Abstract
We have optimized the induction of antiviral cytotoxic T lymphocytes (CTL) in rhesus macaques by a lipopeptide vaccine containing seven peptides from simian immunodeficiency virus (SIV) Nef and Gag proteins and a strong T-helper peptide from tetanus toxoid (TT) that is promiscuous in humans (peptide TT 830-846). Two of the eight immunized macaques showed T-helper (Th) cell proliferation and a specific synthesis of gamma interferon in response to TT 830-846 peptide. They also showed multispecific cytotoxic activity against three to five of the immunizing SIV peptides. These results show the importance of a strong specific type 1 Th response for inducing a multispecific CTL response in vivo, which is essential for the development of an anti-human immunodeficiency virus vaccine.
Virus-specific CD8+ responses are essential for immune protection against several viruses (8, 34, 37). Hence, successful vaccines must induce cellular immunity mediated by CD8+. Cytotoxic T lymphocytes (CTLs) are involved in the control of the viral load during human immunodeficiency virus (HIV) infection (4, 7, 17, 18) and seem to be very important in vaccine-induced protection (12). However, as they exert considerable selective pressure in both primary and late-stage HIV infections, they may select for escape mutant viruses (5, 14, 28). We have demonstrated that the induction by lipopeptides of CD8+ CTLs recognizing only one epitope in the simian immunodeficiency virus (SIV)-infected macaque model is not sufficient to protect against SIV and may favor the selection of variant viruses and the emergence of escape mutant viruses (23). Thus, the induction of multispecific CTLs that recognize several virus isolates by giving an appropriate vaccine is likely to be essential to prevent selection of mutant or variant viruses.
We have now immunized eight rhesus macaques (Macaca mulatta) (92102, 92105, 92109, 92117, 92120, 92125, 92127, and 92129) with tetanus toxoid (TT) (500 μg per monkey) in incomplete Freund adjuvant (IFA) (three subcutaneous and intramuscular injections given at 1-month intervals). Indeed, initial vaccination with a carrier protein induces helper T (Th) memory cells that may then be exploited by using selected relevant T epitopes from the same protein to boost B cell and CTL responses (15, 31). The proliferation of peripheral blood mononuclear cells (PBMCs) against TT was measured by monitoring 3H-labeled thymidine incorporation 1 month after the last immunization; it was significant for all macaques (data not shown). Five months later, there was a significant Th-specific response to lipopeptide TT 830-846 in only two macaques, 92109 and 92129 (data not shown).
Seven months after the last TT immunization, the macaques were given three subcutaneous injections of a mixed-micelle formulation of eight lipopeptides in sterile water (500 μg of each lipopeptide) without any adjuvant at 1-month intervals. This immunization procedure was thus compatible with human vaccination. Five sequences of lipopeptides were selected from the SIV Nef (LP1, amino acids [aa] 101 to 126; LP2, aa 125 to 147; LP3, aa 155 to 178; LP4, aa 201 to 225; and LP5, aa 221 to 247), and two were selected from the Gag protein (LP6, aa 165 to 195, and LP7, aa 246 to 281). These were identical to sequences previously reported (6, 7, 10, 23) except for the introduction of an additional Nɛ-palmitoyl-lysylamide residue at the C terminus (23). In addition, to improve CTL induction, a lipopeptide containing promiscuous human Th epitope from TT-derived peptide, aa 830 to 846, Ac-QYIKANSKFIGITELKK, referred to herein as LP-TT; this was preferred to peptide 830-843 because of its greater solubility and was synthesized with a modification of the N-terminal extremity by an acetyl group, to avoid any heterogeneity that might be produced by formation of the pyroglutamyl analog upon storage. The mixed-micelle formulation was obtained by dissociating each component in concentrated acetic acid (80%) before mixing and sterilization by filtration. Dilution resulted in the formation of mixed micelles or aggregates that, statistically, contained each of the constituents.
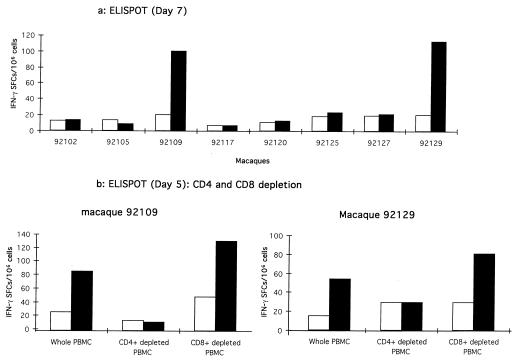
Lipopeptide TT 830-846 Th-specific responses were assessed 6 months after the last lipopeptide immunization; they showed that the same two macaques (92109 and 92129) always had strong responses to lipopeptide TT 830-846 (data not shown). We therefore investigated whether these results depended upon a particular type 1 or type 2 profile and analyzed the CD4+ or CD8+ nature of these T cells. We first assessed lymphokine production by PBMCs which had undergone a short stimulation in vitro with LP-TT by using a sensitive enzyme-linked immunospot (ELISPOT) assay for gamma interferon (IFN-γ) adapted from Scheibenbogen et al. (33). Only the PBMCs from the two macaques showing proliferative assay response (92109 and 92129) specifically synthesized IFN-γ (Fig. 1a). We then confirmed the CD4+ nature of the effector T cells that secreted IFN-γ in response to lipopeptide TT 830-846 by using the ELISPOT assay to measure IFN-γ levels in PBMCs, CD4+-depleted PBMCs, and CD8+-depleted PBMCs (Fig. 1b). The LP-TT-specific response of the CD8+-enriched population was completely abrogated. Conversely, depletion of CD8+ cells did not decrease the number of IFN-γ spot-forming cells (SFCs), indicating that the IFN-γ secretion induced by LP-TT was mediated by CD4+ T cells.
FIG. 1.
Synthesis of IFN-γ by CD4+ lymphocytes after LP-TT stimulation in macaques 92109 and 92129. (a) Helper peptide-specific IFN-γ SFCs in PBMCs from the eight macaques were analyzed after the third mixed-micelle lipopeptide immunization. The ELISPOT assay was performed 7 days after one short in vitro stimulation. Briefly, PBMCs (2.5 × 106/ml) were cultured for 3 days in 24-well microtiter plates (Costar, Cambridge, Mass.) in complete medium, with 5 μM LP-TT or with 5 μM irrelevant LP (Nef HIV 66-97) (LP-i). IL-2 (Boehringer, Mannheim, Germany) was then added to each well (10 IU/ml), and incubation was continued for 4 days. Effector cells were then washed, counted, and seeded in duplicate in 96-well nitrocellulose plates (Multi-Screen HA; Millipore, Bedford, Mass.) that had been coated with the mouse anti-human IFN-γ capture monoclonal antibody (Genzyme, Russelheim, Germany) (8 μg/ml in carbonate buffer) and blocked with complete medium. Effector cells were incubated at 105 and 2 × 105 per well with 5 μM LP-i (white columns) or 5 μM LP-TT (black columns) for 48 h in complete medium containing 20 IU of IL-2/ml. The ELISPOT assay was performed as previously described (33). Responses were considered significant if there were a minimum of five SFCs per well and if this number was at least twice that obtained with the negative control. (b) A depletion assay was done using anti-CD4 or anti-CD8 monoclonal antibodies (MAbs) on the Th cell responder macaques (90109 and 92129) to obtain CD4+-depleted PBMCs and CD8+-depleted PBMCs, together with whole PBMCs. PBMCs were incubated for 30 min with cocktails of anti-human CD8 (DAKO, Glostrup, Denmark; Becton Dickinson, Mountain View, Calif.; and Ortho Diagnostic Systems, Raritan, N.J.) (1 μl of each/106 cells) or cocktails of human anti-CD4 (DAKO; Sigma Chemical Co., St. Louis, Mo.; and Ortho Diagnostic Systems) (1 μl of each/106 cells) MAbs coated on Dynabeads in 500 μl of complete medium on ice (BioMag goat anti-mouse immunoglobulin G; PerSeptive Biosystems, Framingham, Mass.). Conjugate-coated cells were then removed with a magnet (Dynal) and cultured in vitro for 5 days. Finally, they were tested in the ELISPOT assay.
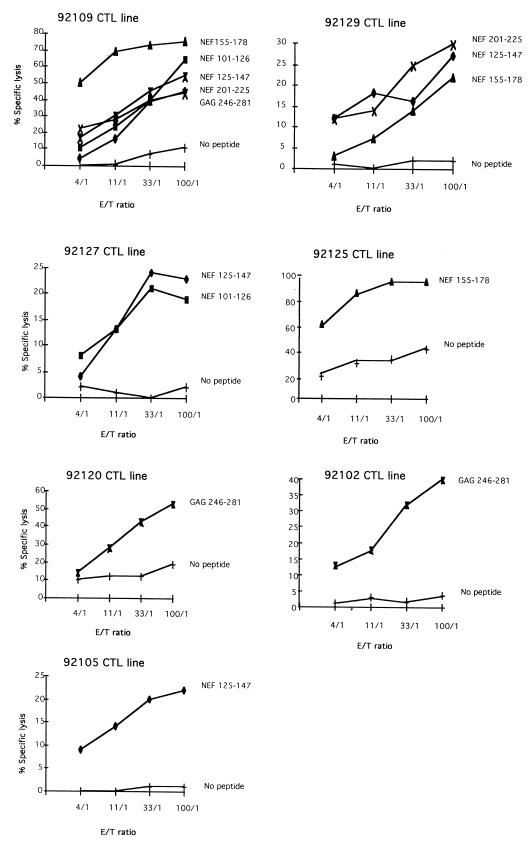
The lipopeptide-induced CTL responses were examined after the last mixed-micelle immunization by stimulating macaque PBMCs with a mixture of the seven long free peptides without the helper TT 830-846 epitope and testing them against autologous B lymphoblastoid cell lines (B-LCLs) sensitized by the same long peptides. On the day of the chromium release test (CRT), effector cells were >70% CD8+ T cells for every test performed, as might be expected for class I-restricted antigen-specific CTLs. Most (seven) of the eight immunized macaques had CTL activity (Fig. 2), and macaques 92109 and 92129 had strong and multispecific CTL responses to five and three long peptides, respectively. We tested overlapping short peptides (8 to 11 aa) spanning the sequence of the long peptides (Table 1) to identify six epitopes recognized by CTLs from macaque 92109, three of which (NEF 169-178, NEF 215-225, and GAG 266-275) were very strongly recognized. Three others caused less lysis. Similarly, the CTLs from macaque 92129 recognized four peptides (NEF 128-136, NEF 201-211, NEF 211-219, and NEF 169-178). Macaque 92127 had CTLs that recognized two long peptides with a cytotoxic activity lower than those of peptides from macaques 92109 and 92129 (Fig. 2). Macaques 92125, 92120, 92102 and 92105 had CTLs that recognized a single peptide, while the CTLs from macaque 92117 recognized no peptide. A maximum of one short peptide in every long peptide recognized was identified for macaques 92125, 92127, and 92105 (Table 1). In contrast, in macaques 92102 and 92120, no short epitopic peptides within long peptides were identified. CTL activities persisted in all the macaques for 9 to 10 months after the last lipopeptide immunization and did not result from in vitro induction of primary CTL responses, since they were not detected after antigen-specific stimulation of naive PBMCs from the seven CTL responders (data not shown). The multispecific activity in the two responder macaques was then associated with significant type 1 Th (Th1) responses to the TT 830-846 peptide after immunization with either TT or lipopeptide (P < 0.05 by the chi-square test). A recent in vivo study has shown that T helper subset cells with Th1 profiles regulate both the sensitivity and the frequency of epitope-specific CTL responses in mice (29). A relationship between significant Th responses and strong, persistent CTLs with high frequencies of CTL precursors (CTLp) in vivo in mice (3, 16, 19, 24, 32, 35) and humans vaccinated with lipopeptides against chronic hepatitis B virus infection (20, 39) has also been described.
FIG. 2.
Cytotoxic activities of the seven responder macaques. Anti-peptide CTL lines were obtained by pulsing for 2 h macaque PBMCs (10 × 106 cells/ml) with a mixture of the five Nef and two Gag free long peptides (10 μM each) corresponding to the immunizing lipopeptides. The cells were then washed and resuspended (106/ml) in complete medium and incubated in 24-well microtiter plates. After 3 days of incubation, IL-2 was added to each well (10 IU/ml). On days 7 and 14, effector cells were washed and diluted to 106/ml, placed in new plates, and stimulated with irradiated peptide-pulsed (10 μM each) autologous PBMCs (10 × 106/ml) for 2 h and then diluted to 106/ml in complete medium containing 20 IU of IL-2/ml (effector/stimulator ratio, 1:1). A CRT was performed after the third stimulation of CTL lines. The target cells were autologous B-LCLs (immortalized by the herpesvirus papio) alone or incubated overnight with various long peptides (10 μM/ml). The CRT was considered positive if the specific 51Cr release observed in the presence of peptide-pulsed target cells exceeded by 10% that observed for B-LCLs without peptide at two effector/target (E/T) ratios. Only the positive cytotoxic responses against peptide-sensitized target cells of the seven responder macaques are shown.
TABLE 1.
Epitopic specificities found in five immunized macaques
| Effector cellsa from macaque | Target cellsb | % Specific lysisc at the E/T ratiod of:
|
|||
|---|---|---|---|---|---|
| 100:1 | 33:1 | 11:1 | 4:1 | ||
| 92109 | None | 25 | 21 | ||
| Nef 169-178 | 89e | 75 | |||
| None | 14 | 11 | 5 | ||
| Nef 215-225 | 41 | 36 | 22 | ||
| None | 19 | 9 | 7 | 3 | |
| Gag 266-275 | 40 | 24 | 16 | 6 | |
| None | 14 | 11 | 11 | 5 | |
| Nef 101-110 | 26 | 22 | 16 | ||
| Nef 128-136 | 28 | 23 | |||
| None | 41 | 34 | 41 | 27 | |
| Nef 116-126 | 57 | 45 | 48 | 34 | |
| 92129 | None | 8 | 3 | 2 | 0 |
| Nef 128-136 | 46 | 34 | 28 | 9 | |
| Nef 201-211 | 22 | 16 | 16 | 5 | |
| Nef 211-219 | 19 | 16 | 10 | 4 | |
| None | 52 | 44 | 30 | ||
| Nef 169-178 | 65 | 54 | 33 | ||
| 92125 | None | 38 | 34 | 25 | 19 |
| Nef 169-178 | 86 | 87 | 70 | 54 | |
| 92127 | None | 28 | 21 | ||
| Nef 116-126 | 43 | 32 | |||
| 92105 | No peptide | 22 | 14 | 12 | 2 |
| Nef 128-136 | 34 | 24 | 13 | 5 | |
CTL cell lines were obtained from PBMCs of the five immunized macaques following specific stimulation with the seven long peptides in vitro.
Target cells were autologous B-LCLs immortalized by the herpesvirus papio and incubated with short peptides (10 μM).
Target cells (5 × 103) were labeled with 51Cr and incubated for 4 h with various numbers of target cells.
E/T ratio, effector-to-target ratio.
CRT was considered positive if the specific 51Cr release observed in the presence of peptide-pulsed target cells exceeded by 10% that observed on B-LCLs without peptide at two E/T ratios.
Synthetic peptides modified at one end by addition of a lipid moiety are highly immunogenic for T and B cell responses in vivo (2, 11, 22). Lipopeptides also facilitate the presentation of peptides by major histocompatibility complex (MHC) class I molecules (11), which may be due to their ability to rapidly cross the cell membrane and enter the cytoplasm of intact cells (21, 38). Last, large synthetic lipopeptides can be processed in a manner similar to whole exogenous proteins and become associated with MHC class II molecules. We chose to use the promiscuous helper peptide TT 830-843 that binds to several HLA-DR molecules (20, 26, 27, 39) in this study. Other promiscuous peptides could be used in humans, including PADRE epitopes, which bind to 14 different HLA-DR molecules (9). This degenerate binding specificity of peptides could overcome the problem of the extreme polymorphism of HLA-DR molecules in humans. We used the promiscuous TT peptide because it provides adequate T cell help to induce CTLs, as shown in a human vaccine trial against chronic hepatitis B virus (20, 39). We believe that an immunizing formulation with mixed micelles that statistically contain each of the constituents allows the physical association of Th and CTL peptides and may favor the presentation of peptides to T helper lymphocytes and CTLs by the same antigen-presenting cell, which is essential for optimal productive interactions and collaboration between Th cells and CTLs (36).
The breadth of the CTL peptide recognition spectrum in only two macaques (92109 and 92129) in this study could have been due to the heterogeneity of their MHC class I molecules. This is unlikely in the light of the results of much larger studies including our previous experiments (7, 23). We obtained 16 CTL responders from 22 macaques immunized with lipopeptides and found that 14 of them had mono- or bispecific CTL responses. In addition, these two macaques belong to the same cohort as the six Th nonresponders, and all these macaques may share the same MHC class I molecules. Therefore, induction of multispecific CTLs is unlikely to be due to particular MHC class I molecules. The mono- and bispecific responder macaques may not have had MHC class II molecules suitable for presenting immunizing TT 830-846 lipopeptide. This problem could be overcome in humans by using several promiscuous peptides that are presented by a majority of MHC class II molecules. Adding another T helper epitope or synthesizing more immunogenic lipopeptides containing modified peptides (25) may better stimulate a Th1 response for inducing anti-HIV multispecific CTL responses. HIV-specific Th epitopes would be more relevant to induce strong helper activity at the time of HIV infection. Few HIV Th epitopes have been described to date, but the restoration of anti-HIV proliferative responses (1, 13, 30) by highly active antiretroviral treatment of primary infected humans may provide a better definition of Th epitopic regions.
Finally, our findings suggest that anti-TT 830-846 T lymphocytes with a Th1 profile are most important and indicate that soluble factors like IFN-γ, and probably interleukin 2 (IL-2), help to induce optimal differentiation of CTL responses, leading to multispecific cytotoxic responses. These results appear to be promising for the future development of peptide vaccines to protect against HIV infection.
Acknowledgments
This work was supported by the Agence Nationale de Recherche sur le SIDA, the Institut Pasteur de Lille and by the Centre National de Recherches Scientifiques. Lorenzo Mortara holds a Sidaction/Ensemble Contre le SIDA fellowship.
We thank Claire Bony and Pascale Villefroy for excellent technical assistance and Isabelle Bouchaert for technical advice on phenotypic analysis. The English text was edited by Owen Parkes.
REFERENCES
- 1.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debré P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science (Washington, DC) 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.BenMohamed L, Gras-Masse H, Tartar A, Daubersies P, Brahimi K, Bossus M, Thomas A, Druilhe P. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur J Immunol. 1997;27:1242–1253. doi: 10.1002/eji.1830270528. [DOI] [PubMed] [Google Scholar]
- 3.Bennett S R M, Carbone F C, Karamalis F, Miller J F A P, Heath W R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B A, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 6.Bourgault I, Chirat F, Tartar A, Lévy J P, Guillet J G, Venet A. Simian immunodeficiency virus as a model for vaccination against HIV. Induction in rhesus macaques of GAG- or NEF-specific cytotoxic T lymphocytes by lipopeptides. J Immunol. 1994;152:2530–2537. [PubMed] [Google Scholar]
- 7.Bourgault-Villada I, Mortara L, Aubertin A M, Gras-Masse H, Lévy J P, Guillet J G. Positive role of macaque cytotoxic T lymphocytes during SIV infection: decrease of cellular viremia and increase of asymptomatic clinical period. FEMS Immunol Med Microbiol. 1997;19:81–87. doi: 10.1111/j.1574-695X.1997.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 8.Byrne J A, Oldstone M B A. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984;51:682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Guercio M F, Alexander J, Kubo R T, Arrhenius T, Maewal A, Appella E, Hoffman S L, Jones T, Valmori D, Sakaguchi K, Grey H M, Sette A. Potent immunogenic short linear peptide constructs composed of B cell epitopes and Pan DR T helper epitopes (PADRE) for antibody responses in vivo. Vaccine. 1997;15:441–448. doi: 10.1016/s0264-410x(97)00186-2. [DOI] [PubMed] [Google Scholar]
- 10.Deprez B, Sauzet J P, Boutillon C, Martinon F, Tartar A, Sergheraert C, Guillet J G, Gomard E, Gras-Masse H. Comparative efficiency of simple lipopeptide constructs for in vivo induction of virus-specific CTL. Vaccine. 1996;14:375–382. doi: 10.1016/0264-410x(95)00220-u. [DOI] [PubMed] [Google Scholar]
- 11.Deres K, Schild H, Wiesmüller K H, Jung G, Rammensee H G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature (London) 1989;342:561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- 12.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, McMichael A, Gotch F. Early suppression of SIV replication by CD8+nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 13.Gorochov G, Neumann A U, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, Debré P. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 14.Goulder P J R, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 15.Herrington D A, Clyde D F, Losonsky G, Cortesia M, Murphy J R, Davis J, Baqar S, Felix A M, Heimer E P, Gillessen D, Nardin E, Nussenzweig R S, Nussenzweig V, Hollingdale M R, Levine M M. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature (London) 1987;328:257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- 16.Keene J A, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P M, Eeftinck-Schattenkerk J K M, Osterhaus A D M E, Schuitemaker H, Miedema F. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasarte J J, Sarobe P, Gullon A, Prieto J, Borras-Cuesta F. Induction of cytotoxic T lymphocytes in mice against the principal neutralizing domain of HIV-1 by immunization with an engineered T-cytotoxic–T-helper synthetic peptide construct. Cell Immunol. 1992;141:211–218. doi: 10.1016/0008-8749(92)90140-k. [DOI] [PubMed] [Google Scholar]
- 20.Livingston B D, Crimi C, Grey H, Ishioka G, Chisari F V, Fikes J, Grey H, Chesnut R W, Sette A. The hepatitis B virus-specific CTL responses induced in humans by lipopeptide vaccination are comparable to those elicited by acute viral infection. J Immunol. 1997;159:1383–1392. [PubMed] [Google Scholar]
- 21.Loing E, Delannoye A, Sergheraert C, Tartar A, Gras-Masse H. Assessing cytoplasm delivery of lipopeptides into intact cells by a functional assay based on PKC inhibition. I. The Jurkat model. Pept Res. 1996;9:229–232. [PubMed] [Google Scholar]
- 22.Martinon F, Gras-Masse H, Boutillon C, Chirat F, Deprez B, Guillet J G, Gomard E, Tartar A, Lévy J P. Immunization of mice with lipopeptides bypasses the prerequisite for adjuvant. Immune response of BALB/c mice to human immunodeficiency virus envelope glycoprotein. J Immunol. 1992;149:3416–3422. [PubMed] [Google Scholar]
- 23.Mortara L, Letourneur F, Gras-Masse H, Venet A, Guillet J-G, Bourgault-Villada I. Selection of virus variants and emergence of virus escape mutants after immunization with an epitope vaccine. J Virol. 1998;72:1403–1410. doi: 10.1128/jvi.72.2.1403-1410.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ossendorp F, Mengedé E, Camps M, Filius R, Melief C J M. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostankovitch M, Guichard G, Connan F, Muller S, Chaboissier A, Hoebeke J, Choppin J, Briand J P, Guillet J G. A partially modified retro-inverso pseudopeptide modulates the cytokine profile of CTL specific for an influenza virus epitope. J Immunol. 1998;161:200–208. [PubMed] [Google Scholar]
- 26.O’Sullivan D, Arrhenius T, Sidney J, Del Guercio M F, Albertson M, Wall M, Oseroff C, Southwood S, Colon S M, Gaeta F C A, Sette A. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991;147:2663–2669. [PubMed] [Google Scholar]
- 27.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 28.Price D A, Goulder P J R, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R M, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romieu R, Baratin M, Kayibanda M, Guillet J G, Viguier M. IFN-γ-secreting T helper cells regulate both the frequency and avidity of epitope-specific CD8+ T lymphocytes induced by peptide immunization: an ex vivo analysis. Int Immunol. 1998;10:1273–1279. doi: 10.1093/intimm/10.9.1273. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science (Washington, DC) 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 31.Rubistein A, Goldstein H, Pettoello-Mantovani M, Mizrachi Y, Bloom B R, Furer E, Althaus B, Que J U, Hasler T, Cryz S J. Safety and immunogenicity of a V3 loop synthetic peptide conjugated to purified protein derivative in HIV-seronegative volunteers. AIDS. 1995;9:243–251. [PubMed] [Google Scholar]
- 32.Sauzet J P, Gras-Masse H, Guillet J G, Gomard E. Influence of strong CD4 epitope on long-term virus-specific cytotoxic T cell responses induced in vivo with peptides. Int Immunol. 1996;8:457–465. doi: 10.1093/intimm/8.4.457. [DOI] [PubMed] [Google Scholar]
- 33.Scheibenbogen C, Lee K H, Stevanovic S, Witzens M, Willhauck M, Waldmann V, Naeher H, Rammensee H G, Keilholz U. Analysis of the T cell response to tumor and viral peptide antigens by an IFNγ-ELISPOT assay. Int J Cancer. 1997;71:932–936. doi: 10.1002/(sici)1097-0215(19970611)71:6<932::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 34.Schulz M, Zinkernagel R M, Hengartner H. Peptide-induced antiviral protection by cytotoxic T cells. Proc Natl Acad Sci USA. 1991;88:991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirai M, Pendleton C D, Ahlers J, Takeshita T, Newman M, Berzofsky J A. Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs. J Immunol. 1994;152:549–556. [PubMed] [Google Scholar]
- 36.Stuhler G, Schlossman S F. Antigen organization regulates cluster formation and induction of cytotoxic T lymphocytes by helper T cell subsets. Proc Natl Acad Sci USA. 1997;94:622–627. doi: 10.1073/pnas.94.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor P M, Askonas B A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986;58:417–420. [PMC free article] [PubMed] [Google Scholar]
- 38.Thiam K, Loing E, Gilles F, Verwaerde C, Quatannens B, Auriault C, Gras-Masse H. Induction of apoptosis by protein kinase C pseudosubstrate lipopeptides in several human cells. Lett Pept Sci. 1997;4:397–402. [Google Scholar]
- 39.Vitiello A, Ishioka G, Grey H M, Rose R, Farness P, LaFond R, Yuan L, Chisari F V, Furze J, Bartholomeuz R, Chesnut R W. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. J Clin Investig. 1995;95:341–349. doi: 10.1172/JCI117662. [DOI] [PMC free article] [PubMed] [Google Scholar]