Abstract
Background:
Although it is well-accepted that increased plasma free fatty acid (FFA) concentration causes lipid overload and muscle insulin resistance in people with obesity, plasma FFA concentration poorly predicts insulin-resistant glucose metabolism. It has been proposed that hyperinsulinemia in people with obesity sufficiently inhibits adipose tissue triglyceride lipolysis to prevent FFA-induced insulin resistance. However, we hypothesized enhanced FFA clearance in people with obesity, compared with lean people, prevents a marked increase in plasma FFA even when FFA appearance is high.
Methods:
We assessed FFA kinetics during basal conditions and during a hyperinsulinemic-euglycemic clamp procedure in 14 lean people and 46 people with obesity by using [13C]palmitate tracer infusion. Insulin-stimulated muscle glucose uptake rate was evaluated by dynamic PET-imaging of skeletal muscles after [18F]fluorodeoxyglucose injection.
Results:
Plasma FFA clearance was accelerated in participants with obesity and correlated negatively with muscle insulin sensitivity without a difference between lean and obese participants. Furthermore, insulin infusion increased FFA clearance and the increase was greater in obese than lean participants.
Conclusions:
Our findings suggest plasma FFA extraction efficiency, not just plasma FFA concentration, is an important determinant of the cellular fatty acid load and the stimulatory effect of insulin on FFA clearance counteracts some of its antilipolytic effect.
Keywords: Obesity, insulin resistance, adipose tissue, fatty acids
1. INTRODUCTION
It is a widely accepted view that increased fatty acid release from adipose tissue in people with obesity causes an increase in plasma free fatty acid (FFA) concentration, which in turn causes insulin resistance in the liver and skeletal muscles (i.e., impaired insulin action on hepatic glucose production and muscle glucose uptake) [1, 2]. And yet, plasma FFA concentration is a poor predictor of insulin-resistant glucose metabolism and plasma FFA are often not elevated in people with obesity and insulin resistance [3–6]. It has been proposed that hyperinsulinemia in people with obesity compensates for reduced adipose tissue insulin sensitivity and sufficiently inhibits adipose tissue triglyceride lipolysis to prevent FFA-induced insulin resistance [3, 7]. However, plasma FFA concentration is determined by both the FFA appearance rate in plasma and the plasma FFA clearance rate. Accordingly, it is possible that a high plasma FFA clearance rate in people with insulin resistance prevents a marked increase in plasma FFA concentration even when FFA release from adipose tissue is high. Results from studies conducted in rats support this idea [8]. Moreover, they demonstrate that insulin increases muscle FFA clearance [8, 9], suggesting the insulin-mediated decrease in plasma FFA concentration is not only due to decreased adipose tissue triglyceride lipolysis — as is commonly thought — but also increased plasma FFA clearance.
Here, we studied the effects of adiposity and insulin on plasma FFA clearance and its relationship with insulin sensitivity in lean people and people with obesity. We hypothesized that plasma FFA clearance would be greater in participants with obesity than lean participants and would be negatively correlated with muscle insulin sensitivity. Furthermore, we hypothesized that an acute increase in plasma insulin would increase plasma FFA clearance. FFA clearance was determined during basal conditions and during a hyperinsulinemic-euglycemic clamp procedure (HECP) by using a stable isotope-labeled palmitate tracer infusion. Muscle insulin sensitivity was assessed as insulin-stimulated glucose uptake rate, which we determined by using [18F]-labeled fluorodeoxyglucose (FDG) infusion in conjunction with positron-emission tomography (PET).
2. EXPERIMENTAL DESIGN AND METHODS
2.1. Study participants
The data included in this analysis were obtained from 14 lean people and 46 people with obesity who participated in two ongoing studies (NCT02994459, NCT03408613) that used the same experimental protocol. All participants provided written informed consent before initiating the study protocols, which were approved by the Institutional Review Board at Washington University in St. Louis, MO. Participants completed a medical examination, after they fasted overnight, that included a medical history and physical examination, standard blood tests, and an oral glucose tolerance test. Potential participants were excluded if they: i) had a disease or were taking medications or dietary supplements that could affect the study outcome measures; ii) consumed excessive amounts of alcohol; iii) had ≥3% body weight changes within the past six months; or iv) participated in structured exercise for more than 90 min/week. Body composition was determined by using dual-energy x-ray absorptiometry (Lunar iDXA, GE Healthcare).
2.2. Metabolic testing, sample processing, and calculations
All participants completed a basal metabolic study and a HECP (50 mU insulin/m2 body surface area/min) after they fasted overnight [10]. To determine FFA kinetics, [U-13C]palmitate was infused intravenously (6.0 nmol/kg fat-free mass/min during basal conditions; 4.5 nmol/kg fat-free mass/min during the HECP) [11]. Approximately 150 min after starting the HECP, [18F]fluorodeoxyglucose (185 MBq) was administered intravenously and muscle glucose uptake was determined by dynamic PET [10]. Blood samples to determine plasma palmitate enrichment, and fatty acid, glucose, and insulin concentrations were obtained before starting the tracer infusions and during the last 30 min of the basal period and the HECP. Plasma glucose concentration was determined by using a glucose analyzer (YSI 2300 STAT, YSI Inc, Yellow Springs, OH). Plasma insulin concentration was determined by using an immunoassay (Elecsys®, Roche Diagnostics). The plasma palmitate tracer-to-tracee ratio and plasma FFA concentrations were determined by using gas-chromatography mass spectrometry, and palmitate flux (appearance rate in and disappearance rate from plasma) was calculated as previously described [7, 11, 12], assuming a steady state, because both plasma palmitate concentration (not shown) and the plasma palmitate enrichment were steady during the sampling periods (Supplemental Figure 1). Plasma palmitate clearance rate, which represents the volume of plasma that is cleared of palmitate per unit of time, was calculated by dividing the palmitate disappearance rate by the plasma palmitate concentration. Palmitate mean residence time, which represents the amount of time each palmitate molecule spends in the circulation, was calculated as the inverse of the palmitate fractional disappearance rate, which is the fraction of the total plasma pool that disappears per unit of time.
2.3. Statistical analysis
Student’s t-test for independent samples was used to evaluate differences in participants’ age, body composition, basic metabolic profile, and insulin sensitivity between the lean and obese groups. Repeated measures analysis of variance was used to evaluate differences in palmitate kinetics during basal conditions and during the HECP between the lean and obese groups. Skewed data sets were log-transformed to achieve normality before analysis. Values are reported as mean ± SEM or median (quartiles). Correlations between variables were evaluated by using the Pearson product moment coefficient. To evaluate potential differences in outcomes between men and women, we also included sex as a covariate in these analyses. However, no differences between men and women were observed. A P-value ≤0.05 was considered statistically significant. Statistical analyses were performed by using STATA, version 16.0 (StataCorp LLC).
3. RESULTS
3.1. Participant general metabolic characteristics
Participants with obesity had more body fat, higher fasting plasma insulin concentration, impaired glucose tolerance, and reduced whole-body and muscle insulin sensitivity (assessed as the glucose infusion rate and muscle glucose uptake rate during the HECP, respectively) than lean participants (Table 1). Fasting plasma glucose concentration and plasma glucose and insulin concentrations during the HECP were not significantly different between the lean and obese groups (Table 1). Therefore, the assessment of whole-body and muscle insulin sensitivity was not confounded by potential differences in plasma insulin between the lean and obese groups. In fact, the unadjusted whole-body and muscle glucose uptake rates (expressed in µmol/min and µmol/kg muscle/min, respectively) correlated strongly (r ≥ 0.95; P < 0.001) with whole-body (data not shown) and muscle (Supplemental Figure 2) glucose uptakes rate adjusted for the plasma insulin concentration (expressed in [µmo/min]/(mU/l)] and [µmol/kg muscle/min]/(mU/l)], respectively).
Table 1.
Participants’ age, body composition, plasma metabolic profile, and insulin sensitivity
| Lean | Obese | P value | |
|---|---|---|---|
| N (M, F) | 14 (5, 9) | 46 (12, 34) | |
| Age (years) | 39.7 ± 3.3 | 45.2 ± 1.6 | 0.113 |
| Height (cm) | 168.4 ± 1.8 | 168.2 ± 1.2 | 0.950 |
| Total body mass (kg) | 64.1 ± 1.5 | 107.2 ± 2.5 | <0.001 |
| Body surface area (m2) | 1.73 ± 0.03 | 2.15 ± 0.03 | <0.001 |
| Body mass index (kg/m2) | 22.6 ± 0.5 | 37.8 ± 0.7 | <0.001 |
| Fat-free mass (kg) | 44.5 ± 1.9 | 55.6 ± 1.6 | 0.001 |
| Fat mass (kg) | 19.0 ± 1.4 | 49.6 ± 1.5 | <0.001 |
| Body fat (%) | 30.1 ± 2.2 | 47.1 ± 0.9 | <0.001 |
| Fasting plasma glucose (mg/dl) | 91.1 ± 1.5 | 95.2 ± 1.5 | 0.144 |
| 2-h OGTT plasma glucose (mg/dl) | 117.1 ± 6.8 | 142.8 ± 4.5 | 0.006 |
| Plasma glucose during the HECP (mg/dl) | 110.9 ± 2.7 | 108.3 ± 1.1 | 0.301 |
| Fasting plasma insulin (mU/l) | 5.8 ± 0.5 | 16.9 ± 1.4 | <0.001 |
| Plasma insulin during the HECP (mU/l) | 108.2 ± 4.4 | 115.3 ± 3 | 0.247 |
| Fasting plasma FFA (µmol/l) | 501.6 ± 38.7 | 596.8 ± 24.0 | 0.053 |
| Plasma FFA during the HECP (µmol/l) | 43.9 ± 3.6 | 53.0 ± 4.2 | 0.102 |
| GIR during the HECP (µmol/min) | 3,724 ± 287 | 2,493 ± 169 | <0.001 |
| GIR/I during the HECP (µmol/min)/(mU/l) | 35.1 ± 3.0 | 22.3 ± 1.5 | <0.001 |
| GIRBM during the HECP (µmol/kg BM/min) | 58.4 ± 4.0 | 23.9 ± 1.7 | <0.001 |
| GIRFFM during the HECP (µmol/kg FFM/min) | 84.3 ± 6.4 | 46.3 ± 3.2 | <0.001 |
| MGU during the HECP (µmol/kg muscle/min) | 97.7 ± 6.7 | 52.8 ± 4.9 | <0.001 |
| MGU/I during the HECP (µmol/kg muscle/min)/(mU/l) | 0.91 ± 0.06 | 0.47 ± 0.04 | <0.001 |
Values are mean ± SEM. Abbreviations: BM, body mass; GIR, glucose infusion rate; GIR/I, glucose infusion rate in relation to plasma insulin concentration; FFM, fat-free mass; HECP, hyperinsulinemic-euglycemic clamp; MGU, muscle glucose uptake rate; MGU/I, muscle glucose uptake rate in relation to plasma insulin concentration; OGTT, oral glucose tolerance test.
3.2. Plasma palmitate and total FFA concentrations
During basal conditions, plasma palmitate and total FFA concentrations were about 15% greater in the obese than in the lean group (Table 1 and Figure 1A). Plasma palmitate concentration correlated strongly with total FFA concentration (r=0.95, P<0.001), confirming that palmitate is a good surrogate for total FFA kinetics [11]. Plasma palmitate and total FFA concentrations during the HECP were several-times lower (P<0.001) compared with basal conditions in both the lean and obese groups and plasma palmitate and total FFA concentrations during the HECP were not different between the lean and obese groups (Table 1 and Figure 1A).
Figure 1.
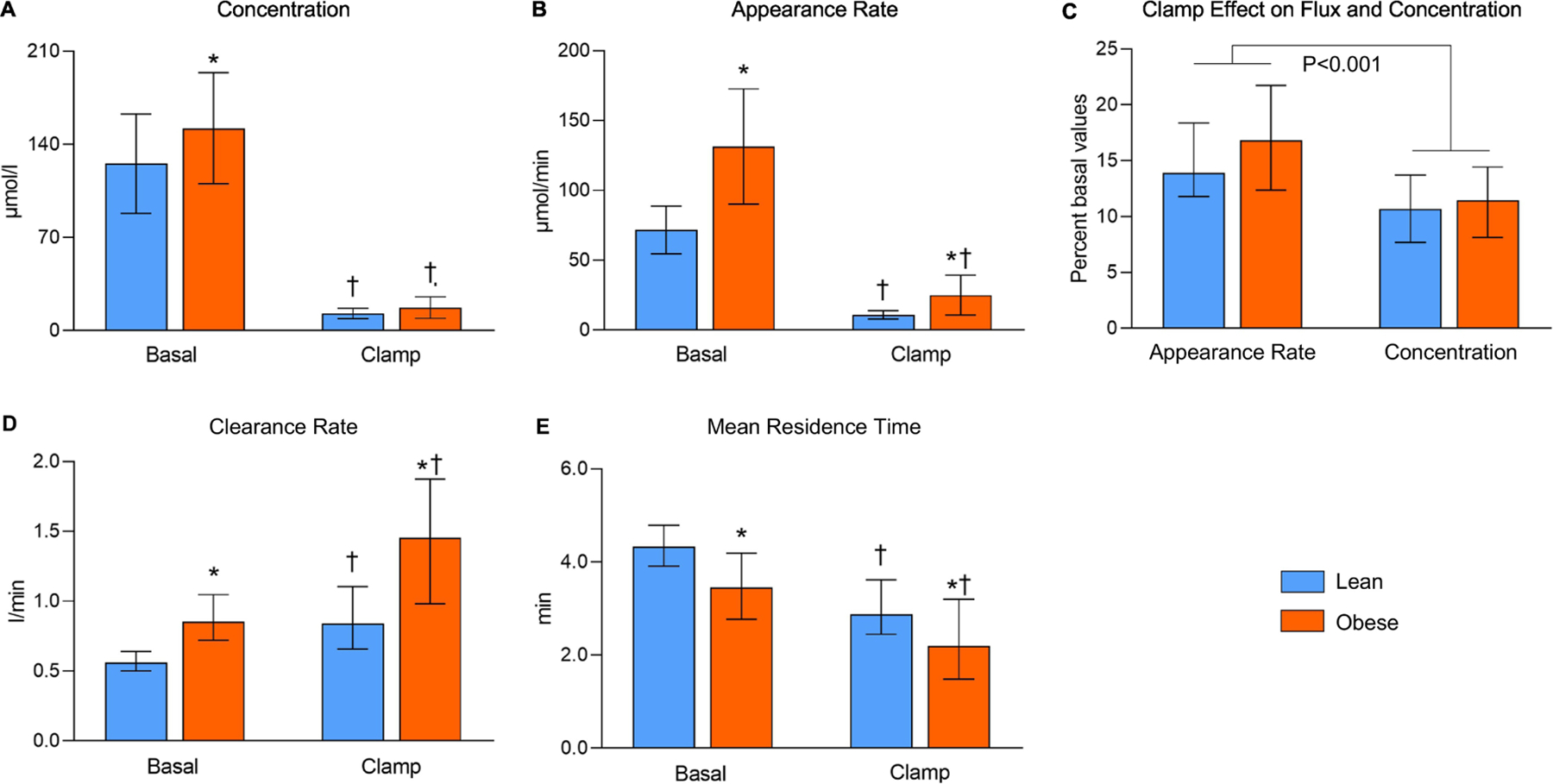
Palmitate concentration in plasma (A), appearance rate in plasma (B), plasma clearance rate (D), and mean residence time in the circulation (E) during basal conditions and during the hyperinsulinemic-euglycemic clamp procedure, and clamp-induced changes in palmitate appearance rate and plasma concentration (C). Values are median and interquartile range. For the outcomes in panels A, B, D, and E, there was a significant group (lean vs obese) × condition (basal vs clamp) interaction, P < 0.001. * Significantly different from the corresponding value in the lean group, P < 0.05. † Significantly different from the corresponding basal value, P < 0.05.
3.3. Palmitate kinetics
Basal palmitate appearance rate in plasma was about twice as great in the obese compared with the lean group, and as expected [7], was several-fold lower (P<0.001) during the HECP compared with basal conditions in both the lean and obese groups (Figure 1B). The difference in palmitate appearance rate between the lean and obese groups (Figure 1B) was much greater than the difference in plasma palmitate concentration (Figure 1A). Furthermore, the relative decrease from basal values in plasma palmitate concentration was greater than the relative decrease in palmitate appearance rate (Figure 1C). These data demonstrate that the difference in appearance rate between the lean and obese groups alone cannot explain the difference in concentration and the decrease in appearance during the HECP compared with basal conditions alone cannot explain the decrease in concentration during the HECP. In fact, basal plasma palmitate clearance rate was about 50% higher and palmitate mean residence time in the circulation was about 20% shorter in the obese compared with the lean group (Figures 1D, 1E). In addition, plasma palmitate clearance rate was about 30% greater (P<0.001) and palmitate mean residence time was about 30% shorter (P<0.001) during the HECP than during basal conditions (Figures 1D, 1E) and the increase in clearance from basal conditions was greater in the obese than the lean group (group × condition interaction, P<0.001). Because we found insulin increases plasma palmitate clearance and basal plasma insulin concentration was significantly higher in the obese compared with the lean group, we also evaluated the effect of obesity on plasma palmitate clearance with statistical adjustment for plasma insulin and found palmitate clearance was significantly higher in the obese than the lean group (P=0.003) even after adjusting for plasma insulin concentration. Furthermore, the greater increase in palmitate clearance from basal values during the HECP in the obese compared with the lean group occurred despite a similar or even smaller increase in plasma insulin.
3.4. Relationships between plasma palmitate concentration and palmitate kinetics
There was a significant negative correlation between basal plasma palmitate concentration and basal plasma palmitate clearance rate in both the lean and obese groups (Figure 2A), demonstrating that the efficiency of palmitate removal from plasma decreases as palmitate concentration increases. Furthermore, at any plasma palmitate concentration, plasma palmitate clearance rate and both total palmitate disappearance rate (µmol/min) and palmitate disappearance rate in relation to fat-free mass were higher (~50% and 30%, respectively) in the obese than the lean group (Figures 2A–C).
Figure 2.
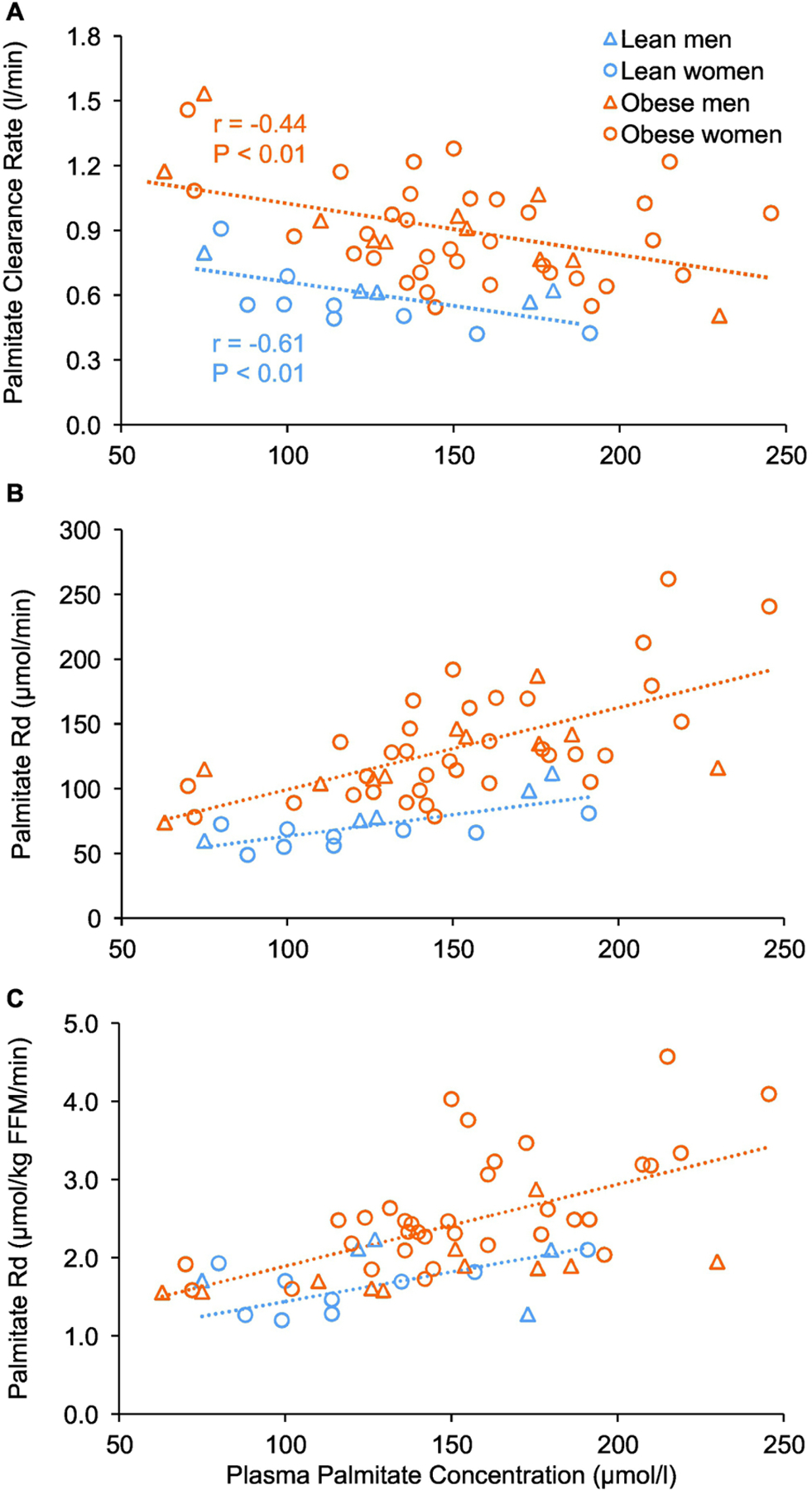
Relationships between basal plasma palmitate concentration and plasma palmitate clearance rate (A), total palmitate disappearance rate (B), and palmitate disappearance rate in relation to fat-free mass (C). Abbreviations: FFM, fat-free mass.
3.5. Relationships between plasma palmitate clearance rate and insulin sensitivity
There was no significant correlation between plasma palmitate concentration or total FFA concentration and muscle insulin sensitivity (Figure 3A, Supplemental Figure 3A) and whole-body insulin sensitivity (glucose infusion rate during the HECP; Supplemental Figure 3B). However, there were strong negative correlations between plasma palmitate clearance rate and whole-body (not shown) and muscle insulin sensitivity (Figure 3B), and also between plasma palmitate disappearance rate and whole-body (not shown) and muscle insulin sensitivity (Figure 3C). Moreover, these relationships were not different (P>0.15) between the lean and obese groups (Figures 3B, 3C).
Figure 3.
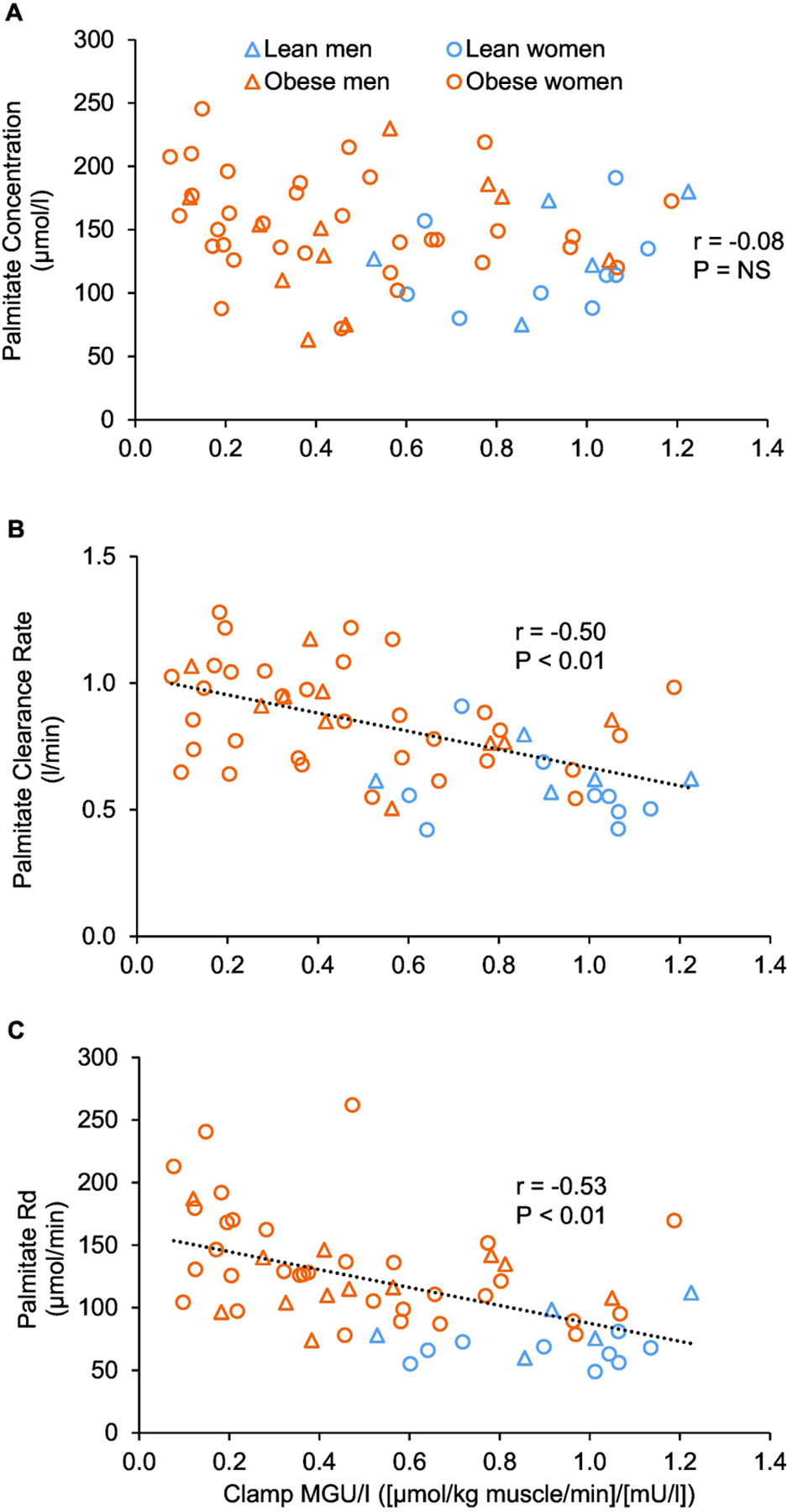
Relationships between muscle insulin sensitivity, assessed as muscle glucose uptake rate in relation to plasma insulin concentration during the hyperinsulinemic-euglycemic clamp procedure, and basal plasma palmitate concentration (A), plasma palmitate clearance rate (B), and palmitate disappearance rate from plasma (C). Abbreviations: MGU/I, muscle glucose uptake rate in relation to plasma insulin concentration; Rd, disappearance rate.
4. DISCUSSION
Plasma FFA are an important fuel source for skeletal muscles and other lean tissues, but FFA delivery in excess of energy needs can cause ectopic lipid accumulation and impair insulin-mediated muscle glucose uptake [1, 2]. Although it is conventional wisdom that excessive adipose tissue FFA release causes an increase in plasma FFA concentration and, in turn lipid overload and muscle insulin resistance in people with obesity [1, 2, 13], we hypothesized alterations in tissue FFA clearance might also be involved. Indeed, we found plasma FFA clearance was enhanced in participants with obesity, compared with lean ones. Furthermore, muscle insulin sensitivity correlated negatively with plasma FFA clearance and the relationship was not different between the lean and obese groups. On the other hand, plasma FFA concentration did not significantly correlate with insulin sensitivity. In addition, we found insulin infusion increases plasma FFA clearance and the insulin-mediated increase is greater in people with obesity compared with lean people. These findings have important implications, because they suggest: i) factors that regulate cellular fatty acid transport, not only plasma FFA concentration, determine the cellular fatty acid load, and ii) the plasma FFA-lowering effect of insulin is not only due to inhibition of adipose tissue triglyceride lipolysis but also due to increased plasma FFA clearance. Accordingly, although insulin decreases FFA supply from adipose tissue to lean tissues, it also promotes the translocation of FFA from plasma into cells where they can presumably interfere with insulin signaling [2].
The exact mechanisms responsible for increased basal plasma FFA clearance associated with insulin resistance are unclear. Results from studies conducted in animals and with giant sarcolemmal vesicles prepared from skeletal muscle biopsies from people demonstrate insulin resistance is associated with increased FFA clearance by skeletal muscles, but not liver or adipose tissue [8, 14]. Furthermore, FFA uptake into adipose tissue contributes only a small fraction (<10%) of total whole-body FFA disposal [12]. Accordingly, muscle was the most likely site of increased FFA clearance in people with obesity and insulin resistance in our study. FFA uptake in myocytes occurs primarily by facilitated diffusion [15, 16]. Both obesity and insulin resistance are associated with increased expression of CD36 and other fatty acid transporter proteins [8, 14, 17–19], which helps explain the increase in muscle fatty acid clearance. However, the exact mechanisms involved in causing this increase are unclear.
Insulin infusion significantly increased plasma FFA clearance above basal values in our study. Furthermore, the ability of insulin to increase plasma FFA clearance was not impaired in people with obesity and was even greater in the obese than the lean group, despite insulin resistance to glucose uptake in the obese. Therefore, the hyperinsulinemia in people with obesity likely contributed to their higher basal FFA clearance. However, it was not solely responsible for it, because basal FFA clearance was greater in the obese than the lean group even after adjusting for the difference in basal plasma insulin concentration. Accordingly, additional mechanisms are responsible for the enhanced plasma FFA clearance in people with obesity and insulin resistance. The dissociation between insulin sensitivity of glucose uptake and FFA clearance in people with obesity suggests different signaling mechanisms are involved in mediating insulin-stimulated glucose and fatty acid uptake in muscle. Results from studies conducted in vitro demonstrate insulin stimulates FFA uptake in muscles and adipose tissue [8, 9, 20], presumably because it inhibits the degradation of fatty acid transporters, stimulates their translocation to the plasma membrane, and activates their enzymatic activity [21–24]. The primary mechanism by which insulin stimulates glucose uptake in muscle involves the translocation of glucose transporter 4 to the plasma membrane [2].
Insulin is also a potent inhibitor of hormone sensitive lipase in adipose tissue and even small increases in plasma insulin concentration above basal values almost completely inhibit release of FFA from adipose tissue in both lean people and people with obesity [7, 12, 25]. Therefore, it is commonly accepted that inhibition of adipose tissue triglyceride lipolysis is the key mechanism responsible for the plasma FFA-lowering effect of insulin [3, 7, 13]. The data from our study mostly support this notion, because we found the FFA appearance rate in plasma is more responsive to insulin than plasma FFA clearance rate. Nonetheless, plasma FFA clearance increased substantially during the HECP and the greater increase in plasma FFA clearance in the obese compared with the lean group was responsible for the greater decrease in plasma FFA concentration in the obese compared with the lean group. Accordingly, insulin is an important regulator of plasma FFA concentration, not only because it inhibits adipose tissue triglyceride lipolysis but also because it increases plasma FFA clearance.
We did not evaluate the metabolic fate of fatty acids that are extracted from plasma, which could have important implications. The results from a previous study suggest obesity is associated with increased non-oxidative, but not oxidative, fatty acid disposal [26]. This observation suggests fatty acids that are extracted “in excess” in people with obesity compared with lean people are preferentially used to synthesize lipids that might be involved in causing insulin resistance [1, 2]. This in turn would support the relationship between plasma FFA clearance and insulin resistance we observed. Another limitation of our study includes the single dose of insulin we used, which was within the range of peak postprandial plasma insulin concentrations [10]. Therefore, we cannot determine the responsiveness of plasma FFA clearance to smaller changes in insulin as they occur during the transition from postabsorptive to postprandial conditions.
5. CONCLUSIONS
The data presented here demonstrate: i) plasma FFA clearance rate is an important determinant of plasma FFA concentration, ii) insulin stimulates FFA clearance, iii) the effect of insulin on FFA clearance is augmented in people with obesity, and iv) FFA clearance is inversely associated with muscle insulin sensitivity. Together, these findings suggest increased FFA extraction efficiency by lean tissues, not only fatty acid delivery from adipose tissue, determines the cellular fatty acid load in people with insulin resistance. Hyperinsulinemia in people with obesity curbs the release of FFA from adipose tissue but also promotes their plasma clearance.
Supplementary Material
Highlights.
Plasma free fatty acid (FFA) clearance is higher in obese than lean people
Insulin increases FFA clearance and the increase is greater in obese than lean people
High FFA clearance in the obese keeps plasma FFA concentration low relative to FFA flux
Plasma FFA clearance correlates negatively with muscle insulin sensitivity
There is no relationship between plasma FFA concentration and muscle insulin sensitivity
ACKNOWLEDGEMENTS
We thank the staff of the Center for Human Nutrition, the Clinical and Translational Research Unit, the Clinical and Translational Imagining Unit, and the Division of Radiological Sciences for assistance in conducting the metabolic studies and their technical assistance in processing the study samples, and the study participants for their participation.
FUNDING
This study was supported by NIH grants R01 DK115400, P30 DK056341 (Washington University School of Medicine Nutrition and Obesity Research Center), P30 DK020579 (Washington University School of Medicine Diabetes Research Center), and UL1 TR002345 (Washington University School of Medicine Institute of Clinical and Translational Sciences), and grants from the American Diabetes Association (1-18-ICTS-119), and the Longer Life Foundation (2019–011).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST
The authors report no conflicts of interest relevant to this article.
CLINICAL TRIALS REGISTRATION NUMBERS
REFERENCES
- [1].Solis-Herrera C, Triplitt C, Cersosimo E, DeFronzo RA. Pathogenesis of Type 2 Diabetes Mellitus. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al. , editors. Endotext South Dartmouth (MA): MDText.com, Inc.; 2000. [Google Scholar]
- [2].Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiological reviews 2018;98:2133–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011;60:2441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011;60:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johnston LW, Harris SB, Retnakaran R, Giacca A, Liu Z, Bazinet RP, et al. Association of NEFA composition with insulin sensitivity and beta cell function in the Prospective Metabolism and Islet Cell Evaluation (PROMISE) cohort. Diabetologia 2018;61:821–30. [DOI] [PubMed] [Google Scholar]
- [6].Arner P, Rydén M. Fatty Acids, Obesity and Insulin Resistance. Obesity facts 2015;8:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes care 2012;35:1316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hegarty BD, Cooney GJ, Kraegen EW, Furler SM. Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes 2002;51:1477–84. [DOI] [PubMed] [Google Scholar]
- [9].Dyck DJ, Steinberg G, Bonen A. Insulin increases FA uptake and esterification but reduces lipid utilization in isolated contracting muscle. American journal of physiology Endocrinology and metabolism 2001;281:E600–7. [DOI] [PubMed] [Google Scholar]
- [10].van Vliet S, Koh HE, Patterson BW, Yoshino M, LaForest R, Gropler RJ, et al. Obesity Is Associated With Increased Basal and Postprandial β-Cell Insulin Secretion Even in the Absence of Insulin Resistance. Diabetes 2020;69:2112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes 2003;52:1641–8. [DOI] [PubMed] [Google Scholar]
- [12].Shadid S, Jensen MD. Pioglitazone increases non-esterified fatty acid clearance in upper body obesity. Diabetologia 2006;49:149–57. [DOI] [PubMed] [Google Scholar]
- [13].Carpentier AC. 100(th) anniversary of the discovery of insulin perspective: insulin and adipose tissue fatty acid metabolism. American journal of physiology Endocrinology and metabolism 2021;320:E653–e70. [DOI] [PubMed] [Google Scholar]
- [14].Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2004;18:1144–6. [DOI] [PubMed] [Google Scholar]
- [15].Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annual review of nutrition 2002;22:383–415. [DOI] [PubMed] [Google Scholar]
- [16].Glatz JFC, Luiken J. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. Journal of lipid research 2018;59:1084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proceedings of the National Academy of Sciences of the United States of America 2009;106:15430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Binnert C, Koistinen HA, Martin G, Andreelli F, Ebeling P, Koivisto VA, et al. Fatty acid transport protein-1 mRNA expression in skeletal muscle and in adipose tissue in humans. American journal of physiology Endocrinology and metabolism 2000;279:E1072–9. [DOI] [PubMed] [Google Scholar]
- [19].Turcotte LP, Swenberger JR, Zavitz Tucker M, Yee AJ. Increased fatty acid uptake and altered fatty acid metabolism in insulin-resistant muscle of obese Zucker rats. Diabetes 2001;50:1389–96. [DOI] [PubMed] [Google Scholar]
- [20].Kelly KR, Abbott MJ, Turcotte LP. Short-term AMP-regulated protein kinase activation enhances insulin-sensitive fatty acid uptake and increases the effects of insulin on fatty acid oxidation in L6 muscle cells. Experimental biology and medicine (Maywood, NJ) 2010;235:514–21. [DOI] [PubMed] [Google Scholar]
- [21].Smith J, Su X, El-Maghrabi R, Stahl PD, Abumrad NA. Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. The Journal of biological chemistry 2008;283:13578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Corpeleijn E, Pelsers MM, Soenen S, Mensink M, Bouwman FG, Kooi ME, et al. Insulin acutely upregulates protein expression of the fatty acid transporter CD36 in human skeletal muscle in vivo. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 2008;59:77–83. [PubMed] [Google Scholar]
- [23].Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Developmental cell 2002;2:477–88. [DOI] [PubMed] [Google Scholar]
- [24].Digel M, Staffer S, Ehehalt F, Stremmel W, Ehehalt R, Füllekrug J. FATP4 contributes as an enzyme to the basal and insulin-mediated fatty acid uptake of C₂C₁₂ muscle cells. American journal of physiology Endocrinology and metabolism 2011;301:E785–96. [DOI] [PubMed] [Google Scholar]
- [25].Coppack SW, Frayn KN, Humphreys SM, Dhar H, Hockaday TD. Effects of insulin on human adipose tissue metabolism in vivo. Clinical science (London, England : 1979) 1989;77:663–70. [DOI] [PubMed] [Google Scholar]
- [26].Groop LC, Bonadonna RC, Simonson DC, Petrides AS, Shank M, DeFronzo RA. Effect of insulin on oxidative and nonoxidative pathways of free fatty acid metabolism in human obesity. The American journal of physiology 1992;263:E79–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


