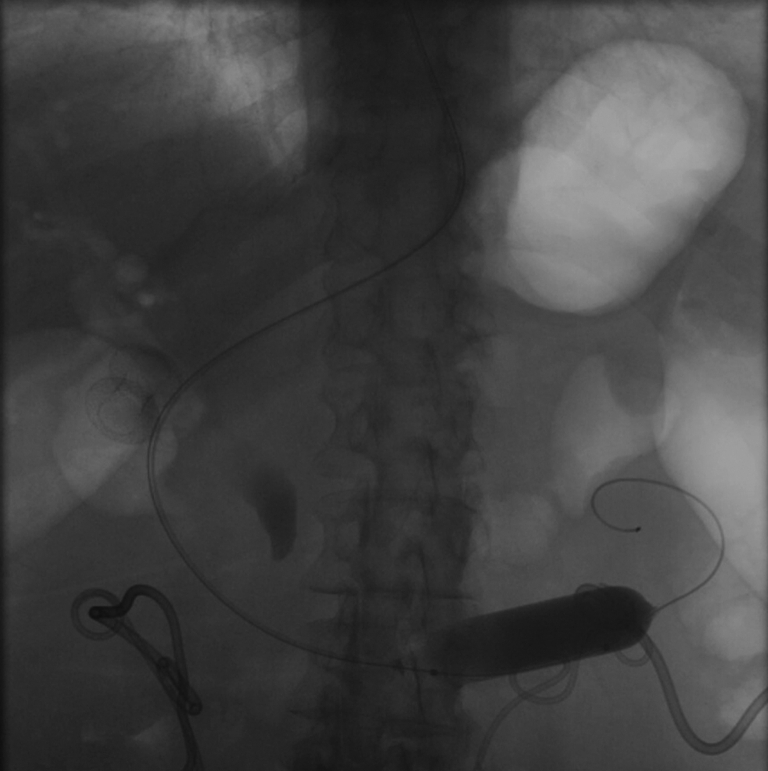
An 18- to 20-mm dilation balloon catheter (CRE Balloon Dilatation Catheter; Boston Scientific) was then advanced over the guidewire under fluoroscopy with the assistant maintaining back pressure to facilitate advancement. Once the catheter was past the obstruction, the balloon was inflated to 20 mm (Fig. 2). The guidewire was then removed.
Figure 2.
Inflation of dilation balloon catheter to 20 mm.
Step 3
The linear echoendoscope was advanced side-by-side the dilation balloon catheter into the stomach.
Step 4
The irrigation foot pedal was connected to the premixed solution of contrast, water, and methylene blue and then attached to the dilation balloon catheter. The endoscopist then irrigated the solution through the balloon catheter, essentially performing a balloon-occlusion enterogram (Fig. 3). This distends the jejunum and prevents backflow of the solution proximally into the stomach. The agitation of the jejunal contents during irrigation also simplifies identification of the jejunum.
Figure 3.
Balloon-occlusion enterogram.
Step 5
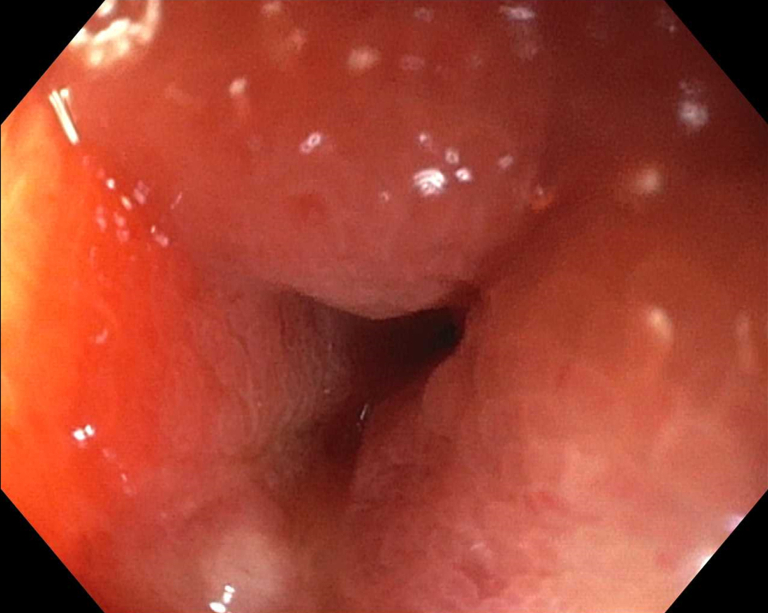
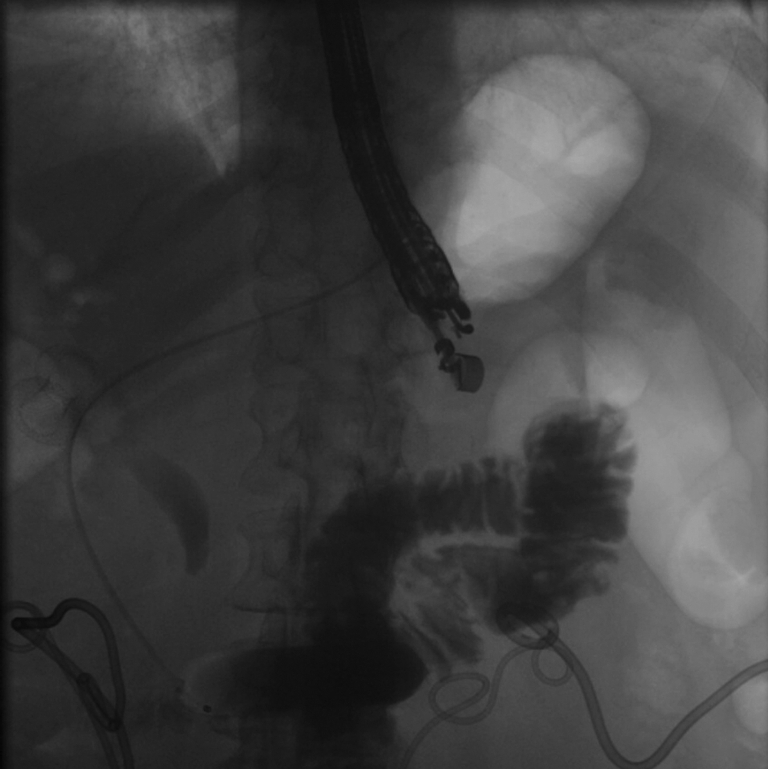
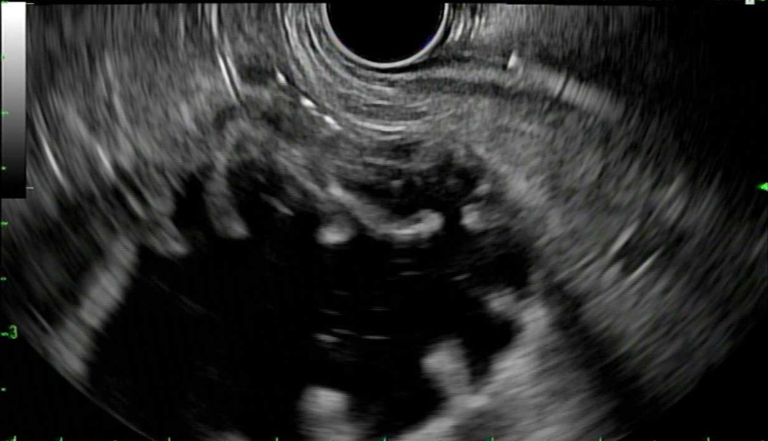
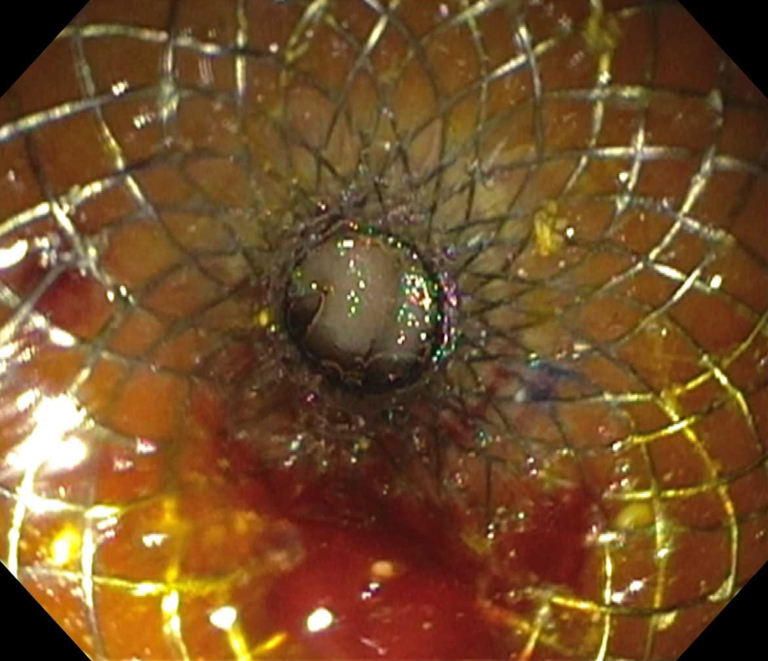
Under EUS and fluoroscopic guidance, a suitable site for LAMS placement was identified (Fig. 4). We then administered 1 mg of glucagon (with the option of giving up to 4 mg) and, using a freehand technique, deployed a 15- or 20-mm diameter electrocautery-enhanced LAMS (AXIOS; Boston Scientific) from the stomach into the jejunum. Deployment of the proximal flange resulted in a blue solution coming through the stent into the stomach, confirming successful placement. After balloon dilation of the LAMS, the jejunum is typically visible endoscopically (Fig. 5).
Figure 4.
EUS image of jejunum prior to stent placement.
Figure 5.
Successful deployment of lumen-apposing metal stent with jejunum visible through stent.
Adverse Events
Stent misdeployment represents the most common adverse event of this procedure, occurring in up to 10% of cases.6,7 Less common adverse events include aspiration pneumonia, postoperative infection, and small-bowel perforation.
Conclusions
This simple technique modification offers a stable window to perform an EUS-GJ by enhancing distention of the jejunum and allowing for its easy identification. This facilitates freehand LAMS deployment, which can reduce the number of instruments required and, most importantly, allows the endoscopist to take advantage of an ideal puncture window before the jejunum moves away (particularly after EUS-guided needle puncture) from the stomach or contracts. Furthermore, by preventing backflow of solution into the stomach, less irrigation solution may be required. While further study is needed to determine the superiority of one method over another, we describe this simplified technique to aid endoscopists in performing EUS-GJ and to offer an alternative method when other methods are not feasible, such as when an endoscope cannot be advanced past the luminal obstruction.
Disclosure
Dr Krishna received funding for investigator-initiated research from Mauna Kea. Dr Lara is a consultant for AbbVie. Dr Papachristou is a consultant for Olympus. All other authors disclosed no financial relationships.
Acknowledgments
Dr Han was supported by the Path to K award from The Ohio State University College of Medicine Office of Research and the Center for Clinical and Translational Science through the Richard P. & Marie R. Bremer Medical Research Fund and William H. Davis Endowment for Basic Medical Research.
Supplementary data
Dilation balloon-occlusion technique for EUS-guided gastrojejunostomy.
References
- 1.Khashab M.A., Kumbhari V., Grimm I.S., et al. EUS-guided gastroenterostomy: the first U.S. clinical experience (with video) Gastrointest Endosc. 2015;82:932–938. doi: 10.1016/j.gie.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Tonozuka R., Tsuchiya T., Mukai S., et al. Endoscopic ultrasonography-guided gastroenterostomy techniques for treatment of malignant gastric outlet obstruction. Clin Endosc. 2020;53:510–518. doi: 10.5946/ce.2020.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Wanrooij R.L.J., Vanella G., Bronswijk M., et al. Endoscopic ultrasound-guided gastroenterostomy versus duodenal stenting for malignant gastric outlet obstruction: an international, multicenter, propensity score-matched comparison. Endoscopy. 2022;54:1023–1031. doi: 10.1055/a-1782-7568. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez-Aldehuelo R., Subtil Iñigo J.C., Martínez Moreno B., et al. EUS-guided gastroenterostomy versus duodenal self-expandable metal stent for malignant gastric outlet obstruction: results from a nationwide multicenter retrospective study (with video) Gastrointest Endosc. 2022;96:1012–1020.e3. doi: 10.1016/j.gie.2022.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Irani S., Itoi T., Baron T.H., et al. EUS-guided gastroenterostomy: techniques from east to west. VideoGIE. 2020;5:48–50. doi: 10.1016/j.vgie.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghandour B., Bejjani M., Irani S.S., et al. Classification, outcomes, and management of misdeployed stents during EUS-guided gastroenterostomy. Gastrointest Endosc. 2022;95:80–89. doi: 10.1016/j.gie.2021.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Bejjani M., Ghandour B., Subtil J.C., et al. Clinical and technical outcomes of patients undergoing endoscopic ultrasound-guided gastroenterostomy using 20-mm vs. 15-mm lumen-apposing metal stents. Endoscopy. 2022;54:680–687. doi: 10.1055/a-1654-6914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dilation balloon-occlusion technique for EUS-guided gastrojejunostomy.
Dilation balloon-occlusion technique for EUS-guided gastrojejunostomy.