Abstract
As a family of cationic host defense peptides, defensins are mainly synthesized by Paneth cells, neutrophils, and epithelial cells, contributing to host defense. Their biological functions in innate immunity, as well as their structure and activity relationships, along with their mechanisms of action and therapeutic potential, have been of great interest in recent years. To highlight the key research into the role of defensins in human and animal health, we first describe their research history, structural features, evolution, and antimicrobial mechanisms. Next, we cover the role of defensins in immune homeostasis, chemotaxis, mucosal barrier function, gut microbiota regulation, intestinal development and regulation of cell death. Further, we discuss their clinical relevance and therapeutic potential in various diseases, including infectious disease, inflammatory bowel disease, diabetes and obesity, chronic inflammatory lung disease, periodontitis and cancer. Finally, we summarize the current knowledge regarding the nutrient-dependent regulation of defensins, including fatty acids, amino acids, microelements, plant extracts, and probiotics, while considering the clinical application of such regulation. Together, the review summarizes the various biological functions, mechanism of actions and potential clinical significance of defensins, along with the challenges in developing defensins-based therapy, thus providing crucial insights into their biology and potential clinical utility.
Subject terms: Immunology, Molecular biology, Diseases
Introduction
Host defense peptides (HDPs) are polypeptides assembled from fewer than 100 amino acids. These peptides tend to have a high proportion of positively charged and hydrophobic residues.1,2 Based on the Host Defense Peptides Database in 2022 (https://wangapd3.com/main.php), scientists have identified or predicted a total of 3257 HDPs. These HDPs are derived from various organisms, including 365 from bacteria, five from archaea, eight from protists, 30 from fungi, 371 from plants, and 2521 from animals. Among these, 348 are classified as defensins and have an average length of 41.26 residues, with an average net charge of 4.61.
Based on the amino acid composition, length and structural characteristics, mammalian HDPs are generally categorized into two prominent families: defensins and cathelicidins. The cathelicidins comprise a conserved gene family, initially thought to produce small proteins with cysteine protease inhibitor activity, as well as antimicrobial activity.3,4 Recently, however, the notion of protease inhibitor activity of cathelicidins has been refuted.5 Although pro-defensins are inactive, pro-cathelicidins and cathelicidins are equally bactericidal.5 Initially, direct activity against microorganisms was deemed to be the primary role of HDPs. For example, mouse cryptdins and human alpha defensin-5 (HD5) directly kill Salmonella, and human alpha defensin 6 (HD6) traps Salmonella in a high-ordered “nanonet” structure to prevent infection.6,7 However, many HDPs lose their antimicrobial potency in some localized microenvironments. Even so, it is becoming increasingly clear that HDPs act as immunomodulatory mediators that regulate the mammalian innate immune response and moderate the establishment of adaptive immunity.8,9 The structure, function and mechanism of action of cathelicidins4,10–13 and defensins14–20 have been reviewed over the past few years. Nonetheless, given the enormous number of defensins known, the diversity of their biological activities, the intricate ways in which they function, and the multitude of targets they interact with, publishing a comprehensive review on this topic is an arduous, if not impossible, feat.
Thus, this review is focused on defensins in host defense. It mainly summarizes and discusses their properties, biological function, related clinical diseases, and therapeutic potential, as well as their nutritional regulation. We will also cover the function of defensins in promoting the chemotaxis of immune cells, their influence on multiple signaling pathways involved in inflammation and immunity, how they maintain gut microbial homeostasis and their regulation of epithelial injury and the promotion of proper organ development and eukaryotic cell death, as well as their contribution to clinical diseases and their therapeutic potential. We will highlight the current knowledge base regarding mammalian defensins and their roles in regulating host health, thus providing a theoretical basis for clinical therapeutic strategies targeting defensins to treat disease.
History of defensins
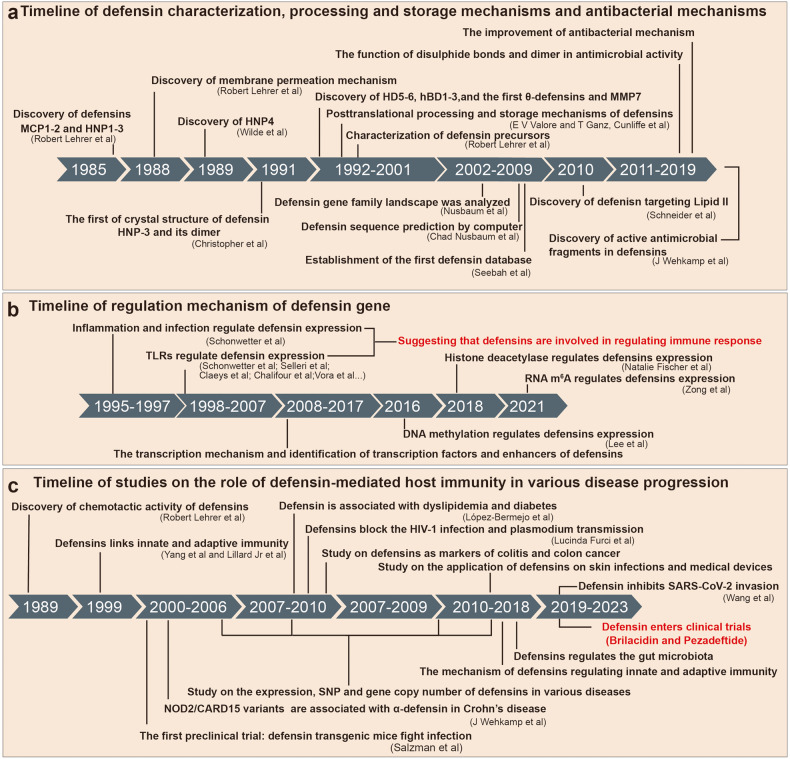
In 1985, Dr. Robert Lehrer from the University of California, Los Angeles, was the first to discover and name defensins. He reported that rabbit defensins MCP-1 and MCP-2 had strong antibacterial and antiviral activities21,22 (Fig. 1a). That same year, he and his team discovered and characterized the structure of human neutrophil peptides (HNP1-3)23 (Fig. 1a). Over time, more defensins were found, such as HNP4 (ref. 24) in 1989, HD5 (ref. 25) and HD6 (ref. 26) in 1992 and 1993, respectively, and human β-defensins (hBD1-3)27–29 in 1995, 1997, and 2001, respectively (Fig. 1a). The first θ-defensin was found in 1999 (ref. 30) (Fig. 1a). Since then, with the widespread use of in silico analyses, researchers have been able to predict the sequence and structure of defensins31 (Fig. 1a). Meanwhile, in the late 20th and early 21th centuries, the scientific community widely studied the processing and storage mechanisms of defensins6,32–36 (Fig. 1a). In addition, from 1988 to 2010, the antibacterial mechanisms of defensins have been established, which involve a membrane penetration mechanism and targeting lipid II by inhibiting cell wall synthesis37,38 (Fig. 1a). During this period, the role of defensin dimers, disulfide bonds and other biochemical structures in their antibacterial function and mechanisms have also been analyzed39–43 (Fig. 1a).
Fig. 1.
Introduction to the history of defensin research. a Timeline of defensin characterization, processing and storage mechanisms and antibacterial mechanisms. b Timeline of regulation mechanism of defensin gene. c Timeline of studies on the role of defensin-mediated host immunity in various disease progression. SNP single-nucleotide polymorphism
In 2007, chromosome 8 was fully sequenced and analyzed by the Human Genome Project, resulting in the description of the first human defensin gene family landscape44–46 (Fig. 1a). The first defensin database was established in the same year, incorporating 350 defensins47 (Fig. 1a). Then, in the early decade of the 21st century, researchers gradually analyzed the regulation pattern of defensin gene expression48–53 (Fig. 1b). These results provide essential data and technical support for the subsequent research into the genetic engineering and drug development of defensins.
Over the last two decades, defensins have been found to regulate immune cell chemotaxis and to be involved in regulating sperm activity, male infertility, thrombosis, melanin deposition, and other essential biological functions.54–58 Further, defensins have also been shown to induce the host’s innate immune response, enhance the host’s adaptive immune response and promote the activation of T cells, macrophages, and other immune cells59–65 (Fig. 1c). Importantly, the role and mechanism of defensins in regulating immune responses have been fully analyzed. Since the 2010s, defensins have been used in the biomedical field.66 For example, they have been applied to the surface of medical instruments to produce long-lasting and broad-spectrum antibacterial activity67,68 (Fig. 1c). Also, with the development of gene editing and peptide chemical synthesis technologies, many preclinical studies have been conducted on a variety of diseases and tumors via the use of transgenic mouse models of defensins and oral or injected recombinant defensins, and their regulatory role and precise mechanisms in different diseases and tumors have been explored in depth69–79 (Fig. 1c). Thus, the interaction network of defensins regulating host immune homeostasis has been constructed, and several reliable drug targets have been identified. In recent years, two defensins have entered clinical trials (Fig. 1c). In conclusion, with the continuous development of science and technology, the study of defensins is deepening, becoming an essential tool and resource to achieve biological protection and human health.
Structural features and evolution
Most defensins are cationic peptides of 18–45 amino acids. They have six conserved cysteines that allow for three intramolecular disulfide bonds that stabilize the peptide.80 The essential information and structural characteristics of most human, mouse, pig, and bovine defensins are listed in Table 1. Mammalian defensins are categorized as α-, β-, and θ-defensins, based on amino acid homology and cysteine residue connectivity19 (Fig. 2). However, humans only produce α- and β-defensins.81,82 Despite having differing covalent structures, α- and β-defensins have similar tertiary structures (Fig. 2 and Table 1). The gene clusters that encode the α-defensin subfamily and most β-defensin subfamily are situated on chromosome 8 (ref. 83) (Table 1), with α-defensin genes deriving from β-defensin genes.84,85 In addition, mammalian defensin genes evolved rapidly, and some newly discovered hBDs are encoded by genes on chromosomes 11 and 20 (Table 1). Through in situ hybridization studies, it has been revealed that the defensin genes clustered on chromosome 20 are transcribed at different locations in the epididymis,86 and there is evidence that they are involved in sperm chemotaxis and maturation and associated with idiopathic infertility.19,56,87–89
Table 1.
Various characteristic features of human, mouse, pig, and bovine defensins
| Defensin name | Abbreviation | Chromosome | UniProt ID | Signal peptide | Mature defensin | Disulfide pattern in mature defensin |
|---|---|---|---|---|---|---|
| Human α-defensin | ||||||
| Neutrophil defensin-1 | HNP1 | 8 | P59665 | 1–19 | 66–94 | 66–94, 68–83, 73–93 |
| Neutrophil defensin 2 | HNP2 | 8 | P59666 | 1–19 | 64–94 | 66–94, 68–83, 73–93 |
| Neutrophil defensin 3 | HNP3 | 8 | P59666 | 1–19 | 65–94 | 66–94, 68–83, 73–93 |
| Neutrophil defensin 4 | HNP4 | 8 | P12838 | 1–19 | 66–96 | 65–93, 67–82, 72–92 |
| Human α-defensin-5 | HD5 | 8 | Q01523 | 1–19 | 63–94 | 65–93, 67–82, 72–92 |
| Human α-defensin 6 | HD6 | 8 | Q01524 | 1–19 | 69–100 | 72–99, 74–88, 78–98 |
| Human β-defensin | ||||||
| Human β-defensin-1 | hBD1 | 8 | P60022 | 1–21 | 33–68 | 37–66, 44–59, 49–57 |
| Human β-defensin 2 | hBD2 | 8 | O15263 | 1–23 | 24–64 | 31–60, 38–53, 43–61 |
| Human β-defensin 103A | hBD3 | 8 | P81534 | 1–22 | 23–67 | 33–62, 40–55, 45–63 |
| Human β-defensin 104A | hBD4 | 8 | Q8WTQ1 | 1–22 | 23–72 | 30–57, 37–51, 41–58 |
| Human β-defensin 105A | hBD5 | 8 | Q8IZN8 | 1–27 | 28–78 | 43–74, 53–67, 57–73 |
| Human β-defensin 6 | hBD6 | 8 | Q496I8 | 1–20 | 21–65 | 26–53, 33–47, 37–54 |
| Human β-defensin 109B | hBD9 | 8 | Q30KR1 | 1–22 | 23–87 | 31–59, 38–53, 43–60 |
| Human β-defensin 127 | hBD27 | 20 | Q9H1M4 | 1–20 | 21–63 | 24–53, 33–47, 37–54 |
| Human β-defensin 119 | DEFB20 | 20 | Q8N690 | 1–21 | 22–84 | 28–55, 35–49, 39–56 |
| Human β-defensin 126 | hBD26 | 20 | Q9BYW3 | 1–20 | 21–111 | 27–58, 34–52, 38–59 |
| Human β-defensin 118 | DEFB18 | 20 | Q8N690 | 1–19 | 20–62 | 27–54, 34–48, 38–55 |
| Human β-defensin 132 | hBD32 | 20 | Q7Z7B7 | 1–22 | 23–95 | 27–55, 35–49, 39–56 |
| Human β-defensin 107A | hBD7 | 8 | Q8IZN7 | 1–20 | 21–65 | 26–53, 33–47, 37–54 |
| Human β-defensin 114 | DEFB14 | 6 | Q30KQ6 | 1–26 | 27–69 | 29–57, 36–60, 40–58 |
| Human β-defensin 108B | hBD8 | 11 | Q496I8 | 1–26 | 27–56 | 27–55, 35–39, 37–56 |
| Mouse α-defensin | ||||||
| Mouse α-defensins-1 | Cryp1 | 8 | P11477 | 1–19 | 59–93 | 64–92, 66–81, 71–91 |
| Mouse α-defensins 2 | Cryp2 | 8 | Q8C1N9 | 1–19 | 20–95 | |
| Mouse α-defensins 3 | Cryp3 | 8 | P28310 | 1–16 | 59–93 | 64–92, 66–81, 71–91 |
| Mouse α-defensins 4 | Cryp4 | 8 | P28311 | 1–19 | 59–92 | 64–89, 66–81, 71–88 |
| Mouse α-defensins 5 | Cryp5 | 8 | L7N230 | 1–19 | 20–93 | |
| Mouse α-defensins 6 | Cryp6 | 8 | P28310 | 1–19 | 61–93 | 64–92, 66–81, 71–91 |
| Mouse α–defensins 17 | Cryp17 | 8 | P28310 | 1–16 | 59–93 | 64–92, 66–81, 71–91 |
| Mouse β-defensin | ||||||
| Mouse β-defensin-1 | mBD1 | 8 | P56386 | 1–21 | 33–69 | 37–66, 44–59, 49–67 |
| Mouse β-defensin 2 | mBD2 | 8 | P82020 | 1–20 | 21–71 | 37–66, 44–59, 49–67 |
| Mouse β-defensin 3 | mBD3 | 8 | Q9WTL0 | 1–20 | 23–63 | 31–59, 38–52, 42–60 |
| Mouse β-defensin 4 | mBD4 | 8 | P82019 | 1–22 | 23–63 | 31–59, 38–52, 42–60 |
| Mouse β-defensin 6 | mBD6 | 8 | Q91VD6 | 1–22 | 23–63 | 31–59, 38–52, 42–60 |
| Mouse β-defensin 7 | mBD7 | 8 | Q91V70 | 1–22 | 26–71 | 31–58, 38–52, 42–59 |
| Mouse β-defensin 9 | mBD9 | 8 | Q8R2I6 | 1–24 | 25–67 | 34–62, 41–55, 45–63 |
| Mouse β-defensin 10 | mBD10 | 8 | Q8R2I8 | 1–23 | 24–73 | 37–66, 44–59, 49–67 |
| Mouse β-defensin 11 | mBD11 | 8 | Q8R2I7 | 1–23 | 24–77 | 37–66, 44–59, 49–67 |
| Mouse β-defensin 12 | mBD12 | 8 | Q8K4N3 | 1–27 | 28–78 | 46–73, 53–67, 57–74 |
| Mouse β-defensin 14 | mBD14 | 8 | Q7TNV9 | 1–22 | 23–67 | 33–62, 40–55, 45–63 |
| Mouse β-defensin 19 | mBD19 | 8 | Q8K3I8 | 1–19 | 20–83 | 27–54, 34–48, 38–55 |
| Mouse β-defensin 20 | mBD20 | 2 | Q30KP3 | 1–21 | 22–96 | 24–52, 32–46, 36–53 |
| Mouse β-defensin 29 | mBD29 | 2 | Q8BGW9 | 1–23 | 24–78 | 40–67, 47–61, 51–68 |
| Mouse β-defensin 30 | mBD30 | 14 | Q30KN4 | 1–22 | 23–75 | 35–62, 42–56, 46–63 |
| Mouse β-defensin 41 | mBD17 | 8 | Q8K3I8 | 1–19 | 20–65 | 35–63, 42–56, 46–64 |
| Mouse β-defensin 42 | mBD42 | 14 | Q8BVB5 | 1–21 | 22–75 | 33–60, 40–54, 44–61 |
| Pig β-defensin | ||||||
| Pig β-defensin-1 | pBD1 | 15 | O62697 | 1–20 | 24–64 | 31–60, 38–53, 43–61 |
| Pig β-defensin 2 | pBD2 | 15 | Q6R953 | 1–21 | 22–69 | 37–65, 44–59,49–66 |
| Pig β-defensin 128 | pBD128 | 17 | A0A287BN95 | 1–19 | 20–94 | 25–52, 32–46, 36–53 |
| Pig β-defensin 121 | pBD123 | 17 | A0A8E8LS78 | 1–19 | 20–60 | 22–49, 29–43, 33–50 |
| Pig β-defensin 110 | pBD110 | 7 | A0A287BBL9 | 1–19 | 20–67 | 35–63, 42–56, 46–64 |
| Bovine β-defensin | ||||||
| Bovine β-defensin 4 | BNBD4 | 27 | P46162 | 1–22 | 23–63 | 31–60, 38–53, 43–61 |
| Bovine β-defensin-5 | BNBD5 | 27 | P46163 | 1–22 | 23–64 | 31–60, 38–53, 43–61 |
| Bovine β-defensin 7 | BNBD7 | 27 | P46165 | 1–22 | 23–62 | 31–60, 38–53, 43–61 |
| Bovine β-defensin 10 | BNBD10 | 27 | P46168 | 1–22 | 23–62 | 31–60, 38–53, 43–61 |
| Bovine β-defensin 119 | BNBD119 | 13 | Q32P86 | 1–20 | 21–83 | 27–54, 34–48, 38–55 |
| Bovine β-defensin 127 | BNBD127 | 13 | A0A3Q1N9G9 | 1–22 | 23–54 | 23–55, 33–49, 37–56 |
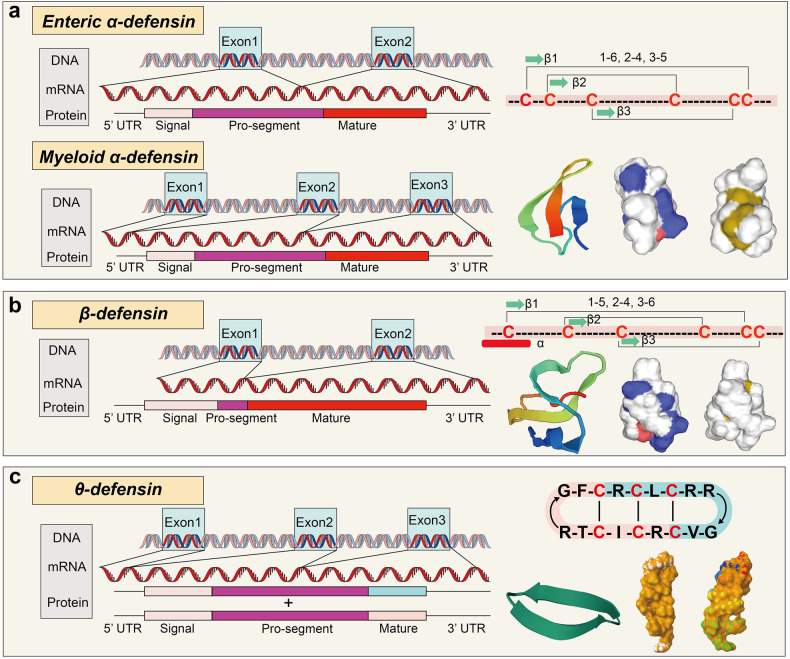
Fig. 2.
Structural characteristics of defensins from gene to mRNA to protein. The structure of defensin genes and peptides, including the alignment of the enteric and myeloid α-defensins (a, UniProt: P59665), β-defensin (b, UniProt: P60022), and θ-defensin (c, UniProt: P82271) genes are indicated, along with the number of exons and the coding of signal peptides, pro-segment and mature peptides, as well as the location and the disulfide pairing of cysteines and the helical wheel plots and three-dimensional structure
All defensins undergo a multi-step synthesis process, beginning with a pre-defensin that contains a signal segment, pro-segment and a mature peptide. Their processing varies depending on expression site and typically involves a fast cleavage of the signal peptide of 20 or so amino acids, generating a pro-defensin (Fig. 2). The pro-fragment is thought to promote pro-defensin charge balance, helping to reduce the toxicity of defensin toward eukaryotic cells.19 β-defensin has a shorter pro-segment than α-defensin. It may be due to differences in the transcription patterns (α-defensins are usually constitutively produced, while most β-defensin expression occurs in response to stimuli15), leading to different processing and intracellular transport requirements for the mature peptides to rapidly react to immune responses. It is worth noting that crystal structure analyses of defensins show that defensins exist as dimers or multimers.90–92 Lu et al. have preliminarily studied the importance of dimerization for the biological roles of defensins. They found that these polymers have stronger antibacterial and membrane destruction activity and can enhance binding to multiple molecular targets compared to monomers.91,93–95
Based on the difference in the coding exons, α-defensins are classified into myeloid and enteric α-defensins (Fig. 2a). HNP1-4 are four of the six known myeloid α-defensins and are expressed primarily in the granules of neutrophils96 and certain lymphocytes,97 as well as natural killer (NK) cells.98 Notably, mouse neutrophils lack defensins.99 HNP1-4 are stored in the azurophilic granules as fully processed mature peptides.34,100 Upon fusing with phagasomes, α-defensins-laden azurophil granules then release large amounts of HDPs in the proximity of the pathogen surface, where they quickly penetrate the cell membrane due to their amphipathic nature.64,101 The other two α-defensins, HD5 and HD6, are enteric α-defensins that are mainly expressed by Paneth cells (PCs).102–105 Unlike pro-HNP1-4 processing and vesicle storage, HD5 and HD6 are stored in secretory vesicles as a pro-peptide and are processed by one or more isoforms of Paneth cell trypsin.32 However, whether the pro-HD5 peptide is converted into its mature form during secretion or within the lumen is unclear. In addition to PCs, the reproductive tract and oral cavity also express HD5 and HD6. Interestingly, these two peptides are functionally different. The antibacterial activity of HD5 is to kill bacteria directly,106 while HD6 does so indirectly by forming self-assembled nanonets in order to trap bacteria and prevent infection.7,107–109 Although mouse neutrophils lack defensins, mouse PCs express more than 20 α-defensins throughout the mouse small intestine,110–112 which are also called cryptdins. Seventeen cryptdins (Cryptdin1-17) have been identified at the protein level.113,114 All the peptides have potent in vitro bactericidal activity,41 with S. aureus appearing to be more susceptible to cryptdin-mediated killing than E. coli.41 Mouse cryptdins are processed into their active form by matrix metalloproteinase 7 (MMP7) during granulogenesis.6,33 Indeed, mice lacking MMP7 cannot process the precursors of pro-cryptdin, leading to a deficiency of mature cryptdins, thus impairing their ability to scavenge infections and regulate immune homeostasis.6 In mouse and human PCs, mature α-defensin is oxidized to prevent internal digestion.115
Compared with α-defensins, the localization of the cysteine residues along the amino acid sequence of β-defensins (BDs), the folding pattern of the peptide chain and the disulfide bond pattern are entirely different (Fig. 2b). The peptide chains of BDs fold to form three β-lamellae with four conserved glycine, proline, threonine and lysine residues. The synthesis and secretion of BDs are also different from α-defensins. BDs are directly secreted into the extracellular space in their mature form to exert immunomodulatory and antibacterial activities.116 BDs mainly display stimulated expression, but constitutive expression patterns also exist. For example, the promoter of DEFB1 does not contain response elements for NF-κB and AP-1, so DEFB1 gene expression is not upregulated in response to inflammatory factors but is physiologically expressed in epithelial cells.117 However, the expression of most BDs is limited to specific tissues or epithelial cells where they perform a particular function. For example, the production of macaque BD126 is confined to the epididymal epithelium, where it is attached to membranes of sperm cells as they traverse through the epididymis. This exclusive function safeguards macaque sperm from being attacked by the immune system within the female reproductive tract.118
Presently, the progress in studying θ-defensins is relatively slow compared with α-defensins and β-defensins. However, from an evolutionary perspective, it is clear that θ-defensin genes arose from mutated α-defensin genes.30,64 θ-defensins are the only cyclic peptides in animals (Fig. 2c) and have been isolated from rhesus macaques and baboons. Rhesus θ-defensins (RTDs) are primarily synthesized in the bone marrow and secreted by neutrophils, PCs and monocytes.119 Intriguingly, θ-defensins are chimeras of 18 residues formed by spliced heads and tails from two separate precursors, each of which contains nine amino acids.30,120 In humans, the θ-defensin gene has an early termination codon that hinders efficient translation of the desired precursor,121,122 indicating that θ-defensins are not exist in the human body and were most likely phased out by natural selection.
Antimicrobial mechanisms of defensins
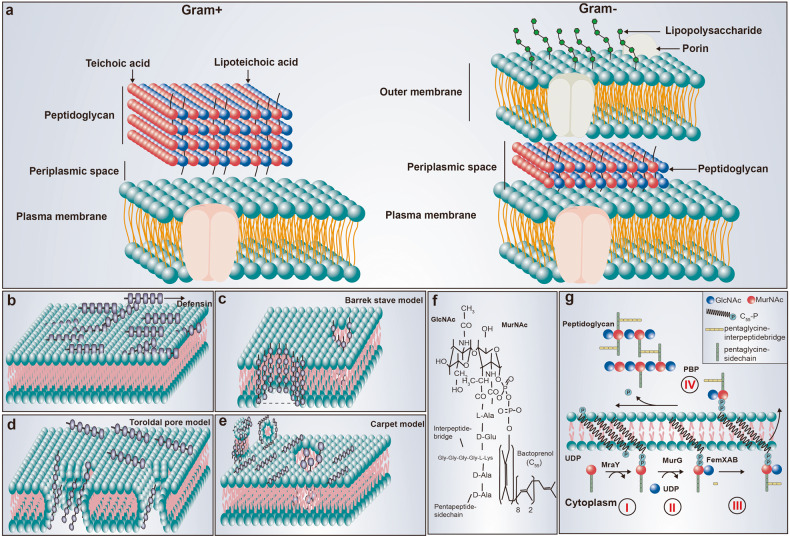
Defensins possess wide-ranging antibacterial activity against both Gram-negative (G−) and Gram-positive (G+) bacteria in vivo and in vitro.123–130 For example, the anti-Staphylococcal and anti-E. coli activity of hBD3 is 1 mg/L and 4 mg/L, respectively.131 However, the cell membrane structure of G− and G+ bacteria differ (Fig. 3a) as the cell membrane of G− bacteria has three layers that include an outer membrane, a peptidoglycan layer and a plasma membrane, whereas G+ bacteria have only a peptidoglycan layer and a plasma membrane. G− surfaces contain many lipopolysaccharides (LPS) with a negative charge.132 By interacting with negatively charged components on the surface of G− bacteria, defensins destroy membrane barrier function. With the accumulation of defensins on the membrane (Fig. 3b), the electrostatic attraction and penetration of defensins bound to the membrane are enhanced, and the defensins freely diffuse and preassemble on the membrane surface,133–135 followed by hydrophobic interactions between the amphipathic peptide domain and the membrane phospholipids.136,137 There are three primary models for defensin-mediated transmembrane pore formation, which are barrel-stave, toroidal pore, and carpet models.135,138–141 The first proposed mechanism for permeabilization was the barrel-stave model, which serves as a prototype for defensin-mediated transmembrane pore formation. Defensins serve as staves that insert themselves vertically into the phospholipid bilayer, yielding barrel-like structures (Fig. 3c), such as HD5 for G− bacteria.128,142–144 The toroidal pore model depicts the insertion of defensins into the membrane, causing a consistent curvature of the phospholipid monolayer from the upper portion to the lower portion (Fig. 3d). In the carpet model, peptide-induced membrane disruption is similar to that of a detergent-like action (Fig. 3e). For example, the cell membrane adsorbs hBD3 through strong electrostatic interaction of Arg12 with POPG lipids in G+ bacteria.145
Fig. 3.
Antimicrobial mechanisms of defensin. a The cell membrane structure of G− and G+ bacteria. b Defensins accumulate on the cell membrane before destroying it. c–e Illustrations of the various modes of defensins-mediated cell killing, including the barrel-stave model, the toroidal pore model and the carpet model. f The structure Lipid II; g Cell wall biosynthesis begins in the cytoplasm where UDP-MurNAc-pentapeptide is formed. This soluble precursor is then linked to the membrane carrier bactoprenolphosphate (C55P) by MraY, yielding Lipid I (reaction I). MurG subsequently adds GlcNAc to form Lipid II (reaction II). After the formation of the interpeptide bridge (as seen in reaction III), the monomeric peptidoglycan unit undergoes translocation across the cytoplasmic membrane for incorporation into the cell wall (reaction III). It is noteworthy that this interpeptide bridge formation is limited to some Gram-positive bacteria, as highlighted by research.38 Note: To better demonstrate the crosstalk mechanism of defensins in regulating immune homeostasis, the intestine containing PCs and mucosal structures was used as the background of the regulatory network
However, the membrane destruction model cannot fully explain the complete mechanism behind the defensin-mediated bacterial killing. Specifically, it is difficult for this model to account for how defensins can swiftly eradicate bacteria in the LPS-deficient outer membrane of G+ bacteria. Thus, it is likely that another mechanism exists for defensin-mediated bacterial killing. One possible alternative mechanism is that defensins disrupt cell wall synthesis (Fig. 3f, g) by targeting the membrane-anchored cell wall precursor, lipid II, which is crucial for the process.146–149 Plectasin, a fungal defensin secreted by Pseudoplectania nigrella, displays strong antibacterial activity against G+ bacteria, even against otherwise resistant clinical isolates.38 Tanja and colleagues found that plectasin does not cause any disruptions to membrane integrity as it had no influence on the typical features of the membrane penetration mechanism, such as membrane potential and intracellular K+ contents.38 Interestingly, plectasin treatment led to an accumulation of the cell wall precursor, UDP-MurNAc-pentapeptide.38 Plectasin effectively prevents the interaction between the lipid I and lipid II carriers and cell wall biosynthetic enzymes by bonding with them in a 1:1 molar ratio. The equilibrium-binding constants for lipid II and lipid I are 1.8 × 10−7 mol and 1.1 × 10−6 mol, respectively, indicating that the second sugar in lipid II, N-acetyl glucosamine (GlcNAc), plays a role in stabilizing this complex.38 In addition to plectasin, researchers have identified other defensins targeting lipid II, such as hBD3 and HNP1, Cg-Defh1-2 from crassostrea gigas, oryzeasin and eurocin from fungi, and lucifensin from maggots.146,147,150–153 For example, in S. aureus treated with hBD3, the UDP-MurNAc-pentapeptide, a cell wall precursor, was also found to accumulate,147 like in the case for plectasin. Further, hBD3 was shown to inhibit the activity of staphylococcal penicillin-binding protein 2 (PBD2) when the molar ratio of hBD3 to lipid II is 2:1. However, hBD3 treatment also resulted in a decreased membrane potential, and transcriptome data indicated that hBD3 treatment was partially like HDPs treatment exposed to membrane-active α-helices.147,150 Thus, hBD3 exhibits a pleiotropic antibacterial mechanism against S. aureus involving cell wall synthesis inhibition via targeting lipid II and effects on membrane permeabilization.
Recently, the Wehkamp lab found that in a reduced physiological environment, the disulfide bridges characteristic of defensins become disrupted, rendering them susceptible to protease degradation. This process liberates novel antimicrobial peptide fragments that enhance the antimicrobial repertoire and may thus be an evolutionary trait enabling the host to mount an effective broad-spectrum response towards invading pathogens with minimal resources. For example, duodenal fluid- and gastrointestinal-derived trypsin degrade full-length HD5, hBD1, and HNP4 into various bioactive fragments with different antibacterial properties. Other fragments showed different antibacterial activity. As an example, HNP41-11 exhibits superior antimicrobial potential in comparison to the intact peptide on mass and molar levels.154 Other fragments, including HD51-9, HD51-13, HD57-32, and HD5fl, substantially affected the growth of all tested bacterium, while others, like HD514-32 and HD510-27, were ineffective against the tested bacteria under the same experimental conditions.107 The minor differences in fragment sequences of HD51-9 and HD51-13 resulted in different antimicrobial activity.107 These results suggest that defensin fragmentation is a fine-tuning mechanism for host-microbe interactions.
The biological functions of defensins
As the number of studies of defensins increases, it has been found that these molecules act in numerous biological processes, including showing immunomodulatory and chemotactic activities, maintaining mucosal barrier function, balancing the gut microbiota and regulating organ development and cell death. Therefore, gradually defensins have been perceived to be innate immune factors. Here, we review the biological functions of defensins that have been discovered to date.
Immunomodulatory activity
Increasing evidence indicates that the direct bactericidal activity of defensins in regulating the antibacterial immune response is not the only essential role of defensins in regulating host immune homeostasis. Specifically, they also modulate both innate and adaptive immune responses as immune regulatory factors.1,14,101,155 Not surprisingly, dysregulation of defensins expression is associated with autoinflammatory and autoimmune diseases, including sepsis, irritable bowel syndrome (IBS), atherosclerosis, thrombosis, rheumatoid arthritis and type 1 diabetes.74,76,156–162 However, the involvement of defensins in immune regulation is very complicated, and their role goes far beyond simply acting as immunomodulators via a singular receptor or linear signaling within the immune system (Fig. 4). A case in point of the complex roles of defensins in the immune response is the protein–protein interaction network of hBD3. Notably, hBD3 interacts with no less than 46 proteins or receptors and 1779 genes show differential expression upon hBD3 stimulation of TLR4 agonist KDO2-lipid A-primed mouse macrophage cells.163 These varied responses suggest that defensins exert their effects mainly by interacting or trans-activating various extracellular and intracellular receptors.
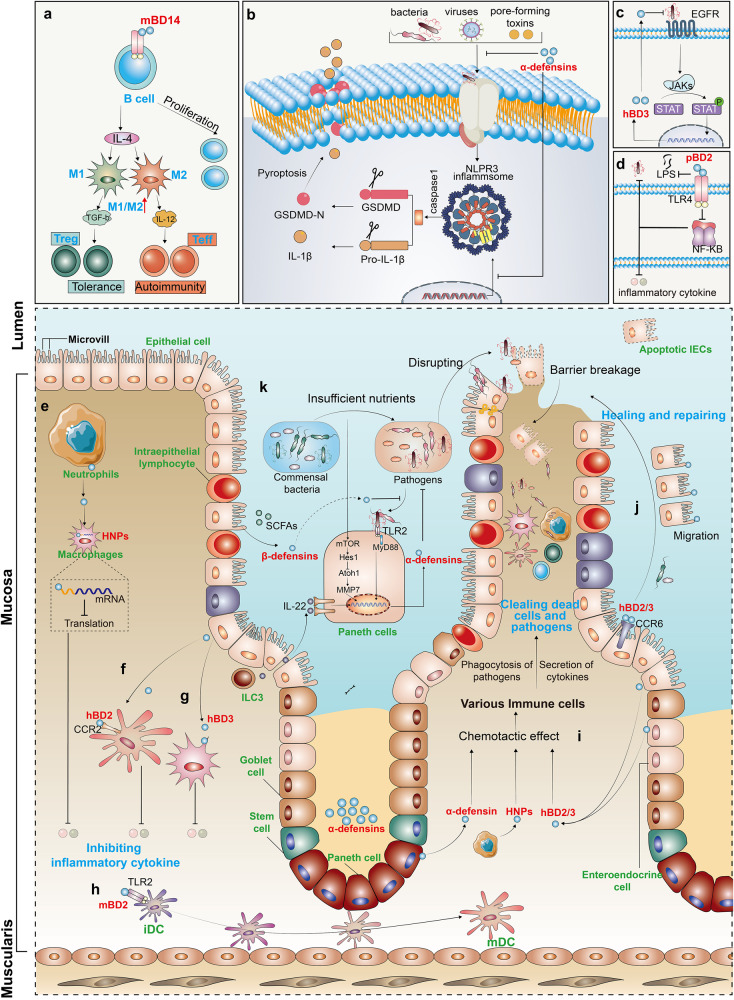
Fig. 4.
Regulation role of defensins in immune homeostasis. a mBD14 promotes B cell proliferation via TLR2 and improves the M1/M2 macrophage balance and induces regulatory T cells. b Mature α-defensins prevent NLRP3 inflammasome activation and the release of IL-1β. c hBD3 is activated by EGFR-mediated MAP kinase and JAK/STAT signaling pathways after H. pylori infection. d By competitively inhibiting the LPS-induced activation of the NF-κB via TLR4, pBD2 can effectively restrict downstream inflammatory cytokine secretion. e HNP1 released by neutrophils enters macrophages to bind to mRNA, and then inhibits mRNA translation of various inflammatory factors. f, g hBD2, hBD3, and HNPs inhibit the secretion of inflammatory cytokine; h mBD2 promotes the maturation of DCs via TLR4 signal. i Defensins recruit various immune cell to clear out dead cells and pathogens. j hBD2 and hBD3 regulates the repair of barrier function via the CCR6-Rho-ROCK signaling pathway. k In a nutrition-deficient state, the continuously activated α-defensins promote the resistance to invasion by enteric pathogens through an mTOR-Hes1-Atoh1-MMP7-α-defensins axis
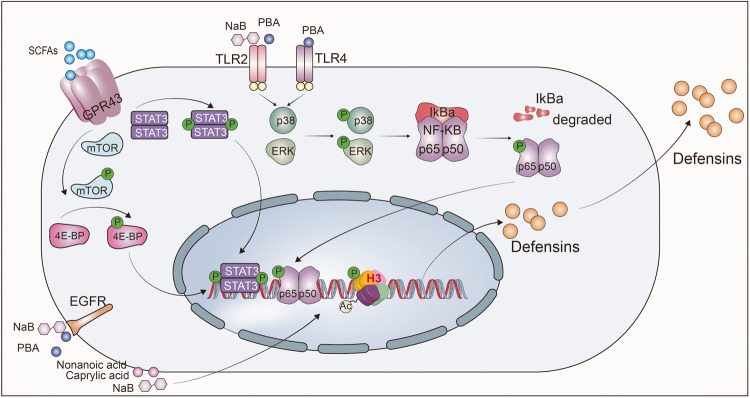
Regulation of autoimmunity is one of the main functions of defensins. Miani et al. found that endocrine cell-expressed mBD14 promotes B cell proliferation and increases their secretion of IL-4 by acting on TLR2 (ref. 159). Subsequently, IL-4 further improves the M1/M2 macrophage balance and induces regulatory T-cell responses to prevent autoimmune diabetes159 (Fig. 4a). In addition, mBD2 functions as an endogenous TLR4 ligand that acts upon immature dendritic cells (iDCs), resulting in the enhanced expression of costimulatory molecules and the maturation of DCs50 (Fig. 4h). These findings indicate that defensins can regulate acquired immune responses. Further, in the nutritionally-deficient state, the continuously activated α-defensins promote resistance to enteric pathogen invasion via an mTOR-Hes1-Atoh1-MMP7-α-defensins axis164 (Fig. 4k). In addition, defensins also regulate the expression of inflammatory factors. Koeninger et al.70 found that hBD2 improves disease activity indices and prevents colitis-associated weight loss in three mouse models (dextran sodium sulfate (DSS), 2,4,6-Trinitrobenzenesulfonic acid (TNBS) and T-cell transfer into immunodeficient recipient mice). Furthermore, they found that hBD2 engages with CCR2 on DCs to inhibit NF-κB activity and to promote CREB phosphorylation, thus reducing the expression of inflammatory factors (Fig. 4f). Our previous studies showed that pBD2, a porcine β-defensin, competitively inhibits LPS- and DSS-induced activation of NF-κB signaling via TLR4, thus dampening the secretion of inflammatory cytokines69,165 (Fig. 4d). Similarly, Zhang et al. and Lian et al. observed that pBD2 decreases the adherence of E. coli to cells and alleviates inflammation via the TAK1-NF-κB pathway.166–168 Semple and colleagues found that hBD3 is a strong inhibitor of TNF-α and IL-6 accumulation, two potent pro-inflammatory cytokines (Fig. 4g).169 Like β-defensins, HNPs are also released from dying neutrophils during apoptosis or necrosis and effectively suppress pro-inflammatory responses by interfering with the production of nitric oxide and inflammatory cytokines from macrophages.170 Neutrophils are the initial and most abundant cells to reach the area of inflammation-induced injury, where they release a large amount of the defensin HNP1 (ref. 101). In this study, it was shown that HNP1 acts as a "molecular brake" to limit macrophage-driven inflammation.101 Notably, neutrophil-derived HNP1 enters macrophages, where its positive charge and amphipathic characteristic help it bind to mRNA to inhibit the translation of various inflammatory factors rather than affecting mRNA transcription and stability (Fig. 4e). In addition, Shi et al. found that defensin-deficient (Mmp7−/−) mice produce more IL-1β in the colon and cecum and are more susceptible to DSS-induced colitis.171 Exogenous supplementation with mature α-defensins, rather than precursor α-defensins or β-defensins, inhibit IL-1β secretion following activation of inflammatory NLRP3 inflammasomes in human and mouse macrophages171,172 (Fig. 4b). The data indicate that α-defensins may have a significant role in maintaining gut homeostasis by modulating the expression of IL-1β. However, defensin-mediated regulation of TLR signaling does not necessarily exhibit an anti-inflammatory effect, as they can also potently amplify the immune cell response to bacterial DNA via a TLR-9-mediated pathway, while hBD2 and hBD3 can induce self-DNA condensation into particles that are endocytosed by plasmacytoid DCs, resulting in the activation of TLR-9-dependent IFN-α production.173,174 Neutrophil-secreted HNP1-3 can also boost bacterial phagocytosis by triggering macrophages to accelerate their expression of TNF and IFNγ.175
Moreover, epigenetics plays a regulating role in the production of defensins. For instance, after deacetylase inhibition, NF-κB is modified by the acetylase p300, which enhances the transcription of Defb2 in colonic primary epithelial cells while decreasing the potential of harmful inflammatory responses.176 Our previous study also found that after enterotoxigenic Escherichia coli infection, METTL3, an N6-adenosine-methyltransferase, interacts with the transcription factor FoxO6 and modulates Gpr161 transcription and subsequent regulation of β-defensin expression.177
The critical role of defensins in host defense via their immunomodulatory activity is also well-studied. Various bacteria, including Vibrio cholerae, Bacteroides fragilis, Pseudomonas aeruginosa, different Pseudomonas species and Salmonella enteritidis, regulate the production of hBD2 (refs. 178–183). Mechanistically, this regulation is related to the interaction between bacterial flagellum and TLR5 (refs. 184,185). Moreover, hBD2 is induced via Nod1-dependent activation of NF-κB after infection by Helicobacter pylori186,187 or P. aeruginosa.180 Similarly, infection with H. pylori upregulates the generation of hBD3 by the EGFR-dependent activation of MAP kinase and JAK/STAT pathway188 (Fig. 4c). Further, an exciting study revealed the existence of a signaling pathway essential for skin resistance to pathogen infection occurs through the interaction between the epithelium and neutrophils via defensins.60 Upon Staphylococcus aureus infection, defensins are released by keratinocytes and activate Mrgpra2 receptors on neutrophils, which results in IL-1β and CXCL2 release to promote infection resistance. Disruption of this signaling cascade can lead to immune deficiency and abscess formation.60
In addition to the pool of defensins secreted by neutrophils and epithelial cells as a response to infection, antigen exposure also triggers the release of defensins in NK cells and PCs.189,190 For example, HD6, released by PCs, blocks enteric bacterial pathogen invasion by ordered self-assembly of microbe-entangling peptide nanonets.7 Mmp7 knockout mice have considerably diminished clearance of E. coli6 and Chlamydia trachomatis191 in the intestine compared to parental wild-type mice. Moreover, the production of cryptdin family types and levels is higher in conventionally-raised mice than in germ-free mice.192 Mechanistically, the NOD2 signaling pathway is essential for PCs to secrete defensins. After bacterial infection, NOD2 recognizes muramyl dipeptide (MDP) and then activates NF-κB, thus upregulating the transcription of defensins.193–195 NOD2-mediated defensin regulation is beneficial in protecting against Haemophilus influenzae-induced otitis media.196 In addition, Nod2-deficient mice show different intestinal microbiota compositions from wild-type mice and increased susceptibility to infection upon challenge with Listeria monocytogenes.197 These studies demonstrate that NOD2-induced secretion of α-defensin plays a vital role in regulating the composition of intestinal microbiota and defending against pathogen invasion.
The COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) has drastically affected both public health and the economy. As of November 2022, over 700 million cases and 6.6 million deaths have been reported globally.198–200 With respect to anti-SARS-CoV-2 infection, defensins have also been considered as potential therapeutic molecules.201–204 For example, HD5 inhibits SARS-CoV-2 S1 binding and thereby prevents pseudovirions entry into enterocytes by competitively binding with angiotensin-converting enzyme 2 (ACE2).205 Similar effects were also found for HNP1 and hBD2, but not for hBD5 and hBD6 (refs. 206–208). For example, by molecular dynamics simulations and by functional studies, it was found that hBD2 interacts with the CoV-2-receptor binding domain (RBD) and obstructs viral entrance of ACE2-expressing cells.207 In contrast, HNP1 inhibits viral fusion but does not affect the binding of the spike RBD to ACE2 (refs. 206,209). In silico approaches also suggested that defensins can physically bind spike surface viral protein (Sgp), thus preventing its interaction with ACE2 (refs. 203,210). Therefore, this evidence suggests that defensins could target ACE2, Sgp or disrupt the viral membrane. Meanwhile, maternal transmission of defensins can protect fetuses from SARS-CoV-2 infection.211 Together, these findings provide substantial evidence that defensins are crucial in safeguarding individuals against various bacterial and viral infections.
In summary, defensins are essential for immune regulation, but many questions remain, including how do defensins, usually considered innate immune factors, activate so many immune pathways? Is the pathway of defensin activation due to an inherent characteristic of the peptides or in response to different immune activation modes? Are they an effector, sensor or activator in immune regulation? Moreover, what are their means of action? Elucidation of these questions is essential if defensins are to become actionable clinical therapeutic targets.
Chemotactic activity
Chemotactic activity is a vital factor in driving the coordinated migration of immune cells in and out of tissues, as well as dictating their spatial organization and interaction in tissues212 (Fig. 4i). Several studies have reported that α-defensins, as well as β-defensins, like chemokines, play essential roles in immune cell activation and recruitment.54,213 Moreover, the concentration of chemotactic defensins is lower than that of bactericidal defensins.214 The earliest clues to the chemotaxis of defensins were the findings that HNP1 and HNP2 induce migration of human monocytes215 and T cells.216 Subsequent studies revealed that HNP1 selectively chemo-attract naive T cells and iDCs.55 The pre-treatment of pertussis toxin could depress the chemotactic activity stimulated by most defensins, suggesting that this activity depends on Gi-protein-coupled receptors (GPCRs)20 (Table 2). It has been reported that hBD2, hBD3, mBD2, mBD3, and mBD29 have chemotactic activity on T cells and iDCs via interacting with the chemokine receptor CCR6 (refs. 54,217–219). Interestingly, hBD2 and hBD3 also utilize CCR2 to regulate monocyte and macrophage trafficking.220,221 This suggests that some defensins use more than one GPCR to induce cell migration. Moreover, Rohrl et al. also demonstrated that mBD4 and mBD14 interact with CCR2 in monocytes, macrophages, and neutrophils.221 A similar phenomenon in β-defensin-1 has also been found in fish.222
Table 2.
Target cells and receptors of defensins-induced cell migration
| Family | Defensins | Target cell | Receptor | Reference | |
|---|---|---|---|---|---|
| GPCR | Non-GPCR | ||||
| α | HNP1 | Mo/Mφ, naive T, memory T, iDC, MC | n.d. | n.d. | 55,215,216,482 |
| α | HNP2 | Mo/Mφ, T cell | n.d. | n.d. | 215,216 |
| α | HNP3 | Mo/Mφ, memory T, MC | n.d. | n.d. | 482 |
| α | HD5 | Mo/Mφ, naive T, memory T, MC | n.d. | n.d. | 482 |
| β | hBD2 | Ep, memory T, iDC, MC, Mo/Mφ | CCR6, CCR2 | EGFR | 54,221,483–485 |
| β | hBD3 | Ep, Mo/Mφ | n.d. | EGFR | 220,221,484 |
| β | hBD4 | Ep | n.d. | EGFR | 483 |
| β | BEBD, BNBD3/9 | iDC | n.d. | n.d. | 486 |
| β | mBD2, mBD3, mBD29 | iDC | CCR6 | n.d. | 217,218 |
| β | mBD4 | Mo/Mφ, DC | CCR6, CCR2 | n.d. | 221,487 |
| β | mBD14 | Mo/Mφ, HEK293 | CCR6, CCR2 | n.d. | 221,488 |
| β | maBD1 | Mo/Mφ | n.d. | n.d. | 222 |
CCR CC chemokine receptor, EC endothelial cell, Ep epithelial cell, Mφ macrophage, Mo monocyte, n.d. not determined, BEBD bovine enteric β-defensin, BNDB bovine neutrophil β-defensin
Chemotaxis of defensins facilitates the flow of inflammatory effector cells and effector molecules to the site of infection, enabling the body to kill pathogenic microorganisms more effectively while providing a bridge between natural and acquired immune responses.223 However, the mechanism of β-defensin’s chemotactic action is better understood than the chemotactic properties of α-defensins, as currently, the receptors responsible for mediating the chemotactic effects of human α-defensins have not been characterized.
Maintaining the mucosal barrier
The mucosal barrier is the initial line of defense. Thus, rapidly promoting the repair and reconstruction of mucosal damage is especially important for organisms to maintain homeostasis. The breakdown of barrier function leads to Crohn’s disease (CD) and atopic dermatitis (AD).224–228 In the past, for both ileal and colonic CD, the absence of defensins was thought to be only associated with a general reduction in mucosal antibacterial activity.224,229 However, presently, studies have found that defensins can repair barrier damage by promoting epithelial cell proliferation. Moreover, they also actively participate in controlling the expression of barrier-specific proteins to maintain barrier function.224,226,229,230 For instance, in the cuticle barrier of the skin, hBD1 and hBD3 through CCR6-aPKC-Rac1 and CCR6-GSK3-PI3K signaling increased the expression and cell membrane positioning of barrier proteins. This leads to elevated trans-epithelial electrical resistance and reduced permeability in keratinocyte layers.226,231 In the intestine, hBD3-induction not only promotes intestinal epithelial cells (IECs) migration and preserves the intestinal barrier through CCR6-Rho-ROCK (Fig. 4j) but also inhibits autophagy through the CXCR4 signaling pathway, which significantly promotes IECs migration and maintains mucosal integrity.232–234 In addition, hBD2 can stimulate migration, proliferation, and tube formation in colonic epithelial and endothelial cells, thereby accelerating the closure of wounds.235–238 Mechanistically, Koeninger et al.70 found that hBD2 engages with CCR2 on DCs, which leads to a reduction in NF-κB and an increase in CREB phosphorylation, ultimately reducing inflammation. Of note, hBD2 has been employed as an indicator of disease severity and skin barrier characteristics in human allergic dermatitis and tinea corporis diseases.239,240 These findings indicate that the function of β-defensins in promoting the mucosal barrier primarily depends on activating the chemokine receptor family.
Like β-defensins, α-defensins also play an essential function in maintaining the mucosal barrier. In a mouse model, an increase in heat stress results in the upregulation of cryptdin2 expression. In addition, the severity of the heat stress-induced injury to intestinal barrier function positively correlates with the levels of cryptdin2 in both serum and the intestine.241 In humans with liver cirrhosis, compromised HD5 and HD6 function inhibits the function of T cells. Subsequently, immune cell deficiency perpetuates the vicious cycle of inflammation, causing elevated intestinal permeability as well as bacterial translocation.242 In patients with CD, TCF1-, and TCF4-mediated regulation of Wnt signaling-driven HD6 secretion by PCs is disrupted, which damages the repair of the mucosal barrier.104,243,244
Surprisingly, defensins can also be negatively regulated by the mucosal barrier. The epidermal growth factors (EGFs), essential for wound repair, can induce the expression of hBD3 after epidermal cell wounding.245 TGF-α, a member of the EGFs, participates in the repair process after mucosal damage.246 When the mucosa is injured, the expression of TGF-α increases rapidly. TGF-α can promote the proliferation of PCs and crypt cells in vivo, which secrete many defensins that maintain immune homeostasis as indicated by the repair of intestinal mucosa and wound healing.246,247 A defective MUC2 mucin barrier, typical in IBD, leads to deficient stimulation of hBD2 and barrier repair.248
Although recent studies and their conclusions, without exception, describe HD5 as a critical molecule in the human gut that fights off microbes and inhibits damage, a recent study provided the opposite conclusion. It showed that HD5 promotes the adhesion of Shigella to destroy the epithelial barrier function by targeting bacterial membrane proteins and that this process depends on the native tertiary structure and the critical residue of Arg28 of HD5 (ref. 249). This finding fundamentally challenges the understanding of the role of defensins as “protectors”, which may be due to the unique properties of HD5 and Shigella, or that Shigella has possibly evolved to highjack this function of HD5.
Balancing the gut microbiota
It is known that the gut microbiota is a highly complex ecosystem that performs crucial physiological functions, including maintaining intestinal barrier integrity, promoting immunological fitness, and maintaining metabolic homeostasis, and that it dynamically responds to intrinsic and extrinsic stimuli. The microbiota community in humans comprises ~1000 species, involving up to 1015 procaryotic cells, with a weight of 1 kg and a ratio to eukaryotic cells that is approximately 1:1 (refs. 250,251). In recent years, it has been found that HDPs, especially defensins, are crucial for intestinal homeostasis and recovery of intestinal microbiota.252–254 For example, PCs directly sense the presence of gut commensals, and they preserve homeostasis of the intestinal-microbial interface by secreting several members of the α-defensin family.255 A new study has provided novel insight into how gut bacteria interact with defensins to prevent non-obesity diabetes (NOD).159 The pancreatic endocrine cells of NOD mice showed almost no expression of mBD14, and treatment with mBD14 significantly reduced autoimmune responses and the incidence of diabetes from 85% to 35% in NOD mice.159 Compared with naive NOD mice, the production of mBD14 was significantly upregulated in NOD mice receiving gut microbiota from normal mice.159 Further studies showed that the aromatic hydrocarbon receptor ligand and butyric acid, products of the gut microbiota, can facilitate the secretion of IL-23 and IL-22 through innate lymphoid cells (ILCs) of the pancreas, and the latter triggers the transcription and secretion of mBD14 in pancreatic endocrine cells.159
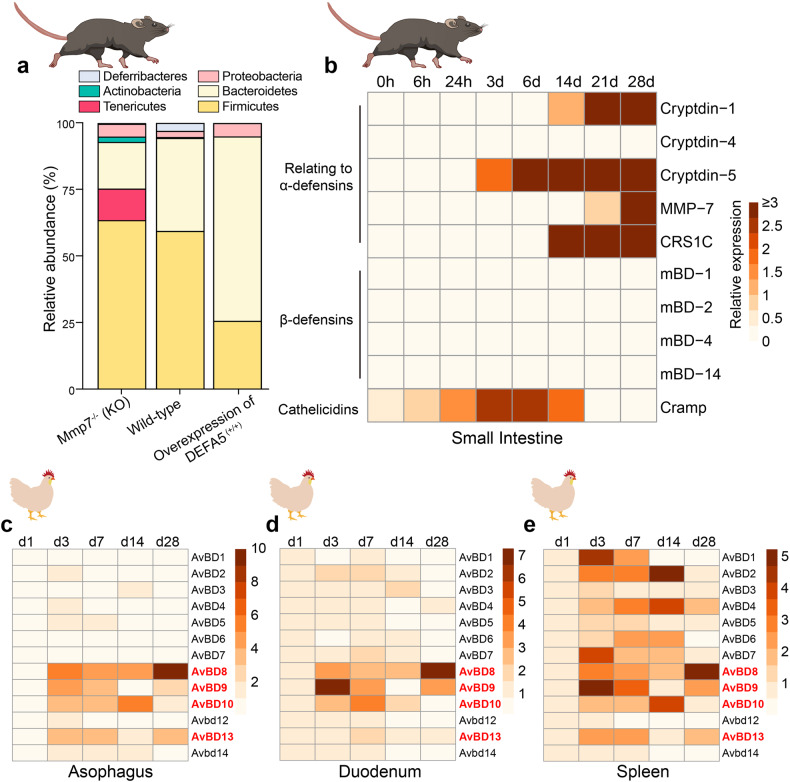
Germ-free and gene-deficient animals are essential tools for studying the function of gene coding and the interaction between organisms and microorganisms.256 For example, by gene editing, Salzman et al. constructed Mmp7 and HD5 transgenic mice. Their research revealed that Mmp7 and HD5 does not affect the total bacterial numbers; however, there is a reduction in the population of Firmicutes and a corresponding enhancement in Bacteroidetes in HD5+/+ mice compared with wild-type mice. In the Mmp7−/− mice, they found an opposite change. We re-analyzed their 16 S ribosomal RNA sequencing data and found that the abundance of Firmicutes increases with the loss of active defensins, whereas the number of Bacteroidetes decreases proportionally, and other mechanisms are responsible for maintaining bacterial numbers257 (Fig. 5a). Further, they found that defensin deficiency significantly increased segmented filamentous bacteria (SFB) colonization in Mmp7 knockout mice. The mice overexpressing HD5 exhibited opposite results, which are associated with the level of lamina propria Th17 cells.257 This provides evidence that defensins can activate acquired immune responses via controlling the intestinal microbiota.
Fig. 5.
Regulation role of defensins in gut microbiota and intestinal development. a Intestinal microbiota composition in HD5 and Mmp7 transgenic mice. b–e Defensins gene expression maps, including for the small intestine of mice during 0–28d after birth, the esophagus of chicken during 1–28d, the duodenum of chicken during 1–28d and the spleen of chicken from 1 to 28d
Not only has the ability of defensins to regulate the composition and metabolic function of intestinal microbiota been directly demonstrated through gene deletion in mice, it has also been found to be true in a human clinical correlation study and a mouse defensin feeding experiment. For example, lower HD5 secretion in older adults compared with middle-aged people is linked to age-related differences in gut microbiota composition.258 Specifically, the study identified a negative correlation between the fecal concentration of HD5 and Alistipes and Christensenellaceae R-7. Previous studies have shown that Alistipes has pathogenic effects on colorectal cancer and is associated with symptoms of depression.259 Furthermore, Christensenellaceae R-7 has a negative correlation with body mass index in various populations and its presence increases with age.260 These findings suggest that low HD5 may contribute to age-related differences in gut microbiota and increase the risk of disease in older adults. In addition, rebamipide, a drug used to protect the gastrointestinal mucosa, has been found to have the ability to regulate the small intestinal microbiota. Specifically, it can upregulate α-defensin-5 in the small intestine while simultaneously downregulating the presence of Bacteroides and Clostridium, while upregulating Lactobacillus, thereby inhibiting indomethacin-induced small intestinal injury.261
Dysbiosis, which refers to the imbalance of the intestinal microbiota composition, is associated with psychological stress and has been known to trigger or worsen symptoms of depression. Psychological stress-induced reductions in α-defensin-5 levels result in microbiota dysbiosis in mice with depression, and α-defensin-5 supplementation attenuates the unbalanced gut microbiota and metabolites.262 Fecal α-defensin-5 concentrations have been significantly correlated with gut microbiota composition, including being positively correlated with the beneficial bacteria Ruminococcaceae, Allobaculum, Sutterella, and Akkermansia, but they have been negatively correlated with the harmful bacteria Erysipelotrichaceae.262 In addition, a recent study reported that hBD2 ameliorates acute graft–versus–host disease (aGvHD) through regulating the gut microbiome to limit ileal neutrophil infiltration and restrain T-cell receptor signaling.263 Tamima and colleagues found that induction of hBD2 is impaired in cases of aGVHD in both humans and mice. However, when hBD2 was administered, the severity and mortality of aGVHD were reduced. This can be traced back to hBD2’s effect on the intestinal microbiome, specifically an increase in multiple Bacteroides species and a reduction in Ruminococcaceae. These changes coincide with a reduction in neutrophil recruitment into the ileum of aGVHD mice. Interestingly, studies have demonstrated that an increase in Bacteroides is linked with lower GVHD severity in mice.264 It is essential to acknowledge that the decreased neutrophil infiltration in the ileum that results from hBD2 treatment was reversed when antibiotics were given to the mice. Thus, the data suggest that hBD2’s effects on intestinal neutrophil infiltration are dependent on intact microbes. In conclusion, hBD2 not only alters the composition of specific intestinal microbiomes, but it is also a critical factor in treating GVHD.
In summary, these studies indicated that PCs and epithelial cells in the intestinal mucosa establish a host immune barrier by secreting defensins, thereby improving the host’s ability to maintain a commensal relationship with microorganisms while allowing an appropriate response to changes in the gut microbial population throughout the host’s life cycle.
Regulation of intestinal development
The intestinal tract is a key site for nutrient digestion and absorption and has gradually been regarded as the largest immune organ. Healthy intestinal development is principally related to the normal functions of all organs and tissues of the body. Internal factors that affect intestinal development include hormone homeostasis, nutrient metabolism, growth factors, and immune effectors. In recent years, it has been found that HDPs, especially defensins, are also closely related to intestinal development. Although existing research suggests that fetuses are in a non-sterile environment, all mammals have far fewer gut microbial communities before birth than after, and the gut microbiome immediately changes after birth as breast milk and other nutrients are ingested.256,265 Collado et al. found that the microbial content of breast milk remains stable over time.266 Likewise, the intestinal microbiome gradually stabilizes,267 and by adulthood it is composed of an established climax community that is chiefly marked by obligate anaerobes.268,269 The expression of HNP1-3 increases with age in one- to three-year-old children, in parallel with their growing microbiome colonization.270 The gut microbiota stimulates ILCs to secrete mBD14 to prevent underdevelopment diseases.159 In intestinal development, PCs physically appear 2 weeks after birth, whose formation and maturation depend on Wnt signals.271 Van et al. found that TCF4 deficiency inhibits PC maturation and epithelial cell proliferation in mice, thus inhibiting the expression of cryptdin1 and cryptdin6 (ref. 271). In addition, an exciting study investigated the dynamic pattern of HDPs during the earliest stages of small intestine development.272 Here, we show the dynamic expression map of HDPs by analyzing their data (Fig. 5b). The data shows that the expression of defensins and related genes could not be detected in newborn mice, whereas they continuously express cathelicidins. By 21 days after birth, the IECs no longer express cathelicidins. The reduced expression of cathelicidins first occurs in the crypts and lower villi and reaches the tip of the villi some days later. In contrast, the expression of α-defensin and its related genes begin at 14 days after birth, which is associated with PC maturation.272 Menard et al. found that global knockout of Cramp, which encodes for cathelicidin, promoted the maturation of PCs, as well as IEC proliferation, in mice.272 These results suggest that cathelicidins maintain intestinal health at the earliest stage of development, while the function of defensins in maintaining intestinal health starts from day 14 after birth. Moreover, when cathelicidins expression is inhibited, PCs mature several days ahead of time, which initiates the expression and secretion of defensins, thus maintaining intestinal development. The developmental expression of HD5 and HD6, human defensin family members, has also been confirmed, where their mRNA levels tend to be lower during fetal life as compared to newborns and adults.273 Notably, it has been demonstrated that the expression of intestinal defensin mRNA during the second trimester of pregnancy is substantially lower, ranging from a 40 to 250-fold difference compared to the levels detected in the adult gut.274 Although BDs (Ct > 30) are less expressed in the intestine than α-defensins, in chicken it has been shown that during the first month of development, the spleen is the predominant site of BDs expression.275 By re-analyzing their data, we demonstrated the dynamic expression of β-defensins (avian β-defensin, AvBD) in different chicken organs (Fig. 5c–e). The results showed that most β-defensins were still low in expression in the esophagus and duodenum, while most β-defensins were highly expressed in the spleen.275 At the same time, AvBD8, AvBD9 and AvBD10 showed similar high expression in the three tissues, indicating that these three β-defensins may serve as critical regulators of tissues and organ development in chicken.
These data suggest that newborn intestinal epithelium lacks complete enteric defensins, and development regulates their expression. However, current research has underestimated the importance of HDPs, especially defensins, in intestinal development. Unfortunately, the precise mechanism behind this phenomenon is still unclear, though it may be linked to either the maturation of PCs or the intestinal microbiota. Whether the HDPs are directly related to the homeostasis and/or the development of the gastrointestinal tract, and is part of the inherent mechanism of such, remains to be further studied.
Regulation of cell death
Unlike bacterial cell membranes, eukaryotic cell membranes are rich in amphiphilic molecules. Negatively charged phospholipids are predominantly present on the cytoplasmic side, while amphiphilic phospholipids are predominantly distributed on the extracellular (or organelle) side. This results in a neutral charge of the overall eukaryotic cell membranes.276–278 Mostly, defensins are not cytotoxic to most eukaryotic cells. Even so, in some situations, recent evidence has shown that defensins are involved in several cell death pathways, such as apoptosis, pyroptosis and necrosis. For example, high-concentration HNP1 enters human bronchial and alveolar epithelial cells, where they quickly translocate to the endoplasmic reticulum and activate caspase-3, the main executioner of apoptosis.279 HNP1 also promotes alcohol-induced hepatic fibrosis and hepatocyte apoptosis.280 However, HNP1 can inhibit the apoptosis of neutrophil cells through P2Y6-mediated Bcl-xL and caspase-3 and decrease mitochondrial membrane potential.281 In addition, it has been discovered that a high concentration of HD5 induce apoptosis in IECs and primary CD4+ T cells,282 whereas hBD3 can trigger apoptosis and the production of IL-8 in airway smooth muscle cells.283 Antigen-presenting cells (APCs), including DCs, monocytes and macrophages, are critical in initiating, modulating and resolving inflammation due to their ability to sense, process and present antigens.284,285 HD5 and mBD2, respectively, interact with tumor necrosis factor receptors (TNFR1 and TNFR2) outside the cell membrane and are subsequently translocated to mitochondria, targeting the mitochondrial membrane to induce apoptosis of macrophages and DCs.286,287 In addition, hBD1 inhibits apoptosis in DCs through CCR6 and promotes the monocyte differentiation to iDCs and the final maturation of DCs stimulated by LPS.288 These findings indicate that defensins have an important immunoregulatory function in controlling the natural process of elimination and maturation of APCs. Defensins have been shown to induce the death of tumor cells.289–296 Ninety percent of renal clear cell carcinomas and eighty-two percent of prostate cancers, specifically, lose expression of hBD1 (ref. 297). However, the synthesized hBD1 inhibits the proliferation of the bladder cancer cell. In addition, the activation of caspase-3 and consequent cell apoptosis is observed in SW156 kidney cancer cell line when DEFB1 gene is overexpressed.298 Jurkat T cells and A549 cells undergo cell death when exposed to HNP1-3, which triggers caspase-3 and caspase-7 activation and ADP-ribose polymerase cleavage in Jurkat cells.295 These studies suggest that defensin-induced or -regulated apoptosis may vary depending on the cell type, immune status and defensin concentration. However, the effect of defensin-promoted apoptosis on the host’s innate or adaptive immune response remains unclear.
Beyond their implications in apoptosis, defensins are also involved in pyroptosis and necroptosis. Using HNP1 and HNP3 transgenic mice with neutrophil-specific expression of the defensins, Chen et al. observed that increased gene copy number of HNP1/HNP3 promotes pyroptosis in an NLRP3-dependent manner mediated by P2X7 (refs. 74,299). Wang et al. used an LPS-primed macrophage model to demonstrate that hBD2 enhanced IL-1β secretion and pyroptosis, and this is mediated by P2X7-dependent expression of NLRP3.300 Ethidium bromide uptake test results, on the other hand, indicated that HNP1-induced P2X7-K+ efflux-caspase-1 signaling contributes to pyroptotic pore formation. This suggests that in macrophages HNP1 promotes pyroptosis and IL-1β secretion by acting on various functions of the NLRP3 inflammasome downstream of P2X7 (ref. 299). Moreover, studies utilizing double-stranded RNA-induced ablation models have suggested that the ADAM10-Notch signaling pathway strengthens skin innate immunity via enhancing mBD6 expression downstream of type I interferon responses, thereby investigating the relationship between the endopeptidase ADAM10 and pyroptosis of hair follicles.301
With respect to necroptosis, research has shown that in atrazine-induced programmed necrosis, as well as immune dysfunction of grass carp hepatocytes, there is a downregulation of β-defensin.302 Notably, reduced α-defensin expression and necroptosis of PCs are both associated with ileal CD.303,304 This indicates that α-defensin, or perhaps other defensins in the ileum, potentially play a crucial role in the disease through a mechanism related to necroptosis.
In addition, increasingly novel types of regulated or programmed cell death, such as ferroptosis,305,306 cuproptosis,307,308 parthanatos309–311 and lysosome-dependent cell death (LCD),312,313 have been discovered. Each of these exhibits distinct molecular cascades and regulatory pathways. However, solid evidence for the specific role of defensins mediating these forms of programmed cell death requires further investigation.
Clinical relevance and therapeutic potential of defensins
The function of defensins in immune regulation has been discussed above. Therefore, it is of great research value for biomedical investigators to use defensins and their derived peptides as a basis to develop and test new therapeutics to treat both infectious and autoimmune diseases. As a starting point for this goal, basic research into defensins over recent decades have resulted in the identification of 348 defensins from animals, plants, and microorganisms, which together provide a sturdy groundwork for further translation of the field. Some of the advances based on the role of defensins in disease pathology (Table 3 and Fig. 6a) and the formulation of therapeutic strategies for defensins or their derived peptides designed based on defensins (DPDs) are summarized below.
Table 3.
Various human diseases associated with defensins
| Disease | Defensins | Level | Defensins mechanism in disease | Reference |
|---|---|---|---|---|
| Periodontitis | hBD1-2 | Low | Low levels of hBD1-2 are associated with periodontitis. | 489–491 |
| Periodontitis | HNP1-3 | High | The pathogenesis of severe periodontitis may be aided by a local deficiency in HNP1-3. | 385,386 |
| Periodontitis | hBD3 | High | By suppressing inflammatory responses in macrophages, hBD3 exhibits the potential to hinder the progress of periodontitis. | 383,387 |
| IBD | HD5 | Low | Low levels of HD5 disrupt the balance of intestinal microbiota, causing the overgrowth of bacteria and the invasion of potentially pathogenic bacteria into the epithelium. This leads to abnormalities in the intestinal tract’s function. | 303,341,492,493 |
| IBD | hBD3 | High | On the one hand, hBD3 may resist microbial attack on the surface of the intestinal cavity. On the other hand, hBD3 enters the lamina propria and performs chemotaxis to recruit immune cells. | 326 |
| IBD | hBD2 | High | hBD2 reduced inflammation and improved disease activity indices, which may have been due to its impact on the activation of the NF-κB pathway. | 237,324–330 |
| IBD | HNP1-3 | High | HNP1-3 may be a risk gene for severe UC, and its high expression in UC patients may induce an over-immune response, but a low dose of HNP1 can relieve colitis. | 78,340,356–358 |
| T1MD | HNP1-3 | High | By activating a c-Src-dependent signaling pathway, HNP1 can reduce hepatic gluconeogenesis and lower blood glucose levels. | 71,160,364 |
| Obesity | HD5 | Low | HD5 enhances glucoregulation and ameliorates the lipid profiles in both the plasma and the liver. | 72,366 |
| COPD | hBD1-2 | High | hBD2 levels are positively correlated with severity of COPD and IL-8 levels. | 372,374,375 |
| Vitiligo | hBD1 | Low | hBD1, along with its gene polymorphisms, could potentially influence an individual’s vulnerability to vitiligo, as well as the level of disease activity. | 494 |
| Infertility | hBD1 | Low | Male infertility caused by leukocytospermia and asthenozoospermia is associated with hBD1 deficiency. hBD1 is capable of elevating sperm motility and egg-penetrating ability via trigging a CCR6-dependent Ca2+ mobilization. | 495,496 |
| Acne | hDB1-2 | High | While defensins are effective against Propionibacterium acnes, their accumulation may lead to the formation of lesions in the epithelial tissue. | 497,498 |
| Psoriasis | hBD2 | High | After exposure to hBD2, Th17 cells were recruited to promote the development of skin pathology in psoriasis patients. | 158,499 |
| Atopic dermatitis | hBD1-3 | Low | Th2 cytokine environment can inhibit the expression of hBD1-3 in AD, which may contribute to increased susceptibility to skin infections and exacerbate the symptoms. | 226,500 |
| Allergic rhinitis | hBD1-3 | Low | Th2 cytokine environment can inhibit the expression of hBD1-3 in AR. The decreased levels of hBD1-3 may increase their susceptibility to respiratory tract infections and exacerbations in the tonsils of these patients. | 501–503 |
Fig. 6.
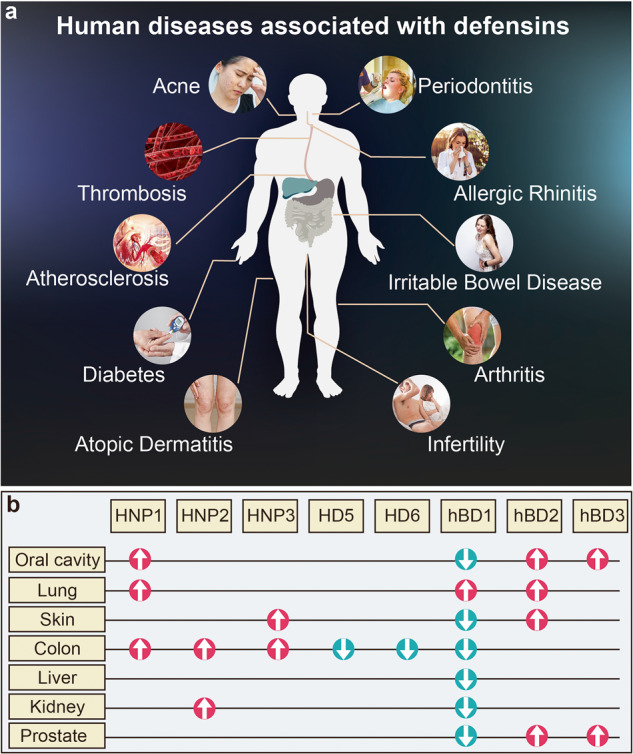
Defensins in disease. a Human diseases directly or indirectly associated with defensins. b HNP1-3, HD5, HD6, and hBD1-3 are either increased (red arrow) or decreased (aqua arrow) in cancers from different anatomical locations within the human body
Clinical relevance and preclinical studies of defensins
Infectious disease and defensins
Although significant progress has been made in understanding the disease-causing nature of pathogens and developing treatments to fight infection, infectious diseases remain a leading cause of death around the world.314 In fact, in 2019 alone, they were responsible for over 13.7 million fatalities.315 Despite advances in medicine, our current antimicrobials have become less effective over the past few decades due to the increasing prevalence of drug resistance, as exemplified by multidrug-resistant tuberculosis.314,316 Notably, the immunomodulatory activity of defensins in clearing pathogenic infections is extensive and challenging for microorganisms to develop resistance to.
Numerous studies have highlighted the therapeutic potential of defensins as a form of treatment for various types of infections. One such example is the prevention of mycobacterium tuberculosis in mice through the subcutaneous injection of HNP1. Moreover, in vitro mechanistic experiments further demonstrated beneficial outcomes to verify using HNP1 as an anti-infective agent for tuberculosis.317 Exogenous supplementation of recombinant hBD1 or hBD2 effectively controlled Salmonella infection. Nearly 50% of infected mice that were inoculated with recombinant hBD1 or hBD2 were still alive 206 h post-inoculation compared to complete lethality within just 24 h for control mice, while in the liver and spleen, the abundance of live Salmonella was remarkably reduced in the treated mice.318,319 Deficiency of mBD2, an analog of hBD2, in a mouse model of local P. aeruginosa-mediated corneal infection showed a worse outcome than control mice, indicating that mBD2 promotes resistance to P. aeruginosa-induced keratitis.79 Likewise, synthetic nine-mer peptides, specifically ALYLAIRRR and ALYLAIRKR, developed based on the active fragment of insect defensins, have been observed to provide protection in mice infected with lethal Methicillin-resistant S. aureus (MRSA).320
Similarly, administering exogenous defensins has also achieved beneficial effects against viral pathogens. For example, HNP4 and HD6 can block herpes simplex virus (HSV) infection.321 In addition, studies have shown that recombinant mBD2, when given before or after exposure to human influenza A virus (IAV), can protect experimental mice from a lethal virus challenge by 70% and 30%, respectively.322 pBD2 inhibits the proliferation of pseudorabies virus in transgenic mice.75 It is worth noting that Zhou Rui’s laboratory constructed the first pBD2 transgenic pig and explored the role and mechanism of pBD2 transgenic pig in swine influenza virus (SIV) infection. Studies have shown that pBD2 transgenic pigs can effectively relieve SIV-related clinical symptoms. Mechanistically, pBD2 enters host cells, mediated by energy-dependent endocytosis, to bind SLC25A4, a pro-apoptotic molecule.80 This interaction inhibits SIV-induced cell apoptosis.80
These experimental data all confirm the excellent therapeutic potential of defensin in anti-infection. Despite these benefits, no clinical trials currently utilize human defensin molecules in infectious disease treatment. Still, several clinical trials have involved the use of two defensin analogs, which will be discussed later (6.2 Clinical Trials of Defensins).
Inflammatory bowel disease and defensins
IBD, including ulcerative colitis (UC) and CD, is a complex barrier disease marked by a loss of tolerance towards commensal microbes, altered microbial composition, barrier dysfunction and chronic inflammation of temporal intensity.323 In the intestine, defensins help strengthen host immunity and help maintain the correct balance between defending against harmful pathogens and tolerating beneficial microorganisms. However, when the expression of defensins decreases, it disrupts immune homeostasis and exacerbates intestinal inflammatory response. Therefore, the alteration of defensin expression is considered an indispensable factor in the pathogenesis of IBD.
β-defensins: focusing on hBD2
The most replicated finding in active IBD is an increase of hBD2. Patients with UC exhibit a ten-fold increase and patients with colonic CD have a 3–4-fold increase compared to controls, and thus both groups express hBD2 at relatively high levels, especially in the inflamed tissue vs the non-inflamed tissue; however, there was no obvious difference in patients with ileal CD.237,324–330 Notably, in UC, hBD2 levels increase with the degree of inflammation, whereas this is not observed in CD.330 Another study found that patients with colonic CD exhibit reduced functional antimicrobial activity against commensal gut microbiota compared to patients with UC,229 but it is unclear if this difference is hBD2-mediated. The differences in hBD2 abundance observed between UC, colonic CD and ileal CD have different mechanisms. The most pronounced genetic risk factor of CD, especially ileal CD, is a frameshift mutation in the Nod2 gene (around one-third of patients with CD carry this mutation), rendering them incapable of proper hBD2 expression.331–334 In contrast, patients with UC exhibit diminished colonic mucin production, which may prevent hBD2 (and other HDPs) from being chemostatically retained in the mucus layer.324,330,335 Thus, enhanced hBD2 expression in UC is likely a counter-response to protect against microbial encroachment caused by diminished barrier function, as well as defects in mucus production, whereas reduced or unaltered hBD2 expression in CD may instead relate to different disease pathology and etiology (such as frameshift mutations).
In addition, hBD2 is distributed differently among the colon cell population. Patients with UC exhibit notably higher hBD2 expression in the luminal/villous compartment (I/v-IEC) compared to the crypt compartment (c-IEC), suggesting that mature IECs facing the intestinal lumen are responsible for producing more hBD2 (ref. 328). The production of defensin by plasma cells is also thought to be clinically relevant in UC since these cells accumulate in large numbers between the distorted crypts and muscular mucosae.336 According to Rahman et al., there is a significant increase in plasma lineage cells observed in colonic samples of patients suffering from UC compared to those with CD and control patients, and hBD2 secreted by plasma cells was upregulated by two- to threefold.336 This highlights the potential mechanism by which plasma cells regulate UC through hBD2 at sites of intestinal inflammation. No independent studies have investigated the difference in hBD2 expression between plasma cells, I/v-IEC and c-IEC. We speculate that the potential mechanism of hBD2 to prevent microbial attack might be related to the distance between cells and the intestinal cavity. The closer the cell is to the intestinal cavity, the higher the expression is. A study involving systemic administration via subcutaneous administration of hBD2 in the scapular region in mice found that recombinant hBD2 reduced inflammation, improved disease activity indices and prevented colitis-associated weight loss.70 And another study demonstrated a potential improvement in DSS-induced changes in paracellular permeability and mucosal lesions through the intrarectal administration of pBD2, which may impact the activation of NF-κB signaling.69 However, to date, there have been no studies of hBD2 in clinical trials in IBD. Given the differences in the expression of hBD2 in cases of UC, ileum CD, and colonic CD, these three clinical phenotypes may respond differently after hBD2 treatment. We speculate from our previous description that a protective effect of hBD2 therapy might be observed more often in UC or colonic CD than in ileal CD. Nonetheless, it appears that no related studies have been conducted thus far.
The expression of hBD1 is constant in the intestinal epithelium, and its expression levels remain unchanged in patients with IBD.337 Despite this, the precise function and mechanism of hBD1 concerning IBD have not been fully elucidated. hBD3 and hBD4 are like hBD2 and are noticeably increased in expression levels within the colon of patients with UC and CD.326 This observation may be because hBD2, hBD3 and hBD4 are inducible rather than constitutively expressed. However, in patients with IBD, the concentration of hBD3 and hBD4 are much lower than hBD2, and there is no significant difference in serum hBD3 and hBD4 (ref. 337). This suggests that hBD3 and hBD4 may be able to regulate local immunity. In addition, Meisch et al. investigated the distribution of hBD3 in the terminal ileum of healthy individuals and patients with CD. According to their findings, in the healthy small intestine, hBD3 is primarily observed in the luminal surface of the intestinal epithelium, as well as inside PC granules. However, in cases of CD, hBD3 relocates to the basolateral surface of the villus epithelium and accumulates in the lamina propria of the terminal ileum.326 We speculate that in patients with CD, hBD3 may, on the one hand, resist the microbial attack on the surface of the intestinal cavity and, on the other hand, enter the lamina propria and perform chemotaxis to recruit immune cells. Like with hBD2, there are still no clinical trials of hBD3 and hBD4 to treat IBD.
α-defensins: focusing on HD5
HD5 and HD6 are secreted mainly by PCs located in the small intestine and ileum, with a small amount coming from IECs.338 PCs continuously express HD5 and HD6 to protect nearby epithelial stem cells situated at the base of the crypts, thereby maintaining barrier integrity.337 Nevertheless, in IBD, the microbes and their metabolites and inflammatory factors interact to destroy PCs and IECs, thus disrupting HD5 and HD6 expression.338,339 Multiple studies have demonstrated a significant reduction in ileal HD5 and HD6 levels in patients with CD.340,341 As a result, antibacterial activity mediated by HD5 and HD6 is disrupted, resulting in a massive microbiome’s severe invasion of the intestinal mucosa and destruction of the epithelial barrier. Interestingly, patients with UC and colonic CD exhibit a significant increase in HD5 levels in their colon.328,342–345 This is mainly due to the absence of PCs in the colon of healthy people. However, after the occurrence of IBD, PC translocation hyperplasia occurs in the colonic crypts of patients with UC and colonic CD.337,344,346 We speculate that the possible mechanism is the colonic defensive response to microorganisms after the occurrence of IBD. Multiple mouse and cell studies have consistently confirmed the therapeutic effect of HD5 on colitis. For example, Shukla et al. found that HD5 administration improved ethanol- and colitis-triggered dysbiosis, inflammation response and barrier defects in the small intestine and colon.347 In addition, Zeng et al. created the recombinant NZ9000SHD-5 strain by transfecting the DEFA5 gene vector of pN8148-SHD-5 into Lactococcus lactis (L. lactis), which continuously produces mature HD5 (ref. 348). They found that NZ9000SHD-5 ameliorates intestinal damage and inflammation in mouse with DSS-induced colitis compared to the L. lactis + DSS group. These direct HD5 supplementation trials suggest that increased defensin expression is a potential avenue to treat colitis. Indeed, in a randomized clinical trial of anti-TNF therapy in patients with UC, HD5 was significantly upregulated in those who responded to the therapy compared to those that did not, with a lower microflora imbalance index in the responders.349 This suggests that the rise of HD5 may play a vital role in successfully treating UC with anti-TNF therapy. However, this needs to be confirmed experimentally; for example, in HD5 transgenic and knockout mice in a colitis model.
Unfortunately, to date, no clinical trials for IBD utilizing HD5 have been reported. However, some clinical retrospective and correlation studies have revealed the mechanism of PC regulation of HD5 and HD6 expression. This is helpful as it would then allow the targeting of the pathway of HD5 secretion by PCs as an additional means to treat IBD, to develop related inhibitors or agonists and to provide a solid foundation for the clinical application of HD5. For example, NOD2, a significant risk factor for ileal CD, is highly expressed in PCs, as shown by genome-wide association studies (GWASs).104,350,351 Economou and colleagues performed a meta-analysis and found that the CD risk is significantly increased in individuals with two mutated Nod2 alleles (17.1-fold) and Nod2 heterozygotes (2.4-fold).352 The mRNA expression of DEF5A (the gene encoding HD5) in PCs is significantly reduced in patients with a Nod2 mutation compared to patients with CD expressing wild-type Nod2 (ref. 62). These data suggest that NOD2 directly regulates HD5 in PCs to prevent CD and enhance mucosal protection. However, the Nod2 mutation does not fully explain the downregulation of HD5. This is because healthy patients with Nod2 mutations have higher HD5 expression levels than patients with CD expressing wild-type Nod2 (ref. 62). In addition, the DEFA5 gene promoter in PCs lacks NF-κB binding sites, indicating NOD2 is not directly involved in DEFA5 gene transcription,62,337 suggesting that other factors also influence the regulation of HD5. Notably, Wnt signaling regulates the positioning, differentiation and maturation of PCs.353 Blocking the Wnt signaling pathway disrupts HD5 production in PCs and induces CD.104 This is because HD5 is a transcriptional target of TCF1 and TCF4, which act downstream in Wnt signaling, and thus is directly regulated by Wnt signaling in PCs.104,271,354 Both adult and child patients with CD exhibit a decrease in the expression of TCF1 and its active isoforms, confirming its role in CD pathology.244,355 Reduced expression of TCF4 is also associated with reduced expression of HD5 in PCs in patients with ileal CD irrespective of the degree of inflammation. Nevertheless, this association is not observed in patients with colonic CD or UC. Moreover, in Tcf-4 knockout mice the α-defensins expression and bacterial killing activity were lower compared to wild-type mice, and in both species the reduced defensins expression occurred independently of the NOD2 genotype.340
Similarly, HNP1-3 expression is also dysregulated in IBD. Multiple studies have repeatedly confirmed that patients with IBD highly express HNP1-3 and patients with UC have significantly higher expression than patients with CD.340,356 It is worth noting that experiments in mice have confirmed that HNP1 has dual effects. On the one hand, low doses of HNP1 (5 μg/day) can ameliorate DSS-induced colitis.78 On the other hand, high doses of HNP1 (100 μg/day) can promote a macrophage-driven inflammatory response and aggravate the progression of DSS-induced colitis.357 In addition, data from clinical samples showed that individuals with active UC have significantly higher expression of HNP1 compared to those with UC in remission. Kanmura et al. confirmed that an increased gene copy number of HNP1-3 and the severity of UC are positively correlated.358 These data suggest that HNP1-3 may be a risk gene for severe UC, and its high expression in patients with UC may induce a hyperinflammatory response. However, it is still challenging to know where the critical concentration of HNP1-3 is for the concentration-transition-dependent effect in patients with UC and whether to consider the concentration between HNP1-3 alone or the concentration of the three in total. These answers will require further studies in patients with UC in remission.
Defensins in diabetes and obesity
Type 2 diabetes is closely linked to obesity, which is expected to affect 1 billion people worldwide by 2030 (ref. 359). Evidence of dyshomeostasis of defensin in serum and tissues of patients with diabetes has been reported. For example, hBD1-3 is down-regulated, and HNP1-3 is upregulated, in the serum of individuals with type 1 diabetes (T1D).360–363 According to a prospective study examining cardiovascular risk factors, individuals belonging to the highest quartile for plasma HNP1-3 show a significant correlation with being leaner, more insulin sensitive and possessing lower levels of total and LDL-cholesterol.364 On the other hand, those belonging to the lowest quartile for circulating HNP1-3 lack these benefits.364 Moreover, even after considering the factors of age, BMI, insulin sensitivity and smoking, the links with serum lipids remain solid.364 Another investigation conducted by Liu et al. found that HNP1 inhibits hepatic gluconeogenesis via a c-Src-dependent pathway, resulting in lowering blood glucose concentration in normal mice and Zucker diabetic fatty rats.71 In addition, a low number of HNP1-3 gene copies may increase the risk for renal dysfunction,83 which is closely related to diabetes.365 These data suggest that HNP1-3 has a practical clinical significance in the control of blood lipid levels and treating diabetes-related diseases. Of note, various studies have pointed to the role of HD5 in both obesity and diabetes. For example, the levels of HD5 in the jejunum have been found to have an inverse correlation with obesity in humans.366 In addition, when mice are fed a high-fat diet and are deficient in vitamin D, there is a decrease in the expression of the murine analog, α-defensin-5. Functionally, mice with α-defensin-5 knockout experience more severe liver steatosis and metabolic disorders than the HFD-fed mice. However, when these mice were given exogenous HD5, observed symptoms improved, indicating that the protein is an essential regulator of metabolic balance.367 In addition, Larsen et al. fed mice a 60% HFD for 13 weeks and treated them with physiologically relevant levels of HD5 (0.001%) or vectors for 10 weeks. They found that HD5-treated mice show better glucoregulatory performance, as well as improved plasma and liver lipid levels in comparison to those treated with vectors.72 These findings demonstrate that the implementation of human defensins may hold promise in enhancing host metabolism, as well as mitigating the commonly related triad of dyslipidemia, obesity and diabetes. Moreover, clinical sample data and in vivo studies in mice and in vitro cell experiments also support the therapeutic benefits of defensins in treating obesity and diabetes. Nevertheless, no trials have been conducted in people with related diseases. The difficulty in producing defensins remains a significant challenge. However, the Wehkamp laboratory recently demonstrated that intestinal proteases digest HD5 to form peptide fragments with potential antimicrobial activity.107 This newly generated peptide fragment may replace full-length peptides, providing a solution for the clinical use of HD5 active fragments.
Chronic inflammatory lung disease and defensins
The lungs inspire numerous pathogens daily. As defensins play a vital role in the fight against pathogens and mediate immune response, the role of specific defensins in regulating inflammatory lung disease has been investigated. Multiple studies have confirmed that single-nucleotide polymorphisms and copy number variations of DEFB1 and DEFB2 are associated with chronic obstructive pulmonary disease (COPD) and asthma.368–373 The ile38 variant (untranslated regions) of hBD1 was detected in 15.0% of patients, while only 2.8% of healthy individuals carried this variant. Its presence has been found to be significantly associated with the disease.369 Furthermore, over 80% of patients with this hBD1 ile38 variant reported experiencing sputum production for more than three months during their follow-up period. This suggests that the ile38 variant of hBD1 exacerbates the disease state of COPD. In addition, Andresen et al. and Baines et al. reported that hBD1 expression is elevated in bronchia biopsies of patients suffering from asthma or COPD.372,374 This rise in hBD1 expression is associated with COPD’s pathological changes and disease severity.372,374 Similar studies were replicated with hBD2. For example, levels of hBD2 were observed to correlate with IL-8 level as well as COPD severity.375 This result implies that it is an effector in the innate immune response involved in COPD’s pathogenesis. However, studies have also reported that hBD2 is decreased in central airways of COPD individuals who smoke, but not in distal ones.376 In addition, the concentration of hBD2 in pharyngeal washing fluid and sputum of smokers or former smokers is markedly lower than individuals who never smoked.377 Upon co-infection with viruses and bacteria, individuals with COPD have shown a decrease in the production of hBD2. Administering recombinant hBD2 has proven to be effective in reducing lung neutrophilia caused by exposure to cigarette smoke, while still maintaining proper immune function and promoting an appropriate response to bacterial stimuli.378 In addition, oral treatment with hBD2 is beneficial in mitigating the effects of house dust mite challenge in a murine asthma model, whether administered prophylactically or therapeutically.373,379 We speculate that the upregulation of hBD2 in COPD will play a pro-inflammatory role in inducing lung cell death. Due to the impaired immune function in patients with COPD, when smoking or when there is a large challenge by bacteria and viruses, hBD2 already expressed in COPD will be neutralized. In such cases apoptotic epithelial cells will not be able to continue to express hBD2. Thus, the immunomodulatory, antibacterial and antiviral effects of hBD2 are inhibited, and the inflammatory response of COPD is further aggravated.
Periodontitis and defensins
Periodontitis, which is responsible for a large percentage of tooth loss among adults, affects approximately 47% of adults.380 Defensins are biomarkers for the early diagnosis of periodontitis and regulate the interaction between the subgingival microbiota and host tissues.381 Research has indicated that the concentration of both α-and β-defensins in the saliva of individuals with chronic periodontitis is higher than in healthy cases.382–387 In addition, a recent bioinformatics study predicted that hBD1 might be able to bind effectively to the virulence factors of red complex bacteria in periodontitis, potentially reducing the severity of the infection.388 In vivo, hBD3 inhibits the severity of periodontitis induced by Porphyromonas gingivalis in mice and decreases osteoclast formation, while less alveolar bone loss was also observed.387
Cancer and defensins
The role of defensins in cancer development and progression has been a topic of intensive research, with some noteworthy findings.389,390 Human tumor tissue clinical samples show remarkable changes in the expression of defensins, while in vivo studies in mice and in vitro studies of related cancer cells show that defensins have anticancer and tumor progression effects. For example, in one study 82% of prostate cancer clinical tissues showed complete loss or minimal expression of hBD1 protein, while adjacent benign epithelial cells expressed it normally.297 Similarly, 90% of clinical renal cell carcinoma tissues show cancer-specific deletion of hBD1 protein.297 In addition, clinical samples of renal and prostate cancer reveal the discovery of three novel hBD1 promoter mutations.298 Synthetic hBD1 and overexpression of hBD1 can promote the death of bladder cancer cell and the renal cancer cell.298 These data suggest that hBD1 could possibly function as a tumor suppressor in urological cancers. In addition, hBD1 inhibits tumor growth of oral squamous cell carcinoma (OSCC) and lung cancer in vitro and in vivo in mice. However, hBD1 production appears to be closely linked to cancer type. For example, hBD1 expression is reduced in prostate, kidney and skin basal cell carcinoma (SBCC) and skin squamous cell carcinoma (SSCC), colon, liver, and OSCC but upregulated in lung squamous cell carcinoma (LSCC) and adenocarcinoma (AC). This pattern is further supported by studies indicating that serum hBD1 levels are notably elevated in patients with lung cancer as opposed to healthy people and patients with pneumonia.297,298,391–395
It should be noted that further confirmation of the potential therapeutic benefits of hBD1 is still lacking in transgenic animal studies and in vivo studies in primates. Phan and colleagues found that the sequence of hBD3 possesses a homologous β2-β3 loop that binds phosphoinositides to promote cytolysis of tumor cells.396 Continuous infusion of hBD3 in mice shows a remarkable inhibition in tumor growth in Lewis lung carcinoma cells and inhibits migration of colon cancer cells.397,398
However, some paradoxical results of hBD3 promoting tumor progression have also been found. For example, hBD3 contributes to the carcinogenesis of cervical cancer, HNSCC and OSCC via the activation of NF-κB signaling.399–403 Notably, defensins may also be regulated by bacteria or viruses before indirectly influencing cancer development. For example, Porphyromonas gingivalis, associated with oral cancer progression, actively triggers the transcription of α-defensins in oral tumor cells, which in turn is thought to promote the proliferation of these cells.389 In contrast, HD5 and HD6 are protective against colon cancer.404–406 For example, HD5 expression is reduced in colon cancer tissues from patients, and prognostic results indicate that patients with high HD5 expression have significantly longer survival than patients with low HD5 expression.405 HD5 overexpression also inhibits tumor growth in nude mice. Similarly, HD5 also inhibits the growth of gastric cancer.407 We summarized the expression of defensins in different cancer types in Fig. 6b.
Overall, the immunomodulatory activity of defensins offers the potential for them to be an effective anticancer therapy. Nonetheless, the development of defensin-based cancer therapies is complicated by the conflicting roles of defensins in different cancers. Future research is required to identify unique active structures of defensins that can be used to develop derived peptides with the discriminatory ability to target specific cancers.
Clinical trials of defensins
Brilacidin
Brilacidin, a synthetic defensin mimetic obtained from plants, has undergone extensive clinical testing involving more than 500 human patients for the treatment of various conditions, such as acute bacterial skin and skin structure infection (ABSSSI), UC, COVID-19 and oral mucositis (OM). For example, in vitro testing has revealed that brilacidin exhibits broad-spectrum antiviral activity, particularly against multiple human coronaviruses, including SARS-CoV-2. However, it does not possess antiviral activity against influenza or enterovirus.408–410 According to previous research, brilacidin has a dual anti-SARS-CoV-2 mechanism of action that involves targeting host cell surface heparan sulfate proteoglycans to prevent viral attachment and to inactivate viral particles.408 In fact, the US FDA has granted Fast Track status for brilacidin for COVID-19 treatment and a Phase 2 clinical trial (NCT04784897) on hospitalized patients has been conducted.411 Although the study did not meet its primary endpoint, the recovery time was significantly reduced for patients who received study treatment less than seven days after showing symptoms of COVID-19. Regarding two secondary endpoints, a higher number of patients treated with brilacidin (5-dose group) experienced clinical improvement by ten days after treatment initiation, as assessed using the National Emergency Warning Score 2 (NEWS2) criteria. The mean change in NEWS2 baseline was more remarkable for the brilacidin-treatment groups at all evaluated time points.
Brilacidin also effectively prevents and controls OM in patients undergoing head and neck cancer (HNC) chemotherapy. A Phase II clinical trial of brilacidin for this circumstance (NCT02324335) showed that patients with HNC who self-administered brilacidin three times a day for 7 weeks significantly reduced the incidence of OM compared with placebo (from 60 to 36.8%).412 Two randomized, phase II trials (NCT02052388 and NCT01211470) indicate that a single dose of intravenous brilacidin is just as safe and effective as FDA-approved daptomycin for the treatment of ABSSSI, with an early clinical response (7-day) rate of 90% (refs. 413–415).
Aside from its use in COVID-19, ABSSSI and OM, brilacidin is currently being developed as a preventative measure for UC. Most patients with UC that were treated with brilacidin achieved induction of clinical remission.416 After administering brilacidin, there were no reports of Serious Adverse Events (SAEs) and it was generally well-tolerated.416 In animal models, brilacidin also demonstrated a potential therapeutic effect in treating keratitis (topical drops) and pulmonary infection (intraperitoneal injection) induced by Aspergillus fumigatus.417,418 These studies all show the clinical potential of brilacidin, although further investigation is needed.
Pezadeftide (HXP124)
HXP124 is a novel plant defensin being clinically developed by Hexima Ltd as a novel topical candidate for treating onychomycosis.419,420 To evaluate its efficacy, a Phase I/IIa clinical trial was conducted using pezadeftide (Australian Clinical Trials ID: ACTRN12618000131257) in a double-blinded, randomized study with multiple ascending doses.420,421 Patients who received daily topical application of pezadeftide for 6 weeks were found to have reduced infection area compared to those receiving current best-in-class therapies, with a shorter treatment time and excellent safety profile.420,421 The clinical data from this trial demonstrated a 69% Mycological Cure rate at 12 weeks, a vast improvement over the 29% rate achieved by the control group.420,421 These results show very promising clinical efficacy. Hexima has since raised $11 million and initiated Phase IIb testing of 2% pezadeftide in three active arms to determine the optimal dosing frequency and evaluate further its safety and efficacy.422
Hurdles to the development of defensins-based therapy
From the extensive evidence presented previously, the role of defensins and targeted therapies offer new hope, but most natural defensins may not be suitable as drugs for direct application. The main challenges lie in the following areas. (1) The effect of defensins in vivo requires a suitable local microenvironment. The direct antibacterial activity requires high local concentrations, and many defensins exhibit specific cytotoxicity and inflammatory responses at high concentrations,17 as described in the Regulation of Cell Death section. In addition, specific disease environments lead to significant changes in mucosal pH and salt ion concentrations that do not allow defensins to function.423–426 (2) Natural defensins are sensitive to protease-mediated inactivation. The human body contains nearly 600 proteases427 that, depending on the structure of the defensin, work together to exert proteolytic activity. The stability of peptidases can be significantly improved through various methods, such as altering specific amino acid residues or modifying the peptide skeleton through techniques like protection or cyclization of the amino and carboxyl terminus.428 Another approach is the utilization of protease inhibitors. (3) The formulation and delivery of defensin drugs have not been fully realized. Most current HDP-based therapies are applied externally; for example, to treat skin and respiratory diseases.17,429,430 This is because current defensin drugs lack appropriate drug-like properties. Without the participation of the formulation and delivery system, the absorption efficiency, delivery efficiency and metabolic cycle of defensin drugs will be directly affected. Therefore, for defensin drugs to be delivered to the body (by oral or injection), a way must be found to overcome these barriers. One way to address this issue is to consider alternative delivery methods such as liposomes, polymer nanoparticles, carbon nanotubes and similar materials. For example, Krishnakumari et al. synthesized the N-terminal myridamylated Phd1-3 peptide, MPhd1-3. They found that it was more active against S. aureus and remained active in the presence of 150 mM NaCl, whereas hBD1-3 was not.431 In addition, Lei et al. designed a nanobiotic component assembled from C-terminally myristoylated HD5 (HD5-myr).432 In vitro and in vivo experiments have revealed that HD5-myr has an extraordinary efficacy in disrupting the structure of bacterial membranes or cell walls, and its antibacterial activity is considerably higher in the presence of sodium chloride or serum when compared to HD5 (ref. 432). According to Yuan et al., a three-dimensional porous structure was introduced onto polyether ether ketone through sulfonation, which was then coated with mBD14 to create a long-lasting antimicrobial coating (SP-mBD). The newly formed coating showed a high efficiency in eradicating a wide range of bacteria while promoting osseointegration.68 (4) The high cost of synthesis or expression. Currently, most of the clinical and experimental applications of defensins are based on chemical synthesis methods, including solid-phase peptide synthesis (SPPS) and liquid-phase peptide synthesis (LPPS).425 Of these techniques, SPPS is the most widely employed. However, SPPS has some technical, cost and environmental challenges. For example, SPPS has difficulty synthesizing defensins containing many hydrophobic side-chain amino acids. This is because the structure of defensins contains many β folds or α helices, which have high hydrophobicity, resulting in high aggregation in water-based solvents, thus producing low solubility intermediates that affect the subsequent purification process.433,434 It is worth noting that with the development of defensins fragmentation research, some small fragments of defensins, such as HD51-9 (refs. 107,435) and HNP41-11 (ref. 154) have been shown to replace full-length defensins in antibacterial activity. We think this could be a pivotal way to overcome the limitations of high synthesis costs and that small fragments are more helpful in finding suitable delivery materials in vivo.
Nutritional regulation strategy
Due to defensins being toxic to mammalian cell membranes at high concentrations, the transcription, translation, and activity of most defensins are strictly controlled to avoid excessive immune responses. Given these mechanisms, developing strategies to regulate the expression and secretion of defensins would be significant. Here, we review interventional methods for handling endogenous defensins, including fatty acids, amino acids, microelements, plant extracts and probiotics (Table 4).
Table 4.
Regulation effects of nutrients on the expression of defensins
| Nutrient | Object | Defensins | Pathway | Reference | |
|---|---|---|---|---|---|
| Fatty acid | SCFAs | HT29 | hBD1-2 | 439 | |
| SCFAs | Mice IEC | mBD1/3/4 | GPR43-mTOR/STAT3 | 442 | |
| Sodium phenylbutyrate | IPEC-J2 | pBD1-3 | TLR2/4-NF-κB | 443,444 | |
| Caprylic acid and nonanoic acid | IPEC-J2 | pBD1-2 | Acetylation H3K9 | 441 | |
| Sodium phenylbutyrate | IPEC-J2 | pBD1-3 | HDAC inhibition | 443 | |
| Butyrate | Macrophage and intestine | pBD2-3 | HDAC inhibition | 446 | |
| Amino acid | Leucine | Mice PCs | Cryptdin1 | 453 | |
| l-isoleucine | IPEC-J2 | pBD1-3 | Sirt1-ERK-90RSK | 455 | |
| l-isoleucine | Caco- | hBD2 | 456 | ||
| l-arginine | Intestine and oral | pBD2-3 | 457 | ||
| Isoleucine | Caco-2 | hBD2 | GPCR-ERK | 456 | |
| l-isoleucine | Bovine kidney EC | β-defensins | NF-κB/rel | 458 | |
| Microelements | 25OH vitamin D3 (25D3) | keratinocytes | hBD4 | TLR2-NF-κB-Cyp271b/Cyp24-VDR | 459,460 |
| 1,25OH vitamin D3 | IEC and monocyte | AvBD3/6/9 | VDR | 461 | |
| Zn2+ | IPEC-J2 | pBD1-3 | 454 | ||
| Plant extracts | ITF | Intestine | mBD1 | 467 | |
| Avocado sugar | keratinocyte | hBD2 | TLR2-ERK/MAPK | 468 | |
| EGCG | IPEC-J2 | pBD2 | ERK1/2/p38-MAPK | 464 | |
| Dehydroandrographolid | HCT-116 | hBD2 | p38-MAPK | 470 | |
| Reishi | Intestine | RD5-6 | TLR4 | 471 | |
| β-Glucan | Ovine ruminal ECs | SBD1 | Dectin-1-Syk-NF-κB | 469 | |
| Black tea extract and theaflavins | Oral ECs | hBD1/2/4 | 466 | ||
| Probiotics | Lactobacillus | Caco-2 | hBD2 | NF-κB and AP-1 | 473 |
| Escherichia coli Nissle 1917 | Fecal | hBD2 | NF-κB and AP-1 | 476–478 | |
| Bacillus subtilis yb-1114246 | Intestine | AvBD1 | TLR2-NF-κB | 435 | |
| Clostridium butyricum | IPEC-J2 and Intestine | pBD1-3 | TLR2 | 479 | |
| Lactobacillus casei strain Shirot | Caco-2 | hBD2 | 504 | ||
| Bacillus subtilis BYS2 | Intestine | AvBD1/6 | 505 | ||
| Lactobacillus salivarius B1 | Intestine | pBD2 | 506 | ||
| Lactobacillus rhamnosus MLGA | Intestine | AvBD9 | 507 | ||
| Bifidobacterium longum spp. S12 | Intestine and cancer cells | hBD2 | 508 | ||
| Lactobacillus helveticus SBT2171 | Caco-2 | hBD2 | TLR2-JNK | 472 |
Fatty acids
Fatty acids (FAs), the main components of lipids, undergo various metabolic processes after absorption in the gut. Proper lipid metabolism in the gut is essential to ensure adequate energy for the body’s organs. In humans, defects in the absorption of lipids can cause severe symptoms, including IBD and IBS.436,437 The length of the aliphatic hydrocarbon chain of free FAs has been found to have a negative correlation with the ability of FAs to induce defensin expression.438 Undigested dietary fiber in the large intestine undergoes bacterial fermentation, yielding short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate. They enhance the expression of hBD1 and hBD2 in IECs439 (Fig. 7). In pigs, caprylic acid and nonanoic acid, both medium-chain fatty acids, significantly increase pBD2-3 and pBD1-2 expression, respectively, in porcine intestinal enterocytes (IPEC-J2) and decrease bacterial translocation, with augmented antibacterial activity440,441 (Fig. 7).
Fig. 7.
Signaling pathways of fatty acids-regulated expression of defensins. SCFAs induce the expression of β-defensins via GPR43-STAT3 and GPR43-mTOR-4E-BP signals; sodium butyrate (NaB) induces the expression of β-defensins via TLR2-p38/ERK- NF-κB, HDAC inhibition and EGFR signals; sodium phenylbutyrate (PBA) induces the expression of β-defensins via TLR2/TLR4-p38/ERK-NF-κB and EGFR signals; Caprylic acid and nonanoic acid induce the expression of β-defensins via HDAC inhibition-acetylation H3K9
The expression of defensins mediated by SCFAs involves multiple signaling pathways. For instance, GPR43 mediates SCFA-regulated β-defensins-1, 3, and 4 expressions in IECs via activating mTOR and STAT3 (ref. 442) (Fig. 7). Sodium phenylbutyrate, an aromatic SCFA, promotes the expression of pBD1-3 through the TLR2/4-mediated NF-κB pathway443,444 (Fig. 7). In addition to TLRs or GPCRs, histone modification is involved in FA-induced expression of defensins. For example, caprylic and nonanoic acid attenuate histone deacetylase (HDAC) activity, leading to an elevation in the acetylation level of H3K9 and an upregulation of pBD1 and pBD2 (ref. 441) (Fig. 7). Sodium phenylbutyrate and butyrate further amplify defensin secretion through histone deacetylation and STATs phosphorylation in IPEC-J2 cells and crypt organoids443,445 (Fig. 7). In addition, in our previous study we found that butyrate upregulates pBD2 and pBD3 to enhance disease resistance, including promoting the removal of harmful bacteria and improving inflammation caused by E. coli O157:H7 infection in piglets via HDAC inhibition446 (Fig. 7). These results indicate that FAs are adequate to induce the expression of defensins via multiple signaling pathways, in which histone acetylation may be the target of FAs to activate these signaling.
Amino acids and microelements
Amino acids are critical regulators in many metabolic processes. Amino acid transport in the intestine is crucial to supply sufficient amino acids to all tissues and to maintain the homeostasis of plasma amino acid levels.447 It has been previously shown that defensins play an essential role in the IBD-induced disorder of amino acid metabolic profile in blood, feces and the intestine.448–451 Consistent with these results, some amino acids promote intestinal barrier function and intestinal endocrine homeostasis via a defensins-related mechanism.452,453 Takakuwa et al. found that leucine administration significantly induces α-defensins secretion from the PCs of the small intestine, compared with phosphate-buffered saline and 19 other amino acids, in a dose-independent manner.453 Moreover, l-isoleucine and branched-chain amino acids (BCAA) administration enhances pBD1-3 levels in the small intestine and epithelial cells,454,455 as well as in human colonic epithelial cells.456 l-arginine administration promotes the expression of pBD2 and pBD3 in the ileum of weaned pigs.457 Interestingly, the ability of amino acids to induce defensins expression was related to not only the type of amino acid but also its isomer. For example, l-isoleucine could induce a 12-fold expression of β-defensins at a low concentration of 3 μg/mL, whereas d-isoleucine required a concentration of 200 μg/mL.458 Mechanistically, the MAPK pathway is related to the amino acid-induced expression of defensins. For instance, the expression of β-defensins induced by l-isoleucine is via the SIRT1/ERK/90RSK signals and GPCRs-ERK pathways in IPEC-J2 cells455,456 (Fig. 8). 25OH vitamin D3 (25D3) activates TLR2, which promotes the expression of CYP27b1 and CYP24, which, in turn, convert 25D3 into active 1,25(OH)2 vitamin D3, a ligand for the vitamin D receptor (VDR), ultimately leading to defensins transcription and therefore mediating an antimicrobial response459,460 (Fig. 8). Moreover, 25D3 can induce AvBD secretion in the avian embryonic gut.461 Like amino acids, zinc remarkably increases the mRNA and protein levels of pBD1-3 in IPEC-J2 cells.454 Nevertheless, the mechanism still needs further investigation.
Fig. 8.
Signaling pathways of amino acids, vitamin D and plant extracts regulate the expression of defensins. Top left: l-isoleucine induces the expression of β-defensins via the SIRT1/ERK/90RSK signals, and G-protein coupled receptor-ERK pathways. Top right: 25OH vitamin D3 (25D3) induces the expression of β-defensins via TLR2-NF-κB-CYP271B/CYP24-VDR signals. Bottom: Avocado sugar via TLR2-ERK1/2, EGCG, and DA via p38, Reishi via TLR4, and b-Glucan via Dectin-1-Syk-Ikk-NF-κB regulate the expression and section of defensins
Plant extracts
Plant extracts are bioactive compounds extracted from plants with one or more biological functions, such as antimicrobial (bacteria, protozoa, and fungi), immunity and antioxidant activities.462,463 For example, green tea and vegetables are rich in epigallocatechin-3-gallate (EGCG), which helps to prevent the breakdown of recombinant hBD1 and hBD2. At the same time, it encourages their secretion in epithelial cells through the activation of ERK1/2 and p38-MAPK signal pathways464 (Fig. 8). Peony, a plant that is commonly used in traditional Chinese medicine, contains paeoniflorin (PF), a compound that increases the expression of hBD2 in bronchial epithelial cells to strengthen epithelial antimicrobial barriers. Mechanistically, this effect is achieved by upregulating the p38-MAPK, ERK, and NF-κB signaling pathways.465 Similarly, black tea extract and theaflavins attenuate IL-8 secretion and induce hBD1, hBD2, and hBD4 secretion in epithelial cells.466 In addition, fiber and carbohydrates, rich in plants, can regulate defensins expression and improve immunity. For example, Chen et al. found that supplementing with inulin-type fructan fibers (ITF) can lead to an increase in the expression of mBD1, which in turn may contribute to protection against autoimmune diabetes by regulating cytokine production and improving the ratio of Treg/Th17 (ref. 467). Avocado sugar modulates the hBD2 and hBD3 expression in human keratinocytes through TLR2 and ERK/MAPK activation468 (Fig. 8). β-Glucan from Saccharomyces cerevisiae activates the Dectin-1-Syk-NF-κB pathway to induce β-defensin-1 expression in the ruminal epithelial cells of sheep469 (Fig. 8).
Furthermore, plant-derived Chinese herbal medicines dehydroandrographolide (DA) and reishi are also effective regulators of defensin expression. Xiong et al. found that DA enhances innate intestinal tract immunity by increasing hBD2 expression in HCT-116 intestinal cells through the p38-MAPK pathway470 (Fig. 8). Reishi, a polypore fungus, enhances IgA secretion and the expression of RD5 and RD6 in the rat intestine via a TLR4-dependent signaling in a concentration-dependent manner (Fig. 8); however, it does not activate TNF-α. Therefore, supplementation with reishi may be a potential therapy to ameliorate intestinal infection.471
Probiotics
Probiotics have shown potential as a therapy for gut inflammation, but their interaction with host defense defensins remains relatively unexplored. For instance, the S layer protein derived from Lactobacillus Swiss SBT2171 promotes the expression of hBD2 via the TLR2-JNK signal, thus providing a protective shield against infection.472 Other strains of Lactobacillus and probiotic cocktails like VSL#3 stimulate hBD2 secretion through the NF-κB and AP-1 signal, helping to reinforce intestinal barrier functions.473 Similarly, the probiotic E. coli Nissle 1917 boosts hBD2 expression via flagellin-mediated NF-κB and AP-1 pathways, enhancing the mucosal barrier against luminal bacteria.474,475 With beneficial outcomes observed in human clinical studies, E. coli Nissle 1917 appears to show promise.476–478
Selenium-enriched Bacillus subtilis yb-1114246 activates the TLR2-NF-κB signal to control intestinal β-defensins expression, thereby improving the immune status of the intestine.435 Our prior research indicates that C. butyricum binds to the adhesion sites of IECs, prompting the secretion of pBD1-3 by IECs.479 C. butyricum and pBD1-3 synergistically positively regulate the composition of intestinal microbiota and SCFA production, culminating in an improvement in intestinal immune function in weaned piglets.479 Over- or under-production of defensins can adversely impact intestinal integrity. However, the beneficial effects of probiotics in adjusting abnormal defensin levels, be it an increase or decrease, have been fairly consistent in aiding host recovery. A deeper understanding of the interactions between probiotics and defensins is necessary. This will facilitate the comprehensive analysis of dysregulation of defensin homeostasis and microbial crosstalk in various gastrointestinal diseases, which is vital for treating gastrointestinal diseases.
Although several nutrients have been shown to regulate the expression of defensins, this screening approach excessively relies on reproducible experiments. A high throughput screening method was recently developed, in which the capacity of up to 584 compounds to induce the expression of specific defensins, such as LL-37 and AvBD9, could be determined in one in vitro experiment.480,481 Such a screening approach will significantly accelerate the speed of discovery of nutrient-induced defensin expression.
Conclusions
As a common defense mechanism among mammals, host-derived defensins comprise a critical innate immune barrier to external insults. A better understanding of the expression site, chemotactic activity, inflammation regulation, damage regulation and secretion regulation of host-derived defensins is critical to comprehending host defense mechanisms and disease processes. Although there is still a lack of solid clinical trials that adequately utilize the immune effectors of defensins in various diseases, both clinical and preclinical data obtained using mouse models highlight the vital role that defensins play in regulating the immune response. Meanwhile, in the future, the field should focus on exploring defensin functions and mechanisms in ameliorating specific diseases by establishing defensin knockout animal models or utilizing clinical samples. Moreover, as multiple defensins are present in the host, better tools and proteomic methodologies must explore how the synergies between defensins improve innate immunity or enhance resistance to infection. But even so, the combined data generated to date in the field point to a bright future for a role of defensins or their derivatives in the treatment of various human diseases.
Acknowledgements
We gratefully acknowledge the financial support provided by the Zhejiang Provincial Key R&D Program of China (2021C02008), the China Agriculture Research System of MOF and MARA (CARS-35), the National Natural Science Foundation of China (Grant Nos. 32002185, 31930057 and U21A20249), the National Key R&D Program (2018YFA0507802), Taishan Industrial Leading Talents Project, and Program from National Center of Technology Innovation for Pigs for this study.
Author contributions
J.F. conceptualized and alone wrote the manuscript and drew all figures; Y.W., F.W., and Z.X. conceptualized and critically commented on the manuscript. J.M. and M.J. critically commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests.
Contributor Information
Fudi Wang, Email: fwang@zju.edu.cn.
Yizhen Wang, Email: yzwang321@zju.edu.cn.
References
- 1.Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013;9:761–768. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, et al. Antimicrobial peptides: promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019;39:831–859. doi: 10.1002/med.21542. [DOI] [PubMed] [Google Scholar]
- 3.Zaiou M, Nizet V, Gallo RL. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Invest. Dermatol. 2003;120:810–816. doi: 10.1046/j.1523-1747.2003.12132.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Wang M, Shan A, Feng X. Avian host defense cathelicidins: structure, expression, biological functions, and potential therapeutic applications. Poult. Sci. 2020;99:6434–6445. doi: 10.1016/j.psj.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pazgier M, et al. Structural and functional analysis of the pro-domain of human cathelicidin, LL-37. Biochemistry. 2013;52:1547–1558. doi: 10.1021/bi301008r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson CL, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 7.Chu H, et al. Human alpha-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science. 2012;337:477–481. doi: 10.1126/science.1218831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Easton DM, Nijnik A, Mayer ML, Hancock REW. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009;27:582–590. doi: 10.1016/j.tibtech.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasupuleti M, Schmidtchen A, Malmsten M. Antimicrobial peptides: key components of the innate immune system. Crit. Rev. Biotechnol. 2012;32:143–171. doi: 10.3109/07388551.2011.594423. [DOI] [PubMed] [Google Scholar]
- 10.Piktel E, et al. The role of cathelicidin LL-37 in cancer development. Arch. Immunol. Ther. Exp. 2016;64:33–46. doi: 10.1007/s00005-015-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheenstra MR, van Harten RM, Veldhuizen EJA, Haagsman HP, Coorens M. Cathelicidins modulate TLR-activation and inflammation. Front. Immunol. 2020;11:1137. doi: 10.3389/fimmu.2020.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young-Speirs M, Drouin D, Cavalcante PA, Barkema HW, Cobo ER. Host defense cathelicidins in cattle: types, production, bioactive functions and potential therapeutic and diagnostic applications. Int. J. Antimicrob. Agents. 2018;51:813–821. doi: 10.1016/j.ijantimicag.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 13.van Harten RM, van Woudenbergh E, van Dijk A, Haagsman HP. Cathelicidins: immunomodulatory antimicrobials. Vaccines. 2018;6:63. doi: 10.3390/vaccines6030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu D, Lu W. Defensins: a double-edged sword in host immunity. Front. Immunol. 2020;11:764. doi: 10.3389/fimmu.2020.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Lu W. Defensins in innate immunity. Curr. Opin. Hematol. 2014;21:37–42. doi: 10.1097/MOH.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 16.Gao X, et al. Defensins: the natural peptide antibiotic. Adv. Drug Deliv. Rev. 2021;179:114008. doi: 10.1016/j.addr.2021.114008. [DOI] [PubMed] [Google Scholar]
- 17.van der Does AM, Hiemstra PS, Mookherjee N. Antimicrobial host defence peptides: immunomodulatory functions and translational prospects. Adv. Exp. Med. Biol. 2019;1117:149–171. doi: 10.1007/978-981-13-3588-4_10. [DOI] [PubMed] [Google Scholar]
- 18.Holly MK, Diaz K, Smith JG. Defensins in viral infection and pathogenesis. Annu Rev. Virol. 2017;4:369–391. doi: 10.1146/annurev-virology-101416-041734. [DOI] [PubMed] [Google Scholar]
- 19.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Han Z, Oppenheim JJ. Alarmins and immunity. Immunol. Rev. 2017;280:41–56. doi: 10.1111/imr.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleischmann J, Selsted ME, Lehrer RI. Opsonic activity of MCP-1 and MCP-2, cationic peptides from rabbit alveolar macrophages. Diagn. Microbiol. Infect. Dis. 1985;3:233–242. doi: 10.1016/0732-8893(85)90035-5. [DOI] [PubMed] [Google Scholar]
- 22.Lehrer RI, Daher K, Ganz T, Selsted ME. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J. Virol. 1985;54:467–472. doi: 10.1128/jvi.54.2.467-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selsted ME, Harwig SS, Ganz T, Schilling JW, Lehrer RI. Primary structures of three human neutrophil defensins. J. Clin. Invest. 1985;76:1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilde CG, Griffith JE, Marra MN, Snable JL, Scott RW. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J. Biol. Chem. 1989;264:11200–11203. [PubMed] [Google Scholar]
- 25.Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 26.Jones DE, Bevins CL. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993;315:187–192. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- 27.Bensch KW, Raida M, Mägert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 28.Harder J, Bartels J, Christophers E, Schröder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 29.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 30.Tang YQ, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 31.Schutte BC, et al. Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc. Natl Acad. Sci. USA. 2002;99:2129–2133. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh D, et al. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat. Immunol. 2002;3:583–590. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 33.Ayabe T, et al. Activation of Paneth cell alpha-defensins in mouse small intestine. J. Biol. Chem. 2002;277:5219–5228. doi: 10.1074/jbc.M109410200. [DOI] [PubMed] [Google Scholar]
- 34.Valore EV, Ganz T. Posttranslational processing of defensins in immature human myeloid cells. Blood. 1992;79:1538–1544. [PubMed] [Google Scholar]
- 35.Ganz T, Liu L, Valore EV, Oren A. Posttranslational processing and targeting of transgenic human defensin in murine granulocyte, macrophage, fibroblast, and pituitary adenoma cell lines. Blood. 1993;82:641–650. [PubMed] [Google Scholar]
- 36.Wu Z, Powell R, Lu W. Productive folding of human neutrophil alpha-defensins in vitro without the pro-peptide. J. Am. Chem. Soc. 2003;125:2402–2403. doi: 10.1021/ja0294257. [DOI] [PubMed] [Google Scholar]
- 37.Yeaman MR, Bayer AS, Koo SP, Foss W, Sullam PM. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J. Clin. Invest. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider T, et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science. 2010;328:1168–1172. doi: 10.1126/science.1185723. [DOI] [PubMed] [Google Scholar]
- 39.Sahl HG, et al. Mammalian defensins: structures and mechanism of antibiotic activity. J. Leukoc. Biol. 2005;77:466–475. doi: 10.1189/jlb.0804452. [DOI] [PubMed] [Google Scholar]
- 40.Hoover DM, et al. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J. Biol. Chem. 2000;275:32911–32918. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, et al. Mouse α-defensins: structural and functional analysis of the 17 cryptdin isoforms identified from a single jejunal crypt. Infect. Immun. 2022;0:e00361–00322. doi: 10.1128/iai.00361-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kvansakul M, et al. Binding of phosphatidic acid by NsD7 mediates the formation of helical defensin-lipid oligomeric assemblies and membrane permeabilization. Proc. Natl Acad. Sci. USA. 2016;113:11202–11207. doi: 10.1073/pnas.1607855113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder BO, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature. 2011;469:419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 44.Nusbaum C, et al. DNA sequence and analysis of human chromosome 8. Nature. 2006;439:331–335. doi: 10.1038/nature04406. [DOI] [PubMed] [Google Scholar]
- 45.Groth M, et al. High-resolution mapping of the 8p23.1 beta-defensin cluster reveals strictly concordant copy number variation of all genes. Hum. Mutat. 2008;29:1247–1254. doi: 10.1002/humu.20751. [DOI] [PubMed] [Google Scholar]
- 46.Logsdon GA, et al. The structure, function and evolution of a complete human chromosome 8. Nature. 2021;593:101–107. doi: 10.1038/s41586-021-03420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seebah S, et al. Defensins knowledgebase: a manually curated database and information source focused on the defensins family of antimicrobial peptides. Nucleic Acids Res. 2007;35:D265–D268. doi: 10.1093/nar/gkl866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schonwetter BS, Stolzenberg ED, Zasloff MA. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 49.Diamond G, Russell JP, Bevins CL. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc. Natl Acad. Sci. USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biragyn A, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 51.Vora P, et al. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J. Immunol. 2004;173:5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 52.Menendez A, et al. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal Paneth cell α-defensins. J. Innate Immun. 2013;5:39–49. doi: 10.1159/000341630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Redfern RL, Reins RY, McDermott AM. Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp. Eye Res. 2011;92:209–220. doi: 10.1016/j.exer.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang D, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 55.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- 56.Li X, et al. β-Defensin 19/119 mediates sperm chemotaxis and is associated with idiopathic infertility. Cell Rep. Med. 2022;3:100825. doi: 10.1016/j.xcrm.2022.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Economopoulou M, et al. Inhibition of pathologic retinal neovascularization by alpha-defensins. Blood. 2005;106:3831–3838. doi: 10.1182/blood-2005-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Candille SI, et al. A -defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–1423. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langhorst J, et al. Activated innate immune system in irritable bowel syndrome? Gut. 2007;56:1325–1326. doi: 10.1136/gut.2007.125005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong X, et al. Keratinocyte-derived defensins activate neutrophil-specific receptors Mrgpra2a/b to prevent skin dysbiosis and bacterial infection. Immunity. 2022;55:1645–1662. doi: 10.1016/j.immuni.2022.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szekeres M, et al. Relevance of defensin β-2 and α defensins (HNP1-3) in Alzheimer’s disease. Psychiatry Res. 2016;239:342–345. doi: 10.1016/j.psychres.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 62.Wehkamp J, et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biswas A, et al. Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc. Natl Acad. Sci. USA. 2010;107:14739–14744. doi: 10.1073/pnas.1003363107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lehrer RI, Lu W. α-Defensins in human innate immunity. Immunol. Rev. 2012;245:84–112. doi: 10.1111/j.1600-065X.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 65.Xie JW, et al. Alpha defensin-1 attenuates surgically induced osteoarthritis in association with promoting M1 to M2 macrophage polarization. Osteoarthr. Cartil. 2021;29:1048–1059. doi: 10.1016/j.joca.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Augustin DK, et al. Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect. Immun. 2011;79:595–605. doi: 10.1128/IAI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pfeufer NY, et al. Bioactive coating of titanium surfaces with recombinant human β-defensin-2 (rHuβD2) may prevent bacterial colonization in orthopaedic surgery. J. Bone Jt. Surg. Am. 2011;93:840–846. doi: 10.2106/JBJS.I.01738. [DOI] [PubMed] [Google Scholar]
- 68.Yuan X, et al. Multifunctional sulfonated polyetheretherketone coating with beta-defensin-14 for yielding durable and broad-spectrum antibacterial activity and osseointegration. Acta Biomater. 2019;86:323–337. doi: 10.1016/j.actbio.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 69.Han F, et al. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J. Immunol. 2015;194:1882–1893. doi: 10.4049/jimmunol.1402300. [DOI] [PubMed] [Google Scholar]
- 70.Koeninger L, et al. Human β-defensin 2 mediated immune modulation as treatment for experimental colitis. Front. Immunol. 2020;11:93–93. doi: 10.3389/fimmu.2020.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu HY, et al. Suppression of hepatic glucose production by human neutrophil alpha-defensins through a signaling pathway distinct from insulin. J. Biol. Chem. 2008;283:12056–12063. doi: 10.1074/jbc.M801033200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Larsen IS, et al. Human Paneth cell α-defensin-5 treatment reverses dyslipidemia and improves glucoregulatory capacity in diet-induced obese mice. Am. J. Physiol. -Endocrinol. Metab. 2019;317:E42–E52. doi: 10.1152/ajpendo.00019.2019. [DOI] [PubMed] [Google Scholar]
- 73.Kokoza V, et al. Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc. Natl Acad. Sci. USA. 2010;107:8111–8116. doi: 10.1073/pnas.1003056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Q, et al. Increased gene copy number of DEFA1/DEFA3 worsens sepsis by inducing endothelial pyroptosis. Proc. Natl Acad. Sci. USA. 2019;116:3161–3170. doi: 10.1073/pnas.1812947116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang J, et al. Porcine β-defensin 2 inhibits proliferation of pseudorabies virus in vitro and in transgenic mice. Virol. J. 2020;17:18. doi: 10.1186/s12985-020-1288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abu-Fanne R, et al. Neutrophil α-defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability. Blood. 2019;133:481–493. doi: 10.1182/blood-2018-07-861237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Canas JJ, et al. Human neutrophil peptides 1-3 protect the murine urinary tract from uropathogenic Escherichia coli challenge. Proc. Natl Acad. Sci. USA. 2022;119:e2206515119. doi: 10.1073/pnas.2206515119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maeda T, et al. Low concentrations of human neutrophil peptide ameliorate experimental murine colitis. Int. J. Mol. Med. 2016;38:1777–1785. doi: 10.3892/ijmm.2016.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu M, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. β-Defensins 2 and 3 together promote resistance to Pseudomonas aeruginosa Keratitis1. J. Immunol. 2009;183:8054–8060. doi: 10.4049/jimmunol.0902140. [DOI] [PubMed] [Google Scholar]
- 80.Huang J, et al. Porcine β-defensin 2 confers enhanced resistance to swine flu infection in transgenic pigs and alleviates swine influenza virus-induced apoptosis possibly through interacting with host SLC25A4. Antivir. Res. 2022;201:105292. doi: 10.1016/j.antiviral.2022.105292. [DOI] [PubMed] [Google Scholar]
- 81.Zhang LJ, Gallo RL. Antimicrobial peptides. Curr. Biol. 2016;26:R14–R19. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 82.Schneider JJ, Unholzer A, Schaller M, Schäfer-Korting M, Korting HC. Human defensins. J. Mol. Model. 2005;83:587–595. doi: 10.1007/s00109-005-0657-1. [DOI] [PubMed] [Google Scholar]
- 83.Ai Z, et al. Low α-defensin gene copy number increases the risk for IgA nephropathy and renal dysfunction. Sci. Transl. Med. 2016;8:345ra388. doi: 10.1126/scitranslmed.aaf2106. [DOI] [PubMed] [Google Scholar]
- 84.Lynn DJ, Bradley DG. Discovery of α-defensins in basal mammals. Dev. Comp. Immunol. 2007;31:963–967. doi: 10.1016/j.dci.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 85.Amid C, et al. Manual annotation and analysis of the defensin gene cluster in the C57BL/6J mouse reference genome. BMC Genomics. 2009;10:606. doi: 10.1186/1471-2164-10-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodríguez-Jiménez FJ, et al. Distribution of new human beta-defensin genes clustered on chromosome 20 in functionally different segments of epididymis. Genomics. 2003;81:175–183. doi: 10.1016/s0888-7543(02)00034-4. [DOI] [PubMed] [Google Scholar]
- 87.Yenugu S, Hamil KG, Radhakrishnan Y, French FS, Hall SH. The androgen-regulated epididymal sperm-binding protein, human beta-defensin 118 (DEFB118) (formerly ESC42), is an antimicrobial beta-defensin. Endocrinology. 2004;145:3165–3173. doi: 10.1210/en.2003-1698. [DOI] [PubMed] [Google Scholar]
- 88.Zhou CX, et al. An epididymis-specific beta-defensin is important for the initiation of sperm maturation. Nat. Cell Biol. 2004;6:458–464. doi: 10.1038/ncb1127. [DOI] [PubMed] [Google Scholar]
- 89.Yudin AI, et al. ESP13.2, a member of the beta-defensin family, is a macaque sperm surface-coating protein involved in the capacitation process. Biol. Reprod. 2003;69:1118–1128. doi: 10.1095/biolreprod.103.016105. [DOI] [PubMed] [Google Scholar]
- 90.Campopiano DJ, et al. Structure-activity relationships in defensin dimers: a novel link between beta-defensin tertiary structure and antimicrobial activity. J. Biol. Chem. 2004;279:48671–48679. doi: 10.1074/jbc.M404690200. [DOI] [PubMed] [Google Scholar]
- 91.Rajabi M, et al. Functional determinants of human enteric α-defensin HD5: crucial role for hydrophobicity at dimer interface. J. Biol. Chem. 2012;287:21615–21627. doi: 10.1074/jbc.M112.367995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao L, et al. Invariant gly residue is important for α-defensin folding, dimerization, and function: a case study of the human neutrophil α-defensin HNP1. J. Biol. Chem. 2012;287:18900–18912. doi: 10.1074/jbc.M112.355255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pazgier M, et al. Sometimes it takes two to tango: contributions of dimerization to functions of human α-defensin HNP1 peptide. J. Biol. Chem. 2012;287:8944–8953. doi: 10.1074/jbc.M111.332205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Lu W, Hong M. The membrane-bound structure and topology of a human α-defensin indicate a dimer pore mechanism for membrane disruption. Biochemistry. 2010;49:9770–9782. doi: 10.1021/bi101512j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lehrer RI, et al. Multivalent binding of carbohydrates by the human α-defensin, HD51. J. Immunol. 2009;183:480–490. doi: 10.4049/jimmunol.0900244. [DOI] [PubMed] [Google Scholar]
- 96.Greenwald GI, Ganz T. Defensins mediate the microbicidal activity of human neutrophil granule extract against Acinetobacter calcoaceticus. Infect. Immun. 1987;55:1365–1368. doi: 10.1128/iai.55.6.1365-1368.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agerberth B, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 98.Chalifour A, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104:1778–1783. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- 99.Eisenhauer PB, Lehrer RI. Mouse neutrophils lack defensins. Infect. Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harwig SS, Park AS, Lehrer RI. Characterization of defensin precursors in mature human neutrophils. Blood. 1992;79:1532–1537. [PubMed] [Google Scholar]
- 101.Brook M, et al. Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc. Natl Acad. Sci. USA. 2016;113:4350–4355. doi: 10.1073/pnas.1601831113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ouellette AJ. Paneth cell alpha-defensins in enteric innate immunity. Cell. Mol. Life Sci. 2011;68:2215–2229. doi: 10.1007/s00018-011-0714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Courth LF, et al. Crohn’s disease-derived monocytes fail to induce Paneth cell defensins. Proc. Natl Acad. Sci. USA. 2015;112:14000–14005. doi: 10.1073/pnas.1510084112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang E, Shen J. The roles and functions of Paneth cells in Crohn’s disease: a critical review. Cell Prolif. 2021;54:e12958. doi: 10.1111/cpr.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ouellette AJ. Paneth cell alpha-defensin synthesis and function. Curr. Top. Microbiol. Immunol. 2006;306:1–25. doi: 10.1007/3-540-29916-5_1. [DOI] [PubMed] [Google Scholar]
- 106.Ericksen B, Wu Z, Lu W, Lehrer RI. Antibacterial activity and specificity of the six human {alpha}-defensins. Antimicrob. Agents Chemother. 2005;49:269–275. doi: 10.1128/AAC.49.1.269-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ehmann D, et al. Paneth cell α-defensins HD-5 and HD-6 display differential degradation into active antimicrobial fragments. Proc. Natl Acad. Sci. USA. 2019;116:3746–3751. doi: 10.1073/pnas.1817376116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chairatana P, Nolan EM. Human α-defensin 6: a small peptide that self-assembles and protects the host by entangling microbes. Acc. Chem. Res. 2017;50:960–967. doi: 10.1021/acs.accounts.6b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chairatana P, Chiang IL, Nolan EM. Human α-defensin 6 self-assembly prevents adhesion and suppresses virulence traits of Candida albicans. Biochemistry. 2017;56:1033–1041. doi: 10.1021/acs.biochem.6b01111. [DOI] [PubMed] [Google Scholar]
- 110.Huttner KM, Selsted ME, Ouellette AJ. Structure and diversity of the murine cryptdin gene family. Genomics. 1994;19:448–453. doi: 10.1006/geno.1994.1093. [DOI] [PubMed] [Google Scholar]
- 111.Ayabe T, et al. Modulation of mouse Paneth cell α-defensin secretion by mIKCa1, a Ca2+-activated, intermediate conductance potassium channel. J. Biol. Chem. 2002;277:3793–3800. doi: 10.1074/jbc.M107507200. [DOI] [PubMed] [Google Scholar]
- 112.Nakamura K, et al. Expression and localization of Paneth cells and their α-defensins in the small intestine of adult mouse. Front. Immunol. 2020;11:570296. doi: 10.3389/fimmu.2020.570296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Selsted ME, Miller SI, Henschen AH, Ouellette AJ. Enteric defensins: antibiotic peptide components of intestinal host defense. J. Cell Biol. 1992;118:929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ouellette AJ, et al. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect. Immun. 1994;62:5040–5047. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gassler N. Paneth cells in intestinal physiology and pathophysiology. World J. Gastrointest. Pathophysiol. 2017;8:150–160. doi: 10.4291/wjgp.v8.i4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tongaonkar P, Selsted ME. SDF2L1, a component of the endoplasmic reticulum chaperone complex, differentially interacts with {alpha}-, {beta}-, and {theta}-defensin propeptides. J. Biol. Chem. 2009;284:5602–5609. doi: 10.1074/jbc.M806664200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li K, et al. CD14 overexpression upregulates TNF-α-mediated inflammatory responses and suppresses the malignancy of gastric carcinoma cells. Mol. Cell. Biochem. 2013;376:137–143. doi: 10.1007/s11010-013-1559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tollner TL, Bevins CL, Cherr GN. Multifunctional glycoprotein DEFB126—a curious story of defensin-clad spermatozoa. Nat. Rev. Urol. 2012;9:365–375. doi: 10.1038/nrurol.2012.109. [DOI] [PubMed] [Google Scholar]
- 119.Pace BT, Lackner AA, Porter E, Pahar B. The role of defensins in HIV pathogenesis. Mediat. Inflamm. 2017;2017:5186904. doi: 10.1155/2017/5186904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lehrer RI, Cole AM, Selsted ME. θ-Defensins: cyclic peptides with endless potential. J. Biol. Chem. 2012;287:27014–27019. doi: 10.1074/jbc.R112.346098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nguyen TX, Cole AM, Lehrer RI. Evolution of primate θ-defensins: a serpentine path to a sweet tooth. Peptides. 2003;24:1647–1654. doi: 10.1016/j.peptides.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 122.Li D, et al. Evolution of primate α and θ defensins revealed by analysis of genomes. Mol. Biol. Rep. 2014;41:3859–3866. doi: 10.1007/s11033-014-3253-z. [DOI] [PubMed] [Google Scholar]
- 123.Lazzaro BP, Zasloff M, Rolff J. Antimicrobial peptides: application informed by evolution. Science. 2020;368:eaau5480. doi: 10.1126/science.aau5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu SS, et al. Antimicrobial and immunomodulatory activity of beta-defensin from the Chinese spiny frog (Quasipaa spinosa) Dev. Comp. Immunol. 2022;126:104264. doi: 10.1016/j.dci.2021.104264. [DOI] [PubMed] [Google Scholar]
- 125.Stambuk F, et al. Big defensin from the scallop Argopecten purpuratus ApBD1 is an antimicrobial peptide which entraps bacteria through nanonets formation. Fish. Shellfish Immunol. 2021;119:456–461. doi: 10.1016/j.fsi.2021.10.037. [DOI] [PubMed] [Google Scholar]
- 126.Yacoub HA, et al. Biocidal activity of chicken defensin-9 against microbial pathogens. Biochem. Cell Biol. 2016;94:176–187. doi: 10.1139/bcb-2015-0121. [DOI] [PubMed] [Google Scholar]
- 127.Bertrams W, et al. Tribolium castaneum defensin 1 kills Moraxella catarrhalisin an in vitro infection model but does not harm commensal bacteria. Virulence. 2021;12:1003–1010. doi: 10.1080/21505594.2021.1908741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jung SW, Lee J, Cho AE. Elucidating the bacterial membrane disruption mechanism of human α-defensin 5: a theoretical study. J. Phys. Chem. B. 2017;121:741–748. doi: 10.1021/acs.jpcb.6b11806. [DOI] [PubMed] [Google Scholar]
- 129.Mathew B, Olli S, Guru A, Nagaraj R. Chimeric analogs of human β-defensin 1 and θ-defensin disrupt pre-established bacterial biofilms. Bioorg. Med. Chem. Lett. 2017;27:3264–3266. doi: 10.1016/j.bmcl.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 130.Soares JR, et al. Interaction between the plant ApDef(1) defensin and Saccharomyces cerevisiae results in yeast death through a cell cycle- and caspase-dependent process occurring via uncontrolled oxidative stress. Biochim. Biophys. Acta-Gen. Subj. 2017;1861:3429–3443. doi: 10.1016/j.bbagen.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 131.Bolatchiev A. Antibacterial activity of human defensins against Staphylococcus aureus and Escherichia coli. PeerJ. 2020;8:e10455. doi: 10.7717/peerj.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Luo Y, Song Y. Mechanism of antimicrobial peptides: antimicrobial, anti-inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021;22:11401. doi: 10.3390/ijms222111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aghamiri S, et al. Antimicrobial peptides as potential therapeutics for breast cancer. Pharmacol. Res. 2021;171:105777. doi: 10.1016/j.phrs.2021.105777. [DOI] [PubMed] [Google Scholar]
- 134.Kumar P, Kizhakkedathu JN, Straus SK. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules. 2018;8:4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ. Antimicrobial host defence peptides: functions and clinical potential. Nat. Rev. Drug Discov. 2020;19:311–332. doi: 10.1038/s41573-019-0058-8. [DOI] [PubMed] [Google Scholar]
- 136.Bin Hafeez A, Jiang X, Bergen PJ, Zhu Y. Antimicrobial peptides: an update on classifications and databases. Int. J. Mol. Sci. 2021;22:11691. doi: 10.3390/ijms222111691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hollmann A, Martinez M, Maturana P, Semorile LC, Maffia PC. Antimicrobial peptides: interaction with model and biological membranes and synergism with chemical antibiotics. Front. Chem. 2018;6:204. doi: 10.3389/fchem.2018.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 140.Wu Q, Patočka J, Kuča K. Insect antimicrobial peptides, a mini review. Toxins. 2018;10:461. doi: 10.3390/toxins10110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tuerkova A, et al. Effect of helical kink in antimicrobial peptides on membrane pore formation. eLife. 2020;9:e47946. doi: 10.7554/eLife.47946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Awang T, Pongprayoon P. The penetration of human defensin 5 (HD5) through bacterial outer membrane: simulation studies. J. Mol. Model. 2021;27:291. doi: 10.1007/s00894-021-04915-w. [DOI] [PubMed] [Google Scholar]
- 143.Kabelka I, Vácha R. Advances in molecular understanding of α-helical membrane-active peptides. Acc. Chem. Res. 2021;54:2196–2204. doi: 10.1021/acs.accounts.1c00047. [DOI] [PubMed] [Google Scholar]
- 144.Matsuzaki K. Membrane permeabilization mechanisms. Adv. Exp. Med. Biol. 2019;1117:9–16. doi: 10.1007/978-981-13-3588-4_2. [DOI] [PubMed] [Google Scholar]
- 145.Lee J, Jung SW, Cho AE. Molecular insights into the adsorption mechanism of human β-defensin-3 on bacterial membranes. Langmuir. 2016;32:1782–1790. doi: 10.1021/acs.langmuir.5b04113. [DOI] [PubMed] [Google Scholar]
- 146.de Leeuw E, et al. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett. 2010;584:1543–1548. doi: 10.1016/j.febslet.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sass V, et al. Human β-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect. Immun. 2010;78:2793–2800. doi: 10.1128/IAI.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moazzezy N, et al. Computational evaluation of modified peptides from human neutrophil peptide 1 (HNP-1) J. Biomol. Struct. Dyn. 2022;40:1163–1171. doi: 10.1080/07391102.2020.1823249. [DOI] [PubMed] [Google Scholar]
- 149.Grein F, Schneider T, Sahl H-G. Docking on lipid II—a widespread mechanism for potent bactericidal activities of antibiotic peptides. J. Mol. Biol. 2019;431:3520–3530. doi: 10.1016/j.jmb.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 150.Wilmes M, Cammue BP, Sahl HG, Thevissen K. Antibiotic activities of host defense peptides: more to it than lipid bilayer perturbation. Nat. Prod. Rep. 2011;28:1350–1358. doi: 10.1039/c1np00022e. [DOI] [PubMed] [Google Scholar]
- 151.Oeemig JS, et al. Eurocin, a new fungal defensin: structure, lipid binding, and its mode of action. J. Biol. Chem. 2012;287:42361–42372. doi: 10.1074/jbc.M112.382028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Essig A, et al. Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J. Biol. Chem. 2014;289:34953–34964. doi: 10.1074/jbc.M114.599878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schmitt P, et al. Insight into invertebrate defensin mechanism of action: oyster defensins inhibit peptidoglycan biosynthesis by binding to lipid II. J. Biol. Chem. 2010;285:29208–29216. doi: 10.1074/jbc.M110.143388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ehmann D, et al. Fragmentation of human neutrophil α-defensin 4 to combat multidrug resistant bacteria. Front. Microbiol. 2020;11:1147. doi: 10.3389/fmicb.2020.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lu W. Antimicrobial peptides. Semin. Cell Dev. Biol. 2019;88:105–106. doi: 10.1016/j.semcdb.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 156.Gremmel T, et al. Human neutrophil α-defensins are associated with adenosine diphosphate-inducible neutrophil-platelet aggregate formation and response to clopidogrel in patients with atherosclerosis. Transl. Res. 2014;164:202–208. doi: 10.1016/j.trsl.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 157.Higazi M, et al. Opposing effects of HNP1 (α-defensin-1) on plasma cholesterol and atherogenesis. PLoS ONE. 2020;15:e0231582. doi: 10.1371/journal.pone.0231582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kolbinger F, et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J. Allergy Clin. Immunol. 2017;139:923–932. doi: 10.1016/j.jaci.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 159.Miani M, et al. Gut microbiota-stimulated innate lymphoid cells support β-defensin 14 expression in pancreatic endocrine cells, preventing autoimmune diabetes. Cell Metab. 2018;28:557–572. doi: 10.1016/j.cmet.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 160.Németh BC, et al. Relevance of α-defensins (HNP1-3) and defensin β-1 in diabetes. World J. Gastroenterol. 2014;20:9128–9137. doi: 10.3748/wjg.v20.i27.9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Antikainen AAV, et al. Genome-wide association study on coronary artery disease in type 1 diabetes suggests beta-defensin 127 as a risk locus. Cardiovasc. Res. 2021;117:600–612. doi: 10.1093/cvr/cvaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen Q, et al. Human neutrophil defensins disrupt liver interendothelial junctions and aggravate sepsis. Mediators Inflamm. 2022;2022:7659282. doi: 10.1155/2022/7659282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hancock REW, Haney EF, Gill EE. The immunology of host defence peptides: beyond antimicrobial activity. Nat. Rev. Immunol. 2016;16:321–334. doi: 10.1038/nri.2016.29. [DOI] [PubMed] [Google Scholar]
- 164.Liang S, et al. Nutrient sensing by the intestinal epithelium orchestrates mucosal antimicrobial defense via translational control of Hes1. Cell Host Microbe. 2019;25:706–718. doi: 10.1016/j.chom.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 165.Huang C, et al. Porcine beta-defensin 2 provides protection against bacterial infection by a direct bactericidal activity and alleviates inflammation via interference with the TLR4/NF-κB pathway. Front. Immunol. 2019;10:1673–1673. doi: 10.3389/fimmu.2019.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang K, et al. Recombinant porcine beta defensin 2 alleviates inflammatory responses induced by Escherichia coli in IPEC-J2 cells. Int. J. Biol. Macromol. 2022;208:890–900. doi: 10.1016/j.ijbiomac.2022.03.178. [DOI] [PubMed] [Google Scholar]
- 167.Lian S, et al. Transcriptome analysis reveals the multiple functions of pBD2 in IPEC-J2 cells against E. coli. Int. J. Mol. Sci. 2022;23:9754. doi: 10.3390/ijms23179754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xie K, et al. The immunomodulatory function of the porcine β-defensin 129: alleviate inflammatory response induced by LPS in IPEC-J2 cells. Int. J. Biol. Macromol. 2021;188:473–481. doi: 10.1016/j.ijbiomac.2021.07.194. [DOI] [PubMed] [Google Scholar]
- 169.Semple F, et al. Human beta-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur. J. Immunol. 2010;40:1073–1078. doi: 10.1002/eji.200940041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Miles K, et al. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J. Immunol. 2009;183:2122–2132. doi: 10.4049/jimmunol.0804187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shi J, et al. A novel role for defensins in intestinal homeostasis: regulation of IL-1beta secretion. J. Immunol. 2007;179:1245–1253. doi: 10.4049/jimmunol.179.2.1245. [DOI] [PubMed] [Google Scholar]
- 172.Galliher-Beckley AJ, Lan LQ, Aono S, Wang L, Shi J. Caspase-1 activation and mature interleukin-1β release are uncoupled events in monocytes. World J. Biol. Chem. 2013;4:30–34. doi: 10.4331/wjbc.v4.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McGlasson SL, et al. Human β-defensin 3 increases the TLR9-dependent response to bacterial DNA. Eur. J. Immunol. 2017;47:658–664. doi: 10.1002/eji.201646799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tewary P, et al. β-Defensin 2 and 3 promote the uptake of self or CpG DNA, enhance IFN-α production by human plasmacytoid dendritic cells, and promote inflammation. J. Immunol. 2013;191:865–874. doi: 10.4049/jimmunol.1201648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Soehnlein O, et al. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J. Clin. Invest. 2008;118:3491–3502. doi: 10.1172/JCI35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fischer N, et al. Histone deacetylase inhibition enhances antimicrobial peptide but not inflammatory cytokine expression upon bacterial challenge. Proc. Natl Acad. Sci. USA. 2016;113:E2993–E3001. doi: 10.1073/pnas.1605997113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zong X, et al. Enterotoxigenic Escherichia coli infection promotes enteric defensin expression via FOXO6-METTL3-m(6)A-GPR161 signalling axis. RNA Biol. 2021;18:576–586. doi: 10.1080/15476286.2020.1820193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shirin T, et al. Antimicrobial peptides in the duodenum at the acute and convalescent stages in patients with diarrhea due to Vibrio cholerae O1 or enterotoxigenic Escherichia coli infection. Microbes Infect. 2011;13:1111–1120. doi: 10.1016/j.micinf.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 179.Yoon YM, et al. Bacteroides fragilis enterotoxin induces human beta-defensin-2 expression in intestinal epithelial cells via a mitogen-activated protein kinase/I kappaB kinase/NF-kappaB-dependent pathway. Infect. Immun. 2010;78:2024–2033. doi: 10.1128/IAI.00118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang F-C. Differential regulation of interleukin-8 and human beta-defensin 2 in Pseudomonas aeruginosa-infected intestinal epithelial cells. BMC Microbiol. 2014;14:275–275. doi: 10.1186/s12866-014-0275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Madi A, et al. Pseudomonas fluorescens can induce and divert the human beta-defensin-2 secretion in intestinal epithelial cells to enhance its virulence. Arch. Microbiol. 2013;195:189–195. doi: 10.1007/s00203-012-0865-3. [DOI] [PubMed] [Google Scholar]
- 182.Fusco A, et al. Beta-defensin-2 and beta-Defensin-3 reduce intestinal damage caused by Salmonella typhimurium modulating the expression of cytokines and enhancing the probiotic activity of Enterococcus faecium. J. Immunol. Res. 2017;2017:6976935. doi: 10.1155/2017/6976935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Garcia JS, Byrd JA, Wong EA. Tissue-, age- and dose-dependent changes in avian β-defensin and LEAP2 mRNA abundance in the intestines of Salmonella Typhimurium challenged broilers. Anim. Biotechnol. 2021;32:637–645. doi: 10.1080/10495398.2020.1738449. [DOI] [PubMed] [Google Scholar]
- 184.Ogushi K, et al. Salmonella enteritidis FliC (flagella filament protein) induces human beta-defensin-2 mRNA production by Caco-2 cells. J. Biol. Chem. 2001;276:30521–30526. doi: 10.1074/jbc.M011618200. [DOI] [PubMed] [Google Scholar]
- 185.Ogushi K, et al. Gangliosides act as co-receptors for Salmonella enteritidis FliC and promote FliC induction of human beta-defensin-2 expression in Caco-2 cells. J. Biol. Chem. 2004;279:12213–12219. doi: 10.1074/jbc.M307944200. [DOI] [PubMed] [Google Scholar]
- 186.Grubman A, et al. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell Microbiol. 2010;12:626–639. doi: 10.1111/j.1462-5822.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 187.Wada A, et al. Helicobacter pylori-mediated transcriptional regulation of the human beta-defensin 2 gene requires NF-kappaB. Cell Microbiol. 2001;3:115–123. doi: 10.1046/j.1462-5822.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 188.Bauer B, et al. The Helicobacter pylori virulence effector CagA abrogates human beta-defensin 3 expression via inactivation of EGFR signaling. Cell Host Microbe. 2012;11:576–586. doi: 10.1016/j.chom.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 189.Mackewicz CE, et al. alpha-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. Aids. 2003;17:F23–F32. doi: 10.1097/00002030-200309260-00001. [DOI] [PubMed] [Google Scholar]
- 190.Ayabe T, et al. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 191.Pal S, Schmidt AP, Peterson EM, Wilson CL, de la Maza LM. Role of matrix metalloproteinase-7 in the modulation of a Chlamydia trachomatis infection. Immunology. 2006;117:213–219. doi: 10.1111/j.1365-2567.2005.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Putsep K, et al. Germ-free and colonized mice generate the same products from enteric prodefensins. J. Biol. Chem. 2000;275:40478–40482. doi: 10.1074/jbc.M007816200. [DOI] [PubMed] [Google Scholar]
- 193.Tan G, Zeng B, Zhi FC. Regulation of human enteric alpha-defensins by NOD2 in the Paneth cell lineage. Eur. J. Cell Biol. 2015;94:60–66. doi: 10.1016/j.ejcb.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 194.Petnicki-Ocwieja T, et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl Acad. Sci. USA. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fruitwala S, El-Naccache DW, Chang TL. Multifaceted immune functions of human defensins and underlying mechanisms. Semin. Cell Dev. Biol. 2019;88:163–172. doi: 10.1016/j.semcdb.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Woo J-I, et al. NOD2/RICK-dependent β-defensin 2 regulation is protective for nontypeable Haemophilus influenzae-induced middle ear infection. PLoS ONE. 2014;9:e90933. doi: 10.1371/journal.pone.0090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 198.Wang F. Fighting the SARS-CoV-2 pandemic: focusing a new lens on COVID-19. Research. 2022;2022:9879646. doi: 10.34133/2022/9879646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fang E, et al. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022;7:94. doi: 10.1038/s41392-022-00950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yan W, Zheng Y, Zeng X, He B, Cheng W. Structural biology of SARS-CoV-2: open the door for novel therapies. Signal Transduct. Target. Ther. 2022;7:26. doi: 10.1038/s41392-022-00884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Laneri S, et al. Antimicrobial peptides and physical activity: a great hope against COVID 19. Microorganisms. 2021;9:1415. doi: 10.3390/microorganisms9071415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Idris MM, Banu S, Siva AB, Nagaraj R. Down regulation of defensin genes during SARS-CoV-2 infection. Acta Virol. 2022;66:249–253. doi: 10.4149/av_2022_306. [DOI] [PubMed] [Google Scholar]
- 203.Banu S, Nagaraj R, Idris MM. Defensins: therapeutic molecules with potential to treat SARS-CoV-2 infection. Indian J. Med. Res. 2022;155:83–85. doi: 10.4103/ijmr.ijmr_2798_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Al-Bayatee NT, Ad’hiah AH. Human beta-defensins 2 and 4 are dysregulated in patients with coronavirus disease 19. Microb. Pathog. 2021;160:105205. doi: 10.1016/j.micpath.2021.105205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang C, et al. Human intestinal defensin 5 inhibits SARS-CoV-2 invasion by cloaking ACE2. Gastroenterology. 2020;159:1145–1147. doi: 10.1053/j.gastro.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xu C, et al. Human defensins inhibit SARS-CoV-2 infection by blocking viral entry. Viruses. 2021;13:1246. doi: 10.3390/v13071246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang L, et al. HBD-2 binds SARS-CoV-2 RBD and blocks viral entry: strategy to combat COVID-19. iScience. 2022;25:103856. doi: 10.1016/j.isci.2022.103856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Deepthi V, Mohanakumar KP, Rajamma U. Efficacy of defensins as neutralizing agents against the deadly SARS-CoV-2. J. Biomol. Struct. Dyn. 2022;41:1–15. doi: 10.1080/07391102.2022.2041487. [DOI] [PubMed] [Google Scholar]
- 209.Kudryashova E, et al. Inhibition of SARS-CoV-2 infection by human defensin HNP1 and retrocyclin RC-101. J. Mol. Biol. 2022;434:167225. doi: 10.1016/j.jmb.2021.167225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liscano Y, Oñate-Garzón J, Ocampo-Ibáñez ID. In silico discovery of antimicrobial peptides as an alternative to control SARS-CoV-2. Molecules. 2020;25:5535. doi: 10.3390/molecules25235535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brancaccio M, et al. Diagnostic and therapeutic potential for HNP-1, HBD-1 and HBD-4 in pregnant women with COVID-19. Int. J. Mol. Sci. 2022;23:3450. doi: 10.3390/ijms23073450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. 2021;54:859–874. doi: 10.1016/j.immuni.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 214.Yang D, Chertov O, Oppenheim JJ. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell. Mol. Life Sci. 2001;58:978–989. doi: 10.1007/PL00000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chertov O, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 217.Conejo-Garcia JR, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat. Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 218.Biragyn A, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J. Immunol. 2001;167:6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 219.Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 220.Jin G, et al. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS ONE. 2010;5:e10993. doi: 10.1371/journal.pone.0010993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Human beta-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J. Immunol. 2010;184:6688–6694. doi: 10.4049/jimmunol.0903984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jiang H, et al. Chemotactic effect of β-defensin 1 on macrophages in Megalobrama amblycephala. Fish. Shellfish Immunol. 2018;74:35–42. doi: 10.1016/j.fsi.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 223.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr. Opin. Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 224.Wehkamp J, Koslowski M, Wang G, Stange EF. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn’s disease. Mucosal Immunol. 2008;1:S67–S74. doi: 10.1038/mi.2008.48. [DOI] [PubMed] [Google Scholar]
- 225.Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019;20:970–979. doi: 10.1038/s41590-019-0415-0. [DOI] [PubMed] [Google Scholar]
- 226.Nguyen HLT, et al. Role of antimicrobial peptides in skin barrier repair in individuals with atopic dermatitis. Int. J. Mol. Sci. 2020;21:7607. doi: 10.3390/ijms21207607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Das P, et al. Keratinocytes: an enigmatic factor in atopic dermatitis. Cells. 2022;11:1683. doi: 10.3390/cells11101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schoultz I, Keita ÅV. Cellular and molecular therapeutic targets in inflammatory bowel disease-focusing on intestinal barrier function. Cells. 2019;8:193. doi: 10.3390/cells8020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nuding S, Fellermann K, Wehkamp J, Stange EF. Reduced mucosal antimicrobial activity in Crohn’s disease of the colon. Gut. 2007;56:1240. doi: 10.1136/gut.2006.118646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fusco A, Savio V, Donniacuo M, Perfetto B, Donnarumma G. Antimicrobial peptides human beta-defensin-2 and -3 protect the gut during candida albicans infections enhancing the intestinal barrier integrity: in vitro study. Front. Cell. Infect. Microbiol. 2021;11:666900. doi: 10.3389/fcimb.2021.666900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kiatsurayanon C, et al. Host defense (Antimicrobial) peptide, human β-defensin-3, improves the function of the epithelial tight-junction barrier in human keratinocytes. J. Invest. Dermatol. 2014;134:2163–2173. doi: 10.1038/jid.2014.143. [DOI] [PubMed] [Google Scholar]
- 232.Chen L, et al. Human β-defensin-3 reduces excessive autophagy in intestinal epithelial cells and in experimental necrotizing enterocolitis. Sci. Rep. 2019;9:19890. doi: 10.1038/s41598-019-56535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Otte JM, et al. Human beta defensin 2 promotes intestinal wound healing in vitro. J. Cell. Biochem. 2008;104:2286–2297. doi: 10.1002/jcb.21787. [DOI] [PubMed] [Google Scholar]
- 234.Sheng Q, et al. Human β-defensin-3 promotes intestinal epithelial cell migration and reduces the development of necrotizing enterocolitis in a neonatal rat model. Pediatr. Res. 2014;76:269–279. doi: 10.1038/pr.2014.93. [DOI] [PubMed] [Google Scholar]
- 235.Baroni A, et al. Antimicrobial human beta-defensin-2 stimulates migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides. 2009;30:267–272. doi: 10.1016/j.peptides.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 236.Vongsa RA, Zimmerman NP, Dwinell MB. CCR6 regulation of the actin cytoskeleton orchestrates human beta defensin-2- and CCL20-mediated restitution of colonic epithelial cells. J. Biol. Chem. 2009;284:10034–10045. doi: 10.1074/jbc.M805289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zilbauer M, et al. Expression of human beta-defensins in children with chronic inflammatory bowel disease. PLoS ONE. 2010;5:e15389. doi: 10.1371/journal.pone.0015389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shulman RJ, Devaraj S, Heitkemper M. Activation of the innate immune system in children with irritable bowel syndrome evidenced by increased fecal human β-defensin-2. Clin. Gastroenterol. Hepatol. 2021;19:2121–2127. doi: 10.1016/j.cgh.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jensen JM, et al. Barrier function, epidermal differentiation, and human beta-defensin 2 expression in tinea corporis. J. Investig. Dermatol. 2007;127:1720–1727. doi: 10.1038/sj.jid.5700788. [DOI] [PubMed] [Google Scholar]
- 240.Clausen ML, et al. Human β-defensin-2 as a marker for disease severity and skin barrier properties in atopic dermatitis. Br. J. Dermatol. 2013;169:587–593. doi: 10.1111/bjd.12419. [DOI] [PubMed] [Google Scholar]
- 241.Ji J, Gu Z, Li H, Su L, Liu Z. Cryptdin-2 predicts intestinal injury during heatstroke in mice. Int. J. Mol. Med. 2018;41:137–146. doi: 10.3892/ijmm.2017.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kaliannan K. Compromise of α-defensin function in liver cirrhosis facilitates the toxic relationship between gut permeability and endotoxemia. Dig. Dis. Sci. 2018;63:2492–2494. doi: 10.1007/s10620-018-5197-y. [DOI] [PubMed] [Google Scholar]
- 243.Hayashi R, et al. Reduced human α-defensin 6 in noninflamed jejunal tissue of patients with Crohn’s disease. Inflamm. Bowel Dis. 2016;22:1119–1128. doi: 10.1097/MIB.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 244.Beisner J, et al. TCF-1-mediated Wnt signaling regulates Paneth cell innate immune defense effectors HD-5 and -6: implications for Crohn’s disease. Am. J. Physiol. Gastroint. Liver Physiol. 2014;307:G487–G498. doi: 10.1152/ajpgi.00347.2013. [DOI] [PubMed] [Google Scholar]
- 245.Sorensen OE, et al. Injury-induced innate immune response in human skin mediated by transactivation of the epidermal growth factor receptor. J. Clin. Invest. 2006;116:1878–1885. doi: 10.1172/JCI28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sukhotnik I, et al. Transforming growth factor-alpha stimulates enterocyte proliferation and accelerates intestinal recovery following methotrexate-induced intestinal mucositis in a rat and a cell culture model. Pediatr. Surg. Int. 2008;24:1303–1311. doi: 10.1007/s00383-008-2271-0. [DOI] [PubMed] [Google Scholar]
- 247.Seno H, et al. Enhanced expression of transforming growth factor (TGF) -alpha precursor and TGF-beta1 during Paneth cell regeneration. Dig. Dis. Sci. 2001;46:1004–1010. doi: 10.1023/a:1010797609041. [DOI] [PubMed] [Google Scholar]
- 248.Cobo ER, Kissoon-Singh V, Moreau F, Chadee K. Colonic MUC2 mucin regulates the expression and antimicrobial activity of β-defensin 2. Mucosal Immunol. 2015;8:1360–1372. doi: 10.1038/mi.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Xu D, et al. Human enteric α-defensin 5 promotes shigella infection by enhancing bacterial adhesion and invasion. Immunity. 2018;48:1233–1244. doi: 10.1016/j.immuni.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jones RM, Neish AS. Gut microbiota in intestinal and liver disease. Annu. Rev. Pathol. Mech. Dis. 2021;16:251–275. doi: 10.1146/annurev-pathol-030320-095722. [DOI] [PubMed] [Google Scholar]
- 251.Hou K, et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022;7:135. doi: 10.1038/s41392-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Franzosa EA, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019;4:293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kumar P, et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity. 2016;44:659–671. doi: 10.1016/j.immuni.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Salzman NH, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl Acad. Sci. USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Salzman NH. Paneth cell defensins and the regulation of the microbiome: détente at mucosal surfaces. Gut Microbes. 2010;1:401–406. doi: 10.4161/gmic.1.6.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Shimizu Y, et al. Lower human defensin 5 in elderly people compared to middle-aged is associated with differences in the intestinal microbiota composition: the DOSANCO Health Study. Geroscience. 2022;44:997–1009. doi: 10.1007/s11357-021-00398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020;11:906. doi: 10.3389/fimmu.2020.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Waters JL, Ley RE. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019;17:83. doi: 10.1186/s12915-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tanigawa T, et al. Rebamipide inhibits indomethacin-induced small intestinal injury: possible involvement of intestinal microbiota modulation by upregulation of α-defensin 5. Eur. J. Pharmacol. 2013;704:64–69. doi: 10.1016/j.ejphar.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 262.Suzuki K, et al. Decrease of α-defensin impairs intestinal metabolite homeostasis via dysbiosis in mouse chronic social defeat stress model. Sci. Rep. 2021;11:9915. doi: 10.1038/s41598-021-89308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rückert T, et al. Human β-defensin 2 ameliorates acute GVHD by limiting ileal neutrophil infiltration and restraining T cell receptor signaling. Sci. Transl. Med. 2022;14:eabp9675. doi: 10.1126/scitranslmed.abp9675. [DOI] [PubMed] [Google Scholar]
- 264.Sofi MH, et al. A single strain of Bacteroides fragilis protects gut integrity and reduces GVHD. JCI Insight. 2021;6:e136841. doi: 10.1172/jci.insight.136841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Walker WA. The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr. Res. 2017;82:387–395. doi: 10.1038/pr.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Collado MC, Delgado S, Maldonado A, Rodríguez JM. Assessment of the bacterial diversity of breast milk of healthy women by quantitative real-time PCR. Lett. Appl. Microbiol. 2009;48:523–528. doi: 10.1111/j.1472-765X.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 267.Jeurink PV, et al. Human milk: a source of more life than we imagine. Benef. Microbes. 2013;4:17–30. doi: 10.3920/BM2012.0040. [DOI] [PubMed] [Google Scholar]
- 268.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 1999;69:1035s–1045s. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 269.Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Malcolm J, et al. Salivary antimicrobial proteins associate with age-related changes in streptococcal composition in dental plaque. Mol. Oral. Microbiol. 2014;29:284–293. doi: 10.1111/omi.12058. [DOI] [PubMed] [Google Scholar]
- 271.van Es JH, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 272.Ménard S, et al. Developmental switch of intestinal antimicrobial peptide expression. J. Exp. Med. 2008;205:183–193. doi: 10.1084/jem.20071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Salzman NH, et al. Enteric defensin expression in necrotizing enterocolitis. Pediatr. Res. 1998;44:20–26. doi: 10.1203/00006450-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 274.Mallow EB, et al. Human enteric defensins. Gene structure and developmental expression. J. Biol. Chem. 1996;271:4038–4045. doi: 10.1074/jbc.271.8.4038. [DOI] [PubMed] [Google Scholar]
- 275.Lyu W, et al. Developmental and tissue patterns of the basal expression of chicken avian β-defensins. Biomed. Res. Int. 2020;2020:2567861. doi: 10.1155/2020/2567861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Virtanen JA, Cheng KH, Somerharju P. Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model. Proc. Natl Acad. Sci. USA. 1998;95:4964–4969. doi: 10.1073/pnas.95.9.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 278.Baxter AA, Lay FT, Poon IKH, Kvansakul M, Hulett MD. Tumor cell membrane-targeting cationic antimicrobial peptides: novel insights into mechanisms of action and therapeutic prospects. Cell. Mol. Life Sci. 2017;74:3809–3825. doi: 10.1007/s00018-017-2604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Liu C-Y, et al. The concentration-dependent chemokine release and pro-apoptotic effects of neutrophil-derived α-defensin-1 on human bronchial and alveolar epithelial cells. Life Sci. 2007;80:749–758. doi: 10.1016/j.lfs.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 280.Ibusuki R, et al. Human neutrophil peptide-1 promotes alcohol-induced hepatic fibrosis and hepatocyte apoptosis. PLoS ONE. 2017;12:e0174913. doi: 10.1371/journal.pone.0174913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nagaoka I, et al. Evaluation of the effect of α-defensin human neutrophil peptides on neutrophil apoptosis. Int. J. Mol. Med. 2010;26:925–934. doi: 10.3892/ijmm_00000544. [DOI] [PubMed] [Google Scholar]
- 282.Lu W, de Leeuw E. Pro-inflammatory and pro-apoptotic properties of Human Defensin 5. Biochem. Biophys. Res. Commun. 2013;436:557–562. doi: 10.1016/j.bbrc.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wang W, et al. Human β-defensin-3 induces IL-8 release and apoptosis in airway smooth muscle cells. Clin. Exp. Allergy. 2017;47:1138–1149. doi: 10.1111/cea.12943. [DOI] [PubMed] [Google Scholar]
- 284.Kashem SW, Haniffa M, Kaplan DH. Antigen-presenting cells in the skin. Annu. Rev. Immunol. 2017;35:469–499. doi: 10.1146/annurev-immunol-051116-052215. [DOI] [PubMed] [Google Scholar]
- 285.Wculek SK, et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 286.Biragyn A, et al. Murine beta-defensin 2 promotes TLR-4/MyD88-mediated and NF-kappaB-dependent atypical death of APCs via activation of TNFR2. J. Leukoc. Biol. 2008;83:998–1008. doi: 10.1189/jlb.1007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Lu W, de Leeuw E. Functional intersection of human defensin 5 with the TNF receptor pathway. FEBS Lett. 2014;588:1906–1912. doi: 10.1016/j.febslet.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wu J, et al. Immunoregulatory effect of human β-defensin 1 on neonatal cord blood monocyte-derived dendritic cells and T cells. Mol. Immunol. 2019;109:99–107. doi: 10.1016/j.molimm.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 289.Flores-Alvarez LJ, et al. PaDef defensin from avocado (Persea americana var. drymifolia) is cytotoxic to K562 chronic myeloid leukemia cells through extrinsic apoptosis. Int. J. Biochem. Cell Biol. 2018;99:10–18. doi: 10.1016/j.biocel.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 290.McKeown ST, et al. The cytotoxic effects of human neutrophil peptide-1 (HNP1) and lactoferrin on oral squamous cell carcinoma (OSCC) in vitro. Oral. Oncol. 2006;42:685–690. doi: 10.1016/j.oraloncology.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 291.Baxter AA, Poon IK, Hulett MD. The plant defensin NaD1 induces tumor cell death via a non-apoptotic, membranolytic process. Cell Death Discov. 2017;3:16102. doi: 10.1038/cddiscovery.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Liu WJ, et al. EGFR-targeting, β-defensin-tailored fusion protein exhibits high therapeutic efficacy against EGFR-expressed human carcinoma via mitochondria-mediated apoptosis. Acta Pharmacol. Sin. 2018;39:1777–1786. doi: 10.1038/s41401-018-0069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Guzmán-Rodríguez JJ, López-Gómez R, Salgado-Garciglia R, Ochoa-Zarzosa A, López-Meza JE. The defensin from avocado (Persea americana var. drymifolia) PaDef induces apoptosis in the human breast cancer cell line MCF-7. Biomed. Pharmacother. 2016;82:620–627. doi: 10.1016/j.biopha.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 294.Jiménez-Alcántar P, López-Gómez R, López-Meza JE, Ochoa-Zarzosa A. PaDef (Persea americana var. drymifolia), a plant antimicrobial peptide, triggers apoptosis, and induces global epigenetic modifications on histone 3 in an acute lymphoid leukemia cell line. Front. Mol. Biosci. 2022;9:801816. doi: 10.3389/fmolb.2022.801816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Aarbiou J, et al. Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm. Res. 2006;55:119–127. doi: 10.1007/s00011-005-0062-9. [DOI] [PubMed] [Google Scholar]
- 296.Agarwal S, et al. Immunomodulatory effects of β-defensin 2 on macrophages induced immuno-upregulation and their antitumor function in breast cancer. BMC Immunol. 2022;23:53. doi: 10.1186/s12865-022-00527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Donald CD, et al. Cancer-specific loss of beta-defensin 1 in renal and prostatic carcinomas. Lab. Invest. 2003;83:501–505. doi: 10.1097/01.lab.0000063929.61760.f6. [DOI] [PubMed] [Google Scholar]
- 298.Sun CQ, et al. Human beta-defensin-1, a potential chromosome 8p tumor suppressor: control of transcription and induction of apoptosis in renal cell carcinoma. Cancer Res. 2006;66:8542–8549. doi: 10.1158/0008-5472.CAN-06-0294. [DOI] [PubMed] [Google Scholar]
- 299.Chen Q, et al. Alarmin HNP-1 promotes pyroptosis and IL-1β release through different roles of NLRP3 inflammasome via P2X7 in LPS-primed macrophages. Innate Immun. 2014;20:290–300. doi: 10.1177/1753425913490575. [DOI] [PubMed] [Google Scholar]
- 300.Wang P, Li G, Gao LI, Zhao C. Human β-defensin 2 enhances IL-1β production and pyroptosis through P2X7-mediated NLRP3 expression in macrophages. Biocell. 2022;46:1197–1207. [Google Scholar]
- 301.Sakamoto K, et al. Disruption of the endopeptidase ADAM10-Notch signaling axis leads to skin dysbiosis and innate lymphoid cell-mediated hair follicle destruction. Immunity. 2021;54:2321–2337. doi: 10.1016/j.immuni.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gao M, et al. Tannic acid through ROS/TNF-α/TNFR 1 antagonizes atrazine induced apoptosis, programmed necrosis and immune dysfunction of grass carp hepatocytes. Fish. Shellfish Immunol. 2022;131:312–322. doi: 10.1016/j.fsi.2022.09.062. [DOI] [PubMed] [Google Scholar]
- 303.Simms LA, et al. Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn’s disease. Gut. 2008;57:903–910. doi: 10.1136/gut.2007.142588. [DOI] [PubMed] [Google Scholar]
- 304.Wehkamp J, Stange EF. An update review on the Paneth cell as key to ileal Crohn’s disease. Front. Immunol. 2020;11:646. doi: 10.3389/fimmu.2020.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Fang X, Ardehali H, Min J, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2022;20:1–17. doi: 10.1038/s41569-022-00735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yan R, et al. The structure of erastin-bound xCT-4F2hc complex reveals molecular mechanisms underlying erastin-induced ferroptosis. Cell Res. 2022;32:687–690. doi: 10.1038/s41422-022-00642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tsvetkov P, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022;7:378. doi: 10.1038/s41392-022-01229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhou Y, et al. Parthanatos and its associated components: promising therapeutic targets for cancer. Pharmacol. Rev. 2021;163:105299. doi: 10.1016/j.phrs.2020.105299. [DOI] [PubMed] [Google Scholar]
- 310.Huang P, et al. Molecular mechanisms of parthanatos and its role in diverse diseases. Int. J. Mol. Sci. 2022;23:7292. doi: 10.3390/ijms23137292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Peng F, et al. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022;7:286. doi: 10.1038/s41392-022-01110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Hu P, et al. Cholesterol-associated lysosomal disorder triggers cell death of hematological malignancy: dynamic analysis on cytotoxic effects of LW-218. Acta Pharm. Sin. B. 2021;11:3178–3192. doi: 10.1016/j.apsb.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Standing up to infectious disease. Nat. Microbiol. 4, 1 (2019). [DOI] [PMC free article] [PubMed]
- 315.Ikuta, K. S. et al. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet400, 2221–2248 (2023). [DOI] [PMC free article] [PubMed]
- 316.Lange C, et al. Management of drug-resistant tuberculosis. Lancet. 2019;394:953–966. doi: 10.1016/S0140-6736(19)31882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sharma S, Verma I, Khuller GK. Therapeutic potential of human neutrophil peptide 1 against experimental tuberculosis. Antimicrob. Agents Chemother. 2001;45:639–640. doi: 10.1128/AAC.45.2.639-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Patro S, Maiti S, Panda SK, Dey N. Utilization of plant-derived recombinant human β-defensins (hBD-1 and hBD-2) for averting salmonellosis. Transgenic Res. 2015;24:353–364. doi: 10.1007/s11248-014-9847-3. [DOI] [PubMed] [Google Scholar]
- 319.Maiti S, et al. Effective control of Salmonella infections by employing combinations of recombinant antimicrobial human β-defensins hBD-1 and hBD-2. Antimicrob. Agents Chemother. 2014;58:6896–6903. doi: 10.1128/AAC.03628-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Saido-Sakanaka H, Ishibashi J, Momotani E, Yamakawa M. Protective effects of synthetic antibacterial oligopeptides based on the insect defensins on Methicillin-resistant Staphylococcus aureus in mice. Dev. Comp. Immunol. 2005;29:469–477. doi: 10.1016/j.dci.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 321.Hazrati E, et al. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 2006;177:8658–8666. doi: 10.4049/jimmunol.177.12.8658. [DOI] [PubMed] [Google Scholar]
- 322.Gong T, et al. Recombinant mouse beta-defensin 2 inhibits infection by influenza A virus by blocking its entry. Arch. Virol. 2010;155:491–498. doi: 10.1007/s00705-010-0608-1. [DOI] [PubMed] [Google Scholar]
- 323.Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine. 2021;66:103293. doi: 10.1016/j.ebiom.2021.103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Langhorst J, et al. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2009;104:404–410. doi: 10.1038/ajg.2008.86. [DOI] [PubMed] [Google Scholar]
- 325.Kolho K-L, Sipponen T, Valtonen E, Savilahti E. Fecal calprotectin, MMP-9, and human beta-defensin-2 levels in pediatric inflammatory bowel disease. Int. J. Colorectal Dis. 2014;29:43–50. doi: 10.1007/s00384-013-1775-9. [DOI] [PubMed] [Google Scholar]
- 326.Meisch JP, et al. Human β-defensin 3 peptide is increased and redistributed in Crohn’s ileitis. Inflamm. Bowel Dis. 2013;19:942–953. doi: 10.1097/MIB.0b013e318280b11a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Chang YY, Ouyang Q. Expression and significance of mucosal beta-defensin-2, TNFalpha and IL-1beta in ulcerative colitis. Zhonghua Nei Ke Za Zhi. 2008;47:11–14. [PubMed] [Google Scholar]
- 328.Fahlgren A, Hammarström S, Danielsson A, Hammarström ML. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin. Exp. Immunol. 2003;131:90–101. doi: 10.1046/j.1365-2249.2003.02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Kapel N, et al. Fecal beta-defensin-2 in children with inflammatory bowel diseases. J. Pediatr. Gastroenterol. Nutr. 2009;48:117–120. doi: 10.1097/MPG.0b013e318174e872. [DOI] [PubMed] [Google Scholar]
- 330.Aldhous MC, Noble CL, Satsangi J. Dysregulation of human beta-defensin-2 protein in inflammatory bowel disease. PLoS ONE. 2009;4:e6285. doi: 10.1371/journal.pone.0006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wehkamp J, et al. Human β-defensin 2 but not β-defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2002;14:745–752. doi: 10.1097/00042737-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 332.Angelberger S, et al. NOD2/CARD15 gene variants are linked to failure of antibiotic treatment in perianal fistulating Crohn’s disease. Am. J. Gastroenterol. 2008;103:1197–1202. doi: 10.1111/j.1572-0241.2007.01741.x. [DOI] [PubMed] [Google Scholar]
- 333.Voss E, et al. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J. Biol. Chem. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 334.Fellermann K, et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am. J. Hum. Genet. 2006;79:439–448. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Gersemann M, Wehkamp J, Stange EF. Innate immune dysfunction in inflammatory bowel disease. J. Intern. Med. 2012;271:421–428. doi: 10.1111/j.1365-2796.2012.02515.x. [DOI] [PubMed] [Google Scholar]
- 336.Rahman A, et al. Beta-defensin production by human colonic plasma cells: a new look at plasma cells in ulcerative colitis. Inflamm. Bowel Dis. 2007;13:847–855. doi: 10.1002/ibd.20141. [DOI] [PubMed] [Google Scholar]
- 337.Ramasundara M, Leach ST, Lemberg DA, Day AS. Defensins and inflammation: the role of defensins in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2009;24:202–208. doi: 10.1111/j.1440-1746.2008.05772.x. [DOI] [PubMed] [Google Scholar]
- 338.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 339.Singh R, Balasubramanian I, Zhang L, Gao N. Metaplastic Paneth cells in extra-intestinal mucosal niche indicate a link to microbiome and inflammation. Front. Physiol. 2020;11:280. doi: 10.3389/fphys.2020.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wehkamp J, et al. The Paneth cell α-defensin deficiency of ileal Crohn’s disease is linked to Wnt/Tcf-41. J. Immunol. 2007;179:3109–3118. doi: 10.4049/jimmunol.179.5.3109. [DOI] [PubMed] [Google Scholar]
- 341.Wehkamp J, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc. Natl Acad. Sci. USA. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zilbauer M, et al. Intestinal alpha-defensin expression in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2011;17:2076–2086. doi: 10.1002/ibd.21577. [DOI] [PubMed] [Google Scholar]
- 343.Taman H, et al. Transcriptomic landscape of treatment-naïve ulcerative colitis. J. Crohns Colitis. 2018;12:327–336. doi: 10.1093/ecco-jcc/jjx139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Williams AD, et al. Human alpha defensin 5 is a candidate biomarker to delineate inflammatory bowel disease. PLoS ONE. 2017;12:e0179710. doi: 10.1371/journal.pone.0179710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wehkamp J, et al. Innate immunity and colonic inflammation: enhanced expression of epithelial alpha-defensins. Dig. Dis. Sci. 2002;47:1349–1355. doi: 10.1023/a:1015334917273. [DOI] [PubMed] [Google Scholar]
- 346.Rana T, et al. Linking bacterial enterotoxins and alpha defensin 5 expansion in the Crohn’s colitis: a new insight into the etiopathogenetic and differentiation triggers driving colonic inflammatory bowel disease. PLoS ONE. 2021;16:e0246393. doi: 10.1371/journal.pone.0246393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Shukla PK, et al. Human defensin-5 blocks ethanol and colitis-induced dysbiosis, tight junction disruption and inflammation in mouse intestine. Sci. Rep. 2018;8:16241. doi: 10.1038/s41598-018-34263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zeng L, et al. An engineering probiotic producing defensin-5 ameliorating dextran sodium sulfate-induced mice colitis via Inhibiting NF-kB pathway. J. Transl. Med. 2020;18:107. doi: 10.1186/s12967-020-02272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Magnusson MK, et al. Anti-TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J. Crohns Colitis. 2016;10:943–952. doi: 10.1093/ecco-jcc/jjw051. [DOI] [PubMed] [Google Scholar]
- 350.Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 351.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 352.Economou M, Trikalinos TA, Loizou KT, Tsianos EV, Ioannidis JP. Differential effects of NOD2 variants on Crohn’s disease risk and phenotype in diverse populations: a metaanalysis. Am. J. Gastroenterol. 2004;99:2393–2404. doi: 10.1111/j.1572-0241.2004.40304.x. [DOI] [PubMed] [Google Scholar]
- 353.Armbruster NS, Stange EF, Wehkamp J. In the Wnt of Paneth cells: immune-epithelial crosstalk in small intestinal Crohn’s disease. Front. Immunol. 2017;8:1204. doi: 10.3389/fimmu.2017.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Andreu P, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 355.Perminow G, et al. Defective Paneth cell-mediated host defense in pediatric ileal Crohn’s disease. Am. J. Gastroenterol. 2010;105:452–459. doi: 10.1038/ajg.2009.643. [DOI] [PubMed] [Google Scholar]
- 356.Yamaguchi N, et al. Concentrations of α- and β-defensins in plasma of patients with inflammatory bowel disease. Inflamm. Res. 2009;58:192–197. doi: 10.1007/s00011-008-8120-8. [DOI] [PubMed] [Google Scholar]
- 357.Hashimoto S, et al. Human neutrophil peptide-1 aggravates dextran sulfate sodium-induced colitis. Inflamm. Bowel Dis. 2012;18:667–675. doi: 10.1002/ibd.21855. [DOI] [PubMed] [Google Scholar]
- 358.Kanmura S, et al. Increased gene copy number of DEFA1A3 is associated with the severity of ulcerative colitis. Clin. Transl. Gastroenterol. 2021;12:e00331. doi: 10.14309/ctg.0000000000000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Davies MJ, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2022;65:1925–1966. doi: 10.1007/s00125-022-05787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Brauner H, et al. Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clin. Exp. Immunol. 2014;177:478–482. doi: 10.1111/cei.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Ertugrul AS, et al. Gingival crevicular fluid levels of human beta-defensins 1 and 3 in subjects with periodontitis and/or type 2 diabetes mellitus: a cross-sectional study. J. Periodont. Res. 2013;48:475–482. doi: 10.1111/jre.12029. [DOI] [PubMed] [Google Scholar]
- 362.Neuwirth A, et al. Eosinophils from patients with type 1 diabetes mellitus express high level of myeloid alpha-defensins and myeloperoxidase. Cell. Immunol. 2012;273:158–163. doi: 10.1016/j.cellimm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 363.Ochoa-Ramirez LA, et al. β-defensin 1 gene polymorphisms are associated with kidney disease in northwestern Mexicans with type 2 diabetes. Immunol. Invest. 2022;51:1398–1406. doi: 10.1080/08820139.2021.1948564. [DOI] [PubMed] [Google Scholar]
- 364.López-Bermejo A, et al. Alpha defensins 1, 2, and 3: potential roles in dyslipidemia and vascular dysfunction in humans. Arterioscler. Thromb. Vasc. Biol. 2007;27:1166–1171. doi: 10.1161/ATVBAHA.106.138594. [DOI] [PubMed] [Google Scholar]
- 365.Oshima M, et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat. Rev. Nephrol. 2021;17:740–750. doi: 10.1038/s41581-021-00462-y. [DOI] [PubMed] [Google Scholar]
- 366.Hodin CM, et al. Reduced Paneth cell antimicrobial protein levels correlate with activation of the unfolded protein response in the gut of obese individuals. J. Pathol. 2011;225:276–284. doi: 10.1002/path.2917. [DOI] [PubMed] [Google Scholar]
- 367.Su D, et al. Vitamin D signaling through induction of Paneth cell defensins maintains gut microbiota and improves metabolic disorders and hepatic steatosis in animal models. Front. Physiol. 2016;7:498. doi: 10.3389/fphys.2016.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Hu RC, Xu YJ, Zhang ZX, Ni W, Chen SX. Correlation of HDEFB1 polymorphism and susceptibility to chronic obstructive pulmonary disease in Chinese Han population. Chin. Med. J. 2004;117:1637–1641. [PubMed] [Google Scholar]
- 369.Matsushita I, et al. Genetic variants of human beta-defensin-1 and chronic obstructive pulmonary disease. Biochem. Biophys. Res. Commun. 2002;291:17–22. doi: 10.1006/bbrc.2002.6395. [DOI] [PubMed] [Google Scholar]
- 370.Janssens W, et al. Genomic copy number determines functional expression of {beta}-defensin 2 in airway epithelial cells and associates with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010;182:163–169. doi: 10.1164/rccm.200905-0767OC. [DOI] [PubMed] [Google Scholar]
- 371.Almutairi M, et al. Human beta-defensin-1 rs2738047 polymorphism is associated with shisha smoking risk among Saudi population. Environ. Sci. Pollut. Res. 2021;28:42916–42933. doi: 10.1007/s11356-021-13660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Andresen E, Günther G, Bullwinkel J, Lange C, Heine H. Increased expression of beta-defensin 1 (DEFB1) in chronic obstructive pulmonary disease. PLoS ONE. 2011;6:e21898. doi: 10.1371/journal.pone.0021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Borchers NS, et al. Human β-defensin 2 mutations are associated with asthma and atopy in children and its application prevents atopic asthma in a mouse model. Front. Immunol. 2021;12:636061. doi: 10.3389/fimmu.2021.636061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Baines KJ, et al. Airway β-defensin-1 protein is elevated in COPD and severe asthma. Mediat. Inflamm. 2015;2015:407271. doi: 10.1155/2015/407271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Liao Z, et al. Enhanced expression of human β-defensin 2 in peripheral lungs of patients with chronic obstructive pulmonary disease. Peptides. 2012;38:350–356. doi: 10.1016/j.peptides.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 376.Pace E, et al. Beta defensin-2 is reduced in central but not in distal airways of smoker COPD patients. PLoS ONE. 2012;7:e33601. doi: 10.1371/journal.pone.0033601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Herr C, Shaykhiev R, Bals R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin. Biol. Ther. 2007;7:1449–1461. doi: 10.1517/14712598.7.9.1449. [DOI] [PubMed] [Google Scholar]
- 378.Milad N, et al. Recombinant human β-defensin 2 delivery improves smoking-induced lung neutrophilia and bacterial exacerbation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022;323:L37–l47. doi: 10.1152/ajplung.00027.2022. [DOI] [PubMed] [Google Scholar]
- 379.Pinkerton JW, et al. Human β-defensin-2 suppresses key features of asthma in murine models of allergic airways disease. Clin. Exp. Allergy. 2021;51:120–131. doi: 10.1111/cea.13766. [DOI] [PubMed] [Google Scholar]
- 380.Teles F, Collman RG, Mominkhan D, Wang Y. Viruses, periodontitis, and comorbidities. Periodontology 2000. 2022;89:190–206. doi: 10.1111/prd.12435. [DOI] [PubMed] [Google Scholar]
- 381.Güncü GN, Yilmaz D, Könönen E, Gürsoy UK. Salivary antimicrobial peptides in early detection of periodontitis. Front. Cell Infect. Microbiol. 2015;5:99. doi: 10.3389/fcimb.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Lu Q, Jin L, Darveau RP, Samaranayake LP. Expression of human beta-defensins-1 and -2 peptides in unresolved chronic periodontitis. J. Periodont. Res. 2004;39:221–227. doi: 10.1111/j.1600-0765.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 383.Yılmaz D, et al. Overexpressions of hBD-2, hBD-3, and hCAP18/LL-37 in gingiva of diabetics with periodontitis. Immunobiology. 2015;220:1219–1226. doi: 10.1016/j.imbio.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 384.Dommisch H, Jepsen S. Diverse functions of defensins and other antimicrobial peptides in periodontal tissues. Periodontology 2000. 2015;69:96–110. doi: 10.1111/prd.12093. [DOI] [PubMed] [Google Scholar]
- 385.Puklo M, Guentsch A, Hiemstra PS, Eick S, Potempa J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral. Microbiol. Immunol. 2008;23:328–335. doi: 10.1111/j.1399-302X.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Dommisch H, Vorderwülbecke S, Eberhard J, Steglich M, Jepsen S. SELDI-TOF-MS of gingival crevicular fluid-a methodological approach. Arch. Oral. Biol. 2009;54:803–809. doi: 10.1016/j.archoralbio.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 387.Cui D, et al. Human β-defensin 3 inhibits periodontitis development by suppressing inflammatory responses in macrophages. Mol. Immunol. 2017;91:65–74. doi: 10.1016/j.molimm.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 388.Venkata Subbiah H, Ramesh Babu P, Subbiah U. In silico targeting of red complex bacteria virulence factors of periodontitis with β-defensin 1. Genet. Eng. Biotechnol. 2022;20:59. doi: 10.1186/s43141-022-00342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Roudi R, Syn NL, Roudbary M. Antimicrobial peptides as biologic and immunotherapeutic agents against cancer: a comprehensive overview. Front. Immunol. 2017;8:1320. doi: 10.3389/fimmu.2017.01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Ghosh SK, McCormick TS, Weinberg A. Human beta defensins and cancer: contradictions and common ground. Front. Oncol. 2019;9:341. doi: 10.3389/fonc.2019.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Gambichler T, et al. Pattern of mRNA expression of beta-defensins in basal cell carcinoma. BMC Cancer. 2006;6:163. doi: 10.1186/1471-2407-6-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Scola N, et al. The expression of antimicrobial peptides is significantly altered in cutaneous squamous cell carcinoma and precursor lesions. Br. J. Dermatol. 2012;167:591–597. doi: 10.1111/j.1365-2133.2012.11110.x. [DOI] [PubMed] [Google Scholar]
- 393.Bonamy C, et al. Expression of the human antimicrobial peptide β-defensin-1 is repressed by the EGFR-ERK-MYC axis in colonic epithelial cells. Sci. Rep. 2018;8:18043. doi: 10.1038/s41598-018-36387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Ling YM, et al. β-defensin 1 expression in HCV infected liver/liver cancer: an important role in protecting HCV progression and liver cancer development. Sci. Rep. 2017;7:13404. doi: 10.1038/s41598-017-13332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Arimura Y, et al. Elevated serum beta-defensins concentrations in patients with lung cancer. Anticancer Res. 2004;24:4051–4057. [PubMed] [Google Scholar]
- 396.Phan TK, et al. Human β-defensin 3 contains an oncolytic motif that binds PI(4,5)P2 to mediate tumour cell permeabilisation. Oncotarget. 2016;7:2054–2069. doi: 10.18632/oncotarget.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Hanaoka Y, et al. In vitro and in vivo anticancer activity of human β-defensin-3 and its mouse homolog. Anticancer Res. 2016;36:5999–6004. doi: 10.21873/anticanres.11188. [DOI] [PubMed] [Google Scholar]
- 398.Uraki S, et al. Human β-defensin-3 inhibits migration of colon cancer cells via downregulation of metastasis-associated 1 family, member 2 expression. Int. J. Oncol. 2014;45:1059–1064. doi: 10.3892/ijo.2014.2507. [DOI] [PubMed] [Google Scholar]
- 399.Xu D, et al. Human beta-defensin 3 contributes to the carcinogenesis of cervical cancer via activation of NF-κB signaling. Oncotarget. 2016;7:75902–75913. doi: 10.18632/oncotarget.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Shuyi Y, et al. Human beta-defensin-3 (hBD-3) upregulated by LPS via epidermal growth factor receptor (EGFR) signaling pathways to enhance lymphatic invasion of oral squamous cell carcinoma. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radio. 2011;112:616–625. doi: 10.1016/j.tripleo.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 401.Du Y, Yang Y, Zhang W, Yang C, Xu P. Human β-defensin-3 and nuclear factor-kappa B p65 synergistically promote the cell proliferation and invasion of oral squamous cell carcinoma. Transl. Oncol. 2023;27:101582. doi: 10.1016/j.tranon.2022.101582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Mburu YK, Abe K, Ferris LK, Sarkar SN, Ferris RL. Human β-defensin 3 promotes NF-κB-mediated CCR7 expression and anti-apoptotic signals in squamous cell carcinoma of the head and neck. Carcinogenesis. 2011;32:168–174. doi: 10.1093/carcin/bgq236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Gomez Hernandez MP, et al. HBD3 induces PD-L1 expression on head and neck squamous cell carcinoma cell lines. Antibiotics. 2019;8:161. doi: 10.3390/antibiotics8040161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Panjeta A, Preet S. Anticancer potential of human intestinal defensin 5 against 1, 2-dimethylhydrazine dihydrochloride induced colon cancer: a therapeutic approach. Peptides. 2020;126:170263. doi: 10.1016/j.peptides.2020.170263. [DOI] [PubMed] [Google Scholar]
- 405.Qiao Q, et al. Human α-defensin 5 suppressed colon cancer growth by targeting PI3K pathway. Exp. Cell Res. 2021;407:112809. doi: 10.1016/j.yexcr.2021.112809. [DOI] [PubMed] [Google Scholar]
- 406.Wei PL, et al. Human α-defensin 6 (HD6) suppresses CRC proliferation and metastasis through abolished EGF/EGFR signaling pathway. Int. J. Med. Sci. 2022;19:34–46. doi: 10.7150/ijms.64850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Wu Z, Ding Z, Cheng B, Cui Z. The inhibitory effect of human DEFA5 in growth of gastric cancer by targeting BMI1. Cancer Sci. 2021;112:1075–1083. doi: 10.1111/cas.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Hu Y, Jo H, DeGrado WF, Wang J. Brilacidin, a COVID-19 drug candidate, demonstrates broad-spectrum antiviral activity against human coronaviruses OC43, 229E, and NL63 through targeting both the virus and the host cell. J. Med. Virol. 2022;94:2188–2200. doi: 10.1002/jmv.27616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Bakovic A, et al. Brilacidin demonstrates inhibition of SARS-CoV-2 in cell culture. Viruses. 2021;13:272. doi: 10.3390/v13020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Xu C, et al. Brilacidin, a non-peptide defensin-mimetic molecule, inhibits SARS-CoV-2 infection by blocking viral entry. EC Microbiol. 2022;18:1–12. [PMC free article] [PubMed] [Google Scholar]
- 411.A study to evaluate the efficacy and safety of brilacidin in hospitalized participants with COVID-19. https://clinicaltrials.gov/show/NCT04784897 (2022).
- 412.Study of the effects of brilacidin oral rinse on radiation-induced oral mucositis in patients with head and neck cancer. https://clinicaltrials.gov/show/NCT02324335 (2017).
- 413.Bassetti M, Del Puente F, Magnasco L, Giacobbe DR. Innovative therapies for acute bacterial skin and skin-structure infections (ABSSSI) caused by methicillin-resistant Staphylococcus aureus: advances in phase I and II trials. Expert Opin. Investig. Drugs. 2020;29:495–506. doi: 10.1080/13543784.2020.1750595. [DOI] [PubMed] [Google Scholar]
- 414.Jorgensen, D., Scott, R., O’Riordan, W. & Tack, K. A randomized, double-blind study comparing single-dose and short-course brilacidin to daptomycin in the treatment of acute bacterial skin & skin structure infections (ABSSSI). https://static1.squarespace.com/static/5715352e20c647639137f992/t/583f8286d2b85731b8713a36/1480557192947/A-Randomized-Double-Blind-Study-Comparing-Single-Dose-and-Short-Course-Brilacidin-to-Daptomycin-in-the-Treatment-of-Acute-Bacterial-Skin-Skin-Structure-Infections-ABSSSI1.pdf (2015).
- 415.Efficacy and safety study of brilacidin to treat serious skin infections. https://clinicaltrials.gov/ct2/show/results/NCT02052388 (2014).
- 416.Innovation Pharmaceuticals Phase 2 PoC Trial for Inflammatory Bowel Disease Achieves Induction of Remission in a Majority of Patients Treated with Brilacidin. www.ipharminc.com/press-release/2017/7/13/innovation-pharmaceuticals-phase-2-poc-trial-for-inflammatory-bowel-disease-achieves-induction-of-remission-in-a-majority-of-patients-treated-with-brilacidin (2017).
- 417.Kowalski RP, Romanowski EG, Yates KA, Mah FS. An independent evaluation of a novel peptide mimetic, brilacidin (PMX30063), for ocular anti-infective. J. Ocul. Pharmacol. Ther. 2016;32:23–27. doi: 10.1089/jop.2015.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Dos Reis TF, et al. A host defense peptide mimetic, brilacidin, potentiates caspofungin antifungal activity against human pathogenic fungi. Nat. Commun. 2023;14:2052. doi: 10.1038/s41467-023-37573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Mercer DK, O’Neil DA. Innate Inspiration: antifungal peptides and other immunotherapeutics from the host immune response. Front. Immunol. 2020;11:2177. doi: 10.3389/fimmu.2020.02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Nicole, v. d. W. et al. The plant defensin HXP124 has the potential to be a new safe and effective topical treatment for onychomycosis. https://hexima.com.au/wp-content/uploads/2018/07/ISHAM-2018-vanderweerden.pdf (2018).
- 421.Nicole, v. d. W. et al. A phase I/IIa, randomized, double-blind, vehicle-controlled study of pezadeftide (HXP124), a novel topical treatment, in patients with onychomycosis. https://hexima.com.au/wp-content/uploads/2021/07/APMA-2021-FINAL-small.pdf (2021).
- 422.Harry, S. Hexima-Pezadeftide-a novel antifungal agent. https://www.edisongroup.com/research/pezadeftide-a-novel-antifungal-agent/30393/ (2022).
- 423.Bucki R, Sostarecz AG, Byfield FJ, Savage PB, Janmey PA. Resistance of the antibacterial agent ceragenin CSA-13 to inactivation by DNA or F-actin and its activity in cystic fibrosis sputum. J. Antimicrob. Chemother. 2007;60:535–545. doi: 10.1093/jac/dkm218. [DOI] [PubMed] [Google Scholar]
- 424.Pezzulo AA, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Luo X, et al. Advancements, challenges and future perspectives on peptide-based drugs: focus on antimicrobial peptides. Eur. J. Pharm. Sci. 2023;181:106363. doi: 10.1016/j.ejps.2022.106363. [DOI] [PubMed] [Google Scholar]
- 426.Zhao C, Yan S, Song Y, Xia X. Roles of antimicrobial peptides in gynecological cancers. Int. J. Mol. Sci. 2022;23:10104. doi: 10.3390/ijms231710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Di L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015;17:134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Bellotti D, Remelli M. Lights and shadows on the therapeutic use of antimicrobial peptides. Molecules. 2022;27:4584. doi: 10.3390/molecules27144584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.de Breij A, et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018;10:eaan4044. doi: 10.1126/scitranslmed.aan4044. [DOI] [PubMed] [Google Scholar]
- 430.Grönberg A, Mahlapuu M, Ståhle M, Whately-Smith C, Rollman O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: a randomized, placebo-controlled clinical trial. Wound Repair Regen. 2014;22:613–621. doi: 10.1111/wrr.12211. [DOI] [PubMed] [Google Scholar]
- 431.Krishnakumari V, Guru A, Adicherla H, Nagaraj R. Effects of increasing hydrophobicity by N-terminal myristoylation on the antibacterial and hemolytic activities of the C-terminal cationic segments of human-β-defensins 1–3. Chem. Biol. Drug Des. 2018;92:1504–1513. doi: 10.1111/cbdd.13317. [DOI] [PubMed] [Google Scholar]
- 432.Lei R, et al. Self-assembling myristoylated human α-defensin 5 as a next-generation nanobiotics potentiates therapeutic efficacy in bacterial infection. ACS Nano. 2018;12:5284–5296. doi: 10.1021/acsnano.7b09109. [DOI] [PubMed] [Google Scholar]
- 433.Mueller LK, Baumruck AC, Zhdanova H, Tietze AA. Challenges and perspectives in chemical synthesis of highly hydrophobic peptides. Front. Bioeng. Biotechnol. 2020;8:162. doi: 10.3389/fbioe.2020.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.da Cunha NB, et al. The next generation of antimicrobial peptides (AMPs) as molecular therapeutic tools for the treatment of diseases with social and economic impacts. Drug Discov. Today. 2017;22:234–248. doi: 10.1016/j.drudis.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Yang J, et al. Selenium enriched Bacillus subtilis yb-1114246 activated the TLR2-NF-κB1 signaling pathway to regulate chicken intestinal β-defensin 1 expression. Food Funct. 2021;12:5913–5926. doi: 10.1039/d1fo01158h. [DOI] [PubMed] [Google Scholar]
- 436.Priyadarshini M, Kotlo KU, Dudeja PK, Layden BT. Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr. Physiol. 2018;8:1091–1115. doi: 10.1002/cphy.c170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Basson AR, et al. Regulation of intestinal inflammation by dietary fats. Front. Immunol. 2021;11:604989. doi: 10.3389/fimmu.2020.604989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Sunkara LT, Jiang W, Zhang G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS ON#. 2012;7:e49558. doi: 10.1371/journal.pone.0049558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Bentley-Hewitt KL, Blatchford PA, Parkar SG, Ansell J, Pernthaner A. Digested and fermented green kiwifruit increases human β-defensin 1 and 2 production in vitro. Plant Food Hum. Nutr. 2012;67:208–214. doi: 10.1007/s11130-012-0305-1. [DOI] [PubMed] [Google Scholar]
- 440.Zeng X, et al. Induction of porcine host defense peptide gene expression by short-chain fatty acids and their analogs. PLoS ONE. 2013;8:e72922. doi: 10.1371/journal.pone.0072922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Wang J, et al. Caprylic acid and nonanoic acid upregulate endogenous host defense peptides to enhance intestinal epithelial immunological barrier function via histone deacetylase inhibition. Int. Immunopharmacol. 2018;65:303–311. doi: 10.1016/j.intimp.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 442.Zhao Y, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11:752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Dou X, et al. TLR2/4-mediated NF-κB pathway combined with the histone modification regulates β-defensins and interleukins expression by sodium phenyl butyrate in porcine intestinal epithelial cells. Food Nutr. Res. 2018;62:1493. doi: 10.29219/fnr.v62.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Dou X, et al. TLR2/EGFR are two sensors for pBD3 and pEP2C induction by sodium butyrate independent of HDAC inhibition. J. Agric. Food Chem. 2020;68:512–522. doi: 10.1021/acs.jafc.9b06569. [DOI] [PubMed] [Google Scholar]
- 445.Beisner J, et al. Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front. Immunol. 2021;12:678360. doi: 10.3389/fimmu.2021.678360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Xiong H, et al. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci. Rep. 2016;6:27070. doi: 10.1038/srep27070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- 448.Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V. Faecal and serum metabolomics in paediatric inflammatory bowel disease. J. Crohns Colitis. 2017;11:321–334. doi: 10.1093/ecco-jcc/jjw158. [DOI] [PubMed] [Google Scholar]
- 449.Bosch S, et al. Fecal amino acid analysis can discriminate de novo treatment-naïve pediatric inflammatory bowel disease from controls. J. Pediatr. Gastroenterol. Nutr. 2018;66:773–778. doi: 10.1097/MPG.0000000000001812. [DOI] [PubMed] [Google Scholar]
- 450.Scoville EA, et al. Alterations in lipid, amino acid, and energy metabolism distinguish crohn’s disease from ulcerative colitis and control subjects by serum metabolomic profiling. Metabolomics. 2018;14:17. doi: 10.1007/s11306-017-1311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Santoru ML, et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci. Rep. 2017;7:9523. doi: 10.1038/s41598-017-10034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Beaumont M, Blachier F. Amino acids in intestinal physiology and health. Adv. Exp. Med. Biol. 2020;1265:1–20. doi: 10.1007/978-3-030-45328-2_1. [DOI] [PubMed] [Google Scholar]
- 453.Takakuwa A, et al. Butyric acid and leucine induce α-defensin secretion from small intestinal Paneth cells. Nutrients. 2019;11:2817. doi: 10.3390/nu11112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Mao X, et al. Zn2+ and l-isoleucine induce the expressions of porcine β-defensins in IPEC-J2 cells. Mol. Biol. Rep. 2013;40:1547–1552. doi: 10.1007/s11033-012-2200-0. [DOI] [PubMed] [Google Scholar]
- 455.Ren M, et al. Different lipopolysaccharide branched-chain amino acids modulate porcine intestinal endogenous β-defensin expression through the Sirt1/ERK/90RSK pathway. J. Agric. Food Chem. 2016;64:3371–3379. doi: 10.1021/acs.jafc.6b00968. [DOI] [PubMed] [Google Scholar]
- 456.Youkou K, et al. Isoleucine, an essential amino acid, induces the expression of human β defensin 2 through the activation of the G-protein coupled receptor-ERK pathway in the intestinal epithelia. Food Nutr. Sci. 2012;3:548–555. [Google Scholar]
- 457.Mao X, et al. Dietary l-arginine supplementation enhances porcine Β-defensins gene expression in some tissues of weaned pigs. Livest. Sci. 2012;148:103–108. [Google Scholar]
- 458.Fehlbaum P, Rao M, Zasloff M, Anderson GM. An essential amino acid induces epithelial beta-defensin expression. Proc. Natl Acad. Sci. USA. 2000;97:12723–12728. doi: 10.1073/pnas.220424597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 460.Schauber J, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Zhang L, et al. 1,25-Dihydroxyvitamin-D3 induces avian β-defensin gene expression in chickens. PLoS ONE. 2016;11:e0154546. doi: 10.1371/journal.pone.0154546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Samanta SK, et al. Phytochemical portfolio and anticancer activity of Murraya koenigii and its primary active component, mahanine. Pharm. Res. 2018;129:227–236. doi: 10.1016/j.phrs.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 463.Caesar LK, Cech NB. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat. Prod. Rep. 2019;36:869–888. doi: 10.1039/c9np00011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Lombardo Bedran TB, Feghali K, Zhao L, Palomari Spolidorio DM, Grenier D. Green tea extract and its major constituent, epigallocatechin-3-gallate, induce epithelial beta-defensin secretion and prevent beta-defensin degradation by Porphyromonas gingivalis. J. Periodont. Res. 2014;49:615–623. doi: 10.1111/jre.12142. [DOI] [PubMed] [Google Scholar]
- 465.Gan Y, et al. Paeoniflorin upregulates β-defensin-2 expression in human bronchial epithelial cell through the p38 MAPK, ERK, and NF-κB signaling pathways. Inflammation. 2014;37:1468–1475. doi: 10.1007/s10753-014-9872-7. [DOI] [PubMed] [Google Scholar]
- 466.Lombardo Bedran TB, Morin M-P, Palomari Spolidorio D, Grenier D. Black tea extract and its theaflavin derivatives inhibit the growth of periodontopathogens and modulate interleukin-8 and β-defensin secretion in oral epithelial cells. PLoS ONE. 2015;10:e0143158. doi: 10.1371/journal.pone.0143158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Chen, K. et al. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 61, 1601006 (2017). [DOI] [PubMed]
- 468.Paoletti I, et al. Patented natural avocado sugar modulates the HBD-2 and HBD-3 expression in human keratinocytes through Toll-like receptor-2 and ERK/MAPK activation. Arch. Dermatol. Res. 2012;304:619–625. doi: 10.1007/s00403-012-1237-1. [DOI] [PubMed] [Google Scholar]
- 469.Zhang M, Jin X, Yang Y-F. β-Glucan from Saccharomyces cerevisiae induces SBD-1 production in ovine ruminal epithelial cells via the Dectin-1–Syk–NF-κB signaling pathway. Cell. Signal. 2019;53:304–315. doi: 10.1016/j.cellsig.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 470.Xiong W-B, et al. Dehydroandrographolide enhances innate immunity of intestinal tract through up-regulation the expression of hBD-2. DARU. 2015;23:37. doi: 10.1186/s40199-015-0119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Kubota A, et al. Reishi mushroom Ganoderma lucidum modulates IgA production and alpha-defensin expression in the rat small intestine. J. Ethnopharmacol. 2018;214:240–243. doi: 10.1016/j.jep.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 472.Kobatake E, Kabuki T. S-layer protein of Lactobacillus helveticus SBT2171 promotes human β-defensin 2 expression via TLR2-JNK signaling. Front. Microbiol. 2019;10:2414. doi: 10.3389/fmicb.2019.02414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Schlee M, et al. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin. Exp. Immunol. 2008;151:528–535. doi: 10.1111/j.1365-2249.2007.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Wehkamp J, et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect. Immun. 2004;72:5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Schlee M, et al. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect. Immun. 2007;75:2399–2407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Möndel M, et al. Probiotic E. coli treatment mediates antimicrobial human β-defensin synthesis and fecal excretion in humans. Mucosal Immunol. 2009;2:166–172. doi: 10.1038/mi.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Zhao Z, Xu S, Zhang W, Wu D, Yang G. Probiotic Escherichia coli NISSLE 1917 for inflammatory bowel disease applications. Food Funct. 2022;13:5914–5924. doi: 10.1039/d2fo00226d. [DOI] [PubMed] [Google Scholar]
- 478.Praveschotinunt P, et al. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat. Commun. 2019;10:5580. doi: 10.1038/s41467-019-13336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Fu J, et al. Clostridium butyricum ZJU-F1 benefits the intestinal barrier function and immune response associated with its modulation of gut microbiota in weaned piglets. Cells. 2021;10:527. doi: 10.3390/cells10030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Lyu W, et al. High throughput screening for natural host defense peptide-inducing compounds as novel alternatives to antibiotics. Front. Cell. Infect. Microbiol. 2018;8:191. doi: 10.3389/fcimb.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Nylén F, et al. Boosting innate immunity: development and validation of a cell-based screening assay to identify LL-37 inducers. Innate Immun. 2014;20:364–376. doi: 10.1177/1753425913493338. [DOI] [PubMed] [Google Scholar]
- 482.Grigat J, Soruri A, Forssmann U, Riggert J, Zwirner J. Chemoattraction of macrophages, T lymphocytes, and mast cells is evolutionarily conserved within the human alpha-defensin family. J. Immunol. 2007;179:3958–3965. doi: 10.4049/jimmunol.179.6.3958. [DOI] [PubMed] [Google Scholar]
- 483.Niyonsaba F, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Investig. Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 484.Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int. Immunol. 2002;14:421–426. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- 485.Niyonsaba F, Ogawa H, Nagaoka I. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology. 2004;111:273–281. doi: 10.1111/j.0019-2805.2004.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Mackenzie-Dyck S, Attah-Poku S, Juillard V, Babiuk LA, van Drunen Littel-van den Hurk S. The synthetic peptides bovine enteric beta-defensin (EBD), bovine neutrophil beta-defensin (BNBD) 9 and BNBD 3 are chemotactic for immature bovine dendritic cells. Vet. Immunol. Immunopathol. 2011;143:87–107. doi: 10.1016/j.vetimm.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 487.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Specific binding and chemotactic activity of mBD4 and its functional orthologue hBD2 to CCR6-expressing cells. J. Biol. Chem. 2010;285:7028–7034. doi: 10.1074/jbc.M109.091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Identification and biological characterization of mouse beta-defensin 14, the orthologue of human beta-defensin 3. J. Biol. Chem. 2008;283:5414–5419. doi: 10.1074/jbc.M709103200. [DOI] [PubMed] [Google Scholar]
- 489.Schaefer AS, et al. A 3′ UTR transition within DEFB1 is associated with chronic and aggressive periodontitis. Genes Immun. 2010;11:45–54. doi: 10.1038/gene.2009.75. [DOI] [PubMed] [Google Scholar]
- 490.Costa LCM, et al. Gingival crevicular fluid levels of human beta-defensin 1 in individuals with and without chronic periodontitis. J. Periodont. Res. 2018;53:736–742. doi: 10.1111/jre.12558. [DOI] [PubMed] [Google Scholar]
- 491.Jaradat SW, et al. Beta-defensin-2 genomic copy number variation and chronic periodontitis. J. Dent. Res. 2013;92:1035–1040. doi: 10.1177/0022034513504217. [DOI] [PubMed] [Google Scholar]
- 492.Coretti L, et al. The interplay between defensins and microbiota in Crohn’s disease. Mediat. Inflamm. 2017;2017:8392523. doi: 10.1155/2017/8392523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Wehkamp J, et al. Inducible and constitutive beta-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2003;9:215–223. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 494.Ochoa‐Ramírez LA, et al. Association of human beta‐defensin 1 gene polymorphisms with nonsegmental vitiligo. Clin. Exp. Dermatol. 2019;44:277–282. doi: 10.1111/ced.13697. [DOI] [PubMed] [Google Scholar]
- 495.Álvarez ÁH, Martínez Velázquez M, Prado Montes de Oca E. Human β-defensin 1 update: potential clinical applications of the restless warrior. Int. J. Biochem. Cell Biol. 2018;104:133–137. doi: 10.1016/j.biocel.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 496.Diao R, et al. Deficient human β-defensin 1 underlies male infertility associated with poor sperm motility and genital tract infection. Sci. Transl. Med. 2014;6:249ra108. doi: 10.1126/scitranslmed.3009071. [DOI] [PubMed] [Google Scholar]
- 497.Tian LM, Ke D. Acne vulgaris is associated with the human β-defensin 1-gene polymorphisms in Han Chinese Ethnic Group patients. Clin. Cosmet. Investig. Dermatol. 2021;14:123–128. doi: 10.2147/CCID.S292797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Silva ON, Porto WF, Ribeiro SM, Batista I, Franco OL. Host-defense peptides and their potential use as biomarkers in human diseases. Drug Discov. Today. 2018;23:1666–1671. doi: 10.1016/j.drudis.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 499.Takahashi T, Yamasaki K. Psoriasis and antimicrobial peptides. Int. J. Mol. Sci. 2020;21:6791. doi: 10.3390/ijms21186791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Chieosilapatham P, Ogawa H, Niyonsaba F. Current insights into the role of human β-defensins in atopic dermatitis. Clin. Exp. Immunol. 2017;190:155–166. doi: 10.1111/cei.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Choi IJ, Rhee C-S, Lee CH, Kim D-Y. Effect of allergic rhinitis on the expression of human β-defensin 2 in tonsils. Ann. Allergy Asthma Immunol. 2013;110:178–183. doi: 10.1016/j.anai.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 502.Bogefors J, Kvarnhammar AM, Höckerfelt U, Cardell LO. Reduced tonsillar expression of human β-defensin 1, 2 and 3 in allergic rhinitis. FEMS Immunol. Med. Microbiol. 2012;65:431–438. doi: 10.1111/j.1574-695X.2012.00959.x. [DOI] [PubMed] [Google Scholar]
- 503.Niyonsaba F, Kiatsurayanon C, Ogawa H. The role of human β-defensins in allergic diseases. Clin. Exp. Allergy. 2016;46:1522–1530. doi: 10.1111/cea.12843. [DOI] [PubMed] [Google Scholar]
- 504.Habil N, Abate W, Beal J, Foey AD. Heat-killed probiotic bacteria differentially regulate colonic epithelial cell production of human β-defensin-2: dependence on inflammatory cytokines. Benef. Microbes. 2014;5:483–495. doi: 10.3920/BM2013.0061. [DOI] [PubMed] [Google Scholar]
- 505.Dong Y, et al. Benefit of dietary supplementation with Bacillus subtilis BYS2 on growth performance, immune response, and disease resistance of broilers. Probiotics Antimicrobial Proteins. 2020;12:1385–1397. doi: 10.1007/s12602-020-09643-w. [DOI] [PubMed] [Google Scholar]
- 506.Deng J, Li Y, Zhang J, Yang Q. Co-administration of Bacillus subtilis RJGP16 and Lactobacillus salivarius B1 strongly enhances the intestinal mucosal immunity of piglets. Res. Vet. Sci. 2013;94:62–68. doi: 10.1016/j.rvsc.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 507.Huang J, et al. Peptidoglycan derived from Lactobacillus rhamnosus MLGA up-regulates the expression of chicken β-defensin 9 without triggering an inflammatory response. Innate Immun. 2020;26:733–745. doi: 10.1177/1753425920949917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Huang F-C, Lu Y-T, Liao Y-H. Beneficial effect of probiotics on Pseudomonas aeruginosa-infected intestinal epithelial cells through inflammatory IL-8 and antimicrobial peptide human beta-defensin-2 modulation. Innate Immun. 2020;26:592–600. doi: 10.1177/1753425920959410. [DOI] [PMC free article] [PubMed] [Google Scholar]