Abstract
Multidrug-resistant (MDR) gram-negative bacteria pose significant challenges to the public health. Various factors are involved in the development and spread of MDR strains, including the overuse and misuse of antibiotics, the lack of new antibiotics being developed, and etc. Efflux pump is one of the most important factors in the emergence of antibiotic resistance in bacteria. Aiming at the introduction of novel plant antibiotic, we investigated the effect of eugenol on the MexA and AcrA efflux pumps in Pseudomonas aeruginosa (P. aeruginosa) and Escherichia coli (E. coli). Molecular docking was performed using PachDock Server 1.3. The effect of eugenol on bacteria was determined by disk diffusion, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). A cartwheel test was also performed to evaluate efflux pump inhibition. Finally, the expression of the MexA and AcrA genes was examined by real-time PCR. The results of molecular docking showed that eugenol interacted with MexA and AcrA pumps at − 29.28 and − 28.59 Kcal.mol−1, respectively. The results of the antibiogram test indicated that the antibiotic resistance of the treated bacteria decreased significantly (p < 0.05). The results of the cartwheel test suggested the inhibition of efflux pump activity in P. aeruginosa and E. coli. Analysis of the genes by real-time PCR demonstrated that the expression of MexA and AcrA genes was significantly reduced, compared to untreated bacteria (p < 0.001). The findings suggest, among other things, that eugenol may make P. aeruginosa and E. coli more sensitive to antibiotics and that it could be used as an inhibitor to prevent bacteria from becoming resistant to antibiotics.
Keywords: Antibiotic resistance, Eugenol, MexA, AcrA
Introduction
Antibiotic resistance is a global problem which affects human health. The imprudent use of antibiotics has resulted in the broader dissemination of resistance (Yadav et al. 2021). Multidrug resistance patterns in both positive and negative bacteria are complex. Some of the most common Gram-negative bacteria that exhibit MDR includeEscherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. These bacteria have been identified as major causes of healthcare-associated infections, and the emergence of MDR strains has made them increasingly difficult to treat (Yusuf et al. 2021). so old antibiotics cannot be used for so many infectious diseases (Biondo 2023).
Currently, there is a lack of effective treatments, effective preventive measures, and new antibiotics, so alternative treatments for these drugs need to be developed (Siala et al. 2017). Many studies have been conducted to find new approaches to overcome this problem. In recent years, several plant extracts have been found to have an antimicrobial effect on microorganisms, and thus the antibacterial activity of their extracts has attracted much attention (Ma et al. 2017). Plant extracts have different degrees of activity. Eugenol is the main component of clove’sessential oil, which has long been known as a systemic and topical analgesic, anti-inflammatory, and antibacterial. Eugenol has been listed as a “Generally Regarded As Safe” substance by the United States Food and Drug Administration (Hemaiswarya et al. 2009).
Microbiologists believe that multidrug-resistant bacteria pose the greatest threat to the community and public health (Kumarasamy et al. 2010). Pseudomonas aeruginosa and Escherichia coli are Gram-negative opportunistic pathogens. Echerichia coli is part of the normal intestinal flora. However, certain strains of Escherichia colican cause serious infections, particulary in the urinary tract and bloodstream (Antonelli et al. 2021). Pseudomonas aeruginosainfects patients with cystic fibrosis, burn wounds, immunodeficiency, chronic obstructive pulmonary disorder,ventilator-associated pneumonia (Qin et al. 2022).
Pseudomonas aeruginosa (P. aeruginosa) and Escherichia coli (E. coli), which are often resistant to amino-glycosides (such as amikacin and gentamicin), β-lactam drugs (imipenem), cephalosporins (ceftazidime) (Tenover and Tenover 2006), and fluoroquinolone (ciprofloxacin), are among the most common causes of nosocomial infections (Ali et al. 2010).
Tthe crisis of MDR in Gram-negative bacteria poses a significant threat to public health. Addressing this crisis will require a multifaceted approach that includes the development of new treatments, the optimization of current therapies, and the implementation of effective antibiotic stewardship and infection prevention and control measures (Bassetti and Garau 2021).One of the most important mechanisms of drug resistance in bacteria is the elimination of antimicrobial and toxic substances with the help of an efflux pump. When the efflux pump is blocked or inhibited, the lowest concentration of the drug has the highest efficac (Negi et al. 2014). Indeed, efflux pumps are bacterial transport proteins that flush out toxins and waste from bacteria into the external environment. Many efflux pumps were identified in Gram-positive and Gram-negative bacteria (Gholampour-Azizi et al. 2015). Multidrug efflux pumps are the major cause of multidrug resistance (Andersen et al. 2015).
Efflux bacterial systems are classified into 5 families: the major facilitator superfamily (MFS), the small multidrug resistance (SMR) family, the resistance-nodulation-division (RND) family, the multidrug and toxic compound extrusion (MATE) family, and the adenosine triphosphate (ATP)-binding cassette (ABC) superfamily. The largest family of these pumps is RND (Askoura et al. 2011). Multidrug pumps are influenced by differentially set expression factors. The use of efflux pump systems in bacteria is naturally expressed. The energy source for the extrusion of toxic compounds for the RND family is PMF-driven (Martinez et al. 2009). They are especially found in Gram-negative bacteria. They form a three-part complex with an inner membrane pump [AcrB (E. coli), MexB (P. aeroginosa)], a periplasmic adaptor [AcrA (E. coli), MexA (P. aeroginosa)], and an outer membrane channel [TolC (E. coli), OprM (P. aeruginosa)]. The pump has three genes that work together to form a three-part structure across the membrane and transport the material out of the bacterial cell. The inner and outer membrane proteins do not interact directly with each other; this is completed by a 3-part complex adapter protein (Nikaido 2010).
The three-part multidrug efflux system MexAB-OprM and AcrAB-OprM plays an important role in P. aeruginosa and E. coli (Glavier et al. 2020). The mechanism of action of these pumps is as follows: the drug enters the inner membrane protein through the periplasmic space and is pumped out of the bacterial cell through a channel in the outer membrane (Shi et al. 2019). Efflux inhibitors can be used to inhibit multi-drug resistance efflux pumps. Efflux pumps show great diversity in identifying different substrates, which leads to different drug resistances in bacteria (Pagès et al. 2005). Therefore, bacterial efflux pumps play an important role in weakening antimicrobial properties, especially for antibiotics and biocides (Nikaido and Takatsuka 2009).
Having the above-mentioned preliminary remarks in mind, the purpose of this study is to describe the antibacterial activity of eugenol in reducing antibiotic resistance, as well as the effect of eugenol on reducing the expression of resistant genes in P. aeruginosa and E. coli at two levels: in silico and in vitro.
Materials and methods
Molecular docking
The three-dimensional (3D) structures of eugenol and the transfer protein were taken from the PubChem BioAssay database (Database of Chemical Molecules and Their Activities in Biological Assays) and the RCSB Protein Data Bank (RCSB PDB; [RCSB PDB: Homepage]), respectively, and minimized using UCSF Chimera version 1.14. The protein code of the efflux pump was 5V5S. The rotating charges and molecular center of mass were determined using AutoDockTools (ADT). After adding polar hydrogen atoms and removing water, the total charge was determined using Kollman charges. Then, both files were uploaded on the PachDock online server, with the existing algorithm as default. Then, 200 poses were generated, but just 10 top-ranked scores were selected for a visual inspection(Jamali et al. 2022).
Eugenol
The eugenol was purchased from Master Dent (USA) with an 850 mg.ml−1 concentration.
Bacterial isolation and identification
In this descriptive study, Gram-negative nosocomial infectious bacteria, E. coli and P. aeruginosa, were collected from Velayat Hospital in Guilan Province, Iran. The bacteria were cultured in Stewart Transport Medium and transferred to the microbial laboratory. After preparing a pure culture of each bacterium, the standard biochemical tests such as triple sugar iron (TSI), sulfide indole motility (SIM), Gram stain, and oxidase test were used for identification at 370 °C.
Antibiogram test against P. aeruginosa and E. coli
To investigate the induction of eugenol susceptibility in isolated strains of P. aeruginosa and E. coli to the antibiotic disks amikacin (30 µg), ceftazidime (30 µg), amipenem (10 µg), ciprofloxacin (5 µg), and gentamicin (10 µg) on Mullerhinton agar, pre- and post-treatment eugenol was tested according to the CLSI standard. A sub-MIC concentration was used to determine the susceptibility of the strains. The concentration was incubated under aerobic conditions at 37 °C for 24 h and evaluated numerically by examining plates that were evaluated according to the CLSI table.
MIC and MBC
MIC and MBC were determined by the macrodilution test. The MIC method was used to determine the susceptibility of strains to eugenol. A suspension of a fresh bacterial culture containing 0.5 McFarland (1.5 × 108 Cells/ml) was prepared to determine the MIC. Eugenol dilutions from 1:2 to 1:1024 were tested three times in Muller-Hinton broth. Then, 100 µl of the microbial suspension were added to each tube and incubated for 24 h. After 24 h, the results were evaluated. The concentration at which 99.99% of the microbes were killed was the minimum lethal concentration, and the transparent tubes were considered MIC.
Cartwheel test
Isolates were tested for efflux pump activity using the ethidium bromide agar cartwheel method. Drug-resistant bacteria were cultured on trypticase soy agar (TSA) with ethidium bromide concentrations (0.5 mg.ml− 1) in strips. Each bacterium was cultured separately in a row (from the center of the environment to the side of the environment). Petri dishes contained bacteria treated with sub-MIC of eugenol and untreated bacteria (as control). The plates were incubated at 37 °C for 24 h. Fluorescence activity for each bacterium was measured using a UV transilluminator.
RNA extraction and quantification of RNA expression by real-time PCR
Real-time PCR was performed to evaluate the expression of mexA and acrA genes under the influence of eugenol. The first two groups were treated with an eugenol sub-MIC concentration of 0.83 mg.mol−1 for P. aeruginosa and 6.64 mg.mol−1 for E. coli. The second group (the control group) included bacteria that were not treated with eugenol. In addition, the housekeeping gene was tested in this 16 S rRNA study. First, a primer design was performed (Table 1).
Table 1.
Primer sequences used in this study
| Gene | Bacteria | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|---|
| MexA | P.aeruginosa | ACCTACGAGGCCGACTACCAGA | GTTGGTCACCAGGGCGCCTTC |
| AcrA | E.coli | CTCTCAGGCAGCTTAGCCCTAA | TGCAGAGGTTCAGTTTTGACTGTT |
| 16 S rRNA | P.aeruginosa | AAGACTAAAACTCAAATGAA | TGGAAAGTTCTTACTATGTC |
| 16 S rRNA | E.coli | CGAGTG GCGGACGGG TGAGT | TCGACATCGTTTACGGCGTGGA |
Total RNA was extracted from bacteria using the relevant kit (Thermo Fisher Scientific, UK) according to the protocol. After the purification of total RNA, it was converted to complementary deoxyribonucleic acid (cDNA) using a synthesis kit (Thermo Fisher Scientific, UK). Real-time PCR was then performed in a thermal cycle (ABI step one, Applied Biosystems, UK) using a master mix reagent (Thermo Fisher Scientific, UK) and SYBER green (Amplicon, Denmank and primers).
Results
Molecular docking
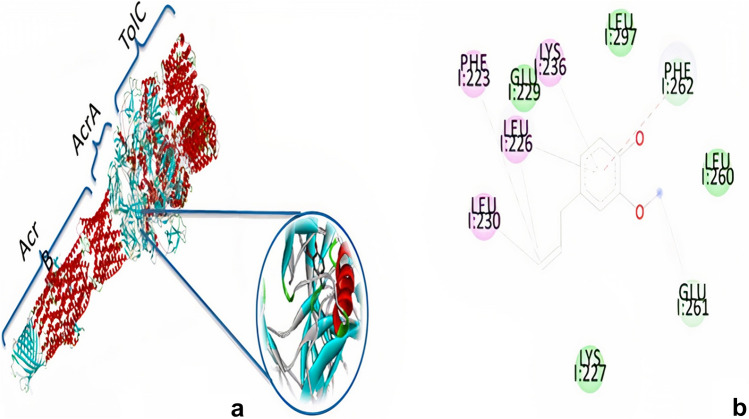
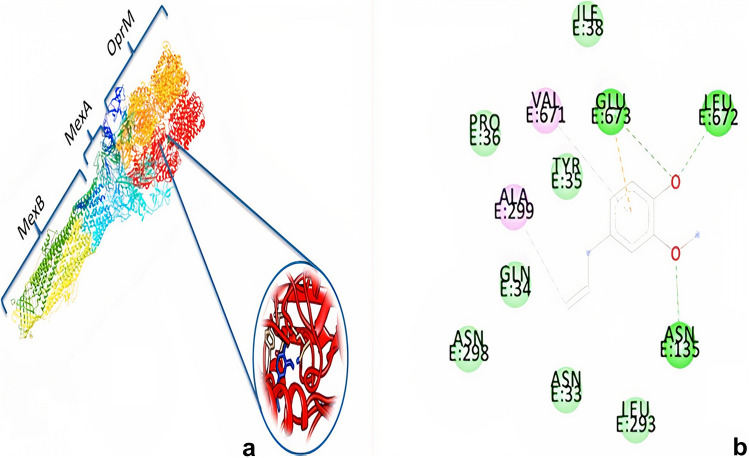
The 3D images (Figs. 1a and 2a) show the interaction of eugenol with the E. coli pump (PDB: 5v5s) and the P. aeruginosa pump (PDB: 6iok) with Discover Studio software, respectively. As shown in Figs. 1b and 2b, the eugenol molecule reacts with amino acids LEU279, LEU260, LEU226, LEU230, GLU229, GLU261, PHE262, PHE223, LYS227, LYS236 in E. coli pump and also GLU673, GLN34, VAL671, TYR35, ALA229, ASN135, ASN33, ASN298, PRO36, LEU672, LEU293, ILE38 amino acid in P. aeruginosa pump. The released energies of the interaction of eugenol with the pumps of E. coli and P. aeruginosa are listed in Table 2.
Fig. 1.
Ineraction between eugenol and AcrAB-TolC multidrug efflux pump. a Three-dimensional figure of the interaction of the Eugenol with Escherichia coli pump, AcrB: inner membrane transporter, AcrA: periplasmic adapter proteins, TolC: outer membrane protein. b Tow- dimensional figure of binding interaction of Eugenol with the residue of AcrA. Molecural docking shows that Eugenol can be fit with peripllasmic part (AcrA) of AcrAB-TolC multidrug efflux pump
Fig. 2.
Ineraction between eugenol and MexAB-OprM multidrug efflux pump. a Three-dimensional figure of the interaction of the Eugenol with Pseudomonas aeruginosa pump, MexB: inner membrane transporter, MexA: periplasmic adapter proteins, OprM: outer membrane protein. b Tow-dimensional figure of binding interaction of Eugenol with the residue of MexA. Molecural docking indicates that Eugenol can be fit with outer membrane (OprM) of MexAB-OprM multidrug efflux pump
Table 2.
Global energy of Eugenol with acrA and mexA
| Protein name | PDB ID | Global energy Kcal.mol− 1 |
Amino acid interacting |
|---|---|---|---|
| AcrA | 5v5s | − 28.59 | PHE223, GLU229, LYS236, ILU230, ILU297, PHE262, ILU262, ILU260, GLU261,, LYS227 |
| MexA | 6iok | − 29.28 | ILE38, PRO36, VAL671, GLU673, ILU672, ALA299, TYR35, GLN34, ASN298, ASN135, IEU293, ASN33 |
Bacterial isolation and identification
Results of diagnostic and biochemical tests from Velayat Hospital confirmed the strains of P. aeruginosa and E. coli.
Antibiogram test results against P. aeruginosa and E. coli
After preparing a microbial suspension of P. aeruginosa and E. coli in the presence and absence of eugenol on Mueller-Hinton Agar (MHA) medium and placing the discs of gentamicin, ciprofloxacin, amikacin, imipenem, and ceftazidime in the incubator for 24 h at 37 °C, the diameter of the zone of inhibition was measured according to the CLSI table. SPSS software (2013 version) was used for data analysis, and results were calculated using one-way analysis of variance (ANOVA) and pairwise mean comparisons at the significance level of P < 0.05 (Table 3).
Table 3.
Results of the average of the inhibition zone in the presence and absence of Eugenol using antibiotics in Escherichia coli and Pseudomonas aeruginosa
| Microorganism\Antibiotics | Inhibition zone(mm) (Mean ± SD) |
|||
|---|---|---|---|---|
| Escherichia coli | Pseudomonas aeruginosa | |||
| Treata | Untreatb | Treata | Untreatb | |
| Gentamicin | 23.50 ± 0.73 | 15 ± 0.70 | 25 ± 0.70 | 10.65 ± 0.49 |
| Ciprofloxacin | 24.50 ± 0.75 | 10 ± 0.70 | 36 ± 0.70 | 24.15 ± 0.20 |
| Amikacin | 24.25 ± 0.49 | 13 ± 0.74 | 37 ± 0.61 | 15.15 ± 0.28 |
| Imipenem | 27.33 ± 0.35 | 12 ± 0.71 | 26 ± 0.89 | 12.60 ± 0.50 |
| Ceftazidime | 25 ± 0.42 | 17 ± 0.36 | 28 ± 0.34 | 15.5 ± 0.70 |
aTreat with Eugenol
bUntreat with Eugenol
MIC/MBC
The MIC and MBC values of eugenol for P. aeruginosa and E. coli are shown in Table 4. The MIC values against P. aeruginosa and E. coli were 1.66 and 13.28 (mg.ml−1), respectively. Moreover, these values for MBC were 3.32 and 26.56 (mg.ml−1), respectively (Table 4).
Table 4.
Results of MIC and MBC of Pseudomonas aeruginosa and Escherichia coli
| Testing\Microorganism | MIC (mg.ml−1) | MBC (mg.ml−1) | Sub-MIC (mg.ml−1) |
|---|---|---|---|
| Pseudomonas aeroginosa | 1.66 | 3.32 | 0.83 |
| Escherichia coli | 13.28 | 26.56 | 6.64 |
Cartwheel test result
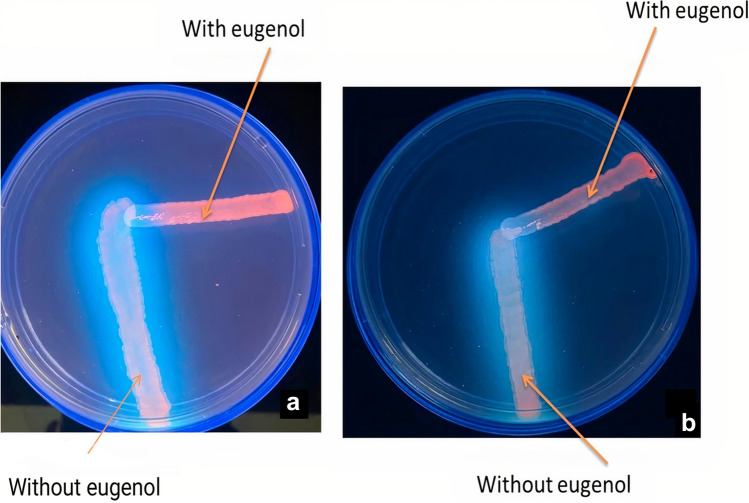
Phenotypic studies of efflux system expression using ethidium bromide in multidrug-resistant clinical strains indicated the presence of an efflux pump. Ethidium bromide in TSA was considered a detector, and its fluorescent property under UV light indicated the presence of an efflux pump. The growth line belonging to treated bacteria with concentrations of sub-MIC and untreated bacteria with eugenol was observed from separate strains by the cartwheel method on TSA medium with ethidium bromide. Blurred lines indicated efflux system activity, and clear lines indicated the absence of the pump (Fig. 3).
Fig. 3.
Cartwheel test. a The EtBr-agar cartwheel method applied to Pseudomonas aeruginosa, b The EtBr-agar cartwheel method applied to Escherichia coli
Expression results of MexA and AcrA genes
To evaluate the function of eugenol on AcrA efflux pumps in E. coli and MexA in P. aeruginosa, two groups were selected and analyzed by real-time PCR. The first group included bacteria treated with a sub-MIC concentration of 6.64 mg.ml−1 for E. coli and 0.83 mg.ml−1 for P. aeruginosa, and the second group (the control group) included bacteria treated with eugenol. For each group, repetition was assessed twice to determine the repeatability of the test. The real-time PCR results were analyzed after normalization with the control group’s internal 16 S rRNA control gene using gene expression and statistical software (Fig. 4).
Fig. 4.
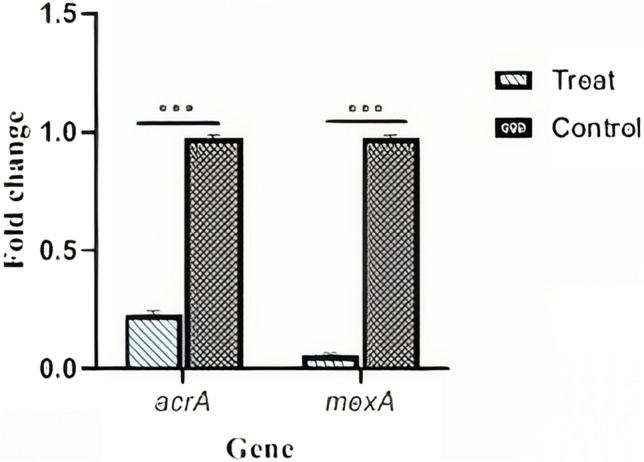
Fold change expression mexA and acrA gene. Eugenol downregulated acrA (p-value < 0.001 and mexA (p-value < 0.001 versus eugenol-untreat bacteria
It was found that eugenol was able to significantly (P < 0.001) reduce the expression of the AcrA and MexA genes in E. coli and P. aeruginosa, respectively. Gene expression results were calculated using SPSS program and statistical analysis of mean ± SD for 2 replicates by paired samples t test.
Discussion
Due to the increasing number of antibiotic-resistant microbial strains, many attempts have been made to use natural medicines to supplement and replace antibiotics. Herbal drugs reduce the dangerous side effects of antibiotics and even have a significant impact on the treatment of bacterial diseases (Wieczorek et al. 2008). Antibiotic resistance is on the rise and has become one of the major public health challenges, and it has several mechanisms (Ebbensgaard et al. 2020). Efflux pumping is one of the mechanisms of antibiotic resistance. It is able to remove waste products and drugs such as antibiotics from the bacterial cell. Resistance-nodulation-division (RND) is one of the families of efflux pumps in Gram-negative bacteria (Colclough et al. 2020). Inhibitors of efflux pumps are compounds that can interfere with the function of efflux pumps and prevent them from pumping out specific compounds. Phenylalanine-arginine beta-naphthylamide (PAβN) is a potent inhibitor of efflux pumps. PAβN was first identified as an inhibitor of the MexAB-OprM efflux pump in Pseudomonas aeruginosa, a Gram-negative bacterium.
PAβN works by binding to the pump’s substrate binding site, thereby blocking the efflux of antibiotics and other compounds from the bacterial cell. PAβN has been shown to inhibit efflux pumps in a wide range of Gram-negative bacteria(Lamers et al. 2013). In the study by askoura et al. 2011 provides valuable insights into the role of efflux pumps in multidrug resistance of Pseudomonas aeruginosa, and the potential use of EPIs as new antimicrobial agents against this pathogen.
In this study, it was shown that eugenol, one of the biochemicals in clove extract, with its antimicrobial properties (Hemaiswarya et al. 2009), can be effective in reducing antibiotic resistance and inhibiting efflux pumps in antibiotic-resistant bacteria such asP. aeruginosa and E. coli .Docking results showed that eugenol had the ability to inhibit the studied proteins. The best interaction between eugenol and the priplasmic part of the efflux pump occurred in P. aeruginosa and E.coli. The inner membrane and outer membrane did not interact directly with each other but were complemented by the tripartite priplasmic adabtor complex. The role of the periplasmic protein was to conduct and transport the drug to a channel in the outer membrane. The drug was pumped out of the bacterial cell through a channel in the outer membrane. The binding energy showed a positive interaction between eugenol and the efflux pump. In addition, the binding energies for the pumps AcrA − 28.59 Kcal.mol−1 and MexA − 29.28 Kcal.mol−1 pumps were observed.
Olso, in this regard, Jamshidi et al. 2018 dynamic studies on the AcrA efflux pump in interaction with PAβN as an efflux pump inhibitor showed that the free energy level resulting from this interaction is equal to − 28 kcal/mol.
Abul et al. (Samreen et al. 2022) consider 19 phytocompounds were screened for efflux pump inhibitory activity againstAcrB protein of E. coli using molecular docking studies. The molecular dynamics simulation provided stability the protein AcrB and its complex with chlorogenic acid under physiological conditions. the best-docked compounds showed interaction with phenylalanine residues except the compound chlorpromazine and 2-hydroxy 1,4 naphthoquinone.
In our study, the residues of amino acids PHE263 and GLU261 for E. coli and ASN33, ASN298, IEU293, and GLU34 for P. aeruginosa were also shown to interact with eugenol via hydrogen bonds, indicating the potent activity of eugenol. The docking results showed that eugenol can inhibit the studied proteins. Our results are further confirmation of the findings of the study on the antimicrobial activity of eugenol by Karumathil et al., which showed that eugenol can decrease resistance to β-lactam antibiotics in bacteria isolated from β-lactam-resistant Acinetobacter baumannii (Karumathil et al. 2018).
Our findings also support Muniz et al. 2021 results, who investigated the antibacterial activity of eugenol and its derivatives allylbenzene, 4-allylanisole, isoeugenol, and 4-allyl-2,6-dimethoxyphenol against the S. aureus NorA efflux pump (EP) in association with norfloxacin and ethidium bromide. They showed that molecular docking was carried out due to the proper interaction between eugenol and the NorA pump with hydrogen bonds, and that most of the energy resulting from the interaction of the pump with 4-allyl-2,6-dimetoxyphenol and isoeugenol was reported to be − 7.11 and − 6.62, respectively.
In our study, when examining the pattern of antibiotic resistance, the growth inhibition zone was larger in eugenol-treated bacteria of P. aeruginosa and E. coli than in bacteria not treated with eugenol. The highest inhibitory zone belonged to ciprofloxacin (36 mm), and the lowest has belonged to imipenem and gentamicin for P. aeruginosa. These amounts for E. coli were 26.67 and 24 mm, respectively.
Considering the sub-MIC concentration values of eugenol for P. aeruginosa and E. coli, 0.83 and 6.64 mg.ml−1, respectively, it is concluded that the concentration was lower than the MIC when the bacterium was growing but has stopped expression of some of its genes. In this regard, Yadav et al. 2013 studied the antimicrobial activity of eugenol on Streptococcus pneumonia by determining the MIC and MBC of 0.32 and 0.64 mg.ml−1, respectively, indicating that eugenol is a good inhibitor. Similar to our study ,Negi et al. 2014 By collecting Pseudomonas aeruginosa samples, antimicrobial sensitivity was determined using disk diffusion test and minimum inhibitory concentration (MIC) against selected drugs before and after addition of known synthetic EPI, PAβN (20 mg/L). Cosider the role of efflux pump in development of drug resistance which can be overcome by use of an efflux pump inhibitor, with more emphasis on compound like curcumin which will have less or no adverse effects if used in vivo.
In the present study, eugenol was used to inhibit the AcrA and MexA pumps. The cartwheel test was performed to induce susceptibility to P. aeruginosa and E. coli bacteria. The red color rate after using UV rays was used as an indicator for effusion pump activity. When these bacteria were unable to pump the ethidium bromide, it accumulated inside them, and the red color rate was high, meaning that eugenol had been able to inhibit the efflux pump and reduce antibiotic resistance. Also, a clear culture streak indicated the inactivity of the efflux pump, and opaque ones indicated the activation of the pump efflux system. This method showed that resistance to antibiotic stains at MIC and sub-MIC concentrations inhibits the efflux pump.
Similar to our study, Christina et al. 2015 tested that copper nanoparticles (CuNPs) could act as an inhibitor of an influx pump and as an antibiofilm. Copper nanoparticles inhibit the efflux activity ofStaphylococcus aureus and P. aeruginosa. Using the vortex method in a culture medium containing TSA and ethidium bromide, each of the strains was cultured in gear and incubated for 24 h at 37 °C. The results showed that at 0.5 mg.ml−1 MIC concentration, CuNP had a significant inhibitory effect on S. aureus and P. aeruginosa strains. The effect of copper nanoparticles has been reported as an inhibitor of the efflux pump. It is also used with antimicrobial properties to reduce multidrug resistance in bacteria.
In addition to the phenotypic study of eugenol on bacteria, the molecular test using real-time PCR for expression of the AcrA gene in E. coli and the MexA gene in P. aeruginosawere also examined with the same eugenol concentration (sub-MIC). The findings indicated that the expression of genes was significantly reduced. In a similar vein, in the study by Adli et al.2014, the effects of eugenol on the expression of biofilm-and quorum-sensing-related genes in Streptococcus mutans were investigated by real-time PCR. The data obtained showed that eugenol in sub-MIC concentrations significantly reduced the expression regulation of the genes tested.
Conclusions
The efflux inhibitors play an important role in reducing bacterial resistance to antibiotics. These inhibitors increase the concentration of antibiotics inside bacterial cells by inhibiting efflux pump activity, which can help prevent bacterial resistance to antibiotics. Furthermore, the use of efflux pump inhibitors can improve the sensitivity of bacteria to antibiotics and increase the therapeutic effectiveness of antibiotics against bacterial infections. Several inhibitors of the MexAB-OprM efflux pump have been identified, including, PaβN, CCCP, Norfloxacin, Piperlongumine and MC-04.
In this study, eugenol was shown to have bactericide properties and inhibitory activity against antibiotic-resistant bacteria and to have the potential to inhibit efflux pumps in antibiotic-resistant bacteria even at concentrations below lethal levels. Also, Eugenol can significantly reduce the expression of efflux pump genes acrA and mexA. Since, medicinal plant bioactives have fewer side effects than synthetic antibiotics. Therefore, nowadays, studies have been consentrated on herbal compounds as inhibitors of efflux pumps. So, it is proposed to introduce eugenol as a candidate for inducing susceptibility in resistant bacteria by conducting more comprehensive studies.
Acknowledgements
The authors thank Islamic Azad University for providing the infrastructure to conduct the present study.
Author contributions
EEA and ZGS prepared figures and tables. MM and BMS prepared manuscript. All authors reviewed the manuscript.
Funding
This research received no specific funding from public, commercial, or nonprofit entities.
Data availability
The datasets generated and/ or analyzed during the current study are avilable from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mirsasan Mirpour, Email: Sassan47@gmail.com, Email: mirsasan_mirpour@yahoo.com.
Bahram Mohammad Soltani, Email: dr.b.soltani@gmail.com, Email: soltanib@modares.ac.ir.
References
- Adil M, Singh K, Verma PK, Khan AU. Eugenol-induced suppression of biofilm-forming genes in Streptococcus mutans: an approach to inhibit biofilms. J Glob Antimicrob Resist. 2014;2(4):286–92. doi: 10.1016/j.jgar.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Ali SQ, Zehra A, Naqvi BS, Shah S, Bushra R. Resistance pattern of ciprofloxacin against different pathogens. Oman Med J. 2010;25(4):294–298. doi: 10.5001/omj.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL, He GX, Kakarla P, Ranjana KC, Kumar S, Lakra WS, et al. Multidrug efflux pumps from enterobacteriaceae, Vibrio cholerae and Staphylococcus aureus bacterial food pathogens. Int J Environ Res Public Health. 2015;12(2):1487–1547. doi: 10.3390/ijerph120201487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli G, Cappelli L, Cinelli P, Cuffaro R, Manca B, Nicchi S, et al. Strategies to tackle antimicrobial resistance: the example of escherichia coli and pseudomonas aeruginosa. Int J Mol Sci. 2021;22(9):4943. doi: 10.3390/ijms22094943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askoura M, Mottawea W, Abujamel T, Taher I. Efflux pump inhibitors (EPIs) as new antimicrobial agents against Pseudomonas aeruginosa. Libyan J Med. 2011;6(1):1–8. doi: 10.3402/ljm.v6i0.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M, Garau J. Current and future perspectives in the treatment of multidrug-resistant Gram-negative infections. J Antimicrob Chemother. 2021;76:IV23–37. doi: 10.1093/jac/dkab352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens. 2023;12(1):1–14. doi: 10.3390/pathogens12010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christena LR, Mangalagowri V, Pradheeba P, Ahmed KBA, Shalini BIS, Vidyalakshmi M, et al. Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RSC Adv. 2015;5(17):12899–909. doi: 10.1039/C4RA15382K. [DOI] [Google Scholar]
- Colclough AL, Alav I, Whittle EE, Pugh HL, Darby EM, Legood SW, et al. RND efflux pumps in Gram-negative bacteria; regulation, structure and role in antibiotic resistance. Future Microbiol. 2020;15(2):143–157. doi: 10.2217/fmb-2019-0235. [DOI] [PubMed] [Google Scholar]
- Ebbensgaard AE, Løbner-Olesen A, Frimodt-Møller J. The role of efflux pumps in the transition from low-level to clinical antibiotic resistance. Antibiotics. 2020;9(12):1–7. doi: 10.3390/antibiotics9120855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholampour-Azizi I, Rouhi S, Yahyayi F. In vitro antifungal activity of Cucumis melo on Candida albicans. Zahedan J Res Med Sci. 2015;17:7. doi: 10.17795/zjrms1019. [DOI] [Google Scholar]
- Glavier M, Puvanendran D, Salvador D, Decossas M, Phan G, Garnier C, et al. Antibiotic export by MexB multidrug efflux transporter is allosterically controlled by a MexA-OprM chaperone-like complex. Nat Commun. 2020;11(1):1–11. doi: 10.1038/s41467-020-18770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemaiswarya S, Doble M. Synergistic interaction of eugenol with antibiotics against Gram negative bacteria. Phytomedicine. 2009;16(11):997–1005. doi: 10.1016/j.phymed.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Jamali N, Soureshjani EH, Mobini GR, Samare-Najaf M, Clark CCT, Saffari-Chaleshtori J. Medicinal plant compounds as promising inhibitors of coronavirus (COVID-19) main protease: an in silico study. J Biomol Struct Dyn. 2022;40(17):8073–84. doi: 10.1080/07391102.2021.1906749. [DOI] [PubMed] [Google Scholar]
- Jamshidi S, Sutton JM, Rahman KM. Mapping the dynamic functions and structural features of AcrB Efflux pump transporter using accelerated molecular dynamics simulations. Sci Rep. 2018;8(1):1–13. doi: 10.1038/s41598-018-28531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karumathil DP, Nair MS, Gaffney J, Kollanoor-Johny A, Venkitanarayanan K. Trans-cinnamaldehyde and eugenol increase Acinetobacter baumannii sensitivity to beta-lactam antibiotics. Front Microbiol. 2018;9(MAY):1–10. doi: 10.3389/fmicb.2018.01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers RP, Cavallari JF, Burrows LL. The efflux inhibitor phenylalanine-arginine Beta-naphthylamide (PAβN) permeabilizes the outer membrane of Gram-Negative Bacteria. PLoS One. 2013;8(3):1–7. doi: 10.1371/journal.pone.0060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Jiang L, Chen Y, Kang J. Activities and mechanisms of eugenol and cinnamaldehyde against Legionella pneumophila. Int J Clin Exp Med. 2017;10(12):16460–16467. [Google Scholar]
- Martinez JL, Sánchez MB, Martínez-Solano L, Hernandez A, Garmendia L, Fajardo A, et al. Functional role of bacterial multidrug efflux pumps in microbial natural ecosystems. FEMS Microbiol Rev. 2009;33(2):430–449. doi: 10.1111/j.1574-6976.2008.00157.x. [DOI] [PubMed] [Google Scholar]
- Muniz DF, dos Santos Barbosa CR, de Menezes IRA, de Sousa EO, Pereira RLS, Júnior JTC, et al. In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem. 2021;337:127776. doi: 10.1016/j.foodchem.2020.127776. [DOI] [PubMed] [Google Scholar]
- Negi N, Prakash P, Gupta ML, Mohapatra TM. Possible role of curcumin as an efflux pump inhibitor in multi drug resistant clinical isolates of Pseudomonas aeruginosa. J Clin Diagnostic Res. 2014;8(10):DC04–7. doi: 10.7860/JCDR/2014/8329.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Structure and mechanism of RND-type multidrug efflux pumps. Adv Enzymol Relat Areas Mol Biol. 2010;77(111):1–60. doi: 10.1002/9780470920541.ch1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H, Takatsuka Y. Mechanisms of RND multidrug efflux pumps. Biochim Biophys Acta - Proteins Proteomics. 2009;1794(5):769–781. doi: 10.1016/j.bbapap.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès JM, Masi M, Barbe J. Inhibitors of efflux pumps in Gram-negative bacteria. Trends Mol Med. 2005;11(8):382–389. doi: 10.1016/j.molmed.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther. 2022;7(1):1–27. doi: 10.1038/s41392-022-01056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samreen, Qais FA, Ahmad I. In silico screening and in vitro validation of phytocompounds as multidrug efflux pump inhibitor against E. coli. J Biomol Struct Dyn. 2022;41:1–13. doi: 10.1080/07391102.2022.2029564. [DOI] [PubMed] [Google Scholar]
- Shi X, Chen M, Yu Z, Bell JM, Wang H, Forrester I, et al. In situ structure and assembly of the multidrug efflux pump AcrAB-TolC. Nat Commun. 2019;10(1):4–9. doi: 10.1038/s41467-019-10512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siala M, Barbana A, Smaoui S, Hachicha S, Marouane C, Kammoun S, et al. Screening and detecting Salmonella in different food matrices in Southern Tunisia using a combined enrichment/real-time PCR method: correlation with conventional culture method. Front Microbiol. 2017;8(DEC):1–10. doi: 10.3389/fmicb.2017.02416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119:S3–10. doi: 10.1016/j.amjmed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Wieczorek P, Sacha P, Hauschild T, Zórawski M, Krawczyk M, Tryniszewska E. Multidrug resistant Acinetobacter baumannii - the role of AdeABC (RND family) efflux pump in resistance to antibiotics. Folia Histochem Cytobiol. 2008;46(3):257–267. doi: 10.2478/v10042-008-0056-x. [DOI] [PubMed] [Google Scholar]
- Yadav S, Kapley A. Antibiotic resistance: global health crisis and metagenomics. Biotechnol Rep. 2021;29:e00604. doi: 10.1016/j.btre.2021.e00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav MK, Park SW, Chae SW, Song JJ, Kim HC. Antimicrobial activities of Eugenia caryophyllata extract and its major chemical constituent eugenol against Streptococcus pneumoniae. Apmis. 2013;121(12):1198–1206. doi: 10.1111/apm.12067. [DOI] [PubMed] [Google Scholar]
- Yusuf E, Bax HI, Verkaik NJ, van Westreenen M. An update on eight “new” antibiotics against multidrug-resistant gram-negative bacteria. J Clin Med. 2021;10(5):1–22. doi: 10.3390/jcm10051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/ or analyzed during the current study are avilable from the corresponding author on reasonable request.