Abstract
Mitochondria have cell-type specific phenotypes, perform dozens of interconnected functions and undergo dynamic and often reversible physiological recalibrations. Given their multifunctional and malleable nature, the frequently used terms ‘mitochondrial function’ and ‘mitochondrial dysfunction’ are misleading misnomers that fail to capture the complexity of mitochondrial biology. To increase the conceptual and experimental specificity in mitochondrial science, we propose a terminology system that distinguishes between (1) cell-dependent properties, (2) molecular features, (3) activities, (4) functions and (5) behaviours. A hierarchical terminology system that accurately captures the multifaceted nature of mitochondria will achieve three important outcomes. It will convey a more holistic picture of mitochondria as we teach the next generations of mitochondrial biologists, maximize progress in the rapidly expanding field of mitochondrial science, and also facilitate synergy with other disciplines. Improving specificity in the language around mitochondrial science is a step towards refining our understanding of the mechanisms by which this unique family of organelles contributes to cellular and organismal health.
To guide scientific progress, medicine has developed a detailed cartography of the multiple functions accomplished by unique body parts and organ systems. The resulting classification system of physiological functions displayed in every medical textbook is the foundation of modern medicine1 (Fig. 1a). Likewise, cell biology recognizes dozens of distinct cellular functions and behaviours that interact to sustain organismal health (Fig. 1b). This detailed cartography of cellular functions and behaviours has guided our theories around how cells divide and differentiate, communicate and interact with one another, has led to discoveries of disease mechanisms and has motivated the development of laboratory methods and specialized instruments to capture the multifaceted nature of different types of living cells. Specificity begets biological insights.
Fig. 1 |. An integrative approach to mitochondrial biology.

a–c, There are organ-specific domains of human health that guide medical investigation and practice (a), cell-type-specific domains of cell biology that guide biological theories and research practices (b) and domains of mitochondrial biology that guide mitochondrial science (c). d, Blind men–here scientists blindfolded by their perspective limited by prevailing theories, training and career history, available instrumentation and/or analytical methods–make valid observations about specific aspects of the animal under study. However, without a global perspective enabling them to perceive the whole animal at once, an error of logic is committed, leading to erroneous induction and conclusions, drawn from its parts, about the nature of the whole organism. e, The same type of limitation exists when examining mitochondria with different instruments that are blind to other aspects of mitochondrial biology, for example, microscopy, biochemistry, genomics and metabolomics. Focused reductionist approaches are necessarily undertaken blind to other domains of function or behaviour, yielding partially valid conclusions and, when taken in isolation, a largely inaccurate picture of the system/organelle. The history of mitochondrial research illustrates the need for interdisciplinary approaches and sufficiently specific terminology to examine mitochondrial biology and its contribution to human health. ROS, reactive oxygen species; MDVs, mitochondria-derived vesicles; TEM, transmission electron microscopy; Cyt c, cytochrome c.
As for organs and cells, several productive decades of mitochondrial research have shown that mitochondria are similarly multifaceted (Fig. 1c). However, despite considerable advances in establishing the molecular machinery and functional diversity among mitochondria, we lack a systematic nomenclature to reflect the full spectrum of functional, morphological and molecular domains of mitochondrial biology. As mitochondria have become the most studied organelle across the biomedical sciences2, establishing a logical framework to teach and study the multifaceted nature of mitochondria becomes paramount. In this Perspective, we examine how infusing further specificity in our classification system for mitochondrial science can propel the field towards increasingly meaningful insights into human health.
The need for specificity in mitochondrial science
The powerful ‘powerhouse of the cell’ analogy3 does not capture the multifunctional and malleable nature of these fantastic organelles; the analogy is too simplistic and incomplete (Fig. 1d,e). Overly simplistic analogies risk impeding progress in mitochondrial science in two main ways. First, incomplete analogies like the ‘powerhouse’ create a monofunctional image of these complex organelles. This narrow mental image limits the spectrum of possible mechanisms by which mitochondria may contribute to the organism’s biology and physiology. In the same way that all cells are recognizable by shared gross anatomical features (for example, cell membrane and nucleus), mitochondria share a recognizable double membrane structure, cristae and a circular genome. Yet at a higher level of resolution, cell subtypes and their mitochondrial subtypes both qualitatively and quantitatively differ in their specific molecular features, activities, functions and behaviours. Unlike powerhouses whose sole function is energy transformation, mitochondria are multifaceted and multifunctional.
The second way in which outdated analogies can impede the field’s progress is by shaping terminology4. For established investigators with decades of historical perspective, some concepts may transcend terminology. However, to newcomers in the field, terminology is the basis of understanding. A powerhouse transforms energy, and the only way in which powerhouses can be ‘dysfunctional’ is by exhibiting diminished energy production capacity. But mitochondria are multifunctional, performing dozens of different functions (see below). As a result, they can malfunction in multiple different ways. Mitochondria also are malleable, capable of dynamically and reversibly adapting to energetic, environmental and other stressors5–7. Temporary increases or decreases among molecular features and functions are the basis of adaptation–not necessarily signs of dysfunctional processes. Thus, letting go and clearing the mind of the simplistic analogy challenges the concept of ‘dysfunction’ and allows investigators to intuitively interpret dynamic changes more physiologically, and therefore more accurately.
Studying ‘human function’ or ‘cellular function’ would strikingly lack specificity and meaning for medicine and cell biology. Similarly, ‘mitochondrial function’ is devoid of the specificity required to advance our science. For the two reasons noted above, we are forced to conclude that the terms ‘mitochondrial function’ and ‘mitochondrial dysfunction’ are flawed, misleading misnomers, and should generally be avoided. To formulate accurate models and hypotheses about the mechanisms in which mitochondrial biology contributes to complex cellular and organismal processes, mitochondrial science needs more specific terminology.
In this Perspective, we first provide quantitative examples of the diversity of mitochondrial phenotypes, highlighting how mitochondria in different organs and cell types molecularly, functionally and morphologically specialize. We then propose a general conceptual framework and hierarchical terminology system to help guide the evolving field of mitochondrial science. Our collective goal should be to develop a logical framework that describes the many ways in which mitochondria dynamically adapt to perturbations, become mismatched to their metabolic environment or acquire specific defects that manifest into one or more quantifiable aspects of mitochondrial biology. The proposed framework can help guide both our theories and measurement approaches to capture the rich diversity of mitochondrial functions, and thus elucidate how they shape health or contribute to disease states.
Mitochondria are multifunctional and essential for life
As a family of organelles, mitochondria perform several functions beyond oxidative phosphorylation (OxPhos) that are essential to multicellular life. Table 1 provides a list of known mitochondrial functions and mitochondrial behaviours. In the same way that all cell types share some fundamental functions (for example, gene expression, protein synthesis and signal transduction), all mitochondria harbour the core OxPhos system. The OxPhos system is a set of five multi-protein complexes, four of which–known as the electron transport chain (ETC)–funnel free electrons from reducing equivalents to molecular oxygen, harnessing in the process the resulting free energy (ΔG) to build an electrochemical gradient (ΔΨm + ΔpH) across the inner mitochondrial membrane (IMM). Electrons are also being injected into the ETC through non-canonical sources including the glycerol phosphate shuttle8 and other enzymes in specific cell types9. The resulting transmembrane potential is the driving force for several mitochondrial functions, including the uptake of ions (Ca2+, Na+, Mn2+ and others)10, import of nuclear-encoded proteins and pre-proteins11, synthesis of iron–sulfur (Fe/S) clusters12–14, antioxidant defences regenerated by nicotinamide nucleotide transhydrogenase (encoded by NNT)15, antiviral signalling16–19, ATP synthesis by the FoF1 ATP synthase (complex V)20, among many others. Membrane potential is also required to maintain the integrity of the mitochondrial permeability transition pore21, to regulate crista shape and for mitochondrial fusion/fission dynamics22.
Table 1 |.
Mitochondrial functions and behaviours
| Description | Reviewed in ref(s). | Methods described in ref(s). | |||
|---|---|---|---|---|---|
| Functions | |||||
| a Membrane potential generation | Formation of the electrochemical gradient (ΔΨm+ΔpH) across the IMM, usually by the electron pumping capacity of the respiratory complexes I, III and IV, but also by other processes including through ATP hydrolysis by the FoF1 ATP synthase (complex V). | 104 | 105,106 | ||
| Amino acid metabolism | Lysine metabolism (lysine-α-ketoglutarate reductase, encoded by AASS). Electrogenic malate–aspartate shuttle system, which is important for balancing pyridine dinucleotide redox states across subcellular compartments. Branched-chain keto and amino acids. Choline and derivatives as structural precursors for lipoproteins, membrane lipids and the neurotransmitter acetylcholine. Betaine as osmoregulator and an intermediate in the cytosolic transulfuration pathway. | 107–111 | 112–119 | ||
| Ascorbate metabolism | l-ascorbate (vitamin C) biosynthesis in many plants and animals, but not in primates, which serves as osmoregulator and antioxidant. Mitochondria may recycle oxidized (dehydro)ascorbic acid. | 120 | 121,122 | ||
| Bicarbonate metabolism | Production of bicarbonate (HCO3−) by mitochondrial carbonic anhydrase V (encoded by CA5A), used as a cofactor for anaplerotic reactions (for example, ureagenesis and gluconeogenesis) and acid–base balance. The TCA cycle is an important contributor to cellular/extracellular acidification due to CO2 production. | 123 | – | ||
| Calcium uptake and extrusion | Uptake of cytoplasmic Ca2+ via the mitochondrial calcium uniporter in a ΔΨm-dependent manner; extrusion by the sodium/calcium exchanger NCLX (encoded by SLC8B1). | 124–126 | 127,128 | ||
| Hydrogen sulfide detoxification | Mitochondrial sulfide quinone oxidoreductase (encoded by SQOR) oxidizes hydrogen sulfide to glutathione persulfide by reducing CoQ. | 129–132 | 133 | ||
| Heat production | Heat generation is stimulated by uncoupling ΔΨm+ΔpH from ATP synthesis (thereby increasing electron flux and respiration) by UCP1 (encoded by UCP1), the ADP/ATP carrier (AAC, also ANT1), or by creatine-dependent substrate cycling and other futile cycles. | 134–137 | 138 | ||
| Intermediate metabolism | Enzymatic interconversion of metabolic intermediates to enable the synthesis of specific macromolecules, including five major anaplerotic ones. This includes the conversion of pyruvate into oxaloacetate by pyruvate carboxylase (encoded by PC), a critical step for de novo glucose synthesis (gluconeogenesis); citrate export to the cytoplasm where it is used for lipid synthesis or converted to acetyl-CoA for acetylation reactions; synthesis of itaconate, a derivative of cis-aconitate; succinate, α-ketoglutarate and others that participate in a variety of signalling process. | 25,139,140 | 141,142 | ||
| Fe/S cluster synthesis | Synthesis of Fe/S clusters, which serve as prosthetic groups of several essential proteins. | 12–14 | 143 | ||
| Light focusing | Mitochondria in the outer segment of the retinal photoreceptors acts as a ‘microlens’ that focuses incoming photons, increasing visual resolution. | 144 | – | ||
| Lipid oxidation | Beta-oxidation of long-chain, medium-chain and short-chain fatty acids into acetyl-CoA. | 145 | 146 | ||
| Lipid synthesis | Synthesis of cardiolipin and phosphatidylethanolamine from ER precursors in the IMM. | 147–150 | – | ||
| mtDNA maintenance and expression | mtDNA replication, transcription, protein synthesis and assembly of the OxPhos system. | 151,152 | 153,154 | ||
| Na+import/export | Sodium (Na+) uptake and release against cytoplasmic Ca2+ by the sodium/calcium exchanger protein NCLX (encoded by SLC8B1) or by Na+/H+ antiporter (molecular identity pending). | 124,155 | 156 | ||
| Neurotransmitter synthesis and degradation | Synthesis of the cofactor BH4 (tetrahydrobiopterin), used by hydrolase enzymes to synthesize catecholamines and neurotransmitters (serotonin, melatonin, norepinephrine and epinephrine) and nitric oxide. Mitochondria with OMM-anchored monoamine oxidases (encoded by MAOA and MAOB, donate electrons and contribute to electron flow in the ETC) also degrade catecholamines. Mitochondria also participate in GABA metabolism. | 9,157 | 158,159 | ||
| One-carbon metabolism and pyrimidine synthesis | The one-carbon metabolism connects the synthesis of nucleotides (purine and pyrimidine), amino acids (methionine, serine and glycine), S-adenosyl-methionine and folate. Ubiquinone-mediated oxidation of dihydroorotate to orotate by dihydroorotate dehydrogenase (encoded by DHODH) is a key step in pyrimidine synthesis. | 160–163 | 164 | ||
| OxPhos | Transduction of generated by the electron transport chain (ETC, also ‘respiratory chain’) into ATP synthesis by the ATP synthase (complex V), abbreviated as OxPhos. | 165 | 166 | ||
| Oxygen sensing | The electron transport and free-radical generation by ETC complexes I and III is modulated by the partial pressure of oxygen, which can limit respiration at very low partial pressures of O2. | 167–170 | – | ||
| Permeability transition | Opening of the high-conductance permeability transition pore (PTP), which dissipates membrane potential and promotes the release of intracristae and matrix-located components into the cytoplasm. | 171,172 | 173–175 | ||
| Protein import | Import, processing and folding of nuclear-encoded polypeptides from the cytoplasm by the translocator of the inner membrane (TIM) and outer membrane (TOM) complexes and associated proteins. | 176 | – | ||
| Redox homeostasis | Re-oxidation of enzymes and/or their redox cofactors (involved in anabolic and catabolic reactions) by the electron acceptors CoQ and cytochrome c (encoded by CYTC) within the mitochondrial respiratory chain, and production of NADPH by NNT. | 177,178 | – | ||
| Respiration | Electrons stored in reducing equivalents NADH and FADH2, or derived from diverse redox reactions are sequentially delivered to respiratory complex I and CoQ, or cytochrome c, respectively, to promote the reduction of molecular oxygen at cytochrome c oxidase (complex IV). | 179,180 | 181 | ||
| ROS production | Production and release of ROS (H2O2, O2•−, others) mainly at respiratory chain complexes I and III. | 182,183 | 184 | ||
| Steroidogenesis | Production of pregnanolone from cholesterol imported via IMM steroidogenic acute regulatory protein (encoded by STAR) followed by enzymatic transformation by P450ssc (encoded by CYP11A1) in the matrix. Intermediate or terminal steps for some steroids occur in the ER. Cytochrome P450 family members participate also in xenobiotic metabolism as well as bile acid and vitamin D biosynthesis. | 33,34,185,186 | 187 | ||
| Behaviours | |||||
| Antiviral signalling | Assembly of the mitochondrial antiviral signal (encoded by MAVS) adaptor protein on the OMM to potentiate downstream signalling, and activation of nuclear interferon pathways in the nucleus by mtDNA release. | 39,188 | – | ||
| Apoptotic signalling | Release of cytochrome c (encoded by CYCS), apoptosis-inducing factor (encoded by AIF), and other proteins that trigger different forms of cell death by acting on cytoplasmic and nuclear effectors. | 189,190 | – | ||
| Cristae remodelling | Dynamic remodelling of IMM cristae junctions, cristae shape and distribution via the combined action of optic atrophy 1 (encoded by OPA1) and mitochondrial contact site and cristae organizing system (MICOS) proteins. | 103,191 | 95 | ||
| DNA signalling | mtDNA extrusion in the cytoplasm, particularly in the form of oxidized mtDNA fragments via proteinaceous pores forming across the IMM and OMM, which trigger inflammasome activation. | 189,190,192,193 | 175 | ||
| Epigenetic remodelling | Transduction of mitochondrial states into changes in epigenome via several functions including metabolic intermediates, DNA release, ROS production and others. | 30,194 | – | ||
| Inter-organelle communication | Exchange of information between mitochondria and other organelles, particular the ER, where mitofusin 2 (encoded by MFN2) plays a key role in tethering organelles. | 195,196 | 197,198 | ||
| Mitochondrial dynamics | Mitochondrial fusion and fission through OMM-anchored and IMM-anchored GTPase proteins capable of merging or constricting mitochondrial membranes to enact fragmentation of larger organelles into smaller ones. |
191,199–201 | 202 | ||
| Mito–mito communication | Exchange of information between mitochondria by soluble signals (for example, ROS-induced ROS release, RIRR), by complete membrane fusion, or by physical extensions of thin protein-carrying OMM and IMM membrane protrusions (that is, nanotunnels) and trans-mitochondrial cristae alignment between energized mitochondria. | 203–206 | 207–209 | ||
| Motility | Movement of energized mitochondria across the cytoplasm via the combined action of motor and adaptor proteins interacting with cytoskeletal elements. | 6,210 | 211 | ||
| Vesicle formation | Release of MDVs destined to different cellular fates by the action of motor and accessory proteins acting on the OMM and IMM. | 212 | 213,214 | ||
Generation of mitochondrial membrane potential is the ‘mother’ of many other functions and behaviours, providing the driving force for the movement of ions, solutes and proteins across the IMM, the driving force for key enzymes and processes, including the phosphorylation of ADP into ATP (OxPhos). Mitochondrial features (that is, molecular components) and activities (individual enzyme and non-enzymatic activities) are too numerous to be comprehensively listed, so only functions and behaviours are included. CoQ, coenzyme Q.
In experimental settings, cells can survive without OxPhos, but cannot survive without energized mitochondria. The fundamental function that requires mitochondria but not OxPhos is Fe/S cluster synthesis23. Fe/S clusters are essential to dozens of enzymatic reactions within the cell, and Fe/S cluster synthesis is in fact maintained in cultured cells whose mitochondria have lost their OxPhos capability24.
Biosynthetic mitochondria
Beyond energy production and Fe/S cluster biosynthesis, mitochondria also perform anaplerosis25. Tricarboxylic acid (TCA) cycle flux connects cataplerosis and anaplerosis25 and release of metabolites (for example, citrate) used in the cytoplasm for the synthesis of lipids and other macromolecules26. Like cells that exhibit metabolic substrate preferences (for example, neurons do not oxidize lipids, mainly lactate and ketone bodies), mitochondria catabolize various substrates (for example, pyruvate, glutamine, fatty acids and other amino acids) with different affinities27. The biosynthetic capacity of some specialized mitochondria contributes to producing molecules required for cell growth and division (for example, nucleotides, amino acid and haem)27, and also to synthesizing endocrine signalling molecules released systemically2.
Signalling mitochondria
Mitochondria are systemic signalling hubs2. They contribute to transducing information both within and between cells28,29. Inside the cell, they release metabolites for signalling in the nucleus where mitochondria-derived molecules are the required substrates and cofactors of epigenetic modifications on the DNA and histones30,31. Succinate for instance is an obligatory metabolic intermediate produced in the mitochondrial matrix, which acts both intracellularly and as an intercellular messenger32. Some mitochondria also specialize in producing systemic signals. Mitochondria in the gonads and adrenal cortex specialize in steroidogenesis, the production of broad-acting messengers including the sex-defining hormones (oestrogens, progesterone and testosterone), and the glucocorticoids and mineralocorticoids that ensure preparedness to metabolic and psychological stressors33,34. In turn, some mitochondria sense extracellular paracrine and endocrine inputs, including succinate, steroid hormones and other molecular signals using DNA-binding receptors and G-protein-coupled receptors on their surface35–38 (reviewed in ref. 2). Organisms are complex communication networks composed of specialized mitochondrial phenotypes.
Mitochondrial signalling also takes other more complete forms involving intercellular mitochondrial transfer. Molecular mitochondrial components, including the mitochondrial DNA (mtDNA), can be incorporated in extracellular vesicles and exosomes39, released as cell-free mtDNA40 and induce responses in target/recipient cells39. Moreover, whole functional mitochondria are exchanged between adjacent cells during normal development41, in response to stress between tissues42,43 and between brain cell types44,45, and as part of normal tissue maintenance in the heart46. Whole mitochondria are also found at high levels in the human circulation47, although their origin and destination(s) remain unclear. The extent of the intercellular cross-talk between mitochondria, and the influence of these processes on the maintenance of health largely remain to be determined.
Mapping mitochondrial diversity across tissues and cell types
Discovering mitochondrial diversity
Mitochondria are remarkably pleiotropic. The notion that mitochondria specialize into distinct tissue-specific mitochondrial phenotypes was first illustrated in two main ways: by imaging mitochondria with different Ca2+ dynamics across two main cytoplasmic compartments in cultured pancreatic acinar cells48, and by quantifying proteomic differences among isolated mitochondria from rat brain, heart, liver and kidney49,50. Subsequent expansion of this latter effort led to Mito-Carta, a curated compendium of mitochondrial proteins in mice that revealed both quantitative and qualitative tissue-based mitochondrial differences51. This dataset, now in its third iteration as MitoCarta3.0 (ref. 52) and other high-confidence databases of mitochondrial proteins (for example, MitoCoP53), have provided rich resources for studying over a hundred biochemical pathways and discovering dozens of new mitochondrial proteins.
In parallel with the elucidation of the mitochondrial proteome, the field has discovered substantial intracellular morphological and molecular heterogeneity of both genetic and non-genetic origin across different cell types and tissues54–59. Mitochondria from the mouse brown adipose tissue, heart, liver and kidney exhibit large variation in the expression and biochemical activity of different OxPhos components and matrix dehydrogenases (which, in turn, shape their differential ability to oxidize different carbon substrates)60,61. Together with our understanding of the divergent bioenergetic requirements and physiological functions of different organ systems62, a systematic understanding of mitochondrial specialization–at the molecular, functional and morphological levels–provides a basis to specify how mitochondrial phenotypes in different organs, cell types and subcellular compartments fulfil specific cellular and organismal functions2.
Next, we provide examples where–as for cell types–mitochondrial phenotypes are quantitatively defined by their (1) functional characteristics, and (2) morphological and ultrastructural features.
Diversity in mitochondrial functions
Mitochondria differ between tissues, cell types and subcellular compartments. Here we provide four well-defined examples of mitochondrial functional specialization.
Blood leucocyte mitochondrial phenotypes.
All leucocytes are derived from the same haematopoietic stem cells but differentiate in a metabolism-instructed manner63 into distinct lineages, cell types and cell subsets that perform unique roles within the organism (Fig. 2a). Two main lineages exist: innate and adaptive immune cells. Within each lineage, say the adaptive lineage, both B and T lymphocytes are produced, and T cells differentiate into at least CD4+helper and CD8+cytotoxic cells. Each subtype also exists in their naïve state (not yet exposed to antigens), in an activated (effector) or in a memory state (holding the memory of antigen exposure).
Fig. 2 |. Different cell types and subcellular compartments contain functionally specialized mitochondrial phenotypes.
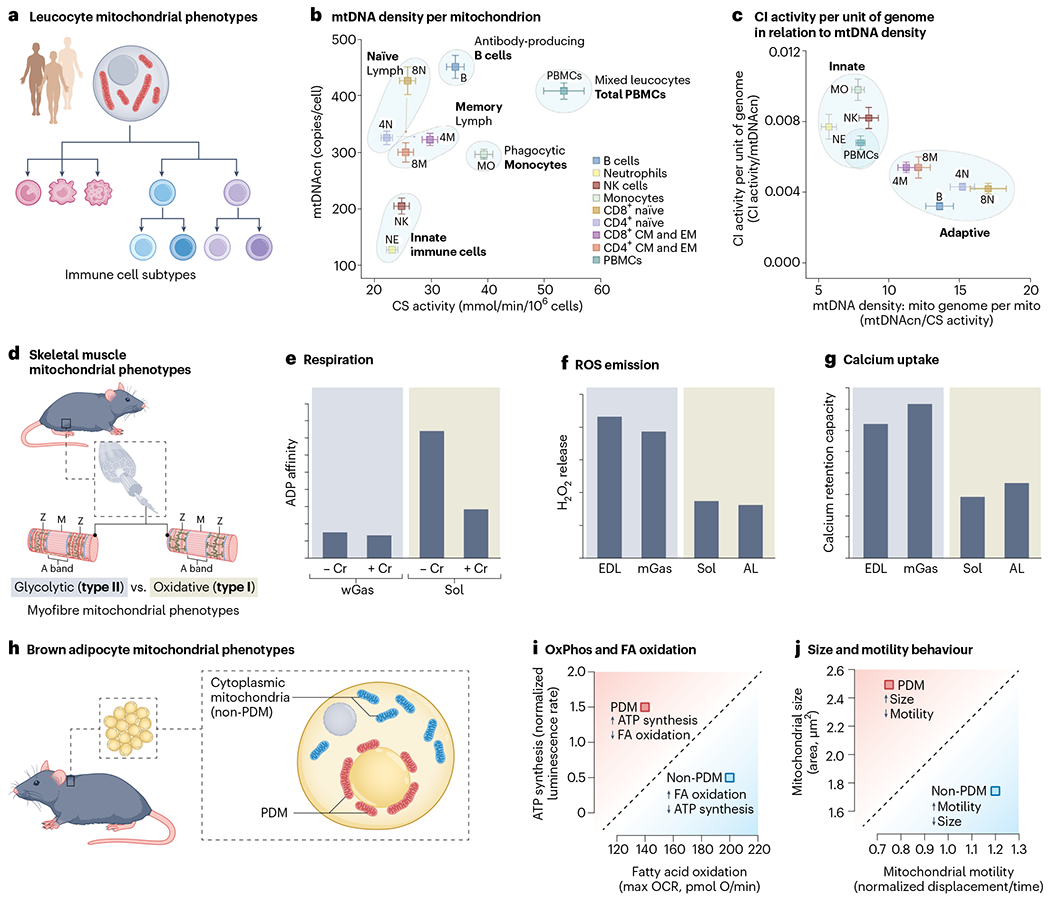
a, Despite originating from the same progenitor haematopoietic stem cell, differentiated human circulating leucocyte subtypes exhibit distinct mitochondrial content and respiratory chain properties. b,c, Bivariate plots illustrating quantitative differences in mtDNAcn, CS activity and OxPhos complex I enzymatic activity. Data for eight different human cell subtypes, plus the heterogeneous cell mixture in peripheral blood mononuclear cells (PBMCs), were isolated using FACS. Data are from ref. 59. Note the clustering of similar mitochondrial phenotypes according to known immunological and immunometabolic differences between naïve and memory cells, or between cell subtypes belonging to the innate and adaptive arms. d, Functional differences among rat skeletal muscle mitochondria between glycolytic (type II, fast-twitch) and oxidative (type I, slow-twitch) muscle fibres. e–g, Three mitochondrial functions are shown: the sensitivity of respiration (e) to ADP concentration and presence of creatine (Cr), where a low Michaelis constant (Km) means that high respiratory rates are driven by little ADP; ROS emission (f), H2O2 release per unit of mitochondrion indexed by CS; the total amount of Ca2+ uptake (g) that mitochondria can sustain before undergoing PTP opening. −Cr, no creatine; +Cr, with creatine; wGas, white gastrocnemius (type II); Sol, soleus (type I); EDL, extensor digitorum longus (type II); AL, adductor longus (type I). Data are from ref. 65. h, Mouse adipocytes contain at least two different types of mitochondria: PDM and non-PDM (cytoplasmic). i,j, Biplots representing (i) the maximal rate of ATP synthesis and maximal oxygen consumption rate (OCR; i) or size versus motility (j). Data are from refs. 71,72. NK, natural killer.
Figure 2b shows the levels of citrate synthase (CS) activity (an imperfect proxy for mitochondrial content per cell), mitochondrial DNA copy number (mtDNAcn; the number of mtDNA copies per cell) and the enzymatic activity of OxPhos complexes within different immune cell types59. Plotting these common mitochondrial features and activities on bivariate plots illustrates the different phenotypic space occupied by each cell subtype. In these plots, the diagonal (where both parameters scale in proportion to each other) reflects the same mitochondrial phenotype, and orthogonal deviations from the diagonal reflects distinct mitochondrial specialization/adaptation.
For example, natural killer cells and neutrophils have similarly low CS activity and mtDNAcn, compared to CD4+ and CD8+ T lymphocytes, which have similar CS activity but about two-to three-fold more mtDNA copies per cell. The naïve-to-memory transition may also lead to a convergence of mitochondrial phenotypes, as expected from their metabolic transitions. Plotting ratios of two features on both x and y axes also reveals distinct types of mitochondria–or mitotypes59–among the two major immune cell lineages (Fig. 2c).
Skeletal muscle mitochondrial phenotypes.
Two major classes of skeletal muscle cell types, or myofibres, coexist: oxidative or myosin heavy chain type I, and glycolytic or myosin heavy chain type II64 (Fig. 2d). Like metabolically divergent immune cells that coexist within the circulation and lymphoid organs, oxidative and glycolytic muscle fibres coexist as a mosaic within the same skeletal muscle65. Nevertheless, their mitochondria functionally differ from one another in three main ways. In rat skeletal muscles, compared to the glycolytic muscle (white gastrocnemius) mitochondrial phenotype, the oxidative muscle (soleus) phenotype (1) has a 50–90% lower affinity for ADP-driven respiration (requires more ADP to drive the same OxPhos flux) and depends heavily on creatine65; (2) emits ~50% less hydrogen peroxide (H2O2)65; and (3) has ~60% lower calcium retention capacity66 (Fig. 2e–g). Thus, mitochondrial populations from closely related skeletal muscle cell types exhibit strikingly different multivariate mitochondrial phenotypes.
A related example concerns the further differentiation of skeletal muscle mitochondria into specialized subpopulations within the same cytoplasm. In each myofibre, two major mitochondrial subpopulations cohabit: subsarcolemmal (SS; also known as perivascular and perinuclear) and intermyofibrillar (IMF), which differ on a number of morpho-functional aspects67. For example, compared to IMF, SS mitochondria have ~60% lower oxidative capacity68, ~25–40% lower enzymatic activities for OxPhos enzymes68, but ~60% higher cardiolipin content68, and exhibit a greater capacity to oxidize lipids via beta-oxidation69. Both subpopulations even undergo differential responses to exercise69. As might be expected, these functional divergences between SS and IMF mitochondria are associated by distinct proteomes, notably highlighting the lower abundance of OxPhos proteins in SS compared to IMF mitochondria70, accounting at least in part for their functional divergences.
Brown adipose tissue mitochondrial phenotypes.
The last example is another instance of intracellular mitochondrial specialization in brown adipocytes where two mitochondrial phenotypes coexist in the same cytoplasm: the peridroplet mitochondria (PDM) surrounding lipid droplets, and non-PDM (that is, cytoplasmic; Fig. 2h)71. Compared to the cytoplasmic type, the PDM have double the respiratory and ATP synthesis rates (pyruvate and malate oxidation), tend to have a higher ΔΨm and contain more of their OxPhos complexes assembled into supercomplexes (Fig. 2i)72. However, PDM have a lower oxidative capacity for fatty acids, are larger, less motile and fuse less often with surrounding mitochondria (Fig. 2j). Thus, functionally defined mitochondrial diversity extends across the cellular and intracellular levels58.
Diversity in mitochondrial morphology
Functional specialization often comes with morphological specialization73. Mitochondria undergo dynamic changes in morphology and function (that is, morphofunction74), where changes in shape trigger downstream changes in functions75–77. Changes in morphology occur within seconds to minutes, triggered by metabolic and endocrine signals78–82. But distinct mitochondria in different cell types also develop relatively stable and sufficiently distinct ultrastructural characteristics (for example, cristae density and anatomy) that can allow a trained investigator to distinguish tissue-specific mitochondrial phenotypes by electron microscopy alone (Fig. 3a).
Fig. 3 |. Diversity in mitochondrial morphology.
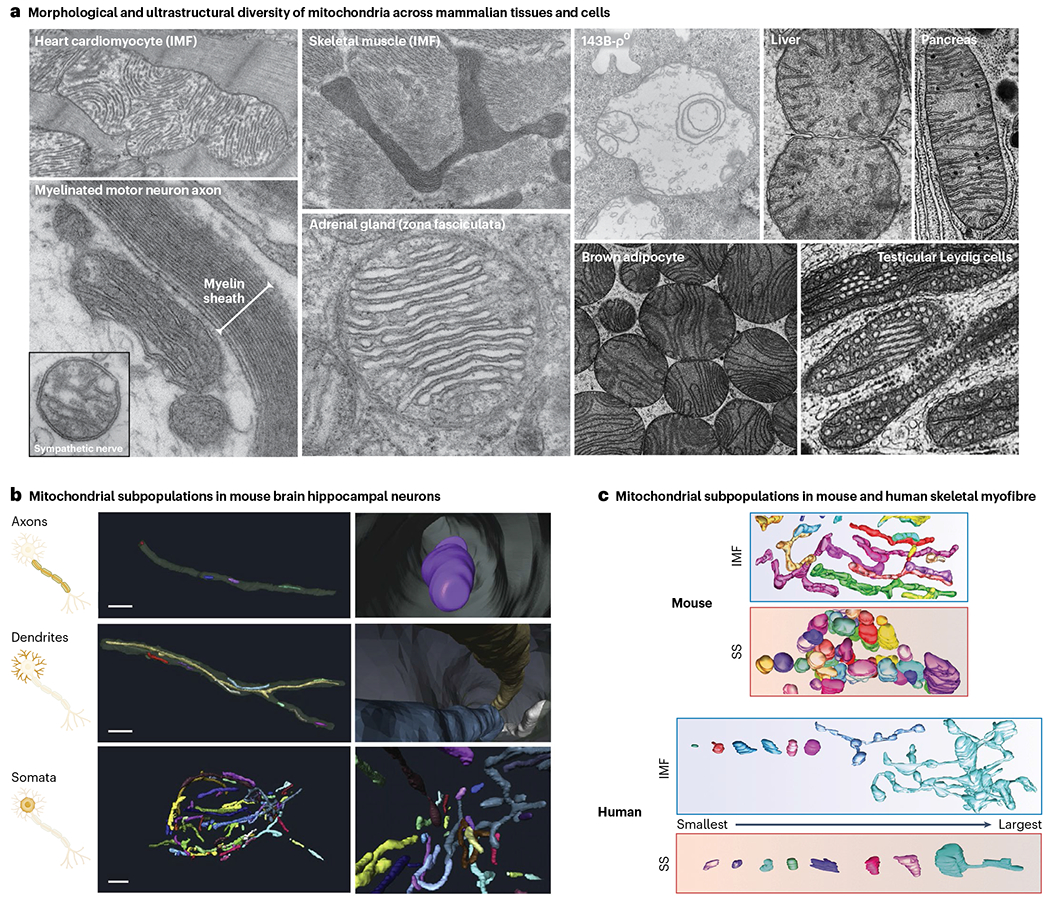
a, TEM micrographs of mitochondria in mammalian tissues and cultured cells. The 143B-ρ0 mitochondrion lacking mtDNA is from ref. 215. Adrenal mitochondrion reproduced with permission from ref. 216. Liver, pancreas, brown adipocyte and Leydig cell mitochondria reproduced with permission from ref. 73; other images are from M.P.’s laboratory). Note the natural variation in morphology (gross shape of mitochondria), in ultrastructure (positioning and organization of internal cristae membranes) and overall electron density (reflecting density of molecular components). b,c, Three-dimensional reconstructions (b) of neural mitochondria from the subcellular compartments of large granule neurons in the mouse dentate gyrus (adapted from ref. 85), and of skeletal muscle (c) mitochondrial phenotypes between the SS and IMF regions of human skeletal muscle fibres (adapted from ref. 86). Note the variation in morphological complexity and volume within the mitochondrial population of the same cell.
The gross morphology (tubular and branched versus rounded and fragmented) of mitochondria in different cell types, or within different subcellular compartments, can also be quite unique (Fig. 3b). For example, neuronal axonal mitochondria must travel long distances and tend to be smaller and shorter organelles, compared to much longer and branched dendritic mitochondria that are less mobile and contribute to local Ca2+ buffering, ATP production and possibly other roles83–85. The neuron somata mitochondria (that is, cell body, where the bulk of mitochondrial biogenesis takes place, and which likely represent the source or ‘stem’ population feeding both axonal and dendritic mitochondrial pools) exhibit intermediate size and morphological complexity85. In skeletal muscle, SS and IMF mitochondria within the same myofibres also are vastly recognizable by their morphologies alone in both mice and humans (Fig. 3c)67,86, and they undergo specific morphological recalibrations to challenges such as exercise87.
In general, as for the transition from stem cells to highly differentiated cellular arbours (for example, neurons), the acquisition of specialized cellular characteristics is associated with a transition from a relatively ‘undifferentiated’, rounded, depolarized and stem-like mitochondrial phenotype, towards specialized elongated and morphologically diverse mitochondrial phenotypes that perform tissue-specific functions and behaviours.
Taken together, the sections above illustrate how mitochondria diverge on at least three quantifiable levels: molecularly, functionally and morphologically. In the context of these rich and diverse tissue-type-specific and cell-type-specific mitochondrial phenotypes, mitochondrial science needs a terminology system that incorporates these notions to effectively guide scientific inquiry.
A new framework for mitochondrial terminology
Why we need more specific terminology
With the goal to best capture and incorporate critical aspects of mitochondrial biology in our daily discourse, we propose some standard terminology. This terminology system: (1) builds on the natural hierarchical organization of biological components from molecules (simple) to organelles (complex); and (2) draws from traditional classification systems around organ-based and cell-type-based terminology in the biomedical and biological sciences (Fig. 4).
Fig. 4 |. Terminology for mitochondrial science organized as a hierarchy of mitochondrial needs.
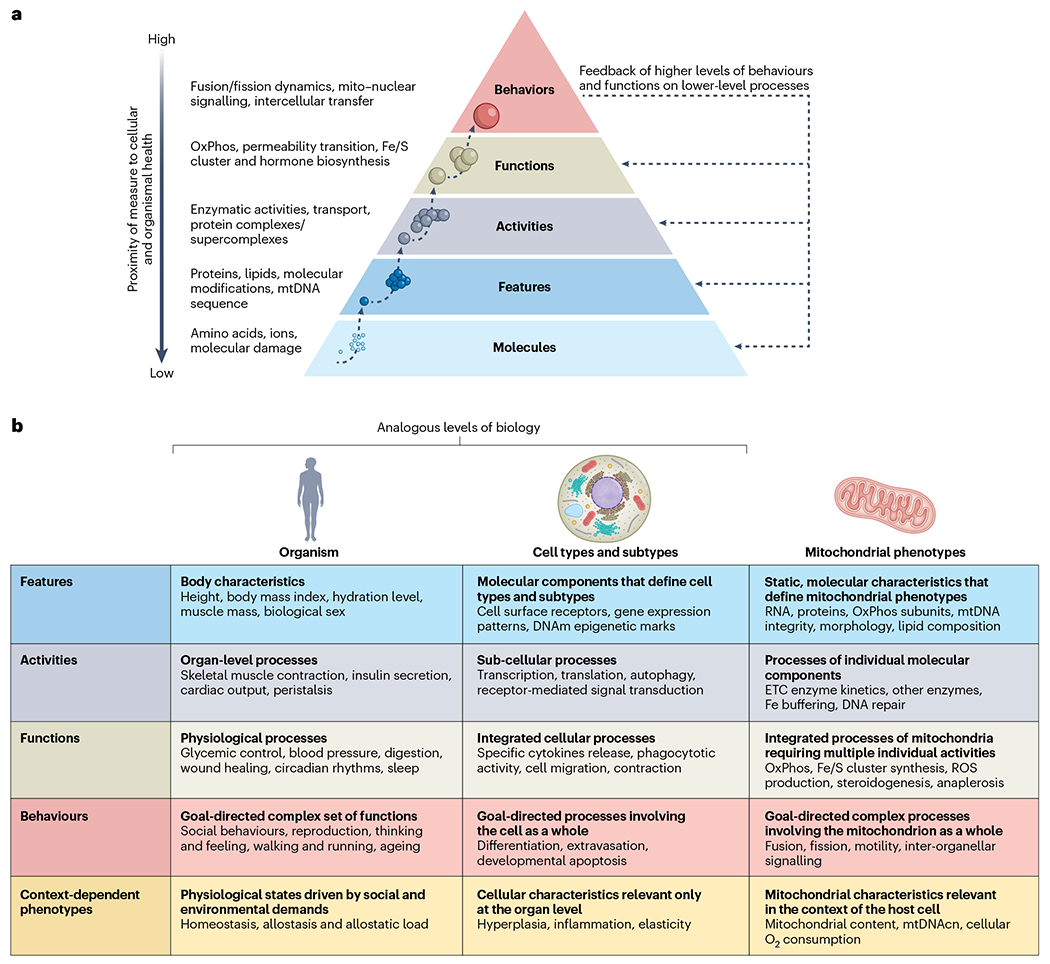
a, inspired by Maslow’s pyramid of human needs217, depicted is a hierarchy of biological organization from molecules to complex organellar behaviours. Lower levels combine to enable higher levels of organization. Each level can be studied using specific types of laboratory methods and analytical approaches. Thus, different approaches provide different types of information about the molecular features, activities, functions and behaviours that define specific mitochondrial phenotypes. b, Operationalization and examples for different levels of organization available to examine and perturb mitochondrial biology. Biomedical terminology related to organismal characteristics (left), and terminology related to cell biology (middle) are provided as parallel illustrative examples at each level of description for mitochondrial biology.
Beyond function and dysfunction.
We see two main steps necessary to increase the level of specificity in mitochondrial science. The first is to deliberately avoid using the terms ‘mitochondrial function’ and ‘mitochondrial dysfunction’. This admittedly uncomfortable exercise forces even the most seasoned mitochondrial investigators towards more specific terminology and a refinement of thought. It also promotes productive exchanges between experts and neophytes4.
By reflexively writing ‘mitochondrial dysfunction’, do we mean a decrease in ATP synthesis rate or maximal respiratory capacity, a metabolic shift from beta-oxidation to glutamine oxidation, a decrease in OxPhos subunit abundance or supercomplex assembly, a change in ROS production, reduced mitochondrial proteostasis, a change in membrane lipid composition, a compensatory increase in mtDNAcn, an increased cytoplasmic citrate export, or a change in motility or fusion dynamics among perinuclear mitochondria? Specificity in language begets specificity of hypotheses. In turn, clear hypotheses clarify the ideal experimental model(s) and approach(es) required to test them. Relinquishing ‘mitochondrial function’ and ‘mitochondrial dysfunction’ from our verbiage appears as a necessary, or at least a useful, step towards a maximally productive future for the growing field.
Towards greater specificity.
Without these catch-all terms, we need a systematic classification system to capture all measurable elements of mitochondrial biology. Therefore, the second step to increase specificity in mitochondrial science is to build such a system.
By analogy, medicine uses organ systems and organ-specific functions to organize medical training, diagnosis and treatment. One must naturally specify whether the electrical rhythm of the heart (electrocardiogram) or brain (electroencephalogram) is to be measured, for these provide different functional information about the organism. Similarly, cell biological processes can be examined at the levels of molecular features that define cell types (for example, cell surface receptors and transcriptional factors), endogenous molecular activities (for example, transcription, translation and intracellular signalling), cellular functions (for example, secretion, migration and contraction) or sets of whole-cell behaviours within their environment (for example, extravasation, phagocytosis and developmentally induced programmed cell death).
Using the same hierarchical logic, we distinguish five main levels of analysis for mitochondrial biology: (1) cell-dependent mitochondrial properties, (2) static molecular features, (3) single-enzyme activities, (4) organellar functions and (5) behaviours. Figure 5 illustrates some examples of each level of analysis, which are described below starting from the smallest and most indirect, to the most complex elements of mitochondrial biology.
Fig. 5 |. Example of measurements across domains of mitochondrial biology.
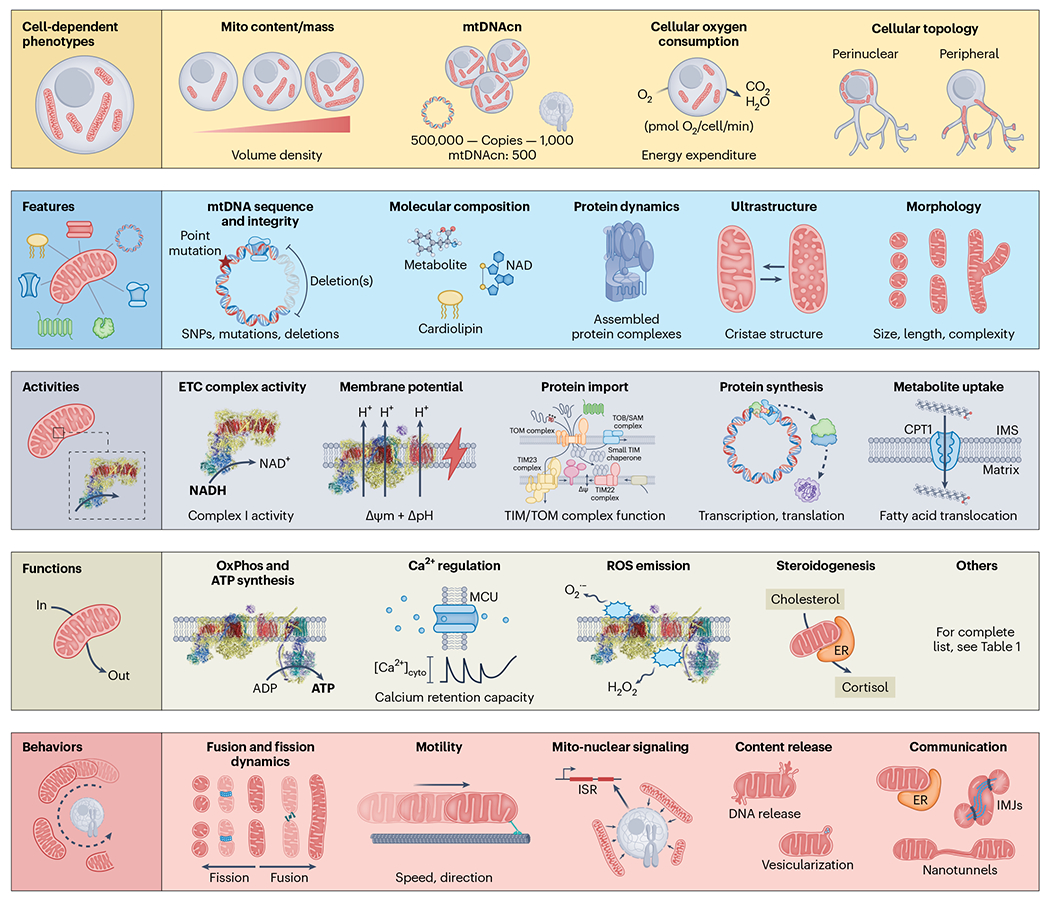
Cell-dependent phenotypes: Frequently used mitochondrial measures such as mitochondrial content (also known as mitochondrial mass), mtDNAcn per cell, and OCR by cells or tissues do not reflect intrinsic mitochondrial properties. Rather, they provide information about cellular energy demand and/or cell-level regulatory processes controlling mitochondrial biology. Features: Features are molecular components that can vary in quantity or quality, generally measurable from frozen or dead cellular material. Activities: Activities emerge from the interaction of multiple features, resulting in specific enzymatic activities or intrinsic properties of mitochondria that change the effective concentration of one or more substrates. Functions: Functions emerge from the combination of several activities, resulting in the transformation of inputs into outputs at the organelle level. Example of activities include energy transformation through the OxPhos system, Ca2+ regulation, macromolecule biosynthesis and the production of signals or outputs. Behaviours: Behaviours emerge from the interaction of multiple functions in collaboration with cytoplasmic and inter-organellar factors. As in cells and organisms, behaviours are best understood as goal driven, meaning that they reflect the coming together of several functions towards an end goal, such as modulating the architecture of the mitochondrial network through dynamics and motility, altering nuclear gene expression through repositioning and signalling, or optimizing cellular and organismal adaptation through inter-organelle and cell–cell communication2. For a list of mitochondrial functions and behaviours, see Table 1. SNP, single-nucleotide polymorphism; MCU, mitochondrial calcium uniporter; ISR, integrated stress response; IMJ intermitochondrial junctions.
The five main levels of analysis in mitochondrial biology
Cell-dependent mitochondrial properties.
Cell-dependent mitochondrial properties are not defined based on intrinsic components of the organelle; they are meaningful only in the context of the cell. As a result, they are the most indirect as they relate to mitochondrial biology. This includes mitochondrial content or volume density, reflecting the proportion of cellular volume occupied by a given mitochondrial phenotype88,89. Similarly, mtDNAcn represents the number of mtDNA copies per cell, typically expressed per copy of diploid nuclear genome. mtDNAcn varies widely between tissues, typically between ~100 to ~5,000 copies per cell59,90. On its own, mtDNAcn says little to nothing about the functional state or specialization of a mitochondrial phenotype but may be interpretable in the context of other features, activities and functions91. Other mitochondrial properties, such as the topology or network distribution of mitochondria within the cell cytoplasm and perinuclear region92,93, or in specialized appendages such as presynaptic terminals94, also bear direct functional significance, but only in the context of the cell (see Fig. 5).
Mitochondrial features.
Features are the intrinsic building blocks of mitochondria. They are generally static molecular components, such as the abundance of specific proteins, membrane lipids, mtDNA integrity, the density and configuration of cristae membranes, and many other quantifiable metrics. Most omics platforms (such as proteomics, lipidomics, transcriptomics and genomics) target static features. As demonstrated in MitoCarta52, profiling mitochondrial features provides rich information on the molecular specialization of mitochondria (that is, the hardware). However, quantifying mitochondrial features does not reflect their functional capacity or behaviours in their cellular context. Static measures of mitochondrial morphology and ultrastructure, which include quantitative measures of size (volume) and morphological features (length, three-dimensional morphological complexity, cristae density, and so on86,95) also belong to the category of mitochondrial features.
Mitochondrial activities.
Activities are single-enzyme activities that are measured as dynamic processes, such as the biochemical activity of monomeric (for example, CS) or multimeric (for example, pyruvate dehydrogenase complex, PDC) enzymes. Activities are made of features but do not classify as mitochondrial functions. Mitochondrial activities include the isolated enzymatic activities of OxPhos complexes96 and any other enzymatic activities, the isolated activity of individual IMM transporters like the ATP/ADP antiporter, proteases, polymerases, helicases, metabolite and ion transport across the IMM, to name a few examples.
Mitochondrial functions.
Functions require at least one step to be physically localized within the mitochondrion, and generally involve multiple activities contributing to the conversion of an input into an output. ATP synthesis, Ca2+ homeostasis, lipid synthesis and many other processes are mitochondrial functions enabled by the interaction of two or more (often dozens) molecular features and activities, cooperating as an integrated system. For example, the conversion of electrons from reducing equivalents into an electrochemical gradient (that is, membrane potential, ΔΨm + ΔpH) is considered a mitochondrial function. Similarly, protein import requires the interaction of multiple proteins and activities to transport and process proteins from the cytoplasm to the mitochondrial matrix. Some functions include complex operations that involve the collaboration of mitochondria with other organelles. A function that illustrates this cooperativity is steroidogenesis within adrenal and gonadal mitochondria. Steroidogenesis requires the import of cholesterol from the cytoplasm to the matrix via the outer mitochondrial membrane (OMM) protein STAR, a redox-dependent side-chain cleavage reaction by the matrix P450ssc enzyme, and in the case of cortisol, several steps in the endoplasmic reticulum (ER) followed by the final enzymatic step by the matrix enzyme 11-beta-hydroxylase34,97. Collectively, these features and activities produce the diffusible endocrine hormone cortisol, making cortisol synthesis a proper mitochondrial function.
Fe/S cluster synthesis also involves several enzymatic and biochemical steps uniquely positioned in mitochondria. Both steroidogenesis and Fe/S cluster synthesis are mitochondrial functions essential to animal life; the former is specific to a few specialized mitochondria in the adrenal glands and gonads, while the latter is essential to the life of all eukaryotic cells. Table 1 provides a developing list of mitochondrial functions and behaviours (not including cell-dependent properties, features or activities, as they would be too numerous), which exhibit variable degrees of mitochondrial specialization across cell types and tissues.
Mitochondrial behaviours.
Behaviours are best understood as a goal-driven series of activities and functions that often involve the organelle as a whole, rather than a specific set of features. Mitochondrial behaviours include motility6, fusion and fission dynamics98, biogenesis99 and mitochondrial–nuclear signalling via an array of metabolic intermediates, ions, proteins and other factors31,100. For example, mitochondrial fission is a complex behaviour requiring the coordination of numerous core proteins and adaptors, in partnership with other organelles101,102, whose goal is to segment individual organelles into two organelles in a non-random, functionally relevant manner103. Like other behaviours, fission is a goal-directed set of activities that morphologically and functionally reshape the whole organelle (and in some cases, the cell itself).
The distinction between mitochondrial functions and behaviours is directly analogous to general classifications of intrinsic functions that take place within cells (gene expression, autophagy, contraction and secretion) compared to goal-directed cellular behaviours that take place within the context of other cells (infiltration, cytotoxic killing and communication). Whereas functions typically refer to processes that take place endogenously within the organelle or in a ‘mitochondrial-autonomous’ manner, behaviours typically involve changes or movement of the whole organelle and/or interactions with other organellar or intercellular partners.
Overcoming mitochondrial jargon
Common mitochondrial science approaches and phrases rest on the long-standing simplification that mitochondria in different cell types either ‘function’ or are ‘dysfunctional’. The evidence reviewed above shows this to be inaccurate. Table 2 highlights some typical phrases together with their limitation, and a reframed statement offering greater specificity. The proposed framework and nomenclature system can guide the design, execution, interpretation, reporting and teaching of mitochondrial science among both experts and non-experts.
Table 2 |.
Infusing specificity into our mitochondrial terminology can enhance how we design and communicate research
| Non-specific notation | Limitation or problem | Specific notation |
|---|---|---|
| In a talk or conversation: A) ‘We measured mitochondrial dysfunction […]’ |
There are dozens of functions, and therefore dozens of ways to exhibit dysfunction. | A’) General: ‘We measured mitochondrial phenotypes […]’ Specific: ‘We measured skeletal muscle mitochondrial OxPhos enzyme activities and citrate production […]’ |
| From an abstract: B) ‘We assessed skeletal muscle mitochondrial function using western blot, lipidomics, biochemical assays, ATP synthesis and live cell microscopy.’ |
These reflect different domains of mitochondrial biology; not all are functions. | B’) ‘We assessed skeletal muscle mitochondrial features including protein abundance and lipid composition, OxPhos enzyme activities using biochemical assays, OxPhos function by monitoring ATP synthesis rates and mitochondrial behaviour by quantifying fusion dynamics by live cell microscopy. |
| From a paper: C) ‘In summary, we have studied the role of mitochondrial dysfunction in heart failure and evaluated mitochondrial boosting strategies as a potential treatment.’ |
Which is the actual nature of the dysfunction? What function is needed to boost? |
C’) ‘Here, we have studied the role of endothelial cell mitochondrial functional recalibrations in heart failure and strategies to boost respiratory chain capacity as a potential treatment.’ |
| From a grant or website: D) ‘The hallmarks of ageing include telomere attrition, mitochondrial dysfunction, […]’ |
In some contexts, a general qualifying term may be needed to refer to molecular, structural, functional and behavioural changes among mitochondria. This term should be plural. Not all changes reflect dysfunction but are rather adaptive recalibrations. |
D’) General: ‘The hallmarks of ageing include telomere attrition, mitochondrial impairments and recalibrations, […]’ Specific: ‘The hallmarks of ageing include telomere attrition, reduced mitochondrial OxPhos capacity, […]’ |
Concluding remarks
Building on previous successful efforts to create highly specific terminology and classification systems to guide scientific inquiry in medicine and in cell biology, we have outlined the beginning of such a framework for mitochondrial science. The integration of molecular, functional and morphological/ultrastructural data highlights the existence of specialized, multifaceted mitochondrial phenotypes, which exist across organs and tissues, cell types and subcellular compartments. Thus, the popular assumption that mitochondria are relatively uniform ‘powerhouses’ passively distributed across the body mainly to subserve energy demands is evidently limited. Rather, specialized mitochondria coexist in a division of labour analogous to that seen among cell types and organs: different types of mitochondria share recognizable mitochondrial hallmarks, but largely subserve different, complementary roles within the organism. Building a systematic, high-resolution cartography of mitochondrial phenotypes is an open challenge for the field.
Because mitochondria are multifunctional and dynamically recalibrate their features, activities, functions and behaviours based on organismal demands and stressors, the terms ‘mitochondrial function’ and ‘mitochondrial dysfunction’ are misleading; the mitochondrial community at large would benefit from adopting more specific and informative terminology to guide teaching and thinking, our experimental designs and future theories. The terminology system outlined in Figs. 4 and 5 is pragmatic, hierarchical and measurement oriented. In the same way that cell biology has benefited from fine-grained classification systems among its subspecialities, the proposed semantic and scientific specificity should deepen our mechanistic models of the role that mitochondria play in health and disease states. Importantly, as mitochondrial science is increasingly poised to contribute meaningful knowledge to other areas of biology and medicine, the proposed classification system also is likely to maximize the interdisciplinary synergy between mitochondrial science and other disciplines.
Acknowledgements
We are grateful to investigators who have contributed to reveal tissue-type-specific and cell-type-specific mitochondrial phenotypes that served as the foundation and motivation for this Perspective. We apologize to authors whose work could not be included due to space constraints. We thank the Twitter mitochondrial community and C. Schmitt who contributed inputs to Table 1, and to members of the Mitochondrial Psychobiology Laboratory, and the Genoxphos Laboratory for stimulating discussions. Work of J.A.E. is supported by Ministerio de Ciencia, Innovación/Agencia Estatal de Investigación (AEI) and Fondo Europeo de Desarrollo Regional (FEDER; RTI2018-099357-B-I00), the Biomedical Research Networking Center on Frailty and Healthy Ageing (CIBERFES-ISCiii-CB16/10/00289) and the Leducq TNE-17CVD. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia, Innovación y Universidades (MCNU) and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (SEV-2015-0505). This work was supported by National Institutes of Health grants R01MH119336, R01MH122706, R01AG066828, R21MH123927 and RF1AG076821, the Wharton Fund and the Baszucki Brain Research Fund (to M.P.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Marieb E & Hoehn K Essentials of Human Anatomy & Physiology (Pearson Education, 2022). [Google Scholar]
- 2.Picard M & Shirihai OS Mitochondrial signal transduction. Cell Metab. 34, 1620–1653 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siekevitz P Powerhouse of the Cell. Sci. Am 197, 131–144 (1957). [Google Scholar]
- 4.Harper ME & Patti ME Metabolic terminology: what’s in a name? Nat. Metab 2, 476–477 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Schmidt CA Prescription drugs and mitochondrial metabolism. Biosci. Rep 42, BSR20211813 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisner V, Picard M & Hajnoczky G Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol 20, 755–765 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand MD & Nicholls DG Assessing mitochondrial dysfunction in cells. Biochem. J 435, 297–312 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mracek T, Drahota Z & Houstek J The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim. Biophys. Acta 1827, 401–410 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Graves SM et al. Dopamine metabolism by a monoamine oxidase mitochondrial shuttle activates the electron transport chain. Nat. Neurosci 23, 15–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell P Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. 1966. Biochim. Biophys. Acta 1807, 1507–1538 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Wiedemann N & Pfanner N Mitochondrial machineries for protein import and assembly. Annu. Rev. Biochem 86, 685–714 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Braymer JJ & Lill R Iron–sulfur cluster biogenesis and trafficking in mitochondria. J. Biol. Chem 292, 12754–12763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinton TV, Batelu S, Gleason N & Stemmler TL Molecular characteristics of proteins within the mitochondrial Fe–S cluster assembly complex. Micron 153, 103181 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Read AD, Bentley RE, Archer SL & Dunham-Snary KJ Mitochondrial iron–sulfur clusters: structure, function, and an emerging role in vascular biology. Redox Biol. 47, 102164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rydstrom J Mitochondrial NADPH, transhydrogenase and disease. Biochim. Biophys. Acta 1757, 721–726 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Bahat A, MacVicar T & Langer T Metabolism and innate immunity meet at the mitochondria. Front. Cell Dev. Biol 9, 720490 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elesela S & Lukacs NW Role of mitochondria in viral infections. Life. 11, 232 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McWhirter SM, Tenoever BR & Maniatis T Connecting mitochondria and innate immunity. Cell 122, 645–647 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Moore CB & Ting JP Regulation of mitochondrial antiviral signaling pathways. Immunity 28, 735–739 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Walker JE The ATP synthase: the understood, the uncertain and the unknown. Biochem. Soc. Trans 41, 1–16 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Bernardi P, Carraro M & Lippe G The mitochondrial permeability transition: recent progress and open questions. FEBS J. 289, 7051–7074 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacVicar T & Langer T OPA1 processing in cell death and disease—the long and short of it. J. Cell Sci 129, 2297–2306 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Roger AJ, Munoz-Gomez SA & Kamikawa R The origin and diversification of mitochondria. Curr. Biol 27, R1177–R1192 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Lill R, Fekete Z, Sipos K & Rotte C Is there an answer? Why are mitochondria essential for life? IUBMB Life 57, 701–703 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Inigo M, Deja S & Burgess SC Ins and outs of the TCA cycle: the central role of anaplerosis. Annu. Rev. Nutr 41, 19–47 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Oates EH & Antoniewicz MR Coordinated reprogramming of metabolism and cell function in adipocytes from proliferation to differentiation. Metab. Eng 69, 221–230 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Spinelli JB & Haigis MC The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol 20, 745–754 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandel NS Mitochondria as signaling organelles. BMC Biol. 12, 34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen K et al. Mitochondria as cellular and organismal signaling hubs. Annu. Rev. Cell Dev. Biol 38, 179–218 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Dai Z, Ramesh V & Locasale JW The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet 21, 737–753 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Reyes I & Chandel NS Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun 11, 102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy MP & Chouchani ET Why succinate? Physiological regulation by a mitochondrial coenzyme Q sentinel. Nat. Chem. Biol 18, 461–469 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassi G, Sidhu SK & Mishra S The expanding role of mitochondria, autophagy and lipophagy in steroidogenesis. Cells 10, 1851 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller WL Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol 379, 62–73 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Beninca C et al. A new non-canonical pathway of Gαq protein regulating mitochondrial dynamics and bioenergetics. Cell. Signal. 26, 1135–1146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hebert-Chatelain E et al. A cannabinoid link between mitochondria and memory. Nature 539, 555–559 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Jimenez-Blasco D et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature 583, 603–608 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Suofu Y et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl Acad. Sci. USA 114, E7997–E8006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torralba D et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun 9, 2658 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trumpff C et al. Stress and circulating cell-free mitochondrial DNA: a systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 59, 225–245 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marti Gutierrez N et al. Horizontal mtDNA transfer between cells is common during mouse development. iScience 25, 103901 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brestoff JR et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab. 33, 270–282 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borcherding N et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood. Cell Metab. 34, 1499–1513 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayakawa K et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aharoni-Simon M et al. Oxidative stress facilitates exogenous mitochondria internalization and survival in retinal ganglion precursor-like cells. Sci. Rep 12, 5122 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicolas-Avila JA et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Al Amir Dache Z et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 34, 3616–3630 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Park MK, Lomax RB, Tepikin AV & Petersen OH Local uncaging of caged Ca2+ reveals distribution of Ca2+-activated Cl− channels in pancreatic acinar cells. Proc. Natl Acad. Sci. USA 98, 10948–10953 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson DT, Harris RA, Blair PV & Balaban RS Functional consequences of mitochondrial proteome heterogeneity. Am. J. Physiol. Cell Physiol 292, 698–707 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Johnson DT et al. Tissue heterogeneity of the mammalian mitochondrial proteome. Am. J. Physiol. Cell. Physiol 292, 689–697 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Pagliarini DJ et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rath S et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgenstern M et al. Quantitative high-confidence human mitochondrial proteome and its dynamics in cellular context. Cell Metab. 33, 2464–2483 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aryaman J, Johnston IG & Jones NS Mitochondrial heterogeneity. Front. Genet 9, 718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fecher C et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat. Neurosci 22, 1731–1742 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Stewart JB & Chinnery PF The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat. Rev. Genet 16, 530–542 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Vincent AE & Picard M Multilevel heterogeneity of mitochondrial respiratory chain deficiency. J. Pathol 246, 261–265 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Pekkurnaz G & Wang X Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat. Metab 4, 802–812 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rausser S et al. Mitochondrial phenotypes in purified human immune cell subtypes and cell mixtures. eLife 10, e70899 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McLaughlin KL et al. Novel approach to quantify mitochondrial content and intrinsic bioenergetic efficiency across organs. Sci. Rep 10, 17599 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lesner NP et al. Differential requirements for mitochondrial electron transport chain components in the adult murine liver. eLife 11, e80919 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jang C et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Kleer I, Willems F, Lambrecht B & Goriely S Ontogeny of myeloid cells. Front. Immunol 5, 423 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harridge SD et al. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch. 432, 913–920 (1996). [DOI] [PubMed] [Google Scholar]
- 65.Picard M, Hepple RT & Burelle Y Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am. J. Physiol. Cell Physiol 302, C629–641 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Picard M, Ritchie D, Thomas MM, Wright KJ & Hepple RT Alterations in intrinsic mitochondrial function with aging are fiber-type-specific and do not explain differential atrophy between muscles. Aging Cell 10, 1047–1055 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Willingham TB, Ajayi PT & Glancy B Subcellular specialization of mitochondrial form and function in skeletal muscle cells. Front. Cell Dev. Biol 9, 757305 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cogswell AM, Stevens RJ & Hood DA Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am. J. Physiol 264, 383–389 (1993). [DOI] [PubMed] [Google Scholar]
- 69.Koves TR et al. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am. J. Physiol. Cell Physiol 288, 1074–1082 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Ferreira R et al. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics 10, 3142–3154 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Benador IY et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 27, 869–885 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benador IY, Veliova M, Liesa M & Shirihai OS Mitochondria bound to lipid droplets: where mitochondrial dynamics regulate lipid storage and utilization. Cell Metab. 29, 827–835 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fawcett DW The Cell Chapter 7: Mitochondria, 410–485 (W. B. Saunders Company, 1966). [Google Scholar]
- 74.Bulthuis EP, Adjobo-Hermans MJW, Willems P & Koopman WJH Mitochondrial morphofunction in mammalian cells. Antioxid. Redox Signal. 30, 2066–2109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cogliati S. et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160–171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Picard M, Shirihai OS, Gentil BJ & Burelle Y Mitochondrial morphology transitions and functions: implications for retrograde signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol 304, 393–406 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong X. et al. Mitochondrial dynamics maintains stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy Cell Stem Cell 29, 1298–1314 (2022). [DOI] [PubMed] [Google Scholar]
- 78.Rambold AS, Kostelecky B, Elia N & Lippincott-Schwartz J Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl Acad. Sci. USA 108, 10190–10195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liesa M & Shirihai OS Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 17, 491–506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Picard M & Turnbull DM Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes 62, 672–678 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu T, Robotham JL & Yoon Y Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl Acad. Sci. USA 103, 2653–2658 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shenouda SM et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124, 444–453 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewis TL Jr., Kwon SK, Lee A, Shaw R & Polleux F MFF-dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nat. Commun 9, 5008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rangaraju V, Lauterbach M & Schuman EM Spatially stable mitochondrial compartments fuel local translation during plasticity. Cell 176, 73–84 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Faitg J. et al. 3D neuronal mitochondrial morphology in axons, dendrites, and somata of the aging mouse hippocampus. Cell Rep. 36, 109509 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vincent AE et al. Quantitative 3D mapping of the human skeletal muscle mitochondrial network. Cell Rep. 26, 996–1009(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang R, Ogden RT, Picard M & Srivastava A Nonparametric k-sample test on shape spaces with applications to mitochondrial shape analysis. J. R. Stat. Soc. Ser. C Appl. Stat 71, 51–69 (2022). [Google Scholar]
- 88.Justs KA et al. Presynaptic mitochondrial volume and packing density scale with presynaptic power demand. J. Neurosci 42, 954–967 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meinild Lundby AK et al. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta. Physiol 222, e12976 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Wachsmuth M, Hubner A, Li M, Madea B & Stoneking M Age-related and heteroplasmy-related variation in human mtDNA copy number. PLoS Genet. 12, e1005939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Picard M. Blood mitochondrial DNA copy number: what are we counting? Mitochondrion 60, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Al-Mehdi AB et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal 5, ra47 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Desai R. et al. Mitochondria form contact sites with the nucleus to couple prosurvival retrograde response. Sci. Adv 6, eabc9955 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun T, Qiao H, Pan PY, Chen Y & Sheng ZH Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 4, 413–419 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Segawa M. et al. Quantification of cristae architecture reveals time-dependent characteristics of individual mitochondria. Life Sci. Alliance 3, e201900620 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frazier AE, Vincent AE, Turnbull DM, Thorburn DR & Taylor RW Assessment of mitochondrial respiratory chain enzymes in cells and tissues. Methods Cell. Biol 155, 121–156 (2020). [DOI] [PubMed] [Google Scholar]
- 97.Bose HS, Lingappa VR & Miller WL Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 417, 87–91 (2002). [DOI] [PubMed] [Google Scholar]
- 98.Friedman JR & Nunnari J Mitochondrial form and function. Nature 505, 335–343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scarpulla RC Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev 88, 611–638 (2008). [DOI] [PubMed] [Google Scholar]
- 100.Cagin U & Enriquez JA The complex cross-talk between mitochondria and the nucleus: What goes in between. Int. J. Biochem. Cell Biol 63, 10–15 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Giacomello M, Pyakurel A, Glytsou C & Scorrano L The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol 21, 204–224 (2020). [DOI] [PubMed] [Google Scholar]
- 102.Kraus F, Roy K, Pucadyil TJ & Ryan MT Function and regulation of the divisome for mitochondrial fission. Nature 590, 57–66 (2021). [DOI] [PubMed] [Google Scholar]
- 103.Kleele T. et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593, 435–439 (2021). [DOI] [PubMed] [Google Scholar]
- 104.Zorova LD et al. Mitochondrial membrane potential. Anal. Biochem 552, 50–59 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramshesh VK & Lemasters JJ Imaging of mitochondrial pH using SNARF-1. Methods Mol. Biol 1782, 351–356 (2018). [DOI] [PubMed] [Google Scholar]
- 106.Teodoro JS, Machado IF, Castela AC, Rolo AP & Palmeira CM The evaluation of mitochondrial membrane potential using fluorescent dyes or a membrane-permeable cation (TPP+) electrode in isolated mitochondria and intact cells. Methods Mol. Biol 2184, 197–213 (2020). [DOI] [PubMed] [Google Scholar]
- 107.Benevenga NJ & Blemings KP Unique aspects of lysine nutrition and metabolism. J. Nutr 137, 1610S–1615S (2007). [DOI] [PubMed] [Google Scholar]
- 108.Borst P. The malate–aspartate shuttle (Borst cycle): how it started and developed into a major metabolic pathway. IUBMB Life 72, 2241–2259 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ananieva EA & Wilkinson AC Branched-chain amino acid metabolism in cancer. Curr. Opin. Clin. Nutr. Metab. Care 21, 64–70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kainulainen H, Hulmi JJ & Kujala UM Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exerc. Sport Sci. Rev 41, 194–200 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Ueland PM Choline and betaine in health and disease. J. Inherit. Metab. Dis 34, 3–15 (2011). [DOI] [PubMed] [Google Scholar]
- 112.Birsoy K. et al. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blemings KP, Crenshaw TD & Benevenga NJ Mitochondrial lysine uptake limits hepatic lysine oxidation in rats fed diets containing 5, 20 or 60% casein. J. Nutr 128, 2427–2434 (1998). [DOI] [PubMed] [Google Scholar]
- 114.Cardaci S. et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat. Cell Biol 17, 1317–1326 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.King MP & Attardi G Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246, 500–503 (1989). [DOI] [PubMed] [Google Scholar]
- 116.LaNoue KF, Bryla J & Bassett DJ Energy-driven aspartate efflux from heart and liver mitochondria. J. Biol. Chem 249, 7514–7521 (1974). [PubMed] [Google Scholar]
- 117.Sullivan LB et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goldberg EJ et al. Tissue-specific characterization of mitochondrial branched-chain keto acid oxidation using a multiplexed assay platform. Biochem. J 476, 1521–1537 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ko JH et al. BCAT1 affects mitochondrial metabolism independently of leucine transamination in activated human macrophages. J. Cell Sci 133, jcs247957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smirnoff N. l-ascorbic acid biosynthesis. Vitam. Horm 61, 241–266 (2001). [DOI] [PubMed] [Google Scholar]
- 121.May JM, Li L, Qu ZC & Cobb CE Mitochondrial recycling of ascorbic acid as a mechanism for regenerating cellular ascorbate. Biofactors 30, 35–48 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Li X, Cobb CE & May JM Mitochondrial recycling of ascorbic acid from dehydroascorbic acid: dependence on the electron transport chain. Arch. Biochem. Biophys 403, 103–110 (2002). [DOI] [PubMed] [Google Scholar]
- 123.Dodgson SJ, Forster RE 2nd, Storey BT & Mela L Mitochondrial carbonic anhydrase. Proc. Natl Acad. Sci. USA 77, 5562–5566 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Palty R. et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl Acad. Sci. USA 107, 436–441 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baughman JM et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Stefani D, Raffaello A, Teardo E, Szabo I & Rizzuto R A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Greotti E & De Stefani D Biosensors for detection of calcium. Methods Cell. Biol 155, 337–368 (2020). [DOI] [PubMed] [Google Scholar]
- 128.De Nadai A, Vajente N, Pendin D & Mattarei A Mt-fura-2, a ratiometric mitochondria-targeted Ca2+ sensor. Determination of spectroscopic properties and Ca2+ imaging assays. Methods Mol. Biol 2275, 187–215 (2021). [DOI] [PubMed] [Google Scholar]
- 129.Cecchini G. Complexities of complex II: sulfide metabolism in vivo. J. Biol. Chem 298, 101661 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kumar R. et al. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase-dependent H2S oxidation. J. Biol. Chem 298, 101435 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Landry AP et al. A catalytic trisulfide in human sulfide quinone oxidoreductase catalyzes coenzyme A persulfide synthesis and inhibits butyrate oxidation. Cell Chem. Biol 26, 1515–1525 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang X. et al. Sulfide-quinone oxidoreductase is required for cysteine synthesis and indispensable to mitochondrial health. Redox Biol. 47, 102169 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Libiad M, Yadav PK, Vitvitsky V, Martinov M & Banerjee R Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem 289, 30901–30910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bertholet AM et al. Mitochondrial uncouplers induce proton leak by activating AAC and UCP1. Nature 606, 180–187 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chouchani ET, Kazak L & Spiegelman BM New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 29, 27–37 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Kazak L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nicholls DG & Locke RM Thermogenic mechanisms in brown fat. Physiol. Rev 64, 1–64 (1984). [DOI] [PubMed] [Google Scholar]
- 138.Cannon B & Nedergaard J Respiratory and thermogenic capacities of cells and mitochondria from brown and white adipose tissue. Methods Mol. Biol 155, 295–303 (2001). [DOI] [PubMed] [Google Scholar]
- 139.Williams NC & O’Neill LAJ A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front. Immunol 9, 141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.O’Neill LAJ & Artyomov MN Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol 19, 273–281 (2019). [DOI] [PubMed] [Google Scholar]
- 141.Cordes T. et al. Itaconate modulates tricarboxylic acid and redox metabolism to mitigate reperfusion injury. Mol. Metab 32, 122–135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ghandour MS, Parkkila AK, Parkkila S, Waheed A & Sly WS Mitochondrial carbonic anhydrase in the nervous system: expression in neuronal and glial cells. J. Neurochem 75, 2212–2220 (2000). [DOI] [PubMed] [Google Scholar]
- 143.Vanlander AV & Van Coster R Clinical and genetic aspects of defects in the mitochondrial iron–sulfur cluster synthesis pathway. J. Biol. Inorg. Chem 23, 495–506 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ball JM, Chen S & Li W Mitochondria in cone photoreceptors act as microlenses to enhance photon delivery and confer directional sensitivity to light. Sci. Adv 8, eabn2070 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Houten SM, Violante S, Ventura FV & Wanders RJ The biochemistry and physiology of mitochondrial fatty acid beta-oxidation and Its genetic disorders. Annu. Rev. Physiol 78, 23–44 (2016). [DOI] [PubMed] [Google Scholar]
- 146.Bennett MJ, Sheng F & Saada A Biochemical assays of TCA cycle and beta-oxidation metabolites. Methods Cell. Biol 155, 83–120 (2020). [DOI] [PubMed] [Google Scholar]
- 147.Schlame M & Greenberg ML Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1862, 3–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eiyama A, Aaltonen MJ, Nolte H, Tatsuta T & Langer T Disturbed intramitochondrial phosphatidic acid transport impairs cellular stress signaling. J. Biol. Chem 296, 100335 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miliara X. et al. Structural determinants of lipid specificity within Ups/PRELI lipid transfer proteins. Nat. Commun 10, 1130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tatsuta T & Langer T Intramitochondrial phospholipid trafficking. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1862, 81–89 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Lechuga-Vieco AV, Justo-Mendez R & Enriquez JA Not all mitochondrial DNAs are made equal and the nucleus knows it. IUBMB Life 73, 511–529 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rackham O & Filipovska A Organization and expression of the mammalian mitochondrial genome. Nat. Rev. Genet 23, 606–623 (2022). [DOI] [PubMed] [Google Scholar]
- 153.Fernandez-Silva P, Acin-Perez R, Fernandez-Vizarra E, Perez-Martos A & Enriquez JA In vivo and in organello analyses of mitochondrial translation. Methods Cell. Biol 80, 571–588 (2007). [DOI] [PubMed] [Google Scholar]
- 154.Fernandez-Vizarra E et al. Isolation of mitochondria for biogenetical studies: an update. Mitochondrion 10, 253–262 (2010). [DOI] [PubMed] [Google Scholar]
- 155.Mitchell P & Moyle J Translocation of some anions cations and acids in rat liver mitochondria. Eur. J. Biochem 9, 149–155 (1969). [DOI] [PubMed] [Google Scholar]
- 156.Baron S. et al. Role of mitochondrial Na+ concentration, measured by CoroNa red, in the protection of metabolically inhibited MDCK cells. J. Am. Soc. Nephrol 16, 3490–3497 (2005). [DOI] [PubMed] [Google Scholar]
- 157.Vasquez-Vivar J, Shi Z & Tan S Tetrahydrobiopterin in cell function and death mechanisms. Antioxid. Redox Signal 37, 171–183 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Besse A. et al. The GABA transaminase, ABAT, is essential for mitochondrial nucleoside metabolism. Cell Metab. 21, 417–427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cavalcanti-de-Albuquerque JP, de-Souza-Ferreira E, de Carvalho DP & Galina A Coupling of GABA metabolism to mitochondrial glucose phosphorylation. Neurochem. Res 47, 470–480 (2022). [DOI] [PubMed] [Google Scholar]
- 160.Bennett CF et al. Peroxisomal-derived ether phospholipids link nucleotides to respirasome assembly. Nat. Chem. Biol 17, 703–710 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fang J. et al. Dihydro-orotate dehydrogenase is physically associated with the respiratory complex and its loss leads to mitochondrial dysfunction. Biosci. Rep 33, e00021 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brosnan ME, MacMillan L, Stevens JR & Brosnan JT Division of labour: how does folate metabolism partition between one-carbon metabolism and amino acid oxidation. Biochem. J 472, 135–146 (2015). [DOI] [PubMed] [Google Scholar]
- 163.Zheng Y & Cantley LC Toward a better understanding of folate metabolism in health and disease. J. Exp. Med 216, 253–266 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao LN & Kaldis P Pairing structural reconstruction with catalytic competence to evaluate the mechanisms of key enzymes in the folate-mediated one-carbon pathway. FEBS J. 10.1111/febs.16439 (2022). [DOI] [PubMed] [Google Scholar]
- 165.Mitchell P & Moyle J Chemiosmotic hypothesis of oxidative phosphorylation. Nature 213, 137–139 (1967). [DOI] [PubMed] [Google Scholar]
- 166.Morciano G. et al. Measurement of ATP concentrations in mitochondria of living cells using luminescence and fluorescence approaches. Methods Cell. Biol 155, 199–219 (2020). [DOI] [PubMed] [Google Scholar]
- 167.Fernandez-Aguera MC et al. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 22, 825–837 (2015). [DOI] [PubMed] [Google Scholar]
- 168.Hernansanz-Agustin P. et al. Na+ controls hypoxic signalling by the mitochondrial respiratory chain. Nature 586, 287–291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lopez-Barneo J & Ortega-Saenz P Mitochondrial acute oxygen sensing and signaling. Crit. Rev. Biochem. Mol. Biol 57, 205–225 (2022). [DOI] [PubMed] [Google Scholar]
- 170.Sommer N. et al. Bypassing mitochondrial complex III using alternative oxidase inhibits acute pulmonary oxygen sensing. Sci. Adv 6, eaba0694 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carrer A. et al. Defining the molecular mechanisms of the mitochondrial permeability transition through genetic manipulation of F-ATP synthase. Nat. Commun 12, 4835 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bonora M, Giorgi C & Pinton P Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol 23, 266–285 (2022). [DOI] [PubMed] [Google Scholar]
- 173.Carraro M & Bernardi P Measurement of membrane permeability and the mitochondrial permeability transition. Methods Cell. Biol 155, 369–379 (2020). [DOI] [PubMed] [Google Scholar]
- 174.Hsu Y-T et al. Apoptosis Techniques and Protocols 2nd edn, vol. 37 (Humana Press, 2002). [Google Scholar]
- 175.Bryant JD, Lei Y, VanPortfliet JJ, Winters AD & West AP Assessing mitochondrial DNA release into the cytosol and subsequent activation of innate immune-related pathways in mammalian cells. Curr. Protoc 2, e372 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pfanner N, Warscheid B & Wiedemann N Mitochondrial proteins: from biogenesis to functional networks. Nat. Rev. Mol. Cell Biol 20, 267–284 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hernansanz-Agustin P & Enriquez JA Functional segmentation of CoQ and cyt c pools by respiratory complex superassembly. Free Radic. Biol. Med 167, 232–242 (2021). [DOI] [PubMed] [Google Scholar]
- 178.Nesci S, Trombetti F & Pagliarani A Nicotinamide nucleotide transhydrogenase as a sensor of mitochondrial biology. Trends Cell Biol. 30, 1–3 (2020). [DOI] [PubMed] [Google Scholar]
- 179.Keilin D. The History of Cell Respiration and Cytochrome (Cambridge University Press, 1966). [Google Scholar]
- 180.Chance B & Williams GR Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem 217, 383–393 (1955). [PubMed] [Google Scholar]
- 181.Schmidt CA, Fisher-Wellman KH & Neufer PD From OCR and ECAR to energy: perspectives on the design and interpretation of bioenergetics studies. J. Biol. Chem 297, 101140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hernansanz-Agustin P & Enriquez JA Generation of reactive oxygen species by mitochondria. Antioxidants 10, 415 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Murphy MP How mitochondria produce reactive oxygen species. Biochem. J 417, 1–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Murphy MP et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab 4, 651–662 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Esteves F, Rueff J & Kranendonk M The central role of cytochrome P450 in xenobiotic metabolism-a brief review on a fascinating enzyme family. J. Xenobiot 11, 94–114 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Omura T & Gotoh O Evolutionary origin of mitochondrial cytochrome P450. J. Biochem 161, 399–407 (2017). [DOI] [PubMed] [Google Scholar]
- 187.Lambeth JD & Stevens VL Cytochrome P450scc: enzymology, and the regulation of intramitochondrial cholesterol delivery to the enzyme. Endocr. Res 10, 283–309 (1984). [DOI] [PubMed] [Google Scholar]
- 188.Koshiba T, Yasukawa K, Yanagi Y & Kawabata S Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci. Signal 4, ra7 (2011). [DOI] [PubMed] [Google Scholar]
- 189.McArthur K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, 6378 (2018). [DOI] [PubMed] [Google Scholar]
- 190.Riley JS et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 37, e99238 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cogliati S, Enriquez JA & Scorrano L Mitochondrial cristae: where beauty meets functionality. Trends Biochem. Sci 41, 261–273 (2016). [DOI] [PubMed] [Google Scholar]
- 192.Xian H. et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 55, 1370–1385 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang C & Youle RJ The role of mitochondria in apoptosis. Annu. Rev. Genet 43, 95–118 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Santos JH Mitochondria signaling to the epigenome: a novel role for an old organelle. Free Radic. Biol. Med 170, 59–69 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Csordas G, Weaver D & Hajnoczky G Endoplasmic reticulum–mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 28, 523–540 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Scorrano L. et al. Coming together to define membrane contact sites. Nat. Commun 10, 1287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Booth DM, Varnai P, Joseph SK & Hajnoczky G Oxidative bursts of single mitochondria mediate retrograde signaling toward the ER. Mol. Cell 81, 3866–3876 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shai N. et al. Systematic mapping of contact sites reveals tethers and a function for the peroxisome–mitochondria contact. Nat. Commun 9, 1761 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sabouny R & Shutt TE Reciprocal regulation of mitochondrial fission and fusion. Trends Biochem. Sci 45, 564–577 (2020). [DOI] [PubMed] [Google Scholar]
- 200.Twig G. et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433–446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pernas L & Scorrano L Mito-morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu. Rev. Physiol 78, 505–531 (2016). [DOI] [PubMed] [Google Scholar]
- 202.Simula L & Campello S Monitoring the mitochondrial dynamics in mammalian cells. Methods Mol. Biol 1782, 267–285 (2018). [DOI] [PubMed] [Google Scholar]
- 203.Santo-Domingo J, Giacomello M, Poburko D, Scorrano L & Demaurex N OPA1 promotes pH flashes that spread between contiguous mitochondria without matrix protein exchange. EMBO J. 32, 1927–1940 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zorov DB, Juhaszova M & Sollott SJ Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev 94, 909–950 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Picard M. et al. Trans-mitochondrial coordination of cristae at regulated membrane junctions. Nat. Commun 6, 6259 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Koren SA et al. All-optical spatiotemporal mapping of ROS dynamics across mitochondrial microdomains. Preprint at bioRxiv 10.1101/2023.01.07.523093 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schauss AC et al. A novel cell-free mitochondrial fusion assay amenable for high-throughput screenings of fusion modulators. BMC Biol. 8, 100 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vincent AE, Turnbull DM, Eisner V, Hajnoczky G & Picard M Mitochondrial Nanotunnels. Trends Cell Biol. 27, 787–799 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Molina AJ & Shirihai OS Monitoring mitochondrial dynamics with photoactivatable green fluorescent protein. Methods Enzymol. 457, 289–304 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nahacka Z, Zobalova R, Dubisova M, Rohlena J & Neuzil J Miro proteins connect mitochondrial function and intercellular transport. Crit. Rev. Biochem. Mol. Biol 56, 401–425 (2021). [DOI] [PubMed] [Google Scholar]
- 211.Silva CA et al. Activity-dependent regulation of mitochondrial motility in developing cortical dendrites. eLife 10, e62091 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sugiura A, McLelland GL, Fon EA & McBride HM A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 33, 2142–2156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cadete VJ et al. Formation of mitochondrial-derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system. J. Physiol 594, 5343–5362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Konig T. et al. MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control. Nat. Cell Biol 23, 1271–1286 (2021). [DOI] [PubMed] [Google Scholar]
- 215.Picard M. et al. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc. Natl Acad. Sci. USA 111, 4033–4042 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Picard M & Sandi C The social nature of mitochondria: implications for human health. Neurosci. Biobehav. Rev 120, 595–610 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Maslow AH A theory of human motivation. Psychol. Rev 50, 370–396 (1943). [Google Scholar]


