Abstract
Case summary
A 10-year-old spayed female domestic medium hair cat presented after sustaining atraumatic insufficiency fractures of the right calcaneus and the left tibia approximately 6 weeks apart. Chronic alendronate therapy had been ongoing for 9 years for the management of previously diagnosed idiopathic hypercalcemia. The right calcaneal fracture was managed non-operatively due to minimal functional impairment. The left tibial fracture was managed via open reduction and internal fixation with orthogonal plating. Alendronate therapy was discontinued at the time of the fracture repair with prednisolone being used to manage the hypercalcemia. Despite rapid clinical improvement, the tibial fracture had a protracted healing course, with clinical union only being achieved 22 weeks postoperatively. At 17 months postoperatively, the idiopathic hypercalcemia remained well controlled. Gait assessment, orthopedic examination and orthogonal radiographs performed at this time revealed resolution of left pelvic limb lameness, a normal orthopedic examination of the left pelvic limb and no evidence of implant-associated complications. Monitoring is ongoing but at the time of publication, no further fractures have occurred.
Relevance and novel information
As reported in humans, this case report gathers evidence of associations between bisphosphonate treatment and the occurrence of insufficiency fractures in cats, and provides evidence that stress reactions may precede their development. If bisphosphonate therapy is utilized in the long term, serial radiographic monitoring for signs of impending fracture may be warranted. Fracture repair can be successful in cats that have received long-term bisphosphonate therapy, but delayed healing should be anticipated and implant choices made accordingly.
Keywords: Bisphosphonate therapy, stress fracture, insufficiency fracture, tibial fracture, calcaneal fracture, patellar fracture and dental anomaly syndrome
Introduction
Bisphosphonate (BP) use in human medicine is widespread; it is highly researched for the treatment of osteoporosis, hypercalcemia of malignancy, bone metastasis and osteogenesis imperfecta.1–3 In veterinary medicine, BPs are gaining favor with treatment for a variety of conditions, including hyperparathyroidism, idiopathic hypercalcemia (IHC), secondary hypercalcemia, tooth resorption and neoplastic bone pain.3–10
BPs are potent osteoclast inhibitors that reduce osteoclast-induced bone resorption and remodeling.1,11 The most widely used BPs are nitrogen-containing, including alendronate, risedronate and zoledronate. These compounds bind to the bone, are endocytosed into osteoclasts and impede metabolic pathways through inhibition of multiple enzymes, predominantly farnesyl diphospate synthase. 12 This metabolic pathway constraint disrupts osteoclast activities involved in bone resorption. 12 Osteoclast apoptosis may also occur but is not required for inhibition of bone resorption.13,14
Of the reported adverse effects from BP use within the human literature, atypical stress fractures or insufficiency fractures have received the most attention.15–26 Insufficiency fractures occur in the absence of trauma when physiological stresses are placed on abnormal bones,27,28 while the term stress fracture is reserved for injuries sustained when repetitive forces act upon normal bone.29,30 Other potential complications from BP use in humans include jaw osteonecrosis,31–34 musculoskeletal pain, 35 atrial fibrillation36–38 and esophageal erosion/irritation. 2 In veterinary medicine, most BP use is transient so many of the side effects appreciated in humans have not been noted. However, the long-termuse of BP therapy has been associated with bilateral atypical patellar fractures in one cat 7 and jaw osteonecrosis.9,39
This case report describes atypical fractures sustained in the absence of trauma in a cat prescribed alendronate for the control of IHC. This is only the second reported case of BP-associated fracture in a cat and the first where surgical stabilization and long-term follow-up are reported.
Case description
A 10-year-old spayed female domestic mediumhair cat was referred for investigation of a 2-month history of right pelvic limb lameness with no inciting trauma. The cat had previously been diagnosed with IHC at the age of 1 year based on a history of sustained hypercalcemia and an absence of salient findings after an appropriate diagnostic work-up. Prednisolone and alendronate were prescribed for management, levels of which were titrated over time based on ionized calcium levels; the last documented period of hypercalcemia was 3 years after the diagnosis of IHC. Current medications consisted of alendronate (10 mg/week), prednisolone (2.5 mg q24h) and gabapentin (100 mg q8h).
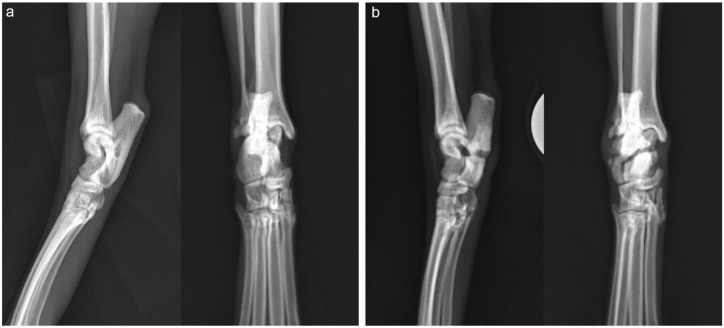
On physical examination, the cat had a moderate weightbearing right pelvic limb lameness with moderate tarsal hyperflexion. The right hock had a normal range of motion with mild palpable crepitus. Orthogonal radiographs of both tibiae and tarsi were performed, revealing a complete transverse right calcaneal body fracture with mild displacement and moderate sclerosis of the fracture ends; there was minimal activity at the fracture site (Figure 1). Although surgical stabilization was recommended, due to perceived improvement over time, the owner elected to trial non-surgical management.
Figure 1.
Mediolateral and dorsoplantar radiographs of both tarsi
(a) Radiographs of the left tarsus are within normal limits. (b) Radiographs of the right tarsus reveal a complete transverse calcaneal body fracture with mild displacement and moderate sclerosis of the fracture ends. There is minimal activity at the fracture site
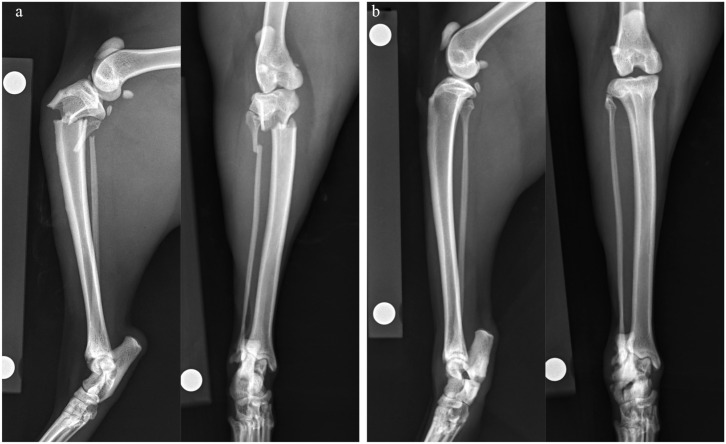
Six weeks later, the cat re-presented for an acute onset non-weightbearing lameness of the left pelvic limb, again with no inciting trauma. Gait analysis confirmed non-weightbearing lameness on the left and mild weightbearing lameness of the right pelvic limb with persistent tarsal hyperflexion. Orthopedic examination revealed swelling and instability at the level of the proximal left tibia. Orthogonal radiographs of both tibiae revealed a complete transverse fracture of the left proximal tibial metaphysis and fibula (Figure 2a). The fracture of the right calcaneus remained static with a persistent fracture line, sclerosis and minimal activity (Figure 2b). Surgical stabilization of the tibial fracture was elected. Complete bloodwork showed no significant findings with the exception of a mild total hypocalcemia (8.2 mg/dl, reference interval [RI] 9.1–10.7), ionized calcium (5.5 mg/dl, RI 5.1–6.0) and phosphorous (5.3 mg/dl, RI 2.7–5.7) were within normal limits.
Figure 2.
Mediolateral and craniocaudal radiographs of both tibiae
(a) Radiographs of the left tibia reveal a complete transverse fracture of the left proximal tibial metaphysis and complete transverse fracture of the proximal fibula. (b) Radiographs of the right tibia are within normal limits, except for the right complete transverse calcaneal body fracture, which remains evident with a persistent fracture line, sclerosis and minimal activity
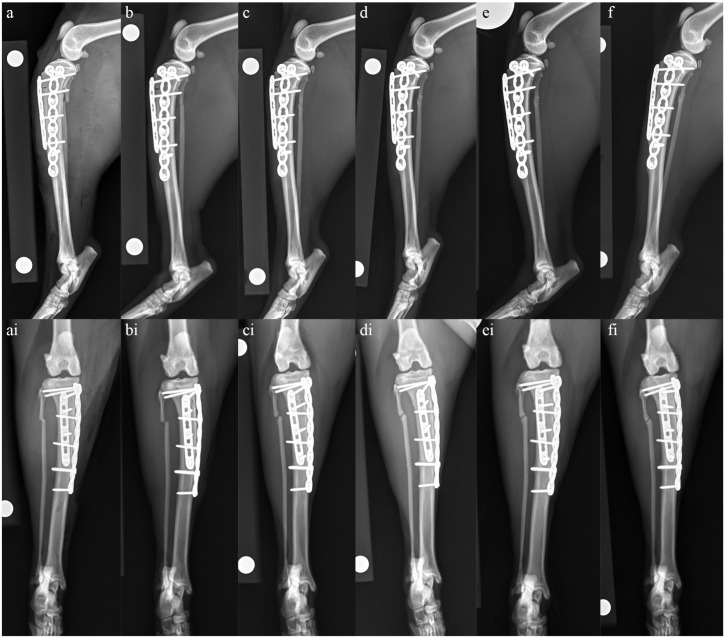
Open reduction and internal fixation via a medial approach 40 was undertaken. A 1.5 mm six-hole locking compression plate (LCP) (DePuy Synthes) was placed cranially and was used to reduce the fracture. A 1.5/2.0 mm split-T seven-hole LCP (DePuy Synthes) was then placed medially. Postoperative radiographs showed anatomic alignment, near-anatomic reduction and appropriate implant placement (Figure 3a and ai). While bone biopsy was considered, as this was not considered likely to impact on therapeutic recommendations, it was not performed due to the brittle nature of the bone and the paucity of bone stock available for repair. Postoperatively, analgesia was managed with methadone (0.2 mg/kg IV q6h) while hospitalized. The cat was discharged the next day with instructions for cage rest, amantadine for additional analgesia (2.9 mg/kg PO q24h) and continuation of current medications (prednisolone 2.5 mg q24h, gabapentin 100 mg q8h), with the exception of alendronate, which was discontinued.
Figure 3.
Mediolateral and craniocaudal radiographs of the left tibia taken (a and ai) immediately postoperatively, (b and bi) 4 weeks postoperatively, (c and ci) 8 weeks postoperatively, (d and di) 14 weeks postoperatively, (e and ei) 22 weeks postoperatively and (f and fi) 17 months postoperatively. (a,ai) Immediately postoperatively, anatomic alignment and near-anatomic reduction have been achieved. Implant placement is appropriate with a 1.5 mm six-hole LCP placed cranially and a 1.5/2.0 mm split-T seven-hole LCP placed medially. (b,bi) At 4 weeks postoperatively, there is minimal evidence of healing at the tibial or fibula fracture sites. Anatomic alignment and near-anatomic reduction have been maintained and there is no evidence of implant-associated complications. (c,ci) At 8 weeks postoperatively, the tibial fracture line is slightly narrower cranially than at the 4-week re-check, and there is evidence of early callus formation caudally and medially. There is also evidence of early remodeling of the fibula fracture. Alignment and apposition have been maintained and implants remain static. (d,di) At 14 weeks postoperatively, there is progressive narrowing of both the tibial and fibula fracture lines when compared with the radiographs taken at 8 weeks postoperatively and evidence of moderate callus formation. Alignment and apposition have been maintained and implants remain static. (e,ei) At 22 weeks postoperatively, clinical union of the tibial fracture has been achieved with no signs of implant-associated complications. There is an oligotrophic nonunion of the fibula. (f,fi) At 17 months postoperatively, radiographs remain static when compared with those taken at 22 weeks postoperatively
Findings at follow-up visits from 4 weeks to 17 months postoperatively are detailed in Table 1. At final follow-up, screening radiographs were performed of the pelvis, both stifles, the right tibia and both tarsi, which revealed no evidence of impending fracture.
Table 1.
Details of follow-up from 4 weeks to 17 months postoperatively, including findings from gait assessment, orthopedic examination, radiography and bloodwork
| Time period postoperatively | Gait assessment | Orthopedic examination findings | Radiographic findings left tibia | Radiographic findings right calcaneus | Complete blood count and serum chemistry results |
|---|---|---|---|---|---|
| 4 weeks | Resolution of left pelvic limb lameness Mild plantigrade stance on right pelvic limb |
No pain response upon palpation over implants Normal stifle range of motion |
Minimal evidence of healing Alignment and apposition maintained Static implants (Figure 3b and bi) |
Not performed at this time | Not performed at this time |
| 8 weeks | Unchanged from 4 weeks | Unchanged from 4 weeks | Narrowing of fracture line with early callus formation (Figure 3c and ci) | Not performed at this time | Not performed at this time |
| 14 weeks | Unchanged from 4 weeks | Unchanged from 4 weeks | Further narrowing of fracture line and moderate callus formation (Figure 3d and di) | Viable, oligotrophic nonunion (Figure 4) | Not performed at this time |
| 22 weeks | Unchanged from 4 weeks | Unchanged from 4 weeks | Clinical union (Figure 3e and ei) | Not performed at this time | No significant findings Both total and ionized calcium, as well as phosphorous, were within the reference interval |
| 17 months | Unchanged from 4 weeks | Unchanged from 4 weeks | No significant changes at 22 weeks (Figure 3f and fi) | Not performed at this time | No significant findings Mild total hypercalcemia (11.2 mg/dl, RI 9.1–10.7) with normal ionized calcium (5.8 mg/dl, RI 5.1–6.0) |
RI = reference interval
Figure 4.
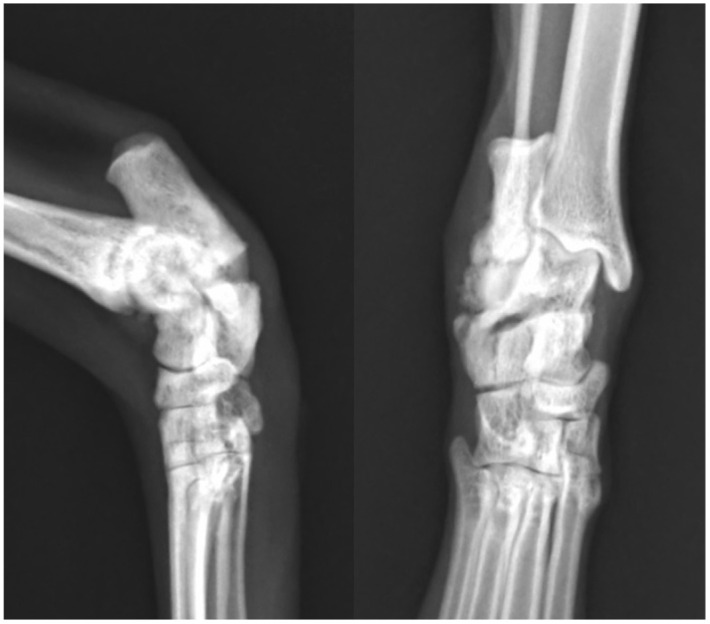
Mediolateral and dorsoplantar radiographs of the right tarsus taken 14 weeks after tibial fracture repair and 28 weeks after calcaneal fracture. There is minimal callus formation, and this is only evident laterally. The fracture ends have become smooth in outline but remain hazy in appearance. This is considered consistent with a viable, oligotrophic non-union
Discussion
Forces are constantly applied to bone with normal activity, which creates microcracks. Bone is constantly remodeling, and therefore these microcracks are repaired in normal tissues. 1 When the balance of bone turnover is compromised, as with BP therapy, this diminishes microfracture healing, which leads to skeletal fragility and fracture formation.2,21 Humans on chronic BP therapy have increased risks of atypical fractures;20,23 these fractures tend to occur at specific locations, most commonly in the subtrochanteric region of the femur, and have distinct characteristics including cortical hypertrophy and a largely transverse configuration. These characteristics are not similar to those seen with other types of pathological fractures, such as those associated with osteoporosis.16,17,19,21,34,41 Based on a previous report 7 and the cat detailed therein, the imbalance of remodeling secondary to chronic BP use may also cause a subset of atypical fractures in cats.
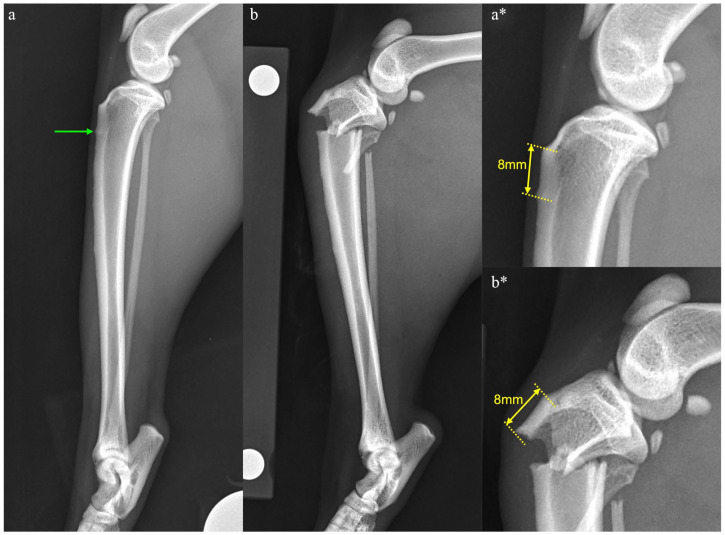
In humans on long-term BP therapy, impending fractures, or stress reactions, can be diagnosed on radiographs, which then progress to a complete fracture. These stress reactions are typically areas of sclerosis or focal increased opacities within the cortex but may also be as subtle as a cortical bulge.18,22 Similar changes have been reported in a cat with a suspected stress fracture of the calcaneus. 42 The cat detailed in this report had radiographs performed of the left tibia as part of a routine work-up for the right calcaneal fracture, 6 weeks before sustaining the left tibial fracture. At that time, no abnormalities were noted associated with the left pelvic limb. Retrospectively, a stress reaction was noted in the proximal cranial cortex of the left tibia, which corresponds with the subsequent site of tibial fracture (Figure 5). As identification of such radiographic features may help predict impending fracture, serial radiographic monitoring of cats on chronic BP therapy may be indicated. The timeline for such monitoring remains uncertain as the accumulation of microdamage leading to fracture takes several years, with microcrack density being linearly related to BP treatment duration. 43 As both cats with BP-related fractures only developed them after 8 years of medication and humans tend to develop them after 3–9 years,17,21,26 monitoring may not need to be started immediately. Obtaining baseline radiographs at the time of commencing therapy and considering annual screening radiographs after 5 years may be a reasonable proposed timeline, with monitoring starting earlier if clinical signs of pain or lameness arise. The half-life of BPs within bone is not currently known in cats, but due to the prolonged half-life in humans (10 years) 44 and dogs (3 years), 45 continued monitoring for several years after stopping therapy may also be indicated. In the cat detailed in this report, radiographic monitoring performed 17 months postoperatively revealed no evidence of stress reaction.
Figure 5.
Mediolateral views of the left tibia taken (a) 6 weeks before tibial fracture and (b) at the time of tibial fracture diagnosis, with magnified images from the same time periods (a* and b*). Note the stress reaction in the cranial cortex of the tibia, evidenced as a focal area of increased opacity seen 8 mm distal to the tibial tuberosity (green arrow). This corresponds to the site of fracture noted 6 weeks later, which was, again, 8 mm distal to the tibial tuberosity
The use of BP in animals is not well studied when compared with its use in humans, but there are case reports and retrospective studies describing their use and associated complications.3–6,10,46–50 Due to the way that BPs are generally used within veterinary medicine, reports of complications associated with long-term use are scarce. In dogs, where BPs tend to be prescribed for short treatment periods, such as for toxicities causing hypercalcemia and palliative treatments for bone neoplasia, there are currently no reports of BP-associated fracture.3,4,48,49 Dogs have been used in studies aiming to evaluate the side effects of BP in humans, but these studies have relatively short therapeutic periods.51–53 This makes atypical fractures less likely, as they tend to be reported after years of therapy in other species.17,21,26 The most common indication for prolonged BP use in veterinary medicine is feline IHC.4–6,10 While the long-term follow-up of chronically treated cases of feline IHC is sparse, rendering the prevalence of adverse effects unknown. This report, in addition to a previous one, 7 indicates that owners should be warned of the risk of atypical fracture formation.
Chronic glucocorticoid treatment reduces bone turnover and has been identified as a risk factor for atypical fracture formation. 20 The long-term administration of steroids inhibits fracture healing in rabbits 54 and is known to decrease osteoblastic activity and therefore matrix synthesis.55–58 It should be considered that the chronic prednisolone therapy may have exaggerated the effect of alendronate in this cat, both in terms of the predisposition to fracture and the delayed healing noted.
In the only other published case report detailing BP-associated fractures in a cat, bilateral, staged, atraumatic patellar fractures were sustained in a Maine Coon receiving chronic alendronate therapy to prevent progression of tooth resorption. 7 In that cat, the cortices of the long bones were shown to be significantly thicker than in the control cats. 7 The relative tibial medullary cavity diameters from each set of radiographs were calculated for the cat detailed in the present report, based on the measurements described previously; 7 there was no evidence of a narrower medullary cavity or thicker cortices in this case.
The atraumatic fractures sustained in this case, and those reported previously in a cat on chronic BP therapy, 7 were located in areas and had characteristics that are uncommon. Interestingly, the fractures that these cats sustained have all been reported in cats with patellar fracture and dental anomaly syndrome (PADS). This syndrome is described in cats that have a combination of dental anomalies and which sustain atraumatic insufficiency fractures to the patellae and multiple other bones.58–62 Excluding patellar fractures, other fracture sites encountered in this syndrome include the acetabulum, tibia, ischium, humeral condyle, calcaneus, ilium and pubis. 61 These fractures tend to have similar characteristics, including a simple fracture with a sclerotic fracture line. 61 In the previous BP-associated fracture case, 7 the patellar fractures were radiographically similar to those sustained in cats with PADS. Tibial fractures described in PADS are predominantly proximal diaphyseal or metaphyseal transverse fractures, while calcaneal fractures are typically short oblique fractures located at the base. 61 These fracture patterns are consistent with what was observed in the case reported here.
Currently, the etiology of PADS remains unknown; however, speculations have arisen, including osteopetrosis, a primary bone disorder or a connective tissue disease process.58–62 The similarities in location and radiographic appearance between fractures encountered in PADS and those detailed secondarily to chronic BP therapy may indicate a common pathogenesis. Indeed, if fractures in cats on long-term BP therapy occur secondarily to osteoclast inhibition, it would hold that a primary dysfunction of osteoclasts (and potentially odontoclasts) could also be involved in PADS. Clearly, this hypothesis requires further research, but due to the similarities, if radiographic monitoring of cases receiving long-term BP therapy is considered, it may be prudent to focus on areas where fractures have been reported in PADS.
While it is not possible to state definitively that the cat reported herein did not suffer from PADS, there are several aspects of presentation that would not be typical for this condition. There was no history, or evidence, of dental or oral abnormalities and there were no patellar fractures. In addition, the signalment of the patient is not typical for PADS as these cats generally present with their first fracture earlier in life, at a mean age of 28 months. 59 Given these inconsistencies and the history of chronic alendronate administration, a BP-related insufficiency fracture was considered more likely.
Delayed healing of insufficiency fractures in patients receiving BPs is well recognized in humans17,20,21 and was observed in this case. In this cat, alendronate therapy was discontinued after tibial fracture repair to reduce the risk of additional fractures and potentially facilitate healing of the current ones. However, based on a previous study in humans, likely due to the long half-life of BPs, there was no difference in fracture healing between patients that discontinued BP therapy and those that continued with it. 20 Based on the very small numbers of cats with BP-related fractures, it is not possible to make a definitive recommendation regarding whether BPs should be discontinued in order to reduce future fracture risk; however, given their long half-life as reported in dogs and humans,44,45 discontinuation of therapy is unlikely to make a significant difference to healing of fractures that have already occurred. As protracted healing is anticipated, stabilization should be planned accordingly. Given the risks of plate bending after tibial fracture stabilization in cats 63 and the lower risk associated with orthogonal plating 64 or use of angle-stable interlocking nails, 65 consideration should be given to the latter two methods in cases with BP-associated tibial fracture. Orthogonal plating in this case resulted in a very satisfactory outcome with no implant-associated complications 17 months after stabilization.
Conclusions
This is the first report describing surgical stabilization and long-term follow-up in a cat with atypical fractures receiving long-term BP therapy as well as steroid treatment. As anticipated, clinical union was delayed and stabilization methods for BP-associated fractures should be chosen specifically to withstand cyclic loading throughout a protracted healing period. Retrospectively, a stress reaction was evident in the tibial cortex, which progressed to complete fracture; serial radiographic monitoring may be indicated in cats on long-term BP therapy as this may allow the early detection of stress reactions that may predispose to complete fracture.
Acknowledgments
The authors would like to acknowledge both Dr Danielle Marturello and Dr Courtney Bartels for their involvement with this case.
Footnotes
Accepted: 1 June 2023
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognized high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS Open Reports. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Karen L Perry  https://orcid.org/0000-0001-6456-1817
https://orcid.org/0000-0001-6456-1817
References
- 1. Rodan GA. Mechanisms of action of bisphosphonates. Annu Rev Pharmacol Toxicol 1998; 38: 375–388. [DOI] [PubMed] [Google Scholar]
- 2. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 2008; 83: 1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suva LJ, Cooper A, Watts AE, et al. Bisphosphonates in veterinary medicine: the new horizon for use. Bone 2021; 142. DOI: 10.1016/j.bone.2020.115711. [DOI] [PubMed] [Google Scholar]
- 4. Hostutler RA, Chew DJ, Jaeger JQ, et al. Uses and effectiveness of pamidronate disodium for treatment of dogs and cats with hypercalcemia. J Vet Intern Med 2005; 19: 29–33. [DOI] [PubMed] [Google Scholar]
- 5. Whitney JL, Barrs VR, Wilkinson MR, et al. Use of bisphosphonates to treat severe idiopathic hypercalcaemia in a young Ragdoll cat. J Feline Med Surg 2011; 13: 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hardy BT, de Brito Galvao JF, Green TA, et al. Treatment of ionized hypercalcemia in 12 cats (2006–2008) using PO-administered alendronate. J Vet Intern Med 2015; 29: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Council N, Dyce J, Drost WT, et al. Bilateral patellar fractures and increased cortical bone thickness associated with long-term oral alendronate treatment in a cat. JFMS Open Rep 2017; 3. DOI: 10.1177/2055116917727137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salwat SM, Farid MHM, Hakam HM. Potential effect of alendronate on tooth eruption and molar root formation in young growing and osteoprotic albino rats (A histiological and histochemical study). Al-Azhar Dent J Girls 2018; 5: 237–242. [Google Scholar]
- 9. Larson MJ, Oakes AB, Epperson E, et al. Medication-related osteonecrosis of the jaw after long-term bisphosphonate treatment in a cat. J Vet Intern Med 2019; 33: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurtz M, Desquilbet L, Maire J, et al. Alendronate treatment in cats with persistent ionized hypercalcemia: a retrospective cohort study of 20 cases. J Vet Intern Med 2022; 36: 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell RG, Muhlbauer RC, Bisaz S, et al. The influence of pyrophosphate, condensed phosphates, phosphonates and other phosphate compounds on the dissolution of hydroxyapatite in vitro and on bone resorption induced by parathyroid hormone in tissue culture and in thyroparathyroidectomised rats. Calcif Tissue Res 1970; 6: 183–196. [DOI] [PubMed] [Google Scholar]
- 12. Rogers MJ, Monkkonen J, Munoz MA. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone 2020; 139. DOI: 10.1016/j.bone.2020.115493. [DOI] [PubMed] [Google Scholar]
- 13. Roelof AJ, Ebetino FH, Reszka AA, et al. Bisphoshonates. Mechanism of action. In: Bilezikian J, Raisz L, Martin TJ. (eds). Principles of bone biology. San Diego, CA: Elsevier, 2008, pp 1737–1767. [Google Scholar]
- 14. Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011; 48: 677–692. [DOI] [PubMed] [Google Scholar]
- 15. Whyte MP, Wenkert D, Clements KL, et al. Bisphosphonate-induced osteopetrosis. N Engl J Med 2003; 349: 457–463. [DOI] [PubMed] [Google Scholar]
- 16. Nalla RK, Kruzic JJ, Kinney JH, et al. Aspects of in vitro fatigue in human cortical bone: time and cycle dependent crack growth. Biomaterials 2005; 26: 2183–2195. [DOI] [PubMed] [Google Scholar]
- 17. Odvina CV, Zerwekh JE, Rao DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 2005; 90: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 18. Kwek EBK, Goh SK, Koh JSB, et al. An emerging pattern of subtrochanteric stress fractures: a long term complication of alendronate therapy?. Injury 2008; 39: 224–231. [DOI] [PubMed] [Google Scholar]
- 19. Geusens P. Bisphosphonates for postmenopausal osteoporosis: determining duration of treatment. Curr Osteoporos Rep 2009; 7: 12–17. [DOI] [PubMed] [Google Scholar]
- 20. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy. A systematic review of case/case series studies. Bone 2010; 47: 169–180. [DOI] [PubMed] [Google Scholar]
- 21. Sellmeyer DE. Atypical fractures as a potential complication of long-term bisphosponate therapy. JAMA 2010; 304: 1480–1484. [DOI] [PubMed] [Google Scholar]
- 22. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab 2010; 95: 1555–1565. [DOI] [PubMed] [Google Scholar]
- 23. Giusti A, Hamdy NAT, Dekkers OM, et al. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48: 966–971. [DOI] [PubMed] [Google Scholar]
- 24. Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364: 1728–1737. [DOI] [PubMed] [Google Scholar]
- 25. Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27: 2544–2550. [DOI] [PubMed] [Google Scholar]
- 26. Imbuldeniya AM, Jiwa N, Murphy JP. Bilateral atypical insufficiency fractures of the proximal tibia and a unilateral distal femoral fracture with long-term intravenous bisphosphonate therapy: a case report. J Med Case Rep 2012; 6: 50. DOI: 10.1186/1752-1947-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daffner RH, Pavlov H. Stress fractures: current concepts. AJR Am J Roentgenol 1992; 159: 245–252. [DOI] [PubMed] [Google Scholar]
- 28. Matcuk GR, Mahanty SR, Skalski MR, et al. Stress fractures: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol 2016; 23: 365–375. [DOI] [PubMed] [Google Scholar]
- 29. Brukner P, Bradshaw C, Khan KM, et al. Stress fractures: a review of 180 cases. Clin J Sport Med 1996; 6: 85–89. [PubMed] [Google Scholar]
- 30. Ohta-Fukushima M, Mutoh Y, Takasugi S, et al. Characteristics of stress fractures in young athletes under 20 years. J Sports Med Phys Fitness 2002; 42: 198. [PubMed] [Google Scholar]
- 31. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 2005; 23: 8580–8587. [DOI] [PubMed] [Google Scholar]
- 32. Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 2005; 63: 1567–1575. [DOI] [PubMed] [Google Scholar]
- 33. Woo SB, Hellstein JW, Kalmar JR. Systemic review: bisphosphonates and osteonecrosis of the jaws [published correction appears in Ann Intern Med 2006; 145: 235]. Ann Intern Med 2006; 144: 753–761. [DOI] [PubMed] [Google Scholar]
- 34. McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 2013; 126: 13–20. [DOI] [PubMed] [Google Scholar]
- 35. US Food and Drug Administration. Information on bisphosphonates (marketed as Actonel, Actonel +Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa). http://wayback.archive-it.org/7993/20170112032106/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124165.htm (2008, accessed 15 December 2022).
- 36. Black DM, Delmas PD, Eastell R, et al.; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356: 1809–1822. [DOI] [PubMed] [Google Scholar]
- 37. Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation [letter]. N Engl J Med 2007; 356: 1895–1896. [DOI] [PubMed] [Google Scholar]
- 38. Heckbert SR, Li G, Cummings SR, et al. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med 2008; 168: 826–831. [DOI] [PubMed] [Google Scholar]
- 39. Rogers-Smith E, Whitley N, Elwood C, et al. Suspected bisphosphonate-related osteonecrosis of the jaw in a cat being treated with alendronate for idiopathic hypercalcaemia. Vet Rec Case Rep 2019; 7. DOI: 10.1136/vetreccr-2018-000798. [DOI] [Google Scholar]
- 40. Piermattei DL, Johnson DL. The pelvis and hip joint. In: Piermattei DL, Johnson KA. (eds). An atlas of surgical approaches to the bones and joints of the dog and cat. 4th ed. Philadelphia, PA: Elsevier, 2004, pp 370–373. [Google Scholar]
- 41. Capeci CM, Tejwani NC. Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg Am 2009; 91: 2556–2561. [DOI] [PubMed] [Google Scholar]
- 42. Cantatore M, Clements DN. Bilateral calcaneal stress fractures in two cats. J Small Anim Pract 2015; 56: 417–421. [DOI] [PubMed] [Google Scholar]
- 43. Pienkowski D, Wood CL, Malluche HH. Trabecular bone microcrack accumulation in patients treated with bisphosphonates for durations up to 16 years. J Orthop Res 2023; 41: 1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khan SA, Kanis JA, Vasikaran S, et al. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res 1997; 12: 1700–1707. [DOI] [PubMed] [Google Scholar]
- 45. Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone 1996; 18: 75–85. [DOI] [PubMed] [Google Scholar]
- 46. Reddy MS, Weatherford TW, Smith A, et al. Alendronate treatment of naturally-occurring periodontitis in beagle dogs. J Periodontol 1995; 66: 211–217. [DOI] [PubMed] [Google Scholar]
- 47. Mohn KL, Jacks TM, Schleim KD, et al. Alendronate binds to tooth root surfaces and inhibits progression of feline tooth resorption: a pilot proof-of-concept study. J Vet Dent 2009; 26: 74–81. [DOI] [PubMed] [Google Scholar]
- 48. Fan TM, de Lorimier LP, Garrett LD, et al. The bone biologic effects of zoledronate in healthy dogs and dogs with malignant osteolysis. J Vet Intern Med 2008; 22: 380–387. [DOI] [PubMed] [Google Scholar]
- 49. Oblak ML, Boston SE, Higginson G, et al. The impact of pamidronate and chemotherapy on survival times in dogs with appendicular primary bone tumors treated with palliative radiation therapy. Vet Surg 2012; 41: 430–435. [DOI] [PubMed] [Google Scholar]
- 50. Wypij JM, Heller DA. Pamidronate Disodium for palliative therapy of feline bone-invasive tumors. Vet Med Int 2014; 2014. DOI: 10.1155/2014/675172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mashiba T, Hirano T, Turner CH, et al. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 2000; 15: 613–620. [DOI] [PubMed] [Google Scholar]
- 52. Burr DB, Allen MR. Mandibular necrosis in beagle dogs treated with bisphosphonates. Orthod Craniofac Res 2009; 12: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burr DB, Miller L, Grynpas M, et al. Tissue mineralization is increased following 1-year treatment with high doses of bisphosphonates in dogs. Bone 2003; 33: 960–969. [DOI] [PubMed] [Google Scholar]
- 54. Waters RV, Gamradt SC, Asnis P. Systemic corticosteroids inhibit bone healing in a rabbit ulnar osteotomy model. Acta Orthop Scand 2000; 71: 316–321. [DOI] [PubMed] [Google Scholar]
- 55. Aaron JE, Francis RM, Peacock M, et al. Contrasting microanatomy of idiopathic and corticosteroid-induced osteoporosis. Clin Orthop Relat Res 1989; 243: 294–305. [PubMed] [Google Scholar]
- 56. LoCascio V, Bonucci E, Imbimbo B, et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner 1990; 8: 39–51. [DOI] [PubMed] [Google Scholar]
- 57. Lukert B, Kream BE. Clinical and basic aspects of glucocorticoid action in bone. In: Bilezikian JP, Raisz LG, Rodan GA. (eds). Principles of bone biology. New York: Academic Press, 1996, pp 533–548. [Google Scholar]
- 58. Langley-Hobbs S. Patella fracture in cats with persistent deciduous teeth knees and teeth syndrome (KaTS). Comp Anim 2016; 21: 620–626. [Google Scholar]
- 59. Langley-Hobbs SJ. Survey of 52 fractures of the patella in 34 cats. Vet Rec 2009; 164: 80–86. [DOI] [PubMed] [Google Scholar]
- 60. Langley-Hobbs SJ, Ball S, Mckee WM. Transverse stress fractures of the proximal tibia in 10 cats with non-union patellar fractures. Vet Rec 2009; 164: 425–430. [DOI] [PubMed] [Google Scholar]
- 61. Reyes NA, Longley M, Bailey S, et al. Incidence and types of preceding and subsequent fractures in cats with patellar fracture and dental anomaly syndrome. J Feline Med Surg 2019; 21: 750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Howes C, Longley M, Reyes N, et al. Skull pathology in 10 cats with patellar fracture and dental anomaly syndrome. J Feline Med Surg 2019; 21: 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morris AP, Anderson AA, Barnes DM, et al. Plate failure by bending following tibial fracture stabilisation in 10 cats. J Small Anim Pract 2016; 57: 472–478. [DOI] [PubMed] [Google Scholar]
- 64. Craig A, Witte PG, Moody T, et al. Management of feline tibial diaphyseal fractures using orthogonal plates performed via minimally invasive plate osteosynthesis. J Feline Med Surg 2018; 20: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marturello DM, Perry KL, Déjardin LM. Clinical application of the small I-Loc interlocking nail in 30 feline fractures: a prospective study. Vet Surg 2021; 50: 588–599. [DOI] [PubMed] [Google Scholar]