Abstract
Objective:
Physical activity (PA) may help maintain brain structure and function in aging. Since the intensity of PA needed to effect cognition and cerebrovascular health remains unknown, we examined associations between PA and cognition, regional white matter hyperintensities (WMH), and regional cerebral blood flow (CBF) in older adults.
Method:
Forty-three older adults without cognitive impairment underwent magnetic resonance imaging (MRI) and comprehensive neuropsychological assessment. Waist-worn accelerometers objectively measured PA for approximately one week.
Results:
Higher time spent in MVPA was uniquely associated with better memory and executive functioning after adjusting for all light PA. Higher MVPA was also uniquely associated with lower frontal WMH volume although the finding was no longer significant after additionally adjusting for age and accelerometer wear time. MVPA was not associated with CBF. Higher time spent in all light PA was uniquely associated with higher CBF but not with cognitive performance or WMH volume.
Conclusions:
Engaging in PA may be beneficial for cerebrovascular health and MPVA in particular may help preserve memory and executive function in otherwise cognitively healthy older adults. There may be differential effects of engaging in lighter PA and MVPA on MRI markers of cerebrovascular health although this needs to be confirmed in future studies with larger samples. Future randomized controlled trials that increase PA are needed to elucidate cause-effect associations between PA and cerebrovascular health.
Keywords: Cerebral Blood Flow, White Matter Hyperintensities, Cognition, Aging, Exercise, Perfusion, Mobile Health, Digital Health
INTRODUCTION
The aging population in the United States is predicted to more than double by the year 2050 (Vincent & Velkoff, 2010). This unprecedented growth will result in increased numbers of individuals living with Alzheimer’s disease (AD) and related dementias (ADRD). Compared to 2020, it is projected that the number of people aged 65 and older diagnosed with AD will increase by 22% by the year 2025 (“2020 Alzheimer’s Disease Facts and Figures,” 2020). Research suggests that approximately 30% of AD cases can be prevented by modifiable behaviors (Livingston et al., 2020), with 21% of AD cases in the US being linked to physical inactivity (Barnes & Yaffe, 2011). In recent decades, physical activity (PA) has been consistently found to bolster cognitive abilities in healthy older adults (Ahlskog, Geda, Graff-Radford, & Petersen, 2011; Beckett, Ardern, & Rotondi, 2015; Colcombe et al., 2006; Domingos, Pêgo, & Santos, 2021; Haeger, Costa, Schulz, & Reetz, 2019; Spartano et al., 2019; Voelcker-Rehage, Godde, & Staudinger, 2011; Zlatar, Godbole, et al., 2019). Supervised intervention studies have shown that PA targeting 60–80% of maximum heart rate are most favorable in changing cognitive performance, brain structure, and brain function (Colcombe et al., 2004; Sanders, Hortobágyi, Gemert, van der Zee, & van Heuvelen, 2019; Vidoni et al., 2015). A study involving older participants without dementia found that moderate intensity aerobic exercise can increase hippocampal volume by 2% and improve memory function (Erickson et al., 2011). Furthermore, a 3-month intervention consisting of 30-minute aerobic exercise intervals three times per week, with a target heart rate starting at 65% of maximum heart rate and increasing by 5% every week, induced neurovascular plasticity among older adults (Maass et al., 2015). These findings suggest that changes in moderate to vigorous intensity physical activity (MVPA) may be necessary to affect cognitive health (Zlatar, Godbole, et al., 2019).
Despite the abundance of literature that supports the benefits of PA on brain structure, function, and cognition in aging, the mechanisms by which PA may preserve cerebrovascular health remain understudied. Both cerebral blood flow (CBF) and white matter hyperintensity (WMH) volume are indicators of cerebrovascular health that have rarely been studied together as a function of accelerometer-measured PA. Zlatar and colleagues (2019) previously showed that accelerometer-measured light PA and MVPA are associated with greater CBF in the frontal lobe of older adults with normal cognition, whereas sedentary time had an inverse association with CBF (Zlatar, Hays, et al., 2019). Similarly, a small intervention study with healthy, sedentary older men demonstrated that aerobic exercise training improves CBF by 27% in the frontal lobe (Kleinloog et al., 2019), while fitness has been associated with CBF in cognitively healthy adults at risk for AD (Dougherty et al., 2020). As such, increased CBF through long-term exercise training may be a mechanism to improve cognitive functioning (Joris, Mensink, Adam, & Liu, 2018).
WMH are frequently observed on T2 FLAIR magnetic resonance imaging (MRI) in older adults and are considered an indicator of small vessel cerebrovascular disease. It is well established that greater WMH reflects an increased risk of developing ADRD (Bangen et al., 2018, 2020; Brickman et al., 2012, 2015; Lee et al., 2016). Systematic reviews have explored associations between WMH and PA in cross-sectional and longitudinal studies, but results have been mixed (Sexton et al., 2016; Torres, Strack, Fernandez, Tumey, & Hitchcock, 2015). One study, which involved ten masters athletes and ten sedentary older adults matched in age and education, showed that masters athletes showed an 83% reduction in deep WMH compared to their sedentary counterparts. In addition, they found an inverse relationship between deep WMH volume and life-long aerobic exercise (Tseng et al., 2013). Higher sedentary time was also associated with greater WMH in those with lower levels of kidney function (Bronas et al., 2019). Another study, however, did not find significant associations between WMH and PA in healthy older adults with memory complaints. The study did find that those who reduced their PA level over three years had a trend towards increased WMH (Moon et al., 2018). Different findings may be attributable to methodological differences, such as the PA assessment method used (i.e., self-report (Best et al., 2017) versus objective measures (Arnardottir et al., 2016)) and the time frame under consideration (PA for the past week, year, lifelong, etc.). Studies that measure PA objectively can provide a more accurate assessment of PA that is free of recall bias.
This study aimed to advance the literature on PA prescriptions to promote brain health by investigating if accelerometer-measured PA is associated with cognitive performance and markers of cerebrovascular health (WMH and CBF) in a sample of cognitively healthy, community-dwelling older adults. We sought to examine whether objectively-measured PA is sensitive to cognitive functioning and cerebrovascular health markers. We examined the associations of continuous measures of two intensities of PA (all light PA and MVPA) with cognitive and brain health variables to help eludicate whether MVPA is necessary to observe an association with cognition and brain markers or whether lighter PA is similarly associated with these variables. We examined WMH and CBF in frontal and temporal regions, which have been implicated in cerebrovascular dysfunction in aging and dementia risk (Bangen et al., 2018; Yew et al., 2017). We examined executive functioning and memory performance, which are subserved by frontal and temporal regions, respectively. We hypothesized that higher MVPA would be associated with better cognition, lower WMH volume, and higher CBF, and would have stronger associations with these variables than less intense PA (i.e., all light PA), given evidence that moderate levels of PA may be necessary to influence brain health (Chapman et al., 2013; Hayes, Hayes, Cadden, & Verfaellie, 2013).
METHOD
Participants
Participants were 43 community-dwelling, English-speaking older adults. Participants were recruited from ongoing studies at the University of California San Diego’s WISE lab and Alzheimer’s Disease Research Center, from ResearchMatch (https://www.researchmatch.org), flyers, community engagement talks, and by word of mouth. Participants were included if they were aged 65+, had no contraindications for MRI, were able to walk independently, had no mild cognitive impairment or dementia based on standard neuropsychological testing (Jak et al., 2009), had no history of head injury with loss of consciousness within the past 6 months or moderate-severe head injury in the past, had no major neurologic or psychiatric disorders, had no history of major vascular events, had no diabetes, had no poorly controlled medical conditions, and had no history of falls in the past year resulting in hospitalization. All participants provided written informed consent. The University of California San Diego’s Institutional Review Board approved the protocols. Data included in this manuscript was obtained in compliance with the Helsinki Declaration.
Procedure
All participants were pre-screened via telephone to ensure they met study criteria. This included administration of the modified Telephone Interview for Cognitive Status (m-TICS) as a first pass screening of cognitive impairment. Those with m-TICS scores ≤34 were disqualified from further participation (Cook, Marsiske, & McCoy, 2009). For those who met basic criteria, an Actigraph accelerometer was mailed to measure PA, and participants were instructed to wear it for 7 days, during waking hours, in their natural environments. After the measurement period, participants brought the accelerometer to their in-person appointment, at which time neuropsychological testing was administered. If participants met our cognitive criteria (no more than two scores <1 standard deviation from age-appropriate norms within one or more cognitive domains (Jak et al., 2009)) they were scheduled for a brain MRI appointment within one week.
Physical activity measurement
PA was objectively measured using tri-axial accelerometers (GT3X + and GT3X-BT, ActiGraph, LLC, Pensacola, FL). Consistent with recent studies (Dohrn, Sjöström, Kwak, Oja, & Hagströmer, 2018; Kerr et al., 2018), participants were instructed not to change their regular activities and to wear the accelerometer on a belt on their hip, during waking hours only, for a minimum of 12 hours per day for one week. To ensure compliance, all participants received two phone calls from study staff (on days 2 and 5 of the monitoring period). Participant data were considered valid at the day level only if they attained a minimum of 600 minutes of wear, consistent with National Health and Nutrition Examination Survey (NHANES) best practices (Troiano et al., 2008). Participants were included only if they wore the device a minimum of 3,000 total minutes of wear spread across at least 4 valid days (Hart, Swartz, Cashin, & Strath, 2011; Jerome, Young, Laferriere, Chen, & Vollmer, 2009; Trost, Mciver, & Pate, 2005). Data were processed using the ActiLife version 6 software (Pensacola, FL). The unit of measurement for accelerometers is counts per minute (CPM), with higher counts indicating greater intensity of movement. Non-wear time was determined using a modified Choi algorithm(Choi, Liu, Matthews, & Buchowski, 2011) in which 90 consecutive minutes of 0 counts with a 2-minute spike tolerance was screened as non-wear. Data were aggregated to 60-second epochs so published cut points could be applied. Consistent with standard practice, sedentary time was defined as time spent at < 100 CPM, all light PA as 100–1951 CPM, and MVPA as ≥ 1952 CPM (Copeland & Esliger, 2009). For the PA variables used in analyses, minutes within each intensity level were averaged across days worn, reflecting the average time in minutes per day spent at each intensity level.
Participants were instructed to wear accelerometers during waking hours only. If sleep was observed (i.e., >20-hour wear periods) data were visually inspected to ensure that behavior was indicative of sleep (i.e., short periods of small amounts of movement consistently through the night with only very brief periods of larger amounts of movement). Data were removed using a method developed by Full and colleagues (2018) that involves looking for the last period of substantial movement to establish go-to-bed time, and the first period of moderate amounts of movement to establish wake up time and removing the time in between from consideration (i.e., it was converted to “non-wear” time) (Full et al., 2018).
Cognitive assessment
Participants completed the Mattis Dementia Rating Scale (DRS), the NIH Toolbox Cognition Battery(Casaletto et al., 2015), Rey Auditory Verbal Learning Test (RAVLT), the Golden Stroop Color Word Interference Test, the Wechsler Memory Scale-Revised (WMS-R) Logical Memory I and II, Trail Making test Parts A and B, and verbal fluency tests (FAS and animals). To ensure participants included in the study were cognitively unimpaired, we applied the comprehensive MCI diagnostic criteria proposed by Jak/Bondi (2009), which requires at least two impaired test scores (>1 SD below normative means) within a cognitive domain. These diagnostic criteria are more strongly related to AD biomarkers and have greater diagnostic stability than typical diagnostic criteria requiring impaired performance on one cognitive test (>1.5 SD) (Wong et al., 2019). Individuals classified as MCI were excluded from this study (Jak et al., 2009). Normative scores were derived from the respective testing manuals and available published norms (J. Heaton, 2004; Ivnik, Malec, Smith, Tangalos, & Petersen, 1996).
Since executive and episodic memory performance scores are most responsive to exercise in intervention trials (Kennedy, Hardman, MacPherson, Scholey, & Pipingas, 2016), we created executive and memory composite scores by converting raw scores into z-scores based on the entire sample, and then averaging across z-scores for the following tests: Executive Composite Score = Trail Making Test Part B minus Trail Making Test Part A (scores were reversed prior to averaging to reflect higher scores=better performance), Stroop Color Word Trial, and verbal fluency FAS. Note that for the Trail Making Test, a difference score (B-A) was because it may reflect a purer measure of the executive functions required to complete Part B by subtracting sequencing, visual scanning, and psychomotor components common to both Parts A and B (R. K. Heaton, Nelson, Thompson, Burks, & Franklin, 1985). Memory Composite Score = WMS-R Logical Memory I and II, RAVLT Trials 1–5, Trial 6 (short delay free recall) and RAVLT delayed recall. The executive and memory composite scores were used as cognitive outcomes in analyses.
Brain image acquisition
Imaging data was acquired on a GE Discovery MR 750 3T whole body system with a body transmit coil and an 8-channel receive-only head coil at the University of California, San Diego’s Center for Functional MRI. The structural brain sequence consisted of (1) a high-resolution T1-weighted Fast Spoiled Gradient Recall (3DFSPGR) scan for anatomy and registration purposes: 172 1mm contiguous sagittal slices, field of view (FOV)=25 cm, repetition time (TR)=8 ms, echo time (TE)=3.1 ms, flip angle=12, inversion time (TI)=600 ms, 256×192 matrix, Bandwidth=31.25 kHz, frequency direction=S-I, NEX=1, scan time=8 min and 13 s and (2) a T2-weighted fluid attenuated inversion recovery (FLAIR) scan to detect white matter hyperintensities: 36 axial slices with no interslice gap at a voxel size of .47x.47×4.00 mm3, FOV=24 cm, TR=8650 ms, TE=136 ms, flip angle = 111, TI=2250 ms, 256×256 matrix, Bandwidth=31.25 kHz, frequency direction=A/P, NEX = 1, scan time = 6 min and 40 s). CBF was quantified with a 2D Pseudo Continuous Arterial Spin Labeling (ASL) MRI (2DPCASL) sequence; TR=4500 ms, TE=3.2 ms, FOV=24 cm, labeling duration=1800 ms, post-labeling delay=2000 ms, with a single shot spiral acquisition and a total scan time of 4:30 min plus a 40.5 s calibration scan. The calibration scan was acquired immediately after the ASL scan using a spiral readout with TR=4.5 s and TE=3.2 ms with 8 dummy radiofrequency (RF) pulses (amplitude set to zero) to generate a 36 s delay followed by a 90-degree RF pulse in the last repetition interval to generate proton density-weighted contrast. Field map scans were collected for off-line field map correction for signal bunching and dropouts in the frontal/medial temporal lobes.
Brain image processing
T1-weighted Anatomical Images
T1-weighted anatomical images were processed using FreeSurfer 6.0 software. Briefly, images underwent skull stripping, B1 bias field correction, gray matter-white matter segmentation, reconstruction of cortical surface models, and parcellation and labeling of regions on the cortical surface as well as segmentation and labeling of subcortical structures (Dale, Fischl, & Sereno, 1999; Fischl et al., 2002). FreeSurfer was used to generate intracranial volume and anatomical regions of interest (ROIs) for the CBF data.
T2-weighted FLAIR Images
Methods for processing the T2-weighted FLAIR images were similar to those previously described (Hoagey, Lazarus, Rodrigue, & Kennedy, 2021).White matter hyperintense voxels were identified on T2-weighted FLAIR images. Lesions were segmented using the lesion prediction algorithm (LPA) (Schmidt, 2017) as implemented in the lesion segmentation toolbox (LST) version 2.0.5 (www.statisticalmodelling.de/lst.html) for SPM12. This algorithm consists of a binary classifier in the form of a logistic regression model trained on the data of 53 individuals with multiple sclerosis who had severe lesion patterns. Covariates in the model include a lesion belief map as well as a spatial covariate considering voxel specific changes in lesion probability. Parameters of this model fit are used to segment lesions in new images producing a lesion probability map wherein each voxel value represents an estimated probability that it is a white matter lesion.
For quality assurance purposes, trained raters visually inspected each participant’s lesion probability map overlaid on the participant’s T2-weighted FLAIR image to ensure that the LST output optimally minimized false positive voxels (e.g., motion artifacts) and false negative voxels (i.e., voxels appearing as legitimate white matter lesions on the FLAIR image that were labeled as 0 in the lesion probability map). To eliminate false positive voxels in regions where white matter lesions are not biologically plausible, multiple regions of avoidance were created and combined to form an exclusion mask. To remove false positive voxels in the choroid plexus of the ventricles, the CSF probability map obtained from processing each participant’s T1-weighted scan using FMRIB’s Automated Segmentation Tool (FAST) (Zhang, Brady, & Smith, 2001) was registered to their native FLAIR space using Advanced Normalization Tools (ANTs) (Avants, Tustison, & Johnson, 2014). The CSF probability maps in each participant’s FLAIR space were thresholded at 0.5 and then binarized to obtain the CSF exclusion mask. Three additional regions of avoidance were derived on the Montreal Neurological Institute (MNI) 1mm space brain and then registered to each participant’s native FLAIR space using ANTs. The regions were (1) a 2mm mid-sagittal exclusion mask to remove false positive voxels in the septum pellucidum, (2) a ventral exclusion mask (below z=34) to eliminate false positive voxels from the cerebellum and brainstem, and (3) a dorsal exclusion mask (above z=130) to remove false positive voxels from exiting blood vessels. The four exclusion masks were summed to create one total exclusion mask and then applied to each participant’s thresholded lesion probability map.
Frontal and temporal lobar masks from the Wake Forest University PickAtlas (Maldjian, Laurienti, & Burdette, 2004; Maldjian, Laurienti, Kraft, & Burdette, 2003) were registered to each participant’s FLAIR space using ANTs. The binary WMH segmentation from LST was then multiplied by the FLAIR-registered binary lobar mask to obtain WMH volume for frontal and temporal lobes.
ASL Images
ASL data were processed using the Cerebral Blood Flow Biomedical Informatics Research Network (CBFBIRN) (Shin, Ozyurt, & Liu, 2013) pipeline established at the University of California San Diego’s Center for Functional Magnetic Resonance Imaging. CBFBIRN uses a combination of custom MATLAB(MathWorks, 1996) routines and various functions from Analysis of Functional Neuroimages (AFNI) (Cox, 1996) and FMRIB Software Library (FSL) (Smith et al., 2004) to quantify CBF and adjust for partial volume effects. MATLAB was used to form a mean ASL image from the average difference of the control and tag images. Voxelwise CBF calibration was performed using the proton density image to convert the ASL difference signal into physiological units (ml/100g/min). In addition, slice timing delays were accounted for, making the post-labeling delay slice specific. Skull stripping of the high-resolution T1-weighted image was performed using AFNI’s 3dSkullStrip. Tissue segmentation was performed using FSL’s Automated Segmentation Tool (FAST) algorithm to define CSF, gray matter (GM), and white matter (WM) regions. The high-resolution T1-weighted image and partial volume segmentations were registered to ASL space using AFNI’s 3dAllineate program. To correct for partial volume effects and ensure that CBF values were not influenced by decreased perfusion in the WM or increased volume of CSF, we used a linear regression method (Asllani, Borogovac, & Brown, 2008) with a 5×5 regression kernel to obtain corrected GM CBF measurements. For each participant’s partial volume corrected quantified CBF map (in units of mL/100 g tissue/min), voxels with negative intensities were replaced with zero.
FreeSurfer was used to generate a priori anatomical ROIs for the CBF data. Briefly, for each participant, the FreeSurfer formatted T1-weighted brain volume was registered to the ASL CBF-aligned T1-weighted anatomical image (the latter was derived as part of the CBFBIRN pipeline). The resulting co-registration matrix was used to align the FreeSurfer aparc+aseg segmentation volume to the ASL CBF-aligned T1-weighted image. The CBF-aligned FreeSurfer volumes were visually inspected to ensure proper alignment and were then downsampled to the resolution of the CBF ASL image. Mean CBF was then extracted for FreeSurfer ROIs.
Statistical Analysis
Multiple linear regression models were used to examine the associations of continuous PA with cognitive functioning and MRI variables. Cognitive variables of interest included the memory and executive function composite scores described above. Cerebrovascular brain health variables of interest included a limited number of a priori ROIs to minimize the number of statistical analyses in an attempt to reduce susceptibility to type 1 errors. For WMH, we examined frontal and temporal WMH volumes. For CBF, we examined four ROIs (two frontal regions and two temporal regions) including: rostral middle frontal gyrus, medial orbitofrontal cortex, hippocampus, and inferior temporal cortices. As described above, we selected these specific ROIs given these regions have been shown to be implicated in cerebrovascular dysfunction in aging and dementia risk (Bangen et al., 2018; Yew et al., 2017).
Frontal and temporal WMH volumes were divided by total intracranial volume to correct for head size. The distribution of WMH volume (divided by total intracranial volume) was positively skewed, so a log-transformation was used to improve distribution normality. Each of the dependent variables (memory, executive function, regional WMH, regional CBF) were assessed in separate models. Two models were run for each dependent variable: one model including MVPA and all light PA as predictors and a second model additionally adjusting for age and accelerometer wear time. We selected covariates for inclusion in our models based on both theoretical and statistical considerations. We considered including sex and education as covariates in our models given expected associations with cognition and brain variables. However, in an effort to maximize statistical power and not overadjust our models, we did not include sex and education as covariates given they did correlate with any of the dependent variables in our sample (all p-values > .05). Potential multicollinearity of the independent variables was assessed for all models. All variance inflation factor (VIF) values were < 1.3 and the all bivariate correlation coefficients between the independent variables were r’s < .6 (Field, 2009). In order to address potential inflation of type I error due to multiple comparisons, false discovery rate (FDR) was controlled at 0.05 using the Benjamini-Hochberg procedure (Benjamini & Hochberg, 1995). Each set of analyses examining the same category of dependent variable was treated as an omnibus test with multiple comparisons correction separately applied for each (i.e., applied separately for the two cognitive measures, two WMH ROIs, and four CBF ROIs). All statistical analyses were conducted using the IBM SPSS Statistics for Macintosh, Version 28.0. Figures were created using R (R Core Team, 2021).
RESULTS
Participant characteristics and accelerometer assessment
Descriptive data for clinical and demographic characteristics is shown in Table 1. On average, the sample was approximately 72 years and well-educated. The sample had relatively low vascular risk burden (i.e., the mean Framingham Stroke Risk Profile (D’Agostino et al., 1994) score indicated a 7% probability of having a stroke within the next 10-years). The mean score on a measure of global cognitive functioning was unimpaired (i.e., Dementia Rating Scale total score of 140 out of 144).
Table 1.
Participant characteristics for the entire sample (N=43)
| Mean | SD | |
|---|---|---|
|
| ||
| Demographics | ||
|
| ||
| Age (years) | 71.77 | 4.19 |
| Education (years) | 16.86 | 2.21 |
| Sex (% female) | 74.40% | — |
| Race (% White) | 93% | — |
| Framingham Stroke Risk Profile (%) | 7.19 | 4.23 |
|
| ||
| Cognitive measures | ||
|
| ||
| Memory Composite z-score | 0.00 | 0.80 |
| Executive Composite z-score | 0.00 | 0.75 |
|
| ||
| MRI measures | ||
|
| ||
| Frontal WMH volume (%) | 0.16 | 0.29 |
| Temporal WMH volume (%) | 0.12 | 0.11 |
| Rostral middle frontal gyrus CBF, ml/100g/min | 45.87 | 13.61 |
| Medial orbitofrontal cortex CBF, ml/100g/min | 40.80 | 17.06 |
| Hippocampal CBF, ml/100g/min | 40.03 | 11.52 |
| Inferior temporal CBF, ml/100g/min | 36.44 | 15.21 |
PA = physical activity; min = minutes; WMH = white matter hyperintensities; CBF = cerebral blood flow. WMH variables expressed as percent are normalized by total intracranial volume to correct for head size and then multiplied by 100. Note that analyses were performed on log transformed WMH variables although descriptive statistics are shown here for non-transformed data.
Participants were compliant with accelerometer wear during the assessment period. Accelerometer metrics and the amount of time participants spent on average within different activity categories are displayed in Table 2.
Table 2.
Accelerometer metrics
| Mean | Standard deviation | Minimum | Maximum | |
|---|---|---|---|---|
|
| ||||
| Variable | ||||
|
| ||||
| Days of wear | 7.40 | 1.28 | 4.00 | 10.00 |
| Daily minutes of wear | 910.82 | 74.19 | 782.50 | 1105.20 |
| Total accelerator wear time (min) | 6733.23 | 1307.11 | 3913.00 | 11052.00 |
| Sedentary time min/day (CPM 0–99) | 585.37 | 109.22 | 350.12 | 844.71 |
| All light PA min/day (CPM 100–1951) | 301.65 | 81.50 | 136.50 | 524.33 |
| Moderate to vigorous PA min/day (CPM ≥1952) | 23.80 | 17.36 | 1.38 | 60.17 |
CPM=Accelerometer counts per minute; min = minutes; PA = physical activity
Associations of continuous PA with measures of cognition
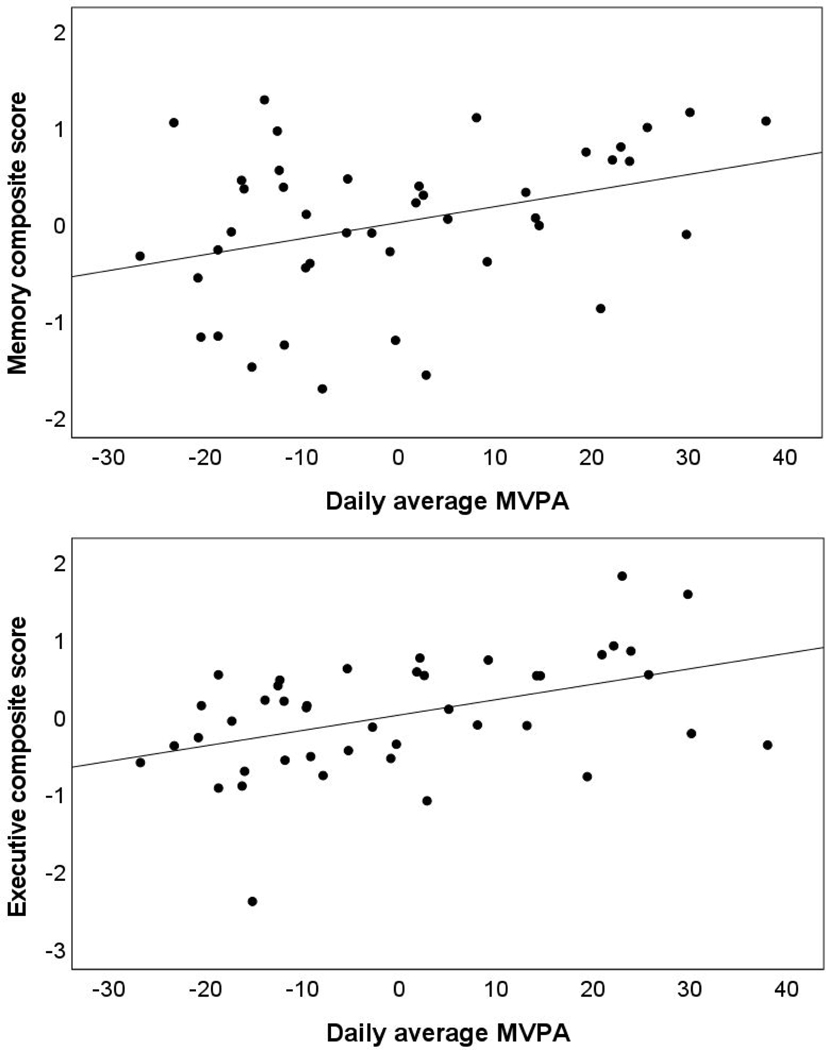
In models including MVPA and all light PA as predictors, higher MVPA was uniquely associated with better performance on memory and executive functioning measures. See Table 3 and Figure 1. As shown in Table 3, all light PA was not uniquely associated with performance on cognitive measures. In models additionally adjusted for age and accelerometer wear time, findings remained similar. That is, higher MVPA was associated with better memory and executive functioning. These results survived correction for multiple comparisons.
Table 3.
Multiple Linear Regression Models for Association of Physical Activity with Cognitive Performance (N=43)
| MEMORY | EXECUTIVE FUNCTION | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| β | t | p | β | t | p | |
| Model 1 | ||||||
| All Light PA | .030 | 0.200 | .843 | −.028 | −.193 | .848 |
| MVPA | .356 | 2.373 | .023 | .456 | 3.170 | .003 |
| Model 2 | ||||||
| All Light PA | .067 | −.361 | .720 | −.004 | −.027 | .979 |
| MVPA | .333 | 2.061 | .046 | .413 | 2.536 | .015 |
Abbreviations: PA = physical activity; MVPA = moderate to vigorous physical activity. Wear time denotes total time the accelerometer was worn. Model 1: All light PA and MVPA as independent variables. Model 2: Additionally adjusted for age and accelerometer wear time. Statistically significant (p < 0.05) results appear in bold font. These results survived correction for multiple comparisons.
Figure 1.
Partial regression plots for the association of moderate to vigorous physical activity (MVPA) and cognitive performance for episodic memory (top panel) and executive function (bottom panel) adjusting for all light physical activity
Associations of PA with measures of brain health
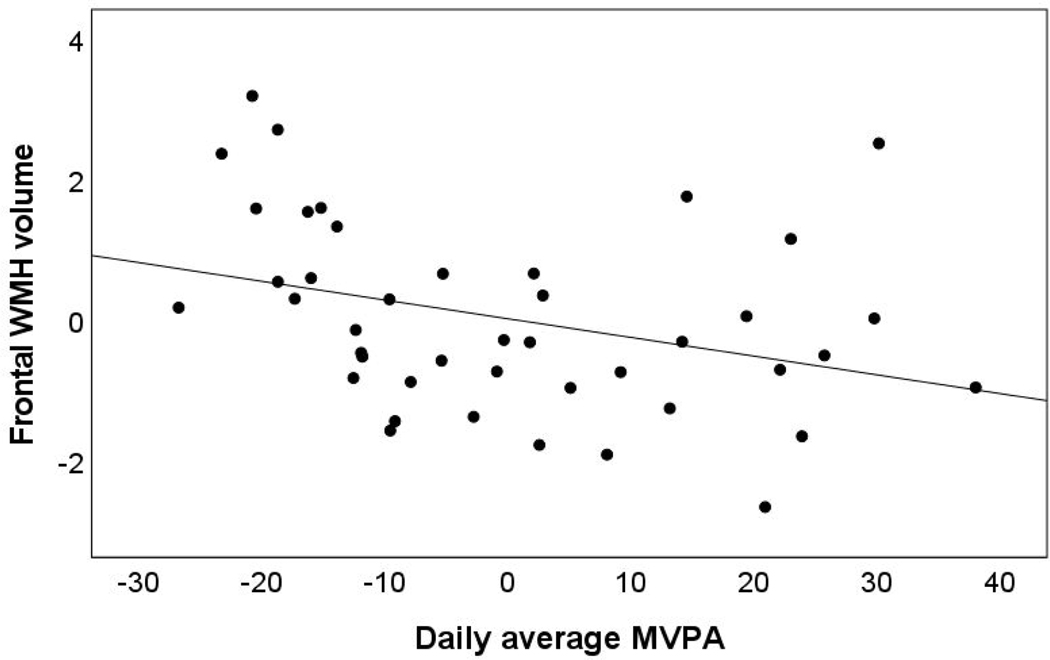
In models including MVPA and all light PA as predictors, higher continuous MVPA was uniquely associated with lower frontal WMH volume but not temporal WMH volume. See Table 4 and Figure 2. In models additionally adjusted for age and accelerometer wear time, findings were attenuated and there was no longer a significant association between MVPA and frontal WMH volume. All light PA was not significantly associated with WMH volume.
Table 4.
Multiple Linear Regression Models for Association of Physical Activity with Regional WMH Volume (N=43)
| FRONTAL WMH | TEMPORAL WMH | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| β | t | p | β | t | p | |
|
| ||||||
| Model 1 | ||||||
| All Light PA | −.013 | −.089 | .930 | .029 | .187 | .852 |
| MVPA | −.342 | −2.262 | .029 | −.277 | −1.784 | .082 |
| Model 2 | ||||||
| All Light PA | −.122 | −.899 | .374 | −.078 | −.535 | .596 |
| MVPA | −.130 | −.907 | .370 | −.076 | −.492 | .626 |
β = standardized coefficient; PA = physical activity; MVPA = moderate to vigorous physical activity; WMH = white matter hyperintensities. Wear time denotes total time the accelerometer was worn. Model 1: All light PA and MVPA as independent variables. Model 2: Additionally adjusted for age and accelerometer wear time. Statistically significant (p < 0.05) results appear in bold font.
Figure 2.
Partial regression plot for the association of moderate to vigorous physical activity (MVPA) and frontal white matter hyperintensities (WMH) volume adjusting for all light physical activity
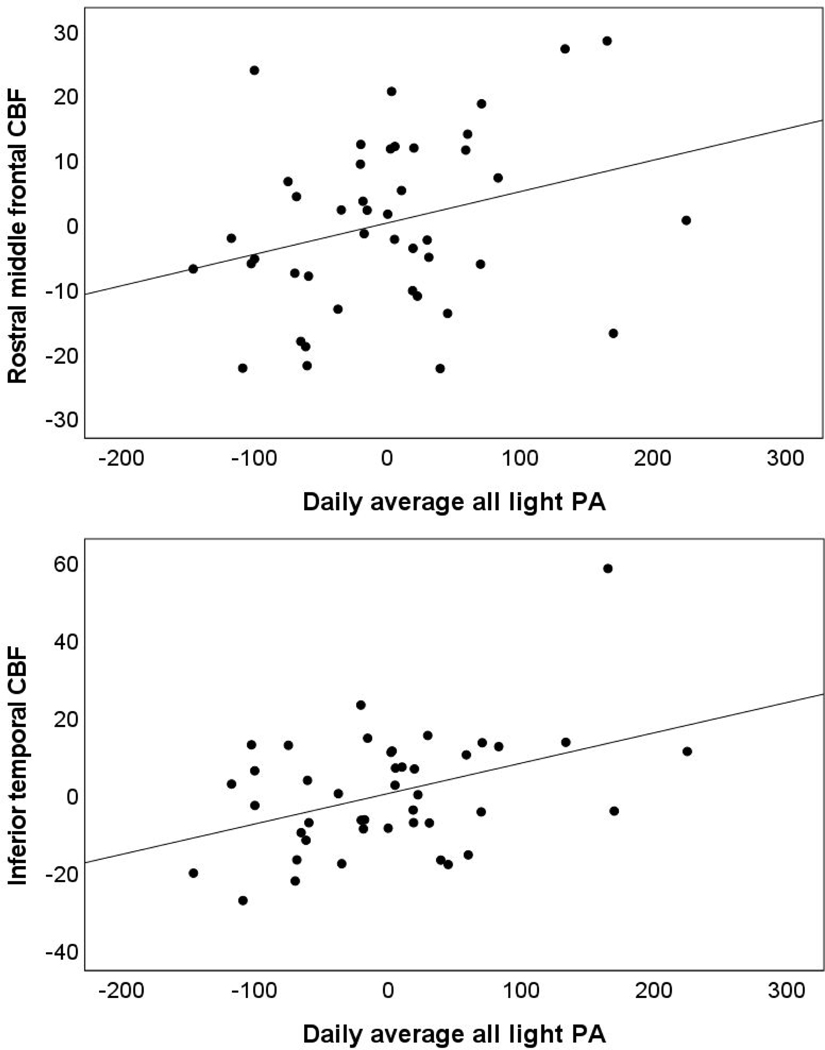
Higher continuous MVPA was not significantly associated with regional CBF. In contrast, there was a unique association between greater all light PA and higher inferior temporal CBF. In models additionally adjusted for age and accelerometer wear time, findings remained similar and there was a unique association between higher all light PA and greater rostral middle frontal and inferior temporal CBF. See Table 4 and Figure 3. The result for inferior temporal CBF survived correction for multiple comparisons although the finding for rostral middle frontal CBF did not. In addition, there was one CBF outlier (i.e., regional CBF values >3 standard deviations from the sample mean), however, excluding this one participant did not change the pattern or significance of results.
Figure 3.
Partial regression plots for the association of all light physical activity (PA) and cerebral blood flow (CBF) in rostral middle frontal (top panel) and inferior temporal regions (bottom panel) adjusting for moderate to vigorous physical activity
DISCUSSION
This study sought to advance the literature on PA prescriptions to promote brain health by investigating if objectively measured PA is associated with cognitive performance and markers of cerebrovascular health (regional CBF and WMH volume) in a sample of cognitively healthy, community-dwelling older adults. Greater time spent in MVPA was uniquely associated with better performance on memory and executive functioning tasks. Higher MVPA was also associated with lower frontal WMH volume although the finding was attenuated and no longer significant after additionally adjusting for age and accelerometer wear time. MVPA was not associated with CBF. Higher time spent in all light PA was uniquely associated with higher inferior temporal CBF. However, higher time spent in light PA was not uniquely associated with cognitive performance or WMH volume. Although findings are preliminary and should be replicated in larger samples with longitudinal follow-up, the pattern of results suggest that MVPA may be beneficial for cognitive functioning and both MVPA and all light PA may be protective against decline in aspects of cerebrovascular health.
WMH are often seen as incidental findings on neuroimaging (Wardlaw, Valdés Hernández, & Muñoz-Maniega, 2015). However, growing research clearly demonstrates their clinical importance. WMH become more common with advanced aging (DeCarli et al., 2005; Morris et al., 2009) and until relatively recently were often considered to be part of normal aging (Wardlaw et al., 2015), although their prevalence is highly variable and increases with vascular risk factors including hypertension (Dufouil, Alperovitch, & Tzourio, 2003; Maillard et al., 2012), diabetes (Werhane et al., 2021) and smoking (Gons et al., 2011). A meta-analysis of 22 studies found that WMH were associated with a faster decline in global cognition, executive function, and processing speed as well as a 2-fold increase in the risk of developing dementia and a 3-fold increase in risk of stroke (Debette & Markus, 2010). In addition, we have previously shown that baseline higher WMH volume predicts conversion from normal cognition to mild cognitive impairment (Bangen et al., 2018) as well as functional decline (Bangen et al., 2020).
Our findings are in line with previous studies suggesting that PA may be protective against WMH (Bronas et al., 2019; Gow et al., 2012; Tseng et al., 2013; Yu et al., 2021). Although it should be noted that the literature linking PA and WMH has been mixed (Sexton et al., 2016; Torres et al., 2015) with some studies finding no association between PA and WMH (Soldan et al., 2022). Notably, although WMH have often been thought of as irreversible and many longitudinal studies focus on slowing or halting progression of WMH, there is some evidence that WMH may not always be permanent. Early WMH may reflect shifts in water content and not just permanent myelin loss or axonal damage. Indeed, some studies of individuals who have had a stroke have shown WMH reduction over time (Moriya, Kozaki, Nagai, & Toba, 2009; Wardlaw et al., 2017). Elucidating the mechanisms by which PA affects white matter health needs further study (Soldan et al., 2022). Some evidence suggests that exercise-induced brain-derived neurotrophic factor (BDNF) and endothelial growth factor (Gaitán et al., 2021; Nicolini, Fahnestock, Gibala, & Nelson, 2021; Soldan et al., 2022) may enhance axon regeneration and also increase CBF and neurogenesis, suggesting multiple potential ways PA could improve white matter integrity (Trigiani & Hamel, 2017). Future randomized clinical trials involving longitudinal interventions are needed to establish whether PA may prevent progression or development of WMH or even possibly reverse WMH, thereby potentially mitigating their effects on cognitive and functional abilities.
It was somewhat unexpected that in the current study MVPA did not relate to CBF. We have previously shown that MVPA is associated with greater frontal CBF whereas sedentary time had an inverse association with CBF (Zlatar, Hays, et al., 2019). Whereas WMH development are generally thought of as a slowly progressive process, results across studies examining ASL MRI among older adults at risk for cognitive impairment have suggested that associations with CBF may be complex, with some studies reporting increases in CBF and others reporting decreases in CBF, while others suggest both depending on the regions or risk factors examined (Bangen et al., 2012; Wierenga et al., 2012). Increased resting CBF among older at-risk adults has often been interpreted as reflecting neurovascular dysregulation or possible compensatory mechanisms (Bangen et al., 2017). Findings from an 8-year longitudinal study using H(2)(15)O positron emission tomographic CBF suggested that CBF may increase to compensate for lower interregional neural communication resulting from white matter disruption (Kraut, Beason-Held, Elkins, & Resnick, 2008).
Given our strict inclusion criteria, our sample was comprised of cognitively unimpaired and medically healthy individuals. Our MVPA-CBF findings may have differed in a sample with greater vascular risk burden or if we had examined different brain regions. As described above, we selected a limited number of a priori ROIs to minimize the number of statistical analyses in an attempt to reduce susceptibility to type 1 errors. The specific a priori regions we examined were selected because they have been implicated in cerebrovascular dysfunction in aging and dementia (Bangen et al., 2018; Yew et al., 2017).
Given the observed associations of all light PA with CBF (but not cognition or WMH volume) and associations of MVPA with cognition and WMH volume (but not CBF), it is possible that different PA intensities have divergent associations with markers of brain health. Alterations in CBF may represent a relatively early or subtle change in cerebrovascular functioning that precedes the development of frank lesions such as WMH (Bangen et al., 2021; Zlokovic, 2011). Our findings suggest that lighter intensity PA may be more sensitive to subtle changes in cerebrovascular function (i.e., CBF), whereas MVPA may be more favorable in protecting against cognitive decline and development of frank lesions (i.e., WMH). Previous studies have reported associations between greater PA and reduced WMH volume (e.g., Tseng et al., 2013). However, in the present study, the association of MVPA and WMH was observed when we accounted for all light PA but attenuated and no longer significant when we additionally adjusted for age and accelerometer wear time. Future studies with larger samples and longitudinal data should further elucidate the association of different PA intensities with cognition, CBF, and WMH as well as potential moderators of MRI-PA associations.
This study has limitations that should be considered when interpreting our findings and should be addressed in future studies. The cross-sectional design limits our ability to make causal interpretations and the relatively small sample size reduces statistical power to detect associations. Strengths of this study include the use of a well-characterized sample of cognitively unimpaired older adults, ASL MRI to assess CBF together with quantification of WMH volume, and accelerometry to objectively measure PA as it occurs in free-living environments.
CONCLUSION
Engaging in PA may be beneficial for cerebrovascular health (WMH, CBF) with greater MPVA in particular possibly preserving memory and executive function in otherwise cognitively healthy older adults. There may be differential effects of engaging in lighter activities and MVPA on MRI markers of cerebrovascular health although this needs to be confirmed in future studies. Specifically, lighter activities may be associated with subtle alterations in CBF whereas MVPA may be more sensitive to WMH. Furthermore, randomized controlled trials that test different levels of PA intensity are needed to elucidate cause-effect associations and dose-response patterns of PA with cognitive and brain health.
Table 5.
Multiple Linear Regression Models for Association of Physical Activity with Regional CBF (N=43)
| rMFG CBF | mOFC CBF | Hippo CBF | InfTemp CBF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| β | t | p | β | t | p | β | t | p | β | t | p | |
| Model 1 | ||||||||||||
| All Light PA | .291 | 1.933 | .060 | .185 | 1.167 | .250 | .112 | .702 | .487 | .419 | 2.861 | .007 |
| MVPA | .163 | 1.086 | .284 | −.107 | −.678 | .501 | −.123 | −.774 | .444 | −.159 | −1.088 | .283 |
| Model 2 | ||||||||||||
| All Light PA | .354 | 2.280 | .028 | .237 | 1.581 | .122 | .031 | .192 | .849 | .477 | 3.372 | .002 |
| MVPA | .049 | .295 | .770 | −.239 | −1.504 | .141 | −.030 | −.176 | .861 | −.290 | −1.932 | .061 |
Abbreviations: PA = physical activity; MVPA = moderate to vigorous physical activity; rMFG = rostral middle frontal gyrus; CBF = cerebral blood flow; mOFC = medial orbitofrontal cortex; hippo = hippocampal; InfTemp = inferior temporal gyrus. Wear time denotes total time the accelerometer was worn. Model 1: All light PA and MVPA as independent variables. Model 2: Additionally adjusted for age and accelerometer wear time. Statistically significant (p < 0.05) results appear in bold font. The results for inferior temporal CBF survived correction for multiple comparisons although the finding for rostral middle frontal CBF did not.
Acknowledgements
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (K23AG049906 to ZZZ, R01AG066657 to ZZZ, and R01 AG063782 to KJB). The REDCap software system provided by the UCSD Clinical and Translational Research Center is supported by Award Number UL1TR001442 from the National Center For Research Resources. We thank David Hoagey, Kristen Kennedy, and Karen Rodrigue for sharing their methods, guidance, and code for WMH quantification. We would like to acknowledge our wonderful research participants for volunteering their time and effort to advance scientific knowledge. We thank the Wellness Initiative for Senior Enrichment (WISE) lab’s students who helped with data collection efforts. Finally, we thank the UC San Diego Shiley-Marcos Alzheimer’s Disease Research Center for their assistance with participant recruitment.
Footnotes
Disclosures
The authors have no conflicts of interest to report.
References
- 2020 Alzheimer’s disease facts and figures. (2020). Alzheimer’s and Dementia, 16(3), 391–460. 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- Ahlskog JE, Geda YE, Graff-Radford NR, & Petersen RC (2011). Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic Proceedings, Vol. 86, pp. 876–884. Elsevier Ltd. 10.4065/mcp.2011.0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnardottir NY, Koster A, van Domelen DR, Brychta RJ, Caserotti P, Eiriksdottir G, … Sveinsson T. (2016). Association of change in brain structure to objectively measured physical activity and sedentary behavior in older adults: Age, Gene/Environment Susceptibility-Reykjavik Study. Behavioural Brain Research, 296, 118–124. 10.1016/j.bbr.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asllani I, Borogovac A, & Brown TR (2008). Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magnetic Resonance in Medicine, 60(6), 1362–1371. 10.1002/mrm.21670 [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison N, & Johnson H. (2014). Advanced Normalization Tools (ANTS) Release 2.x. Retrieved from https://brianavants.wordpress.com/2012/04/13/updated-antscompile-instructions-april-12-2012/ [Google Scholar]
- Bangen KJ, Clark AL, Edmonds EC, Evangelista ND, Werhane ML, Thomas KR, … Delano-Wood L. (2017). Cerebral Blood Flow and Amyloid-β Interact to Affect Memory Performance in Cognitively Normal Older Adults. Frontiers in Aging Neuroscience, 9. 10.3389/fnagi.2017.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Preis SR, Delano-Wood L, Wolf PA, Libon DJ, Bondi MW, … Brickman AM (2018). Baseline White Matter Hyperintensities and Hippocampal Volume are Associated with Conversion from Normal Cognition to Mild Cognitive Impairment in the Framingham Offspring Study. Alzheimer Disease and Associated Disorders, 32(1), 50–56. 10.1097/WAD.0000000000000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Restom K, Liu TT, Wierenga CE, Jak AJ, Salmon DP, & Bondi MW (2012). Assessment of Alzheimer’s Disease Risk with Functional Magnetic Resonance Imaging: An Arterial Spin Labeling Study. Journal of Alzheimer’s Disease, 31(s3), S59–S74. 10.3233/JAD-2012-120292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Thomas KR, Sanchez DL, Edmonds EC, Weigand AJ, Delano-Wood L, … Initiative, for the A. D. N. (2021). Entorhinal Perfusion Predicts Future Memory Decline, Neurodegeneration, and White Matter Hyperintensity Progression in Older Adults. Journal of Alzheimer’s Disease, 81, 1711–1725. 10.3233/JAD-201474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Thomas KR, Weigand AJ, Sanchez DL, Delano-Wood L, Edmonds EC, … Bondi MW (2020). Pattern of regional white matter hyperintensity volume in mild cognitive impairment subtypes and associations with decline in daily functioning. Neurobiology of Aging, 86, 134–142. 10.1016/J.NEUROBIOLAGING.2019.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, & Yaffe K. (2011, September). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology, Vol. 10, pp. 819–828. 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett MW, Ardern CI, & Rotondi MA (2015). A meta-analysis of prospective studies on the role of physical activity and the prevention of Alzheimer’s disease in older adults. BMC Geriatrics, 15(1). 10.1186/s12877-015-0007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y. (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Best JR, Rosano C, Aizenstein HJ, Tian Q, Boudreau RM, Ayonayon HN, … LiuAmbrose T. (2017). Long-term changes in time spent walking and subsequent cognitive and structural brain changes in older adults. Neurobiology of Aging, 57, 153–161. 10.1016/j.neurobiolaging.2017.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Provenzano FA, Muraskin J, Manly JJ, Blum S, Apa Z, … Mayeux R. (2012). Regional White Matter Hyperintensity Volume, Not Hippocampal Atrophy, Predicts Incident Alzheimer Disease in the Community. Archives of Neurology, 69(12), 1621–1627. 10.1001/ARCHNEUROL.2012.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, … Mayeux R. (2015). Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiology of Aging, 36(1), 27–32. 10.1016/J.NEUROBIOLAGING.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronas UG, Steffen A, DIon C, Boots EA, Arfanakis K, Marquez DX, & Lamar M. (2019). Sedentary Time and White Matter Hyperintensity Volume in Older Adults. Medicine and Science in Sports and Exercise, 51(8), 1613–1618. 10.1249/MSS.0000000000001957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Umlauf A, Beaumont J, Gershon R, Slotkin J, Akshoomoff N, & Heaton RK (2015). Demographically Corrected Normative Standards for the English Version of the NIH Toolbox Cognition Battery. Journal of the International Neuropsychological Society, 21(5), 378–391. 10.1017/S1355617715000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, DeFina LF, Keebler MW, Didehbani N, & Lu H. (2013). Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Frontiers in Aging Neuroscience, 5. 10.3389/fnagi.2013.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi L, Liu Z, Matthews CE, & Buchowski MS (2011). Validation of accelerometer wear and nonwear time classification algorithm. Medicine and Science in Sports and Exercise, 43(2), 357–364. 10.1249/MSS.0b013e3181ed61a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, Mcauley E, … Kramer AF (2006). Exercise: An Active Route to Healthy Aging Aerobic Exercise Training Increases Brain Volume in Aging Humans. Retrieved from https://academic.oup.com/biomedgerontology/article/61/11/1166/630432 [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, … Elavsky S. (2004). Cardiovascular fitness, cortical plasticity, and aging. Retrieved from www.pnas.orgcgidoi10.1073pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SE, Marsiske M, & McCoy KJM (2009). The use of the modified telephone interview for cognitive status (Tics-M) in the detection of amnestic mild cognitive impairment. Journal of Geriatric Psychiatry and Neurology, 22(2), 103–109. 10.1177/0891988708328214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland JL, & Esliger DW (2009). Accelerometer Assessment of Physical Activity in Active, Healthy Older Adults. Journal of Aging and Physical Activity, 17(1), 17–30. 10.1123/japa.17.1.17 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical Surface-Based Analysis I. Segmentation and Surface Reconstruction. Retrieved from http://www.idealibrary.com [DOI] [PubMed] [Google Scholar]
- Debette S, & Markus HS (2010). The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ, 341(jul26 1), c3666–c3666. 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, … Wolf PA (2005). Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiology of Aging, 26(4), 491–510. 10.1016/J.NEUROBIOLAGING.2004.05.004 [DOI] [PubMed] [Google Scholar]
- Dohrn IM, Sjöström M, Kwak L, Oja P, & Hagströmer M. (2018). Accelerometermeasured sedentary time and physical activity—A 15 year follow-up of mortality in a Swedish population-based cohort. Journal of Science and Medicine in Sport, 21(7), 702–707. 10.1016/j.jsams.2017.10.035 [DOI] [PubMed] [Google Scholar]
- Domingos C, Pêgo JM, & Santos NC (2021, March 26). Effects of physical activity on brain function and structure in older adults: A systematic review. Behavioural Brain Research, Vol. 402. Elsevier B.V. 10.1016/j.bbr.2020.113061 [DOI] [PubMed] [Google Scholar]
- Dougherty RJ, Boots EA, Lindheimer JB, Stegner AJ, van Riper S, Edwards DF, … Cook DB (2020). Fitness, independent of physical activity is associated with cerebral blood flow in adults at risk for Alzheimer’s disease. Brain Imaging and Behavior, 14(4), 1154–1163. 10.1007/s11682-019-00068-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, Alperovitch A, & Tzourio C. (2003). Influence of education on the relationship between white matter lesions and cognition. Neurology, 60(5), 831–836. 10.1212/01.WNL.0000049456.33231.96 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, … Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. (2009). Discovering statistics using SPSS (3rd ed.). Sage Publications. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron, 33(3), 341–355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Full KM, Kerr J, Grandner MA, Malhotra A, Moran K, Godoble S, … Soler X. (2018). Validation of a physical activity accelerometer device worn on the hip and wrist against polysomnography. Sleep Health, 4(2), 209–216. 10.1016/j.sleh.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitán JM, Moon HY, Stremlau M, Dubal DB, Cook DB, Okonkwo OC, & van Praag H. (2021). Effects of Aerobic Exercise Training on Systemic Biomarkers and Cognition in Late Middle-Aged Adults at Risk for Alzheimer’s Disease. Frontiers in Endocrinology, 12. 10.3389/fendo.2021.660181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gons RAR, van Norden AGW, de Laat KF, van Oudheusden LJB, van Uden IWM, Zwiers MP, … de Leeuw F-E (2011). Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain, 134(7), 2116–2124. 10.1093/brain/awr145 [DOI] [PubMed] [Google Scholar]
- Gow AJ, Bastin ME, Munoz Maniega S, Valdes Hernandez MC, Morris Z, Murray C, … Wardlaw JM (2012). Neuroprotective lifestyles and the aging brain: Activity, atrophy, and white matter integrity. Neurology, 79(17), 1802–1808. 10.1212/WNL.0b013e3182703fd2 [DOI] [PubMed] [Google Scholar]
- Haeger A, Costa AS, Schulz JB, & Reetz K. (2019, January 1). Cerebral changes improved by physical activity during cognitive decline: A systematic review on MRI studies. NeuroImage: Clinical, Vol. 23. Elsevier Inc. 10.1016/j.nicl.2019.101933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart TL, Swartz AM, Cashin SE, & Strath SJ (2011). How many days of monitoring predict physical activity and sedentary behaviour in older adults? International Journal of Behavioral Nutrition and Physical Activity, 8(1), 62. 10.1186/1479-5868-862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Hayes JP, Cadden M, & Verfaellie M. (2013). A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Frontiers in Aging Neuroscience, 5. 10.3389/fnagi.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton J. (2004). Reworking qualitative data. Sage. [Google Scholar]
- Heaton RK, Nelson LM, Thompson DS, Burks JS, & Franklin GM (1985). Neuropsychological findings in relapsing-remitting and chronic-progressive multiple sclerosis. Journal of Consulting and Clinical Psychology, 53(1), 103–110. 10.1037/0022-006X.53.1.103 [DOI] [PubMed] [Google Scholar]
- Hoagey DA, Lazarus LTT, Rodrigue KM, & Kennedy KM (2021). The effect of vascular health factors on white matter microstructure mediates age-related differences in executive function performance. Cortex, 141, 403–420. 10.1016/j.cortex.2021.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, & Petersen RC (1996). Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, STROOP, TMT, and JLO. Clinical Neuropsychologist, 10(3), 262–278. 10.1080/13854049608406689 [DOI] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, & Delis DC (2009). Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry, 17(5), 368–375. 10.1097/JGP.0b013e31819431d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome GJ, Young DR, Laferriere D, Chen C, & Vollmer WM (2009). Reliability of RT3 Accelerometers among Overweight and Obese Adults. Medicine & Science in Sports & Exercise, 41(1), 110–114. 10.1249/MSS.0b013e3181846cd8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PJ, Mensink RP, Adam TC, & Liu TT (2018, May 1). Cerebral blood flow measurements in adults: A review on the effects of dietary factors and exercise. Nutrients, Vol. 10. MDPI AG. 10.3390/nu10050530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy G, Hardman RJ, MacPherson H, Scholey AB, & Pipingas A. (2016). How Does Exercise Reduce the Rate of Age-Associated Cognitive Decline? A Review of Potential Mechanisms. Journal of Alzheimer’s Disease, 55(1), 1–18. 10.3233/JAD-160665 [DOI] [PubMed] [Google Scholar]
- Kerr J, Rosenberg D, Millstein RA, Bolling K, Crist K, Takemoto M, … Buchner D. (2018). Cluster randomized controlled trial of a multilevel physical activity intervention for older adults. International Journal of Behavioral Nutrition and Physical Activity, 15(1). 10.1186/s12966-018-0658-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinloog JPD, Mensink RP, Ivanov D, Adam JJ, Uludağ K, & Joris PJ (2019). Aerobic Exercise Training Improves Cerebral Blood Flow and Executive Function: A Randomized, Controlled Cross-Over Trial in Sedentary Older Men. Frontiers in Aging Neuroscience, 11. 10.3389/fnagi.2019.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut MA, Beason-Held LL, Elkins WD, & Resnick SM (2008). The Impact of Magnetic Resonance Imaging-Detected White Matter Hyperintensities on Longitudinal Changes in Regional Cerebral Blood Flow. Journal of Cerebral Blood Flow & Metabolism, 28(1), 190–197. 10.1038/sj.jcbfm.9600512 [DOI] [PubMed] [Google Scholar]
- Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TLS, … Brickman AM (2016). White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Annals of Neurology, 79(6), 929–939. 10.1002/ANA.24647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, … Mukadam N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413–446. 10.1016/S01406736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, … Düzel E. (2015). Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular Psychiatry, 20(5), 585–593. 10.1038/mp.2014.114 [DOI] [PubMed] [Google Scholar]
- Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, … DeCarli C. (2012). Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. The Lancet Neurology, 11(12), 1039–1047. 10.1016/S1474-4422(12)70241-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, & Burdette JH (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21(1), 450–455. 10.1016/j.neuroimage.2003.09.032 [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- MathWorks I. (1996). MATLAB: The Language of Technical Computing: Computation, Visualization, Programming. Installation Guide for UNIX Version 5. Math Works Incorporated. [Google Scholar]
- Moon SY, de Souto Barreto P, Cesari M, Chupin M, Mangin JF, Bouyahia A, … Vellas B. (2018). Physical Activity and Changes in White Matter Hyperintensities over Three Years. Journal of Nutrition, Health and Aging, 22(3), 425–430. 10.1007/s12603-017-0959-3 [DOI] [PubMed] [Google Scholar]
- Moriya Y, Kozaki K, Nagai K, & Toba K. (2009). Attenuation of Brain White Matter Hyperintensities after Cerebral Infarction. American Journal of Neuroradiology, 30(3), e43–e43. 10.3174/ajnr.A1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris Z, Whiteley WN, Longstreth WT, Weber F, Lee Y-C, Tsushima Y, … AlShahi Salman R. (2009). Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ, 339(aug17 1), b3016–b3016. 10.1136/bmj.b3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolini C, Fahnestock M, Gibala MJ, & Nelson AJ (2021). Understanding the Neurophysiological and Molecular Mechanisms of Exercise-Induced Neuroplasticity in Cortical and Descending Motor Pathways: Where Do We Stand? Neuroscience, 457, 259–282. 10.1016/j.neuroscience.2020.12.013 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Sanders LMJ, Hortobágyi T, Gemert S. la B. van, van der Zee, E. A., & van Heuvelen, M. J. G. (2019, January 1). Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. PLoS ONE, Vol. 14. Public Library of Science. 10.1371/journal.pone.0210036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P. (2017, January). Bayesian inference for structured additive regression models for large-scale problems with applications to medical imaging. Ludwig-Maximilians-Universität München. Retrieved from http://nbn-resolving.de/urn:nbn:de:bvb:19-203731 [Google Scholar]
- Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, & Johansen-Berg H. (2016). A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. NeuroImage, 131, 81–90. 10.1016/j.neuroimage.2015.09.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DD, Ozyurt IB, & Liu TT (2013). The Cerebral Blood Flow Biomedical Informatics Research Network (CBFBIRN) database and analysis pipeline for arterial spin labeling MRI data. Frontiers in Neuroinformatics, 7(OCT). 10.3389/fninf.2013.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, … Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Soldan A, Alfini A, Pettigrew C, Faria A, Hou X, Lim C, … Albert M. (2022). Actigraphy-estimated physical activity is associated with functional and structural brain connectivity among older adults. Neurobiology of Aging, 116, 32–40. 10.1016/j.neurobiolaging.2022.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartano NL, Demissie S, Himali JJ, Dukes KA, Murabito JM, Vasan RS, … Seshadri S. (2019). Accelerometer-determined physical activity and cognitive function in middle-aged and older adults from two generations of the Framingham Heart Study. Alzheimer’s and Dementia: Translational Research and Clinical Interventions, 5, 618–626. 10.1016/j.trci.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres ER, Strack EF, Fernandez CE, Tumey TA, & Hitchcock ME (2015). Physical activity and white matter hyperintensities: A systematic review of quantitative studies. Preventive Medicine Reports, 2, 319–325. 10.1016/j.pmedr.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigiani LJ, & Hamel E. (2017). An endothelial link between the benefits of physical exercise in dementia. Journal of Cerebral Blood Flow & Metabolism, 37(8), 2649–2664. 10.1177/0271678X17714655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, & McDowell M. (2008). Physical Activity in the United States Measured by Accelerometer. Medicine & Science in Sports & Exercise, 40(1), 181–188. 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- Trost SG, Mciver KL, & Pate RR (2005). Conducting Accelerometer-Based Activity Assessments in Field-Based Research. Medicine & Science in Sports & Exercise, 37(11), S531–S543. 10.1249/01.mss.0000185657.86065.98 [DOI] [PubMed] [Google Scholar]
- Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, … Zhang R. (2013). White matter integrity in physically fit older adults. NeuroImage, 82, 510–516. 10.1016/j.neuroimage.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Johnson DK, Morris JK, van Sciver A, Greer CS, Billinger SA, … Burns JM (2015). Dose-response of aerobic exercise on cognition: A community-based, pilot randomized controlled trial. PLoS ONE, 10(7). 10.1371/journal.pone.0131647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent GK, & Velkoff VA (2010). THE NEXT FOUR DECADES The Older Population in the United States: 2010 to 2050 Population Estimates and Projections Current Population Reports. Retrieved from www.census.gov/population/www [Google Scholar]
- Voelcker-Rehage C, Godde B, & Staudinger UM (2011). Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Frontiers in Human Neuroscience, (MARCH). 10.3389/fnhum.2011.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Chappell FM, Valdés Hernández M. del C., Makin SDJ, Staals J, Shuler K, … Dennis MS (2017). White matter hyperintensity reduction and outcomes after minor stroke. Neurology, 89(10), 1003–1010. 10.1212/WNL.0000000000004328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Valdés Hernández MC, & Muñoz-Maniega S. (2015). What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. Journal of the American Heart Association, 4(6), 1140. 10.1161/JAHA.114.001140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhane ML, Thomas KR, Bangen KJ, Weigand AJ, Edmonds EC, Nation DA, … Delano-Wood L. (2021). Arterial Stiffening Moderates the Relationship Between Type2 Diabetes Mellitus and White Matter Hyperintensity Burden in Older Adults With Mild Cognitive Impairment. Frontiers in Aging Neuroscience, 13. 10.3389/fnagi.2021.716638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Dev SI, Shin DD, Clark LR, Bangen KJ, Jak AJ, … Bondi MW (2012). Effect of Mild Cognitive Impairment and APOE Genotype on Resting Cerebral Blood Flow and its Association with Cognition. Journal of Cerebral Blood Flow & Metabolism, 32(8), 1589–1599. 10.1038/jcbfm.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CG, Thomas KR, Edmonds EC, Weigand AJ, Bangen KJ, Eppig JS, … Bondi MW (2019). Neuropsychological criteria for mild cognitive impairment in the framingham heart study’s old-old. Dementia and Geriatric Cognitive Disorders, 46(5–6), 253–265. 10.1159/000493541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Mathiason MA, Han S, Gunter JL, Jones D, Botha H, & Jack C. (2021). Mechanistic Effects of Aerobic Exercise in Alzheimer’s Disease: Imaging Findings From the Pilot FIT-AD Trial. Frontiers in Aging Neuroscience, 13. 10.3389/fnagi.2021.703691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, & Smith S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
- Zlatar ZZ, Godbole S, Takemoto M, Crist K, Sweet CMC, Kerr J, & Rosenberg DE (2019). Changes in Moderate Intensity Physical Activity Are Associated With Better Cognition in the Multilevel Intervention for Physical Activity in Retirement Communities (MIPARC) Study. American Journal of Geriatric Psychiatry, 27(10), 1110–1121. 10.1016/j.jagp.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatar ZZ, Hays CC, Mestre Z, Campbell LM, Meloy MJ, Bangen KJ, … Wierenga CE (2019). Dose-dependent association of accelerometer-measured physical activity and sedentary time with brain perfusion in aging. Experimental Gerontology, 125. 10.1016/j.exger.2019.110679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B. v. (2011). Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neuroscience 2011 12:12, 12(12), 723–738. 10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]