Abstract
Objective
For acute cholecystitis, the treatment of choice is laparoscopic cholecystectomy. In mild-to-moderate cases, the use of antibiotic prophylaxis for the prevention of postoperative infectious complications (POICs) lacks evidence regarding its cost-effectiveness when compared with no prophylaxis. In the context of rising antimicrobial resistance, there is a clear rationale for a cost-effectiveness analysis (CEA) to determine the most efficient use of National Health Service resources and antibiotic routine usage.
Design
16 of 226 patients (7.1%) in the single-dose prophylaxis group and 29 of 231 (12.6%) in the non-prophylaxis group developed POICs. A CEA was carried out using health outcome data from thePerioperative antibiotic prophylaxis in the treatment of acute cholecystitis (PEANUTS II) multicentre, randomised, open-label, non-inferiority, clinical trial. Costs were measured in monetary units using pound sterling, and effectiveness expressed as POICs avoided within the first 30 days after cholecystectomy.
Results
This CEA produced an incremental cost-effectiveness ratio of −£792.70. This suggests a modest cost-effectiveness of antibiotic prophylaxis being marginally less costly and more effective than no prophylaxis. Three sensitivity analyses were executed considering full adherence to the antibiotic, POICs with increased complexity and break-point analysis suggesting caution in the recommendation of systematic use of antibiotic prophylaxis for the prevention of POICs.
Conclusion
The results of this CEA point to greater consensus in UK-based guidelines surrounding the provision of antibiotic prophylaxis for mild-to-moderate cases of acute cholecystitis.
Keywords: cost-effectiveness, laparoscopic cholectstectomy, antibiotics, surgical infections, surgical complications
WHAT IS ALREADY KNOWN ON THIS TOPIC
Guidelines recommending the use of antibiotic prophylaxis in emergency cholecystectomy for cases of mild-to-moderate acute cholecystitis are based on low quality evidence.
Although the National Institute for Health and Care Excellence (NICE) recommend antibiotic prophylaxis for all clean-contaminated procedures such as cholecystectomies, there are no data relating to its cost-effectiveness for cases of mild-to-moderate acute cholecystitis.
WHAT THIS STUDY ADDS
Findings from our cost-effectiveness study suggest that antibiotic prophylaxis is modestly more cost-effective than no prophylaxis for emergency cholecystectomy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The study’s findings are in line with recommendations by NICE.
However, with antimicrobial resistance declared by WHO as one of the top ten global public health threats, antibiotic stewardship is increasingly important.
The modest cost-effectiveness of antibiotic prophylaxis suggests that this recommendation should be interpreted with caution in the future.
Introduction
The gallbladder is a small organ that sits beneath the liver. It contains bile, a digestive liquid that aids in the digestion of fatty foods.1 Acute cholecystitis describes the sudden-onset inflammation of the gallbladder. Patients may present with pain, localised tenderness and a palpable mass in the right upper quadrant of their abdomen.2 This condition commonly occurs following cholelithiasis (gallstones), which affects 15% of the UK population.3 Gallstones can obstruct ducts within the gallbladder causing calculous cholecystitis which accounts for 90%–95% of cases. The remaining 5%–10% of cases, termed acalculous cholecystitis, are not caused by gallstones.4
For acute calculous cholecystitis, the treatment of choice is a laparoscopic cholecystectomy (LC)—a minimally invasive surgical removal of the gallbladder.5 Over 50 000 LCs are performed every year in the UK, making it one of the most performed National Health Service (NHS) procedures.6 Among the complications of LCs are postoperative infectious complications (POICs), including surgical-site infections (SSIs).7 8
For severe acute cases, there is clear guidance for the commencement of preoperative antibiotic therapy.2 However, UK-based guidelines are contradictory for mild-to-moderate cases of acute cholecystitis. The most recent National Institute for Health and Care Excellence (NICE) guidelines (2019) recommend antibiotic prophylaxis in all clean-contaminated procedures, such as cholecystectomies.9 The Scottish Intercollegiate Guidelines Network does not explicitly recommend prophylaxis, stating that prophylaxis should be highly considered.10 Despite the lack of consensus, 36% of surgeons prescribed antibiotic therapy before gallbladder surgery in 2014.11
Some UK surgeons follow international guidelines, such as Tokyo Guidelines 2018 and Surgical Infection Society Guidelines (2017),12 13 which favour antibiotic prophylaxis in the management of the American Society of Anesthesiologists (ASA) grade I and grade II (mild-to-moderate) acute cholecystitis. However, the evidence base for these recommendations is poor due to trials having small sample sizes14 and low adherence to these guidelines in Europe.15
The NHS Long Term Plan and UK government are promoting antibiotic stewardship,16 17 with a goal set for 15% reduction in human antibiotic use by 2024.17 Unnecessary prescription of prophylactic antibiotics can lead to antimicrobial resistance (AMR), which costs the NHS approximately £180 million a year.18
The use of antibiotic prophylaxis for the prevention of POICs is not well informed due to the lack of definitive research regarding its effectiveness and cost data when compared with no prophylaxis. As a result, there is a clear rationale for this study as it will help to determine the most efficient use of NHS resources in an era of rising AMR.
The aim of this study was to conduct a cost-effectiveness analysis (CEA) of antibiotic prophylaxis versus no antibiotic prophylaxis in patients with mild-to-moderate acute calculous cholecystitis undergoing an emergency LC. The primary objective was to determine whether antibiotic prophylaxis is economically justifiable for the NHS.
Literature review
A systematic search of the literature was conducted on the following databases: Ovid MEDLINE, Embase and Scopus, on 8 February 2022. The following search string was implemented on all three databases (“antibiotic” OR “antimicrob* OR “prophyla*”) AND (“cholecystitis” OR “cholecystectomy”). The major international guidelines on antibiotic prophylaxis for acute cholecystitis, TG18,12 are based off clinical trials and research before 2018. Due to the vast amount of research on this topic and to capture further advancements since the publication of TG18,12 we chose to limit our search to articles published in 2018 and onwards. We accepted randomised controlled trials (RCTs), non-randomised controlled trials, cohort studies and economic evaluations. The full inclusion and exclusion criteria can be found in online supplemental table S1. Fourteen articles underwent full-text screening, from which three RCTs were selected to be included in this systematic review. Our search did not yield any economic evaluations or cohort studies of relevance. This review was conducted in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A further scoping review was conducted to identify economic evaluations published prior to 2018, which yielded no eligible papers.
bmjgast-2023-001162supp001.pdf (388.9KB, pdf)
The TG18 guidelines concluded that antibiotic prophylaxis should be given to low-risk and medium-risk patients based on prior literature.12 Since then, two RCTs have concluded that prophylaxis is not necessary. First, Jaafar et al19 compared the effect of a prophylactic dose of 4 g piperacillin/tazobactam with no prophylaxis on POICs. Results showed no significant difference in incidence of POICs between the two groups. Second, the RCT by Guler et al20 aimed to investigate the effect of 1 g of perioperative intravenous cefazolin on POICs. They concluded that for low-risk LCs, antibiotic prophylaxis is not recommended due to there being no significant difference in incidence of POICs. However, limitations of this study include 11.2% of the population being ASA grade III (high-risk), and patients who received antibiotics during surgery for gallbladder perforation being included in the prophylaxis group. van Braak et al14 investigated whether a single dose (2 g) of the antibiotic cefazolin given as a prophylactic would reduce POICs in the PEANUTS II trial.
Due to notable limitations in the study by Guler et al,20 as well as a much larger sample size and recency compared with Jaafar et al, the PEANUTS II trial by Braak et al14 was chosen as the basis for our CEA.
Although CEAs of antibiotic prophylaxis have been carried out for a vast range of surgeries,21 to date, no economic evaluations have been conducted in the context of an LC for mild-to-moderate acute cholecystitis. This study is the first to evaluate the cost-effectiveness of antibiotic prophylaxis for this procedure in the context of the British healthcare system.
Methods
Study design
This economic evaluation estimated the cost-effectiveness of antibiotic prophylaxis for patients undergoing LC secondary to acute mild-to-moderate cholecystitis using data from the PEANUTS II trial and NHS costing.
The PEANUTS II trial was a multicentre (five teaching hospitals, one academic hospital), randomised non-inferiority clinical trial based in the Netherlands. It involved 457 patients presenting with acute calculous cholecystitis for whom immediate cholecystectomy was indicated. Participants were randomised to a single-dose prophylaxis group (n=226) and no-prophylaxis group (n=231). With a non-inferiority margin of 10%, the trial concluded that omitting antibiotic prophylaxis was not recommended.
Costs were measured in monetary units, pound sterling, and effectiveness expressed in terms of POICs avoided within the first 30 days after cholecystectomy. Considering there were no utility measures reported in the PEANUTS II trial, a cost-utility analysis, giving outcomes in terms of quality-adjusted life-years, was not chosen.
This analysis was conducted from the NHS perspective; the patient perspective was not considered. A 30-day time horizon was chosen to mirror the timeframe of the PEANUTS II trial.22 Outcomes beyond this were not reported in the trial data. This coincides with the 30-day surveillance period for gallbladder surgery given by guidelines adhered to in the UK, in addition to statistical evidence suggesting that the majority of SSIs are identified within 30 days after surgery.7 22
Application to British healthcare system
While outcomes for this economic analysis were obtained from a Dutch trial, they were applied to the UK perspective by factoring in similarities between the British and Dutch healthcare systems. Both the UK and the Netherlands have universal healthcare coverage, which ensures that all residents have access to essential medical care irrespective of their financial circumstances. The British public are automatically entitled to free healthcare through the NHS, which is mostly funded by general taxation.23 Likewise, all Dutch residents have basic healthcare coverage through statutory health insurance offered by private insurers, as mandated by the Health Insurance Act of 2006 (Zorgverzekeringswet).24 Private insurers are obligated to accept all applicants, and means-tested subsidies (healthcare allowances) available from the government help cover insurance premiums for low-income residents.25
Second, as is the case in the UK, the Dutch government are responsible for setting healthcare priorities and monitoring access, quality and costs.25 The NHS and the Dutch healthcare system are united by their core principles of providing consistently high-quality, accessible healthcare that is available to all, regardless of their background or financial limitations.26 27 In addition, both countries occupy top positions (second for the Netherlands; fourth for the UK) in overall healthcare system performance rankings when compared with other high-income countries.28
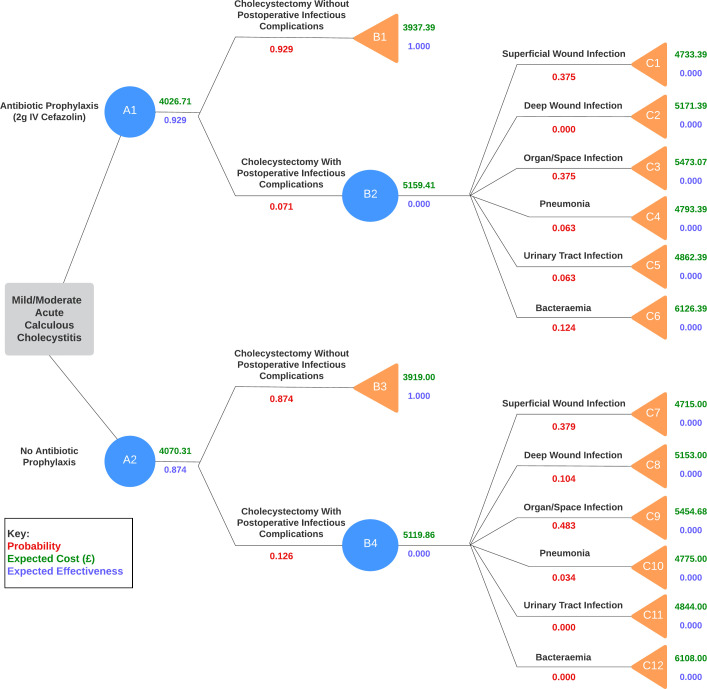
Description of the model
The main outcome of the study is the absence of POICs. A decision tree (figure 1) was constructed comparing the incidence of POICs in patients undergoing cholecystectomy with and without antibiotic prophylaxis. The parameter values were based on the reported probability of POICs obtained from the PEANUTS II trial. The estimated costs of treating these complications are summarised in online supplemental table S2. It was decided that intention-to-treat analysis data would be used to determine the effect of antibiotic prophylaxis on the incidence on POICs. In addition, it was assumed that each POIC extending from a decision node was mutually exclusive to another.
Figure 1.
Decision tree showing incidence of postoperative infectious complications (POICs) in antibiotic prophylaxis group and no antibiotic prophylaxis group.
For the antibiotic prophylaxis arm of the tree, the intervention was considered successful if the patient did not develop any POICs following cholecystectomy (n=226). POICs were stratified into SSIs: superficial wound infection, deep wound infection and organ/space infection; and distant infections: pneumonia, urinary tract infections and bacteraemia. For the non-prophylaxis arm, outcomes were deemed unsuccessful if patients developed POICs (n=231). The break down of complications is summarised in online supplemental table S3 which was obtained from the PEANUTS II trial.14
Despite non-infectious complications occurring more commonly than infectious complications, these were omitted from the decision tree since there was no statistically significant difference observed between the antibiotic prophylaxis and non-prophylaxis groups. Prior literature has shown that antibiotic prophylaxis does not significantly impact the incidence of non-infectious complications, such as biloma and bile duct injury, following a cholecystectomy.29
Moreover, death was not modelled as the PEANUTS II trial only reported one death due to severe sepsis (secondary to bile leakage), representing a mortality rate of 2 in 100 000 which was deemed negligible. This is reinforced by a recent systematic review and pooled analysis which found the mortality rate of an LC was 0.08%–0.14% internationally.30
According to the trial, all POICs were treated successfully within the 30-day period of the study. Any adverse effects of antibiotic treatment were not accounted for due to their rare incidence, occurring only 0.39% of the time.31
Costs
Costs were obtained from British national sources: NHS 2021/2022 National Tariff Payment System32 and British National Formulary (BNF).33 A comprehensive breakdown of costs can be found in online supplemental table S2. In the decision tree, cost values at decision nodes were generated by calculating weighted averages from endpoint nodes using probability values provided by the PEANUTS II trial. All the costs in this study were taken from 2022 databases and therefore did not require discounting.
Cholecystectomy
The cost of a cholecystectomy in this case comprised the cost of the surgical procedure in addition to a single outpatient follow-up appointment. NICE guidelines recommend that patients are to be seen in the outpatient setting 2 weeks postsurgery, so that they can be assessed for POICs.34
Surgical costs were exclusively accounted for by the NHS national tariff for an LC in adult patients with the lowest score for complexity and comorbidity. This was despite 1.97% of patients in the PEANUTS II trial undergoing open surgery, since the authors identified no association between mode of surgery and incidence of POICs. National tariffs include factors, such as duration of hospital stay, cost of required staff, tests, procedures and medications into their total price.32 In this case, the ‘non-elective spell’ tariff was used to reflect patients undergoing an emergency procedure as reported in the trial.
Antibiotic prophylaxis
The PEANUTS II trial specified that antibiotic prophylaxis consisted of a single dose of 2 g cefazolin administered intravenously. This cost was estimated at £18.39 according to BNF.
Complications
All six types of POICs have been assigned costs based on NHS national tariffs. Since UK guidelines do not outline specific management strategies for different types of SSIs, superficial and deep wound infections have been modelled in accordance with the healthcare resource group (HRG) codes,32 or tariff payments, that they are most closely correlated with. Organ/Space infection was modelled as intra-abdominal abscess given this condition most resembles the description provided by the PEANUTS II trial. In line with NICE guidelines and the trial, management of this condition consists of percutaneous drainage and antibiotic therapy. Costs of medications were obtained from BNF. The sum of antibiotic therapy was added to the national tariff for percutaneous (single) drainage of abdominal abscess.
Considering patients in the PEANUTS II trial were mostly managed conservatively, or with antibiotics, the lowest complication and comorbidity (CC) scores were chosen from national tariffs to reflect the presumed low complexity of POICs in this study. Where relevant, tariffs stratified into ‘without intervention’ were selected over those with interventions. Since the trial did not report the duration of hospital stay for each POIC, a ‘best-case scenario’ approach was taken to mean all POICs were treated within the trim point (length of stay beyond which the excess bed day tariff applies) of their respective tariffs. Additionally, the lowest dose and shortest duration of antibiotics were taken where guidelines offered ranges.
Effectiveness
Effectiveness was represented by either POICs avoided per cholecystectomy performed or no incidence of POIC (equivalent to effectiveness of 1, and 0 otherwise). The probabilities of developing POICs were obtained from the PEANUTS II trial, which recorded the development of infectious complications within the 30 days following the cholecystectomy. This included complications developed during the inpatient stay as well as those reported after discharge via an outpatient appointment.
Discounting the effectiveness measure was not performed as the outcomes are independent of the time period in which they occur.
Results
Cost outcomes
Patients in the antibiotic prophylaxis group accessed healthcare resources costing an estimated £4026.71, whereas expected costs for patients in the non-prophylaxis group were £4070.31. Therefore, this represents a cost-saving of £43.59 per patient on average.
Effectiveness outcomes
In terms of effectiveness, the chosen measure was the proportion of POICs averted. The effectiveness in the non-prophylaxis group was 87.4% of POICs averted, whereas in the intervention group, with antibiotic prophylaxis, it was 92.9%. Thus, the use of antibiotic prophylaxis represented on average a 5.5% reduction in the incidence of POICs after LC.
Cost-effectiveness
Antibiotic prophylaxis is proven to be more clinically effective and at a lower cost with our calculated incremental cost-effectiveness ratio (ICER) of −£792.70. The generated ICER is portrayed on a cost-effectiveness plane diagram (figure 2).
Figure 2.
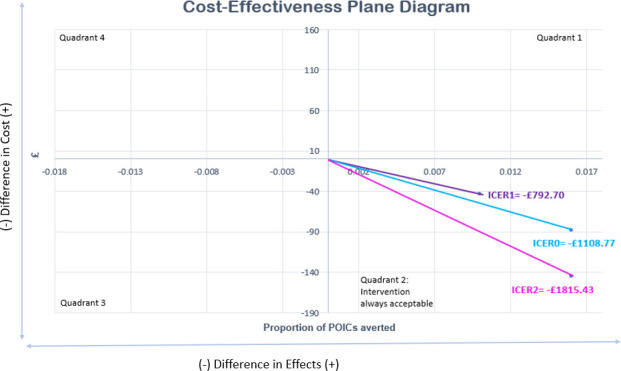
Cost-effectiveness plane diagram. ICER0—original, ICER1—generated from sensitivity analysis 1, ICER2—generated from sensitivity analysis 2. ICER, incremental cost-effectiveness ratio; POIC, postoperative infectious complication.
Since the antibiotic prophylaxis regimen represents the dominant strategy, it is unnecessary to source a willingness-to-pay threshold. Hence, monetary net benefit and health net benefit ratios were not calculated.
Sensitivity analyses
Three one-way sensitivity analyses were conducted to assess the robustness and influence of the assumptions made on the overall conclusions drawn from the PEANUTS II trial. The newly generated ICERs are also displayed on figure 2.
Sensitivity analysis 1
In the original CEA model, results from the intention-to-treat analysis (ITTA) of the study were used. The ITTA includes “all randomised patients in the groups to which they are randomly assigned (…) regardless of the treatment they actually received”.35 The attrition rate was relatively small and similar across both groups (9.7% and 9.1% in the prophylaxis and non-prophylaxis groups, respectively). This presumably underestimates the effect of antibiotic prophylaxis on POICs, but the majority school of thought agrees that it more closely represents the reality of clinical practice. Online supplemental appendix 1 provides further details on adherence to study protocol extracted from the PEANUTS II trial.
A sensitivity analysis was carried out using results from the per-protocol analysis (PPA), which reflects a reality where patients are fully compliant with protocol.36 Data from patients who did not receive the treatment they were originally allocated to were excluded from the interpretation of results. This identifies the cost-saving that can be entirely attributed to the effect of antibiotic prophylaxis on POICs. See online supplemental figure S1 for the corresponding decision tree.
It is worth noting that the PPA introduces an attrition bias by excluding a subset of participants who deviated from protocol meaning the groups of patients being compared no longer have similar characteristics.
The generated ICER1 of −£1108.77 remains negative, suggesting that antibiotic prophylaxis is still cost-effective.
Sensitivity analysis 2
The second sensitivity analysis was conducted using the tariff prices of POICs with increased complexity. In the original model, costs of complications were calculated assuming a ‘best-case scenario’, whereby the lowest CC scoring HRG codes were used from the national tariffs. Nevertheless, this would rarely be the case in clinical practice.
Naturally, POICs in patients with additional complications or comorbidities (ie, complex) correspond to HRG codes with a higher CC score, and subsequently higher tariff. A single incremental rise in complexity of HRG codes was made for each of the POICs; except superficial wound infection, for which added complexity would become equivalent to deep wound infection given the assumption made in this study. For deep wound infections, a new HRG code was selected on the basis of patients undergoing a ‘single intervention’ to manage their more severe condition. See online supplemental table S4 for the cost break down of this sensitivity analysis, and online supplemental figure S2 for its corresponding decision tree.
This generated an ICER2 of −£1815.43 (as cost-saving increased to £145.23) suggesting once again that antibiotic prophylaxis is the superior regimen despite adjustment for this uncertainty.
Sensitivity analysis 3
The third sensitivity analysis describes by how much the proportion of POICs averted must change to render antibiotic prophylaxis neither cost-effective nor cost-ineffective (ICER=0). The calculation demonstrates that the probability of POICs averted with antibiotic prophylaxis must decrease by 0.035 from 0.929 to 0.894 for ICER=0.
Discussion
The PEANUTS II trial concluded that omitting antibiotic prophylaxis should not be recommended for patients with mild-to-moderate acute cholecystitis undergoing an LC. The results of this CEA demonstrate that antibiotic prophylaxis is the dominant strategy: both cost-saving, due to decreased consumption of healthcare resources, and more clinically effective in terms of reduced rate of POICs when compared with no prophylaxis. However, the differences are not large, suggesting caution in the generalisation of the results. The PEANUTS II trial data found no significant difference between the groups for individual POICs. The extensive sensitivity analyses in this study suggest that prophylaxis remains cost-effective despite changes in costs of POIC treatment. It should be noted that a modest change in effectiveness from 0.942 to 0.894 would no longer support the routine use of prophylaxis for mild-to-moderate cases of acute cholecystitis. Therefore, there is a trade-off to be made. Antibiotic overprescription is known to result in the spread of AMR. AMR could take the form of an additional cost of £20 000 per patient episode in hospital due to increased severity of illness from infection with resistant bacteria.37 It also results in increased hospital stay and mortality rate. LCs are one of the most performed abdominal surgical procedures in high-income countries, so offers a valuable opportunity to reduce unnecessary consumption of antibiotics. Future cost-savings from omission of antibiotic prophylaxis may be much greater than those derived from reduction in POICs. Clinicians will face difficult decisions in the future regarding the provision of antibiotic prophylaxis.
There have been no CEAs of antibiotic prophylaxis versus no antibiotic prophylaxis for emergency LC from an NHS perspective. The most comparable literature, conducted by Matsui et al,31 found that antibiotic prophylaxis for elective LC reduced postoperative medical costs and overall incidence of POIC. This is in line with our findings. However, the comparability to our CEA is limited. The study was conducted in Japan, which has a notably different healthcare system to that of the UK, and the study population were patients undergoing an elective LC, thus at significantly lower infection risk compared with patients in the PEANUTS II trial. Therefore, one of the main strengths of this analysis is that it is the first economic evaluation of antibiotic prophylaxis for emergency cholecystectomy based on the results of a multicentre randomised clinical trial.
Online supplemental table S5 highlights the key assumptions made in this economic evaluation. The findings of this analysis must be considered in the context of the following limitations. First, in some cases, specific POIC treatment pathways were unable to be matched to tariffs in the NHS tariff payment system. In these instances, costs were estimated with the closest matching HRG codes. This may have resulted in some bias in the final costs calculated per intervention. Second, the trial data did not include reported confidence intervals. This impacted our ability to develop a comprehensive sensitivity analysis using upper and lower limits to manipulate probabilities. Furthermore, according to ITTA data from the study, there was no statistically significant difference in the incidence of POICs between the groups (p=0.052), although prophylaxis remained cost-effective. This suggests that even small changes in sample size can strongly influence overall conclusions drawn from the trial. Third, while costs related to hospital stay and procedures performed were calculated, the chosen time horizon of 30 days may be too short. A meta-analysis concluded that 76% of readmission rates after cholecystectomies were due to postsurgical complications.38 Patients who were not successfully treated for POICs and experienced further infectious complications which required readmission beyond the 30-day period, have not been included in this study. Fourth, we assumed the cost of treating the complications were mutually exclusive and did not benefit from economies of scale. Lastly, we were not able to account for AMR’s true impact over time on the prophylaxis efficacy.
The PEANUTS II trial was conducted in the Netherlands. Based on similarities between the Dutch healthcare system and the NHS, the outcomes of the PEANUTS II trial have been generalised to the UK population. Both healthcare systems are founded on the principles of fairness and equity, striving for access to care for all citizens.26 27 Although some patient characteristics are similar, such as life expectancy (82 for the Netherlands; 81 for the UK), other factors can vary between the two countries.39 Higher proportions of the population are obese in the UK compared with the Netherlands (64% vs 47%).40 As obesity is a major risk factor for POICs, this may limit generalisability to the UK population.41 Furthermore, the prescribed antibiotic regimen is dependent on disease severity and isolates observed in the local area. The PEANUTS II trial only accounts for a single dose of 2 g cefazolin. Thus, the findings may not be generalisable to other populations where alternative prophylactic doses may be in use. Finally, the costs reported in this study are derived from national sources in the UK, such as the NHS and BNF, and thus are limited to the NHS perspective. This limits the generalisability of the findings of this CEA to other healthcare systems, where patient demographics and access to care can vary greatly.
Conclusion
This economic evaluation suggests that single-dose antibiotic prophylaxis to reduce the incidence of POICs after emergency LC for mild-to-moderate acute cholecystitis is modestly more cost-effective than no prophylaxis. This is in line with current NICE guidelines. However, this recommendation may be subject to change in the future due to costs associated with the rising prevalence of AMR which have not been accounted for in this study.
Footnotes
MS, AH and AA contributed equally.
Contributors: MS, AH and AA are joint first authors who contributed equally to the study design, conception, data curation, data analysis and manuscript creation and critical revision. LdP was the supervisor for this project. All authors (MS, AH, AA and LdP) have read and agreed to the published version of the manuscript. MS is the guarantor for the overall content of the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article or uploaded as supplementary information. There is no additional unpublished data from the study.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.NHS Choices . Gallbladder removal [online]. 2019. Available: https://www.nhs.uk/conditions/gallbladder-removal
- 2.BMJ Best Practice . Acute cholecystitis summary. 2022. Available: https://bestpractice.bmj.com/topics/en-gb/3000084?q=Acute%20cholecystitis&c=recentlyviewed
- 3.NICE . Gallstones. 2019. Available: https://cks.nice.org.uk/topics/gallstones/
- 4.NHS . Acute cholecystitis. 2019. Available: https://www.nhs.uk/conditions/acute-cholecystitis/
- 5.NICE . Gallstone disease: diagnosis and management. 2014. Available: https://www.nice.org.uk/guidance/cg188/chapter/recommendations#laparoscopic-cholecystectomy
- 6.Clifford RE, Rajput K, Naing CY, et al. Reducing waiting lists for Laparoscopic Cholecystectomy: an intensive approach to aid COVID-19 recovery. Eur Surg 2022;54:113–6. 10.1007/s10353-021-00722-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren DK, Nickel KB, Wallace AE, et al. Risk factors for surgical site infection after Cholecystectomy. Open Forum Infect Dis 2017;4:ofx036. 10.1093/ofid/ofx036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen MA, Nickel KB, Wallace AE, et al. Surgical site infection after Cholecystectomy: rates and operative risk factors. Value in Health 2014;17:A35. 10.1016/j.jval.2014.03.214 [DOI] [Google Scholar]
- 9.NICE . Surgical site infections: prevention and treatment. 2019. Available: https://www.nice.org.uk/guidance/ng125/chapter/recommendations
- 10.Scottish International Guidelines Network . SIGN 104 antibiotic prophylaxis in surgery. 2014. Available: http://medicinainterna.net.pe/images/guias/GUIA_PARA_LA_PROFILAXIS_ANTIBIOTICA_EN_CIRUGIA.pdf
- 11.Pasquali S, Boal M, Griffiths EA, et al. Meta-analysis of perioperative antibiotics in patients undergoing Laparoscopic Cholecystectomy. Br J Surg 2016;103:27–34; 10.1002/bjs.9904 [DOI] [PubMed] [Google Scholar]
- 12.Gomi H, Okamoto K, Ukai T, et al. Tokyo guidelines 2018: antimicrobial therapy for acute cholangitis and Cholecystitis. J Hepatobiliary Pancreat Sci 2018;25:3–16.:E6. 10.1002/jhbp.560 [DOI] [PubMed] [Google Scholar]
- 13.Mazuski JE, Tessier JM, May AK, et al. The surgical infection society revised guidelines on the management of intra-abdominal infection. Surgical Infections 2017;18:1–76. 10.1089/sur.2016.261 [DOI] [PubMed] [Google Scholar]
- 14.van Braak WG, Ponten JEH, Loozen CS, et al. Antibiotic prophylaxis for acute Cholecystectomy: PEANUTS II Multicentre randomized non-inferiority clinical trial. Br J Surg 2022;109:267–73. 10.1093/bjs/znab441 [DOI] [PubMed] [Google Scholar]
- 15.Bass GA, Gillis AE, Cao Y, et al. Self‐Reported and actual adherence to the Tokyo guidelines in the European Snapshot audit of complicated Calculous biliary disease. BJS Open 2020;4:622–9. 10.1002/bjs5.50294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Service . Long term plan. 2022. Available: https://www.longtermplan.nhs.uk/
- 17.Gove M, Hancock M. HM government: tackling antimicrobial resistance 2019–2024 the UK’s five-year national action plan. 2019. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_plan.pdf [DOI] [PubMed]
- 18.House of Commons Health and Social Care Committee . House of Commons health and social care committee. antibiotic resistance. eleventh report of session 2017-2019. 2018. Available: https://publications.parliament.uk/pa/cm201719/cmselect/cmhealth/962/962.pdf
- 19.Jaafar G, Sandblom G, Lundell L, et al. Antibiotic prophylaxis in acute Cholecystectomy Revisited: results of a double-blind randomised controlled trial. Langenbecks Arch Surg 2020;405:1201–7. 10.1007/s00423-020-01977-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guler Y, Karabulut Z, Sengul S, et al. The effect of antibiotic prophylaxis on wound infections after Laparoscopic Cholecystectomy: A randomised clinical trial. Int Wound J 2019;16:1164–70. 10.1111/iwj.13175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen J, David M, Veerman JL. Systematic review of the cost-effectiveness of preoperative antibiotic prophylaxis in reducing surgical-site infection. BJS Open 2018;2:81–98. 10.1002/bjs5.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Healthcare Safety Network . Surgical site infection event. 2023. Available: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf
- 23.The Commonwealth Fund . International health care system profiles: England. 2020. Available: https://www.commonwealthfund.org/international-health-policy-center/countries/england
- 24.Kuipers T, van de Pas R, Krumeich A. Is the Healthcare provision in the Netherlands compliant with universal health coverage based on the right to health?: A narrative literature review. Global Health 2022;18:38. 10.1186/s12992-022-00831-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Commonwealth fund International health care system profiles: Netherlands. 2020. Available: https://www.commonwealthfund.org/international-health-policy-center/countries/netherlands
- 26.Zorginstituut Nederland . About us. 2022. Available: https://english.zorginstituutnederland.nl/
- 27.Department of Health and Social Care . The NHS Constitution for England. 2021. Available: https://www.gov.uk/government/publications/the-nhs-constitution-for-england/the-nhs-constitution-for-england
- 28.The Commonwealth Fund . Improving health care quality: mirror, mirror 2021: reflecting poorly. 2021. Available: https://www.commonwealthfund.org/publications/fund-reports/2021/aug/mirror-mirror-2021-reflecting-poorly
- 29.van Dijk AH, van der Hoek M, Rutgers M, et al. Efficacy of antibiotic agents after spill of bile and gallstones during Laparoscopic Cholecystectomy. Surg Infect (Larchmt) 2019;20:298–304. 10.1089/sur.2018.195 [DOI] [PubMed] [Google Scholar]
- 30.Pucher PH, Brunt LM, Davies N, et al. Outcome trends and safety measures after 30 years of Laparoscopic Cholecystectomy: a systematic review and pooled data analysis. Surg Endosc 2018;32:2175–83. 10.1007/s00464-017-5974-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsui Y, Satoi S, Kaibori M, et al. Antibiotic prophylaxis in Laparoscopic Cholecystectomy: a randomized controlled trial. PLoS One 2014;9:e106702. 10.1371/journal.pone.0106702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NHS . National tariff payment system. 2021. Available: https://www.england.nhs.uk/publication/past-national-tariffs-documents-and-policies/
- 33.British national formulary. 2022. Available: https://bnf.nice.org.uk/
- 34.National Institute for Health and Care Excellence . Gallstone disease: diagnosis and management. n.d. Available: https://www.nice.org.uk/guidance/cg188/chapter/1-Recommendations#managing-gallbladder-stones [PubMed]
- 35.Fisher LD, Dixon DO, Herson J, et al. Intention to treat in clinical trials. In Statistical Issues in Drug Research and Development 2017:331–50. 10.1201/9780203738610 [DOI] [Google Scholar]
- 36.Shah PB. Intention-to-treat and per-protocol analysis. CMAJ 2011;183:696. 10.1503/cmaj.111-2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P&T 2015;40:277–83. [PMC free article] [PubMed] [Google Scholar]
- 38.McIntyre C, Johnston A, Foley D, et al. Readmission to hospital following Laparoscopic Cholecystectomy: a meta-analysis. Anaesthesiol Intensive Ther 2020;52:47–55. 10.5114/ait.2020.92967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Institut National D’études Démographiques . Life expectancy at birth in Europe and other developed OECD countries. 2019. Available: https://www.ined.fr/en/everything_about_population/data/europe-developed-countries/life-expectancy/
- 40.Papanicolas I, Mossialos E, Gundersen A, et al. Performance of UK national health service compared with other high income countries: observational study. BMJ 2019;367:l6326. 10.1136/bmj.l6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tjeertes EKM, Hoeks SE, Beks SBJC, et al. Erratum to: obesity--a risk factor for postoperative complications in general surgery BMC Anesthesiol 2015;15:155. 10.1186/s12871-015-0136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2023-001162supp001.pdf (388.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article or uploaded as supplementary information. There is no additional unpublished data from the study.