Abstract
Neurodegenerative diseases (ND) are an entire spectrum of clinical conditions that affect the central and peripheral nervous system. There is no cure currently, with treatment focusing mainly on slowing down progression or symptomatic relief. Cellular therapies with various cell types from different sources are being conducted as clinical trials for several ND diseases. They include neural, mesenchymal and hemopoietic stem cells, and neural cells derived from embryonic stem cells and induced pluripotent stem cells. In this review, we present the list of cellular therapies for ND comprising 33 trials that used neural stem progenitors, 8 that used differentiated neural cells ,and 109 trials that involved non-neural cells in the 7 ND. Encouraging results have been shown in a few early-phase clinical trials that require further investigations in a randomized setting. However, such definitive trials may not be possible given the relative cost of the trials, and in the setting of rare diseases.
Keywords: neural stem cells, Parkinson’s, cell therapy, clinical trials
In this review, we present the list of cellular therapies for 7 neurodegenerative diseases including 33 trials that utilized neural stem progenitors and 8 that utilized differentiated neural cells, discussing the outcomes and challenges. While encouraging results have been shown in a few of them, more work is required for precise differentiation into cells of interest, development of highly precise surgical techniques, and development of earlier diagnostic tools. Finally, trials have to stand the test of randomized placebo-controlled trials, measured against standardized disease rating scales with long-term outcomes measured to establish effectiveness of the treatment.
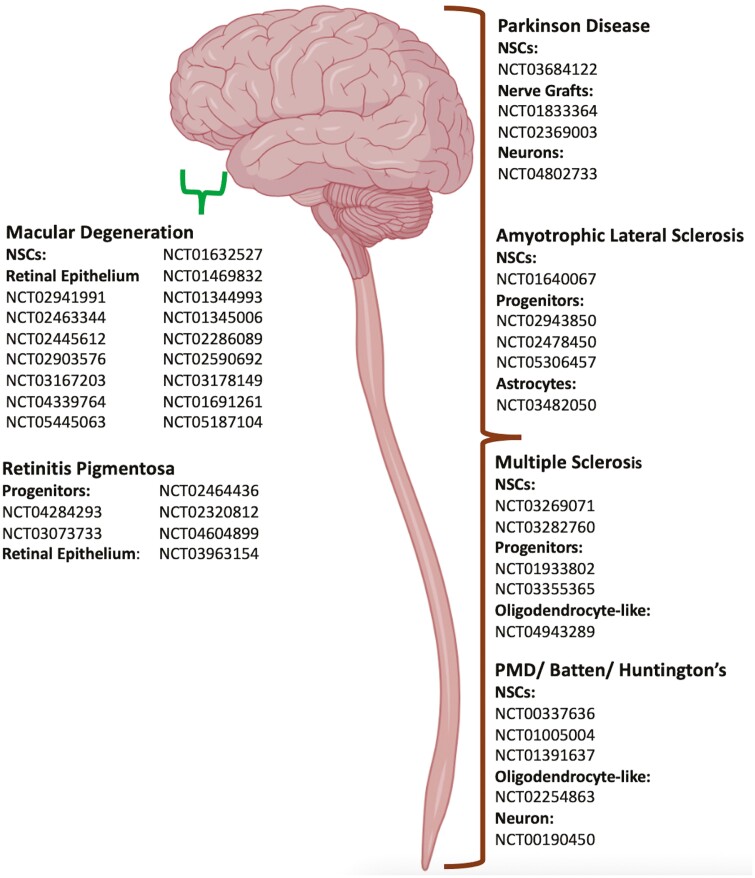
Graphical abstract
Graphical Abstract.
Introduction
Neural stem cells (NSCs) are multipotent cells found within the central nervous system (CNS) and can differentiate into neural lineages of neurons, glial, and oligodendrocytes.1-5 Sources of NSCs for clinical transplantation range from fetal neural tissues, transdifferentiated cells from embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs). Neurodegenerative diseases (ND) are disorders characterized by progressive loss of neurons associated with deposition of proteins showing altered physicochemical properties in the brain and in peripheral organs.6 Affecting hundreds of millions worldwide, they are debilitating and increasingly represent a major global health challenge worldwide today, with limited management options and mostly non-curative. A cellular therapy approach has been proposed to address the clinical gap to effect a cure for ND. The first clinical trials using stem cells in ND took place in mid-1980s with 3-400 participants treated with fetal cell transplantation for Parkinson’s disease (PD), in the open-label format. Subsequently, patients with Huntington’s have also experienced clinical benefits with implants of fetal neural grafts,7-9 although temporal. Since then, many other neural and non-neural cell transplantation has been investigated for a range of neurological conditions.
Cell transplantation of neural lineages can exert clinical benefits through multiple mechanisms, such as (1) working synergistically with the endogenous microenvironment to upregulate intrinsic cell proliferation or neuroprotection enhancing the overall regenerative capacity of the transplanted tissue and (2) integrating into the endogenous host network, replacing the cells of interests/injured cells10 as well as form functional synapses.11 As such, the focus of this review is to detail the latest clinical trials using NSCs and cells of neural lineage for the treatment of ND. Clinical trials involving stem cells from other sources have also been listed but not elaborated upon.
Methods
For each disease, a search of the disease name (Parkinson’s Disease, Amyotrophic Lateral Sclerosis, Multiple Sclerosis, Batten Disease, Pelizaeus-Merzbacher Disease, Huntington’s Disease, Macular Degeneration, Retinitis Pigmentosa, Alzheimer Disease, Niemann-Pick Diseases, Krabbe Disease, Gaucher Disease, Tay-Sachs Disease, Sandhoff Disease, Mucopolysaccharidoses, Spinocerebellar Ataxias, Dementia With Lewy Bodies, Frontotemporal Lobar Degeneration, Multiple System Atrophy), neural stem cells, neural cells, and cell therapy were performed on clinicaltrials.gov. Diseases with cell therapy using cells of neural lineage are discussed. They will also be listed in the tables, followed by the non-neural lineage cell clinical trials for each disease type. For this purpose, only interventional and not observational studies are included. Studies that were terminated, withdrawn, suspended, or unknown (past completion dates but status not verified for more than 2 years) were excluded. Similarly, studies not registered in clinicaltrials.gov were omitted from this study.
Parkinson’s Disease
Parkinson’s disease is a chronic ND characterized by the progressive loss of dopaminergic (DA) neurons in the substantia nigra. It is manifested by motor movement impairment such as bradykinesia, tremors, postural instability, and muscle rigidity. It can be effectively treated with medications such as levodopa, dopamine agonists, and monoamine oxidase inhibitors in the early stages but these medications are unable to stop the underlying neurodegeneration.12,13 To date, no curative therapies are available to reverse the progression of the disease processes.14 Since the late 80s, several groups have transplanted human fetal ventral mesencephalic (fVM) tissues into the caudate and putamen of PD patients, resulting in moderate amelioration of PD symptoms.15-19 Further analysis of the human clinical trials with fVM transplantation demonstrated clinical improvements in various parameters14 with the best outcomes for milder PD patients with a short disease duration. However, such grafts are associated with the development of dyskinesis,20,21 postulated to be due to the presence of serotonergic cells in the donated grafts.22 Han et al. have summarized in a book chapter the clinical outcomes of earlier trials of fetal brain-derived neural stem cell transplantation into PD patients. With the patients (n between 1 and 40) observed between 12 months and 24 years, symptom improvement was observed in 11 of the 13 trials, and dyskinesia was reported in 3 out of the 12 trials.23 Moreover, graft survival is low, which necessitates multiple donors per patient. With the advancements made in the DA neurons differentiation protocols, DA neurons can be differentiated from various cell sources (ESCs, iPSCs, and MSCs) and these more defined and differentiated populations of cells, being more homogenous have led to new waves of initiatives for cell transplantation in PD patients.14
There have been 18 trials listed on clinicaltrials.gov with 4 being neural lineage and directly delivered into the brain (Table 1). There is 1 trial utilizing NSCs derived from Wharton’s jelly (WJ) MSCs through in vitro differentiation (NCT03684122). The multipotency characteristic of MSCs enables them to differentiate into many cell types, including neurons and other neuronal cells.24 WJ-MSCs differentiated into NSCs were found to have enhanced therapeutic potential compared to undifferentiated WJ-MSCs. These NSCs preserve their immunomodulatory properties while displaying neuroectodermal characteristics.25 There are 2 other clinical trials using neural progenitor cells (NPCs)(NCT03309514 and NCT01329926) but both were withdrawn due to insufficient funding. Another 3 studies involved differentiated neural cells, specifically peripheral nerve grafts containing Schwann cells, and DA neurons differentiated from human embryonic stem cells. The 2 trials that implant autologous peripheral nerve graft directly into the substantia nigra showed that it is safe, feasible, and associated with clinical benefits.26,27 The challenge with the use of peripheral nerve grafts is that they contain heterogenous populations of cell types. While it has been proven feasible and safe, more data awaits on its efficacy.27
Table 1.
Clinical Trials listed on clinicaltrials.gov for Parkinson’s disease.
| No | Disease | Sponsor | Cell type | Route | Cell dose | Plannedparticipants | Year | Phase | Clinical trial # |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Parkinson disease | Craig Van Horne | Autologous peripheral nerve graft | Implanted to substantia nigra | Not mentioned | 8 | 2012 | na | NCT01833364 |
| 2 | Parkinson disease | Craig Van Horne | Autologous peripheral nerve graft | Implanted to substantia nigra | Not mentioned | 70 | 2015 | 1 | NCT02369003 |
| 3 | Parkinson disease | University of Jordan | Allogenic WJSC and its derived NSC | MSC (IV, IT), NSCs (IT) | MSC for iv (120-180 M), MSC for IT (240-260 M), NSC (24-36 MM) | 10 | 2018 | 1/2 | NCT03684122 |
| 4 | Advanced Parkinson disease | BlueRock Therapeutics | ESC-derived dopamine neurons (MSK-DA01) | Surgical transplanted into putamen | Not mentioned | 12 | 2021 | 1 | NCT04802733 |
| 5 | Parkinson disease | The Foundation for Orthopaedics and Regenerative Medicine | Allogenic UC-MSC | IV | 100M | 20 | 2011 | 1 | NCT05152394 |
| 6 | Neurodegenerative disease | Elliot Lander | Autologous adipose-derived stromal vascular fraction | IV, intra-articular, and soft-tissue injections. | Not mentioned | 3000 | 2013 | na | NCT01953523 |
| 7 | Stroke/TBI/Parkinson/ALS/Alzheimer/Dementia | MD Stem Cells | Autologous BMSC | IV and intranasal | Not mentioned | 500 | 2016 | na | NCT02795052 |
| 8 | TBI/Alzheimer’s/Dementia/Parkinson-Dementia/Late Encephalopathy | MD Stem Cells | Autologous BMSC | iv and intranasal | Not mentioned | 100 | 2018 | na | NCT03724136 |
| 9 | Parkinson disease | The University of Texas Health Science Center | Allogenic BM MSC | IV | 1,3,6, and 10 M/kg | 20 | 2017 | 1 | NCT02611167 |
| 10 | Parkinson disease | The University of Texas Health Science Center | Allogenic BM MSC | IV | 20 and 30 M/KG | 45 | 2020 | 2a | NCT04506073 |
| 11 | Parkinson disease | Belarusian Medical Academy of Post-Graduate Education | Autologous BM MSC | IV and intranasal | IV(0.5-2 M/kg), intranasal (5-12.6M) + IV (10-50 M) | 50 | 2017 | 2,3 | NCT04146519 |
| 12 | Parkinson disease | Hebei Newtherapy Bio-Pharma Technology Co., Ltd | MSC | IV | 10-20 M | 20 | 2018 | 1 | NCT03550183 |
| 13 | Multiple system atrophy/ Parkinsonism | Indonesia University | Autologous adipose MSC, allogeneic UC MSC | IT | Adipose MSC (100M), uc MSC (100M) | 15 | 2020 | na | NCT04876326 |
| 14 | Parkinson disease | Shanghai East Hospital | Human amniotic epithelial stem cells | Stereotactic implantation into lateral ventricle | 50 M | 3 | 2020 | 1 | NCT04414813 |
| 15 | Parkinson disease | Shanghai East Hospital | Human amniotic epithelial stem cells | Stereotactic implantation into lateral ventricle | 50 M | 12 | 2022 | 1 | NCT05435755 |
| 16 | Parkinson disease | Hope Biosciences Stem Cell Research Foundation | Allogenic adipose-derived MSC (HB-adMSCs) | IV | Not mentioned | 60 | 2021 | 2 | NCT04995081 |
| 17 | Parkinson disease | Hope Biosciences Stem Cell Research Foundation | Autologous adipose-derived MSC (HB-adMSCs) | IV | Not mentioned | 24 | 2021 | 2 | NCT04928287 |
| 18 | Idiopathic Parkinson disease | Taiwan Mitochondrion Applied Technology Co., Ltd | Autologous adipose-derived MSC (Mitocell) | Stereotactic intrastriatal implantation | 60 M and 200M | 9 | 2022 | 1 | NCT05094011 |
Abbreviations: ALS, amyotrophic lateral sclerosis; BM-MSC, bone marrow mesenchymal stem cells; BMSC, bone marrow stem cells; IM, intramuscular; IT, intrathecal; IV, intravenous; M, million; na, not applicable TBI, traumatic brain injury; UC, umbilical cord.
Amyotrophic Lateral Sclerosis
Amyotrophic Lateral Sclerosis (ALS) is a progressive motor neuron disease affecting motoneurons in the cortex, brain stems, and spinal cord with a worldwide incidence of 2-3 per 100 000.28 Affected patients suffers from progressive muscle weakness, atrophy, spasticity, and paralysis, culminating in death within 3-5 years. The majority (90%) of the cases are sporadic with the remaining 10% being familial ALS.29 As yet, there is neither a cure nor effective therapy to stop the progression of the disease. Stem cell transplantation has been proposed as a promising therapeutic option11,30 as they are equipped with the complex cellular machinery to modify the local environment through secretions of neurotrophic factors31,32 as well as possibly differentiate into astrocytes to increase the efficiency of glutamate re-uptake, a process disrupted in ALS.33 In addition, transplanted stem cells that differentiate into neuronal cells may form synapses with the native motor neurons, providing trophic, and/ or contact-mediated support.34 Several studies have demonstrated the safety and efficacy of stem cells from different sources such as fetal NSCs,35-37 peripheral blood stem cells,38,39 MSCs,40-46 and olfactory ensheathing cells, a differentiated cell of neural lineage47 in ALS patients.
Among the 32 cell transplantation clinical trials recorded, 5 are with cells that are neural-related and are delivered through direct injections (Table 2). Only one uses human fetal NSCs injected intraspinally, which showed the absence of progression of disease of all 6 enrolled participants for 18 months, with 2 experiencing improvements in ambulation scores.48 This work, extended to another 12 participants showed neither severe adverse effects nor increased disease progression over 60 months, and improvement in ALS functional rating scale between 1st and 4th month after transplantation.49 Next, Kadimastem et al. demonstrated that intrathecal injection of 100-250 million ESCs-derived astrocyte, AstroRx, significantly reduced the rate of disease progression by 6 months (NCT03482050).50 There are another 2 trials, 1 using glial restricted progenitor cells (Q cell) that has yet to initiate recruitment and another using human NPCs genetically engineered to hyper-express glial-derived neurotrophic factor (GDNF) that showed a single administration of these cells was safe and are able to provide new support cells and deliver GDNF up to 42 months post-transplantation.51
Table 2.
Clinical trials listed on clinicaltrials.gov for amyotrophic lateral sclerosis
| No | Disease | Sponsor | Cell type | Route | Cell dose | Planned participants | Year | Phase | Clinical trial # |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ALS | Azienda Ospedaliera Santa Maria, Terni, Italy | Human fetal NSC | Intraspinal | 2.25 or 4.5 M | 18 | 2011 | 1 | NCT01640067 |
| 2 | ALS | Cedars-Sinai Medical Center | Human neural progenitor cells expressing GDNF (CNS10-NPC-GDNF) | Unilateral lumbar spinal cord injections | 0.2 M, 0.5 M | 18 | 2017 | 1 | NCT02943850 |
| 3 | ALS | Kadimastem | ESC-derived astrocyte (AstroRx) | IT | 100 M, 250 M | 16 | 2018 | 1,2 | NCT03482050 |
| 4 | ALS | Q Therapeutics | Glial restricted progenitor cells (Q cell) | Transplantation into lumbar or cervical spinal cord | Lumbar (dose 1), cervical (dose 1,2,3,4,5) | 30 | 2021 | 1,2 | NCT02478450 |
| 5 | ALS | Cedars-Sinai Medical Center | Human neural progenitor cells expressing GDNF (CNS10-NPC-GDNF) | Injection into motor cortex | 5.25M, 10.5M | 16 | 2022 | 1 | NCT05306457 |
| 6 | ALS | Fundacion para la Formacion e Investigacion Sanitarias de la Region de Murcia | Autologous BMSC | Intraspinal | Not mentioned | 11 | 2007 | 1/2 | NCT00855400 |
| 7 | ALS | Fundacion para la Formacion e Investigacion Sanitarias de la Region de Murcia | Autologous BMSC | IT | Not mentioned | 63 | 2010 | 1/2 | NCT01254539 |
| 8 | ALS | The First Affiliated Hospital of Dalian Medical University | Autologous peripheral blood MNC | Transplant into subarachnoid space | Not mentioned | 14 | 2010 | na | NCT03085706 |
| 9 | ALS | Mayo Clinic | Autologous adipose MSC | Intraspinal | 1 M | 1 | 2010 | 1 | NCT01142856 |
| 10 | ALS | Mayo Clinic | Autologous adipose MSC | IT | 10, 50, 100 M, 200 M | 27 | 2012 | 1 | NCT01609283 |
| 11 | ALS | Mayo Clinic | Autologous adipose MSC | IT | 60-150M | 60 | 2017 | 2 | NCT03268603 |
| 12 | ALS | Corestem | Autologous BM MSC (HYNR-CS inj) | IT | 2 M/kg | 71 | 2011 | 1,2 | NCT01363401 |
| 13 | ALS | Corestem | Autologous BM MSC [Lenzumestrocel (Neuronata-R Inj.)] | IT | 2 M/kg or 5 M/kg | 115 | 2021 | 3 | NCT04745299 |
| 14 | ALS | Brainstorm-cell therapeutics | Autologous BM MSC secreting neurotrophic factors (MSC-NTF) | IT and IM | IT (24 M), I M (60 M) | 12 | 2011 | 1,2 | NCT01051882 |
| 15 | ALS | Brainstorm-cell therapeutics | autologous BM MSC secreting neurotrophic factors (MSC-NTF) | IM | 94 M, 141 M or 188 M | 14 | 2012 | 2 | NCT01777646 |
| 16 | ALS/ND | Hanyang University Seoul Hospital | Autologous BM MSC (HYNR-CS inj) | IT | 0.5,1 or 2 M/kg | 6 | 2012 | 1 | NCT01758510 |
| 17 | ALS | Bioinova, s.r.o. | Autologous BM MSC | IT | 15 ± 4.5 M cells | 26 | 2012 | 1,2 | NCT03828123 |
| 18 | ALS | Hospital Universitario Dr. Jose E. Gonzalez | Autologous HSC | IT | Not mentioned | 14 | 2012 | 2/3 | NCT01933321 |
| 19 | ALS | Royan Institute | Autologous BM MSC | IV | 2 M/kg | 6 | 2013 | 1 | NCT01759797 |
| 20 | ALS | Royan Institute | Autologous BM MSC | IT | 2 M/kg | 8 | 2013 | 1 | NCT01771640 |
| 21 | ALS | Royan Institute | Allogenic adipose MSC | IV | 2 M/kg | 19 | 2014 | 1 | NCT02492516 |
| 22 | ALS | Brainstorm-cell therapeutics | Autologous BM MSC (NurOwn) | IT and IM | Not mentioned | 48 | 2014 | 2 | NCT02017912 |
| 23 | ALS | Brainstorm-cell therapeutics | Autologous BM MSC(NurOwn) | IT | Not mentioned | 263 | 2017 | 3 | NCT03280056 |
| 24 | ALS | Andalusian initiative for advanced therapies - Fundación Pública Andaluza Progreso y Salud | Autologous adipose MSC | IV | 1,2 or 4 M/kg | 52 | 2014 | 1,2 | NCT02290886 |
| 25 | ALS | China Medical University Hospital | Autologous adipose MSC | IV and intracerebral | IV(200-800 M), IC (100-400 M) | 1 | 2015 | 1 | NCT02383654 |
| 26 | ALS | Hospital e Maternidade Dr. Christóvão da Gama | Autologous MSC | IT | 100 M | 3 | 2015 | 1 | NCT02987413 |
| 27 | ALS | Mossakowski Medical Research Centre Polish Academy of Sciences | Autologous adipose regenerative cells | Intraspinal/IT | 44 M | 30 | 2015 | 1 | NCT03296501 |
| 28 | ALS | Hadassah Medical Organization | Autologous BM MSC | IT | 4 M/kg | 20 | 2016 | 1,2 | NCT04821479 |
| 29 | Stroke/TBI/Parkinson/ALS/ Alzheimer/ Dementia | MD Stem Cells | Autologous BMSC | IV and intranasal | Not mentioned | 500 | 2016 | na | NCT02795052 |
| 30 | ALS | Polski Bank Komorek Macierzystych JSC (PBKM) | WJ MSC | IT | Not mentioned | 20 | 2020 | 1,2 | NCT04651855 |
| 31 | ALS | Rapa Therapeutics LLC | Autologous hybrid TREG/Th2 Cell (RAPA-501) | IV | 20 M or 80 M | 21 | 2020 | 1/2 | NCT04220190 |
| 32 | ALS | Fundacion para la Formacion e Investigacion Sanitarias de la Region de Murcia | Autologous BM MNC | IM | Not mentioned | 100 | 2021 | 2 | NCT04849065 |
Abbreviations: ALS, amyotrophic lateral aclerosis; BM, bone marrow; GDNF, glial-derived neurotrophic factors; HSC, hemopoietic stem cells; IM, intramuscular; IT, intrathecal; IV, intravenous; M, million; MNC, mononuclear cells; MSC, mesenchymal stem cells; na, not applicable NSC, neural stem cells; SC, stem cells; TBI, traumatic brain injury; UC, umbilical cord; WJ, Wharton Jelly.
MSCs are the most common cells used for the treatment of ALS (24 out of the 32 trials). Across the trials, 24 (73%) are phase I/II trials which are mainly safety trials with small patient samples, with 11 out of the 32 trials not specifying the cell dose. More studies are required to elucidate the most effective cells source and dose, method of administration, and frequency of transplantation,29 along with the ability to diagnose the disease earlier to improve outcomes of cell therapy before the onset of irreversible damage.52
Multiple Sclerosis
Multiple sclerosis (MS) is an immune-mediated demyelinating disease of the CNS resulting in CNS neurodegeneration and axonal loss. It is characterized by early acute lesions composed of discrete areas of inflammatory demyelination that either resolve by remyelination or evolve into chronic lesions with associated axonal loss, oligodendroglial cell loss, and glial scarring.25 Current disease modifying therapies may prevent or delay disease progression through immunosuppression and immunomodulation53-56 although beset with potentially serious adverse events.57 Once progressive disability is established, there is currently no cure.58
A total of 48 studies involving cell therapy for MS was retrieved (Table 3), with 5 cell sources being of neural lineage (10.4%), 22 using MSCs as donor cells, and 10 studies utilizing dendritic and T cells as an immunotherapy approach. Among the clinical trials using cells of neural lineage, 2 used autologous sources while 3 were allogenic (2 fetal and 1 umbilical cord blood). Intrathecal/intraventricular routes were the mode of administration for those cells. The first phase I trial commenced in 2014, with intrathecal autologous MSCs-derived NPCs given in 3 doses of 10 million cells 3 months apart.25 MSCs-derived NPCs are a subpopulation of MSCs that exhibit neuroectodermal lineage characteristics with reduced capacity to undergo mesodermal differentiation,59-63 minimizing risks of ectopic differentiation.64 Similar to MSCs, these NPCs exhibit immunoregulatory and trophic properties both in vitro and in vivo along with upregulation of trophic factors including hepatocyte growth factor,59,60,65 with encouraging results of improved muscle strength, and bladder function in half of the participants. Additionally, four-tenth of participants had a reduction in expanded disability status scale scores compared to baseline, with no serious adverse events reported.25
Table 3.
Clinical trials listed on clinicaltrials.gov for multiple sclerosis.
| No | Disease | Sponsor | Cell type | Route | Cell dose | Planned participants | Year | Phase | Clinical trial # |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MS | Tisch Multiple Sclerosis Research Center of New York | Autologous MSC-derived neural progenitors (MSC-NP) | IT | 6-30M | 20 | 2014 | 1 | NCT01933802 |
| 2 | MS | Tisch Multiple Sclerosis Research Center of New York | Autologous MSC-derived neural progenitors (MSC-NP) | IT | Not mentioned | 50 | 2018 | 2 | NCT03355365 |
| 3 | Progressive Multiple Sclerosis | IRCCS San Raffaele | Fetal-derived NSC | IT | 0.7 M, 1.4 M, 2.8 M, 5.7 M | 4 | 2017 | 1 | NCT03269071 |
| 4 | Secondary progressive MS | Casa Sollievo della Sofferenza IRCCS | Fetal-derived NSC | Intraventricular | 5, 10, 16, and 24 M | 24 | 2017 | 1 | NCT03282760 |
| 5 | Primary progressive MS | Joanne Kurtzberg | UCB-derived oligodendrocyte-like cells (DUOC-01) | IT | 10 M (3 pax), 10-25 M (3 pax), 25-50 M (16pax) | 20 | 2021 | 1a | NCT04943289 |
| 6 | MS | Baylor College of Medicine | Autologous CD34+ HSC | IV | 3 M | 10 | 1999 | 2 | NCT00040482 |
| 7 | MS | Ottawa Hospital Research Institute | Autologous CD34+ HSC | IV | Not mentioned | 24 | 2001 | 2 | NCT01099930 |
| 8 | MS | Hadassah Medical Organization | Autologous T cell | SubC | 10-30 M cells (×4 doses) | 30 | 2002 | 1/2 | NCT01448252 |
| 9 | Relapse Remitting MS/secondary Progressive MS | Opexa Therapeutics | Autologous myelin reactive T cell (Tovaxin) | SubC | 6-9 M, 30-45 M, or 60-90 M | 16 | 2002 | 1/2 | NCT00587691 |
| 10 | Relapse Remitting MS | Opexa Therapeutics | Autologous myelin reactive T cell (Tovaxin) | SubC | 30-45 M | 150 | 2006 | 2 | NCT00245622 |
| 11 | MS/secondary Progressive MS | Opexa Therapeutics | Autologous T cell (Tcelna/ Imilecleucel-T) | SubC | 30-45M (×5 a yr) ×2 yrs | 183 | 2012 | 2 | NCT01684761 |
| 12 | MS | Northwestern University | Autologous mobilized HSC | IV | Not mentioned | 110 | 2005 | 2 | NCT00273364 |
| 13 | Relapse Remitting MS | National Institute of Allergy and Infectious Diseases (NIAID) | Autologous CD34+ HSC | IV | Not mentioned | 25 | 2006 | 2 | NCT00288626 |
| 14 | Relapsing MS/relapsing remitting MS/secondary progressive MS | National Institute of Allergy and Infectious Diseases (NIAID) | Autologous HSC | IV | 4-7.5M CD34+ cells/kg | 156 | 2019 | 3 | NCT04047628 |
| 15 | MS | Hadassah Medical Organization | Autologous BM MSC | IT and IV | IT (1M/kg) and IV (0.3-1M/kg) | 24 | 2006 | 1/2 | NCT00781872 |
| 16 | MS | Hadassah Medical Organization | Autologous BM MSC | IT and IV | IT (1M/kg) and IV (1M/kg) up to 8 times | 24 | 2013 | 1/2 | NCT04823000 |
| 17 | MS | Talaris Therapeutics | Allogenic HSC | IV | Not mentioned | 3 | 2007 | 1/2 | NCT00497952 |
| 18 | MS | University of Cambridge | Autologous BM MSC | IV | up to 2 M/kg | 10 | 2008 | 1/2 | NCT00395200 |
| 19 | MS/autoimmune disease/cerebellar degeneration/ | Fred Hutchinson Cancer Center | Autologous or syngeneic peripheral blood stem cell | IV | At least 4 M CD34+ cells/kg | 80 | 2008 | 2 | NCT00716066 |
| 20 | Autoimmune disease/nervous system disease | Andalusian Initiative for Advanced Therapies - Fundación Pública Andaluza Progreso y Salud | Autologous adipose MSC | IV | 4 M/kg | 30 | 2010 | 1/2 | NCT01056471 |
| 21 | MS | Andalusian Initiative for Advanced Therapies - Fundación Pública Andaluza Progreso y Salud | Autologous BM MSC | IV | 1-2 M/kg | 26 | 2013 | 1/2 | NCT01745783 |
| 22 | MS | Royan Institute | Autologous BM MSC | IV | Not mentioned | 22 | 2011 | 1/2 | NCT01377870 |
| 23 | Relapse remitting MS/secondary progressive MS/progressive relapsing MS | The Cleveland Clinic | Autologous BM MSC | IV | 1-2 M/kg | 24 | 2011 | 1 | NCT00813969 |
| 24 | MS | University of Jordan | Autologous BM MSC | IT | 13 | 2012 | 2 | NCT01895439 | |
| 25 | MS | University of Jordan | Allogenic WJ MSC | IT and IV | IT(200 M) with 100 M (iv) | 60 | 2017 | 1/2 | NCT03326505 |
| 26 | Progressive MS | North Bristol NHS Trust | Autologous BM cells | IV | Not mentioned | 80 | 2012 | 2 | NCT01815632 |
| 27 | MS | North Bristol NHS Trust | Autologous BM cells | IV | Not mentioned | 4 | 2014 | na | NCT01932593 |
| 28 | Relapse remitting MS | Germans Trias i Pujol Hospital | Autologous BM MSC | IV | 1 M/kg | 9 | 2012 | 1/2 | NCT02035514 |
| 29 | MS | Antonio Uccelli | Autologous BM MSC | IV | 1-2m M/kg | 20 | 2012 | 1/2 | NCT01854957 |
| 30 | MS | Imperial College London | Autologous BM MSC | IV | 1-2 M/kg | 21 | 2012 | 1/2 | NCT01606215 |
| 31 | MS | Karolinska Institutet | Autologous BM MSC | IV | 1-2 M/kg | 7 | 2012 | 1 | NCT03778333 |
| 32 | MS | Translational Biosciences | Allogenic WJ MSC | IV | 140 M (over 7 days, 20 M/day) | 20 | 2014 | 1/2 | NCT02034188 |
| 33 | MS | Sara Varea | Autologous tolerogenic dendritic cells | IV | Not mentioned | 20 | 2015 | 1 | NCT02283671 |
| 34 | MS | Ottawa Hospital Research Institute | Autologous BM MSC | IV | 1-2 M/kg | 31 | 2015 | 2 | NCT02239393 |
| 35 | Relapse remitting MS/secondary progressive MS | Banc de Sang i Teixits | Autologous BM MSC (XCEL-MC-ALPHA) | IV | Not mentioned | 8 | 2015 | 1/2 | NCT02495766 |
| 36 | MS | Dimitrios Karussis | Autologous BM MSC | IT or IV | 1 M/kg | 48 | 2015 | 2 | NCT02166021 |
| 37 | Relapse remitting MS/secondary progressive MS | Centro de Hematología y Medicina Interna | Autologous peripheral blood stem cells | IV | At least 1 M/kg CD34+ cells | 1000 | 2015 | na | NCT02674217 |
| 38 | Relapse-remitting/chronic progressive MS | Fundació Institut Germans Trias i Pujol | Autologous VitD3 tolerogenic monocyte derived dendritic cells (tolDC-VitD3) | intranodal | 5 M, 10 M, 15 M | 16 | 2017 | 1 | NCT02903537 |
| 39 | MS | University Hospital, Antwerp | Autologous tolerogenic dendritic cells (tolDC) | intradermal | 5 M, 10 M, 15 M | 9 | 2017 | 1 | NCT02618902 |
| 40 | Primary and secondary progressive MS | Atara Biotherapeutics | Allogenic T Cell (ATA188) | IV | Not mentioned | 265 | 2017 | 1/2 | NCT03283826 |
| 41 | Chronic progressive MS | Brainstorm-Cell Therapeutics | Autologous BM MSC (NurOwn) | IT | Not mentioned (3 doses) | 20 | 2019 | 2 | NCT03799718 |
| 42 | Relapse-remitting MS | Judith Pich | Autologous peripheral blood differentiated adult tolerogenic dendritic cells | IV | Not mentioned | 45 | 2020 | 2 | NCT04530318 |
| 43 | Relapse remitting MS | FibroBiologics | Tolerogenic fibroblasts | IV | 100 M | 5 | 2020 | 1 | NCT05080270 |
| 44 | MS | Nantes University Hospital | Autologous EBV-specific cytotoxic T-cell lymphocytes | IV | Not mentioned | 7 | 2021 | 1 | NCT02912897 |
| 45 | Relapse remitting MS | Uppsala University | Autologous HSC | IV | Not mentioned | 200 | 2021 | na | NCT05029206 |
| 46 | MS | Hope Biosciences Stem Cell Research Foundation | Adipose MSC (HB-adMSCs) | IV | Not mentioned | 24 | 2021 | 2 | NCT05116540 |
| 47 | Progressive MS | Haukeland University Hospital | Autologous BM MSC | IT | Not mentioned | 18 | 2021 | 1/2 | NCT04749667 |
| 48 | MS | ImStem Biotechnology | ESC MSC (IMS001) | IV | Low or high dose | 30 | 2021 | 1 | NCT04956744 |
Abbreviations: BM, bone marrow; BMSC, bone marrow stem cells; ESC; embryonic stem cells; HSC, hemopoietic stem cells; IT, intrathecal; IV, intravenous; M, million; MS. Multiple sclerosis; MSC, mesenchymal stem cells; na, not applicable NSC, neural stem cells; SubC, subcutaneous; UCB, umbilical cord blood; WJ, Wharton Jelly.
There had been some success reported with immunotherapy, such as the use of autologous T cells primed against myelin basic protein, myelin oligodendrocyte glycoprotein, and proteolipid protein (PLP) resulting in a reduced annualized relapse rate in a randomized controlled trial.57 The large majority of trials using MSCs were of small number (n = 7-60), which showed similar outcomes with either intravenous or intrathecal approaches.40,66-69
Rare Neurodegenerative Diseases
A group of rare ND has been grouped into this section including Pelizaeus-Merzbacher disease (PMD), Batten disease, and a few enzymes deficiency disorders (Table 4). PMD is an X-linked hypomyelinating disorder that progressively degenerate the white matter of the brain causing problems in learning, coordination, and motor skills.70-72 Gene duplications and point mutation of the PLP1 gene result in abnormal myelin production or abnormal trafficking in the endoplasmic reticulum of oligodendrocytes.73,74 Neuronal Ceroid Lipofuscinosis (NCL), also known as Batten disease is a heterogenous class of lysosomal storage disease characterized by neurodegeneration that share common clinical features of progressive visual and cognitive decline.75
Table 4.
Clinical trials listed on clinicaltrials.gov for Batten and Pelizaeus-Merzbacher diseases.
| No | Disease | Sponsor | Cell type | Route | Cell dose | Planned participants | Year | Phase | Clinical trial # |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Batten disease | StemCells, Inc | CNS-SC | Stereotactic injection into subcortical sites and lateral ventricles | Low dose (700M), high dose (1400M) | 6 | 2006 | 1 | NCT00337636 |
| 2 | PMD | StemCells, Inc. | CNS-SC | Intracerebral | 300 M | 4 | 2009 | 1 | NCT01005004 |
| 3 | PMD | StemCells, Inc. | CNS-SC | Intracerebral | 300 M | 4 | 2011 | 1 | NCT01391637 |
| 4 | Batten/MPS II/NP/PMD/inborn metabolic brain disease/ | Joanne Kurtzberg | UCB derived oligodendrocyte-like cells (DUOC-01) | IT | 50.1-350.1 M/kg | 12 | 2014 | 1 | NCT02254863 |
| 5 | Batten/NP/Gaucher’s/ | Masonic Cancer Center | allogenic BM and UCB HSC | IV | Not mentioned | 135 | 1995 | 2,3 | NCT00176904 |
| 6 | NP/PMD/Tay Sachs/Krabbe/Batten/Sandhorf disease | Talaris Therapeutics Inc | allogenic HSC | IV | Not mentioned | 30 | 2011 | 1,2 | NCT01372228 |
| 7 | Batten/MPS I and IV/NP/Gauchers/Krabbe/Fucosidosis | New York Medical College | placental derived SC and cord blood | IV | Not mentioned | 43 | 2013 | 1 | NCT01586455 |
Abbreviations: BM, bone marrow; CNS SC, central nervous system neural stem cells; HSC, hemopoietic stem cells; IT, intrathecal; IV, intravenous; M, million; MPS, Mucopolysaccharidosis; NP, Niemann-Pick; PMD, Pelizaeus-Merzbacher disease; SC, stem cells; UCB, umbilical cord blood.
To date, there have been a few stem cell based clinical trials, with cell sources ranging from human fetal CNS-derived NSCs to hemopoietic stem cells (HSCs) from BM and perinatal tissues including placental, cord, and cord blood. Direct CNS delivery of allogeneic CNS-derived NSCs was used in 3 trials and umbilical cord blood-derived oligodendrocyte-like cells in one. The use of human CNS stem cells for treatment of NCL is a first-in-human clinical trial involving a purified population of human NSCs, for a ND (NCT00337636). Results concluded that neural cell transplantation in NCL is largely safe, with evidence of long-term engraftment determined at post-mortem, suggesting a therapeutic role for NSCs in ND. Nevertheless, it remains crucial to target earlier disease stages for a better runway to curtail disease progression and potential clinical improvement.76
In the case of PMD, a phase I trial with fetal NSCs in 4 PMD participants showed successful engraftment and donor-derived myelination in 2009.[71,72] However, there was evidence of disease progression in 2 subjects at long-term follow-up. There was evidence of donor-specific alloantibodies, which may necessitate immunosuppressive therapies for such allogeneic cell therapy. Kurtzberg et al. performed a clinical trial using cord blood-derived oligodendrocyte-like cells (DUOC-01) for inherited metabolic diseases (NCT02254863).77 Preliminary results on participants (n = 7) with Batten disease demonstrated safety and feasibility parameters for transplantation of DUOC-01, and promising results in 4 out of the 5 survivors showing no evidence of the disease on magnetic resonance imaging.78
The rarity of such ND makes it challenging to recruit patients for any trials. As the use of autologous cells is precluded in genetic diseases, unless in the context of gene-edited approaches, the immediate focus should be the development of effective immunosuppressive protocols, and early administration of allogeneic stem cells.75,79
Huntington’s Disease
Huntington’s disease (HD) is an autosomal dominant ND where cognitive, motor, and psychiatric abilities progressively decline over 2-3 decades. HD mainly affects the medium spiny striatal neurons, making it an attractive target for cell therapy.80 Of the 4 trials retrieved, only 1 used fetal neuronal tissue (Table 5). In that seminal French study, human fetal-derived graft demonstrated survival and significant improvements in both motor and cognitive function in 3 participants over a 6-year period7,81 but faded off thereafter suggesting the lack of a permanent cure. The other 3 trials involved the use of MSCs, specifically Cellavita-HD, with the latest phase II/III trials initiated in 2020.
Table 5.
Clinical Trials listed on clinicaltrials.gov for Huntington’s disease
| No | Disease | Sponsor | Cell type | Route | Cell dose | Planned participants | Year | Phase | Clinical trial # |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Huntington’s disease | Assistance Publique - Hôpitaux de Paris | Fetal neurons | Intracerebral | Not mentioned | 54 | 2002 | 2 | NCT00190450 |
| 2 | Huntington’s disease’s | Azidus Brasil | Immature dental pulp MSC (NestaCell-HDTM) | IV | 3 M/kg or 6 M/kg | 6 | 2017 | 1 | NCT02728115 |
| 3 | Huntington’s disease | Azidus Brasil | Immature dental pulp MSC (NestaCell-HDTM) | IV | 9 M/kg or 18 M/kg | 35 | 2018 | 2 | NCT03252535 |
| 4 | Huntington‘s disease | Azidus Brasil | Immature dental pulp MSC (NestaCell-HDTM) | IV | 24 M/kg | 35 | 2020 | 2,3 | NCT04219241 |
Abbreviations: IV, intravenous; M, million; MSC, mesenchymal stem cells.
There are many other active clinical trials that aim to treat HD, as reviewed by Kim et al.,82 with stem cell therapies accounting for around 10% (3/28). The advantage of stem cells is that the new neurons can replace degenerating cells in the affected brain regions and, consequently, ameliorate the disease profile. This efficacy might be further improved by engineering the cells to hyper-secrete BDNF, GDNF, or other neurotropic factors or combining stem cells with genetic modification therapy for HD.
Macular Degeneration
There are 2 main forms of macular degeneration (MD); Stargardt disease (STGD1) being the most common cause of MD in children and young adults,83 and age-related macular degeneration affecting up to 8.7% of the world’s population84 and Stargardt disease results from ABCA4 gene mutations85 and results in progressively severe impairment of sight. Atrophic age-related MD shares the key features of progressive atrophy of retinal pigment epithelium (RPE) and overlying photoreceptor cells as STGD1,83 while age-related MD is due to exposure to immune-mediated and oxidative stresses from genetic and environmental factors.86 They are currently incurable although replenishment of degenerating RPE cells with healthy cells offers the possibility of benefit by supporting the function and survival of overlying photoreceptor cells.86
There are 22 trials listed of which 16 involved neural-related cells (Table 6). ESCs-derived RPE cells from the ESCs line MA09 were used in 6 of these trials. The first ESCs-derived RPE cells were initiated in 2011 with 12 participants (NCT01469832) where subretinal injections of 200 000 ESCs-RPE cells were found to be safe, although no benefit was established.83 Another 2 trials (NCT01345006 for STGD1 and NCT01344993 for age-related MD) demonstrated similar reassuring safety outcomes using the same hESCs-RPE source for up to 37 months post-transplantation.86,87 In these 2 trials where only 1 eye was treated, best-corrected visual acuity (BCVA) improved in the majority of treated eyes (10 out of 17) in addition to improvements in vision-related quality-of-life measures.86 Since then, there have been at least 4 different ESCs derived RPE cell products (CPCB-RPE1, ASP7316, ASP7317, and PF-05206388) developed by 3 companies that are currently in phase I/II clinical trials for different forms of MD (Table 6). Among them, CPCB-RPE1 has been shown to be safe and well tolerated by participants with advanced dry age-related MD at 1-year post-transplantation with improvements of BCVA in 4 out of 15 participants.88 Instances of focally reduced sensitivity and thinning in the hyperpigmented retina at higher doses of hESCs derived RPE has been observed which suggest the potential for harm and indicate that intervention at earlier stages of degeneration should be approached with caution.83
Table 6.
Clinical trials listed on clinicaltrials.gov for macular degeneration.
| No | Disease | Sponsor | Cell type | Route | Cell dose | Planned participants | Year | Phase | Clinical trial # |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Stargartd disease | Astellas Institute for Regenerative Medicine | ESC RPE (MA09-hRPE) | Subretinal | 50 000, 100 000, 150 000 and 200 000 | 12 | 2011 | 1/2 | NCT01469832 |
| 2 | Stargartd disease | Astellas Institute for Regenerative Medicine | ESC RPE (MA09-hRPE) | Subretinal | 50 000, 100 000, 150 000 and 200 000 | 12 | 2013 | 1/2 | NCT02941991 |
| 3 | AMD | Astellas Institute for Regenerative Medicine | ESC RPE (MA09-hRPE) | Subretinal | 50 000, 100 000, 150 000 and 200 000 | 13 | 2011 | 1/2 | NCT01344993 |
| 4 | AMD | Astellas Institute for Regenerative Medicine | ESC RPE (MA09-hRPE) | Subretinal | 50 000, 100 000, 150 000 and 200 000 | 11 | 2013 | 1/2 | NCT02463344 |
| 5 | Stargartd disease | Astellas Institute for Regenerative Medicine | ESC RPE (MA09-hRPE) | subretinal | 50 000, 100 000, 150 000 and 200 000 | 13 | 2011 | 1/2 | NCT01345006 |
| 6 | Stargartd disease | Astellas Institute for Regenerative Medicine | ESC RPE (MA09-hRPE) | Subretinal | 50 000, 100 000, 150 000 and 200 000 | 13 | 2012 | 1/2 | NCT02445612 |
| 7 | AMD | StemCells, Inc | CNS SC | Subretinal | 200 000/ 1 M | 15 | 2012 | 1/2 | NCT01632527 |
| 8 | AMD | Lineage Therapeutics/Hoffmann-La Roche | ESC RPE (Opregen) | Subretinal | 50 000-200 000 | 24 | 2015 | 1/2 | NCT02286089 |
| 9 | AMD/Stargartd | Federal University of São Paulo | ESC RPE | Subretinal | 100 000 | 15 | 2015 | 1/2 | NCT02903576 |
| 10 | Dry MD/GA | Regenerative Patch Technologies, LLC | ESC RPE (CPCB-RPE1) | Subretinal | 100 000 | 16 | 2016 | 1/2 | NCT02590692 |
| 11 | Macular degenerative disease | Astellas Institute for Regenerative Medicine | ESC RPE (ASP7316) | Subretinal | Not mentioned | 36 | 2018 | 1/2 | NCT03167203 |
| 12 | AMD | Astellas Institute for Regenerative Medicine | ESC RPE (ASP7317) | Into the macular | Low, med, high cell dose | 18 | 2018 | 1 | NCT03178149 |
| 13 | AMD | National Eye Institute | Autologous iPSC RPE | Subretinal | Not mentioned | 20 | 2020 | 1/2a | NCT04339764 |
| 14 | AMD | Moorfields Eye Hospital NHS Foundation Trust | ESC RPE (PF-05206388) | Intraocular | Not mentioned | 10 | 2021 | 1 | NCT01691261 |
| 15 | Macular degeneration | Beijing Tongren Hospital | Autologous iPSC RPE | Subretinal | Not mentioned | 10 | 2022 | 1 | NCT05445063 |
| 16 | AMD | Institute of Biophysics and Cell Engineering of National Academy of Sciences of Belarus | Autologous cultured retinal stem and progenitor cells | Subretinal | Not mentioned | 20 | 2022 | 1/2 | NCT05187104 |
| 17 | AMD | Janssen Research & Development | Umbilical tissue cells (CNTO2476) | Subretinal | 60 K, 120 K, 300 K, 560 K | 39 | 2010 | 1/2 | NCT01226628 |
| 18 | GA MD | Janssen Research & Development | Umbilical tissue cells (CNTO2476) | Subretinal | 0.3 M | 21 | 2015 | 2 | NCT02659098 |
| 19 | AMD/Stargartd disease | University of San Paulo | Autologous BM SC | Intravitreal | 10 M | 20 | 2011 | 1/2 | NCT01518127 |
| 20 | Retinopathy/Age-related and hereditary macular degeneration | University of California | Autologous BM SC | Intravitreal | Not mentioned | 15 | 2012 | 1 | NCT01736059 |
| 21 | Retinopathy/Age-related and hereditary macular degeneration | MD Stem Cells | Autologous BM SC | Retrobulbar, intravitreal, subretinal, intraoptic | 240 M (retrobulbar), 4 M (intravitreal), 8 M (subretinal, intraoptic) | 500 | 2016 | na | NCT03011541 |
| 22 | Macular degeneration/RP | Foundation of Orthopaedics and Regenerative Medicine | UC MSC | IV, subtenon | 100 M | 20 | 2022 | 1 | NCT05147701 |
Abbreviations: AMD, age-related macular degeneration; BM SC, bone marrow stem cells; CNS SC, human central nervous system neural stem cells; ESC, embryonic stem cells; GA, geographic atrophy; iPSC, induced pluripotent stem cells; IV, intravenous; million; na, not applicable RP, retinitis pigmentosa; RPE, retinal pigment epithelium; UC MSC, umbilical cord mesenchymal stem cells.
The first clinical trial using autologous human iPSCs derived RPE for MD was led by Dr Takahashi in 1 patient in 2013.89 The patient suffered no severe adverse event or rejection aside from cystoid macular edema, with BCVA remaining stable at 1-year post-transplantation (JPRN-UMIN000011929).89 There are currently 2 other autologous iPSCs-RPE trials for MD registered (NCT04339764 in 2020 and NCT05445063 in 2022).
The majority of the donor cells for treatment of MD are generally differentiated RPE cell types. Nonetheless, we found 6 trials using autologous bone marrow stem cells (BMSCs), human umbilical tissue-derived cells, and allogenic umbilical cord MSC for cell therapy through various routes (Table 6). There are currently no phase III trials using cell therapy for MD despite the first trial taking place back in 2011, alluding to the difficulties in developing effective cell therapies for MD.
Retinitis Pigmentosa
Retinitis pigmentosa (RP) is an inherited retinal dystrophy that is characterized by the onset of night blindness, loss of peripheral vision, and loss of central vision in the later stages through the death of photoreceptors.90 There is no effective treatment for RP as once photoreceptors are lost, they do not regenerate, with most being legally blind by age 40.
Six of the 19 cell therapy trials are performed with cells of neural lineage (Table 7). Five of them used retinal progenitor cells (RPCs) that can be expanded and differentiated into retinal cells, and have been derived from either fetal ocular tissues (n = 4) or ESCs (n = 1). Transplanted RPCs have not been rejected immunologically, nor formed tumors in the absence of immunosuppressants.91,92 Another trial transplanting 3-6 million fetal derived-RPCs intravitreally (n = 54) was shown to be safe at 12 months follow-up. Based on the results published on clinicaltrials.gov, there seems to have beneficial effects at the higher cell dose with significant improvement of BCVA and other measures between treated and non-treated eyes.92 There is a single trial that utilizes human fetal NPCs directly instead of more differentiated and lineage-restricted retinal progenitor cells (NCT04284293), while others used BM-derived HSCs, or MSCs from different sources (Table 7). More research needs to be focused on (1) improving the efficacy of transplants with photoreceptor potential, (2) improve survival of cells implanted in the subretinal space in areas of geographic atrophy, and (3) factors impacting the immunological readiness of the subretinal space to facilitate longer survival of the transplanted cells.93
Table 7.
Clinical trials listed on clinicaltrials.gov for retinitis pigmentosa.
| No | Disease | Sponsor | Cell type | Route | Cell dose | Planned participants | Year | Phase | Clinical trial # |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Retinitis Pigmentosa | ReNeuron Limited | fetal RPC | Subretinal | 200K, 500K, 1M | 29 | 2015 | 1/2 | NCT02464436 |
| 2 | Retinitis Pigmentosa | jCyte, Inc | Fetal RPC (jCell) | Intravitreal | 0.5-3M | 38 | 2015 | 1/2 | NCT02320812 |
| 3 | Retinitis Pigmentosa | jCyte, Inc | Fetal RPC (jCell) | Intravitreal | 3 or 6M | 84 | 2017 | 2 | NCT03073733 |
| 4 | Retinitis Pigmentosa | jCyte, Inc | Fetal RPC (jCell) | Intravitreal | 6M | 30 | 2020 | 2 | NCT04604899 |
| 5 | Retinitis Pigmentosa due to monogenic mutation | Centre d’Etude des Cellules Souches | ESCs retinal pigment epithelium | Subretinal | not mentioned | 12 | 2019 | 1/2 | NCT03963154 |
| 6 | Retinitis Pigmentosa | Cedars-Sinai Medical Center | Fetal neural progenitor cells (CNS10-NPC) | Subretinal | 300K and 1M | 16 | 2021 | 1/2 | NCT04284293 |
| 7 | Retinitis Pigmentosa | University of Sao Paulo | Autologous BM SC | Intravitreal | 10M | 5 | 2009 | 1 | NCT01068561 |
| 8 | Retinitis Pigmentosa | University of Sao Paulo | Autologous BM SC | Intravitreal | 10M | 50 | 2011 | 2 | NCT01560715 |
| 9 | Retinitis Pigmentosa | University of California, Davis | Autologous BM CD34+ cells | Intravitreal | 1-7 M | 15 | 2012 | 1 | NCT01736059 |
| 10 | Retinitis Pigmentosa | University of California, Davis | Autologous BM CD34+ cells | Intravitreal | Not mentioned | 4 | 2021 | 1 | NCT04925687 |
| 11 | Retinitis Pigmentosa | Mahidol University | Autologous BM MSC | Intravitreal | 1 M | 14 | 2012 | 1 | NCT01531348 |
| 12 | Retinitis Pigmentosa | Red de Terapia Celular | Autologous BM SC | Intravitreal | 5-60 M | 8 | 2014 | 1 | NCT02280135 |
| 13 | Retinitis Pigmentosa | MD Stem Cells | Autologous BM SC | Retrobulbar, Intravitreal, Subretinal, Intraoptic | 240 M (retrobulbar), 4M(intravitreal), 8 M (subretinal, intraoptic) | 500 | 2017 | na | NCT03011541 |
| 14 | Retinitis Pigmentosa | PT. Prodia Stem Cell Indonesia | UC MSC | Peribulbar | 1 M | 18 | 2018 | 1/2 | NCT04315025 |
| 15 | Retinitis Pigmentosa | Ankara Universitesi Teknokent | WJ MSC | Subtenon | 2-6 M | 32 | 2019 | 3 | NCT04224207 |
| 16 | Retinitis Pigmentosa | Jinnah Burn and Reconstructive Surgery Centre, Lahore | UC MSC | Subtenon, suprachoroidal | Not mentioned | 50 | 2021 | 2 | NCT04763369 |
| 17 | Retinitis Pigmentosa | University of California, Davis | Autologous BM CD34+ cells | Intravitreal | Not mentioned | 4 | 2021 | 1 | NCT04925687 |
| 18 | Retinitis Pigmentosa | TC Erciyes University | WJ MSC or exosomes | Subtenon | Not mentioned | 135 | 2022 | 2,3 | NCT05413148 |
| 19 | Retinitis Pigmentosa | The Foundation for Orthopaedics and Regenerative Medicine | UC MSC | IV and subtenon | 100 M | 20 | 2022 | 1 | NCT05147701 |
Abbreviations BM, bone marrow; ESC, embryonic stem cells; IV, intravenous; MSC, mesenchymal stem cells; na, not applicable RP, retinitis pigmentosa; RPC, retinal progenitor cells; SC, stem cells; UC, umbilical cord; WJ, Wharton Jelly.
Discussion
We found 8 clinical trials involving NSCs, 10 involving neural progenitors, and 23 involving differentiated neural cells (Supplementary Table S1). However, only clinical trials listed on clinicaltrials.gov were covered and while it is the largest clinical trials registry, this would inevitably exclude those only listed on other trials’ databases such as EU Clinical Trials Register and Japan Registry for Clinical Trials and others. NSCs have shown potential for the treatment of ND but have yet to be fully exploited. The mammalian brain has a limited ability for repair and regeneration through neurogenesis and gliogenesis. Of the available cell sources for therapy, NSCs have an advantage over the other cell sources as it can not only contribute to multipotent differentiation in replacing neural and non-neural cell types but also participate in modulating inflammatory damage through the secretion of trophic factors. Fetal tissue grafts on the other hand require multiple donors due to the small quantity of stem cells per donor and poor graft survival, with the need to coordinate collection, processing, and analysis before transplantation, and thus presents a significant logistical challenge. While tissue grafts serve to replace the required cells and produce cytokine/growth hormones etc., more such as repair of pathways, axonal outgrowth restorations are existing challenges that hamper the engraftment of tissue grafts, and their overall survival.94 Bioengineered scaffolds that reproduce the conditions of the extracellular matrix, enhance exchange, and crosstalk between different cell types, engrafted and hosts’ cells, and healthy and injured tissues while providing cell support will be a step forward. The use of advanced cryopreservation techniques such as vitrification may help address some logistical issues with the scheduling of neurosurgical transplantation.95 The use of hESCs-derived RPE has been extensively used for MD, which presents a theoretical risk of immune rejection. It is hoped that the advent of autologous-iPSCs-derived RPE/RPCs may circumvent the immune considerations although both hESCs and iPSCs-derived cells have tumorigenic potential albeit a small risk. To err on the side of caution, differentiated cells are preferred (Supplementary Table S1), where a very consistent and high purity cells of interest can be obtained for clinical use, doing away with the dangers of tumorigenicity and/or unintended differentiation or proliferation of stem cells. Further improvements in precise differentiation of the desired cells from different cell sources, with minimal risk of tumorigenicity remain a priority80,96 with standardization in the characterization of these cells to define its multi-potency or lineage-restriction. For cell therapy via direct neurotransplantation, the need to develop minimally invasive but highly precise surgical techniques is also crucial and it is timely for now, with the higher resolution imaging currently available, to allow accurate graft placement, and post-surgery observation.14 It is also important to design randomized placebo-controlled trials while standardizing the disease rating scales and long-term measurable outcomes to establish effectiveness of the treatment. Finally, we will need to push forward with earlier detection and intervention for the best potential of cellular therapy.
Supplementary Material
Contributor Information
Yiping Fan, Department of Reproductive Medicine, KK Women’s and Children’s Hospital, Singapore, Singapore; Experimental Fetal Medicine Group, Department of Obstetrics and Gynaecology, Yong Loo Lin School of Medicine, National University Health System, Singapore, Singapore; Academic Clinical Program in Obstetrics and Gynaecology, Duke-NUS Medical School, Singapore, Singapore.
Eyleen L K Goh, Neuroscience and Mental Health Faculty, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore.
Jerry Kok Yen Chan, Department of Reproductive Medicine, KK Women’s and Children’s Hospital, Singapore, Singapore; Experimental Fetal Medicine Group, Department of Obstetrics and Gynaecology, Yong Loo Lin School of Medicine, National University Health System, Singapore, Singapore; Academic Clinical Program in Obstetrics and Gynaecology, Duke-NUS Medical School, Singapore, Singapore.
Acknowledgments
The graphical abstract was created with BioRender.com.
Funding
JKYC is supported by Singapore’s Ministry of Health (MOH-001221-01, MOH-000932-00,) and National Medical Research Council (CIRG/1484/2018, NMRC CSA (SI)/008/2016, NMRC STaR22jul-0004).
Conflict of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
YP: conceptualizing and design of the paper, data acquisition, analysis of data and drafting the manuscript, EG and JC: critical review of the manuscript. All authors: final approval of manuscript.
Data Availability
All data are incorporated into the article and its online supplementary material.
References
- 1. Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433-1438. 10.1126/science.287.5457.1433 [DOI] [PubMed] [Google Scholar]
- 2. Ray J, Peterson DA, Schinstine M, Gage FH.. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci USA. 1993;90(8):3602-3606. 10.1073/pnas.90.8.3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Temple S. The development of neural stem cells. Nature. 2001;414(6859):112-117. 10.1038/35102174 [DOI] [PubMed] [Google Scholar]
- 4. Reynolds BA, Weiss S.. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707-1710. 10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- 5. Fan Y, Marcy G, Lee ES, et al. Regionally-specified second trimester fetal neural stem cells reveals differential neurogenic programming. PLoS One. 2014;9(9):e105985. 10.1371/journal.pone.0105985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kovacs GG. Concepts and classification of neurodegenerative diseases. Handb Clin Neurol. 2017;145:301-307. 10.1016/B978-0-12-802395-2.00021-3 [DOI] [PubMed] [Google Scholar]
- 7. Bachoud-Levi AC, Gaura V, Brugieres P, et al. Effect of fetal neural transplants in patients with Huntington’s disease 6 years after surgery: a long-term follow-up study. Lancet Neurol. 2006;5(4):303-309. 10.1016/S1474-4422(06)70381-7 [DOI] [PubMed] [Google Scholar]
- 8. Cicchetti F, Saporta S, Hauser RA, et al. Neural transplants in patients with Huntington’s disease undergo disease-like neuronal degeneration. Proc Natl Acad Sci USA. 2009;106(30):12483-12488. 10.1073/pnas.0904239106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keene CD, Chang RC, Leverenz JB, et al. A patient with Huntington’s disease and long-surviving fetal neural transplants that developed mass lesions. Acta Neuropathol. 2009;117(3):329-338. 10.1007/s00401-008-0465-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fink KD, Deng P, Torrest A, et al. Developing stem cell therapies for juvenile and adult-onset Huntington’s disease. Regen Med. 2015;10(5):623-646. 10.2217/rme.15.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen KS, Sakowski SA, Feldman EL.. Intraspinal stem cell transplantation for amyotrophic lateral sclerosis. Ann Neurol. 2016;79(3):342-353. 10.1002/ana.24584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finberg JP, Rabey JM.. Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology. Front Pharmacol. 2016;7:340. 10.3389/fphar.2016.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo X, Tang L, Tang X.. Current developments in cell replacement therapy for Parkinson’s disease. Neuroscience. 2021;463:370-382. 10.1016/j.neuroscience.2021.03.022 [DOI] [PubMed] [Google Scholar]
- 14. Jang SE, Qiu L, Chan LL, Tan E-K, Zeng Li.. Current status of stem cell-derived therapies for Parkinson’s disease: from cell assessment and imaging modalities to clinical trials. Front Neurosci. 2020;14:558532. 10.3389/fnins.2020.558532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindvall O, Rehncrona S, Gustavii B, et al. Fetal dopamine-rich mesencephalic grafts in Parkinson’s disease. Lancet. 1988;2(8626-8627):1483-1484. 10.1016/s0140-6736(88)90950-6 [DOI] [PubMed] [Google Scholar]
- 16. Madrazo I, Leon V, Torres C, et al. Transplantation of fetal substantia nigra and adrenal medulla to the caudate nucleus in two patients with Parkinson’s disease. N Engl J Med. 1988;318(1):51. 10.1056/NEJM198801073180115 [DOI] [PubMed] [Google Scholar]
- 17. Freed CR, Breeze RE, Rosenberg NL, et al. Transplantation of human fetal dopamine cells for Parkinson’s disease. Results at 1 year. Arch Neurol. 1990;47(5):505-512. 10.1001/archneur.1990.00530050021007 [DOI] [PubMed] [Google Scholar]
- 18. Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710-719. 10.1056/NEJM200103083441002 [DOI] [PubMed] [Google Scholar]
- 19. Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54(3):403-414. 10.1002/ana.10720 [DOI] [PubMed] [Google Scholar]
- 20. Olanow CW, Gracies JM, Goetz CG, et al. Clinical pattern and risk factors for dyskinesias following fetal nigral transplantation in Parkinson’s disease: a double blind video-based analysis. Mov Disord. 2009;24(3):336-343. 10.1002/mds.22208 [DOI] [PubMed] [Google Scholar]
- 21. Carlsson T, Winkler C, Lundblad M, et al. Graft placement and uneven pattern of reinnervation in the striatum is important for development of graft-induced dyskinesia. Neurobiol Dis. 2006;21(3):657-668. 10.1016/j.nbd.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 22. Politis M, Wu K, Loane C, et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med. 2010;2(38):38ra46. 10.1126/scitranslmed.3000976 [DOI] [PubMed] [Google Scholar]
- 23. Han F, Hu B.. Stem cell therapy for Parkinson’s disease. Adv Exp Med Biol. 2020;1266:21-38. 10.1007/978-981-15-4370-8_3 [DOI] [PubMed] [Google Scholar]
- 24. Urrutia DN, Caviedes P, Mardones R, et al. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS One. 2019;14(3):e0213032. 10.1371/journal.pone.0213032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harris VK, Stark J, Vyshkina T, et al. Phase I trial of intrathecal mesenchymal stem cell-derived neural progenitors in progressive multiple sclerosis. EBioMedicine. 2018;29:23-30. 10.1016/j.ebiom.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Horne CG, Quintero JE, Slevin JT, et al. Peripheral nerve grafts implanted into the substantia nigra in patients with Parkinson’s disease during deep brain stimulation surgery: 1-year follow-up study of safety, feasibility, and clinical outcome. J Neurosurg. 2018;129(6):1550-1561. 10.3171/2017.8.JNS163222 [DOI] [PubMed] [Google Scholar]
- 27. Quintero JE, Slevin JT, Gurwell JA, et al. Direct delivery of an investigational cell therapy in patients with Parkinson’s disease: an interim analysis of feasibility and safety of an open-label study using DBS-Plus clinical trial design. BMJ Neurol Open. 2022;4(2):e000301. 10.1136/bmjno-2022-000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mao Z, Zhang S, Chen H.. Stem cell therapy for amyotrophic lateral sclerosis. Cell Regen. 2015;4:11. 10.1186/s13619-015-0026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sykova E, Rychmach P, Drahoradova I, et al. Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transplant. 2017;26(4):647-658. 10.3727/096368916X693716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pandya RS, Mao LL, Zhou EW, et al. Neuroprotection for amyotrophic lateral sclerosis: role of stem cells, growth factors, and gene therapy. Cent Nerv Syst Agents Med Chem. 2012;12(1):15-27. 10.2174/187152412800229152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lunn JS, Sakowski SA, Kim B, Rosenberg AA, Feldman Eva L.. Vascular endothelial growth factor prevents G93A-SOD1-induced motor neuron degeneration. Dev Neurobiol. 2009;69(13):871-884. 10.1002/dneu.20747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu L, Yan J, Chen D, et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82(7):865-875. 10.1097/01.tp.0000235532.00920.7a [DOI] [PubMed] [Google Scholar]
- 33. Lepore AC, Rauck B, Dejea C, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11(11):1294-1301. 10.1038/nn.2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hefferan MP, Galik J, Kakinohana O, et al. Human neural stem cell replacement therapy for amyotrophic lateral sclerosis by spinal transplantation. PLoS One. 2012;7(8):e42614. 10.1371/journal.pone.0042614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glass JD, Boulis NM, Johe K, et al. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;30(6):1144-1151. 10.1002/stem.1079 [DOI] [PubMed] [Google Scholar]
- 36. Riley J, Glass J, Feldman EL, et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I trial, cervical microinjection, and final surgical safety outcomes. Neurosurgery. 2014;74(1):77-87. 10.1227/NEU.0000000000000156 [DOI] [PubMed] [Google Scholar]
- 37. Feldman EL, Boulis NM, Hur J, et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75(3):363-373. 10.1002/ana.24113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Appel SH, Engelhardt JI, Henkel JS, et al. Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology. 2008;71(17):1326-1334. 10.1212/01.wnl.0000327668.43541.22 [DOI] [PubMed] [Google Scholar]
- 39. Cashman N, Tan LY, Krieger C, et al. Pilot study of granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells in amyotrophic lateral sclerosis (ALS). Muscle Nerve. 2008;37(5):620-625. 10.1002/mus.20951 [DOI] [PubMed] [Google Scholar]
- 40. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187-1194. 10.1001/archneurol.2010.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prabhakar S, Marwaha N, Lal V, et al. Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis: a pilot study. Neurol India. 2012;60(5):465-469. 10.4103/0028-3886.103185 [DOI] [PubMed] [Google Scholar]
- 42. Baek W, Kim YsFau - Koh SH, Koh ShFau - Lim SWet al. . Stem cell transplantation into the intraventricular space via an Ommaya reservoir in a patient with amyotrophic lateral sclerosis. (0390-5616 (Print)). [PubMed]
- 43. Mazzini L, Mareschi K, Ferrero I, et al. Autologous mesenchymal stem cells: clinical applications in amyotrophic lateral sclerosis. Neurol Res. 2006;28(5):523-526. 10.1179/016164106X116791 [DOI] [PubMed] [Google Scholar]
- 44. Mazzini L, Mareschi K, Ferrero I, et al. Stem cell treatment in amyotrophic lateral sclerosis. J Neurol Sci. 2008;265(1-2):78-83. 10.1016/j.jns.2007.05.016 [DOI] [PubMed] [Google Scholar]
- 45. Mazzini L, Mareschi K, Ferrero I, et al. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy. 2012;14(1):56-60. 10.3109/14653249.2011.613929 [DOI] [PubMed] [Google Scholar]
- 46. Mazzini L, Ferrero I, Luparello V, et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp Neurol. 2010;223(1):229-237. 10.1016/j.expneurol.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 47. Chen L, Chen D, Xi H, et al. Olfactory ensheathing cell neurorestorotherapy for amyotrophic lateral sclerosis patients: benefits from multiple transplantations. Cell Transplant. 2012;21(Suppl 1):S65-S77. 10.3727/096368912X633789 [DOI] [PubMed] [Google Scholar]
- 48. Mazzini L, Gelati M, Profico DC, et al. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med. 2015;13:17. 10.1186/s12967-014-0371-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mazzini L, Gelati M, Profico DC, et al. ; ALS-NSCs Trial Study Group. Results from phase I clinical trial with intraspinal injection of neural stem cells in amyotrophic lateral sclerosis: a long-term outcome. Stem Cells Transl Med. 2019;8(9):887-897. 10.1002/sctm.18-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Balfour H. AstroRx® shows promise in early phase amyotrophic lateral sclerosis trial. Available at https://doi.org/https://www.europeanpharmaceuticalreview.com/news/137141/astrorx-shows-promise-in-early-phase-amyotrophic-lateral-sclerosis-trial/. Published 2020. Accessed 27 September, 2022.
- 51. Baloh RH, Johnson JP, Avalos P, et al. Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: a phase 1/2a trial. Nat Med. 2022;28(9):1813-1822. 10.1038/s41591-022-01956-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sykova E, Cizkova D, Kubinova S.. Mesenchymal stem cells in treatment of spinal cord injury and amyotrophic lateral sclerosis. Front Cell Dev Biol. 2021;9:695900. 10.3389/fcell.2021.695900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Comi G, Radaelli M, Soelberg Sorensen P.. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet. 2017;389(10076):1347-1356. 10.1016/S0140-6736(16)32388-1 [DOI] [PubMed] [Google Scholar]
- 54. Kieseier BC, Hartung HP.. Current disease-modifying therapies in multiple sclerosis. Semin Neurol. 2003;23(2):133-146. 10.1055/s-2003-41138 [DOI] [PubMed] [Google Scholar]
- 55. Johnson KP. Natalizumab (Tysabri) treatment for relapsing multiple sclerosis. Neurologist. 2007;13(4):182-187. 10.1097/01.nrl.0000263760.53418.5b [DOI] [PubMed] [Google Scholar]
- 56. Jeffery DR, Markowitz CE, Reder AT, Weinstock-Guttman B, Tobias K.. Fingolimod for the treatment of relapsing multiple sclerosis. Expert Rev Neurother. 2011;11(2):165-183. 10.1586/ern.10.193 [DOI] [PubMed] [Google Scholar]
- 57. Fox E, Wynn D, Cohan S, et al. A randomized clinical trial of autologous T-cell therapy in multiple sclerosis: subset analysis and implications for trial design. Mult Scler. 2012;18(6):843-852. 10.1177/1352458511428462 [DOI] [PubMed] [Google Scholar]
- 58. Ontaneda D, Thompson AJ, Fox RJ, Cohen JA.. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389(10076):1357-1366. 10.1016/S0140-6736(16)31320-4 [DOI] [PubMed] [Google Scholar]
- 59. Harris VK, Faroqui R, Vyshkina T, Sadiq SA.. Characterization of autologous mesenchymal stem cell-derived neural progenitors as a feasible source of stem cells for central nervous system applications in multiple sclerosis. Stem Cells Transl Med. 2012;1(7):536-547. 10.5966/sctm.2012-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harris VK, Yan QJ, Vyshkina T, et al. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J Neurol Sci. 2012;313(1-2):167-177. 10.1016/j.jns.2011.08.036 [DOI] [PubMed] [Google Scholar]
- 61. Fu L, Zhu L, Huang Y, et al. Derivation of neural stem cells from mesenchymal stemcells: evidence for a bipotential stem cell population. Stem Cells Dev. 2008;17(6):1109-1121. 10.1089/scd.2008.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hermann A, Gastl R, Liebau S, et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117(Pt 19):4411-4422. 10.1242/jcs.01307 [DOI] [PubMed] [Google Scholar]
- 63. Mareschi K, Novara M, Rustichelli D, et al. Neural differentiation of human mesenchymal stem cells: evidence for expression of neural markers and eag K+ channel types. Exp Hematol. 2006;34(11):1563-1572. 10.1016/j.exphem.2006.06.020 [DOI] [PubMed] [Google Scholar]
- 64. Grigoriadis N, Lourbopoulos A, Lagoudaki R, et al. Variable behavior and complications of autologous bone marrow mesenchymal stem cells transplanted in experimental autoimmune encephalomyelitis. Exp Neurol. 2011;230(1):78-89. 10.1016/j.expneurol.2011.02.021 [DOI] [PubMed] [Google Scholar]
- 65. Cristofanilli M, Harris VK, Zigelbaum A, et al. Mesenchymal stem cells enhance the engraftment and myelinating ability of allogeneic oligodendrocyte progenitors in dysmyelinated mice. Stem Cells Dev. 2011;20(12):2065-2076. 10.1089/scd.2010.0547 [DOI] [PubMed] [Google Scholar]
- 66. Mohyeddin Bonab M, Yazdanbakhsh S Fau - Lotfi J, Lotfi J Fau - Alimoghaddom K, et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007;4(1735-1383 (Print):50-57. [PubMed] [Google Scholar]
- 67. Yamout B, Hourani R, Salti H, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010;227(1-2):185-189. 10.1016/j.jneuroim.2010.07.013 [DOI] [PubMed] [Google Scholar]
- 68. Connick P, Kolappan M, Patani R, et al. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials. 2011;12:62. 10.1186/1745-6215-12-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bonab MM, Sahraian MA, Aghsaie A, et al. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr Stem Cell Res Ther. 2012;7(6):407-414. 10.2174/157488812804484648 [DOI] [PubMed] [Google Scholar]
- 70. Helman G, Van Haren K, Bonkowsky JL, et al. ; GLIA Consortium. Disease specific therapies in leukodystrophies and leukoencephalopathies. Mol Genet Metab. 2015;114(4):527-536. 10.1016/j.ymgme.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gupta N, Henry RG, Kang SM, et al. Long-term safety, immunologic response, and imaging outcomes following neural stem cell transplantation for Pelizaeus-Merzbacher disease. Stem Cell Rep. 2019;13(2):254-261. 10.1016/j.stemcr.2019.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gupta N, Henry RG, Strober J, et al. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. 2012;4(155):155ra137. 10.1126/scitranslmed.3004373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pouwels PJ, Vanderver A, Bernard G, et al. Hypomyelinating leukodystrophies: translational research progress and prospects. Ann Neurol. 2014;76(1):5-19. 10.1002/ana.24194 [DOI] [PubMed] [Google Scholar]
- 74. Schneider A, Montague P, Griffiths I, et al. Uncoupling of hypomyelination and glial cell death by a mutation in the proteolipid protein gene. Nature. 1992;358(6389):758-761. 10.1038/358758a0 [DOI] [PubMed] [Google Scholar]
- 75. Kruer MC, Pearce DA, Orchard PJ, Steiner RD.. Prospects for stem cell therapy in neuronal ceroid lipofuscinosis. Regen Med. 2013;8(5):527-529. 10.2217/rme.13.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Selden NR, Al-Uzri A, Huhn SL, et al. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. J Neurosurg Pediatr. 2013;11(6):643-652. 10.3171/2013.3.PEDS12397 [DOI] [PubMed] [Google Scholar]
- 77. Kurtzberg J, Buntz S, Gentry T, et al. Preclinical characterization of DUOC-01, a cell therapy product derived from banked umbilical cord blood for use as an adjuvant to umbilical cord blood transplantation for treatment of inherited metabolic diseases. Cytotherapy. 2015;17(6):803-815. 10.1016/j.jcyt.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brondon J, Page K, Polishchuk V, et al. Outcomes of umbilical cord blood transplantation in children with batten disease. Stem Cells Transl Med. 2019;8:S12. 10.1002/sctm.12557 [DOI] [Google Scholar]
- 79. Miller WP, Rothman SM, Nascene D, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. 2011;118(7):1971-1978. 10.1182/blood-2011-01-329235 [DOI] [PubMed] [Google Scholar]
- 80. Precious SV, Zietlow R, Dunnett SB, Kelly CM, Rosser AE.. Is there a place for human fetal-derived stem cells for cell replacement therapy in Huntington’s disease? Neurochem Int. 2017;106:114-121. 10.1016/j.neuint.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bachoud-Levi AC, Remy P, Nguyen JP, et al. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet. 2000;356(9246):1975-1979. 10.1016/s0140-6736(00)03310-9 [DOI] [PubMed] [Google Scholar]
- 82. Kim A, Lalonde K, Truesdell A, et al. New avenues for the treatment of Huntington’s disease. Int J Mol Sci. 2021;22(16):8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mehat MS, Sundaram V, Ripamonti C, et al. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells in macular degeneration. Ophthalmology. 2018;125(11):1765-1775. 10.1016/j.ophtha.2018.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 85. Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236-246. 10.1038/ng0397-236 [DOI] [PubMed] [Google Scholar]
- 86. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509-516. 10.1016/S0140-6736(14)61376-3 [DOI] [PubMed] [Google Scholar]
- 87. Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713-720. 10.1016/S0140-6736(12)60028-2 [DOI] [PubMed] [Google Scholar]
- 88. Kashani AH, Lebkowski JS, Rahhal FM, et al. One-year follow-up in a phase 1/2a clinical trial of an allogeneic rpe cell bioengineered implant for advanced dry age-related macular degeneration. Transl Vis Sci Technol. 2021;10(10):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mandai M, Kurimoto Y, Takahashi M.. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;377(8):792-793. 10.1056/NEJMc1706274 [DOI] [PubMed] [Google Scholar]
- 90. Hartong DT, Berson EL, Dryja TP.. Retinitis pigmentosa. Lancet. 2006;368(9549):1795-1809. 10.1016/S0140-6736(06)69740-7 [DOI] [PubMed] [Google Scholar]
- 91. Liu Y, Chen SJ, Li SY, et al. Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Res Ther. 2017;8(1):209. 10.1186/s13287-017-0661-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Beathard R. Safety and Efficacy of Intravitreal Injection of Human Retinal Progenitor Cells in Adults With Retinitis Pigmentosa. Available at https://doi.org/https://clinicaltrials.gov/ct2/show/results/NCT03073733. Published 2022. Accessed 12 October, 2022.
- 93. Cotrim CC, Jorge R, Oliveira MC, et al. Clinical studies using stem cells for treatment of retinal diseases: state of the art. Arquivos Brasileiros de Oftalmologia. 2020;83(2):160-167. [DOI] [PubMed] [Google Scholar]
- 94. Martinez B, Peplow PV.. Biomaterial and tissue-engineering strategies for the treatment of brain neurodegeneration. Neural Regen Res. 2022;17(10):2108-2116. 10.4103/1673-5374.336132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chong YK, Toh TB, Zaiden N, et al. Cryopreservation of neurospheres derived from human glioblastoma multiforme. Stem Cells. 2009;27(1):29-39. 10.1634/stemcells.2008-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fan Y, Tong ZP, Goh EL, et al. Regionally-derived second-trimester primary hfNSCs have different neurogenic capacity for neuronal differentiation. J Stem Cell Res Ther. 2019;9(5):1000451. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online supplementary material.